Abstract
Background
In 2016, according to the German Federal Statistical Office, 178 425 cases of intoxication (poisoning) were treated in German hospitals. The poison control centers in the German-speaking countries gave advice in a total of 268 787 instances of poisoning in that year, and use of activated charcoal was recommended in 4.37% of cases. The application of activated charcoal plays a major role in both primary and secondary detoxification. This article serves as an overview of the mechanism of action, indications, contraindications, modes of application, and dosing of activated charcoal.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed. The opinions of experts from the poison control centers in the German-speaking countries were considered in the interpretation of the data.
Results
The administration of activated charcoal is indicated to treat moderately severe to life-threatening intoxication. It should be carried out as soon as possible, within the first hour of the ingestion; timed-release preparations can be given up to 6 hours after the ingestion. An important contraindication is impaired consciousness with the danger of aspiration in a patient whose airway has not yet been secured. Activated charcoal is ineffective or inadequately effective in cases of poisoning with acids or bases, alcohols, organic solvents, inorganic salts, or metals. The proper dosage consists of an amount that is 10 to 40 times as much as that of the intoxicating substance, or else 0.5–1 g/kg body weight in children or 50 g in adults. Repeated application is indicated for intoxications with agents that persist for a longer time in the stomach and for intoxications with timed-release drugs or drugs with a marked enterohepatic or entero-enteric circulation. The routine combination of activated charcoal with a laxative is not recommended.
Conclusion
Even though intoxications are common, there is still no internationally valid guideline concerning the administration of activated charcoal. A precise analysis of the risks and benefits is needed for each administration, and a poison control center should be consulted for this purpose.
Intoxication is a commonly encountered phenomenon. The etiology varies widely, and many different toxins can be involved. According to data from the Federal Statistical Office, 178 425 cases of poisoning were treated in German hospitals in 2016 (1, 2). A poison control center (PCC) is often consulted by medical and paramedical personnel (emergency rescue services, office-based physicians, hospital physicians, and pharmacists) or by members of the population for toxicological advice. Determination of the indication for treatment with activated charcoal—also known as activated carbon—plays a major role in eliminating the toxic capability of a potentially hazardous substance.
Activated charcoal was given in 0.89% of cases of poisoning in childhood registered in the USA in 2013. In that year, it was recommended in circa 50 000 patients across all age groups (3, 4). In Germany, the network of PCC gave advice in a total of 268 787 instances of poisoning across all age groups in 2016, recommending administration of activated charcoal in 4.37% of cases. Activated charcoal is included in the WHO Model List of Essential Medicines (5). Inclusion of activated charcoal among the standard antidotes carried by the emergency rescue services is recommended, for example, in Bavaria (6). Activated charcoal is one of the substances in the so-called Bremen List, a list compiled by the poison control center in northern Germany (GIZ-Nord) of five antidotes that emergency rescue service workers should always have at hand (7). Anyone can obtain activated charcoal from their local pharmacy without a prescription and administer it under strict guidelines after consultation with a PCC (8).
There is no internationally accepted guideline for the administration of activated charcoal. The most comprehensive publications are the position papers of the American Academy of Clinical Toxicology (AACT) and the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) on single-dose activated charcoal (1997 and 2005; 9, 10) and multi-dose activated charcoal in the treatment of acute poisoning (1999; 11). Ethical considerations make it practically impossible to conduct randomized controlled trials, so most of the data stem from in-vitro studies, animal experiments, studies with human volunteers, case reports, clinical case series, or observational studies. The only large human studies on the administration of activated charcoal have been carried out in developing countries, with somewhat contradictory results (12– 14).
In this review we discuss the mechanism of action of activated charcoal, indications and contraindications for its use, the time window in which it can be given, the mode of administration, and the adverse effects.
Method
Using the search terms “activated charcoal + poisoning,” we selectively surveyed the PubMed database for publications that had appeared at any time up to October 2018. In analyzing the data, we also took account of experts from the Society for Clinical Toxicology (Gesellschaft für Klinische Toxikologie e.V., GfKT), a professional body representing the PCC and clinical toxicologists in Germany, Austria, and Switzerland (website, in German: www.klinitox.de).
Mechanism of action
Activated charcoal adsorbs many noxious substances—medical drugs, phytotoxins and poisonous chemicals—onto its surface, preventing their absorption from the gastrointestinal tract. As a secondary decontamination mechanism, it interrupts a potential enterohepatic and/or enteroenteric circulation (8, 15, 16). The capacity for binding to the toxic substance depends on several factors, including (17– 19):
The particle size of the substance
The solubility of the substance
The ionization of the substance
The pH of the substance
The stomach contents
Substances that are adsorbed insufficiently or not at all by activated charcoal, owing to their physical properties, are listed in Box 1 (4).
BOX 1. Drugs/toxins that are known to be adsorbed or known not to be adsorbed onto activated charcoal (9– 11, 37, 38, e1– e9).
-
Substances that are adsorbed
ACE inhibitors
Amphetamines
Antidepressants (except lithium)
Antiepileptics
Antihistamines
Aspirin, salicylates
Atropine
Barbiturates
Benzodiazepines (NB: somnolence)
Beta blockers
Calcium-channel blockers
Quinine, quinidine
Chloroquine and primaquine
Dapsone
Digoxin, Digitoxin
Diuretics (especially furosemide, torasemide)
Nonsteroidal antirheumatics (NSAR)
Neuroleptics
Oral antidiabetics
(especially glibenclamide, glipizide)
Opiates, dextromethorphan (NB: somnolence)
Paracetamol
Piroxicam
Tetracyclines
Theophylline
-
Phytotoxins that are adsorbed
Amatoxin (death cap)
Aconitine (aconite)
Colchicine (autumn crocus)
Cucurbitacin (courgette, Cucurbitaceae)
Ergotamine, ergot alkaloids
Ibotenic acid, muscarine (fly agaric, panther cap)
Nicotine (tobacco)
Ricin (castor oil plant)
Strychnine (nux vomica)
Taxanes (yew)
Digitalis glycosides (foxglove)
-
Substances that are adsorbed insufficiently or not at all
Alcohols (e.g., ethanol, methanol, and glycols [for instance ethyleneglycol])
Anorganic salts (e.g., sodium chloride)
Metals and their anorganic compounds (e.g., lithium, iron, or other heavy metals [for instance lead or mercury])
Organic solvents (e.g., acetone, dimethylsulfoxide)
Acids and bases
Cyanides
Indication
Activated charcoal should be given only after ingestion of poisons that bind adequately to carbon.
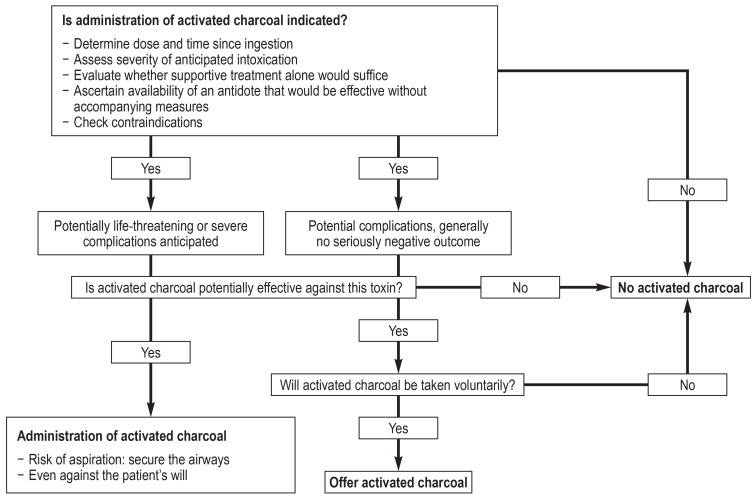
Administration of activated charcoal should be preceded by weighing up the potential risks and benefits (Figure). This analysis depends on the anticipated severity of the poisoning, as assessed by means of the Poisoning Severity Score (20). This scoring system categorizes the symptoms for each relevant organ system and for the acid–base balance according to their severity (none = 1, mild = 2, moderate = 3, severe = 4, fatal = 5). The most severely affected organ system determines the degree of severity (0–4). In the case of mild intoxication activated charcoal is only exceptionally given, i.e., when it is directly at hand and the occurrence of unpleasant symptoms of intoxication can be prevented. From moderate intoxication onwards, administration of activated charcoal is at least recommended, observing the efficacy and contraindications within the time window described below. In the event that the toxin is unknown (e.g., ingestion of vegetable or fungal material), administration of activated charcoal is still recommended if severe poisoning cannot be ruled out and there is no elevated risk of aspiration.
Figure.
Decision algorithm for administration of activated charcoal, modified from Isbister et al. (39)
Administration
Activated charcoal exists in the form of carbon tablets or as a powder or granulate. In clinical practice it seems more practical to use powder or granulate, which is available in doses of 10 to 100 g. Carbon tablets, in contrast, contain only 250 mg each, so a very large number of tablets would have to be used to achieve an adequate carbon surplus. To maximize the adsorption area (the surface area of modern charcoal granulate preparations is as much as 2500 to 3000 m2/g), carbon tablets have to be suspended in water.
Activated charcoal can be suspended in any liquid, but preferably in still water. Other liquids can be used for children, for instance sweetened tea or fruit juice. Yoghurt and milk have also been used (21– 24). It makes sense to have children drink through a straw out of an opaque container or a wrapped glass. It must always be remembered that the suspension liquid may also bind to the surface of the activated charcoal, thus reducing the available binding capacity. If a given patient cannot swallow the activated charcoal, it can be administered via a stomach tube.
Single-dose treatment: time window, indication, and dosage
For activated charcoal to be effective in primary elimination of the poison, it must come into contact with the substance concerned. Therefore, it must be given promptly. Clinically relevant adsorption can be anticipated with administration within 1 to 2 h of ingestion. In a total of 115 studies on volunteers, it was shown for 43 medications (including paracetamol and salicylates) that administration of activated charcoal within 30 min after ingestion lowered the bioavailability of the trial substance by an average of 69.1%; for administration after 1 h, the corresponding figure was 34.4% (9, 10).
In individual cases, later administration of activated charcoal may still be effective, e.g., following ingestion of slow-release preparations or substances that inhibit gastrointestinal motility, such as opiates, salicylates, or anticholinergics. There are a few other medications—typically nonpolar lipophilic substances with a late plasma peak and low endogenous clearance (e.g., amlodipine)—for which late administration of activated charcoal may be effective (19). However, a meta-analysis of volunteer studies showed no greater efficacy of delayed activated charcoal administration for cholinergic drugs than for anticholinergic drugs (19).
Clinical efficacy of delayed administration of activated charcoal (well over an hour after ingestion) has been found for the following medications:
Quetiapine in its rapid-release formulation: reduction of resorption by 35% with activated charcoal administration 0.5 to 6 h (median 3 h) after ingestion (25)
Citalopram: less frequent occurrence of QTc prolongation (4.2% versus 11.2%, relative risk 0.28) with activated charcoal administration 0.5 to 6.25 h (median 2 h) after ingestion (26)
Paracetamol: significantly less severe liver damage with activated charcoal administration 4 to 16 h after ingestion and simultaneous treatment with acetylcysteine (27)
Promethazine: lowering of the likelihood of delirium by 20% (absolute risk reduction) for activated charcoal administration within 2 h and by 9% for administration within 4 h after ingestion (28)
Protracted administration of activated charcoal also seems advisable after ingestion of fungi or of vegetable materials that are not readily digested, e.g., yew needles (Box 1).
The dosage is determined either by the amount of toxin ingested—if known—or by the body weight (BW) of the person concerned. When the amount of toxin to be bound is known, 10 times more activated charcoal should be given (29). According to Jürgens et al., administration of a 40-fold amount is best (19). This is practicable only with toxin intakes in the milligram range; otherwise the dose is 0.5 to 1 g/kg BW, to a maximum of 30 to 50 g. In adults, a single dose of 50 g activated charcoal is generally recommended independent of BW, in exceptional circumstances up to 100 g (Table).
Table. Dosage and administration of activated charcoal.
| Single-dose treatment | Multi-dose treatment | |
| Children | 0.5 to 1 g/kg BW to a maximum of 30 to 50 g | Initial dose 0.5 to 1 g/kg BW, followed by 0.125 to 0.25 g/kg BW/h every 1 to 4 h for 24 h |
| Adults >50 kg | 50 g | Initial dose 50 g, followed by 12.5 g/h every 1 to 4 h for 24 h |
| Known amount of toxin | Ten to 40 times more AC than toxin | No specific dose recommendation |
AC = Activated charcoal; BW = body weight
Multi-dose treatment: time window, indication, dosage, and administration
Multi-dose administration of activated charcoal has two effects: First, toxins are primarily eliminated even in distal sections of the gastrointestinal tract. Moreover, repeated administration is effective against substances that stay in the stomach for longer. Therefore, multi-dose administration of activated charcoal can still be started more than 1 h after ingestion. There are no precise recommendations for protracted multi-dose administration. With dapsone, for instance, accelerated elimination was observed even when multi-dose activated charcoal administration was not initiated until day 5 after ingestion (11). Toxic substances that stay in the stomach for longer periods of time include bezoar-forming drugs such as carbamazepine and slow-release quetiapine. These substances generate a dense mass of incompletely digested or undigested material (bezoar). Acetylsalicylic acid also has a longer retention time due to precipitation in the acid stomach contents, with clumping and possibly pylorospasm. This may also occasionally occur after ingestion of large amounts of other slow-release preparations.
The second effect is secondary elimination of toxin in the case of active substances that are subject to enterohepatic and/or enteroenteric circulation, both of which are interrupted by activated charcoal. In the case of enteroenteric circulation, this effect is termed “gastrointestinal dialysis”. In these circumstances the intestinal wall functions as a semipermeable membrane: toxins can diffuse out of the blood from serosal to mucosal onto the charcoal in the intestinal lumen. These substances usually have a long half-life, a low distribution volume, and low plasma protein binding (11, 15, 16).
The efficacy of multi-dose administration of activated charcoal against severe intoxication with carbamazepine, quinine, phenobarbital, and theophylline has been demonstrated clinically and/or in animal experiments (11). A special feature of theophylline is that activated charcoal is effective even after intravenous intoxication (11). Multi-dose activated charcoal administration is also effective against digitoxin and digoxin, although digitalis antitoxin is the treatment of choice in the event of severe intoxication. Amatoxin and colchicine, for example, are subject to an effective enterohepatic circulation. Volunteer studies have shown accelerated elimination of amitriptyline, dextropropoxyphene, disopyramide, nadolol, phenylbutazone, phenytoin, piroxicam, sotalol, and salicylates. This was true also for oxcarbazepine and lamotrigine (11, 30). For some active substances, clearance of the toxin can be accelerated, with subsequent excretion via the intestine. This was observed in studies with citalopram (31) and venlafaxine (32) (Box 2).
Box 2. Toxins for which multi-dose treatment with active charcoal is indicated.
-
Definite indications
Carbamazepine, quinine, dapsone, phenobarbital, theophylline
Digoxin/digitoxin (pronounced enterohepatic circulation, if no antidote available)
Slow-release quetiapine (bezoar-forming preparation)
Amatoxin, colchicine (pronounced enterohepatic circulation)
-
Acceleration of elimination described
Acetylsalicylic acid
Amitriptyline, phenytoin, piroxicam, sotalol, salicylates, oxcarbazepine, lamotrigine
Citalopram, venlafaxin
The first dose of a multi-dose activated charcoal treatment regimen corresponds to the amount that would be given as a single dose. The initial dose is followed by further doses of 12.5 g/h in adults and 0.125 to 0.25 g/kg BW/h in children at successive intervals of 1 h, 2 h, and 4 h (11) (Table). Regurgitation may be a problem, so that the dose may have to be reduced or the interval between doses extended. Combination with a prokinetic agent, e.g., intravenous metoclopramide, is possible provided the contraindications are observed, and insertion of a stomach tube can be considered.
The duration of activated charcoal administration depends on the plasma concentration of the toxin and/or the degree of clinical improvement. If the plasma level is used as the criterion, administration of activated charcoal should not be discontinued until the concentration has been reduced to near the therapeutic range. Usually the activated charcoal treatment does not need to be given for more than 24 h, but in exceptional cases administration for 48 h may be necessary.
Intestinal motility must be monitored in patients receiving multi-dose activated charcoal, as must the electrolyte balance and water balance. Repeated determination of the plasma concentration of the toxin is, if feasible, worthwhile. Stomach emptying must be checked before each successive dose of activated charcoal. If a stomach tube has been inserted, one must check for reflux into a sealed bag or aspirate the contents of the tube with a syringe. In the case of a large amount of residual charcoal or reflux >80–100 mL, the remaining charcoal is removed and the next dose of activated charcoal should be given with caution or at a later time.
Contraindications
Activated charcoal can only be given to a cooperative patient. Administration is contraindicated if the patient is not fully conscious and therefore has inadequate swallowing reflexes and a high risk of aspiration. This is also true for threatened severe intoxication, where swift clouding of consciousness can be anticipated, and for toxins with a high aspiration risk such as gasoline or lamp oil. In any of these cases the airways must first be secured by intubation, then activated charcoal can be given via stomach tube. Other contraindications are (9):
Direct danger of cerebral seizures
Swallowing disorders
Any known (current or previous) disorder of gastrointestinal transit
Injury, bleeding, corrosion, and/or (known or suspected) perforation of the gastrointestinal tract
Activated charcoal is not recommended in the event of repeated vomiting, but it can be given after a single episode of vomiting that stops without medication (Box 3).
Box 3. Contraindications to activated charcoal administration.
Patient not fully conscious, with no swallowing reflex and no safeguarding of airways
Gastrointestinal corrosion or bleeding, impaired gastrointestinal passage or suspected perforation
Ingestion of gasoline/oil or nonadsorbable substances
Recurring vomiting
The express wish of the patient (adult or child) not to receive activated charcoal
If gastroscopy is planned, e.g., to retrieve toxic materials, activated charcoal should not be administered until after the procedure, when it can be given through the working channel of the gastroscope or via a stomach tube.
After consultation with a PCC, activated charcoal can also be given by a person with no medical training. However, any such action must not be allowed to delay transport to hospital or the performance of elementary measures to stabilize vital functions, insofar as necessary (8). Administration of activated charcoal against the express will of the patient, whether an adult or a minor, is justified only in the event of a life-threatening intoxication. In the case of a life-threatening suicide attempt, forced administration of activated charcoal via a stomach tube can be considered even against the patient’s will.
Combination with a laxative
For many years routine simultaneous administration of a laxative (e.g., sodium sulfate) was common practice, but it is no longer recommended (33). A laxative can be given in individual cases after ingestion of a substance that slows down gastrointestinal motor activity. This can also be done in the event of constipation, possibly preceded by medicinal bowel stimulation. Administering polyethyleneglycol electrolyte solution at the same time as activated charcoal would result in the former binding to the latter and reducing its adsorption capacity, so they are given separately (generally 2 h apart).
Laxatives are also not routinely recommended together with multi-dose activated charcoal treatment (33). Administration of a laxative can be considered in the case of slow-release preparations or reduced gastrointestinal motility, but only after consultation with a PCC.
Adverse effects and complications
The adverse effects of activated charcoal in patients who have ingested poisons are difficult to measure because of the higher than usual dosage. With regular use, vomiting, constipation, diarrhea, nausea, urge to defecate, and anal irritation are all common. There have been a few reports—all in connection with repeated administration of high doses of activated charcoal—of either small intestinal (pseudo-)occlusion requiring surgical intervention or charcoal stercoliths perforating a loop of sigmoid colon (34).
A rare but serious complication is aspiration of charcoal leading to pulmonary failure, with a potentially fatal outcome. However, only isolated instances have been reported (9, 35, 36).
Further procedure
Depending on the degree of intoxication, patients treated with activated charcoal should be monitored in the hospital or at home. One can normally assume that there will be no further symptoms after the first excretion of charcoal, except for bezoar-forming substances, which require a longer observation period. After all symptoms still present have worn off, the treatment can be declared successful. Patients who have ingested poison with suicidal intent must always undergo psychiatric evaluation.
Summary
Activated charcoal is indicated for primary elimination of the toxin in moderate to severe cases of poisoning. It should be given as soon as possible (generally within 30 to 60 min of ingestion), and the patient must be alert and cooperative. The most important contraindication is a not fully conscious patient with no swallowing reflex. Furthermore, the toxin must display adequate binding to activated charcoal, which is not the case for acids/bases, alcohols, glycols, organic solvents, or metals. The adult dose of activated charcoal is usually 50 g, while in children the amount administered is determined by body weight (0.5 to 1 g/kg BW). Multi-dose treatment is required for effective elimination of some toxins. In most cases no additional laxative should be given.
Because of the frequent need for application of activated charcoal and the potentially narrow time window for effective administration, physicians should be familiar with the basic principles of activated charcoal treatment and, if necessary, always have a supply at hand. This applies particularly to primary-care physicians and pediatricians, as well as emergency physicians and emergency rescue personnel. It should be discussed whether households with children, facilities for children (e.g., kindergartens, after-school care, schools), homes for the disabled, and care homes for the elderly should be officially recommended to keep a supply of activated charcoal. We, the authors, and the experts at the PCC support such a recommendation (8).
Before activated charcoal is administered by a person with no medical training, a local physician or a PCC should be contacted to confirm the indication and advise on the exact procedure.
Key Messages.
Activated charcoal should be given as soon as possible (preferably not more than 1 h) after ingestion of the toxic substance. For slow-release preparations, activated charcoal can be administered up to 6 h after ingestion.
The contraindications to treatment with activated charcoal are reduced consciousness with the danger of aspiration (without previous airway management) and known or suspected gastrointestinal bleeding, corrosion, or perforation.
Forced administration of activated charcoal via a stomach tube against the patient’s will is justified only in the case of life-threatening intoxication (e.g., in attempted suicide).
Reviews of studies with volunteer probands show that the bioavailability of the substance ingested (43 different preparations tested) is reduced by an average of 69.1% if the activated charcoal is given within 30 min and by 34.4% if it is administered within 1 h.
Activated charcoal can be given by persons with no medical training after consultation with a poison control center. However, other measures (e.g., transport to the hospital or stabilization of vital functions) must not be delayed as a result.
Acknowledgments
Translated from the original German by David Roseveare
Acknowledgments
We are grateful to the members of Working Group I of the Society for Clinical Toxicology (Gesellschaft für Klinische Toxikologie) for compiling a consensus paper on behalf of the poison control centers of Germany, Austria, and Switzerland (“Gabe von Aktivkohle bei Vergiftungen – Konsensuspapier der deutschsprachigen Giftinformationszentren”), which formed the basis for this article.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Müller D, Desel H. Common causes of poisoning—etiology, diagnosis and treatment. Dtsch Arztebl Int. 2013;110:690–700. doi: 10.3238/arztebl.2013.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Statistisches Bundesamt. Gesundheitsberichterstattung des Bundes. www.destatis.de/ 2018 (last accessed on 18 December 2018) [Google Scholar]
- 3.Mowry JB, Spyker DA, Cantilena LR Jr., McMillan N, Ford M. 2013 Annual report of the American Association of Poison Control Centers‘ National Poison Data System (NPDS): 31st annual report. Clin Toxicol (Phila) 2014;52:1032–1283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juurlink DN. Activated charcoal for acute overdose: a reappraisal. Br J Clin Pharmacol. 2016;81:482–487. doi: 10.1111/bcp.12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. WHO model list of essential medicines, 18th list. www.who.int/medicines/publications/essentialmedicines/en/. 2013 (last accessed on 18 December 2018) [Google Scholar]
- 6.Rettungsdienstausschuss Bayern, Bayerisches Staatsministerium des Innern, für Bau und Verkehr. Empfehlung 2/03-2018 vom 13.03.2018 des Rettungsdienstausschuss Bayern; Antidota - Empfehlung für eine einheitliche Vorhaltung. www.aelrd-bayern.de/images/stories/pdf/rda/Empfehlung_Antidota.pdf. 2018 (last accessed on 18 December 2018) [Google Scholar]
- 7.Schaper A, Bandemer G, Callies A, et al. Vorhaltung von Antidota im Notarztdienst [Die Bremer Antidota-Liste als Diskussionsgrundlage für eine minimale Vorhaltung von Antidota] Notarzt. 2012;28:114–118. [Google Scholar]
- 8.Pfab R, Schmoll S, Dostal G, Stenzel J, Hapfelmeier A, Eyer F. Single dose activated charcoal for gut decontamination: application by medical non-professionals—a prospective study on availability and practicability. Toxicol Rep. 2016;4:49–54. doi: 10.1016/j.toxrep.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chyka PA, Seger D. American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists: Position statement: single-dose activated charcoal. J Toxicol Clin Toxicol. 1997;35:721–741. doi: 10.3109/15563659709162569. [DOI] [PubMed] [Google Scholar]
- 10.Chyka PA, Seger D, Krenzelok EP, Vale JA. American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists: Position paper: single-dose activated charcoal. Clin Toxicol (Phila) 2005;43:61–87. doi: 10.1081/clt-200051867. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. J Toxicol Clin Toxicol. 1999;37:731–751. doi: 10.1081/clt-100102451. [DOI] [PubMed] [Google Scholar]
- 12.de Silva HA, Fonseka MM, Pathmeswaran A, et al. Multiple-dose activated charcoal for treatment of yellow oleander poisoning: a single-blind, randomised, placebo-controlled trial. Lancet. 2003;361:1935–1938. doi: 10.1016/s0140-6736(03)13581-7. [DOI] [PubMed] [Google Scholar]
- 13.Eyer P, Eyer F. Is this the epitaph for multiple-dose activated charcoal? Lancet. 2008;371:538–539. doi: 10.1016/S0140-6736(08)60248-2. [DOI] [PubMed] [Google Scholar]
- 14.Eddleston M, Juszczak E, Buckley NA, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–587. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyer F, Jung N, Neuberger H, et al. Seromucosal transport of intravenously administered carbamazepine is not enhanced by oral doses of activated charcoal in rats. Basic Clin Pharmacol Toxicol. 2008;102:337–346. doi: 10.1111/j.1742-7843.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 16.Eyer F, Jung N, Neuberger H, et al. Enteral exsorption of acetaminophen after intravenous injection in rats: influence of activated charcoal on this clearance path. Basic Clin Pharmacol Toxicol. 2007;101:163–171. doi: 10.1111/j.1742-7843.2007.00107.x. [DOI] [PubMed] [Google Scholar]
- 17.Watson WA. Factors influencing the clinical efficacy of activated charcoal. Drug Intell Clin Pharm. 1987;21:160–166. [PubMed] [Google Scholar]
- 18.Andersen AH. Experimental studies on the pharmacology of activated charcoal; the effect of pH on the adsorption by charcoal from aqueous solutions. Acta Pharmacol Toxicol (Copenh) 1947;3:119–218. [PubMed] [Google Scholar]
- 19.Jürgens G, Hoegberg LC, Graudal NA. The effect of activated charcoal on drug exposure in healthy volunteers: a meta-analysis. Clin Pharmacol Ther. 2009;85:501–505. doi: 10.1038/clpt.2008.278. [DOI] [PubMed] [Google Scholar]
- 20.Persson HE, Sjöberg GK, Haines JA, Pronczuk de Garbino J. Poisoning severity score: grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36:205–213. doi: 10.3109/15563659809028940. [DOI] [PubMed] [Google Scholar]
- 21.Hoegberg LC, Angelo HR, Christophersen AB, Christensen HR. The effect of food and ice cream on the adsorption capacity of paracetamol to high surface activated charcoal: in vitro studies. Pharmacol Toxicol. 2003;93:233–237. doi: 10.1046/j.1600-0773.2003.pto930506.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoegberg LC, Christophersen AB, Christensen HR, Angelo HR. Comparison of the adsorption capacities of an activated-charcoal—yogurt mixture versus activated-charcoal—water slurry in vivo and in vitro. Clin Toxicol (Phila) 2005;43:269–275. [PubMed] [Google Scholar]
- 23.Rangan C, Nordt SP, Hamilton R, Ingels M, Clark RF. Treatment of acetaminophen ingestion with a superactivated charcoal-cola mixture. Ann Emerg Med. 2001;37:55–58. doi: 10.1067/mem.2001.111572. [DOI] [PubMed] [Google Scholar]
- 24.Bonner AB. Does the addition of chocolate milk reduce the absorption capacity of orally administered activated charcoal for GI decontamination of acetaminophen ingestion? Clin Toxicol. 2005;43 [Google Scholar]
- 25.Isbister GK, Friberg LE, Hackett LP, Duffull SB. Pharmacokinetics of quetiapine in overdose and the effect of activated charcoal. Clin Pharmacol Ther. 2007;81:821–827. doi: 10.1038/sj.clpt.6100193. [DOI] [PubMed] [Google Scholar]
- 26.Isbister GK, Friberg LE, Stokes B, et al. Activated charcoal decreases the risk of QT prolongation after citalopram overdose. Ann Emerg Med. 2007;50:593–600, e1-46. doi: 10.1016/j.annemergmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Spiller HA, Winter ML, Klein-Schwartz W, Bangh SA. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. J Emerg Med. 2006;30:1–5. doi: 10.1016/j.jemermed.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Page CB, Duffull SB, Whyte IM, Isbister GK. Promethazine overdose: clinical effects, predicting delirium and the effect of charcoal. QJM. 2009;102:123–131. doi: 10.1093/qjmed/hcn153. [DOI] [PubMed] [Google Scholar]
- 29.Olson KR. Activated charcoal for acute poisoning: one toxicologist‘s journey. J Med Toxicol. 2010;6:190–198. doi: 10.1007/s13181-010-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keranen T, Sorri A, Moilanen E, Ylitalo P. Effects of charcoal on the absorption and elimination of the antiepileptic drugs lamotrigine and oxcarbazepine. Arzneimittelforschung. 2010;60:421–426. doi: 10.1055/s-0031-1296306. [DOI] [PubMed] [Google Scholar]
- 31.Friberg LE, Isbister GK, Hackett LP, Duffull SB. The population pharmacokinetics of citalopram after deliberate self-poisoning: a Bayesian approach. J Pharmacokinet Pharmacodyn. 2005;32:571–605. doi: 10.1007/s10928-005-0022-6. [DOI] [PubMed] [Google Scholar]
- 32.Kumar VV, Oscarsson S, Friberg LE, Isbister GK, Hackett LP, Duffull SB. The effect of decontamination procedures on the pharmacokinetics of venlafaxine in overdose. Clin Pharmacol Ther. 2009;86:403–410. doi: 10.1038/clpt.2009.114. [DOI] [PubMed] [Google Scholar]
- 33.Barceloux D, McGuigan M, Hartigan-Go K. Position statement: cathartics American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:743–752. doi: 10.3109/15563659709162570. [DOI] [PubMed] [Google Scholar]
- 34.Merck. Fachinformation Ultracarbon. https://s3.eu-central-1.amazonaws.com/prod-cerebro-ifap/media_all/70679.pdf. Mai 2014 (last accessed on 26 March 2018) [Google Scholar]
- 35.Menzies DG, Busuttil A, Prescott LF. Fatal pulmonary aspiration of oral activated charcoal. BMJ. 1988;297:459–460. doi: 10.1136/bmj.297.6646.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack MM, Dunbar BS, Holbrook PR, Fields AI. Aspiration of activated charcoal and gastric contents. Ann Emerg Med. 1981;10:528–529. doi: 10.1016/s0196-0644(81)80009-1. [DOI] [PubMed] [Google Scholar]
- 37.Eckert K, Eyer P, Zilker T. [Activated charcoal—first aid treatment in oral poisoning] Dtsch Arztebl. 1999;96:A-2826–A-2830. [Google Scholar]
- 38.Neuvonen PJ, Olkkola KT. Oral activated charcoal in the treatment of intoxications Role of single and repeated doses. Med Toxicol Adverse Drug Exp. 1988;3:33–58. doi: 10.1007/BF03259930. [DOI] [PubMed] [Google Scholar]
- 39.Isbister GK, Kumar VV. Indications for single-dose activated charcoal administration in acute overdose. Curr Opin Crit Care. 2011;17:351–357. doi: 10.1097/MCC.0b013e328348bf59. [DOI] [PubMed] [Google Scholar]
- E1.Hojer J, Troutman WG, Hoppu K, et al. Position paper update: ipecac syrup for gastrointestinal decontamination. Clin Toxicol (Phila) 2013;51:134–139. doi: 10.3109/15563650.2013.770153. [DOI] [PubMed] [Google Scholar]
- E2.Benson BE, Hoppu K, Troutman WG, et al. Position paper update: gastric lavage for gastrointestinal decontamination. Clin Toxicol (Phila) 2013;51:140–146. doi: 10.3109/15563650.2013.770154. [DOI] [PubMed] [Google Scholar]
- E3.Merigian KS, Woodard M, Hedges JR, Roberts JR, Stuebing R, Rashkin MC. Prospective evaluation of gastric emptying in the self-poisoned patient. Am J Emerg Med. 1990;8:479–483. doi: 10.1016/0735-6757(90)90146-q. [DOI] [PubMed] [Google Scholar]
- E4.Buckley NA, Whyte IM, O‘Connell DL, Dawson AH. Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose. J Toxicol Clin Toxicol. 1999;37:753–757. doi: 10.1081/clt-100102452. [DOI] [PubMed] [Google Scholar]
- E5.Hillman RJ, Prescott LF. Treatment of salicylate poisoning with repeated oral charcoal. Br Med J (Clin Res Ed) 1985;291 doi: 10.1136/bmj.291.6507.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Chyka PA, Holley JE, Mandrell TD, Sugathan P. Correlation of drug pharmacokinetics and effectiveness of multiple-dose activated charcoal therapy. Ann Emerg Med. 1995;25:356–362. doi: 10.1016/s0196-0644(95)70295-4. [DOI] [PubMed] [Google Scholar]
- E7.Arimori K, Nakano M. Accelerated clearance of intravenously administered theophylline and phenobarbital by oral doses of activated charcoal in rats A possibility of the intestinal dialysis. J Pharmacobiodyn. 1986;9:437–441. doi: 10.1248/bpb1978.9.437. [DOI] [PubMed] [Google Scholar]
- E8.Neuvonen PJ, Elonen E. Effect of activated charcoal on absorption and elimination of phenobarbitone, carbamazepine and phenylbutazone in man. Eur J Clin Pharmacol. 1980;17:51–57. doi: 10.1007/BF00561677. [DOI] [PubMed] [Google Scholar]
- E9.Wason S, Baker RC, Carolan P, Seigel R, Druckenbrod RW. Carbamazepine overdose—the effects of multiple dose activated charcoal. J Toxicol Clin Toxicol. 1992;30:39–48. doi: 10.3109/15563659208994444. [DOI] [PubMed] [Google Scholar]