Abstract
Passive force enhancement is defined as the increase in passive, steady-state, isometric force of an actively stretched muscle compared with the same muscle stretched passively to that same length. Passive force enhancement is long lasting, increases with increasing muscle length and increasing stretch magnitudes, contributes to the residual force enhancement in skeletal and cardiac muscle, and is typically only observed at muscle lengths at which passive forces occur naturally. Passive force enhancement is typically equal to or smaller than the total residual force enhancement, it persists when a muscle is deactivated and reactivated, but can be abolished instantaneously when a muscle is shortened quickly from its stretched length. There is strong evidence that the passive force enhancement is caused by the filamentous sarcomeric protein titin, although the detailed molecular mechanisms underlying passive force enhancement remain unknown. Here I propose a tentative mechanism based on experimental evidence that associates passive force enhancement with the shortening of titin’s free spring length in the I-band region of sarcomeres. I suggest that this shortening is accomplished by titin binding to actin and that the trigger for titin-actin interactions is associated with the formation of strongly bound cross bridges between actin and myosin that exposes actin attachment sites for titin through movement of the regulatory proteins troponin and tropomyosin.
Keywords: cross bridge theory, residual force enhancement, sarcomere nonuniformity, sliding filament theory, titin
INTRODUCTION/BACKGROUND
Striated muscles are thought to have three basic mechanical properties: the force-length relationship (e.g., 15), the force-velocity relationship (e.g., 28), and the history-dependent properties of residual force enhancement and force depression (e.g., 1). These properties are typically considered to be associated, in one way or another, with the active force production of the cross bridges. However, passive properties play a significant role in the force-length relationship of skeletal and cardiac muscles, and passive properties have also received renewed attention for their potential role in the force enhancement property (23, 25).
In this brief opinion paper, I focus on the passive force enhancement in striated muscle. However, before doing that, it is essential to define and understand the residual force enhancement property for appropriate context. Residual force enhancement is defined as the increase in active, steady-state, isometric force of a muscle following active stretching (eccentric action) compared with the corresponding (same length and same activation) active, steady-state, isometric force of a muscle whose length was not changed (Fig. 1).
Fig. 1.
Example of force as a function of time for 3 different contractile conditions for a cat soleus muscle (35°C). Bottom trace (at 4 s) is a passive stretch of the soleus, where stretching occurs between ~2.5 and 3.5 s. Middle trace (at 4 s) represents an isometric contraction at the stretched length. Finally, top trace (at 4 s) represents stretching of the activated muscle in the same manner as the passive stretch (same magnitude and same speed of stretching). FE indicates the residual force enhancement, which is the increase in steady-state isometric force after active muscle stretching compared with the corresponding (same length, same activation) isometric force for a purely isometric contraction. PFE indicates the passive force enhancement, which corresponds to the increase in steady-state passive force following stretching of an activated muscle compared with the same stretching of the passive muscle.
Residual force enhancement was first studied systematically by Abbott and Aubert (1952) (1) in isolated frog, toad, and dogfish muscles. Their results were then reproduced and confirmed for all structural levels of muscle including voluntary and electrically activated human skeletal muscles (e.g., 17, 47, 69), isolated muscle fibers (e.g., 10, 45), single myofibrils (e.g., 37, 49, 65), and single sarcomeres (e.g., 48).
Residual force enhancement has been shown to increase with increasing magnitudes of stretching but is largely independent of the speed of stretching (e.g., 6, 10, 30). Furthermore, force enhancement is long lasting (minutes in skinned fibers and single myofibrils) but can be abolished instantaneously when a muscle is deactivated and active force is allowed to drop to zero (e.g., 1, 60). Finally, force enhancement is associated with a decrease in metabolic cost (per unit of force produced) compared with the cost of force for a purely isometric contraction (36) but is not associated with a change in muscle stiffness (e.g., 72), indicating that force enhancement is not achieved by a greater proportion of attached cross bridges.
Several molecular and subcellular mechanisms have been proposed for explaining the residual force enhancement properties of skeletal muscles. For a detailed review, the interested reader is referred to Herzog (2018) (27) and Herzog et al. (2016) (26). However, two mechanisms have received more attention than the others. The first of these, the so-called sarcomere length nonuniformity theory (e.g., 59, 60), explains residual force enhancement within the boundaries of the two-filament cross bridge theory (31, 33, 34, 66). Briefly, according to the sarcomere length nonuniformity theory, residual force enhancement is caused by the development of vast sarcomere length nonuniformities when a muscle is actively stretched on the descending limb of the force-length relationship (29, 58). However, many primary predictions of this theory (e.g., force enhancement cannot occur on the ascending part of the force-length relationship, force enhancement cannot exceed the plateau force of the force-length relationship, force enhancement cannot occur in a single sarcomere or half-sarcomere, isometric reference forces occur with essentially uniform sarcomere lengths, and sarcomere length nonuniformity increases with active muscle stretching) have been shown to be violated (e.g., 32, 35, 45, 48, 56, 57, 63). Therefore, it appears untenable to support the sarcomere length nonuniformity theory as the mechanism explaining the residual force enhancement property.
The second mechanism that has been proposed for explaining the residual force enhancement property of striated muscles is associated with the engagement of a passive structural element. Proponents of this mechanism have argued that residual force enhancement can be explained readily with the “engagement” of a passive structural muscle element upon muscle activation (11, 12, 62). How this engagement might occur, or what structures may be involved, had not been identified for a long time. As a consequence of this engagement, forces arising from this passive structure were thought to be greater when a muscle was stretched actively compared with when it was stretched passively.
One of the first scientists to mention such a possibility was Paul Edman, who argued that the dependence of force enhancement on stretch magnitude and independence from stretch speed could readily be explained with the engagement of a parallel elastic element upon activation (10). However, Edman’s own experiments on shortening-stretch cycles performed in single, intact frog fibers did not support the existence of such a passive mechanism (10). Noble (62) was among the first to suggest that the passive structural muscle component that could cause residual force enhancement was the sarcomeric, filamentous, spring protein titin. Noble realized that titin was perfectly placed in the sarcomere to fulfill such a role, but direct evidence of an “engagement of titin upon activation” was lacking at the time. Theoretical modeling before the experimental discovery of titin’s role in residual force enhancement revealed that, indeed, the engagement of a parallel elastic element upon muscle activation could readily explain many of the basic features of residual force enhancement and force depression (12). However, there was no direct evidence of the involvement of a passive element contributing to force enhancement until we discovered, rather serendipitously, what is now referred to as the “passive force enhancement” (23).
PASSIVE FORCE ENHANCEMENT
Discovery
Passive force enhancement was first discovered and described in detail for the cat soleus muscle (23). We performed experiments aimed at quantifying the amount of force enhancement at different muscle lengths, including long muscle lengths at which the muscle produced passive forces naturally. When stretching the soleus muscle actively to lengths of naturally occurring passive forces, we observed that after deactivation these actively stretched muscles had a greater passive force than the muscles that were stretched passively to that same lengths or muscles that were activated isometrically at the long lengths (Fig. 1). I remember vividly that we thought we had made a mistake and pulled the muscle to a length greater than the isometric reference length, as we could not explain otherwise why the passive force was systematically greater after active compared with passive stretching of a muscle. However, once such an error had been eliminated, we studied this increased passive force following active stretching carefully and concluded that it was part of the total force enhancement observed in these tests (24, 25, 38, 39, 46). We called this newly discovered property of muscle “passive force enhancement,” and the name has been adopted universally by the scientific community. After publication of our 2002 results, we searched for evidence of this property and found that it had been observed many times before (e.g., 60) but had been ignored, probably because it was “just” passive force that occurred after the “real” experiment was long done. Through the discovery of the passive force enhancement, I learned that it is crucial in science to have an open mind and be ready to pay attention to unexpected results, even if they may not be a part of the objectives you were pursuing with your experiments. Serendipity and chance favor the prepared mind.
Definition
We defined passive force enhancement as the increase in steady-state passive force observed after stretching of an active muscle and subsequent deactivation, compared with the corresponding (same length) passive force observed after stretching of a passive muscle (Fig. 1) (23).
Properties
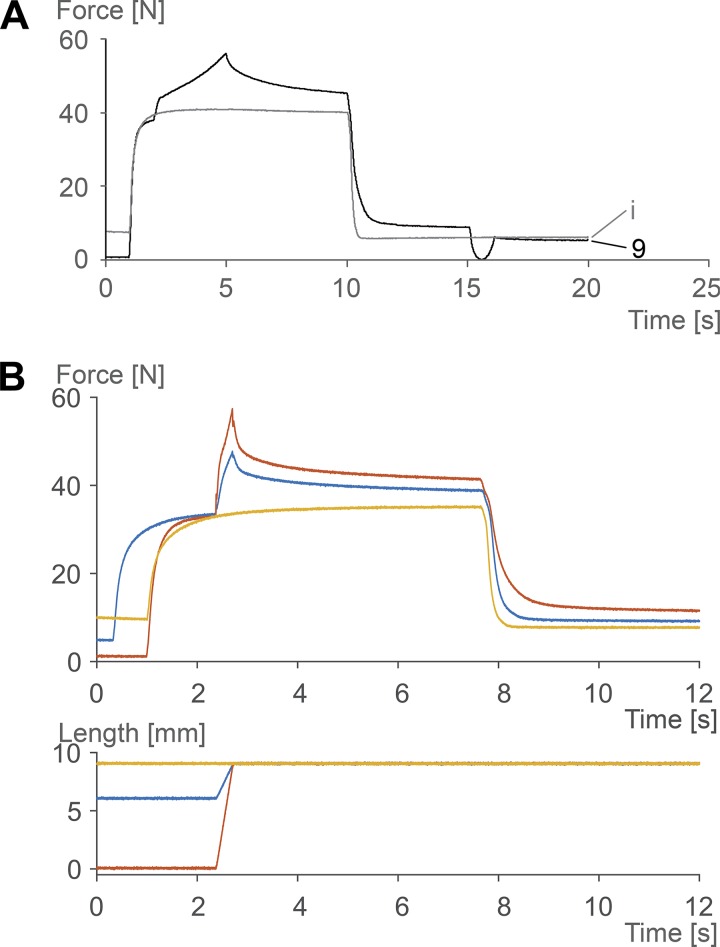
Passive force enhancement has been shown to occur primarily at muscle lengths where passive forces occur naturally (23), has been shown to be long lasting, but can be abolished instantaneously when a muscle is shortened to its initial (prestretch) length and pulled back to its final (poststretch) length (25) (Fig. 2A). Passive force enhancement increases with increasing stretch magnitudes (Fig. 2B), is typically equal to or smaller than the total residual force enhancement (24), suggesting that it is a component, but not the exclusive cause, of the residual force enhancement, and increases with the amount of force during stretch (24). Passive force enhancement has been observed on all structural levels of muscles, including intact human muscles (e.g., 47), isolated muscle preparations activated through electrical stimulation (23, 25), single intact and skinned fibers (e.g., 46), single myofibrils (38, 39, 64), and single sarcomeres and half-sarcomeres (48).
Fig. 2.
Force-time and muscle length change-time histories of cat soleus muscle for a variety of experimental conditions (at 35°C). A: passive force enhancement is long lasting but can be abolished instantaneously by a shortening-stretch cycle (at ~15 s) that brings the muscle to its prestretch length in the shortening phase and back to its original stretched length in the stretch phase. i refers to the isometric reference contraction; 9 refers to the muscle that was stretched by 9 mm. B: passive force enhancement (like the total residual force enhancement) increases with increasing stretch magnitudes. The 9-mm stretch of the activated soleus muscle produces more force enhancement and passive force enhancement (difference between yellow and red lines at 12 s) than the 3-mm stretch (difference between yellow and blue lines).
The discovery that passive force enhancement occurs in single myofibrils and isolated sarcomeres was important insofar as passive forces in these structures originate virtually exclusively from the structural protein titin (4, 38, 49, 51, 54). Therefore, titin (Fig. 3) became the main target for explaining passive force enhancement properties. There are two primary mechanisms that have been put forward in the literature for how titin might cause passive force enhancement following active muscle stretching. The first of these mechanisms is associated with an activation-induced stiffening of the titin molecule and the second with a shortening of the free spring length of titin and corresponding increase in its stiffness. These mechanisms are discussed briefly below, and the interested reader is referred to recent reviews for greater detail (20).
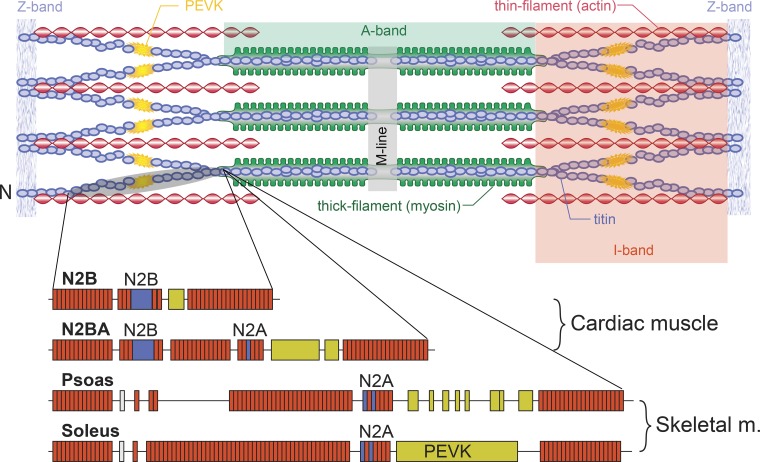
Fig. 3.
Schematic illustration of a sarcomere with Z band, actin, myosin, and titin filaments in their supposed arrangement. Titin runs from the middle of the sarcomere (M line) in either direction toward the Z band. Titin is thought to be firmly attached to myosin and then run freely across the I-band region of the sarcomere until ~50–100 nm away from the Z band, where it is thought to attach to actin and then enter the Z band bound to actin. Below the schematic sarcomere are illustrations of the structure of titin in cardiac muscle and in 2 selected skeletal muscles (psoas and soleus). Note that cardiac titin isoforms are much smaller than those of skeletal muscles and that skeletal muscles have different isoforms that are thought to reflect the passive requirements of the muscle. Specific segments (N2B, N2A, N2BA, and PEVK) are indicated. The brownish rectangles represent immunoglobulin domains. Adapted from Granzier and Labeit (16) with permission from Wolters Kluwer Health, Inc.
Theories
The first mechanism of titin-based passive force enhancement is associated with the idea that titin becomes stiffer upon activation and when stretched actively produces a greater “passive” force compared with when a muscle is stretched passively. These increased titin stiffness and force are then maintained when the muscle is deactivated, thus producing the passive force enhancement. The most obvious way that titin stiffness might be increased upon muscle activation (i.e., the release of calcium from the sarcoplasmic reticulum and the increase in calcium concentration near the contractile filaments actin and myosin) is if one assumes that, upon activation, calcium binds to specific sites on titin, thereby increasing the number or strength of molecular bonds that must be broken when an active muscle (and thus titin) is being stretched. Labeit et al. (44) were the first to provide compelling evidence that calcium activation in single fibers increases titin-based stiffness and force in single molecule and single skinned fiber experiments. Specifically, they showed that calcium activation of skinned fibers, in which actin-myosin interactions were inhibited with 2,3-butanedione monoxime, showed significantly increased titin-based force compared with fibers that were stretched passively (in the absence of calcium). Molecular experiments using the functionally relevant PEVK region of titin (Fig. 3) showed that E-rich motives in this region bound calcium and increased the stiffness of the PEVK segment. These results were confirmed by later experiments in single myofibrils (38, 39) and single sarcomeres (48).
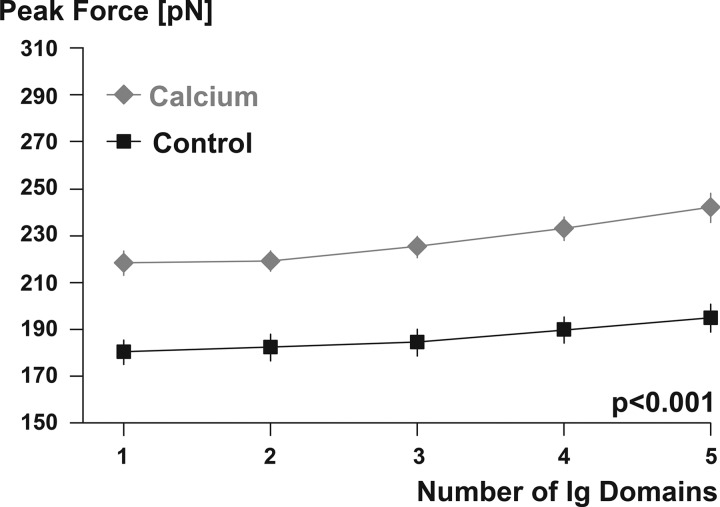
Titin contains a large number of repeat immunoglobulin domains (Fig. 3) that make up a substantial part of this protein’s length and molecular weight. Although the functional role of the immunoglobulin domains of titin is not clear and their physiological significance is widely debated (7, 18, 19, 40, 55, 67), their abundance makes them an interesting target for calcium binding and associated stiffness changes. We used recombinant techniques to obtain eight tandem I27 cardiac titin immunoglobulin domains and determined their capacity for binding calcium with fluorescence spectroscopy (9). Once satisfied that these immunoglobulin domains bind calcium, we tested their unfolding strength and found that in the presence of calcium the force required to unfold and stretch these domains was ~20% greater than in the absence of calcium (Fig. 4) (9). These results suggested that, indeed, immunoglobulin domains can bind calcium upon muscle activation, thereby increasing their unfolding strength and making titin stiffer in the activated state (presence of calcium) compared with the nonactivated, passive state (absence of calcium). However, when calcium was removed from the immunoglobulin environment, calcium bound to the I27 domains was released, and the effect of calcium binding was reversed. Therefore, one would expect that upon deactivation of a muscle, and its associated removal of calcium from the titin environment, the increased titin stiffness achieved upon activation should be transient and reversed upon deactivation.
Fig. 4.
Peak force required to unfold immunoglobulin (Ig) domains in the absence (Control) and presence (Calcium) of calcium, simulating activation of a muscle. Note the substantially increased force required to unfold and stretch immunoglobulin domains in the presence of calcium. Only the first 5 (of 8) immunoglobulin domains are shown, and only results where at least 5 domains unfolded were included in this analysis. Unfolding of 6 or more immunoglobulin domains was rare, and thus the number of observations was deemed inappropriate for statistical testing. Unfolding of 5 of the 8 immunoglobulin domains was observed in >300 individual test specimens for each of the calcium and the control conditions. Adapted from DuVall et al. (9) with permission from Springer Nature.
Calcium has been shown to bind to areas of titin other than just immunoglobulin domains [for example, the glutamate-rich region of the PEVK segment; Labeit et al. 2003 (44)]. However, it has not been shown whether calcium binding to areas other than the I27 domain is reversible. When myofibrils are stretched at low (resting) and at high (active) calcium concentrations (and in the absence of cross-bridge binding achieved by troponin C deletion) differences in titin-based forces persist (39), but when calcium is removed titin-based forces are indistinguishable between the two conditions, suggesting that the effect of calcium binding is reversed not only for titin’s immunoglobulin domains but for all calcium binding sites on titin, with no apparent effect on titin force. Consequently, it appears that calcium binding to titin is not a good candidate for explaining the passive force enhancement in striated muscles. Rather, calcium binding to titin and its associated mechanical implications appear to be a transient phenomenon that exists only in the presence of calcium, that is, when the muscle is activated, but not when the muscle is deactivated (passive).
In summary, these findings of calcium binding to immunoglobulin domains of titin, and corresponding increase in force required to extend these domains, led to the hypothesis that calcium binding to titin contributes to the total residual force enhancement observed in muscles after active stretching but does not contribute to the passive force enhancement observed after deactivation of an actively stretched muscle.
Aside from calcium binding, titin has been found to change its inherent stiffness in a variety of other ways. For example, protein kinase G and A have been found to modulate cardiac passive stiffness by phosphorylation of titin (41, 42). Therefore, it has been suggested that titin is a molecular spring whose stiffness can be changed in a variety of ways and that some of these changes can be produced by muscle activation (20, 22, 26, 68).
The second mechanism by which titin has been thought to contribute to the residual force enhancement property of striated muscle is a shortening of its effective spring length (e.g., 21, 22). Titin runs from the center of the sarcomere along the thick filament to which it is “firmly” bound. It then runs freely across the I-band segment of the sarcomere and inserts into the thin, actin, filament ~50–100 nm away from inserting into the Z band (73, 74). It has been suggested that titin’s free spring length in the I-band region could be shortened readily by further attachment of titin to the “rigid” actin filament, thereby rendering the proximal (bound) titin segments inextensible and just leaving the distal (unbound) segments for elongation (e.g., 27). Titin has been found to bind to actin in vitro (2, 5, 8, 43, 50, 52, 53, 61, 73, 75), thus providing support for this theory. However, direct evidence for titin binding to actin in intact muscles remains elusive.
In recent experiments (9a), we divided titin into separate segments using fluorescently labeled, titin-specific antibodies in rabbit psoas myofibril preparations. We then stretched these myofibrils passively (pCa = 8.5) and actively (pCa = 3.5). During passive stretching from average sarcomere lengths of 1.8 µm to average sarcomere lengths of 4.0 µm, all labeled titin segments elongated as one would expect based on their stiffness properties. However, during active myofibril stretching, proximal segments were only elongated for part of the stretch cycle and then remained at a nearly constant length (Fig. 5). Elongation stopped at sarcomere lengths where the proximal segments had not reached their fully extended length. I interpreted these results as showing that the proximal titin segment had bound to actin and thus elongation of the bound segment stopped while all sarcomere (and associated I band) elongations were taken up exclusively by the free distal segments of titin (Fig. 5). If proximal titin segments indeed bind to actin when an active muscle is stretched, titin’s free spring length that can be used to accommodate sarcomere elongations would be shortened and thus would be stiffer and produce more force upon stretching compared with a passively stretched muscle where the entire titin length is available for accommodating sarcomere length changes.
Fig. 5.
Titin segmental elongations as a function of sarcomere lengths. Titin is divided into a proximal (close to the Z band) and a distal (close to myosin) segment by a fluorescently labeled antibody in the PEVK domain. For passive stretching (not shown), the distal and proximal segments elongate continuously when sarcomeres are stretched and sarcomere length increases. During active stretching shown here, the proximal segment of titin only elongates for part of the sarcomere stretching and then stops elongating. This occurs at a sarcomere length of ~3.0 µm for the particular myofibril shown here as an example from >120 experiments. In other myofibrils, titin proximal segment elongation stopped occurring at sarcomere lengths ranging from 2.2 to 3.4 µm, and this occurrence seemed to depend crucially on the sarcomere lengths at which myofibril activation occurred. When the proximal segment stops elongating, all sarcomere elongation is taken up by the distal segment of titin. I interpret this result as showing that titin binds to actin during active stretching. This binding of titin to actin does not occur through activation (calcium release) but seems to require cross-bridge binding to actin. Therefore, this phenomenon is independent of calcium and is thought to persist when the muscle is deactivated and calcium is sequestered back into the sarcoplasmic reticulum of muscle fibers.
Residual force enhancement and passive force enhancement in single myofibrils activated with calcium while cross-bridge attachment is completely inhibited (for example, with 2,3-butanedione monoxime or troponin C deletion) are negligible (49) or small (38, 39, 64). However, when cross-bridge interactions are allowed, force enhancement and passive force enhancement are substantial, with passive forces that can increase up to a factor of four for certain conditions in actively compared with passively stretched myofibrils (48, 49, 64). These results suggest that residual force enhancement and passive force enhancement do not rely (much) on activation (calcium) but rely in some way on active, cross bridge-based force production. Since calcium binding to titin seems to be transient and reversible (9, 44), and thus is not expected to play a role in passive muscle, passive force enhancement is likely not a calcium (activation)-dependent property. Rather, titin binding to actin (or some other structural sarcomere component), which seems to depend on strongly bound cross-bridge configurations, appears to be a more likely candidate for the passive force enhancement properties in striated muscle.
In light of these findings, I propose the following tentative theory for passive force enhancement (Fig. 6). When a muscle is actively stretched (eccentric muscle action), strong cross-bridge binding to actin results in a configurational change of the regulatory proteins tropomyosin and troponin. This configurational change, in turn, exposes titin attachment sites on actin. Some proximal (toward the Z band) segments of titin (the exact segments need to be determined) bind firmly to actin, thereby leaving only the unbound (distal) titin segments for accomplishing the increases in I-band length with increasing muscle (sarcomere) length. This binding of titin to actin results in increased titin-based forces in the actively stretched muscle. When the muscle is deactivated, titin remains attached to actin, thereby providing a long-lasting increase in passive muscle force: the passive force enhancement observed after deactivation of an actively stretched muscle. This passive force enhancement is typically smaller than the total residual force enhancement, presumably because upon muscle deactivation calcium bound to titin is released and the associated increase in stiffness is lost. However, deactivation of isometric muscles is associated with a lengthening of fibers and sarcomeres, and associated lengthening of titin, which may lead to an overestimation of the passive force enhancement, reaching values equal to or greater than the residual force enhancement (for example in Fig. 1) for some exceptional experimental conditions. Passive force enhancement diminishes slowly and is lost within ~3–4 min (in cat soleus muscles; unpublished observation). However, passive force enhancement can be abolished instantaneously if a muscle is quickly shortened from its stretched position (25), suggesting that there is a mechanical “release mechanism” for titin bound to actin.
Fig. 6.
Proposed mechanism for the passive force enhancement property of skeletal muscle. In the initial condition, the sarcomere is short and in the passive state (A). If stretched passively from that initial configuration, the sarcomere will become longer and all titin segments are stretched and produce a force in accordance with the titin properties (B). If the sarcomere is activated via calcium but cross-bridge binding to actin is inhibited (for example, by 2,3-butanedione monoxime or by troponin C deletion) and then stretched, calcium will bind to titin (indicated by the brownish color in C) but titin will not bind to actin. Titin is stiffer in this configuration because of the bound calcium. If the sarcomere is stretched while activated and cross-bridge forces are allowed to develop (normal activation and force production, eccentric muscle action), titin will bind calcium and will bind to actin (I propose somewhere in the PEVK region), and will produce more force than in C, because of the titin binding to actin that is made possible by the cross-bridge binding to actin (D). When the actively stretched muscle (in which cross bridges were allowed to bind to actin) is now deactivated, calcium is released from titin and titin will remain bound to actin, thereby producing the passive force enhancement observed in skeletal muscles after stretching of an activated muscle (E). If now, in the deactivated state, the muscle is quickly shortened to its initial length and immediately stretched back to its original length (indicated by double arrow in F), titin is released from actin and the passive force enhancement is immediately abolished, while without this quick shortening-stretch cycle, the passive force enhancement would persist for minutes.
SUMMARY
Passive force enhancement is observed after deactivation of an actively stretched (eccentric action) muscle. The origins of the passive force enhancement remain unknown. However, there is compelling evidence that the passive force enhancement is associated with the structural, filamentous protein titin. I propose tentatively that passive force enhancement is not activation (calcium) dependent but depends crucially on force production in eccentric muscle action by actin-myosin-based cross bridges. Specifically, I hypothesize that strong cross-bridge binding results in a configurational change of troponin and tropomyosin that frees up attachment sites for titin on actin. Titin then binds to actin, thereby shortening its free spring length and making itself stiffer, thus increasing titin-based force when a muscle is stretched. Upon deactivation, titin remains bound to actin but can be released immediately, and all passive force enhancement is abolished, when the muscle shortens quickly from its stretched length.
CONCLUDING REMARKS
The hypotheses proposed above about the function of titin in active and passive muscle, and particularly the proposed mechanism for the passive force enhancement property, may appear far-fetched. However, there are mechanisms for passive force production in animal systems that have been shown to work in a way similar to that proposed above for titin in passive force enhancement. For example, in muscles of mollusks there is a titin analog called twitchin. Twitchin is a titinlike protein that has been shown to form connections between actin and myosin in the so-called catch state of these muscles (3, 13, 70, 71). In the catch state, muscles can produce great forces at virtually no metabolic cost and in the absence of calcium (passive muscle) (14). Twitchin attachment between actin and myosin is regulated by phosphorylation, the catch state corresponding to the dephosphorylated twitchin state (14). The idea that a passive structural element engages between actin and myosin when long-lasting and great forces are required is intriguing, because maintaining such forces with cross bridges would be metabolically expensive, whereas attachment and detachment of a structural protein require virtually no metabolic energy. Maybe the passive force enhancement observed in mammalian skeletal muscle is an evolutionary remnant of the role played by twitchin in molluscan muscles.
GRANTS
This work was funded by the Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada), the Killam Foundation, and the Canada Research Chair Programme.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
W.H. conceived and designed research; performed or supervised experiments; analyzed and supervised analysis of data; interpreted results of experiments; prepared figures; drafted manuscript; edited and revised manuscript; and approved final version of manuscript.
REFERENCES
- 1.Abbott BC, Aubert XM. The force exerted by active striated muscle during and after change of length. J Physiol 117: 77–86, 1952. [PMC free article] [PubMed] [Google Scholar]
- 2.Astier C, Raynaud F, Lebart MC, Roustan C, Benyamin Y. Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett 429: 95–98, 1998. doi: 10.1016/S0014-5793(98)00572-9. [DOI] [PubMed] [Google Scholar]
- 3.Avrova SV, Shelud’ko NS, Borovikov YS, Galler S. Twitchin of mollusc smooth muscles can induce “catch”-like properties in human skeletal muscle: support for the assumption that the “catch” state involves twitchin linkages between myofilaments. J Comp Physiol B 179: 945–950, 2009. doi: 10.1007/s00360-009-0375-z. [DOI] [PubMed] [Google Scholar]
- 4.Bartoo ML, Linke WA, Pollack GH. Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Physiol Cell Physiol 273: C266–C276, 1997. doi: 10.1152/ajpcell.1997.273.1.C266. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P, Nagy A, Kengyel A, Szatmári D, Mártonfalvi Z, Huber T, Kellermayer MS. Interaction forces between F-actin and titin PEVK domain measured with optical tweezers. Biophys J 93: 2102–2109, 2007. doi: 10.1529/biophysj.107.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullimore SR, Leonard TR, Rassier DE, Herzog W. History-dependence of isometric muscle force: effect of prior stretch or shortening amplitude. J Biomech 40: 1518–1524, 2007. doi: 10.1016/j.jbiomech.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Yuan G, Winardhi RS, Yao M, Popa I, Fernandez JM, Yan J. Dynamics of equilibrium folding and unfolding transitions of titin immunoglobulin domain under constant forces. J Am Chem Soc 137: 3540–3546, 2015. doi: 10.1021/ja5119368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CS, Bogomolovas J, Gasch A, Hidalgo CG, Labeit S, Granzier HL. Titin-actin interaction: PEVK-actin-based viscosity in a large animal. J Biomed Biotechnol 2011: 310791, 2011. doi: 10.1155/2011/310791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuVall MM, Gifford JL, Amrein M, Herzog W. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J 42: 301–307, 2013. doi: 10.1007/s00249-012-0875-8. [DOI] [PubMed] [Google Scholar]
- 9a.DuVall MM, Jinha A, Schappacher-Tilp G, Leonard TR, Herzog W. Differences in titin segmental elongation between passive and active stretch in skeletal muscle. J Exp Biol 220: 4418–4425, 2017. doi: 10.1242/jeb.160762. [DOI] [PubMed] [Google Scholar]
- 10.Edman KA, Elzinga G, Noble MI. Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol 80: 769–784, 1982. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edman KA, Tsuchiya T. Strain of passive elements during force enhancement by stretch in frog muscle fibres. J Physiol 490: 191–205, 1996. doi: 10.1113/jphysiol.1996.sp021135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forcinito M, Epstein M, Herzog W. Can a rheological muscle model predict force depression/enhancement? J Biomech 31: 1093–1099, 1998. doi: 10.1016/S0021-9290(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 13.Funabara D, Hamamoto C, Yamamoto K, Inoue A, Ueda M, Osawa R, Kanoh S, Hartshorne DJ, Suzuki S, Watabe S. Unphosphorylated twitchin forms a complex with actin and myosin that may contribute to tension maintenance in catch. J Exp Biol 210: 4399–4410, 2007. doi: 10.1242/jeb.008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galler S. Molecular basis of the catch state in molluscan smooth muscles: a catchy challenge. J Muscle Res Cell Motil 29: 73–99, 2008. doi: 10.1007/s10974-008-9149-6. [DOI] [PubMed] [Google Scholar]
- 15.Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol 184: 170–192, 1966. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzier HL, Labeit S. The giant muscle protein titin is an adjustable molecular spring. Exerc Sport Sci Rev 34: 50–53, 2006. doi: 10.1249/00003677-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Hahn D, Seiberl W, Schwirtz A. Force enhancement during and following muscle stretch of maximal voluntarily activated human quadriceps femoris. Eur J Appl Physiol 100: 701–709, 2007. doi: 10.1007/s00421-007-0462-3. [DOI] [PubMed] [Google Scholar]
- 18.Herzog JA, Leonard TR, Jinha A, Herzog W. Titin (visco-) elasticity in skeletal muscle myofibrils. Mol Cell Biomech 11: 1–17, 2014. [PubMed] [Google Scholar]
- 19.Herzog JA, Leonard TR, Jinha A, Herzog W. Are titin properties reflected in single myofibrils? J Biomech 45: 1893–1899, 2012. doi: 10.1016/j.jbiomech.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Herzog W. Mechanisms of enhanced force production in lengthening (eccentric) muscle contractions. J Appl Physiol (1985) 116: 1407–1417, 2014. doi: 10.1152/japplphysiol.00069.2013. [DOI] [PubMed] [Google Scholar]
- 21.Herzog W, Duvall M, Leonard TR. Molecular mechanisms of muscle force regulation: a role for titin? Exerc Sport Sci Rev 40: 50–57, 2012. doi: 10.1097/JES.0b013e31823cd75b. [DOI] [PubMed] [Google Scholar]
- 22.Herzog W, Lee EJ, Rassier DE. Residual force enhancement in skeletal muscle. J Physiol 574: 635–642, 2006. doi: 10.1113/jphysiol.2006.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog W, Leonard TR. Force enhancement following stretching of skeletal muscle: a new mechanism. J Exp Biol 205: 1275–1283, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Herzog W, Leonard TR. The role of passive structures in force enhancement of skeletal muscles following active stretch. J Biomech 38: 409–415, 2005. doi: 10.1016/j.jbiomech.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Herzog W, Schachar R, Leonard TR. Characterization of the passive component of force enhancement following active stretching of skeletal muscle. J Exp Biol 206: 3635–3643, 2003. doi: 10.1242/jeb.00645. [DOI] [PubMed] [Google Scholar]
- 26.Herzog W, Schappacher G, DuVall M, Leonard TR, Herzog JA. Residual force enhancement following eccentric contractions: a new mechanism involving titin. Physiology (Bethesda) 31: 300–312, 2016. doi: 10.1152/physiol.00049.2014. [DOI] [PubMed] [Google Scholar]
- 27.Herzog W. The multiple roles of titin in muscle contraction and force production. Biophys Rev 10: 1187–1199, 2018. doi: 10.1007/s12551-017-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Bio Sci 126: 136–195, 1938. doi: 10.1098/rspb.1938.0050. [DOI] [Google Scholar]
- 29.Hill AV. The mechanics of active muscle. Proc R Soc Lond B Biol Sci 141: 104–117, 1953. doi: 10.1098/rspb.1953.0027. [DOI] [PubMed] [Google Scholar]
- 30.Hisey B, Leonard TR, Herzog W. Does residual force enhancement increase with increasing stretch magnitudes? J Biomech 42: 1488–1492, 2009. doi: 10.1016/j.jbiomech.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 31.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Chem 7: 255–318, 1957. doi: 10.1016/S0096-4174(18)30128-8. [DOI] [PubMed] [Google Scholar]
- 32.Huxley AF, Peachey LD. The maximum length for contraction in vertebrate striated muscle. J Physiol 156: 150–165, 1961. doi: 10.1113/jphysiol.1961.sp006665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature 233: 533–538, 1971. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 34.Huxley HE. The mechanism of muscular contraction. Science 164: 1356–1366, 1969. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 35.Johnston K, Jinha A, Herzog W. The role of sarcomere length non-uniformities in residual force enhancement of skeletal muscle myofibrils. R Soc Open Sci 3: 150657, 2016. doi: 10.1098/rsos.150657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joumaa V, Herzog W. Energy cost of force production is reduced after active stretch in skinned muscle fibres. J Biomech 46: 1135–1139, 2013. doi: 10.1016/j.jbiomech.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Joumaa V, Leonard TR, Herzog W. Residual force enhancement in myofibrils and sarcomeres. Proc Biol Sci 275: 1411–1419, 2008. doi: 10.1098/rspb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joumaa V, Rassier DE, Leonard TR, Herzog W. Passive force enhancement in single myofibrils. Pflugers Arch 455: 367–371, 2007. doi: 10.1007/s00424-007-0287-2. [DOI] [PubMed] [Google Scholar]
- 39.Joumaa V, Rassier DE, Leonard TR, Herzog W. The origin of passive force enhancement in skeletal muscle. Am J Physiol Cell Physiol 294: C74–C78, 2008. doi: 10.1152/ajpcell.00218.2007. [DOI] [PubMed] [Google Scholar]
- 40.Kellermayer MS, Smith SB, Granzier HL, Bustamante C. Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science 276: 1112–1116, 1997. doi: 10.1126/science.276.5315.1112. [DOI] [PubMed] [Google Scholar]
- 41.Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104: 87–94, 2009. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 42.Krüger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil 27: 435–444, 2006. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 43.Kulke M, Fujita-Becker S, Rostkova E, Neagoe C, Labeit D, Manstein DJ, Gautel M, Linke WA. Interaction between PEVK-titin and actin filaments: origin of a viscous force component in cardiac myofibrils. Circ Res 89: 874–881, 2001. doi: 10.1161/hh2201.099453. [DOI] [PubMed] [Google Scholar]
- 44.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100: 13716–13721, 2003. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee EJ, Herzog W. Residual force enhancement exceeds the isometric force at optimal sarcomere length for optimized stretch conditions. J Appl Physiol (1985) 105: 457–462, 2008. doi: 10.1152/japplphysiol.01109.2006. [DOI] [PubMed] [Google Scholar]
- 46.Lee EJ, Joumaa V, Herzog W. New insights into the passive force enhancement in skeletal muscles. J Biomech 40: 719–727, 2007. doi: 10.1016/j.jbiomech.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Lee HD, Herzog W. Force enhancement following muscle stretch of electrically stimulated and voluntarily activated human adductor pollicis. J Physiol 545: 321–330, 2002. doi: 10.1113/jphysiol.2002.018010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leonard TR, DuVall M, Herzog W. Force enhancement following stretch in a single sarcomere. Am J Physiol Cell Physiol 299: C1398–C1401, 2010. doi: 10.1152/ajpcell.00222.2010. [DOI] [PubMed] [Google Scholar]
- 49.Leonard TR, Herzog W. Regulation of muscle force in the absence of actin-myosin-based cross-bridge interaction. Am J Physiol Cell Physiol 299: C14–C20, 2010. doi: 10.1152/ajpcell.00049.2010. [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Jin JP, Granzier HL. The effect of genetically expressed cardiac titin fragments on in vitro actin motility. Biophys J 69: 1508–1518, 1995. doi: 10.1016/S0006-3495(95)80021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linke WA, Granzier H. A spring tale: new facts on titin elasticity. Biophys J 75: 2613–2614, 1998. doi: 10.1016/S0006-3495(98)77706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linke WA, Ivemeyer M, Labeit S, Hinssen H, Rüegg JC, Gautel M. Actin-titin interaction in cardiac myofibrils: probing a physiological role. Biophys J 73: 905–919, 1997. doi: 10.1016/S0006-3495(97)78123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linke WA, Kulke M, Li H, Fujita-Becker S, Neagoe C, Manstein DJ, Gautel M, Fernandez JM. PEVK domain of titin: an entropic spring with actin-binding properties. J Struct Biol 137: 194–205, 2002. doi: 10.1006/jsbi.2002.4468. [DOI] [PubMed] [Google Scholar]
- 54.Linke WA, Popov VI, Pollack GH. Passive and active tension in single cardiac myofibrils. Biophys J 67: 782–792, 1994. doi: 10.1016/S0006-3495(94)80538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Linke WA. Titin gene and protein functions in passive and active muscle. Annu Rev Physiol 80: 389–411, 2018. doi: 10.1146/annurev-physiol-021317-121234. [DOI] [PubMed] [Google Scholar]
- 56.Llewellyn ME, Barretto RP, Delp SL, Schnitzer MJ. Minimally invasive high-speed imaging of sarcomere contractile dynamics in mice and humans. Nature 454: 784–788, 2008. doi: 10.1038/nature07104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moo EK, Fortuna R, Sibole SC, Abusara Z, Herzog W. In vivo sarcomere lengths and sarcomere elongations are not uniform across an intact muscle. Front Physiol 7: 187, 2016. doi: 10.3389/fphys.2016.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J 57: 209–221, 1990. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan DL. An explanation for residual increased tension in striated muscle after stretch during contraction. Exp Physiol 79: 831–838, 1994. doi: 10.1113/expphysiol.1994.sp003811. [DOI] [PubMed] [Google Scholar]
- 60.Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. J Physiol 522: 503–513, 2000. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagy A, Cacciafesta P, Grama L, Kengyel A, Málnási-Csizmadia A, Kellermayer MS. Differential actin binding along the PEVK domain of skeletal muscle titin. J Cell Sci 117: 5781–5789, 2004. doi: 10.1242/jcs.01501. [DOI] [PubMed] [Google Scholar]
- 62.Noble MI. Enhancement of mechanical performance of striated muscle by stretch during contraction. Exp Physiol 77: 539–552, 1992. doi: 10.1113/expphysiol.1992.sp003618. [DOI] [PubMed] [Google Scholar]
- 63.Peterson DR, Rassier DE, Herzog W. Force enhancement in single skeletal muscle fibres on the ascending limb of the force-length relationship. J Exp Biol 207: 2787–2791, 2004. doi: 10.1242/jeb.01095. [DOI] [PubMed] [Google Scholar]
- 64.Powers K, Schappacher-Tilp G, Jinha A, Leonard T, Nishikawa K, Herzog W. Titin force is enhanced in actively stretched skeletal muscle. J Exp Biol 217: 3629–3636, 2014. doi: 10.1242/jeb.105361. [DOI] [PubMed] [Google Scholar]
- 65.Rassier DE, Herzog W, Pollack GH. Stretch-induced force enhancement and stability of skeletal muscle myofibrils. Adv Exp Med Biol 538: 501–515, 2003. doi: 10.1007/978-1-4419-9029-7_45. [DOI] [PubMed] [Google Scholar]
- 66.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science 261: 58–65, 1993. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 67.Rivas-Pardo JA, Eckels EC, Popa I, Kosuri P, Linke WA, Fernández JM. Work done by titin protein folding assists muscle contraction. Cell Rep 14: 1339–1347, 2016. doi: 10.1016/j.celrep.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schappacher-Tilp G, Leonard T, Desch G, Herzog W. A novel three-filament model of force generation in eccentric contraction of skeletal muscles. PLoS One 10: e0117634, 2015. doi: 10.1371/journal.pone.0117634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seiberl W, Hahn D, Herzog W, Schwirtz A. Feedback controlled force enhancement and activation reduction of voluntarily activated quadriceps femoris during sub-maximal muscle action. J Electromyogr Kinesiol 22: 117–123, 2012. doi: 10.1016/j.jelekin.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 70.Shelud’ko NS, Matusovsky OS, Permyakova TV, Matusovskaya GG. “Twitchin-actin linkage hypothesis” for the catch mechanism in molluscan muscles: evidence that twitchin interacts with myosin, myorod, and paramyosin core and affects properties of actomyosin. Arch Biochem Biophys 466: 125–135, 2007. doi: 10.1016/j.abb.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Siegman MJ, Funabara D, Kinoshita S, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a twitchin-related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc Natl Acad Sci USA 95: 5383–5388, 1998. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugi H, Tsuchiya T. Stiffness changes during enhancement and deficit of isometric force by slow length changes in frog skeletal muscle fibres. J Physiol 407: 215–229, 1988. doi: 10.1113/jphysiol.1988.sp017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trombitás K, Granzier H. Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am J Physiol Cell Physiol 273: C662–C670, 1997. doi: 10.1152/ajpcell.1997.273.2.C662. [DOI] [PubMed] [Google Scholar]
- 74.Trombitás K, Pollack GH. Elastic properties of connecting filaments along the sarcomere. Adv Exp Med Biol 332: 71–79, 1993. doi: 10.1007/978-1-4615-2872-2_7. [DOI] [PubMed] [Google Scholar]
- 75.Yamasaki R, Berri M, Wu Y, Trombitás K, McNabb M, Kellermayer MS, Witt C, Labeit D, Labeit S, Greaser M, Granzier H. Titin-actin interaction in mouse myocardium: passive tension modulation and its regulation by calcium/S100A1. Biophys J 81: 2297–2313, 2001. doi: 10.1016/S0006-3495(01)75876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]