Abstract
Atherosclerosis is a chronic inflammatory pathology that precipitates substantial morbidity and mortality. Although initiated by physiological patterns of low and disturbed flow that differentially prime endothelial cells at sites of vessel branch points and curvature, the chronic, smoldering inflammation of atherosclerosis is accelerated by comorbidities involving inappropriate activation of the adaptive immune system, such as autoimmunity. The innate contributions to atherosclerosis, especially in the transition of monocyte to lipid-laden macrophage, are well established, but the mechanisms underpinning the infiltration, persistence, and effector dynamics of CD8 T cells in particular are not well understood. Adaptive immunity is centered on a classical cascade of antigen recognition and activation, costimulation, and effector cytokine secretion upon recall of antigen. However, chronic inflammation can generate alternative cues that supplant this behavior pattern and promote the retention and activation of peripherally activated T cells. Furthermore, the atherogenic foci that activated immune cell infiltrate are unique lipid-laden environments that offer a diverse array of stimuli, including those of survival, antigen hyporesponsiveness, and inflammatory cytokine expression. This review will focus on how known cardiovascular comorbidities may be influencing CD8 T-cell activation and how, once infiltrated within atherogenic foci, these T cells face a multitude of cues that skew the classical cascade of T-cell behavior, highlighting alternative modes of activation that may help contextualize associations of autoimmunity, viral infection, and immunotherapy with cardiovascular morbidity.
Keywords: adaptive immunity, antigen-independent responses, atherosclerosis, CD8, inflammation, T cells
INTRODUCTION
Atherosclerosis is a pathological disease process highlighted by a chronic accumulation of lipids, immune cells, and necrotic debris at nonrandom sites of curvature and branch points within the vasculature (37). These sites are associated with low and disturbed flow (LDF) patterns sensed as shear stress by the vascular endothelium. Within the endothelium, a conversion from steady laminar shear to disturbed flow results in dynamic alterations in endothelial transcriptional and posttranscriptional programs, sensitizing the endothelium to subsequent cytokine activation. In this way, systemic cytokine responses to IL-1β, for example, lead to a focal activation of the endothelium, upregulation of proinflammatory cytokines (e.g., IL-6 and CCL5), and the expression of leukocyte adhesion molecules (e.g., P-, L-, and E-selectins, ICAM-1, and VCAM) (31). The ensuing recruitment of monocytes and their conversion to foam cells beneath the leaky arterial endothelium is a hallmark of disease. Notably, the endothelium covers the growing plaque, providing a thin line of defense against the most devastating consequences of atherosclerosis, sudden thrombotic rupture of unstable plaque or slower erosion and thrombosis of well-encapsulated plaque (39).
The resultant pathology of advanced atherosclerosis manifests as cardiovascular disease (CVD), the leading cause of death, and is compounded by the increasing rates of obesity, physical inactivity, and metabolic disease in the United States. Strikingly, autopsy studies have shown that nearly all people harbor atherosclerotic plaque (11). However, even when lipids have been suppressed and plaque has effectively stopped growing, substantial residual risk, due to sudden thrombotic events leading to myocardial infarction and stroke, still remains (39). In the early 2000s, results from multiple patient-controlled studies revealed systemic inflammation, indexed as elevations in high-sensitivity C-reactive protein (CRP), to be as clinically predictive for cardiovascular events as circulating levels of low-density lipid. CRP is an acute-phase reactant produced peripherally to the site of atherosclerotic plaque buildup in response to inflammatory stimuli (77), and its utility as a biomarker of atherosclerotic disease has helped shift the paradigm of atherosclerotic pathology away from passive deposition of lipids into a disease contingent on the integration of inflammatory cell processes. This “inflammatory hypothesis” has been tested most recently within the two large multicentered trials: the Cardiovascular Inflammation Reduction Trial (CIRT) and Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) trial. The results of these trials converted the association of increased CRP into an independent, targetable biomarker of disease activity, as blockade of IL-1β decreased recurrence of CVD events, while failure to systematically influence biomarkers of inflammation, as in the case of the CIRT, yielded no significant effect on rate of CVD (79, 80).
Although atherosclerotic pathology now has broadly recognized foundations in inflammation, how each arm of the immune system intersects with the cascade of disease progression is not fully understood. The immune system is categorized into two domains based on speed and specificity of response. Comprising the innate response are cells of the myeloid lineage that broadly recognize “foreign” material and respond within seconds with nonspecific inflammatory secretion of cytokines and enzymes. Monocytes, upon taking residence within developing atheroma, transition into macrophages that phagocytose lipid at which point they are termed foam cells. This process, which includes foam cell death within atherosclerotic lesions, further elicits inflammation. Deservedly, this has been extensively researched and links between macrophage activation, lipid uptake, and atherosclerotic pathology are considered well established.
In contrast, the adaptive immune system, which requires a period of lymphoid-derived cellular programming that involves antigen-presenting cells (APCs) and costimulatory signals, is much less understood in the context of atherosclerotic disease progression. T cells participate in the pathological process of atherosclerotic plaque development as early as monocytes (38), and it is suggested that their status in circulation and their residence in plaque may serve as biomarkers of disease volatility (40, 102). Thus it is critical to not only understand how the smoldering process of atherosclerotic pathology interfaces with T-cell activation but also how the plaque microenvironment may also be modulating activation events that have significant mortality consequences in the general population.
Classical activation of T cells entails steps of antigen processing, presentation, and costimulation, resulting in T cells that are equipped with inflammatory programs with memory to produce rapid and specific responses should the same antigenic stimulus be presented at a later time. The role for T-cell subsets, especially of CD4 T cells, has been studied and reviewed (96, 117), as have the classical modes of T- and B-cell activation within the context of atherosclerosis (48). Thus this review will highlight the role of activated CD8 T cells with effector memory and alternative activation potential within the atherogenic cascade, as recent histoanalyses have suggested CD8 T cells outnumber CD4 T cells in prethrombotic fibroatheromas (103). Although the concept of T cells in atherosclerosis is not new, how autoimmunity or systemic chronic infection interfaces with the pathology of atherosclerosis from initial infiltration at sites of activated endothelium, to persistence within the plaque microenvironment is an underdeveloped area of research.
FROM NAÏVE TO INFILTRATED: GENERATING AN EFFECTOR T CELL
T-cell immunity is highlighted by the specificity of T-cell responses against specific antigens, small peptide fragments recognized by a cell’s T-cell receptor (TCR). Recognition of antigen by TCR is genetically driven through gene rearrangements in the V, D, and J domains that result in a range of unique TCRs capable of recognizing a tremendous range of antigens (87). This diversity in TCR is pruned by positive and then negative selection within the thymus to produce a pool of T cells that are CD4+ or CD8+ and not self-reactive. These mature, naïve T cells then circulate through the body, sampling a variety of organs in search of their cognate antigen that will trigger clonal proliferation of their specific TCR configuration and differentiated effector programs (88).
Priming T Cells to Antigens and Beyond
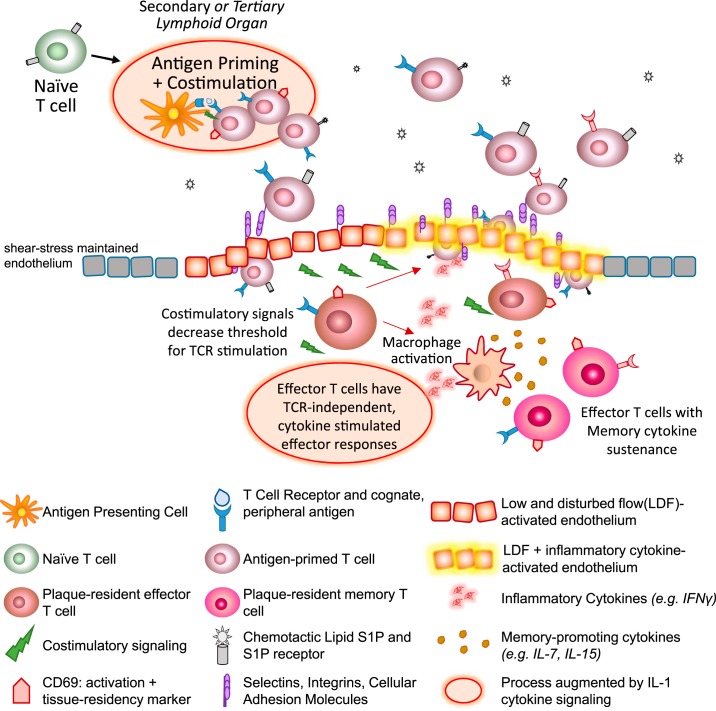
T cells that are developmentally mature but have not yet recognized antigen are classically maintained within peripheral lymphoid organs, where dendritic cells present them with processed protein fragments bound to major histocompatibility complexes and provide costimulatory signals. In “homeostatic health,” this occurs within secondary lymphoid tissues: the spleen, which specializes in presentation of blood-borne antigens, and lymph nodes, which present an array of antigens obtained from skin or mucosal surfaces via lymphatic drainage. If a T cell does not encounter antigen that its TCR recognizes, it egresses and reenters the circulatory circuit, perhaps surveying another node or lymphoid tissue. Only upon cognate antigen recognition is lymphocyte egress blocked, through internalization of the receptor for the chemotactic lipid sphingosine-1-phosphate (S1P). S1P is expressed at high levels in blood, and upon maturation in the thymus, naïve T cells express high levels of its receptor. Thus the high gradient of S1P drives migration back into circulation and the lymphatic system. However, upon TCR recognition and costimulatory signaling, CD69 expression induces its internalization, trapping the cell as the programs of clonal expansion and activation take place (Fig. 1).
Fig. 1.
Alternative cues for T cell activation within atherogenic foci. Naïve, mature T cells with a wide range of T-cell receptors (TCRs) are generated in the thymus and are antigen primed and costimulated by antigen-presenting cells (APCs) within lymphoid organs. T cells undergo clonal proliferation and upregulate CD69 upon antigen activation, which in turns facilitate degradation of the receptor for sphingosine-1 phosphate (S1P), a chemotactic lipid concentrated within circulating blood and lymph. Once clonal expansion and differentiated effector programs occur, the antigen-primed T cells egresses upon reexpression of S1P receptors, rolling on the vascular endothelium. Upon encountering low and disturbed flow (LDF)-activated endothelium, which upregulates several adhesion molecules (ICAMs, VCAMs, and integrins), T cells infiltrate and undergo diapedesis. Within the plaque microenvironment, they receive a multitude of costimulatory cues (e.g., CD28 and tumor necrosis factor families). In addition to costimulatory signals that decrease the threshold needed for TCR stimulation, LDF foci are also rich in IL-1 cytokines, which can promote TCR-independent, innate-like responses from effector T cells. The inflammatory cytokines (e.g., IFNγ) secreted as a result of these effector T-cell programs can then 1) further induce LDF-activated endothelium to upregulate adhesion molecules, facilitating entry of more T cells; and 2) activate macrophages to secrete memory T-cell-sustaining cytokines (e.g., IL-7 and IL-15). Thus the initial seeding of effector T cells within LDF-foci can augment the inflammatory environment of atherosclerotic plaque through alternative modes of T-cell activation.
Chronic inflammatory states have been linked to the development of tertiary lymphoid structures, which would suggest alternative locations and means for T cells to acquire effector programs in the setting of viral infections or autoimmunity. Chronic and acute inflammatory events are often accompanied by systemic elevations in inflammatory cytokines, such as those that comprise the IL-1 family. The CANTOS trial highlighted the significance of the IL-1 cytokines, a group of at least 11 proinflammatory cytokines (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ) that share transmembrane receptors and intracellular signaling pathways (110). The balance of their expression against four antagonist cytokines (IL-1Ra, IL-36Ra, IL-37, and IL-38) has systemic and targeted proinflammatory roles in maintaining homeostasis and potentiating disease (30, 74). Expressed primarily by monocytes, these cytokines have significant intersection with and influence over adaptive immunity, including the potentiation of effector programs induced by APC and T-cell interactions and the support of stromal organization of inducible lymphoid tissues.
Clonal expansion of antigen-recognized TCRs is dependent on IL-2, a cytokine termed the T-cell growth factor. This results in expansion of the antigen-recognized TCR configuration and is critical to mounting effective defenses against foreign antigens. Not only do IL-1 cytokines augment production of the T-cell growth cytokine IL-2 (65) and promote robust expansion (9, 86), but they also promote dendritic cell priming during pathogenic viral infections (75) and skew T-cell programs into more inflammatory subsets (108). Within the lung, IL-1 signaling through stromal cells supports the formation of inducible bronchus-associated lymphoid tissue after influenza virus infection, which enabled efficient viral clearance (68). Recently, another IL-1 family member, IL-36, was implicated in controlling the formation of tertiary lymphoid structures in mouse models (111), and its expression is found in human high endothelial venules, a critical component of lymphoid structures that recruit naïve and memory T cells from blood (112). Tertiary lymphoid structures with delineated T-cell areas and B-cell follicles have been proposed to exist within the adventitia in chronic hyperlipidemic mouse models, as staining of adventitial endothelium-associated VCAM-1 was localized to areas of lymphoid aggregates (20, 64). While additional antigen priming centers in the setting of viral clearance or tumor antigen presentation may be beneficial, the organization of such structures within the normally immune-privileged vessel wall would seem to promote plaque-destabilizing inflammatory cell inputs. However, functional analysis of these structures in ApoE−/− mice has suggested effects on the regulatory T cells that help maintain aortic T-cell homeostasis (42). Thus, while the role for tertiary lymphoid organs to promote auto-antigen recognition and thus promote the deleterious inflammation of atherosclerosis is a salient hypothesis, they may also have tolerizing roles against plaque-antigen that warrant further exploration.
Increased Invasive Potential
Mechanistically, T-cell recruitment to the inflamed vascular endothelium at atherogenic regions is thought to be similar to the recruitment of innate immune cells. Activated CD4 and CD8 T cells roll on the endothelium through the interaction of glycosylated carbohydrate PSGL-1 with lectins on the endothelial cell surface, mainly P- and E-selectin. Under high shear (>6 dyn/cm2), rolling is aided by a tether and sling system on activated T cells, similar to that observed in neutrophils (1, 92). Firm adhesion to the endothelium is mediated by the binding of integrins LFA-1 (aL/b2) and VLA-4 (a4/b1) to ICAM1, ICAM2, and VCAM (8, 90). Once bound to the endothelium, activated T cells crawl along the endothelial cell surface (8). Interestingly, activated T cells more often migrate against the direction of flow than with it, an effect that appears in activated effector T cells and not primary T cells or neutrophils (101). The activated atherogenic endothelium upregulates P- and L-selectin and ICAM1 and VCAM, providing ample adhesion molecules for activated T cells (28), and rolling and crawling behavior by myeloid cells has been described in vivo by multiphoton imaging (18). Although T-cell recruitment was also examined, little specific recruitment was observed 6 wk after a high-fat diet in this ApoE−/− model, suggesting that T cells are recruited later in plaque progression (18). However, it is possible that T cells activated by TCR stimulation (e.g., by virus, or autoimmune response) would be more frequently recruited to atherogenic regions.
Binding and crawling of effector T cells precede their diapedesis. While intravital imaging of T-cell interactions with dendritic cells in the arterial wall has not yet been performed, there may be some similarities to behavior in experimental autoimmune encephalitis, which has been imaged in detail (8). In experimental autoimmune encephalitis, activated effector T cells pass into the subendothelium, where they encounter APCs. This restimulation, indicated by interferon-γ (IFNγ), promotes increased crawling of T cells on the endothelial cell surface, followed by increased tissue infiltration and cytokine expression of TNF, IL-17, and IL-2 (8). Interestingly, a transfer of antigen-specific T cells (e.g., myelin specific) with nonspecific (e.g., Ova-specific) T cells promoted increased crawling and infiltration of nonspecific T cells not seen when they are injected alone. This suggests that reactivation and likely cytokine release from locally reactivated T cells stimulate the endothelium and local environment to encourage the crawling and recruitment of additional cells (8) (Fig. 1). Whether T-cell recruitment and reactivation in plaque play a similar role in driving the subsequent recruitment of other T cells and other immune cell subpopulations remains to be shown.
ATHEROSCLEROTIC MICROENVIRONMENT: A MULTITUDE OF ALTERNATIVE T-CELL CUES
Within human atherosclerotic plaque, histoanalyses have revealed that T cells cluster in shoulder regions of developing plaque with congregations of T cells at sites of atheroma rupture (82). Specifically, CD8 T cells are almost fivefold higher in number per vessel area in unstable versus stable plaques. Unstable plaques also had substantially less infiltration of T-regulatory cells (82). Whereas antigen-specific responses have been observed within plaque (50), it is likely that many of the T cells that arrive in the plaque have been activated by an antigen peripheral to the atherogenic microenvironment. As previously mentioned, any activated T cell will express the surface molecules to interact with the cell adhesion molecules expressed on the atherogenic endothelium. Viral responses can induce massive expansions of both antigen-specific and bystander T-cell populations, and similar chronic expansions of effector T cells have been observed in the autoimmune diseases strongly correlated with cardiovascular risk. Additionally, endothelial cells are “semiprofessional” APCs, as they can present not only antigen (84) but also several costimulatory molecules, especially at sites of LDF (17, 71). These sites of LDF become stiffened with age, and advanced atherosclerotic pathology and the potential for this to further amplify T-cell activation are interesting (85). Thus a critical question is what becomes of these activated T cells in tissue, whether or not they see a plaque-specific antigen. The microenvironment of an atherogenic foci is rich in diversity of cues that modulate immune cell interactions, phenotypes, and effector mechanisms.
Cultivating Lasting Memory
Human histology studies have suggested that the infiltrated cells maintain an activated phenotype, with some conversion into memory T cells (114). In peripheral tissues, maintenance of a viable memory population relies on retention signals and availability of IL-7 and IL-15 (43). Retention signals can include antigenic stimuli but also extend to macrophage interactions and CD69-mediated degradation of the S1P receptor. Because CD69 is upregulated upon TCR signaling and other cytokine stimuli, it is often measured as a marker of solely T-cell activation status. However, the consistent finding of substantial CD69 expression by T cells within the plaque microenvironment may also indicate T-cell retention and residency (40). Interestingly, S1P is carried in circulation by HDL molecules (51), and the beneficial effects of high HDL levels in circulation have been postulated to be due to limited vascular inflammation (29).
T-Cell Survival: Costimulation and Cytokine Signals
The effects of costimulatory cues in atherosclerosis have been reviewed (32). In brief, costimulatory molecules are intimately involved in the pathophysiology of adaptive immunity and atherosclerosis and are upregulated by differential flow patterns. The upregulated signals include CD40 ligand, CD137 (4–1BB), CD134 (OX40), and CD27 (24). Not only are costimulatory signals requisite for effector T-cell generation, but signals such as CD137 also endow survival potential (94) and the presence of multiple costimulatory signals can actually lower the threshold for antigen mimicry, thus increasing the inflammatory potential of resident effector memory cells (49).
In addition to costimulatory signals, the atherosclerotic microenvironment is rich in other cytokine cues that support survival and memory retention. Effector memory populations rely on IL-7 and IL-15 for homeostatic proliferation and long-term survival. Without these signals, they undergo apoptosis and the recall response to antigen is lost. Not only do human endothelial cells produce IL-15, but monocytes and ox-LDL positive macrophages do as well (41). IL-15 has been identified as a TCR-independent signal for T cells within atherosclerotic plaque (41), and serum IL-15 has been identified as an independent predictor of CVD (46) with direct IL-15 administration shown to aggravate atherosclerosis in mouse models (104). Furthermore, human gene polymorphisms have been associated positively with subclinical atherosclerosis and cardiovascular risk (3). Overall, these findings suggest that the atherogenic environment is not only prone to the infiltration of activated T cells but that environment of LDF-activated endothelium is uniquely equipped with multiple cues that enable their homeostatic existence and sustain their inflammatory programs.
Alternative Means of Modulating Effector T-cell Responses
The atherosclerotic plaque is a cholesterol-rich environment. Interestingly, cholesterol metabolism is typically associated with less inflammatory T-cell programs and cholesterol as a ligand has been associated with TCR hyporesponsiveness and CD1c stabilization (12). Cellular cholesterol in young versus aged patients differs by as much as twofold (27), and this increased membrane cholesterol has been associated with dampened TCR responses to anti-CD3-activating beads in the absence of lipid raft organization defects (69). Although this has direct correlation with age-related senescence of immune functions, it is interesting to postulate that the lipid-rich microenvironment may influence the cholesterol content of resident immune cells. As a ligand, cholesterol has also been shown to hinder TCR signaling by allosterically binding the TCR complex and maintaining the TCR in an inactive conformation that is incapable of receiving phosphorylation, which is necessary to transmit a TCR signaling event into the cytoplasm (93). Alternatively, cholesteryl esters have also been recognized as a cofactor for CD1c presentation (59), a class of proteins related to major histocompatibility class I glycoproteins that present lipid antigens. CD1c-reactive T cells have been elevated in certain autoimmune diseases with extra risk, and the high levels of both cholesterol esters and CD1c upregulation on foamy macrophages suggest that this self-reactivity that is antigen independent may play a role in the T-cell inflammatory mechanisms with plaque (62, 98).
In addition to lipid recognition, T cells that have been previously stimulated by a specific antigen, perhaps peripheral to any antigen it would reencounter within plaque, are responsive to innate-like cytokine cues (114a). Cytokines in the IL-1 family, such as IL-36, are implicated in such responses (97, 107, 108). IL-12 is a proinflammatory cytokine expressed within human plaques with signaling cascades shown to promote CD8 T-cell survival (100). Additionally, it has been reported that T cells are responsive to the macrophage-produced cytokine in the absence of TCR stimulation (116). Thus plaque-specific antigens may indeed instigate relevant TCR-dependent responses. However, the plaque microenvironment is also rich with cues that have the alternative potential to promote pathological effects (Fig. 1).
CORRELATES OF ATHEROSCLEROSIS WITH SYSTEMIC ACTIVATION OF THE ADAPTIVE IMMUNE SYSTEM
In the 1940s, CVD was the leading cause of death in the United States, yet the etiology of the disease and the risk factors associated with its mortality were unknown. With the enactment of the National Heart Act spurring longitudinal examination of large population cohorts as in the Framingham Heart study, the now traditional risk factors of hypertension, hyperlipidemia, and diabetes were uncovered (57). Epidemiological analyses of CVD have continued, and follow-up studies and retroactive analyses continue to implicate other diseases in conferring increased risk for CVD. Among the identified diseases are autoimmunity and a variety of chronic viral infections (26). Autoimmune diseases have a complex disease etiology but ultimately result in inappropriate activation of the adaptive immune system against self. In contrast, viral infections have an identifiable “beginning” and initiate a cascade of antiviral defenses, but their incomplete eradication can also lead to smoldering, chronic activation of the adaptive immune system. Thus these two health correlates are complex, with questionable etiology and resolution, and their link to increased CVD events is interesting and perhaps similar to that seen during obesity. A disease and reservoir of metainflammation, obesity not only instigates chronic and maladaptive inflammatory programs in macrophages (55) that contribute to a heightened CVD risk (105), but it also has the potential to exacerbate the pathological adaptive immune responses in both autoimmunity and viral infection (19, 106).
Autoimmunity
Systemic erythematous lupus (SLE) is an autoimmune disease highlighted by malar rash, production of antinuclear antibodies, and most notably, nephritic complications that present in women of reproductive age, a population not normally susceptible to substantial CVD. Thus it was striking to uncover in the 1970s that SLE patients, particularly women under the age of 44, had almost a 50-fold elevated risk ratio for myocardial infarction when compared retrospectively to the Framingham study (60). In fact, CVD is the leading cause of premature mortality in SLE patients, but the underlying mechanisms are not well distinguished. This increased prevalence of CVD in SLE patients was initially attributed to the anti-inflammatory, steroid-based SLE therapies that have the known side-effects of hypertension, diabetes, and dyslipidemia. However, multiple cohort studies since have established SLE-associated inflammation to confer independent risks for developing not only CVD complications but also experience accelerated, asymptomatic atherosclerosis. In a cohort study comparing SLE patients to control subjects with no prior CVD history through electron-beam computed chromatography to screen for arterial calcification, it was found the SLE did not significantly correlate with LDL or HDL levels and that SLE patients had statistically younger age of presenting with coronary artery calcification (6). Another larger cohort study was concurrently published in 2003 and corroborated the accelerated rate of atherosclerotic development in SLE patients at every age group (83). Using ultrasound to measure premature atherosclerotic focal protrusions within the carotid vasculature, the researchers additionally correlated length of SLE duration, less aggressive immunosuppressive therapies, and higher damage-index scores to be independent predictors of plaque presence (83). Thus SLE disease, regardless of lipid level and other “traditional” comorbidities of hypertension or diabetes, correlated significantly with not only CVD end-point measures of myocardial infarction but also asymptomatic intermediary attributes of atherosclerotic pathology. Studies have since aimed to characterize which disease characteristics are associated with accelerated atherosclerosis. Repeatedly, serum measurements of circulating autoreactive antibodies have been analyzed, hypothesizing that plaque presence might correlate with increased circulation of antibodies. However, there has been a consistent lack of correlation of autoantibody presence and measured end point, including anti-smith antibodies (83), anti-double stranded DNA (6), anti-phospholipid antibodies (6, 35), or Sjogren’s syndrome antigens A or B (35).
Overall, the theme of autoimmunity posing independent, increased risk for CVD is extended to autoimmune psoriasis (109), rheumatoid arthritis (21), and irritable bowel diseases (36, 115). Each are examples of diseases where overt TCR reactivity is directed toward antigens that are enriched peripheral to atherosclerotic plaque environments but may have chronic elevations in IL-1 family cytokines (5, 47, 52, 89) (Fig. 2). Interestingly, the association of memory T-cell phenotypes in circulation in RA has been associated with subclinical atherosclerosis, as measured by carotid artery calcification (113). The memory pool associated with increased carotid atherosclerosis had also acquired alternative natural killer cell receptor expression, a phenotype ascribed to cells that have previously received TCR activation (45).
Fig. 2.
Peripheral activation of the adaptive immune system confers increased risk for cardiovascular disease. Several autoimmune diseases with major pathological manifestations external to the vascular system confer independent elevated risk for accelerated atherosclerosis and cardiovascular disease (CVD). These include autoimmune psoriasis, rheumatoid arthritis, systemic lupus erythematous, and irritable bowel disease (Crohn’s and ulcerative colitis). While the inciting antigen is not known for each disease, suggested antigens that may contribute to systemic autoreactivity are noted in boxes. In addition to autoimmune disease, certain viral infections with chronic latency [cytomegalovirus (CMG), hepatitis C virus (HCV), and human immunodeficiency virus (HCV)] also confer increased risk for CVD complications, especially in the setting of cardiac and vessel transplants. These infections generate long-lasting pools of memory T cells that reside in barrier tissues and secondary lymphoid organs and, possibly, the vessel wall and are susceptible to inflammatory reactivation.
Viral Infection
The correlation of viral infection to atherosclerotic disease was initiated by the study of Marek’s disease, a herpes virus, being causative of overt plaque development within chickens (63). Since then, multiple viruses have been located latent within human arteries, including herpes simplex virus, which was tested for in patients undergoing coronary bypass (10). Cytomegalovirus (CMV) is a herpes family virus with lifelong latency and reactivations that are asymptomatic in the healthy patient. However, in patients undergoing cardiac transplant, recipients with CMV reactivation, evidenced by IgG titers and positive viral cultures, had increased rates of atherosclerosis-related deaths, as well as greater rates of graft rejection (33).
In other instances of persistent viral infection, such as hepatitis C virus (HCV), there are ties between systemic cytokine release and increased oxidative stress exacerbating atherosclerotic inflammation (2). However, there is also correlation between the localization of positive-stranded HCV RNA within carotid plaque tissue and CVD. Not only was HCV RNA localization specific, as it was never found in non-HCV patients, but it was sensitive, as it was sometimes localized to plaque tissue when undetectable in serum (14). When contextualized with findings that HCV RNA has been correlated with atherosclerotic complications in larger population studies, it is plausible that latent viral infections may take up local residence in atherogenic foci, where they remain resident until local infection unleashes inflammatory effector responses that coalesce in CVD (53).
Perhaps the most common viral infection in the general population is the influenza virus. Strikingly, mouse models showed that influenza infections have overt inflammatory effects in ApoE−/− mice that exacerbate atherosclerosis (67). Just 10 days after infection with influenza virus, mice experienced increased intimal cellularity, including increased CD3 T-cell infiltration. In this case, influenza virus was not located within the murine plaque and systemic release of cytokines was believed to be mediating the increase of plaque cellularity. Human epidemiological studies have been conducted comparing the rates of CVD in patients receiving, or not, the flu vaccine (58). Strikingly, vaccination drastically reduced CVD mortality (25, 34).
Acquired immune deficiency syndrome is a disease of immunodeficiency; thus it is at first puzzling that human immunodeficiency virus (HIV) patients have increased CVD. Yet, despite depleting the CD4 T-cell compartment, HIV causes chronic inflammation that results in bystander activation of CD8 T-cell specific to Epstein Barr virus, CMV, and the flu (4, 22, 70, 73). In fact, the rates of CMV infection in HIV+ patients have been determined to be an independent risk factor for CVD (56, 76). This is explained, perhaps, by the increased monocyte presentation of soluble CD14 and CD163 (16), which expands CMV-specific CD8 T-cell responses. Given the ubiquity of atherosclerosis, patients with HIV and depletion of their adaptive immune compartment, except for certain virus-specific CD8 T cells (66), exemplify the power of reactivated CD8 memory responses, even in the absence of recall antigen, in potentiating atherosclerotic inflammation (13).
The role for chronic viral infections and even vaccination against certain viral infections to influence CVD through immunological mechanisms is complex, but has great biomedical significance. The correlations of systemic increases or decreases in type 1 interferons may be affecting attrition (61, 91) or expansion (95) of certain memory CD8 T-cell subsets. However, provided the consistent demonstration of memory T-cell pools correlating with CVD, both in circulation, and isolated from within plaques, it is a process that warrants further investigation.
DISCUSSION
Topics for Further Development
Atherosclerotic plaque is a unique microenvironment in which T cells are classically and perhaps alternatively influenced by chronic elevation of cytokines. Blockade of certain IL-1 cytokine family members have demonstrated therapeutic impact in mitigating atherosclerosis. However, to further bridge the gap between epidemiological findings of diseases that confer accelerated CVD risk and mechanistic understanding of the pathology, several fields require further exploration.
First is the impact of peripheral-antigen experienced T cells. While local antigen cues certainly have inflammatory and regulatory roles in potentiating and protecting against atherosclerotic inflammation, the diseases correlated with increased CVD often highlight antigens located peripheral to atherogenic foci or vessel wall (Fig. 2). Additionally, the atherosclerotic microenvironment is rich in signals that may mitigate TCR activation and activate innate-like, TCR-independent effector programs. Furthermore, in diseases where T-cell-specific responses can be measured, such as through antibody production, there is often little association of antigen or antibody in circulation with CVD risk (83). Some of these diseases might be tied to inherent allelic variations in IL-1 cytokines (44), which may pervasively skew responses into inflammatory programs that result in accelerated atherosclerotic progression.
Recently, strong associations have been made between the ratio of naïve:memory T cells and CVD risk (7, 99). Though these memory subsets may be maintained by an environment rich in survival signals, they may also be surrounded by TCR-hyporesponsive cues, as has been established in other sites of chronic inflammation, such as the tumor microenvironment. However, even if hyporesponsive to TCR signals, these memory T cells may still be permissive to cytokine signals and can still have robust inflammatory responses (15, 54) (Fig. 1). While some studies have found T-cell memory infiltration to positively correlate with detection of subclinical atherosclerosis (72), others have identified an opposite effect on CVD events (114). It is not clear if this may be attributed to differential effects at different states of pathological progression, but it certainly warrants further investigation. In studies of posttransplant atherosclerosis, it has previously been suggested to be mediated through reactivation of CD8 T-cell memory (23). Recently, associations have been made between diabetic patients with increased effector memory T cells in circulation and CVD disease (26 vs. 18%, P < 0.05) (78). Thus contextualizing how activated effector T-cell transition into memory phenotypes is influenced by various disease processes or therapeutic regimens has major clinical relevance. This is especially true as CVD remains the leading cause of death, traditional risk factors are only increasing in the general population, and immunomodulatory therapies are being implemented as anti-cancer regimens.
Tools for Further Exploration
The cross-sectional epidemiological studies that have uncovered chronic inflammatory diseases conferring independent risk for CVD have been paramount to the study of atherosclerosis. However, further resolution into how the different arms of immunity interface and are affected by both acute and chronic inflammatory insults will require longitudinal analyses. Additionally, it had been suggested in 2006 that contributions of T cells to atherosclerosis was limited by isolation methods and a small number of cells isolated from lesions within mouse models (81). However, isolation and characterization methods have since advanced and it is now possible to make comparisons of circulating and plaque-resident cells with rigor. As immune cells rely on their environment for constant feedback, effector programs, and survival, studying their function and potential within their resident environment is key. Thus isolating T cells from distinct regions within the plaque (shoulder regions, adjacent to necrotic cores, or underlying a stable fibrotic cap) will reveal significant spatial and temporal information as to their dynamic states of interaction and activation. With the development of laser capture technology and single cell sequencing, the resolution of these environmental influences of metabolites and cytokines on cell interactions will be enhanced.
In summary, inflammation worsens CVD and the role of effector T cells is only beginning to be understood, although they are likely to play a substantial role perhaps in promoting disease. This might certainly occur by T-cell recognition of antigen in plaque, but we propose that there is also room for a TCR-independent response for plaque-residing T cells. Intervention at both points will constitute a more complete assessment of their pathological contributions and lead to the development of potentially more efficacious therapy.
GRANTS
The work was supported in part by American Heart Association Clinical Health Profession Grant Association Wide 17CPRE33660241 (to M. M. Xu), National Institutes of Health Grant R00-HL-125727 (to P. A. Murphy), and the Boehringer Ingelheim Endowed Chair in Immunology, UCONN Health School of Medicine (to A. T. Vella).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.X. prepared figures; M.M.X. and P.A.M. drafted manuscript; M.M.X., P.A.M., and A.T.V. edited and revised manuscript; M.M.X., P.A.M., and A.T.V. approved final version of manuscript.
REFERENCES
- 1.Abadier M, Pramod AB, McArdle S, Marki A, Fan Z, Gutierrez E, Groisman A, Ley K. Effector and regulatory T cells roll at high shear stress by inducible tether and sling formation. Cell Reports 21: 3885–3899, 2017. doi: 10.1016/j.celrep.2017.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol 20: 3410–3417, 2014. doi: 10.3748/wjg.v20.i13.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeles-Martínez J, Posadas-Sánchez R, Pérez-Hernández N, Rodríguez-Pérez JM, Fragoso JM, Bravo-Flores E, Posadas-Romero C, Vargas-Alarcón G. IL-15 polymorphisms are associated with subclinical atherosclerosis and cardiovascular risk factors. The Genetics of Atherosclerosis Disease (GEA) Mexican Study. Cytokine 99: 173–178, 2017. doi: 10.1016/j.cyto.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol 214: 231–241, 2008. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa A, Siewert K, Stöhr J, Besgen P, Kim SM, Rühl G, Nickel J, Vollmer S, Thomas P, Krebs S, Pinkert S, Spannagl M, Held K, Kammerbauer C, Besch R, Dornmair K, Prinz JC. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med 212: 2203–2212, 2015. doi: 10.1084/jem.20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asanuma Y, Oeser A, Shintani AK, Turner E, Olsen N, Fazio S, Linton MF, Raggi P, Stein CM. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med 349: 2407–2415, 2003. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 7.Baragetti A, Ramirez GA, Magnoni M, Garlaschelli K, Grigore L, Berteotti M, Scotti I, Bozzolo E, Berti A, Camici PG, Catapano AL, Manfredi AA, Ammirati E, Norata GD. Disease trends over time and CD4+CCR5+ T-cells expansion predict carotid atherosclerosis development in patients with systemic lupus erythematosus. Nutr Metab Cardiovasc Dis 28: 53–63, 2018. doi: 10.1016/j.numecd.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462: 94–98, 2009. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA 106: 7119–7124, 2009. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci USA 80: 6386–6389, 1983. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 338: 1650–1656, 1998. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 12.Bietz A, Zhu H, Xue M, Xu C. Cholesterol metabolism in T cells. Front Immunol 8: 1664, 2017. doi: 10.3389/fimmu.2017.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, Capeau J, Cohen A. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol 61: 511–523, 2013. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 14.Boddi M, Abbate R, Chellini B, Giusti B, Giannini C, Pratesi G, Rossi L, Pratesi C, Gensini GF, Paperetti L, Zignego AL. Hepatitis C virus RNA localization in human carotid plaques. J Clin Virol 47: 72–75, 2010. doi: 10.1016/j.jcv.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Broderick L, Bankert RB. Memory T cells in human tumor and chronic inflammatory microenvironments: sleeping beauties re-awakened by a cytokine kiss. Immunol Invest 35: 419–436, 2006. doi: 10.1080/08820130600755066. [DOI] [PubMed] [Google Scholar]
- 16.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 204: 1227–1236, 2011. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carman CV, Martinelli R. T lymphocyte-endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front Immunol 6: 603, 2015. doi: 10.3389/fimmu.2015.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chèvre R, González-Granado JM, Megens RT, Sreeramkumar V, Silvestre-Roig C, Molina-Sánchez P, Weber C, Soehnlein O, Hidalgo A, Andrés V. High-resolution imaging of intravascular atherogenic inflammation in live mice. Circ Res 114: 770–779, 2014. doi: 10.1161/CIRCRESAHA.114.302590. [DOI] [PubMed] [Google Scholar]
- 19.Damouche A, Lazure T, Avettand-Fènoël V, Huot N, Dejucq-Rainsford N, Satie AP, Mélard A, David L, Gommet C, Ghosn J, Noel N, Pourcher G, Martinez V, Benoist S, Béréziat V, Cosma A, Favier B, Vaslin B, Rouzioux C, Capeau J, Müller-Trutwin M, Dereuddre-Bosquet N, Le Grand R, Lambotte O, Bourgeois C. Adipose tissue is a neglected viral reservoir and an inflammatory site during chronic HIV and SIV infection. PLoS Pathog 11: e1005153, 2015. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 171: 223–229, 1993. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 21.del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44: 2737–2745, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Doisne JM, Urrutia A, Lacabaratz-Porret C, Goujard C, Meyer L, Chaix ML, Sinet M, Venet A. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J Immunol 173: 2410–2418, 2004. doi: 10.4049/jimmunol.173.4.2410. [DOI] [PubMed] [Google Scholar]
- 23.Ducloux D, Bamoulid J, Crepin T, Rebibou JM, Courivaud C, Saas P. Posttransplant Immune Activation: Innocent Bystander or Insidious Culprit of Posttransplant Accelerated Atherosclerosis. Cell Transplant 26: 1601–1609, 2017. doi: 10.1177/0963689717735404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ensminger SM, Witzke O, Spriewald BM, Morrison K, Morris PJ, Rose ML, Wood KJ. CD8+ T cells contribute to the development of transplant arteriosclerosis despite CD154 blockade. Transplantation 69: 2609–2612, 2000. doi: 10.1097/00007890-200006270-00022. [DOI] [PubMed] [Google Scholar]
- 25.Estabragh ZR, Mamas MA. The cardiovascular manifestations of influenza: a systematic review. Int J Cardiol 167: 2397–2403, 2013. doi: 10.1016/j.ijcard.2013.01.274. [DOI] [PubMed] [Google Scholar]
- 26.Frostegård J. Atherosclerosis in patients with autoimmune disorders. Arterioscler Thromb Vasc Biol 25: 1776–1785, 2005. doi: 10.1161/01.ATV.0000174800.78362.ec. [DOI] [PubMed] [Google Scholar]
- 27.Fülöp T Jr, Douziech N, Goulet AC, Desgeorges S, Linteau A, Lacombe G, Dupuis G. Cyclodextrin modulation of T lymphocyte signal transduction with aging. Mech Ageing Dev 122: 1413–1430, 2001. doi: 10.1016/S0047-6374(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 28.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27: 165–197, 2009. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlbäck B, Kono M, Proia RL, Smith JD, Hla T. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal 400: er8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity 39: 1003–1018, 2013. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res 103: 1220–1231, 2008. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 261: 3561–3566, 1989. doi: 10.1001/jama.1989.03420240075030. [DOI] [PubMed] [Google Scholar]
- 34.Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J 25: 25–31, 2004. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson JT, Herlitz Lindberg M, Gunnarsson I, Pettersson S, Elvin K, Öhrvik J, Larsson A, Jensen-Urstad K, Svenungsson E. Excess atherosclerosis in systemic lupus erythematosus–a matter of renal involvement: case control study of 281 SLE patients and 281 individually matched population controls. PLoS One 12: e0174572, 2017. doi: 10.1371/journal.pone.0174572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haapamäki J, Roine RP, Turunen U, Färkkilä MA, Arkkila PE. Increased risk for coronary heart disease, asthma, and connective tissue diseases in inflammatory bowel disease. J Crohn’s Colitis 5: 41–47, 2011. doi: 10.1016/j.crohns.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685–1695, 2005. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 38.Hansson GK, Libby P, Schönbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res 91: 281–291, 2002. doi: 10.1161/01.RES.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 39.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med 278: 483–493, 2015. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosono M, de Boer OJ, van der Wal AC, van der Loos CM, Teeling P, Piek JJ, Ueda M, Becker AE. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis 168: 73–80, 2003. doi: 10.1016/S0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 41.Houtkamp MA, van Der Wal AC, de Boer OJ, van Der Loos CM, de Boer PA, Moorman AF, Becker AE. Interleukin-15 expression in atherosclerotic plaques: an alternative pathway for T-cell activation in atherosclerosis? Arterioscler Thromb Vasc Biol 21: 1208–1213, 2001. doi: 10.1161/hq0701.092162. [DOI] [PubMed] [Google Scholar]
- 42.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, Burton FL, Ialenti A, Sabir SR, McInnes IB, Brewer JM, Garside P, Weber C, Lehmann T, Teupser D, Habenicht L, Beer M, Grabner R, Maffia P, Weih F, Habenicht AJ. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42: 1100–1115, 2015. doi: 10.1016/j.immuni.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends Immunol 36: 556–564, 2015. doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Italiani P, Manca ML, Angelotti F, Melillo D, Pratesi F, Puxeddu I, Boraschi D, Migliorini P. IL-1 family cytokines and soluble receptors in systemic lupus erythematosus. Arthritis Res Ther 20: 27, 2018. doi: 10.1186/s13075-018-1525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jabri B, Selby JM, Negulescu H, Lee L, Roberts AI, Beavis A, Lopez-Botet M, Ebert EC, Winchester RJ. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity 17: 487–499, 2002. doi: 10.1016/S1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 46.Kaibe M, Ohishi M, Ito N, Yuan M, Takagi T, Terai M, Tatara Y, Komai N, Rakugi H, Ogihara T. Serum interleukin-15 concentration in patients with essential hypertension. Am J Hypertens 18: 1019–1025, 2005. doi: 10.1016/j.amjhyper.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 47.Kawaguchi T, Mori M, Saito K, Suga Y, Hashimoto M, Sako M, Yoshimura N, Uo M, Danjo K, Ikenoue Y, Oomura K, Shinozaki J, Mitsui A, Kajiura T, Suzuki M, Takazoe M. Food antigen-induced immune responses in Crohn’s disease patients and experimental colitis mice. J Gastroenterol 50: 394–405, 2015. doi: 10.1007/s00535-014-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ketelhuth DF, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res 118: 668–678, 2016. doi: 10.1161/CIRCRESAHA.115.306427. [DOI] [PubMed] [Google Scholar]
- 49.Kissler S, Anderton SM, Wraith DC. Antigen-presenting cell activation: a link between infection and autoimmunity? J Autoimmun 16: 303–308, 2001. doi: 10.1006/jaut.2000.0498. [DOI] [PubMed] [Google Scholar]
- 50.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, Ley K. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest 122: 3114–3126, 2012. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurano M, Yatomi Y. Sphingosine 1-phosphate and atherosclerosis. J Atheroscler Thromb 25: 16–26, 2018. doi: 10.5551/jat.RV17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, Chamilos G, Feldmeyer L, Marinari B, Chon S, Vence L, Riccieri V, Guillaume P, Navarini AA, Romero P, Costanzo A, Piccolella E, Gilliet M, Frasca L. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun 5: 5621, 2014. [Erratum in Nat Commun 6: 6595, 2015.] doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 53.Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke 41: 2894–2900, 2010. doi: 10.1161/STROKEAHA.110.598136. [DOI] [PubMed] [Google Scholar]
- 54.Lehman HK, Simpson-Abelson MR, Conway TF Jr, Kelleher RJ Jr, Bernstein JM, Bankert RB. Memory T cells in the chronic inflammatory microenvironment of nasal polyposis are hyporesponsive to signaling through the T cell receptor. J Assoc Res Otolaryngol 13: 423–435, 2012. doi: 10.1007/s10162-012-0313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res 191: 29–44, 2018. doi: 10.1016/j.trsl.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lurain NS, Hanson BA, Hotton AL, Weber KM, Cohen MH, Landay AL. The association of human cytomegalovirus with biomarkers of inflammation and immune activation in HIV-1-infected women. AIDS Res Hum Retroviruses 32: 134–143, 2016. doi: 10.1089/aid.2015.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383: 999–1008, 2014. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol 130: 304–309, 2008. doi: 10.1016/j.ijcard.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 59.Mansour S, Tocheva AS, Cave-Ayland C, Machelett MM, Sander B, Lissin NM, Molloy PE, Baird MS, Stübs G, Schröder NW, Schumann RR, Rademann J, Postle AD, Jakobsen BK, Marshall BG, Gosain R, Elkington PT, Elliott T, Skylaris CK, Essex JW, Tews I, Gadola SD. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci USA 113: E1266–E1275, 2016. doi: 10.1073/pnas.1519246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 145: 408–415, 1997. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 61.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J Virol 75: 5965–5976, 2001. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melián A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol 155: 775–786, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minick CR, Fabricant CG, Fabricant J, Litrenta MM. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol 96: 673–706, 1979. [PMC free article] [PubMed] [Google Scholar]
- 63a.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med 117: 1367–1381, 1993. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63b.Mitsuyama K, Niwa M, Takedatsu H, Yamasaki H, Kuwaki k, Yoshioka S, Yamauchi R, Fukunaga S, Torimura T. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol 22: 1304–1310, 2016. doi: 10.3748/wjg.v22.i3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, Weih F, Weber C, Gerdes N, Habenicht AJ. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res 114: 1772–1787, 2014. doi: 10.1161/CIRCRESAHA.114.301137. [DOI] [PubMed] [Google Scholar]
- 65.Muegge K, Williams TM, Kant J, Karin M, Chiu R, Schmidt A, Siebenlist U, Young HA, Durum SK. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science 246: 249–251, 1989. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- 66.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, Hsue P, McCune JM, Deeks SG. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One 5: e8886, 2010. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E-deficient mice. Circulation 107: 762–768, 2003. doi: 10.1161/01.CIR.0000048190.68071.2B. [DOI] [PubMed] [Google Scholar]
- 68.Neyt K, GeurtsvanKessel CH, Deswarte K, Hammad H, Lambrecht BN. Early IL-1 signaling promotes iBALT induction after influenza virus infection. Front Immunol 7: 312, 2016. doi: 10.3389/fimmu.2016.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen DH, Espinoza JC, Taub DD. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mech Ageing Dev 125: 641–650, 2004. doi: 10.1016/j.mad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 30: 1495–1509, 2016. doi: 10.1097/QAD.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 117: 1292–1301, 2008. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 72.Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP. Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 8: e71498, 2013. doi: 10.1371/journal.pone.0071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev 254: 78–101, 2013. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family–balance between agonists and antagonists in inflammatory diseases. Cytokine 76: 25–37, 2015. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 75.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat Immunol 14: 246–253, 2013. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel EU, Gianella S, Newell K, Tobian AA, Kirkpatrick AR, Nalugoda F, Grabowski MK, Gray RH, Serwadda D, Quinn TC, Redd AD, Reynolds SJ. Elevated cytomegalovirus IgG antibody levels are associated with HIV-1 disease progression and immune activation. AIDS 31: 807–813, 2017. doi: 10.1097/QAD.0000000000001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 111: 1805–1812, 2003. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rattik S, Engelbertsen D, Wigren M, Ljungcrantz I, Östling G, Persson M, Nordin Fredrikson G, Bengtsson E, Nilsson J, Björkbacka H. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res, 2018. doi: 10.1177/1479164118817942. [DOI] [PubMed] [Google Scholar]
- 79.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators . Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 380: 752–762, 2019. doi: 10.1056/NEJMoa1809798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJ, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PR, Troquay RP, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 81.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol 26: 2421–2432, 2006. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 82.Rohm I, Atiskova Y, Drobnik S, Fritzenwanger M, Kretzschmar D, Pistulli R, Zanow J, Krönert T, Mall G, Figulla HR, Yilmaz A. Decreased regulatory T cells in vulnerable atherosclerotic lesions: imbalance between pro- and anti-inflammatory cells in atherosclerosis. Mediators Inflamm 2015: 1, 2015. doi: 10.1155/2015/364710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, Crow MK, Schwartz JE, Paget SA, Devereux RB, Salmon JE. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med 349: 2399–2406, 2003. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 84.Rothermel AL, Wang Y, Schechner J, Mook-Kanamori B, Aird WC, Pober JS, Tellides G, Johnson DR. Endothelial cells present antigens in vivo. BMC Immunol 5: 5, 2004. doi: 10.1186/1471-2172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saitakis M, Dogniaux S, Goudot C, Bufi N, Asnacios S, Maurin M, Randriamampita C, Asnacios A, Hivroz C. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. eLife 6: e23190, 2017. doi: 10.7554/eLife.23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarkar S, Yuzefpolskiy Y, Xiao H, Baumann FM, Yim S, Lee DJ, Schenten D, Kalia V. Programming of CD8 T cell quantity and polyfunctionality by direct IL-1 signals. J Immunol 201: 3641–3650, 2018. doi: 10.4049/jimmunol.1800906. [DOI] [PubMed] [Google Scholar]
- 87.Schatz DG, Ji Y. Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11: 251–263, 2011. doi: 10.1038/nri2941. [DOI] [PubMed] [Google Scholar]
- 88.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol 8: 1295–1301, 2007. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 89.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, Lundberg K, Engström A, Venables PJ, Toes RE, Holmdahl R, Klareskog L, Malmström V. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum 62: 44–52, 2010. doi: 10.1002/art.25036. [DOI] [PubMed] [Google Scholar]
- 90.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol 185: 4846–4855, 2010. doi: 10.4049/jimmunol.0903732. [DOI] [PubMed] [Google Scholar]
- 91.Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali MA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ. Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity 40: 801–813, 2014. doi: 10.1016/j.immuni.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sundd P, Gutierrez E, Koltsova EK, Kuwano Y, Fukuda S, Pospieszalska MK, Groisman A, Ley K. ‘Slings’ enable neutrophil rolling at high shear. Nature 488: 399–403, 2012. doi: 10.1038/nature11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Swamy M, Beck-Garcia K, Beck-Garcia E, Hartl FA, Morath A, Yousefi OS, Dopfer EP, Molnár E, Schulze AK, Blanco R, Borroto A, Martín-Blanco N, Alarcon B, Höfer T, Minguet S, Schamel WW. A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity 44: 1091–1101, 2016. doi: 10.1016/j.immuni.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol 162: 5037–5040, 1999. [PubMed] [Google Scholar]
- 95.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272: 1947–1950, 1996. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 96.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol 25: 615–622, 2013. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsurutani N, Mittal P, St Rose MC, Ngoi SM, Svedova J, Menoret A, Treadway FB, Laubenbacher R, Suárez-Ramírez JE, Cauley LS, Adler AJ, Vella AT. Costimulation endows immunotherapeutic CD8 T cells with IL-36 responsiveness during aerobic glycolysis. J Immunol 196: 124–134, 2016. doi: 10.4049/jimmunol.1501217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med 199: 417–422, 2004. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ugarte-Gil MF, Sánchez-Zúñiga C, Gamboa-Cárdenas RV, Aliaga-Zamudio M, Zevallos F, Tineo-Pozo G, Cucho-Venegas JM, Mosqueira-Riveros A, Perich-Campos RA, Alfaro-Lozano JL, Medina M, Rodríguez-Bellido Z, Alarcón GS, Pastor-Asurza CA. Circulating naive and memory CD4+ T cells and metabolic syndrome in patients with systemic lupus erythematosus: data from a primarily Mestizo population. Rheumatology (Oxford) 54: 1302–1307, 2015. doi: 10.1093/rheumatology/keu434. [DOI] [PubMed] [Google Scholar]
- 100.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J Immunol 174: 600–604, 2005. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 101.Valignat MP, Theodoly O, Gucciardi A, Hogg N, Lellouch AC. T lymphocytes orient against the direction of fluid flow during LFA-1-mediated migration. Biophys J 104: 322–331, 2013. doi: 10.1016/j.bpj.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van der Wal AC, Piek JJ, de Boer OJ, Koch KT, Teeling P, van der Loos CM, Becker AE. Recent activation of the plaque immune response in coronary lesions underlying acute coronary syndromes. Heart 80: 14–18, 1998. doi: 10.1136/hrt.80.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Dijk RA, Duinisveld AJ, Schaapherder AF, Mulder-Stapel A, Hamming JF, Kuiper J, de Boer OJ, van der Wal AC, Kolodgie FD, Virmani R, Lindeman JH. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc 4: e001403, 2015. doi: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Es T, van Puijvelde GH, Michon IN, van Wanrooij EJ, de Vos P, Peterse N, van Berkel TJ, Kuiper J. IL-15 aggravates atherosclerotic lesion development in LDL receptor deficient mice. Vaccine 29: 976–983, 2011. doi: 10.1016/j.vaccine.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 105.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 444: 875–880, 2006. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 106.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 13: 981–1000, 2014. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE, Gabay C. IL-36R ligands are potent regulators of dendritic and T cells. Blood 118: 5813–5823, 2011. doi: 10.1182/blood-2011-05-356873. [DOI] [PubMed] [Google Scholar]
- 108.Vigne S, Palmer G, Martin P, Lamacchia C, Strebel D, Rodriguez E, Olleros ML, Vesin D, Garcia I, Ronchi F, Sallusto F, Sims JE, Gabay C. IL-36 signaling amplifies Th1 responses by enhancing proliferation and Th1 polarization of naive CD4+ T cells. Blood 120: 3478–3487, 2012. doi: 10.1182/blood-2012-06-439026. [DOI] [PubMed] [Google Scholar]
- 109.Wakkee M, Thio HB, Prens EP, Sijbrands EJ, Neumann HA. Unfavorable cardiovascular risk profiles in untreated and treated psoriasis patients. Atherosclerosis 190: 1–9, 2007. doi: 10.1016/j.atherosclerosis.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 110.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal 3: cm1, 2010. [DOI] [PubMed] [Google Scholar]
- 111.Weinstein AM, Chen L, Brzana EA, Patil PR, Taylor JL, Fabian KL, Wallace CT, Jones SD, Watkins SC, Lu B, Stroncek DF, Denning TL, Fu YX, Cohen PA, Storkus WJ. Tbet and IL-36γ cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment. OncoImmunology 6: e1322238, 2017. doi: 10.1080/2162402X.2017.1322238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weinstein AM, Giraldo NA, Petitprez F, Julie C, Lacroix L, Peschaud F, Emile JF, Marisa L, Fridman WH, Storkus WJ, Sautès-Fridman C. Association of IL-36γ with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol Immunother 68: 109–120, 2019. doi: 10.1007/s00262-018-2259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A, Zartoshti A, Bokhari S, Bathon JM. Association of elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheumatol 68: 92–102, 2016. doi: 10.1002/art.39419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K, Wolf D. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass Cytometry. Circ Res 122: 1675–1688, 2018. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114a.Xu MM, Ménoret A, Nicholas SE, Günther S, Sundberg EJ, Zhou B, Rodriguez A, Murphy PA, Vella AT. Direct CD137 costimulation of CD8 T cells promotes retention and innate-like function within nascent atherogenic foci. Am J Physiol Heart Circ 2019 Apr 12. [Epub ahead of print] doi: 10.1152/ajpheart.00088.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol 106: 741–747, 2011. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 116.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res 98: 524–531, 2006. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 117.Zhou X. CD4+ T cells in atherosclerosis. Biomed Pharmacother 57: 287–291, 2003. doi: 10.1016/S0753-3322(03)00082-9. [DOI] [PubMed] [Google Scholar]