Abstract
Background
Electronic health records (EHR) from primary care are emerging in Alzheimer’s disease (AD) research, but their accuracy is a concern. We aimed to validate AD diagnoses from primary care using additional information provided by general practitioners (GPs), and a register of dementias.
Patients and methods
This retrospective observational study obtained data from the System for the Development of Research in Primary Care (SIDIAP). Three algorithms combined International Statistical Classification of Diseases (ICD-10) and Anatomical Therapeutic Chemical codes to identify AD cases in SIDIAP. GPs evaluated dementia diagnoses by means of an online survey. We linked data from the Register of Dementias of Girona and from SIDIAP. We estimated the positive predictive value (PPV) and sensitivity and provided results stratified by age, sex and severity.
Results
Using survey data from the GPs, PPV of AD diagnosis was 89.8% (95% CI: 84.7–94.9). Using the dataset linkage, PPV was 74.8 (95% CI: 73.1–76.4) for algorithm A1 (AD diagnoses), and 72.3 (95% CI: 70.7–73.9) for algorithm A3 (diagnosed or treated patients without previous conditions); sensitivity was 71.4 (95% CI: 69.6–73.0) and 83.3 (95% CI: 81.8–84.6) for algorithms A1 (AD diagnoses) and A3, respectively. Stratified results did not differ by age, but PPV and sensitivity estimates decreased amongst men and severe patients, respectively.
Conclusions
PPV estimates differed depending on the gold standard. The development of algorithms integrating diagnoses and treatment of dementia improved the AD case ascertainment. PPV and sensitivity estimates were high and indicated that AD codes recorded in a large primary care database were sufficiently accurate for research purposes.
Keywords: dementia, family physician, survey, algorithm, data accuracy, real-world data, validation, electronic medical records
Introduction
Alzheimer’s disease (AD) entails a heavy burden for patients, their families and public health systems. Its prevalence and economic costs are forecasted to increase dramatically in coming decades due to worldwide population aging.1,2 Electronic health records (EHR) might be useful to update the AD epidemiology, especially in primary care settings. Paradoxically, although general practitioners (GPs) are the gatekeepers of health care services and thus play a pivotal role in the recognition of AD, primary care EHR have scarcely been applied in dementia studies, and even less frequently in AD studies.3,4
A concern about using EHR is the accuracy of diagnoses, as reported in research on dementia diagnoses in primary care,5 but recent studies report high positive predictive value (PPV), indicating that dementia codes in primary care databases were sufficiently accurate for research use.3,6 Nonetheless, literature about the accuracy of EHR in AD is mainly focused on hospital records7–9 and data from primary care is scant.4,10
We hypothesized that EHR from primary care would be a valid tool to study AD, since dementia diagnoses are accurate6 and AD is the most prevalent dementia subtype. The present study sought to validate AD diagnoses recorded in a primary care database by comparing AD from primary care with information from an online survey of GPs and with data from a registry of dementia diagnoses.
Materials and methods
We conducted a retrospective observational study, approved by the Clinical Research Ethics Committee of the Primary Care Research Institute IDIAP Jordi Gol (IDIAPJGol). We followed the STARDS 2015 guidelines11 and the RECORD statement.12
We used data from the Information System for Research in Primary Care (SIDIAP database), which contains anonymized longitudinal medical records related to demographics including deprivation index,13 symptoms, diagnoses, laboratory tests, prescriptions, and pharmacy dispensing from about 6 million patients (>80% of the Catalan population) attended in the primary care settings of the Catalan Health Service.14,15 Accuracy of SIDIAP data have been analyzed for several conditions16–18 including overall dementia,6 but not yet for AD.
Using SIDIAP data, we identified AD cases using algorithms that combined EHR, a method previously applied to identify dementia cases.10,19 We followed Imfeld et al 2013 to define three algorithms that combine information about diagnoses and pharmacological treatment to identify AD cases (Table 1). We considered treated patients as cases because in Catalonia the prescription of anti-dementia drugs can be requested by the GP but requires approval from a geriatrician, a psychiatrist, or a neurologist.
Table 1.
Algorithms to identify Alzheimer's disease (AD) cases in electronic health records from primary care, using codes from the international statistical classification of diseases and related health problems, version 10 (ICD-10) and from the Anatomical Therapeutic Chemical (ATC)
| Algorithm | Definition of AD case |
|---|---|
| A1 | Diagnosed patients: have an ICD10 code for AD (F00 or G30). |
| A2 | Diagnosed or treated patients: have a code for AD (ICD10: F00 or G30) or for prescription or billing of anti-dementia drugs (ATC: N06DA, N06DX01). |
| A3 | Diagnosed or treated patients without previous conditions: have a code for AD (ICD10: F00 or G30) or for prescription or billing of anti-dementia drugs (ATC: N06DA, N06DX01). Treated patients were included if they had no code of dementia diagnosis or had a code of unspecified dementia (F03), and were excluded if they had a code for: a specific subtype of dementia such as Lewy bodies dementia, vascular or frontotemporal dementia (ICD10: F01, F02); Parkinson (ICD10: G20-G22); anti-Parkinson drugs (ATC: N04); or cerebrovascular disease (ICD10:I60- I69, G45, G46) within two years prior to AD diagnosis. |
We followed two approaches. First, we asked the GPs to complete an online survey to provide additional information about dementia diagnoses.6 Our previously published validation study focused on diagnoses of overall dementia recorded in SIDIAP; here, we provide a sub-analysis restricted to AD diagnoses (International Statistical Classification of Diseases, 10th revision (ICD-10): F00, G30). Second, we linked SIDIAP data with a dementia-specific registry for the province of Girona.
Survey sent to GPs
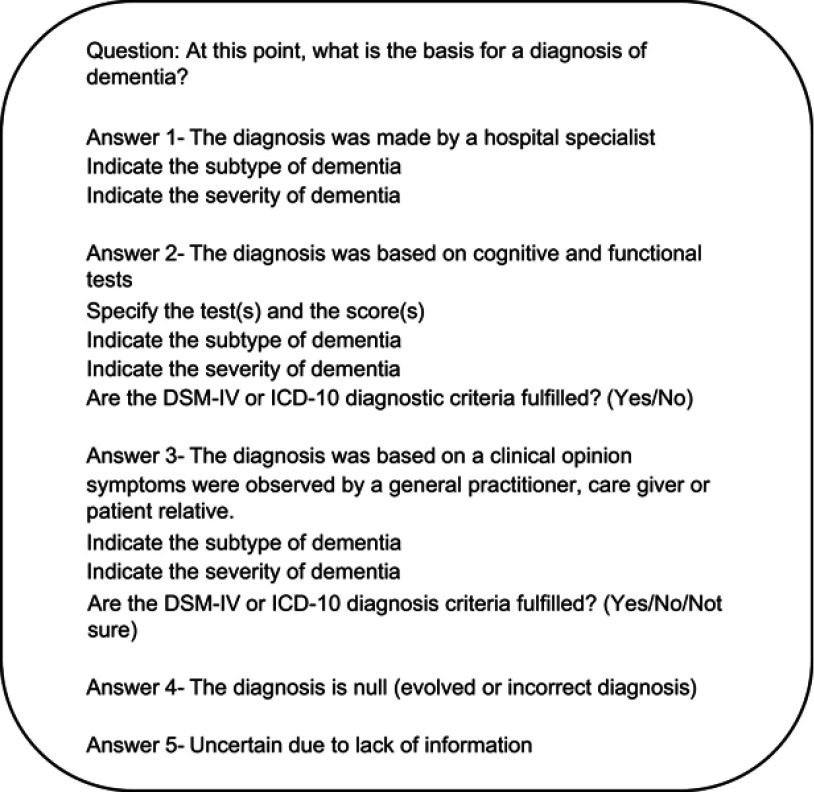
Survey questions, design and administration are described elsewhere.6 Briefly, GPs from the IDIAPJGol Agency of Clinical Research Management in Primary Care (AGICAP) network20 were invited to participate. Those who agreed to participate were sent the online survey in January 2018 and given 1 month to evaluate a consecutive series of their patients with a dementia diagnosis recorded in the EHR.6 The survey asked about the current basis for a dementia diagnosis, the fulfillment of DSM-IV or ICD-10 diagnosis criteria, and the dementia subtype (Figure S1). Patients were defined as AD cases if they were identified as a true dementia case (diagnosed by a specialist; or based on cognitive and functional tests or a clinical opinion) and the GP confirmed the dementia subtype as AD. Such confirmation of dementia subtype was based on the fulfillment of the DSM-IV or the ICD10 diagnostic criteria. The inclusion of both sets of diagnostic criteria aimed to facilitate the identification of true cases, because some physicians might be more familiarized with one of them.
Linkage between SIDIAP and the registry of dementias
The Registry of Dementias of Girona (ReDeGi) contains demographic and clinical data of all the incident cases of dementia diagnosed in the seven hospitals of the public health care system within the province of Girona.21 The ReDeGi uses standardized criteria for case definition, and follows the Center for Disease Control and Prevention guidelines on surveillance systems.22 The ReDeGi includes the Clinical Dementia Rating score,23 a measure of disease severity (mild, moderate and severe). A recent study found high adherence to the clinical practice guidelines among physicians in memory clinics in the ReDeGi catchment area.24
We included patients recorded in ReDeGi between 2007 and 2016, and ascribed to primary care settings of the Catalan Health Service.The linkage was carried out using the national health identifier from the Catalan Health Service, which was available in both databases. We encoded the national health identifier and we used this ciphered identifier in all linkage procedures (Figure 1). Researchers did not have access to the national health identifier or any other kind of identifying data.
Figure 1.

Register of dementias of Girona (ReDeGi) and SIDIAP linkage flow diagram. Data confidentiality measures were applied during information transfer (encoded data in grey arrows; password in dotted grey lines).Abbreviation: SIDIAP, Information System for Research in Primary Care.
In ReDeGi, AD cases were identified according to ICD-10 codes, excluding mixed dementia cases. In SIDIAP, we identified AD cases using the above-mentioned algorithms combining EHR (Table 1).
Statistical analysis
We used percentages to describe categorical variables and mean (SD) for continuous variables. Using data from the survey of GPs, we calculated the PPV including the number of confirmed diagnoses of AD in the numerator, against all the diagnoses of AD evaluated by GP, in the denominator. We replicated the PPV estimate considering AD diagnoses evaluated and not evaluated by GPs, as sensitivity analysis: we included AD diagnoses confirmed by the GPs or with evidence of treatment with anti-dementia drugs in the numerator, and all AD diagnoses (evaluated or not by GPs) in the denominator. Using data from the linkage between SIDIAP and ReDeGi, we estimated the PPV and sensitivity of AD cases. We calculated the PPV considering the number of AD cases identified in both ReDeGi and SIDIAP in the numerator, and the AD cases identified in SIDIAP, in the denominator. Sensitivity was defined as the number of AD cases identified in both ReDeGi and SIDIAP in the numerator, and the AD cases identified in ReDeGi in the denominator. For both PPV and sensitivity, results were stratified by AD definition (Table 1), age, sex and severity. We provided 95% CI assuming a normal distribution for all estimates. The level of significance was defined as p-value=0.05. All analyses were performed using R software v3.5.2.25
Results
Survey sent to GPs
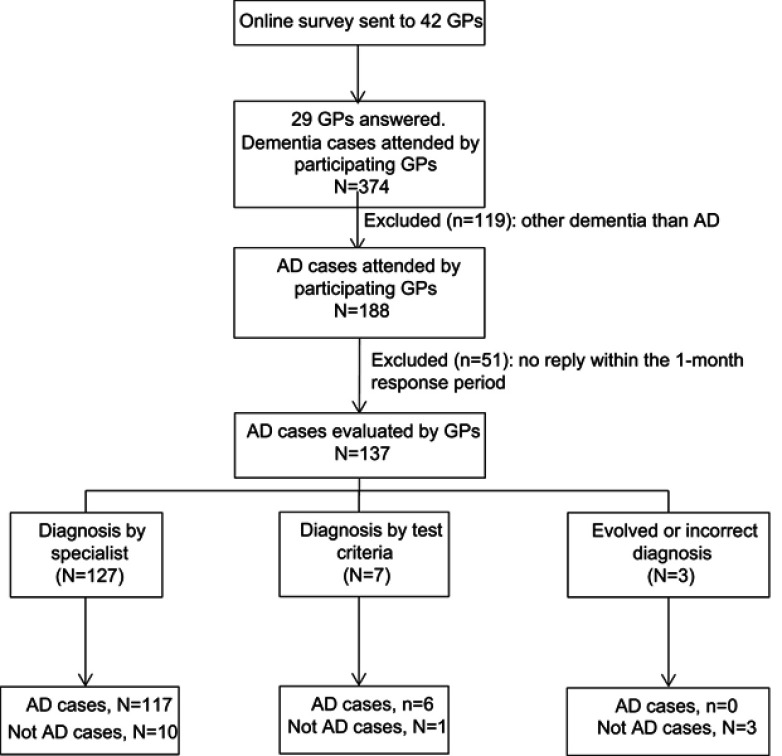
We sent the survey to the 42 GPs who agreed to participate, of which 29 (69%) provided feedback. The quota of patients assigned to these 29 GPs is described in Table S1. Of 188 patients with a diagnosis of AD, 137 were evaluated within the 1-month response period (Figure 2). Table 2 provides a description of patient characteristics.
Table S1.
Description of the quota of patients from the 29 general practitioners who answered the survey
| Sociodemographic characteristics | Quota of patients (n=29) |
|---|---|
| Percentage of men, (sd) | 49.5 (3.4) |
| Age, (sd) | 47.5 (2.0) |
| Percentage of people aged ≥65 years, (sd) | 21.4 (4.9) |
| Number of patients, (sd) | 1426 (331) |
| Rural areas, n (%) | 5 (17.1) |
| Socioeconomic status in urban areas, n (%) | |
| Status 1: less deprived | 2 (8.3) |
| Status 2 | 2 (8.3) |
| Status 3 | 6 (25) |
| Status 4: more deprived | 14 (58.3) |
Figure 2.
Flow of Alzheimer's disease (AD) diagnoses evaluated by general practitioners (GPs) responding to an online survey.
Table 2.
Baseline demographic and clinical characteristics of patients according to the validation method: survey of general practitioners (GPs) to evaluate recorded AD diagnoses or linkage between datasets from primary (SIDIAP) and secondary care (ReDeGi)
| Survey | Dataset | ||
|---|---|---|---|
| Evaluated patients (n=137) | Not-evaluated patients (n=51) | All selected patients (n=4,966) | |
| Age, (sd) | 80.9 (7.7) | 83.5 (6.9) | 80.2 (6.9) |
| Women, n (%) | 92 (67%) | 36 (70%) | 3151 (63%) |
| Deprivation index, n (%) | |||
| Quintile 1: less deprived | 12 (9%) | 1 (2%) | 629 (22.64) |
| Quintile 2 | 32 (23%) | 14 (27%) | 472 (16.99) |
| Quintile 3 | 21 (15%) | 8 (16%) | 545 (19.62) |
| Quintile 4 | 34 (25%) | 13 (26%) | 653 (23.51) |
| Quintile 5: more deprived | 38 (28%) | 15 (29%) | 479 (17.24) |
| Antidementia drugs, n (%) | 92 (67%) | 32 (63%) | 3275 (66%) |
| Severity of dementia, n (%) | |||
| Mild | - | - | 2955 (60%) |
| Moderate | - | - | 1532 (31%) |
| Severe | - | - | 437 (9%) |
Of the diagnoses evaluated by GPs using the survey, 123 were considered as AD cases, and 14 were not confirmed as AD cases (Figure 2). We estimated the PPV in 89.8% (95% CI: 84.7–94.9). The supplementary materials show specific numerators and denominators used to estimate PPVs. Sensitivity analysis considered the 137 AD diagnoses evaluated by GPs using the survey questions and the 51 not evaluated, of which 32 were treated patients. Sensitivity analysis provided a PPV of 82.4 (95%CI: 77.0–87.8) (Supplementary materials). Stratified analyses are shown in Table 3.
Table 3.
Positive predictive value (PPV) of Alzheimer's disease (AD) diagnoses recorded in SIDIAP using information provided by the survey of general practitioners as reference. Sensitivity analysis included all AD patients of participating general practitioners (whether their diagnoses were evaluated or not)
| Population | n | Main analysis | n | Sensitivity analysis | ||||
|---|---|---|---|---|---|---|---|---|
| TP | FP | PPV (95% CI) | TP | FP | PPV (95% CI) | |||
| Women | 92 | 84 | 8 | 91.3 (85.5–97.1) | 128 | 106 | 22 | 82.8 (77.0–87.8) |
| Men | 45 | 39 | 6 | 86.7 (76.7–96.6) | 60 | 49 | 11 | 81.6 (71.9–91.4) |
| <80 years | 84 | 74 | 10 | 88.1 (79.9–94.2) | 105 | 89 | 16 | 84.8 (77.9–91.6) |
| ≥80 years | 54 | 49 | 4 | 92.4 (87.8–100.5) | 83 | 66 | 17 | 79.5 (70.8–88.2) |
| Total | 137 | 123 | 14 | 89.8 (84.7–94.9) | 188 | 155 | 33 | 82.4 (77.0–87.8) |
Abbreviations: TP, true positive; FP, false positive; SIDIAP, Information System for Research in Primary Care.
Linkage between SIDIAP and the registry of dementias
This linkage provided data for 4,996 patients, as described in Table 2. A specific AD diagnosis was found for 2,728 patients according to ReDeGi, 2,603 in SIDIAP and 1,947 in both databases.
PPV and sensitivity of AD diagnoses were 74.8 (95% CI: 73.1–76.4) and 71.4 (95% CI: 69.6–73.0), respectively (Supplementary materials). When using algorithm A2 (where cases included diagnosed or treated patients), PPV declined and sensitivity increased; when using algorithm A3 (excluding cases with previous conditions), PPV was unchanged and sensitivity increased compared to algorithm A1 (Table 4). No differences were observed when estimates were stratified by age, but men had lower PPV estimates than women, and sensitivity decreased with severity in all AD case definitions (Table 4).
Table 4.
Positive predictive value (PPV) and sensitivity of Alzheimer's disease (AD) diagnoses in SIDIAP using three algorithms to define AD cases and linkage with the register of dementias of Girona as reference
| Algorithm | Group | Subgroup | TP | FP | FN | PPV (95% CI) | Sensitivity (95% CI) |
|---|---|---|---|---|---|---|---|
| A1. Diagnosed patients | |||||||
| Sex | Women | 1,365 | 398 | 528 | 77.4 (75.4–79.3) | 72.1 (70.0–74.1) | |
| Men | 582 | 258 | 253 | 69.3 (66.1–72.3) | 69.3 (66.1–72.3) | ||
| Age | <80 years | 843 | 285 | 275 | 74.9 (72.3–77.4) | 75.4 (72.8–77.8) | |
| ≥80 years | 1,104 | 371 | 506 | 74.8 (72.5–77.0) | 68.6 (66.3–70.8) | ||
| Severity | Mild | 1346 | 386 | 434 | 77.7 (75.7–79.6) | 75.6 (73.6–77.6) | |
| Moderate | 472 | 220 | 263 | 68.2 (64.6–71.6) | 64.2 (60.7–67.7) | ||
| Severe | 123 | 43 | 83 | 74.1 (66.9–80.2) | 59.7 (52.9–66.2) | ||
| Total population | 1,947 | 656 | 781 | 74.8 (73.1–76.4) | 71.4 (69.6–73.0) | ||
| A2. Diagnosed or treated patients | |||||||
| Sex | Women | 1,623 | 715 | 270 | 69.4 (67.5–71.3) | 85.7 (84.1–87.2) | |
| Men | 711 | 484 | 124 | 59.5 (56.7–62.2) | 85.1 (82.6–87.4) | ||
| Age | <80 years | 992 | 531 | 126 | 65.1 (62.7–67.5) | 88.7 (86.7–90.4) | |
| ≥80 years | 1,342 | 668 | 268 | 66.8 (64.7–68.8) | 83.4 (81.4–85.1) | ||
| Severity | Mild | 1585 | 703 | 195 | 69.3 (67.4–71.1) | 89.0 (87.5–90.4) | |
| Moderate | 590 | 391 | 145 | 60.1 (57.0–63.2) | 80.3 (77.2–83.0) | ||
| Severe | 152 | 87 | 54 | 63.6 (57.3–69.4) | 73.8 (67.4–79.3) | ||
| Total population | 2,334 | 1,199 | 394 | 66.1 (64.5–67.6) | 85.6 (84.2–86.8) | ||
| A3. Diagnosed or treated patients without previous conditions | |||||||
| Sex | Women | 1,599 | 558 | 294 | 74.6 (72.7–76.4) | 83.9 (82.2–85.5) | |
| Men | 687 | 346 | 148 | 67.5 (64.5–70.3) | 81.8 (79.0–84.3) | ||
| Age | <80 years | 973 | 378 | 145 | 72.7 (70.3–75.0) | 86.7 (84.6–88.6) | |
| ≥80 years | 1,313 | 527 | 297 | 72.1 (69.9–74.1) | 80.9 (78.9–82.7) | ||
| Severity | Mild | 1552 | 519 | 228 | 74.9 (73.0–76.7) | 87.2 (85.6–88.7) | |
| Moderate | 569 | 280 | 166 | 67.0 (63.8–70.1) | 77.4 (74.3–80.3) | ||
| Severe | 144 | 59 | 62 | 70.9 (64.3–76.7) | 69.9 (63.3–75.8) | ||
| Total population | 2,286 | 905 | 442 | 72.3 (70.7–73.9) | 83.3 (81.8–84.6) | ||
Abbreviations: TP, true positive; FP, false positive; FN, false negative; SIDIAP, Information System for Research in Primary Care.
Discussion
We provided a comprehensive overview of the accuracy of AD diagnoses routinely recorded in primary care. Our PPV and sensitivity estimates indicated high accuracy of AD codes in both global and stratified analyses, similar to the one observed in other conditions such as cancer16 or cardiovascular disease.26,27 We enhanced the robustness of our findings by using two gold standards: a survey to GPs and a linkage with a provincial register of dementia. Estimates differed depending on the gold standard, and values were higher using data from the survey than from the ReDeGi linkage.
When the survey to GPs was the gold standard, our PPV estimate was similar to or slightly higher than previously reported in primary care studies on overall dementia4 or AD.10,28 In other words, patients with an AD diagnosis in EHR were very likely to have the disease. When the linkage with ReDeGi was the gold standard, PPV estimates varied between 66% and 74% depending on the applied algorithm. The lowest PPV was achieved when using algorithm A2, revealing that some treated patients might not actually have AD. The indications for anti-dementia drugs (anticholinesterases and memantine) are relatively specific for AD, but also can be used for dementia with Lewy bodies or other conditions.3 Indeed, when we restricted the treated patients considered as AD (algorithm A3), our PPV improved, likely because the exclusion of patients who had a code indicating other subtypes of dementia, previous cerebrovascular disease or Parkinsonism decreased the false positives. For example, to consider previous cerebrovascular diseases might help discern between AD and vascular dementia, in line with previous EHR-based epidemiological studies of AD prevalence.10
Our PPV estimates differed depending on the gold standard, and were higher when using data from the survey (82–89%) than from the ReDeGi linkage (66–74%). These differences were unlikely to be related with the different study populations (ie, Catalonia in the survey, and Girona province in the linkage), since clinical practices are homogeneous within the primary care services of the Catalan Health Service.16 The variability might be related to differences in validation methods and in diagnostic procedures in primary and secondary care. We cannot discard that selection bias affected PPV values based on the survey, since GPs enrolled voluntarily and not all AD diagnoses were evaluated. However, sensitivity analysis provided similar results, suggesting a low level of overestimation. In parallel, some underestimation of the PPV values based on the linkage may have occurred, because we used a conservative AD definition. Previous studies considered diagnoses of unspecified dementia as AD cases,7 but we restricted this criterion to treated patients, following Imfeld et al (2013). We also used pure AD cases recorded in ReDeGi as a gold standard, while other studies included mixed dementia in the AD case definition.7 In primary care databases, the mixed dementia code is seldom used;10 therefore, if AD codes were used instead, the number of false positives would be increased.
Sensitivity estimates obtained using the linkage analysis showed that more than two-thirds of AD cases had received a diagnosis. Identification of AD cases – treated or not – depends greatly on the GP’s role. Some level of under-recording of AD might occur when GPs use free-text instead of ICD-10 codes to record AD diagnoses, do not discern between dementia subtypes because they consider the diagnosis and treatment of dementia useless,29,30 or identify general dementia in a first visit and specify the subtype in a later visit.7 Our sensitivity estimates improved when patients treated with anti-dementia drugs were included as AD cases (algorithms A2 and A3). Anti-dementia drugs might have been prescribed in primary care without recording a diagnostic code for AD in the EHR, or a diagnosis of AD might have been made in secondary care or in a private clinic, with no record of diagnosis in the primary care database of the Catalan Health Service.31
Our findings disagree with previous evidence suggesting that less than half of the expected number of patients with dementia are recognized in primary care.32 Centers with an active approach to dementia diagnosis are likely to record a higher proportion of community cases on their health care databases.33 Thus, the quality of records regarding subtypes of dementia might substantially depend on the dementia policies implemented in each primary care setting, and their implementation or modifications over time.3 For example, dementia diagnosis rates improved substantially during 10 years in the UK.31 In the Catalan Health Service, dementia is actively considered; eg, a cognitive test is administered in about 25% of people aged at least 65 years – with or without memory complaints.6 Moreover, most of the AD diagnoses recorded in SIDIAP were done by a specialist, suggesting that primary and secondary care are well coordinated in the Catalan Health System.34,35
Finally, we succeeded in identifying AD cases using algorithms. The integration of data related to AD diagnoses, treatment and comorbidities enhanced case identification. We recommend that future EHR studies develop AD case algorithms depending on their research objectives. Large prospective studies might benefit from the definition of AD cases as diagnosed patients to avoid a biased risk estimate due to a large number of false positives.3 In epidemiological studies, such as prevalence estimates, a definition of AD cases as diagnosed or treated patients would ensure identification of the highest number of individuals having the condition.
We acknowledge several limitations. First, the PPV could be overestimated because the validation study was carried out with GPs from the AGICAP network, who are regularly involved in clinical trials and therefore they could tend to be more accurate in registering diagnosis in the electronic history of patients. Second, GPs voluntarily answered the survey, which could affect the representativeness of the study sample. Third, we used ReDeGi as gold standard, but this is a register based on cases recorded in secondary care settings and therefore, it might not capture all AD cases occurred in the Girona province. However, the ReDeGi has proved to cover about 75% of the expected cases of dementia in the area under surveillance.36 Even more, ReDeGi provides good-quality diagnoses made by specialists in neurology, geriatrics, psychiatry or internal medicine from outpatient consultation offices in specialized care or in the hospital memory clinics.
Conclusion
Our findings suggested that EHR from primary care were accurate to identify AD cases, but efforts can be made to improve diagnostic accuracy in men and in severe AD cases. Algorithms combining information on diagnosis and treatment for AD might be a powerful tool in primary care research. Sensitivity estimates differed depending on the method used – survey or linkage with a register,-but were sufficiently high to suggest that the primary care data were accurate for research purposes.
Acknowledgments
We acknowledge all the general practitioners from the AGICAP network who participated in this study. We thank Eduardo Hermosilla for data management support, Dr Vilalta-Franch for his expertise in the diagnosis of dementia, and Elaine Lilly, Ph.D., for the revision of the English text. This work was supported by the Real world Outcomes across the AD spectrum for better care: Multi-modal data Access Platform (ROADMAP) from the Innovative Medicines Initiative (Grant Agreement number 116020). This project was also supported by clinical research grants from Carlos III Health Institute, within the Net for Research in Preventive Activities and Health Enhancement (RedIAPP RD16/0007/0004) framework, and from the Agency for Management of University and Research Grants (2017 SGR 1146).
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Estimates of positive predictive value (PPV) and sensitivity of Alzheimer’s disease (AD) diagnoses recorded in primary care
● When using data from the online survey of general practitioners (GPs):
Sensitivity analysis: considering AD diagnoses evaluated and not evaluated by GPs.
-
When using data from the linkage between the Register of Dementias of Girona (ReDeGi) and Information System for Research in Primary Care (SIDIAP) (algorithm A1):
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 2.Martin Prince A, Wimo A, Guerchet M, et al. World Alzheimer report 2015 the global impact of dementia an analysis of prevalence, incidence, cost and trends; 2015. Availble from: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf. Accessed October14, 2018.
- 3.Wilkinson T, Ly A, Schnier C, et al. Identifying dementia cases with routinely collected health data: a systematic review. Alzheimers Dement. 2018. doi: 10.1016/j.jalz.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuinness LA, Warren-Gash C, Moorhouse LR, Thomas SL. The validity of dementia diagnoses in routinely collected electronic health records in the United Kingdom: a systematic review. Pharmacoepidemiol Drug Saf. 2017;2019:1–12. doi: 10.1002/pds.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zunzunegui Pastor MV, Del Ser T, Rodríguez LA, García YMJ, Domingo J, Otero PA. Demencia no detectada y utilización de los servicios sanitarios: implicaciones para la atención primaria TT - non-detected dementia and use of the health services: implications for primary care. Aten Primaria. 2003;31(9):581–586. Availble from: http://pesquisa.bvsalud.org/portal/resource/pt/ibc-29693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponjoan A, Garre-Olmo J, Blanch J, et al. Epidemiology of dementia: prevalence and incidence estimates using validated electronic health records from primary care. Clin Epidemiol. 2018. In prepara:217–228. doi: 10.2147/CLEP.S186590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van De Vorst IE, Vaartjes I, Sinnecker LF, Beks LJM, Bots ML, Koek HL. The validity of national hospital discharge register data on dementia: a comparative analysis using clinical data from a university medical centre. Neth J Med. 2015;73(2):69 Available from: http://www.njmonline.nl/getpdf.php?id=1540. Accessed December 18, 2018. [PubMed] [Google Scholar]
- 8.Sommerlad A, Perera G, Singh-Manoux A, Lewis G, Stewart R, Livingston G. Accuracy of general hospital dementia diagnoses in England: sensitivity, specificity, and predictors of diagnostic accuracy 2008–2016. Alzheimer’s Dement. 2018;14(7):933–943. doi: 10.1016/j.jalz.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon A, Ngandu T, Soininen H, Hallikainen MM, Kivipelto M, Laatikainen T. Validity of dementia and Alzheimer disease diagnoses in Finnish national registers. Alzheimers Dement. 2014;10(3):303–309. doi: 10.1016/j.jalz.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Imfeld P, Brauchli Pernus YB, Jick SS, Meier CR. Epidemiology, co-morbidities, and medication use of patients with Alzheimer’s disease or vascular dementia in the UK. J Alzheimers Dis. 2013;35(3):565–573. doi: 10.3233/JAD-121819 [DOI] [PubMed] [Google Scholar]
- 11.Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6(11). doi:10.1136/bmjopen-2016-012799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benchimol EI, Smeeth L, Guttmann A, et al. The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domínguez-Berjón MF. Construcción de un índice de privación a partir de datos censales en grandes ciudades españolas (Proyecto MEDEA). Gac Sanit. 2008;22(3):179–187. [DOI] [PubMed] [Google Scholar]
- 14.Bolíbar B, Fina Avilés F, Morros R, et al. [SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research]. Med Clin (Barc). 2012;138(14):617–621. doi: 10.1016/j.medcli.2012.01.020 [DOI] [PubMed] [Google Scholar]
- 15.García-Gil MDM, Hermosilla E, Prieto-Alhambra D, et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Qual Prim Care. 2012;20(2):135–145. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22688222. Accessed June 22, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Gil M, Elorza J-M, Banque M, et al. Linking of primary care records to census data to study the association between socioeconomic status and cancer incidence in Southern Europe: a Nation-wide ecological study. PLoS One. 2014;9(10):e109706. doi: 10.1371/journal.pone.0109706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos R, Balló E, Marrugat J, et al. Validity for use in research on vascular diseases of the SIDIAP (Information system for the development of research in primary care): the EMMA study. Rev Española Cardiol. 2012;65(1):29–37. doi: 10.1016/j.recesp.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 18.Fina-Aviles F, Medina-Peralta M, Mendez-Boo L, et al. The descriptive epidemiology of rheumatoid arthritis in Catalonia: a retrospective study using routinely collected data. Clin Rheumatol. 2016;35(3):751–757. doi: 10.1007/s10067-014-2801-1 [DOI] [PubMed] [Google Scholar]
- 19.Pujades-Rodriguez M, Assi V, Gonzalez-Izquierdo A, et al. The diagnosis, burden and prognosis of dementia: a record-linkage cohort study in England. PLoS One. 2018. doi: 10.1371/journal.pone.0199026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinyoles Bargalló E, Pujol Ribó C, Red AGICAP. [Clinical trials in primary care. The experience of the AGICAP network in Catalonia]. Atención Primaria. 2004;34(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garre-Olmo J, Flaqué M, Gich J, et al. A clinical registry of dementia based on the principle of epidemiological surveillance. BMC Neurol. 2009;9:5. doi: 10.1186/1471-2377-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German RR, Lee LM, Horan JM, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2001;50(RR–13):1–35; quiz CE1–7 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18634202. Accessed June 22, 2012. [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 2012;43(11):2412. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 24.Turró-Garriga O, Calvó-Perxas L, Vilalta-Franch J, et al. Adherence to clinical practice guidelines during dementia work-up in a real-world setting: a study from the registry of dementias of Girona. Callahan B, ed J Alzheimer’s Dis. 2017;59(3):997–1007. doi: 10.3233/JAD-170284 [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Found stat comput Vienna, Austria; 2018. Availble from: https://www.r-project.org/. Accessed September8, 2018.
- 26.Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National patient registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of cardiovascular disease event ascertainment using linkage to UK hospital records. Epidemiology. 2017;28(5):735–739. doi: 10.1097/EDE.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimer’s Dis. 2016;54(1):337–349. doi: 10.3233/JAD-160105 [DOI] [PubMed] [Google Scholar]
- 29.Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(e011146):1–8. doi: 10.1136/bmjopen-2016-011146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond N, Birtwhistle R, Williamson T, Khan S, Garies S, Molnar F. Prevalence and management of dementia in primary care practices with electronic medical records: a report from the Canadian primary care sentinel surveillance network. C Open. 2016;4(2):E177–E184. doi: 10.9778/cmajo.20150050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donegan K, Fox N, Black N, Livingston G, Banerjee S, Burns A. Trends in diagnosis and treatment for people with dementia in the UK from 2005 to 2015: a longitudinal retrospective cohort study. Lancet Public Heal. 2017;2(3):e149–e156. doi: 10.1016/S2468-2667(17)30031-2 [DOI] [PubMed] [Google Scholar]
- 32.Connolly A, Gaehl E, Martin H, Morris J, Purandare N. Underdiagnosis of dementia in primary care: variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health. 2011;15(8):978–984. doi: 10.1080/13607863.2011.596805 [DOI] [PubMed] [Google Scholar]
- 33.Perera G, Pedersen L, Ansel D, et al. Dementia prevalence and incidence in a federation of European electronic health record databases: the European medical informatics framework resource. Alzheimer’s Dement. 2018;14(2):130–139. doi: 10.1016/j.jalz.2017.06.2270 [DOI] [PubMed] [Google Scholar]
- 34.Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Garre-Olmo J. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2012. doi: 10.1097/JGP.0b013e31826ce617 [DOI] [PubMed] [Google Scholar]
- 35.Vilalta-Franch J, Garre-Olmo J, López-Pousa S, Llinàs-Reglà J, Calvó-Perxas L, Cubí-Montfort R. [Feasibility of a telemedicine support system for diagnosing dementia in primary care]. Rev Neurol. 2012;55(5):263–269. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22930137. Accessed December 16, 2014. [PubMed] [Google Scholar]
- 36.Calvó-Perxas L, Aguirregomozcorta M, Casas I, et al. Rate of dementia diagnoses according to the degree of aging of the population. Int Psychogeriatrics. 2015;27(03):419–427. doi: 10.1017/S1041610214002130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.