Abstract
The cochlear summating potential (SP) to a tone is a baseline shift that persists for the duration of the burst. It is often considered the most enigmatic of cochlear potentials because its magnitude and polarity vary across frequency and level and its origins are uncertain. In this study, we used pharmacology to isolate sources of the SP originating from the gerbil cochlea. Animals either had the full complement of outer and inner hair cells (OHCs and IHCs) and an intact auditory nerve or had systemic treatment with furosemide and kanamycin (FK) to remove the outer hair cells. Responses to tone bursts were recorded from the round window before and after the neurotoxin kainic acid (KA) was applied. IHC responses were then isolated from the post-KA responses in FK animals, neural responses were isolated from the subtraction of post-KA from pre-KA responses in NH animals, and OHC responses were isolated by subtraction of post-KA responses in FK animals from post-KA responses in normal hearing (NH) animals. All three sources contributed to the SP; OHCs with a negative polarity and IHCs and the auditory nerve with positive polarity. Thus the recorded SP in NH animals is a sum of contributions from different sources, contributing to the variety of magnitudes and polarities seen across frequency and intensity. When this information was applied to observations of the SP recorded from the round window in human cochlear implant subjects, a strong neural contribution to the SP was confirmed in humans as well as gerbils.
NEW & NOTEWORTHY Of the various potentials produced by the cochlea, the summating potential (SP) is typically described as the most enigmatic. Using combinations of ototoxins and neurotoxins, we show contributions to the SP from the auditory nerve and from inner and outer hair cells, which differ in polarity and vary in size across frequency and level. This complexity of sources helps to explain the enigmatic nature of the SP.
Keywords: auditory nerve, cochlear implants, electrocochleography, inner hair cells, outer hair cells
INTRODUCTION
The summating potential (SP) is one of several potentials produced by the cochlea in response to sound. To stimulation with tone bursts, the SP appears as a baseline shift that persists for the duration of the burst and thus represents the stimulus envelope (Dallos et al. 1972; Davis et al. 1958). The SP has clinical significance due to changes associated with endolymphatic hydrops, the pathophysiological correlate of Meniere’s disease (Eggermont 2017; Hornibrook 2017; Hornibrook et al. 2012; Iseli and Gibson 2010), and may provide an indication of cochlear synaptopathy, or “hidden hearing loss” (Liberman et al. 2016). The SP is also highly variable in cochlear implant (CI) subjects (Riggs et al. 2017). The sources of the SP are typically considered “enigmatic” because changes in its polarity across frequency and level are thought to reflect multiple origins (Dallos et al. 1972; Davis et al. 1958). Typically, it is described as a hair cell potential produced by asymmetries in cochlear transduction to the two directions of stereociliary movement (Whitfield and Ross 1965). A neural contribution is rarely considered in descriptions of the SP (Eggermont 2017; Hornibrook 2017; Russell 2008), although it has been suggested in the past (Davis et al. 1950; Kupperman 1966), and animal experiments using neurotoxins have shown a reduction in the SP magnitude (Forgues et al. 2014; Sellick et al. 2003; van Emst et al. 1995). To better understand the underlying processes, we report in this article the results of pharmacological experiments in gerbils that are intended to isolate the individual contributions of outer hair cells (OHCs), inner hair cells (IHCs), and neural sources to the SP. We also show that the properties of the different sources help to interpret recordings from the round window (RW) in human subjects receiving CIs.
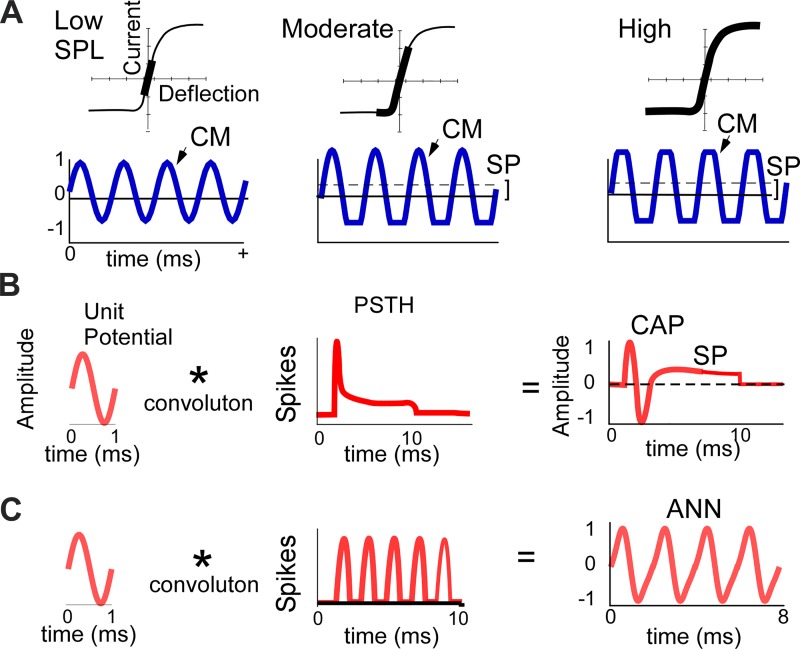
The sources of the responses seen with electrocochleography (ECochG) are the OHCs, IHCs, and spiral ganglion neurons. Dissection of these sources depends on differences in the biophysical properties that produces them (Fig. 1). For hair cells, the cochlear microphonic (CM) is derived from currents flowing through transduction channels in the stereocilia as they follow the basilar membrane (Davis 1965; Russell 1983; Santos-Sacchi 1993). For low-intensity tones, the CM is a relatively undistorted sinusoid (Fig. 1A, left). For higher intensities, the input-output function is limited by saturation of channel openings or closings. The saturation is asymmetric, with the operating point, or proportion of channels open at rest, usually less than 50% (Russell 2008) Thus, for moderate intensities (Fig. 1A, middle), saturation occurs only to one direction, producing a non-zero average response, seen as a contribution to the SP. For high intensities (Fig. 1A, right), saturation occurs for both directions. The hair cell function in Fig. 1, A–C, is close to that of OHCs, with ~40% open channels compared with ~10% for IHCs (Russell 2008), at least in the basal cochlea (Johnson 2015). Thus a key difference between OHCs and IHCs is the differential degree of saturation to the input.
Fig. 1.
Schematics of the sources of cochlear potentials, including the cochlear monophonic (CM), compound action potential (CAP), auditory nerve neurophonic (ANN), and summating potential (SP). A: input-output function of hair cell transduction (top row) and CM waveforms produced (bottom row) as a function of intensity (across rows). For low intensities, the transduction is within the linear range, producing a sinusoidal CM. For moderate intensities, the asymmetric input-output function saturates only in the hyperpolarizing direction of stereociliary movement, producing a flattened waveform and an SP (mean of the response). For high intensities, saturation occurs in both directions but the SP remains the same, because it is a function of the degree of asymmetry. B: the CAP is produced by the convolution (*) of a unit potential, or shape of an action potential as it appears at the round window, and the well-timed onset responses in the poststimulus time histogram (PSTH). A contribution to the SP during the sustained portion would appear if the unit potential were asymmetric. C: the ANN is produced by the convolution (*) of the unit potential and the cyclic PSTH to low-frequency tones. SPL, sound pressure level.
The auditory nerve contributions can be represented by the convolution of the “unit potential,” or shape of a single action potential at the RW, with the poststimulus time histogram (PSTH), or pattern of the population of auditory nerve fibers firing during the tone burst (Chertoff 2004; Goldstein and Kiang 1958; Kiang et al. 1976; Wang 1979). Traditionally, this convolution has been applied to describe the compound action potential (CAP; Fig. 1B), but it is equally valid for the auditory nerve neurophonic (ANN; Fig. 1C) and possibly also for the SP (Fig. 1B). The CAP is produced by the synchronized responses of auditory nerve fibers to onsets and is most prominent to high frequencies. In the PSTH to high frequencies (above the range of neural phase-locking), it appears as an onset followed by a steady state derived from asynchronous firing of action potentials. The ANN is produced to low frequencies by the phase-locked firing over a restricted portion of a stimulus cycle. Because a neural contribution to the SP is not considered in most reports, there is no consensus on a possible source. Unlike the CAP and ANN, the PSTH does not provide a time structure to support an SP. To be incorporated into this theoretical framework, any SP is therefore likely to arise from asymmetry in the unit potential. Measurements of the unit potential are made through spike-triggered averaging based on firing in auditory nerve single units. Although noisy, many such measurements show asymmetries (Kiang et al. 1976; Prijs 1986; Versnel et al. 1992; Wang 1979). In addition, a neural component can be detected at the RW from spontaneous or driven activity in the form of energy near that of the unit potential, broadly centered at 700–1,000 Hz (Dolan et al. 1990; McMahon and Patuzzi 2002).
Our interest in the SP extends primarily from observations in CI subjects, where it has a highly variable morphology (Riggs et al. 2017). There is growing technology to enable use of ECochG to monitor functional integrity of the cochlea during implantation in efforts to reduce surgically induced trauma (Acharya et al. 2016; Adunka et al. 2010; Calloway et al. 2014; Campbell et al. 2015; Dalbert et al. 2015; Giardina et al. 2018; Giardina et al. In press), and the SP may be particularly informative of the site of lesion (Helmstaedter et al. 2018). Thus a more comprehensive understanding of the sources of the SP is of importance for understanding the cochlear responses from ECochG in CI subjects, and possibly for other clinical uses, as well.
MATERIALS AND METHODS
A total of 57 Mongolian gerbils (Meriones unguiculatus) were used in the experiments. All animal protocols were prospectively approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill, following the standards of the National Institutes of Health and Committee on Care and Use of Laboratory Animals. Human ECochG was performed in subjects receiving CIs under a protocol prospectively approved by the University of North Carolina’s Institutional Review Board for research involving human subjects. Written consent was obtained for all adults, written parental consent was obtained for all pediatric subjects, and written patient assent was obtained from children between 7 and 18 yr of age. Inclusion criteria for ECochG were that subjects were scheduled to receive a CI after the medical and audiological evaluation had established candidacy. Potential candidates were excluded if they required an English interpreter, were undergoing revision surgery, or presented with severe inner ear malformations. The subject pool therefore consisted of the typical mix of subjects of all ages seen at a large implant center.
Experimental design.
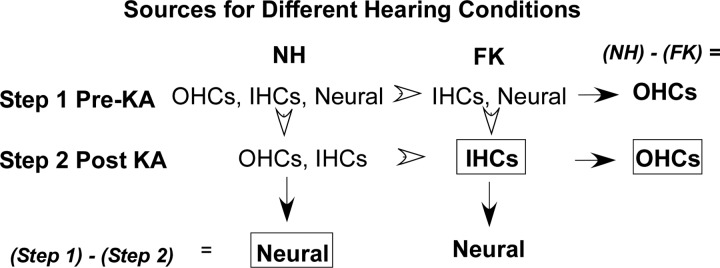
The research design of the experiments and data analysis to isolate sources contributing to the SP is shown in Fig. 2. The experiments used animals with two hearing conditions: untreated, normal hearing animals (NH) or animals treated with a combination of furosemide and kanamycin (FK), which has ototoxic properties causing the death of hair cells. The goal was to identify and use a combination of FK that would selectively remove OHCs and leave IHCs intact. Next, by recording before and after the neurotoxin kainic acid (KA) was applied to the RW (steps 1 and 2, respectively, in Fig. 2), the individual sources could be isolated. The contribution from IHCs was obtained from the post-KA FK animals. The neural contribution was obtained from the NH animals by subtracting the post-KA from the pre-KA responses. The OHC contribution was obtained by subtracting the post-KA responses across the hearing conditions. An important consideration is that the IHC contribution is obtained in the absence of OHCs. The lack of the :cochlear amplifier” provided by OHCs will affect the measured and calculated responses using this value, especially to low intensities. Most of the results in this study used suprathreshold stimuli (60–90 dB SPL), so the effect of OHC loss was minimized, but there were still smaller neural responses in the FK animals, so we used the post-KA subtraction to reduce this confound.
Fig. 2.
Experimental design. The goal was to isolate outer hair cells (OHCs), inner hair cells (IHCs), and neural sources of the summating potential (SP). To do this, animals with two hearing conditions were used. In step 1, recordings were made from normal hearing (NH) animals with the full complement of OHCs, IHCs, and neural elements or from animals treated with a combination of furosemide and kanamycin (FK), which had OHCs removed. In step 2, the neurotoxin kainic acid (KA) was applied and the recordings repeated. The response contributed by IHCs was isolated directly in FK animals after KA. Subtractions within groups (step 1 – step 2; arrows) yield the neural responses, whereas subtractions between groups (NH – FK; arrowheads) yield the OHC responses. The boxes indicate the resulting subtractions used to quantify the neural, IHC, and OHC responses (see text).
Development of a gerbil model to selectively remove OHCs.
Previous work on guinea pigs (Dallos and Cheatham 1976), mice (Oesterle et al. 2008), and chinchillas (Dallos and Harris 1978; Santi et al. 1982) has shown that varying the doses of kanamycin alone or in combination with a diuretic, such as furosemide or bumetanide, can selectively remove OHCs while leaving IHCs intact. The use of a diuretic allows for ototoxicity from a single dose of kanamycin, rather than sequential injections over many days. In gerbils, a combination of furosemide at 100 mg/kg and kanamycin at 500 mg/kg removed OHCs but also removed most IHCs (Abbas and Rivolta 2015). Thus our approach was to vary the dosages of these two compounds to produce maximal loss of OHCs combined with maximal preservation of IHCs. Gerbils were injected with subcutaneous doses of 200–500 mg/kg kanamycin (Diamondback Drugs, Scottsdale, AZ) followed 20–30 min later by an intraperitoneal dose of 50–150 mg/kg furosemide (Merck NADA no. 34-478). Controls were animals injected with furosemide alone, which by itself is not ototoxic but does reduce the endocochlear potential through its diuretic action affecting the stria vascularis. This typically resolves within 24 h (Sewell 1984; Syka and Melichar 1985). The recordings from FK animals were done 7–10 days after drug administration.
For both NH and FK animals, baseline ECochG recordings were followed by treatment with KA (60 or 100 mM; Sigma no. K0250). The KA was dissolved in artificial perilymph, heated to 37°C, and applied to the RW niche for 1 h. The formula for the artificial perilymph was (in mM) 127.5 NaCl, 3.5 KCl, 25 NaHCO3, 1.3 CaCl2, 1.2 MgCl2, 0.75 NaH2PO4, and 11 glucose, and the pH was adjusted to 7.3 with HCl (Mikulec et al. 2009). The KA was sometimes reapplied within the 60 min to account for fluid displacement by seepage from surrounding structures. After the hour, the KA solution was wicked from the RW, which was then flushed with artificial perilymph. An identical ECochG recording series was then performed.
Following experimental completion, animals were euthanized by an overdose of pentobarbital sodium, followed by decapitation. The temporal bones were removed, fixed in 4% paraformaldehyde for 3 days, and then decalcified in 10% EDTA. The basilar membrane was dissected in toto and prepared as a whole-mount. Hair cells were counted in two ways: early cases were stained with iron hematoxylin or toluidine blue, mounted, and coverslipped. Hair cells were counted in 250-μm increments using a Zeiss Axioscope with a ×40 objective (Carl Zeiss, Thornwood, NY). In later cases, OHCs and IHCs were immunolabeled with a mouse antibody to parvalbumin (Sigma no. P3088, RRID:AB_477329; 1:20,000) and neural processes with a goat antibody to Na+-K+ ATPase α3 (Santa Cruz Biotechnology no. sc-48345, RRID:AB_626712), and viewed under a confocal microscope (Zeiss LSM 710). The parvalbumin was visualized using a biotinylated secondary antibody (horse anti-mouse, no. BA-2000; Vector Laboratories; RRID:AB2313571) and a tertiary treatment with streptavidin bound to Alexa Fluor 568 (no. S11226, RRID:AB_2315774). The Na+-K+ ATPase was visualized with a secondary antibody conjugated with Alexa Fluor 488 (donkey anti-goat, no. A11055; Molecular Probes; RRID:AB_142672).
ECochG in gerbils and humans.
The acoustic stimulation and recordings of cochlear responses were performed using a Bio-logic Navigator Pro (Natus Medical, San Carlos, CA) in both animals and humans, as described previously (Choudhury et al. 2012; Fontenot et al. 2018; Forgues et al. 2014; Riggs et al. 2017). For all recordings, the electrode attached to the noninverting input of the differential amplifier was a stainless steel probe of the type used for facial nerve monitoring during CI surgeries (Neurosign 3602-00-TE; Magstim, Whitland, UK) and placed in the RW niche. The use of similar equipment facilitates comparisons between the animal and human data sets.
Gerbils were anesthetized with 1.5 g/kg urethane and 10 mg/kg pentobarbital sodium. The small dose of pentobarbital provides sedation so that an anesthetic plane with urethane is reached more quickly. The head was shaved, and the animal was placed on a heating pad and kept at an internal temperature near 38°C, monitored via rectal probe. The animal was then injected subcutaneously with 0.05 mg/kg atropine to control respiratory secretions and 0.5 ml of normal saline for fluid replacement. Lidocaine/epinephrine was injected in the subcutaneous tissue above the right ear for topical analgesia and hemostasis (right side was used in all surgeries). The right auricle was dissected and reflected, and a sound tube was placed at the external auditory canal, using remnant auricular tissue to ensure a seal. The RW niche was visualized through a small opening in the bulla. To ensure low impedances, a small drop of artificial perilymph solution was applied to the RW before the first recording. A needle electrode (no. 102516; Natus Medical) placed in the contralateral neck muscles provided the inverting input, and both inputs were referenced to a needle electrode at the base of the tail.
Stimuli used in gerbils were tone bursts (2, 3, 4, 6, and 8 kHz), alternating in condensation and rarefaction phases, with 100 repetitions to each phase. Calibration was performed in a test animal using a ¼-in. microphone and measuring amplifier ((Bruel & Kjaer, Naerum, Denmark) attached to a small probe tube near the end of the speaker in the closed field. Subsequently, the calibration tables in the clinical device were adjusted to produce the correct SPL to each tone frequency. Intensities used were 0–90 dB SPL for 4 kHz and 60–90 dB SPL for the other frequencies. Rise/fall times were one cycle or 1 ms, whichever was longer, shaped by a Blackman window. Plateau durations were 10 ms (2 kHz) or 5 ms (3 to 8 kHz). Recordings were 512 points, and recording epochs were 32 ms (2 kHz) or 10.66 ms (3 to 8 kHz). Tone presentation rate was 11.1 s−1. The signals were amplified with a gain of 1,000 times, with a high-pass filter setting of 1 Hz and low-pass settings of 10 kHz (2 kHz stimuli) or 15 kHz (3 to 8 kHz).
For human subjects, recordings were done intraoperatively during CI surgery, after exposure of the RW niche and before insertion of the CI (see Choudhury et al. 2012; Fontenot et al. 2018; Riggs et al. 2017). Surface electrodes over the contralateral mastoid and on the forehead served as the inverting and reference electrodes, respectively. Differences in stimulation and recording parameters compared with the gerbil setup were 1) the repetition rates were higher (17.33 s−1), 2) the high-pass filter setting was increased from 1 to 10 Hz, and 3) the gain was increased to 50,000 times. The increase in the high-pass setting was done to reduce the effects of low frequencies in the noisier operating room environment; however, doing so causes attenuation of the SP as the duration of the stimulus increases.
In some cases, for both gerbils and humans, we crimped or removed the sound tube to demonstrate that the response was not electrical artifact. In addition, in one human recording the speaker’s ground shielding was removed and the artifact recorded directly; these recordings could subsequently be used with any later recording in either preparation to see if the response was associated with electrical artifact. An additional useful indication is that electrical artifact starts before the acoustic delay.
Data analysis.
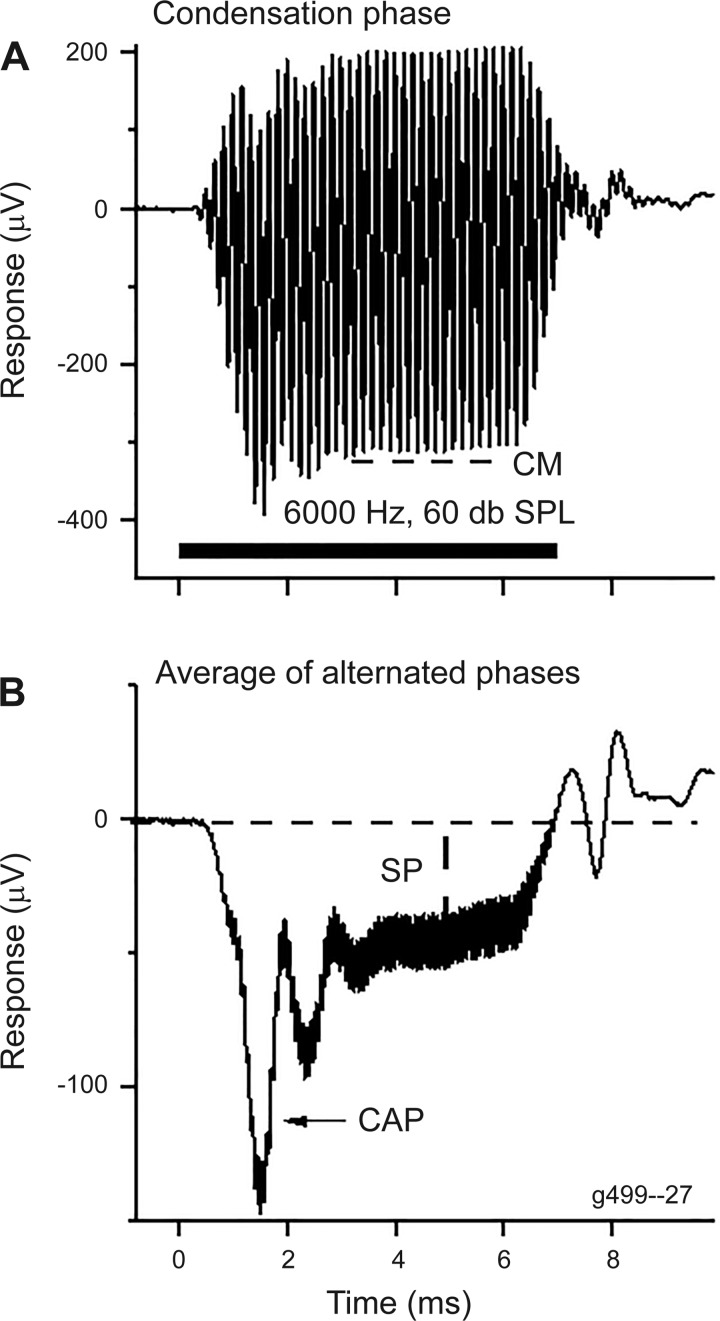
The temporal and spectral analyses of the ECochG signal were performed using custom routines in MATLAB (version R2017a; The MathWorks, Natick, MA). An example of the recordings and analysis in a NH animal is shown in Fig. 3. The stimulus was a 6-kHz tone burst at 60 dB peak SPL. The averaged responses are shown to the condensation-phase stimulus (Fig. 3A) and for the sum of the two phases (condensation and rarefaction), divided by two (Fig. 3B). The CM was measured as the root mean square value for condensation-phase stimuli over the time window indicated. The CAP and SP are most visible in the sum of the two phases, which removes the largest part of the CM as it alternates with the stimulus phase, whereas the CAP and SP do not alternate with phase. For the gerbils, where a 0.1-Hz high-pass filter was used, the SP in the steady state was constant for the duration of the tone burst stimulus and was taken as the average deviation from baseline over a period of 1 ms just before the beginning of the fall of the stimulus. In humans, the measurement was taken immediately after the CAP, because the 10-Hz high-pass filter caused the response to decay.
Fig. 3.
Example of a response obtained using electrocochleography and its analysis. Recordings are from a gerbil to a 6-kHz and 60-dB SPL tone burst. A: the average of 100 responses to a condensation-phase stimulus (horizontal bar at bottom). The cochlear monophonic (CM) was measured from a time window during the steady state (dashed line). B: the summating potential (SP) was measured from the sum of the alternated phases (rarefaction and condensation) as the deviation from baseline in the steady-state response following the compound action potential (CAP).
RESULTS
Development of a model to selectively remove OHCs.
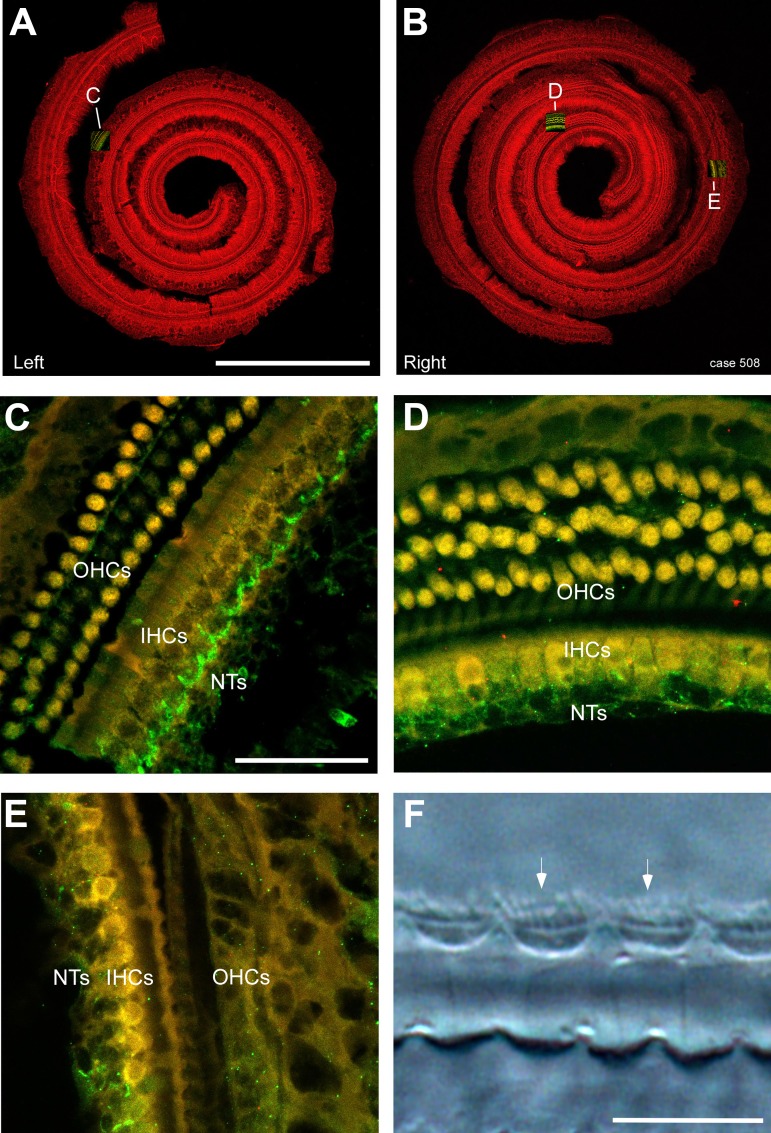
The requirement for inclusion was for IHCs to be preserved from the base of the cochlea through the point of CF for a given tone frequency and for OHCs to be lost over this same cochlear span. This criterion was used because the traveling wave excites all parts of the cochlea basal to the characteristic frequency (CF) region of the tone but does not continue past the point of CF, so OHCs that are apical to the CF region of the stimulus do not contribute to the response. An example with this desired pattern is shown in Fig. 4. The dissected basilar membranes from the left and right sides are shown in Fig. 4, A and B; the red fluorescence is from an antibody to parvalbumin and viewed with a confocal microscope under low power. The right side was used for ECochG recordings before and after treatment with KA to remove nerve terminals. Insets show the locations of Fig. 4, C–E. In Fig. 4C, the image has both the red channel for parvalbumin and green for Na+-K+ ATPase. The hair cells appear yellow after the red and green channels are combined, due to background levels of autofluorescence present in hair cells (in the green channel), whereas the nerve terminals (NTs) are bright green. There is a full complement of hair cells at this relatively apical location (note the middle row of OHCs is faint but labeled) and a regular pattern of NTs closely opposed to IHCs. In Fig. 4D, the side treated with KA, the hair cells are normal but the NTs are disrupted, even at this apical location. In Fig. 4E, located more basally, the OHCs are entirely removed but the IHCs remain with the NTs disrupted, thus isolating the IHCs at this location. Higher magnification (Fig. 4F), viewed with differential interference contrast microscopy, shows that the stereocilia (arrow) of IHCs are intact.
Fig. 4.
Histology from a case with the desired pattern of hair cell loss. A and B: the dissected basilar membranes from the left and right sides, respectively, in a case treated with 100 mg/kg furosemide and 300 mg/kg kanamycin. The red fluorescence is from an antibody to parvalbumin. The right side was treated with kainic acid (KA) to remove nerve terminals (NTs) during the electrocochleography. Insets show locations of C–E. Scale bar = 1 mm. C: higher magnification image from the second turn of the left side showing labeling with anti-parvalbumin (yellow due to combination of red fluorescence from parvalbumin and green autofluorescence) for hair cells and an antibody to a Na+-K+ ATPase (green) for NTs. The outer hair cells (OHCs), inner hair cells (IHCs), and NTs are all intact; note the chalice-like appearance of terminals coalescing at the base of IHCs. Scale bar in C = 50 μm and also applies to D and E. D: from the apical part of the right side. OHCs and IHCs are present, but the NTs have been affected by the KA and no longer form chalices under the hair cells. E: from a basal part of the right side. OHCs are entirely absent, but IHCs remain. The NTs have been affected by the KA. F: higher magnification from the basal turn of the right side, viewed with differential interference contrast microscopy to show that the stereocilia (arrows) of IHCs are intact. Scale bar = 15 μm.
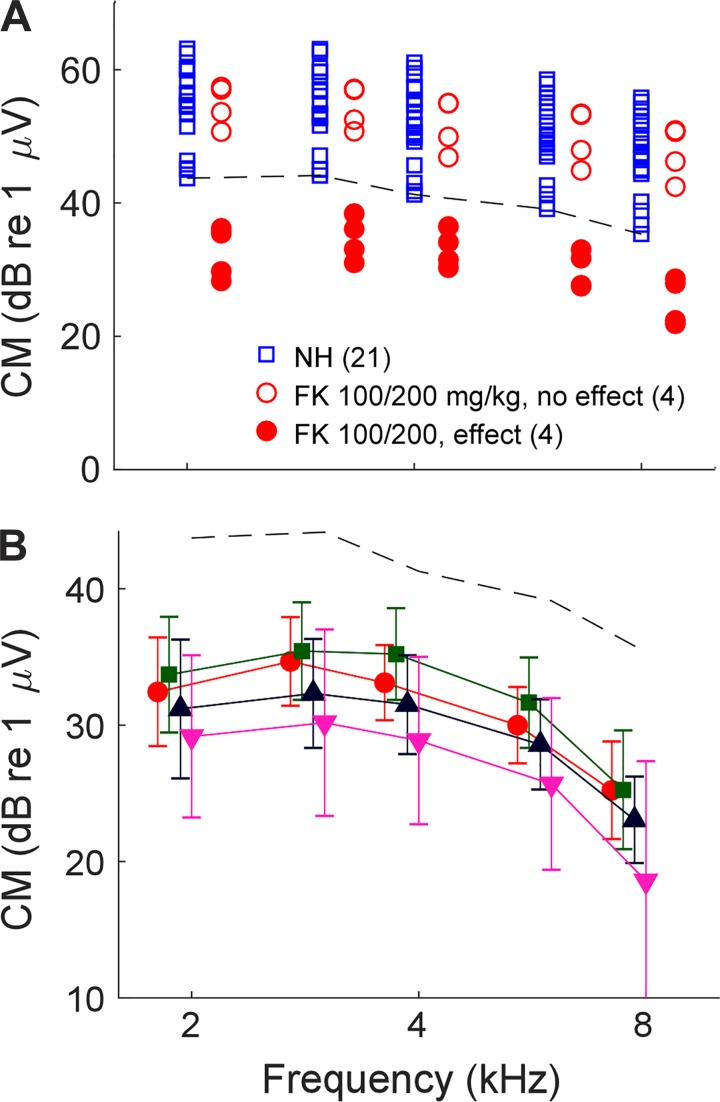
To develop a preparation with the desired features required a parametric evaluation of the effects of different FK treatments on the ECochG and survival of hair cells. Physiologically, the effectiveness of the drug combinations was determined by their effect on the CM, which is a purely hair cell potential. The results are summarized in Table 1 and Figs. 5 and 6. For NH animals, the magnitude of the CM varied over about a 20-dB range across 21 animals (Fig. 5A). For FK animals, responses within this range were considered “unchanged,” whereas any responses less than the lower limit of NH animals were considered “reduced.” As shown in Table 1, responses were consistently unchanged if only furosemide was used (5 animals) or if the dosage of furosemide was 50 mg/kg (2 animals). For furosemide dosages of 100 mg, the effects were highly variable across the range of kanamycin dosages, except for the highest dose (500 mg/kg), where all three animals showed reduced responses. A furosemide dosage of 150 mg/kg resulted in reduced responses across three animals tested at two dosages of kanamycin. In Fig. 5A, variability in the effectiveness of FK is shown for eight cases receiving one dosage level (100/200 mg/kg FK). Of the eight cases, four were in the unchanged range and four were reduced. Figure 5B shows the effects of different dosages of kanamycin that produced cases with reduced responses. There was considerable overlap, but in general the higher doses showed a smaller CM. Statistically, a two-way ANOVA revealed significant effects of frequency (F = 13.8, df = 4, P < 0.001) and dose of kanamycin (F = 6.32, df = 3, P = 0.001), with no interaction (F = 0.07, df = 12, P = 0.99). Thus the dosage makes a difference on average, but high variability across animals makes the outcome difficult to control.
Table 1.
Distributions of furosemide and kanamycin treatment effects
| Drug Parameters, mg/kg |
No. of CMs (pre-KA) |
Hair Cell Losses, % |
||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | F | K | Normal | Lower | OHCs | IHCs | Overall |
| None | 21 | 0 | 0 | 21 | 0 | |||
| F only | 4 | 100 | 0 | 4 | 0 | |||
| 1 | 150 | 0 | 1 | 0 | ||||
| FK | 2 | 50 | 200 | 2 | 0 | |||
| 2 | 50 | 400 | 2 | 0 | ||||
| 8 | 100 | 200 | 4 | 4 | 85 ± 8.90 | 2.8 ± 5.57 | 65 ± 10.1 | |
| 10 | 100 | 300 | 4 | 6 | 88 ± 16.9 | 34 ± 29.8 | 77 ± 18.9 | |
| 5 | 100 | 400 | 3 | 2 | 89 ± 21.6 | 56 ± 33.3 | 82 ± 23.6 | |
| 3 | 100 | 500 | 0 | 3 | 81 ± 32.8 | 57 ± 49.2 | 76 ± 36.0 | |
| 1 | 150 | 200 | 0 | 1 | 89 | 22 | 76 | |
| 2 | 150 | 400 | 0 | 2 | 99 ± 0.15 | 67 ± 13.5 | 93 ± 2.79 | |
| Total | 59 | 41 | 18 | |||||
Values are no. of subjects (n), dosages of furosemide (F) and kanamycin (K), no. of cochlear monophonics (CM) before treatment with kainic acid (pre-KA) for cases with normal (within the range of 21 cases with no treatment) and fewer than normal CMs, and percent loss of outer hair cells (OHCs), inner hair cells (IHCs), and overall hair cell loss (means ± SD). Data for hair cell losses are only for cases with fewer than normal CMs.
Fig. 5.
Cochlear microphonic (CM) from untreated, normal hearing (NH) animals and animals treated with different doses of furosemide and kanamycin (FK). A: the magnitude of the CM from the NH animals (blue squares) varied over about a 20-dB range. We used the bottom of this range (dashed line) as the threshold for “normal hearing,” and any responses lower than this were considered to be “reduced,” i.e., affected by the FK treatment. The effects of FK were highly variable. For the dosage shown (100/200 mg/kg), some cases had CMs within the normal range (open red circles), whereas others had reduced responses (closed red circles). B: means and SD of cases with reduced responses (below dashed line) using different dosages of FK. Despite the large SDs, there were significant effects of both frequency and FK dosage (2-way ANOVA; see text for values from the test).
Fig. 6.
Cochleograms from cases in animals treated with different doses of furosemide and kanamycin (FK) with reduced responses. A: cases that had the desired loss of outer hair cells (OHCs; solid lines) and survival of inner hair cells (IHCs; dashed lines). The locations for characteristic frequencies of 2, 4, and 8 kHz (arrowheads) and the round window (RW; arrow) are shown in A1 and A4. The full frequency axis for the gerbil is given in A3 and A6 (Müller 1996). Dosages (furosemide/kanamycin) in mg/kg are indicated in parentheses. B: examples of cases with either too much (B1–B3) or too little (B4) hair cell loss to be suitable for the goals of our experiment.
In parallel with the effects on the CM, the effects of FK on hair cell losses were also highly variable and required close inspection to identify cases with the desired pattern of OHC loss and IHC preservation. Among the 18 cases with reduced responses, we identified 7 that had the appropriate pattern of hair cell loss (Fig. 6A). Five of these seven cases (Fig. 6, A1–A4) had a complete complement of IHCs and loss of OHCs to about the 2-kHz region or lower. The remaining two cases had nearly as good a pattern of hair cell loss, but with some loss of IHCs in the most basal cochlea (Fig. 6A5) or a relatively small number of missing IHCs elsewhere (Fig. 6A6). The most basal IHCs should be responding at a relatively negligible level for the frequencies used compared with other parts of the cochlea. These seven cases received FK doses of 100/200 (n = 3), 150/200 (n = 1), or 100/300 mg/kg (n = 3).
The remaining cases (n = 9; Fig. 6B) had a variety of patterns of hair cell loss. The most common type was too much loss of IHCs, starting in the apex but extending into the frequency range of interest (Fig. 6, B1–B3). These patterns were typically obtained with higher doses of kanamycin, confirming results described previously for the gerbil (Abbas and Rivolta 2015). One case was unusual (Fig. 6B3) because the IHC loss was greatest at the base of the cochlea. Two cases (Fig. 6B4) had too little OHC loss, with preserved OHCs basal to about the 8-kHz location in the cochlea.
Variability: effects of FK and KA across frequency and level.
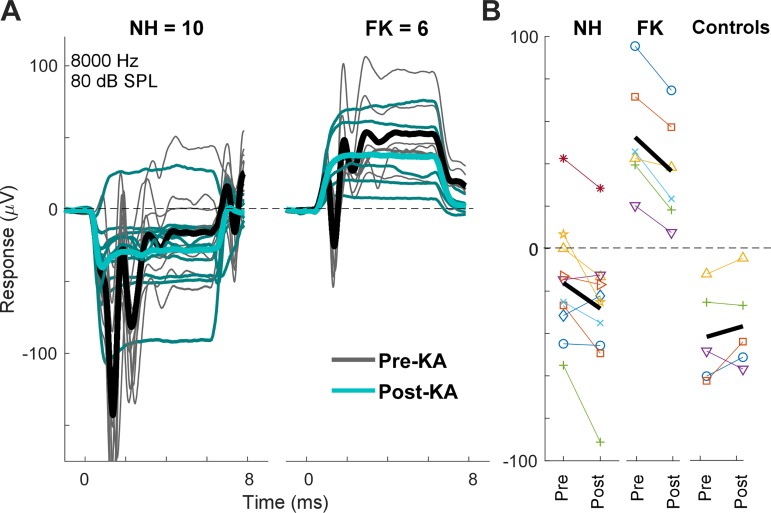
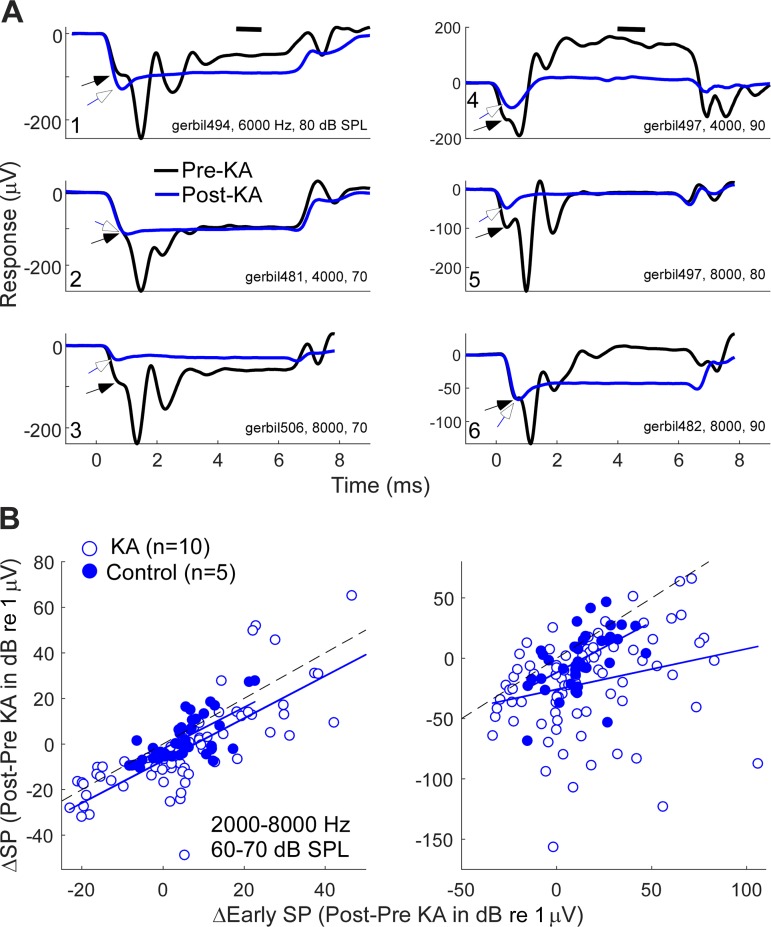
The second step in the design shown in Fig. 2 is to remove the neural component from both NH and FK animals using KA. To control for the possibility that a smaller SP after KA could be due to a general metabolic decline during the delay between pre- and post-KA measurements (~1 h) or to nonspecific effects of KA on OHCs or IHCs, animals were excluded if the magnitude of the CM decreased or the threshold increased by >3 dB. This criterion removed four NH animals and one FK animal. Thus, in the following analyses, there were 10 NH cases that were a subset of 14 cases where KA was used, and 6 FK cases that were a subset of the 7 cases with suitable anatomy. Five control animals without KA (i.e., artificial perilymph only) also met these criteria. To illustrate the variability, we show data at one frequency (8,000 Hz) and level (80 dB SPL; Fig. 7A). Each curve is the sum of the averaged responses to the two phases of stimulation (as in Fig. 3B), with thin lines being individual cases and thick lines the grand average across cases. There was wide variation for both groups both before and after the KA. However, the SP for the NH animals was negative in most cases, whereas for the FK animals, it was uniformly positive. After KA, the average SP moved in the negative direction for both groups. This negative movement was consistent across cases; for the NH animals, 8/10 cases had more negative SPs after KA, as did all 6 of the FK cases (Fig. 7B). Control cases (n = 5) did not show this negative shift; instead, the average the shift was slightly in the positive direction. The negative shift in SP after KA was significant for the NH cases (paired t-test, t = −2.63, df = 9, P = 0.028) and FK cases (t = −5.54, df = 5, P = 0.003), whereas the shift for the control cases was not significant (t = 1.07, df = 4, P = 0.35).
Fig. 7.
Waveforms and summating potentials (SPs) in individual cases for an 8-kHz tone burst at 80 dB SPL A: examples are shown for the pre- and post-kainic acid (KA) responses for both normal hearing (NH; n = 10) animals and animals treated with different doses of furosemide and kanamycin (FK; n = 6). There is a distinct difference in the polarity of responses between most NH and FK animals (thin lines). For both groups, the grand average of the responses after KA were more negative (thick lines). B: differences in the pre- and post-KA responses. For both NH and FK animals, the change in response was consistently negative with few exceptions. For 5 control cases where only artificial perilymph was applied, there was no systematic changes in the SP. Thick black line represents the mean for each condition.
Across all frequencies and intensities (2 to 8 kHz, 60–90 dB SPL), a linear mixed model showed large main effects of frequency, level, and group (Table 2). The groups were NH or FK animals and included both pre- and post-KA recordings. There were significant interactions between frequency, level, and group in each two-way comparison, but not in the three-way comparison. To test for the effects of KA, a repeated-measures linear mixed model (Table 3) showed large main effects of frequency, level, and group, where the groups were the pre- and post-KA comparison and included both NH and FK animals. The two-way interaction with level was significant, showing that level affected the SP differently in the two groups, but not of frequency. The three-way comparison was not significant. These results show that both FK and KA produced systematic deviations in the SP compared with NH animals, and the effects of frequency and level could differ between groups. Thus removal of OHC and neural contributions significantly affect the SP across frequencies and intensities.
Table 2.
Linear mixed model to test for significant effect of furosemide and kanamycin treatment on the SP
| Factor | Numerator df | Denominator df | F Statistic | P Value |
|---|---|---|---|---|
| Frequency | 4 | 472 | 17.06 | <0.001 |
| Intensity | 3 | 472 | 27.86 | <0.001 |
| Group (NH or FK) | 1 | 472 | 367.26 | <0.001 |
| Frequency × level | 12 | 472 | 3.99 | <0.001 |
| Frequency × group | 4 | 472 | 3.34 | 0.01 |
| Level × group | 3 | 472 | 18.86 | <0.001 |
| Frequency × level × group | 12 | 472 | 1.30 | 0.217 |
Statistical results indicate main effects of frequency, level, and group [normal hearing (NH) animals or furosemide and kanamycin (FK)-treated animals] on the summating potential (SP), as well as two- and three-way interactions.
Table 3.
Repeated-measures linear mixed model to test for significant effects of kainic acid on the SP
| Factor | Numerator df | Denominator df | F Statistic | P Value |
|---|---|---|---|---|
| Frequency | 4 | 455 | 52.14 | <0.001 |
| Level | 3 | 455 | 38.69 | <0.001 |
| Group (pre- and post-KA) | 1 | 454 | 33.38 | <0.001 |
| Frequency × level | 12 | 455 | 17.34 | <0.001 |
| Frequency × group | 4 | 454 | 0.51 | 0.73 |
| Level × group | 3 | 454 | 4.32 | 0.005 |
| Frequency × level × group | 12 | 454 | 0.05 | 1 |
Statistical results indicate main effects of frequency, level, and group [pre- or post-kainic acid (KA) treatment] on the summating potential (SP), as well as two- and three-way interactions.
Isolation of IHC, OHC, and neural responses.
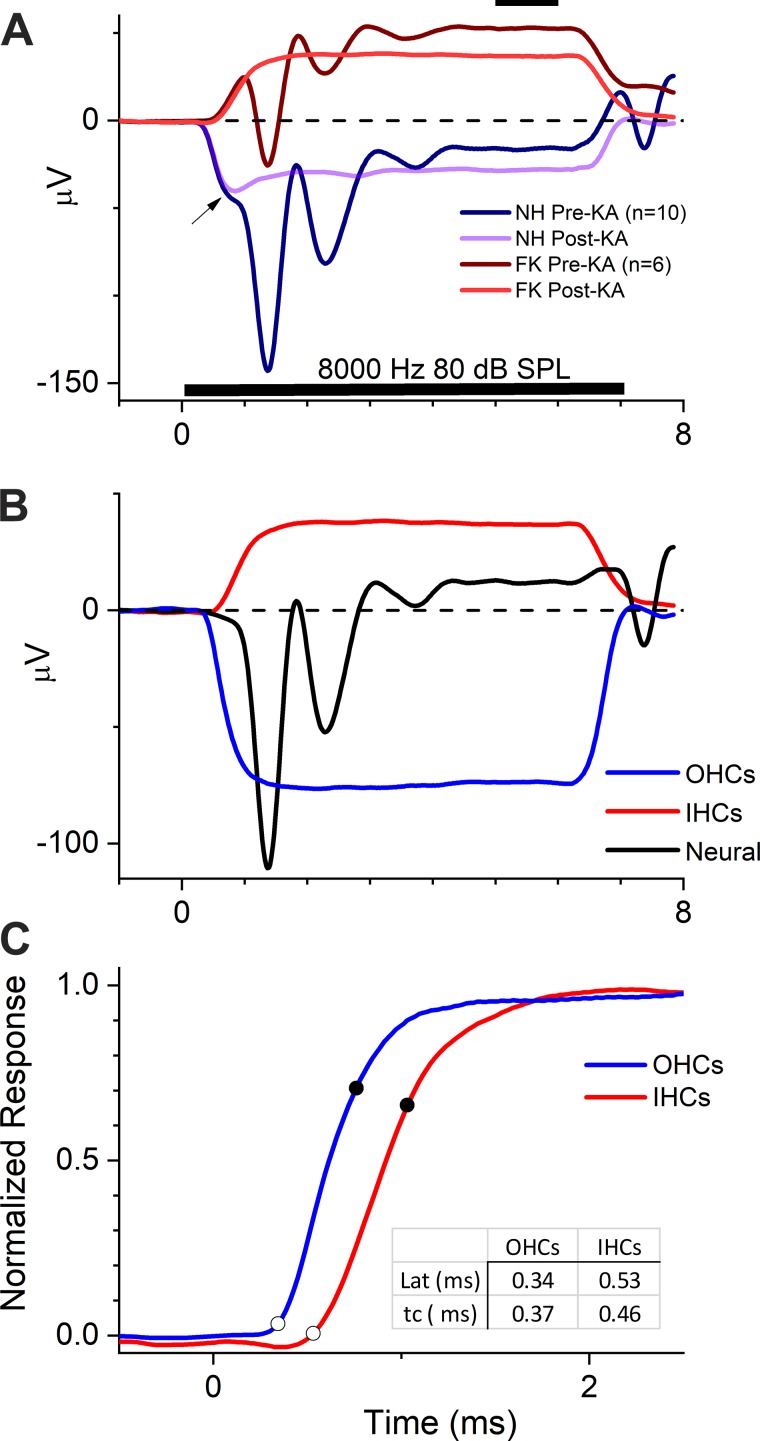
In Fig. 8, we implement the subtraction procedures as described in Fig. 2. In Fig. 8A, we show the grand averages from the NH (n = 10) and FK (n = 6) animals shown in Fig. 7. The grand averages provide the best estimate of the relative sizes of the contribution from each source, given the variability across individual cases. For both the NH and FK animals, the effect of the KA was to remove the CAP and make the SP more negative, as previously illustrated. The change in the SP after KA suggests that a neural contribution with a positive polarity was removed. For the NH animal, the post-KA response, which consists of IHCs and OHCs, had an initial negative decline (Fig. 8A, arrow), followed by a more positive SP in the steady state. The initial decline was unchanged from its pre-KA state, where it preceded the CAP. This part of the response can be considered an “early SP,” as distinct from the steady-state SP. For the FK animals, the post-KA response was derived from IHCs only. The curve is not complex, having no early SP, consistent with a contribution from a single source.
Fig. 8.
Contributions of outer hair cells (OHCs), inner hair cells (IHCs), and neural sources to the summating potential (SP). A: grand average responses in normal hearing (NH) animals and animals treated with different doses of furosemide and kanamycin (FK) that passed criteria for inclusion at this frequency–level combination (see text). The compound action potential (CAP) was removed by treatment with kainic acid (KA) in both cases. For the NH case, the post-KA response is the combined hair cell response (both OHCs and IHCs), whereas for the FK case, the post-KA response is the isolated response from IHCs. The SP was measured as the average response from 5 to 6 ms (solid bar at top). In the NH animal, the combined OHC and IHC response post-KA was complex, with an initial negative decline (arrow; also seen before KA) that became less negative in the steady state. B: responses after subtractions of the responses in A that reveal IHC, OHC, and neural contributions. The IHC response is the same as the post-KA response in FK animals shown in A. The neural response is the subtraction of the post-KA from the pre-KA response in the NH animals in A. The OHC response is the subtraction of the post-KA response in the FK animals from the post-KA response in the NH animals. C: difference in time courses of the OHC and IHC responses. The curve for the OHC is inverted, and both are normalized to the maximum response and plotted on an expanded timescale. Spline fits to the data were used to define the latencies (point of maximum slope change, open circles) and time constant (time from latency to 63.2% of the maximum responses, closed circles). The responses of OHC occurred earlier than in IHCs, and the time constant was shorter. Lat, latency; tc, time constant.
The potentials obtained for each source after the subtractions are shown in Fig. 8B. The neural component was obtained from subtraction of the post- from the pre-KA response in NH animals. It had a CAP followed by modulations that are likely to be due to subsequent components of the auditory nerve and brain stem responses. The SP of the neural contribution had a positive polarity. The OHC contribution was obtained by subtraction of the post-KA responses of the FK animals (consisting of IHCs only) from those of NH animals (consisting of the OHCs and IHCs). The SP contribution from OHCs had a negative polarity, in contrast to the IHCs.
The curves for both OHCs and IHCs were not individually complex, in contrast to the sum of their contributions, which interacted to produce an early SP followed by a more positive SP (Fig. 8A, arrow). The shape of the combined OHC and IHC responses derives from the different time courses of the OHC and IHC contributions, as shown in Fig. 8C. There are the same data as in Fig. 8B but normalized to the maximum responses with an expanded timescale and with the OHC contribution inverted. The OHC response began earlier than the IHC response and had a steeper rise to maximum. To measure these differences, the curves were fit with a smoothed spline function. The point of maximum change in slope, occurring on the knee of the resulting function’s initial rise, was defined as the latency. A line that included 63.2% of the rise (i.e., 1 – 1/e, an estimate of the impulse response) was fit and the time between the end points defined as the time constant. The initial negative deflection in the combined response (Fig. 8A, arrow) was therefore due to the earlier onset of the OHCs, followed by the more positive, steady-state SP when combined with the later arriving IHC response.
Polarities and magnitudes of each SP contribution across frequency and level.
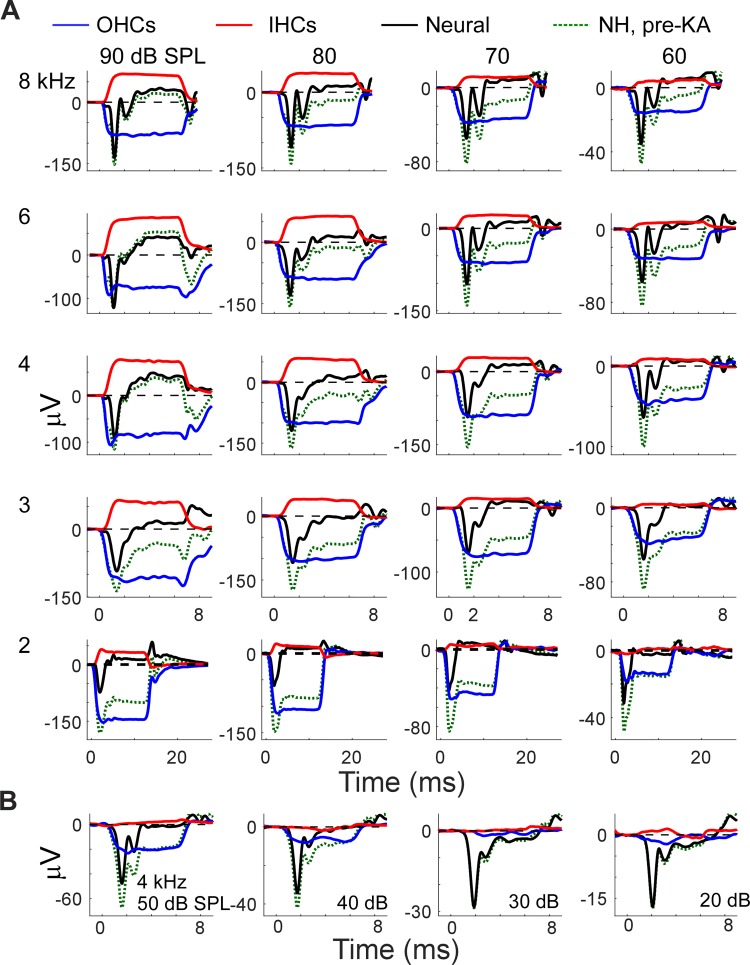
Responses of each of the three components, OHCs, IHCs and neural, were assessed over the range of 2–8 kHz and from 60 to 90 dB SPL. The limitation to frequencies to 2 kHz or higher was due to FK animals that retained a complement of apical OHCs that would be excited by lower frequency stimuli. The upper limit of 8 kHz was due to limitations imposed by the use of clinical equipment. To 4 kHz, sound level was tested down to 0 dB SPL.
The time waveforms for each component are shown in Fig. 9, following the design of Fig. 8B. The original pre-KA response for the NH animals is also shown. For each frequency–level combination in Fig. 9A, the basic pattern of negative SPs from OHCs and positive SPs from the nerve and IHCs was followed. Note that the scales vary in each panel, to emphasize patterns rather than changes in magnitudes. Overall trends were that to lower frequencies and intensities, the OHC contribution dominated so that the SPs in the original responses were negative. To higher frequencies and intensities (90 dB SPL, 4–8 kHz), the IHC and the auditory nerve contributions were dominant, producing a positive SP in the original responses. With lowered level, the SP from IHCs decreased to small values and could become smaller than the neural contribution, whereas an SP from OHCs was seen across the ranges of frequencies and intensities shown. Note, however, that the IHCs were measured in the absence of OHCs, so their thresholds would be raised due to loss of the cochlear sensitivity provided by the OHCs. At 4 kHz, as the level was further lowered (Fig. 9B), the IHC contribution to the SP was negligible by 50 dB SPL, the OHC contribution extended to 40 dB SPL, and the neural contribution extended to 20 dB SPL. Interestingly, the neural contribution switched to negative polarity for these lower intensities.
Fig. 9.
Curves for each isolated component across frequency (2 to 8 kHz) and level (60–90 dB). A: grand averages of contributions from outer hair cells (OHCs), inner hair cells (IHCs), and neural sources, derived as in Fig. 8B, plotted across intensities (columns) and frequencies (rows). The raw responses in the normal hearing (NH) animal are also shown (dotted lines). B: similar data for the responses to lower intensities (20–50 dB) of 4-kHz tone bursts. Note changes in y-axis across each row as the level decreases.
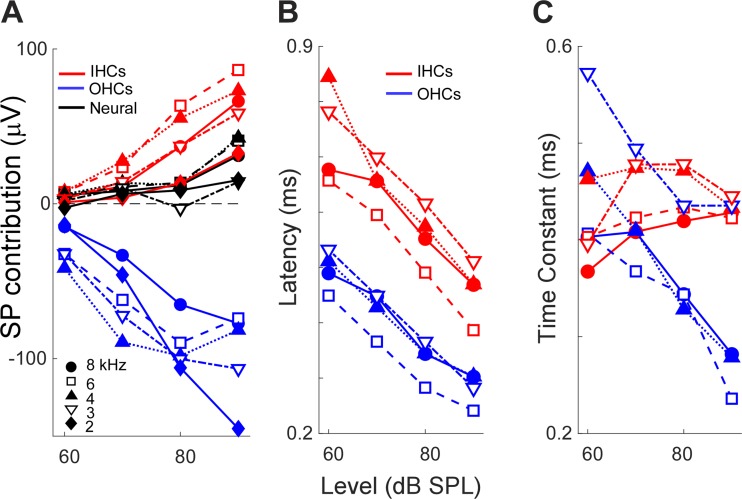
The change in magnitude and time course of the SP as functions of frequency and level are quantified in Fig. 10. The absolute magnitude of the SP from each component increased as the level was raised, with the neural contribution changing over a smaller range than IHCs or OHCs. The trends for frequency were less clear.
Fig. 10.
Magnitudes and time courses of the isolated components. A: measurements of the summating potential (SP) from each component as a function of sound level, with frequency as a parameter. B: measurements of the latency of the SP from outer hair cells (OHCs) and inner hair cells (IHCs). C: measurements of the time constants of the SP from OHCs and IHCs.
Two parameters, latency and time constant, were measured for OHCs and IHCs (see Fig. 8C). Unfortunately, the responses to 2 kHz were not suitable for comparison because of an error in setting the rise time in some cases. For the range of 3–8 kHz, the latency of IHCs was consistently longer than that of OHCs. Both decreased with level, over the range of ~0.8 to 0.4 ms for IHCs and 0.5 to 0.3 ms for OHCs (Fig. 10B). The mean latency of IHCs was 0.21 ± 0.057 (SD) ms longer than OHCs. The time constants of OHCs and IHCs followed different patterns (Fig. 10C). For IHCs, the time constants were relatively constant across frequencies and intensities, at ~0.4 ms. In contrast, the time constants of OHCs decreased with level and showed more spread across frequencies. Although these values could have been affected by a delay inherent to a 1-Hz high-pass filter, this should have been uniform for each measurement. Thus, at high intensities, the time constants of OHCs were shorter than for IHCs, whereas for moderate intensities, the time constants could be similar for both hair cell types.
Relationship between the CM and the SP.
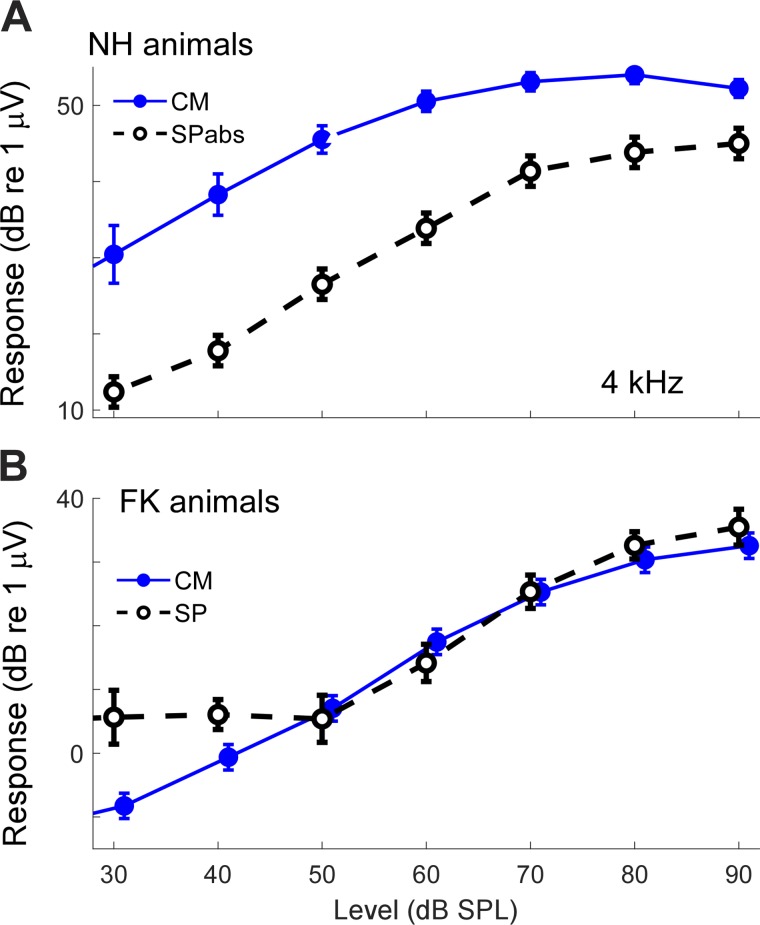
Once the nerve is removed with KA, the SP magnitudes should reflect the degree of asymmetry in the hair cell responses. As schematized in Fig. 1A, asymmetry in the two directions of stereociliary motion produces a non-zero absolute magnitude of response, seen as an SP in ECochG. The larger the asymmetry, the greater the SP, so the SP provides information about the degree of asymmetry in OHC and IHCs. In NH animals, the CM recorded from the RW reflects the action of both OHCs and IHCs, with the OHCs thought to provide the larger contribution (Dallos 1973; Russell and Sellick 1983). For FK animals, the CM is derived from IHCs. The asymmetries in IHCs are thought to be greater than those in OHCs (see Russell 2008 for a review), so the SP should be proportionally greater compared with the CM. These relationships are shown for a single frequency (4,000 Hz, Fig. 11). All curves are post-KA so that only hair cells are present. In the NH animals, the overall asymmetry is reflected in the magnitudes of the contributions from OHCs and IHCs, so the absolute values of each were summed (SPabs). This SPabs was much smaller than the CM. In contrast, for the FK animals, the SP was derived purely from IHCs, was positive in polarity, and was larger than the CM to higher intensities. These results support greater asymmetry in IHCs than in OHCs.
Fig. 11.
Relationship between cochlear monophonic (CM) and summating potential (SP) in normal hearing (NH) animals and animals treated with different doses of furosemide and kanamycin (FK). A: post-KA response in NH animals (n = 10). The CM is much larger than the SP. Here the SP represents the summed absolute magnitudes (SPabs) of the outer hair cell (OHC) and inner hair cell (IHC) response. The observed SP was much smaller because the OHCs and IHCs had different polarities. B: in contrast, the post-KA response for FK animals (n = 6) was from IHCs alone. The CM from the IHCs was smaller than the SP, which indicates a greater degree of asymmetry in IHCs than in OHCs. Error bars are SE.
Effects of changes in the early SP.
It is possible that the generally more negative SP following KA could be attributed to changes in the efferent activity after loss of neural input, rather than to a direct contribution from neural sources. The early SP occurs before contributions from the IHCs or CAP, and thus should reflect changes in the asymmetry of the operating point for OHCs alone (Fig. 1). Thus a comparison of changes in early and steady-state SP can help assess the degree to which changes in OHC asymmetry, which might occur due to changes in efferent activity after loss of neural input, affect the change in the steady-state SP. That is, if change in the early SP accounted for all of the change in the steady-state SP, loss of efferent activity could be considered the major cause. In contrast, if changes in the early and steady-state SPs were uncorrelated, it would suggest that the loss of neural activity itself had an effect. In Fig. 12A, the effect of KA could be to make the early SPs more negative (Fig. 12A1), positive (Fig. 12, A3 and A5), or have no change (Fig. 12, A2 and A6). The steady-state SPs followed these changes in some cases (Fig. 12, A1–A3) but not others (Fig. 12, A4–A6). In Fig. 12B, the changes in the early and steady-state SPs are compared for animals treated with KA and for control animals where artificial perilymph was applied rather than KA. Because there are only five controls, a regression analysis was significant for only a few frequency–level combinations, so the data were pooled across frequencies and intensities to identify trends in the relationships. For both level ranges shown, the change in the early SP (x-axis) was much greater for the KA than for the control animals, as is clear by the difference in the lengths of the lines. For the control animals, the changes in the steady-state SP followed those of the early SP, with a slope near 1 (slopes = 0.84 and 0.91 for lower and higher intensities, respectively; r = 0.52 and 0.70, respectively; both P < 0.001 with the caveat of multiple samples/case). For the KA animals, the slope was also near 1 for the lower intensities (slope = 0.93, r = 0.79, P < 0.001), but not for the higher intensities (slope = 0.30, r = 0.23, P = 0.03). These data indicate that 1) the change in operating point of OHCs, as reflected in the early SP of the SP, shows great variability after removal of neural activity, possibly due to the loss of efferent control, and that 2) for control animals, the change in the operating point had a substantial influence on the SP, but 3) for the KA animals, this was only true at lower intensities; at the higher intensities, the change in the steady-state SP was little influenced by the changes in early SP.
Fig. 12.
Comparison of early and steady-state summating potential (SP). The early SP should represent the asymmetry in outer hair cells, because it occurs before responses from inner hair cells or the auditory nerve. A: examples of variation in the early SP pre- and post-kainic acid (KA; closed and open arrows, respectively) and the SP (horizontal bar at top). All of the curves shown are from normal hearing animals and are the average of alternating polarities. Note the variety of patterns: the early SP could increase after KA (A1), stay the same (A2 and A6), or become smaller (A3–A5). The change in the SP from pre- to post-KA could either follow the change in the early SP (A1–A3) or deviate from it (A4–A6). B: regressions of the change in the early SP (Δearly SP) with the change in the SP (ΔSP). Frequencies (2–8 kHz are grouped) and two intensity ranges are shown. For control animals where artificial perilymph only was applied to the round window, ΔSP was proportional to Δearly SP for both intensity ranges. For animals where KA was used, the range of the early SP was generally much greater, as shown by the longer lines (blue line) compared with controls (black line); this greater variability was presumably due to the loss of neural input and subsequent loss of effective efferent control. For lower intensities (left), the SP for KA animals was more negative than controls, but Δearly SP still exerted a strong effect. For higher intensities (right), Δearly SP had less of an effect, presumably indicating an increased influence of neural activity.
SP in human CI subjects.
In the current literature, the interpretation of the SP to tones in humans generally does not include consideration of the effect from the auditory nerve. In addition, much of the focus has been related to changes thought to be associated with endolymphatic hydrops (recently reviewed by Eggermont 2017; Hornibrook 2017). However, the SP in most CI subjects is unlikely to be affected by hydrops, and the results in animals described in this article give basis for interpretations of the variety of SPs observed. Our human sample consists of ECochG recordings from the RW of 334 CI subjects, of all ages. We will focus on the larger responses to 2 and 4 kHz, because these are most comparable to the results reported here in gerbils. Most CI subjects have responses only to lower frequencies, but significant responses to 2,000 Hz were seen in 143 subjects, and to 4,000 Hz in 93 of these. If the CM was small, the SP and CAP were typically also negligible, so we only included cases where the CM response was >0.5 µV (−6 dB re 1 µV). This included 55 cases with responses to 2,000 Hz and 37 to 4,000 Hz, for a total of 92 responses. All were to a high sound level (90 dB above normal hearing level).
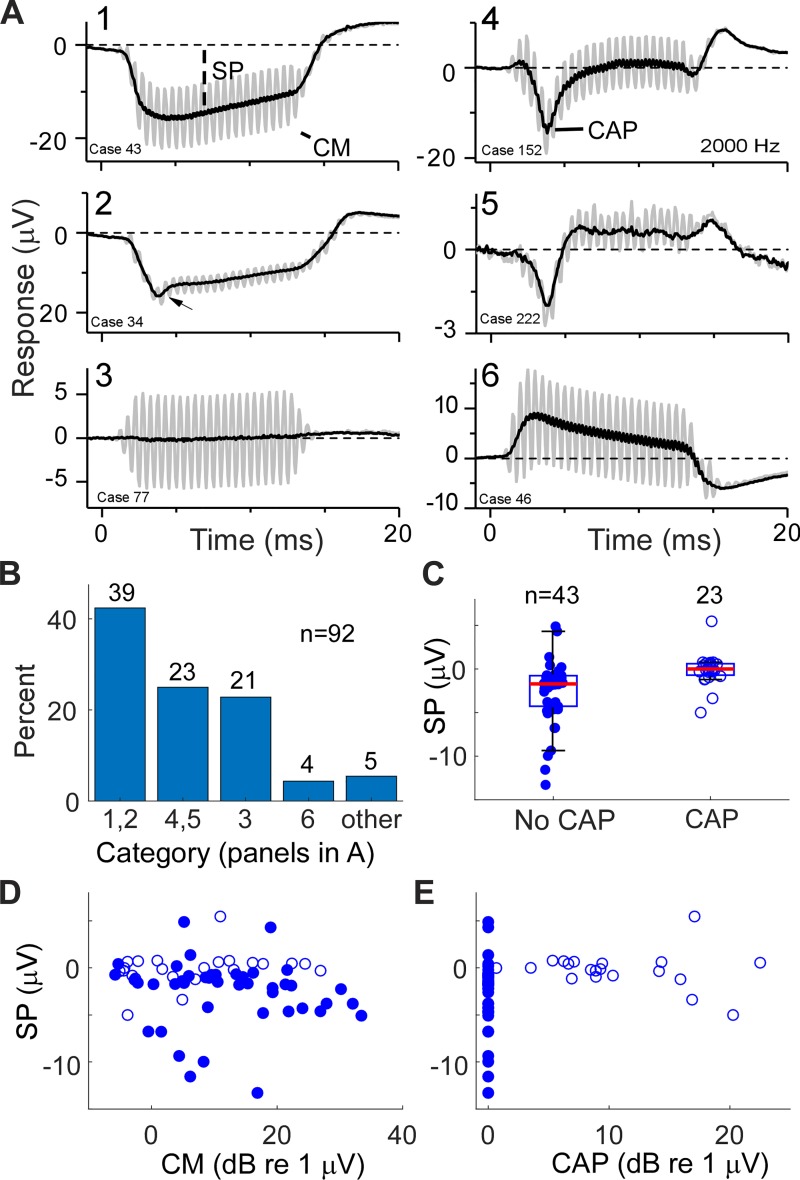
Examples of the variations in SP in relation to the CM and CAP are shown in Fig. 13A. The curves in gray are to condensation-phase stimuli showing the CM, whereas those in black are the average of condensation and rarefaction phases that emphasize the SP and CAP. Information about the subjects (age and etiology of hearing loss) are provided in the legend. Note than in several cases the SP declined in magnitude during the stimulus; this decline was presumably related to the high-pass filter setting of 10 Hz instead of 1 Hz, as used in the gerbils. The case in Fig. 13A1 had a large, negative SP and no CAP. The case in Fig. 13A2 also had a negative SP, but the curve is more complex, with a negativity (arrow) followed by a more positive value at the steady state. The case in Fig. 13A3 had a large CM but no CAP or SP. The cases in Fig. 13, A4 and A5, both had large CAPs and SPs that were near zero or positive. Finally, the case in Fig. 13A6 had a positive SP and no CAP.
Fig. 13.
Patterns of responses in human cochlear implant (CI) subjects, including the cochlear monophonic (CM), compound action potential (CAP), and summating potential (SP). A: examples of responses from human CI subjects. A1–A6 are each from a different subject and show the responses to condensation-phase stimuli (gray) to highlight the CM and the average of alternated phases (black) to highlight the CAP and SP. A1: a case with no CAP and a strongly negative SP. Subject age: 43 yr; etiology of hearing loss: auditory neuropathy spectrum disorder (ANSD). A2: an interesting case with a negative peak that could be neural (a CAP) or from a mixture of outer hair cells and inner hair cells (see the post-kainic acid response from the normal hearing animal in Fig. 8A for comparison). Subject age: 65 yr; etiology: Meniere’s disease. A3: a case with a large CM but no CAP or SP. Subject age: 2 yr; etiology: ANSD with cochlear nerve deficiency from imaging. A4: a case with a large CAP and small SP. Subject age: 66 yr; etiology: Meniere’s. A5: a case with a large CAP and positive SP. Subject age: 73 yr; etiology: sensorineural hearing loss. A6: a case with no CAP and a positive SP. Subject age: 2 yr; etiology: ANSD with cochlear nerve deficiency from imaging. B: distributions of the different response types illustrated by A1–A6. C: box and scatter plots of SP for cases with and without a CAP. Cases without a CAP had a wider range, particularly with negative polarities, than cases with a CAP. Red line indicates median, box indicates the semi-interquartile range, and whiskers indicate the range not considered outliers. D: CM vs. SP, showing that cases without a CAP (closed circles) could have a large, negative SP even though the CM was small. Open circles represent cases with a CAP. E: CAP vs. SP, providing another view of these relationships.
These panels were ordered by the relative amounts of positivity and negativity associated with OHCs, IHCs, and the auditory nerve. That is, a possible interpretation for the negative polarity of the SP for the case in Fig. 13A1 is of a dominant OHC contribution to the SP, with small or absent IHC and neural contributions. For the case in Fig. 13A2, it is unlikely that the small negative decline (arrow) is a CAP (but see Scott et al. 2016); instead, the more likely case is that it is a mix of OHC followed by IHC contributions, as shown in Fig. 8. For the case in Fig. 13A3, the interpretation is that the neural contributions were negligible, and the hair cell response was within the linear range of the input-output functions such that no appreciable SP was produced. The cases in Fig. 13, A4 and A5, both had large neural contribution, as shown by the large CAPs, and the SPs were near zero or positive, a pattern that suggests each source (OHCs, IHCs, and the auditory nerve) was contributing. For the case in Fig. 13A6, the positive SP was without a CAP or a complex shape, suggesting that IHCs dominated. These interpretations must be only tentative, because there are of course caveats related to differences in the two systems studied, including species and hearing condition. However, they do indicate that the SP is influenced by different sources and may therefore be useful in characterizing the state of residual function in individual cases.
In terms of these responses as exemplars, the largest group (n = 39/92) were those with a negative deflection and no CAP, as in Fig. 13, A1 and A2, and in the histogram in Fig. 13B. Most (n = 22) of these were children diagnosed with auditory neuropathy spectrum disorder (ANSD) on the basis of the presence of a CM with a degraded wave V in the auditory brain stem response (ABR) (Berlin et al. 1998; Kaga et al. 1996; Rance et al. 1999; Starr et al. 1996; Teagle et al. 2010). Examples with a complex response not attributable to a CAP, as in Fig. 13A2, were few (n = 2). The second largest group (n = 23) had a CAP and small SP, as in Fig. 13, A4 and A5. The third most common group (n = 21) had a CM but no CAP or SP, as in Fig. 13A3. Only a handful of cases had no CAP and a positive polarity response (n = 4), as in Fig. 136, and five cases had SPs that rose or fell slowly and were classed as “other.”
As mentioned, a neural contribution to the SP is not usually considered in clinical studies. However, in our sample, a major distinction was between responses with a CAP and those without. Cases with a CAP must have intact IHCs and neural responses, whereas those without a CAP lack a neural response and may have impaired IHCs and/or OHCs, as well. The main characteristic of the cases with a CAP was that the SPs were tightly clustered near zero, whereas those without a CAP were spread more widely, especially in the negative direction (Fig. 13C). The difference in the distributions of the SP between cases with and without a CAP was significant (Mann-Whitney U-test: n = 43, 23; medians = −1.71, 0.01; U = 806, 183; z = 4.18, P < 0.001). When plotted as a function of the size of the CM (Fig. 13D) or CAP (Fig. 13E), there were no clear trends for the SP to increase systematically with either feature.
DISCUSSION
Using pharmacological manipulation of the gerbil cochlea we were able to show that the IHCs, OHCs and neural sources all contribute to the SP. This information should enhance interpretations of the SP in research and clinical settings. It also helps explain the variability in the SP that is typically reported. When applied to CI subjects, the new interpretation indicates a strong influence of neural contributions that would not have been recognized without these animal studies.
The ototoxic and neurotoxic model.
By titrating the amounts of furosemide and kanamycin, it was possible to produce nearly complete loss of OHCs and preservation of IHCs. Remaining OHCs were primarily located in the apex, so by stimulating only at frequencies >2 kHz, no OHCs would have participated in generating the responses. Similar patterns of hair cell loss have been obtained in guinea pig (Dallos and Cheatham 1976), mice (Oesterle et al. 2008), and chinchillas (Dallos and Harris 1978; Santi et al. 1982), although the effective dosages vary by species. In the gerbil the effects of the dosages were variable across animals, reducing the yield of successful cases.
The results depend on the assumptions that KA removes neural contributions without materially affecting hair cell responses and that the FK treatment selectively damages OHCs and leaves IHCs functionally intact. To improve the likelihood that these assumptions were met, we accepted only cases that showed no change in CMs after KA or those without the correct cytocochleograms for the frequency ranges tested. However, it is difficult to prove that the KA and resulting loss of synapses have no effect on IHCs or that there is no loss of function in stereocilia of the IHCs after FK treatment. Furthermore, there may be effects on the endocochlear potential through cells involved in potassium recycling to the stria vascularis, and the efferent system may be differentially activated after loss of auditory input. The cochlea is a complex system, and network effects of any manipulation are to be expected.
An important consideration is that IHC activity will be altered after the OHC contribution is removed, due to loss of the cochlear amplifier, resulting in loss of IHC and neural activity compared with the normal hearing condition. The neural component determined by application of the KA was indeed smaller in the FK animals. A smaller IHC contribution would affect the subtraction used to obtain the OHC response, causing the IHC contribution to be underestimated and the OHC component to be correspondingly overestimated. A different approach would be to remove the IHCs, but a method for this has yet to be demonstrated in gerbils.
Neural contribution to the SP.
A neural contribution to the SP to tones is generally not considered in clinical applications (Ferraro 2010; Hornibrook et al. 2012; Iseli and Gibson 2010) or to play a significant role in most animal studies (Dallos et al. 1972; Durrant et al. 1998; Zheng et al. 1997). However, in addition to the current study, removal of a contribution to the SP with positive polarity originating from neural elements has been previously seen after application of KA, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), or TTX (Forgues et al. 2014; Sellick et al. 2003; van Emst et al. 1995). In this report, we show systematic effects of KA across a range of frequency and level combinations.
An important consideration for the change in response observed after KA is that the loss of auditory input to the brain will affect the olivocochlear system that includes medial olivocochlear efferents to the OHCs. In principle, changes in efferent activity could change the SP through effects on the operating point of OHCs and hence on the SP produced by reduced or increased asymmetry. In urethane-anesthetized animals, the role of descending systems on the OHCs appears to be small, based on weaker contralateral suppression of distortion product otoacoustic emissions compared with awake animals (Chambers et al. 2012; Guitton et al. 2004). A factor that argues against the change in SP being due to efferents was provided in Fig. 12, which was that change in the SP was often independent of the change in the overall operating point, which could, in principle, be estimated by the early SP in the SP. The rationale is that because this early SP occurs before the CAP or the SP at steady state, it represents the asymmetry from isolated responses from OHCs. We found that the early SP was more variable in cases after KA than was the case in controls, which is consistent with a system where the operating point is no longer influenced by auditory input. In control animals with no KA, the change in the SP was correlated with the early SP, suggesting that the two are both related by the asymmetry in the OHCs. However, in KA animals to high intensities the correlation decreased, indicating that the loss of neural activity had an effect independent of this change in OHCs. In addition, a change in the SP after KA was most commonly in the negative direction, whereas the change in the early SP was most often positive. More directly, the same negative shift in the SP was observed after KA in the FK animals without functioning OHCs. It is still possible that the KA had a direct effect on IHCs. However, the same results have been seen with TTX (van Emst et al. 1995) and CNQX (Sellick et al. 2003), which block action potentials and the postsynaptic glutamate receptors, respectively. These do not have the traumatic effects on the nerve terminals seen with KA. Thus we think our results and those of others demonstrate a direct neural contribution to the SP.
Another possible reason for the neglect of a neural contribution to the SP may be that the mechanism for its production from the sustained, asynchronous firing of auditory nerve fibers to high-frequency tone bursts is not clear. The CAP is understood to be produced by the convolution of a unit potential, or action potential shape as it appears at the RW, with the distribution of action potentials in the population PSTH (Earl and Chertoff 2010; Goldstein and Kiang 1958; Lichtenhan and Chertoff 2008). The CAP is the result of the early peak followed by the smaller steady-state response consistently seen in PSTHs of single auditory nerve fibers to high frequencies. This model was then extended to the ANN, where the convolution is between the unit potential and the cycle histogram, because of the cyclic PSTH produced by neural phase-locking to low frequencies (Fontenot et al. 2017; Forgues et al. 2014; Riggs et al. 2017; Snyder and Schreiner 1984). However, the population activity evoked by high-level, high-frequency stimuli is sustained and asynchronous, so there is no time structure to produce an SP, unlike with the CAP and ANN. Instead, the neural SP is most likely to be the consequence of asymmetry in the unit potential. Measurements of the unit potential, made by spike-triggered averaging of the RW response while simultaneously recording from single auditory nerve fibers, often shows greater negative than positive phase contributions (Kiang et al. 1976; Prijs 1986; Versnel et al. 1992). With the very large number of action potentials produced in response to intense sounds, even a small asymmetry of the unit potential would be expected to produce an SP.
In addition to eliminating spiking activity, KA also removes the postsynaptic synapse, thus eliminating any contribution from a depolarization produced in NTs. It is therefore possible that part of the neural SP is the cumulative effect of these excitatory postsynaptic potentials, as reported by Dolan et al. (1989). However, similar effects to those reported presently were seen with TTX (van Emst et al. 1995), which blocks spiking activity but leaves ligand-gated channels intact. Thus a role for spiking activity as part of the SP seems clear, but the neural contribution may have multiple components.
Hair cell contributions to the SP.
Hair cells are most often considered as the source of the SP, with contributions from both OHCs and IHCs (Dallos et al. 1972; Durrant et al. 1998; Zheng et al. 1997). As a consequence of their greater asymmetry, the SP from IHCs should have lower thresholds and contribute a greater proportion to the overall SP than OHCs on a per hair cell basis. These properties have been demonstrated in chinchillas where the IHC and neural contributions were removed by carboplatin (Durrant et al. 1998) or nerve sectioning (Zheng et al. 1997). In the current study, the isolated responses from IHCs in the gerbil did not have a lower threshold than the OHC response (Fig. 9B). This result is presumably because the IHC threshold was determined in the FK model, in the absence of OHCs and the cochlear amplifier.
The SP contributions from both IHCs and OHCs were simple in form, although opposite in polarity. The more complex waveforms, before steady state was reached when both IHCs and OHCs were present, indicate different time courses, as in Fig. 8A (arrow) and Fig. 10, B and C. This timing difference is too large (~02–0.4 ms) and too variable with frequency to be the difference between stereociliary deflection derived from direct contact with the tectorial membrane, as in OHCs, and that derived from viscous flow between the reticular laminar and tectorial membrane, as in IHCs. Instead, the different time courses could be related to the different channel properties of the basolateral membrane in these two cell types. At resting membrane potential, OHCs are essentially permeable to K+ (Housley and Ashmore 1992) and thus have minimal delay between current entering the cell at the apex and leaving at the base. In contrast, K+ is removed from IHCs by two voltage-sensitive channel currents (Kros and Crawford 1990). The “fast” channel, which can be inactivated by tetraethylammonium (TEA), has a time constant of ~0.25 ms and has the larger conductance, whereas the “slow” channel, which is inactivated by 4-aminopyridine, has a time constant >2 ms (Lopez-Poveda and Eustaquio-Martín 2006; van Emst et al. 1998). Of the two channels, the time constant of the fast, TEA-sensitive channel is similar to the time constant and delay of IHCs compared with OHCs (both ~0.2–0.4 ms; Fig. 9). Thus the difference in timing could indicate that the early SP is derived purely from CM asymmetries, whereas the SP from hair cells at steady state is influenced by both CM asymmetries and currents produced by direct current components of receptor potentials.
In contrast to the IHCs, where the time constant was unchanging with frequency and level, for OHCs the time constant was shorter at high intensities and decreased as level was lowered. A lowering time constant suggests a multiplicity of control parameters rather than a single-channel property. A possible source is the transduction operating point. At high frequencies and intensities, the main generators will be located closer to the basal turn of the cochlea than for low frequencies and intensities. Systematic changes in latency with frequency and level in both OHCs and IHCs indicate this shift in the locus of response. The degree of saturation should increase with level such that the response rapidly goes from minimum to maximum, providing a faster time constant. An alternative or supplemental possibility is changes in channel kinetics of OHCs along the tonotopic axis. Channels at the base are faster acting with higher conductance than those at the apex (Johnson et al. 2011; Mammano and Ashmore 1996). For IHCs, the trend with tonotopy is reversed, with faster kinetics from IHCs in the apex (Johnson 2015). At present, this trend is not reflected in our data, because the IHC time constants remained the same across frequency and level. For the gerbil, it may be that we have not yet explored a wide enough range of frequencies for the trend to be revealed.
Finally, how can the difference in polarity between OHCs and IHCs be explained? It is important to note that the polarity of the SP at the RW is to some degree species dependent. In the chinchilla, the SP has a uniformly positive polarity at the RW (Durrant et al. 1998; Zheng et al. 1997), in contrast to the guinea pig, gerbil (Gans 1983; Ohlemiller and Siegel 1992), mouse (Harvey and Steel 1992), and humans (Dauman et al. 1988; Ferraro et al. 1994). In this report, we show polarity of the SP to be variable in both gerbils and humans. A difference in geometry between source and recording location will affect the polarity, because a given location can act as a current sink or current source relative to the generators. This effect can be seen as a reversed polarity from an electrode placed on a small cochleostomy at the apex of the cochlea compared with at the RW (Honrubia and Ward 1969; van Deelen and Smoorenburg 1986). Relative to the RW, OHCs and IHCs could have different geometries as a consequence of the different operating points between the two cell types. The IHCs, being the more asymmetric, will have an increased representation from the base of the cochlea compared with OHCs, because even for low levels of stimulation presented, each IHC will be within its range of asymmetry. In contrast, for OHCs, the point of asymmetry near the CF region will be attained only at moderate and high stimulus levels so that the basal spread of the asymmetric elements with increases in level will be less than for IHCs. Thus a more apical locus of activity for OHCs compared with IHCs is a possible basis for the different polarities in SPs recorded. Another possibility would be that the operating points of OHCs are at a position greater than 50% open channels at rest, whereas IHCs are at less than 50% open. This result has been shown in intracellular recordings using low-frequency stimuli at low intensities (Cody and Russell 1987; Dallos 1985), but in most studies, the asymmetry for OHCs at the frequencies and intensities used in the current study was determined to be near 50% open (Cody and Russell 1987; Johnson et al. 2011). Because an operating point of 50% should result in no SP (Fig. 10A), it has been suggested that IHCs must be the major source of the SP (Johnson et al. 2011). Yet, our results and those of others show that at moderate and high intensities, the OHCs contribute as well, and can in fact dominate, so some asymmetry appears to be present, possibly opposite to that of IHCs.
Clinical uses and interpretations of the SP.
Our interest in the SP extends primarily from observations in CI subjects, where the SP has a highly variable morphology. Currently, the ECochG is being used to describe the residual function of each subject before implantation to help account for speech perception outcomes (Fitzpatrick et al. 2014; Formeister et al. 2015; McClellan et al. 2014) and as a tool to monitor functional integrity of the cochlea during insertion (Acharya et al. 2016; Adunka et al. 2010; Calloway et al. 2014; Campbell et al. 2015; Dalbert et al. 2015). In this latter regard, the SP was recently studied in guinea pigs during cochlear implantation and found to be more indicative of location of trauma than the CM (Helmstaedter et al. 2018). To make full use of the ECochG in CI subjects requires the ability to use and accurately interpret all of the information contained within the complex response to tones.
Much of the variability in the SP seen in CI subjects is related to the presence or absence of neural activity. In cases without a CAP, the SP was typically large and negative. By extension from the gerbil results, these would be primarily from OHCs, and the presence of functioning IHCs could be revealed by use of a stimulus with a complex envelope. In cases where a CAP was present, the SP was typically small and could be of either polarity, suggesting the IHCs and/or neural contributions were counterbalancing or even overpowering the OHC contributions. Similar patterns were seen when the SP in children with ANSD receiving CIs were compared with the SP in children and adults without ANSD (Riggs et al. 2017). Thus it appears that in humans, as in gerbils, a neural contribution to the SP is likely to be present. In addition, the lack of correlation between the size of the SP and either the CM or CAP is further evidence that the measured SPs are often a balance of contributions and do not scale directly with input level received. We do fully recognize that details between humans and gerbils are unlikely to be exact (species difference in polarities recorded at the RW were previously noted), but it does appear that similar general principles, including a neural contribution and a balance of sources with different polarities, are applicable in humans as well as in gerbils.
Historically, the main clinical application of the SP has been in patients with Meniere’s disease (recently reviewed by Eggermont 2017; Hornibrook 2017). With tones, a more negative SP to low frequencies is considered an indication of endolymphatic hydrops (Arenberg et al. 1993; Dauman et al. 1988; Gibson 2009; Iseli and Gibson 2010). The interpretation is that the CM shows an increased asymmetry in the hydropic condition, producing a larger SP (see Fig. 1A). On the basis of the present results, an increase in the negative potential would indeed be expected with increased asymmetry in the CM produced by OHCs, so this is a plausible interpretation. However, an alternative source of an increased negative potential is the loss of a positive potential from IHCs and/or the auditory nerve. A relative loss of IHC or neural activity would also reduce the CAP. Thus a possible explanation for the increased SP in cases with Meniere’s disease is associated with neural loss rather than endolymphatic hydrops.
The SP evoked by clicks is more commonly used than tones for the identification of hydrops. To clicks, the SP is an initial increasing response that precedes the CAP (both potentials being positive in the recording configurations typically used). It is thus similar to the initial negative deflections seen before the CAP both before and after KA in NH animals (Fig. 8A, arrow). The SP to clicks differs from the SP to tones in that there can be no neural spiking contribution, because these would only be present during and after the CAP. Thus the SP to clicks must be composed of contributions from OHCs, IHCs, and dendritic potentials (if present), which precede the CAP in that order. Because the SP to clicks is both a mixture of sources with different polarities and is changing rapidly over a small interval of time, the interpretation of the SP to clicks must be approached cautiously.
GRANTS
This work was supported by National Institutes of Health Grant F30 DC015168 (to C. K. Giardina) and a research contract from the Med El Corporation.
DISCLOSURES
D. C. Fitzpatrick has had past and current research contracts with MED-El Corporation and Advanced Bionics and Cochlear Corporation.
AUTHOR CONTRIBUTIONS
A.K.P., K.A.H., and D.C.F. conceived and designed research; A.K.P., K.A.H., W.C.S., J.D.W., K.E.F., M.M.M., and S.H.P. performed experiments; A.K.P., K.A.H., C.K.G., G.D.G., and D.C.F. analyzed data; A.K.P., K.A.H., C.A., and D.C.F. interpreted results of experiments; A.K.P., K.A.H., and D.C.F. prepared figures; A.K.P. and D.C.F. drafted manuscript; K.A.H. and D.C.F. edited and revised manuscript; K.A.H. and D.C.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Kristen White, Victoria Madden, and Pablo Ariel for advice and instruction on both confocal and differential interference contrast microscopy at the Microscopy Services Laboratory, UNC Pathology and Laboratory Medicine.
REFERENCES
- Abbas L, Rivolta MN. Aminoglycoside ototoxicity and hair cell ablation in the adult gerbil: A simple model to study hair cell loss and regeneration. Hear Res 325: 12–26, 2015. doi: 10.1016/j.heares.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya AN, Tavora-Vieira D, Rajan GP. Using the implant electrode array to conduct real-time intraoperative hearing monitoring during pediatric cochlear implantation: preliminary experiences. Otol Neurotol 37: e148–e153, 2016. doi: 10.1097/MAO.0000000000000950. [DOI] [PubMed] [Google Scholar]
- Adunka OF, Mlot S, Suberman TA, Campbell AP, Surowitz J, Buchman CA, Fitzpatrick DC. Intracochlear recordings of electrophysiological parameters indicating cochlear damage. Otol Neurotol 31: 1233–1241, 2010. doi: 10.1097/MAO.0b013e3181f1ffdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenberg IK, Kobayashi H, Obert AD, Gibson WP. Intraoperative electrocochleography of endolymphatic hydrops surgery using clicks and tone bursts. Acta Otolaryngol Suppl 113, Suppl 504: 58–67, 1993. doi: 10.3109/00016489309128124. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Bordelon J, St John P, Wilensky D, Hurley A, Kluka E, Hood LJ. Reversing click polarity may uncover auditory neuropathy in infants. Ear Hear 19: 37–47, 1998. doi: 10.1097/00003446-199802000-00002. [DOI] [PubMed] [Google Scholar]
- Calloway NH, Fitzpatrick DC, Campbell AP, Iseli C, Pulver S, Buchman CA, Adunka OF. Intracochlear electrocochleography during cochlear implantation. Otol Neurotol 35: 1451–1457, 2014. doi: 10.1097/MAO.0000000000000451. [DOI] [PubMed] [Google Scholar]
- Campbell L, Kaicer A, Briggs R, O’Leary S. Cochlear response telemetry: intracochlear electrocochleography via cochlear implant neural response telemetry pilot study results. Otol Neurotol 36: 399–405, 2015. doi: 10.1097/MAO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Otolaryngol 13: 209–217, 2012. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertoff ME. Analytic treatment of the compound action potential: estimating the summed post-stimulus time histogram and unit response. J Acoust Soc Am 116: 3022–3030, 2004. doi: 10.1121/1.1791911. [DOI] [PubMed] [Google Scholar]
- Choudhury B, Fitzpatrick DC, Buchman CA, Wei BP, Dillon MT, He S, Adunka OF. Intraoperative round window recordings to acoustic stimuli from cochlear implant patients. Otol Neurotol 33: 1507–1515, 2012. doi: 10.1097/MAO.0b013e31826dbc80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Russell IJ. The response of hair cells in the basal turn of the guinea-pig cochlea to tones. J Physiol 383: 551–569, 1987. doi: 10.1113/jphysiol.1987.sp016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbert A, Sim JH, Gerig R, Pfiffner F, Roosli C, Huber A. Correlation of electrophysiological properties and hearing preservation in cochlear implant patients. Otol Neurotol 36: 1172–1180, 2015. doi: 10.1097/MAO.0000000000000768. [DOI] [PubMed] [Google Scholar]
- Dallos P. The Auditory Periphery Biophysics and Physiology. New York: Academic, 1973. [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci 5: 1591–1608, 1985. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Cheatham MA. Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am 60: 510–512, 1976. doi: 10.1121/1.381086. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol 41: 365–383, 1978. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dallos P, Schoeny ZG, Cheatham MA. Cochlear summating potentials. Descriptive aspects. Acta Otolaryngol Suppl 302: 1–46, 1972. [PubMed] [Google Scholar]
- Dauman R, Aran JM, Charlet de Sauvage R, Portmann M. Clinical significance of the summating potential in Menière’s disease. Am J Otol 9: 31–38, 1988. [PubMed] [Google Scholar]
- Davis H. A model for transducer action in the cochlea. Cold Spring Harb Symp Quant Biol 30: 181–190, 1965. doi: 10.1101/SQB.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- Davis H, Deatherage BH, Eldredge DH, Smith CA. Summating potentials of the cochlea. Am J Physiol 195: 251–261, 1958. doi: 10.1152/ajplegacy.1958.195.2.251. [DOI] [PubMed] [Google Scholar]
- Davis H, Fernandez C, McAuliffe DR. The excitatory process in the cochlea. Proc Natl Acad Sci USA 36: 580–587, 1950. doi: 10.1073/pnas.36.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Nuttall AL, Avinash G. Asynchronous neural activity recorded from the round window. J Acoust Soc Am 87: 2621–2627, 1990. doi: 10.1121/1.399054. [DOI] [PubMed] [Google Scholar]
- Dolan DF, Xi L, Nuttall AL. Characterization of an EPSP-like potential recorded remotely from the round window. J Acoust Soc Am 86: 2167–2171, 1989. doi: 10.1121/1.398477. [DOI] [PubMed] [Google Scholar]
- Durrant JD, Wang J, Ding DL, Salvi RJ. Are inner or outer hair cells the source of summating potentials recorded from the round window? J Acoust Soc Am 104: 370–377, 1998. doi: 10.1121/1.423293. [DOI] [PubMed] [Google Scholar]
- Earl BR, Chertoff ME. Predicting auditory nerve survival using the compound action potential. Ear Hear 31: 7–21, 2010. doi: 10.1097/AUD.0b013e3181ba748c. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Ups and downs in 75 years of electrocochleography. Front Syst Neurosci 11: 2, 2017. doi: 10.3389/fnsys.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro JA. Electrocochleography: a review of recording approaches, clinical applications, and new findings in adults and children. J Am Acad Audiol 21: 145–152, 2010. doi: 10.3766/jaaa.21.3.2. [DOI] [PubMed] [Google Scholar]
- Ferraro JA, Thedinger BS, Mediavilla SJ, Blackwell WL. Human summating potential to tone bursts: observations on tympanic membrane versus promontory recordings in the same patients. J Am Acad Audiol 5: 24–29, 1994. [PubMed] [Google Scholar]
- Fitzpatrick DC, Campbell AP, Choudhury B, Dillon MT, Forgues M, Buchman CA, Adunka OF. Round window electrocochleography just before cochlear implantation: relationship to word recognition outcomes in adults. Otol Neurotol 35: 64–71, 2014. doi: 10.1097/MAO.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, Dillon MT, Rooth MA, Teagle HF, Park LR, Brown KD, Adunka OF, Buchman CA, Pillsbury HC, Fitzpatrick DC. Residual cochlear function in adults and children receiving cochlear implants: correlations with speech perception outcomes. Ear Hear 1, 2018. doi: 10.1097/AUD.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot TE, Giardina CK, Fitzpatrick DC. A model-based approach for separating the cochlear microphonic from the auditory nerve neurophonic in the ongoing response using electrocochleography. Front Neurosci 11: 592, 2017. doi: 10.3389/fnins.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgues M, Koehn HA, Dunnon AK, Pulver SH, Buchman CA, Adunka OF, Fitzpatrick DC. Distinguishing hair cell from neural potentials recorded at the round window. J Neurophysiol 111: 580–593, 2014. doi: 10.1152/jn.00446.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formeister EJ, McClellan JH, Merwin WH 3rd, Iseli CE, Calloway NH, Teagle HF, Buchman CA, Adunka OF, Fitzpatrick DC. Intraoperative round window electrocochleography and speech perception outcomes in pediatric cochlear implant recipients. Ear Hear 36: 249–260, 2015. doi: 10.1097/AUD.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Gans DP. Effects of acoustic trauma on the cochlear potentials. J Acoust Soc Am 74: 1742–1746, 1983. doi: 10.1121/1.390257. [DOI] [PubMed] [Google Scholar]
- Giardina CK, Brown KD, Adunka OF, Buchman CA, Hutson KA, Pillsbury HC, Fitzpatrick DC. Intracochlear electrocochleography: response patterns during cochlear implantation and hearing preservation. Ear Hear. In press. doi: 10.1097/AUD.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina CK, Khan TE, Pulver SH, Adunka OF, Buchman CA, Brown KD, Pillsbury HC, Fitzpatrick DC. Response changes during insertion of a cochlear implant using extracochlear electrocochleography. Ear Hear 39: 1146–1156, 2018. doi: 10.1097/AUD.0000000000000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson WP. A comparison of two methods of using transtympanic electrocochleography for the diagnosis of Meniere’s disease: click summating potential/action potential ratio measurements and tone burst summating potential measurements. Acta Otolaryngol Suppl 129, Suppl 560: 38–42, 2009. doi: 10.1080/00016480902729843. [DOI] [PubMed] [Google Scholar]
- Goldstein MH Jr, Kiang NY. Synchrony of neural activity in electric responses evoked by transient acoustic stimuli. J Acoust Soc Am 30: 107–114, 1958. doi: 10.1121/1.1909497 [DOI] [Google Scholar]
- Guitton MJ, Avan P, Puel JL, Bonfils P. Medial olivocochlear efferent activity in awake guinea pigs. Neuroreport 15: 1379–1382, 2004. doi: 10.1097/01.wnr.0000131672.15566.64. [DOI] [PubMed] [Google Scholar]
- Harvey D, Steel KP. The development and interpretation of the summating potential response. Hear Res 61: 137–146, 1992. doi: 10.1016/0378-5955(92)90044-N. [DOI] [PubMed] [Google Scholar]
- Helmstaedter V, Lenarz T, Erfurt P, Kral A, Baumhoff P. The summating potential is a reliable marker of electrode position in electrocochleography: cochlear implant as a theragnostic probe. Ear Hear 39: 687–700, 2018. doi: 10.1097/AUD.0000000000000526. [DOI] [PubMed] [Google Scholar]
- Honrubia V, Ward PH. Properties of the summating potential of the guinea pig’s cochlea. J Acoust Soc Am 45: 1443–1450, 1969. doi: 10.1121/1.1911622. [DOI] [PubMed] [Google Scholar]
- Hornibrook J. Tone burst electrocochleography for the diagnosis of clinically certain Meniere’s disease. Front Neurosci 11: 301, 2017. doi: 10.3389/fnins.2017.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornibrook J, Kalin C, Lin E, O’Beirne GA, Gourley J. Transtympanic electrocochleography for the diagnosis of Ménière’s disease. Int J Otolaryngol 2012: 852714, 2012. doi: 10.1155/2012/852714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Ionic currents of outer hair cells isolated from the guinea-pig cochlea. J Physiol 448: 73–98, 1992. doi: 10.1113/jphysiol.1992.sp019030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli C, Gibson W. A comparison of three methods of using transtympanic electrocochleography for the diagnosis of Meniere’s disease: click summating potential measurements, tone burst summating potential amplitude measurements, and biasing of the summating potential using a low frequency tone. Acta Otolaryngol 130: 95–101, 2010. doi: 10.3109/00016480902858899. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Membrane properties specialize mammalian inner hair cells for frequency or intensity encoding. eLife 4: e08177, 2015. doi: 10.7554/eLife.08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti W, Fettiplace R. Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70: 1143–1154, 2011. doi: 10.1016/j.neuron.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaga K, Nakamura M, Shinogami M, Tsuzuku T, Yamada K, Shindo M. Auditory nerve disease of both ears revealed by auditory brainstem responses, electrocochleography and otoacoustic emissions. Scand Audiol 25: 233–238, 1996. doi: 10.3109/01050399609074960. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC, Kahn AR. The relationship of gross potentials recorded from cochlea to single unit activity in the auditory nerve. In: Electrocochleography, edited by Ruben RJ, Elberling C, Salomon G. Baltimore, MD: University Park, 1976, p. 95–115. [Google Scholar]
- Kros CJ, Crawford AC. Potassium currents in inner hair cells isolated from the guinea-pig cochlea. J Physiol 421: 263–291, 1990. doi: 10.1113/jphysiol.1990.sp017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupperman R. The dynamic DC potential in the cochlea of the guinea pig (summating potential). Acta Otolaryngol 62: 465–480, 1966. doi: 10.3109/00016486609119591. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Epstein MJ, Cleveland SS, Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 11: e0162726, 2016. doi: 10.1371/journal.pone.0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenhan JT, Chertoff ME. Temporary hearing loss influences post-stimulus time histogram and single neuron action potential estimates from human compound action potentials. J Acoust Soc Am 123: 2200–2212, 2008. doi: 10.1121/1.2885748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martín A. A biophysical model of the inner hair cell: the contribution of potassium currents to peripheral auditory compression. J Assoc Res Otolaryngol 7: 218–235, 2006. doi: 10.1007/s10162-006-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammano F, Ashmore JF. Differential expression of outer hair cell potassium currents in the isolated cochlea of the guinea-pig. J Physiol 496: 639–646, 1996. doi: 10.1113/jphysiol.1996.sp021715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan JH, Formeister EJ, Merwin WH 3rd, Dillon MT, Calloway N, Iseli C, Buchman CA, Fitzpatrick DC, Adunka OF. Round window electrocochleography and speech perception outcomes in adult cochlear implant subjects: comparison with audiometric and biographical information. Otol Neurotol 35: e245–e252, 2014. doi: 10.1097/MAO.0000000000000557. [DOI] [PubMed] [Google Scholar]
- McMahon CM, Patuzzi RB. The origin of the 900 Hz spectral peak in spontaneous and sound-evoked round-window electrical activity. Hear Res 173: 134–152, 2002. doi: 10.1016/S0378-5955(02)00281-2. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Plontke SK, Hartsock JJ, Salt AN. Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol Neurotol 30: 131–138, 2009. doi: 10.1097/MAO.0b013e318191bff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. The cochlear place-frequency map of the adult and developing Mongolian gerbil. Hear Res 94: 148–156, 1996. doi: 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Campbell S, Taylor RR, Forge A, Hume CR. Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. J Assoc Res Otolaryngol 9: 65–89, 2008. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Siegel JH. The effects of moderate cooling on gross cochlear potentials in the gerbil: basal and apical differences. Hear Res 63: 79–89, 1992. doi: 10.1016/0378-5955(92)90076-Y. [DOI] [PubMed] [Google Scholar]
- Prijs VF. Single-unit response at the round window of the guinea pig. Hear Res 21: 127–133, 1986. doi: 10.1016/0378-5955(86)90034-1. [DOI] [PubMed] [Google Scholar]
- Rance G, Beer DE, Cone-Wesson B, Shepherd RK, Dowell RC, King AM, Rickards FW, Clark GM. Clinical findings for a group of infants and young children with auditory neuropathy. Ear Hear 20: 238–252, 1999. doi: 10.1097/00003446-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Riggs WJ, Roche JP, Giardina CK, Harris MS, Bastian ZJ, Fontenot TE, Buchman CA, Brown KD, Adunka OF, Fitzpatrick DC. Intraoperative electrocochleographic characteristics of auditory neuropathy spectrum disorder in cochlear implant subjects. Front Neurosci 11: 416, 2017. doi: 10.3389/fnins.2017.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis’ theory. Nature 301: 334–336, 1983. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Russell IJ. Cochlear receptor potentials. In: The Senses, A Comprehensive Reference, edited by Basbaum AI, Kaneko A, Shepherd GM, Westheimer G. San Diego, CA: Elsevier, 2008, p. 319–358. [Google Scholar]
- Russell IJ, Sellick PM. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol 338: 179–206, 1983. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA, Ruggero MA, Nelson DA, Turner CW. Kanamycin and bumetanide ototoxicity: anatomical, physiological and behavioral correlates. Hear Res 7: 261–279, 1982. doi: 10.1016/0378-5955(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Harmonics of outer hair cell motility. Biophys J 65: 2217–2227, 1993. doi: 10.1016/S0006-3495(93)81247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott WC, Giardina CK, Pappa AK, Fontenot TE, Anderson ML, Dillon MT, Brown KD, Pillsbury HC, Adunka OF, Buchman CA, Fitzpatrick DC. The compound action potential in subjects receiving a cochlear implant. Otol Neurotol 37: 1654–1661, 2016. doi: 10.1097/MAO.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellick P, Patuzzi R, Robertson D. Primary afferent and cochlear nucleus contributions to extracellular potentials during tone-bursts. Hear Res 176: 42–58, 2003. doi: 10.1016/S0378-5955(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res 14: 305–314, 1984. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Schreiner CE. The auditory neurophonic: basic properties. Hear Res 15: 261–280, 1984. doi: 10.1016/0378-5955(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain 119: 741–753, 1996. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Syka J, Melichar I. The effect of loop diuretics upon summating potentials in the guinea pig. Hear Res 20: 267–273, 1985. doi: 10.1016/0378-5955(85)90031-0. [DOI] [PubMed] [Google Scholar]
- Teagle HF, Roush PA, Woodard JS, Hatch DR, Zdanski CJ, Buss E, Buchman CA. Cochlear implantation in children with auditory neuropathy spectrum disorder. Ear Hear 31: 325–335, 2010. doi: 10.1097/AUD.0b013e3181ce693b. [DOI] [PubMed] [Google Scholar]
- van Deelen GW, Smoorenburg GF. Electrocochleography for different electrode positions in guinea pig. Acta Otolaryngol 101: 207–216, 1986. doi: 10.3109/00016488609132829. [DOI] [PubMed] [Google Scholar]
- van Emst MG, Giguère C, Smoorenburg GF. The generation of DC potentials in a computational model of the organ of Corti: effects of voltage-dependent K+ channels in the basolateral membrane of the inner hair cell. Hear Res 115: 184–196, 1998. doi: 10.1016/S0378-5955(97)00192-5. [DOI] [PubMed] [Google Scholar]
- van Emst MG, Klis SF, Smoorenburg GF. Tetraethylammonium effects on cochlear potentials in the guinea pig. Hear Res 88: 27–35, 1995. doi: 10.1016/0378-5955(95)00095-L. [DOI] [PubMed] [Google Scholar]
- Versnel H, Prijs VF, Schoonhoven R. Round-window recorded potential of single-fibre discharge (unit response) in normal and noise-damaged cochleas. Hear Res 59: 157–170, 1992. doi: 10.1016/0378-5955(92)90112-Z. [DOI] [PubMed] [Google Scholar]
- Wang B. The Relation Between the Compound Action Potential and Unit Discharges of the Auditory Nerve (ScD Thesis). Cambridge, MA: Massachusetts Institute of Technology, 1979, p. 219. [Google Scholar]
- Whitfield IC, Ross HF. Cochlear-microphonic and summating potentials and the outputs of individual hair-cell generators. J Acoust Soc Am 38: 126–131, 1965. doi: 10.1121/1.1909586. [DOI] [PubMed] [Google Scholar]
- Zheng XY, Ding DL, McFadden SL, Henderson D. Evidence that inner hair cells are the major source of cochlear summating potentials. Hear Res 113: 76–88, 1997. doi: 10.1016/S0378-5955(97)00127-5. [DOI] [PubMed] [Google Scholar]