Abstract
Effector CD8 T cells infiltrate atherosclerotic lesions and are correlated with cardiovascular events, but the mechanisms regulating their recruitment and retention are not well understood. CD137 (4–1BB) is a costimulatory receptor induced on immune cells and expressed at sites of human atherosclerotic plaque. Genetic variants associated with decreased CD137 expression correlate with carotid-intimal thickness and its deficiency in animal models attenuates atherosclerosis. These effects have been attributed in part to endothelial responses to low and disturbed flow (LDF), but CD137 also generates robust effector CD8 T cells as a costimulatory signal. Thus, we asked whether CD8 T cell-specific CD137 stimulation contributes to their infiltration, retention, and IFNγ production in early atherogenesis. We tested this through adoptive transfer of CD8 T cells into recipient C57BL/6J mice that were then antigen primed and CD137 costimulated. We analyzed atherogenic LDF vessels in normolipidemic and PCSK9-mediated hyperlipidemic models and utilized a digestion protocol that allowed for lesional T-cell characterization via flow cytometry and in vitro stimulation. We found that CD137 activation, specifically of effector CD8 T cells, triggers their intimal infiltration into LDF vessels and promotes a persistent innate-like proinflammatory program. Residence of CD137+ effector CD8 T cells further promoted infiltration of endogenous CD8 T cells with IFNγ-producing potential, whereas CD137-deficient CD8 T cells exhibited impaired vessel infiltration, minimal IFNγ production, and reduced infiltration of endogenous CD8 T cells. Our studies thus provide novel insight into how CD137 costimulation of effector T cells, independent of plaque-antigen recognition, instigates their retention and promotes innate-like responses from immune infiltrates within atherogenic foci.
NEW & NOTEWORTHY Our studies identify CD137 costimulation as a stimulus for effector CD8 T-cell infiltration and persistence within atherogenic foci, regardless of atherosclerotic-antigen recognition. These costimulated effector cells, which are generated in pathological states such as viral infection and autoimmunity, have innate-like proinflammatory programs in circulation and within the atherosclerotic microenvironment, providing mechanistic context for clinical correlations of cardiovascular morbidity with increased CD8 T-cell infiltration and markers of activation in the absence of established antigen specificity.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/effector-cd8-t-cells-seed-atherogenic-foci/.
Keywords: atherosclerosis, CD137, CD8 T cell, inflammation, interferon-γ
INTRODUCTION
Atherosclerosis is a complex disease laced by not only passive lipid accumulation but also immune cell recruitment and endothelial cell (EC) activation (22, 56). Low and disturbed flow (LDF) is an established activator of ECs, instigating transcriptional programs that facilitate pathological infiltration of T cells, macrophages, dendritic cells, and others through upregulation of adhesion receptors, cytokines, and chemokines (16, 18, 37). Once resident within the vascular wall, these foci of immune infiltrates contribute to the development of atherosclerotic lesions at sites of LDF: geometric curvatures such as the aortic arch, branch points along the abdominal aorta, and the junction of the right subclavian and brachiocephalic arteries (9, 22). Accelerated by systemic inflammation, including activation of the adaptive immune system, these lesions develop into plaques that are prone to rupture and endothelial erosion, leading to the adverse health events associated with atherosclerosis (24).
Although CD8 T cells are among the first to infiltrate atherogenic foci (22) and their frequency within human plaques has been correlated with intima-media carotid thickness and rate of cardiovascular events (31, 71), their role in the pathological progression of atherosclerosis is not well understood. This is in part because they are not as abundant within developed atherosclerotic plaques as macrophages but also because their characterization has been limited largely to histoanalyses due to lack of dedicated isolation protocols that allow for single-cell characterization of lesion-infiltrated cells (54). Nonetheless, immunostaining of a human aortic biobank has revealed that CD8 T cells accumulate within plaque intima during prethrombotic development of pathological fibroatheromas and that CD8 T cells are the dominant CD3+ population within early fibroatheromas, outnumbering CD4+ T cells in a ratio of 5:1 (64). Additionally, histoanalyses of human atherosclerotic plaque suggest that lesional T cells sustain an activated phenotype based on their expression of surface molecules and proximal location to IFNγ cytokine staining (23, 29, 61). In patients with chronic infections, such as human immunodeficiency virus or cytomegalovirus, markers of activated CD8 T cells in circulation are also associated with increased plaque severity (4, 26, 39, 63). Furthermore, increased circulation of activated T cells, as in autoimmunity, is an independent risk factor for cardiovascular events (58). Patients with type 1 diabetes (10), psoriasis (17), inflammatory bowel disease (49), multiple sclerosis (6), and systemic lupus erythematous (12) face accelerated pathological progression of atherosclerosis and increased rates of cardiovascular morbidity.
Within hyperlipidemic mouse models, CD8 T cells similarly infiltrate plaques at early stages of atherosclerosis (2, 32) and the pathological role of CD8 T cells in the inflammatory cascade has been established through antibody depletion (8, 35). In mice, the lesional area of individual atherogenic foci and number of infiltrated T cells is small, but protocols have recently been developed to address the need for flow cytometric characterization of murine-infiltrated cells (19). These studies have focused on assessing the infiltration dynamics of leukocytes and T-cell populations as a whole, and thus subtype-specific analyses warrant further investigation (15). In the current absence of phenotypical characterization of specific T-cell subtypes from atherosclerotic lesions, it has been conventionally believed that they maintain a classical antigen-dependent effector function. Although plaque-specific byproducts may have significant roles in the activation and expansion of infiltrated T cells, the heterogeneity of T-cell receptors (TCRs) within plaque is suggestive of a broader T-cell infiltrate than one resulting from antigen-dependent clonal expansion alone (47, 61). In antiviral immunity, CD8 T cells are responsive to cytokine stimulation, secreting proinflammatory cytokines as a “bystander effect” in the absence of cognate antigen restimulation (3, 11). These cells are often identified by their expression of nonconventional T-cell surface molecules, including those classically associated with natural killer cells (e.g., CD161) or dendritic cells (e.g., CD11c), and are generated after antigen priming, as in viral infections (30, 34). Costimulated effector CD8 T cells similarly produce IFNγ in response to cytokine signals (62), and thus, it is plausible that peripherally activated CD8 T cells infiltrate atherosclerotic plaque and mediate its pathological progression through “innate like” production of cytokines such as IFNγ (46, 51).
CD137 (4-1BB) is a tumor necrosis factor costimulatory receptor induced on a range of immune cells but also expressed at sites of human atherosclerotic plaque and genetically correlated with atherosclerotic disease (48, 59). CD137 was first identified as an inducible T-cell gene expressed upon antigen activation (72), and now, CD137-agonistic regimens are being employed to generate durable-effector CD8 T-cell responses against certain cancers (40). Within human plaque, CD137 colocalizes with both ECs and CD8 T cells (48). Additionally, soluble CD137, which is released by leukocytes after activation and is elevated in autoimmunity (42, 57), correlates with increased risk of cardiovascular events in patients with acute coronary syndrome (73, 74). In hyperlipidemic mouse models, global CD137 deficiency decreases plaque lesion size and diminishes monocyte/macrophage infiltration (27), whereas agonistic CD137 activation facilitates plaque development (38, 60). Bone marrow transplant experiments have hinted at a proatherogenic role for CD137 in circulating blood cells (27), but the specific circulating populations responsible have not yet been defined.
Previous studies have laid the framework for CD137 mediating atherosclerotic pathology, but elucidating the specific cell types that CD137 pathologically affects would provide mechanistic context for why autoimmunity and viral infection confer increased atherosclerotic risk and could also inform long-term risk profiles for patients undergoing CD137-agonistic cancer immunotherapies. Given the dual significance of CD137 activation at sites of atherosclerotic plaque and in activating antigen-primed CD8 T cells into effector cells with inflammatory cytotoxic potential, we wondered how CD137 activation of effector CD8 T cells specifically impacts their pathological infiltrative and inflammatory programs. In particular, we focused on characterizing the inflammatory phenotype of lesional T cells and questioned whether, as we had observed in other contexts (62), CD137 activation generates TCR-independent programs. To test the effect of CD137 specifically on effector CD8 T cells, we used adoptive transfer of congenically marked wild-type (WT) or CD137-deficient CD8 T cells specific for nonplaque antigen. Immunization and CD137 costimulation of the host mouse allowed us to then isolate the effect of CD137 costimulation on effector CD8 T cells from previously reported proinflammatory effects of the host vasculature. Through this adoptive transfer technique and the application of a new digestion protocol for isolating lesional T cells, we were able to study how CD137 costimulation triggers effector CD8 T cells to infiltrate, persist, and sustain innate-like inflammatory programs within LDF-mediated atherogenic foci.
MATERIALS AND MEHTODS
Mice.
All animal studies were performed in accordance with UConn Health (Farmington, CT) Institutional Animal Care and Use Committee regulations and were approved by the committee. CD45.2 C57BL/6J (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Ova (SIINFEKL257–264)-specific OT-I TCR transgenic, recombination activating 1-deficient (Rag1−/−) mice that were WT or CD137−/− on C57BL/6J CD45.1 or CD45.1/2 Het background were bred in house. All mice were maintained in the UConn Health Animal Facility in accordance with National Institutes of Health guidelines. All mice used in these studies were between 1.5 and 8 mo of age.
Hyperlipidemia.
Mice received 100-µl intraperitoneal injections of 1 × 1011 viral particles of AAV8-encoding mutant PCSK9 (pAAV/D377Y-mPCSK9) produced at the Gene Transfer Vector Core (Grousbeck Gene Therapy Center, Harvard Medical School). Considering the differential response of male versus female mice to viral production (66), only male mice were used for hyperlipidemia studies to achieve a consistent response to virally produced mPCSK9 protein. Between 1 and 5 days after AAV injection, mice were placed on the Clinton/Cybulsky high-fat rodent diet (HFD) with regular casein and 1.25% added cholesterol (D12108C; Research Diets). To measure cholesterol levels, blood was collected from the right ventricle into lithium-heparinized tubes (365965; BD Biosciences) and centrifuged at 5,000 g for 10 min to obtain serum. Samples were stored at −80°C and analyzed by the Total Cholesterol Assay Kit (INC, cat. no. STA-384; Cell Biolabs).
Surgical induction of LDF.
Partial carotid artery ligation (PCAL) (45) was performed as previously described (43, 44). In brief, mice were anesthetized with isoflurane, and the left external carotid, internal carotid, and occipital artery were ligated with 9-0 Ethilon suture (US stock 2813G; Ethicon). Sham operation controls were conducted in separate mice where the left carotid branches were encircled with suture as in PCAL, but vessels were not ligated. High-resolution Doppler ultrasound (Vevo 2100) was performed 1 wk after ligations to confirm vessel patency and reduction in flow.
Adoptive transfer and immunizations.
Naïve splenic OT-I cells (5 × 105 viable CD8+ Vα2+ Vβ5+ CD45.1+ CD44low) were intravenously transferred into WT recipients. Recipient mice were injected intraperitoneally with 100 µg SIINFEKL257–264 peptide (InvivoGen, San Diego, CA) + 100 µg of rat IgG control or agonist anti-CD137 (Clone 3H3 mAb; Bio X Cell) 4–24 h after adoptive transfer.
Vessel Harvests.
Vessels were flushed with phosphate-buffered saline (PBS) through the left ventricle and out the right atrium and then dissected free of adventitial tissue, minced with scissors into 1.5-ml Eppendorfs, and incubated for 1 h at 37°C with gentle rotation (20 rpm) in balanced salt solution (BSS) media containing 150 U/ml collagenase type IV (Sigma-Aldrich C5138), 60 U/ml DNase I (Sigma-Aldrich), 1 µM MgCl2 (Sigma Aldrich), 1 µM CaCl2 (Sigma-Aldrich), and 5% fetal bovine serum (FBS). Digested tissues were crushed through 35-µm cell-strainer caps (BD Biosciences) and quenched with 5 ml of cold BSS + 10% FBS in round-bottom tubes. Supernatant was removed after a 5-min, 320-g centrifuge, and the cell pellet was resuspended and quantified using a Z1 particle counter (Beckman Coulter).
In vitro stimulations.
All stimulations were conducted at 37°C and 5% CO2 in 200 µl of complete tumor medium consisting of modified Eagle’s medium with 5% FBS, amino acids, salts, and antibiotics in 96-well plates. Carotid and aortic arch vessels were seeded at 7.5 × 103 cells/well; blood and spleen cells were seeded at 1.5 × 104 cells/well. Stimulations were with phorbol 12-myristate 13-acetate (PMA; 50 ng/ml Calbiochem, Darmstadt, Germany) + ionomycin (1 µg/ml Invitrogen), murine cytokines IL-2 (5 ng/ml) and NH2-terminally processed IL-36 [1 µg/ml; produced according to previous protocols (21) and R & D Systems], or SIINFEKL peptide (1 ng/ml, InvivoGen) for 60–65 h. Secreted IFNγ was analyzed in culture supernatants by ELISA (R&D Systems, Minneapolis, MN).
Flow Cytometry.
Surface staining: naïve OT-I splenocytes for adoptive transfer were identified as (catalog/clone): LIVE/DEAD Fixable Blue Dead− (L23105; ThermoFischer), CD8+ (BD Biosciences 558106/53–6.7), CD4− (Tonbo 60–0042/RM4–5), B220− (BD Bioscience 553093/RA3–6B2), CD45.1+ (eBioscience 17-0453-82/A20), CD45.2 (11-0454-81/104; Invitrogen), Vα2+ (46-5812-80/B20.1; eBioscience), Vβ5+ (553190/MR9-4; BD Bioscience), and CD44lo (80-0441/IM7; Tonbo). Analysis of vessel-infiltrated cells was conducted using LIVE/DEAD Fixable, CD4 (Invitrogen), CD45.1 (eBioscience), CD45.2 (Invitrogen), Vα2 (eBioscience), Vβ5+ (BD Bioscience), B220 (BD Bioscience), CD3 (562600/145-2011; BD Bioscience), CD8 (MA5-17595/CT-CD8a; Invitrogen), Streptavidin (554063; BD PharMingen), and CD11c (80-0032/17A2; Tonbo). LSR Aria IIa (BD Biosciences) was used for acquisition. Viable cell gate is representative of a size gate, single-cell gate, and viability gate.
For intracellular staining, cells were incubated for the last 5 h in the presence of GolgiStop (BD Biosciences), surface stained, permeabilized and fixed via FoxP3 fixation kit (eBioscience), and stained at 4°C overnight with IFNγ (554412/XMG1.2; BD PharMingen). Acquisition was performed by MACSQuant (Miltenyi Biotec). All flow cytometry data were analyzed with FlowJo (Tree Star, Ashland, OR).
Immunohistofluoresence.
Vessels were flushed in situ with PBS before resection and then fixed in 4% buffered paraformaldehyde overnight at 4°C, dehydrated in 30% wt/vol sucrose overnight, and snap-frozen in OCT and stored at −80°C. Specimens were sectioned at 10 µm and fixed for 2–10 min in ice-cold acetone before staining at room temperature. Slides were air-dried before PBS rehydration and blocked for 1 h with 0.01% Triton X-100 (BioRad), 2.5% wt/vol bovine serum albumin (Sigma-Aldrich), and 2.5% goat serum (Invitrogen). Sections were stained with CD31 (102502/Mec13.3 at 1 µg/ml; Biolegend) and CD45.1 (110720/A20 at 1 µg/ml; Biolegend) for 2 h, followed by goat anti-rat IgG Alexa Fluor 594 (A-11007 at 2 µg/ml; Invitrogen) and DAPI staining for 1 h. Fluoromount-G (ThermoFischer) was used to mount slides before imaging on Zeiss LSM 800 confocal at ×20 and ×63 magnification. ImageJ Cell Counter was used to quantify images taken at ×20 with CD45.1 threshold set to 100. DAPI split channel images where the threshold was between 90 and 255. Lumen surface was defined as a CD45.1+ cell in contact with the luminal CD31 EC lining and intimal infiltration as CD45.1+ staining between the EC and medial elastin layers of the vessel wall.
CyTOF.
After PBS-flushed arteries were digested to liberate infiltrated cells, cells isolated from five ligated or contralateral control carotid vessels were respectively pooled to obtain sufficient cell number for CyTOF processing. Analyses and labeling were conducted as previously described (41). The pooled samples were analyzed by a mass cytometer (Helios; Fluidigm, South San Francisco, CA); data were debarcoded using Fluidigm Debarcoder version 1.04 and then merged to create viSNE maps using Matlab (The MathWorks, Natick, MA). All antibodies (Supplemental Table S1; Supplemental Material for this article is available at http://doi.org/10.5281/zenodo.2598759) were from Fluidigm .
Statistics.
Unless otherwise indicated, dots represent an individual biological replicate, and summary graphs represent means ± SD. Analyses were performed with GraphPad Prism version 7 (GraphPad Software). For comparisons within groups, we used paired, two-tailed Student’s t-tests (“paired t-test” in text), and for comparisons between groups, we used two-group, two-tailed Student’s t-tests (“2-group t-test” in text). When there was a noticeable departure from normality, nonparametric tests based on ranks were used (e.g., Figs. 5 and 8 and Supplemental Fig. S6, which used Mann-Whitney tests for 2-group analyses and Wilcoxon-matched pair tests for paired analyses, as appropriate). P values of <0.05 were considered statistically significant and are further defined as indicated.
Fig. 5.
T-cell CD137 expression is critical for infiltration of effector CD8 T cells into low and disturbed flow (LDF) foci. A: experimental setup for adoptive transfer of CD45.1 Ova-specific CD8 T cells that are either wild-type (WT) or CD137−/− into normolipidemic CD45.2 WT mice immunized with SIINFEKL and agonist anti-CD137 mAb. Vessels were harvested 4 days postimmunization (dpi). B: percentage (gated on viable cells) of Ova-specific transferred CD8 T cells within physiological areas LDF (aortic arch) and high laminar flow (linear carotid). Data are pooled from 2 experiments, 2–3 mice/group/experiment. nsP = 0.3, **P = 0.004 by 2-group t-test; n = 5/group. C: cell counts of T cells isolated from the aortic arch and linear carotid 4 dpi. Only the population of transferred CD8 T cells achieved statistical significance. Population of “endogenous T cells” is inclusive of endogenous CD8 T cells that are Vα2+β5+ (and thus likely Ova-specific) and non-Vα2β5 as well as endogenous CD4 T cells. For cells isolated from aortic arch: nsP = 0.5 (endogenous T cells) and *P = 0.01 (transferred CD8 T cells); for cells isolated from the linear carotid: nsP > 0.1 (transferred and endogenous CD8 T cells) via Mann-Whitney test. Data are pooled from 2 experiments, 2–3 mice/group/experiment; n = 5 mice/group. Data points from individual mice are as follows: ◆, mice with transferred WT cells; ◇, mice with transferred CD137−/− cells
Fig. 8.
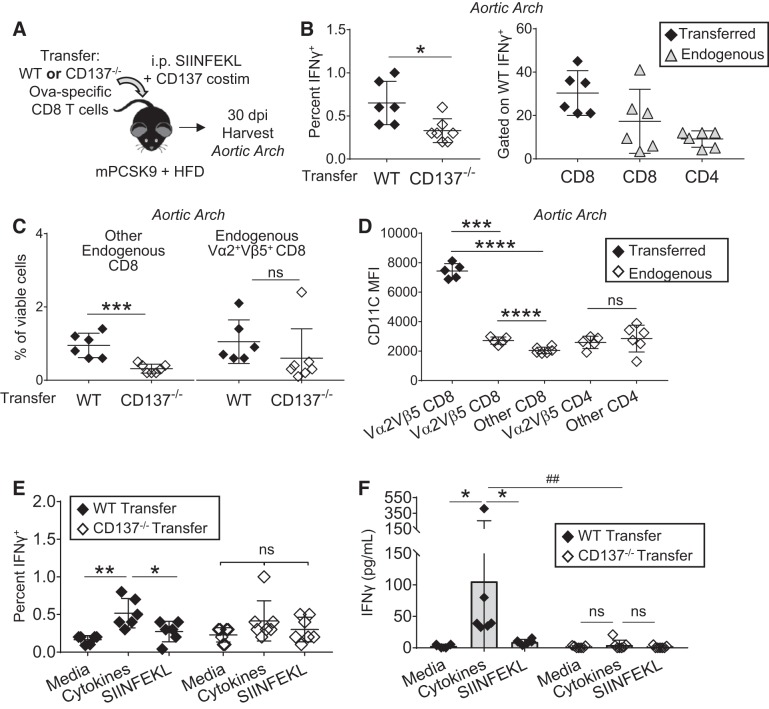
Infiltration of effector CD8 T cells promotes a diverse proinflammatory infiltrate within nascent atherogenic foci. A: hyperlipidemic mice received transfer of wild-type (WT) or CD137−/− Ova-specific CD8 T cells and were immunized with SIINFEKL and agonist anti-CD137 mAb. Thirty days postimmunization (dpi), aortic arches were analyzed by flow cytometry for infiltration of transferred CD8 T cells. B: cells isolated from aortic arch were stimulated for 40 h with phorbol 12-myristate 13-acetate + ionomycin and 5 h with brefeldin A. Intracellular IFNγ analyzed through flow cytometry (gated on total events) and T-cell distribution of WT transfer IFNγ+ response (gated on IFNγ+ cells). *P = 0.01 by 2-group t-test. C: infiltration of endogenous CD8 T cells that do not express the Ova-specific TCR (Vα2Vβ5) have increased infiltration of the aortic arch 30 dpi when the atherogenic foci is first seeded with WT effector CD8 T cells. Infiltration of endogenous, CD137-sufficient Ova-specific CD8 T cells was not statistically significant. nsP = 0.3, ***P = 0.0006 by 2-group t-test. D: CD11c MFI expression from indicated T-cell subsets isolated from aortic arch of mice that received WT Ova-specific CD8 T cells. nsP = 0.8, ***P = 0.0005, and ****P < 0.0001 via paired t-tests. E: intracellular cytokine staining after stimulation with media, cytokines (IL-2 + IL-36), or SIINFEKL gated on total events on cells isolated from the aortic arch of mice that received WT or CD137−/− transfer of CD8 T cells. nsP > 0.1, *P = 0.01, and **P = 0.006 by paired t-test. F: cells isolated from the aortic arch 30 dpi secrete IFNγ in response to the cytokine combination of IL-2 + IL-36, but not SIINFEKL cognate antigen restimulation. *P = 0.03 and nsP > 0.2 via Wilcoxon matched-pairs test, ##P = 0.001 via Mann-Whitney test; n = 6–7 mice/group for all experiments in this figure.
RESULTS
Effector CD8 T cells infiltrate low and disturbed flow-activated endothelium.
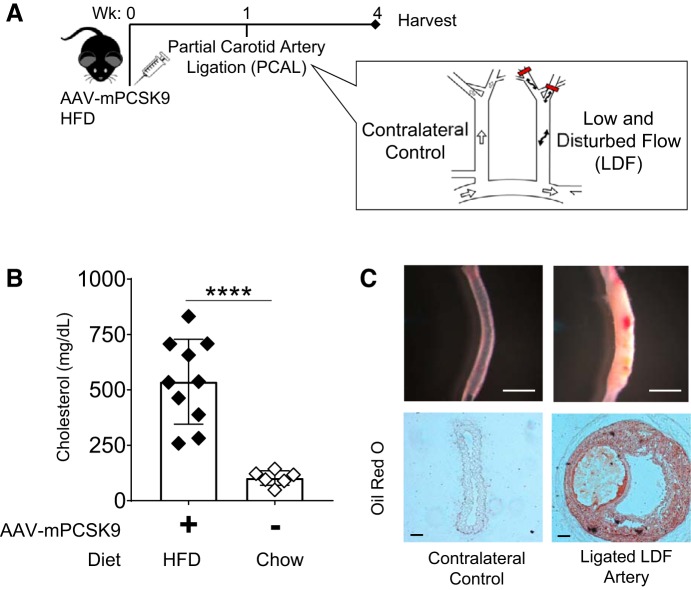
To examine pathological immune infiltration into nascent atherogenic foci, we combined partial carotid artery ligation (PCAL) with AAV-mPCSK9, surgically inducing LDF within the geometrically straight left carotid in the setting of rapid LDL receptor deficiency-mediated hyperlipidemia (Fig. 1A) (33, 55). PCAL entails ligation of all but one branch of the left carotid, generating LDF throughout the left carotid artery and leaving the contralateral right carotid artery as an internal control subject to its physiological laminar flow patterns. When combined with high-fat diet (HFD), WT C57BL/6J mice developed hyperlipidemia within days that was sustained at an experimental end point of 3 wk (Fig. 1B). Consistent with previous studies (33), we found that PCAL induces lipid-laden plaque within 4 wk (Fig. 1C). This plaque is also cellularized in a LDF-dependent manner (Supplemental Figure S1A). Thus, PCAL induces lipid and cell accumulation at atherogenic foci and serves as an experimental model of nascent, LDF-dependent cell infiltration.
Fig. 1.
Partial carotid artery ligation (PCAL) induces low and disturbed flow (LDF)-dependent formation of atherosclerotic plaque. A: wild-type (WT) mice were injected with AAV-mPCSK9 and placed on a high-fat diet (HFD). PCAL was performed within 1 wk, introducing LDF within the left carotid and maintaining physiological laminar flow patterns within the contralateral right. Vessels were harvested 3 wk after ligation. B: AAV-mPCSK9 injection and HFD feeding of WT mice induce hyperlipidemia with cholesterol in mg/dl 5-fold higher than mice fed normal chow; mean: 537 (SD 191) vs. 102 mg/dl (SD 33). ****P < 0.0001 by 2-group t-test; n = 6–10 mice/group. C: carotid vessels 3 wk after PCAL showing LDF-mediated atherosclerotic plaque development. Unstained carotid vessels (white scale bar, 1 mm) and cross-sectional histopathology of indicated carotid vessels stained with Oil Red O (black scale bar, 50 µm).
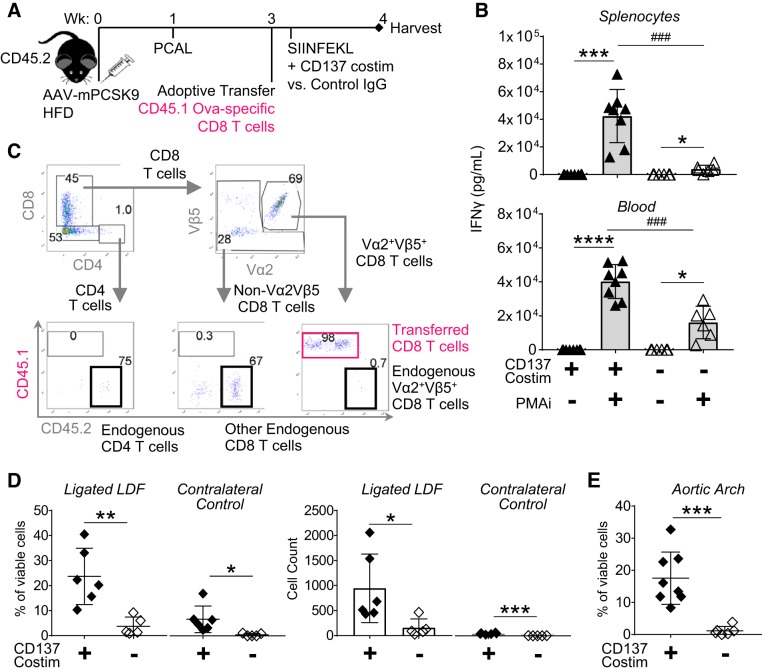
To study the infiltration of peripherally generated effector CD8 T cells into PCAL-ligated vessels, we transferred TCR-transgenic CD45.1 (OT-I, Ova-specific) cells into recipient WT mice with established mPCSK9-mediated hyperlipidemia (Fig. 2A). Activated effector CD8 T cells were generated by immunizing recipient mice with cognate SIINFEKL peptide and costimulatory agonist anti-CD137 mAb 4–24 h after adoptive transfer. When compared with mice that received rat IgG control, increased IFNγ responsiveness of both splenocytes and blood cells to the mitogen PMA + ionomycin 4 days postimmunization (dpi) was dependent on prior in vivo CD137 costimulation (Fig. 2B). Vessel infiltration of transferred and endogenous T cells was analyzed through digest of PBS-flushed arteries and flow cytometry (Fig. 2C). A digest protocol was developed to maximize isolation of lymphocytes while also preserving cell viability for not only flow cytometry characterization but also in vitro restimulation experiments (detailed in materials and methods). Consistent with previous reports (48), CD137 costimulation did not significantly affect infiltration of CD4 T cells (Supplemental Fig. S1B). Yet CD137 costimulation significantly increased infiltration of transferred effector CD8 T cells into PCAL-ligated carotids by both percentage of viable cells and total count of transferred CD8 T cells isolated per vessel (Fig. 2D). This CD137-mediated infiltration was also observed within regions of physiological LDF such as the aortic arch (Fig. 2E). Parallel to the clinical observation that systemic inflammation predisposes autoimmune patients to atherosclerotic pathology regardless of lipid levels (52, 58), normolipidemic mice fed chow diet and mPCSK9-hyperlipidemic mice on HFD had no obvious difference in effector cell infiltration, by either percentage or total cell count, into physiological areas of LDF 4 dpi, and cholesterol level in hyperlipidemic mice had no significant correlation with effector cell infiltration into ligated and physiological LDF foci (Supplemental Fig. S2). Thus, effector CD8 T cells accumulate at induced and physiological sites of LDF independent of cholesterol levels; their distribution in situ could reveal parallels with human CD8 T-cell plaque residence.
Fig. 2.
CD137 costimulation increases recruitment of activated effector CD8 T cells into low and disturbed flow (LDF) foci. A: hyperlipidemic CD45.2 wild-type (WT) mice received adoptive transfer of CD45.1 Ova-specific splenocytes 2 wk after partial carotid artery ligation (PCAL) and were immunized with SIINFEKL + rat IgG control or agonist anti-CD137 mAb. B: in vivo CD137 costimulation induces an activated effector population that is resident in spleen and circulates in blood 4 days postimmunization (dpi). Splenocytes and blood cells were stimulated in vitro with media or phorbol 12-myristate 13-acetate + ionomycin (PMAi). *P = 0.02, **P = 0.009, ***P = 0.0004, and ****P < 0.0001 by paired t-test; ###P < 0.0007 by 2-group t-test; n = 6–8 mice/group. C: gating strategy for identifying T cells from PBS-flushed vessels by flow cytometry. CD45.1 indicates transferred population, and CD45.2 indicates an endogenous population and Ova specificity through TCR expression of Vα2β5. Gating scheme is enlarged in Supplemental Fig. S1C, with isotype stains used to establish gates. D: percentage (gated on viable cells) and total cell count of transferred CD8 T cells isolated from PBS-flushed contralateral control vs. ligated carotids 4 dpi. *P ≤ 0.03, **P = 0.004, and ***P = 0.0002 by 2-group t-test; n = 5–6 mice/group. E: %infiltrated transferred CD8 T cells isolated from PBS-flushed aortic arch. Data shown are 1 experiment representative of 2 experimental replicates. ***P = 0.0004 by 2-group t-test n = 6–8 mice/group. Data points from individual mice are as follows: ▲ and ◆, mice with CD137 costimulation; △ and ◇, IgG-treated controls
Localization of effector CD8 T cells was visualized by immunofluorescent staining of carotid artery cross-sections (Fig. 3, A and B). The EC layer was demarcated by CD31 (platelet endothelial cell adhesion molecule) and transferred CD8 T cells by CD45.1. Total infiltration of transferred CD8 T cells per cross-section was increased upon CD137 costimulation, and this was due to increased numbers deep within the intima, not at the abluminal surface (Fig. 3C). The underlying medial and adventitial layers were minimally infiltrated by effector CD8 T cells (Supplemental Fig. S3), suggesting that the immune-privileged medial status had not been compromised, consistent with early-stage human atherosclerotic lesions (64). Having established that effector CD8 T cells infiltrate neointima at sites of induced and physiological LDF in a manner similar to human plaque, we sought to gain mechanistic insight into their potential role within atherosclerotic pathology.
Fig. 3.
CD137 costimulation instigates luminal infiltration of activated effector CD8 T cells. A: hyperlipidemic CD45.2 wild-type (WT) mice received adoptive transfer of CD45.1 Ova-specific splenocytes 2 wk after PCAL and were immunized with SIINFEKL + rat IgG control or agonist anti-CD137 mAb. B: confocal microscopy of ligated carotid vessels 4 days postimmunization (dpi) with either control IgG treatment or CD137 costimulation stained for endothelial cells (CD31; green), Ova-specific transferred CD8 T cells (CD45.1; red), and DAPI (blue). White squares demarcate magnified images below, and arrows indicate infiltrating effector CD8 T cells. (top scale bar, 50 µm; bottom scale bar, 20µm). Intima (I), medial (M), and adventitial (A) layers of the developing plaque are indicated by dashed lines. C: total count of transferred CD8 T cells per carotid vessel cross-section (left) and localization of cells either within intima (top right) or at the lumen surface (bottom right) in partial carotid artery ligation (PCAL)-carotid cross-sections of mice that received transfer of CD45.1 WT Ova-specific CD8 T cells and immunization with SIINFEKL + rat IgG control or agonist anti-CD137 mAb. nsP = 0.09, **P < 0.006 by 2-group t-test; n = 5 mice/group. Data shown are from 1 experiment representative of 2 experimental replicates. HFD, high-fat diet. Data points from individual mice are as follows: ◆, mice with CD137 co-stimulation; ◇, IgG-treated controls.
Infiltrated effector CD8 T cells have robust inflammatory potential.
To test for differences in infiltrative cell phenotype downstream of both surgically induced and physiological LDF patterns, we performed cytometry by time of flight (CyTOF) analysis on cells isolated from PCAL carotids, contralateral control carotids, and physiological LDF vessels 4 dpi with cognate antigen and CD137 costimulation (Fig. 2A). ViSNE maps of PCAL-ligated and control carotid vessels revealed two islands of cells dominated by cells isolated from PCAL-ligated vessels (Supplemental Fig. S4). LDF group 1 appeared to be dominated by endogenous monocytes and a small population of CD4+ T cells, whereas LDF group 2 was dominated CD45.1+ cells that were largely CD8+, representing the transferred Ova-specific population of effector CD8 T cells. This group also expressed Granzyme B and markers associated with innate-like CD8 T-cell responses, i.e,. the conventional dendritic cell marker CD11c (5, 50, 65) and natural killer cell marker CD161 (13). Thus, not only do effector CD8 T cells infiltrate surgically induced and physiological atherogenic foci, but they also express enzymes and surface markers suggestive of responsiveness to nonantigen stimuli.
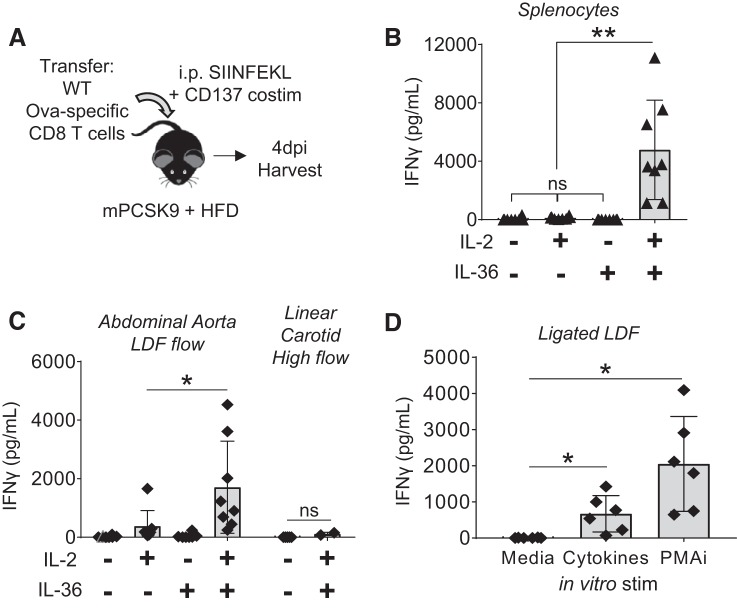
To assess whether the local cytokine environment could potentially play a role in antigen-independent activation of infiltrated effector CD8 T cells, we tested for expression of all proinflammatory IL-1 cytokines in the intimal response to LDF (43), as we had previously described effector T cells having innate-like responses to IL-1 cytokines when combined with IL-2 (62), a cytokine found within plaques (14). This revealed LDF-mediated upregulation of IL-36γ and IL-1β (Supplemental Fig. S5A). Like other IL-1 cytokines, IL-36 requires NH2-terminal cleavage for full biological activity, and the associated protease Cathepsin S (1) was similarly upregulated by LDF. IL-1β has a well-studied role in mediating atherosclerosis (53) and IL-36γ a prominent role in psoriasis, but IL-36γ has not yet been studied in atherosclerosis. Thus, informatics analysis (Supplemental Fig. S5) suggested a possible role for IL-1β and IL-36γ in mediating antigen-independent CD8 T-cell activation within the inflamed intima.
To determine whether IL-1β and/or IL-36γ could elicit innate-like secretion of IFNγ from dual-costimulated effector CD8 T cells, as we had previously studied (62), we exposed splenocytes in vitro to these cytokines in combination with IL-2. IL-1β, both alone and in combination with IL-2, did not elicit IFNγ production (Supplemental Fig. S5C). However, IL-36γ + IL-2 induced a dose-dependent production of IFNγ from both dual-costimulated CD8 T cells and whole splenocytes harvested 4 dpi with SIINFEKL and CD137 mAb (Fig. 4, A and B). To determine whether the intima-infiltrated transferred effector CD8 T cells were similarly capable of responding to cytokines (IL-2 and IL-36γ), we compared in vitro IFNγ secretion from cells isolated from LDF regions (surgically induced and physiological) with cells isolated from linear carotid arteries as a control. We observed increased IFNγ secretion from cultures of LDF infiltrates, both within the abdominal aorta and PCAL carotids (Fig. 4, C and D), whereas cells isolated from the contralateral control carotids failed to produce detectable amounts of IFNγ. Furthermore, IFNγ secretion trended with number of infiltrated effector CD8 T cells per vessel (Supplemental Fig. S5B), suggesting that these are the cells responsible for producing the majority of IFNγ, a concept further defined by intracellular staining studies shown in Fig. 8. Having established that peripherally activated effector CD8 T cells exhibit innate-like proinflammatory programs in situ, our goal was then to resolve the role of T cell-CD137 signaling in mediating this process.
Fig. 4.
Infiltrated T cells have innate-like inflammatory potential. A: hyperlipidemic CD45.2 wild-type (WT) mice received adoptive transfer of CD45.1 Ova-specific splenocytes 2 wk after partial carotid artery ligation (PCAL) and were immunized with SIINFEKL and agonist anti-CD137 mAb. Vessels were harvested 4 days postimmunization (dpi). B: 4 dpi with SIINFEKL and agonist anti-CD137 mAb, splenocytes have a robust innate-like response to the cytokine combination of IL-2 + IL-36. Cells were stimulated in vitro, and secreted IFNγ was measured by ELISA analysis of supernatant. nsP > 0.1, **P < 0.03 by series of paired t-tests; n = 5–8/group. C: this innate-like secretion was also seen in cells isolated from the abdominal aorta, a physiological site of low and disturbed flow (LDF) at intercostal branch points, but not in cells isolated from the linear carotid, a vessel subjected to laminar high flow (right). nsP = 0.2, *P = 0.015 by paired t-test; n = 2–8 mice/group. D: cells isolated from PCAL-ligated carotids have innate-like responses to cytokines (IL-2 + IL-36) and also secrete IFNγ in response to phorbol 12-myristate 13-acetate + ionomycin (PMAi), as measured by ELISA. *P < 0.02 by paired t-tests; n = 6 mice/group. ▲ and ◆, Data points from individual mice.
T-cell CD137 expression is critical for robust infiltration of effector CD8 T cells to LDF-activated endothelium.
To delineate the requirement for CD137 on CD8 T cells versus other CD137-expressing cells within LDF atherogenic intima, the infiltrative capacity of transferred CD137−/− CD8 T cells was examined in WT recipient mice immunized with cognate SIINFEKL peptide and agonistic anti-CD137 mAb (Fig. 5A). T-cell CD137 expression was required for robust infiltration into the aortic arch by both percentage (Fig. 5B) and cell count (Fig. 5C). Similar trends were observed in the PCAL model of LDF, although statistical significance was not reached (Supplemental Fig. S6A). Thus, even when the recipient environment of LDF-activated endothelium is CD137 sufficient [including other T-cell subtypes (68), monocytes (36), dendritic cells (69), and natural killer cells (70)], significant infiltration of effector CD8 T cells into neointima requires their own activation through CD137 costimulation. If this infiltration is persistent, these innate-like CD8 T cells could have biological relevance within atherosclerosis.
Persistence of effector CD8 T cells requires T-cell CD137.
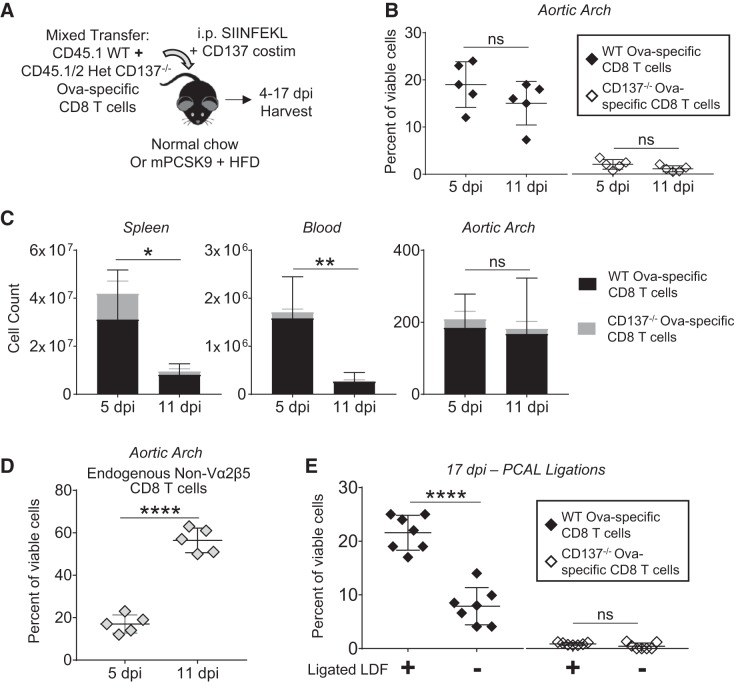
To test whether CD137 expression on transferred CD8 T cells impacted persistence within atherosclerotic plaque, we transferred a 1:1 mixture of WT (CD45.1+) and CD137−/− (CD45.1/45.2 Het) Ova-specific CD8 T cells into WT recipient mice (CD45.2+) that then received cognate SIINFEKL peptide and CD137 costimulation (Fig. 6A). The distribution of each congenically marked population within blood and their infiltration into LDF vessels was assessed through flow cytometry (Supplemental Fig. S6B). Between 5 and 11 dpi, the percentage of viable cells (Fig. 6B) and cell count (Fig. 6C) of transferred CD8 T cells isolated from the aortic arch did not significantly change despite a significant drop in the proportion of transferred WT Ova-specific CD8 T cells circulating within spleen and blood over the same time course (Fig. 6C). Accompanying the increased infiltration of WT transferred effector CD8 T cells were endogenous populations of CD8 T cells (Fig. 6D). To further assess the persistence of the transferred CD137-costimulated effector CD8 T cells within LDF foci, we used hyperlipidemic PCAL mice (as in Fig. 2A) and administered a 1:1 WT and CD137−/− mixture of Ova-specific CD8 T cells. Strikingly, only the infiltration of WT Ova-specific CD8 T cells was preferential to LDF-ligated carotids 17 dpi (Fig. 6E). Thus, CD137-costimulated effector T cells persist for weeks within LDF-activated foci independent of plaque-antigen specificity, and their infiltration is accompanied by the infiltration of other T cells, which is perhaps indicative of progressive plaque pathology.
Fig. 6.
Infiltration of CD137-costimulated effector CD8 T cells is persistent within low and disturbed flow (LDF) foci. A: experimental setup for mixed adoptive transfer of wild-type (WT) (CD45.1) and CD137−/− (Het CD45.1/2) Ova-specific CD8 T cells. Total no. of transferred cells is kept consistent throughout all adoptive transfer experiments. B: normolipidemic mice were harvested 5 and 11 days postimmunization (dpi) with cognate SIINFEKL and agonist anti-CD137 mAb. Percentage (gated on viable cells) of each transferred population isolated from the aortic arch; nsP > 0.12 by unpaired 2-tailed Student’s t-test; n = 5 mice/time point. C: count of transferred CD8 T cells per spleen and per 400 µl of blood decreases dramatically from 5 to 11 dpi (*P < 0.03, **P < 0.01), whereas infiltration of transferred WT Ova-specific CD8 T cells within the aortic arch does not (nsP ≥ 0.3). Significance determined by 2-group t-test; n = 5 mice/time point. D: percentage (gated on viable cells) of endogenous CD8 populations isolated from the aortic arch. ****P < 0.0001 by 2-group t-test; n = 5 mice/time point. E: hyperlipidemic partial carotid artery ligation (PCAL) mice [AAV-mPCSK9 mice on high-fat diet (HFD) as in Fig. 2A] received mixed transfer of WT and CD137−/− Ova-specific CD8 T cells. Mice were harvested 17 dpi, and the percentage of each indicated population isolated from either ligated LDF carotids or the contralateral control right carotids are shown. nsP > 0.1, ****P < 0.0001 via paired t-test; n = 7 mice/group.
Infiltration of effector CD8 T cells promotes a diverse, proinflammatory CD8 T-cell infiltrate.
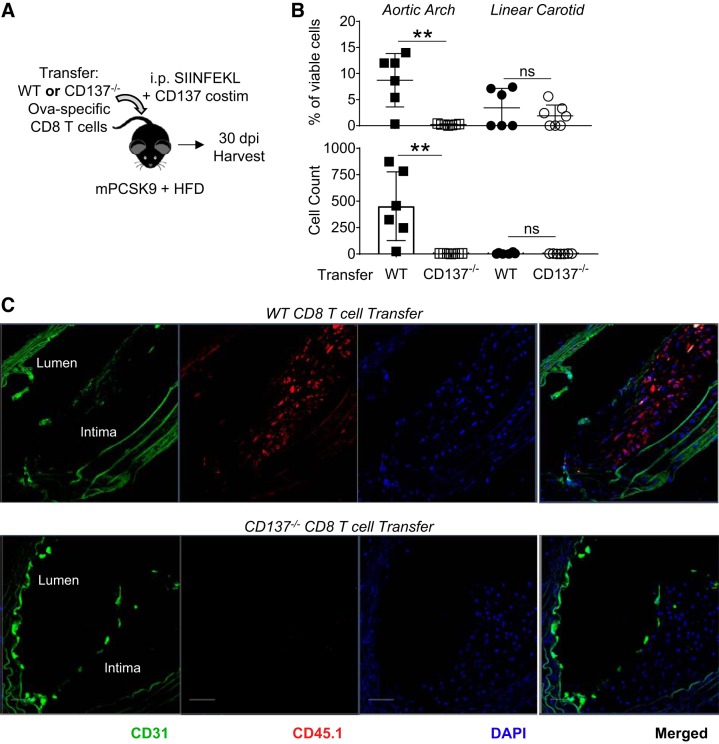
Atherosclerosis is a chronic disease in which LDF regions are repetitively exposed to T cells activated by environmental antigen or costimulation signals, exposures that could pathologically seed atherogenic foci with effector CD8 T cells. To assess how T-cell CD137 influences the persistence of these infiltrated T cells, WT or CD137−/− Ova-specific CD8 T cells were transferred into hyperlipidemic recipient mice, provided a single injection of cognate SIINFEKL peptide and agonist anti-CD137 mAb, and then analyzed 30 dpi (Fig. 7A). At this time point, the overt cell expansion induced by antigen priming and costimulation has subsided in spleen and in circulation (Supplemental Fig. S7A). Through both percentage and total cells isolated per vessel, T-cell expression of CD137 was necessary for effector CD8 T-cell persistence within the aortic arch (Fig. 7B). Immunohistofluoresence of another physiological foci of LDF, the branch point of the right subclavian artery from the brachiocephalic artery, revealed that the transferred CD8 T-cell population infiltrates and persists within plaque intima at physiological sites of LDF-mediated atherogenesis (Fig. 7C). Thus, a single, acute activation of CD8 T cells may indeed lead to their chronic persistence within plaque intima, perhaps imprinting an inflammatory signature and progressive plaque phenotype.
Fig. 7.
Infiltration of effector CD8 T cells promotes a diverse proinflammatory infiltrate within nascent atherogenic foci. A: hyperlipidemic mice received transfer of wild-type (WT) or CD137−/− Ova-specific CD8 T cells and were immunized with SIINFEKL and agonist anti-CD137 mAb. Thirty days postimmunization (dpi), aortic arches were analyzed by flow cytometry for infiltration of transferred CD8 T cells. B: percentage (gated on viable cells) and cell count of transferred CD8 per vessel are shown. nsP > 0.2, **P < 0.004 by 2-group t-test; n = 6–7 mice/group. C: immunohistofluoresence of the branch point of the right subclavian artery from the brachiocephalic artery 30 dpi shows intimal persistence of transferred CD8 T cells (CD45.1 in red) within atherogenic foci.
We then tested whether these T cells retained inflammatory responses, as we had observed 4 dpi during their phase of acute expansion (Figs. 2 and 4). Cells isolated from the aortic arch were stimulated in vitro with PMA + ionomycin to broadly assess their IFNγ-producing potential. Through both ELISA (Supplemental Fig. S7B) and intracellular cytokine staining (Fig. 8B), it was confirmed that expression of CD137 on transferred CD8 T cells endows developing atherogenic foci with inflammatory cell infiltrates. To define these proinflammatory plaque-resident populations, we analyzed surface expression of the IFNγ+ population from mice receiving WT CD8 T cells and found that the majority were transferred Ova-specific cells (CD45.1+), suggesting that even 30 dpi, an inflammatory program is sustained (Fig. 8B). Parallel to this persistence of transferred effector CD8 T cells was the infiltration of endogenous (CD45.2+) CD8 T cells, which composed a substantial compartment of the PMA + ionomycin inflammatory response. This endogenous CD8 population was composed of both “other” (non-Vα2Vβ5) and endogenous Vα2+Vβ5+ CD8 T cells, which are largely Ova specific (Fig. 8C) (25). Notably, infiltration of non-Vα2Vβ5 CD8 T cells was significantly higher in mice that received transfer of WT, CD137+ Ova-specific CD8 T cells (Fig. 8C). Infiltration of CD4 T cells into the aortic arch was not significantly affected by CD137 expression of the transferred CD8 T cells (Supplemental Fig. S7C). Thus, not only is T-cell CD137 requisite for effector CD8 T-cell persistence within LDF vessels, but it also promotes the infiltration of additional CD8 T cells that have proinflammatory, IFNγ-producing programs.
To test whether the immune infiltrate directed by intimal infiltration of WT CD137+ effector CD8 T cells into LDF foci maintained an innate-like inflammatory program, we assessed their surface expression and functional capacity to respond to cognate antigen or instead, cytokines. CD11c expression was analyzed, as it was a marker initially identified to be expressed 4 dpi (Supplemental Fig. S4). Not only did the transferred CD8 T cells have significantly higher CD11c MFI than all other T-cell subtypes isolated from the aortic arch (Fig. 8D), but they also had preserved innate-like IFNγ responses to the cytokines IL-2 and IL-36, as supported by intracellular cytokine staining (Fig. 8E). Whereas the percentage of IFNγ+ T cells was not substantially different through this assay, which is limited in time course by the toxicity of the agent used to prevent secretion of IFNγ, analysis of cell culture supernatant for cumulative IFNγ secretion was significantly higher in mice that received transfer of WT Ova-specific CD8 T cells (Fig. 8F). Furthermore, this innate-like production of IFNγ was stronger than cognate antigen restimulation, whereas splenocytes from the same mice had a stronger response to SIINFEKL peptide restimulation than to the cytokines (Supplemental Fig. S7D). Mice that received CD137−/− Ova-specific CD8 T cells did not have cytokine-mediated or antigen restimulation responses that were significant over media control. Thus, CD137 activation of effector CD8 T cells promotes their infiltration into vascular sites of LDF, and once seeded within atherogenic foci, they are responsive to the inflammatory cytokine environment where their chronic intimal persistence facilitates subsequent infiltration of other CD8 T cells.
DISCUSSION
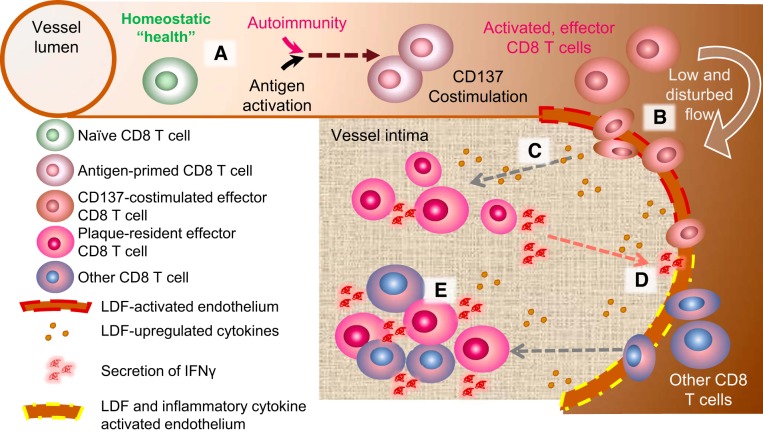
Using in vivo mouse models of surgically induced and physiological LDF, we show that activation of CD137 on CD8 T cells promotes their neointimal infiltration within developing atherogenic foci independent of plaque-specific antigen recognition. Once resident, these CD137-stimulated effector CD8 T cells are retained and express markers and phenotypes akin to innate-like cells. Mechanistically, we show that both IL-36γ and the enzyme required for its activating cleavage, cathepsin S, are upregulated at atherogenic foci. Furthermore, the combination of IL-36γ with IL-2, a cytokine native to neointimal environments, is sufficient to induce innate-like cytokine production from plaque-derived CD8 T cells in vitro. The presence of these innate-like CD8 T cells in the atherogenic intima promotes infiltration of other endogenous CD8 T cells, thereby altering plaque immune cell composition. Thus, through phenotypic and in vitro characterization of plaque-lesional T cells, our data support a model in which generation of effector CD8 T cells in circulation, perhaps physiologically induced by systemic viral infection or autoimmunity, drives their infiltration into nascent atherogenic intima where they persist, secrete proinflammatory cytokines, and orchestrate additional recruitment of immune cells into nascent atherogenic foci (Fig. 9).
Fig. 9.
Proposed model for how CD137 costimulation promotes the seeding of activated effector CD8 T cells into nascent atherogenic foci. A: under conditions of homeostatic health, naive CD8 T cells do not readily infiltrate the vessel wall, even at sites of low and disturbed flow (LDF)-activated endothelium, where adhesion receptors or chemokines are upregulated (red dashed line). B: increased circulation of effector CD8 T cells by CD137 costimulation prompts their infiltration at LDF foci independent of cognate TCR recognition. C: LDF activates intimal cells at atherogenic foci to secrete cytokines, such as IL-36γ, that promote innate-like secretion of IFNγ from infiltrated CD8 T cells. D: proinflammatory cytokines produced in C further alter the plaque microenvironment, generating an intimal niche (yellow dashed line) that is permissive to infiltration of other CD8 T cells in circulation. E: together, the initial seeding of CD137-stimulated effector CD8 T cells and the subsequent infiltration of other CD8 T cells constitute a plaque with increased inflammatory potential, potentially resulting in accelerated atherosclerotic pathology and vascular events.
Our studies shed light on the complicated role of CD137 in both atherosclerotic plaque development and activation of multiple immune cell types by specifically defining the effects of CD137 on effector CD8 T cells. The significance of delineating this cell type-specific response is reinforced by the apparently contradictory observation that CD137 deficiency in animal models leads to reduced atherogenesis, whereas a human genetic SNP linked with decreased CD137 mRNA is associated with increased intimal medial thickness (59). Through comparisons of CD137 mAb administration versus rat IgG control (Figs. 2 and 3), followed by adoptive transfer of WT and CD137-deficient Ova-specific CD8 T cells (Figs. 5–8), our studies establish CD137 costimulation of effector CD8 T cells as pathological in promoting their infiltration and retention within atherogenic foci in mice. In light of the emergence of CD137-agonistic cancer immunotherapies (40), understanding how CD137 activation impacts the progression of atherosclerosis, perhaps through the pathological seeding of CD8 T cells and survival advantage within the plaque microenvironment, is of great clinical importance. Other costimulatory members of the tumor necrosis family have been linked to atherosclerosis (20), and it would be interesting to examine whether CD134 (OX40), LIGHT, and CD70 have similar means of orchestrating additional inflammatory infiltrate or whether CD137 is unique in its specificity for promoting effector CD8 T-cell infiltration into atherogenic foci.
Plaque-specific antigens have clear significance in CD4 T cell-mediated atherosclerotic pathology (22), but our data support a model in which circulation of effector CD8 T cells, even if generated peripheral to the atherosclerotic lesion, leads to plaque infiltration from the vessel lumen that is independent of classic lesional antigen recognition. Shortly after direct CD137 costimulation of Ova-specific CD8 effector T cells, we observed their accumulation within the neointima of atherogenic foci (Fig. 3). This infiltration was persistent, and their intimal localization could be visualized even 30 dpi (Fig. 7). Recent single-cell immune profiling of atherosclerotic lesions has reported not only that T cells are a significant component of atherosclerotic lesions but that they also have surprisingly high sublineage heterogeneity, equivalent in cluster number to those identified within the spleen (71). Whether antigen-independent infiltration of atherogenic foci is limited to effector CD8 T cells or is applicable to all T-cell subsets has not to our knowledge been explored. However, we consistently observed no substantial effect of CD137 costimulation on the infiltration of endogenous CD4 T cells into atherosclerotic lesions. Thus, our data suggest that CD137 costimulation, especially when layered with antigen activation, is pathologic in seeding atherogenic foci with effector CD8 T cells, which has implications in autoimmunity and chronic viral infection, where local presentation of CD137-ligand could be constantly generating autoreactive effector CD8 T cells that are infiltrative and supportive of an intimal niche that is permissive to further inflammatory immune cell infiltration.
Human histoanalyses have identified a clear CD8 infiltrate “footprint” that precedes the formation of advanced and unstable plaque (64), but how these intimal-localized CD8 T cells contribute to destabilizing inflammation is not fully understood. Although lipid byproducts and heat shock proteins have been described as autoantigens local to the atherosclerotic plaque that are capable of generating effector T-cell responses, our prior work with effector T cells highlights other mechanisms of activation (62). Through stimulation of vessel-infiltrated cells with the plaque cytokine IL-36, we show that innate-like responses may be induced by simple proximity to intimal cells upregulating this potent cytokine within LDF-exposed regions of the vasculature (Figs. 4 and 8). IL-36 is a member of the proinflammatory IL-1 family of cytokines and has only recently been identified as expressed by ECs in inflamed vasculature (67) and now by LDF-activated atherogenic intima (Supplemental Fig. S5A). IL-1β blockade shows therapeutic promise in patients with previous myocardial infarction (53), but IL-36 has distinct signaling mechanisms (21), and our studies suggest that IL-36 is unique among the IL-1 family in that it is upregulated by atherogenic intima and is capable of activating innate-like inflammatory programs from plaque-resident CD8 T cells. These responses, which include IFNγ production, are elicited regardless of plaque TCR specificity and may further promote infiltration of other inflammatory cells into nascent atherogenic foci. IL-36 is additionally upregulated in several autoimmune diseases with increased cardiovascular morbidity (7, 28), suggesting that innate-like stimulation of intimal CD8 T cells may indeed have mechanistic contributions to the destabilizing progression of fatty streaks to pathological plaque.
In conclusion, we show that CD137 stimulation of effector CD8 T cells drives their infiltration specifically into regions of LDF, where they are capable of responding to cytokines within the intimal environment and exhibiting innate-like phenotypes that further promote the recruitment of other CD8 T cells into atherogenic foci. These antigen-independent responses may form the basis for yet unexplored but more direct mechanisms of pathologic communication with the well-studied cells of the innate immune system. They also suggest that the generation of CD8 effector T cells through a variety of systemic stimuli could drive acute seeding and long-lasting retention of these cells within the atherosclerotic plaque, one of the earliest immunological signatures of unstable plaque progression.
GRANTS
The work was supported in part by an American Heart Association Clinical Health Profession Grant to M. M. Xu (Association Wide 17CPRE33660241); the Boehringer Ingelheim Endowed Chair in Immunology, UConn Health School of Medicine (Farmington, CT), to A. T. Vella; National Institutes of Health grants to P. A. Murphy (R00-HL-125727) and A. Rodriguez (5R01HL131862-02); and the Linda and David Roth Chair of Cardiovascular Research to A. Rodriguez.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.X., P.A.M., and A.T.V. conceived and designed research; M.M.X., A.M., S.-A.E.N., and P.A.M. performed experiments; M.M.X. and P.A.M. analyzed data; M.M.X., S.G., E.J.S., B.Z., A.R., P.A.M., and A.T.V. interpreted results of experiments; M.M.X. prepared figures; M.M.X. drafted manuscript; M.M.X., A.M., S.G., E.J.S., B.Z., A.R., P.A.M., and A.T.V. edited and revised manuscript; M.M.X., A.M., S.-A.E.N., S.G., E.J.S., B.Z., A.R., P.A.M., and A.T.V. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the technical support of Brent Heineman (Center for Vascular Biology), who ran the cholesterol measurement assays. We also thank the Institute for Systems Genomics at UConn Health and JAX (Farmington, CT) for providing help with the CyTOF studies.
Present address of S. Günther: Deutsches Elektronen-Synchrotron DESY, Hamburg, Germany.
REFERENCES
- 1.Ainscough JS, Macleod T, McGonagle D, Brakefield R, Baron JM, Alase A, Wittmann M, Stacey M. Cathepsin S is the major activator of the psoriasis-associated proinflammatory cytokine IL-36γ. Proc Natl Acad Sci USA 114: E2748–E2757, 2017. doi: 10.1073/pnas.1620954114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts-Grill N, Rezvan A, Son DJ, Qiu H, Kim CW, Kemp ML, Weyand CM, Jo H. Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: an expanded flow cytometry method. Arterioscler Thromb Vasc Biol 32: 623–632, 2012. doi: 10.1161/ATVBAHA.111.242180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198: 1583–1593, 2003. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betjes MG, Litjens NH, Zietse R. Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant 22: 3298–3303, 2007. doi: 10.1093/ndt/gfm348. [DOI] [PubMed] [Google Scholar]
- 5.Beyer M, Wang H, Peters N, Doths S, Koerner-Rettberg C, Openshaw PJ, Schwarze J. The beta2 integrin CD11c distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Respir Res 6: 70, 2005. doi: 10.1186/1465-9921-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain 127: 844–850, 2004. doi: 10.1093/brain/awh104. [DOI] [PubMed] [Google Scholar]
- 7.Chu M, Wong CK, Cai Z, Dong J, Jiao D, Kam NW, Lam CW, Tam LS. Elevated Expression and Pro-Inflammatory Activity of IL-36 in Patients with Systemic Lupus Erythematosus. Molecules 20: 19588–19604, 2015. doi: 10.3390/molecules201019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, Zernecke A. CD8+ T Cells Regulate Monopoiesis and Circulating Ly6C-high Monocyte Levels in Atherosclerosis in Mice. Circ Res 117: 244–253, 2015. doi: 10.1161/CIRCRESAHA.117.304611. [DOI] [PubMed] [Google Scholar]
- 9.Combadière C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117: 1649–1657, 2008. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 10.de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B, Eckel RH. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation 130: 1110–1130, 2014. doi: 10.1161/CIR.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 11.Ehl S, Hombach J, Aichele P, Hengartner H, Zinkernagel RM. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J Exp Med 185: 1241–1251, 1997. doi: 10.1084/jem.185.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Côte R, Grover SA, Fortin PR, Clarke AE, Senécal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 44: 2331–2337, 2001. doi:. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson JR, Smith KE, Fleming VM, Rajoriya N, Newell EW, Simmons R, Marchi E, Björkander S, Kang YH, Swadling L, Kurioka A, Sahgal N, Lockstone H, Baban D, Freeman GJ, Sverremark-Ekström E, Davis MM, Davenport MP, Venturi V, Ussher JE, Willberg CB, Klenerman P. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Reports 9: 1075–1088, 2014. doi: 10.1016/j.celrep.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145: 33–43, 1999. doi: 10.1016/S0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 15.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med 203: 1273–1282, 2006. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu Rev Immunol 27: 165–197, 2009. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA 296: 1735–1741, 2006. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 18.Gimbrone MA Jr, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjurich BN, Taghavie-Moghadam PL, Galkina EV. Flow Cytometric Analysis of Immune Cells Within Murine Aorta. Methods Mol Biol 1339: 161–175, 2015. doi: 10.1007/978-1-4939-2929-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res 103: 1220–1231, 2008. doi: 10.1161/CIRCRESAHA.108.182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günther S, Sundberg EJ. Molecular determinants of agonist and antagonist signaling through the IL-36 receptor. J Immunol 193: 921–930, 2014. doi: 10.4049/jimmunol.1400538. [DOI] [PubMed] [Google Scholar]
- 22.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 12: 204–212, 2011. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135: 169–175, 1989. [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med 278: 483–493, 2015. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell 76: 17–27, 1994. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 26.Hsue PY, Hunt PW, Sinclair E, Bredt B, Franklin A, Killian M, Hoh R, Martin JN, McCune JM, Waters DD, Deeks SG. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS 20: 2275–2283, 2006. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 27.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation 121: 1124–1133, 2010. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 28.Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, Kahlenberg JM, Harms PW, Gudjonsson JE. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol 140: 109–120, 2017. doi: 10.1016/j.jaci.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6: 131–138, 1986. doi: 10.1161/01.ATV.6.2.131. [DOI] [PubMed] [Google Scholar]
- 30.Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, Kwon BS. 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol 238: 76–86, 2005. doi: 10.1016/j.cellimm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Kolbus D, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Björkbacka H, Nilsson J. Association between CD8+ T-cell subsets and cardiovascular disease. J Intern Med 274: 41–51, 2013. doi: 10.1111/joim.12038. [DOI] [PubMed] [Google Scholar]
- 32.Kolbus D, Ramos OH, Berg KE, Persson J, Wigren M, Björkbacka H, Fredrikson GN, Nilsson J. CD8+ T cell activation predominate early immune responses to hypercholesterolemia in Apoe−(/)− mice. BMC Immunol 11: 58, 2010. doi: 10.1186/1471-2172-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar S, Kang DW, Rezvan A, Jo H. Accelerated atherosclerosis development in C57Bl6 mice by overexpressing AAV-mediated PCSK9 and partial carotid ligation. Lab Invest 97: 935–945, 2017. doi: 10.1038/labinvest.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurioka A, Klenerman P, Willberg CB. Innate-like CD8+ T-cells and NK cells: converging functions and phenotypes. Immunology 154: 547–556, 2018. doi: 10.1111/imm.12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation 127: 1028–1039, 2013. doi: 10.1161/CIRCULATIONAHA.112.001347. [DOI] [PubMed] [Google Scholar]
- 36.Langstein J, Schwarz H. Identification of CD137 as a potent monocyte survival factor. J Leukoc Biol 65: 829–833, 1999. doi: 10.1002/jlb.65.6.829. [DOI] [PubMed] [Google Scholar]
- 37.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Yan J, Wu C, Wang Z, Yuan W, Wang D. CD137-CD137L interaction regulates atherosclerosis via cyclophilin A in apolipoprotein E-deficient mice. PLoS One 9: e88563, 2014. doi: 10.1371/journal.pone.0088563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med 14: 385–390, 2013. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch DH. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol Rev 222: 277–286, 2008. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 41.Ménoret A, Buturla JA, Xu MM, Svedova J, Kumar S, Rathinam VA, Vella AT. T cell-directed IL-17 production by lung granular γδ T cells is coordinated by a novel IL-2 and IL-1β circuit. Mucosal Immunol 11: 1398–1407, 2018. doi: 10.1038/s41385-018-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michel J, Schwarz H. Expression of soluble CD137 correlates with activation-induced cell death of lymphocytes. Cytokine 12: 742–746, 2000. doi: 10.1006/cyto.1999.0623. [DOI] [PubMed] [Google Scholar]
- 43.Murphy PA, Butty VL, Boutz PL, Begum S, Kimble AL, Sharp PA, Burge CB, Hynes RO. Alternative RNA splicing in the endothelium mediated in part by Rbfox2 regulates the arterial response to low flow. eLife 7: e29494, 2018. doi: 10.7554/eLife.29494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy PA, Hynes RO. Alternative splicing of endothelial fibronectin is induced by disturbed hemodynamics and protects against hemorrhage of the vessel wall. Arterioscler Thromb Vasc Biol 34: 2042–2050, 2014. doi: 10.1161/ATVBAHA.114.303879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison DG, Giddens DP, Jo H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J Vis Exp (40): 1861, 2010. doi: 10.3791/1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng CT, Fong LY, Sulaiman MR, Moklas MA, Yong YK, Hakim MN, Ahmad Z. Interferon-Gamma Increases Endothelial Permeability by Causing Activation of p38 MAP Kinase and Actin Cytoskeleton Alteration. J Interferon Cytokine Res 35: 513–522, 2015. doi: 10.1089/jir.2014.0188. [DOI] [PubMed] [Google Scholar]
- 47.Oksenberg JR, Stavri GT, Jeong MC, Garovoy N, Salisbury JR, Erusalimsky JD. Analysis of the T-cell receptor repertoire in human atherosclerosis. Cardiovasc Res 36: 256–267, 1997. doi: 10.1016/S0008-6363(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 48.Olofsson PS, Söderström LA, Wågsäter D, Sheikine Y, Ocaya P, Lang F, Rabu C, Chen L, Rudling M, Aukrust P, Hedin U, Paulsson-Berne G, Sirsjö A, Hansson GK. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 117: 1292–1301, 2008. doi: 10.1161/CIRCULATIONAHA.107.699173. [DOI] [PubMed] [Google Scholar]
- 49.Papa A, Danese S, Urgesi R, Grillo A, Guglielmo S, Roberto I, Bonizzi M, Guidi L, De Vitis I, Santoliquido A, Fedeli G, Gasbarrini G, Gasbarrini A. Early atherosclerosis in patients with inflammatory bowel disease. Eur Rev Med Pharmacol Sci 10: 7–11, 2006. [PubMed] [Google Scholar]
- 50.Qualai J, Li LX, Cantero J, Tarrats A, Fernández MA, Sumoy L, Rodolosse A, McSorley SJ, Genescà M. Expression of CD11c Is Associated with Unconventional Activated T Cell Subsets with High Migratory Potential. PLoS One 11: e0154253, 2016. doi: 10.1371/journal.pone.0154253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranjbaran H, Sokol SI, Gallo A, Eid RE, Iakimov AO, D’Alessio A, Kapoor JR, Akhtar S, Howes CJ, Aslan M, Pfau S, Pober JS, Tellides G. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J Immunol 178: 592–604, 2007. doi: 10.4049/jimmunol.178.1.592. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators . C-reactive protein levels and outcomes after statin therapy. N Engl J Med 352: 20–28, 2005. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 54.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol 26: 2421–2432, 2006. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 55.Roche-Molina M, Sanz-Rosa D, Cruz FM, García-Prieto J, López S, Abia R, Muriana FJ, Fuster V, Ibáñez B, Bernal JA. Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler Thromb Vasc Biol 35: 50–59, 2015. doi: 10.1161/ATVBAHA.114.303617. [DOI] [PubMed] [Google Scholar]
- 56.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med 340: 115–126, 1999. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 57.Sharief MK. Heightened intrathecal release of soluble CD137 in patients with multiple sclerosis. Eur J Neurol 9: 49–54, 2002. doi: 10.1046/j.1468-1331.2002.00323.x. [DOI] [PubMed] [Google Scholar]
- 58.Sherer Y, Shoenfeld Y. Mechanisms of disease: atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol 2: 99–106, 2006. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 59.Söderström LA, Gertow K, Folkersen L, Sabater-Lleal M, Sundman E, Sheikine Y, Goel A, Baldassarre D, Humphries SE, de Faire U, Watkins H, Tremoli E, Veglia F, Hamsten A, Hansson GK, Olofsson PS. Human genetic evidence for involvement of CD137 in atherosclerosis. Mol Med 20: 456–465, 2014. doi: 10.2119/molmed.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Söderström LA, Jin H, Caravaca AS, Klement ML, Li Y, Gisterå A, Hedin U, Maegdefessel L, Hansson GK, Olofsson PS. Increased Carotid Artery Lesion Inflammation Upon Treatment With the CD137 Agonistic Antibody 2A. Circ J 81: 1945–1952, 2017. doi: 10.1253/circj.CJ-17-0230. [DOI] [PubMed] [Google Scholar]
- 61.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol 25: 615–622, 2013. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsurutani N, Mittal P, St Rose MC, Ngoi SM, Svedova J, Menoret A, Treadway FB, Laubenbacher R, Suárez-Ramírez JE, Cauley LS, Adler AJ, Vella AT. Costimulation Endows Immunotherapeutic CD8 T Cells with IL-36 Responsiveness during Aerobic Glycolysis. J Immunol 196: 124–134, 2016. doi: 10.4049/jimmunol.1501217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Berg PJ, van Stijn A, Ten Berge IJ, van Lier RA. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J Clin Virol 41: 213–217, 2008. doi: 10.1016/j.jcv.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 64.van Dijk RA, Duinisveld AJ, Schaapherder AF, Mulder-Stapel A, Hamming JF, Kuiper J, de Boer OJ, van der Wal AC, Kolodgie FD, Virmani R, Lindeman JH. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc 4: e001403, 2015. doi: 10.1161/JAHA.114.001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinay DS, Kim CH, Choi BK, Kwon BS. Origins and functional basis of regulatory CD11c+CD8+ T cells. Eur J Immunol 39: 1552–1563, 2009. doi: 10.1002/eji.200839057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vozenilek AE, Blackburn CM, Schilke RM, Chandran S, Castore R, Klein RL, Woolard MD. AAV8-mediated overexpression of mPCSK9 in liver differs between male and female mice. Atherosclerosis 278: 66–72, 2018. doi: 10.1016/j.atherosclerosis.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinstein AM, Giraldo NA, Petitprez F, Julie C, Lacroix L, Peschaud F, Emile JF, Marisa L, Fridman WH, Storkus WJ, Sautes-Fridman C. Association of IL-36gamma with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol Immunother 68: 109–120, 2019. doi: 10.1007/s00262-018-2259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J Immunol 168: 4897–4906, 2002. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 69.Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, Tamada K, Mittler RS, Tsuchiya H, Pardoll DM, Chen L. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol 168: 4262–4267, 2002. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 70.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol 169: 4230–4236, 2002. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 71.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, Zernecke A, Pramod AB, Ghosh AK, Anto Michel N, Hoppe N, Hilgendorf I, Zirlik A, Hedrick CC, Ley K, Wolf D. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res 122: 1675–1688, 2018. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, Greenberg PD. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110: 201–210, 2007. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan J, Gong J, Liu P, Wang C, Chen G. Positive correlation between CD137 expression and complex stenosis morphology in patients with acute coronary syndromes. Clin Chim Acta 412: 993–998, 2011. doi: 10.1016/j.cca.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 74.Yan J, Wang C, Chen R, Yang H. Clinical implications of elevated serum soluble CD137 levels in patients with acute coronary syndrome. Clinics (São Paulo) 68: 193–198, 2013. doi: 10.6061/clinics/2013(02)OA12. [DOI] [PMC free article] [PubMed] [Google Scholar]