Abstract
Background
Craniopharyngioma is a tumor of low histological malignancy resulting from an anomaly of embryonic development. Affected children and adolescents are being studied with respect to their quality of life, progression-free survival, and overall survival in the framework of the ongoing KRANIOPHARYNGEOM 2007 project.
Methods
This prospective, multicenter project consists of a randomized trial with an adaptive design combined with a purely observational study. The randomized, unblinded trial includes patients whose tumors have been incompletely resected and is intended to compare the outcomes of immediate postoperative radiotherapy versus radiotherapy on progression. Its primary endpoint is quality of life as assessed subjectively by the patients themselves with the “Pediatric Quality of Life” questionnaire (PEDQOL). In exploratory analyses, linear mixed models were used to study the effect of further factors on quality of life.
Results
An interim intention-to-treat analysis of the randomized trial revealed only minor differences between the treatment arms with respect to quality of life (n = 24). The exploratory analyses (n = 131) showed that preoperative involvement of, or operative damage to, the anterior and posterior regions of the hypothalamus was associated with a lower quality of life. Complete resection was followed by a lower quality of life than incomplete resection. Radiotherapy, a common treatment for tumors that progress after incomplete resection, was also associated with a lower quality of life.
Conclusion
Hypothalamus-sparing treatment approaches are recommended to optimize the quality of life of children and adolescents with cranio-pharyngioma. The available evidence does not support any recommendation as to when radiotherapy should be performed after incomplete resection so that the best quality of life can be achieved.
Craniopharyngioma is a tumor of the sellar region that arises because of an anomaly in embryonic development. It is treated with neurosurgical procedures and radiotherapy. Because tumors of this type lie in the immediate vicinity of the optic chiasm, pituitary gland, and hypothalamus, the patient’s quality of life can be impaired over the long term by dysfunction of these structures induced either by the tumor itself or by its treatment; hypothalamic obesity is a particular problem (1). Complete resection was once considered the goal of surgery, but neurosurgeons now increasingly prefer incomplete resection in order to avoid surgical damage to the anatomical structures of the hypothalamic-pituitary axis.
The KRANIOPHARYNGEOM 2007 study (Clinical Trials.gov, identifier NCT01272622) is a prospective multicenter study of children and adolescents with craniopharyngioma. Its goal is to investigate the quality of life, progression-free survival, and overall survival of the affected patients as a function of the treatment. The study is based on a multidisciplinary approach involving all the medical specialties that collaborate in these patients’ diagnostic evaluation, treatment, and long-term care. For patients whose tumors have been incompletely resected, the KRANIOPHARYNGEOM 2007 project also includes a randomized substudy that was intended to determine the optimal timing of postoperative radiotherapy.
Methods
Study design
Recruitment for the KRANIOPHARYNGEOM 2007 study began in 2007 and includes children and adolescents from across Germany who received an initial diagnosis of craniopharyngioma before their 18th birthday. In the randomized trial, patients who were at least five years old and had undergone radiologically confirmed incomplete tumor resection were randomized to receive postoperative radiotherapy either right after surgery or only on progression of disease. Patients were not included in the randomized trial if they did not meet the inclusion criteria or did not consent to randomization, but these patients’ further course was nonetheless longitudinally observed and documented.
Endpoints
Quality of life was assessed with the “Pediatric Quality of Life” questionnaire (PEDQOL) (4, 5), which yields a numerical score reflecting the subjective assessment—by the patients themselves and their parents—of quality of life in seven distinct domains. The subscore in each area can range from 25 to 100 points, with a low score reflecting a higher quality of life (for details, cf. eMethods). The primary endpoint for the randomized trial was the change in the PEDQOL physical function subscore from a first time point three months after surgery to another time point three years after surgery. The secondary endpoints were progression-free and overall survival. For the entire patient group, the PEDQOL score in all domains was analyzed one year and three years after surgery in order to assess long-term quality of life after the patients’ postoperative condition had become stable.
Neuroradiological diagnostic studies
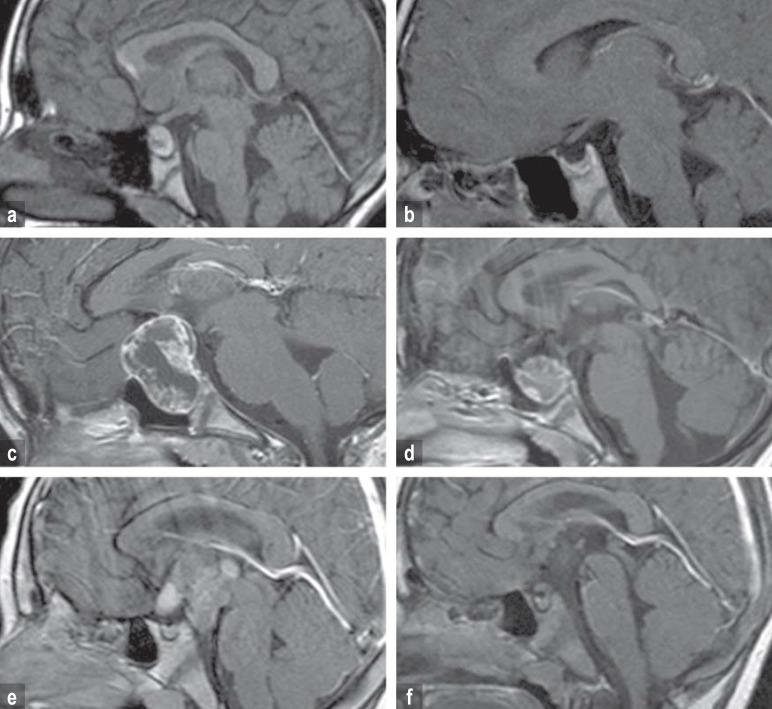
The degree of preoperative hypothalamic involvement (HI) and of operative hypothalamic damage (HD) was determined neuroradiologically according to published criteria (5, 6). In brief, a score of 0 corresponded to no demonstrable HI or HD, a score of 1 corresponded to involvement or damage of anterior hypothalamic structures not including the mammillary bodies, and a score of 2 corresponded to involvement or damage of anterior and posterior hypothalamic areas including the mammillary bodies (figure 1).
Figure 1.
MRI on diagnosis and 36 months after surgery in three children with craniopharyngioma with different degrees of preoperative hypothalamic involvement and operative hypothalamic damage.
Figures 1a, b: sellar tumor (no hypothalamic involvement [a]/no operative hypothalamic damage [b])
Figures 1c, d: preoperative involvement (c) and operative damage (d) of the anterior hypothalamus (not including the mammillary bodies).
Figures 1e, f: preoperative involvement (e) und operative damage (f) of the anterior and posterior hypothalamus (including the mammillary bodies).
Statistical methods
The primary evaluation of the results of the randomized trial was carried out with an intention-to-treat analysis. The main hypothesis was tested in an adaptive study design with a two-tailed significance level set at 5%. All further analyses, including the analysis of quality of life in the overall patient cohort, can only be considered exploratory; the significance level was, therefore, not adjusted for multiple testing. P-values less than or equal to 0.05 were considered statistically noticeable. Based on the data from the overall patient cohort, exploratory analyses employing linear mixed models were carried out to study the effects of age, sex, degree of resection, radiotherapy, preoperative HI, and operative HD on self-assessed quality of life (for details of the statistical methods, see eMethods).
Results
Randomized trial
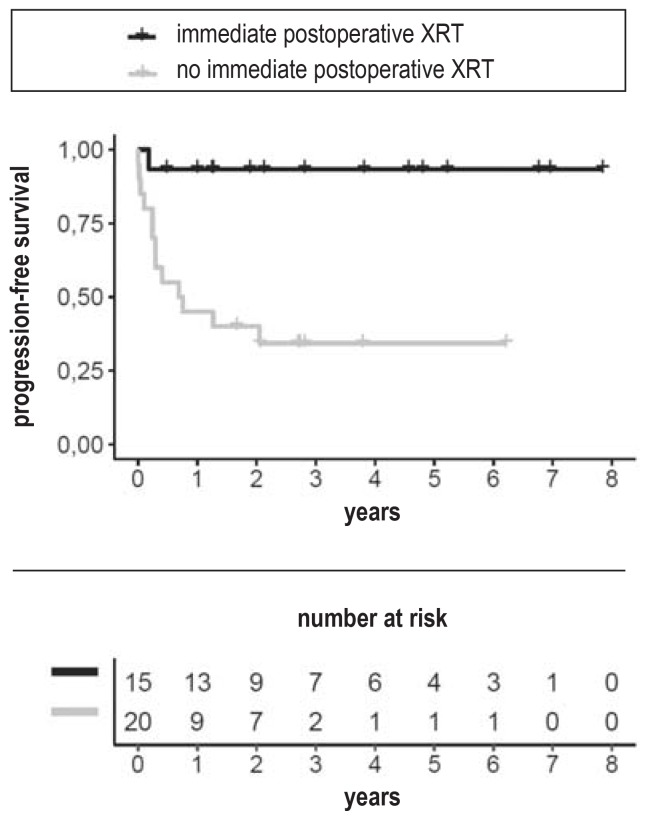
24 patients were included in the analysis of the primary endpoint (Figure 2, eFigure 1). The PEDQOL physical function subscore in the 10 patients who underwent radiotherapy immediately after surgery increased by +2.1 ± 16.6 points (mean ± standard deviation), reflecting a mild worsening. The change in the 14 patients who only underwent radiotherapy on progression was -0.9 ± 15.5 (two-tailed p-value by the Mann–Whitney U test = 0.63). Seven of these 14 patients underwent radiotherapy within three years of surgery. Randomization was terminated in September 2016, as required by the underlying adaptive design, because the probability of detecting a significant difference would then have been too low even if recruitment had been extended for the longest practically achievable time (“stop for futility”). Since the end of recruitment, the project has been continued as a purely observational study. The analysis of progression-free survival (PFS) reveals good tumor control after primary radiotherapy: the estimated PFS at one year is 93.3% (95% confidence interval [81.5; 100]), as opposed to 45.0% [27.7; 73.1]) in the patients who did not undergo radiotherapy immediately after surgery (efigure 2). This difference had been anticipated in the planning of the study; the central question of the randomized trial was the extent to which delayed radiotherapy might provide a benefit with respect to quality of life. None of the randomized patients died, so there was no evidence of any difference in overall survival. Further details of the analysis of the randomized part of the study are provided in the eMethods section.
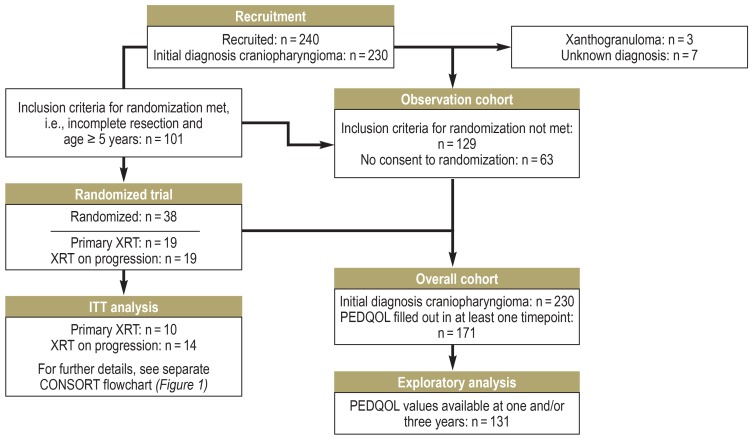
Figure 2.
Flowchart of recruitment and randomization in the KRANIOPHARYNGEOM 2007 study.
CONSORT, Consolidated Standards of Reporting Trials; PEDQOL, Pediatric Quality of Life Questionnaire; XRT, radiotherapy
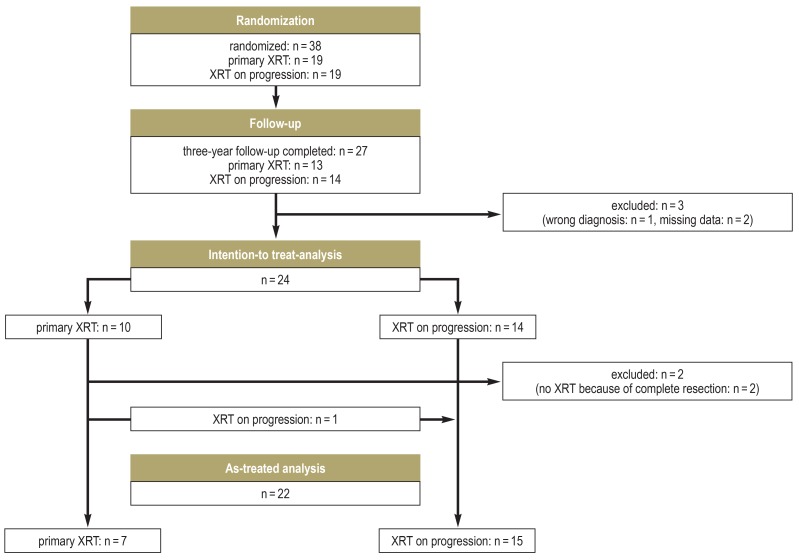
eFigure 1.
CONSORT flowchart for the randomized part of the study.
CONSORT, Consolidated Standards of Reporting Trials; XRT, radiotherapy
eFigure 2.
Kaplan–Meier estimate of progression-free survival
p = 0.0013 (log-rank test).
XRT, radiotherapy
Patient cohort for exploratory analyses
As the randomized comparison yielded no conclusion about the optimal timing of radiotherapy for the patients’ subjectively assessed quality of life (QoL), further parameters were studied in the overall patient cohort for their possible effect on postoperative QoL. 240 patients had been recruited as of June 2018, of whom 230 had a craniopharyngioma. PEDQOL responses one and/or three years after surgery were available from 131 patients; data from these patients were included in the analysis (Figure 2, eFigure 3).
eFigure 3.
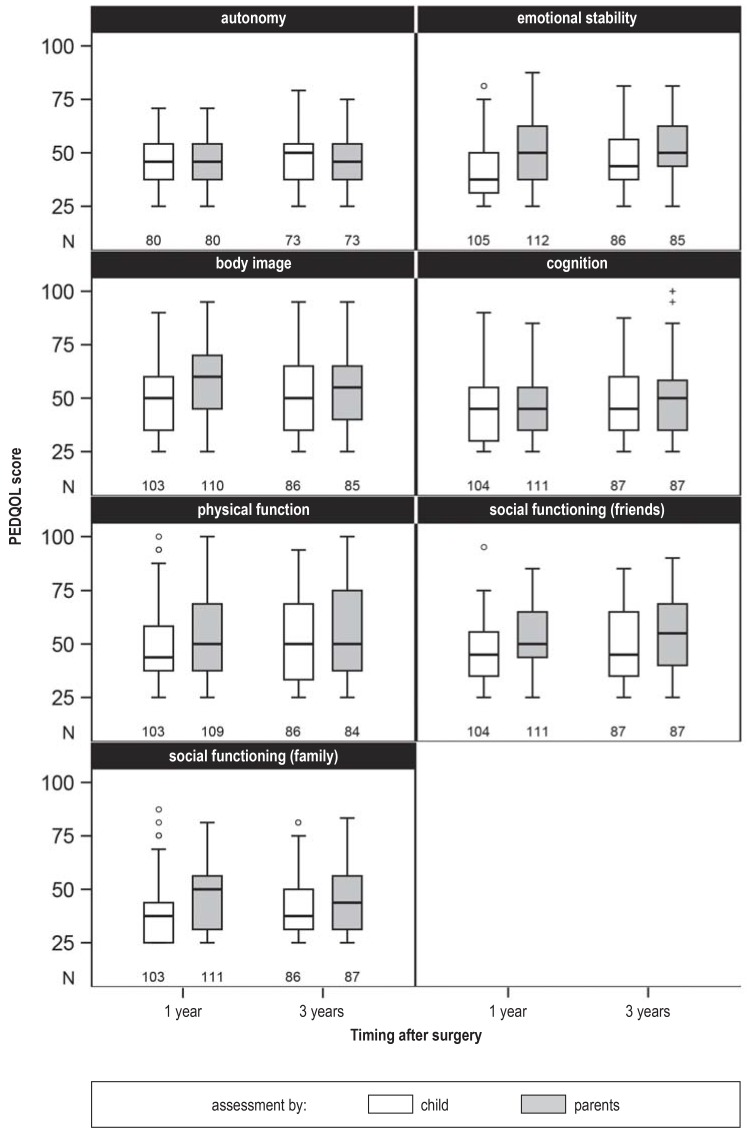
The number of completed questionnaires and the distribution of PEDQOL scores in the different domains, one and three years after surgery. PEDQOL, Pediatric Quality of Life Questionnaire
Information on the investigated patient cohort is presented in the Table. The documentation of some variables was incomplete; e.g., the extent of resection was unknown for some patients. It was decided, however, to carry out each QoL analysis with the largest possible number of patients. The median age at diagnosis was 10 years (range, 1–18), and 66 of 131 patients (50%) were female. Only 6% of the patients had no hypothalamic involvement at the time of diagnosis; 21% had anterior HI, and 73% had both anterior and posterior HI. Tumor resection was complete in 18% and incomplete in 82%. Radiotherapy was documented for 41 of the 91 patients who had undergone incomplete resection; one additional patient underwent radiotherapy after complete resection. The type of radiotherapy was not predetermined in the study protocol. 47% of the patients who underwent radiotherapy had photon radiotherapy, and another 47% had proton-beam radiotherapy.
Table. Patient cohort for exploratory analysis of the quality of life*.
|
Overall cohort (n = 131) |
Extent of resection (unknown: n = 16) | |||
| Incomplete (n = 94) | Complete (n = 21) | |||
| Parameter | Category |
Number (percent) or median (minimum; maximum) |
Number (percent) or median (minimum; maximum) |
Number (percent) or median (minimum; maximum) |
| Sex n = 131 |
Female Male |
66 (50%) 65 (50%) |
51 (54%) 43 (46%) |
11 (52%) 10 (48%) |
| Age on diagnosis (years) n = 131 |
– < 7 years ≥ 7 years |
9.7 (1.3; 17.6) 40 (31%) 91 (69%) |
9.8 (1.3; 17.5) 31 (33%) 63 (67%) |
11.5 (3.1; 17.6) 4 (19%) 17 (81%) |
| BMI-SDS on diagnosis n = 115 |
– | 0.4 (−3.8; 10.0) | 0.3 (−3.0; 8.7) | 1.5 (−3.8; 10.0) |
| Tumor site n = 113 |
Intrasellar Intra-/extrasellar Extrasellar |
2 (2%) 91 (81%) 20 (18%) |
2 (2%) 69 (84%) 11 (13%) |
0 (0%) 12 (63%) 7 (37%) |
| Preoperative hypothalamic involvement n = 131 |
None Anterior Anterior and posterior |
8 (6%) 28 (21%) 95 (73%) |
5 (5%) 23 (24%) 66 (70%) |
2 (10%) 2 (10%) 17 (81%) |
| Extent of resection n = 115 |
Complete Incomplete |
21 (18%) 94 (82%) |
0 (0%) 94 (100%) |
21 (100%) 0 (0%) |
| Postoperative hypothalamic damage n = 130 |
None Anterior Anterior and posterior |
42 (32%) 40 (31%) 48 (37%) |
35 (37%) 34 (36%) 25 (27%) |
2 (10%) 3 (14%) 16 (76%) |
| Radiotherapy n = 121 |
No Yes |
74 (61%) 47 (39%) |
50 (55%) 41 (45%) |
19 (95%) 1 (5%) |
| Radiotherapy within one year of surgery n = 121 |
No Yes |
90 (74%) 31 (26%) |
63 (69%) 28 (31%) |
20 (100%) 0 (0%) |
| Type of radiotherapy n = 47 |
Photons Proton beam Other (seeds, stereotactic radiosurgery) |
22 (47%) 22 (47%) 3 (6%) |
19 (46%) 19 (46%) 3 (7%) |
1 (100%) 0 (0%) 0 (0%) |
*The available values are indicated for every parameter. The figures given are either the number (and percentage of the relevant total) or the median (minimum; maximum).
Instead of the body-mass index itself (BMI, kg/m2), the BMI is reported as a Standard Deviation Score (BMI-SDS) representing the deviation from the age- and sex-specific mean BMI (7).
To check whether the PEDQOL responses of these 131 patients were representative of the overall cohort, the groups with and without PEDQOL values at one or three years were descriptively compared. The two groups displayed no statistically noticeable differences in any of the main baseline characteristics (age on diagnosis, sex, tumor site, hypothalamic involvement or hypothalamic damage). A small difference was seen with respect to treatment: information on quality of life was available for 72% (94/131) of the patients who underwent incomplete resection, but for only 50% (21/42) of those who underwent complete resection (p = 0.014).
Factors affecting patients’ postoperative quality of life
Patients who were aged seven years or older when craniopharyngioma was diagnosed rated their own quality of life one or three years after surgery worse than younger children did, in six of the seven domains of assessment (efigure 4). Male and female patients differed only slightly in self-assessed quality of life.
eFigure 4.
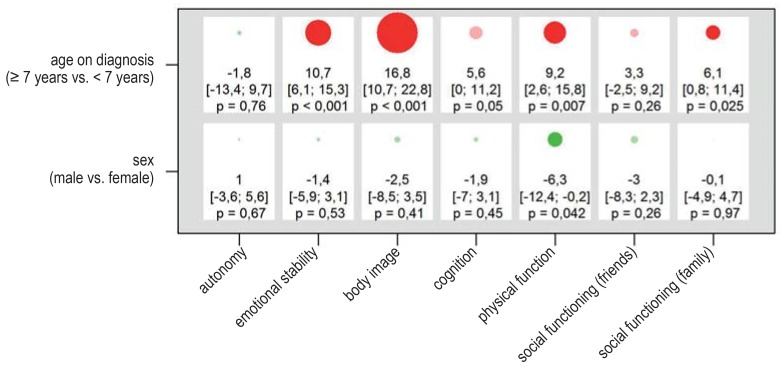
Results of the linear mixed model for self-assessed quality of life in the overall patient cohort (n = 131). The PEDQOL score was modeled separately in each domain as a function of time, the factor in question, and the interaction between time and the factor in question. The results are displayed as differences of least-square estimates, with the associated 95% confidence intervals and p-values, describing the mean difference in PEDQOL scores between the two categories of each factor. The size of the circles is proportional to the mean difference; circles representing positive and negative effects on the quality of life are colored green and red, respectively (opaque when p = 0.05). A box corresponds to the results of a model. The higher the PEDQOL score, the lower the subjective quality of life; a minus sign thus indicates a beneficial influence on the quality of life.
PEDQOL, Pediatric Quality of Life Questionnaire
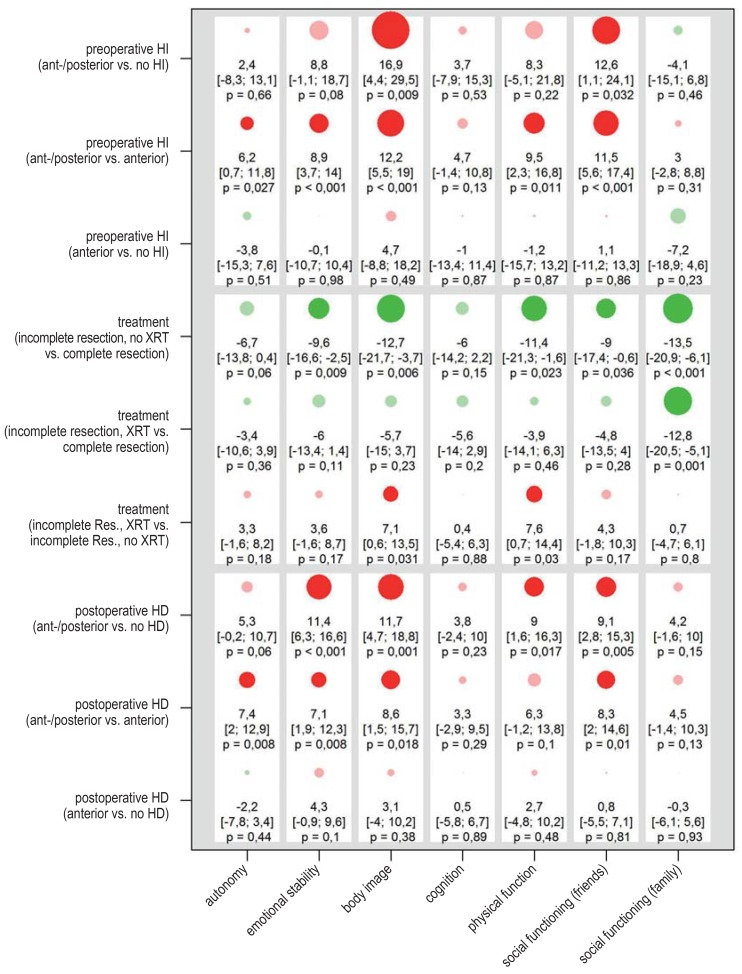
Patients who preoperatively had anterior and posterior HI rated their quality of life markedly worse than patients without HI or with only anterior HI (Figure 3, eFigure 5). For example, the PEDQOL body image subscore in patients with second-degree HI was 16.9 points higher than in those without HI ([4.4; 29.5], p = 0.009). When interpreting such differences, one should bear in mind that different responses to a single domain-specific question can lead to an approximately five-point difference in the corresponding subscore, with higher scores indicating a lower quality of life. In both comparisons, patients with anterior and posterior HI had higher PEDQOL subscores relating to body image and to social behavior (as determined from questions about friendships); a comparison of first- and second-degree HI revealed statistically noticeable differences in three further domains. On the other hand, no relevant difference was seen between patients with anterior HI and patients without any preoperative HI.
Figure 3.
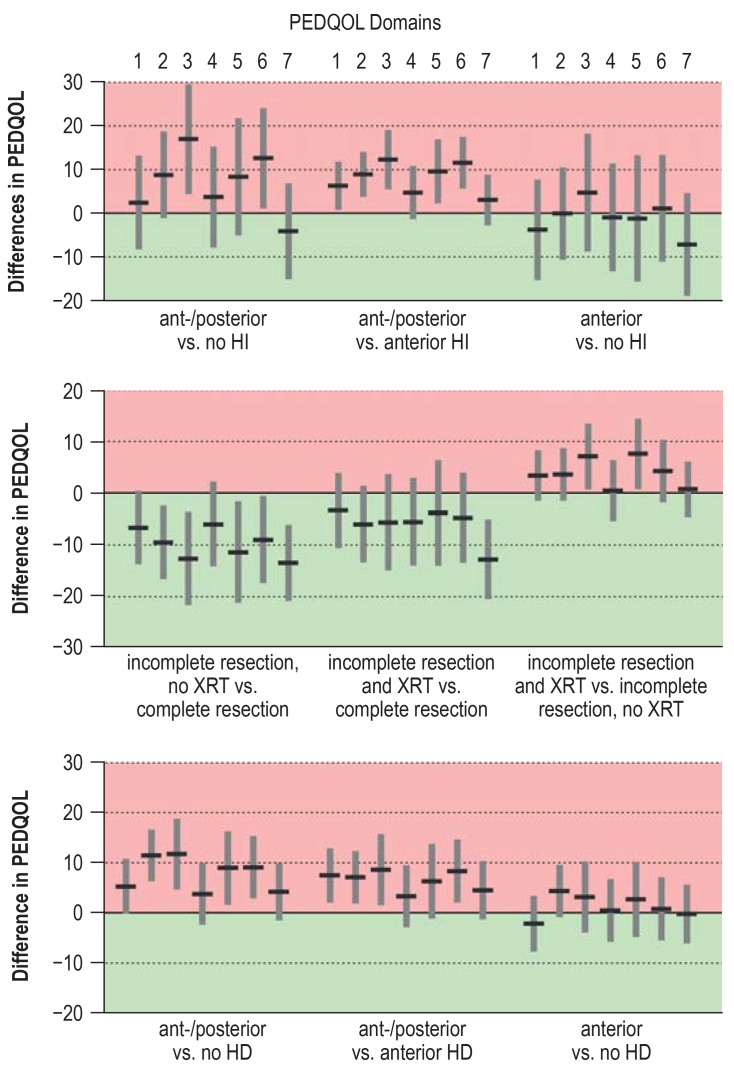
Results of the exploratory analses of self-assessed quality of life in the overall cohort (n = 131). The diagram shows mean PDQOL differences between the two categories of each of the three individual factors (preoperative HI, treatment, postoperative HD) one and three years after surgery, with corresponding 95% confidence intervals. The seven domains of PEDQOL are: 1) autonomy, 2) emotional stability, 3) body image, 4) cognition, 5) physical function, 6) social functioning (friends), and 7) social functioning (family). The higher the PEDQOL score, the lower the self-assessed quality of life; a negative change thus represents a beneficial effect on quality of life.
A detailed listing of the results, including p-values (efigure 5) and details of the calculation, can be found in eMethods.
HD, hypothalamic damage; HI, hypothalamic involvement; PEDQOL, Pediatric Quality of Life Questionnaire; XRT, radiotherapy
The degree of postoperative hypothalamic damage also affects the quality of life. The self-assessed QoL of patients with anterior and posterior HD was worse in all domains than that of patients with only anterior HD or no HD; the difference was statistically noticeable in four of the seven domains. Patients with anterior HD did not differ from patients without HD in their self-assessed QoL.
The effects of preoperative HI and operative/postoperative HD on patients’ quality of life cannot be assessed independently, because these two entities are strongly correlated with each other (p<0.0001, eTable 1). For example, all 48 patients with second-degree postoperative HD had second-degree HI before surgery.
eTable 1. The association of preoperative hypothalamic involvement, extent of surgical resection, and postoperative hypothalamic damage: Comparison of preoperative hypothalamic involvement and postoperative hypothalamic damage*.
|
Preoperative hypothalamic involvement |
Postoperative hypothalamic damage | Total | |||
| None |
Anterior (first-degree) |
Anterior and posterior (second-degree) |
|||
| None | Number | 7 | 0 | 0 | 7 |
| Row percent | 100% | 0% | 0% | 100% | |
| Column percent | 17% | 0% | 0% | 5% | |
|
Anterior (first-degree) |
Number | 15 | 13 | 0 | 28 |
| Row percent | 54% | 46% | 0% | 100% | |
| Column percent | 36% | 33% | 0% | 22% | |
|
Anterior and posterior (second-degree) |
Number | 20 | 27 | 48 | 95 |
| Row percent | 21% | 28% | 51% | 100% | |
| Column percent | 48% | 68% | 100% | 73% | |
| Total | Number | 42 | 40 | 48 | 130 |
| Row percent | 32% | 31% | 37% | 100% | |
| Column percent | 100% | 100% | 100% | 100% | |
*p <0.0001 by Fisher’s exact test
The temporal dependence of radiotherapy was considered in the analysis of treatment modalities: PEDQOL responses were assigned to the “Radiotherapy” category if the patient had undergone radiotherapy before answering the corresponding questions (table). Whether or not they were treated with radiotherapy, patients who underwent incomplete resection uniformly rated their quality of life better than those who underwent complete resection. The effects of the extent of resection and of HD could not be assessed independently, as these two parameters were closely linked (p = 0.0001, eTables 2, 3). Thus, most (16/21) patients who underwent complete resection had second-degree hypothalamic damage thereafter.
eTable 2. The association of preoperative hypothalamic involvement, extent of surgical resection, and postoperative hypothalamic damage: comparison of preoperative hypothalamic involvement and extent of resection*.
|
Preoperative hypothalamic involvement |
Extent of resection | Total | ||
| Incomplete | Complete | |||
| None | Number | 5 | 2 | 7 |
| Row percent | 71% | 29% | 100% | |
| Column percent | 5% | 10% | 6% | |
|
Anterior (first-degree) |
Number | 23 | 2 | 25 |
| Row percent | 92% | 8% | 100% | |
| Column percent | 24% | 10% | 22% | |
|
Anterior and posterior (second-degree) |
Number | 66 | 17 | 83 |
| Row percent | 80% | 20% | 100% | |
| Column percent | 70% | 81% | 72% | |
| Total | Number | 94 | 21 | 115 |
| Row percent | 82% | 18% | 100% | |
| Column percent | 100% | 100% | 100% | |
*p = 0.26 by Fisher’s exact test
Quality of life after incomplete tumor resection
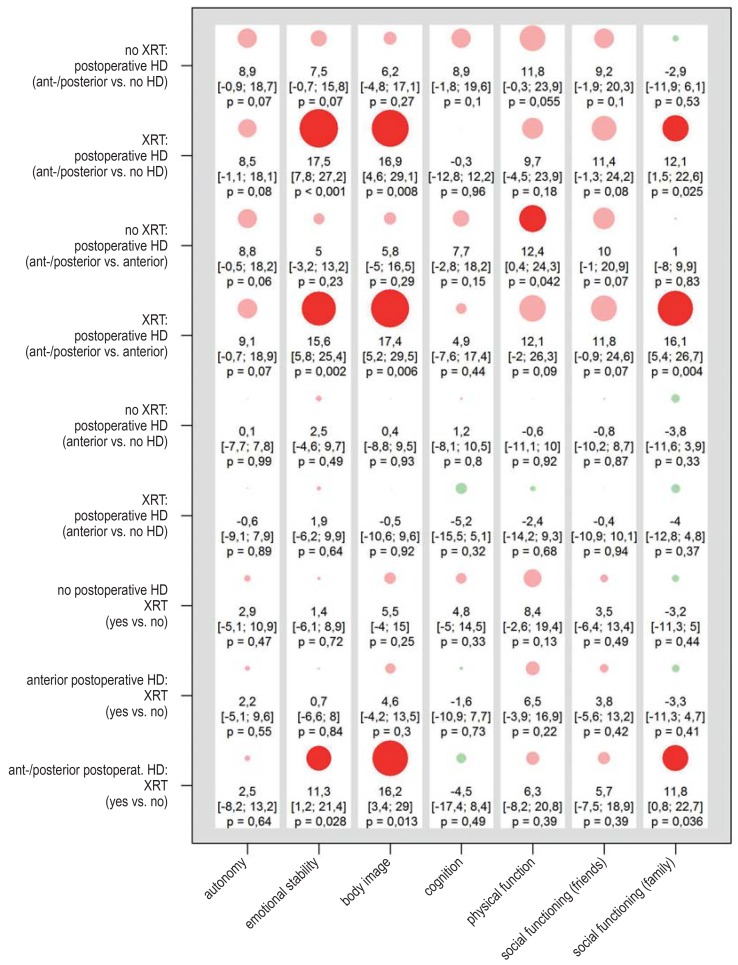
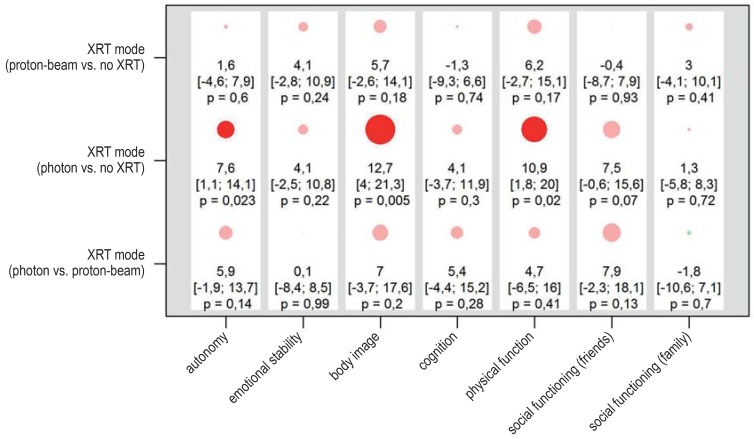
Among the 94 patients whose tumors were incompletely resected, those who underwent radiotherapy had a marginally lower self-assessed quality of life than those who did not, with statistically noticeable differences in two PEDQOL subscores—body image and physical function (figure 3). Radiotherapy was performed after progression in most cases (27/41). The analysis was repeated with simultaneous consideration of possible hypothalamic damage and adjustment for age. The deleterious effect of anterior and posterior hypothalamic damage was confirmed and was found to be even stronger in patients who had also received radiotherapy (efigure 6). Quality of life as a function of the radiotherapeutic modality (efigure 7) was also investigated. A statistically noticeable worsening of the quality of life in the domains of autonomy, body image, and physical function was found after photon radiotherapy, but a direct comparison of photon radiotherapy versus proton-beam radiotherapy revealed no statistically noticeable difference. The degree of hypothalamic damage could not be considered in this analysis because of the small number of cases. The patient cohorts who underwent photon radiotherapy and proton-beam radiotherapy were compared in descriptive fashion with respect to their main features. There were no relevant differences other than the year of diagnosis: the patients who underwent proton-beam radiotherapy received their diagnosis two years earlier (comparison of medians, p = 0.047). Despite this, an analysis of the effect of radiotherapeutic modality adjusted for year of diagnosis or age at the time of diagnosis (not shown), confirmed the results of the unadjusted analyses.
eFigure 6.
Results of the linear mixed models for the self-assessed quality of life for patients who underwent incomplete resection (n = 94). The PEDQOL score was modeled separately in each domain as a function of time, the factor in question, and the interaction between time and the factor in question. The results are displayed as differences of least-square estimates, with the associated 95% confidence intervals and p-values, describing the mean difference in PEDQOL scores between the two categories of each factor. The size of the circles is proportional to the mean difference; circles representing positive and negative effects on the quality of life are colored green and red, respectively (opaque when p = 0.05). A box corresponds to the results of a model. The higher the PEDQOL score, the lower the subjective quality of life; a minus sign thus indicates a beneficial influence on the quality of life. HD, hypothalamic damage; HI, hypothalamic involvement, PEDQOL, Pediatric Quality of Life Questionnaire; XRT, radiotherapy
eFigure 7.
Results of the linear mixed models for the self-assessed quality of life for patients who underwent incomplete resection (n = 94). The PEDQOL score was modeled separately in each domain as a function of time, the factor in question, and the interaction between time and the factor in question. The results are displayed as differences of least-square estimates, with the associated 95% confidence intervals and p-values, describing the mean difference in PEDQOL scores between the two categories of each factor. The size of the circles is proportional to the mean difference; circles representing positive and negative effects on the quality of life are colored green and red, respectively (opaque when p = 0.05). A box corresponds to the results of a model. The higher the PEDQOL score, the lower the subjective quality of life; a minus sign thus indicates a beneficial influence on the quality of life. PEDQOL, Pediatric Quality of Life Questionnaire; XRT, radiotherapy
Discussion
Treatment strategies for craniopharyngioma in childhood and adolescence are a matter of controversy (8, 9). Radical neurosurgical procedures with the goal of complete resection of the tumor often have serious long-term adverse effects because of operative damage to the hypothalamus (1, 10, 11). One complication, in particular, that markedly impairs the quality of life of the surviving patients is severe obesity as part of a hypothalamic syndrome (12, 13). More conservative surgical strategies intended to preserve the integrity of hypothalamic structures are often associated with the need for further surgery or for postoperative radiotherapy after incomplete primary resection of the craniopharyngioma (1, 14).
Radical surgery was the treatment of choice for decades, with tumor control rates of 65–90%, compared to 10–50% after incomplete resection (15– 18). Yet the radical neurosurgical approach, even in expert hands, carries the risk of postoperative damage to hypothalamic-pituitary structures, which can become clinically manifest as endocrine deficits, obesity as part of a hypothalamic syndrome, neurocognitive deficits, and behavioral abnormalities (1). For this reason, more conservative, hypothalamus-sparing surgical strategies with subsequent radiotherapy are now being increasingly used (5, 6, 19– 26). Our randomized trial unfortunately yielded no answer to the question of the ideal timing of radiotherapy after the incomplete resection of a craniopharyngioma. Good tumor control was seen after primary radiotherapy, but the data do not support any conclusion about possible differences between the randomized groups with respect to quality of life. The interim analysis enabled a check of the assumptions under which the study was planned, leading to a cessation of randomization because of the low chance of success. Thus, despite low case numbers and little advance knowledge, the use of an adaptive design permitted a randomized trial to be performed with the option of premature termination of the study or adaptation of case numbers after an interim analysis. The structured, prospective acquisition of data also made it possible to draw conclusions about the quality of life of children and adolescents with craniopharyngioma as a function of various factors, in both the randomized and the observational cohort.
The special prognostic significance of the posterior hypothalamic area is also indicated by experimental studies in animals, which have shown that changes in posteriorly located hypothalamic nuclei (the dorsomedial nucleus, the dorsal hypothalamic nucleus, and the ventromedial nucleus) are primarily responsible for the hypothalamic syndrome (27). Roth et al. (24) confirmed these results in a study of imaging findings in patients with sellar masses of various types and their association with the outcome. In contrast, the preoperative involvement of, or operative damage to, anterior hypothalamic areas has a much less deleterious effect on the postoperative quality of life. In view of these facts and the beneficial effect of restricted surgical strategies (i.e., incomplete tumor resection) on quality of life, hypothalamus-sparing surgery is now recommended as a means of avoiding posterior hypothalamic damage. Moreover, our analyses of the subgroup of craniopharyngioma patients who underwent incomplete tumor resection confirmed the deleterious effect of posterior hypothalamic damage on quality of life. The particular radiotherapeutic modality used (photon vs. proton-beam radiotherapy) was not found to make any detectable difference; in any case, no such difference could have been expected in view of the short follow-up interval.
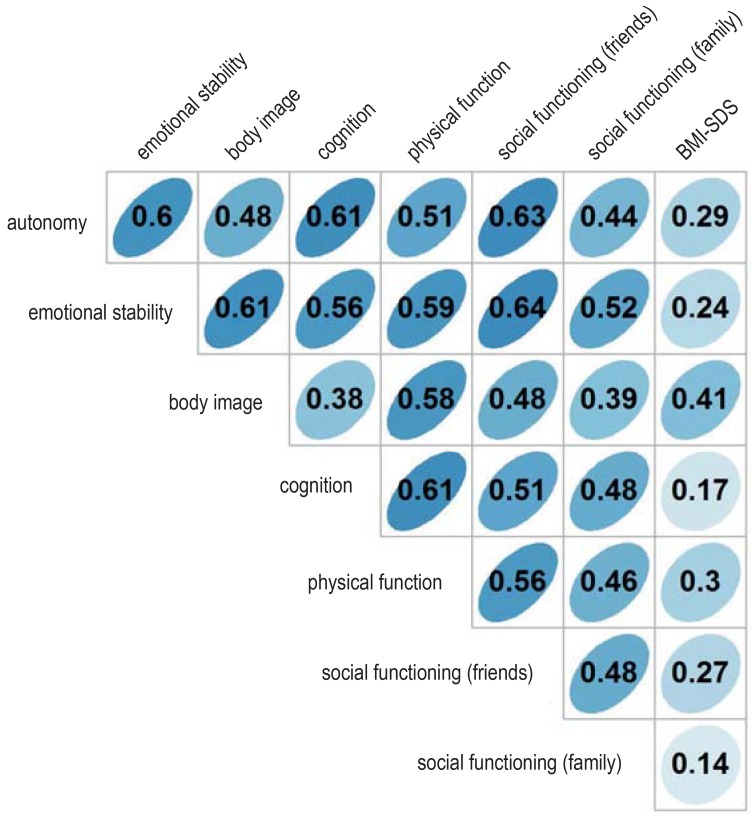
Our study has certain limitations that affect the interpretation of the results. Because of the rarity of the disease, the case numbers were small, despite the near-completeness of the study sample. Because of the short follow-up interval, analyses and conclusions about late sequelae and long-term impairment of patients’ quality of life are not yet possible. Other studies have shown, however, that comorbidites and risk factors for impaired quality of life in craniopharyngioma patients generally exert their lasting prognostic effects early on, i.e., in the first three years after diagnosis (28). A fully reliable assessment of the long-term quality of life will only be possible in future analyses. Moreover, it must be kept in mind that the analyses presented here are based on the patients’ own assessments of their quality of life; a repeated analysis based on information supplied by their parents did, however, yield very similar results (not shown). There was also a very high correlation between self-assessed quality of life in the domains of body image and physical function and the body-mass index (an objectively measurable quantity) (efigure 8); this supports the validity of the results with respect to quality of life.
eFigure 8.
Association between subjectively self-assessed quality of life and body-mass index
Correlation between subjective quality of life (self-assessment by the patient in the seven domains of the PEDQOL) and body-mass index (BMI, kg/m²) for the overall patient cohort (n = 131). The BMI is expressed as a standard deviation score (SDS) in relation to the age- and sex-specific mean BMI (7). The repeated measurements of the PEDQOL and the BMI one and three years after surgery were averaged for each patient (e5). Pearson‘s correlation coefficients are shown.
PEDQOL, the Pediatric Quality of Life Questionnaire
Particularly with respect to the posterior hypothalamic region, our findings support the recommendation for a hypothalamus-sparing neurosurgical approach. In case of progression of the residual tumor after incomplete resection, conservative treatment is recommended, e.g., radiotherapy, in order to achieve effective tumor control while avoiding further surgery, which would carry a high risk of further hypothalamic damage adversely affecting the clinical outcome. Children and adolescents with craniopharyngioma should only be treated by experienced multidisciplinary teams.
Supplementary Material
eMethods
Methods of the randomized trial
Adaptive design
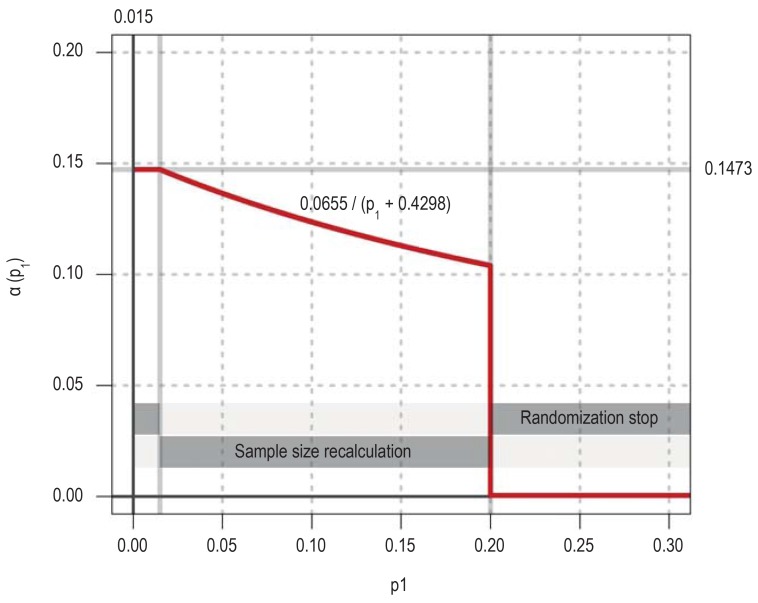
A two-phase adaptive design was used because the trial was expected to run for a long period of time and there could be no certainty about the assumptions made in the planning stage. This type of design has the advantage that it can be adapted after an interim analysis, e.g., by recalculation of the required case numbers, without detracting from the confirmatory character of the trial. The interim analysis was to be carried out when ten patients in each treatment group had reached the primary endpoint. The confirmatory error function (CEF) shown in eFigure 9 was defined in order implement the adaptive design. The CEF shows, depending on the p-value after the first phase, what the maximal value of the p-value after the second phase must be (a[p1]) to enable rejection of the null hypothesis (e1).
The primary endpoint for the randomized trial was the change, from three months to three years after surgery, of the self-assessed quality of life in the physical function domain of the Pediatric Quality of Life Questionnaire (PEDQOL). The null hypothesis (two-tailed) was that this change would be the same in both treatment groups. To test the null hypothesis, two one-tailed p-values were used; these were calculated with the exact Mann–Whitney U test for the alternative hypotheses of either the superiority or the inferiority of immediate postoperative radiotherapy (XRT) compared to the wait-and-watch strategy of XRT only on progression. The trial protocol specified that, in case a randomized patient died in the first three years after randomization, the primary endpoint would be replaced by the value 2000 minus the number of days from randomization to death of any cause. This was to ensure that, in case any patient died, the change in quality of life would be replaced by a value greater than any possible change in PEDQOL (maximum possible change, 75 points), with the change score inversely related to the duration of survival after randomization. Any patients who died would have been taken into account in this manner in the calculation of the p-value for the primary endpoint with the Mann–Whitney U test. In fact, none of the randomized patients died, so this method did not need to be applied.
Randomization was to be terminated in case the interim analysis revealed a large difference between the two groups (smaller one-sided p-value = 0.015). Whenever this happened, however, there would have been another subset of patients who had been recruited by the time of the interim analysis, but had not yet had the full intended three-year follow-up for the primary endpoint. The incorporation of data from these so-called interim patients is desirable from the ethical viewpoint and would also increase the efficiency of the trial (e2). Therefore, for such a case, the adaptive design provides that the analysis of the primary endpoint should be repeated with the interim patients one year after the conclusion of the interim analysis. The null hypothesis can then be rejected only if the one-tailed p-value in this analysis is less than or equal to 0.1473.
In case of a moderate effect, i.e., in case the lower of the two one-tailed p-values lies in the range 0.015 < p1= 0.2, randomization is supposed to be continued. This approach enables design modifications, e.g., data-driven recalculation of required case numbers for the independent second phase.
Randomization should be terminated if both of the one-tailed p-values for the first phase are greater than 0.2, because preliminary power analyses showed that, in this case, no acceptable prolongation of the recruitment period would yield an adequate probability of demonstrating a difference between the two treatment groups (stop for futility).
Sample size calculation
On the basis of preliminary data, there was an estimated 25% probability that the difference in the self-assessed quality of life score with respect to physical function would be smaller (i.e., that the quality of life would be better) in the patients who did not receive XRT immediately after surgery, compared to those who did. Proceeding from this assumption and from the adaptive design described above, the probability of obtaining a p-value below the futility threshold of 0.2 (cf. eFigure 9) in an interim analysis of ten patients per treatment group is calculated to be 86.3% (e3).
The case numbers for the second phase of the trial were not fixed in advance. If a moderate effect had been found, the required case number for the second phase was supposed to be determined on the basis of the results from the first phase and any further relevant outside information. The significance level for the analysis of the second phase of the trial would then have been the error level given by the CEF, i.e., a(p1).
Randomization
Quality of life was assessed three months after surgery in all patients who met the inclusion criteria for the randomized trial and agreed to participate in it. Depending on the PEDQOL physical function subscore three months after surgery, two strata were defined (score = 56 points and >56 points), within each of which the patients were randomly allotted in a 1:1 ratio to the two treatment arms—XRT immediately after surgery and XRT only on progression. The outcome of randomization was communicated to the trial sites by telephone from the trial headquarters in Oldenburg. For this purpose, the trial headquarters made use of randomization lists generated by the biostatistical institute. The randomized trial was not blinded.
Results of the interim analysis of the randomized trial
It was estimated, on the basis of data from the earlier KRANIOPHARYNGEOM 2000 study, that a sufficient number of patients for the interim analysis would be recruited in 2.5 years, and thus that the interim analysis would be feasible 5.5 years after the start of recruitment, which was in 2007. In fact, however, the potential participants were, in general, much less willing to consent to randomization than had been expected, with the result that the interim analysis could not be carried out until 2016. From 2007 to 2016, 38 patients were randomized (efigure 1). 27 patients had completed three years of follow-up by the time of the interim analysis. Three of these 27 patients were excluded from the intention-to-treat analysis, one because of an error in diagnosis and two because of missing data with respect to the primary endpoint. Protocol deviations were detected in four of the remaining 24 patients. In view of the low case numbers, the data monitoring committee decided to raise the number of patients for the first phase from 20 to 24, with the result that the cohort to be evaluated consisted of 20 patients who had received the treatment allotted to them by randomization.
The mean change (± standard deviation) in the PEDQOL physical function subscore of patients who received XRT immediately after surgery was +2.08 ± 16.58 points, reflecting a mild worsening of quality of life (eFigure 10a). The corresponding change in patients who received XRT only on progression was -0.89 ± 15.48. The one-tailed p-values in the exact Mann–Whitney U tests for the comparison of self-assessed quality of life relating to physical function were 0.3153 and 0.6933. Randomization was, therefore, stopped for futility, according to the advance provisions of the adaptive trial design.
A sensitivity analysis was carried out in which the patients were analyzed according to the treatment they had actually received (i.e., an as-treated, rather than intention-to-treat, analysis). Two patients were excluded from this analysis because their tumor resections were judged retrospectively to have been complete, with the consequence that they received no XRT (efigure 1). In a further patient, a wait-and-watch strategy for XRT was adopted despite randomization to immediate XRT. One patient who was randomized to the XRT-on-progression group was found to have progressive disease immediately after randomization. Data from this patient were entered into the analysis in the category to which he had been randomized, even though the patient, in fact, received XRT immediately after randomization, because of early progression. The as-treated analysis comparing the two treatment groups revealed a slightly greater mean worsening of quality of life after immediate XRT compared to XRT on progression (4.76 ± 12.6 versus 0.83 ± 16.34 points) (eFigure 10b). Even with this difference, however, the one-tailed p-values—0.3075 and 0.7049—would have justified a stop for futility. Further intention-to-treat and as-treated analyses, employing linear mixed models, with the inclusion of two patients who had missing PEDQOL values over the course of the trial (efigure 1) likewise led to the same result.
Secondary endpoints of the randomized trial: progression-free survival and overall survival
Progression is defined as neuroradiologically confirmed progression of residual tumor by more than 20%, or else a tumor recurrence confirmed by a reference neuroradiologist, after a complete resection likewise confirmed by a reference neuroradiologist. The analyses reported in what follows are all according to the as-treated principle. 35 of the 38 randomized patients were included—a higher number than in the analysis of the primary endpoint, because patients were included in the analysis of progression-free survival (PFS) whether or not they had completed the three years of follow-up required for the primary endpoint. PFS was defined as the time from randomization to a first event. Patients without progression were included in the evaluation as right-censored patients. One patient was excluded because of a wrong diagnosis, and two further patients were excluded who received no XRT because their tumors had been completely resected.
The two treatment groups differed noticeably with respect to PFS. The patients who received XRT only on progression had a shorter PFS (p = 0.0013 by the log-rank test, eFigure 2). The median PFS in this group was 0.72 years, with 13 progression events recorded; in contrast, among the patients who received XRT immediately after surgery, only one experienced progression. This difference had been anticipated in the planning of the trial, which was designed to test whether the disadvantage of the XRT-on-progression strategy with respect to PFS might be compensated by a better quality of life. The dilemma can be stated as follows: although immediate postoperative XRT may prevent progression and thereby obviate the need for further surgery, patients without progression might be unnecessarily exposed to the adverse sequelae of XRT if they were given XRT right after the primary resection. On the other hand, watching and waiting for progression may worsen the patient’s prognosis after XRT, because XRT will only be performed in the face of progression, rather than preventively.
None of the randomized patients have died to date, and there is thus no evidence yet of any difference in overall survival. Only one death among all patients in the study had been documented by the time of the analysis.
Exploratory analyses of the PEDQOL in the overall cohort
Quality of life (QoL) was assessed with the PEDQOL (2001 version), a scoring instrument enabling subjective assessment (or self-assessment) by the affected children and their parents. The PEDQOL yields subscores in seven different domains: autonomy, emotional stability, body image, physical function, social functioning with respect to friends, and social functioning in the family. In each domain, there are 4 to 6 questions on the patient’s competencies or feelings relating to that domain, with a possible score of 25, 50, 75, or 100 points on each question. These scores are averaged to yield a score in each domain ranging from 25 to 100 points. Lower scores correspond to a higher QoL, higher scores to a lower QoL. A slightly different version of the questionnaire is used for children under 7.
The PEDQOL scores one and three years after surgery were analyzed. The number of completed questionnaires and the distribution of scores in the different domains are shown in eFigure 3. The number of cases was lower for the autonomy domain, because questions about autonomy were only asked of children aged 7 and up.
Statistical methods for the exploratory analysis of the PEDQOL
In exploratory analyses, the effects of demographic features (age, sex), treatment (extent of resection, XRT), preoperative hypothalamic involvement, and postoperative hypothalamic damage on self-assessed QoL were studied on the basis of data from the overall patient cohort. Differences in self-assessed QoL between the categories of each of the factors assessed were estimated with linear mixed models. The PEDQOL score was modeled separately in each domain as a function of the time of data acquisition, the influencing factor(s) in question, and the interaction between the time of data acquisition and the influencing factor or factors. The advantage of this procedure was that it enabled patients to be included in the analysis whose PEDQOL scores were missing from one of the two timepoints. It is known that mixed models still yield valid results in the presence of missing data of the type “missing completely at random” or “missing at random” (e4). In order to take account of the correlation between PEDQOL scores in each individual patient at differing time points, the patient was considered in these models as a random effect. The findings are displayed as differences resulting from least-square estimates, with corresponding 95% confidence intervals (CI), representing the average difference in PEDQOL scores between two categories of each factor over all timepoints of data acquisition.
Change in subjectively assessed quality of life over time
The children’s self-assessed quality of life worsened over time in nearly all domains; this deterioration was statistically noticeable in two domains, autonomy (3.52; 95% confidence interval [0.69; 6.35], p = 0.016) and emotional stability (5.58 [2.35; 8,80], p = 0.001). In the remaining domains, no noticeable difference was seen between PEDQOL scores at one and three years. No statistically noticeable difference over time was seen in any domain in the parents’ assessment of their children’s quality of life.
Self-assessment versus parental assessment
In five of seven domains, the parents considered their children’s quality of life to be worse than the children themselves did (efigure 3). This difference was statistically noticeable at both timepoints in five domains: emotional stability (6.57 [4.71; 8.42], p <0.0001), body image (4.85 [2.90; 6.79], p <0.0001), physical function (4.65 [2.35; 6.95], p <0.0001), social functioning with respect to friends (5.24 [3.15; 7.34], p <0.0001), and social functioning in the family (3.71 [1.56; 5.86], p = 0.0008). No noticeable differences were found in the other two domains, autonomy (-0.48 [-2.27; 1.31]) and cognition (1.91 [-0.21; 4.03], p = 0.08).
The effect of age and sex
Children who were aged 7 or older at the time of diagnosis of a craniopharyngioma were found to have a lower self-assessed quality of life than younger children, in various QoL domains, at one and three years after surgery (efigure 4). To simplify the presentation of the findings, the patients were compared across the age classes that were differentiated in the administration of the PEDQOL; yet, even when the age of the patient is considered as a continuous independent variable, the same finding is obtained, i.e., that self-assessed quality of life is worse with increasing age on diagnosis. Older children rate their quality of life noticeably worse than younger children in the domains emotional stability, body image, physical function, and social functioning in the family. For example, the PEDQOL body image subscore of older patients was an average of 16.8 points higher ([10.7; 22.8], p <0.001) than that of younger patients. Only slight differences in self-assessed QoL were seen in male vs. female patients; male patients had a somewhat more favorable self-assessment only in the domain of physical function.
BOX. Craniopharyngioma.
Craniopharyngioma is a tumor of the sellar region with low histological malignancy, resulting from an anomaly of embryonic development. Its incidence is 0.5 to 2 cases per million persons per year, with peaks in childhood/adolescence (8 years) and in adulthood (40–50 years). Craniopharyngioma in children is usually of the adamantinous type, with cyst formation (2, 3). Approximately 40 children and adolescents receive a diagnosis of craniopharyngioma in Germany each year. The diagnosis is often late because of the nonspecific manifestations, including intracranial hypertension (headache, nausea/vomiting), visual disturbances, growth disturbances, and diabetes insipidus. The prognosis of craniopharyngioma is generally poor without treatment (surgery, radiotherapy) because of progression of the disease. The overall survival 10 years after surgery and/or radiotherapy is over 90%, but many patients suffer from serious long-term sequelae that markedly impair their quality of life because of either preoperative involvement of the hypothalamus by the tumor or operative hypothalamic damage. These include:
Endocrine deficits (diabetes insipidus, growth-hormone deficiency)
Obesity and eating disorders
Neuropsychological deficits (emotional lability, deficient impulse control, cognitive impairment)
Fatigue and disturbances of the circadian rhythm
Visual disturbances.
Key Messages.
Craniopharyngiomas are tumors that arise from an anomaly of embryonic development. They are adjacent to important structures in the brain that can be damaged both by the tumor and by its treatment, with ensuing deficits that impair the affected patients’ quality of life.
Preoperative involvement and operative damage of posterior hypothalamic areas impair the quality of life.
A hypothalamus-sparing neurosurgical strategy is recommended for primary resection in order to avoid posterior hypothalamic damage.
Children and adolescents with craniopharyngioma should be treated by experienced multidisciplinary teams.
An adaptive study design enables the performance of confirmatory studies even in small patient cohorts, with the opportunity for interim checking of the assumptions on which the study was planned.
eFigure 5.
Results of the linear mixed models for the self-assessed quality of life in the overall patient cohort (n = 131); alternative representation of Figure 3. The PEDQOL score was modeled separately in each domain as a function of time, the factor in question, and the interaction between time and the factor in question. The results are displayed as differences of least-square estimates, with the associated 95% confidence intervals and p-values, describing the mean difference in PEDQOL scores between the two categories of each factor. The size of the circles is proportional to the mean difference; circles representing positive and negative effects on the quality of life are colored green and red, respectively (opaque when p = 0.05). A box corresponds to the results of a model. The higher the PEDQOL score, the lower the subjective quality of life; a minus sign thus indicates a beneficial influence on the quality of life.
HD, hypothalamic damage; HI, hypothalamic involvement, PEDQOL, Pediatric Quality of Life Questionnaire; Res., resection;
XRT, radiotherapy
eFigure 9.
Conditional error function for the randomized trial component of the KRANIOPHARYNGEOM 2007 project
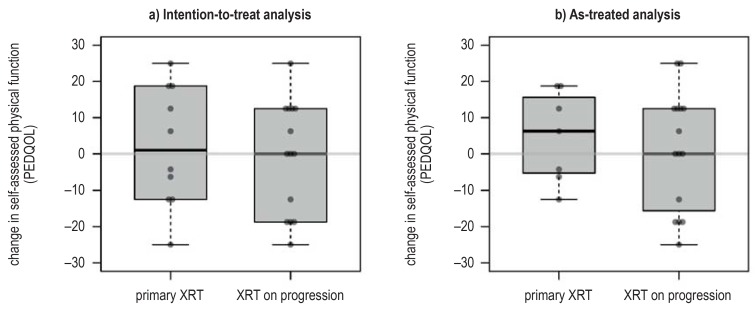
eFigure 10.
Results of the interim assessment of the primary endpoint of the randomized trial. A negative difference indicates improvement of the quality of life, and a positive difference indicates worsening.
a) Intention-to-treat analysis: the two-tailed p-value by the Mann–Whitney U test is p = 0.63.
b) As-treated analysis: the two-tailed p-value by the Mann–Whitney U test is p = 0.61.
PEDQOL, Pediatric Quality of Life questionnaire; XRT, radiotherapy.
eTable 3. The association of preoperative hypothalamic involvement, extent of surgical resection, and postoperative hypothalamic damage: comparison of extent of resection and postoperative hypothalamic damage*.
|
Extent of resection |
Postoperative hypothalamic damage | Total | |||
| None |
Anterior (first-degree) |
Anterior and posterior (second-degree) |
|||
| Incomplete | Number | 35 | 34 | 25 | 94 |
| Row percent | 37% | 36% | 27% | 100% | |
| Column percent | 95% | 92% | 61% | 82% | |
| Complete | Number | 2 | 3 | 16 | 21 |
| Row percent | 10% | 14% | 76% | 100% | |
| Column percent | 5% | 8% | 39% | 18% | |
| Total | Number | 37 | 37 | 41 | 115 |
| Row percent | 32% | 32% | 36% | 100% | |
| Column percent | 100% | 100% | 100% | 100% | |
* p = 0.0001 by Fisher’s exact test
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Funding
KRANIOPHARYNGEOM 2007 is supported financially by the German Childhood Cancer Foundation (Deutsche Kinderkrebsstiftung), Bonn, Germany.
Data sharing
The data cannot yet be made available because the study has not yet been concluded. Moreover, the small number of cases would likely make it difficult to preserve the full anonymity of the data.
Footnotes
Conflict of interest statement
Prof. Müller has received reimbursement of participation fees for scientific meetings and continuing medical education events from the following companies: Ferring, Lilly, Pfizer, Sandoz/Hexal, Novo Nordisk, Ipsen, and Merck Serono. He has received reimbursement of travel expenses from Ipsen and lecture honoraria from Pfizer.
The other authors state that they have no conflict of interest.
References
- 1.Müller HL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab. 2011;96:1981–1991. doi: 10.1210/jc.2011-0174. [DOI] [PubMed] [Google Scholar]
- 2.Müller HL, Merchant TE, Puget S, Martinez-Barbera JP. New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol. 2017;13:299–312. doi: 10.1038/nrendo.2016.217. [DOI] [PubMed] [Google Scholar]
- 3.Müller HL, Sörensen N. Kraniopharyngeom im Kindes- und Jugendalter-Perspektiven in Diagnostik, Therapie und Nachsorge durch interdisziplinäre und multizentrische Kooperation. Dtsch Arztebl. 2006;103:2634–2640. [Google Scholar]
- 4.Calaminus G, Weinspach S, Teske C, Gobel U. Quality of life in children and adolescents with cancer First results of an evaluation of 49 patients with the PEDQOL questionnaire. Klin Padiatr. 2000;212:211–215. doi: 10.1055/s-2000-9679. [DOI] [PubMed] [Google Scholar]
- 5.Müller HL, Gebhardt U, Teske C, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol. 2011;165:17–24. doi: 10.1530/EJE-11-0158. [DOI] [PubMed] [Google Scholar]
- 6.Müller HL, Gebhardt U, Faldum A, et al. Xanthogranuloma, Rathke’s cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J Clin Endocrinol Metab. 2012;97:3935–3943. doi: 10.1210/jc.2012-2069. [DOI] [PubMed] [Google Scholar]
- 7.Rolland-Cachera MF, Cole TJ, Sempé M, Tichet J, Rossignol C, Charraud A. Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr. 1991;43:13–21. [PubMed] [Google Scholar]
- 8.Müller HL. More or less? Treatment strategies in childhood craniopharyngioma. Childs Nerv Syst. 2006;22:156–157. doi: 10.1007/s00381-005-1192-7. [DOI] [PubMed] [Google Scholar]
- 9.Sainte-Rose C, Puget S, Wray A, et al. Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst. 2005;21:691–695. doi: 10.1007/s00381-005-1209-2. [DOI] [PubMed] [Google Scholar]
- 10.de Vile CJ GD, Hayward RD, Kendall BE, Neville BG, Stanhope R. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J Clin Endocrinol Metab. 1996;81:2734–2737. doi: 10.1210/jcem.81.7.8675604. [DOI] [PubMed] [Google Scholar]
- 11.Ozyurt J, Müller HL, Thiel CM. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J Neurooncol. 2015;125:9–21. doi: 10.1007/s11060-015-1885-z. [DOI] [PubMed] [Google Scholar]
- 12.Müller HL. Paediatrics: surgical strategy and quality of life in craniopharyngioma. Nat Rev Endocrinol. 2013;9:447–449. doi: 10.1038/nrendo.2013.125. [DOI] [PubMed] [Google Scholar]
- 13.Müller HL, Bruhnken G, Emser A, et al. Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv Syst. 2005;21:975–980. doi: 10.1007/s00381-004-1124-y. [DOI] [PubMed] [Google Scholar]
- 14.Kortmann RD. Different approaches in radiation therapy of craniopharyngioma. Front Endocrinol (Lausanne) 2011;2 doi: 10.3389/fendo.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajan B, Ashley S, Gorman C, et al. Craniopharyngioma—long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26:1–10. doi: 10.1016/0167-8140(93)90019-5. [DOI] [PubMed] [Google Scholar]
- 16.Fahlbusch R, Honegger J, Paulus W, Huk W, Buchfelder M. Surgical treatment of craniopharyngioma: experience with 168 patients. J Neurosurg. 1999;90:237–250. doi: 10.3171/jns.1999.90.2.0237. [DOI] [PubMed] [Google Scholar]
- 17.Stripp DC, Maity A, Janss AJ, et al. Surgery with or without radiation therapy in the management of craniopharyngioma in children and young adults. Int J Rad Oncol Biol Phys. 2004;58:714–720. doi: 10.1016/S0360-3016(03)01570-0. [DOI] [PubMed] [Google Scholar]
- 18.Steno J, Bizik I, Steno A, Matejcik V. Craniopharyngiomas in children: how radical should the surgeon be? Childs Nerv Syst. 2011;27:41–54. doi: 10.1007/s00381-010-1330-8. [DOI] [PubMed] [Google Scholar]
- 19.Elowe-Gruau E, Beltrand J, Brauner R, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98:2376–2382. doi: 10.1210/jc.2012-3928. [DOI] [PubMed] [Google Scholar]
- 20.Fjalldal S, Holmer H, Rylander L, et al. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab. 2013;98:3253–3262. doi: 10.1210/jc.2013-2000. [DOI] [PubMed] [Google Scholar]
- 21.van Gompel JJ, Nippoldt TB, Higgins DM, Meyer FB. Magnetic resonance imaging-graded hypothalamic compression in surgically treated adult craniopharyngioma determining postoperative obesity. Neurosurg Focus. 2010;28 doi: 10.3171/2010.1.FOCUS09303. [DOI] [PubMed] [Google Scholar]
- 22.Mallucci C, Pizer B, Blair J, et al. Management of craniopharyngioma: the Liverpool experience following the introduction of the CCLG guidelines Introducing a new risk assessment grading system. Childs Nerv Syst. 2012;28:1181–1192. doi: 10.1007/s00381-012-1787-8. [DOI] [PubMed] [Google Scholar]
- 23.Garre ML, Cama A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr Opin Pediatr. 2007;19:471–479. doi: 10.1097/MOP.0b013e3282495a22. [DOI] [PubMed] [Google Scholar]
- 24.Roth CL, Eslamy H, Werny D, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring) 2015;23:1226–1233. doi: 10.1002/oby.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puget S, Garnett M, Wray A, et al. Pediatric craniopharyngioma: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106:3–12. doi: 10.3171/ped.2007.106.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Flitsch J, Müller HL, Burkhardt T. Surgical strategies in childhood craniopharyngioma. Front Endocrinol (Lausanne) 2011;2 doi: 10.3389/fendo.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bereket A, Kiess W, Lustig RH, et al. Hypothalamic obesity in children. Obes Rev. 2012;13:780–798. doi: 10.1111/j.1467-789X.2012.01004.x. [DOI] [PubMed] [Google Scholar]
- 28.Müller HL, Bueb K, Bartels U, et al. Obesity after childhood craniopharyngioma–German multicenter study on pre-operative risk factors and quality of life. Klin Padiatr. 2001;213:244–249. doi: 10.1055/s-2001-16855. [DOI] [PubMed] [Google Scholar]
- E1.Proschan MA, Hunsberger SA. Designed extension of studies based on conditional power. Biometrics. 1995;51:1315–1324. [PubMed] [Google Scholar]
- E2.Faldum A, Hommel G. Strategies for including patients recruited during interim analysis of clinical trials. J Biopharm Stat. 2007;17:1211–1225. doi: 10.1080/10543400701645439. [DOI] [PubMed] [Google Scholar]
- E3.Noether GE. Sample size determination for some common nonparametric tests. J Am Stat Assoc. 1987;82:645–647. [Google Scholar]
- E4.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer; 2000 [Google Scholar]
- E5.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 2 - correlation between subjects. BMJ. 1995;310 doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Methods of the randomized trial
Adaptive design
A two-phase adaptive design was used because the trial was expected to run for a long period of time and there could be no certainty about the assumptions made in the planning stage. This type of design has the advantage that it can be adapted after an interim analysis, e.g., by recalculation of the required case numbers, without detracting from the confirmatory character of the trial. The interim analysis was to be carried out when ten patients in each treatment group had reached the primary endpoint. The confirmatory error function (CEF) shown in eFigure 9 was defined in order implement the adaptive design. The CEF shows, depending on the p-value after the first phase, what the maximal value of the p-value after the second phase must be (a[p1]) to enable rejection of the null hypothesis (e1).
The primary endpoint for the randomized trial was the change, from three months to three years after surgery, of the self-assessed quality of life in the physical function domain of the Pediatric Quality of Life Questionnaire (PEDQOL). The null hypothesis (two-tailed) was that this change would be the same in both treatment groups. To test the null hypothesis, two one-tailed p-values were used; these were calculated with the exact Mann–Whitney U test for the alternative hypotheses of either the superiority or the inferiority of immediate postoperative radiotherapy (XRT) compared to the wait-and-watch strategy of XRT only on progression. The trial protocol specified that, in case a randomized patient died in the first three years after randomization, the primary endpoint would be replaced by the value 2000 minus the number of days from randomization to death of any cause. This was to ensure that, in case any patient died, the change in quality of life would be replaced by a value greater than any possible change in PEDQOL (maximum possible change, 75 points), with the change score inversely related to the duration of survival after randomization. Any patients who died would have been taken into account in this manner in the calculation of the p-value for the primary endpoint with the Mann–Whitney U test. In fact, none of the randomized patients died, so this method did not need to be applied.
Randomization was to be terminated in case the interim analysis revealed a large difference between the two groups (smaller one-sided p-value = 0.015). Whenever this happened, however, there would have been another subset of patients who had been recruited by the time of the interim analysis, but had not yet had the full intended three-year follow-up for the primary endpoint. The incorporation of data from these so-called interim patients is desirable from the ethical viewpoint and would also increase the efficiency of the trial (e2). Therefore, for such a case, the adaptive design provides that the analysis of the primary endpoint should be repeated with the interim patients one year after the conclusion of the interim analysis. The null hypothesis can then be rejected only if the one-tailed p-value in this analysis is less than or equal to 0.1473.
In case of a moderate effect, i.e., in case the lower of the two one-tailed p-values lies in the range 0.015 < p1= 0.2, randomization is supposed to be continued. This approach enables design modifications, e.g., data-driven recalculation of required case numbers for the independent second phase.
Randomization should be terminated if both of the one-tailed p-values for the first phase are greater than 0.2, because preliminary power analyses showed that, in this case, no acceptable prolongation of the recruitment period would yield an adequate probability of demonstrating a difference between the two treatment groups (stop for futility).
Sample size calculation
On the basis of preliminary data, there was an estimated 25% probability that the difference in the self-assessed quality of life score with respect to physical function would be smaller (i.e., that the quality of life would be better) in the patients who did not receive XRT immediately after surgery, compared to those who did. Proceeding from this assumption and from the adaptive design described above, the probability of obtaining a p-value below the futility threshold of 0.2 (cf. eFigure 9) in an interim analysis of ten patients per treatment group is calculated to be 86.3% (e3).
The case numbers for the second phase of the trial were not fixed in advance. If a moderate effect had been found, the required case number for the second phase was supposed to be determined on the basis of the results from the first phase and any further relevant outside information. The significance level for the analysis of the second phase of the trial would then have been the error level given by the CEF, i.e., a(p1).
Randomization
Quality of life was assessed three months after surgery in all patients who met the inclusion criteria for the randomized trial and agreed to participate in it. Depending on the PEDQOL physical function subscore three months after surgery, two strata were defined (score = 56 points and >56 points), within each of which the patients were randomly allotted in a 1:1 ratio to the two treatment arms—XRT immediately after surgery and XRT only on progression. The outcome of randomization was communicated to the trial sites by telephone from the trial headquarters in Oldenburg. For this purpose, the trial headquarters made use of randomization lists generated by the biostatistical institute. The randomized trial was not blinded.
Results of the interim analysis of the randomized trial
It was estimated, on the basis of data from the earlier KRANIOPHARYNGEOM 2000 study, that a sufficient number of patients for the interim analysis would be recruited in 2.5 years, and thus that the interim analysis would be feasible 5.5 years after the start of recruitment, which was in 2007. In fact, however, the potential participants were, in general, much less willing to consent to randomization than had been expected, with the result that the interim analysis could not be carried out until 2016. From 2007 to 2016, 38 patients were randomized (efigure 1). 27 patients had completed three years of follow-up by the time of the interim analysis. Three of these 27 patients were excluded from the intention-to-treat analysis, one because of an error in diagnosis and two because of missing data with respect to the primary endpoint. Protocol deviations were detected in four of the remaining 24 patients. In view of the low case numbers, the data monitoring committee decided to raise the number of patients for the first phase from 20 to 24, with the result that the cohort to be evaluated consisted of 20 patients who had received the treatment allotted to them by randomization.
The mean change (± standard deviation) in the PEDQOL physical function subscore of patients who received XRT immediately after surgery was +2.08 ± 16.58 points, reflecting a mild worsening of quality of life (eFigure 10a). The corresponding change in patients who received XRT only on progression was -0.89 ± 15.48. The one-tailed p-values in the exact Mann–Whitney U tests for the comparison of self-assessed quality of life relating to physical function were 0.3153 and 0.6933. Randomization was, therefore, stopped for futility, according to the advance provisions of the adaptive trial design.
A sensitivity analysis was carried out in which the patients were analyzed according to the treatment they had actually received (i.e., an as-treated, rather than intention-to-treat, analysis). Two patients were excluded from this analysis because their tumor resections were judged retrospectively to have been complete, with the consequence that they received no XRT (efigure 1). In a further patient, a wait-and-watch strategy for XRT was adopted despite randomization to immediate XRT. One patient who was randomized to the XRT-on-progression group was found to have progressive disease immediately after randomization. Data from this patient were entered into the analysis in the category to which he had been randomized, even though the patient, in fact, received XRT immediately after randomization, because of early progression. The as-treated analysis comparing the two treatment groups revealed a slightly greater mean worsening of quality of life after immediate XRT compared to XRT on progression (4.76 ± 12.6 versus 0.83 ± 16.34 points) (eFigure 10b). Even with this difference, however, the one-tailed p-values—0.3075 and 0.7049—would have justified a stop for futility. Further intention-to-treat and as-treated analyses, employing linear mixed models, with the inclusion of two patients who had missing PEDQOL values over the course of the trial (efigure 1) likewise led to the same result.
Secondary endpoints of the randomized trial: progression-free survival and overall survival
Progression is defined as neuroradiologically confirmed progression of residual tumor by more than 20%, or else a tumor recurrence confirmed by a reference neuroradiologist, after a complete resection likewise confirmed by a reference neuroradiologist. The analyses reported in what follows are all according to the as-treated principle. 35 of the 38 randomized patients were included—a higher number than in the analysis of the primary endpoint, because patients were included in the analysis of progression-free survival (PFS) whether or not they had completed the three years of follow-up required for the primary endpoint. PFS was defined as the time from randomization to a first event. Patients without progression were included in the evaluation as right-censored patients. One patient was excluded because of a wrong diagnosis, and two further patients were excluded who received no XRT because their tumors had been completely resected.
The two treatment groups differed noticeably with respect to PFS. The patients who received XRT only on progression had a shorter PFS (p = 0.0013 by the log-rank test, eFigure 2). The median PFS in this group was 0.72 years, with 13 progression events recorded; in contrast, among the patients who received XRT immediately after surgery, only one experienced progression. This difference had been anticipated in the planning of the trial, which was designed to test whether the disadvantage of the XRT-on-progression strategy with respect to PFS might be compensated by a better quality of life. The dilemma can be stated as follows: although immediate postoperative XRT may prevent progression and thereby obviate the need for further surgery, patients without progression might be unnecessarily exposed to the adverse sequelae of XRT if they were given XRT right after the primary resection. On the other hand, watching and waiting for progression may worsen the patient’s prognosis after XRT, because XRT will only be performed in the face of progression, rather than preventively.
None of the randomized patients have died to date, and there is thus no evidence yet of any difference in overall survival. Only one death among all patients in the study had been documented by the time of the analysis.
Exploratory analyses of the PEDQOL in the overall cohort
Quality of life (QoL) was assessed with the PEDQOL (2001 version), a scoring instrument enabling subjective assessment (or self-assessment) by the affected children and their parents. The PEDQOL yields subscores in seven different domains: autonomy, emotional stability, body image, physical function, social functioning with respect to friends, and social functioning in the family. In each domain, there are 4 to 6 questions on the patient’s competencies or feelings relating to that domain, with a possible score of 25, 50, 75, or 100 points on each question. These scores are averaged to yield a score in each domain ranging from 25 to 100 points. Lower scores correspond to a higher QoL, higher scores to a lower QoL. A slightly different version of the questionnaire is used for children under 7.
The PEDQOL scores one and three years after surgery were analyzed. The number of completed questionnaires and the distribution of scores in the different domains are shown in eFigure 3. The number of cases was lower for the autonomy domain, because questions about autonomy were only asked of children aged 7 and up.
Statistical methods for the exploratory analysis of the PEDQOL
In exploratory analyses, the effects of demographic features (age, sex), treatment (extent of resection, XRT), preoperative hypothalamic involvement, and postoperative hypothalamic damage on self-assessed QoL were studied on the basis of data from the overall patient cohort. Differences in self-assessed QoL between the categories of each of the factors assessed were estimated with linear mixed models. The PEDQOL score was modeled separately in each domain as a function of the time of data acquisition, the influencing factor(s) in question, and the interaction between the time of data acquisition and the influencing factor or factors. The advantage of this procedure was that it enabled patients to be included in the analysis whose PEDQOL scores were missing from one of the two timepoints. It is known that mixed models still yield valid results in the presence of missing data of the type “missing completely at random” or “missing at random” (e4). In order to take account of the correlation between PEDQOL scores in each individual patient at differing time points, the patient was considered in these models as a random effect. The findings are displayed as differences resulting from least-square estimates, with corresponding 95% confidence intervals (CI), representing the average difference in PEDQOL scores between two categories of each factor over all timepoints of data acquisition.
Change in subjectively assessed quality of life over time
The children’s self-assessed quality of life worsened over time in nearly all domains; this deterioration was statistically noticeable in two domains, autonomy (3.52; 95% confidence interval [0.69; 6.35], p = 0.016) and emotional stability (5.58 [2.35; 8,80], p = 0.001). In the remaining domains, no noticeable difference was seen between PEDQOL scores at one and three years. No statistically noticeable difference over time was seen in any domain in the parents’ assessment of their children’s quality of life.
Self-assessment versus parental assessment
In five of seven domains, the parents considered their children’s quality of life to be worse than the children themselves did (efigure 3). This difference was statistically noticeable at both timepoints in five domains: emotional stability (6.57 [4.71; 8.42], p <0.0001), body image (4.85 [2.90; 6.79], p <0.0001), physical function (4.65 [2.35; 6.95], p <0.0001), social functioning with respect to friends (5.24 [3.15; 7.34], p <0.0001), and social functioning in the family (3.71 [1.56; 5.86], p = 0.0008). No noticeable differences were found in the other two domains, autonomy (-0.48 [-2.27; 1.31]) and cognition (1.91 [-0.21; 4.03], p = 0.08).
The effect of age and sex
Children who were aged 7 or older at the time of diagnosis of a craniopharyngioma were found to have a lower self-assessed quality of life than younger children, in various QoL domains, at one and three years after surgery (efigure 4). To simplify the presentation of the findings, the patients were compared across the age classes that were differentiated in the administration of the PEDQOL; yet, even when the age of the patient is considered as a continuous independent variable, the same finding is obtained, i.e., that self-assessed quality of life is worse with increasing age on diagnosis. Older children rate their quality of life noticeably worse than younger children in the domains emotional stability, body image, physical function, and social functioning in the family. For example, the PEDQOL body image subscore of older patients was an average of 16.8 points higher ([10.7; 22.8], p <0.001) than that of younger patients. Only slight differences in self-assessed QoL were seen in male vs. female patients; male patients had a somewhat more favorable self-assessment only in the domain of physical function.