Abstract
Type 2 diabetes (T2D) and increased liver fat content (LFC) alter lipoprotein profile and composition and impair liver substrate uptake. Exercise training mitigates T2D and reduces LFC, but the benefits of different training intensities in terms of lipoprotein classes and liver substrate uptake are unclear. The aim of this study was to evaluate the effects of moderate-intensity continuous training (MICT) or sprint interval training (SIT) on LFC, liver substrate uptake, and lipoprotein profile in subjects with normoglycemia or prediabetes/T2D. We randomized 54 subjects (normoglycemic group, n = 28; group with prediabetes/T2D, n = 26; age = 40–55 yr) to perform either MICT or SIT for 2 wk and measured LFC with magnetic resonance spectroscopy, lipoprotein composition with NMR, and liver glucose uptake (GU) and fatty acid uptake (FAU) using PET. At baseline, the group with prediabetes/T2D had higher LFC, impaired lipoprotein profile, and lower whole body insulin sensitivity and aerobic capacity compared with the normoglycemic group. Both training modes improved aerobic capacity (P < 0.001) and lipoprotein profile (reduced LDL and increased large HDL subclasses; all P < 0.05) with no training regimen (SIT vs. MICT) or group effect (normoglycemia vs. prediabetes/T2D). LFC tended to be reduced in the group with prediabetes/T2D compared with the normoglycemic group posttraining (P = 0.051). When subjects were divided according to LFC (high LFC, >5.6%; low LFC, <5.6%), training reduced LFC in subjects with high LFC (P = 0.009), and only MICT increased insulin-stimulated liver GU (P = 0.03). Short-term SIT and MICT are effective in reducing LFC in subjects with fatty liver and in improving lipoprotein profile regardless of baseline glucose tolerance. Short-term MICT is more efficient in improving liver insulin sensitivity compared with SIT.
NEW & NOTEWORTHY In the short term, both sprint interval training and moderate-intensity continuous training (MICT) reduce liver fat content and improve lipoprotein profile; however, MICT seems to be preferable in improving liver insulin sensitivity.
Keywords: lipoprotein profile and exercise, liver fat content, liver glucose uptake, sprint interval training
INTRODUCTION
Liver is an important determinant of plasma glucose and fatty acid metabolism (34). In obesity and insulin resistance, impairments in hepatic metabolism and endogenous glucose production (EGP; 15, 26) as well as increased hypertriglyceridemia increase the risk for type 2 diabetes (T2D; 4, 47). Moreover, in sedentary subjects with obesity and overweight, accumulation of excessive triglycerides in liver, known as hepatic steatosis, leads to impaired liver function [decreased hepatic insulin clearance (22) and increased EGP (36)], decreased liver insulin-stimulated glucose uptake (GU; 4), and decreased liver blood flow (33). In obesity and insulin resistance, excess visceral fat mass increases free fatty acid (FFA) delivery to the liver. The increased FFA delivery to the liver further contributes to the increased liver FFA uptake that has been shown to be associated with hepatic steatosis (47). Furthermore, hepatic steatosis contributes in the development of metabolic syndrome (50) and cardiovascular diseases (43).
The accumulation of fat in the liver has been shown to be associated with dyslipidemia both in normoglycemic subjects (20) and in subjects with T2D (19). The dyslipidemia associated with hepatic steatosis affects both the lipoprotein subclass profile and composition. In fact, it has been shown by Toledo et al. that in subjects with T2D, the severity of dyslipidemia depends on the degree of hepatic steatosis (44). Moreover, recent studies have shown that the distribution of high-density lipoprotein (HDL) subclasses predicts the risk of acquiring T2D, with small HDLs having higher risk and large HDLs protecting against it (28).
Exercise training reduces liver fat content (LFC) both in subjects with and subjects without T2D even in the absence of weight loss (10, 18). In addition, training improves liver function and the lipoprotein profile independently of weight reduction (11, 18, 23, 41). Several studies have suggested a dose-response relationship between physical activity and health benefits (8), with increasing volume of physical activity achieving the most beneficial outcomes. Even though the health benefits of regular physical exercise training on chronic diseases have been long known (24), adherence among the general population still remains low with lack of time being one of the main constraints (45). Therefore, many studies are focused on establishing a time-efficient dose of exercise training, which can be implemented and accepted by the general population on a larger scale.
Gibala and colleagues first demonstrated that 2 wk of sprint interval training improves exercise performance similarly to moderate-intensity continuous training (MICT; 9a). Thereafter, we and others have shown that sprint interval training (SIT) rapidly induces marked improvements in aerobic capacity (6, 25), skeletal muscle performance (25), and whole body insulin sensitivity in healthy subjects as well as in patients with cardiometabolic diseases (3, 48). Recently, with the same data set as in this study, we showed that only MICT improved intestinal insulin sensitivity, whereas both SIT and MICT decreased fatty acid uptake (FAU) in the intestine (29). To our knowledge, it is unclear how SIT challenges liver and whether it leads to positive exercise training-induced responses in liver metabolism and function.
The purpose of the present study was to compare the effects of 2 wk of SIT and MICT on LFC, liver substrate uptake, and lipoprotein subclasses in subjects with normoglycemia and prediabetes/T2D. We hypothesized that there would be impairments in the lipoprotein profile and liver GU in the group with prediabetes/T2D compared with the normoglycemic group at baseline and that exercise training would reduce LFC and FAU in the group with prediabetes/T2D. In addition, we hypothesized that MICT would induce more significant improvements in liver metabolism because of the higher training volume-induced demands compared with SIT.
MATERIALS AND METHODS
The present study is part of a larger study entitled “The effects of short-time high-intensity interval training on tissue glucose and fat metabolism in healthy subjects and patients with type 2 diabetes” (NCT01344928). The study was approved by the local ethical committee of the Hospital District of Southwest Finland (decision 95/180/2010 section 228) and performed in compliance with the Declaration of Helsinki. The purpose and nature of the study and potential risks involved were explained in detail, and written informed consent was obtained before any measurements were performed.
Subjects.
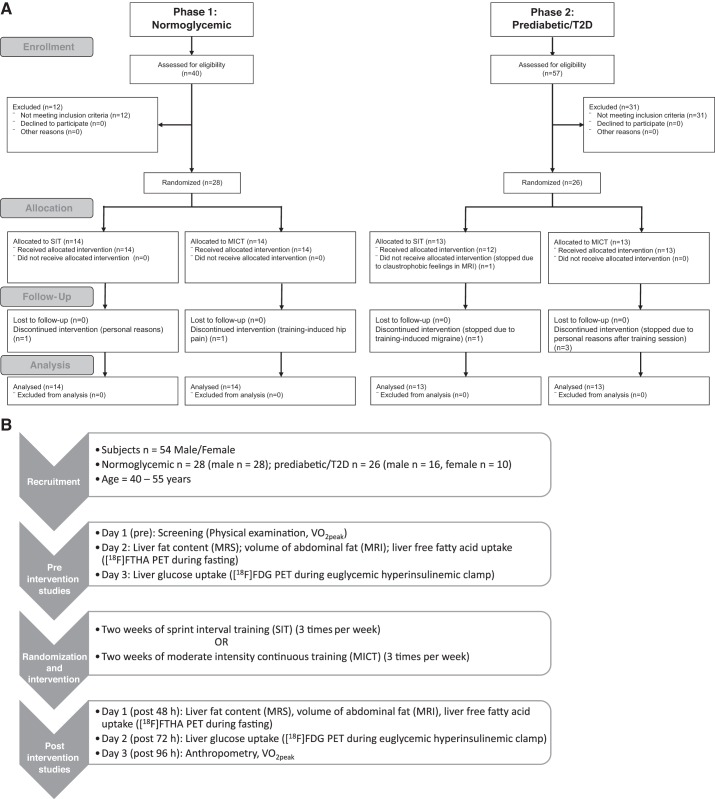
The study subjects were recruited in two phases. In the first phase, untrained normoglycemic (healthy) men were recruited, and in the second phase, untrained subjects with prediabetes/T2D (men and women) were recruited. The inclusion criteria for the normoglycemic group has been described in detail previously (21). In the second phase (recruitment of the group with prediabetes/T2D), because of the lack of male volunteers, women were also included in the study. The inclusion and exclusion criteria for the group with prediabetes/T2D have been explained in detail previously (12). The groups were randomized into SIT and MICT as previously described (12). Given the nature of the intervention, no blinding was used. In total, 54 sedentary 40–55-yr-old subjects, of whom 28 were normoglycemic men and 26 were men or women with prediabetes/T2D, were recruited in this study (Fig. 1A). Of 26 subjects with prediabetes/T2D (men, n = 16; women, n = 10), 17 met the criteria for T2D, and 9 had impaired fasting glucose concentrations and/or impaired glucose tolerance (2). Of 17 subjects with T2D, 13 were treated with oral hypoglycemic medication [11 with metformin, 5 with dipeptidyl peptidase-4 (sitagliptin), and 1 with sulfonylurea], whereas 4 subjects were newly diagnosed and did not take any medication for T2D. None of the subjects with prediabetes/T2D were on insulin, and two of women were on contraceptives. In addition, seven subjects with prediabetes/T2D were taking statins. In total, seven subjects dropped out during the intervention, one because of exercise-induced hip pain, one because of training-induced migraine, one because of claustrophobic feeling within the MRI scanner, and four because of personal reasons.
Fig. 1.
A: consort flow diagram showing the total number of subjects recruited and analyzed. B: study design. [18F]FDG, 2-[18F]fluoro-2-deoxy-d-glucose; [18F]FTHA, 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid; MICT, moderate-intensity continuous training; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; PET, positron emission tomography; SIT, sprint interval training; T2D, type 2 diabetes; V̇o2peak, aerobic capacity (peak oxygen consumption).
Study design.
Measurements were performed during three different visits before and after the training intervention as detailed in Fig. 1B. An overnight fast for at least 10 h was required before PET measurements. Participants were also asked to abstain from any caffeinated or alcoholic drinks, avoid strenuous physical exercise, and stop all oral hypoglycemic medication 48 h before the measurements. After 2 wk of exercise-training intervention, follow-up studies were repeated, starting on the second day (∼48 h) after the last exercise-training session. The measurements performed are described in Fig. 1B.
Exercise interventions.
Both SIT and MICT groups exercised three times a week for 2 wk. All six training sessions were performed under supervision. The training protocols have been explained previously (21). Briefly, each SIT session consisted of 4–6 × 30-s exercise bouts of all-out cycling effort (Wingate protocol) with 4 min of recovery between the bouts (during the recovery period, subjects remained still or continued to do unloaded cycling). Each bout of SIT started with 5 s of acceleration to maximal cadence followed by a sudden increase in load, which was 7.5% of whole body weight in kilograms for normoglycemic subjects and 10% of fat-free mass in kilograms for subjects with prediabetes/T2D (Monark Ergomedic 828E; Monark, Vansbro, Sweden). MICT training consisted of 40–60 min of cycling at moderate intensity [60% of peak oxygen consumption (V̇o2peak); Tunturi E85; Tunturi Fitness, Almere, The Netherlands]. The cycling duration was increased by 10 min after every other session until 60 min was reached in the last two sessions.
Maximal exercise test.
An incremental bicycle ergometer test (Ergoline 800s; VIASYS Healthcare) with direct respiratory measurements using ventilation and gas exchange (Jaeger Oxycon Pro; VIASYS Healthcare) was used to measure V̇o2peak, as described previously (21). Initial exercise intensity was 50 W, which was increased by 30 W after every 2 min until volitional exhaustion. Mean oxygen consumption at the highest 1 min was expressed as V̇o2peak. The workload in the last 2 min of the test was averaged and used as a measure for maximal performance. The peak respiratory exchange ratio was ≥1.15, and peak blood lactate concentration, measured from capillary samples obtained immediately and 1 min after exhaustion (YSI 2300 Stat Plus; YSI Life Sciences), was ≥8.0 mmol/l for all the tests. A peak heart rate (RS800CX; Polar Electro, Kempele, Finland) within 10 beats of the age-appropriate reference value (220 − age) was true in all except 1 participant in both groups and in both the pretraining and posttraining tests. Therefore, the highest value of oxygen consumption was expressed as V̇o2peak and not maximal oxygen consumption.
Lipoprotein subclasses.
Lipid and lipoprotein metabolic biomarkers were quantified from fasting serum samples using high-throughput proton NMR metabolomics (Nightingale Health, Helsinki, Finland). This technique provides quantification of 14 lipoprotein subclasses. These 14 lipoprotein subclass sizes were defined as follows: extremely large very low density lipoprotein (VLDL) with particle diameters from 75 nm upward and a possible contribution of chylomicrons, 5 VLDL subclasses (extra-large, large, medium, small, and extra-small), intermediate-density lipoprotein (IDL), 3 low-density lipoprotein (LDL) subclasses (large, medium, and small), and 4 HDL subclasses (extra-large, large, medium, and small). The following components of the lipoprotein subclasses were quantified: total lipids, phospholipids, triglycerides, cholesterol, free cholesterol, and cholesterol esters. The details of the experimentation have been described previously (39).
PET scanning.
PET studies were conducted after an overnight fast. Radiotracers 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid ([18F]FTHA) and 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) were used to measure liver FAU and GU, respectively. On the first PET scan session, liver FAU was measured using [18F]FTHA PET (35) during the fasting state. [18F]FTHA radiotracer (156 ± 1.1 MBq, mean ± SE) was injected, and dynamic imaging of the abdominal region (3 × 300-s frames) was acquired starting at 46 min after the tracer injection. On the second day, liver GU was measured using [18F]FDG under euglycemic-hyperinsulinemic clamp. The euglycemic-hyperinsulinemic clamp technique was used as previously described (5). On average, 91 ± 2 min after the start of the clamp and after 47 min of [18F]FDG (157 ± 0.9 MBq) injection, dynamic imaging of the abdominal region (3 × 300-s frames) was acquired. Arterialized blood samples were obtained during [18F]FTHA and [18F]FDG scans to measure the plasma radioactivity for calculating the tracer input function. During [18F]FTHA scans, blood samples were also collected to measure [18F]FTHA metabolites for correcting the plasma input function (27). An automatic gamma counter (Wizard 1480; Wallac, Turku, Finland) was used to measure plasma radioactivity. Computed tomography imaging was acquired for anatomical references.
Image analysis.
The PET imaging data were corrected for dead time, decay, and photon attenuation and were reconstructed using the three-dimensional ordered-subset expectation maximization method. Carimas 2.7 software (http://turkupetcentre.fi/carimas/) was used for image analysis. Three-dimensional volumes of interest were drawn on the liver, being cautious about the movement of the diaphragm and avoiding major vessels in the liver. Tissue time-activity curves were obtained from the three-dimensional volumes of interest, and graphical analysis was used to quantify the fractional uptake rate (31). GU and FAU rates were calculated by multiplying corresponding fractional uptake rate values by the mean plasma glucose or FFA level during the imaging period, respectively. Whole liver GU and FAU were obtained by multiplying liver GU and FAU by liver volume, respectively. Whole lean liver GU and FAU were obtained by subtracting the LFC from liver volume and multiplying it by liver GU and liver FAU. Because of technical problems in the PET scanner and tracer production, the final numbers of subjects for liver glucose and FFA uptake analyses were 20 and 17 in the high-LFC group and 19 and 16 for the low-LFC group, respectively.
Magnetic resonance spectroscopy and MRI measurements.
Magnetic resonance spectroscopy and MRI studies were performed using a Philips Gyroscan Intera 1.5T CV Nova Dual scanner (Philips Medical Systems). LFC and volume were measured as previously described (32). Abdominal subcutaneous adipose tissue and visceral adipose tissue depots were determined according to the classification by Abate et al. (1). Abdominal fat masses were analyzed from the image slice in which the xiphoid process was seen to the image slice in which both the femur heads were visible using sliceOmatic version 4.3 software (https://www.tomovision.com/products/sliceomatic.html). To obtain the mass, the pixel surface area was multiplied by the slice thickness and the density of adipose tissue, 0.9196 kg/l (1).
Glycemic status, insulin sensitivity, and body composition.
Whole body insulin-stimulated GU (M-value) was determined during the euglycemic-hyperinsulinemic clamp as previously described (5). Insulin was infused at a continuous rate of 1 mU·kg−1·min−1 (Actrapid; Novo Nordisk, Copenhagen, Denmark), and blood samples were taken every 5–10 min to adjust the exogenous glucose infusion and to maintain the plasma glucose concentration as close as possible to the level of 5 mmol/l. Insulin (100 U/ml) infusion (Actrapid) was started at a rate of 120 mU·min−1·m−2 during the first 4 min. After 4 min and up to 7 min, infusion rate was reduced to 80 mU·min−1·m−2, and from 7 min to the end of the clamp, it was kept constant at 40 mU·min−1·m−2. Glucose (20%) infusion was started 4 min after the start of the insulin infusion with a rate of 0.5 times the subject’s weight in kilograms. At 10 min, glucose infusion was doubled, and after that it was further adjusted according to plasma glucose levels to maintain a steady-state level of 5 mmol/l. Arterialized venous blood samples were collected before the clamp and every 5–10 min to measure the plasma glucose concentration for adjusting the glucose infusion rate. Arterialized plasma glucose was determined in duplicate by the glucose oxidase method (Analox GM9 Analyzer; Analox Instruments, London, United Kingdom). Whole body insulin-stimulated GU rate (M-value) was calculated from the measured glucose values collected when the subjects had reached steady state during the PET scan that was started 91 ± 2 min after the start of the clamp. The [18F]FDG PET study was performed when the subject had reached stable glucose concentrations at a level of 5 mmol/l (within 5% range for at least 15 min) after positioning in the PET scanner. EGP was calculated from the PET data (14). Alanine transaminase (ALAT), aspartate aminotransferase (ASAT), total cholesterol, triacylglycerols, and HDL concentrations were measured by automated enzymatic method (Cobus 8000; Roche Diagnostics, Mannheim, Germany). LDL was calculated using the Friedewald equation (7). Finally, whole body fat percentage was measured using a bioimpedance monitor (InBody 720; Mega Electronics, Kuopio, Finland).
Statistics.
The sample size for the whole study (NCT01344928) was based on skeletal muscle GU (quadriceps femoris) (6, 37). No sample size calculation was performed specifically for the parameters of liver. Normal distribution of the variables was tested using the Shapiro-Wilk test and evaluated visually. Logarithmic or square root transformations were done when appropriate to achieve normal distribution. Statistical analyses were performed using hierarchical mixed linear models with compound symmetry covariance structure for repeated measurements. The model included one within-subjects factor (time: overall mean change between baseline and measurement after intervention), two between-subjects factors [diabetic status (Dia): normoglycemic vs. prediabetic/T2D; training: SIT vs. MICT], and all their interactions. In the comparison between the normoglycemic group and the group with prediabetes/T2D, women were excluded to avoid mixing the effects of sex and glucose intolerance (Tables 2–4 and Fig. 2). In the comparison between high-LFC and low-LFC groups, all subjects (men and women) were pooled together using a model that included one within-subjects factor (time) and between-subjects factor group (high LFC vs. low LFC; SIT vs. MICT) and interaction terms (LFC × time: difference between the high-LFC and low-LFC groups; training × time: difference between training modes; Table 5 and Figs. 3 and 4). We also took both medication status (taking/not taking oral hypoglycemic medication; taking/not taking statins) and sex into account in all the analyses. Subjects with one value but another missing (dropouts, technical problems) are accounted for by restricted maximum likelihood estimation within the linear mixed models. Therefore, model-based means (SAS least squares means) and 95% confidence intervals are reported for all the parameters. Correlations are reported as Pearson’s correlation coefficients.
Table 2.
Subject characteristics of the normoglycemic group and the group with prediabetes/T2D before and after exercise intervention
| Parameter | Normoglycemic |
Prediabetic/T2D |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Baseline | Time | Dia × time | Training × time | |
| n | 28 | 26* | ||||||
| Age | 48 (46, 50) | 49 (48, 51) | ||||||
| Men/women,* n | 28/0 | 16/0 | ||||||
| Prediabetes/T2D, n | 5/11 | |||||||
| SIT/MICT, n | 14/14 | 9/7 | ||||||
| Anthropometrics | ||||||||
| Weight, kg | 83.6 (79.7, 87.5) | 83.3 (79.4, 87.2) | 96.3 (91.2, 101.5) | 96.2 (91.0, 101.3) | <0.001§ | 0.22 | 0.80 | 0.36 |
| BMI, kg/m2 | 26.1 (25.1, 27.1) | 26.0 (25.0, 27.0) | 30.4 (29.1, 31.8) | 30.4 (29.0, 31.7) | <0.001§ | 0.17§ | 0.70 | 0.30 |
| Whole body fat,† % | 22.6 (20.9, 24.3) | 21.7 (20.0, 23.3) | 28.8 (26.5, 31.2) | 28.1 (25.7, 30.4) | <0.001§ | <0.001§ | <0.001§ | 0.62 |
| Subcutaneous fat mass,† kg | 4.1 (3.7, 4.5) | 4.0 (3.6, 4.4) | 5.6 (4.9, 6.4) | 5.5 (4.9, 6.4) | <0.001§ | 0.03§ | 0.93 | 0.65 |
| Visceral fat mass,‡ kg | 3.1 (2.7, 3.4) | 3.0 (2.6, 3.4) | 4.2 (5.0, 3.6) | 4.1 (4.8, 3.5) | <0.001§ | 0.002§ | 0.54 | 0.60 |
| V̇o2peak, ml·kg−1·min−1 | 34.2 (32.7, 35.7) | 35.7 (34.2, 37.2) | 29.3 (27.2, 31.4) | 30.0 (27.9, 32.1) | <0.001§ | 0.003§ | 0.23 | 0.005§ |
| Liver volume,† ml | 1,366 (1,282, 1,455) | 1,373 (1,289, 1,464) | 1,773 (1,628, 1,932) | 1,730 (1,587, 1,886) | <0.001§ | 0.32 | 0.12 | 0.95 |
| Glucose profile | ||||||||
| Glucosefasting,† mmol/l | 5.6 (5.4, 5.8) | 5.5 (5.3, 5.7) | 6.6 (6.3, 7.0) | 6.6 (6.3, 7.0) | <0.001§ | 0.86 | 0.71 | 0.83 |
| Glucoseclamp, mmol/l | 4.9 (4.8, 5.1) | 4.9 (4.8, 5.1) | 4.8 (4.6, 5.1) | 5.0 (4.7, 5.2) | 0.40 | 0.35 | 0.34 | 0.86 |
| Insulinfasting FDGday,† mU/l | 5.5 (4.3, 7.0) | 5.4 (4.2, 6.9) | 13.1 (9.3, 18.3) | 12.0 (8.5, 17.0) | <0.001§ | 0.46 | 0.66 | 0.14 |
| Insulinclamp, mU/l | 75.4 (69.6, 81.2) | 76.5 (70.5, 82.5) | 87.6 (79.9, 95.4) | 86.0 (77.8, 94.2) | 0.02§ | 0.92 | 0.57 | 0.46 |
| EGP,‡ µmol·min−1·kg−1 | 5.5 (2.4, 8.5) | 4.2 (1.2, 7.2) | 18.6 (13.5, 23.5) | 13.0 (7.8, 18.4) | <0.001§ | 0.38 | 0.10 | 0.60 |
| Whole body insulin sensitivity (M-value),† µmol·min−1·kg−1 | 35.3 (30.0, 40.6) | 38.7 (33.3, 44.1) | 17.5 (10.3, 24.8) | 21.6 (14.2, 29.0) | <0.001§ | <0.001§ | 0.11 | 0.06 |
| HbA1c, mmol/mol | 36.9 (35.2, 38.6) | 34.8 (33.0, 36.5) | 39.6 (37.3, 41.8) | 37.5 (35.2, 39.9) | 0.08 | <0.001§ | 0.75 | 0.38 |
| Lipid profile | ||||||||
| FFAfasting, mmol/l | 0.70 (0.62, 0.77) | 0.62 (0.54, 0.70) | 0.69 (0.60, 0.78) | 0.68 (0.58, 0.78) | 0.86 | 0.04§ | 0.11 | 0.01§ |
| FFAclamp,‡ mmol/l | 0.065 (0.05, 0.08) | 0.060 (0.05, 0.07) | 0.093 (0.07, 0.12) | 0.082 (0.06, 0.10) | 0.02§ | 0.15 | 0.70 | 0.76 |
| Cholesterol, mmol/l | 5.0 (4.7, 5.3) | 4.5 (4.1, 4.8) | 4.8 (4.4, 5.3) | 4.4 (3.9, 4.9) | 0.51 | <0.001§ | 0.57 | 0.12 |
| HDL,† mmol/l | 1.4 (1.2, 1.5) | 1.3 (1.2, 1.4) | 1.2 (1.1, 1.4) | 1.1 (1.0, 1.2) | 0.10 | <0.001§ | 0.66 | 0.19 |
| LDL, mmol/l | 3.1 (2.9, 3.4) | 2.8 (2.5, 3.1) | 2.7 (2.3, 3.1) | 2.6 (2.2, 3.0) | 0.09 | 0.001§ | 0.16 | 0.12 |
| Triglycerides,† mmol/l | 0.9 (0.8, 1.1) | 0.8 (0.7, 1.0) | 1.7 (1.4, 2.1) | 1.5 (1.2, 1.9) | <0.001§ | 0.08 | 0.96 | 0.63 |
| Inflammatory markers | ||||||||
| CRP,† mg/l | 1.0 (0.6, 1.7) | 0.5 (0.3, 0.9) | 1.9 (1.1, 3.5) | 0.8 (0.4, 1.6) | 0.81 | 0.001§ | 0.78 | 0.75 |
| ALAT,† U/l | 27.1 (23.1, 31.9) | 23.3 (19.7, 27.6) | 42.2 (34.0, 52.3) | 34.5 (27.5, 43.2) | <0.001§ | 0.001§ | 0.62 | 0.27 |
| ASAT,† U/l | 26.0 (23.2, 29.1) | 22.7 (20.1, 25.7) | 31.8 (27.3, 37.0) | 25.3 (21.4, 29.8) | 0.047§ | 0.003§ | 0.40 | 0.23 |
| GT,† U/l | 24.0 (19.0, 30.3) | 19.0 (15.0, 24.1) | 47.7 (35.0, 65.0) | 36.2 (26.3, 49.7) | <0.001§ | <0.001§ | 0.70 | 0.59 |
| PET data | ||||||||
| Whole lean liver GU, µmol/min | 57 (50, 64) | 59 (52, 67) | 66 (57, 75) | 67 (58, 76) | 0.10 | 0.32 | 0.77 | 0.09 |
| Whole lean liver FAU, µmol/min | 129 (105, 153) | 121 (95, 147) | 137 (111, 163) | 143 (115, 171) | 0.60 | 0.93 | 0.50 | 0.25 |
Values are model-based means with 95% confidence intervals in parentheses; number of subjects n = 44 (all men). The P value for baseline indicates the difference between the normoglycemic group and the group with prediabetes/type 2 diabetes (T2D). The P value for time indicates the change between pretraining (Pre) and posttraining (Post) measurements in the whole study group. The P value for diabetic status (Dia) × time interaction indicates whether the change in the parameter was different between the normoglycemic group and the group with prediabetes/T2D. The P value for training × time interaction indicates whether the change in the parameter was different between the sprint interval training and moderate-intensity continuous training modes. ALAT, alanine transaminase; ASAT, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; EGP, endogenous glucose production; FAU, fatty acid uptake; FFAclamp and FFAfasting, free fatty acid concentration during euglycemic-hyperinsulinemic clamp and during fasting, respectively; Glucoseclamp and Glucosefasting, glucose concentration during euglycemic-hyperinsulinemic clamp and during fasting, respectively; GT, γ-glutamyltranspeptidase; GU, glucose uptake; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; Insulinclamp and Insulinfasting FDGday, insulin concentration during euglycemic-hyperinsulinemic clamp and during fasting on the day of 2-[18F]fluoro-2-deoxy-d-glucose injection, respectively; LDL, low-density lipoprotein; V̇o2peak, aerobic capacity (peak oxygen consumption).
Women were excluded from the analysis to avoid mixing effects of sex.
Log transformation was performed to achieve normal distribution.
Square root transformation was performed to achieve normal distribution.
P value is statistically significant (P ≤ 0.05).
Table 4.
Correlations at baseline between the subcomponents in lipoprotein subclasses and general parameters
| Component | Lipoprotein Component | General Parameter | r | P |
|---|---|---|---|---|
| Lipids | Extra-large HDL* | M-value* | 0.49 | <0.001 |
| Extra-large HDL* | ALAT* | −0.38 | 0.01 | |
| Extra-large HDL* | Liver volume* | −0.50 | <0.001 | |
| Small HDL | M-value* | −0.46 | <0.001 | |
| Small HDL | V̇o2peak | −0.37 | 0.01 | |
| Small HDL | LFC† | 0.35 | 0.02 | |
| Small HDL | Liver volume* | 0.36 | 0.01 | |
| Phospholipids | Extra-large HDL† | M-value* | 0.35 | 0.01 |
| Extra-large HDL† | Liver volume* | −0.39 | <0.001 | |
| Small HDL* | M-value* | −0.59 | <0.001 | |
| Small HDL* | V̇o2peak | −0.54 | <0.001 | |
| Small HDL* | LFC† | 0.50 | <0.001 | |
| Small HDL* | Liver volume* | 0.46 | <0.001 | |
| Cholesterol | Extra-large HDL* | M-value* | 0.42 | <0.01 |
| Extra-large HDL* | ALAT* | −0.37 | 0.01 | |
| Extra-large HDL* | Liver volume* | −0.43 | <0.001 | |
| Cholesterol esters | Extra-large HDL* | Liver volume* | −0.29 | 0.03 |
| Free cholesterol | Extra-large HDL* | M-value* | 0.44 | <0.01 |
| Extra-large HDL* | ALAT* | −0.29 | 0.03 | |
| Extra-large HDL* | Liver volume* | −0.44 | <0.001 | |
| Small HDL* | M-value* | −0.50 | <0.001 | |
| Small HDL* | LFC† | 0.41 | 0.01 | |
| Small HDL* | Liver volume* | 0.40 | <0.01 | |
| Triglycerides | Small HDL* | M-value* | −0.66 | <0.001 |
| Small HDL* | V̇o2peak | −0.42 | <0.001 | |
| Small HDL* | ALAT* | 0.41 | <0.001 | |
| Small HDL* | LFC† | 0.54 | <0.001 | |
| Small HDL* | Liver volume* | 0.52 | <0.001 |
Number of subjects n = 44 (all men). Women were excluded from the analysis to avoid mixing effects of sex. ALAT, alanine transaminase; HDL, high-density lipoprotein; LFC, liver fat content; M-value, whole body insulin sensitivity; V̇o2peak, aerobic capacity (peak oxygen consumption).
Log transformation was performed to achieve normal distribution.
Square root transformation was performed to achieve normal distribution.
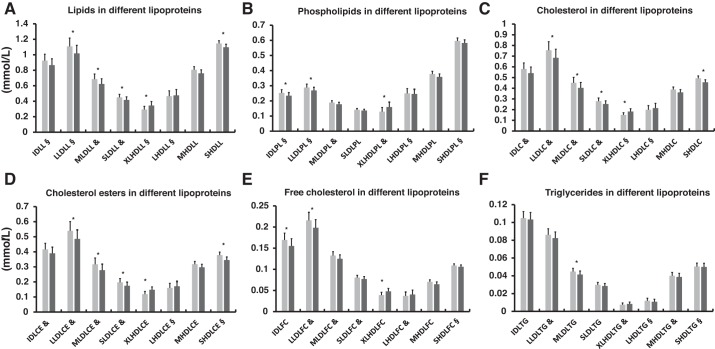
Fig. 2.
Effects of 2 wk of exercise training on subcomponents of lipoproteins in the normoglycemic group and the group with prediabetes/type 2 diabetes (men only, n = 44). A: lipids. B: phospholipids. C: cholesterol. D: cholesterol esters. E: free cholesterol. F: triglycerides. C, cholesterol; CE, cholesterol esters; FC, free cholesterol; IDL, intermediate-density lipoprotein; L, lipids; LHDL, large high-density lipoprotein; LLDL, large low-density lipoprotein; MHDL, medium high-density lipoprotein; MLDL, medium low-density lipoprotein; PL, phospholipids; SHDL, small high-density lipoprotein; SLDL, small low-density lipoprotein; TG, triglycerides; XLHDL, extra-large high-density lipoprotein. Pretraining, light gray; posttraining, dark gray. All values are expressed as model-based means, and bars are 95% confidence intervals. §Log transformation or &square root transformation was performed to achieve normal distribution. *P < 0.05 value for time interaction (i.e., the groups behaved similarly for the change).
Table 5.
Subject anthropometrics, glucose lipid profiles, and inflammatory markers for SIT and MICT exercise-training modes
| SIT |
MICT |
||||
|---|---|---|---|---|---|
| Parameter | Pre | Post | Pre | Post | Training × Time P Value |
| n | 27 | 27 | |||
| Men/women | 23/4 | 21/6 | |||
| Anthropometrics | |||||
| Weight, kg | 86.6 (82.3, 90.8) | 86.1 (81.8, 90.4) | 88.1 (83.9, 92.3) | 87.8 (83.6, 92.0) | 0.63 |
| BMI, kg/m2 | 27.8 (26.6, 29.0) | 27.6 (26.4, 28.8) | 28.7 (27.5, 29.9) | 28.6 (27.4, 29.7) | 0.60 |
| Whole body fat,† % | 26.2 (23.5, 29.3) | 25.1 (22.5, 28.1) | 27.1 (24.3, 30.3) | 26.2 (23.4, 29.2) | 0.65 |
| Subcutaneous fat mass,† kg | 5.5 (4.6, 6.4) | 5.3 (4.4, 6.3) | 5.7 (4.8, 6.7) | 5.6 (4.7, 6.6) | 0.57 |
| Visceral fat mass,‡ kg | 3.0 (2.4, 3.7) | 2.9 (2.3, 3.6) | 3.1 (2.5, 3.7) | 2.9 (2.3, 3.5) | 0.43 |
| V̇o2peak, ml·kg−1·min−1 | 31.1 (28.9, 33.2) | 32.7 (30.5, 34.9) | 30.6 (28.5, 32.8) | 31.1 (28.9, 33.3) | 0.053 |
| Liver volume,† ml | 1,417 (1,329, 1,510) | 1,398 (1,311, 1,491) | 1,547 (1,455, 1,646) | 1,543 (1,450, 1,642) | 0.56 |
| Glucose profile | |||||
| Glucosefasting,† mmol/l | 6.1 (5.7, 6.4) | 6.0 (5.7, 6.4) | 6.0 (5.7, 6.4) | 5.9 (5.6, 6.2) | 0.44 |
| Glucoseclamp, mmol/l | 4.9 (4.7, 5.1) | 4.9 (4.7, 5.1) | 4.9 (4.7, 5.1) | 5.0 (4.8, 5.2) | 0.56 |
| Insulinfasting FDGday,† mU/l | 7.9 (6.2, 10.0) | 7.0 (5.5, 8.9) | 7.4 (5.8, 9.3) | 7.6 (6.0, 9.7) | 0.19 |
| Insulinclamp, mU/l | 80.7 (74.7, 86.7) | 80.5 (74.2, 86.7) | 80.7 (74.8, 86.5) | 82.8 (76.6, 89.0) | 0.56 |
| EGP,‡ µmol·min−1·kg−1 | 5.8 (4.2, 7.7) | 5.9 (4.2, 7.8) | 6.6 (4.9, 8.5) | 7.1 (5.2, 9.3) | 0.76 |
| Whole body insulin sensitivity (M-value),† µmol·min−1·kg−1 | 25.0 (20.5, 30.5) | 30.6 (25.0, 37.4) | 20.6 (16.9, 25.1) | 23.6 (19.2, 28.9) | 0.47 |
| HbA1c, mmol/mol | 37.3 (35.7, 38.9) | 35.9 (34.3, 37.5) | 38.4 (36.9, 40.0) | 35.9 (34.2, 37.5) | 0.14 |
| Lipid profile | |||||
| FFAfasting, mmol/l | 0.69 (0.61, 0.77) | 0.68 (0.59, 0.76) | 0.81 (0.73, 0.88) | 0.71 (0.63, 0.79)* | 0.03§ |
| FFAclamp,‡ mmol/l | 0.071 (0.06, 0.09) | 0.063 (0.05, 0.08) | 0.082 (0.07, 0.10) | 0.067 (0.05, 0.08) | 0.58 |
| Cholesterol, mmol/l | 5.0 (4.7, 5.4) | 4.4 (4.0, 4.7) | 4.9 (4.6, 5.3) | 4.6 (4.2, 4.9) | 0.07 |
| HDL,† mmol/l | 1.3 (1.2, 1.5) | 1.2 (1.1, 1.3) | 1.3 (1.2, 1.5) | 1.3 (1.1, 1.4) | 0.23 |
| LDL, mmol/l | 3.0 (2.7, 3.3) | 2.6 (2.3, 2.9) | 3.0 (2.6, 3.3) | 2.7 (2.4, 3.1) | 0.09 |
| Triglycerides,† mmol/l | 1.2 (1.0, 1.4) | 1.1 (0.9, 1.3) | 1.2 (1.0, 1.4) | 1.0 (0.9, 1.3) | 0.83 |
| Inflammatory markers | |||||
| CRP,† mg/l | 1.2 (0.8, 2.0) | 0.6 (0.4, 1.0) | 1.6 (1.0, 2.4) | 0.7 (0.4, 1.0) | 0.64 |
| ALAT,† U/l | 31.3 (26.3, 37.2) | 25.2 (21.1, 30.0) | 29.8 (25.2, 35.3) | 26.4 (22.2, 31.5) | 0.27 |
| ASAT,† U/l | 27.1 (24.0, 30.5) | 21.7 (19.2, 24.6) | 26.3 (23.4, 29.5) | 24.7 (21.8, 28.1) | 0.10 |
| GT,† U/l | 28.3 (22.0, 36.3) | 22.6 (17.5, 29.1) | 29.8 (23.3, 38.1) | 22.6 (17.6, 29.0) | 0.52 |
Values are model-based means with 95% confidence intervals in parentheses; number of subjects n = 54 (men and women). The P value for training × time interaction indicates whether the change in the parameter was different between the sprint interval training (SIT) and moderate-intensity continuous training (MICT) modes. ALAT, alanine transaminase; ASAT, aspartate transaminase; BMI, body mass index; CRP, C-reactive protein; EGP, endogenous glucose production; FFAclamp and FFAfasting, free fatty acid concentration during euglycemic-hyperinsulinemic clamp and during fasting, respectively; Glucoseclamp and Glucosefasting, glucose concentration during euglycemic-hyperinsulinemic clamp and during fasting, respectively; GT, γ-glutamyltranspeptidase; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; Insulinclamp and Insulinfasting FDGday, insulin concentration during euglycemic-hyperinsulinemic clamp and during fasting on the day of 2-[18F]fluoro-2-deoxy-d-glucose injection, respectively; LDL, low-density lipoprotein; Post, posttraining; Pre, pretraining; V̇o2peak, aerobic capacity (peak oxygen consumption).
P value was significant for the MICT group.
Log transformation was performed to achieve normal distribution.
Square root transformation was performed to achieve normal distribution.
P value is statistically significant (P ≤ 0.05).
Fig. 3.
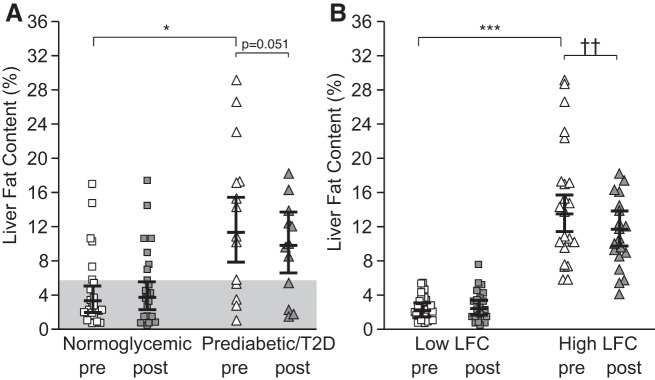
Liver fat content (LFC) before and after 2 wk of intervention. A: effects of 2 wk of sprint interval training (SIT) and moderate-intensity continuous training (MICT) on LFC in normoglycemic men and men with prediabetes/type 2 diabetes (T2D; men only, n = 44). B: effects of SIT and MICT on LFC in high-LFC and low-LFC groups (men and women; n = 54). The shaded area in A denotes normal liver fat content (≤5.6%). Post, posttraining; Pre, pretraining. All values are expressed as model-based means, and bars are 95% confidence intervals. *P ≤ 0.05 baseline differences between the normoglycemic men and men with prediabetes/T2D. ***P ≤ 0.001 baseline differences between low-LFC and high-LFC groups. ††P ≤ 0.01 time effect for the high-LFC group.
Fig. 4.
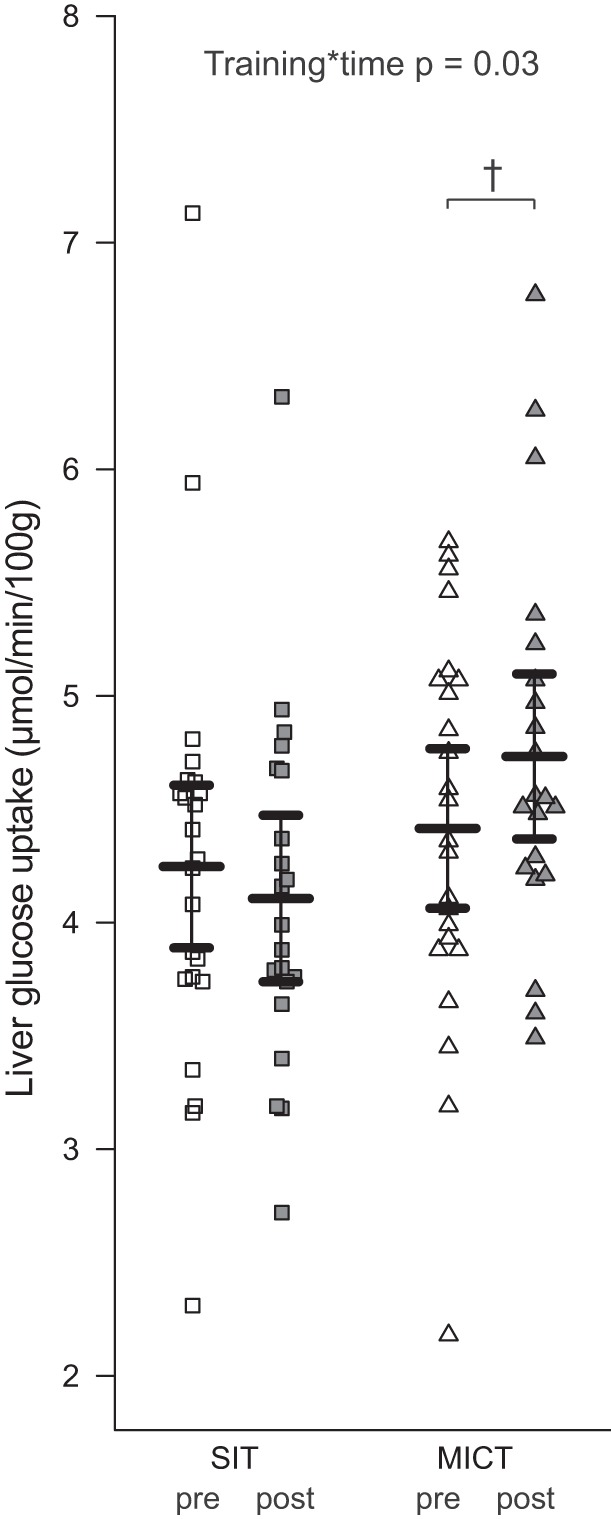
Insulin-stimulated liver glucose uptake in sprint interval training (SIT) and moderate-intensity continuous training (MICT) groups before (pre) and after the training intervention (post) in all subjects (men and women, n = 54). All values are expressed as model-based means, and bars are 95% confidence intervals. P value for training × time indicates that the change in liver glucose uptake was different between the training groups. †P value for the improvement in liver glucose uptake in the MICT group compared with SIT was significant.
All tests were performed as two sided, with a significance level set at 0.05. The analyses were performed using SAS version 9.3 for Windows (SAS Institute, Cary, NC).
RESULTS
The effects of exercise training were analyzed separately between men with prediabetes and men with T2D (Table 1). As most of the changes in the variables were similar, the men with prediabetes and the men with T2D were combined into one group. Consequently, the effects of exercise training have been compared between normoglycemic men and men with prediabetes/T2D. The effects of exercise training between men and women are shown in Supplemental Table S1 (Supplemental Material for this article is available online at https://doi.org/10.5281/zenodo.2642678).
Table 1.
Effects of exercise training on liver fat content, liver volume, liver substrate uptake, and liver enzyme inflammatory profile in men with prediabetes and T2D
| Parameter | Prediabetic |
T2D |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Baseline | Time | Dia × time | |
| n | 5 | 4 | 11 | 9 | |||
| Men/women,* n | 5/0 | 11/0 | |||||
| Anthropometrics | |||||||
| LFC, % | 15.0 (6.8, 23.1) | 14.1 (5.9, 22.2) | 11.8 (6.0, 17.5) | 9.9 (4.1, 15.7) | 0.52 | 0.06 | 0.48 |
| Liver volume, ml | 1,713 (1,370, 2,056) | 1,712 (1,367, 2,056) | 1,835 (1,592, 2,078) | 1,775 (1,532, 2,018) | 0.55 | 0.23 | 0.24 |
| PET data | |||||||
| Liver GU, µmol· 100 g−1·min−1 | 4.3 (3.9, 4.8) | 4.2 (3.7, 4.7) | 4.2 (3.9, 4.5) | 4.4 (4.0, 4.7) | 0.64 | 0.91 | 0.39 |
| Liver FAU, µmol· 100 g−1·min−1 | 10.1 (7.3, 12.9) | 10.7 (7.6, 13.8) | 8.1 (6.2, 10.0) | 8.7 (6.6, 10.9) | 0.22 | 0.52 | 0.99 |
| EGP, µmol·min−1·kg−1 | 18.1 (8.5, 27.8) | 10.3 (0.4, 20.2) | 18.6 (11.4, 25.9) | 13.5 (6.1, 20.9) | 0.85 | 0.01‡ | 0.48 |
| Whole lean liver GU, µmol/min | 78 (58, 99) | 83 (67, 98) | 76 (55, 97) | 83 (68, 99) | 0.70 | 0.84 | 0.73 |
| Whole lean liver FAU, µmol/min | 185 (134, 236) | 157 (121, 193) | 192 (137, 248) | 156 (116, 195) | 0.39 | 0.88 | 0.79 |
| Inflammatory markers | |||||||
| CRP,† mg/l | 3.1 (1.1, 8.6) | 1.1 (0.4, 3.3) | 1.5 (0.7, 2.9) | 0.7 (0.3, 1.5) | 0.17 | 0.01‡ | 0.56 |
| ALAT, U/l | 46.8 (32.8, 60.8) | 39.7 (24.6, 54.9) | 43.9 (34.5, 53.4) | 36.5 (26.4, 46.7) | 0.74 | 0.10 | 0.97 |
| ASAT,† U/l | 36.0 (26.9, 48.2) | 29.4 (21.2, 40.8) | 29.8 (24.5, 36.3) | 23.4 (18.8, 29.1) | 0.41 | 0.06 | 0.85 |
| GT,† U/l | 80.9 (42.7, 153.4) | 36.2 (23.5, 55.8) | 54.8 (28.1, 106.7) | 29.1 (18.6, 45.6) | 0.052 | 0.05 | 0.54 |
Values are model-based means with 95% confidence intervals in parentheses; number of subjects n = 16 (all men). The P value for baseline indicates the difference between the men with prediabetes and those with type 2 diabetes (T2D). The P value for time indicates the change between pretraining (Pre) and posttraining (Post) measurements in the whole study group. The P value for diabetic status (Dia) × time interaction indicates whether the change in the parameter was different between men with prediabetes and those with T2D. ALAT, alanine transaminase; ASAT, aspartate transaminase; CRP, C-reactive protein; EGP, endogenous glucose production; FAU, fatty acid uptake; GT, γ-glutamyltranspeptidase; GU, glucose uptake; LFC, liver fat content.
Women were excluded from this analysis.
Log transformation was performed to achieve normal distribution.
P value is statistically significant (P ≤ 0.05).
At baseline, men with prediabetes/T2D were heavier and had higher body adiposity, impaired glucose and lipid profiles, and lower whole body insulin sensitivity and aerobic capacity than the normoglycemic men (all baseline P < 0.05, Table 2). Both SIT and MICT improved whole body insulin sensitivity similarly in both the normoglycemic men and men with prediabetes/T2D and slightly but significantly decreased HbA1c, whole body fat percentage, and depot specific adiposity (all time P < 0.05, Table 2). Both SIT and MICT improved aerobic capacity (V̇o2peak; time P < 0.001), but the improvement in the SIT group was significantly different compared with the MICT group, with SIT inducing a greater increase (training × time P = 0.005, Table 2).
At baseline the group with prediabetes/T2D had a significantly impaired lipoprotein profile, both in terms of subclass distribution (VLDL, IDL, LDL, and HDL) and composition (lipids, phospholipids, cholesterol, cholesterol esters, free cholesterol, and triglycerides), compared with the normoglycemic group (Table 3). Both SIT and MICT increased lipid, phospholipid, free cholesterol, cholesterol, and cholesterol ester concentrations in extra-large HDLs, whereas there was a significant reduction in various components of IDL, LDL, and small HDL subclasses (Fig. 2, A–F). There were significant correlations between the lipoprotein subcomponents and M-value, V̇o2peak, and liver parameters (Table 4). After the training intervention, there was no significant change in the VLDL subclasses and composition. In the analyses, both diabetes medication and statins were taken as covariate to see whether medication affected the training response, but it did not have any effect on the results.
Table 3.
Baseline differences between the normoglycemic group and the group with prediabetes/T2D in different lipoprotein components and subclasses
| Parameter | Normoglycemic | Prediabetic/T2D* | P Value |
|---|---|---|---|
| Lipids | |||
| Extremely large VLDL | 0.01 (0.01, 0.02) | 0.04 (0.03, 0.05) | <0.0001 |
| Extra-large VLDL | 0.03 (0.02, 0.05) | 0.09 (0.06, 0.13) | <0.001 |
| Large VLDL | 0.17 (0.13, 0.22) | 0.35 (0.26, 0.48) | <0.0001 |
| Medium VLDL | 0.41 (0.34, 0.50) | 0.67 (0.52, 0.87) | <0.01 |
| Small VLDL | 0.047 (0.42, 0.54) | 0.61 (0.51, 0.72) | 0.047 |
| IDL | 1.06 (0.95, 0.17) | 0.81 (0.70, 0.93) | <0.01 |
| Large LDL | 1.29 (1.15, 1.44) | 0.95 (0.82, 1.11) | <0.001 |
| Medium LDL | 0.79 (0.71, 0.87) | 0.59 (0.50, 0.69) | <0.01 |
| Small LDL | 0.51 (0.46, 0.56) | 0.40 (0.34, 0.46) | <0.01 |
| Small HDL | 1.10 (1.06, 1.14) | 1.19 (1.14, 1.25) | <0.01 |
| Phospholipids | |||
| Extremely large VLDL | 0.001 (0.001, 0.001) | 0.004 (0.003, 0.007) | <0.0001 |
| Extra-large VLDL | 0.005 (0.003, 0.009) | 0.012 (0.007, 0.022) | <0.0001 |
| Large VLDL | 0.03 (0.02, 0.04) | 0.06 (0.04, 0.08) | <0.0001 |
| Medium VLDL | 0.006 (0.004, 0.009) | 0.015 (0.009, 0.025) | <0.001 |
| Small VLDL | 0.11 (0.10, 0.13) | 0.15 (0.13, 0.17) | 0.01 |
| Extra-small VLDL | 0.14 (0.13, 0.15) | 0.12 (0.10, 0.13) | 0.02 |
| IDL | 0.29 (0.27, 0.33) | 0.22 (0.19, 0.25) | <0.0001 |
| Large LDL | 0.33 (0.30, 0.36) | 0.25 (0.23, 0.29) | <0.001 |
| Medium LDL | 0.20 (0.19, 0.22) | 0.17 (0.15, 0.19) | 0.02 |
| Extra-large HDL | 0.16 (0.13, 0.20) | 0.10 (0.07, 0.14) | 0.02 |
| Small HDL | 0.54 (0.52, 0.56) | 0.65 (0.62, 0.69) | <0.0001 |
| Cholesterol | |||
| Extremely large VLDL | 0.001 (0.001, 0.002) | 0.004 (0.002, 0.007) | <0.001 |
| Extra-large VLDL | 0.005 (0.003, 0.008) | 0.011 (0.007, 0.018) | <0.001 |
| Large VLDL | 0.03 (0.02, 0.04) | 0.06 (0.04, 0.09) | <0.001 |
| Extra-small VLDL | 0.21 (0.19, 0.23) | 0.17 (0.15, 0.19) | <0.001 |
| IDL | 0.68 (0.61, 0.75) | 0.49 (0.41, 0.57) | <0.01 |
| Large LDL | 0.90 (0.80, 1.01) | 0.62 (0.51, 0.74) | <0.0001 |
| Medium LDL | 0.54 (0.48, 0.61) | 0.37 (0.30, 0.45) | <0.01 |
| Small LDL | 0.33 (0.29, 0.37) | 0.23 (0.19, 0.27) | <0.01 |
| Cholesterol esters | |||
| Extra-large VLDL | 0.003 (0.002, 0.004) | 0.006 (0.004, 0.010) | <0.001 |
| Large VLDL | 0.02 (0.01, 0.03) | 0.03 (0.02, 0.04) | <0.001 |
| Extra-small VLDL | 0.14 (0.13, 0.16) | 0.11 (0.10, 0.13) | <0.001 |
| IDL | 0.48 (0.43, 0.53) | 0.36 (0.30, 0.42) | <0.01 |
| Large LDL | 0.64 (0.57, 0.73) | 0.44 (0.36, 0.53) | <0.001 |
| Medium LDL | 0.39 (0.34, 0.45) | 0.25 (0.20, 0.31) | <0.01 |
| Small LDL | 0.24 (0.21, 0.27) | 0.16 (0.13, 0.19) | <0.001 |
| Small HDL | 0.40 (0.38, 0.43) | 0.36 (0.33, 0.39) | 0.02 |
| Free cholesterol | |||
| Extremely large VLDL | 0.0004 (0.0003, 0.0007) | 0.0024 (0.0014, 0.0043) | <0.0001 |
| Extra-large VLDL | 0.002 (0.001, 0.004) | 0.005 (0.003, 0.009) | <0.001 |
| Large VLDL | 0.01 (0.01, 0.02) | 0.04 (0.02, 0.05) | <0.001 |
| Medium VLDL | 0.05 (0.03, 0.06} | 0.08 (0.06, 0.10) | <0.001 |
| Small VLDL | 0.07 (0.06, 0.07) | 0.08 (0.07, 0.10) | 0.04 |
| Extra-small VLDL | 0.07 (0.06, 0.07) | 0.06 (0.05, 0.07) | 0.03 |
| IDL | 0.20 (0.18, 0.22) | 0.13 (0.11, 0.16) | <0.0001 |
| Large LDL | 0.26 (0.23, 0.28) | 0.18 (0.15, 0.21) | <0.0001 |
| Medium LDL | 0.15 (0.14, 0.16) | 0.12 (0.10, 0.13) | <0.0001 |
| Small LDL | 0.09 (0.08, 0.10) | 0.07 (0.06, 0.08) | <0.01 |
| Extra-large HDL | 0.05 (0.04, 0.06) | 0.03 (0.02, 0.04) | <0.01 |
| Large HDL | 0.05 (0.04, 0.06) | 0.03 (0.02, 0.04) | 0.04 |
| Small HDL | 0.10 (0.10, 0.11) | 0.12 (0.11, 0.12) | <0.0001 |
| Triglycerides | |||
| Extremely large VLDL | 0.01 (0.008, 0.013) | 0.03 (0.020, 0.039) | <0.0001 |
| Extra-large VLDL | 0.02 (0.02, 0.03) | 0.06 (0.04, 0.09) | <0.0001 |
| Large VLDL | 0.10 (0.08, 0.13) | 0.22 (0.17, 0.30) | <0.0001 |
| Medium VLDL | 0.22 (0.18, 0.27) | 0.39 (0.31, 0.51) | <0.0001 |
| Small VLDL | 0.19 (0.17, 0.22) | 0.28 (0.24, 0.34) | <0.0001 |
| Extra-small VLDL | 0.09 (0.08, 0.10) | 0.12 (0.10, 0.13) | 0.02 |
| Medium HDL | 0.03 (0.03, 0.04) | 0.05 (0.04, 0.05) | <0.001 |
| Small HDL | 0.04 (0.04, 0.05) | 0.06 (0.05, 0.07) | <0.0001 |
Values are model-based means with 95% confidence intervals in parentheses and are given in mmol/l; number of subjects n = 44 (all men). HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LDL, low-density lipoprotein; T2D, type 2 diabetes; VLDL, very low density lipoprotein.
Women were excluded from the analysis to avoid mixing effects of sex.
LFC, liver volume, whole liver GU, EGP, and liver enzyme levels [ALAT, ASAT, and γ-glutamyltranspeptidase (GT)] were higher in the men with prediabetes/T2D compared with the normoglycemic men (Fig. 3A and Table 2). After training, there was a significant reduction in liver enzyme (ALAT, ASAT, and GT) and C-reactive protein levels without any differences between the groups or training modes (Table 2). No training response was observed in liver GU, FAU, or EGP in either of the groups.
Regarding LFC, the training response differed between the normoglycemic men and men with prediabetes/T2D (Dia × time P = 0.03), with a tendency to reduce LFC in the men with prediabetes/T2D (P = 0.051 time effect for men with prediabetes/T2D; Fig. 3A). During further data analysis, we observed that in the normoglycemic group, seven subjects had LFC above 5.6%, which has been recommended as the cutoff value for normal LFC (42), whereas seven subjects with prediabetes/T2D had LFC below 5.6%. Next, we pooled all subjects (men and women) together and divided them into low (<5.6%)- and high (>5.6%)-LFC groups. The high-LFC group had 522% higher LFC (Fig. 3B) compared with the low-LFC group. After training, LFC was reduced (by −13%, P = 0.009) only in the high-LFC group (Fig. 3B). LFC correlated negatively with whole body insulin sensitivity in all subjects before and after the intervention (preintervention: r = −0.67, P < 0.001; postintervention: r = −0.62, P < 0.001). Interestingly, in the same comparison between high-LFC and low-LFC groups we saw that MICT improved insulin-stimulated liver GU by 7%, whereas no change was observed after SIT (Fig. 4). There were no differences between SIT and MICT in any other parameters except fasting plasma FFA levels, which were reduced significantly only after MICT (Table 5). We found no differences in EGP. In the low-LFC group, EGP correlated inversely with aerobic capacity (r = −0.62, P < 0.01).
DISCUSSION
We studied the effects of high-intensity, low-volume SIT and low-intensity, high-volume MICT on LFC, lipoprotein subclasses, and liver metabolism in untrained, middle-aged subjects with normoglycemia or prediabetes/T2D using magnetic resonance spectroscopy, NMR, and PET. As expected, the group with prediabetes/T2D had higher LFC and liver enzyme levels and an impaired lipoprotein profile compared with normoglycemic subjects (men only) at baseline. However, contrary to our hypothesis, no differences were found in the liver substrate uptake between the normoglycemic group and the group with prediabetes/T2D. After a 2-wk training intervention, both SIT and MICT reduced LFC, liver enzyme levels, and inflammatory markers in subjects with prediabetes/T2D (men only) or subjects with high LFC (men and women). Training improved lipoprotein subclass profile similarly in all subjects regardless of training mode. MICT increased liver insulin-stimulated GU, and there was a nonsignificant reduction in liver FFA uptake, whereas no changes were found after SIT (men and women). The effects of training on liver substrate uptake were independent of baseline glucose tolerance or LFC.
MICT improves liver insulin sensitivity and leads to a nonsignificant reduction in liver FFA uptake.
Contrary to our hypothesis, we did not find baseline differences in insulin-stimulated liver GU when expressed per 100 g of tissue between the normoglycemic group and the group with prediabetes/T2D (men only) or between the high-LFC and low-LFC groups (men and women). Previous data from our and other groups have shown both similar (47) and impaired insulin-stimulated liver GU in subjects with T2D (15) and increased LFC (15, 33). In the studies showing impaired insulin-stimulated liver GU the subjects were older than the subjects in this study and the LFC was higher than 20%, which may explain discrepancies between this and previous studies (15, 33).
One of the key findings in the present study was the improvement in liver GU (men and women) after training in the MICT, but not the SIT, group. This finding agrees with our recent data regarding intestinal insulin sensitivity with the same subjects (29), where the insulin-stimulated colonic GU improved only after MICT and not after SIT. One explanation for this finding might be the difference in energy expenditure between SIT and MICT. When we calculated the energy consumption for all training sessions based on the effective training time, MICT had ∼691% higher total energy consumption compared with SIT [SIT 392 (355, 429) and MICT 2,710 (2,474, 2,946) kcal; means (95% confidence intervals)]. However, according to Skelly et al. the 24-h energy expenditure is comparable between SIT and MICT because of the higher posttraining energy expenditure after SIT (38). This might explain the similar results we found for most of the parameters in our study.
Another explanation for the difference between the training modes could be the negative association between liver GU and the plasma FFA level (16). It has been shown that increase in the plasma FFA level impairs the insulin-stimulated liver GU (16). This is because plasma FFAs have an allosteric inhibitory effect on glucokinase enzyme (which phosphorylates glucose), which leads to less trapping of glucose inside the liver cells resulting in a lower liver GU. Interestingly, in our study, plasma FFA levels were reduced only in the MICT group, possibly explaining the increase in liver GU in the MICT group only. Moreover, change in fasting plasma FFA levels correlated inversely with the change in liver GU only in the MICT group (r = −0.60, P = 0.01).
Liver plays a very important role in the whole body FFA metabolism; each milliliter of liver has been shown to utilize almost 50 times more FFAs compared with 1 g of muscle (17). Therefore, even small changes in liver FFA metabolism warrant attention. There was a nonsignificant decrease in liver FAU (P = 0.10) after MICT, but not after SIT, in our study (men and women). This decrease in FAU only after MICT is probably due to the greater reduction in the circulating fasting FFA levels due to higher training volumes and energy expenditure than in SIT (6). As most of the liver FAU occurs during the postprandial period (13, 46), it is possible that the training period was too short to induce training responses great enough to be detectable under fasting conditions. Unfortunately, because of radiation dose limitations, liver FAU was measured only under fasting conditions in the present study.
Reduction in LFC.
Regarding LFC, the training response differed between the normoglycemic group and the group with prediabetes/T2D (men), with a nonsignificant reduction in LFC in the group with prediabetes/T2D (P = 0.051), whereas no change was observed in the normoglycemic group. Interestingly, when we further divided the subjects (men and women) into high (LFC >5.6%)- and low (LFC <5.6%)-LFC groups (42), we saw that just 2 wk of exercise training reduced LFC by −13% in subjects with high LFC to start with. However, there was no reduction in LFC in the low-LFC group. In the present study the training intervention was short, consisting only of six training sessions and thus probably not long enough to reduce LFC in subjects with LFC already at a normal level. The tendency to decrease LFC in the group with prediabetes/T2D (men) and the significant decrease in LFC in the high-LFC group (men and women) were independent of the training mode in both comparisons. This finding agrees with a recent study done on subjects with obesity and nonalcoholic fatty liver disease in which the subjects performed 4 wk of either high-intensity interval training or MICT; the study showed that both training modes reduced LFC without any differences between training modes (49). Overall notable in this study is that people who are not that obese (body mass index below or slightly above 30) already have high LFC and complications of diabetes.
Improvement in lipoprotein profile and protection against diabetes.
At baseline, the lipoprotein profile was significantly impaired in the group with prediabetes/T2D compared with the normoglycemic group (men only). However, no changes were observed in VLDL subclasses and their components. This is probably because VLDLs have a faster turnover rate compared with LDLs and HDLs and the effects of acute and long-term exercise training on VLDLs have been shown to be temporary and disappear within a few hours after the last exercise session (30, 40). However, all the IDL (except the IDL triglyceride content) and LDL subclasses and components decreased without any differences between the normoglycemic group and the group with prediabetes/T2D (men only; Fig. 2, A–F). Thus, short-term exercise training improves the lipoprotein profile not only in subjects with a normal lipoprotein profile but also in subjects with an impaired lipoprotein profile regardless of their baseline glucose tolerance. Interestingly, both SIT and MICT had a protective effect for diabetes by efficiently reducing the concentration of lipoproteins associated with risk of acquiring diabetes and improving the concentration of those associated with diabetes prevention. In our study, the reduction in the small-LDL composition is noteworthy as it has been shown that smaller LDL particles are associated with the risk of acquiring diabetes (28). Meanwhile, for HDL subclasses, we saw an improvement in the concentration of large-HDL subclasses and reduction in the concentration of the small-HDL subclasses. The improvement in concentration of large HDLs is vital as it has been shown in previous studies that the larger HDLs carry a lower risk of acquiring diabetes, whereas the smaller HDLs carry a high risk of acquiring diabetes (28). The changes in these subfractions are significant, as Garvey et al. have also demonstrated an association between the progression of insulin resistance and the increase in VLDL, LDL, and small-HDL concentrations (9). Additionally, we found a positive correlation between extra-large HDLs and M-value and negative correlations between extra-large HDLs and liver volume and ALAT, whereas with small HDLs we found an interesting positive correlation with LFC (Table 4).
Limitations.
There are some limitations in this study that warrant consideration. Subjects were only asked to maintain their normal dietary habits, and no diet control was performed; thus the effect of diet on weight reduction and body adiposity posttraining cannot be ruled out when critically interpreting the data. Also, the findings for V̇o2peak need to be interpreted in relation to possible measurement error (12). Liver GU and FAU were studied in different metabolic environments, [18F]FDG during euglycemic-hyperinsulinemic clamp and [18F]FTHA during fasting, corresponding to the conditions under which GU and FAU are at their highest, respectively. Because of radiation dose limitations, it was not possible to perform [18F]FDG and [18F]FTHA studies both during fasting and during clamp. No control subjects were included in the study. The power calculations were made for the whole study (NCT01344928) based on its primary outcome, skeletal muscle GU, and no sample size calculation was performed specifically for the measures of the present study. Finally, the duration of the training intervention was only 2 wk, and likely more differences would be revealed with a longer training period.
Conclusion.
In conclusion, training reduced LFC in subjects with prediabetes/T2D and subjects with fatty liver but not in subjects with normoglycemia or low LFC. Training improved lipoprotein profile, by reducing the concentration of lipoproteins associated with risk of acquiring T2D and improving the concentration of those associated with diabetes prevention, and liver insulin sensitivity regardless of baseline glucose tolerance. Regarding the training modes, MICT was more effective in improving liver insulin sensitivity compared with SIT, whereas the training mode had no effect on LFC or lipoprotein profile.
GRANTS
This work was supported by grants from Orion Research Foundation to K. K. Motiani and J. C. Hannukainen, Suomen Kulttuurirahasto (Finnish Cultural Foundation) to K. K. Motiani, Juho Vainion Säätiö (Juho Vainio Foundation) to K. K. Motiani, and Finnish Cultural Foundation Varsinais-Suomen Rahasto (Varsinais-Suomi Regional Fund) to K. K. Motiani, Suomen Akatemia (Academy of Finland) Grants 251399, 251572, 256470, 281440, and 283319 to K. K. Kalliokoski, and grants from European Foundation for the Study of Diabetes to J. C. Hannukainen, Diabetesliitto (Diabetes Research Foundation) to J. C. Hannukainen, Hospital District of Southwest Finland to J. C. Hannukainen, Ministry of Education of the State of Finland to J. C. Hannukainen, Paavo Nurmi Foundation to K. K. Kalliokoski, Novo Nordisk to K. K. Kalliokoski, Paulo Foundation to K. K. Kalliokoski, and Turku University Foundation to J.-J. Eskelinen. The study also received funding from the Emil Aaltonen Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.K. and J.C.H. conceived and designed research; A.M.S., J.T., J.-J.E., M.Y.-K., R.P., M.H.-S., O.S., N.S., K.K.K., and J.C.H. performed experiments; K.K.M., J.-J.E., and V.S. analyzed data; K.K.M., E.L., K.K.K., and J.C.H. interpreted results of experiments; K.K.M. prepared figures; K.K.M. drafted manuscript; K.K.M., A.M.S., J.T., J.-J.E., M.Y.-K., K.A.V., M.A.H., P.N., K.K.K., and J.C.H. edited and revised manuscript; K.A.V., P.N., K.K.K., and J.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the staff at Turku PET Centre, University of Turku, Åbo Akademi University, and Paavo Nurmi Centre for their excellent contributions in the completion of this study.
REFERENCES
- 1.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35: 1490–1496, 1994. [PubMed] [Google Scholar]
- 2.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care 38, Suppl 1: S8–S16, 2015. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 3.Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. BMC Endocr Disord 9: 3, 2009. doi: 10.1186/1472-6823-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borra R, Lautamäki R, Parkkola R, Komu M, Sijens PE, Hällsten K, Bergman J, Iozzo P, Nuutila P. Inverse association between liver fat content and hepatic glucose uptake in patients with type 2 diabetes mellitus. Metabolism 57: 1445–1451, 2008. doi: 10.1016/j.metabol.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 6.Eskelinen JJ, Heinonen I, Löyttyniemi E, Saunavaara V, Kirjavainen A, Virtanen KA, Hannukainen JC, Kalliokoski KK. Muscle-specific glucose and free fatty acid uptake after sprint interval and moderate-intensity training in healthy middle-aged men. J Appl Physiol (1985) 118: 1172–1180, 2015. doi: 10.1152/japplphysiol.01122.2014. [DOI] [PubMed] [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. [PubMed] [Google Scholar]
- 8.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP; American College of Sports Medicine . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 43: 1334–1359, 2011. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 9.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 52: 453–462, 2003. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 9a.Gibala MJ, Little JP, Van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short‐term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol 575: 901–911, 2006. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60: 1278–1283, 2011. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halverstadt A, Phares DA, Wilund KR, Goldberg AP, Hagberg JM. Endurance exercise training raises high-density lipoprotein cholesterol and lowers small low-density lipoprotein and very low-density lipoprotein independent of body fat phenotypes in older men and women. Metabolism 56: 444–450, 2007. doi: 10.1016/j.metabol.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Heiskanen MA, Sjöros TJ, Heinonen IH, Löyttyniemi E, Koivumäki M, Motiani KK, Eskelinen JJ, Virtanen KA, Knuuti J, Hannukainen JC, Kalliokoski KK. Sprint interval training decreases left-ventricular glucose uptake compared to moderate-intensity continuous training in subjects with type 2 diabetes or prediabetes. Sci Rep 7: 10531, 2017. doi: 10.1038/s41598-017-10931-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh J, Hayashi AA, Webb J, Adeli K. Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atheroscler Suppl 9: 7–13, 2008. doi: 10.1016/j.atherosclerosissup.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Iozzo P, Gastaldelli A, Järvisalo MJ, Kiss J, Borra R, Buzzigoli E, Viljanen A, Naum G, Viljanen T, Oikonen V, Knuuti J, Savunen T, Salvadori PA, Ferrannini E, Nuutila P. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: a validation study. J Nucl Med 47: 1016–1022, 2006. [PubMed] [Google Scholar]
- 15.Iozzo P, Hallsten K, Oikonen V, Virtanen KA, Kemppainen J, Solin O, Ferrannini E, Knuuti J, Nuutila P. Insulin-mediated hepatic glucose uptake is impaired in type 2 diabetes: evidence for a relationship with glycemic control. J Clin Endocrinol Metab 88: 2055–2060, 2003. doi: 10.1210/jc.2002-021446. [DOI] [PubMed] [Google Scholar]
- 16.Iozzo P, Lautamaki R, Geisler F, Virtanen KA, Oikonen V, Haaparanta M, Yki-Jarvinen H, Ferrannini E, Knuuti J, Nuutila P. Non-esterified fatty acids impair insulin-mediated glucose uptake and disposition in the liver. Diabetologia 47: 1149–1156, 2004. doi: 10.1007/s00125-004-1443-2. [DOI] [PubMed] [Google Scholar]
- 17.Iozzo P, Turpeinen AK, Takala T, Oikonen V, Solin O, Ferrannini E, Nuutila P, Knuuti J. Liver uptake of free fatty acids in vivo in humans as determined with 14(R,S)-[18F]fluoro-6-thia-heptadecanoic acid and PET. Eur J Nucl Med Mol Imaging 30: 1160–1164, 2003. doi: 10.1007/s00259-003-1215-0. [DOI] [PubMed] [Google Scholar]
- 18.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112, 2009. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 19.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 285: E906–E916, 2003. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med 164: 2169–2175, 2004. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 21.Kiviniemi AM, Tulppo MP, Eskelinen JJ, Savolainen AM, Kapanen J, Heinonen IH, Huikuri HV, Hannukainen JC, Kalliokoski KK. Cardiac autonomic function and high-intensity interval training in middle-age men. Med Sci Sports Exerc 46: 1960–1967, 2014. doi: 10.1249/MSS.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 22.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 293: E1709–E1715, 2007. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 347: 1483–1492, 2002. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 24.Kujala UM. Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med 43: 550–555, 2009. doi: 10.1136/bjsm.2009.059808. [DOI] [PubMed] [Google Scholar]
- 25.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588: 1011–1022, 2010. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackey RH, Greenland P, Goff DC Jr, Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 60: 508–516, 2012. doi: 10.1016/j.jacc.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mäki MT, Haaparanta M, Nuutila P, Oikonen V, Luotolahti M, Eskola O, Knuuti JM. Free fatty acid uptake in the myocardium and skeletal muscle using fluorine-18-fluoro-6-thia-heptadecanoic acid. J Nucl Med 39: 1320–1327, 1998. [PubMed] [Google Scholar]
- 28.Mora S, Otvos JD, Rosenson RS, Pradhan A, Buring JE, Ridker PM. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 59: 1153–1160, 2010. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motiani KK, Savolainen AM, Eskelinen JJ, Toivanen J, Ishizu T, Yli-Karjanmaa M, Virtanen KA, Parkkola R, Kapanen J, Grönroos TJ, Haaparanta-Solin M, Solin O, Savisto N, Ahotupa M, Löyttyniemi E, Knuuti J, Nuutila P, Kalliokoski KK, Hannukainen JC. Two weeks of moderate-intensity continuous training, but not high-intensity interval training, increases insulin-stimulated intestinal glucose uptake. J Appl Physiol (1985) 122: 1188–1197, 2017. doi: 10.1152/japplphysiol.00431.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nellemann B, Christensen B, Vissing K, Thams L, Sieljacks P, Larsen MS, Jørgensen JO, Nielsen S. Ten weeks of aerobic training does not result in persistent changes in VLDL triglyceride turnover or oxidation in healthy men. Eur J Endocrinol 171: 603–613, 2014. doi: 10.1530/EJE-14-0333. [DOI] [PubMed] [Google Scholar]
- 31.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3: 1–7, 1983. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 32.Rigazio S, Lehto HR, Tuunanen H, Någren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P. The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab 295: E413–E419, 2008. doi: 10.1152/ajpendo.00744.2007. [DOI] [PubMed] [Google Scholar]
- 33.Rijzewijk LJ, van der Meer RW, Lubberink M, Lamb HJ, Romijn JA, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Liver fat content in type 2 diabetes: relationship with hepatic perfusion and substrate metabolism. Diabetes 59: 2747–2754, 2010. doi: 10.2337/db09-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenson RS, Underberg JA. Systematic review: evaluating the effect of lipid-lowering therapy on lipoprotein and lipid values. Cardiovasc Drugs Ther 27: 465–479, 2013. doi: 10.1007/s10557-013-6477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savisto N, Viljanen T, Kokkomäki E, Bergman J, Solin O. Automated production of [18 F]FTHA according to GMP. J Labelled Comp Radiopharm 61: 84–93, 2018. doi: 10.1002/jlcr.3589. [DOI] [PubMed] [Google Scholar]
- 36.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87: 3023–3028, 2002. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 37.Sjoros TJ, Heiskanen MA, Motiani KK, Loyttyniemi E, Eskelinen JJ, Virtanen KA, Savisto NJ, Solin O, Hannukainen JC, Kalliokoski KK. Increased insulin-stimulated glucose uptake in both leg and arm muscles after sprint interval and moderate-intensity training in subjects with type 2 diabetes or prediabetes. Scand J Med Sci Sports 28: 77–87, 2018. doi: 10.1111/sms.12875. [DOI] [PubMed] [Google Scholar]
- 38.Skelly LE, Andrews PC, Gillen JB, Martin BJ, Percival ME, Gibala MJ. High-intensity interval exercise induces 24-h energy expenditure similar to traditional endurance exercise despite reduced time commitment. Appl Physiol Nutr Metab 39: 845–848, 2014. doi: 10.1139/apnm-2013-0562. [DOI] [PubMed] [Google Scholar]
- 39.Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 8: 192–206, 2015. doi: 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 40.Sondergaard E, Rahbek I, Sørensen LP, Christiansen JS, Gormsen LC, Jensen MD, Nielsen S. Effects of exercise on VLDL-triglyceride oxidation and turnover. Am J Physiol Endocrinol Metab 300: E939–E944, 2011. doi: 10.1152/ajpendo.00031.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.St. George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology 50: 68–76, 2009. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 42.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–E468, 2005. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 43.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 54: 3541–3546, 2005. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 44.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care 29: 1845–1850, 2006. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 45.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Med Sci Sports Exerc 34: 1996–2001, 2002. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 46.van Hees AM, Jans A, Hul GB, Roche HM, Saris WH, Blaak EE. Skeletal muscle fatty acid handling in insulin resistant men. Obesity (Silver Spring) 19: 1350–1359, 2011. doi: 10.1038/oby.2011.10. [DOI] [PubMed] [Google Scholar]
- 47.Viljanen AP, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, Järvisalo M, Parkkola R, Rönnemaa T, Guiducci L, Lehtimäki T, Raitakari OT, Mari A, Nuutila P. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab 94: 50–55, 2009. doi: 10.1210/jc.2008-1689. [DOI] [PubMed] [Google Scholar]
- 48.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. doi: 10.1136/bjsports-2013-092576. [DOI] [PubMed] [Google Scholar]
- 49.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity: a randomized trial. Metabolism 78: 128–140, 2018. doi: 10.1016/j.metabol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Yki-Järvinen H. Fat in the liver and insulin resistance. Ann Med 37: 347–356, 2005. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]