Abstract
All mammals must breathe and breathe continuously from birth. Similarly, all mammals, including infants, have high functional demands for feeding. However, the pathway that food takes through the pharynx interrupts respiration. The coordination between swallowing and breathing is therefore critical for all infant mammals. Clinically, this coordination differs between term and preterm infants. However, the neurological mechanisms underlying this coordination and how it matures as infants grow are poorly understood. Here, we integrate high-resolution data from multiple physiologic processes across a longitudinal time frame to study suck-swallow-breathe dynamics in a preterm animal model, the infant pig. In doing so, we test the hypothesis that preterm birth will have an impact on some, but not all, behaviors associated with suck-swallow-breath performance. We hypothesize that coordination will be disrupted, reflecting incomplete connections in the brainstem. We found that preterm pigs became rhythmic and mature in sucking and swallowing behaviors, suggesting substantial postnatal maturation in the coordination of these behaviors. However, their ability to coordinate swallowing and breathing never developed. These results have implications for the nature of clinical care of human infants, as well as for how feeding processes develop in mammals. Clinically, they provide a foundation for developing interventions for preterm infants. Additionally, these results suggest that the lack of coordination between swallowing and breathing may be a significant factor in determining the minimum gestation time across mammals.
NEW & NOTEWORTHY Preterm infants face a variety of challenges associated with safe feeding, but obtaining high-resolution longitudinal data to understand these challenges in humans is challenging. We used a pig model to acquire high-speed videofluoroscopic and respiratory inductance plethysmograph data throughout the nursing period to show that preterm birth does not have substantial impacts on the ability of infants to perform isolated behaviors. However, it does decrease the ability of preterm infants to coordinate among behaviors during feeding.
Keywords: aerodigestive, dysphagia, feeding, neonate, premature
INTRODUCTION
Endothermy in mammals requires near continuous respiration from birth. Because the pathways for air and food cross in the pharynx, swallowing must include brief pauses of respiration to protect the airway. Thus coordination between respiration and swallowing is critical for mammals at all developmental stages. Yet the coordination between these two critical behaviors for survival is often reduced at birth, especially in preterm infants (14, 15, 17, 24). Understanding the mechanisms driving developmental patterns of aerodigestive coordination in preterm infants has physiologic implications for the survival of infants and the clinical care of human infants. Furthermore, testing the mechanisms driving poor aerodigestive coordination in preterm infants has implications for mammalian life-history evolution, as the development of aerodigestive coordination may be one of the primary components determining the minimum time of gestation across mammals.
Our biomechanical understanding of sucking, swallowing, and breathing as distinct behaviors is fairly well developed. Sucking and swallowing are mammal synapomorphies that begin before birth (9, 28), whereas respiration is obligate for mammals within minutes of birth (8). Postnatally, these behaviors are primarily controlled via central pattern generators (CPGs), and within a few hours of birth most mammals have some form of coordination between sucking and swallowing, and swallowing and breathing (27, 30). Yet, how this coordination differs from, as well as matures into, a stable juvenile or adult pattern is not known (26). Clinically, preterm human infants exhibit signs of dysfunction in all three behaviors, as well as decreased coordination among them, although clinical studies are limited both in the temporal resolution of their data and in their ability to follow patients through ontogeny (10, 14, 22, 24).
Here, we used a validated infant pig model (2) to determine the longitudinal changes in sucking, swallowing, and respiratory behaviors, and the coordination among them, in preterm and term infants. We test the null hypothesis that coordination merely requires a certain amount of development to achieve stereotyped levels and that shifting the duration of prenatal versus postnatal development will be irrelevant as a factor in the infant performance throughout nursing (preterm and term infants will perform similarly throughout nursing). We test two alternative possibilities about functional development within infancy before weaning, based on physiologic measurements only possible in an animal model: first, preterm infants will initially differ from term infants in feeding but before weaning will not differ from term infants. In other words, the greater plasticity of the preterm neural system will permit these infants to develop equivalent feeding patterns to term infants at the same age. Second, preterm infants will differ from term infants throughout the weaning period, i.e; that the requirements of obligate respiration after birth will prevent preterm feeding patterns within and between behaviors from reaching term behavioral patterns. To test these predictions, we measured 1) the maturation of individual behaviors (respiratory, sucking, and swallowing rates) as well as 2) how integrated behaviors (the relationships between sucking and swallowing and between swallowing and breathing) change throughout the nursing period using a repeated measures design, comparing performance at 7 and 17 days of age [2–4 mo and 9–12 mo human age (7)]. By simultaneously measuring multiple physiologic processes throughout infancy, we were able to gain insight into the development of neural control in behaviors requiring single CPGs, as well as those requiring coordination between CPGs. Our ultimate goal is to answer questions about the development of feeding and respiration in mammals.
METHODS
All experiments included in this manuscript were approved by Northeast Ohio Medical University (NEOMED) Institutional Animal Care and Use Committee 17-04-071.
Animal housing and care.
Experimental Sus scrofa used in experiments were acquired via Caesarean section either at term (2 litters, n = 6 individuals) or 6–8 days preterm [2 litters, n = 5 individuals, 107–109 days of gestation; human equivalent 30–32 wk gestation (7)] from Yorkshire/Landrace cross sows (Shoup Farms, Wooster, OH). Each litter was spaced ~6 mo apart, and surgical delivery ensured that differences in performance between litters and term/preterm were due to the date of delivery rather than by any effect of vaginal delivery.
Detailed methods for the Caesarean section can be found in Ref. 2, but in short, sows were sedated (Telazol, 10 ml im), placed on a surgical table, and anesthetized with isofluorane before the Caesarean section was performed following standard aseptic technique. Individual neonatal pigs were removed by making an incision in the uterus of the sow. Each umbilical cord was clamped and cut, and the neonate was wrapped in a warm towel. Fluid in the airways was allowed to drain or be removed by suction if necessary. Newborn pigs were placed in a warmed incubator set at 30°C (Dräger Medical Isolette Infant Incubator C2000, Telford, PA), with strong breathers intermixed with those that had slowed breathing to encourage spontaneous ventilation. Neonates were fed colostrum within 2 h of birth, followed by infant pig formula (Solustart Pig Milk Replacement; Land O’ Lakes, Arden Mills, MN) from a nipple bottle (NASCO Farm & Ranch, Fort Atkinson, WI). Infants were monitored 24 h a day for the first week of life, at which point care followed validated and standard care for infant pigs (2, 12, 13, 33).
Data acquisition.
When the pigs were 7 days old, swallowing was assessed using simultaneous highspeed videofluoroscopy with respiration measured through a thoracic inductance plethysmograph band (18, 19). Pigs were fed infant formula mixed with barium to visualize milk through the fluoroscope (GE9400 C-Arm, 85 kV, 4 MA) that digitally recorded images at 100 frames/s using a high-speed camera (XC1M digital camera; XCitex, Cambridge, MA). Video was undistorted and calibrated following standard X-Ray Reconstruction for Moving Morphology (XROMM) protocol (5).
Respiration during feeding sequences was monitored through a respiratory inductance plethysmograph band (Powerlab; ADInstruments, Colorado Springs, CO) placed around the thorax. The plethysmograph and video signals were synchronized via a digital synchronization signal recorded on an ADInstruments Powerlab 36/16 at 10 kHz. During data collection, pigs were allowed to feed ad libitum, which typically lasted at least 60 s and included at least 50 swallows. We recorded data when the pigs were 7 and 17 days old, following previously validated protocols (2). Seven-day-old pigs are the youngest age where pigs have developed suitable levels of thermoregulatory ability to be safely transported out of the housing facility to the videofluoroscopy suite. Pigs wean starting at 21 days old, so by 17 days they have begun to show interest in solid food and are highly efficient at consuming milk (29).
Data processing.
For each video feeding sequence, we identified the first set of swallows, with synchronized respiratory data, that occurred following the first 5 s of feeding, as swallows in the first few seconds of feeding occur at a faster rate than the rest of a feeding sequence (16). Swallows were identified by the frame at which the bolus was accumulated in the supraglottic space before passing the epiglottis (2). All individuals identifying swallows were trained on swallow identification using single-blind procedures until inter- and intrarater reliability reached at least 95%. The timing of the suck was determined by identifying the time at which the middle tongue reached its maximal point of elevation to seal with the hard palate (34). The number of sucks per swallow were calculated as the number of sucks that occurred after a swallow and before the next suck that was accompanied by a swallow. The delay of the swallow from a suck was calculated as the percentage of the suck cycle in which the swallow occurred (Supplemental Fig. S1; All supplemental material are available at https://doi.org/10.6084/m9.figshare.7982240.v1). Because we recorded at 100 Hz we were able to assess small, but significant, timing differences that change with development. In a previous study on infant animals, we found that the relative timing of tongue movement to the swallow was predictive of dysfunction (19). Thus a swallow occurring sooner after the suck (small percentage) indicates that transport is occurring more quickly. The variation in this parameter within an individual is also important, as it indicates how consistently the suck and the swallow are paced relative to each other.
Respiratory inductance plethysmograph signals were downsampled to 1 kHz and were synchronized with swallow timing via custom MATLAB code. We then identified a 200-ms window from the swallow of the synchronized respiratory data and measured the onset of inspiration following the swallow as the time difference from the swallow to the local zero of the phethysmograph signal (2). We calculated suck, swallow, and respiratory rates by dividing the duration of a single cycle by 60 s.
To calculate the amount of milk acquired per suck, we outlined the area of the bolus the frame before the swallow following published protocols (20). We converted the raw bolus area to a body size-standardized area by dividing the area of the bolus by the square of the length of the palate for each pig. Following this, we divided the cube of the square root of the standardized area by the number of sucks per swallow.
Statistical analyses.
All statistical analyses were performed in R (v. 3.5.0, http://www.r-project.org/). We use linear mixed-effects models to test for the effect of preterm birth (term or preterm; hereafter: treatment) and age (7 or 17 days) on performance. Treatment, days of postnatal maturation (hereafter: age), and their interaction were treated as fixed effects and individual pig was treated as a random effect [Response ~ Treatment + Age + Treatment × Age + (1|Individual); lmer4 (3)]. These analyses enabled us to test the impact of preterm birth (treatment) and postnatal maturation (age) on performance and further tested whether term and preterm infants changed differently as they aged (treatment × age). P values were obtained using likelihood ratio tests of the full model with the effect in question against the model without the effect in question. For analyses with significant interactions, Tukey’s post hoc analyses were performed to test for specific differences between groups (R package emmeans). Data used in statistical analyses are available upon request.
RESULTS
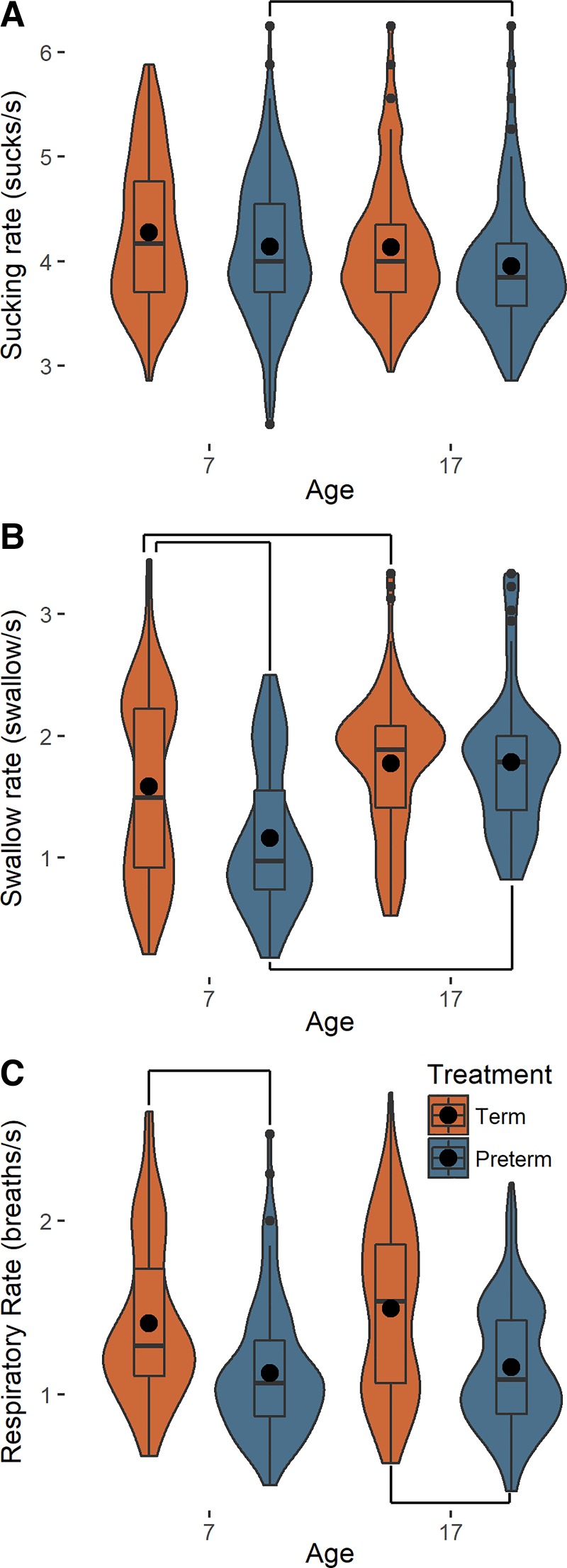
Term pigs displayed regular and stable breathing, sucking, and swallowing rates by the time they were 7 days old, and those rates remained constant through ontogeny (Fig. 1, A–C). The variation among these rates was consistent across groups. Preterm pigs exhibited consistent breathing and sucking patterns through ontogeny, although their respiratory rate was always lower than term pigs (Fig. 1, A–C, and Supplemental Table S1). Preterm pigs had a lower swallow rate than term pigs (~1 swallow/s) at day 7 (Fig. 1B and Supplemental Table S1), although by day 17 their swallowing rate had developed to be similar to term pigs (~2 swallows/s).
Fig. 1.
Maturation of sucking and swallowing relationships in term (orange) and preterm (blue) pigs through ontogeny. A; sucking rate during feeding (day 7: term n = 386 sucks, preterm n = 420 sucks; day 17: term n = 450 sucks, preterm n = 374 sucks). B: swallow rate (day 7: term n = 158 swallows, preterm n = 96 swallows; day 17: term n = 196 swallows, preterm n = 136 swallows). C: respiratory rate during feeding (day 7: term n = 159 breaths, preterm n = 122 breaths; day 17: term n = 178 breaths, preterm n = 158 breaths) on days 7 and 17. Small black dots are outliers; large black circles are means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis; and lines connecting plots show significant differences identified by mixed models or Tukey’s post hoc analyses when interactions were significant (P < 0.05).
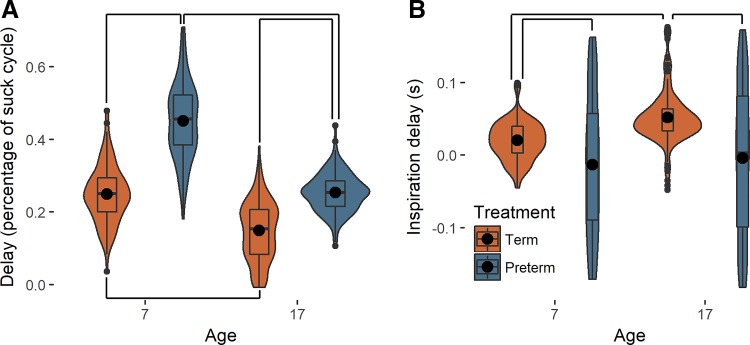
An effect of postnatal maturation was observed for both term and preterm pigs in the coordination between sucking and swallowing, although there were differences between the groups at both ages. At 7 days old, term pigs swallowed 25 ±7% SD of the way through the suck/swallow cycle, but by day 17 the swallow occurred at 15 ± 8% SD of the suck cycle (Fig. 2A and Supplemental Table S1). Term pigs also possessed a stereotyped relationship between the number of pure sucks between a suck/swallow by the time they were 7 days old that remained constant through ontogeny [Supplemental Table S2 (11)]. Preterm pigs also decreased the latency of the swallow from the suck, from 45 ± 10 to 25 ± 5% (Fig. 2A), and the amount of variation in this timing decreased to be near term levels by day 17 (Levene’s test P < 0.001, Supplemental Table S2). However, despite this dramatic change, preterm pigs exhibited a longer delay between the suck and the swallow at both ages tested (Fig. 2A), and they did not reach term equivalent performance in the number of sucks they took per swallow until 17 days of age (Supplemental Table S2). Furthermore, preterm pigs acquired a smaller amount of size-normalized milk volume per suck at both ages, although both term and preterm pigs did acquire more milk per suck at day 17 than day 7 (Supplemental Fig. S2).
Fig. 2.
The coordination of different behaviors in term and preterm pigs. A: term (orange) and preterm (blue) pigs both decrease the delay of the swallow relative to the beginning of the suck cycle, and preterm pigs exhibit a greater delay than term pigs throughout ontogeny (day 7: term n = 143 swallows, preterm n = 88 swallows; day 17: term n = 174 swallows, preterm n = 158 swallows). B: term pigs begin inspiration shortly after a swallow, and this delay increases through ontogeny, whereas preterm pigs show no coordination between respiration and swallowing through ontogeny (day 7: term n = 106 breaths, preterm n = 65 breaths; day 17: term n = 143 breaths, preterm n = 104 breaths). Small black dots are outliers; large black circles are means; box and whisker plots show median and interquartile range; width of each plot indicate the frequency distribution of the data along the y-axis; and lines connecting plots show significant differences identified by mixed models or Tukey’s post hoc analyses when interactions were significant (P < 0.05).
At day 7, term pigs began inspiration shortly after swallowing (0.02 ± 0.03 s), and by day 17, they increased this delay to 0.05 ± 0.04 s. However, preterm pigs displayed no stereotypy in the relationship between the swallow and inspiration and instead appear to breathe at any point in time before or following a swallow at both days 7 and 17 (Fig. 2B), resulting in significant effects of treatment (P < 0.001) and age (P < 0.001), the latter of which is driven by maturation of coordination in term pigs.
DISCUSSION
Our results are the first to integrate high-resolution data from multiple physiologic processes across a longitudinal time frame to study suck-swallow-breathe dynamics, and by doing so we are able to answer questions about the neurological development of these integrated behaviors. Preterm infants had high levels of developmental plasticity through ontogeny in oral behaviors (sucking, swallowing, and coordinating the two), but displayed lower respiratory rates and exhibited decreased coordination between swallowing and respiration throughout ontogeny. Many rhythmic processes in the body are controlled via CPGs, including aspects of locomotion, sucking, swallowing, and respiration (4, 27, 30). These patterns appear to reach stable levels in term infants shortly after birth (by 7 days), and by the time preterm pigs are 17 days old (human equivalent ~6–9 mo), their CPGs controlling sucking, swallowing, and respiration appear to have reached similar levels of stereotypy as term infants. However, preterm birth appears to fundamentally alter respiratory rates during feeding, as preterm infants consistently had slower respiratory rates during feeding than term pigs.
Although preterm infants are generally able to eventually develop neural control to reach term levels of performance in isolated behaviors, preterm birth appears to disrupt the development of CPG connectivity. Preterm infants developed coordination in their suck-swallow relationship well after term pigs and well past the equivalent age from conception. The fact that they do show maturation in these behaviors suggests that there is substantial postnatal development in the relationship between sucking and swallowing as infants mature (14, 24). Additionally, preterm infants never developed stereotyped or coordinated swallow-breathe dynamics, which suggests that many of the problems they face in feeding lie at the interface of this coordination (24).
Clinical implications.
Our results suggest that the problems preterm infants face to safely and effectively feed are due to the coordination of different behaviors rather than the reduced ability to perform any one behavior. Preterm infant pigs had a delayed swallow onset compared with term infant pigs. A delayed pharyngeal swallow has been associated with dysphagia in human infants, which is thought to arise in part due to inefficient feeding that results in increased fatigue and decreased milk consumption (22, 29). Furthermore, the fact that our preterm infants acquired less volume-normalized milk per suck than term infants, and that they did not get better at acquiring milk with time, suggests that despite significant postnatal development of suck-swallow dynamics occurring between the CPGs for sucking (i.e., hypoglossal motoneurons from the reticular formation) and swallowing (the nucleus tractus solitarius), preterms still exhibit substantial problems acquiring and processing milk.
Additionally, the lack of any maturation of coordination between swallowing and breathing in preterm infants suggests that a significant mechanism behind dysphagia in preterm infants lies at the interface of this coordination. Previous work on humans has speculated that swallow-breathe coordination is reduced in preterm infants (14, 24), but the reduced temporal resolution of data collection and the inability to follow infants longitudinally through nursing in a controlled environment have made it difficult to follow the maturation of the coordination between these two critical processes. Our results suggest that few synaptic connections between the CPG in the nucleus tractus solitarius generating the pattern generator for swallowing and the CPG in the prebötzinger complex generating respiratory rhythms develop postnatally and provide a foundation for understanding how and when different systems develop during mammal ontogeny.
Developmental explanations.
One possibility for the observed differences in postnatal development of coordination between behaviors could be that sucking, swallowing, and breathing begin at different times in development. Sucking and swallowing behaviors arise earlier in development than respiratory behaviors, as human fetuses suck and swallow starting between weeks 11 and 13 of prenatal development (28) but are not performing gas exchange through the lungs via breathing until after birth. Thus the early natal development of sucking and swallowing behaviors could allow for the brain to develop the basis for these behaviors early enough in utero for preterm infants to be only minimally affected. The interlinking between swallowing and respiratory neural pathways occurs shortly after birth, and preterm infants may never develop substantial synaptic connections between the swallowing pathway and the interneurons of the brainstem controlling respiration (25). The development of these connections may act as a regulator driving the minimum amount of time of gestation across mammals, as premature birth will impact aerodigestive coordination and result in decreased offspring survival (1, 14, 23). Although there is extensive variation in morphological development of features such as limbs and the olfactory organs across mammals (31, 32), all mammals must be able to coordinate swallowing and breathing to survive.
Conclusions.
We found that single behaviors, controlled by isolated CPGs, show substantial postnatal development in preterm infants, especially in sucking and swallowing. In contrast, preterm birth has a substantial impact on respiratory performance (6, 21), and the coordination between swallowing and breathing never developed in preterm pigs, suggesting that preterm birth fundamentally impacts some, but not all, aspects of feeding. These results provide novel insights into the neurological development of the brain, highlight potential areas of focus for developing appropriate interventions focused on developing coordination between behaviors for preterm infant feeding, and show that preterm birth impacts the development of suck-swallow-breathe relationships in mammals and may drive the length of gestation across mammals.
GRANTS
This project was funded by National Institute of Child Health and Human Development Grant R01-HD-088561 (to R. Z. German).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.J.M., F.D.H.G., R.K.B., and R.Z.G. conceived and designed research; C.J.M., F.D.H.G., L.E.B., B.M.S., and R.Z.G. performed experiments; C.J.M. analyzed data; C.J.M. and R.Z.G. interpreted results of experiments; C.J.M. prepared figures; C.J.M. drafted manuscript; C.J.M., F.D.H.G., and R.Z.G. edited and revised manuscript; C.J.M., F.D.H.G., L.E.B., B.M.S., R.K.B., and R.Z.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Wu, E. Catchpole, C. Lewis, J. Irizarry, and K. McGrattan for assistance caring for piglets and collecting data, as well as S. Dannemiller for expertise in delivering and caring for the piglets. We thank the musculoskeletal research group at Northeast Ohio Medical University (NEOMED) for comments on a draft of the manuscript.
REFERENCES
- 1.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr 97: 61–67, 2008. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballester A, Gould F, Bond L, Stricklen B, Ohlemacher J, Gross A, DeLozier K, Buddington R, Buddington K, Danos N, German R. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia 33: 627–635, 2018. doi: 10.1007/s00455-018-9881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 4.Biewener AA, Patek SN. Animal Locomotion (2nd ed.). Oxford, UK: Oxford University Press, 2018. [Google Scholar]
- 5.Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, Crisco JJ. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol 313: 262–279, 2010. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- 6.Caminita F, van der Merwe M, Hance B, Krishnan R, Miller S, Buddington K, Buddington RK. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 308: L118–L129, 2015. doi: 10.1152/ajplung.00173.2014. [DOI] [PubMed] [Google Scholar]
- 7.Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, Keates HL, Lumbers ER, Colditz PB, Lingwood BE. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One 8: e68763, 2013. doi: 10.1371/journal.pone.0068763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman JL. Looking forward to breathing. Prog Brain Res 188: 213–218, 2011. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- 9.German RZ, Crompton AW. The ontogeny of feeding in mammals. In: Feeding: Form, Function and Evolution in Tetrapod Vertebrates, edited by Schwenk K, editor. San Diego, CA: Academic, 2000, p. 449–457. [Google Scholar]
- 10.German RZ, Crompton AW, Gould FD, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia 32: 73–77, 2017. doi: 10.1007/s00455-016-9778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia). Dysphagia 19: 147–154, 2004. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 12.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. J Comp Physiol A 182: 539–547, 1998. doi: 10.1007/s003590050201. [DOI] [PubMed] [Google Scholar]
- 13.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neurol 48: 589–594, 2006. doi: 10.1017/S001216220600123X. [DOI] [PubMed] [Google Scholar]
- 15.Gewolb IH, Vice FL, Schweitzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol 43: 22–27, 2001. doi: 10.1017/S0012162201000044. [DOI] [PubMed] [Google Scholar]
- 16.Gierbolini-Norat EM, Holman SD, Ding P, Bakshi S, German RZ. Variation in the timing and frequency of sucking and swallowing over an entire feeding session in the infant pig Sus scrofa. Dysphagia 29: 475–482, 2014. doi: 10.1007/s00455-014-9532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldfield EC, Wolff PH, Schmidt RC. Dynamics of oral-respiratory coordination in full-term and preterm infants: 1. Comparisons at 38-40 weeks postconceptional age. Dev Sci 2: 363–373, 1999. doi: 10.1111/1467-7687.00081. [DOI] [Google Scholar]
- 18.Gould FD, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, German RZ. The physiologic impact of unilateral recurrent laryngeal nerve (RLN) lesion on infant oropharyngeal and esophageal performance. Dysphagia 30: 714–722, 2015. doi: 10.1007/s00455-015-9648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould FD, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, German RZ. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol (1985) 120: 495–502, 2016. doi: 10.1152/japplphysiol.00946.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould FD, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal swallow effects of recurrent laryngeal nerve lesion on bolus shape and airway protection in an infant pig model. Dysphagia 32: 362–373, 2017. doi: 10.1007/s00455-016-9762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med 165: 83–87, 2002. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 22.Jadcherla S. Dysphagia in the high-risk infant: potential factors and mechanisms. Am J Clin Nutr 103: 622S–628S, 2016. doi: 10.3945/ajcn.115.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau C, Sheena HR, Shulman RJ, Schanler RJ. Oral feeding in low birth weight infants J Pediatr 130: 561–569, 1997. doi: 10.1016/S0022-3476(97)70240-3. [DOI] [PubMed] [Google Scholar]
- 24.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants Acta Paediatr 92: 721–727, 2003. doi: 10.1111/j.1651-2227.2003.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Harris B. Coordination of Respiration and Swallowing (Online) GI Motility Online, Springer Nature. [2006]. doi: 10.1038/gimo10. [DOI] [Google Scholar]
- 26.McGrattan KE, McFarland DH, Dean JC, Hill E, White DR, Martin-Harris B. Effect of single-use, laser-cut, slow-flow nipples on respiration and milk ingestion in preterm infants. Am J Speech Lang Pathol 26: 832–839, 2017. doi: 10.1044/2017_AJSLP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev 14: 77–86, 2008. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 28.Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal and swallowing activity in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev 71: 61–87, 2003. doi: 10.1016/S0378-3782(02)00110-X. [DOI] [PubMed] [Google Scholar]
- 29.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterol Motil 23: e401–e408, 2011. doi: 10.1111/j.1365-2982.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 30.Samson N, Praud JP, Quenet B, Similowski T, Straus C. New insights into sucking, swallowing and breathing central generators: a complexity analysis of rhythmic motor behaviors. Neurosci Lett 638: 90–95, 2017. doi: 10.1016/j.neulet.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Schneider NY. The development of the olfactory organs in newly hatched monotremes and neonate marsupials. J Anat 219: 229–242, 2011. doi: 10.1111/j.1469-7580.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider NY, Gurovich Y. Morphology and evolution of the oral shield in marsupial neonates including the newborn monito del monte (Dromiciops gliroides, Marsupialia Microbiotheria) pouch young. J Anat 231: 59–83, 2017. doi: 10.1111/joa.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol (1985) 102: 587–600, 2007. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 34.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol (1985) 112: 1512–1519, 2012. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]