Abstract
Purpose of review
There is currently increased focus on improved understanding of how dendritic cell (DC) tolerogenicity is determined and maintained, and on their therapeutic potential. We review recent progress in profiling of regulatory DC (DCreg), innovative approaches to enhancing DC tolerogenicity in situ, ex vivo-generation of DCreg and initial clinical testing of these cells in organ transplantation.
Recent findings
“Omics” studies indicate that the distinctive properties of DCreg are the result of a specific transcriptional program characterized by activation of tolerance-enhancing genes, rather than the retention of an immature state. In situ DC-directed targeting of nanovesicles bearing immune regulatory molecules can trigger in vivo expansion of Ag-specific regulatory cells. Innovative approaches to ex vivo modification of DC to enhance their regulatory function and capacity to migrate to secondary lymphoid organs have been described. Cross-dressing (with donor MHC molecules) of graft-infiltrating host DC that regulate anti-donor T cell responses has been implicated in “spontaneous” liver transplant tolerance. Clinical trials of DCreg therapy have begun in living donor renal and liver transplantation.
Summary
Further definition of molecules that can be targeted to promote the function and stability of DCreg in vivo may lead to standardization of DCreg manufacturing for therapeutic application.
Keywords: Dendritic cells, immune regulation, tolerance, therapy
Introduction
Dendritic cells (DC) are a heterogeneous group of bone marrow (BM)-derived professional antigen (Ag)-presenting cells (APC) that promote self-tolerance in the healthy steady-state [1] and regulate innate and adaptive immunity [2,3]. There is therefore a strong rationale for targeting DC in situ or for using DC as cellular therapies to improve long-term outcomes in organ transplantation. Challenges to optimizing these approaches induce (i) the diversity and plasticity of DC, (ii) incomplete understanding of the molecular pathways that induce a stable, enduring regulatory profile, (iii) establishing optimal strategies for selective targeting of DC in vivo, (iv) ex vivo generation of regulatory DC (DCreg), (v) combination of DCreg therapy with appropriate immunosuppressive (IS) agents, and clinical trial design.
Human DC comprise multiple subsets,- i.e. CD141+ (cDC1) and CD1c+ (cDC2) myeloid cells that originate from a common DC precursor [4], inflammatory DC that differentiate from monocytes under inflammatory conditions and non-conventional CD123+ plasmacytoid DC [5,6]. Indeed, multiple subsets of rodent and human DC [7–9] with the ability to regulate (in rodents) organ transplant rejection and graft-versus-host disease following hematopoietic stem cell transplantation [10–12] have been characterized. Recently, transcription factors that differentially guide DC subset development have been reviewed [13] and transcriptional determinants of tolerogenic and immunogenic states during DC maturation described [14]. Mechanisms that underlie the roles of DC as inducers of peripheral tolerance have also been reviewed [15], as have methods and protocols for the ex vivo generation of human DCreg and their mechanisms of tolerance induction [16]. Some of these protocols have been incorporated into clinical trials, but with no current consensus regarding the optimal approach [17]. Herein, we consider recent developments in DCreg profiling, targeting and use of DCreg as therapeutic agents in transplantation.
Transcriptional profiling/signatures of DCreg
Intracellular pathways and transcriptional regulators that determine DCreg differentiation and function are poorly understood and few attempts have been made to better comprehend DCreg biology based on transcriptomic or proteomic (“omics”) profiles. Studies to date (reviewed by Schinnerling et al [18]) indicate that tolerogenic properties of DC emerge as the result of a specific transcriptional program rather than from the retention of an immature state. The transcription factor interferon regulatory factor 4 promotes regulatory T cell (Treg) generation by augmenting expression of genes required for Ag presentation, together with those that promote T cell tolerance [14]. Further omics studies are needed to define which molecules induce a regulatory profile and can therefore be targeted to render DC tolerogenic and enhance their stability, longevity and resistance to pro-inflammatory stimuli for therapeutic application. Investigation has been hampered by the fact that many different protocols that target distinct signaling pathways are used to generate DCreg, e.g. use of IL-10, transforming growth factor (TGF)β, vitamin (Vit)D3 or dexamethasone (Dex), or a combination of IL-10 or Dex with a stimulatory agent, such as the Toll-like receptor (TLR) 4 agonist bacterial lipopolysaccharide or CD40L. Recently, Garcia-Gonzalez et al [19,20] examined the transcriptional profile of human monocyte-derived DCreg generated with Dex and activated with the clinically-used TLR4 agonist monophosphoryl lipid A (MPLA). Dex and MPLA jointly induced a distinct transcriptional profile in DC characterized by the activation of tolerance-associated genes and suppression of inflammatory genes, conferring the potential to regulate immune responses. The pathways that showed greatest regulation were those involving cellular chemotactic responses, cell-cell signaling and interaction, as well as zinc and reactive oxygen species metabolism, favoring the recruitment and proliferation of Treg, while suppressing effector T cell responses. This and other recent innovative strategies to promote DCreg function discussed below are depicted in Fig. 1.
Figure 1. Recent approaches to targeting/modification of DC to promote tolerance.
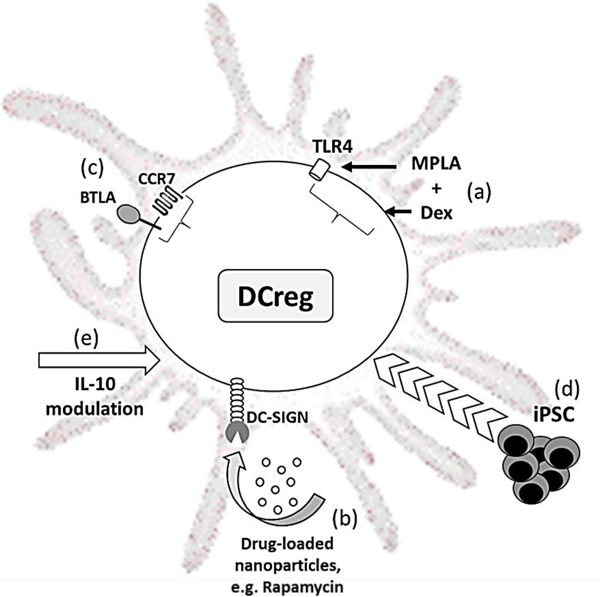
(a) Activation of dexamethasone (Dex)-generated DCreg with the Toll-like receptor 4 ligand MPLA (monophosphoryl lipid A) is associated with upregulation of tolerance-associated genes and downregulation of inflammatory genes. (b) Rapamycin-loaded silicon nanoparticles displaying anti-DC-SIGN are taken up by myeloid DC that, in turn, suppress allogeneic T cell proliferation. (c) Overexpression of the chemokine receptor type 7 (CCR7) and BTLA (B and T lymphocyte attenuator) enhances immature mouse DC migration and tolerogenic function. (d) Infusion of donor-derived DCreg generated from induced pluripotential stem cells (iPSC) is associated with prolonged allograft survival. (e) IL-10 induces a CD83hi CCR7+ human DCreg subset that exhibits efficient CCR7-directed migration and generates potently suppressive CD4+ Treg.
In vivo function of infused and endogenous DCreg in organ transplantation tolerance
The therapeutic effect of pre-transplant infusion of donor-derived DCreg in heart-allografted mice does not appear to depend on the in vivo persistence of intact donor DCreg [21] that are likely killed by host natural killer (NK) cells, but on the function of quiescent, conventional host DC in secondary lymphoid organs (SLO). Indeed, as shown by Morelli and colleagues [22], deletion of host DC prevents the therapeutic effect of donor-derived DCreg. Host DC acquire donor MHC Ag via the direct pathway of allorecognition by cross-dressing [23,24] or via the indirect pathway by Ag transfer from the donor DCreg (cross-presentation) [25] (Fig. 2). A role for donor-derived microvesicles (exosomes) released by the donor DCreg and acquired by host DC may be an advantage, since it amplifies the effect of the infused DCreg. Consequently, T cell activation is reduced, indirect pathway T cell deletion occurs, CD4+ T cell-B cell help is impaired, and anti-donor antibody (Ab) production is suppressed [26]. Independence of the immune regulatory effect of donor-derived DCreg on their persistence following systemic administration offers a potential advantage over other cell therapy approaches (e.g. infusion of Treg) the success of which may depend on in vivo persistence/replication/function of the adoptively-transferred cells. Also, DCreg have the important ability to regulate CD4+ and CD8+ memory T cell (Tmem) responses [27–30],- a major barrier to long-term allograft survival in humans.
Figure 2. Acquisition of donor Ag may underly host DCreg regulation of alloimmunity and promotion of tolerance.
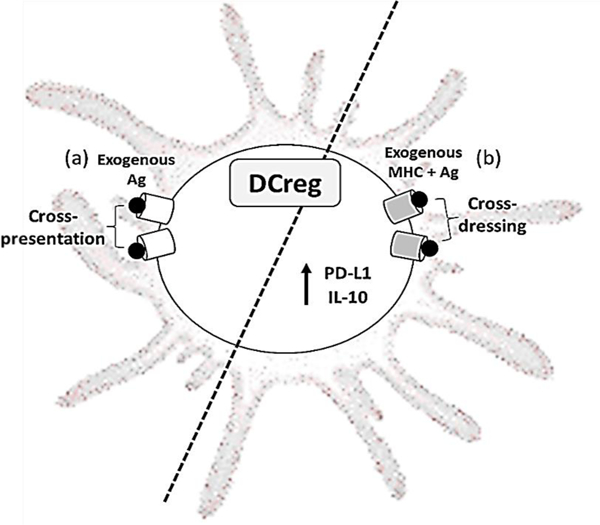
(a) Cross-presentation of processed exogenous donor Ag (peptide) and endogenous MHC I by quiescent host DCs and (b) cross-dressing of host DCs to express exogenous Ag and exogenous MHC I/II, together with high levels of programed death ligand 1 (PD-L1) and IL-10 may play key roles in the regulation of alloimmunity and the promotion of tolerance. For further information and discussion, see refs [22,25,32].
In both preclinical (mouse) and clinical studies, total lymphoid irradiation and anti-thymocyte globulin administration promotes hematopoietic cell chimerism and tolerance in organ transplant recipients. In studying the mechanistic basis of these effects, Hongo et al [31] have established that CD8+ DC are likely responsible for the initiation of tolerance induction since they ingest apoptotic cells and suppress anti-donor T cell responses via mechanisms including indoleamine dioxygenase production. Moreover, reciprocal interactions between CD8+ DC and invariant NKT cells were required for tolerance induction.
The role of cross-dressing of host DC in organ transplant tolerance
Cross-dressing has only recently been implicated in the instigation of transplant tolerance. Thus, Ono et al [32] have investigated the role of graft-infiltrating, cross-dressed DC in mouse fully MHC-mismatched liver transplant tolerance that occurs without use of IS agents [33]. While donor interstitial DC diminished rapidly following liver transplantation, they were replaced in the graft by host DC that peaked on postoperative day 7 and persisted indefinitely. About 60% of these recipient DC displayed donor MHC class I, indicating cross-dressing. By contrast, only a very minor fraction (0–2%) of cross-dressed DC (CD-DC) was evident in the spleen. CD-DC sorted from the liver grafts expressed much higher levels of the T cell coinhibitory molecule programed death ligand 1 (PD-L1) and high levels of anti-inflammatory IL-10 compared with graft non-CD-DC. Concomitantly, high incidences of programed death protein 1 (PD-1)hi T cell immunoglobulin and mucin domain containing-3 (TIM-3)+ exhausted graft-infiltrating CD8+ T cells were observed. Importantly, unlike non-CD-DC, the CD-DC did not stimulate proliferation of allogeneic T cells, but markedly suppressed anti-donor host T cell proliferation. CD-DC were much less evident in allografts from donors lacking the transmembrane adaptor protein DNAX-activating protein of 12kDa that are rejected acutely [34]. These findings suggest that graft-infiltrating PD-L1hi CD-DC play a key role in the regulation of alloimmunity and induction of liver transplant tolerance.
Cross-dressing of host DC has also been implicated in allospecific tolerance in a different context. Thus, maternal hematopoietic microchimerism, that has been linked to the development of allospecific tolerance, has been shown recently [35] to be associated with membrane alloAg acquisition by host DC. In the mouse model examined, cross-dressing was associated with enhanced expression of PD-L1 on myeloid DC and reduced presentation of allopeptide + self-MHC complexes together with increased PD-L1 on plasmacytoid DC that was associated with PD-L1-dependent CD4+ T cell anergy. Thus, acquisition of exosomes bearing alloAg by host DC may provide an important link between microchimerism and the induction of tolerance.
Innovative approaches to in situ targeting of DC
Prolonged ischemia enhances DC maturation and potentiates adaptive immunity. In the post-ischemic period, CD4+ T cells recruited to the liver are closely associated with hepatic DC. Using 2-photon intravital microscopy together with confocal microscopy, Funken et al have shown [36] that in situ targeting of hepatic DC with a VitD analog (paricalcitol) attenuates their maturation, promotes their tolerogenicity and ameliorates CD4+ Th1 cell responses post-ischemia. This beneficial effect was abolished by blocking DC-T cell interactions mediated by the cell surface glycoprotein CD44. In a recent report, Zheng et al [37] found that injected exosomes derived from DC could attenuate ischemia-reperfusion injury by modulating the balance between Treg and Th17 cells.
Important pathways and molecular mechanisms that regulate peripheral tolerance are being uncovered by combining methods that target delivery of defined T cell Ags to DC in vivo with genetic modification of the DC [15]. These approaches are determining the roles of specific immunomodulatory pathways and different DC subsets in maintaining immunological tolerance. In a recent example, Reeves et al [38] have shown that APC-targeted expression of proinsulin coverts insulin-specific CD8+ T cell priming to tolerance in autoimmune-prone, non-obese diabetic mice. This shift in T cell priming to tolerance exemplifies the tolerogenic capacity of autoAg expression by APC in situ and the ability to overcome defects in pathways controlling peripheral tolerance.
Nanoparticle-based drug delivery systems that enable directed, cell type-specific targeting in vivo in combination with delivery of multiple drugs in a single formulation have emerged as a promising approach to DC-based immunotherapy. Receptors that have been used to target DC include DEC205 (CD205), CD11c, DC-specific intracellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), the mannose receptor, Fc receptors and CD40. While most approaches have focused on induction of immunity in the context of tumor immunotherapy, nanoparticle delivery is also a promising approach to induce DC with tolerogenic capacity. Thus, polymeric synthetic nanoparticles (‘nanocarriers’) that target DC induce stable Ag (ovalbumin)-specific tolerance by delivery of rapamycin [39]. Recently, biodegradable porous silicon nanoparticles displaying anti-DC-SIGN and loaded with rapamycin were shown [40] to target and be taken up more efficiently (compared with isotype-coated nanoparticles) by myeloid DC in human blood. Moreover, DC pre-conditioning with the rapamycin-loaded nanoparticles suppressed allogeneic T cell proliferation. Notably, Clemente-Casdares et al [41] have shown that systemic delivery of nanoparticles coated with autoAg bound to MHC II molecules triggers in vivo expansion of Ag-specific type-1 Treg that, in turn, inhibit autoAg-loaded APCs and drive differentiation of regulatory B cells in experimental autoimmune disease.
Ex vivo generation of DCreg
Myeloid DC with tolerogenic properties can be generated readily from BM precursors or blood monocytes in response to granulocyte-macrophage colony-stimulating factor ± IL-4, with addition of one or more pharmacologic/biologic agents that stably inhibit their maturation and promote their tolerogenicity [42]. These agents include anti-inflammatory cytokines (IL-10; TGFβ), anti-sense oligonucleotides targeting costimulatory molecules, anti-inflammatory/IS drugs (calcineurin inhibitors [CNI], rapamycin, mycophenolate mofetil [MMF], corticosteroids), VitD3 and cyclic AMP inducers, in particular prostaglandin E2. Currently, there is no consensus as to the optimal agent(s) or combination of agents to use for generation of clinical grade DCreg. Phenotypic characteristics of DCreg include low levels of MHC class I and II and T cell costimulatory molecule (CD80; CD86) expression and enhanced expression of T cell co-inhibitory (e.g. PD-L1) and death-inducing ligands (e.g. FasL). Their functional characteristics include low secretion of bioactive IL-12p70 and comparatively high levels of anti-inflammatory cytokine (IL-10; TGFβ) production. In addition, DCreg can expand or induce the de novo generation of Treg [10,43,44].
An important consideration regarding generation of DCreg is their ability to migrate to SLO. Genetic modification of DC to enhance their tolerogenic function and promote their in vivo migration to SLO has been documented [45–47]. In a more recent study, Dong et al [48] co-transfected donor-derived immature DC with an adenovirus bearing the chemokine receptor type 7 (CCR7) gene and a small interfering RNA targeting the nuclear factor-κB transcription factor subunit RelB to concurrently overexpress CCR7 and downregulate RelB expression. The co-transfected cells displayed enhanced migratory ability, resistance to apoptosis and enhanced ability (compared with CCR7 or RelB knockdown alone) to upregulate Treg and improve mouse skin allograft survival, suggesting that this might be a promising approach in skin (including vascularized composite allograft) transplantation. In a related study, Xin et al [49] reported that overexpression of CCR7 and BTLA (B and T lymphocyte attenuator, an Ig superfamily coinhibitory receptor similar structurally to PD-1 and CTLA4) in immature mouse DC enhanced their tolerogenic function and migration to the SLO chemokine CCL19.
The potential of donor-derived DCreg generated from (murine) induced pluripotential stem cells (iPSC) to function as therapeutic cellular vaccines to generate Treg and induce donor-specific tolerance has been examined recently by Cai et al [50]. Mouse heart allografts were accepted permanently in a donor-specific manner, accompanied by differentiation of donor-specific CD4+ Treg, illustrating the promising potential of iPSC-DCreg.
DCreg subsets for therapeutic application
Human IL-10-modulated DC (IL-10 DC) [51] that potently induce Treg [52] have been the focus of research on DCreg for many years. A recent report by Steinbrink and colleagues [53] identified two populations of human monocyte-derived IL-10 DC with tolerogenic properties, - CD83hi CCR7+ IL-10 DC and CD83lo CCR7- IL-10 DC. The CD83hi CCR7+ IL-10 DC generated more potently suppressive CD4+ Treg, exhibited efficient CCR7-directed migration (towards the SLO chemokine CCL21) and retained stable function under inflammatory conditions, thus satisfying important criteria for prospective clinical application.
Donor-versus recipient-derived DCreg for therapy of graft rejection
The first reports suggesting that DCreg of donor origin could be used to inhibit allograft rejection appeared in 1995/1996 [54,55]. Subsequently, many reports have shown that donor-derived DCreg or syngeneic/autologous DCreg (either pulsed or unpulsed with donor Ag) infused alone or together with an IS agent(s) can induce indefinite organ allograft survival/donor-specific tolerance in rodents [10,56]. In more recent studies in a pre-clinical, MHC-mismatched non-human primate (NHP) renal allograft model, graft survival was prolonged significantly in rhesus macaques given VitD3- and IL-10-conditioned DCreg one week before transplant, in combination with a minimal IS regimen of costimulation blockade and rapamycin [57]. No evidence of host sensitization (donor-specific Abs) was observed. Median graft survival time was also prolonged in this model when autologous DCreg pulsed with donor Ag (cell membrane microvesicles) were infused a day before transplantation [58]. These important translational studies demonstrate both the safety and efficacy of a single (donor-derived) DCreg infusion. They also provide novel mechanistic insights. Thus, infusion of donor-derived DCreg is associated with selective attenuation of anti-donor Tmem responses, Eomesoderminlo CTLA4hi alloreactive CD8+ Tmem [59] and maintenance of donor-reactive CD4+CTLA4hi T cells with a regulatory phenotype [60]. These observations in NHP provide a compelling basis for clinical testing of DCreg in organ transplantation.
Innovative approaches to combination therapy
Many pharmacologic and biologic agents promote DC tolerogenicity in rodents [42]. Following observations that exosomes derived from immature donor DC (immDex) prolonged experimental graft survival and that their effects were associated with Treg, Ma et al [61] combined the exosomes with donor Ag-specific Treg and achieved tolerance without IS therapy in a rat liver allograft model. The immDex amplified Treg in vivo most likely through binding/fusing with host DC and being presented by these DC, which could be augmented by IL-2 administration. Also, in a rat liver allograft model, combined infusion of donor-derived immature DC and CD4+ Treg, 7 days before transplantation, was more effective than either regulatory cell population alone and appeared to maintain a feedback loop between the DCreg and Treg in vivo [62].
Clinical trials of DCreg therapy in renal and liver transplantation
The potential of DCreg for the prevention of rejection after clinical solid organ transplantation has been discussed extensively in recent reviews [17,63]. Clinical trials of DCreg therapy in renal or liver transplantation have begun in Europe and the US. Autologous DCreg infusion, 1 day before transplant, is under examination at the University of Nantes, France, in live donor renal transplantation with standard of care (SOC) triple drug IS (azathroprine, steroid, tacrolimus) (ClinicalTrials.gov Identifier: NCT0225055 [64]). An NIH-supported clinical trial to test the safety of donor-derived DCreg infusion in living donor renal transplantation will begin at the University of Pittsburgh, US in 2018 [65].
The possibility that DCreg administration as a novel adjunct induction therapy may promote immunological mechanisms conducive to induction of donor-specific T cell hyporesponsiveness (tolerance) and enable early withdrawal of all IS after liver transplantation carries the potential important advantage of sparing patients the side effects of long-term IS, particularly CNI. Recently, in a multi-center study of early post-transplant IS drug withdrawal (CNI-based therapy; no induction) in liver transplantation [66], IS minimization starting 12–14 months post-transplant was tolerated by the majority of patients, while complete IS withdrawal was achieved in 13% of those that qualified for the minimization protocol. This degree of success provides a potential basis for assessment of the impact of innovative strategies, including DCreg infusion, aimed at improving the incidence of safe withdrawal of IS therapy and operational tolerance in human liver transplantation.
At the University of Pittsburgh, a first-in-human, single center, open-label, phase I/II study ( NCT03164265) to test the safety and preliminary efficacy of a single infusion of donor-derived DCreg in de novo adult living donor liver transplant recipients [67] has been initiated. Patients receive SOC IS (MMF, steroid and tacrolimus), without Ab induction. GMP grade DCreg are generated [68] in VitD3 and IL-10 from monocytes obtained by leukapheresis from prospective living donors and infused as induction therapy into their respective recipients, 7 days before transplant. The DCreg dose range (2.5–10 × 106/kg) corresponds to the range for which both safety and efficacy were demonstrated in the preclinical NHP renal transplant model [57]. A half dose of MMF is administered concomitant with DCreg infusion and until the time of transplant to minimize any potential risk of sensitization. In eligible patients, determined by permissive liver function tests and (at 12 months post-transplant) a permissive liver biopsy, IS drug weaning begins at month 6 and continues through month 18. Follow-up continues for 3 years after the last dose of IS.
CONCLUSIONS AND FUTURE PROSPECTS
Omics studies are better defining the molecules that enhance the tolerogenic phenotype, stability and longevity of DCreg and their resistance to proinflammatory stimuli. This is likely to instruct improved and more standardized design of protocols for generation of clinical grade DCreg for therapeutic application. Strategies that target DC selectively in situ to enhance their immune regulatory function and that enhance migration of DCreg to SLO show promise for potential therapeutic application. Acquisition of donor MHC by host DC (cross-dressing) and the immune regulatory function of these cross-dressed DC in allograft recipients in relation to development of transplant tolerance is a key topic for future mechanistic studies. Clinical trials of DCreg in organ transplantation have been instigated and will provide valuable insights into the value of these novel regulatory immune cells for improved outcomes in organ transplantation.
Acknowledgements
We thank our many colleagues in the laboratory and clinic whose invaluable contributions have made our studies possible.
Funding for the authors’ work is from the National Institutes of Health (grant numbers R01 AI18777, U01 AI136779 and U19 AI131453) and from the US Department of Defense (grant number W81XWH-15–2-0027).
Financial support and sponsorship
Funding for the authors’ work is from the National Institutes of Health (grant numbers R01 AI18777, U01 AI136779 and U19 AI131453), the US Department of Defense (grant number W81XWH-15–2-0027) and University of Pittsburgh Medical Center Enterprises (Immune Transplant and Therapy Center).
Footnotes
Conflicts of interest
No conflicts of interest.
REFERENCES AND RECOMMENDED READING
- 1.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D: Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med 2009, 206:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM: Dendritic cells and the control of immunity. Nature 1998, 392:245–252. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Nussenzweig MC: Tolerogenic dendritic cells. Annu Rev Immunol 2003, 21:685–711. [DOI] [PubMed] [Google Scholar]
- 4.Satpathy AT, Wu X, Albring JC, Murphy KM: Re(de)fining the dendritic cell lineage. Nat Immunol 2012, 13:1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, Dalod M, Soumelis V, Amigorena S: Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity 2013, 38:336–348. [DOI] [PubMed] [Google Scholar]
- 6.Collin M, Bigley V: Human dendritic cell subsets: an update. Immunology 2018, 154:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu YJ: Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 2001, 106:259–262. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, et al. : Dendritic cell subsets in health and disease. Immunol Rev 2007, 219:118–142. [DOI] [PubMed] [Google Scholar]
- 9.Macri C, Pang ES, Patton T, O’Keeffe M: Dendritic cell subsets. Semin Cell Dev Biol 2017, In press. [DOI] [PubMed] [Google Scholar]
- 10.Morelli AE, Thomson AW: Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 2007, 7:610–621. [DOI] [PubMed] [Google Scholar]
- 11.Stenger EO, Turnquist HR, Mapara MY, Thomson AW: Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood 2012, 119:5088–5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rogers NM, Isenberg JS, Thomson AW: Plasmacytoid dendritic cells: no longer an enigma and now key to transplant tolerance? Am J Transplant 2013, 13:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devi KS, Anandasabapathy N: The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol 2017, 39:137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. **.Vander Lugt B, Riddell J, Khan AA, Hackney JA, Lesch J, DeVoss J, Weirauch MT, Singh H, Mellman I: Transcriptional determinants of tolerogenic and immunogenic states during dendritic cell maturation. J Cell Biol 2017, 216:779–792.An analysis of the transcriptional determinants, in particular IRF4 versus NFκB, that enable DC to exhibit tolerogenic versus immunogenic activity
- 15. *.Iberg CA, Jones A, Hawiger D: Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol 2017, 38:793–804.A review of the tolerogenic functions of DC in vivo revealed by targeted delivery of Ag in combination with various genetic modifications of the DC.
- 16.Domogalla MP, Rostan PV, Raker VK, Steinbrink K: Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front Immunol 2017, 8:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. *.Marin E, Cuturi MC, Moreau A: Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand? Front Immunol 2018, 9:274.This review discusses the characteristics and mechanisms of action of human, NHP and mouse tolerogenic DC, recent phase I/II clinical trials of DCreg in autoimmune disease, and trials that are emerging in organ transplantation.
- 18.Schinnerling K, Garcia-Gonzalez P, Aguillon JC: Gene Expression Profiling of Human Monocyte-derived Dendritic Cells - Searching for Molecular Regulators of Tolerogenicity. Front Immunol 2015, 6:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Gonzalez PA, Schinnerling K, Sepulveda-Gutierrez A, Maggi J, Hoyos L, Morales RA, Mehdi AM, Nel HJ, Soto L, Pesce B, et al. : Treatment with Dexamethasone and Monophosphoryl Lipid A Removes Disease-Associated Transcriptional Signatures in Monocyte-Derived Dendritic Cells from Rheumatoid Arthritis Patients and Confers Tolerogenic Features. Front Immunol 2016, 7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Gonzalez PA, Schinnerling K, Sepulveda-Gutierrez A, Maggi J, Mehdi AM, Nel HJ, Pesce B, Larrondo ML, Aravena O, Molina MC, et al. : Dexamethasone and Monophosphoryl Lipid A Induce a Distinctive Profile on Monocyte-Derived Dendritic Cells through Transcriptional Modulation of Genes Associated With Essential Processes of the Immune Response. Front Immunol 2017, 8:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, Erdos G, Larregina AT, Morelli AE: Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood 2010, 116:2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Divito SJ, Shufesky WJ, Sumpter T, Wang H, Tkacheva OA, Wang W, Liu C, Larregina AT, Morelli AE: Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant 2012, 12:1398–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G, Sullivan ML, Gibson GA, Watkins SC, Larregina AT, et al. : Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Invest 2016, 126:2805–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, Abdi R, Uehara M, Kim JI, Markmann JF, et al. : Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol 2016, 1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. *.Zeng F, Morelli AE: Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol 2018. In press.An informative discussion of the role of MHC cross-dressing of antigen-presenting cells via extracellular vesicles in positive or negative regulation of T cell responses in the steady state, transplantation and other conditions.
- 26.Morelli AE, Thomson AW: Orchestration of transplantation tolerance by regulatory dendritic cell therapy or in-situ targeting of dendritic cells. Curr Opin Organ Transplant 2014, 19:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenna TJ, Thomas R, Steptoe RJ: Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood 2008, 111:2091–2100. [DOI] [PubMed] [Google Scholar]
- 28.Kenna TJ, Waldie T, McNally A, Thomson M, Yagita H, Thomas R, Steptoe RJ: Targeting antigen to diverse APCs inactivates memory CD8+ T cells without eliciting tissue-destructive effector function. J Immunol 2010, 184:598–606. [DOI] [PubMed] [Google Scholar]
- 29.Nasreen M, Waldie TM, Dixon CM, Steptoe RJ: Steady-state antigen-expressing dendritic cells terminate CD4+ memory T-cell responses. Eur J Immunol 2010, 40:2016–2025. [DOI] [PubMed] [Google Scholar]
- 30.Kleijwegt FS, Jansen DT, Teeler J, Joosten AM, Laban S, Nikolic T, Roep BO: Tolerogenic dendritic cells impede priming of naive CD8(+) T cells and deplete memory CD8(+) T cells. Eur J Immunol 2013, 43:85–92. [DOI] [PubMed] [Google Scholar]
- 31. *.Hongo D, Tang X, Zhang X, Engleman EG, Strober S: Tolerogenic interactions between CD8(+) dendritic cells and NKT cells prevent rejection of bone marrow and organ grafts. Blood 2017, 129:1718–1728.A report showing that reciprocal tolerogenic interactions between CD8+ DC and invariant natural killer cells are required for tolerance induction following combined donor bone marrow and heart transplantation in mice.
- 32. **.Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O, Yokota S, Stolz DB, Ross MA, Morelli AE, et al. : Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology 2018, 67:1499–1515.This study describes the normal finding that cross-dressed, graft-infiltrating host DC regulate anti-donor T cell responses associated with T cell exhaustion in experimental mouse liver transplantation.
- 33.Yokota S, Yoshida O, Ono Y, Geller DA, Thomson AW: Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance. Liver Transpl 2016, 22:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida O, Kimura S, Dou L, Matta BM, Yokota S, Ross MA, Geller DA, Thomson AW: DAP12 deficiency in liver allografts results in enhanced donor DC migration, augmented effector T cell responses and abrogation of transplant tolerance. Am J Transplant 2014, 14:1791–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. **.Bracamonte-Baran W, Florentin J, Zhou Y, Jankowska-Gan E, Haynes WJ, Zhong W, Brennan TV, Dutta P, Claas FH, van Rood JJ, et al. : Modification of host dendritic cells by microchimerism-derived extracellular vesicles generates split tolerance. Proc Natl Acad Sci U S A 2017, 114:1099–1104.This study reveals that cross-dressing of host DC by maternal microchimerism-derived exosomes promotes T cell tolerance.
- 36.Funken D, Ishikawa-Ankerhold H, Uhl B, Lerchenberger M, Rentsch M, Mayr D, Massberg S, Werner J, Khandoga A: In situ targeting of dendritic cells sets tolerogenic environment and ameliorates CD4(+) T-cell response in the postischemic liver. FASEB J 2017, 31:4796–4808. [DOI] [PubMed] [Google Scholar]
- 37.Zheng L, Li Z, Ling W, Zhu D, Feng Z, Kong L: Exosomes Derived from Dendritic Cells Attenuate Liver Injury by Modulating the Balance of Treg and Th17 Cells After Ischemia Reperfusion. Cell Physiol Biochem 2018, 46:740–756. [DOI] [PubMed] [Google Scholar]
- 38.Reeves PLS, Rudraraju R, Wong FS, Hamilton-Williams EE, Steptoe RJ: Antigen presenting cell-targeted proinsulin expression converts insulin-specific CD8(+) T-cell priming to tolerance in autoimmune-prone NOD mice. Eur J Immunol 2017, 47:1550–1561. [DOI] [PubMed] [Google Scholar]
- 39.Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’Neil C, Altreuter DH, Browning E, et al. : Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A 2015, 112:E156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. *.Stead SO, McInnes SJP, Kireta S, Rose PD, Jesudason S, Rojas-Canales D, Warther D, Cunin F, Durand JO, Drogemuller CJ, et al. : Manipulating human dendritic cell phenotype and function with targeted porous silicon nanoparticles. Biomaterials 2018, 155:92–102.This study reports that targeted delivery of anti-DC-SIGN expressing nanoparticles loaded with rapamycin to DC renders a maturation-resistant phenotype that suppresses allogeneic T cell proliferation.
- 41.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. : Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016, 530:434–440. [DOI] [PubMed] [Google Scholar]
- 42.Hackstein H, Thomson AW: Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004, 4:24–35. [DOI] [PubMed] [Google Scholar]
- 43.Huang H, Dawicki W, Zhang X, Town J, Gordon JR: Tolerogenic dendritic cells induce CD4+CD25hiFoxp3+ regulatory T cell differentiation from CD4+CD25-/loFoxp3- effector T cells. J Immunol 2010, 185:5003–5010. [DOI] [PubMed] [Google Scholar]
- 44.Raker VK, Domogalla MP, Steinbrink K: Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front Immunol 2015, 6:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takayama T, Nishioka Y, Lu L, Lotze MT, Tahara H, Thomson AW: Retroviral delivery of viral interleukin-10 into myeloid dendritic cells markedly inhibits their allostimulatory activity and promotes the induction of T-cell hyporesponsiveness. Transplantation 1998, 66:1567–1574. [DOI] [PubMed] [Google Scholar]
- 46.Lu L, Gambotto A, Lee WC, Qian S, Bonham CA, Robbins PD, Thomson AW: Adenoviral delivery of CTLA4Ig into myeloid dendritic cells promotes their in vitro tolerogenicity and survival in allogeneic recipients. Gene Ther 1999, 6:554–563. [DOI] [PubMed] [Google Scholar]
- 47.Xin HM, Peng YZ, Yuan ZQ, Guo H: In vitro maturation and migration of immature dendritic cells after chemokine receptor 7 transfection. Can J Microbiol 2009, 55:859–866. [DOI] [PubMed] [Google Scholar]
- 48.Dong Z, Chen Y, Peng Y, Wang F, Yang Z, Huang G, Chen Y, Yuan Z, Cao T, Peng Y: Concurrent CCR7 Overexpression and RelB Knockdown in Immature Dendritic Cells Induces Immune Tolerance and Improves Skin-Graft Survival in a Murine Model. Cell Physiol Biochem 2017, 42:455–468. [DOI] [PubMed] [Google Scholar]
- 49. *.Xin H, Zhu J, Miao H, Gong Z, Jiang X, Feng X, Tong Y: Adenovirus-Mediated CCR7 and BTLA Overexpression Enhances Immune Tolerance and Migration in Immature Dendritic Cells. Biomed Res Int 2017, 2017:3519745.This study reports that co-overexpression of CCR7 and BTLA (B and T lymphocyte attenuator) enhances immature mouse DC tolerogenicity and CCL19-directed migration.
- 50. *.Cai S, Hou J, Fujino M, Zhang Q, Ichimaru N, Takahara S, Araki R, Lu L, Chen JM, Zhuang J, et al. : iPSC-Derived Regulatory Dendritic Cells Inhibit Allograft Rejection by Generating Alloantigen-Specific Regulatory T Cells. Stem Cell Reports 2017, 8:1174–1189.These findings describe the capacity of pluripotential stem cell-derived murine DCreg to inhibit heart graft rejection and induce donor-specific Treg.
- 51.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH: Induction of tolerance by IL-10-treated dendritic cells. J Immunol 1997, 159:4772–4780. [PubMed] [Google Scholar]
- 52.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG: Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood 2010, 116:935–944. [DOI] [PubMed] [Google Scholar]
- 53. **.Kryczanowsky F, Raker V, Graulich E, Domogalla MP, Steinbrink K: IL-10-Modulated Human Dendritic Cells for Clinical Use: Identification of a Stable and Migratory Subset with Improved Tolerogenic Activity. J Immunol 2016, 197:3607–3617.This study reports the superior tolerogenic function and secondary lymphoid organ migrating potential of a human IL-10 DC subset (CD83+CCR7+) compared with CD83loCCR7- IL-10 DC.
- 54.Rastellini C, Lu L, Ricordi C, Starzl TE, Rao AS, Thomson AW: Granulocyte/macrophage colony-stimulating factor-stimulated hepatic dendritic cell progenitors prolong pancreatic islet allograft survival. Transplantation 1995, 60:1366–1370. [PMC free article] [PubMed] [Google Scholar]
- 55.Fu F, Li Y, Qian S, Lu L, Chambers F, Starzl TE, Fung JJ, Thomson AW: Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+, CD80dim, CD86-) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation 1996, 62:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beriou G, Moreau A, Cuturi MC: Tolerogenic dendritic cells: applications for solid organ transplantation. Curr Opin Organ Transplant 2012, 17:42–47. [DOI] [PubMed] [Google Scholar]
- 57.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ, Lakkis FG, Wijkstrom M, Murase N, Humar A, et al. : Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant 2013, 13:1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. *.Ezzelarab MB, Raich-Regue D, Lu L, Zahorchak AF, Perez-Gutierrez A, Humar A, Wijkstrom M, Minervini M, Wiseman RW, Cooper DKC, et al. : Renal Allograft Survival in Nonhuman Primates Infused With Donor Antigen-Pulsed Autologous Regulatory Dendritic Cells. Am J Transplant 2017, 17:1476–1489.This report shows that autologous DCreg preloaded with donor microvesicles and administered one day before renal transplantation together with a minimal immunosuppressive regimen prolongs median graft survival time in nonhuman primates.
- 59.Ezzelarab MB, Lu L, Guo H, Zahorchak AF, Shufesky WF, Cooper DK, Morelli AE, Thomson AW: Eomesodermin(lo) CTLA4(hi) Alloreactive CD8+ Memory T Cells Are Associated With Prolonged Renal Transplant Survival Induced by Regulatory Dendritic Cell Infusion in CTLA4 Immunoglobulin-Treated Nonhuman Primates. Transplantation 2016, 100:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. *.Ezzelarab MB, Lu L, Shufesky WF, Morelli AE, Thomson AW: Donor-Derived Regulatory Dendritic Cell Infusion Maintains Donor-Reactive CD4(+)CTLA4(hi) T Cells in Non-Human Primate Renal Allograft Recipients Treated with CD28 Co-Stimulation Blockade. Front Immunol 2018, 9:250.This study shows that pre-transplant infusion of donor-derived DCreg promotes and maintains donor-reactive CD4+CTLA4hi T cells with a regulatory phenotype, even in the presence of CD28 costimulation blockade.
- 61.Ma B, Yang JY, Song WJ, Ding R, Zhang ZC, Ji HC, Zhang X, Wang JL, Yang XS, Tao KS, et al. : Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci Rep 2016, 6:32971.In this report, the authors show that infusion of immature DC-derived exosomes combined with donor Ag-specific Treg promotes rat liver allograft tolerance.
- 62.He W, Chen L, Zheng L, Luo L, Gao L: Prolonged survival effects induced by immature dendritic cells and regulatory T cells in a rat liver transplantation model. Mol Immunol 2016, 79:92–97. [DOI] [PubMed] [Google Scholar]
- 63.Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D: Update on Dendritic Cell-Induced Immunological and Clinical Tolerance. Front Immunol 2017, 8:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreau A, Alliot-Licht B, Cuturi MC, Blancho G: Tolerogenic dendritic cell therapy in organ transplantation. Transpl Int 2017, 30:754–764. [DOI] [PubMed] [Google Scholar]
- 65.Thomson AW, Zahorchak AF, Ezzelarab MB, Butterfield LH, Lakkis FG, Metes DM: Prospective Clinical Testing of Regulatory Dendritic Cells in Organ Transplantation. Front Immunol 2016, 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaked A, Feng S, Punch J, Reyes J, Levitsky J, Klintmalm G, Kopetskie H, DesMarais M, Priore A, Bridges ND, et al. : Early post-transplant immunosuppression (IS) withdrawal - final outcomes of the ITN030ST AWISH Study. Am J Transplant 2016, 16:269. [Google Scholar]
- 67. *.Thomson AW, Humar A, Lakkis FG, Metes DM: Regulatory dendritic cells for promotion of liver transplant operational tolerance: Rationale for a clinical trial and accompanying mechanistic studies. Hum Immunol 2018, 79:314–321.This article describes the rationale for a clinical trial of donor-derived DCreg and immunosuppressive drug withdrawal in live donor liver transplantation that is underway at the University of Pittburgh.
- 68.Zahorchak AF, Macedo C, Hamm DE, Butterfield LH, Metes DM, Thomson AW: High PD-L1/CD86 MFI ratio and IL-10 secretion characterize human regulatory dendritic cells generated for clinical testing in organ transplantation. Cell Immunol 2018, 323:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]


