Abstract
Alterations in perinatal conditions (such as preterm birth) is linked to adult health and disease, in particular, the cardiovascular system. Neddylation, a novel posttranslational modification through which the ubiquitin-like protein NEDD8 is conjugated to protein substrates, has emerged as an important mechanism regulating embryonic cardiac chamber maturation. However, the importance of neddylation in postpartum cardiac development has not been investigated. Here, we aimed to determine whether transient, postnatal inhibition of neddylation has immediate and prolonged impact on the structure and function of the neonatal and adult hearts. Sprague-Dawley pups were given three intraperitoneal injections of MLN4924 (MLN), a specific neddylation inhibitor, at postnatal days (P)1, 3, and 5. Cardiac structure and function were temporally assessed during aging and after 2 wk of isoproterenol (ISO) infusion in adulthood. MLN treatment resulted in modest reduction of neddylated proteins in neonatal hearts. The MLN-treated rats developed cardiac hypertrophy and dysfunction by P7, which was accompanied by significantly reduced cardiomyocyte proliferation. At 3 mo of age, cardiac contractile function was restored in MLN-treated rats, but MLN-treated hearts displayed hypertrophic phenotype. Whereas ISO infusion triggered compensatory cardiac hypertrophy without impairing cardiac contractility in the control rats, the MLN-treated rats displayed a similar degree of hypertrophy, which quickly progressed to decompensation with ventricular wall thinning, chamber dilatation, and reduced ejection fraction as well as exacerbated pathological cardiac remodeling. Our findings suggest that neddylation is required for postnatal cardiac development and that perturbation of neddylation during development predisposes adult hearts to cardiac failure under stress conditions.
NEW & NOTEWORTHY Our study demonstrates that perinatal perturbation of neddylation induces cardiomyopathy, impairs postnatal cardiac development, and increases susceptibility to catecholamine-induced cardiac dysfunction. The results reveal a previously unappreciated role of neddylation in postnatal cardiac maturation and call for close monitoring for the potential cardiotoxicity of MLN4924 (pevonedistat) and other agents that modify neddylation, especially in pregnant women and preadolescents.
Keywords: cardiomyocyte proliferation, heart failure, isoproterenol, MLN4924, neddylation
INTRODUCTION
Heart failure affects ~1–2% of the population worldwide and is a frequent source of hospital-related morbidity and mortality (15). As the first organ formed during organogenesis, the fetal heart undergoes a series of structural and functional modifications in the early postnatal stage to form a functionally competent adult heart. This postnatal cardiac maturation process involves a switch in the mode of cardiac growth from hyperplasia to hypertrophy (9, 30), a metabolic transition from fetal-type glycolysis to adult-type fatty acid oxidation (20), a robust mitochondrial biogenesis (39), and a reprogramming of the expression of fetal and adult genes (25), among other changes. Failure to enact such transitions has been shown to cause cardiac structural defects, heart failure, and early mortality in mice (2) (16). Alterations in perinatal conditions such as preterm birth and changes in oxygen exposure have also been linked to increased cardiac vulnerability in adulthood (3, 4). Discovering the novel mechanisms regulating postnatal cardiac development and maturation is important for understanding the pathogenesis of neonatal cardiomyopathy and for developing therapeutic approaches for heart failure.
Over the past few decades, the posttranslational modification of proteins by ubiquitin (UB) and ubiquitin-like proteins has emerged as a novel and crucial regulatory mechanism of multiple cellular functions, including differentiation and developmental processes (18, 42). NEDD8 is a small ubiquitin-like protein that shares ~60% homology with ubiquitin (13). Conjugation of NEDD8 to protein substrates, termed neddylation, occurs in a process analogous to ubiquitination and employs its own specific E1 (NEDD8-activating enzyme, NAE, consisting of heterodimeric UBA3 and NAE1), E2 (UBC12 or UBE2F), and E3 enzymes (8). This process can be reversed, appropriately termed deneddylation, by NEDD8 proteases such as the COP9 signalosome (CSN) and NEDD8 protease-1 (NEDP1). Functional studies focused on the cullin family of proteins and activity of cullin-RING ubiquitin ligases, the most common NEDD8 substrate, have suggested a wide range of cellular processes regulated by neddylation, including transcriptional regulation, cell cycle and differentiation, ribosome biogenesis, apoptosis, inflammatory response, and targeted proteolysis (7, 14).
Emerging evidence from mice lacking neddylation and deneddylation enzymes demonstrates the importance of balance between neddylation and deneddylation in embryonic development. Abrogation of neddylation in mice by deleting UBA3, which encodes the catalytic subunit of NAE, results in embryonic lethality at the peri-implantation stage (37). Inhibition of deneddylation by germline mutations in any CSN subunits (CSN2, CSN3, CSN5, CSN6, and CSN8) also lead to early embryonic lethality (22, 23, 38, 43, 45). Defective cell cycle progression and cell death were implicated in the developmental arrest, highlighting the critical role of an intact NEDD8 pathway in cell differentiation and proliferation.
We (46) recently identified an essential role for neddylation in cardiac development and maturation. We demonstrated that neddylation is highly active in embryonic and fetal hearts but is developmentally downregulated in adult hearts. Conditional deletion of NAE1, the regulatory subunit of NAE, in the developing heart by using αMHCCre transgene results in ventricular noncompaction by embryonic day (E)16.5, heart failure by E18.5, and ultimately perinatal lethality of mice. Mechanistically, neddylation is required for the fine-tuning of Hippo-YAP signaling during cardiac development, which is accomplished by temporally downregulating Hippo kinases and consequently sustaining YAP signaling in a cullin-7-dependent manner.
While our findings from NAE1-deficient hearts demonstrated the physiological function of neddylation in mid- to late-gestational cardiac development, the early lethality of the NAE1 mutant hearts precludes the use of this mouse model to study the importance of neddylation in preadolescent cardiac development. Meanwhile, since these findings were gained from a loss-of-function mouse model that permanently and completely inactivates neddylation in affected cardiomyocytes, there remains a knowledge gap regarding whether transient and modest inhibition of neddylation has any impact on cardiac development and maturation.
MLN4924 (pevonedistat) is a specific and potent inhibitor of NAE and has proven effective in limiting tumor growth in both animal studies and preclinical trials (31, 36, 40, 41). By temporarily modulating neddylation in perinatal rats, we report here that modest inhibition of neddylation using MLN4924 disrupted the last wave of cardiomyocyte proliferation and impaired cardiac function in perinatal hearts. Moreover, although adult MLN4924-treated rats recovered from transient cardiomyopathy, they developed cardiac hypertrophy in adulthood and were more susceptible to adrenergic agonist-induced heart failure. Our findings suggest that short-term perturbation of neddylation can compromise the anatomy and function of the developing heart. Furthermore, impaired neddylation during the perinatal period is linked to cardiac hypertrophy and heart failure in mature animals. As a result of these studies, clinical studies using pevonedistat and other neddylation-modifying compounds should proceed with caution, particularly in pregnant women and preadolescences.
MATERIALS AND METHODS
Animal models and delivery of MLN4924 and isoproterenol.
Sprague-Dawley rats were administered three doses of MLN4924 (30 mg/kg) or vehicle (0.9% NaCl) at postnatal days (P)1, 3, and 5 via intraperitoneal (ISO) injection. At 3 mo of age, a cohort was subcutaneously infused with ISO (Sigma; 30 mg·kg−1·day−1 for 14 days) via osmotic pumps (Alzet, model 1002). The osmotic pump was implanted on the dorsal surface, slightly posterior to the scapulae and was removed at the end of the 14th day. All animal experiments were approved by the Augusta University Institutional Animal Care and Use Committee.
Echocardiography.
Adult rats were anesthetized by inhalation of isoflurane (2.5% for induction and 1.5% for maintenance) via a nose cone. The adequacy of the anesthesia was monitored by toe pinch. Cardiac images and loops were recorded using a VEVO 2100 echocardiography system with a 13- to 24-MHz transducer (MS250, Visual Sonics). The left ventricular (LV) morphometric and functional parameters were analyzed offline using VEVO 2100 software. Echocardiography of conscious P7 neonates was performed by gently securing the rats on the station with tapes and was recorded with an 18- to 38-MHz transducer (MS400, Visual Sonics).
Histology and immunohistochemistry analysis.
For histology analysis, 5-μm myocardium or embryo sections were deparaffinized, rehydrated, and subjected to hematoxylin-eosin (H&E) staining or Masson trichrome staining, respectively. For immunohistochemistry analysis, these sections were incubated in preheated sodium citrate buffer (pH 6.0, 98°C) for 10 min with the PT Link system (Dako). After preincubation with 10% nonimmune goat serum (Thermo Fisher Scientific) to prevent nonspecific binding, tissue sections were incubated with primary antibodies [phospho-Histone 3, Cell Signaling Technology; wheat germ agglutinin (WGA), Thermo Fisher Scientific; troponin T (TnT), Developmental Studies Hybridoma Bank] at 4°C overnight and subsequently incubated with appropriate Alexa Fluor-conjugated secondary antibodies (Thermo Fisher Scientific) for 1 h at room temperature. Finally, sections were stained with DAPI (Sigma) and mounted on Vectashield antifade mounting medium (Vector). Images were captured with an Olympus BX41 (Olympus) or Zeiss Upright 780 confocal microscope (Zeiss).
5-Ethynyl-2′-deoxyuridine incorporation assay.
5-Ethynyl-2′-deoxyuridine (EdU) was used to label cardiomyocytes undergoing mitosis in vitro and in vivo. For in vivo labeling, EdU was diluted in 20% cyclodextrin and injected intraperitoneally into the rats (20 mg/kg) at indicated time points. Collected hearts were embedded in Tissue-Tek OCT compound (Sakura Finetek), and the cryosections were subjected to EdU staining, which was performed using a Click-iT EdU Imaging Kit (Thermo Fisher Scientific). To identify cardiomyocytes, the tissue sections were counterstained with cardiac TnT and DAPI.
Protein extraction and Western blot analysis.
Protein was extracted from ventricular myocardium tissues or cultured cells, concentration were determined with BCA reagents (Thermo Fisher Scientific), and SDS-PAGE, immunoblotting, and densitometry were performed as previously described (46).
RNA preparation and real-time PCR.
Isolation of total RNA and reverse transcription into single-stranded cDNA was performed as previously described (46). Gene expression in each sample was measured in triplicate by quantitative real-time polymerase chain reaction (qRT-PCR; StepOnePlus Real-Time PCR System, Thermo Fisher Scientific) using the SYBR Green assay with gene-specific primers at a final concentration of 200 nM. Relative gene expression was calculated using the 2−ΔΔCT method against the rat housekeeping gene acidic ribosomal phosphoprotein P0 (RPLP0) as appropriate. Each experiment was repeated at least three times independently. The primers used for qPCR are listed in Table 1.
Table 1.
qPCR primers
| Rat | Forward Sequence | Reverse Sequence |
|---|---|---|
| Cdc20 | CAAGCGGTGGCAATGATAAC | GCACCTTGATGTTGAGTGAATG |
| Ctgf | AGGGACACGAACTCATTTAGAC | CAGCAGTTAGGAACCCAGATT |
| Cyr61 | GAAGGCAGACCCTGTGAATATAA | CCACAGCACCGTCAATACA |
| Gadd45a | GACTTTGCAGAAGGAGAGAGAG | GTAACCCGGCCATCCTAAAT |
| Myh6 | GCCCTCCGGGGTTCGGCGG | GCGTGGCGCTCCTTGGGGTT |
| Myh7 | GTGGCGGGTCGGGGGGTG | GGTCGGGGGGCCTGGTCT |
| Nppa | CGGCGCGGGTCTGGTGGGTTTCG | CCTCGTCTTCTGCCGGCGTC |
| RPLP0 | TTGAAATCCTGAGCGATGTGCAGC | GCCATTGTCAAACACCTGCTGGAT |
Statistical analysis.
Results are shown as means (bar graph) ± SE (error bar) with specific data (circle/triangle symbols). Differences between two groups of data were evaluated using an unpaired two-tailed Student’s t-test. Comparison of two groups of data among more than two groups was performed by one-way ANOVA F-test. Comparison among more than two groups was conducted by two-way ANOVA multiple comparison. A P value of <0.05 was considered statistically significant. More details can be found in individual figure legends.
RESULTS
Effect of MLN4924 administration on neddylation in neonatal rat hearts.
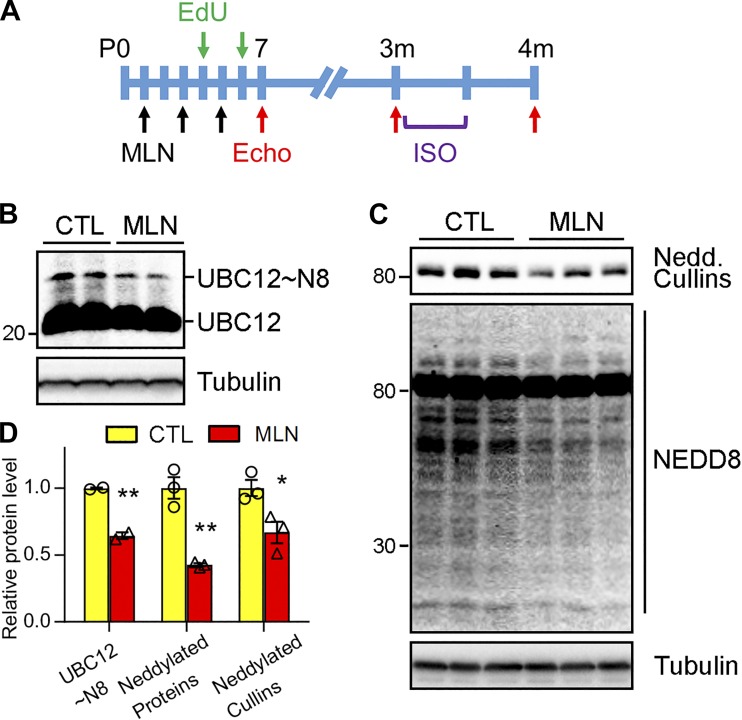
Chronic inhibition of neddylation arising from lack of NAE1-impaired ventricular chamber maturation during mid- to late gestation (46). To investigate whether transient perturbation of neddylation interferes with cardiac development, we used MLN4924 to modulate neddylation in the neonatal hearts. MLN4924 occupies the ATP binding site on UBA3 and forms an irreversible covalent adduct to UBA3, thereby preventing the activation of NEDD8 and neddylation (31). Previous studies showed that, when given at doses ranging from 30 to 90 mg/kg, once or twice daily for 21 days, MLN4924 is well tolerated in adult mice (24, 31). A preliminary screening of different doses (30, 40, and 60 mg/kg) revealed that three injections of MLN4924 to neonatal rats at a dose higher than 30 mg/kg every other day caused mortality of neonatal rats. Therefore, neonatal rats were treated with MLN4924 (30 mg/kg) by intraperitoneal injection at P1, P3, and P5 (Fig. 1A). Importantly, NAE-activated NEDD8 is relayed to the E2 enzyme UBC12, forming an intermediate UBC12-NEDD8 thioester complex (13). To determine whether the employed MLN4924 treatment regimen effectively suppressed neddylation in the heart, we measured the formation of NEDD8-UBC12 conjugate under a nonreducing condition. Western blot of myocardial lysates showed that MLN4924 reduced NEDD8-conjguated UBC12 by 40% (Fig. 1B). Moreover, MLN4924 treatment led to an ~50% reduction of overall neddylated proteins (Fig. 1, C and D). These results indicated that our regimen modestly suppressed neddylation in the hearts of neonatal rats.
Fig. 1.
MLN4924 (MLN, pevonedistat) inhibited neddylation in neonatal hearts. A: schematic diagram depicting the timeline of MLN administration and experimental analyses. Neonatal rats received vehicle (Ctl) or MLN (30 mg·kg−1·day−1) via intraperitoneal injections at perinatal days (P)1, 3, and 5. At 3 mo of age, these rats were infused with β-adrenergic receptor agonist isoproterenol (ISO, 30 mg·kg−1·day−1) through osmotic minipump for 2 wk. To analyze cardiomyocyte proliferation, 5-ethynyl-2′-deoxyuridine (EdU) was intraperitoneally injected to the animals 3 h before harvest. Echocardiography (Echo) was performed at indicated times. B and C: Western blot of indicated proteins in Ctl or MLN-treated rat hearts at P7. UBC12~N8, NEDD8-conjugated UBC12; UBC12, native form of UBC12; Nedd. Cullins, neddylated cullins. D: quantification of neddylated UBC12, total neddylated proteins, and cullins in B and C. Unpaired Student’s t-test, *P < 0.05, **P < 0.01 vs. Ctl.
MLN4924 led to growth retardation and induced cardiomyopathy in neonates.
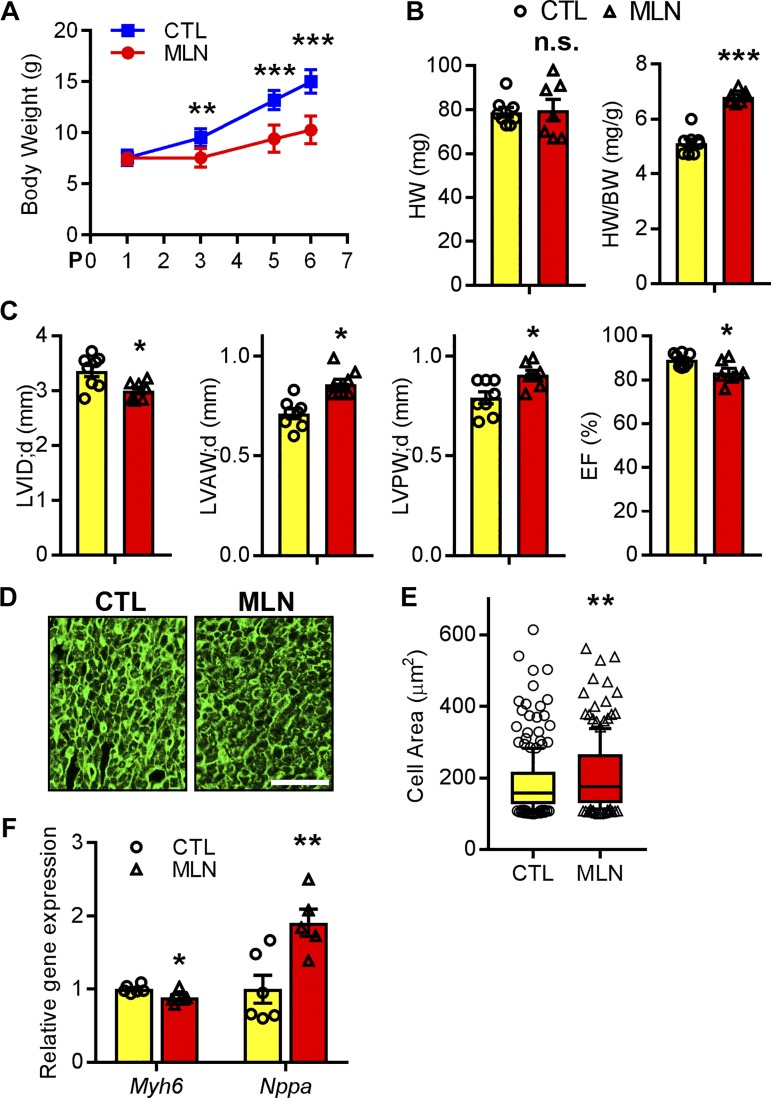
The impact of neddylation inhibition on neonates is not known. Gravimetric analysis showed that body weight of rat pups receiving MLN4924 were significantly decreased from P3 to P6 compared with rat pups injected with vehicle (Fig. 2A), suggesting that MLN4924 impairs perinatal growth. Despite its impact on body weight (BW), heart weight (HW) was preserved in MLN4924-treated pups. Consequently the HW/BW ratio was significantly increased by P7 (Fig. 2B), suggesting an increase in heart size relative to body size. We next performed echocardiography immediately after MLN4924 treatment to examine cardiac function. MLN4924-treated hearts had significantly increased left ventricle (LV) anterior (AW) and posterior wall thickness (PW) at diastole (LVAW;d and LVPW;d), decreased LV internal diameter at diastole (LVID;d), and significantly decreased fractional shortening (FS, 57.6 ± 1.3 vs. 49.0 ± 2.4% of controls, P < 0.05; Fig. 2C and Table 2), indicating that MLN4924 administration in the perinatal period leads to early cardiac hypertrophy with compromised contractility. In agreement with the echocardiography data, cardiomyocytes from MLN4924-treated hearts had significantly increased cross-sectional areas (Fig. 2, D and E). Pathological cardiac remodeling is often accompanied by reactivation of genes transiently expressed in fetal hearts and the downregulation of genes expressed in adulthood. Indeed, quantitative real-time PCR analysis showed that myosin heavy chain 6 (Myh6), which encodes a sarcomeric protein highly expressed in adult hearts, was downregulated in MLN4924-treated hearts, whereas the fetal gene natriuretic peptide precursor A (Nppa) was upregulated. Taken together, these results suggest that inhibition of neddylation in neonates induces cardiomyopathy and impairs cardiac function.
Fig. 2.
MLN4924 (MLN, pevonedistat) induced neonatal cardiomyopathy. A: body weight of rat pups injected with MLN or control (Ctl) at indicated time points. B: heart weight (HW)-to-body weight (BW) ratio and HW of perinatal day (P)7 rat hearts. C: echocardiography parameters at P7; n = 8 for Ctl and 7 for MLN. LV, left ventricle; LVID;d, LV diastolic internal diameter; LVAW;d, LV diastolic anterior wall thickness; LVPW;d, LV diastolic posterior wall thickness; FS, fractional shortening. D and E: representative images (D) of wheat germ agglutinin (WGA, green)-stained myocardium sections from rats at P7 and quantification (E) of cross-sectional area. Bar, 100 μm. F: relative gene expression levels assessed by qPCR analysis of P7 hearts. Unpaired Student’s t-test for A, B, and E. One-way ANOVA followed by post hoc F-test for C and F. *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant vs. Ctl.
Table 2.
Echocardiography measurements of MLN-treated rats and their littermate controls at P7 (related to Fig. 2)
| P7 | Ctl | MLN |
|---|---|---|
| n | 8 | 7 |
| Body weight, g | 15.0 ± 0.4 | 10.3 ± 0.5*** |
| LVAW;d, mm | 0.76 ± 0.02 | 0.82 ± 0.02* |
| LVAW;s, mm | 1.41 ± 0.05 | 1.43 ± 0.04 |
| LVID;d, mm | 3.30 ± 0.07 | 3.03 ± 0.05** |
| LVID;s, mm | 1.37 ± 0.06 | 1.48 ± 0.06 |
| LVPW;d, mm | 0.79 ± 0.02 | 0.88 ± 0.02** |
| LVPW;s, mm | 1.37 ± 0.04 | 1.45 ± 0.05 |
| EF, % | 88.3 ± 1.0 | 81.2 ± 2.1** |
| FS, % | 57.6 ± 1.3 | 49.0 ± 2.4** |
| LV Vol;d, µl | 44.4 ± 3.1 | 36.1 ± 2.1* |
| LV Vol;s, µl | 5.0 ± 0.7 | 6.1 ± 0.9 |
| Heart rate, beats/min | 347 ± 6 | 323 ± 4** |
Values are means ± SE. P7, perinatal day 7; Ctl, control; MLN, MLN4924, pevonedistat; LV, left ventricle; LVAW;d, LV anterior wall thickness at end-diastole; LVPW;s, LV anterior wall thickness at end-systole; LVID;d, LV, internal dimension at end-diastole; LVID;s, LV internal dimension at end-systole; LVPW;d, LV posterior wall thickness at end-diastole; LVPW;s, LV posterior wall thickness at end-systole; EF, ejection fraction; FS, fraction shortening; LV Vol;d, LV volume at end-diastole; LV Vol;s, LV volume at end-systole. One-way ANOVA followed by post hoc F-test.
P < 0.05,
P < 0.01,
P < 0.001 vs. Ctl.
Transient inhibition of neddylation in the perinatal period impairs cardiomyocyte proliferation.
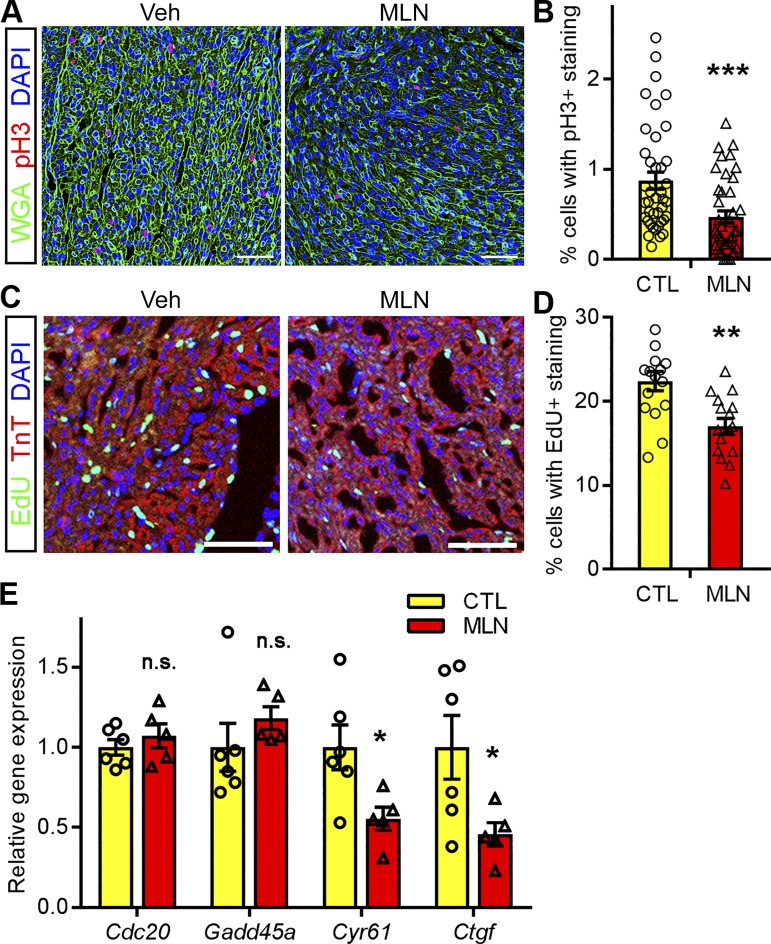
Recently, we (46) showed that neddylation is required for cardiomyocyte proliferation in developing fetal hearts. To determine whether transient inhibition of neddylation in neonatal hearts influences the last round of cardiomyocyte proliferation before they exit the cell cycle, we assessed cell proliferation in control and MLN4924-expoxed hearts by using the mitotic marker phospho-histone H3 (pH3). MLN4924 treatment significantly reduced pH3-positive cells in the hearts at P7 (Fig. 3, A and B). We sought to confirm these results by labeling proliferating cardiomyocytes with EdU. Control and MLN4924-treated rats were provided EdU via intraperitoneal injection 3 h before euthanization and tissue collection at P7 (Fig. 1A). Similarly, coimmunostaining of EdU with the cardiomyocyte marker TnT identified fewer EdU-positive cardiomyocytes in MLN4924-treated rat hearts compared with vehicle-treated controls (Fig. 3, C and D). These data suggest that transient inhibition of neddylation in neonatal hearts impairs cardiomyocyte proliferation. This hypothesis is supported by our observation that positive regulators of cell proliferation, Cyr61 and Ctgf, were significantly downregulated in MLN4924-treated hearts. These effects were specific, as other cell cycle genes such as Cdc20 and Gadd45a did not differ between MLN4924-treated and control animals (Fig. 3E). Taken together, these data suggest that transient inhibition of neddylation in neonatal hearts impairs cardiomyocyte proliferation by suppressing the expression of genes involved in cell cycle regulation.
Fig. 3.
Impaired cardiomyocyte proliferation in MLN4924 (MLN, pevonedistat)-treated neonatal rats. Rat hearts that received MLN or vehicle (Veh, Ctl) were collected at perinatal day (P)7. A and B: immunostaining of phospho-histone H3 (pH3); red) in myocardium sections. Representative images (A) and quantification (B) of pH3-positive cardiomyocytes are shown. Sections were counterstained with wheat germ agglutinin (WGA, green) and DAPI (blue); n = 4 per group; bars, 200 μm. C and D: 5-ethynyl-2′-deoxyuridine (EdU, green) incorporation assay. EdU was injected into rats 3 h before harvest. Sections were counterstained with troponin T (TnT, red) and DAPI (blue). Representative images (C) and quantification (D) of EdU-positive cardiomyocytes are shown. Bars, 200 μm; n = 4 per group. E: relative gene expression levels assessed by qPCR analysis of P6 rat hearts; n = 5 per group. Unpaired Student’s t-test for B and D; one-way ANOVA followed by post hoc F-test for E. *P < 0.05, **P < 0.01, ***P < 0.001; n.s. not significant vs. Ctl.
MLN4924-treated rats developed cardiac hypertrophy in adulthood.
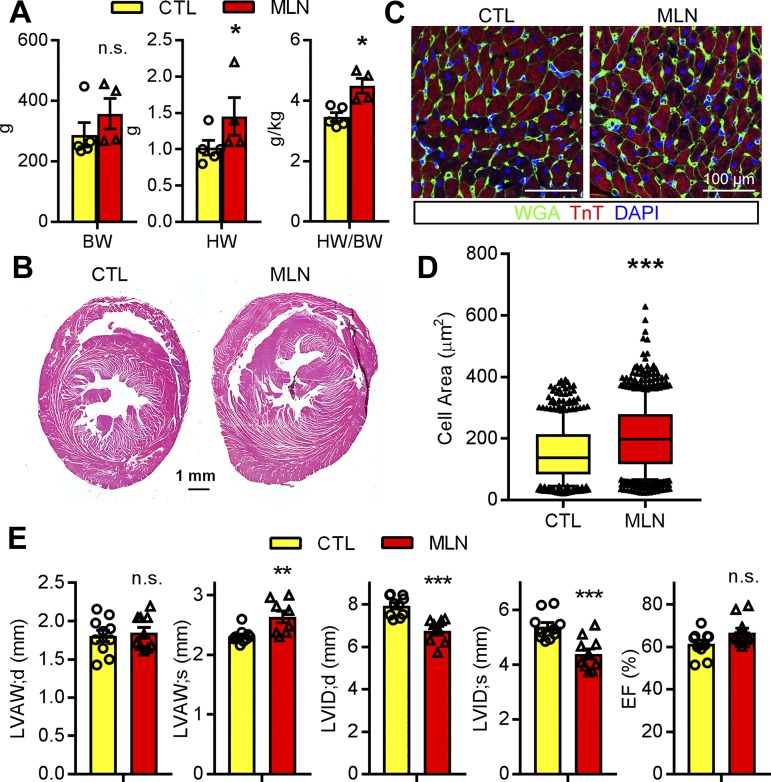
We next sought to identify whether short-term administration of MLN4924 to neonatal rats has an impact on their postnatal growth and cardiac development. Although the MLN4924-treated rats exhibited delayed postnatal growth, they were able to achieve their somatic growth potential and were indistinguishable from control rats by 3 mo of age (Fig. 4A). However, the hearts of MLN4924-treated rats were much larger at 3 mo of age, and HW/BW ratio was significantly increased (Fig. 4, A and B). Moreover, immunohistochemical staining of myocardial sections revealed that cross-sectional cardiomyocyte area was significantly increased in MLN4924-treated rats compared with control rat hearts (Fig. 4, C and D). Echocardiography examination performed at 3 mo after MLN4924 treatment demonstrated that MLN4924 treated-rats had significantly increased LVPW thickness and decreased LVID in both systolic and diastolic phases compared with control rats (Fig. 4E). Ejection fraction and fractional shortening did not differ between groups. These data suggest that administration of MLN4924 during perinatal development leads to cardiac hypertrophy with preserved cardiac contractility in adulthood.
Fig. 4.
MLN4924 (MLN, pevonedistat)-treated rats developed cardiac hypertrophy. A: body weight (BW) and heart weight (HW)-to-BW ratio. B and C: hematoxylin-eosin staining and immunostaining of cross-sectioned paraffin-embedded rat hearts at 3 mo of age treated with vehicle (Ctl) or MLN. Antibodies against wheat germ agglutinin (WGA, green) and troponin T (TnT, red) were used in C. D: quantification of cross-sectional cell area identified by WGA staining (n > 500 cells/heart, 4 hearts per group). E: echocardiography of Ctl (n = 5) and MLN (n = 5)-treated rats at 3 mo of age. LVAW;d, left ventricular (LV) diastolic anterior wall thickness; LVAW;s, systolic LV anterior wall thickness; LVID;d, diastolic LV internal diameter; LVID;s, sysstolic LV internal diameter; EF, injection fraction. Unpaired Student’s t-test for A and D; one-way ANOVA followed by post hoc F-test for E. *P < 0.05, **P < 0.01, ***P < 0.001; n.s. not significant vs. Vehicle.
MLN4924-treated rats are sensitized to ISO-induced heart failure.
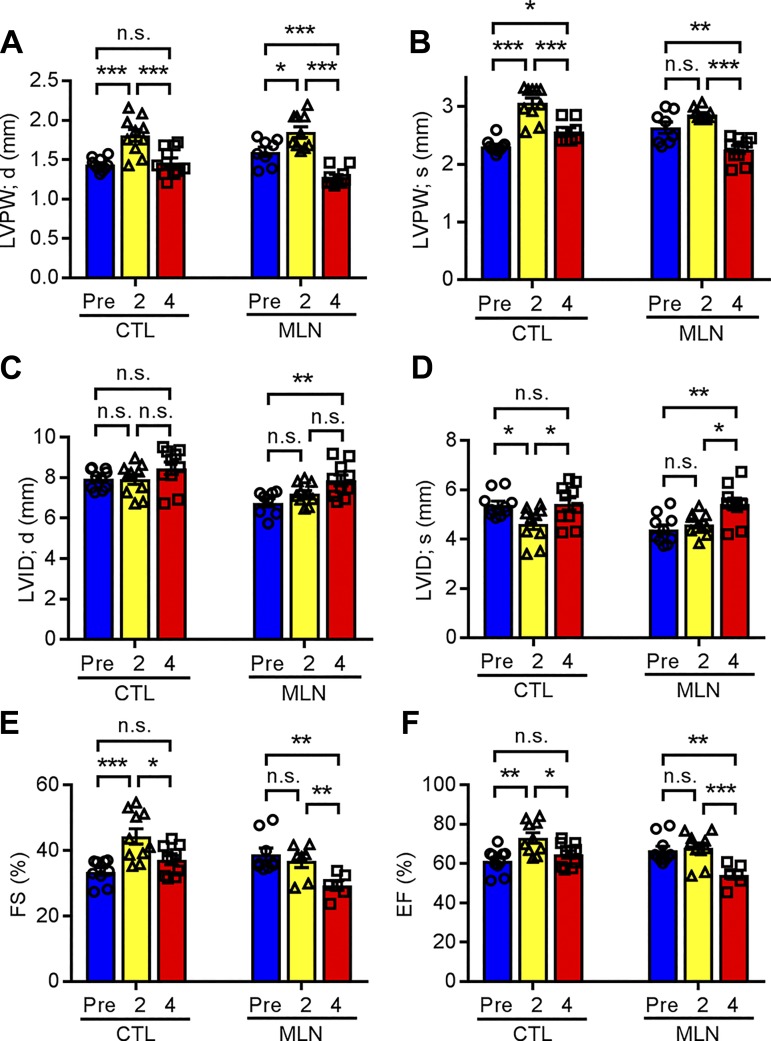
On the basis of our observations that inhibition of neddylation during the perinatal period resulted in cardiac hypertrophy in adulthood, we sought to determine whether administration of MLN4924 during the perinatal period impacts cardiac adaptation to pathological stress in adulthood. ISO is a β-adrenoreceptor agonist that induces cardiac hypertrophy associated with myocyte cell death and fibrosis with eventual progression to heart failure (5). As illustrated in Fig. 1A, 3-mo-old rats that received either vehicle or MLN4924 after birth were subcutaneously infused with ISO (30 mg·kg−1·day−1) via osmatic minipump for 2 wk. Echocardiography was performed to trace the changes in cardiac morphometry and function before ISO administration, and at 2 and 4 wk post-minipump implantation. In response to ISO infusion, vehicle-treated rats developed compensatory cardiac hypertrophy after 2 wk of ISO exposure, as evidenced by increased LVPW;s and LVPW;d thickness, decreased LVID;s, and increased ejection fraction (73.1 ± 3.8 vs. 61.4 ± 2.7% pre-ISO infusion, P < 0.01) and fractional shortening (44.3 ± 3.5 vs. 34.4 ± 2.0% pre-ISO infusion, P < 0.01) (Fig. 5, E and F, and Table 3). By 4 wk, these parameters were mostly restored to the levels comparable to those of pre-ISO treatment, suggesting that the vehicle-treated hearts had preserved cardiac function. Similarly, MLN4924-treated hearts developed cardiac hypertrophy after 2 wk of ISO exposure, as evidenced by increased LVPW;s and LVPW;d thickness (Fig. 5, A and B). While the extent of cardiac hypertrophy was indistinguishable between vehicle and MLN4924 groups as judged by comparable LV wall thickness, the MLN4924 group did not show increased ejection fraction (68.0 ± 3.6 vs. 66.6 ± 3.0% pre-ISO infusion, P = 0.677) and fractional shortening (39.5 ± 2.9 vs. 38.3 ± 2.6% pre-ISO infusion, P = 0.649) after 2 wk of ISO infusion (Fig. 5F and Table 3). These findings suggest that ISO infusion failed to induce a hypercontractile function in MLN4924-treated rats as it did to the control group. Moreover, by 4 wk, the MLN4924+ISO hearts exhibited LV wall thinning and chamber dilatation, as revealed by significantly reduced LVPW;s and LVPW;d thickness and increased LVID;s and LVID;d compared with measurements after 2 wk of ISO exposure and baseline measurements (Fig. 5, A–D). Consequently, the MLN4924+ISO hearts had significantly decreased LV fractional shortening (32.6 ± 2.3 vs. 38.3 ± 2.6% pre-ISO infusion, P < 0.05) and ejection fraction (58.8 ± 3.3 vs. 66.6 ± 3.1% pre-ISO infusion, P < 0.05) compared with pre-ISO measurements (Fig. 5, E and F and Table 3). Compared with the Vehicle+ISO hearts at 4 wk post-ISO treatment, the MLN4924+ISO hearts had significantly reduced LV wall thickness, EF, and FS (Fig. 5, E and F). Together, these data suggest that transient inhibition of neddylation in the perinatal period may impair adrenergic responses in adulthood and predispose these animals to cardiac dysfunction.
Fig. 5.
MLN4924 (MLN, pevonedistat)-treated rats were sensitized to isoproterenol (ISO)-induced heart failure. Control (Ctl; n = 5) and MLN (n = 5)-treated rats were infused with ISO (30 mg·kg−1·day−1) through osmatic minipump for 2 wk. Echocardiography was performed at indicated time points. A–F: left ventricular (LV) diastolic posterior wall thickness (LVPW;d), LV systolic posterior wall thickness (LVPW;s), LV diastolic internal diameter (LVID;d), LV systolic internal diameter (LVID;s), fractional shortening (FS), and ejection fraction (EF). Two-way ANOVA multiple comparison. *P < 0.05, **P < 0.01, ***P < 0.001. n.s., not significant.
Table 3.
Echocardiography measurements of MLN-treated rats and their littermates at indicated time (related to Fig. 4)
| Ctl |
MLN |
|||||
|---|---|---|---|---|---|---|
| ISO | Pre | 2-wk post | 4-wk post | Pre | 2-wk post | 4-wk post |
| LVAW;d, mm | 1.38 ± 0.02 | 1.79 ± 0.06*** | 1.43 ± 0.05### | 1.54 ± 0.04 | 1.87 ± 0.05*** | 1.25 ± 0.03***###$ |
| LVAW;s, mm | 2.35 ± 0.03 | 3.01 ± 0.07*** | 2.50 ± 0.07### | 2.60 ± 0.10 | 2.82 ± 0.04 | 2.30 ± 0.06*###$ |
| LVID;d, mm | 7.93 ± 0.15 | 7.90 ± 0.24 | 8.46 ± 0.31 | 7.07 ± 0.26 | 7.20 ± 0.16 | 7.85 ± 0.26 |
| LVID;s, mm | 5.39 ± 0.15 | 4.61 ± 0.22* | 5.42 ± 0.25# | 4.39 ± 0.18 | 4.60 ± 0.14 | 5.42 ± 0.24**## |
| LVPW;d, mm | 1.43 ± 0.02 | 1.80 ± 0.07*** | 1.46 ± 0.05## | 1.56 ± 0.06 | 1.85 ± 0.06** | 1.27 ± 0.03***##$ |
| LVPW;s, mm | 2.31 ± 0.03 | 3.06 ± 0.09*** | 2.50 ± 0.06*### | 2.56 ± 0.08 | 2.86 ± 0.03** | 2.25 ± 0.06*###$ |
| EF, % | 61.3 ± 2.72 | 73.1 ± 3.82* | 64.7 ± 2.73 | 66.6 ± 3.09 | 68.0 ± 3.63 | 58.7 ± 3.33*#$ |
| FS, % | 34.3 ± 1.97 | 44.2 ± 3.48* | 37.0 ± 2.06 | 38.2 ± 2.63 | 39.4 ± 2.85 | 32.5 ± 2.31*#$ |
| LV Vol;d, µl | 339 ± 21 | 339 ± 32 | 396 ± 45 | 265 ± 32 | 273 ± 19 | 335 ± 38 |
| LV Vol;s, µl | 142 ± 14 | 101 ± 15 | 146 ± 22 | 89 ± 13 | 98 ± 10 | 146 ± 21*# |
| Heart rate, beats/min | 349 ± 18 | 445 ± 22* | 302 ± 14### | 367 ± 6 | 451 ± 19** | 346 ± 11### |
Values are means ± SE. Ctl, control (n = 5); MLN, MLN4924 (n = 5), pevonedistat; ISO, isoproterenol; LV, left ventricle; LVAW;d, LV anterior wall thickness at end-diastole; LVPW;s, LV anterior wall thickness at end-systole; LVID;d, LV internal dimension at end-diastole; LVID;s, LV internal dimension at end-systole; LVPW;d, LV posterior wall thickness at end-diastole; LVPW;s, LV posterior wall thickness at end-systole; EF, ejection fraction; FS, fraction shortening; LV Vol;d, LV volume at end-diastole; LV Vol;s, LV volume at end-systole. Two-way ANOVA multiple comparison.
P < 0.05,
P < 0.01,
P < 0.001 vs. Pre in Ctl or MLN group;
P < 0.05,
P < 0.01,
P < 0.001 vs. 2 wk in Ctl or MLN group;
P < 0.05 vs. Ctl at 4 wk post-ISO.
Exacerbated pathological cardiac remodeling in MLN4924-treated rats following ISO infusion.
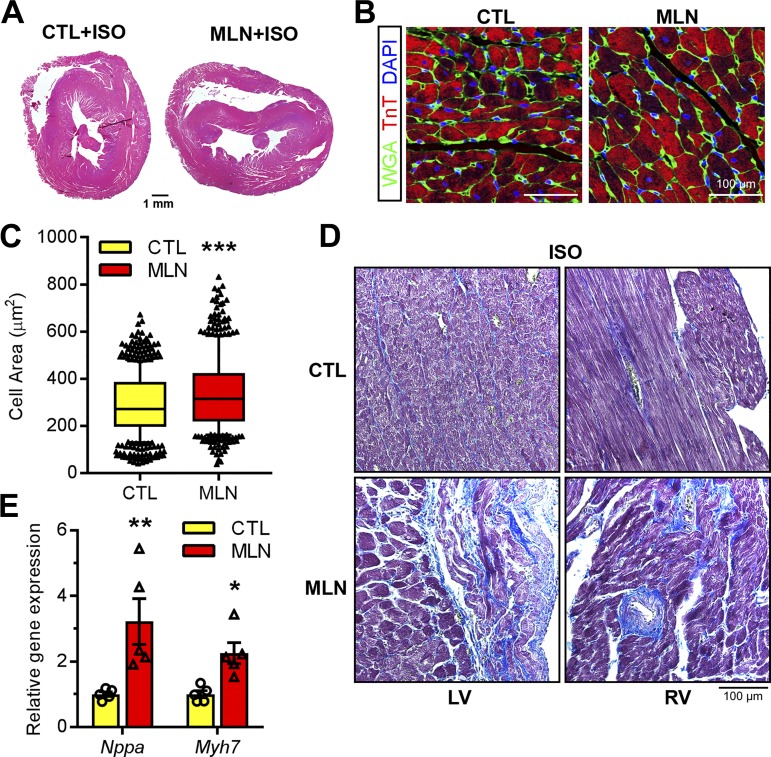
We next assessed the impact of ISO infusion on cardiac remodeling in control and MLN4924-treated animals. H&E-stained myocardial sections showed that MLN4924 hearts were enlarged and dilated after ISO infusion (Fig. 6A), which is consistent with echocardiographic data. Moreover, cardiomyocyte cross-sectional area was significantly increased in MLN4924-treated hearts, suggesting augmented cardiomyocyte hypertrophy after completion of ISO treatment (Fig. 6, B and C). qRT-PCR analysis revealed significant upregulation of cardiac fetal genes such as Nappa and Myh7 in MLN4924+ISO hearts compared with the Vehicle+ISO hearts (Fig. 6D). Finally, interstitial fibrosis was commonly identified in the left and right ventricles of MLN-4924-treated animals after ISO exposure (Fig. 6E). Taken together, these data suggested that transient inhibition of neddylation in the perinatal period propagates pathological remodeling, which presumably contributes to impaired cardiac function.
Fig. 6.
Isoproterenol (ISO) aggravated pathological cardiac remodeling in MLN4924 (MLN, pevonedistat)-treated rats. Hearts from control (Ctl) and MLN-treated rats were harvested 4 wk post-ISO infusion and subjected to analyses. A, B, and D: hematoxylin-eosin staining (A), immunostaining (B), and Masson's trichrome staining (D) of paraffin-embedded myocardium sections. LV, left ventricle; RV right ventricle. Alexa Fluor 488-conjugated wheat germ agglutinin (WGA, green) was used in B. C: quantification of cross-sectional cell area (n > 500 cells/heart, 4 hearts per group). E: relative gene expression levels assessed by qPCR (n = 5 per group). Unpaired Student’s t-test for C; one-way ANOVA followed by post hoc F-test for E. *P < 0.05, **P < 0.01, ***P < 0.001 vs. Ctl.
DISCUSSION
The eukaryotic ubiquitin superfamily contains a series of ubiquitin-like proteins (UBLs) including NEDD8, SUMO, ISG15, Ufm1, and Urm1 among others (10). Modification of protein substrates by these modifiers, which can occur in a temporally controlled and compartment-specific manner, offer important regulatory mechanisms by modulating the functional activity, stability, and localization of target proteins. In contrast to ubiquitin, the function of NEDD8 in the heart is relatively unknown. Dysregulation of neddylation is linked to dilated cardiomyopathy and desmin-related cardiomyopathy (19). Inactivation of deneddylation by deletion of a CSN subunit 8 impaired proteasomal and autophagic proteolysis and caused heart failure in both embryonic and adult hearts (32–34). Furthermore, CSN8 hypomorphism leads to accumulation of neddylated proteins and promotes desmin-related cardiomyopathy (35). Using MLN4924, which has ~100- to 1,000-fold higher inhibitory effect on NAE than other ubiquitin-like activating enzymes (31), our study reveals a critical role for neddylation in postnatal cardiac development. We demonstrate that intact neddylation is required for the last burst of cardiomyocyte proliferation before adolescence and that suppression of neddylation in the early postnatal period induces a hypertrophic cardiomyopathy with impaired recovery from β-adrenergic stimulation in adult animals. Together, these lines of evidence demonstrate that a balanced neddylation-deneddylation pathway is crucial throughout heart development, maturation, remodeling, and disease progression. Given that MLN4924 (pevonedistat) has potent antitumor activity and is currently undergoing clinical trials for multiple malignancies (31, 36, 40, 41), our findings raise concern regarding potential cardiotoxicity with this class of compounds, especially in pregnant women and children.
Neddylation activity remains high in the developing heart until cardiomyocytes exit the cell cycle after the first postnatal week (46). By deleting NAE1 specifically in cardiomyocytes, we (46) have previously reported an indispensable role for neddylation in embryonic cardiac chamber maturation. In our previous study, NAE1 deletion was discernable as early as E12.5, and, since NAE1 is necessary for NEDD8 activation, NAE1 deletion led to complete and irreversible inhibition of neddylation in cardiomyocytes (46). Here, we interrogate the role of neddylation in a late phase of cardiac development using a potent inhibitor of NAE. In comparison to genetic models with constitutive deletion of NAE1, administration of MLN4924 on P1, P3, and P5 resulted in a modest inhibition of neddylation (~30% decrease in neddylated UBC12 and ~50% decrease in total neddylated protein). Hence, the neonatal cardiomyopathy resulting from MLN4924 suggests that modest, transient inhibition of neddylation during key phases of heart development has a detrimental impact on cardiac structure and function. To date, regulation of neddylation has been poorly understood. Identification of environmental and genetic factors that influence neddylation activity may shed light on the development of congenital heart disease and neonatal cardiomyopathy.
Previous studies have suggested that short-term systematic inhibition of neddylation using MLN4924 has no discernable effect on heart or brain function in adult mice (28). In contrast, exposure to MLN4924 during key windows of cardiac development promotes a hypertrophic cardiomyopathy, suggesting a central role for neddylation in heart maturation. Although the exact mechanisms remain unclear, we speculate that MLN4924-induced cardiotoxicity could be linked to its impact on cell proliferation and protein homeostasis. Cullins are the most studied NEDD8 targets (14, 27). Neddylation of cullin family proteins (cullin 1–9) triggers the assembly and activation of ~400 multisubunit cullin-RING ubiquitin ligases (CRLs), which account for the degradation of ~20% cellular proteins (31). Previous studies showed that MLN4924 induces cell cycle arrest by causing the accumulation of several critical CRL substrates, including CDT1, WEE1, p21, and p27 (21, 31), which are well-known cell cycle inhibitors. We (37) recently reported that neddylation inhibition causes cardiomyocyte proliferation arrest, which is attributable to the inactivation of Cul7 ubiquitin ligase and consequently the accumulation of its substrate Hippo kinase Mst1. Consistent with these reports, we found that administration of MLN4924 inhibits cardiomyocyte expansion in the quickly developing heart (Fig. 3), which could have an immediate impact on cardiac performance. Interestingly, although MLN4924 has been shown to promote apoptosis in cancer cells and vascular smooth muscle cells (1, 31), we did not identify differences in TUNEL-positive cardiomyocytes or cleavage of caspase-3 in the hearts of MLN4924-treated neonatal rats (data not shown), suggesting that the proapoptotic effects of MLN4924 could be cell type specific. Meanwhile, neddylation has been shown to regulate protein homeostasis, which is essential for cellular integrity. MLN4924 robustly inhibits cullin neddylation in cardiomyocytes (34, 46). Thus, it is possible that MLN4924 impairs the activity of CRLs and disrupts protein homeostasis in MLN4924-treated hearts, thereby contributing to the cardiotoxicity of MLN4924. Supporting this notion, impaired cullin neddylation through the deletion of CSN8 or cardiac-restricted deletion of cullin 3 perturbs protein homeostasis, leading to dilated cardiomyopathy and perinatal lethality (26, 33). Nevertheless, whether MLN4924 treatment affects the global protein turnover and the expression of CRL targets in the heart remain to be determined. Last, administration of MLN4924 caused an ~30% loss of body weight in the neonates, indicating its possible toxicity in other developing organs. Whether the potential damage on these organs could in turn impact cardiac function is not clear.
While neddylation clearly plays an important role in cardiac development, short-term inhibition of neddylation during the final phases of cardiac maturation permanently alters cardiomyocyte shape and function and may increase susceptibility to heart failure in later life. Although pharmacokinetic studies have demonstrated that MLN4924 is completely eliminated from the plasma within 24 h after infusion (29, 36), administration during the immediate postnatal period promotes cardiac hypertrophy and enhances pathological remodeling and cardiac dysfunction in response to ISO (Figs. 5 and 6). Notably, MLN4924-treated rats failed to develop positive an inotropic response to ISO infusion as control rats did, and the underlying mechanisms remains elusive. We speculate that the increased cardiac vulnerability to ISO infusion is consequent to disrupted maturation from neonatal to adult cardiomyocytes. In mice, the last burst of cardiomyocyte proliferation and nonreplicative DNA synthesis occurs in the first 2 wk after birth and is critical to achieving appropriate cardiomyocyte number and binucleation of existing cardiomyocytes for the adult heart (9, 30). Our observation that MLN4924 leads to an arrest of cardiomyocyte proliferation (Fig. 3) may lead to a reduction in total cardiomyocyte number in the adult heart, triggering compensatory cardiomyocyte hypertrophy (Fig. 4) and sensitizing the adult heart to pathological insults. Simultaneously with these phenotypic changes, cardiomyocytes experience rapid changes in oxygen tension and undergo a critical metabolic transition by shifting from fetal-type glycolytic to adult-type oxidative metabolism as the major source of ATP (20). This process involves temporal downregulation of hypoxia-inducible factor 1-α (HIF-1α) signaling (11, 17) to repress glycolysis and promote fatty acid oxidation and mitochondrial biogenesis (12, 44). Interestingly, neddylation of cullin 2 activates cullin 2-von Hippel-Lindau (VHL) ubiquitin ligase and promotes HIF-1α degradation (6). Therefore, it is possible that inhibition of neddylation results in HIF-1α accumulation and disrupts the metabolic transition in cardiomyocytes, leading to an insufficient metabolic capacity of adult heart when responding to stress. Studies to understand how neddylation controls the maturation of cardiomyocytes are underway.
In summary, our study reveals an unappreciated role of neddylation in perinatal cardiac maturation and suggests that perturbation of neddylation during development induces cardiomyopathy and predisposes the heart to failure under stressed conditions. Nevertheless, our understanding of the biological function of neddylation in the heart is still in its infancy. Future studies are warranted to understand which myocardial proteins are modified by NEDD8, how neddylation controls cardiomyocyte function and survival, and how disruption of neddylation contributes to the development and pathogenesis of cardiac disease.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J. Zou, W.M., R.L., and H.S. conceived and designed research; J. Zou, W.M., R.L., and J. Li performed experiments; J. Zou, W.M., R.L., J. Li, and H.S. analyzed data; J. Zou, J. Li, B.K.S., I.-m.K., J. Liu, J. Zhou, N.L.W., and H.S. interpreted results of experiments; J. Zou and H.S. prepared figures; J. Zou and H.S. drafted manuscript; J. Zou, R.L., B.K.S., I.-m.K., J. Liu, J. Zhou, N.L.W., and H.S. edited and revised manuscript; J. Zou, W.M., R.L., J. Li, B.K.S., I.-m.K., J. Liu, J. Zhou, N.L.W., and H.S. approved final version of manuscript.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants R01-HL-124248 (to H. Su) and F31-HL-139079 (to R. Littlejohn) and American Heart Association Grants 17POST33410592 (to J. Zou) and 16SDG30940002 (to J. Li).
REFERENCES
- 1.Ai TJ, Sun JY, Du LJ, Shi C, Li C, Sun XN, Liu Y, Li L, Xia Z, Jia L, Liu J, Duan SZ. Inhibition of neddylation by MLN4924 improves neointimal hyperplasia and promotes apoptosis of vascular smooth muscle cells through p53 and p62. Cell Death Differ 25: 319–329, 2018. doi: 10.1038/cdd.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguère V, Evans RM. ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6: 13–24, 2007. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Aye CY, Lewandowski AJ, Lamata P, Upton R, Davis E, Ohuma EO, Kenworthy Y, Boardman H, Wopperer S, Packham A, Adwani S, McCormick K, Papageorghiou AT, Leeson P. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res 82: 36–46, 2017. doi: 10.1038/pr.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertagnolli M, Huyard F, Cloutier A, Anstey Z, Huot-Marchand JE, Fallaha C, Paradis P, Schiffrin EL, Deblois D, Nuyt AM. Transient neonatal high oxygen exposure leads to early adult cardiac dysfunction, remodeling, and activation of the renin-angiotensin system. Hypertension 63: 143–150, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01760. [DOI] [PubMed] [Google Scholar]
- 5.Chang SC, Ren S, Rau CD, Wang JJ. Isoproterenol-induced heart failure mouse model using osmotic pump implantation. Methods Mol Biol 1816: 207–220, 2018. doi: 10.1007/978-1-4939-8597-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis VF, Ehrentraut SF, Campbell EL, Glover LE, Bayless A, Kelly CJ, Kominsky DJ, Colgan SP. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB J 29: 208–215, 2015. doi: 10.1096/fj.14-259663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enchev RI, Schulman BA, Peter M. Protein neddylation: beyond cullin-RING ligases. Nat Rev Mol Cell Biol 16: 30–44, 2015. doi: 10.1038/nrm3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong L, Yeh ET. Identification of the activating and conjugating enzymes of the NEDD8 conjugation pathway. J Biol Chem 274: 12036–12042, 1999. doi: 10.1074/jbc.274.17.12036. [DOI] [PubMed] [Google Scholar]
- 9.Hirai M, Cattaneo P, Chen J, Evans SM. Revisiting preadolescent cardiomyocyte proliferation in mice. Circ Res 118: 916–919, 2016. doi: 10.1161/CIRCRESAHA.115.308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature 458: 422–429, 2009. doi: 10.1038/nature07958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hölscher M, Schäfer K, Krull S, Farhat K, Hesse A, Silter M, Lin Y, Pichler BJ, Thistlethwaite P, El-Armouche A, Maier LS, Katschinski DM, Zieseniss A. Unfavourable consequences of chronic cardiac HIF-1α stabilization. Cardiovasc Res 94: 77–86, 2012. doi: 10.1093/cvr/cvs014. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Hickey RP, Yeh JL, Liu D, Dadak A, Young LH, Johnson RS, Giordano FJ. Cardiac myocyte-specific HIF-1α deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J 18: 1138–1140, 2004. doi: 10.1096/fj.04-1510fje. [DOI] [PubMed] [Google Scholar]
- 13.Kamitani T, Kito K, Nguyen HP, Yeh ET. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J Biol Chem 272: 28557–28562, 1997. doi: 10.1074/jbc.272.45.28557. [DOI] [PubMed] [Google Scholar]
- 14.Kandala S, Kim IM, Su H. Neddylation and deneddylation in cardiac biology. Am J Cardiovasc Dis 4: 140–158, 2014. [PMC free article] [PubMed] [Google Scholar]
- 15.Khera R, Pandey A, Ayers CR, Agusala V, Pruitt SL, Halm EA, Drazner MH, Das SR, de Lemos JA, Berry JD. Contemporary epidemiology of heart failure in fee-for-service medicare beneficiaries across healthcare settings. Circ Heart Fail 10: e004402, 2017. doi: 10.1161/CIRCHEARTFAILURE.117.004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev 22: 1948–1961, 2008. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol 28: 3790–3803, 2008. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Johnson JA, Su H. Ubiquitin and ubiquitin-like proteins in cardiac disease and protection. Curr Drug Targets 19: 989–1002, 2018. doi: 10.2174/1389450117666151209114608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Ma W, Li H, Hou N, Wang X, Kim IM, Li F, Su H. NEDD8 ultimate buster 1 long (NUB1L) protein suppresses atypical neddylation and promotes the proteasomal degradation of misfolded proteins. J Biol Chem 290: 23850–23862, 2015. doi: 10.1074/jbc.M115.664375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56: 130–140, 2010. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L, Chu Y, Yi J, Wang X, Sun Y, Jeong LS, Liu J, Jia L. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res 72: 3360–3371, 2012. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 22.Lykke-Andersen K, Schaefer L, Menon S, Deng XW, Miller JB, Wei N. Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E, and early embryonic death. Mol Cell Biol 23: 6790–6797, 2003. doi: 10.1128/MCB.23.19.6790-6797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menon S, Chi H, Zhang H, Deng XW, Flavell RA, Wei N. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor-induced entry into the cell cycle from quiescence. Nat Immunol 8: 1236–1245, 2007. doi: 10.1038/ni1514. [DOI] [PubMed] [Google Scholar]
- 24.Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res 71: 3042–3051, 2011. doi: 10.1158/0008-5472.CAN-10-2122. [DOI] [PubMed] [Google Scholar]
- 25.Nandi SS, Mishra PK. Harnessing fetal and adult genetic reprograming for therapy of heart disease. J Nat Sci 1: e71, 2015. [PMC free article] [PubMed] [Google Scholar]
- 26.Papizan JB, Vidal AH, Bezprozvannaya S, Bassel-Duby R, Olson EN. Cullin-3-RING ubiquitin ligase activity is required for striated muscle function in mice. J Biol Chem 293: 8802–8811, 2018. doi: 10.1074/jbc.RA118.002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20, 2005. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 28.Reihe CA, Pekas N, Wu P, Wang X. Systemic inhibition of neddylation by 3-day MLN4924 treatment regime does not impair autophagic flux in mouse hearts and brains. Am J Cardiovasc Dis 7: 134–150, 2017. [PMC free article] [PubMed] [Google Scholar]
- 29.Sarantopoulos J, Shapiro GI, Cohen RB, Clark JW, Kauh JS, Weiss GJ, Cleary JM, Mahalingam D, Pickard MD, Faessel HM, Berger AJ, Burke K, Mulligan G, Dezube BJ, Harvey RD. Phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with advanced solid tumors. Clin Cancer Res 22: 847–857, 2016. doi: 10.1158/1078-0432.CCR-15-1338. [DOI] [PubMed] [Google Scholar]
- 30.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis during hypertrophy in adult mice. Am J Physiol Heart Circ Physiol 266: H1439–H1445, 1994. doi: 10.1152/ajpheart.1994.266.4.H1439. [DOI] [PubMed] [Google Scholar]
- 31.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S, Cullis CA, Doucette A, Garnsey JJ, Gaulin JL, Gershman RE, Lublinsky AR, McDonald A, Mizutani H, Narayanan U, Olhava EJ, Peluso S, Rezaei M, Sintchak MD, Talreja T, Thomas MP, Traore T, Vyskocil S, Weatherhead GS, Yu J, Zhang J, Dick LR, Claiborne CF, Rolfe M, Bolen JB, Langston SP. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458: 732–736, 2009. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 32.Su H, Li F, Ranek MJ, Wei N, Wang X. COP9 signalosome regulates autophagosome maturation. Circulation 124: 2117–2128, 2011. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional Csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res 108: 40–50, 2011. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su H, Li J, Osinska H, Li F, Robbins J, Liu J, Wei N, Wang X. The COP9 signalosome is required for autophagy, proteasome-mediated proteolysis, and cardiomyocyte survival in adult mice. Circ Heart Fail 6: 1049–1057, 2013. doi: 10.1161/CIRCHEARTFAILURE.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su H, Li J, Zhang H, Ma W, Wei N, Liu J, Wang X. COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity. Circ Res 117: 956–966, 2015. doi: 10.1161/CIRCRESAHA.115.306783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M, Hua Z, Blakemore SJ, Faessel H, Sedarati F, Dezube BJ, Giles FJ, Medeiros BC. Pevonedistat (MLN4924), a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 169: 534–543, 2015. doi: 10.1111/bjh.13323. [DOI] [PubMed] [Google Scholar]
- 37.Tateishi K, Omata M, Tanaka K, Chiba T. The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J Cell Biol 155: 571–579, 2001. doi: 10.1083/jcb.200104035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomoda K, Yoneda-Kato N, Fukumoto A, Yamanaka S, Kato JY. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J Biol Chem 279: 43013–43018, 2004. doi: 10.1074/jbc.M406559200. [DOI] [PubMed] [Google Scholar]
- 39.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res 79: 208–217, 2008. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Medeiros BC, Erba HP, DeAngelo DJ, Giles FJ, Swords RT. Targeting protein neddylation: a novel therapeutic strategy for the treatment of cancer. Expert Opin Ther Targets 15: 253–264, 2011. doi: 10.1517/14728222.2011.550877. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zhang W, Yan Z, Liang Y, Li L, Yu X, Feng Y, Fu S, Zhang Y, Zhao H, Yu J, Jeong LS, Guo X, Jia L. Radiosensitization by the investigational NEDD8-activating enzyme inhibitor MLN4924 (pevonedistat) in hormone-resistant prostate cancer cells. Oncotarget 7: 38380–38391, 2016. doi: 10.18632/oncotarget.9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6: 599–609, 2005. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 43.Yan J, Walz K, Nakamura H, Carattini-Rivera S, Zhao Q, Vogel H, Wei N, Justice MJ, Bradley A, Lupski JR. COP9 signalosome subunit 3 is essential for maintenance of cell proliferation in the mouse embryonic epiblast. Mol Cell Biol 23: 6798–6808, 2003. doi: 10.1128/MCB.23.19.6798-6808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11: 407–420, 2007. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Zhao R, Yeung SC, Chen J, Iwakuma T, Su CH, Chen B, Qu C, Zhang F, Chen YT, Lin YL, Lee DF, Jin F, Zhu R, Shaikenov T, Sarbassov D, Sahin A, Wang H, Wang H, Lai CC, Tsai FJ, Lozano G, Lee MH. Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers. J Clin Invest 121: 851–865, 2011. doi: 10.1172/JCI44111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou J, Ma W, Li J, Littlejohn R, Zhou H, Kim IM, Fulton DJR, Chen W, Weintraub NL, Zhou J, Su H. Neddylation mediates ventricular chamber maturation through repression of Hippo signaling. Proc Natl Acad Sci USA 115: E4101–E4110, 2018. doi: 10.1073/pnas.1719309115. [DOI] [PMC free article] [PubMed] [Google Scholar]