Abstract
Noncanonical roles for caspase-3 are emerging in the fields of cancer and developmental biology. However, little is known of nonapoptotic functions of caspase-3 in most cell types. We have recently demonstrated a disassociation between caspase-3 activation and execution of apoptosis with accompanying cytoplasmic caspase-3 sequestration and preserved endothelial barrier function. Therefore, we tested the hypothesis that nonapoptotic caspase-3 activation promotes endothelial barrier integrity. Human lung microvascular endothelial cells were exposed to thrombin, a nonapoptotic stimulus, and endothelial barrier function was assessed using electric cell-substrate impedance sensing. Actin cytoskeletal rearrangement and paracellular gap formation were assessed using phalloidin staining. Cell stiffness was evaluated using magnetic twisting cytometry. In addition, cell lysates were harvested for protein analyses. Caspase-3 was inhibited pharmacologically with pan-caspase and a caspase-3-specific inhibitor. Molecular inhibition of caspase-3 was achieved using RNA interference. Cells exposed to thrombin exhibited a cytoplasmic activation of caspase-3 with transient and nonapoptotic decrease in endothelial barrier function as measured by a drop in electrical resistance followed by a rapid recovery. Inhibition of caspases led to a more pronounced and rapid drop in thrombin-induced endothelial barrier function, accompanied by increased endothelial cell stiffness and paracellular gaps. Caspase-3-specific inhibition and caspase-3 knockdown both resulted in more pronounced thrombin-induced endothelial barrier disruption. Taken together, our results suggest cytoplasmic caspase-3 has nonapoptotic functions in human endothelium and can promote endothelial barrier integrity.
Keywords: barrier function, caspase-3, cytoskeleton, endothelium, human lung microvascular endothelial cells, nonapoptotic, thrombin
INTRODUCTION
Apoptosis is an active form of programmed cell death that leads to cellular dismantling without eliciting an inflammatory response (23). Caspases are a family of cysteine proteases that regulate the complex biological process of apoptosis. Caspases are expressed as inactive zymogens but, upon activation (e.g., cleavage), amplify death stimuli via initiator caspases, caspase-2 and -9 (intrinsic pathway) and caspase-8 and -10 (extrinsic pathway), and lead to dismantling of the cell via caspase-3 and -7 (7). Whereas caspase-3 and -7 are both executioner caspases, they have distinct substrate preferences, with caspase-3 being recognized as the major executioner caspase during apoptosis (53). Traditionally, activation of caspase-3 has heralded cell death (7, 25, 30, 53). A pathogenic role for exuberant apoptosis has been suggested in many disease states, including pulmonary vascular diseases (24, 28, 31).
Endothelial cells create a selective barrier isolating the contents of the vascular compartment from the interstitium. In lungs, injury to this barrier leads to increased extravasation of proteinaceous fluid and inflammatory cell recruitment from the intravascular space in interstitial and alveolar spaces, causing profound hypoxemia (19, 21, 42, 51), the hallmark of the acute respiratory distress syndrome (ARDS) (54). Sepsis, an overwhelming bloodstream infection, is a major cause of ARDS, contributing up to ~80% of ARDS cases with mortality approaching 40% (47). Endothelial barrier dysfunction in sepsis is a complex phenomenon involving cytoskeletal changes in response to proinflammatory mediators, where the actin cytoskeleton plays a pivotal role in mediating endothelial barrier function and permeability (19, 42a). Complementarily, endothelial cell apoptosis has been identified as a key feature in sepsis-induced vascular dysfunction and demonstrated to be a key determinant of vascular permeability (24, 28, 31). We have identified endothelial cells as the main cellular compartment undergoing apoptotic cell death during endotoxemia-induced lung injury (16, 48). To that end, inhibition of caspases, or caspase-3 specifically, attenuates apoptosis and can protect against many forms of lung injury (16, 24, 31, 40). These data would suggest a completely pathogenic role for caspase-3 activation. However, caspase-3-deficient mice are not protected from endotoxin-induced mortality (39, 52), suggesting possible alternative roles for caspase-3.
Our group has demonstrated in an endotoxemia model of lung injury that caspase-3 activation is required for induction of lung injury (16). Interestingly, loss of mitogen-activated protein kinase-activated protein kinase 2 (MK2) signaling, in vivo and in vitro (in endothelial cells), disassociated caspase-3 activation from execution of apoptosis (16). Specifically, loss of MK2 signaling did not prevent caspase-3 activation but rather led to cytoplasmic sequestration of active/cleaved caspase-3 that was associated with prevention of apoptosis and endotoxemia-induced lung injury (16). These findings suggest that preventing nuclear translocation of caspase-3 reduces apoptosis and resultant lung injury, or, more intriguingly, when apoptosis is absent, cytoplasmic caspase-3 may function in a barrier-protective manner.
Therefore, we sought to test the hypothesis that, during nonapoptotic endothelial barrier disruption, caspase-3 promotes barrier integrity.
EXPERIMENTAL PROCEDURES
Reagents and Tissues
Human lung microvascular endothelial cells.
Primary human lung microvascular endothelial cells (HLMVECs) were derived from individual donors (Lonza, Walkersville, MD) and maintained according to the manufacturer's recommendations. Cells were analyzed between passages 4 and 6 and were cultured in complete media with 10% FBS (Lonza). Cells from Lonza were used for experiments in Fig. 1, A and B.
Fig. 1.
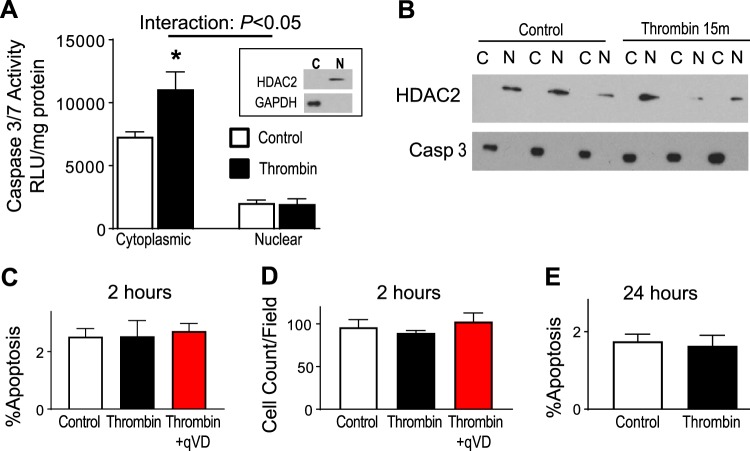
Thrombin induces cytoplasmic caspase-3 (Casp 3) activation without cell death. A: human lung microvascular endothelial cells (HLMVECs) were exposed to 1.25 U/ml of thrombin for 15 min, and cells were harvested for cytoplasmic and nuclear preparations for assessment of caspase-3 activity. Cytoplasmic fractions demonstrate higher caspase-3 activity than nuclear fractions. Thrombin stimulation leads to an increase in caspase-3 activity only within the cytoplasmic fraction. Purity of cytosolic (C) and nuclear (N) fractions was assessed by exclusion of GAPDH and histone deacetylase (HDAC)-2 immunoblotting, respectively (inset); n = 3. B: nuclear and cytosolic fractions were immunoprobed for caspase-3 expression. Caspase-3 is present within the cytosolic fraction under control conditions and remains in the cytosol even after thrombin stimulation. C and D: HLMVECs were exposed to 1.25 U/ml of thrombin for 2 h, and Hoechst 33342 staining was used to measure apoptosis (by nuclear condensation and fragmentation) and cell dropout (by cell counts). Caspase inhibition in HLMVECs did not result in increased apoptosis with thrombin exposure (C) or cell dropout (D); n = 4; 4,925–5,502 cells counted/group. E: HLMVECs were exposed to 1.25 U/ml of thrombin for 24 h, and Hoechst 33342 staining was used to measure apoptosis. Thrombin exposure did not result in increased apoptosis; n = 4. RLU, relative light units; qVD, q-VD-OPH. *P < 0.05 vs. control.
Because of a shortage of proprietary culture media (Lonza), subsequent experiments were performed using primary cells obtained from Cell Biologics (Chicago, IL). As these cells are only isolated using CD31 selection, we performed further subselection using Griffonia simplicifolia lectin 1, to obtain microvascular endothelial cells (HLMVECs), as previously described (50). Based on availability, our experiments are performed on HLMVECs derived from a total of three human donors, with one purchased from Lonza and two from Cell Biologics.
Pharmacological inhibitors.
Inhibition of caspase-3 was achieved using two inhibitors, q-VD-OPH (qVD; APExBIO, Houston, TX) and z-DEVD-FMK (DEVD; Cayman Chemical, Ann Arbor, MI). Although qVD can inhibit other caspases, it has higher specificity for caspase-3 than other caspases, with 17.2-fold higher specificity than for caspase-9 (12). DEVD is a caspase-3-specific inhibitor (29). Both agents are cell permeable and irreversibly bind the active site to inhibit substrate cleavage. Doses were chosen based on previous publications.
siRNA.
Duplex RNAs encoding nontargeting negative control small-interfering (si) RNA (On-Target Plus, NonTargeting Pool) and siRNA directed against human caspase-3 were used for RNA interference (RNAi) and were manufactured by Dharmacon (Lafayette, CO). Four duplex siRNAs that target caspase-3 were screened to detect suppression of total caspase-3. The transfection of duplex RNA was performed using Geneporter B reagent (Genelantis, San Diego, CA), according to the manufacturer's recommendations. HLMVECs were plated at a density of 1 × 105/cm2 and transfected as previously described (17). The final concentration of RNA duplexes was 50 nM. siRNA number J-004307–06–0002 (target sequence, CCGACAAGCUUGAAUUUAU) had the most effect in suppression of caspase-3 (data not shown) and was subsequently used for all knockdown studies. Cells were plated in six-well dishes and transfected with siRNA. The following day cells were trypsinized, pooled, and replated into Electrical Cell-substrate Impedance Sensing System (ECIS) plates (see below). An aliquot of cells was harvested for confirmation of caspase-3 knockdown.
Nuclear and cytoplasmic fractions of cells were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Rockford, IL) as per the manufacturer’s instructions. Immunoblotting to detect protein expression was performed using standard techniques. Antibodies directed against total caspase-3 (no. 9662) and β-actin (no. 12620) (Cell Signaling, Boston, MA) were used as per the manufacturer’s recommendations. Caspase-3 activity was measured using the Caspase-Glo 3/7 Assay (Promega, Madison, WI) as per the manufacturer’s instructions.
Exposure to thrombin.
HLMVECs were plated, and the following morning culture media was changed to include pharmacological inhibitors, as noted in each experiment. After a 2-h stabilization period, thrombin (product no. T4393; Sigma, St. Louis, MO) was added at a concentration of 1.25 U/ml. Previously, thrombin dosing was expressed as a concentration, typically in the nanomolar range (32, 34). More recently, in hopes of standardizing dosing based on its activity, thrombin is sold as National Institutes of Health (NIH) units per milligram of protein. Thrombin activity is expressed in NIH units obtained by direct comparison with NIH thrombin reference standard units. We have identified dosing of thrombin from 0.2 to 1.0 U/ml in the literature (6, 13, 22). We empirically chose a higher dose of 1.25 U/ml.
Measurements
Endothelial barrier function.
Twenty thousand cells were plated on 0.1% gelatin-coated gold-plated electrodes, and agonist-induced electrical resistance, as a marker of barrier integrity, was measured using an ECIS (Applied Biophysics, Troy, NY), as previously described (20). Pooled data from individual wells are shown as summation plots, and the maximum drop in transendothelial electrical resistance (TERMAX) from individual wells is used for statistical analyses.
Paracellular gaps.
Cells were grown on 0.1% gelatin-coated MatTek glass-bottom plates (Ashland, MA) and exposed to thrombin, described above. After exposures, cells were fixed with paraformaldehyde and permeabilized with Triton X. Polymerized actin was stained using Texas Red-phalloidin at a ratio of 1:200. A blinded investigator (Carino) obtained random images of each condition. Paracellular gaps were quantified using ImageJ.
Magnetic twisting cytometry.
Cells were grown to confluence in 0.1% gelatin-coated glass-bottom 96-well strip-well plates and incubated with arginine-glycine-aspartate-coated ferrimagnetic beads (~5 μm diameter) for 20 min. Wells were washed three times with media to remove unattached beads. After a 2-h stabilization period in basal media, thrombin was added. Beads are magnetized in a plane parallel to the stage and twisted perpendicularly. Beads are directly visualized, and their displacement is measured and used to calculate the overall stiffness of the cells, as previously described (2).
Statistics
Data are shown as means ± SE. Nonparametric tests were used to not assume normal distribution of data. Comparison between two groups was performed using Mann-Whitney tests. We performed multiple comparisons by analysis of variance with Tukey’s post hoc testing. A P value of <0.05 was considered significant. Data were analyzed using GraphPad Prism 7.
RESULTS
Thrombin Induces Cytoplasmic Caspase-3 Activation Without Cell Death
Because we wanted to explore a potential barrier-protective effect for caspase-3, we sought an injury model not mediated by apoptosis to avoid confounding by the canonical functions of caspase-3 (53). Thrombin-induced endothelial barrier disruption is a well-described model that induces rapid cytoskeletal reorganization, resulting in barrier disruption, with maximal barrier disruption at ~15–30 min (4, 5, 19). This model system is ideally suited for our experimental design, since thrombin stimulation also rapidly activates caspase-3 (1, 46). As shown in Fig. 1A, human lung microvascular endothelial cells (HLMVECs) exposed to thrombin for 15 min (time of maximal barrier disruption) exhibited minimal caspase-3 activity within the nuclear fractions of HLMVECs compared with cytosolic fractions. Additionally, only cytosolic caspase-3 activity increases with thrombin stimulation. This would imply that any inhibition of caspase-3 would inhibit the cytosolic fraction, as activity within this pool increases with stimulation. To further confirm this finding, nuclear and cytosolic fractions were immunoprobed for caspase-3 expression. As shown in Fig. 1B, caspase-3 expression is restricted to the cytosolic fraction in HLMVECs at baseline conditions and does not translocate to the nucleus following thrombin stimulation. These results suggest any manipulation to caspase-3 will only affect cytosolic caspase-3. This rise in cytosolic caspase-3 activity was not associated with subsequent apoptosis. Only 2.52% of cells under control conditions had condensed and fragmented nuclei, characteristic for apoptosis. Importantly, thrombin stimulation for 2 h had no effect on HLMVEC apoptosis (2.53%), shown in Fig. 1B. Because cellular detachment may also lead to changes in barrier function, we measured total cell counts of endothelial monolayers. As shown in Fig. 1C, there is no difference in total cell counts after 2 h of thrombin stimulation. Because thrombin-induced caspase-3 activation may have apoptotic effects at later time points, we investigated the effects of thrombin on apoptosis at 24 h. As shown in Fig. 1E, low amount of apoptosis is present under control conditions (1.75%), and after 24 h of thrombin stimulation there is essentially no change in apoptosis (1.64%).
Caspases Enhance Endothelial Barrier Integrity During Thrombin Stimulation
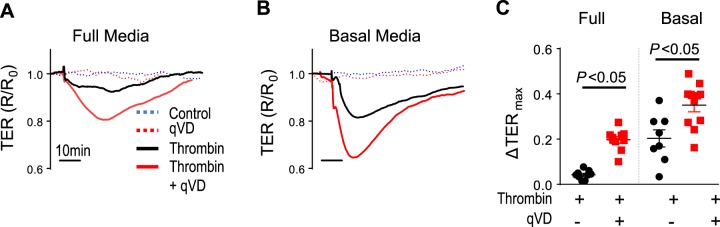
As shown in Fig. 2, A and B, HLMVECs exposed to thrombin exhibited a drop in TER, which recovers back to baseline by ~1 h, further demonstrating the transient nature of thrombin-induced endothelial barrier disruption. Having demonstrated the transient and nonapoptotic nature of thrombin-induced endothelial barrier disruption, we next tested the role of caspase-3. HLMVECs pretreated with the pan-caspase inhibitor qVD (25 μg/ml, 2 h) were exposed to thrombin, as described above. There were no mean baseline differences in electrical resistance of endothelial monolayers treated with vehicle or qVD (1,304 and 1,309 Ω, respectively). However, caspase inhibition with qVD led to worsened thrombin-induced endothelial barrier disruption (Fig. 2A), suggesting a potential protective role of caspases. Furthermore, this effect was magnified when serum concentration was reduced from full (Fig. 2, A and C) to basal (Fig. 2, B and C) media conditions (10 and 2.5% fetal bovine serum, respectively). The worsened thrombin-induced endothelial barrier disruption with caspase inhibition is likely not because of apoptosis or cellular dropout because 1) the monolayer integrity, as measured by electrical resistance, rapidly recovers back to baseline within ~1 h (Fig. 2, A and B) and 2) percentage of apoptotic cells and total cell counts was not different (Fig. 1, B and C). These data suggest an endothelial barrier-enhancing role for caspases.
Fig. 2.
Caspase inhibition worsens thrombin-induced endothelial barrier disruption. Human lung microvascular endothelial cells (HLMVECs) were exposed to 1.25 U/ml of thrombin, and endothelial barrier integrity was measured. A and B: HLMVECs grown in full media or basal media (10 and 2.5% fetal bovine serum, respectively) show a drop in transendothelial resistance (TER) in response to thrombin. Caspase inhibition with q-VD-OPH (qVD) leads to a more significant drop in TER compared with thrombin alone. Representative tracing for each condition is plotted. C: quantification of maximal drop in TER; n = 5 separate experiments; 8–11 individual wells/condition. R/R0, resistance/resistance at baseline.
Caspases Prevent Actin Cytoskeletal Rearrangement During Thrombin Stimulation
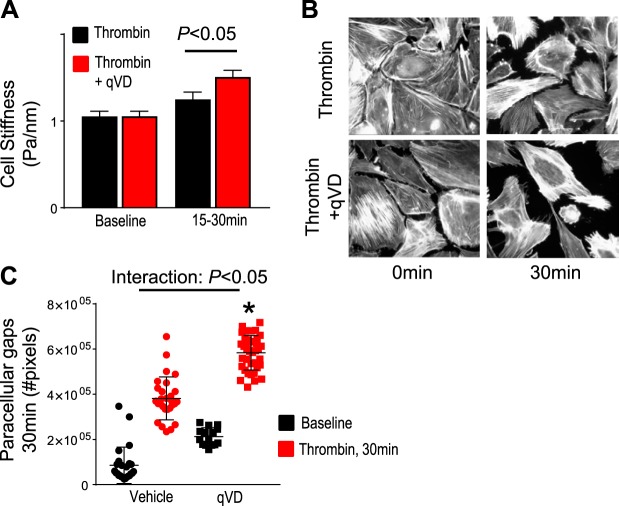
Thrombin leads to a rapid and transient cytoskeletal reorganization inducing stress fiber formation (4, 19), leading to increased tensile forces, with increased cell stiffness (15, 36), which promotes cellular contraction and ultimately results in paracellular gap formation and endothelial barrier disruption (4, 5, 19, 34, 36). To test if the endothelial barrier-protective effects of caspases were mediated by cytoskeletal changes, we measured cell stiffness using magnetic twisting cytometry at the time point of maximal endothelial barrier disruption (~15–30 min; see Fig. 2, A and B), as previously described (2). As shown in Fig. 3A, exposing HLMVECs to thrombin increased cellular stiffness. The thrombin-induced increase in stiffness was significantly greater when caspases were inhibited, suggesting that caspases play a role in reducing tensile forces generated by thrombin. Because thrombin-induced contractile forces ultimately result in paracellular gap formation (5, 34, 36), we tested the role of caspases in mediating paracellular gaps. As shown in Fig. 3B, exposure of HLMVECs to thrombin for 30 min results in paracellular gap formation, which is significantly exaggerated when caspases are inhibited, quantified in Fig. 3C, suggesting that caspases play a role in attenuating thrombin-induced paracellular gap formation. These data show that caspases promote endothelial barrier integrity by reducing tensile forces and ultimately reducing paracellular gap formation during thrombin stimulation.
Fig. 3.
Caspase inhibition exacerbates thrombin-induced endothelial cellular contraction and paracellular gap formation. A: human lung microvascular endothelial cells (HLMVECs) were grown in basal media, and magnetic twisting cytometry was used to assess cellular stiffness at baseline and after thrombin stimulation (1.25 U/ml). Caspase inhibition with q-VD-OPH (qVD) leads to stiffer cells compared with thrombin alone. Summation of individual cell stiffness for each condition is plotted; n = 5 separate experiments; 148–235 cells analyzed/condition. B and C: HLMVECs grown in basal media and after 30 min of thrombin stimulation (time point of maximum drop in TER and increased cell stiffness); cells were fixed and stained using phalloidin, and paracellular gaps were quantified. B: fluorescent microscopy demonstrates at baseline there is strong cortical actin staining, and in response to thrombin there is marked actin stress fiber formation with resultant paracellular gaps. In caspase-inhibited cells, there is increased paracellular gap formation following thrombin. C: quantification of paracellular gaps demonstrates a significant increase in paracellular gaps in caspase-inhibited cells compared with thrombin alone. Representative images of two experiments taken at ×10 magnification. More than 105 images were analyzed. R/R0, resistance/resistance at baseline; TERmax, maximum drop in transendothelial electrical resistance; Casp7, caspase-7; Casp3, caspase-3. *P < 0.05 vs. thrombin alone.
Caspase-3 Promotes Endothelial Barrier Integrity During Thrombin Stimulation
Because thrombin activates caspase-3 and not other caspases (46) and qVD has a markedly higher affinity for inhibiting caspase-3, as opposed to other caspases (12), our data suggest that caspase-3 is barrier enhancing. However, to confirm the role of caspase-3 during thrombin-induced endothelial barrier disruption, we used DEVD, a well-established inhibitor more specific to caspase-3 (29). There were no mean baseline differences in electrical resistance of endothelial monolayers treated with vehicle or DEVD (1,418 and 1,393 Ω, respectively). Similar to pan-caspase inhibition with qVD (Fig. 2, A–C), caspase-3-specific inhibition with DEVD (50 μg/ml, 2 h pretreatment) augmented thrombin-induced endothelial barrier disruption (Fig. 4, A and C). This effect was further magnified when serum concentration was reduced from basal media (Fig. 4, A and C) to serum-free media (Fig. 4, B and C) conditions (2.5 and 0% fetal bovine serum, respectively). These data suggest caspase-3 promotes endothelial barrier integrity during thrombin-mediated injury.
Fig. 4.
Caspase-3 promotes endothelial barrier integrity during thrombin-induced disruption. Human lung microvascular endothelial cells (HLMVECs) were exposed to 1.25 U/ml of thrombin, and endothelial barrier integrity was measured. A and B: HLMVECs grown in basal media or serum-free media (2.5 and 0% fetal bovine serum, respectively) show a drop in transendothelial resistance (TER) in response to thrombin. Caspase-3 inhibition with z-DEVD-FMK (DEVD) leads to a more significant drop in TER compared with thrombin alone. Summation of all individual wells for each condition is plotted. C: quantification of maximal drop in TER. D: HLMVECs were grown in 6-well plates and treated with nontargeting small-interfering RNA (siRNA) or siRNA against caspase-3. Following siRNA exposure (24 h), HLMVECs were plated on Electrical Cell-substrate Impedance Sensing System (ECIS) plates; an aliquot of cells was replated for immunoblotting. Cells were harvested at 48 h after siRNA treatment, at the time of ECIS experiments. Left, immunoblotting for caspase-7 shows no nonspecific knockdown of caspase-7 expression. Right, immunoblotting for caspase-3 shows significant knockdown of caspase-3 expression. E: quantification of maximal drop in TER shows knockdown of caspase-3 leads to significant thrombin-induced endothelial barrier disruption; n = 3–4 separate experiments; 6–14 individual wells/condition.
After showing that pharmacological inhibition of caspase-3 worsened thrombin-induced endothelial barrier integrity, we sought to confirm these findings using molecular silencing of caspase-3. As shown in Fig. 4D, using siRNA targeting caspase-3, we achieved significant knockdown of caspase-3 (~57%) with no effect on caspase-7 protein expression. Interestingly, transfection of cells resulted in higher baseline electrical resistances. The electrical resistances of endothelial monolayers treated with nontargeting siRNA (si-Scramble) and siRNA targeting caspase-3 were similar, with 2,274 and 2,177 Ω, respectively. Similar to pharmacological inhibition of caspase-3 with qVD or DEVD, thrombin-induced drop in endothelial monolayer resistance is further exaggerated with caspase-3 knockdown (Fig. 4E), proportional to the degree of caspase-3 expression. These experiments were performed in basal media, since cells did not tolerate replating in serum-free media after transfection reactions (data not shown).
DISCUSSION
While caspase-3 is a canonical executioner caspase and plays a pivotal role in execution of apoptosis, recent work suggests that cells can survive caspase-3 activation (18). Ding et al. (18) demonstrated that, during fruit fly development, numerous cells and cell types survive caspase-3 activation. These data not only suggest normal development follows cellular caspase-3 activation but, more importantly, implicate nonapoptotic functions for caspase-3. In cancer cell lines and undifferentiated tissues, investigators have identified nonapoptotic functions of caspase-3 involving cellular adhesion, migration, tumor invasion, tissue regeneration, and cellular proliferation (8, 10, 14, 41). In neuronal tissues, caspase-3 has a role not only in development and differentiation but also in fully differentiated cells where caspase-3 is important for neuronal plasticity (27). A nonapoptotic role for caspase-3 in endothelial cells has not been previously reported, to our knowledge. This may be in part due to the critical role apoptosis plays in endothelial barrier disruption in many disease states, such as ARDS (16, 24, 31, 40, 48, 54).
An important aspect of our studies is the specific stimulus used. Our usage of thrombin to test our hypotheses was quite deliberate and was based on key factors associated with thrombin: 1) known disruptor of endothelial barrier integrity; 2) endothelial barrier disruption that is nonapoptotic in nature; and 3) most importantly, activates caspase-3. We attempted to use platelet-activating factor (PAF) as a second agent to test our hypotheses. PAF is a well-described endothelial barrier disruptor (33, 37). The predominant mechanism of endothelial barrier disruption seems to occur via paracellular gap formation (33, 37). Although measurements of apoptosis were not end points for effects of PAF, apoptosis seems unlikely at the time points of maximal endothelial barrier disruption, ~10–15 min (9, 33, 37). To our knowledge, there is no description of caspase-3 effects with PAF treatment in endothelial cells. We first sought to test the effect of PAF on caspase-3 activity. As shown in Supplemental Fig. S1 (Supplemental Material is available online at https://doi.org/10.5281/zenodo.2667839), we did not see an increase in caspase-3 activity with PAF treatment. Given the lack of an effect of PAF on caspase-3 activity, we elected not to interrogate the role of caspase-3 inhibition on PAF-induced endothelial barrier disruption. In contrast, thrombin stimulation of HLMVECs results in nonapoptotic activation of caspase-3, thereby avoiding confusion based on the canonical functions of caspase-3. Our data demonstrate that, during nonapoptotic insults, as with thrombin, caspase-3 exerts barrier-protective effects in HLMVECs by mediating cytoskeletal changes. It is not clear if a similar barrier-protective role for caspase-3 exists during apoptotic insults. To that end, Petrache et al. (45) showed that tumor necrosis factor (TNF)-α induces bovine pulmonary artery endothelial cell apoptosis by 4 h and that inhibiting caspases with z-VAD did not alter endothelial barrier disruption at later time points. Interestingly, the caspase-inhibited monolayers seem to demonstrate a lower electrical resistance in the first several hours following TNF-α, suggesting an initial worsened barrier integrity when caspases were inhibited (45). However, given the apoptotic nature of TNF-α, a specific effect of caspase-3, independent of apoptosis, on endothelial barrier integrity is difficult to ascertain (44). Our group has previously demonstrated that, in response to lipopolysaccharide (apoptosis-inducing stimulus), caspase-3 is activated in the cytoplasm and subsequently translocates to the nucleus, with resultant apoptosis and loss of endothelial barrier integrity at later time points (16). This raises the possibility that cytoplasmic caspase-3 may exert barrier-protective effects even during apoptotic insults but may be temporal in nature and lost when caspase-3 translocates to the nucleus (Fig. 5).
Fig. 5.
Schematic of dual and divergent location-specific functions of caspase-3 (Casp 3). Cytoplasmic activation of caspase-3 promotes endothelial barrier integrity although the molecular targets are unknown and warrant further investigation. Nuclear translocation of caspase-3 promotes apoptosis and endothelial barrier disruption; the molecular mechanism by which caspase-3 translocates to the nucleus is still under investigation.
The dismantling of cells during the execution phase of apoptosis results in caspase-3-mediated cleavage of many cytoskeletal structures (35). Indeed, caspase-3-mediated cleavage has been shown to include actin (49), myosin light chain (44), and proteins involved in maintenance of junctional complex integrity, notably focal adhesion kinase (55). In contrast to apoptosis, cytoskeletal remodeling and cell-cell interactions via junctional proteins represent two major mechanisms by which paracellular gap formation can be initiated and sustained in endothelial cells (19). The temporal dynamics and reversibility of our barrier dysfunction data suggest that the effects of caspase-3 on the cytoskeletal response to thrombin are not related to apoptosis and most likely posttranslational rather than transcriptional. Of particular relevance to this work, in platelets, thrombin stimulation increased activation and cytoskeletal localization of caspase-3 (1, 3). Additionally, in failing cardiac myocytes, Moretti et al. (43) found that caspase-3 cleaved ventricular essential myosin light chain (vMLC1) at a noncanonical cleavage site, leading to depressed myocyte contraction. These works implicate caspase-3 in potentially nonapoptotic cytoskeletal changes in other cell types (1, 3, 43). It must be noted that thrombin in human platelets (1, 3) and the tachycardia pacing model used to obtain failing cardiac myocytes (43) both can lead to cellular apoptosis, suggesting that the role of caspase-3 in these cytoskeletal changes may signify a precursor to apoptosis. In contrast, our current work clearly shows a role for caspase-3 in nonapoptotic regulation of cytoskeletal function in HLMVECs.
Several limitations accompany our studies. First, the total number of biological replicates (human donors) in our experiments is limited to three, based on availability at commercial sources. Additionally, we had to change vendors for the supply of our HLMVECs because of a lack of reliable availability of culture media (see experimental procedures). This led to some notable findings, namely the magnitude of thrombin effects. To investigate this further, we calculated the time to TERMAX for all of our experiments (shown in Supplemental Fig. S2); we noted that almost all experiments involving thrombin and caspase-3 inhibition, pharmacological or molecular knockdown, were equivalent to thrombin alone. The one exception was that the time to TERMAX with thrombin alone was longer than thrombin and caspase inhibition during full serum conditions (Fig. 2A). Interestingly, the time to TERMAX for thrombin during full serum conditions was at times beyond the classic description of thrombin responses and at the end of our experiments (Supplemental Fig. S2). This likely represents an overall drift in the TER of the cultures, rather than an effect of thrombin. These findings highlight the following two main points: 1) the protective effect of serum on thrombin responses and 2) the relevance of caspase-3 function on endothelial barrier integrity, since caspase-3 inhibition can worsen endothelial barrier function during the thrombin stimulation during full serum conditions, where there is muted endothelial barrier disruption. Additionally, HLMVECs from Cell Biologics (Fig. 4A) had a dampened thrombin response in basal media, similar to full media conditions for Lonza HLMVECs (Fig. 2A). These observed differences are likely based on a combination of genetic differences based on individual donors and proprietary concentrations of growth factors in the differing culture media. Although the magnitude of the effect of thrombin may have led us to perform potentially duplicative experiments of varying conditions (full media, basal media, and serum free), our data are consistent and essentially show a dose titration of effect. Additionally, the similar trend across multiple vendors of HLMVECs, and multiple conditions of serum, further verifies the important role of caspase-3 in maintaining endothelial barrier integrity during thrombin-induced injury.
Another limitation is that we do not know the exact downstream target(s) of caspase-3 in mediating endothelial barrier-protective effects. Our data show that with caspase inhibition there are increased paracellular gaps (Fig. 3, B and C) at baseline, suggesting a possible barrier maintenance role for caspase activity. However, the electrical resistance with pan-caspase inhibition, caspase-3 inhibition, or caspase-3 knockdown is nearly identical to control conditions at baseline. Additionally, cell stiffness is not changed at baseline with caspase inhibition (Fig. 3A). Given the known effects of caspase-3 on myosin light chain (43, 44), we evaluated changes in phosphorylation or abundance of myosin light chain but were unable to detect differences (data not shown). It is important to note that caspase-3-mediated cleavage of many cytoskeletal structures is known in the context of apoptotic stimuli (43, 44). These associations may not hold true during nonapoptotic conditions. We additionally examined whether the barrier-protective effects of caspase-3 occurred via Rac1-mediated mechanisms by sphingosine 1-phosphate (S-1-P) stimulation (19). Caspase inhibition did not alter S-1-P-induced endothelial barrier enhancement (Supplemental Fig. S1), suggesting alternative signaling pathways or that caspase-3 is upstream of S-1-P-induced endothelial barrier enhancement (19). This is consistent with the available literature in human umbilical vein endothelial cells and intestinal epithelial cells showing S-1-P by itself does not affect caspase-3 expression or activity (26, 38). Although the molecular mechanism(s) is not clear at present, these pathways function to increase tensile forces within endothelial cells and ultimately result in increased paracellular gap formation. Our data clearly show that caspase inhibition results in increased cell stiffness and paracellular gap formation.
In summary, our data demonstrate that caspase-3 has nonapoptotic functions in lung microvascular endothelium and that caspase-3 functions to enhance the endothelial barrier during nonapoptotic injury. These data imply that broadly inhibiting apoptosis may not be a practical strategy in treating/preventing lung injury, since caspase-3, the terminal executioner of apoptosis, may also protect against lung injury. In conjunction with our previous data demonstrating nuclear translocation of caspase-3 is associated with increased apoptosis and endothelial barrier disruption (16), our data bring about the possibility of a transition point linking reversible (cytoskeletal) and irreversible (apoptotic) modes of endothelial barrier disruption that, if identified, could be exploited for therapeutic intervention. Identifying the molecular pathways that promote nuclear translocation of caspase-3 may lead to therapeutic targets that can harness the protective effects of caspase-3 and prevent the deleterious consequences of endothelial apoptosis (Fig. 5). Additionally, effector proteins cleaved by caspase-3 in response to injurious stimuli are likely cell type and stimulus specific; identification of the specific cytoskeletal proteins being affected by caspase-3 warrants further investigation.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-133413.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.S. and M.D. conceived and designed research; K.S., K.C., L.J., L.S., H.L., S.S.A., and M.D. performed experiments; K.S., L.J., C.E.M., T.M.K., S.M.D., S.S.A., and M.D. analyzed data; K.S., C.E.M., T.M.K., S.M.D., S.S.A., M.J.R., L.A.S., and M.D. interpreted results of experiments; K.S., L.J., S.S.A., and M.D. prepared figures; K.S., H.L., S.S.A., and M.D. drafted manuscript; K.S., L.J., C.E.M., T.M.K., H.L., S.M.D., S.S.A., L.A.S., and M.D. edited and revised manuscript; K.S., K.C., L.J., L.S., C.E.M., T.M.K., H.L., S.M.D., S.S.A., M.J.R., L.A.S., and M.D. approved final version of manuscript.
REFERENCES
- 1.Amor NB, Pariente JA, Salido GM, Rosado JA, Bartegi A. Thrombin-induced caspases 3 and 9 translocation to the cytoskeleton is independent of changes in cytosolic calcium in human platelets. Blood Cells Mol Dis 36: 392–401, 2006. doi: 10.1016/j.bcmd.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 2.An SS, Mitzner W, Tang WY, Ahn K, Yoon AR, Huang J, Kilic O, Yong HM, Fahey JW, Kumar S, Biswal S, Holgate ST, Panettieri RA Jr., Solway J, Liggett SB. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol 138: 294–297.e4, 2016. doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Amor N, Pariente JA, Salido GM, Bartegi A, Rosado JA. Caspases 3 and 9 are translocated to the cytoskeleton and activated by thrombin in human platelets. Evidence for the involvement of PKC and the actin filament polymerization. Cell Signal 18: 1252–1261, 2006. doi: 10.1016/j.cellsig.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 7.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725–731, 2003. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Boland K, Flanagan L, Prehn JH. Paracrine control of tissue regeneration and cell proliferation by Caspase-3. Cell Death Dis 4: e725, 2013. doi: 10.1038/cddis.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brailoiu E, Barlow CL, Ramirez SH, Abood ME, Brailoiu GC. Effects of platelet-activating factor on brain microvascular endothelial cells. Neuroscience 377: 105–113, 2018. doi: 10.1016/j.neuroscience.2018.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brentnall M, Weir DB, Rongvaux A, Marcus AI, Boise LH. Procaspase-3 regulates fibronectin secretion and influences adhesion, migration and survival independently of catalytic function. J Cell Sci 127: 2217–2226, 2014. doi: 10.1242/jcs.135137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis 8: 345–352, 2003. doi: 10.1023/A:1024116916932. [DOI] [PubMed] [Google Scholar]
- 13.Chiang ET, Camp SM, Dudek SM, Brown ME, Usatyuk PV, Zaborina O, Alverdy JC, Garcia JG. Protective effects of high-molecular weight polyethylene glycol (PEG) in human lung endothelial cell barrier regulation: role of actin cytoskeletal rearrangement. Microvasc Res 77: 174–186, 2009. doi: 10.1016/j.mvr.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly PF, Jäger R, Fearnhead HO. New roles for old enzymes: killer caspases as the engine of cell behavior changes. Front Physiol 5: 149, 2014. doi: 10.3389/fphys.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuerrier CM, Gagner A, Lebel R, Gobeil F Jr, Grandbois M. Effect of thrombin and bradykinin on endothelial cell mechanical properties monitored through membrane deformation. J Mol Recognit 22: 389–396, 2009. doi: 10.1002/jmr.953. [DOI] [PubMed] [Google Scholar]
- 16.Damarla M, Parniani AR, Johnston L, Maredia H, Serebreni L, Hamdan O, Sidhaye VK, Shimoda LA, Myers AC, Crow MT, Schmidt EP, Machamer CE, Gaestel M, Rane MJ, Kolb TM, Kim BS, Damico RL, Hassoun PM. Mitogen-activated protein kinase-activated protein kinase 2 mediates apoptosis during lung vascular permeability by regulating movement of cleaved caspase 3. Am J Respir Cell Mol Biol 50: 932–941, 2014. doi: 10.1165/rcmb.2013-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damico RL, Chesley A, Johnston L, Bind EP, Amaro E, Nijmeh J, Karakas B, Welsh L, Pearse DB, Garcia JG, Crow MT. Macrophage migration inhibitory factor governs endothelial cell sensitivity to LPS-induced apoptosis. Am J Respir Cell Mol Biol 39: 77–85, 2008. doi: 10.1165/rcmb.2007-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding AX, Sun G, Argaw YG, Wong JO, Easwaran S, Montell DJ. CasExpress reveals widespread and diverse patterns of cell survival of caspase-3 activation during development in vivo (Abstract). eLife 5: e10936, 2016. doi: 10.7554/eLife.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 20.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JGN. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 279: 24692–24700, 2004. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 21.Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA 294: 2889–2896, 2005. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- 22.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 280: 17286–17293, 2005. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 23.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916, 2005. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Kuwano K, Kunitake R, Hagimoto N, Miyazaki H, Kaneko Y, Kawasaki M, Maeyama T, Hara N. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol 117: 202–208, 1998. doi: 10.1159/000024011. [DOI] [PubMed] [Google Scholar]
- 25.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19: 107–120, 2012. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenspon J, Li R, Xiao L, Rao JN, Marasa BS, Strauch ED, Wang JY, Turner DJ. Sphingosine-1-phosphate protects intestinal epithelial cells from apoptosis through the Akt signaling pathway. Dig Dis Sci 54: 499–510, 2009. doi: 10.1007/s10620-008-0393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 68: 1171–1180, 2003. doi: 10.1023/B:BIRY.0000009130.62944.35. [DOI] [PubMed] [Google Scholar]
- 28.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, Edwards CK III, Schuchman EH, Fuks Z, Kolesnick R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med 186: 1831–1841, 1997. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z, Hendrickson EA, Bremner TA, Wyche JH. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem 272: 13432–13436, 1997. doi: 10.1074/jbc.272.20.13432. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med 361: 1570–1583, 2009. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, Maeyama T, Hara N. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol 157: 597–603, 2000. doi: 10.1016/S0002-9440(10)64570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawkitinarong K, Linz-McGillem L, Birukov KG, Garcia JG. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am J Respir Cell Mol Biol 31: 517–527, 2004. doi: 10.1165/rcmb.2003-0432OC. [DOI] [PubMed] [Google Scholar]
- 33.Knezevic II, Predescu SA, Neamu RF, Gorovoy MS, Knezevic NM, Easington C, Malik AB, Predescu DN. Tiam1 and Rac1 are required for platelet-activating factor-induced endothelial junctional disassembly and increase in vascular permeability. J Biol Chem 284: 5381–5394, 2009. doi: 10.1074/jbc.M808958200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knezevic N, Tauseef M, Thennes T, Mehta D. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med 206: 2761–2777, 2009. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278: 294–298, 1997. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300: C146–C154, 2011. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuebler WM, Yang Y, Samapati R, Uhlig S. Vascular barrier regulation by PAF, ceramide, caveolae, and NO - an intricate signaling network with discrepant effects in the pulmonary and systemic vasculature. Cell Physiol Biochem 26: 29–40, 2010. doi: 10.1159/000315103. [DOI] [PubMed] [Google Scholar]
- 38.Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem 276: 10627–10633, 2001. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 39.Lamkanfi M, Moreira LO, Makena P, Spierings DC, Boyd K, Murray PJ, Green DR, Kanneganti TD. Caspase-7 deficiency protects from endotoxin-induced lymphocyte apoptosis and improves survival. Blood 113: 2742–2745, 2009. doi: 10.1182/blood-2008-09-178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le A, Damico R, Damarla M, Boueiz A, Pae HH, Skirball J, Hasan E, Peng X, Chesley A, Crow MT, Reddy SP, Tuder RM, Hassoun PM. Alveolar cell apoptosis is dependent on p38 MAP kinase-mediated activation of xanthine oxidoreductase in ventilator-induced lung injury. J Appl Physiol (1985) 105: 1282–1290, 2008. doi: 10.1152/japplphysiol.90689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu YR, Sun B, Zhao XL, Gu Q, Liu ZY, Dong XY, Che N, Mo J. Basal caspase-3 activity promotes migration, invasion, and vasculogenic mimicry formation of melanoma cells. Melanoma Res 23: 243–253, 2013. doi: 10.1097/CMR.0b013e3283625498. [DOI] [PubMed] [Google Scholar]
- 42.MacIntyre NR. Current issues in mechanical ventilation for respiratory failure. Chest 128, Suppl 2: 561S–567S, 2005. doi: 10.1378/chest.128.5_suppl_2.561S. [DOI] [PubMed] [Google Scholar]
- 42a.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem 92: 1075–1085, 2004. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 43.Moretti A, Weig HJ, Ott T, Seyfarth M, Holthoff HP, Grewe D, Gillitzer A, Bott-Flügel L, Schömig A, Ungerer M, Laugwitz KL. Essential myosin light chain as a target for caspase-3 in failing myocardium. Proc Natl Acad Sci USA 99: 11860–11865, 2002. doi: 10.1073/pnas.182373099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrache I, Birukov K, Zaiman AL, Crow MT, Deng H, Wadgaonkar R, Romer LH, Garcia JG. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 17: 407–416, 2003. doi: 10.1096/fj.02-0672com. [DOI] [PubMed] [Google Scholar]
- 45.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JGN. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 280: L1168–L1178, 2001. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 46.Rosado JA, Lopez JJ, Gomez-Arteta E, Redondo PC, Salido GM, Pariente JA. Early caspase-3 activation independent of apoptosis is required for cellular function. J Cell Physiol 209: 142–152, 2006. doi: 10.1002/jcp.20715. [DOI] [PubMed] [Google Scholar]
- 47.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 48.Singer BD, Mock JR, D’Alessio FR, Aggarwal NR, Mandke P, Johnston L, Damarla M. Flow-cytometric method for simultaneous analysis of mouse lung epithelial, endothelial, and hematopoietic lineage cells. Am J Physiol Lung Cell Mol Physiol 310: L796–L801, 2016. doi: 10.1152/ajplung.00334.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sokolowski JD, Gamage KK, Heffron DS, Leblanc AC, Deppmann CD, Mandell JW. Caspase-mediated cleavage of actin and tubulin is a common feature and sensitive marker of axonal degeneration in neural development and injury. Acta Neuropathol Commun 2: 16, 2014. doi: 10.1186/2051-5960-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suresh K, Servinsky L, Jiang H, Bigham Z, Yun X, Kliment C, Huetsch J, Damarla M, Shimoda LA. Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 314: L893–L907, 2018. doi: 10.1152/ajplung.00430.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang PS, Mura M, Seth R, Liu M. Acute lung injury and cell death: how many ways can cells die? Am J Physiol Lung Cell Mol Physiol 294: L632–L641, 2008. doi: 10.1152/ajplung.00262.2007. [DOI] [PubMed] [Google Scholar]
- 52.Vanden Berghe T, Goethals A, Demon D, Bogaert P, Mak TW, Cauwels A, Vandenabeele P. An inactivating caspase-11 passenger mutation muddles sepsis research. Am J Respir Crit Care Med 188: 120–121, 2013. doi: 10.1164/rccm.201210-1775LE. [DOI] [PubMed] [Google Scholar]
- 53.Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci USA 105: 12815–12819, 2008. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 55.Wen L-P, Fahrni JA, Troie S, Guan J-L, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem 272: 26056–26061, 1997. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]