Abstract
A growing body of data provides strong evidence that intracellular angiotensin II (ANG II) plays an important role in mammalian cell function and is involved in the pathogenesis of human diseases such as hypertension, diabetes, inflammation, fibrosis, arrhythmias, and kidney disease, among others. Recent studies also suggest that intracellular ANG II exerts protective effects in cells during high extracellular levels of the hormone or during chronic stimulation of the local tissue renin-angiotensin system (RAS). Notably, the intracellular RAS (iRAS) described in neurons, fibroblasts, renal cells, and cardiomyocytes provided new insights into regulatory mechanisms mediated by intracellular ANG II type 1 (AT1Rs) and 2 (AT2Rs) receptors, particularly, in mitochondria and nucleus. For instance, ANG II through nuclear AT1Rs promotes protective mechanisms by stimulating the AT2R signaling cascade, which involves mitochondrial AT2Rs and Mas receptors. The stimulation of nuclear ANG II receptors enhances mitochondrial biogenesis through peroxisome proliferator-activated receptor-γ coactivator-1α and increases sirtuins activity, thus protecting the cell against oxidative stress. Recent studies in ANG II-induced preconditioning suggest that plasma membrane AT2R stimulation exerts protective effects against cardiac ischemia-reperfusion by modulating mitochondrial AT1R and AT2R signaling. These studies indicate that iRAS promotes the protection of cells through nuclear AT1R signaling, which, in turn, promotes AT2R-dependent processes in mitochondria. Thus, despite abundant data on the deleterious effects of intracellular ANG II, a growing body of studies also supports a protective role for iRAS that could be of relevance to developing new therapeutic strategies. This review summarizes and discusses previous studies on the role of iRAS, particularly emphasizing the protective and counterbalancing actions of iRAS, mitochondrial ANG II receptors, and their implications for organ protection.
Keywords: angiotensin II receptors, cardioprotection, heart, intracellular renin-angiotensin system, mitochondria
INTRODUCTION
A fundamental process that paved the success of multicellular organisms in a dry, terrestrial environment was the development of the renin-angiotensin system (RAS) (27). The system allowed organisms to control their internal milieu by regulating the extracellular fluid volume and the pressure of circulating body fluids: two factors critical for organ perfusion and capillary fluid dynamics. Chronic RAS activation has been implicated in cardiovascular and renal pathologies (11, 62), among others, and has been the subject of enormous interest by researchers in the field. The discovery and characterization of the RAS started in 1898 with the finding by Tigerstedt and Bergman (97), who demonstrated that protein extracts from the kidney increase blood pressure in rabbits. Since then, a substantial body of data has accumulated indicating the presence of not only the circulating RAS, but also of a local or tissue RAS in several organs including the heart and the kidney, and more recently of an intracellular RAS. The classification of the circulating, local, and intracellular systems is defined by the sites of ANG II synthesis at the respective locations, independently of the site of action of the hormone (53). ANG II generated in the circulation (or endocrine RAS) does have tissue effects, but these are not considered part of local RAS systems that depend on the production of the hormone by the tissue (or organ). Similarly, the ANG II produced in tissues could act intracellularly (intracrine effects). The intracellular RAS generates ANG II that may have intracrine effects or could be secreted to exert paracrine or autocrine actions. These systems operate mainly through two G-protein coupled receptors (GCPRs): the angiotensin II (ANG II) type 1 (AT1R) and type 2 receptors (AT2R). However, new enzymes, peptides, and receptors associated with the RAS have been recently described in several tissues and organs adding complexity to the system in terms of interplay and regulation. Because the circulating and local RAS together with new members of this system have been extensively reviewed elsewhere, this review will focus primarily on the intracellular RAS, in particular looking at the role of mitochondrial ANG II receptors in cell and organ protection.
CIRCULATING RAS: THE ENDOCRINE SYSTEM
The RAS is one of the most important and studied hormonal systems of the human body. Commonly known as the circulating RAS, the hormonal system depends on ANG II generation to release aldosterone from the adrenal cortex, which, in turn, controls the NaCl balance by the kidney. This regulatory system works in concert with the antidiuretic hormone to determine the extracellular fluid volume and, together with the action of the heart and the vasculature, generate the pressure gradient and blood flow necessary for body function. Dysregulation of RAS or its chronic activation has been associated with the pathogenesis of several conditions including hypertension, cardiac hypertrophy, and diabetic nephropathy, among others. Hence, RAS suppressors or blockers have been of paramount importance in the treatment and prevention of these conditions (11, 17, 25, 62).
A substantial body of research has contributed to the characterization of the circulating RAS (25, 91). ANG II, the effector of RAS, is derived from angiotensinogen, an α2-globulin produced and released to the circulation by the liver. Angiotensinogen is cleaved by renin, an aspartyl protease, to produce the inactive decapeptide angiotensin I (ANG I), which is further cleaved to the active eight-amino acid peptide ANG II [Ang-(1–8)] by the angiotensin-converting enzyme (ACE). ACE is a matrix metalloproteinase that is present in the endothelium of the vasculature, particularly in the lungs. In the heart, the ACE-like enzyme chymase also generates ANG II. Renin, the rate-limiting step of ANG II production, is released from the granular cells of afferent arterioles of the kidney in response to low extracellular fluid volume or blood pressure. Under these conditions, renin secretion is promoted by activation of the sympathetic nervous system, local baroreflex mechanisms, reductions in NaCl transport at the macula densa of the juxtaglomerular apparatus, and low levels of locally acting hormones (i.e., ANG II and atrial natriuretic peptide) (75). The cellular signaling mechanisms involved in the control of renin release have been the subject of recent reviews (75, 82, 85). With the release of renin, and hence ANG II generation, the extracellular fluid volume and blood pressure are reestablished.
ORGAN RAS
In addition to the cardiovascular effects of the classical circulating RAS, substantial data indicate the presence of an organ-based RAS that carries out diverse physiological functions. Indeed, RAS components have been identified in several organs (72), including kidney, brain, liver, pancreas, vasculature, and heart, among others. Although in its early start the existence of the organ RAS was questioned because of difficulties in separating its components from those of the circulating RAS (i.e., renin, ANG II, and ACE), subsequent studies revealed the presence of genes and synthesis of several of its components in tissues including angiotensinogen, renin, and ACE (50). In the kidney (50, 72), the local RAS appears to be responsible for the higher levels of ANG II in tissues compared with plasma, which are maintained independently from circulating ANG II levels. In the heart, it has been estimated that the tissue generation of ANG II accounts for ~75% of the measured tissue hormone (15).
Locally synthesized ANG II exerts paracrine and autocrine effects on cell growth, proliferation and differentiation, and apoptosis as well. It promotes fibroblast activation and the synthesis of extracellular matrix components (e.g., collagen), cell hypertrophy, and inflammation (62). Increased levels of ANG II in the heart have been associated with heart failure (86), hypertrophy, and fibrosis in hypertensive animals (105). In the latter study, cardiac abnormalities were associated with inflammation, oxidative stress, and cell death. Organ ANG II also plays an important role in the control of renal hemodynamics and tubular transport (50) and controls tissue blood flow, duct cell Na+ transport, and islet β-cell (pro)insulin biosynthesis in the pancreas (56). In the brain, the local RAS mediates dopamine release and could be involved in inflammation, oxidative stress, and dopaminergic cell death (29, 102). However, the relevance of the local RAS to physiology and the pathogenesis of disease has been recently questioned (9) because of difficulties in demonstrating its functional independence from the circulating RAS. However, this is an evolving area of research, and a better understanding of these local systems and their implications in clinical medicine will emerge in the near future.
ANG II-DERIVED METABOLITES
The elucidation of bioactive ANG II-derived peptides is an emerging area of great interest. The discovery of new ANG II-derived peptides has added complexity to the mechanisms involved in the actions of circulating and organ-based RAS (25, 40). Two of these peptides Ang-(1–7) and alamandine provide important modulation of RAS. Ang-(1–7) can be generated by ACE2 from ANG II (26, 71). Decarboxylation of the aspartate residue of Ang-(1–7), in turn, generates the heptapeptide alamandine. The latter can also be generated by ACE2 from the octapeptide Ang A (54, 60, 77).
Ang-(1–7) and alamandine are endogenous negative modulators of RAS activation that act through a GPCR, the Mas receptor (40), and the Mas-related GPCR member D (MrgD) (41), respectively. The Mas receptor is antagonized by A-779, and the MrGD receptor by D-Pro7-Ang-(1–7). The Ang-(1–7)/Mas and the alamandine/MrgD axis are particularly evident when ACE activity is inhibited (81) or AT1R activity is blocked (44). Both Ang-(1–7) and alamandine antagonize most of the cardiovascular and renal actions of ANG II/AT1R-dependent processes (25, 26, 54, 60) such as vasoconstriction, proliferative and proinflammatory actions, fibrosis, and Na+ reabsorption by kidney tubules. Indeed, reduced expression of these countermodulatory arms, however, has been associated with the pathogenesis of diseases (26, 58) such as essential hypertension, remodeling of the heart and the vasculature, and renal disease, among others. However, the relevance of the alamandine/MrGD pathway has been questioned because the AT2R antagonist PD123319 displaces the binding of alamandine from its receptor and inhibits its vasorelaxant action in the aorta (58). This suggested that alamandine could operate through the ANG II/ANG III/AT2R/nitric oxide (NO)-cGMP axis. However, in AT2R knockout mice, PD123319 inhibited the vasorelaxant action of alamandine and its binding to MrGD-transfected Chinese hamster ovary cells, validating the alamandine/MrGD-protective pathway (54).
Other products of ANG II include ANG III [Ang-(2–8)] generated from ANG II by aminopeptidase A and ANG IV [(Ang-(3–8)] produced from ANG III by aminopeptidase N. ANG III can bind and activate both AT1R and AT2R with a lower affinity that ANG II (58). As a consequence, it may have vasopressor or vasodepressor effects depending on experimental conditions (i.e., ANG III levels and the receptor-type involved). In the kidney, however, ANG III is the predominant agonist for the AT2R pathway, thus inducing natriuresis and contributing to blood pressure control (58). By contrast, ANG IV through AT4R induces vasorelaxation in the brain, kidney, and vascular beds (5, 40, 92). Finally, the dipeptide Ang-(3–4) can be generated in kidney proximal tubule membranes from Ang-(1–7) or ANG IV and from ANG III in plasma (18). Ang-(3–4) is a negative regulator of both circulating and local RAS with natriuretic and antihypertensive properties. In renal epithelial cells, it appears to act as an allosteric enhancer of AT2R that through the cAMP-dependent PKA suppresses the inhibition of plasma membrane Ca2+ pumps and the stimulation of Na+-ATPase by the ANG II/AT1R pathway (18). Notably, the Ang-(3–4) levels in plasma inversely correlate with the upright systolic blood pressure in patients (61), suggesting a role of the endogenous dipeptide in the control of blood pressure. Therefore, Ang-(3–4) could provide an important mechanism for integrating information on body-fluid homeostasis from both the endocrine and paracrine RAS.
In summary, the protective arms of RAS (58) against the ANG II/AT1R axis now include the following: the ANG II/AT2R/NO-cGMP, Ang A/ACE2/alamandine/MrGD, ANG I/ANG II/ACE2/Ang-(1–7)/Mas, ANG III/ANG IV/AT4R, and ANG III/ANG IV/Ang-(3–4) pathways. Both ANG III and Ang-(3–4) operate through the AT2R/NO-cGMP cascade. It is likely that not all of these pathways are operational in a given cell type. The expression of key enzymes in tissues probably determines the protective pathway available in cells. For example, in the kidney, the presence of aminopeptidase A leads to the formation of ANG III, which aminopeptidase N converts to ANG IV. This assures the presence in tissues of the ANG III/ANG IV/AT4R pathway but also reinforces the NO-cGMP signaling cascade because of ANG III binding to AT2R (58). The latter induces natriuresis, reduces blood pressure, and ameliorates kidney damage (10). The presence of redundant protective arms of RAS illustrates the complexity of this system to date. How these protective pathways interact to counteract or modulate the ANG II/AT1R axis in tissues is under intense research. However, these pathways offer new possibilities to develop pharmacological drugs to treat medical conditions caused by chronic activation of RAS.
RECEPTORS: ANG II AND DERIVATIVES
ANG II and its metabolites act on target tissues through two main GPCRs: the AT1R that mediates most of its hemodynamic and nonhemodynamic effects and the AT2R that exerts a counterbalance or modulatory effect on AT1R-dependent actions (11, 25, 62). Both receptors bind ANG II with high affinity. Bosnyak et al. (7), evaluated the relative affinities of angiotensin peptides and novel ligands in human embryonic kidney (HEK)-293 cells transfected with either AT1R or AT2R. The values of ANG II concentrations that displace 50% the binding (IC50) of 125I-[SarI-IIe8]ANG II in HEK-293 cells transfected with AT1R and AT2R were 7.9 and 0.52 nM, respectively. The IC50 value for candesartan at the AT1R was 1.56 nM (IC50 at AT2R >1 µM), whereas the AT2R was inhibited by PD123319 with an IC50 of 5.6 nM (IC50 at AT1R >1 µM). The Mas receptor agonist AVE0991 and antagonist A-779 did not bind AT1R or AT2R when evaluated up to 1 µM. Finally, compound 21 and CGP42112, two agonists specific for AT2R, had inhibitory constants (IC50) of 2.29 nM and 0.23 nM, respectively (7). Similar inhibitory constants of compound 21 for the AT2R (Ki = 0.4 nM) have been reported in other preparations (58).
Ang-(1–7), ANG IV, and ANG III also show significant selectivity for AT2R over AT1R (7), suggesting that they could also act as endogenous AT2R ligands. Indeed, in the kidney, the effects of Ang-(1–7) are mediated through different receptors depending on the cell type. For example, in proximal and distal tubular cells, Ang-(1–7) works through AT2R and AT1R to inhibit the Na+-K+-ATPase activity, respectively, while in isolated proximal tubule cells it affects Na+/H+ exchange activity through the Mas receptor (25). Thus, although it is generally recognized that Ang-(1–7) specifically acts through the Mas receptor [ACE2-/Ang-(1–7)/Mas pathway], some effects could be attributed to its “promiscuity” on other receptor types including the Mrg family (31). Indeed, it was recently shown that Ang-(1–7) could bind to both Mas and MrGD receptors in HEK-293 cells in a PD123319-sensitive manner (96). Also, recent studies show that AT2R and Mas receptors colocalized, form heterodimers, and interact to generate their physiological responses, adding more complexity to the regulation of these protective axes (70). The complexity in the interaction between GPCRs has also been reported for the AT1R (76), and a functional cross talk between Mas receptors and AT1R has been described (73). Finally, Ang A, a product of decarboxylation of ANG II that carries a substitution of Asp1 by Ala1, induces AT1R-dependent vasoconstrictor responses in the kidney circulation of rats and mice (31, 106). These effects are inhibited by alamandine acting on the MrgD receptor (54).
The (pro)renin receptor (PRR), an accessory subunit of the vacuolar H+ pump (V-ATPase) is a new member of RAS (65). It binds renin and prorenin with high affinity, enhances ANG II generation, and activates mitogen-activated protein kinases ERK1/2. The PRR has been associated with the control of renin synthesis and release by the macula densa (82) and with the negative modulation (cyclooxygenase-2 dependent) of ANG II/AT1R actions in the renal medulla (32). The PRR is also associated with pathological conditions such as glomerulosclerosis and diabetic nephropathy in mice and rats (42, 43, 47). The stimulation of transforming growth factor-β (TGF-β) by PRR could be an important factor in the genesis of these conditions (99). However, the PRR could also play an important role in renal morphogenesis and development through the canonical Wnt/β-catenin signaling (90).
INTRACELLULAR RAS
Early suggestions of an intracellular RAS date back to 1971 (83) when labeled ANG II infused into the left ventricle of adult rats was found to be localized in the nuclear zone of vascular and cardiac muscle cells. Subsequently, high-affinity binding sites for ANG II were demonstrated in isolated rat hepatic nuclei, and their activation was associated with increased RNA synthesis (20, 78) and the expression of both renin and angiotensinogen (20, 80). More recently, immunohistochemical studies have demonstrated the presence of RAS components in various cells types including glomerulosa cells of rat adrenal cortex, dopaminergic neurons and glial cells, cardiomyocytes, and kidney tubule epithelial cells (35, 74, 98). Intracellular localization of RAS components was to be expected because, upon binding of ANG II, AT1Rs internalize (62) releasing the receptor protein and ANG II in the cytosol. Furthermore, a growing number of studies provide strong evidence in favor of intracellular colocalization of ANG II and renin in various cell types (11, 25, 72, 75). Cellular synthesis of ANG II has been demonstrated in cardiac cells, fibroblasts, vascular smooth muscle, and kidney cells, among others, and found to be physiologically relevant in gene expression, secretion of extracellular matrix components, cell proliferation, and vascular contraction (52). Indeed, the injection of ANG II in vascular smooth muscle cells leads to AT1R-dependent Ca2+ mobilization (37). In addition, intracellular ANG II affects nuclear AT1Rs and AT2Rs in cardiac fibroblasts promoting inositol 1,4,5-trisphosphate receptor (IP3R)-dependent Ca2+ increase and NO generation, respectively, which, in turn, modulates RNA synthesis, cell proliferation, and collagen secretion (95). Also, neonatal cardiac myocytes transfected with an adenoviral vector expressing ANG II demonstrate an increased rate of cellular hypertrophy independent of plasma membrane AT1Rs (4). The effects likely involve nuclear AT1Rs and AT2Rs that through a Gi-dependent process increase de novo RNA synthesis as well as rRNA and NF-кB mRNA (94). Notably, ANG II induces alterations in cell volume and communication and ion-channel activity in cardiomyocytes (15). These alterations in cardiomyocytes together with those induced by intracellular ANG II in cardiac fibroblasts lead to conduction disturbances in the heart (95). The intracellular actions of ANG II are insensitive to extracellular ANG II receptor blockers (ARBs) suggesting that the effects of intracellular ANG II are independent of plasma membrane receptors (16, 95). Indeed, AT2Rs in the nucleus of kidney cortical cells promote endothelial nitric oxide synthase-dependent NO synthesis that is coupled with the soluble guanylyl cyclase (35). These intracellular actions are mediated by ANG II originating either from cellular sources or internalization of the hormone (25). Altogether, these studies support the presence of an intracellular or intracrine RAS that controls cell function independently from the local or circulating systems.
Although the intracellular RAS has been implicated in critical physiological processes, evidence is available for the deleterious effects of high intracellular ANG II in different cell types including cardiomyocytes, cardiac fibroblasts, kidney cells, and neurons, among others. These effects, which include stimulation of hypertrophy, apoptosis, oxidative stress, and TGF-β, and NF-кB expression, have been implicated in organ damage, cardiac hypertrophy, fibrosis (4, 88), conduction abnormalities (15, 16), and inflammation (5, 94). Indeed, high intracellular ANG II in diabetes has been associated with myocardial oxidative stress, cardiomyocyte apoptosis and necrosis, and cardiac fibrosis in rats (87, 88). Similar effects have been observed in diabetic patients where increased intracellular ANG II labeling has been associated with augmented myocardial apoptosis, necrosis, and oxidative stress compared with nondiabetic subjects (28). The upregulation of intracellular RAS in diabetes (or by high-glucose conditions) is mediated through a chymase-dependent renin-mediated mechanism in cardiac myocytes (88), but similar processes increase intracellular ANG II in vascular smooth muscle cells and renal mesangial cells in hyperglycemic conditions (13, 55). In cardiac fibroblasts, however, intracellular ANG II is synthesized by renin and ACE-dependent pathways (87).
In cortical kidney cells, intracellular ANG II is associated with nuclear AT1R-dependent transcription of mRNAs for monocyte chemoattractant protein-1, NHE-3, and TGF-β1 (59), angiotensinogen generation via MAPK/NF-кB signaling pathways (108), and Ca2+ mobilization from intracellular stores (109). These actions mediate cell growth, proinflammatory responses, and Na+-retaining effects, which could lead to high blood pressure (25). Finally, studies suggest that local RAS hyperactivity enhances microglial-derived oxidative stress and neuroinflammation, which play a major role in the degeneration of dopaminergic neurons and possibly the progression of Parkinson’s disease (30). However, intracellular RAS could also play an important role in these events because persistent stimulation of the ANG II- AT1R induces oxidative stress in these cells. These effects reduce the Ang-(1–7)/MAS receptor-dependent increase in SIRT3 levels, further enhancing oxidative stress and promoting mitochondrial dysfunction by unopposed intracellular AT1R signaling (12, 33). In fact, these effects could lead to increased vulnerability to oxidative stress and neurodegeneration in aging (19). Altogether, accumulating data indicate that while intracellular RAS is important for the normal function of cells, its upregulation could lead to ANG II-dependent cellular dysfunction and organ pathology.
MITOCHONDRIAL ANGIOTENSIN RECEPTORS
The presence of a mitochondrial angiotensin system was initially suggested on the basis of renin, ACE, and ANG II localization in intramitochondrial dense bodies of the rat adrenal cortex (74). Subsequently, ANG II binding sites were reported in a purified mitochondrial fraction from rat brain (89), and ANG II was found in mitochondria by immunogold in rat cerebellar cortex (22). Studies also revealed uptake of circulating [125I]ANG II by mitochondrial fractions in the kidney and adrenal glands (100). The presence of ANG II receptors in this organelle was also supported by studies on Percoll-purified heart mitochondria from rat heart that demonstrated the expression of AT1R and AT2R proteins (69).
In 2011, Abadir et al. (1) described a functional mitochondrial angiotensin system. The group used a variety of cells of human and mouse origin (i.e., skeletal muscle cells, mouse cardiac myocytes, renal tubular cells, neuronal cells, and hepatocytes, among others) and demonstrated colocalization of ANG II and AT2R on the inner mitochondrial membrane by immunogold electron microscopy and immunofluorescence. It is reported that mitochondrial AT2Rs are functionally coupled with NO generation and that their stimulation inhibits oxygen consumption in isolated mitochondria. These results were validated using AT2R agonists (i.e., CGP421140) and the NO synthetase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME), indicating a functionally active angiotensin system in the mitochondria that controls respiration through an AT2R/NO-dependent mechanism. Notably, AT2R expression in mitochondria from mouse renal tubular cells was decreased by aging (70 wk old) in mice while AT1R expression, found low in young (20 wk old) mice, was increased. In old animals, these changes were associated with increasing superoxide generation by AT1R. Overall, these studies indicate that aging through alterations in the balance of AT1R to AT2R expression in the mitochondria could affect the survival of organisms by reducing the protective AT2R-mediated NO generation and augmenting the reactive oxygen species (ROS) levels. The latter are generated through stimulation of respiration chain activity secondary to AT1R activation. Indeed, it has been shown (6) that the disruption of AT1R promotes longevity in mice, possibly through the attenuation of oxidative stress and overexpression of prosurvival genes (i.e., SIRT3). Therefore, these studies implicate the mitochondrial AT1Rs and AT2Rs in cell survival, but whether they are causally related to senescence remains to be elucidated.
Recently, Valenzuela et al. (98) confirmed the presence of both AT1R and AT2R in mitochondria from dopaminergic neurons by immunofluorescence and confocal microscopy. In agreement with previous findings, these investigators found a high ratio of AT2R to AT1R protein levels in mitochondria. Fluorescently labeled ANG II, AT1R, and AT2R colocalized with mitochondria. Furthermore, activation of AT1R in the presence of ANG II and PD123319 increased oxidative phosphorylation and maximal respiratory rate and augmented ROS production via NAD(P)H oxidase 4 (NOX4). These effects are reduced in AT1R knockout mice. By contrast, AT2R stimulation in the presence of ANG II and losartan decreased respiratory function and maximal respiratory activity, both of which are enhanced in AT2R knockout mice compared with wild-type animals. NO appears to play a major role in this action of AT2R because inhibition of NOS using l-NAME, reduced NO and increased respiratory function. Notably, AT2R-YFP labeling was increased in mitochondria by 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress while the antioxidant N-acetyl cysteine enhanced mitochondrial AT1R in comparison with untreated controls. These studies suggest modulation of ANG II receptors by the level of oxidative stress in the cell. In mitochondria from aged animals, an increased AT1R/AT2R mRNA expression ratio was observed independently of changes in mitochondrial mass, suggesting that the regulation of ANG II receptors by oxidative stress is lost in aging animals. These results support the existence of a subcellular, compartmentalized RAS, whereby mitochondrial AT1R and AT2R exert tight control over respiratory function in cells. Stimulation of AT1R promotes the respiratory function of mitochondria through NOX4, while AT2R stimulation antagonizes this action through NO generation.
MITOCHONDRIAL ANGIOTENSIN RECEPTORS AND CARDIOPROTECTION
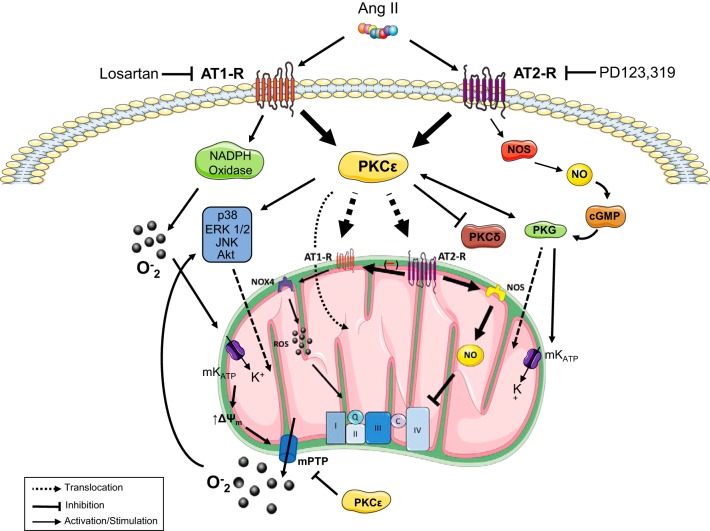
The relevance of AT1R and AT2R in the control of mitochondrial, cell, and organ function is underscored by recent studies from our group elucidating cardioprotective pathways during ANG II-preconditioning (APC) (66–68). APC was induced in isolated Langendorff-perfused rat hearts by infusion of ANG II (4 cycles, 5 min each) before global ischemia followed by reperfusion (I/R). We found that APC significantly protects the heart from I/R damage, improves cardiac postischemic recovery, and reduces infarct size (66) in a manner consistent with the ischemic preconditioning (IPC) protocol originally described by Murry et al. (64). Our studies (68) also revealed a high AT2R/AT1R ratio in cardiac mitochondria from young rats (7 wk old) that was suppressed by ischemia but reversed by APC, suggesting that ANG II promotes a high AT2R/AT1R ratio. Mitochondria from nonischemic hearts without APC also demonstrated a high AT2R/AT1R ratio consistent with previous studies (1, 98). At variance with the AT2R that was abolished by PD123319 but not by losartan, AT1R levels were abrogated by both blockers. This suggested that mitochondrial AT2Rs exert a permissive action over AT1R protein levels because coronary resistance in the perfused heart, an AT1R-dependent process, was not significantly affected by PD123319. Whether the permissive action of AT2R on AT1R involves the Mas or MrGD receptors has not been determined. Notably, the AT1R response profile to losartan and PD123319 was also shared by mitochondrial protein kinase C (PKC), protein kinase Cε (PKCε), Akt, MAPK, and PKG-1 but not by PKCδ suggesting that these kinases are distal to AT1R-dependent PKCε-activation and susceptible to inhibition by AT2R antagonism. In addition, chelerythrine, a pan-PKC inhibitor, reproduced the response profile of losartan on mitochondrial kinases (67).
Most importantly, inhibition of AT2R with PD123319 induced a marked stimulation of mitochondrial (state 3) respiration that was observed even after losartan treatment (68). The stimulatory effect of the AT2R antagonist resulted in a pronounced increase in respiratory control index indicating the activation of the respiratory chain and ATP production. These effects of PD123319 suggest that AT2R maintains a tonic suppression of mitochondrial respiration and coupling independent of possible AT1R-mediated effects. Notably, the postischemic cardiac function also increased with the blockade of AT2R, suggesting that I/R promotes the suppression of both mitochondrial and cardiac function through this receptor. These findings support the idea that following I/R, mitochondrial AT2Rs attenuate myocardial damage by decreasing the energy and oxygen demands of the heart while promoting cell survival through signaling pathways involving AT1R-dependent PKCε stimulation or through the NO/cGMP pathway. Indeed, the blockade of AT1R in APC with losartan, which supports selective AT2R activation, reduces infarct size to a larger extent than APC alone. These effects of APC and losartan were prevented by PD123319. However, ARBs did not affect hearts without APC (68). These observations indicate that AT2R activity in APC is responsible for the beneficial effects of the AT1R blockade on infarct size. These data agree with previous results (51) indicating that blockade of AT2R with PD123319 prevented the cardioprotection against I/R injury in valsartan-treated hearts. Altogether, these studies (66–68) demonstrate that during APC, extracellular ANG II affects mitochondrial function through AT2R signaling, protecting the heart and reducing infarct size in the course of I/R injury (68). Therefore, these findings are consistent with and extend previous reports (1, 98) on functional ANG II receptors in isolated mitochondria from various cell preparations.
The proposed mechanism by which extracellular ANG II affects mitochondrial and cardiac function is shown in Fig. 1. The mechanism includes a permissive action of AT2R signaling on the AT1R/PKCε-dependent pathway in mitochondria, suggesting that AT2Rs exert critical control over AT1R-mediated pathways. Furthermore, these studies indicate that AT2Rs tonically suppress mitochondrial respiration and cardiac function following I/R in APC hearts. Although these two processes can be regulated independently of AT2R, the concomitant stimulation of respiration and cardiac function by PD123319 demonstrates a strong association between these two events. Possible mediators of this interaction are AT2R-sensitive, mitochondrial ROS and cytosolic redox-sensitive protein kinases (i.e., ERK1/2-, p90RSK-, PKC-dependent cascades) that promote contractility through changes in cell calcium (14). Notably, the regulation of these processes is relevant in APC-mediated cardioprotection because AT2R inhibition augments infarct size while AT1R blockade reduces it. Therefore, it appears that AT2Rs are cardioprotective through their permissive action on mitochondrial AT1R signaling and the suppression of cardiac function following I/R. These effects of ANG II in mitochondria add up to the protection conferred by IPC (23), which involves among others activation of prosurvival pathways (phosphatidylinositol 3-kinase-Akt and RasRaf-MEK1/2-ERK1/2). Activation and translocation to the mitochondria of kinases involved in these pathways exert protective action.
Fig. 1.
Proposed mechanisms of mitochondria-mediated cardioprotection by ANG II-preconditioning (APC). APC operates through plasma membrane ANG II type 1 (AT1Rs) and 2 (AT2Rs) receptors that are blocked by losartan and PD123319, respectively. Stimulation of these receptors leads to PKCε activation and translocation of AT1R, AT2R, Akt, ERK1/2, JNK, p38, and PKG to the mitochondria where they exert their protective role. AT1Rs also stimulate NAD(P)H oxidase (NOX) activity leading to superoxide generation, mitoKATP channel activity, membrane depolarization, and mitochondrial permeability transition pores (mPTP) opening. This process enhances the efflux of superoxide into the cytoplasm and promotes the activation of prosurvival kinases. By contrast, AT2R stimulation leads to nitric oxide synthase (NOS)-nitric oxide (NO)-PKG stimulation that further stimulates PKCε. In the mitochondria, the upregulation of AT2R/AT1R protein levels controls the respiratory chain activity through a stimulatory mechanism composed of NOX4-reactive oxygen species (ROS) and an inhibitory pathway involving NOS-NO. The latter is proposed to exert a master control on respiratory function as it modulates AT1Rs while tonically suppressing electron transport chain activity.
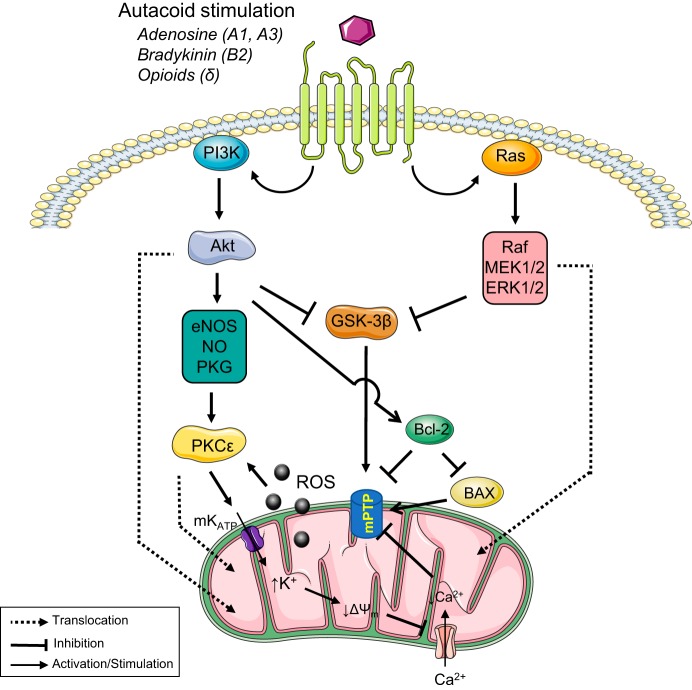
In addition to prosurvival pathways, inhibition of GSK-3β has been shown to block mitochondrial permeability transition pores (mPTP) and activate PKCє through the endothelial nitric oxide synthase-NO-PKG pathway (Fig. 2). Besides, the stimulation of the mitoKATP channel leads to reduced mitochondrial potential and inhibition of mPTP opening. IPC has also been shown to inhibit mPTP by reducing the sensitivity of mitochondria to Ca2+ (46). These findings suggest that cardioprotection induced by APC alone or in combination with IPC could be due to an AT1R-dependent translocation of PKCε and survival kinases to the mitochondria leading to mPTP inhibition and the suppression of mitochondrial respiration and cardiac function by AT2R (68). Also, APC could contribute to improved heart function after I/R by direct effects of ANG II/AT1R on the contractility of myocardial fibers through Ca2+ mobilization, phosphorylation of myosin light chain, and inhibition of phosphatases, among others (62, 101).
Fig. 2.
Main signaling pathways involved in mitochondria-mediated cardioprotection by ischemic preconditioning (IPC). IPC is triggered by ligands (i.e., adenosine, bradykinin, and opioids, among others) that through receptor-mediated processes stimulate signaling pathways involved in cardioprotection. Prosurvival kinases involved in these pathways [phosphatidylinositol 3-kinase (PI3K)-Akt and Ras-Raf-MEK1/2-ERK1/2] are activated and translocated to the mitochondria to exert protective action. Both pathways lead to inhibition of GSK-3β, which blocks the mitochondrial permeability transition pores (mPTP). Also, the activation of the PI3K-Akt signaling mechanism, promote activation of PKCє through the endothelial nitric oxide synthase (eNOS)-nitric oxide (NO)-PKG pathway. Stimulation of mitoKATP channel leads to reduced mitochondrial potential and inhibition of mPTP opening. Reactive oxygen species (ROS) generation by stimulation of respiratory chain activity promotes the continuous stimulation of PKCε and cardioprotection.
An important aspect that needs elucidation is the mechanism(s) mediating the translocation of ANG II receptors to the mitochondria in APC studies. There is no evidence that ANG II receptors are encoded by mitochondrial DNA (8, 93). However, mitochondrial translocation of the protein kinases involved in ANG II signaling (i.e., MAPKs) is involved in mitochondria-mediated cell survival and cell death (45), indicating its relevance in cardioprotection by APC. Intriguingly, both AT1R and AT2R lack a subcellular targeting sequence (2), and whether they are bound to molecular chaperones and TOM70 (38) remains unknown. A translocation process, however, is supported by the inhibitory effect of chelerythrine, which reportedly inhibits the activation/translocation of PKC from the cytosol to subcellular or particulate fractions (3, 57).
NUCLEAR PATHWAYS REGULATING MITOCHONDRIAL ANG II RECEPTORS
Studies on the perfused heart subjected to APC support a critical role of the mitochondrial ANG II receptors in controlling mitochondrial function. However, they did not establish the mechanisms by which plasma membrane and nuclear ANG II receptors affect ANG II signaling in mitochondria. Recent studies by Villar-Cheda et al. (102) provide clues for a possible mechanism involving nuclear AT1R in this action. Using transfection of fluorescent ANG II and ANG II receptors in a MES-23.5 dopaminergic neuron cell line, these authors showed the presence of ANG II receptors and ANG II in nuclei as evidenced by nuclear Hoechst staining. Notably, treatment of cells with ANG II increased the nuclear fluorescence of AT1R, but not AT2R fluorescence, relative to controls. Except for mRNA AT1R, all RAS components including mRNA AT2R, angiotensinogen, mRNA renin, and prorenin receptors were increased by ANG II in a losartan-sensitive pattern (102). The mRNA for peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and IGF-1 was also augmented. These findings indicate that AT1R mediates the nuclear activation of the RAS, including AT2R pathways. An AT1R/IP3-dependent Ca2+ increase appears to mediate these effects since they are inhibited by losartan and the IP3R blocker 2-aminoethoxydiphenyl borate. Although SIRT1 mRNA was not significantly affected by ANG II, overexpression of this marker in isolated nuclei from transgenic mice significantly decreased AT1R and increased AT2R.
Moreover, ANG II treatment of brain-isolated nuclei demonstrated increased levels of nuclear ROS that were inhibited by diphenyleneiodonium and losartan but not PD123319, indicating that ANG II activates the nuclear AT1R/NOX4 pathway to promote ROS production independently of AT2R or PGC-1α mRNA. Notably, treatment of isolated nuclei with ANG II and losartan significantly increased NO levels that were blocked by PD123319 and l-NAME. These data suggest that stimulation of AT2R in the nucleus, like mitochondria (1, 98), leads to NO generation and, likewise, AT1R signaling induces ROS production. Finally, in aged animals (18–20 mo of age), the nuclear AT1R and AT2R levels and their signaling pathways were significantly reduced, suggesting disruption of the nuclear AT1R/AT2R control in aging. It is proposed (102) that activation of nuclear receptors by intracellular or internalized ANG II activates pathways that protect cells from the activation of receptors by the extracellular hormone. Cell protection is performed by activating AT2R/NO-cGMP-mediated pathways, generation of intracellular ANG II, PGC-1α, and IGF-1/SIRT1, and synthesis and trafficking of AT2R to the mitochondria.
The study by Villar-Cheda et al. (102) confirmed earlier reports in hepatic cells demonstrating upregulation of nuclear renin, angiotensinogen, IGF-2, PDGF, and c-Fos by ANG II (20, 21). It also agrees with studies in renal cortex nuclei (34) demonstrating an ANG II/AT1R/Ca2+ release and a phosphatidylinositol 3-kinase-PKC-NOX4 pathway. These cells also express the AT2R/NOS-NO pathway that probably influences transcription and antagonizes ROS. In addition, nuclei from sheep renal cortex also express an ANG I/ANG II/ACE2/Ang-(1–7)/Mas-axis, which attenuates the ANG II/AT1R-dependent ROS generation and is antagonized by A-779 but not by PD123319. Indeed, inhibition of ACE2 with MLN-4760 or the Mas receptor with d-Ala7-Ang-(1–7) exacerbates the ANG II-induced ROS generation. These findings point to an active ACE2/Ang-(1–7) axis that together with AT2R, counterbalances the ANG II/AT1R-mediated oxidative stress in the nuclei of kidney cells. Similar findings concerning the Ang-(1–7)/Mas receptor axis have been reported in the dopaminergic system (12). The axis is downregulated in aging, which may contribute to the aging-related vulnerability to neurodegenerative diseases. Notably, the ACE2/Ang-(1–7) axis is operational in the nucleus and mitochondria of dopaminergic cells where it modulates superoxide production.
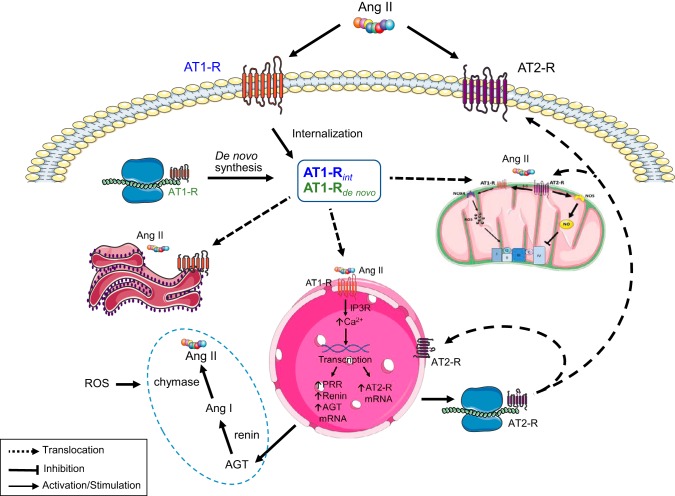
The studies elucidating the nuclear angiotensin system (102) support the idea that the control of mitochondrial AT2R-dependent pathways in the heart is determined by the activation of the nuclear AT1R (Fig. 3). However, AT1Rs in the nuclear membrane of cardiomyocytes do not seem to arise directly from the endocytic internalization of the ANG II-AT1R complex (88, 94) as reported in dopaminergic neurons (102). Rather, AT1R and AT2R in the nucleus of cardiomyocytes appear to originate from intracellular synthesis or a “processing pool.” On the basis of the data available, it appears that this intracellular pool with internalized ANG II-AT1R complex and de novo synthesized AT1R provides for the trafficking of these receptors to the nucleus (88, 94), the endoplasmic reticulum (24), and the mitochondria (1, 98). Posttranscriptional modification of AT1R, such as glycosylation or signal peptides bound to the receptors can facilitate its intracellular trafficking to target cell organelles (39, 53, 88). In the nucleus, activation of AT1R by intracellular ANG II induces upregulation of AT2R mRNA (102), and then, synthesized receptors traffic to the mitochondria, the plasma membrane, and the nucleus. Nuclear AT1R and AT2R in cardiomyocytes mediate de novo RNA synthesis, affecting the abundance of rRNA and NF-κB mRNA through a pertussis toxin-sensitive Gi protein (94). However, nuclear AT1R, but not AT2R stimulation, increased the IP3-dependent Ca2+ release (94, 102) and promoted de novo AT2R mRNA synthesis (102), supporting the relevance of nuclear Ca2+ signaling in gene transcription. It is likely that once activated, the ANG II/AT1R-IP3-dependent Ca2+ pathway also directs the transcription and synthesis of intracellular RAS components (i.e., angiotensinogen, renin, AT1R, and AT2R) (102). An ANG II/AT1R-Ca2+-independent pathway could also be involved in the control of gene expression in cardiomyocytes because rRNA and NF-кB mRNA are sensitive to both valsartan (AT1R blocker) and PD123177 (AT2R blocker) (94).
Fig. 3.
Proposed mechanism of regulation of mitochondrial function by extracellular ANG II receptors. Activation of plasma membrane receptors of ANG II leads to endocytic internalization and trafficking of the ANG II-ANG II type 1 receptor (AT1R) complex to an intracellular (“processing”) pool that also contains the synthesized AT1Rs. ANG II from intracellular synthesis binds to translocated AT1Rs in the nuclear membrane and the mitochondria. At the nucleus, the complex stimulates transcription and synthesis of ANG II type 2 receptors (AT2Rs) that are also translocated to the mitochondria, the plasma membrane and the nucleus. Nuclear AT1Rs also lead to the synthesis of intracellular renin-angiotensin system (RAS) components and ANG II generation. The process can also be stimulated by oxidative stress. ANG II receptors localized at the plasma membrane, nucleus, endoplasmic reticulum, and mitochondria regulate cell metabolism through signaling pathways including reactive oxygen species (ROS), nitric oxide (NO), and PKCε-dependent cascades, among others (see Fig. 1). AGT, angiotensinogen.
The stimulation of the synthesis of intracellular RAS components in cardiomyocytes could also occur through oxidative stress in response to ANG II/AT1R activation of NOX (62) or from mitochondrial ROS as observed in cells exposed to high-glucose media (88). It is likely that during chronic ANG II exposure the cardiomyocyte intracellular RAS is significantly activated by both nuclear and cytosolic ROS-dependent mechanisms that require de novo synthesis of proteins. By contrast, during normal or acute exposure to ANG II, the internalization of AT1R coupled with its processing and redistribution to target organelles suffices to mediate hormonal action. Thus the balance between internalized AT1R and the de novo synthesized receptors in the pool would depend on the strength and duration of extracellular ANG II stimulus. Because of the complexity of these mechanisms and the scarcity of information available, further studies are necessary for their clarification and the elucidation of factors regulating the synthesis and trafficking of intracellular RAS components between intervening cell structures, particularly in the cardiomyocyte. Nevertheless, the proposed scheme could help explain the effect of extracellular ANG II and ARBs on mitochondrial protein content (ANG II receptors and kinases) and regulation by APC in the heart (68). Indeed, it is possible that APC recruits both nuclear and cytosolic ROS-dependent mechanisms of intracellular RAS control, probably leading to increased expression of cardioprotective AT2R in the nucleus and the mitochondria. PKC and Ca2+ release from stimulation of internalized ANG II-AT1R complex in the endoplasmic reticulum (24) and ROS (14, 49) could provide for other regulatory processes involving survival kinase activation and translocation to the mitochondria. The proposed model also provides mechanistic insights on the effects of intracellular ANG II on cardiomyocyte growth and cardiac remodeling (4). The recognition that mitochondrial protein import mechanisms (38) are regulated by metabolism and stress could add another level of complexity to the control of the intracellular RAS mechanism.
Recent studies also demonstrate the presence of renin, ANG I, ANG II, and Ang-(1–7) in mitochondria isolated from renal cortex (104). The mitochondrial preparation processed ANG I to Ang-(1–7) by the endopeptidases neprilysin and thimet oligopeptidase and expressed the Ang-(1–7)/Mas receptor. At variance with previous studies (1, 98), this study demonstrated a complete mitochondrial RAS that includes an ACE2/ANG II-independent pathway for Ang-(1–7) formation because no conversion of ANG II to Ang-(1–7) was observed in isolated mitochondria (77, 104). These studies raised the possibility that a functional Ang-(1–7)/Mas axis in the mitochondria also contributes to AT1R antagonism and cell protection. Indeed, chronic administration of Ang-(1–7) in rats ameliorates ANG II-induced hypertrophy and fibrosis by a mitochondrial ROS-dependent mechanism involving the SIRT3-FoxO3a-SOD2 pathway (33). In addition, Ang-(1–7) ameliorates ANG II-induced generation of mitochondrial ROS and apoptosis in renal NRK-52E epithelial cells (49). Notably, in streptozotocin-diabetic rats with nephropathy, a large-dose treatment of Ang-(1–7) exerted more protection against the progression of diabetes-induced renal disease than valsartan. It has been suggested that reduced renal oxidative stress and inhibited TGF-β1/Smad3 and VEGF signaling could be involved in Ang-(1–7)-induced protection (107). However, whether these factors are causally related to the mitochondrial Ang-(1–7)/Mas axis remains to be elucidated.
It is evident that the presence of an Ang-(1–7)/Mas axis in mitochondria from renal cortex suggests redundant systems for antagonizing ANG II/AT1R-dependent processes involved ROS generation and apoptosis. However, it is possible that the expression of these protective systems is tissue specific. For example, rat adrenal cortex mitochondria possess several components of RAS including renin, ACE, and angiotensinogen (74), different from the sheep renal cortex mitochondria, which appear to rely on both the Ang-(1–7)/Mas axis and AT2Rs (34, 107). Therefore, tissue or species differences could underlie variations in the expression of these RAS components and protective mechanisms in the mitochondria. Studies will be necessary to determine the relevance of redundant systems for ANG II/AT1R antagonism based on cell, tissue, organ, or species differences.
The idea that the intracrine RAS is a protective mechanism against the deleterious effects chronic or high extracellular ANG II has been recently questioned (79) on the basis that the protective pathways could be tissue or cell specific, context dependent (i.e., depending on the level, duration, or nature of the particular stimulus), or determined by the profile of nuclear receptors in the cell. Indeed, studies in sheep kidney demonstrate that AT1Rs are the predominant (~90%) ANG II receptors expressed by nuclei of renal medulla, while those in the renal cortex express AT2R (~60%) primarily (35). These data suggest that, in the absence of other protective pathways, the sheep renal medulla would be more susceptible to damage by chronic stimulation of AT1R-dependent processes due to the low expression of AT2Rs. Similarly, in nuclei isolated from sheep exposed during the fetal stage to betamethasone (36), the ratio of AT1R to AT2R and the ANG II-induced ROS production increased, while NO generation and Ang-(1–7) receptors diminished compared with unexposed animals. Also, blockade of AT2R with PD123319 augmented the generation of ROS and decreased NO by ANG II in controls or treated animals. These studies support the idea that the profile of nuclear receptor subtypes does induce functional changes in NO- and ROS-dependent signaling pathways and could play a role in the end effect of chronic ANG II exposure to cells (protective versus deleterious).
A high ratio of AT1R to AT2R in mitochondria is also associated with oxidative stress and aging (1, 98). Indeed, rats in the early stages of diabetic nephropathy show high AT1R/AT2R ratios in mitochondria from proximal tubule epithelial cells, which are associated with pathological changes (i.e., disruption of mitochondrial bioenergetics and enhanced oxidative stress) (63). By contrast, AT2R overexpression in transgenic rats led to increased mitochondrial AT2R density and attenuated diabetes-induced renal changes (63). Also, the data presented here on the suppression of mitochondrial signaling, respiration, and cardiac function by AT2R in young rats (68) support a protective role of the intracrine RAS on I/R-induced injury. In aged animals, however, low expression of AT2R (1, 98) could increase the susceptibility to oxidative stress-induced damage. These studies support the idea that the balance of AT1R and AT2R present in the nucleus and the mitochondria is a critical factor in the fate (protection or injury) of ANG II-stimulated cells. In the mitochondria, the profile of ANG II receptors could be determined by both nuclear-dependent processes and alterations of mitochondrial protein import processes. The latter could be modulated by metabolism and stress (38). However, additional studies are necessary to elucidate how these factors interact to determine the profile mitochondrial ANG II receptors under different conditions of hormone levels, duration of exposure, cell-type, and aging.
MITOCHONDRIAL ANGIOTENSIN RECEPTORS: CLINICAL RELEVANCE
The protection conferred by AT2R-dependent pathways (1, 6, 102) and possibly the Ang-(1–7)/Mas receptor axis in mitochondria (12, 49, 73) leads to enhanced NO release, suppression of mitochondrial respiration and oxidative stress, and the activation of antiapoptotic pathways. AT2Rs also appear to control mitochondrial AT1R expression, thereby counterbalancing the possible negative effects of chronic RAS activation. In the heart, these pathways lead to the suppression of cardiac function and reduced infarct area after I/R, indicating their relevance to tissue and organ function particularly under conditions of oxidative stress. The loss of these protective pathways could accelerate deleterious AT1R-dependent mitochondrial pathways that promote oxidative stress, inflammation, and apoptosis in aging and during pathological conditions (i.e., ischemic heart disease, diabetic nephropathy, neuropathy). Indeed, it is likely that the success of AT1R blockade on organ protection and survival (17) is due in part to the preservation of the AT2R-dependent defense pathways. This appears to be the case in cardiovascular and renal disease where studies with compound 21 (48, 58, 84) support a protective role of AT2R stimulation. Reports also indicate protection against pancreatic dysfunction and disease by compound 21 (103). However, whether these effects are due to the protection of mitochondrial function is unknown. Enhanced oxidative stress, cardiac cell apoptosis, and necrosis are present in the diabetic heart or during high-glucose conditions (88), but whether alterations in mitochondrial AT1R and AT2R are involved in these events has not been determined. Finally, downregulation of the Ang-(1–7)/Mas axis could contribute to neurodegeneration in aging (12) where the mitochondrial AT2R protective axis is depressed. These findings indicate the critical role that intracellular RAS plays in maintaining normal organ function through the counterbalance of AT1R activation by both nuclear and mitochondrial protective pathways. The presence of antagonistic pathways for AT1R-dependent pathways in cardiac, renal, brain, and pancreas could represent novel therapeutic targets to attenuate the progression of disease affecting these organs and aging.
CONCLUSIONS
In summary, the accumulated data discussed in this review support the idea that the intracellular RAS, through a nuclear-mediated mechanism, modulates the balance of mitochondrial ANG II receptors and exerts control over cell and organ function. This mechanism appears critical to counterbalance the deleterious effects of chronic increases in extracellular ANG II and the activation of AT1R-dependent pathways associated with oxidative stress and inflammation. Indeed, in the heart, the modulation of mitochondrial AT2R by APC is associated with cardioprotection against I/R injury indicating the relevance of these mechanisms for cell, tissue, and organ survival. Thus, despite abundant data on the deleterious effects of intracellular ANG II, a growing body of studies also support a protective role for intracellular RAS in cells. However, the factors responsible for determining the balance between the deleterious and protective roles of intracellular ANG II are not clear and require further studies.
GRANTS
This study was supported by the National Center for Research Resources Grant G12-MD-007600 and National Institute of General Medical Sciences Grant SC1-GM-128210 (to S. Javadov) and the University of Puerto Rico Medical Sciences Campus.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.E., R.E.N., and S.J. analyzed data; N.E. interpreted results of experiments; R.E.N. prepared figures; N.E. drafted manuscript; N.E., R.E.N., and S.J. edited and revised manuscript; N.E., R.E.N., and S.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Keisha Rodriguez for technical assistance. We apologize to all colleagues whose important studies were not cited due to space restriction. Art was created using Servier Medical Art (https://smart.servier.com/).
REFERENCES
- 1.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011. doi: 10.1073/pnas.1101507108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abadir PM, Walston JD, Carey RM. Subcellular characteristics of functional intracellular renin-angiotensin systems. Peptides 38: 437–445, 2012. doi: 10.1016/j.peptides.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaud C, Joyeux-Faure M, Bottari S, Godin-Ribuot D, Ribuot C. New insight into the signalling pathways of heat stress-induced myocardial preconditioning: protein kinase Cepsilon translocation and heat shock protein 27 phosphorylation. Clin Exp Pharmacol Physiol 31: 129–133, 2004. doi: 10.1111/j.1440-1681.2004.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept 120: 5–13, 2004. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2: 247–257, 2010. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the ANG II type 1 receptor promotes longevity in mice. J Clin Invest 119: 524–530, 2009. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121: 297–303, 2011. doi: 10.1042/CS20110036. [DOI] [PubMed] [Google Scholar]
- 8.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44: D1251–D1257, 2016. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell DJ. Clinical relevance of local renin angiotensin systems. Front Endocrinol (Lausanne) 5: 113, 2014. doi: 10.3389/fendo.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey RM. Update on angiotensin AT2 receptors. Curr Opin Nephrol Hypertens 26: 91–96, 2017. doi: 10.1097/MNH.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn JN. Role of the renin-angiotensin system in cardiovascular disease. Cardiovasc Drugs Ther 24: 341–344, 2010. doi: 10.1007/s10557-010-6230-3. [DOI] [PubMed] [Google Scholar]
- 12.Costa-Besada MA, Valenzuela R, Garrido-Gil P, Villar-Cheda B, Parga JA, Lanciego JL, Labandeira-Garcia JL. Paracrine and intracrine angiotensin 1-7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol Neurobiol 55: 5847–5867, 2018. doi: 10.1007/s12035-017-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristovam PC, Arnoni CP, de Andrade MC, Casarini DE, Pereira LG, Schor N, Boim MA. ACE-dependent and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 233: 1035–1043, 2008. doi: 10.3181/0708-RM-229. [DOI] [PubMed] [Google Scholar]
- 14.De Giusti VC, Caldiz CI, Ennis IL, Pérez NG, Cingolani HE, Aiello EA. Mitochondrial reactive oxygen species (ROS) as signaling molecules of intracellular pathways triggered by the cardiac renin-angiotensin II-aldosterone system (RAAS). Front Physiol 4: 126, 2013. doi: 10.3389/fphys.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Mello WC, Frohlich ED. Clinical perspectives and fundamental aspects of local cardiovascular and renal renin-angiotensin systems. Front Endocrinol (Lausanne) 5: 16, 2014. doi: 10.3389/fendo.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Mello WC, Monterrubio J. Intracellular and extracellular angiotensin II enhance the L-type calcium current in the failing heart. Hypertension 44: 360–364, 2004. doi: 10.1161/01.HYP.0000139914.52686.74. [DOI] [PubMed] [Google Scholar]
- 17.Dézsi CA. The different therapeutic choices with ARBs. Which one to give? When? Why? Am J Cardiovasc Drugs 16: 255–266, 2016. doi: 10.1007/s40256-016-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dias J, Axelband F, Lara LS, Muzi-Filho H, Vieyra A. Is angiotensin-(3-4) (Val-Tyr), the shortest angiotensin II-derive d peptide, opening new vistas on the renin-angiotensin system? J Renin Angiotensin Aldosterone Syst 18: 1470320316689338, 2017. doi: 10.1177/1470320316689338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz-Ruiz C, Villar-Cheda B, Dominguez-Meijide A, Garrido-Gil P, Guerra MJ, Labandeira-Garcia JL. Aging-related overactivity of the angiotensin/AT1 axis decreases sirtuin 3 levels in the Substantia Nigra, which induces vulnerability to oxidative stress and neurodegeneration. J Gerontol A Biol Sci Med Sci 2018 Nov 9. [Epub ahead of print]. doi: 10.1093/gerona/gly259. [DOI] [PubMed] [Google Scholar]
- 20.Eggena P, Zhu JH, Clegg K, Barrett JD. Nuclear angiotensin receptors induce transcription of renin and angiotensinogen mRNA. Hypertension 22: 496–501, 1993. doi: 10.1161/01.HYP.22.4.496. [DOI] [PubMed] [Google Scholar]
- 21.Eggena P, Zhu JH, Sereevinyayut S, Giordani M, Clegg K, Andersen PC, Hyun P, Barrett JD. Hepatic angiotensin II nuclear receptors and transcription of growth-related factors. J Hypertens 14: 961–968, 1996. doi: 10.1097/00004872-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann B, Fuxe K, Ganten D. Subcellular localization of angiotensin II immunoreactivity in the rat cerebellar cortex. Hypertension 28: 818–824, 1996. doi: 10.1161/01.HYP.28.5.818. [DOI] [PubMed] [Google Scholar]
- 23.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59: 418–458, 2007. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 24.Ferrão FM, Cardoso LH, Drummond HA, Li XC, Zhuo JL, Gomes DS, Lara LS, Vieyra A, Lowe J. Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells. Am J Physiol Renal Physiol 313: F440–F449, 2017. doi: 10.1152/ajprenal.00261.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrão FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: what is new? World J Nephrol 3: 64–76, 2014. doi: 10.5527/wjn.v3.i3.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrario CM, Ahmad S, Joyner J, Varagic J. Advances in the renin angiotensin system focus on angiotensin-converting enzyme 2 and angiotensin-(1-7). Adv Pharmacol 59: 197–233, 2010. doi: 10.1016/S1054-3589(10)59007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fournier D, Luft FC, Bader M, Ganten D, Andrade-Navarro MA. Emergence and evolution of the renin-angiotensin-aldosterone system. J Mol Med (Berl) 90: 495–508, 2012. doi: 10.1007/s00109-012-0894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000. doi: 10.1161/01.RES.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 29.Garrido-Gil P, Rodriguez-Perez AI, Fernandez-Rodriguez P, Lanciego JL, Labandeira-Garcia JL. Expression of angiotensinogen and receptors for angiotensin and prorenin in the rat and monkey striatal neurons and glial cells. Brain Struct Funct 222: 2559–2571, 2017. doi: 10.1007/s00429-016-1357-z. [DOI] [PubMed] [Google Scholar]
- 30.Garrido-Gil P, Valenzuela R, Villar-Cheda B, Lanciego JL, Labandeira-Garcia JL. Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: an intracellular renin-angiotensin system in the nigra. Brain Struct Funct 218: 373–388, 2013. doi: 10.1007/s00429-012-0402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem 319: 115–123, 2008. doi: 10.1007/s11010-008-9884-4. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013. doi: 10.1161/HYPERTENSIONAHA.112.196303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo L, Yin A, Zhang Q, Zhong T, O’Rourke ST, Sun C. Angiotensin-(1-7) attenuates angiotensin II-induced cardiac hypertrophy via a Sirt3-dependent mechanism. Am J Physiol Heart Circ Physiol 312: H980–H991, 2017. doi: 10.1152/ajpheart.00768.2016. [DOI] [PubMed] [Google Scholar]
- 34.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012. doi: 10.1152/ajpregu.00525.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol 296: F1484–F1493, 2009. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension 57: 620–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haller H, Lindschau C, Quass P, Luft FC. Intracellular actions of angiotensin II in vascular smooth muscle cells. J Am Soc Nephrol 10, Suppl 11: S75–S83, 1999. [PubMed] [Google Scholar]
- 38.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C. The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metab 19: 357–372, 2014. doi: 10.1016/j.cmet.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem 73: 1019–1049, 2004. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 40.Herichova I, Szantoova K. Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocr Regul 47: 39–52, 2013. doi: 10.4149/endo_2013_01_39. [DOI] [PubMed] [Google Scholar]
- 41.Hrenak J, Paulis L, Simko F. Angiotensin A/alamandine/MrgD axis: another clue to understanding cardiovascular pathophysiology. Int J Mol Sci 17: 1098, 2016. doi: 10.3390/ijms17071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H. Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens 2: 332–340, 2008. doi: 10.1016/j.jash.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950–1961, 2006. doi: 10.1681/ASN.2006010029. [DOI] [PubMed] [Google Scholar]
- 44.Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 289: H1013–H1019, 2005. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- 45.Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: therapeutic perspectives. Pharmacol Ther 144: 202–225, 2014. doi: 10.1016/j.pharmthera.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol 549: 513–524, 2003. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007. doi: 10.1681/ASN.2006091062. [DOI] [PubMed] [Google Scholar]
- 48.Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Tschöpe C, Hallberg A, Alterman M, Hucko T, Paetsch I, Dietrich T, Schnackenburg B, Graf K, Dahlöf B, Kintscher U, Unger T, Steckelings UM. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 118: 2523–2532, 2008. doi: 10.1161/CIRCULATIONAHA.108.784868. [DOI] [PubMed] [Google Scholar]
- 49.Kim SM, Kim YG, Jeong KH, Lee SH, Lee TW, Ihm CG, Moon JY. Angiotensin II-induced mitochondrial Nox4 is a major endogenous source of oxidative stress in kidney tubular cells. PLoS One 7: e39739, 2012. doi: 10.1371/journal.pone.0039739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 51.Koid SS, Ziogas J, Campbell DJ. Aliskiren reduces myocardial ischemia-reperfusion injury by a bradykinin B2 receptor- and angiotensin AT2 receptor-mediated mechanism. Hypertension 63: 768–773, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02902. [DOI] [PubMed] [Google Scholar]
- 52.Kumar R, Boim MA. Diversity of pathways for intracellular angiotensin II synthesis. Curr Opin Nephrol Hypertens 18: 33–39, 2009. doi: 10.1097/MNH.0b013e32831a9e20. [DOI] [PubMed] [Google Scholar]
- 53.Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin-angiotensin system. Clin Sci (Lond) 123: 273–284, 2012. doi: 10.1042/CS20120089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Soares E, Barbosa C, Kjeldsen F, Oliveira A, Braga J, Savergnini S, Maia G, Peluso AB, Passos-Silva D, Ferreira A, Alves F, Martins A, Raizada M, Paula R, Motta-Santos D, Klempin F, Pimenta A, Alenina N, Sinisterra R, Bader M, Campagnole-Santos MJ, Santos RA. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res 112: 1104–1111, 2013. [Erratum in Circ Res 11: e156, 2013.] doi: 10.1161/CIRCRESAHA.113.301077. [DOI] [PubMed] [Google Scholar]
- 55.Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res 101: 455–464, 2007. doi: 10.1161/CIRCRESAHA.107.151852. [DOI] [PubMed] [Google Scholar]
- 56.Leung PS. The physiology of a local renin-angiotensin system in the pancreas. J Physiol 580: 31–37, 2007. doi: 10.1113/jphysiol.2006.126193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H, Yang T, Long Z, Cheng J. Effect of mitochondrial ATP-sensitive potassium channel opening on the translocation of protein kinase C epsilon in adult rat ventricular myocytes. Genet Mol Res 13: 4516–4522, 2014. doi: 10.4238/2014.June.17.3. [DOI] [PubMed] [Google Scholar]
- 58.Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res 125: 21–38, 2017. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C, Yang CX, Chen XR, Liu BX, Li Y, Wang XZ, Sun W, Li P, Kong XQ. Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids 50: 1071–1081, 2018. doi: 10.1007/s00726-018-2583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsui T, Tamaya K, Matsumoto K, Osajima Y, Uezono K, Kawasaki T. Plasma concentrations of angiotensin metabolites in young male normotensive and mild hypertensive subjects. Hypertens Res 22: 273–277, 1999. doi: 10.1291/hypres.22.273. [DOI] [PubMed] [Google Scholar]
- 62.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 63.Micakovic T, Papagiannarou S, Clark E, Kuzay Y, Abramovic K, Peters J, Sticht C, Volk N, Fleming T, Nawroth P, Hammes HP, Alenina N, Gröne HJ, Hoffmann SC. The angiotensin II type 2 receptors protect renal tubule mitochondria in early stages of diabetes mellitus. Kidney Int 94: 937–950, 2018. doi: 10.1016/j.kint.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 64.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. doi: 10.1161/01.CIR.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen G, Contrepas A. Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol 8: 127–132, 2008. doi: 10.1016/j.coph.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 66.Nuñez RE, Castro M, Javadov S, Escobales N. Angiotensin II and ischemic preconditioning synergize to improve mitochondrial function while showing additive effects on ventricular postischemic recovery. J Cardiovasc Pharmacol 64: 172–179, 2014. doi: 10.1097/FJC.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nuñez RE, Javadov S, Escobales N. Angiotensin II-preconditioning is associated with increased PKCε/PKCδ ratio and prosurvival kinases in mitochondria. Clin Exp Pharmacol Physiol 44: 1201–1212, 2017. doi: 10.1111/1440-1681.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nuñez RE, Javadov S, Escobales N. Critical role of angiotensin II type 2 receptors in the control of mitochondrial and cardiac function in angiotensin II-preconditioned rat hearts. Pflugers Arch 470: 1391–1403, 2018. doi: 10.1007/s00424-018-2153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parodi-Rullan R, Barreto-Torres G, Ruiz L, Casasnovas J, Javadov S. Direct renin inhibition exerts an anti-hypertrophic effect associated with improved mitochondrial function in post-infarction heart failure in diabetic rats. Cell Physiol Biochem 29: 841–850, 2012. doi: 10.1159/000178526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel S, Hussain T. Dimerization of AT2 and mas receptors in control of blood pressure. Curr Hypertens Rep 20: 41, 2018. [Erratum in Curr Hypertens Rep 20: 41, 2018.] doi: 10.1007/s11906-018-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res 118: 1313–1326, 2016. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 73.Pernomian L, Pernomian L, Gomes MS, da Silva CH. Pharmacological significance of the interplay between angiotensin receptors: MAS receptors as putative final mediators of the effects elicited by angiotensin AT1 receptors antagonists. Eur J Pharmacol 769: 143–146, 2015. doi: 10.1016/j.ejphar.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Peters J, Kränzlin B, Schaeffer S, Zimmer J, Resch S, Bachmann S, Gretz N, Hackenthal E. Presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. Am J Physiol Endocrinol Physiol 271: E439–E450, 1996. doi: 10.1152/ajpendo.1996.271.3.E439. [DOI] [PubMed] [Google Scholar]
- 75.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol 21: 1093–1096, 2010. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prokop JW, Santos RA, Milsted A. Differential mechanisms of activation of the Ang peptide receptors AT1, AT2, and MAS: using in silico techniques to differentiate the three receptors. PLoS One 8: e65307, 2013. doi: 10.1371/journal.pone.0065307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qaradakhi T, Apostolopoulos V, Zulli A. Angiotensin (1-7) and alamandine: similarities and differences. Pharmacol Res 111: 820–826, 2016. doi: 10.1016/j.phrs.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 78.Re R, Parab M. Effect of angiotensin II on RNA synthesis by isolated nuclei. Life Sci 34: 647–651, 1984. doi: 10.1016/0024-3205(84)90228-5. [DOI] [PubMed] [Google Scholar]
- 79.Re RN. Role of intracellular angiotensin II. Am J Physiol Heart Circ Physiol 314: H766–H771, 2018. doi: 10.1152/ajpheart.00632.2017. [DOI] [PubMed] [Google Scholar]
- 80.Re RN, Vizard DL, Brown J, LeGros L, Bryan SE. Angiotensin II receptors in chromatin. J Hypertens Suppl 2: S271–S273, 1984. [PubMed] [Google Scholar]
- 81.Ribeiro-Oliveira A Jr, Nogueira AI, Pereira RM, Boas WW, Dos Santos RA, Simões e Silva AC. The renin-angiotensin system and diabetes: an update. Vasc Health Risk Manag 4: 787–803, 2008. doi: 10.2147/VHRM.S1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riquier-Brison AD, Sipos A, Prókai Á, Vargas SL, Toma L, Meer EJ, Villanueva KG, Chen JC, Gyarmati G, Yih C, Tang E, Nadim B, Pendekanti S, Garrelds IM, Nguyen G, Danser AH, Peti-Peterdi J. The macula densa prorenin receptor is essential in renin release and blood pressure control. Am J Physiol Renal Physiol 315: F521–F534, 2018. doi: 10.1152/ajprenal.00029.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robertson AL Jr, Khairallah PA. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science 172: 1138–1139, 1971. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 84.Sampson AK, Irvine JC, Shihata WA, Dragoljevic D, Lumsden N, Huet O, Barnes T, Unger T, Steckelings UM, Jennings GL, Widdop RE, Chin-Dusting JP. Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br J Pharmacol 173: 729–740, 2016. doi: 10.1111/bph.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology (Bethesda) 22: 310–319, 2007. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 86.Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, Bandinelli B, Bertolozzi I, Polidori G, Toscano T, Maccherini M, Modesti PA. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res 88: 961–968, 2001. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 87.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol 294: H1675–H1684, 2008. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 88.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes 57: 3297–3306, 2008. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sirett NE, McLean AS, Bray JJ, Hubbard JI. Distribution of angiotensin II receptors in rat brain. Brain Res 122: 299–312, 1977. doi: 10.1016/0006-8993(77)90296-7. [DOI] [PubMed] [Google Scholar]
- 90.Song R, Janssen A, Li Y, El-Dahr S, Yosypiv IV. Prorenin receptor controls renal branching morphogenesis via Wnt/β-catenin signaling. Am J Physiol Renal Physiol 312: F407–F417, 2017. doi: 10.1152/ajprenal.00563.2016. [DOI] [PubMed] [Google Scholar]
- 91.Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol 4: 1201–1228, 2014. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stragier B, De Bundel D, Sarre S, Smolders I, Vauquelin G, Dupont A, Michotte Y, Vanderheyden P. Involvement of insulin-regulated aminopeptidase in the effects of the renin-angiotensin fragment angiotensin IV: a review. Heart Fail Rev 13: 321–337, 2008. doi: 10.1007/s10741-007-9062-x. [DOI] [PubMed] [Google Scholar]
- 93.Taanman JW. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta 1410: 103–123, 1999. doi: 10.1016/S0005-2728(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 94.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J Biol Chem 285: 22338–22349, 2010. doi: 10.1074/jbc.M110.121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tadevosyan A, Xiao J, Surinkaew S, Naud P, Merlen C, Harada M, Qi X, Chatenet D, Fournier A, Allen BG, Nattel S. Intracellular angiotensin-II interacts with nuclear angiotensin receptors in cardiac fibroblasts and regulates RNA synthesis, cell proliferation, and collagen secretion. J Am Heart Assoc 6: e004965, 2017. doi: 10.1161/JAHA.116.004965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tetzner A, Gebolys K, Meinert C, Klein S, Uhlich A, Trebicka J, Villacañas Ó, Walther T. G-protein-coupled receptor MrgD is a recept or for angiotensin-(1-7) involving adenylyl cyclase, cAMP, and phosphokinase A. Hypertension 68: 185–194, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07572. [DOI] [PubMed] [Google Scholar]
- 97.Tigerstedt R, Bergman P. Niere und Kreislauf. Scand Arch Physiol 8: 223–271, 1898. doi: 10.1111/j.1748-1716.1898.tb00272.x. [DOI] [Google Scholar]
- 98.Valenzuela R, Costa-Besada MA, Iglesias -Gonzalez J, Perez-Costas E, Villar-Cheda B, Garrido-Gil P, Melendez-Ferro M, Soto-Otero R, Lanciego JL, Henrion D, Franco R, Labandeira-Garcia JL. Mitocho ndrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis 7: e2427, 2016. doi: 10.1038/cddis.2016.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van den Heuvel M, Batenburg WW, Danser AH. Diabetic complications: a role for the prorenin-(pro)renin receptor-TGF-beta1 axis? Mol Cell Endocrinol 302: 213–218, 2009. doi: 10.1016/j.mce.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 100.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 19, Suppl: 583–589, 2001. doi: 10.1097/00004872-200103001-00010. [DOI] [PubMed] [Google Scholar]
- 101.Vila Petroff MG, Mattiazzi AR. Angiotensin II and cardiac excitation-contraction coupling: questions and controversies. Heart Lung Circ 10: 90–98, 2001. doi: 10.1046/j.1444-2892.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- 102.Villar-Cheda B, Costa-Besada MA, Valenzuela R, Perez-Costas E, Melendez-Ferro M, Labandeira-Garcia JL. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis 8: e3044, 2017. doi: 10.1038/cddis.2017.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang L, Wang Y, Li XY, Leung PS. Angiotensin II type 2 receptor activation with compound 21 augments islet function and regeneration in streptozotocin-induced neonatal rats and human pancreatic progenitor cells. Pancreas 46: 395–404, 2017. doi: 10.1097/MPA.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 104.Wilson BA, Nautiyal M, Gwathmey TM, Rose JC, Chappell MC. Evidence for a mitochondrial angiotensin-(1-7) system in the kidney. Am J Physiol Renal Physiol 310: F637–F645, 2016. doi: 10.1152/ajprenal.00479.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Xu J, Liu TS, Yang JJ, Reudelhuber TL, Yang XP. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol 299: H1328–H1338, 2010. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang R, Smolders I, Vanderheyden P, Demaegdt H, Van Eeckhaut A, Vauquelin G, Lukaszuk A, Tourwé D, Chai SY, Albiston AL, Nahmias C, Walther T, Dupont AG. Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent. Hypertension 57: 956–964, 2011. doi: 10.1161/HYPERTENSIONAHA.110.161836. [DOI] [PubMed] [Google Scholar]
- 107.Zhang K, Meng X, Li D, Yang J, Kong J, Hao P, Guo T, Zhang M, Zhang Y, Zhang C. Angiotensin(1-7) attenuates the progression of streptozotocin-induced diabetic renal injury better than angiotensin receptor blockade. Kidney Int 87: 359–369, 2015. doi: 10.1038/ki.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhuo JL, Kobori H, Li XC, Satou R, Katsurada A, Navar LG. Augmentation of angiotensinogen expression in the proximal tubule by intracellular angiotensin II via AT1a/MAPK/NF-кB signaling pathways. Am J Physiol Renal Physiol 310: F1103–F1112, 2016. doi: 10.1152/ajprenal.00350.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006. doi: 10.1152/ajprenal.00269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]