Abstract
Age-related alterations in endothelium and the resulting vascular dysfunction critically contribute to a range of pathological conditions associated with old age. To develop therapies rationally that improve vascular health and thereby increase health span and life span in older adults, it will be essential to understand the cellular and molecular mechanisms contributing to vascular aging. Preclinical studies in model organisms demonstrate that NAD+ availability decreases with age in multiple tissues and that supplemental NAD+ precursors can ameliorate many age-related cellular impairments. Here, we provide a comprehensive overview of NAD+-dependent pathways [including the NAD+-using silent information regulator-2-like enzymes and poly(ADP-ribose) polymerase enzymes] and the potential consequences of endothelial NAD+ deficiency in vascular aging. The multifaceted vasoprotective effects of treatments that reverse the age-related decline in cellular NAD+ levels, as well as their potential limitations, are discussed. The preventive and therapeutic potential of NAD+ intermediates as effective, clinically relevant interventions in older adults at risk for ischemic heart disease, vascular cognitive impairment, and other common geriatric conditions and diseases that involve vascular pathologies (e.g., sarcopenia, frailty) are critically discussed. We propose that NAD+ precursors [e.g., nicotinamide (Nam) riboside, Nam mononucleotide, niacin] should be considered as critical components of combination therapies to slow the vascular aging process and increase cardiovascular health span.
Keywords: endothelial dysfunction, geroscience, microcirculation, oxidative stress, senescence
SUCCESSFUL VASCULAR AGING DETERMINES LIFE SPAN AND HEALTH SPAN
Over the coming decades, the average age of the population of the Western world will continue to grow. Because of the significant increase in the average life expectancy, combined with unfavorable trends in fertility, those aged ≥65 years will become a much larger share of the population [e.g., in the European Union rising from 19 to 29% (51a)]. The share of those aged ≥80 years will increase from 5 to 13% of the population of the European Union by 2070. Similar trends will be manifested both in Japan and the United States. The increasing fiscal strain linked to pensions, health care, and long-term care, combined with the increases in the old-age dependency ratio (people aged 65 years and above relative to those aged 15–64 years; in the European Union, 29.6% in 2016 and 51.2% in 2070), is expected to be a significant challenge to the societies of each industrialized nation (64).
Although aging affects physiology and pathophysiology throughout the body, the consequences of age-related alterations of the cardiovascular system are especially relevant to the life spans and health spans of the populations of the developed countries. Cardiovascular and cerebrovascular diseases are the most common cause of death among older people in these nations (168c), accounting for approximately one-third of all deaths at the age of 65 years and nearly two-thirds at age 85 years (164). In addition, aging-induced functional and structural alterations of the vasculature contribute to the pathogenesis of a wide range of age-related diseases that limit health span, contributing to decreased workforce participation, increased dependency, and institutionalization in older adults. These age-related diseases include coronary heart disease, myocardial infarction, vascular contributions to cognitive impairment and dementia (including stroke), Alzheimer’s disease, hypertension, peripheral artery disease, sarcopenia, and kidney and eye diseases (164). Aging promotes endothelial apoptosis, impairs endothelial angiogenic capacity, and promotes capillary regression (13, 36, 40, 45). A decline in capillary density [“microvascular rarefaction” (13, 142, 149, 157, 168, 169)] contributes to decreased tissue perfusion with age, which is a major contributor to mortality and morbidity. Vascular pathologies also contribute to gait and balance disorders (57, 145, 151, 165) that promote falls. Age-related, proinflammatory changes in the vasculature contribute to the pathogenesis of chronic inflammatory diseases associated with old age, including atherosclerotic diseases (including coronary heart disease, stroke, peripheral artery disease, and renal artery stenosis), osteoarthritis (2), metabolic disease, and diseases of the gastrointestinal tract. Age-related endothelial changes promote increased coagulation and impair stem cell biology [e.g., by altering the local microenvironment in vascular stem cell niches (81, 129)]. Aging-induced dysfunction of microvascular barrier and transport function (e.g., promoting the leakage of microbial breakdown products to the systemic circulation) likely promotes chronic, systemic, low-grade sterile inflammation and distant organ damage (135). Age-related alterations in the endothelial phenotype alter the secretion of growth factors, chemokines, and enzymes that can degrade the extracellular matrix, likely promoting tumor progression, intravasation, and cancer metastases (173). Finally, impaired release of gaseotransmitters [including nitric oxide (NO)] from the microvessels negatively impacts mitochondrial function and cellular bioenergetics in the skeletal muscle, heart, and central nervous system (105, 106).
Therefore, it is critical to understand mechanisms underlying vascular aging (83) to predict and prevent better vascular contributions to the pathogenesis of multiple diseases associated with old age. A better mechanistic understanding of macro- and microvascular aging processes is also critical to develop and evaluate dietary, lifestyle, and pharmacological countermeasures to address this growing health issue.
ROLE OF OXIDATIVE STRESS AND ENDOTHELIAL DYSFUNCTION IN VASCULAR AGING
Impairment of endothelium-dependent, NO-mediated vasodilation (“endothelial dysfunction”) is a frequently used indicator of vascular health (29, 35, 60, 120, 132). Endothelial dysfunction associating with cardiovascular events [reviewed in Lerman and Zeiher (86)] is an early feature of atherosclerotic vascular diseases and significantly contributes to impaired microvascular perfusion (149, 164, 166). Importantly, clinical and preclinical studies demonstrate that aging is a major cause for endothelial dysfunction (9, 44, 51) and that beneficial effects of anti-aging interventions are predicted by their ability to restore endothelial NO mediation in aging (36, 37, 40, 42, 50, 114, 152). In many cases, the loss of NO signaling with age or disease is a direct reflection of oxidative stress, since the superoxide readily reacts with NO to generate peroxynitrite, a free-radical-containing molecule that lacks the signaling ability of NO and damages other molecules. The sources of the superoxide include mitochondrial production and NAD(P)H oxidase activation (36, 37, 44, 136, 143, 151). NO released from the vascular endothelium is a potent vasodilator, which regulates vascular resistance and thereby, tissue perfusion. In addition, endothelium-derived NO confers important vasoprotective, cardioprotective, anti-inflammatory, and anti-aging effects. For instance, NO was demonstrated to regulate cell division and survival, inhibit platelet aggregation and inflammatory cell adhesion to endothelial cells, promote angiogenesis, disrupt proinflammatory signaling pathways, and regulate mitochondrial function and cellular energy metabolism (149, 164, 166). Endothelial dysfunction contributes to the pathogenesis of cardiovascular disease, stroke and hypertension, vascular cognitive impairment and dementia, and a range of pathological conditions, from erectile dysfunction to impaired exercise tolerance, in older adults (164, 166). The critical role of endothelium-derived NO in aging is underscored by the findings that mice genetically deficient for endothelial NO synthase (eNOS) exhibit premature vascular, metabolic, brain, and cardiac aging phenotypes associated with early mortality (89, 150), many of which can be reversed by the supply of NO through exogenous nitrite (147). The mechanisms underlying age-related endothelial dysfunction prominently involve increased oxidative stress (1, 44, 53, 140, 164, 166). Previous preclinical and clinical studies have tested various experimental interventions designed to attenuate oxidative stress and interfere with oxidative stress-mediated pathways to improve endothelial function in animal models of aging (40, 61, 87, 88, 92, 110, 112, 114, 143, 148, 152, 164, 166). Despite these exciting studies, the molecular mechanisms that lie upstream of age-associated, increased oxidative stress remain elusive.
Key objectives of geroscience research are to understand the biology of aging and to translate scientific insight obtained in models of aging into translationally relevant interventions that improve late-life health, including cardiovascular health. The prevailing view in the field of geroscience is that fundamental aging processes are causally upstream of—and the cause of—all age-related pathologies, including cardiovascular diseases. The intervention in these fundamental cellular and molecular processes of aging thus should provide protection against a wide range of age-related diseases and conditions, including endothelial dysfunction. What is currently identifiable about organismal and tissue aging is that it is a very complex process, involving diverse biological mechanisms. However, the exact roles of fundamental cellular and molecular processes of aging in the genesis of increased oxidative stress and consequential endothelial dysfunction in the aging vasculature remain to be elucidated.
ROLE OF NAD+ DEFICIENCY AND CELLULAR ENERGETIC IMPAIRMENT IN AGING-INDUCED ENDOTHELIAL DYSFUNCTION
There is strong evidence that with advanced age, there is decreased availability of cellular NAD+ (62, 95, 177), which may be a common contributor to aging processes across tissues and in evolutionarily distant organisms. In support of this theory, it was demonstrated that the enhancement of NAD+ biosynthesis extends life span in yeast, worms, and flies (3, 4, 12, 102, 103) and improves both general health and longevity in mice (100, 181). Here, we review the evidence supporting the concept that an age-related decline in [NAD+] plays a critical role in vascular aging.
Biological Functions of NAD+
NAD and its phosphorylated form NADP have central roles in the cellular metabolism, energy production, and survival (15). Over 400 enzymes require the NAD+ and NADP+, predominantly to accept or donate electrons for redox reactions. NADP is synthesized by NAD+ kinase, which phosphorylates NAD+. Although both NAD and NADP participate as electron carriers in a multitude of redox reactions, they support distinct functions. NAD+ participates primarily in energy-producing reactions, requiring an electron exchange, including the catabolism of carbohydrates, fatty acids, proteins, and alcohol (e.g., glycolysis, pyruvate-to-lactate and pyruvate-to-acetyl-CoA interconversions, β-oxidation, citric acid cycle, and oxidative phosphorylation). NADP predominantly participates in anabolic pathways, including the synthesis of fatty acids, cholesterol, and DNA. NADP is also critical for the regeneration of components of antioxidant systems. To support these distinct functions, mammalian cells maintain NAD predominantly in the oxidized state to serve as an oxidizing agent for catabolic reactions, whereas NADP exists predominantly in a reduced state (NADPH) to be able to donate electrons readily for reductive cellular biochemical reactions. The cycling of NAD and NADP between oxidized and reduced forms in redox reactions is easily reversible, since when NAD(P)H reduces another molecule, it is reoxidized to NAD(P)+. Thus these coenzymes can continuously cycle between the reduced and oxidized forms without being consumed. The alteration of the availability of these coenzymes, either through a shift in the redox ratio or via changes in cellular synthesis and/or degradation of NAD(H) and NADP(H), will likely affect the function of hundreds of NADH- and NADPH-dependent enzymes.
NAD+ is also the substrate for at least four classes of enzymes important for cellular survival, aging, and normal physiological functioning. These include enzymes with mono ADP-ribosyltransferase and poly(ADP-ribose) polymerase (PARP) activities, which catalyze ADP-ribosyltransfer reactions. NAD+ is a rate-limiting cosubstrate for silent information regulator-2-like enzymes (sirtuins), which are key regulators both of prosurvival pathways and mitochondrial function and catalyze the removal of acyl groups from acylated proteins, using ADP-ribose from NAD as an acceptor. Importantly, both NAD+-dependent PARP enzymes and sirtuins are involved in DNA repair pathways. Finally, ADP-ribosylcyclases, such as CD38, which have relevance for calcium signaling and endothelial NO-mediated vasodilation (180), also require NAD+.
Biosynthesis of NAD+
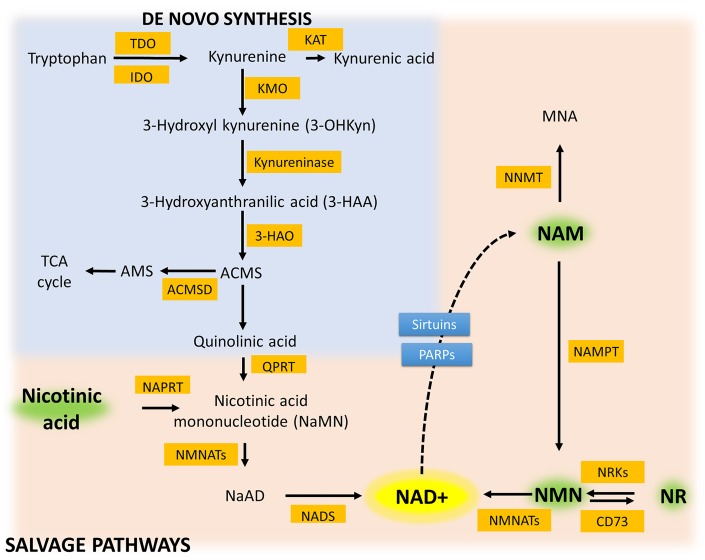
In mammals, NAD+ can be synthesized de novo in the cytosol from the amino acid tryptophan or from nicotinic acid (NA) or salvaged from nicotinamide (Nam) or intermediates containing this moiety (Fig. 1). In the first step of the de novo pathway, tryptophan is converted into N-formylkynurenine by either of two different enzymes: tryptophan-2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO). TDO is critical for NAD+ biosynthesis in the liver, whereas IDO is expressed in many extrahepatic tissues, including endothelial cells (19), and is known to be upregulated in response to inflammatory cytokines. N-formylkynurenine is converted into kynurenine by formamidase. Kynurenine is metabolized in one of two ways: one pathway yields kynurenic acid, whereas the other yields 3-hydroxykynurenine and quinolinic acid, precursors of NAD+.
Fig. 1.
Schematic representation of de novo and salvage pathways for NAD+ biosynthesis. The figure summarizes the key features of both the de novo pathway, whereby l-tryptophan (Trp) is metabolized to NAD+, and the salvage pathway, whereby NAD+ is synthesized from the NAD+ precursors nicotinic acid (NA), nicotinamide (Nam) riboside (NR), and Nam. The de novo biosynthesis of NAD+ starts from Trp, which is enzymatically converted in a series of reactions to quinolinic acid (QA). QA is converted by quinolinate phosphoribosyltransferase (QPRT) to NA mononucleotide (NaMN), which is then converted to NA adenine dinucleotide (NaAD) by Nam mononucleotide (NMN) adenylyltransferase (NMNAT) enzymes. NAD synthase (NADS) generates NAD+ by the amidation of NaAD. In the salvage pathway, NMN is synthesized from Nam by the rate-limiting enzyme, Nam phosphoribosyltransferase (NAMPT). NMN is also synthesized from NR via phosphorylation by NR kinase (NRK). NMN is converted into NAD+ by NMNATs. NA, the other substrate of the NAD+ salvage pathway, is converted by NA phosphoribosyltransferase (NAPRT) to NaMN, which is then converted into NaAD by NMNATs and lastly into NAD by NADS. Multiple enzymes break down NAD+ to produce Nam and ADP-ribosyl moiety, including silent information regulator-2-like enzymes (sirtuins) and poly(ADP-ribose) polymerase 1 and 2 (PARP-1/2). NMN is a substrate of ectoenzyme CD73, with generation of NR. 3-HAO, 3-hydroxyanthranilate-3,4-dioxygenase; ACMS, α-amino-β-carboxymuconate-ε-semialdehyde; ACMSD, ACMS decarboxylase; AMS, aminomuconic semialdehyde; IDO, indoleamine 2,3-deoxygenase; KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase; MNA, 1-methylnicotinamide; NNMT, nicotinamide N-methyltransferase; TCA, tricarboxylic acid cycle; TDO, tryptophan-2,3-dioxygenase.
The Preiss-Handler and NAD+ salvage pathways recycle components of NAD+ that are taken up from food or released by biochemical reactions that break down NAD+. Three vitamin precursors containing a pyridine base that are used in these pathways are NA, Nam, and Nam riboside (NR; Fig. 1). These compounds are termed vitamin B3 or niacin (although niacin may also refer to NA specifically). NAD+ synthesis from Nam requires two steps: Nam is first converted into Nam mononucleotide (NMN) by Nam phosphoribosyltransferase (NAMPT) (69), and then the production of NAD+ from NMN and ATP is catalyzed by NMN adenylyltransferases (NMNATs). NMNAT1 is a nuclear enzyme, NMNAT2 is located in the cytosol and Golgi apparatus, whereas NMNAT3 is located in the mitochondria in most cell types (76). NAMPT is considered the rate-limiting component in this NAD+ biosynthesis pathway (123). In the Preiss-Handler pathway, NA is converted into NA mononucleotide (NaMN) by the addition of ribose-phosphate (from phosphoribosyl pyrophosphate by NA phosphoribosyltransferase). NaMN is then converted into NA adenine dinucleotide (NaAD) by NMNATs and lastly into NAD+ with the presence of ATP and ammonia by NAD synthase. In mammals, which lack nicotinamidase, NA seems to be derived primarily from extracellular sources. Exogenously administered NA has been demonstrated to be a good precursor of NAD biosynthesis, significantly increasing tissue NAD+ levels (34, 71, 90), in addition to its better-known effect of a lipid-lowering agent via direct inhibition of triglyceride synthesis and decreasing secretion of VLDL and LDL particles from hepatocytes (74). Important for the present review is that treatment with niacin is associated with improved endothelial function (126). NR and NA riboside are converted to NMN and NaMN, respectively, by NR kinase 1 (NRK1) and NRK2 (15, 16, 121).
Despite the presence of the de novo pathway, the NAD+ salvage pathway is essential in mammals: a lack of niacin in the diet results in a significant decline in tissue NAD+ (122), and mice lacking NAMPT constitutively are not viable (124). Even with an intact salvage pathway, the lack of niacin in the diet causes the severe vitamin deficiency disease pellagra (84), which is characterized by dermatitis, diarrhea, dementia, and ultimately, death. Data derived from the 1995 Continuing Survey of Food Intakes by Individuals indicate that in the United States, the greatest contribution to the niacin intake of the adult population comes from mixed dishes high in meat, fish, or poultry, enriched and whole-grain breads, and fortified cereals (70). Fish, such as tuna (niacin content: 18.4 mg/100 g), sardines (168a), and salmon (niacin content: 7.8 mg/100 g), and chicken meat (niacin content: 13.9 mg/100 g) and liver (niacin content: 11 mg/100 g) are relatively rich in NAD+ precursors. One of the best food sources of niacin is yeast (niacin content: 40.2 mg/100 g) (168b). Milk and milk products also contain NAD+ precursors (60% as Nam, 40% as NR) (156), although the niacin content in them is significantly lower relative to the aforementioned food items (niacin content in milk: 0.089 mg/100 g). Several food items contain particularly high concentrations of NMN, including edamame, avocado, and broccoli (100).
It should be noted that niacin intake in the adult population in the United States is generous compared with the Estimated Average Requirement (EAR) (70). For instance, the median intake by adult women is 17–20 mg niacin, which exceeds the EAR of 11 mg niacin equivalents needed to prevent pellagra. The Boston Nutritional Status Survey reported that people over age 60 years in this cohort have a median niacin intake of 21 mg/day for men and 17 mg/day for women (70). Niacin intake from supplements is also significant. Over one-third of adults participating in the National Health and Nutrition Examination Survey (1999–2000) reported taking a multivitamin dietary supplement containing niacin in the previous month (119). Data from the Boston Nutritional Status Survey indicates that in elderly individuals taking supplements, the 50th percentile of supplemental niacin intake was 20 mg for men and 30 mg for women (70). Of note, supplements containing up to ~400 mg niacin are available without a prescription. It should also be noted that NA has been used as a lipid-lowering agent since the 1970s, based on its inhibitory effect of triglyceride synthesis, accelerated intracellular hepatic apo B degradation, and the decreased secretion of VLDL and LDL particles.
Endothelial cells abundantly express the enzymes required to metabolize NAD+ precursors (A. Csiszar and Z. Ungvari, unpublished observations), suggesting that endothelial NAD+ levels are likely to be responsive to exogenous administration or dietary intake of NAD+ precursors. For a more extensive review on the biosynthesis of NAD+, the reader is directed to Belenky et al. (15) and Katsyuba and Auwerx (76).
Mechanisms of Age-Related Decline in Cellular NAD+ Levels
NAD+ concentration decreases in multiple tissues over the course of normal aging. Although the dispersion of endothelial cells within a given tissue makes it difficult to measure their NAD+ pools directly in situ, studies on endothelial cells isolated from the brains of young and aged animals provide evidence that [NAD+] also falls in the endothelial compartment (S. Tarantini, A. Csiszar, and Z. Ungvari, unpublished observations).
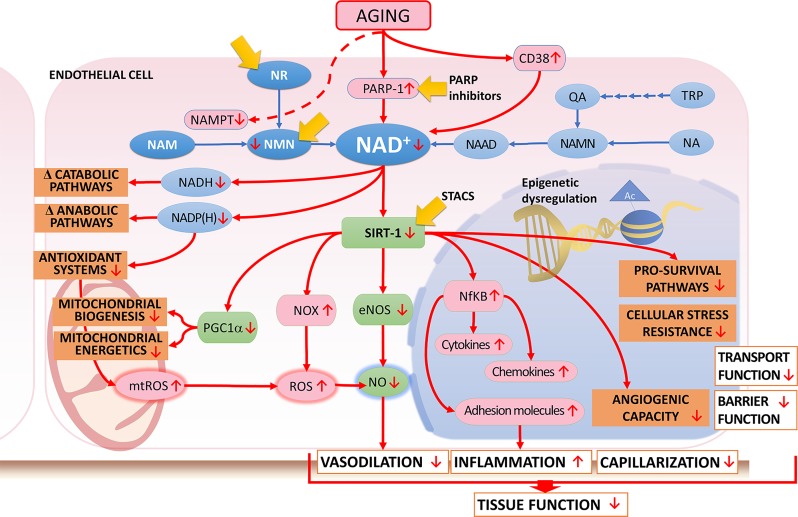
The mechanisms underlying the age-related decline in [NAD+] are likely multifaceted (127) and may include decreased expression of NAMPT (which catalyzes the rate-limiting step in the biosynthesis of NAD+) (178), increased use of NAD+ by activated PARP1 (110), and increased activity and expression of the NADase CD38 (23, 146) (Fig. 2). The functional relevance of these pathways is shown by the findings that genetic depletion of NAMPT and/or pharmacological inhibition of NAMPT (by the inhibitor FK866) decrease cellular NAD+ levels and mimic aspects of the aging phenotype in endothelial cells (171), skeletal muscle (131), and neuronal cells (138, 139). PARP1 is a constitutive factor of the DNA damage-surveillance network. In aged cells, PARP1 is activated in response to DNA damage induced by increased oxidative/nitrative stress. PARP1 cleaves NAD+ and transfers the resulting ADP-ribose moiety onto target nuclear proteins and onto subsequent polymers of ADP-ribose, depleting cellular NAD+ pools in the process. There is evidence that in human tissues (skin samples), advanced aging results in increased DNA damage, which correlates with increased PARP activity and decreased NAD+ levels (95). Importantly, genetic depletion (11) and/or pharmacological inhibition of PARP1 were shown to increase tissue NAD+ levels in rodent models of accelerated aging. Pharmacological inhibition of PARP1 was also shown to improve endothelial function in aged rodents (110–112). Two recent studies demonstrated that the expression and activity of the NADase CD38 increase with age and that the blocking of CD38 activity is sufficient to increase [NAD+] and prevent the age-related decline in multiple tissues, including skeletal muscle, liver, and adipose tissue (23, 146). Endothelial cells are known to express CD38, and CD38-mediated NAD+ depletion in this cell type has been linked to loss of eNOS-mediated NO generation (22, 125).
Fig. 2.
Role of NAD+ deficiency in aging-induced endothelial dysfunction. Aging-induced mechanisms contributing to an age-related decline in NAD+ content may include upregulation of pathways consuming NAD+ [poly(ADP-ribose) polymerase 1 (PARP-1) activation, CD38] and decreased biosynthesis of NAD+ [e.g., due to downregulation of nicotinamide (Nam) phosphoribosyltransferase (NAMPT)]. PARP-1 is a key NAD+-consuming enzyme, competing with silent information regulator-2-like enzymes (sirtuins) for NAD+ availability. In aging, increased DNA damage results in nuclear PARP-1 activation, lowering NAD+ availability. The consequences of age-related NAD+ depletion in endothelial cells include decreased activation of sirtuins (SIRT-1, -2, -6, and -7 in the nucleus; SIRT-3, -4, and -5 in mitochondria; and SIRT-1 and -2 in the cytosol), which contribute to dysregulation of mitochondrial biogenesis; impaired mitochondrial energetics; increased mitochondrial production of reactive oxygen species (mtROS); upregulation of NADPH oxidases (NOX); decreased endothelial nitric oxide (NO) synthase (eNOS) activity and impaired bioavailability of NO; increased activity of NF-κB-driven, proinflammatory pathways; and downregulation of prosurvival and stress-resilience pathways and pathways involved in angiogenesis. Decreased NAD+ supply also altered NADH levels and synthesis of NADP/NADPH, contributing to age-related changes in a wide range of NADH- and NADPH-dependent catabolic and anabolic pathways, as well as impairment of NADP(H)-dependent regeneration of antioxidant systems (e.g., glutathione). These changes impair endothelium-dependent vasodilation, promote inflammation, decrease capillarization and tissue blood flow, and impair transport and barrier function of the endothelial cells. The multifaceted impairment of microvascular endothelial function contributes significantly to the age-related dysfunction of multiple organs. Yellow arrows highlight potential targets for intervention to rescue the function of the NAD+/SIRT-1 axis in aged endothelial cells. These anti-aging interventions include the rescuing of NAD+ levels by treatment with NAD+ precursors [Nam riboside (NR), Nam mononucleotide (NMN)], pharmacological inhibition of NAD+ using PARP-1 activation, or treatment with sirtuin-activating compounds (STACs). NA, nicotinic acid; NAAD, NA adenine dinucleotide; NAMN, NA mononucleotide; PGC1α, peroxisome proliferator-activated receptor-γ coactivator-1α; QA, quinolinic acid; TRP, l-tryptophan.
In addition to the intrinsic effects of age, cardiovascular risk factors that promote accelerated vascular aging result in cellular NAD+ depletion. Accordingly, there is evidence linking high-fat diet-induced obesity (27, 59), high homocysteine levels (20), and diabetes (133, 134) to a decline in cellular NAD+ levels, which would likely contribute to endothelial dysfunction.
Anti-Aging Effects of Treatment with NAD+ Boosters
Cellular NAD+ levels can be increased by the upregulation of the enzymes involved in NAD+ biosynthesis, by inhibition of NAD+ consumers (76), or by treatment with NAD+ precursors (26), including niacin, NMN (48, 107, 159), and NR. Whereas overexpression of enzymes that catalyze NAD+ biosynthesis (NAMPT or NMNATs) effectively boosts NAD+ levels (54, 76), the translational potential of this approach is limited. Significant data are available to support the efficacy and translational relevance of NMN and NR treatment (177). NMN is considered an especially promising candidate as an anti-aging therapeutic approach, due to its multi-targeted effect (80).
Administration of NMN or NR to aged mice increases tissue NAD+ levels (100, 177, 181). The rise in NAD was detected within minutes in some studies, indicating that NMN is quickly absorbed in the gut and is either efficiently transported in the circulation and readily converted by the cells to NAD+ or alternatively, is converted to another NAD+ precursor in the liver, which then circulates to peripheral tissues, increasing cellular NAD+ levels. Recent findings support the latter view, showing that there is a significant first-pass effect, and orally administered NMN and NR are readily metabolized to Nam in the liver, which then can get into the circulation, increasing NAD+ levels in other organs (91). There are strong data to show that human blood NAD+ can rise as much as 2.7-fold with a single oral dose of NR and that oral NR elevates tissue NAD+ in the mouse liver with superior pharmacokinetics to those of NA and Nam (154). Additionally, single doses of 100, 300, and 1,000 mg NR were demonstrated to result in dose-dependent increases in the blood NAD+ metabolome in humans (154). Note that the doses of NAD+ precursors used in preclinical and clinical studies to reverse the adverse effects of aging are significantly higher than the EAR (70) of ~11 mg niacin equivalents needed to prevent pellagra in humans, even if allometric scaling is used.
There is increasing evidence that restoration of cellular NAD+ levels by treatment with NAD+ precursors in aged mice exerts multifaceted anti-aging effects, reversing age-related dysfunction in multiple organs, including the eye (100), skeletal muscle (62), and brain (73). Even short-term administration of NMN or NR has been demonstrated to exert significant protecting effects in a wide range of age-related pathophysiological conditions, improving skeletal muscle energetics and function (62), protecting neuronal stem cells, and increasing the mouse life span (181). The NAD+ booster acipimox, a niacin derivative used for treatment of hyperlipidemia in type 2 diabetic patients, was also shown to improve mitochondrial function in the skeletal muscle (170). NR was also shown to exert protective effects against high-fat diet-induced metabolic abnormalities (27, 155).
Importantly, chronic treatment of aged mice with NAD+ boosters was shown to improve endothelial function in the aorta [Z. Ungvari and S. Tarantini, unpublished observations, and de Picciotto et al. (50)] and in the cerebral circulation (Z. Ungvari and S. Tarantini, unpublished observations). Studies are currently underway to determine whether chronic treatment with NR improves cerebral blood flow (ClinicalTrials.gov, Identifier NCT03482167) in older adults with mild cognitive impairment. More recently, treatment of aged mice with NMN was shown to reverse age-related capillary rarefaction and increase blood flow in the skeletal muscle (48), likely by the increase of the angiogenic capacity of endothelial cells (21, 48). There is also evidence suggesting that in old mice, NMN treatment restores fenestration of liver sinusoidal endothelial cells (66). Fenestration of liver sinusoidal endothelial cells enables the bidirectional exchange of substrates (including insulin, lipoproteins, and pharmacological agents) between the blood and hepatocytes and thereby importantly, contributes to metabolic homeostasis. With increasing age, the frequency and diameter of fenestrations significantly decrease, likely due to age-related disruption of VEGF and NO-dependent signaling pathways, which promote pathologic remodeling of the actin cytoskeleton and cell membrane lipid rafts (32, 72, 108). It is likely that NMN treatment exerts its protective effects on the liver sinusoidal endothelial cells by the restoration of endothelial NO mediation. The available evidence suggests that higher dietary niacin intake is also associated with improved vascular endothelial function in older adults (75). Yet, niacin as add-on treatment to high-dose statins in patients with established coronary artery disease does not appear to improve endothelial function (116). Consistent with the protective effects of diverse NAD+ boosters, treatment of aged rodents with PARP1 inhibitors, which should spare NAD+ (25, 28), was also shown to improve endothelial function (110–112).
Mitochondrial dysfunction and elevated mitochondrial oxidative stress play a critical role in aging-induced cardiovascular dysfunction (47, 136, 161) and vascular impairment (61, 143). The mechanisms contributing to mitochondrial oxidative stress in the aged endothelium are likely multifaceted and involve a dysfunctional electron transport chain. Reduced electron flow through the electron transport chain, in particular, due to aging-induced dysregulation of complex I and complex III (82), likely promotes electron leak and favors increased mitochondrial production of reactive oxygen species. A key mechanism underlying the anti-aging action of NMN treatment is the improvement of cellular energetics by the rescuing of mitochondrial function (62), at least in part, by activating sirtuin deacylases (SIRT1–SIRT7; Fig. 2). Sirtuins are known to mediate beneficial anti-aging (33, 102, 174) and vasoprotective effects (36, 37, 42) of caloric restriction as well. In support of this concept, knockdown of SIRT1 in aged cerebromicrovascular endothelial cells was shown to abolish the antioxidative and mitochondrial-protective effects of NMN treatment (Z. Ungvari and A. Csiszar, unpublished observation). There is direct evidence that activation of SIRT1 underlies NMN-induced restoration of the endothelial angiogenic capacity and increased capillarization in aged mice (141). Previous studies suggest that the age-related decline in oxidative phosphorylation (OXPHOS) and/or increased mitochondrial oxidative stress may be due, at least in part, to the specific loss of mitochondrially encoded transcripts (62). In that regard, it is important that NMN treatment was shown to restore expression of mitochondrial-encoded OXPHOS subunits in aged mice in a SIRT1-dependent manner (62). Treatment with NR was also shown to upregulate mitochondrial gene expression and promote mitochondrial biogenesis in the mouse skeletal muscle (27). Moreover, recent studies show that pharmacological inhibition of α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase (115), the enzyme that limits spontaneous cyclization of α-amino-β-carboxymuconate-ε-semialdehyde in the de novo NAD+ synthesis pathway, can also boosts de novo NAD+ synthesis and SIRT1 activity, ultimately enhancing mitochondrial function in the kidney and liver (77). We posit that rescue of vascular mitochondrial function by the restoration of the expression of mitochondrial-encoded OXPHOS subunits contributes to the vasoprotective effects of treatment with NAD boosters. These observations accord with findings from earlier studies, demonstrating that many of the health benefits of SIRT1 activation are linked to improved mitochondrial function (14). Furthermore, SIRT1-activating compounds (STACs), such as resveratrol and SRT1720, have been demonstrated to exert significant vasoprotective effects in aging and models of accelerated vascular aging (30, 39, 56, 101, 114, 161–163, 179), similar to NAD+ boosters, including upregulation of mitochondrial biogenesis (38), attenuation of mitochondrial oxidative stress (43, 160), activation of antioxidant defense mechanisms (41), and inhibition of apoptosis (114), in endothelial and vascular smooth muscle cells. STACs were also shown to increase capillary density (109), improve endothelial function and blood flow regulation (152), and prevent microvascular fragility (151) in the aged mouse brain and to exert similar vasoprotective effects in nonhuman primate models as well (18, 96). Future studies should determine whether NAD+ boosters also confer similar vascular health benefits. In addition to sirtuin-mediated effects, because mitochondrial ATP production and membrane potential require NAD as an essential coenzyme, the restoration of an optimal NAD/NADH ratio itself should promote efficient mitochondrial function in vascular cells.
PERSPECTIVES
Taken together, progress in geroscience research, investigating the role of fundamental aging processes in the development of age-related chronic diseases (55, 79, 94, 130), including cardiovascular pathologies, has been rapid in recent years (10, 46, 52, 55, 85, 98, 104, 117, 164), from both the basic science and clinical perspectives. The field of vascular aging research matured and expanded when researchers started to apply breakthrough discoveries in biogerontology to the development of new therapeutic strategies to prevent/reverse age-related pathologic functional and phenotypic alterations of blood vessels. In particular, NAD+-boosting strategies were shown to confer multifaceted health benefits in aging, including potential translationally relevant vasoprotective effects. However, the understanding of the cellular and molecular mechanisms by which age-related NAD+ deficiency contributes to age-related vascular pathologies, elucidating the exact mechanisms by which NAD+-boosting strategies exert their anti-aging vascular effects and translating the preclinical findings to the clinics, remains a substantial challenge and an active area of research with numerous open questions.
It remains unclear what downstream mechanisms mediate the beneficial vascular effects of NAD+ boosters. In addition to the role of established NAD+ biosynthetic pathways, new research may reveal new aspects of the NAD+ metabolism, including novel pathways that use NAD+ [e.g., NAD+ addition to RNAs (76)], which contribute to the biological effects of NAD+ boosters in the aged vasculature.
Although NMN and NR have been tested in diverse disease models, no side-by-side comparisons have been conducted between NMN and NR in the context of macrovascular and microvascular aging. Future pharmacological and nutraceutical strategies to rescue vascular NAD+ levels in aging will also need to take into account the limited oral bioavailability of NR and NMN, as well as the tissue specificity of important pathways in the NAD+ metabolism (91). Furthermore, a recent meta-analysis of all randomized studies that compared niacin with placebo, either alone or in combination with statin treatment or other treatments that lower LDL cholesterol levels, also showed that niacin does not affect significantly all-cause mortality rates and does not lower the risk of cardiovascular mortality, nonfatal myocardial infarction, or stroke or the need for revascularization (58). With that regard, studies aimed at the understanding of the differential biological effects of treatment with niacin, NMN, and NR will be highly informative.
Compartmentalization of NAD+ biosynthesis is also not well understood. Subcellular compartments (e.g., the nucleus, cytosol, and mitochondria) appear to express distinct pathways to synthesize NAD+ (176). However, it is not clear what the relevance of this spatial organization is, given that individual enzymes appear to be dispensable in most cases (24, 175), and tracer studies suggest that intact NAD+ can move between the cytosol and mitochondria (49). It is presently unclear how NAD+ intermediates are transported across cell membranes and shared among different subcellular compartments in endothelial cells. Novel isotope-tracer methods to analyze NAD synthesis-breakdown fluxes have been developed (91), which could be adapted to study the endothelial cell-specific NAD+ metabolism.
In 2009, Imai (67) proposed an interesting concept, named the “NAD World,” which implicated the NAD+ metabolism and SIRT1 in systemic regulation of mammalian aging and longevity. Since then, the concept has evolved, and now NMN is hypothesized to function as a systemic signaling molecule that participates in inter-tissue communications among three key tissues, namely, the hypothalamus, adipose tissue, and skeletal muscle, for regulation of aging processes and longevity control (68). The concept implies that the hypothalamus is a high-order control center of systemic aging processes and that inter-tissue communication among the adipose tissue, skeletal muscle, and hypothalamus, mediated by circulating factors (including myokines and adipokines), comprises a critical feedback loop. Importantly, transport and uptake of circulating NMN, as well as inter-tissue communication via circulating factors, depend on the function of the (micro)vasculature. Endothelial cells also express key components of pathways involved in NAD+ biosynthesis and degradation (including PARP1 and CD38). Additionally, SIRT1 is known to regulate several aspects of endothelial function, including angiogenesis and vasodilatory function. Furthermore, NMN appears to impact significantly the function and phenotype of endothelial cells in aging. Thus it would be interesting to incorporate in the model the function and age-related changes of the microvascular endothelial cells and consider the role of endothelial cells (which represent the largest endocrine organ) in systemic regulation of aging within the framework of the NAD World.
With the translation of the protective effects of NAD+-boosting strategies into clinical benefits, several challenges should be considered, including the side-effect profiles of such treatments. Treatment with l-tryptophan is known to cause a range of unwanted side effects (belching and gas, blurred vision, diarrhea, dizziness, drowsiness, dry mouth, headache, heartburn), including the potentially severe eosinophilia-myalgia syndrome (for which it was recalled in 1990). Niacin treatment can cause a flushing reaction (17), as well as gastrointestinal side effects and liver problems, and may promote impaired glucose tolerance (99, 128) at high doses (e.g., ~3 g/day NA). Adverse effects (nausea, vomiting, and signs of liver toxicity) have been reported at Nam intakes of 3 g/day (118) and intakes of NA of 1.5 g/day (97). The niacin derivative lipid-lowering agent acipimox (Olbetam) also causes flushing and gastrointestinal side effects in 10% of the patients. Individuals with liver disease, diabetes mellitus, and alcoholism are more susceptible to the adverse effects of excess niacin intake. Unlike other NAD+ boosters, Nam has the capacity to exert end-product inhibition on SIRT1 deacetylase activity, which may result in unwanted side effects as well. Importantly, chronic administration of NMN resulted in no apparent toxicity in mice (100). Similarly, chronic treatment of laboratory mice with NR for 5–6 (63), 10 (181), or 12 mo (158) was not associated with any obvious toxic adverse effects. It is promising that small-scale clinical studies with NR treatment have not reported adverse effects in humans (154). A small, randomized, placebo-controlled, crossover clinical trial of NR supplementation (2 × 500 mg/day for 2 × 6 weeks) in older adults (93) also reported no major adverse effects. Nevertheless, subsequent clinical trials on larger cohorts should carefully monitor adverse events associated with NMN and NR treatment. It is expected that soon, reliable information will be available on the pharmacokinetics, dosing, and side-effect profiles of NMN and NR treatments in older adults. Multiple clinical studies are ongoing, investigating the effects of treatment with NAD+ boosters in humans, including the effects of NMN on metabolic health in women (ClinicalTrials.gov, Identifier NCT03151239). Ongoing clinical trials with NR treatment include studies to investigate the effects of NR on mitochondrial biogenesis and mitochondrial function (ClinicalTrials.gov, Identifiers NCT03432871 and NCT02835664). Importantly, many of the NAD+ precursors are considered vitamins and are widely available to the public as dietary supplements. New studies should also determine which pharmacological strategies that aim to boost cellular NAD+ levels by the inhibition of degradation of NAD+ would be the most appropriate for vasoprotection in older adults. Several PARP inhibitors are currently available or are undergoing clinical trials for oncologic indications. One important consideration is that PARP inhibitors are potentially genotoxic, which may limit their use in patients with nononcologic diseases.
The effects of an initial study, using longer treatment with NR (2 × 500 mg/day for 6 weeks) on endothelium-dependent dilation and arterial stiffness (ClinicalTrials.gov, Identifier NCT02921659), were recently reported (93). However, the results of the effects of NR on endothelial function and vascular health were inconclusive. Whereas NR was found to elicit small decreases in blood pressure and somewhat reduce aortic stiffness, it did not improve endothelium-dependent, flow-mediated dilation of brachial arteries (93). However, this initial clinical trial had important limitations, which necessitate targeted follow-up studies with fewer outcomes based on two-sided statistical inference to confirm the effects of NR treatment on vascular health. It is becoming evident that in addition to the testing of the effects of NAD+ boosters in healthy adults who exhibit near-normal vascular function, future investigations should also include older patients with cardiovascular and metabolic diseases, characterized by significantly impaired endothelial function. Additional research is also needed to develop sensitive NAD+ quantification methods, preferably the assessment of the entire NAD+ metabolome in relevant tissues, that could be used in the clinical setting to evaluate treatment efficiency (31).
Research over the past two decades has broadened our view of the multifactorial nature and heterogeneity of cellular aging processes (78) that contribute to age-related cardiovascular pathologies (164). Furthermore, there is considerable crosstalk between signaling pathways involved in the vascular aging process. With age, multiple regulatory and homeostatic mechanisms become dysfunctional, and impairment of these compensatory mechanisms significantly decrease cellular resilience to other stressors as well. Because of the complexity of age-related physiological dysfunction, there is a strong, scientific rationale for the pursuance of multiple targets to delay cardiovascular aging. The development of rationally “anti-aging” interventions that target multiple steps in the vascular aging process will likely require a combination therapy approach. Future studies should explore how NAD-boosting strategies can be combined with selective inhibitors of other cellular pathways involved in the aging process (e.g., mammalian target of rapamycin) and determine the dose-limiting toxicities of such combination-targeted therapies.
Finally, the understanding of NAD+ depletion in smooth muscle cell pathophysiology is also a promising area for research. There is evidence that NAD+ levels affect vascular smooth muscle cell contractility and impact structural integrity of the vascular wall (65). For example, vascular smooth muscle-specific, Nampt-deficient mice exhibit an ~40% reduction in aortic NAD+, which appears to promote pathogenesis of aortic aneurysms (172). It will be interesting to determine whether treatment with NAD+ boosters can reverse/prevent alterations in vascular structure and function, which are secondary to aging-induced phenotypic changes in smooth muscle cells (136, 137, 144, 151, 153, 165).
Collectively, we are entering a new era of vascular aging research, and it will change the way we approach prevention and treatment of age-related cardiovascular pathologies. Pharmaceutical companies that prepare for this paradigm shift will realize tremendous benefits for years to come. NAD+-boosting therapeutic strategies have the potential to delay/reverse the age-associated physiological decline in the cardiovascular system, and therefore, we predict that they will be useful components in future anti-aging treatment protocols for prevention of aging-related diseases and extension of the cardiovascular health span.
GRANTS
This work was supported by grants from the American Heart Association (to S. Tarantini), National Institute on Aging (NIA; R01-AG055395 to Z. Ungvari, R01-AG047879 and R01-AG038747 to A. Csiszar, and R01-AG043483 to J. A. Baur), National Heart, Lung, and Blood Institute (R01HL132553), National Institute of Neurological Disorders and Stroke (R01-NS056218 to A. Csiszar and R01-NS100782 to Z. Ungvari), National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK098656 to J. A. Baur), NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), NIA-supported Oklahoma Nathan Shock Center (3P30AG050911-02S1 to Z. Ungvari and A. Csiszar), U.S. National Institutes of Health-supported Oklahoma Shared Clinical and Translational Resources (to A. Yabluchanskiy), National Institute of General Medical Sciences (U54GM104938), Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar, Z. Ungvari, and A. Yabluchanskiy), Presbyterian Health Foundation (to Z. Ungvari, A. Csiszar, and A. Yabluchanskiy), European Union-funded Hungarian grant (EFOP-3.6.1-16-2016-00008), and Reynolds Foundation (to Z. Ungvari and A. Csiszar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C. and S.T. prepared figures; A.C., S.T., and Z.U. drafted manuscript; A.C., S.T., A.Y., P.B., T.K., E.F., J.A.B., and Z.U. edited and revised manuscript; A.C., S.T., A.Y., P.B., T.K., E.F., J.A.B., and Z.U. approved final version of manuscript.
REFERENCES
- 1.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol 285: H1015–H1022, 2003. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khazraji BK, Appleton CT, Beier F, Birmingham TB, Shoemaker JK. Osteoarthritis, cerebrovascular dysfunction and the common denominator of inflammation: a narrative review. Osteoarthritis Cartilage 26: 462–470, 2018. doi: 10.1016/j.joca.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem 277: 18881–18890, 2002. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423: 181–185, 2003. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol 20: 1493–1499, 2000. doi: 10.1161/01.ATV.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 10.Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 39: 129–145, 2017. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab 13: 461–468, 2011. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MF, Arking R, Freeman DC, Maiese K, Tzivion G. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem 283: 27810–27819, 2008. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banki E, Sosnowska D, Tucsek Z, Gautam T, Toth P, Tarantini S, Tamas A, Helyes Z, Reglodi D, Sonntag WE, Csiszar A, Ungvari Z. Age-related decline of autocrine pituitary adenylate cyclase-activating polypeptide impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 70: 665–674, 2015. doi: 10.1093/gerona/glu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci 32: 12–19, 2007. doi: 10.1016/j.tibs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Belenky P, Christensen KC, Gazzaniga F, Pletnev AA, Brenner C. Nicotinamide riboside and nicotinic acid riboside salvage in fungi and mammals. Quantitative basis for Urh1 and purine nucleoside phosphorylase function in NAD+ metabolism. J Biol Chem 284: 158–164, 2009. doi: 10.1074/jbc.M807976200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benyó Z, Gille A, Kero J, Csiky M, Suchánková MC, Nüsing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest 115: 3634–3640, 2005. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 8: 899–916, 2016. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beutelspacher SC, Tan PH, McClure MO, Larkin DF, Lechler RI, George AJ. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant 6: 1320–1330, 2006. doi: 10.1111/j.1600-6143.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 20.Blundell G, Jones BG, Rose FA, Tudball N. Homocysteine mediated endothelial cell toxicity and its amelioration. Atherosclerosis 122: 163–172, 1996. doi: 10.1016/0021-9150(95)05730-7. [DOI] [PubMed] [Google Scholar]
- 21.Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell 8: 100–112, 2009. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 22.Boslett J, Hemann C, Christofi FL, Zweier JL. Characterization of CD38 in the major cell types of the heart: endothelial cells highly express CD38 with activation by hypoxia-reoxygenation triggering NAD(P)H depletion. Am J Physiol Cell Physiol 314: C297–C309, 2018. doi: 10.1152/ajpcell.00139.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, Chini EN. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab 23: 1127–1139, 2016. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD+. Science 352: 1474–1477, 2016. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cantó C, Auwerx J. Interference between PARPs and SIRT1: a novel approach to healthy ageing? Aging (Albany NY) 3: 543–547, 2011. doi: 10.18632/aging.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantó C, Auwerx J. Targeting sirtuin 1 to improve metabolism: all you need is NAD(+)? Pharmacol Rev 64: 166–187, 2012. doi: 10.1124/pr.110.003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 15: 838–847, 2012. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantó C, Sauve AA, Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol Aspects Med 34: 1168–1201, 2013. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 30.Chen YX, Zhang M, Cai Y, Zhao Q, Dai W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE−/− mice through inhibiting vascular inflammatory response. Biochem Biophys Res Commun 465: 732–738, 2015. doi: 10.1016/j.bbrc.2015.08.066. [DOI] [PubMed] [Google Scholar]
- 31.Clement J, Wong M, Poljak A, Sachdev P, Braidy N. The plasma NAD+ metabolome is dysregulated in “normal” aging. Rejuvenation Res 22: 121–130, 2019. doi: 10.1089/rej.2018.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cogger VC, Mohamad M, Solon-Biet SM, Senior AM, Warren A, O’Reilly JN, Tung BT, Svistounov D, McMahon AC, Fraser R, Raubenheimer D, Holmes AJ, Simpson SJ, Le Couteur DG. Dietary macronutrients and the aging liver sinusoidal endothelial cell. Am J Physiol Heart Circ Physiol 310: H1064–H1070, 2016. doi: 10.1152/ajpheart.00949.2015. [DOI] [PubMed] [Google Scholar]
- 33.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 34.Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J Biol Chem 247: 778–783, 1972. [PubMed] [Google Scholar]
- 35.Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience 40: 337–346, 2018. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-tumor necrosis factor-α treatment in aging. Am J Pathol 170: 388–698, 2007. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci 70: 303–313, 2015. doi: 10.1093/gerona/glu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the nonhuman primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci 68: 235–249, 2013. doi: 10.1093/gerona/gls158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820, 2012. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002. doi: 10.1161/01.RES.0000020401.61826.EA. [DOI] [PubMed] [Google Scholar]
- 45.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham GM, Flores LC, Roman MG, Cheng C, Dube S, Allen C, Valentine JM, Hubbard GB, Bai Y, Saunders TL, Ikeno Y. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. Geroscience 40: 453–468, 2018. doi: 10.1007/s11357-018-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, Kim LJ, Osborne B, Joshi S, Lu Y, Treviño-Villarreal JH, Kang MJ, Hung TT, Lee B, Williams EO, Igarashi M, Mitchell JR, Wu LE, Turner N, Arany Z, Guarente L, Sinclair DA. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell 173: 74–89.e20, 2018. [Comment in Cell 173: 8–10, 2018.] 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davila A, Liu L, Chellappa K, Redpath P, Nakamaru-Ogiso E, Paolella LM, Zhang Z, Migaud ME, Rabinowitz JD, Baur JA. Nicotinamide adenine dinucleotide is transported into mammalian mitochondria. eLife 7: e33246, 2018. doi: 10.7554/eLife.33246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15: 522–530, 2016. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol 589: 4545–4554, 2011. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.European Commission’s Directorate-General for Economic and Financial Affairs . The 2018 Ageing Report: Economic & Budgetary Projections for the 28 EU Member States (2016–2070). Luxembourg City, Luxembourg: Publications Office of the European Union, 2018, Institutional Paper 079. https://ec.europa.eu/info/publications/economy-finance/2018-ageing-report-economic-and-budgetary-projections-eu-member-states-2016-2070_en. [Google Scholar]
- 52.Fang Y, McFadden S, Darcy J, Hill CM, Huber JA, Verhulst S, Kopchick JJ, Miller RA, Sun LY, Bartke A. Differential effects of early-life nutrient restriction in long-lived GHR-KO and normal mice. Geroscience 39: 347–356, 2017. doi: 10.1007/s11357-017-9978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Lüscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110: 2889–2895, 2004. doi: 10.1161/01.CIR.0000147731.24444.4D. [DOI] [PubMed] [Google Scholar]
- 54.Frederick DW, Davis JG, Dávila A Jr, Agarwal B, Michan S, Puchowicz MA, Nakamaru-Ogiso E, Baur JA. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem 290: 1546–1558, 2015. doi: 10.1074/jbc.M114.579565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40: 513–521, 2018. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gano LB, Donato AJ, Pasha HM, Hearon CM Jr, Sindler AL, Seals DR. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am J Physiol Heart Circ Physiol 307: H1754–H1763, 2014. doi: 10.1152/ajpheart.00377.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner AW, Montgomery PS, Casanegra AI, Silva-Palacios F, Ungvari Z, Csiszar A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age (Dordr) 38: 64, 2016. doi: 10.1007/s11357-016-9925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garg A, Sharma A, Krishnamoorthy P, Garg J, Virmani D, Sharma T, Stefanini G, Kostis JB, Mukherjee D, Sikorskaya E. Role of niacin in current clinical practice: a systematic review. Am J Med 130: 173–187, 2017. doi: 10.1016/j.amjmed.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 59.Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, Kim B, Park YK, Piersigilli A, Pham TX, Yang Y, Ku CS, Koo SI, Fomitchova A, Cantó C, Schoonjans K, Sauve AA, Lee JY, Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology 63: 1190–1204, 2016. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol (1985) 102: 63–71, 2007. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 61.Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol 592: 2549–2561, 2014. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155: 1624–1638, 2013. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gong B, Pan Y, Vempati P, Zhao W, Knable L, Ho L, Wang J, Sastre M, Ono K, Sauve AA, Pasinetti GM. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol Aging 34: 1581–1588, 2013. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research . Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 65.Humphrey JD, Milewicz DM. Aging, smooth muscle vitality, and aortic integrity. Circ Res 120: 1849–1851, 2017. doi: 10.1161/CIRCRESAHA.117.311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunt NJ, Lockwood G, Warren A, Mao H, McCourt P, Le Couteur DG, Cogger VC. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am J Physiol Gastrointest Liver Physiol 316: G144–G154, 2019. doi: 10.1152/ajpgi.00179.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imai S. The NAD World: a new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem Biophys 53: 65–74, 2009. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Imai SI. The NAD World 2.0: the importance of the inter-tissue communication mediated by NAMPT/NAD+/SIRT1 in mammalian aging and longevity control. NPJ Syst Biol Appl 2: 16018, 2016. doi: 10.1038/npjsba.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Imai SI, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech Dis 2: 16017, 2016. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998. [PubMed] [Google Scholar]
- 71.Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J Nutr 125: 1455–1461, 1995. [DOI] [PubMed] [Google Scholar]
- 72.Jamieson HA, Hilmer SN, Cogger VC, Warren A, Cheluvappa R, Abernethy DR, Everitt AV, Fraser R, de Cabo R, Le Couteur DG. Caloric restriction reduces age-related pseudocapillarization of the hepatic sinusoid. Exp Gerontol 42: 374–378, 2007. doi: 10.1016/j.exger.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson S, Wozniak DF, Imai S. CA1 Nampt knockdown recapitulates hippocampal cognitive phenotypes in old mice which nicotinamide mononucleotide improves. NPJ Aging Mech Dis 4: 10, 2018. doi: 10.1038/s41514-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol 101: S20–S26, 2008. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 75.Kaplon RE, Gano LB, Seals DR. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J Appl Physiol (1985) 116: 156–163, 2014. doi: 10.1152/japplphysiol.00969.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katsyuba E, Auwerx J. Modulating NAD+ metabolism, from bench to bedside. EMBO J 36: 2670–2683, 2017. doi: 10.15252/embj.201797135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katsyuba E, Mottis A, Zietak M, De Franco F, van der Velpen V, Gariani K, Ryu D, Cialabrini L, Matilainen O, Liscio P, Giacchè N, Stokar-Regenscheit N, Legouis D, de Seigneux S, Ivanisevic J, Raffaelli N, Schoonjans K, Pellicciari R, Auwerx J. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 563: 354–359, 2018. doi: 10.1038/s41586-018-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell 159: 709–713, 2014. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim S, Wyckoff J, Morris AT, Succop A, Avery A, Duncan GE, Jazwinski SM. DNA methylation associated with healthy aging of elderly twins. Geroscience 40: 469–484, 2018. doi: 10.1007/s11357-018-0040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klimova N, Kristian T. Multi-targeted effect of nicotinamide mononucleotide on brain bioenergetic metabolism. Neurochem Res. 2019 Jan 19. [Epub ahead of print] doi: 10.1007/s11064-019-02729-0. [DOI] [PubMed] [Google Scholar]
- 81.Kusumbe AP, Ramasamy SK, Itkin T, Mäe MA, Langen UH, Betsholtz C, Lapidot T, Adams RH. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532: 380–384, 2016. doi: 10.1038/nature17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys 373: 16–22, 2000. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- 83.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 84.Lanska DJ. The discovery of niacin, biotin, and pantothenic acid. Ann Nutr Metab 61: 246–253, 2012. doi: 10.1159/000343115. [DOI] [PubMed] [Google Scholar]
- 85.Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience 40: 163–176, 2018. doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 111: 363–368, 2005. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 87.Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J Gerontol A Biol Sci Med Sci 66A: 409–418, 2011. doi: 10.1093/gerona/glq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lesniewski LA, Zigler MC, Durrant JR, Donato AJ, Seals DR. Sustained activation of AMPK ameliorates age-associated vascular endothelial dysfunction via a nitric oxide-independent mechanism. Mech Ageing Dev 133: 368–371, 2012. doi: 10.1016/j.mad.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol 37: 671–680, 2004. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Lin LF, Henderson LM. Pyridinium precursors of pyridine nucleotides in perfused rat kidney and in the testis. J Biol Chem 247: 8023–8030, 1972. [PubMed] [Google Scholar]
- 91.Liu L, Su X, Quinn WJ 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 27: 1067–1080.e5, 2018. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lund DD, Chu Y, Miller JD, Heistad DD. Protective effect of extracellular superoxide dismutase on endothelial function during aging. Am J Physiol Heart Circ Physiol 296: H1920–H1925, 2009. doi: 10.1152/ajpheart.01342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martens CR, Denman BA, Mazzo MR, Armstrong ML, Reisdorph N, McQueen MB, Chonchol M, Seals DR. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat Commun 9: 1286, 2018. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masser DR, Hadad N, Porter H, Stout MB, Unnikrishnan A, Stanford DR, Freeman WM. Analysis of DNA modifications in aging research. Geroscience 40: 11–29, 2018. doi: 10.1007/s11357-018-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One 7: e42357, 2012. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab 20: 183–190, 2014. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA 271: 672–677, 1994. doi: 10.1001/jama.1994.03510330050033. [DOI] [PubMed] [Google Scholar]
- 98.Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 39: 7–18, 2017. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miettinen TA, Taskinen MR, Pelkonen R, Nikkilä EA. Glucose tolerance and plasma insulin in man during acute and chronic administration of nicotinic acid. Acta Med Scand 186: 247–253, 1969. doi: 10.1111/j.0954-6820.1969.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 100.Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, Yoshino J, Imai SI. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab 24: 795–806, 2016. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martín-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep 1: 70, 2011. [Erratum in Sci Rep 3: 1131, 2013.] doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD+ -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell 13: 1075–1085, 2014. doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154: 430–441, 2013. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience 40: 243–256, 2018. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 106.Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, Cantoni O, Carruba MO, Moncada S, Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA 101: 16507–16512, 2004. [Erratum in Proc Natl Acad Sci USA 102: 5635, 2005.] doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, Baker DJ, Cen Y, Wu LE, Sauve AA, van Deursen JM, Rosenzweig A, Sinclair DA. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J 33: 1438–1453, 2014. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Reilly JN, Cogger VC, Le Couteur DG. Old age is associated with ultrastructural changes in isolated rat liver sinusoidal endothelial cells. J Electron Microsc (Tokyo) 59: 65–69, 2010. doi: 10.1093/jmicro/dfp039. [DOI] [PubMed] [Google Scholar]
- 109.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 1: 4, 2009. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjáti K, Szabó C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol 135: 1347–1350, 2002. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabó C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med 9: 659–664, 2002. doi: 10.3892/ijmm.9.6.659. [DOI] [PubMed] [Google Scholar]
- 112.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Haskó G, Marton A, Bátkai S, Kollai M, Szabó C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther 311: 485–491, 2004. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pellicciari R, Liscio P, Giacchè N, De Franco F, Carotti A, Robertson J, Cialabrini L, Katsyuba E, Raffaelli N, Auwerx J. α-Amino-β-carboxymuconate-ε-semialdehyde decarboxylase (ACMSD) inhibitors as novel modulators of de novo nicotinamide adenine dinucleotide (NAD+) biosynthesis. J Med Chem 61: 745–759, 2018. doi: 10.1021/acs.jmedchem.7b01254. [DOI] [PubMed] [Google Scholar]
- 116.Philpott AC, Hubacek J, Sun YC, Hillard D, Anderson TJ. Niacin improves lipid profile but not endothelial function in patients with coronary artery disease on high dose statin therapy. Atherosclerosis 226: 453–458, 2013. doi: 10.1016/j.atherosclerosis.2012.10.067. [DOI] [PubMed] [Google Scholar]
- 117.Podlutsky A, Valcarcel-Ares MN, Yancey K, Podlutskaya V, Nagykaldi E, Gautam T, Miller RA, Sonntag WE, Csiszar A, Ungvari Z. The GH/IGF-1 axis in a critical period early in life determines cellular DNA repair capacity by altering transcriptional regulation of DNA repair-related genes: implications for the developmental origins of cancer. Geroscience 39: 147–160, 2017. doi: 10.1007/s11357-017-9966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rader JI, Calvert RJ, Hathcock JN. Hepatic toxicity of unmodified and time-release preparations of niacin. Am J Med 92: 77–81, 1992. doi: 10.1016/0002-9343(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 119.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol 160: 339–349, 2004. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 120.Rajagopalan S, Brook R, Mehta RH, Supiano M, Pitt B. Effect of losartan in aging-related endothelial impairment. Am J Cardiol 89: 562–566, 2002. doi: 10.1016/S0002-9149(01)02297-4. [DOI] [PubMed] [Google Scholar]
- 121.Ratajczak J, Joffraud M, Trammell SA, Ras R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud ME, Auwerx J, Yanes O, Brenner C, Cantó C. NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells. Nat Commun 7: 13103, 2016. doi: 10.1038/ncomms13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rawling JM, Jackson TM, Driscoll ER, Kirkland JB. Dietary niacin deficiency lowers tissue poly(ADP-ribose) and NAD+ concentrations in Fischer-344 rats. J Nutr 124: 1597–1603, 1994. doi: 10.1093/jn/124.9.1597. [DOI] [PubMed] [Google Scholar]
- 123.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279: 50754–50763, 2004. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 124.Revollo JR, Körner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, Milbrandt J, Kiess W, Imai S. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6: 363–375, 2007. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reyes LA, Boslett J, Varadharaj S, De Pascali F, Hemann C, Druhan LJ, Ambrosio G, El-Mahdy M, Zweier JL. Depletion of NADP(H) due to CD38 activation triggers endothelial dysfunction in the postischemic heart. Proc Natl Acad Sci USA 112: 11648–11653, 2015. doi: 10.1073/pnas.1505556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sahebkar A. Effect of niacin on endothelial function: a systematic review and meta-analysis of randomized controlled trials. Vasc Med 19: 54–66, 2014. doi: 10.1177/1358863X13515766. [DOI] [PubMed] [Google Scholar]
- 127.Schultz MB, Sinclair DA. Why NAD(+) declines during aging: it’s destroyed. Cell Metab 23: 965–966, 2016. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwartz ML. Severe reversible hyperglycemia as a consequence of niacin therapy. Arch Intern Med 153: 2050–2052, 1993. doi: 10.1001/archinte.1993.00410170142014. [DOI] [PubMed] [Google Scholar]
- 129.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3: 289–300, 2008. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Snider TA, Richardson A, Stoner JA, Deepa SS. The Geropathology Grading Platform demonstrates that mice null for Cu/Zn-superoxide dismutase show accelerated biological aging. Geroscience 40: 97–103, 2018. doi: 10.1007/s11357-018-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song J, Ke SF, Zhou CC, Zhang SL, Guan YF, Xu TY, Sheng CQ, Wang P, Miao CY. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J Gerontol A Biol Sci Med Sci 69: 44–57, 2014. doi: 10.1093/gerona/glt122. [DOI] [PubMed] [Google Scholar]
- 132.Sorensen KE, Dorup I, Hermann AP, Mosekilde L. Combined hormone replacement therapy does not protect women against the age-related decline in endothelium-dependent vasomotor function. Circulation 97: 1234–1238, 1998. doi: 10.1161/01.CIR.97.13.1234. [DOI] [PubMed] [Google Scholar]
- 133.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabó C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res 89: 684–691, 2001. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 134.Soriano FG, Virág L, Szabó C. Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly(ADP-ribose) polymerase activation. J Mol Med (Berl) 79: 437–448, 2001. doi: 10.1007/s001090100236. [DOI] [PubMed] [Google Scholar]
- 135.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350: 830–834, 2015. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 136.Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci 70: 1355–1359, 2015. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab 35: 527–530, 2015. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J 33: 1321–1340, 2014. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stein LR, Wozniak DF, Dearborn JT, Kubota S, Apte RS, Izumi Y, Zorumski CF, Imai S. Expression of Nampt in hippocampal and cortical excitatory neurons is critical for cognitive function. J Neurosci 34: 5800–5815, 2014. doi: 10.1523/JNEUROSCI.4730-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tarantini S, Tran CH, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 94: 52–58, 2017. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, Sonntag WE, Ungvari Z, Csiszar A. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 38: 273–289, 2016. doi: 10.1007/s11357-016-9931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17: e12731, 2018. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 16: 469–479, 2017. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. Geroscience 39: 601–614, 2017. [Erratum in Geroscience 40: 219, 2018.] doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tarragó MG, Chini CCS, Kanamori KS, Warner GM, Caride A, de Oliveira GC, Rud M, Samani A, Hein KZ, Huang R, Jurk D, Cho DS, Boslett JJ, Miller JD, Zweier JL, Passos JF, Doles JD, Becherer DJ, Chini EN. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab 27: 1081–1095.e10, 2018. doi: 10.1016/j.cmet.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tenopoulou M, Doulias PT, Nakamoto K, Berrios K, Zura G, Li C, Faust M, Yakovishina V, Evans P, Tan L, Bennett MJ, Snyder NW, Quinn WJ 3rd, Baur JA, Atochin DN, Huang PL, Ischiropoulos H. Oral nitrite restores age-dependent phenotypes in eNOS-null mice. JCI Insight 3: e122156, 2018. doi: 10.1172/jci.insight.122156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 14: 1034–1044, 2015. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 312: H1–H20, 2017. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 309: H1837–H1845, 2015. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell 14: 400–408, 2015. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun 7: 12948, 2016. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trammell SA, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci Rep 6: 26933, 2016. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Trammell SA, Yu L, Redpath P, Migaud ME, Brenner C. Nicotinamide riboside is a major NAD+ precursor vitamin in cow milk. J Nutr 146: 957–963, 2016. doi: 10.3945/jn.116.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tummala KS, Gomes AL, Yilmaz M, Graña O, Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V, Rodriguez-Justo M, Pisano DG, Wagner EF, Djouder N. Inhibition of de novo NAD(+) synthesis by oncogenic URI causes liver tumorigenesis through DNA damage. Cancer Cell 26: 826–839, 2014. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 159.Uddin GM, Youngson NA, Sinclair DA, Morris MJ. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 weeks of exercise in obese female mice. Front Pharmacol 7: 258, 2016. doi: 10.3389/fphar.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 162.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 163.Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A. Mitochondrial protection by resveratrol. Exerc Sport Sci Rev 39: 128–132, 2011. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res 123: 849–867, 2018. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol 312: H1128–H1143, 2017. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15: 555–565, 2018. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE, Csiszar A. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68: 877–891, 2013. doi: 10.1093/gerona/gls242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168a.United States Department of Agriculture, Agricultural Research Service . National Nutrient Database for Standard Reference Legacy Release: Basic Report: 15127, Fish, Tuna, Fresh, Yellowfin, Raw (Online). https://ndb.nal.usda.gov/ndb/foods/show/15127. [12 December 2018].
- 168b.United States Department of Agriculture, Agricultural Research Service . National Nutrient Database for Standard Reference Legacy Release: Full Report (All Nutrients): 18375, Leavening Agents, Yeast, Baker’s, Active dry (Online). https://ndb.nal.usda.gov/ndb/foods/show/18375?fgcd=&manu=&format=Full&count=&max=25&offset=&sort=default&order=asc&qlookup=18375&ds=&qt=&qp=&qa=&qn=&q=&ing=. [12 Dec 2018].
- 168c.United States Department of Health and Human Services . Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed] [Google Scholar]
- 169.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67: 821–829, 2012. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van de Weijer T, Phielix E, Bilet L, Williams EG, Ropelle ER, Bierwagen A, Livingstone R, Nowotny P, Sparks LM, Paglialunga S, Szendroedi J, Havekes B, Moullan N, Pirinen E, Hwang JH, Schrauwen-Hinderling VB, Hesselink MK, Auwerx J, Roden M, Schrauwen P. Evidence for a direct effect of the NAD+ precursor acipimox on muscle mitochondrial function in humans. Diabetes 64: 1193–1201, 2015. doi: 10.2337/db14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang P, Du H, Zhou CC, Song J, Liu X, Cao X, Mehta JL, Shi Y, Su DF, Miao CY. Intracellular NAMPT-NAD+-SIRT1 cascade improves post-ischaemic vascular repair by modulating Notch signalling in endothelial progenitors. Cardiovasc Res 104: 477–488, 2014. doi: 10.1093/cvr/cvu220. [DOI] [PubMed] [Google Scholar]
- 172.Watson A, Nong Z, Yin H, O’Neil C, Fox S, Balint B, Guo L, Leo O, Chu MW, Gros R, Pickering JG. Nicotinamide phosphoribosyltransferase in smooth muscle cells maintains genome integrity, resists aortic medial degeneration, and is suppressed in human thoracic aortic aneurysm disease. Circ Res 120: 1889–1902, 2017. doi: 10.1161/CIRCRESAHA.116.310022. [DOI] [PubMed] [Google Scholar]
- 173.Wieland E, Rodriguez-Vita J, Liebler SS, Mogler C, Moll I, Herberich SE, Espinet E, Herpel E, Menuchin A, Chang-Claude J, Hoffmeister M, Gebhardt C, Brenner H, Trumpp A, Siebel CW, Hecker M, Utikal J, Sprinzak D, Fischer A. Endothelial Notch1 activity facilitates metastasis. Cancer Cell 31: 355–367, 2017. doi: 10.1016/j.ccell.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 174.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689, 2004. [Erratum in Nature 431: 107, 2004.] doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 175.Yamamoto M, Hikosaka K, Mahmood A, Tobe K, Shojaku H, Inohara H, Nakagawa T. Nmnat3 is dispensable in mitochondrial NAD level maintenance in vivo. PLoS One 11: e0147037, 2016. doi: 10.1371/journal.pone.0147037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yoshida T, Tanaka M, Okamoto K. Immunoglobulin G induces microglial superoxide production. Neurol Res 24: 361–364, 2002. doi: 10.1179/016164102101200140. [DOI] [PubMed] [Google Scholar]
- 177.Yoshino J, Baur JA, Imai SI. NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab 27: 513–528, 2018. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zarzuelo MJ, López-Sepúlveda R, Sánchez M, Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85: 1288–1296, 2013. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 180.Zhang G, Teggatz EG, Zhang AY, Koeberl MJ, Yi F, Chen L, Li PL. Cyclic ADP ribose-mediated Ca2+ signaling in mediating endothelial nitric oxide production in bovine coronary arteries. Am J Physiol Heart Circ Physiol 290: H1172–H1181, 2006. doi: 10.1152/ajpheart.00441.2005. [DOI] [PubMed] [Google Scholar]
- 181.Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D’Amico D, Ropelle ER, Lutolf MP, Aebersold R, Schoonjans K, Menzies KJ, Auwerx J. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352: 1436–1443, 2016. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]