Abstract
Activation of the brain renin-angiotensin system (RAS) is a pivotal step in the pathogenesis of hypertension. The paraventricular nucleus (PVN) of the hypothalamus is a critical part of the angiotensinergic sympatho-excitatory neuronal network involved in neural control of blood pressure and hypertension. However, the importance of the PVN (pro)renin receptor (PVN-PRR)—a key component of the brain RAS—in hypertension development has not been examined. In this study, we investigated the involvement and mechanisms of the PVN-PRR in DOCA-salt-induced hypertension, a mouse model of hypertension. Using nanoinjection of adeno-associated virus-mediated Cre recombinase expression to knock down the PRR specifically in the PVN, we report here that PVN-PRR knockdown attenuated the enhanced blood pressure and sympathetic tone associated with hypertension. Mechanistically, we found that PVN-PRR knockdown was associated with reduced activation of ERK (extracellular signal-regulated kinase)-1/2 in the PVN and rostral ventrolateral medulla during hypertension. In addition, using the genetically encoded Ca2+ biosensor GCaMP6 to monitor Ca2+-signaling events in the neurons of PVN brain slices, we identified a reduction in angiotensin II type 1 receptor-mediated Ca2+ activity as part of the mechanism by which PVN-PRR knockdown attenuates hypertension. Our study demonstrates an essential role of the PRR in PVN neurons in hypertension through regulation of ERK1/2 activation and angiotensin II type 1 receptor-mediated Ca2+ activity.
NEW & NOTEWORTHY PRR knockdown in PVN neurons attenuates the development of DOCA-salt hypertension and autonomic dysfunction through a decrease in ERK1/2 activation in the PVN and RVLM during hypertension. In addition, PRR knockdown reduced AT1aR expression and AT1R-mediated calcium activity during hypertension. Furthermore, we characterized the neuronal targeting specificity of AAV serotype 2 in the mouse PVN and validated the advantages of the genetically encoded calcium biosensor GCaMP6 in visualizing neuronal calcium activity in the PVN.
Keywords: calcium, central nervous system, GCaMP6, hypertension, paraventricular nucleus of the hypothalamus, (pro)renin receptor, renin-angiotensin system
INTRODUCTION
Hypertension is one of the most important risk factors for cardiovascular diseases, including stroke, myocardial infarction, congestive heart failure, and chronic kidney disease, and remains the number one cause of morbidity and mortality in the United States (91). The mechanisms leading to hypertension are complex and only partially understood (37, 66, 68) but integrated neural, humoral, and renal mechanisms that collectively mediate increased sympathetic nervous system activity and renal sodium retention have been implicated in its pathogenesis (4, 49, 66). An increase in brain renin-angiotensin system (RAS) activity in cardiovascular regulatory nuclei is a pivotal step in the pathogenesis of hypertension (65, 89). Angiotensinergic sympathoexcitatory pathways from neurons in circumventricular organs in the forebrain project to the paraventricular nucleus (PVN) in the hypothalamus. From the PVN, these pathways project to the rostral ventrolateral medulla (RVLM) in the brain stem or directly to the intermediolateral cell column in the spinal cord (10, 50, 84). The PVN acts as a critical networking center of this angiotensinergic neural circuit in the central regulation of blood pressure (BP).
The (pro)renin receptor (PRR) is a key component of the RAS (58). In vitro, binding of renin or prorenin to the PRR promotes formation of angiotensin II (ANG II), the major bioactive peptide in the RAS (19, 28, 57, 70). In the central nervous system (CNS), ANG II acts through the angiotensin II type 1 receptor (AT1R) to regulate neuronal excitability, sympathetic activity, and BP. Activation of the PRR also directly mediates downstream signaling via MAPK/ERK, phosphoinositide 3-kinase/AKT, Wnt, and other pathways independent of ANG II (6, 7, 61, 85, 90). The PRR is expressed in multiple organs throughout the body, including the brain, heart, lung, pancreas, and placenta (58). We and others have previously reported that the PRR is present in brain regions involved in the central regulation of BP (52, 71), and its expression is elevated in BP regulatory brain regions, including the PVN, in the DOCA-salt hypertensive model (53) and spontaneously hypertensive animals (71). Moreover, polymorphisms of the PRR gene are associated with increased systolic BP in humans (36, 59), highlighting the clinical significance of the PRR in hypertension. However, the importance of the PVN-PRR and the specific mechanisms involved in hypertension are not fully understood. In this study, we tested the hypothesis that the PVN-PRR plays a critical role in hypertension development and explored potential underlying mechanisms.
MATERIALS AND METHODS
Animals
The PRR-floxed mouse used in these studies was generated by Dr. Atsuhiro Ichihara’s laboratory (42). A colony of these mice has been established and maintained in the Feng laboratory, as described previously (53). Wild-type (WT) C57Bl/6J and CX3CR1-GFP reporter (B6.129P-Cx3cr1tm1litt/J) mice used as breeders were purchased from Jackson Laboratory and were maintained in the animal facility at the University of Nevada, Reno. Both male and female mice, age 8–10 wk, were used. All procedures were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee, and the Institutional Biosafety Committee at the University of Nevada, Reno. The use of adeno-associated virus (AAV) was approved by the Institutional Biosafety Committee at the University of Nevada, Reno.
Telemetry Study
Mice were anesthetized using 4–5% isoflurane in 100% O2, flushed at 1 l/min for 2 min. Anesthesia was then maintained using 0.75–1.5% isoflurane. After the neck was shaved and sterilized with alcohol swabs, an incision (~1 cm) was made to separate the oblique and tracheal muscles and expose the left carotid artery. The catheter of a radio telemetry transmitter (PA-C10; Data Science International, St. Paul, MN) was surgically implanted into the left carotid artery and secured. The body of the radio transmitter was embedded in a subcutaneous pocket under the right arm. Mice were allowed to recover for 14 days, and then baseline BP, heart rate (HR), and locomotor activity were recorded for 48 h as described previously (51, 52). Site-specific knockdown of the PRR in the PVN in mice was achieved by bilateral injection of AAV serotype 2 expressing Cre recombinase and enhanced green fluorescent protein (AAV2-Cre-eGFP) into the PVN; control animals were injected with AAV2 expressing eGFP only (AAV2-eGFP). Mice were allowed 3 days to recover and then were implanted with a 50-mg DOCA or sham pellet as previously described (53). Mice were returned to their cages and provided ad libitum access to standard chow and 0.9% saline or water. BP and HR were recorded daily. On days 20 and 21 following DOCA implantation, autonomic functions were assessed using a standard pharmacological method involving randomized intraperitoneal injections of a beta-blocker (propranolol, 6 mg/kg), a muscarinic receptor blocker (methylatropine, 1 mg/kg), and a ganglionic blocker (chlorisondamine, 6 mg/kg) (24). Changes in HR in response to injections of propranolol and methylatropine, and changes in BP following chlorisondamine injection, were analyzed (54).
Bilateral AAV Injections into the PVN
Mice were anesthetized as described above, and the tops of their heads were shaved and sterilized with alcohol wipes. Mice were placed in a digital stereotaxic apparatus (Stoelting, Wood Dale, IL) and held in place by ear bars secured just above the ear canal. An incision (~1 cm) in the skin along the top of the head was made to expose the skull. The skull was cleaned with 3% hydrogen peroxide using cotton swabs, after which holes were drilled into the skull at the stereotaxic coordinates, 0.6 mm posterior to bregma and 0.4 mm to either side of the midline. An outer cannula was lowered 5.1 mm from the surface of the brain, and an inner injector was placed in the cannula. The outer cannula was made from a blunted 25-gauge needle, 30 mm in length, and the inner injector was made from 32-gauge tubing (Microgroup, Medway, MA), 30.1 mm in length. Mice received AAV injections bilaterally into both paraventricular nuclei, as illustrated in Fig. 2A. The injector was secured to a 10-μl Hamilton syringe (Hamilton, Reno, NV) using polyethylene (PE)-50 tubing, and the system was charged with artificial cerebrospinal fluid (aCSF; 127 mM NaCl, 1.6 mM KCl, 1.24 mM KH2PO4, 1.3 mM MgSO4, 2.4 mM CaCl2, 26 mM NaHCO3, 10 mM glucose). Mice then received a 100 nl (1 × 107 viral genomes/nl) injection of AAV, after which the injector and cannula were left in place for an additional 5 min before removing to prevent leakage of injectate. The wound was sutured, and the mice were allowed to recover. PRR-floxed mice were injected with either AAV2-Cre-GFP (hereafter AAV2-Cre) to knock down the PRR or with AAV2-eGFP (hereafter AAV2-Con) as a control. The cell type transduced was characterized by monitoring eGFP fluorescence. AAV2-eGFP and AAV2-Cre were purchased from the Viral Vector Core Facility at the University of Iowa. All AAV2s used in our study employed a CAG promoter.
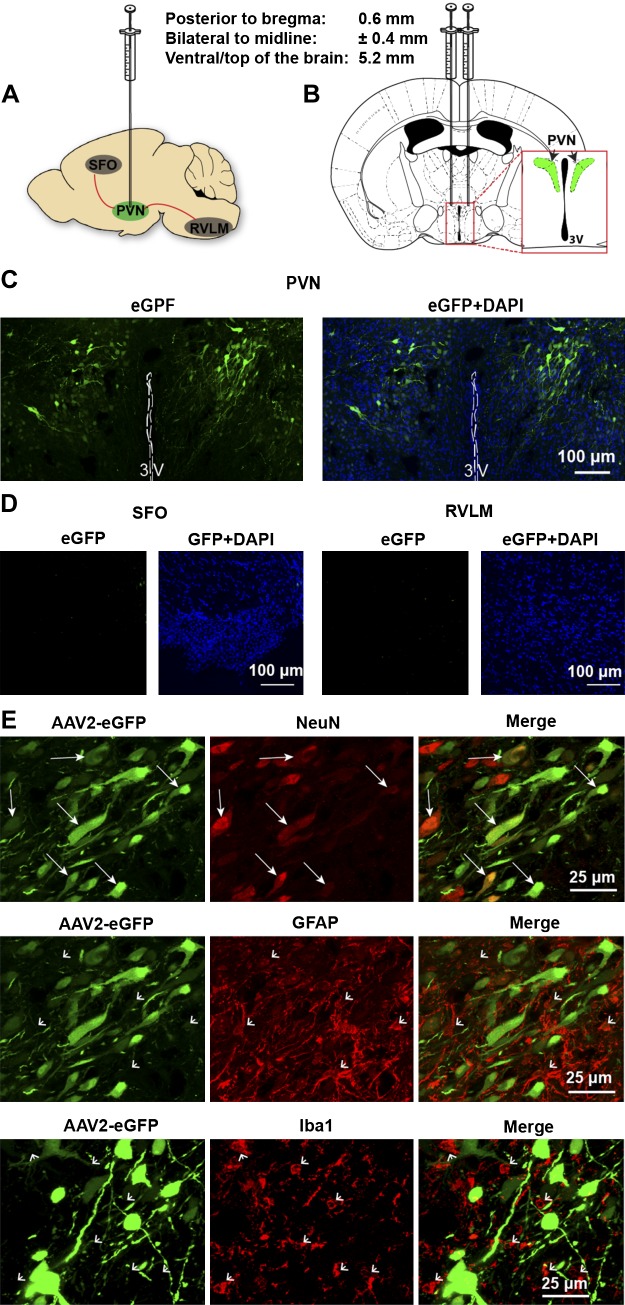
Fig. 2.
Characterization of AAV2 targeting in the PVN of the hypothalamus. AAV2-eGFP was bilaterally injected into the PVN of PRR-floxed mice; 7 days later, mouse brains were collected for imaging. A and B: schematic showing the bilateral PVN injection site and stereotaxic injection coordinates. C: representative images of eGFP fluorescence following bilateral injection of AAV2-eGFP into the PVN. D: representative images showing the specificity of PVN injection in slices from the SFO and RVLM. E: representative images of immunolabeling for the neuronal cell marker NeuN (red), astrocyte marker GFAP (red), and microglial marker IBA1 (red), and their colocalization with eGFP (green). White arrows indicate colocalization of eGFP with NeuN, and arrowheads indicate no colocalization of eGFP with either GFAP or Iba1. 3V, third ventricle; AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; GFAP, glial fibrillary acidic protein; PRR, (pro)renin receptor; PVN, paraventricular nucleus; RVLM, rostral ventrolateral medulla; SFO, subfornical organ.
Immunofluorescence Labeling
For immunofluorescence labeling, mice were anesthetized with isoflurane and transcardially perfused first with ~50 ml of ice-cold phosphate-buffered saline (PBS) and then with ~50 ml of ice-cold 4% paraformaldehyde. Brains were removed, postfixed in 4% paraformaldehyde at 4°C for 2 h, and cryoprotected by incubating in 30% sucrose in PBS for 24 h at 4°C. Brains were then frozen in tissue freezing medium (General Data, Cincinnati, OH) at −20°C, sectioned (30 µm), and floated in PBS in a 24-well plate. Sections were washed twice in PBS and blocked by incubating in PBS containing 0.3% Triton X-100 and 10% normal house serum (NHS) for 1 h at room temperature. Sections in PBS containing 0.3% Triton X-100 and 3% NHS were incubated overnight at 4°C with one or more of the following primary antibodies, as indicated in the text: guinea pig anti-NeuN (1:1000, cat. no. ABN-90; Millipore, Burlington MA), targeting the neuronal marker NeuN; goat anti-IBA-1 (1:200, cat. no. NB100-1028; Novus, Littleton, CO), targeting the microglial marker ionized calcium binding adapter molecule 1 (IBA-1); chicken anti-glial fibrillary acidic protein (GFAP) (1:200, cat. no. ab-4674; Abcam, Cambridge, MA), targeting the astrocyte marker GFAP; and rabbit anti-PRR (1:200, cat. no. ab-40790; Abcam, Cambridge, MA). Sections were washed four times in PBS and incubated for 2 h at room temperature with Alexa 647-conjugated donkey anti-guinea pig (1:1,000; Jackson ImmunoResearch, West Grove, PA), Alexa 594-conjugated rabbit anti-goat (1:1,000; Invitrogen, Carlsbad, CA), Alexa 488-conjugated goat anti-chicken (1:1,000; cat. no. A-11039; Thermo-Scientific, Waltham, MA), Alexa 594-conjugated donkey anti-rabbit (1:1,000; cat. no. A-21207; Thermo-Scientific), or Alexa 647-conjugated donkey anti-guinea pig (1:500, cat. no. 706-605-148; Jackson ImmunoResearch, Carlsbad, CA) secondary antibody, diluted in PBS containing 0.3% Triton X-100 and 3% NHS. Sections were then incubated in PBS containing 1 µg/ml DAPI for 5 min and washed four times in PBS. The specificity of each primary antibody was confirmed using controls lacking primary antibody and imaging with the exact same settings. The specificity of the PRR antibody was further confirmed by Western blot analysis of Neuro-2A cells expressing our previously characterized, AAV2-delivered mouse PRR shRNA (52) using a Wes automated Western blot system (ProteinSimple, San Jose, CA). All sections were mounted on Colorfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and coverslip-mounted with Vectashield hard-set mounting medium (Vector Laboratories, Burlingame, CA). Sections were stored at 4°C and imaged on an SP8 confocal microscope (Leica Biosystems, Wetzlar, Germany).
The efficiency of PRR knockdown in the PVN by AAV2-Cre was assessed by quantifying PRR labeling in images imported into ImageJ (NIH). Regions of interests (ROIs) generated using the GFP channel were used to analyze average PRR fluorescence intensity in the red channels for both AAV2-GFP (AAV-Con) and AAV2-Cre-GFP (AAV-Cre) groups. The background fluorescence of each image was subtracted from the average intensity of that image during the analysis. The average fluorescence intensity was normalized to that in the AAV-Con group and presented as fold change.
p-ERK1/2 immunofluorescence labeling and quantification.
PRR-floxed mice were bilaterally injected in the PVN with either AAV2-Cre or AAV2-Con virus and allowed to recover for 3 days. A separate set of WT mice was chronically infused with either losartan (3 mg·kg−1·day −1) or aCSF via an intracerebroventricular (ICV) cannula, as described (54). Three days after surgery, mice were implanted with a 50-mg DOCA or sham pellet and given access to 0.9% saline or water. After 14 days of treatment, mice were perfused and fixed as described above. Sections (30 µm) were blocked by incubating in PBS containing 0.3% Triton X-100 and 10% NHS, and then incubated overnight with rabbit anti-Thr-202/Tyr-204-phosphorylated (activated) ERK1/2 (p-ERK1/2) (1:500, 4376s; Cell Signaling, Beverly, MA) in PBS containing 0.3% Triton X-100 and 3% NHS. Sections were washed four times with PBS and incubated with Alexa 594-conjugated donkey anti-rabbit secondary antibody (1:1,000; Thermo-Scientific, Waltham, MA) for 2 h at room temperature. Sections were then counterstained with DAPI and mounted as described above. p-ERK1/2Thr202/Tyr204 staining in Fig. 5 was imaged on an SP8 confocal microscope (Leica Biosystems, Wetzlar, Germany). During image acquisition, gain and offset were set at 873.3 and −0.19, respectively, for all subfornical organs (SFOs), and 831.1 and −0.19, respectively, for all PVNs and RVLMs.
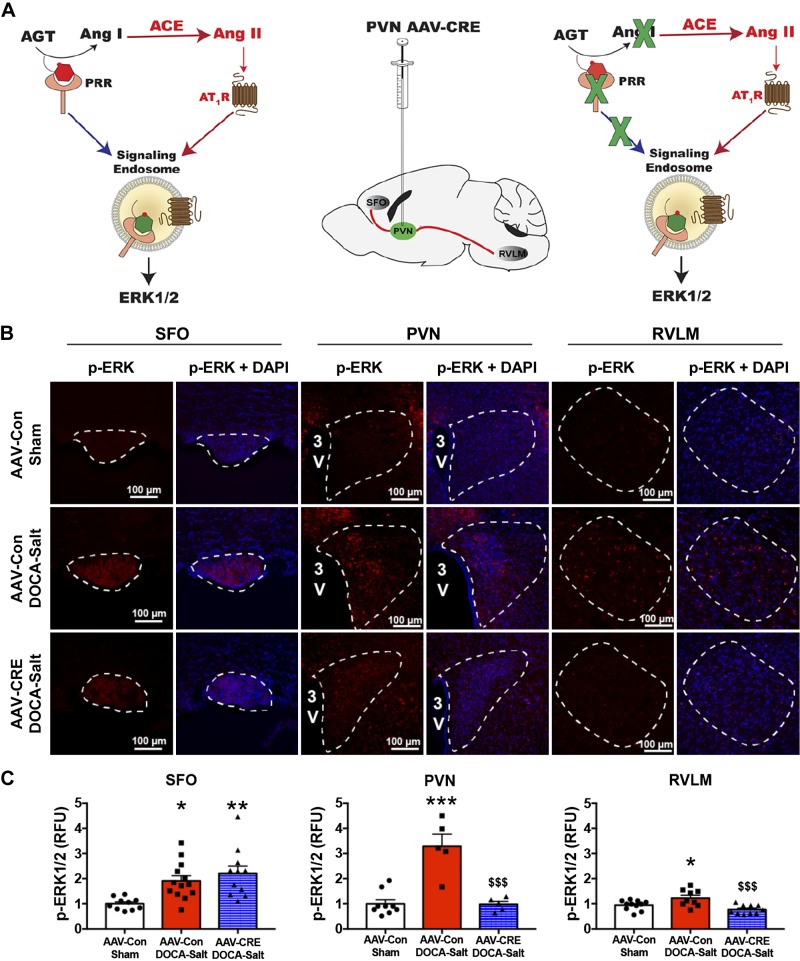
Fig. 5.
PVN-targeted knockdown of the PRR reduces ERK1/2 activation in the PVN and RVLM. Schematics illustrating the activation of ERK1/2 by the PRR and effect of PVN-PRR knockdown on ERK1/2 activation (A). Representative images (B) and summary data (C) quantifying p-ERK1/2 immunostaining intensity in the SFO, PVN, and RVLM of AAV-Con sham, AAV-Con DOCA-Salt, and AAV-Cre DOCA-Salt groups following 2 wk of DOCA-salt or sham treatment. *P < 0.05 vs. AAV-Con Sham; **P < 0.01 vs. AAV-Con sham; ***P < 0.001 vs. AAV-Con Sham; $$$P < 0.001 vs. AAV-Con DOCA-Salt (n = 2–5 brain sections from each mouse, 3–5 mice/group). AAV, adeno-associated virus; PRR, (pro)renin receptor; PVN, paraventricular nucleus; RFU, relative fluorescence units; RVLM, rostral ventrolateral medulla; SFO, subfornical organ.
p-ERK1/2Thr202/Tyr204 staining in both groups shown in Fig. 6 was imaged on a Keyence BZ-X700 microscope with a ×20 objective at the same time using the same setting [numerical aperture (NA) = 0.75]. p-ERK1/2 labeling was quantified by importing images into ImageJ (NIH) and identifying the SFO, PVN, and RVLM using the DAPI channel.
Fig. 6.
Effects of ICV-infused losartan on ERK1/2 activation in the SFO, PVN, and RVLM. Schematics illustrating activation of ERK1/2 by the PRR and the pathways by which chronically ICV-infused losartan inhibits ERK1/2 activation (A). Representative images (B) and summary data (C) quantifying p-ERK1/2 immunostaining in the SFO, PVN, and RVLM following 2 wk of chronic ICV infusion of aCSF or losartan (3 mg·kg−1·day−1) (n = 3–5 brain sections per mouse, 3 mice/group). Data normalized and replotted from Fig. 5C (dotted gray squares) and Fig. 6C (D). *P < 0.05 vs. ICV aCSF; **P < 0.01 vs. ICV aCSF; ****P < 0.001 vs. ICV aCSF. $$$P < 0.001 vs. AAV-Con DOCA-Salt; ##P < 0.01 vs. ICV aCSF; ###P < 0.001 vs. PVN AAV-Cre DOCA-Salt. aCSF, artificial cerebrospinal fluid; ICV, intracerebroventricular; PRR, (pro)renin receptor; PVN, paraventricular nucleus; RFU, relative fluorescence units; RVLM, rostral ventrolateral medulla; SFO, subfornical organ.
ROIs defined using the DAPI channel were overlaid on the red channel (p-ERK), and the fluorescence intensity of the red channel in the ROI was measured. The fluorescence intensity of each ROI was normalized to the area to obtain the average fluorescence intensity of each brain region. The average fluorescence intensity was normalized to the same brain region in sham controls and presented as fold change.
Quantitative Reverse-Transcription Polymerase Chain Reaction
PRR-floxed mice were bilaterally injected with either AAV2-Cre or AAV2-Con, as described above, and allowed to recover for 3 days (to assess early onset of PRR mRNA reduction) or 24 days (to assess whether PRR mRNA levels remained low at the end of the 21-day treatment protocol). Mice were euthanized by cervical dislocation, and their brains were rapidly removed and flash-frozen. Total RNA from tissue micropunches of the PVN, cortex, and brain stem was isolated with TRIzol reagent (Thermo Fisher, Waltham, MA) following the manufacturer’s protocols. Briefly, tissue was homogenized in 500 µl of TRIzol using a Tissuemizer (Tekmar, Vernon, Canada) and incubated for 5 min at room temperature. Samples were centrifuged at 10,000 g for 5 min, and the supernatant was transferred to a fresh tube. Chloroform (100 µl) was added to each sample, after which samples were vortexed for 15 s and incubated at room temperaturefor 5 min. Samples were centrifuged at 12,000 g for 15 min, and the clear aqueous phase was transferred to a fresh tube. RNA was precipitated by adding 250 µl of isopropyl alcohol, and the samples were incubated for 10 min at room temperature. Samples were centrifuged at 12,000 g for 10 min and the supernatant was discarded. The RNA pellet was washed twice by briefly vortexing in 500 µl of 75% ethanol and centrifuging at 7,500 g for 5 min. Ethanol was removed and the pellet was allowed to air dry for 10 min at room temperature. RNA was dissolved in 30 µl of DNAse/RNAse-free H2O (Invitrogen, Carlsbad, CA), and its concentration and purity were determined using a NanoDrop spectrophotometer (Thermo Fisher). Samples were stored at −80°C. cDNA was reverse transcribed using an Applied Biosystems high-capacity cDNA reverse transcription kit (Thermo Fisher), and quantitative PCR was performed using Fast SYBR Green master mix (Thermo Fisher). mRNA levels of the (pro)renin receptor (PRR, ATP6AP2 gene) and the angiotensin receptors, ANG II receptor subtype 1a (AT1aR, AGTR1 gene), ANG II receptor subtype 2 (AT2R, AGTR2 gene), and proto-oncogene Mas (MasR, MAS1 gene), normalized to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH gene), were determined using the ∆∆CT relative quantification method. The following primer pairs were used: mouse ATP6AP2, 5′-TCT CTC CGA ACT GCA AGT GCT ACA-3′ (forward) and 5′-CCA AAC CTG CCA GCT CCA ATG AAT-3′ (reverse); mouse AGTR1, 5′-TCA CCA GAT CAA GTG CAT TTT GA-3′ (forward) and 5′-AGA GTT AAG GGC CAT TTT GCT TT-3′ (reverse); mouse AGTR2, 5′-CAG CAG CCG TCC TTT TGA TAA-3′ (forward) and 5′-TTA TCT GAT GGT TTG TGT GAG CAA-3′ (reverse); mouse MAS1, 5′-CGG TCT ACA TTA CCC ACT TGT C-3′ (forward) and 5′-GAT GGC CAG AAG AGA GTT CAT AG-3′ (reverse); and mouse GAPDH, 5′-AAT GTG TCC GTC GTG GAT CTG A-3′ (forward) and 5′-GAT GCC TGC TTC ACC ACC TTC T-3′ (reverse).
Live-Cell Ca2+ Imaging in PVN Brain Slices
AAV2/9-delivered GCaMP6f (Penn Vector Core, University of Pennsylvania) was used as a genetically encoded Ca2+ biosensor to evaluate Ca2+-signaling activity in the PVN. Because GCaMP6f has the same excitation and emission wavelength as eGFP, in this experiment, we used an AAV2-Cre virus without the eGFP reporter to knock down the PRR in PRR-floxed mice, and AAV2-mCherry (Penn Vector Core, University of Pennsylvania) as the control virus (AAV-Con).
PRR-floxed mice were bilaterally injected into the PVN with either AAV2/9-GCaMP6 + AAV2-Cre or AAV2/9-GCaMP6 + AAV2-mCherry (50 nl each virus, 2 × 107 viral genomes/nl), as described above, and allowed to recover for 3 days. Mice were then implanted with a 50-mg DOCA or sham pellet and given access to 0.9% saline or water for 14 days. At 14 days, mice were euthanized by cervical dislocation, and their brains were removed and placed in ice-cold slicing buffer (125 mM NaCl, 2.5 mM KCl, 25 mM NaHCO3, 1.25 mM NaH2PO4, 3.0 mM 3-myo-inositol, 2.0 mM Na-pyruvate, 0.4 mM ascorbic acid, 0.1 mM CaCl2, 3.0 mM MgCl2, 25 mM glucose). The pia matter was removed, and the hind brain was cut away. The brain was fixed to the cutting stage and submerged in ice-cold slicing buffer. Sections (175-µm thick) through the PVN were cut with a Leica VT1200s vibratome at 0.10 mm/s with 0.1-mm lateral movement of the blade. PVN sections were then transferred to a chamber containing gassed 37°C recording buffer (125 mM NaCl, 2.5 mM KCl, 25.0 mM glucose, 25.0 mM NaHCO3, 1.25 mM NaH2PO4, 3.0 mM 3-myo-inositol, 2.0 mM Na-pyruvate, 0.4 mM ascorbic acid, 1.0 mM MgCl2, 2.0 mM CaCl2) for 30 min. Sections were placed on a 35-mm glass-bottom dish (MatTek, Ashland, MA) containing recording buffer, and Ca2+ events were imaged with an Olympus IX81 microscope (Olympus, Burlingame, CA) equipped with a ×20 objective (NA = 0.45) and an iXon Ultra camera (Andor, Belfast, UK), and analyzed using Andor IQ5 software. A 512 × 512 pixel field of view (413 µm × 413 µm) of the PVN along the third ventricle was imaged, and Ca2+ events were recorded at a rate of 10 frames/s. A baseline was recorded for 45 s, after which ANG II (100 nM) was added, and events were recorded for an additional 75 s. KCl (100 mM) was added to induce a maximal Ca2+ response in neurons. The role of AT1R activation in mediating Ca2+ events was confirmed by preincubating PVN sections for 30 min in gassed recording buffer containing losartan (1 µM) before and during incubation with ANG II and KCl. Images were analyzed using ImageJ (NIH).
For data analysis, the frequency of Ca2+ events was measured as events per second, and amplitude was determined as F – F0, expressed as a percentage of the maximal response to KCl (F – F0/Fm – F0m) as described (63, 64, 75). Briefly, F0 was taken as the least intense, uniform basal fluorescence intensity value obtained during the baseline period (i.e., between spontaneous Ca2+ events in the absence of KCl or ANG II). In cases where baseline recordings fluctuated slightly before application of KCl or ANG II, minimum F0 (F0m) was taken as the least intense and uniform basal fluorescence intensity value immediately before KCl or ANG II application. Ca2+ events were defined using a threshold intensity criterion. Dynamic Ca2+ events with amplitudes greater than 1% of the maximal response were considered valid Ca2+ events and were included in the frequency analysis. Ca2+ signaling sites, defined as regions in which dynamic changes in Ca2+ were elicited, were quantified as the number of sites per field of view (FOV) during the recording period.
Three-dimensional (3-D) reconstructions of Ca2+-imaging files were created using ImageJ (NIH, version 1.5a https://imagej.nih.gov/ij) (2, 25, 26). Initially, Ca2+-imaging files were converted to 32-bit TIFF files to increase image quality. A color-coded look-up table (QUBPalette.lut, Queens University, Belfast, UK) was then imported into the movie file to display intense regions of Ca2+ fluorescence as warm colors (red, orange) and regions of low Ca2+ fluorescence as cold colors (black, blue). Stacked 3-D reconstructions of the movies were then created using the “Stack 3D Surface Plot” plugin, and representative frames were selected for display using the “Interactive 3D Surface Plot” plugin.
Statistical Analysis
Data are presented as means ± SE and were analyzed using Students t-test, one-way (one variant) or two-way (two variants) analysis of variance (ANOVA) for multiple groups, followed by a Bonferroni post hoc test to compare replicate means, as appropriate. Outliers were identified using ROUT and/or Grubbs’ methods, and statistical comparisons were performed using Prism7 software (GraphPad, La Jolla, CA); a P value ≤ 0.05 was considered statistically significant.
RESULTS
Cellular Localization of PRR Expression in the PVN of the Hypothalamus in the Mouse Brain
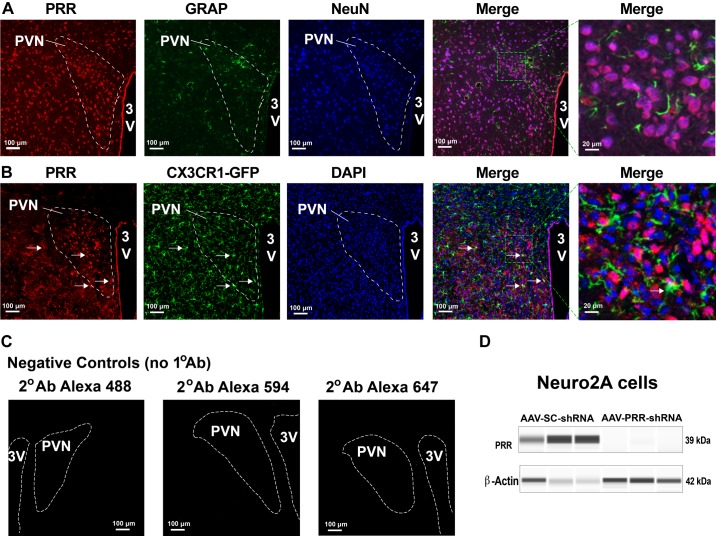
We previously reported that the PRR is widely expressed in neurons located in many cardiovascular regulatory neurons, including the PVN (85). In the current study, we further characterized PRR expression in different cell types in this brain region. Using immunofluorescence labeling, we found that the PRR is mainly expressed in neurons but not astrocytes in the mouse PVN (Fig. 1A). To determine the presence of the PRR in microglial cells in the PVN, we performed immunolabeling for the PRR in microglia reporter mice (CX3CR1-GFP). We found that very few CX3CR1-GFP-expressing cells were positive for PRR immunostaining (Fig. 1B, white arrows). Negative controls showed no fluorescence signals with any of the three secondary antibodies used in these experiments (Fig. 1C), and the specificity of the primary antibody was further confirmed in PRR knockdown and control Neuro2A cells (Fig. 1D). These data indicate that in the PVN of normotensive mice, PRR protein is primarily expressed in neurons, with potentially minor expression in microglia but not astrocytes.
Fig. 1.
Expression and cellular localization of the PRR in the PVN of the mouse hypothalamus. The PVNs of WT C57Bl/6J mice were immunofluorescently labeled for the PRR (red), GFAP (green), and NeuN (blue) (A). The PVNs of CX3CR1-GFP reporter mice were immunofluorescently labeled for the PRR (red) and counterstained with DAPI (blue); white arrows indicate colocalization of the PRR with GFP (B). No primary antibody controls using secondary antibodies conjugated with Alexa 594 (red), Alexa 488 (green), or Alexa 647 (blue) (C). Validating the specificity of the PRR antibody by Western blot analysis of whole cell extracts of Neuro2A cells infected with scrambled (SC) or PRR shRNA (D). PVNs are shown in a dashed outline; the green dashed boxes are areas that appear in higher magnification on the right. 3V, third ventricle; AAV, adeno-associated virus; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; PRR, (pro)renin receptor; PVN, paraventricular nucleus; WT, wild-type.
PRR Knockdown in PVN Neurons by AAV2-Mediated Delivery of Cre Recombinase
To determine whether the PRR in PVN neurons plays a regulatory role in neurogenic hypertension, we knocked down PRR expression in the PVN using virally mediated delivery of Cre recombinase in mice harboring floxed PRR alleles. As a Cre carrier, we used AAV2, which has been shown to target primarily neurons in cortical and hippocampal neurons (43, 67). We first characterized AAV2-mediated expression in the PVN by injecting AAV2-eGPF and monitoring eGFP fluorescence, as shown schematically in Fig. 2, A and B. Seven days after microinjection of AAV2-eGPF into the PVN, eGFP fluorescence was found to be limited to the PVN, as shown in Fig. 2C. No eGFP fluorescence was detected in cell bodies in forebrain regions, such as the SFO, or brain stem regions, such as the RVLM (Fig. 2D). Immunostaining for the neuron-specific marker NeuN, astrocyte-specific marker GFAP, and microglia-specific marker IBA1 showed that AAV2-mediated eGFP expression was colocalized with neurons in the PVN but not with astrocytes or microglia (Fig. 2E).
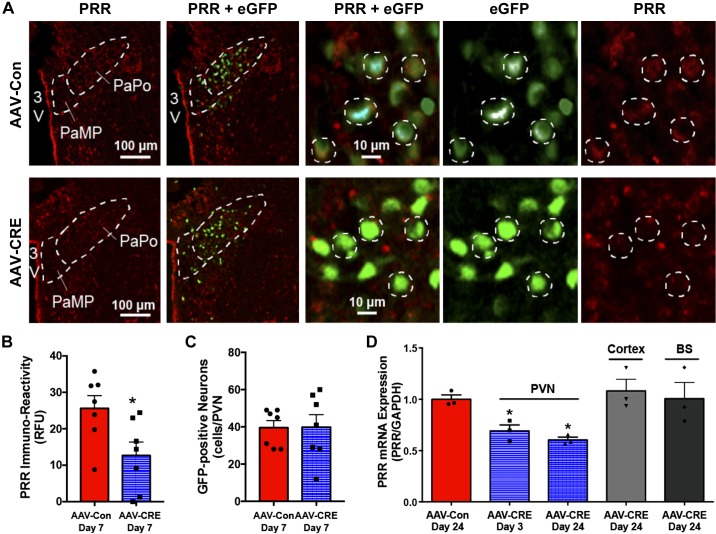
Seven days after injection of AAV-Cre or control AAV2-eGPF virus (hereafter AAV-Con) into the PVN, PRR immunoreactivity in eGFP-positive, Cre recombinase-expressing neurons in mice injected with AAV-Cre was reduced compared with that in eGPF-positive cells in mice injected with AAV-Con virus (Fig. 3A). A semiquantitative analysis of the intensity of PRR immunoreactivity confirmed this (Fig. 3B), revealing a significant reduction in PRR signal intensity in PVN neurons infected with Cre (fold change: 0.38 ± 0.14) compared with those infected with control virus (fold change: 1.11 ± 0.13, P = 0.002). An analysis of PRR mRNA expression in the PVN further showed that AAV-Cre injection induced a significant reduction in PRR mRNA levels, normalized to those in control virus-treated mice, that was detectable on day 3 (fold change: 0.69 ± 0.06, P = 0.01) and was maintained through day 24 (fold change: 0.60 ± 0.03, P = 0.001). By contrast, PRR mRNA measured on day 24 was unchanged in the cerebral cortex (fold change: 1.08 ± 0.11) and brain stem (fold change: 1.00 ± 0.16), confirming the specificity of PVN-targeted injection (Fig. 3C). These data suggest that localized injection of AAV-Cre is an efficient approach for knocking down PRR expression in PVN neurons.
Fig. 3.
PRR knockdown in the PVN by AAV2-delivered Cre recombinase. A: representative images of mouse PRR (red) immunostaining and eGFP fluorescence (green) in the PVN. White dashed circles in higher magnification images (10 μm) indicate representative cells expressing both PRR and eGFP. B: semiquantitative analysis of the relative intensity of PRR fluorescence signals in eGFP-positive cells. C: number of eGFP-positive cells in each PVN (n = 7 PVNs from 3 mice/group). D: PRR mRNA levels in the PVN, cortex, and brain stem (BS), determined by RT-qPCR (*P < 0.05 vs. AAV-Con; n = 3 mice/group, 2 PVNs from each mouse were combined). 3V, third ventricle; AAV, adeno-associated virus; eGFP, enhanced green fluorescent protein; PaPo, paraventricular hypothalamic nucleus, posterior part; PaMP, paraventricular hypothalamic nucleus, medial parvicellular part; PRR, (pro)renin receptor; PVN, paraventricular nucleus; RFU, relative fluorescence units; RT-qPCR, reverse-transcription quantitative PCR.
PRR Knockdown in the PVN Attenuates DOCA-Salt-Induced Hypertension
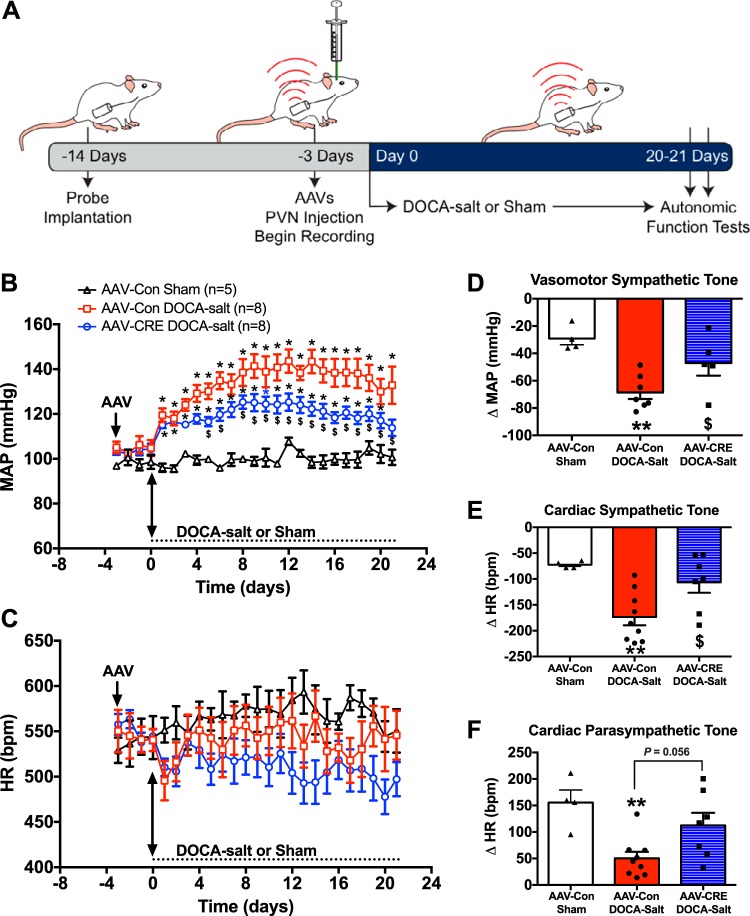
To examine the functional importance of the PVN-PRR in hypertension development, we induced hypertension using DOCA-salt (3, 53, 54), and monitored BP and HR and evaluated sympathetic and parasympathetic tone in unanesthetized mice using a telemetry recording system. As depicted schematically in Fig. 4A, mice were injected with AAV-Cre or AAV-Con 3 days before DOCA-salt or sham treatment. We found that PRR knockdown in the PVN did not alter baseline BP or HR in PRR-floxed mice compared with control mice injected with AAV-Con (Fig. 4, B and C). In control mice, DOCA-salt induced a gradual, and significant, increase in BP compared with sham-treated mice that reached a plateau at ~2 wk. Importantly, BP was significantly lower throughout the protocol in DOCA-salt mice injected with AAV-Cre compared with that in DOCA-salt mice injected with AAV-Con. However, BP remained significantly elevated in AAV2-Cre DOCA-salt mice compared with that in AAV-Con sham mice, indicating that PRR knockdown in the PVN does not completely normalize BP. Differences in HR among the three groups of mice, analyzed by two-way ANOVA, were not significant throughout the protocol (Fig. 4C).
Fig. 4.
Knockdown of the PRR in the PVN attenuates the development of DOCA-salt-induced hypertension and dysautonomia. A: schematic of the experimental protocol. B and C: mean arterial pressure (MAP) and HR at baseline and during 21 days of DOCA-salt treatment. D: vasomotor sympathetic tone, measured as changes in MAP in response to intraperitoneal injection of 6 mg/kg chlorisondamine. E: cardiac sympathetic tone, measured as changes in HR in response to intraperitoneal injection of 5 mg/kg propranolol. F: cardiac parasympathetic tone, measured as changes in HR in response to intraperitoneal injection of 1 mg/kg methylatropine. n = 4–8 mice/group; *P < 0.05 vs. AAV-Con sham; **P < 0.01 vs. AAV-Con Sham; $P < 0.05 vs. AAV-Con DOCA-salt. AAV, adeno-associated virus; HR, heart rate; PRR, (pro)renin receptor; PVN, paraventricular nucleus.
At the end of the protocol, there was a significant increase in vasomotor (ΔBP: −68 ± 4.7 mmHg) and cardiac (ΔHR: −173 ± 16.1 beats/min) sympathetic tone in AAV-Con mice following DOCA-salt compared with sham-treated AAV-Con mice (ΔBP: −29 ± 5 mmHg; ΔHR: −72 ± 3.3 beats/min) (Fig. 4, D and E). PRR knockdown in PVN neurons (AAV2-Cre mice) attenuated the increase in vasomotor (ΔBP: −47 ± 9.2 mmHg) and cardiac (ΔHR: −106 ± 20.2 beats/min; P < 0.05) sympathetic tone induced by DOCA-salt compared with that in mice injected with AAV-Con. Conversely, cardiac parasympathetic tone (Fig. 4F) was impaired in AAV-Con mice that received DOCA-salt stimulation compared with sham-treated AAV2-Con mice (ΔHR: 50 ± 12.2 vs. 156 ± 23.7 beats/min; P = 0.006). Notably, PVN-PRR knockdown attenuated this decrease (ΔHR: 112 ± 23.6 beats/min), restoring cardiac parasympathetic tone to levels that were not significantly different from those in sham-treated AAV-Con mice. These data indicate the pathophysiological importance of the PVN-PRR in neurogenic hypertension and show that it is associated with modulation of autonomic function in mice.
PVN-PRR Regulates ERK1/2 Activation in the PVN and RVLM in DOCA-Salt-Induced Hypertension
It was previously reported that ERK1/2 activation in the PVN is critical for the development of hypertension or heart failure and contributes to elevated sympathetic outflow (74, 87, 88). To examine the effect of PVN-PRR knockdown on ERK1/2 activation status, we immunostained brain slices containing the PVN for p-ERK1/2 at 2 wk of DOCA-salt treatment, a time point chosen because it represents the plateau of BP/hypertension. Figure 5A shows a simplified schematic of the signaling cascade linking the PRR to ERK1/2 activation and an illustration of proposed pathways for PRR deletion in the PVN that affect ERK1/2 activation in the SFO, PVN, and RVLM. As shown in Fig. 5, B and C, p-ERK1/2 signals following 2 wk of DOCA-salt treatment were significantly increased (measured as fold change) in the SFO (1.9 ± 0.2 vs. 1.0 ± 0.1), PVN (3.3 ± 0.2 vs. 1.0 ± 0.2), and RVLM (1.3 ± 0.1 vs. 0.9 ± 0.1) of control (AAV-Con-injected) mice compared with sham-treated mice. PRR knockdown in the PVN did not alter DOCA-salt-induced changes in p-ERK1/2 levels in the SFO (2.2 ± 0.2) compared with that in mice that received AAV-Con (1.9 ± 0.2) but did significantly reduce ERK1/2 activation in both the PVN (1.0 ± 0.1 vs. 3.3 ± 0.2) and RVLM (0.8 ± 0.1 vs. 1.3 ± 0.1).
We and others have previously reported that the PRR mediates both ANG II-dependent and -independent ERK1/2 activation in vitro and in vivo (46, 60, 61). However, it is not known whether this phenomenon also occurs in the PVN of hypertensive mice. To understand further whether the reduction in ERK1/2 activation following PVN-PRR knockdown is mediated by ANG II-dependent or -independent mechanisms (or both), we examined the effects of ICV-infused losartan on p-ERK1/2 immunostaining intensity. Figure 6A illustrates targeted brain regions and the pathway linking ICV-infused losartan and ERK1/2 phosphorylation. As shown in representative images in Fig. 6B and summary data in Fig. 6C, ICV-infused losartan significantly reduced p-ERK1/2 immunostaining intensity in the SFO (fold change: 0.59 ± 0.05 vs. 1.0 ± 0.07), PVN (0.71 ± 0.04 vs. 1.0 ± 0.05), and RVLM (0.65 ± 0.01 vs. 1.0 ± 0.04) in WT mice following 2 wk of DOCA-salt treatment compared with ICV infusion of aCSF, confirming the importance of AT1R in this hypertension model.
To understand better whether PRR-dependent ERK phosphorylation in these brain regions is AT1R-dependent or -independent, we reanalyzed and replotted the data by normalizing the p-ERK1/2 levels in AAV-Cre to AAV-GFP in the PVN targeting experiment or p-ERK1/2 levels in ICV losartan to ICV aCSF in the ICV infusion experiment following DOCA-salt treatment and compared the percent reduction in p-ERK1/2, as shown in Fig. 6D. PVN-PRR knockdown did not affect p-ERK1/2 in the SFO, whereas ICV infusion of losartan significantly reduced p-ERK1/2 in this region as shown in original data (Fig. 5C and Fig. 6C), supporting the appropriateness of our analysis. In the PVN, p-ERK1/2 signal intensity was lower in PVN-PRR knockdown mice (0.29 ± 0.04 vs. 1.0 ± 0.15, normalized to DOCA-salt AAV-Con) compared with that in mice that received ICV losartan (0.71 ± 0.04, normalized to DOCA-salt aCSF). These data suggest that both ANG II-dependent and -independent signaling cascades are involved in the regulation of ERK1/2 activation by the PRR in the PVN. Interestingly, relative ERK1/2 activation in the RVLM following DOCA-salt treatment was similar between losartan-infused WT mice (0.65 ± 0.01 vs. 1.0 ± 0.04 normalized DOCA-salt aCSF) and AAV-Cre mice (0.63 ± 0.05 vs. 1.0 ± 0.09 normalized to DOCA-salt AAV-Con mice).
Knockdown of the PRR Attenuates AT1R Expression and Activity in the PVN in DOCA-Salt Hypertension
To investigate the molecular mechanisms by which PVN-PRR knockdown reduces hypertension further, we measured mRNAs for the key angiotensin receptors, AT1aR, AT2R, and MasR. As shown in Fig. 7, which depicts fold changes in angiotensin receptor mRNAs following PRR knockdown in the PVN, DOCA-salt treatment significantly increased AT1aR (2.5 ± 0.2) and AT2R (1.6 ± 0.1) mRNA levels and decreased MasR mRNA levels (0.06 ± 0.01) in the PVN of AAV-con mice compared with sham treatment (1.0 ± 0.1). Notably, AAV-Cre injection into the PVN attenuated (P < 0.01) the DOCA-salt-induced increase in AT1aR (1.6 ± 0.1) and AT2R (0.8 ± 0.1) mRNA levels, without affecting (P > 0.05) MasR (0.07 ± 0.01) mRNA levels, compared with DOCA-salt-treated AAV-con mice. AT1aR is the major angiotensin receptor in the PVN involved in mediating BP elevation, whereas AT2R and MasR counterbalance the action of AT1aR (20, 30, 50, 69). Accordingly, we hypothesized that a reduction in AT1aR expression/activity in the PVN may be one of the key mechanisms by which PVN-PRR knockdown attenuates DOCA-salt hypertension. To validate our hypothesis functionally and more directly measure AT1aR activity, we evaluated ANG II-induced Ca2+ activity in the PVN in brain slices from DOCA-salt or sham-treated AAV-con mice and DOCA-salt-treated AAV-Cre mice using the Ca2+ biosensor, GCaMP6, delivered via AAV2.
Fig. 7.
PVN-PRR knockdown regulates angiotensin receptor expression. AT1aR (A), AT2R (B), and MasR (C) mRNA levels. **P < 0.01 vs. AAV-Con Sham; ***P < 0.001 vs. AAV-Con sham; $$P < 0.01 vs. AAV-Con DOCA-Salt; $$$P < 0.001 vs. AAV-Con DOCA-Salt (n = 3–4 mice/group). AAV, adeno-associated virus; AT1aR, angiotensin II type 1a receptor; AT2R, angiotensin II type 2 receptor; PRR, (pro)renin receptor; PVN, paraventricular nucleus.
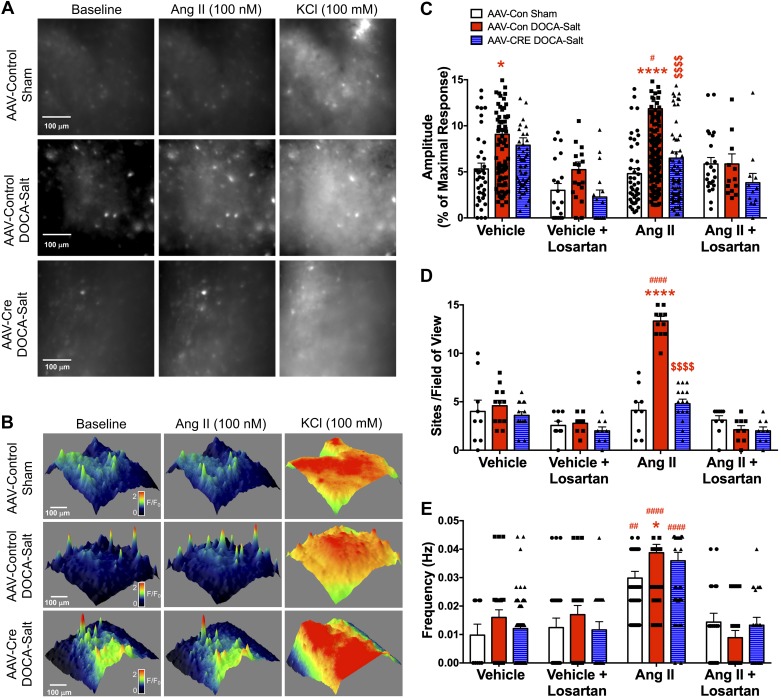
As shown in a snapshot from a live-cell Ca2+-imaging video (Fig. 8A) and 3-D-reconstructed images (Fig. 8B), ANG II (100 nM) had no apparent effect on Ca2+ signal amplitude or number of activation sites in AAV-Con sham mice (white bars) but increased the frequency of Ca2+-signaling events within an FOV. This treatment had no effect on the viability of neurons in the brain slice, as evidenced by the retention of a robust response to a depolarizing concentration of KCl (100 mM). In AAV-con DOCA-salt animals (red bars), ANG II treatment led to a significant increase in the amplitude, number of activation sites, and frequency of Ca2+-signaling events. Knockdown of the PRR in the PVN (AAV-Cre-injected mice) abrogated this DOCA-salt-induced excitatory response to ANG II (blue bars). This ANG II-induced Ca2+ activity was blocked by pretreatment with losartan, suggesting the involvement of AT1Rs.
Fig. 8.
PVN-PRR knockdown regulates AT1R-mediated Ca2+-signaling events in the PVN. Representative snapshot images (A) and 3-D images from 3-D FOV reconstructions (B) of Ca2+ activity within PVN slices of AAV-Con Sham, AAV-Con DOCA-Salt, and AAV-Cre DOCA-Salt mice under control conditions and following treatment with ANG II (100 nM) or KCl (100 mM). Summary data for live-cell Ca2+ imaging showing amplitude (C), sites/FOV (D), and frequency (E) in response to ANG II, detected using the Ca2+ biosensor GCaMP6, in slices containing the PVN from AAV-Con sham, AAV-Con DOCA-Salt, and AAV-Cre DOCA-Salt mice. *P < 0.05 vs. AAV-Con sham in the same treatment group (vehicle or ANG II); ****P < 0.0001 vs. AAV-Con sham in the same treatment group (vehicle or ANG II); $$$$P < 0.0001 vs. AAV-Con DOCA-salt in the ANG II group; #P < 0.05 ANG II vs. vehicle or ANG II vs. ANG II + losartan within the same treatment group; ##P < 0.01 ANG II vs. Vehicle or ANG II vs. ANG II + losartan within the same treatment group; ####P < 0.0001 ANG II vs. vehicle or ANG II vs. ANG II + losartan within the same treatment group (AAV-Con sham, AAV-Con DOCA-salt, AAV-Cre DOCA-Salt) (n = 4 mice/group). 3-D, three-dimensional; AAV, adeno-associated virus; ANG II, angiotensin II; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; FOV, field of view; PRR, (pro)renin receptor; PVN, paraventricular nucleus.
A quantitative analysis of Ca2+ signals in video recordings (Fig. 8, C–E) revealed that ANG II induced a significant increase in both the amplitude (11.8 ± 0.8% of maximal response) and number (13.3 ± 0.5 sites/FOV) of Ca2+ events in the PVN of AAV-Con mice that received 2 wk of DOCA-salt but had minimal effects on the corresponding sham-treated mice (P > 0.05), consistent with an increase in AT1R expression with DOCA-salt treatment. Interestingly, ANG II increased the frequency of Ca2+ events in the PVN of mice from both sham (0.03 ± 0.002 Hz) and AAV-Con DOCA-salt (0.039 ± 0.003 Hz) groups. Notably, Ca2+ event frequency was higher in AAV-Con DOCA-salt-treated mice compared with sham-treated mice. Importantly, PVN-PRR knockdown normalized the ANG II-induced increase in the amplitude (6.5 ± 0.7% of maximal response) and number (4.8 ± 0.5 sites/FOV) of Ca2+ events following DOCA-salt treatment compared with the AAV-con group, without affecting their frequency (0.036 ± 0.003 Hz) (Fig. 8E), indicating that PRR knockdown specifically reduces AT1R-mediated Ca2+ signal amplitude and number of activation sites.
Interestingly, under baseline (vehicle) conditions, we observed a small but significant increase in the amplitude of Ca2+ events in the PVN of DOCA-salt mice (9.1 ± 0.5% of maximal response) that received AAV-Con compared with the corresponding sham mice (5.3 ± 0.6% of maximal response), whereas there was no difference in the number of active sites (4.6 ± 0.6 vs. 4.0 ± 1.2 sites/FOV) or frequency (0.01 ± 0.004 vs. 0.02 ± 0.003 Hz) between these two groups. To our surprise, we found that pretreatment of brain slices from the vehicle group with losartan prevented the DOCA-salt-induced increase in Ca2+ event amplitude, indicating that basal Ca2+ activity in the PVN is losartan sensitive and possibly depends on the AT1R.
DISCUSSION
The PRR is a recently discovered receptor for both renin and prorenin that increases renin activity or nonproteolytically activates prorenin (58). The importance of the PRR is underscored by recent reports of its role in mediating endogenous ANG II formation in the CNS (38, 52, 53, 71). It is well established that the PVN is part of neural circuits that are sensitive to ANG II (10, 11, 44, 50). Expression of the PRR in the PVN and the functional role of the PVN in modulating neuronal activity have been demonstrated previously (53, 62, 85). For example, we showed that PRR knockdown globally in neurons using a neuron-specific, synapsin1 promoter-driven Cre-loxP system decreases ANG II formation in the brain, improves autonomic function, and blunts development of DOCA-salt hypertension (53). However, the brain regions that are key to this effect and the mechanism by which the PRR contributes to the DOCA-salt hypertension remained incompletely understood. The present study provides mechanistic insight into this unresolved question, specifically establishing that the PRR is important in the development of DOCA-salt hypertension through regulation of AT1R-dependent Ca2+ activity in PVN neurons. In summary, our findings indicate that 1) PVN delivery of AAV2 primarily targets neurons, 2) knockdown of the neuronal PVN-PRR in adult mice attenuates the development of DOCA-salt-induced hypertension and is associated with improved autonomic function, 3) knockdown of the PVN-PRR diminishes ERK1/2 activation in the PVN and RVLM neurons, and 4) knockdown of the PVN-PRR reduces AT1aR mRNA expression levels and functionally attenuates AT1R-dependent Ca2+ activity in the PVN, providing a mechanistic basis for the role of the PRR in angiotensinergic signaling during DOCA-salt-induced hypertension.
The PRR is expressed in many cardiovascular regulatory regions of the brain and is localized to neurons and microglia in rat, primate, and human brains (1, 12, 13, 53, 62, 71, 77, 80). In the mouse brain, the PRR is expressed in neurons but not in astrocytes (53, 85). Although evidence for PRR expression in a mouse microglial cell line was reported previously (72), whether PRR protein is also expressed in microglia in the mouse PVN was not known. Using microglia reporter mice (CX3CR1-GFP), we showed here that the PRR is expressed in a very small number of microglial cells in the PVN at the normotensive stage. Similarly, we showed that the PRR is primarily expressed in neurons and not astrocytes. Accordingly, we chose to investigate the role of the neuronal PRR in the PVN in this study. AAVs have come to be used as common tools for genetic manipulation owing to their lack of immunogenicity and effective neuron targeting in the CNS (39). The cell-targeting specificity is determined by the serotype of the AAV. In this study, we characterized AAV2 infection in the PVN and showed that the AAV2 serotype infects mostly neurons, and not astrocytes or microglia, a tropism similar to that reported in previous studies on neurons in other brain regions (43, 67). We further validated that AAV2-Cre sufficiently knocked down the PRR in neurons (Fig. 3). Accordingly, the effect of PRR knockdown in the current study can be inferred to reflect neuronal PRR knockdown. However, considering the importance of brain hypothalamic inflammation in cardiovascular metabolic diseases (27, 72, 79), we recognize that PRRs in microglia may also have a role in the PVN, a question of interest for our future studies.
To evaluate the neuronal activation states that might be altered by PVN-PRR knockdown, we examined the levels of p-ERK1/2Thr202/Tyr204 —a marker of neuronal activation (15, 87)—in three key brain regions: the SFO, PVN, and RVLM. The SFO, which is located outside the blood-brain barrier, senses inputs from the circulation and receives projections from other brain regions. In this latter context, the SFO lies upstream of and projects to the PVN in the hypothalamus (4, 23, 78, 81). Thus, it is not surprising that PRR knockdown in the PVN does not affect ERK1/2 activation in the SFO. Interestingly, however, we found that PVN-PRR knockdown reduced ERK1/2 activation in both the PVN and RVLM, the latter of which is the presympathetic center of BP regulation (14, 45, 47). This finding is consistent with our observation that PVN-PRR knockdown reduces BP, as well as cardiac and vasomotor sympathetic tone (Fig. 4). Following ICV infusion of losartan, ERK1/2 phosphorylation was uniformly reduced in the SFO, PVN, and RVLM, an observation in agreement with a previous finding on the importance of AT1R in mediating ERK1/2 activation in the brain (61). Interestingly, ERK1/2 activation in the PVN was lower with PVN-PRR knockdown than with ICV infusion of losartan. Our data suggest that both ANG II-dependent and direct PRR signaling independent of ANG II are involved in mediating ERK1/2 activation in DOCA-salt hypertension. Surprisingly, however, the degree of ERK1/2 activation in the RVLM was similar between PRR knockdown and losartan treatment. Although the underlying mechanism is not clear, we speculate that angiotensinergic projections from the PVN to the RVLM are dominant in ERK1/2 activation in the RVLM in this hypertension model.
We noted that BP was not completely normalized in PVN-targeted PRR-knockdown mice during DOCA-salt hypertension development. In this context, it is important to highlight that, although the PVN-PRR contributes to increased BP, neural autonomic and neural endocrine dysregulation during hypertension (8, 53, 60, 85), a role for the PRR in other important cardiovascular regulatory areas of the brain, including the SFO (9, 52), supraoptic nucleus (71), and nucleus of the solitary tract (NTS) (90), in regulating BP has been reported. Moreover, other key BP regulating systems in addition to RAS also contribute to DOCA-salt-induced hypertension in mice (3, 31, 86). Thus, it is not surprising that knockdown of PRR in the PVN did not completely normalize BP and autonomic function.
In this study, we also showed that PVN-PRR knockdown differentially affected sympathetic and parasympathetic input to the heart, attenuating the former while increasing the latter, thus producing beneficial effects. It is well known that PVN projections to the RVLM are predominantly excitatory, whereas those to the NTS are primarily inhibitory (18). We showed that PVN-PRR knockdown decreased the levels of p-ERK1/2, a marker of neuronal activity, in the PVN and RVLM, supporting the inference that PVN-PRR knockdown decreases neuronal activity in the PVN, and thus its excitatory effect in the RVLM, which explains the decreased sympathetic activity to the heart and the periphery. It is possible that PVN-PRR knockdown also decreases inhibitory output from the PVN to the NTS, likely leading to increased neuronal activity in the NTS and thus increased cardiac parasympathetic activity.
To decipher the mechanisms of the PRR in hypertension development, we took advantage of the DOCA-salt-induced hypertensive mouse model, which is a model of neurogenic hypertension with upregulated brain RAS activity (3, 53, 54). Accumulating evidence indicates that angiotensin receptors in the PVN, including AT1R, AT2R, and MasR, play key roles in modulating cardiovascular function by interacting with angiotensin peptides (16, 17, 41, 72a, 82). Here, we showed that PRR knockdown in the PVN attenuates hypertension and is associated with improved autonomic function. Furthermore, PRR knockdown in the PVN reduced mRNA levels of both AT1aR and AT2R mRNA, but interestingly had no effect on MasR. AT2R was recently reported to localize in peri-PVN GABAergic neurons that project to both autonomic and AVP-positive neurons in the PVN (22). Activation of these AT2R neurons leads to inhibition of PVN-AVP-positive neurons (20). In rodent models of ANG II-induced hypertension or chronic heart failure, AT2R levels are decreased in the PVN (33, 73); however, in DOCA-salt-induced hypertension, AT2R expression levels in the PVN were reported to be elevated (17), a result similar to our findings (Fig. 7B). The increase in AT2R expression in DOCA-salt-hypertensive mice may be a compensatory mechanism that protects against elevated BP in this model.
Because ANG II/AT1R represents the major vasoconstrictor axis of the brain RAS, whereas ANG II/AT2R and ANG (1–7)/MasR constitute the protective vasodilator axis (20, 21), we proposed that PRR knockdown exerts its beneficial effects on BP, in part, by reducing AT1R signaling in this brain region and tested this hypothesis using Ca2+ imaging. Ca2+ signaling is a critically important aspect of neuronal activity as it participates in the transmission of depolarizing signals and thereby contributes to synaptic activity (5, 35). By acting on AT1Rs, ANG II increases intracellular Ca2+ concentration through various signaling pathways (40). Using the genetically encoded Ca2+ biosensor GCaMP6 (55), we confirmed that PRR knockdown in the PVN alters AT1R-mediated Ca2+ activity in neurons. Because of the cell-specific targeting of AAV2, discussed above (see also Fig. 2), AAV2-mediated GCaMP6 expression is superior to conventional Ca2+-binding fluorescent dyes (55), because it allows intracellular Ca2+ to be recorded in neurons without interference from other cell types in the brain, such as astrocytes and microglia. Importantly, using this Ca2+ biosensor, we showed that PRR knockdown eliminated the augmented AT1R-mediated Ca2+ activity induced by ANG II in PVN neurons of DOCA-salt-treated mice, supporting the conclusion that PRR knockdown reduces AT1R activity.
Because the amplitude of Ca2+ events was increased in the PVN of DOCA-salt-hypertensive mice compared with sham-treated normotensive mice at baseline (vehicle-treated group), and this change was inhibited by treatment with losartan, we speculate that this is an ANG II/AT1R-sensitive mechanism. Among the best-characterized Ca2+ signaling pathways activated by G-protein coupled receptors is the release of intracellular Ca2+ stored in the endoplasmic reticulum (76). The release of Ca2+ from the endoplasmic reticulum causes a depletion of Ca2+ in this cell compartment that can be sensed by stromal interaction molecule 1, which triggers influx of extracellular Ca2+ by store-operated Ca2+ channels, such as the Ca2+ release-activated Ca2+ modulators, Orai1–3, and members of the canonical transient receptor potential cation channel family. Some groups have shown that this store-operated Ca2+ entry) mechanism plays an important role in astrocyte activity (48) and dorsal root ganglion neuron excitability (83). The expression of Orai channels has not been investigated in PVN neurons or microglia, but transient receptor potential cation channel expression in PVN neurons has been previously demonstrated (29, 56). Further studies are needed to determine if these mechanisms play a role in PVN neurons and are responsible for increased excitability and sympathetic activity in hypertension, and to assess the importance of PVN-AT1R in this process.
In summary, we reported here that knockdown of PRR in the PVN attenuates the development of DOCA-salt-induced hypertension and is associated with improved autonomic function. Mechanistically, PVN-PRR knockdown reduces ERK1/2 activation in the PVN and RVLM. In addition, PRR knockdown reduces AT1R-mediated Ca2+ activity in PVN neurons. We conclude that, in the PVN, the PRR makes an important contribution to DOCA-salt-induced hypertension, in part by regulating AT1R expression and function.
Perspectives and Limitations
This study demonstrates an essential role of the PRR in PVN neurons in the development of neurogenic hypertension. The PVN is a complex brain nucleus composed of excitatory and inhibitory neurons, and neurosecretory neurons. Although we observed an overall beneficial effect of PRR knockdown in PVN neurons, the contributions and roles of specific types of neurons in hypertension remain incompletely understood. Further studies should examine the roles and mechanisms of the PRR in specific neuron types.
GRANTS
Research reported in this publication used imaging core facility supported by the National Institute of General Medical Sciences of the National Institutes of Health (NHI/NIGMS grant no. P20-GM103650), and the Transgenic Genotyping and Phenotyping Core facility supported by National Institute of General Medical Sciences of the National Institutes of Health (NHI/NIGMS grant no. 1P20-GM130459). This work was also supported, in part, by grants from the National Heart, Lung, and Blood Institute grant nos. R01-HL122770, R01-HL091905, and NIH/NIGMS (1P20-GM130459) and the American Heart Association National Center (17IRG33370128) to Y. Feng.
DISCLAIMERS
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.L. conceived and designed research; L.A.C.S., C.J.W., W.L., F.T., T.W., A.J.B.G., E.Y., S.G.C., and B.T.D. performed experiments; L.A.C.S., C.J.W., W.L., F.T., T.W., A.J.B.G., E.Y., S.G.C., B.T.D., and Y.F. analyzed data; L.A.C.S., C.J.W., W.L., F.T., T.W., A.J.B.G., E.Y., S.G.C., B.T.D., and Y.F. interpreted results of experiments; L.A.C.S., C.J.W., W.L., B.T.D., and Y.F. prepared figures; L.A.C.S., C.J.W., B.T.D., and Y.F. drafted manuscript; L.A.C.S., C.J.W., W.L., F.T., T.W., A.J.B.G., E.Y., S.G.C., B.T.D., and Y.F. edited and revised manuscript; L.A.C.S., C.J.W., W.L., F.T., T.W., A.J.B.G., E.Y., S.G.C., B.T.D., and Y.F. approved final version of manuscript.
REFERENCES
- 1.Alcazar O, Cousins SW, Striker GE, Marin-Castano ME. (Pro)renin receptor is expressed in human retinal pigment epithelium and participates in extracellular matrix remodeling. Exp Eye Res 89: 638–647, 2009. doi: 10.1016/j.exer.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Baker SA, Drumm BT, Cobine CA, Keef KD, Sanders KM. Inhibitory neural regulation of the Ca 2+ transients in intramuscular interstitial cells of cajal in the small intestine. Front Physiol 9: 328, 2018. doi: 10.3389/fphys.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basting T, Lazartigues E. DOCA-salt hypertension: an update. Curr Hypertens Rep 19: 32, 2017. doi: 10.1007/s11906-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaustein MP, Leenen FHH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 302: H1031–H1049, 2012. doi: 10.1152/ajpheart.00899.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71: 2787–2814, 2014. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol 20: 1263–1268, 2010. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Burcklé C, Bader M. Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006. doi: 10.1161/01.HYP.0000241132.48495.df. [DOI] [PubMed] [Google Scholar]
- 8.Cao T, Feng Y. The (pro)renin receptor and body fluid homeostasis. Am J Physiol Regul Integr Comp Physiol 305: R104–R106, 2013. doi: 10.1152/ajpregu.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao T, Li W, Seth DM, Navar GL, Feng Y. Brain-targeted (pro) renin receptor knockdown modulates body fluid homeostasis. J Hypertens 3: 2014. doi: 10.4172/2167-1095.1000160. [DOI] [Google Scholar]
- 10.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005. doi: 10.1152/jn.01055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- 12.Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, Corvol P, Schwartz CE, Nguyen G. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol 297: R250–R257, 2009. doi: 10.1152/ajpregu.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper SG, Trivedi DP, Yamamoto R, Worker CJ, Feng C-Y, Sorensen JT, Yang W, Xiong Z, Feng Y. Increased (pro)renin receptor expression in subfornical organ neurons in hypertensive humans. Am J Physiol Heart Circ Physiol 314: H796–H804, 2018. doi: 10.1152/ajpheart.00616.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cravo SL, Possas OS, Ferreira-Neto ML. Rostral ventrolateral medulla: an integrative site for muscle vasodilation during defense-alerting reactions. Cell Mol Neurobiol 23: 579–595, 2003. doi: 10.1023/A:1025076130854. [DOI] [PubMed] [Google Scholar]
- 15.Cruz CD, Cruz F. The ERK 1 and 2 pathway in the nervous system: from basic aspects to possible clinical applications in pain and visceral dysfunction. Curr Neuropharmacol 5: 244–252, 2007. doi: 10.2174/157015907782793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culman J, Höhle S, Qadri F, Edling O, Blume A, Lebrun C, Unger T. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin Exp Hypertens 17: 281–293, 1995. doi: 10.3109/10641969509087071. [DOI] [PubMed] [Google Scholar]
- 17.Dai SY, Peng W, Zhang YP, Li JD, Shen Y, Sun XF. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflammation 12: 47, 2015. doi: 10.1186/s12974-015-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 19.Danser AHJ, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension 46: 1069–1076, 2005. doi: 10.1161/01.HYP.0000186329.92187.2e. [DOI] [PubMed] [Google Scholar]
- 20.de Kloet AD, Pitra S, Wang L, Hiller H, Pioquinto DJ, Smith JA, Sumners C, Stern JE, Krause EG. Angiotensin type-2 receptors influence the activity of vasopressin neurons in the paraventricular nucleus of the hypothalamus in male mice. Endocrinology 157: 3167–3180, 2016. doi: 10.1210/en.2016-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Kloet AD, Steckelings UM, Sumners C. Protective angiotensin type 2 receptors in the brain and hypertension. Curr Hypertens Rep 19: 46, 2017. doi: 10.1007/s11906-017-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, Steckelings UM, Scheuer DA, Sumners C, Krause EG. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct 221: 891–912, 2016. doi: 10.1007/s00429-014-0943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dellmann HD. Structure of the subfornical organ: a review. Microsc Res Tech 41: 85–97, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 24.do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic-vagal balance and baroreflex sensitivity in diabetic rats. Am J Physiol Heart Circ Physiol 295: H1974–H1981, 2008. doi: 10.1152/ajpheart.00265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drumm BT, Hennig GW, Baker SA, Sanders KM. Applications of spatio-temporal mapping and particle analysis techniques to quantify intracellular Ca2+ signaling in situ. J Vis Exp 143: e58989, 2019. doi: 10.3791/58989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM, Baker SA. Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J Gen Physiol 149: 703–725, 2017. [Erratum in J Gen Physiol 149: 751, 2017]. doi: 10.1085/jgp.201711771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffy CM, Yuan C, Wisdorf LE, Billington CJ, Kotz CM, Nixon JP, Butterick TA. Role of orexin A signaling in dietary palmitic acid-activated microglial cells. Neurosci Lett 606: 140–144, 2015. doi: 10.1016/j.neulet.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Jan Danser AH, Bader M, Nguyen G, Luft FC, Muller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51: 682–688, 2008. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 29.Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One 2: e573, 2007. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman KL, Brooks VL. AT(1) and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 31.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol 25: 1148–1155, 2014. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res 1447: 28–37, 2012. doi: 10.1016/j.brainres.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 33.Gao L, Wang WZ, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension 52: 708–714, 2008. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron 73: 862–885, 2012. doi: 10.1016/j.neuron.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 36.Hirose T, Hashimoto M, Totsune K, Metoki H, Asayama K, Kikuya M, Sugimoto K, Katsuya T, Ohkubo T, Hashimoto J, Rakugi H, Takahashi K, Imai Y. Association of (pro)renin receptor gene polymorphism with blood pressure in Japanese men: the Ohasama study. Am J Hypertens 22: 294–299, 2009. doi: 10.1038/ajh.2008.357. [DOI] [PubMed] [Google Scholar]
- 37.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol 21: 295–304, 2006. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- 38.Huber MJ, Basu R, Cecchettini C, Cuadra AE, Chen QH, Shan Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am J Physiol Heart Circ Physiol 309: H880–H887, 2015. doi: 10.1152/ajpheart.00095.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther 10: 955–963, 2003. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 40.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PML, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev 67: 754–819, 2015. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khanmoradi M, Nasimi A. Functions of AT1 and AT2 angiotensin receptors in the paraventricular nucleus of the rat, correlating single-unit and cardiovascular responses. Brain Res Bull 132: 170–179, 2017. doi: 10.1016/j.brainresbull.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Kinouchi K, Ichihara A, Sano M, Sun-Wada G-H, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M, Tamai Y, Sato H, Fukuda K, Itoh H. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res 107: 30–34, 2010. doi: 10.1161/CIRCRESAHA.110.224667. [DOI] [PubMed] [Google Scholar]
- 43.Klein RL, Dayton RD, Tatom JB, Henderson KM, Henning PP. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther 16: 89–96, 2008. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight WD, Saxena A, Shell B, Nedungadi TP, Mifflin SW, Cunningham JT. Central losartan attenuates increases in arterial pressure and expression of FosB/ΔFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1051–R1058, 2013. doi: 10.1152/ajpregu.00541.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koganezawa T, Shimomura Y, Terui N. The role of the RVLM neurons in the viscero-sympathetic reflex: a mini review. Auton Neurosci 142: 17–19, 2008. doi: 10.1016/j.autneu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Krop M, Lu X, Danser AH, Meima ME. The (pro)renin receptor. A decade of research: what have we learned? Pflugers Arch 465: 87–97, 2013. doi: 10.1007/s00424-012-1105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumagai H, Oshima N, Matsuura T, Iigaya K, Imai M, Onimaru H, Sakata K, Osaka M, Onami T, Takimoto C, Kamayachi T, Itoh H, Saruta T. Importance of rostral ventrolateral medulla neurons in determining efferent sympathetic nerve activity and blood pressure. Hypertens Res 35: 132–141, 2012. doi: 10.1038/hr.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon J, An H, Sa M, Won J, Shin JI, Lee CJ. Orai1 and Orai3 in combination with Stim1 mediate the majority of store-operated calcium entry in astrocytes. Exp Neurobiol 26: 42–54, 2017. doi: 10.5607/en.2017.26.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leenen FH. The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim Biophys Acta 1802: 1132–1139, 2010. doi: 10.1016/j.bbadis.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 23: 5041–5049, 2003. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W, Liu J, Hammond SL, Tjalkens RB, Saifudeen Z, Feng Y. Angiotensin II regulates brain (pro)renin receptor expression through activation of cAMP response element-binding protein. Am J Physiol Regul Integr Comp Physiol 309: R138–R147, 2015. doi: 10.1152/ajpregu.00319.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Peng H, Cao T, Sato R, McDaniels SJ, Kobori H, Navar LG, Feng Y. Brain-targeted (pro)renin receptor knockdown attenuates angiotensin II-dependent hypertension. Hypertension 59: 1188–1194, 2012. doi: 10.1161/HYPERTENSIONAHA.111.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Peng H, Mehaffey EP, Kimball CD, Grobe JL, van Gool JM, Sullivan MN, Earley S, Danser AH, Ichihara A, Feng Y. Neuron-specific (pro)renin receptor knockout prevents the development of salt-sensitive hypertension. Hypertension 63: 316–323, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W, Sullivan MN, Zhang S, Worker CJ, Xiong Z, Speth RC, Feng Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 65: 352–361, 2015. [Erratum in Hypertension 70: 2017]. doi: 10.1161/HYPERTENSIONAHA.114.04458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knöpfel T, Boyden ES, Reid RC, Carandini M, Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85: 942–958, 2015. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nedungadi TP, Cunningham JT. Differential regulation of TRPC4 in the vasopressin magnocellular system by water deprivation and hepatic cirrhosis in the rat. Am J Physiol Regul Integr Comp Physiol 306: R304–R314, 2014. doi: 10.1152/ajpregu.00388.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen G, Burcklé CA, Sraer JD. Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep 6: 129–132, 2004. doi: 10.1007/s11906-004-0088-3. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. doi: 10.1172/JCI0214276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ott C, Schneider MP, Delles C, Schlaich MP, Hilgers KF, Schmieder RE. Association of (pro)renin receptor gene polymorphism with blood pressure in Caucasian men. Pharmacogenet Genomics 21: 347–349, 2011. doi: 10.1097/FPC.0b013e328344cdd2. [DOI] [PubMed] [Google Scholar]
- 60.Peng H, Jensen DD, Li W, Sullivan MN, Buller SA, Worker CJ, Cooper SG, Zheng S, Earley S, Sigmund CD, Feng Y. Overexpression of the neuronal human (pro)renin receptor mediates angiotensin II-independent blood pressure regulation in the central nervous system. Am J Physiol Heart Circ Physiol 314: H580–H592, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng H, Li W, Seth DM, Nair AR, Francis J, Feng Y. (Pro)renin receptor mediates both angiotensin II-dependent and -independent oxidative stress in neuronal cells. PLoS One 8: e58339, 2013. doi: 10.1371/journal.pone.0058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pitra S, Feng Y, Stern JE. Mechanisms underlying prorenin actions on hypothalamic neurons implicated in cardiometabolic control. Mol Metab 5: 858–868, 2016. doi: 10.1016/j.molmet.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pritchard HAT, Gonzales AL, Pires PW, Drumm BT, Ko EA, Sanders KM, Hennig GW, Earley S. Microtubule structures underlying the sarcoplasmic reticulum support peripheral coupling sites to regulate smooth muscle contractility. Sci Signal 10: eaan2694, 2017. doi: 10.1126/scisignal.aan2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pritchard HAT, Pires PW, Yamasaki E, Thakore P, Earley S. . Nanoscale remodeling of ryanodine receptor cluster size underlies cerebral microvascular dysfunction in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 115: E9745.–, 2018. doi: 10.1073/pnas.1804593115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rassler B. The renin-angiotensin system in the development of salt-sensitive hypertension in animal models and humans. Pharmaceuticals (Basel) 3: 940–960, 2010. doi: 10.3390/ph3040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez-Iturbe B, Vaziri ND. Salt-sensitive hypertension--update on novel findings. Nephrol Dial Transplant 22: 992–995, 2007. doi: 10.1093/ndt/gfl757. [DOI] [PubMed] [Google Scholar]
- 67.Royo NC, Vandenberghe LH, Ma JY, Hauspurg A, Yu L, Maronski M, Johnston J, Dichter MA, Wilson JM, Watson DJ. Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res 1190: 15–22, 2008. doi: 10.1016/j.brainres.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep 13: 55–66, 2011. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santos RAS, Sampaio WO, Alzamora AC, Motta-Santos D, Alenina N, Bader M, Campagnole-Santos MJ. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol Rev 98: 505–553, 2018. doi: 10.1152/physrev.00023.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saris JJ, ’t Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006. doi: 10.1161/01.HYP.0000240064.19301.1b. [DOI] [PubMed] [Google Scholar]
- 71.Shan Z, Shi P, Cuadra AE, Dong Y, Lamont GJ, Li Q, Seth DM, Navar LG, Katovich MJ, Sumners C, Raizada MK. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ Res 107: 934–938, 2010. doi: 10.1161/CIRCRESAHA.110.226977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLoS One 9: e92937, 2014. doi: 10.1371/journal.pone.0092937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72a.Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1-7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension 46: 341–348, 2005. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 73.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92: 401–408, 2011. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted ACE2 overexpression attenuates neurogenic hypertension by inhibiting COX mediated inflammation. Hypertension 65: 577–586, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. doi: 10.1126/scisignal.2005659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sumners C, Fleegal MA, Zhu M. Angiotensin AT1 receptor signalling pathways in neurons. Clin Exp Pharmacol Physiol 29: 483–490, 2002. doi: 10.1046/j.1440-1681.2002.03660.x. [DOI] [PubMed] [Google Scholar]
- 77.Takahashi K, Hiraishi K, Hirose T, Kato I, Yamamoto H, Shoji I, Shibasaki A, Kaneko K, Satoh F, Totsune K. Expression of (pro)renin receptor in the human brain and pituitary, and co-localisation with arginine vasopressin and oxytocin in the hypothalamus. J Neuroendocrinol 22: 453–459, 2010. doi: 10.1111/j.1365-2826.2010.01980.x. [DOI] [PubMed] [Google Scholar]
- 78.Tiruneh MA, Huang BS, Leenen FH. Role of angiotensin II type 1 receptors in the subfornical organ in the pressor responses to central sodium in rats. Brain Res 1527: 79–86, 2013. doi: 10.1016/j.brainres.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 79.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9: 2124–2138, 2014. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valenzuela R, Barroso-Chinea P, Villar-Cheda B, Joglar B, Muñoz A, Lanciego JL, Labandeira-Garcia JL. Location of prorenin receptors in primate substantia nigra: effects on dopaminergic cell death. J Neuropathol Exp Neurol 69: 1130–1142, 2010. doi: 10.1097/NEN.0b013e3181fa0308. [DOI] [PubMed] [Google Scholar]
- 81.Wang HW, Huang BS, White RA, Chen A, Ahmad M, Leenen FH. Mineralocorticoid and angiotensin II type 1 receptors in the subfornical organ mediate angiotensin II - induced hypothalamic reactive oxygen species and hypertension. Neuroscience 329: 112–121, 2016. doi: 10.1016/j.neuroscience.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Hiller H, Smith JA, de Kloet AD, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol Genomics 48: 667–676, 2016. doi: 10.1152/physiolgenomics.00029.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei D, Mei Y, Xia J, Hu H. Orai1 and Orai3 mediate store-operated calcium entry contributing to neuronal excitability in dorsal root ganglion neurons. Front Cell Neurosci 11: 400, 2017. doi: 10.3389/fncel.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wright JW, Roberts KA, Stubley LA, Hanesworth JM, Harding JW. Hypothalamic angiotensin release in response to AII or glutamic acid stimulation of the SFO in rats. Brain Res Bull 31: 649–654, 1993. doi: 10.1016/0361-9230(93)90136-Y. [DOI] [PubMed] [Google Scholar]
- 85.Xu Q, Jensen DD, Peng H, Feng Y. The critical role of the central nervous system (pro)renin receptor in regulating systemic blood pressure. Pharmacol Ther 164: 126–134, 2016. doi: 10.1016/j.pharmthera.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yemane H, Busauskas M, Burris SK, Knuepfer MM. Neurohumoral mechanisms in deoxycorticosterone acetate (DOCA)-salt hypertension in rats. Exp Physiol 95: 51–55, 2010. doi: 10.1113/expphysiol.2008.046334. [DOI] [PubMed] [Google Scholar]
- 87.Yu Y, Wei SG, Zhang ZH, Weiss RM, Felder RB. ERK1/2 MAPK signaling in hypothalamic paraventricular nucleus contributes to sympathetic excitation in rats with heart failure after myocardial infarction. Am J Physiol Heart Circ Physiol 310: H732–H739, 2016. doi: 10.1152/ajpheart.00703.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Y, Xue BJ, Zhang ZH, Wei SG, Beltz TG, Guo F, Johnson AK, Felder RB. Early interference with p44/42 mitogen-activated protein kinase signaling in hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension. Hypertension 61: 842–849, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao X, White R, Huang BS, Van Huysse J, Leenen FH. High salt intake and the brain renin–angiotensin system in Dahl salt-sensitive rats. J Hypertens 19: 89–98, 2001. doi: 10.1097/00004872-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 90.Zubcevic J, Jun JY, Lamont G, Murça TM, Shi P, Yuan W, Lin F, Carvajal JM, Li Q, Sumners C, Raizada MK, Shan Z. Nucleus of the solitary tract (pro)renin receptor-mediated antihypertensive effect involves nuclear factor-κB-cytokine signaling in the spontaneously hypertensive rat. Hypertension 61: 622–627, 2013. doi: 10.1161/HYPERTENSIONAHA.111.199836. [DOI] [PubMed] [Google Scholar]
- 91.Zubcevic J, Waki H, Raizada MK, Paton JFR. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension 57: 1026–1033, 2011. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]