Abstract
Background:
African Americans (AAs) present with cardiovascular disease (CVD) risk factors at younger ages than whites. Consequently, CVD and stroke occur at a higher incidence and at earlier decades in life in AA populations. Arterial stiffness is a predictor of CVD outcomes and partially explains the CVD risk experienced by racial minorities. We evaluated the differences in arterial stiffness observed in AAs and whites through a systematic review and meta-analysis.
Methods:
We searched PubMed and SCOPUS for comparative studies published March 1995 to November 29, 2017 comparing arterial stiffness assessments (pulse wave velocity, augmentation index, and central blood pressure) between AAs and whites. Two independent reviewers examined 195 titles/abstracts, 85 full text articles and 11 articles were included in the meta-analysis using random effects modeling approaches.
Main results:
A total of 5060 white and 3225 AAs were included across 11 relevant studies. Carotid-femoral pulse wave velocity (cfPWV) measures were statistically different between AAs and whites (mean difference = −0.44, 95% confidence interval [CI]: -[−0.67, −0.21], p = 0.0002). Aortic femoral pulse wave velocity was significantly different between AAs and whites (mean difference = −0.21, [95% CI] −0.35, −0.07, p = 0.003) regardless of sex. Augmentation index (AIx) and Augmentation index at a 75 beats per minutes heart rate (AIx @75) was also significantly different between AA and whites (mean difference = −4.36 [95% CI] = −6.59, −2,12, p = 0.0001 and −6.26, [95% CI] = −9.19, −3.33, p < 0.0001, respectively).
Conclusions:
Racial disparities in arterial stiffness persist among African American racial groups in the United States. The lack of homogeneity in studies capturing racial disparities in cfPWV suggest that additional studies are needed to understand the magnitude of racial differences in African Americans and whites that might be clinically relevant.
Keywords: Arterial stiffness, African American, Pulse wave velocity, Augmentation index, Cardiovascular disease, Disparities
INTRODUCTION
African American (AA) life expectancy is 3.4 years shorter than whites and CVDs explain over one-third of this disparity.1 Hypertension (HTN) impacts over 40% of AA >20 years old residing in the US and poses the greatest threat to AA cardiovascular health.2 African Americans develop HTN as early as the second decade of life.3 One consequence of chronically elevated blood pressure is the development of arterial stiffness. Aortic pulse wave velocity (aPWV) is higher in AAs compared to non-Hispanic whites. Moreover, a 1 m/s increase in aPWV raises the number of total CVD events, CVD mortality, and all-cause mortality risk, adjusted for age, sex, and other risk factors by 14%, 15%, and 15%, respectively.4 However, a number of the aforementioned studies included study populations who had compounding cardiovascular disease risk. Racial differences in arterial stiffness in young, asymptomatic individuals is not well understood. Assembly and function of elastin and collagen fibers within the vascular wall supports optimal oxygen delivery to organs and tissues.5 However, HTN causes abnormalities in the structure and function of elastin yielding stiffened vessel walls and non-compliant blood vessels.6 Arterial compliance is a complex process dependent upon the delicate balance of collagen and elastin production and degradation.6 Dysregulation of this process perturbs normal blood vessel dilation and contraction in response to cardiac systole and diastole which allows blood flow to transition from pulsatile to laminar flow and replenish oxygen to organs.7,8
Elastic central arteries, such as the aorta and carotid artery, are more likely to be dysfunctional in African-Americans because of a higher prevalence of CVD risk factors.9 This results in higher levels of end-organ damage.10 However, a comprehensive comparison of arterial stiffness amongst healthy, young AAs and whites is currently lacking. To further understand this paradigm, we conducted a systematic review of the literature and meta-analysis with the goal of identifying and understanding differences in PWV, central blood pressure (CBP) and augmentation index (AIx). These measurements are all important indices of arterial stiffness and general vascular health and might explain disparities observed in the AA populations in the United States.
METHODS
Study design
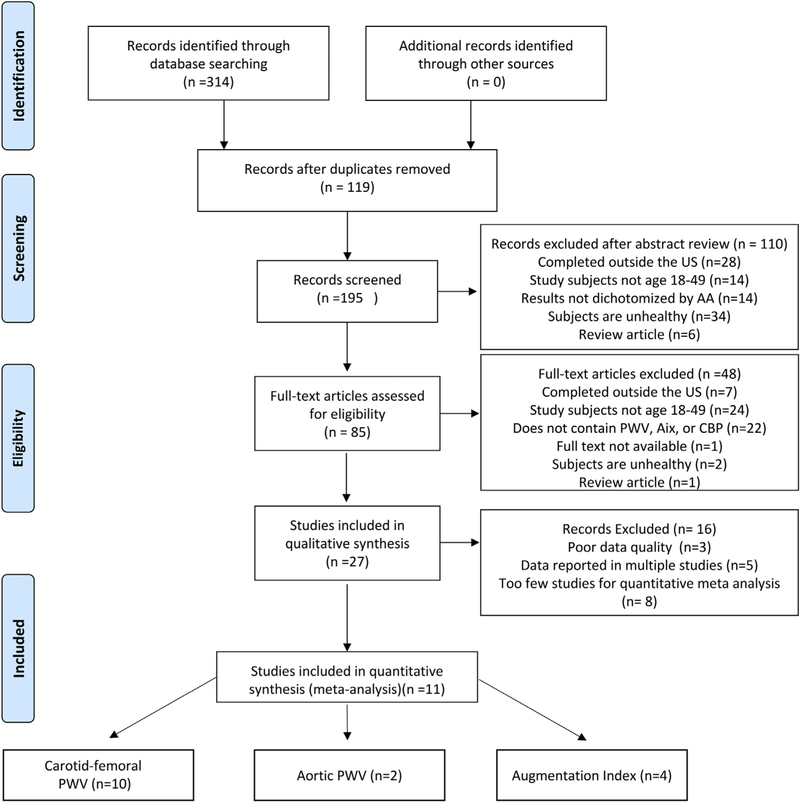
Our team conducted a systematic review of the literature based on methodological recommendations from the Cochrane Handbook for Systematic Reviews and Meta-analysis,11 Table 1. We reported our data according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Figure 1).
Table 1.
Structured question.
| Terms of PICO | Definition of terms |
|---|---|
| Population | African Americans or African Americans between 18 and 49 years of age |
| Exposure | Race in the United States |
| Comparator | Whites between 18 and 49 years of age |
| Outcomes | Pulse wave velocity, central blood pressure, augmentation index |
| Study design | Cross-sectional, case-control, longitudinal, retrospective, and prospective studies |
Fig. 1. PRISMA flow diagram.
A PRISMA flow diagram of studies selected for inclusion in this systematic review and meta-analysis.
Eligibility criteria
Inclusion criteria.
Qualified publications were required to meet the following inclusion criteria: (i) study subjects had to be healthy adults with a mean age between 18 and 49 years; (ii) measures of CBP or PWV or AIx were available; (iii) the study population included non-Hispanic African Americans or African Americans (black Americans of African descent living in the United States) and non-Hispanic Whites as a comparator group; (v) the study was completed in the United States; (vi) and the full-text had to be available.
Exclusion criteria.
Publications were excluded from the study if they met the following criteria: (i) the participants were unhealthy; (ii) the study did not stratify results by African Americans or non-Hispanic Whites; (iii) the published article was an editorial; (iv) and if the published article was a review.
Information sources and search strategy
Based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a systematic search of SCOPUS and PubMed databases was conducted to find articles published up to November 29, 2017. Search terms used to identify eligible studies included: “augmentation index”, “central blood pressure”, “pulse wave velocity”, “African American”, and “African American” (Table 2). The articles were observational, longitudinal, retrospective, and prospective designs. Languages other than English were not considered for review. All retrieved publications were managed using EndNote X8 software.
Table 2.
Search strategy for PubMed/Medline.
| Terms of PICO | Terms and synonyms | Connection |
|---|---|---|
| Population | 2. African American | |
| 6. Pulse wave velocity [mesh or text word] | ||
| Combination | 8. 3 AND 11 | |
| Additional selection criteria | Articles in English |
Study selection and data management
Based on the above inclusion and exclusion criteria, an independent review process of the title and abstract was conducted by two investigators independent of each other. If the two independent investigators experienced uncertainty or ambiguity about one of the manuscripts, a third reviewer was consulted for resolution. Once these publications were identified, a full text review was conducted by the two aforementioned investigators independently. Once the articles are identified based on the inclusion/exclusion criteria, the manuscript quality assessment and data abstraction process was conducted.
Data extraction
A comprehensive data collection table was designed in Microsoft Excel (version 15.21.1) to collect information about each publication. The variables included in this table were author, year, type of participants, mean age for each racial/sex group, the total number of people in each race/sex group, the relevant measures (means, standard deviations, standard errors) for PWV, AIx, CBP, and the type of instrument used. Only one author was responsible for this data extraction process. However, the data quality was examined by the team biostatistician.
Grading of the evidence and assessing bias.
We used the Newcastle-Ottawa Scale to assess the quality of the nonrandomized study groups. Studies were judged on three broad categories including: the selection of study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest. Studies included in the analysis were cross-sectional. We assessed for publication bias using funnel plots and the Egger and Begg-Masumdar: Kendall’s tau bias indicators.
Data synthesis and analysis
Review Manager 5.3 (Cochrane IKMD-Copenhagen, Denmark; Freiburg, Germany; London, UK; USA) was used to conduct all comparisons of mean aorta-femoral PWV (afPWV), carotid-femoral PWV (cfPWV), aortic systolic and diastolic blood pressures (aSBP/aDBP), augmentation index (AIx) and augmentation index @ HR 75 (AIx @75) within these meta-analyses. The Mantel-Haenszel Chi2 test was used to assess heterogeneity within the data. We used I2 to determine the percentage of variability in effect estimates resulting from heterogeneity where we considered an I2 <25% as low, <50% as moderate, and <75% as high heterogeneity. Random-effects models were used for all analysis, as these allow for the possibility of racial differences to vary from study to study. Effect sizes were compared using z-tests. Each meta-analysis included a test (using 2-sided hypothesis testing) of whether the mean PWVs were significantly different (i.e. p < 0.05) between whites and AAs and whether there was significant heterogeneity in the racial difference across studies. Analyses also included 95% confidence intervals for the mean differences between whites and AAs and forest plots of mean differences. A total of 10 meta-analyses were conducted, including a total of 6 comparisons (overall and gender-specific) for cfPWV and AIx. Analyses were also conducted for aSBP and aDBP, AIx @75, and afPWV. When data on males and females were reported separately within a given study, pooled means and standard deviations were calculated using SAS v. 9.4 (SAS Institute, Cary, NC) by incorporating gender-specific sample sizes, means, and standard deviations (or standard errors).
RESULTS AND DISCUSSION
Study selection and description of included studies
The PRISMA flow diagram of selection of papers in the systematic review and meta-analysis is shown in Figure 1. Using predefined keywords, our search returned a total of 314 publications, (SCOPUS = 195 and PubMed = 119) that met our initial inclusion criteria. The search covered from March 1995 November, 2017. After accounting for 119 duplicates, two independent reviewers assessed the eligibility of 195 articles by reviewing title/abstract content against the review inclusion criteria, with disagreements resolved by discussion and if necessary, a third reviewer. Eighty-five full text articles met the inclusion criteria based on information presented in the title/abstract and were reviewed accordingly. Among the potential 85 full text articles reviewed, 27 full-text articles were used for qualitative analysis. Out of the studies excluded from the qualitative analysis, 24 included study participants outside of the defined age range, 22 did not include the specified measures of arterial stiffness, 7 studies were completed outside the US, 1 study was a review article, 1 study was not accessible, and 2 articles included subjects who were unhealthy. Studies potentially reporting on the same population were examined and the study with the highest number of participants captured was included in the meta-analysis. This eliminated 5 studies that included information from the Bogalusa Heart Study. Although articles assessing brachial-ankle PWV, femoral-dorsalis pedis PWV, and carotid PWV met the article inclusion criteria, there were too few studies on each measurement to conduct a meta-analysis using these articles. Other articles were excluded as a result of missing variables such as specific age of the study population and/or data presented in graph form without actual values provided. Eleven articles were included in the final quantitative meta-analysis. There were 1645 whites and 1888 AA included in the analysis for cfPWV analysis. There were 762 whites and 316 AA, included in the analysis for aortic-femoral PWV studies. The AIx studies included 605 whites and 525 AA while the AIx@75 meta-analysis included 130 whites and 122 AA. The aSBP and aDBP meta-analysis included 123 whites and 129 AA. Participants were from the general population and free of documented disease. All of the included studies were published from 2006 to 2017. The sample sizes of studies ranged from 24 participants to 1029 participants. The baseline characteristics of the individual studies are shown in Table 3. The Newcastle-Ottawa Scale (NOS)12 was used to determine the quality of the articles included in the meta-analysis. Most studies provided moderate-high quality data as a whole with scores that ranged between 5 stars to 6 stars (Table 3).
Table 3.
Demographics and characteristics of individual studies.
| Study | Population | White, Sample Size (age +SD) | AA, Sample size (age ± SD) | Male (%), white | Male (%), AA | NOS score |
|---|---|---|---|---|---|---|
| Heffernan, K., 200721 | Healthy Men | 12 (22 ± 3.5) | 12 (22 ± 3.5) | 100.0% | 100.0% | 5 |
| Heffernan, K., 200813 | Healthy Men | 30 (23.6 ± 3.8) | 25 (23.6 ± 2) | 100.0% | 100.0% | 5 |
| Ruan, L., 200935 | Community-based cohort | 735 (36.5 ± 4) | 294 (36.0 ± 4) | 45.7% | 36.7% | 6 |
| Heffernan, K., 200916 | Healthy men | 18 (23 ± 3.0) | 19 (22 ± 1.7) | 100.0% | 100.0% | 5 |
| Ashraf, A., 201214 | Healthy adults | 26 (28 ± 9.9) | 26 (32 ± 10.5) | 23.0% | 7.7% | 5 |
| Morris, A., 201219 | Healthy adults | 469 {49 ± 11) | 386 (47 ± 10) | 41.4% | 48.4% | 5 |
| Yan, H., 201417 | Healthy Adults | 48 (24 ± 4.9) | 52 (24.9 ± 5.0) | 52.0% | 53.9% | 5 |
| Yun, M., 201536 | Smokers | 661 (36.5) | 284 (36.5) | 44.6% | 35.9% | 5 |
| RanadiveS., 201620 | Healthy Adults | 42 (24.3 ± 5.2) | 49 (24.5 ± 4.9) | 52.4% | 38.8% | 5 |
| Wendell, C, 201715 | Low SES, Healthy Adults | 287 (48.2 ± 9.4) | 592 (46.7 ± 9.1) | 37.6% | 42.2% | 5 |
| Wendell, C, 201715 | High SES, Healthy Adults | 686 (47.1 ± 9.5) | 705 (48.3 ± 9.4) | 46.4% | 45.0% | 5 |
| Yan, H., 201718 | Healthy Adults | 27 (21 ± 5.2) | 22 (23 ± 4.7) | 51.9% | 41.0% | 5 |
Racial differences in measures of arterial stiffness
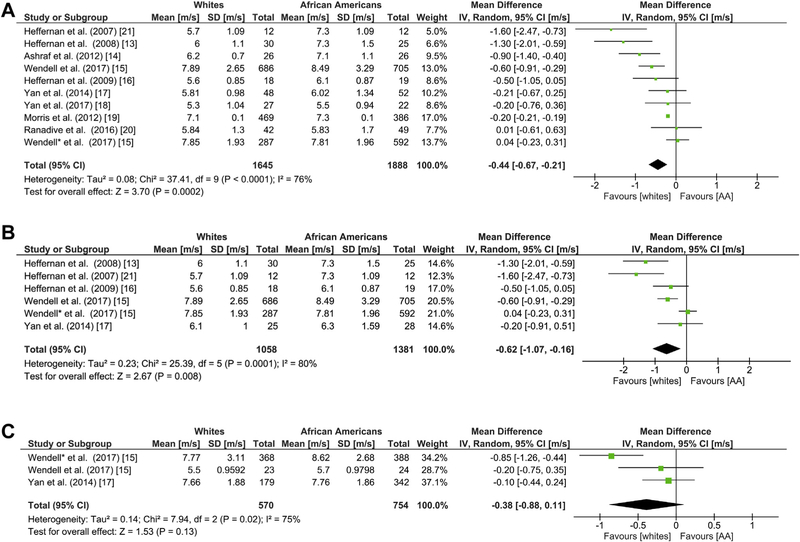
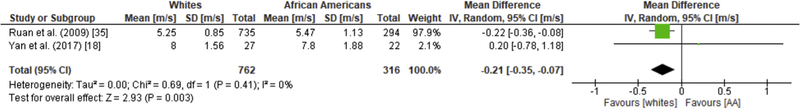
Carotid-femoral PWV is a surrogate measure of aortic stiffness (aPWV). Ten studies examined the association of race with differences in cfPWV13–21. White race was associated with a difference of −0.44 m/s (95% CI: −0.67, −0.21, Figure 2A) in cfPWV. Given that marked heterogeneity was detected (I2 = 76%), we conducted a subgroup analysis based on sex to identify sources of heterogeneity. Six studies were pooled to examine differences in males.13,15–17,21 The mean difference in AA and white male cfPWV was statistically significant but the heterogeneity in this subgroup was slightly higher (I2 = 80%). Two studies, one with two different subset populations based on socioeconomic status, were used for the analysis of cfPWV in AA and white females which showed high heterogeneity (I2 = 75%) and a mean difference of −0.38 m/s (95% CI: −0.88, 0.11; Z = 1.53, p = 0.13) suggesting that cfPWV was higher in AA females compared to whites; however, these differences were not statistically significant (Figure 2B and C).15,17 Two studies were pooled to explore the association between afPWV and race.18,22 Whites had a different mean afPWV compared to AAs (–0.21; 95% CI –0.35, –0.07) corresponding to an overall effect ofZ = 2.93 (p = 0.003), Figure 3.
Fig. 2. Racial Differences in cfPWV.
Forest plots showing the mean differences in cfPWV pulse wave velocity as measured in meters/second (m/s) between African Americans and whites in (a) all sexes (b) male, and (c) female groups. The estimated mean difference for each group is presented as a square with the corresponding 95% confidence interval (CI). The pooled estimated mean difference is depicted as a diamond and derived from the random effects model.
Fig. 3. Racial differences in afPWV.
Forest plots showing the mean differences aortic-femoral pulse wave velocity as measured in meters/second (m/s) between African Americans and whites. The estimated mean difference foreach group is presented as a square with the corresponding 95% confidence interval (CI). The pooled estimated mean difference is depicted as a diamond and derived from the random effects model.
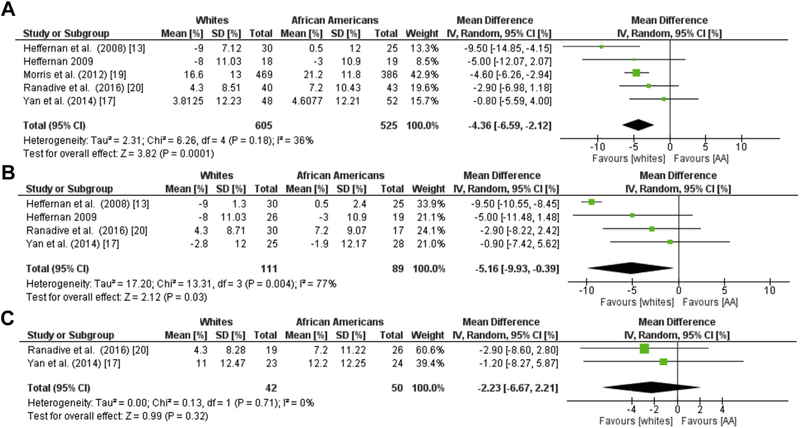
Augmentation index, a systemic measure of arterial stiffness derived from the ascending aortic pressure wave-form, was evaluated in five of the included studies. In 605 whites and 525 AA, a lower AIx was observed in whites compared to AA with a mean difference of −4.36% (95% CI: −6.59, −2.12, I2 = 36%, Z = 3.83, p = 0.0001; Figure 4A).13,16,17,19,20 We observed moderate heterogeneity in this meta-analysis and conducted additional sex based subgroup analysis. A meta-analysis based on sex revealed that male differences in AIx followed the same trends with a mean difference in AIx of−2.95% (95% CI: −5.91, −0.00, I2 = 0%, Z = 1.96, p = 0.05; Figure 4B).13,16,17,20 However, these differences did not persist amongst females (Figure 4C).17,20 An additional meta-analysis of four studies was used to examine racial differences in AIx @75.13,14,16,17 This analysis included 130 whites and 122 AA, and the studies were homogeneous (I2 = 0%). Whites had a lower mean difference in AIx@75 compared to AA (−6.26, 95% CI: −9.19, −3.33; I2 = 0%; Z = 4.10, p < 0.0001; Figure 5).
Fig. 4. Racial Differences in Aix.
Forest plots showing the mean differences in augmentation index (%) between African Americans and whites in (a) all sexes (b) male, and (c) female groups. The estimated mean difference for each group is presented as a square with the corresponding 95% confidence interval. The pooled estimated mean difference is depicted as a diamond and derived from the random effects model.
Fig. 5. Racial Differences in Aix@75.
Forest plots showing the mean differences in augmentation index normalized to 75 bpm (%) between African Americans and whites. The estimated mean difference for each group is presented as a square with the corresponding 95% confidence interval. The pooled estimated mean difference is depicted as a diamond and derived from the random effects model. BPM, beats per minute.
Racial differences in measures of central blood pressure
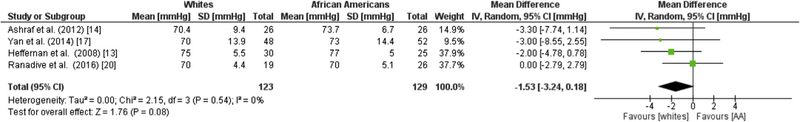
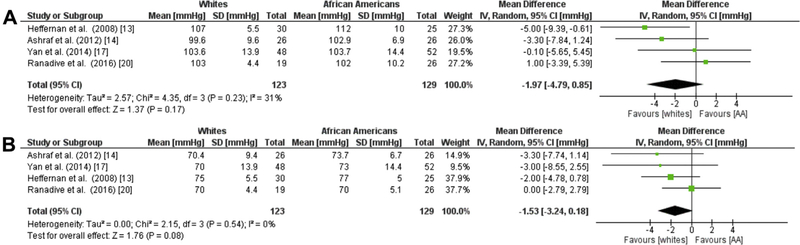
Four studies evaluating a total of 123 whites and 129 AAs showed no significant difference in aSBP or aDBP in AAs compared to whites (SBP −1.97 mmHg; 95% CI = −4.79,0.85; I2 = 31%) and (DBP −1.53 mmHg; 95 CI = −3.24, 0.18; I2 = 0%; Figure 6A, B).13,14,17,20
Fig. 6. Racial Differences in cBP.
Forest plots showing the mean differences central blood pressure (%) between African Americans and whites for (a) aortic SBP and (b) aortic DBP. The estimated mean difference for each group is presented as a square with the corresponding 95% confidence interval. The pooled estimated mean difference is depicted as a diamond and derived from the random effects model. SBP, systolic blood pressure; DBP, diastolic blood pressure.
Publication bias.
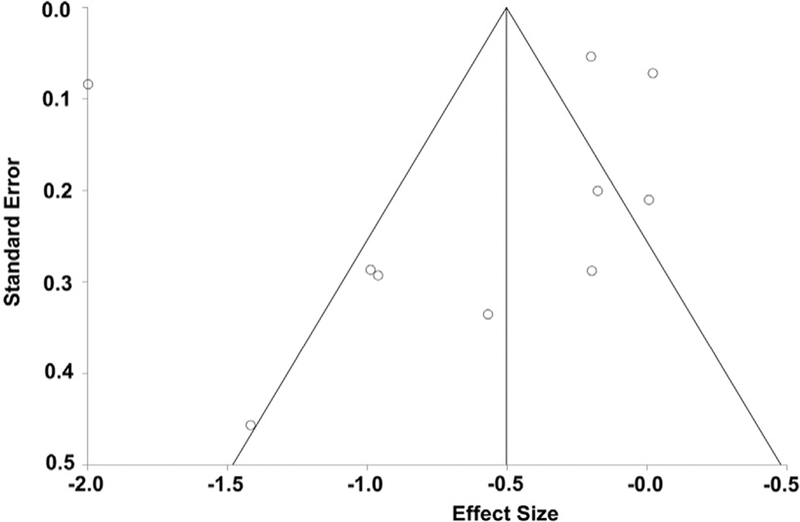
To assess the quality of the data, we used the Newcastle-Ottawa Scale (NOS). The median NOS value of quality assessments was 5.1 which suggest that the quality of the publications was moderate quality. To investigate potential publication bias, we utilized a funnel plot of included studies in the meta-analysis of the cfPWV (Figure 7) because it was the most inclusive. The vertical axis represents standard error in means. Based on the relative symmetry of this plot, we concluded that there was no evidence of gross publication bias, and Egger’s test result was not statistically significant (Egger’sbias = −1.42;p = 0.71).
Fig. 7. Funnel plot of publication effect size in cfPWV studies.
The outer lines outline the triangular region where 95% of studies are expected to fall in the absence of publication biases and heterogeneity.
Systematic review results.
We observed several additional outcome measurements that we have excluded from our study as a result of the insufficient availability of data to perform a meaningful meta-analysis. For example, Heffernan et al. and Yan et al. measured femoral-superior dorsalis pedis (peripheral PWV) in studies included in this meta-analysis.13,17,21 However, the combined number of participants between the three studies were insufficient. Heffernan et al.13,21 and Yan et al.17 showed that there was no difference in peripheral PWV among AAs and whites. Carotid-radial PWV (radial) was also measured in several studies included in the qualitative analysis but was not reported in the quantitative meta-analysis. In a study examining arterial stiffness amongst monozygotic and dizygotic twins who were normotensive (excluded based on age) or prehypertensive, Zhu et al. showed that radial PWV was not different amongst prehypertensive whites and AAs.23 This same group of investigators examined radial PWV in healthy weight, at risk of overweight and overweight adults and found no significant differences based on race.23 They also examined cfPWV (aortic) and found similar trends in both studies.24
Our analysis also included measures of brachial-ankle PWV. Li et al. observed racial differences (p < 0.001) in brachial-ankle PWV (baPWV) in 835 participants who were registered for the Bogalusa Heart Study.25 Additionally, Guo et al. measured baPWV and found that no differences existed amongst AAs and whites.22 However, this study included other populations as well which may have increased the statistical rigor for identifying true differences.
Goel et al. assessed aortic arch PWV and showed a log difference amongst black and white participants in the Dallas Heart Study.26 However, there were no other studies in our qualitative analysis that assessed aortic arch PWV. Other parameters such peripheral AIx were statistically different between AAs and whites in this study; however, the number of studies reporting on this measure was insufficient for a meta-analysis.
Summary of results
In this meta-analysis we examined vascular measures in 2841 white and 2466 black study participants. We observed a significant increase in arterial stiffness as measured by cfPWV and afPWV and an increase in wave reflection as measured by central AIx in healthy AA. In a subgroup analysis based on participant sex, the increase in cfPWV was significant in males but not females. In a subgroup analysis of AIx, the increase in wave reflection trended towards significance in males but not females. We also observed that central AIx@75 bpm arterial wave reflection measurements were significantly different between the two racial groups. Therefore, these assessments may be considered a potential tool for predicting and understanding premature cardiovascular disease risk beyond brachial blood pressure measurements in AA.
Individuals of African descent living in the United States have a higher burden of mortality associated with CVD compared to whites.27 Moreover, disparities in CVD are most prominent at younger ages between AA and whites.28,29 Strategies to detect the early onset of CVD risk in healthy populations not presenting with conventional CVD risk factors are missing. Evidence has accumulated demonstrating that arterial stiffness is an important CVD risk factor for cardiovascular disease independent of traditional risk factors30 and that aortic stiffness measures such as cfPWV serve as the ‘gold standard’ for predicting cardiovascular disease risk amongst younger individuals.31,32 Although assessments of arterial stiffness are included in the European Society of Hypertension and European Society of Cardiology (ESC) guidelines,33 the new ACC/AHA Hypertension guidelines do not include recommendations for these assessments as authors articulated a lack of evidence showing benefit for arterial stiffness screenings in asymptomatic individuals.34 However, strategies that address CVD disparities in younger, asymptomatic AA are needed to change the trajectory of health disparities in the US.
Herein, we analyzed previously published studies examining differences in vascular measures between AA and white populations. This summary provides additional evidence that racial differences in vascular stiffness measures persist in young, healthy, asymptomatic individuals. Moreover, measures including cfPWV, afPWV, and AIx may be suitable targets for reducing CVD disparities.
Sources of heterogeneity.
We observed statistical heterogeneity in the pooled data for cfPWV derived from various sources. First, different instruments and methodologies were used to assess cfPWV (see Table 4). Tonometry-based techniques using the SphyghmoCor device, (AtCor Medical, Australia) are reproducible based on Bland-Altman plot-analysis and were used for 70% of the studies included in this analysis but only 35% of the participants.31 The other methods were tonometry based but utilized non-directional transcutaneous Doppler probes and electrocardiogram monitoring. In addition, the aorta is an elastic capacitive vessel and elasticity of these vessels is directly impacted by age. Although we limited the mean reported age for each study to 18–49, we did not limit the standard deviation ranges nor did we conduct subgroup analysis based on age. However, the age range did not appear to impact other meta-analysis included in this report. Studies from 2007 to 2017 were included for cfPWV measures. In addition to variations in instrumentation, a comprehensive guideline of recommendations on how to measure PWV were released in 2015 by Townsend et al.31 Thus, techniques used to assess PWV may have varied between research teams. Despite high heterogeneity, a publication bias analysis demonstrated that bias did not exist for the cfPWV meta-analysis.
Table 4.
Study outcomes and assessment tools.
| Study | Outcomes | Tools Used for Assessment |
|---|---|---|
| Heffernan, K., 2007 | cfPWV | Applanation tonometry high-fidelity strain-gauge transducer (Millar Instruments, Houston TX, USA) and electrocardiogram monitoring |
| Heffernan, K., 2008 | cfPWV, Aortic Alx and AiX@75, Aortic SBP/DBP | Applanation tonometry (Millar Instruments, Houston, TX) and electrocardiogram monitoring SphygmoCor (AtCor Medical, Sydney Australia) |
| Ruan, L., 2009 | afPWV | Echocardiograph (ECG, Power Vision Toshiba SSH-380 Digital Ultrasound System, Toshiba America Medical Systems Carrollton, TX) Nondirectional transcutaneous doppler flow probe (Toshiba PCK3AT, 7.5 MHz) |
| Heffernan, K., 2009 | cfPWV, Aortic Alx and Alx@75 aortic SBP,DBP | Applanation tonometry high-fidelity strain gauge transducer (Millar Instruments, Houston TX, USA) and SphygmoCor (AtCor Medical, Sydney, Australia) |
| Ashraf, A., 2012 | cfPWV, Aortic Alx and Alx@75, aortic SBP/DBP | SphygmoCor (AtCor Medical, Sydney, Australia) |
| Morris, A., 2012 | cfPWV, Aortic Alx and Alx@75 | SphygmoCor (AtCor Medical, Australia), tonometry and electrocardiographic gating |
| Yan, H., 2014 | Alx, cfPWV, Aortic Alx and Alx@75, aortic SBP/DBP, | Applanation tonometry high-fidelity strain gauge transducer(Millar Instruments, Houston TX, USA) and SphygmoCor (AtCor Medical, Sydney, Australia) |
| RanadiveS., 2016 | cfPWV, Aortic Alx, | Applanation tonometry high-fidelity strain gauge transducer(Millar Instruments, Houston TX, USA) and SphygmoCor (AtCor Medical, Sydney, Australia) |
| Wendell, C, 2017 | cfPWV | Non-directional transcutaneous doppler probe (Model 810A, 9–10-Mhz probes, Parks Medical Electronics, Inc.) |
| Wendell, C, 2017 | cfPWV | Non-directional transcutaneous doppler probe (Model 810A, 9–10-Mhz probes, Parks Medical Electronics, Inc.) |
| Yan, H., 2017 | cfPWV and afPWV | Applanation tonometry high-fidelity strain gauge transducer(Millar Instruments, Houston TX, USA) and SphygmoCor (AtCor Medical, Sydney, Australia) |
Strengths and limitations.
The strengths of this review include the novelty of the pooled groups of data. To our knowledge, this is the first meta-analysis to assess racial differences in vascular stiffness measures in healthy study subjects. Another strength of this review includes the validity of the search, and the statistical approach including random effects. We also used multiple measures of PWV and AIx as well as CBP measures to examine racial differences in the vasculature.
However, we are limited in the interpretation of the results from this meta-analysis. First, the heterogeneity in pooled studies from the cfPWV analysis impact the validity of the analysis and suggest that additional studies should be performed in order to limit the heterogeneity. There were a limited number of studies included in the quantitative analysis for afPWV. This assessment was primarily used in the Bogalusa Heart Study. We eliminated 5 articles from this study to avoid analyzing the same population multiple times. The number of individuals included in the analysis for the AIx, AIx@ 75, and the central SBP/DBP measures were also limited as these measures were not frequently used in studies indexed based on our search criteria. Whether or not these tools would be useful for understanding disparities in premature loss of arterial compliance in the future is unknown.
IMPLICATIONS
The results of the present study may have important clinical implications. Brachial pressure, the current standard for cardiovascular disease risk, is similar among young AAs and whites. However, to-date, measurements of arterial stiffness have not been exploited as predictors of cardiovascular disease risk as they are not currently utilized for treatment decisions in a clinical setting. Utilization of these measurements might provide early insight on disease development in young AAs, leading to early intervention and subsequent prevention of cardiovascular disease. Additional prospective studies and specifically focused research is needed to clarify associations between race and arterial stiffness measures.
Acknowledgements:
Funding for this project was provided by the American Heart Association #15SFDRN25870000; #15SFDRN24480016 (JB, GM, RA) and the National Institutes of Health (National Institute of Arthritis and Musculoskeletal and Skin Diseases grant number P30-AR072582 (PN), National Center for Advancing Translational Science grant number UL1-TR001450 (PN), and National Institute of General Medical Sciences U54-GM104941) (PN).
Footnotes
Declarations of interest: none.
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jnma.2018.06.001.
REFERENCES
- 1.Gillespie CD, Wigington C, Hong Y, &, Centers for Disease, C. and Prevention. (2009). (2013) Coronary heart disease and stroke deaths - United States. MMWR Suppl, 62, 157–160. [PubMed] [Google Scholar]
- 2.Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities, Hyattsville (MD) (2016). [PubMed] [Google Scholar]
- 3.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, & Sorlie P (2004). The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension, 44, 398–404. [DOI] [PubMed] [Google Scholar]
- 4.Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, & Stefanadis C (2010). Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J, 31, 1865–1871. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, & Shi GP (2014). Vascular wall extracellular matrix proteins and vascular diseases. Biochim Biophys Acta, 1842, 2106–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohn JC, Lampi MC, & Reinhart-King CA (2015). Age-related vascular stiffening: causes and consequences. Front Genet, 6, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson KA, Lindholt JS, Hoskins PR, Heickendorff L, Vammen S, & Bradbury AW (2001). The relationship between abdominal aortic aneurysm distensibility and serum markers of elastin and collagen metabolism. Eur J Vasc Endovasc Surg, 21, 175–178. [DOI] [PubMed] [Google Scholar]
- 8.Tsamis A, & Stergiopulos N (2009). Arterial remodeling in response to increased blood flow using a constituent-based model. J Biomech, 42, 531–536. [DOI] [PubMed] [Google Scholar]
- 9.Zieman SJ, Melenovsky V, & Kass DA (2005). Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol, 25, 932–943. [DOI] [PubMed] [Google Scholar]
- 10.Din-Dzietham R, Couper D, Evans G, Arnett DK, & Jones DW (2004). Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens, 17, 304–313. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT (2011). G.S. (Updated March 2011) Cochrane Handbook for Systematic Reviews of Inverventions Version 5.1.0. The Cochrane Collaboration. [Google Scholar]
- 12.Stang A (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol, 25, 603–605. [DOI] [PubMed] [Google Scholar]
- 13.Heffernan KS, Jae SY, Wilund KR, Woods JA, & Fernhall B (2008). Racial differences in central blood pressure and vascular function in young men. Am J Physiol Heart Circ Physiol, 295, H2380–H2387. [DOI] [PubMed] [Google Scholar]
- 14.Ashraf AP, Fisher G, Alvarez J, et al. (2012). Associations of C-reactive protein to indices of vascular health and the influence of serum 25(OH)D status in healthy adults. J Nutr Metab, 2012, 475975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendell CR, Waldstein SR, Evans MK, & Zonderman AB (2017). distributions of subclinical cardiovascular disease in a socioeconomically and racially diverse sample. Stroke, 48,850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan KS, Fahs CA, Iwamoto GA, et al. (2009). Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis, 207, 220–226. [DOI] [PubMed] [Google Scholar]
- 17.Yan H, Ranadive SM, Heffernan KS, et al. (2014). Hemodynamic and arterial stiffness differences between African-Americans and Caucasians after maximal exercise. Am J Physiol Heart Circ Physiol, 306, H60–H68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan H, Ranadive SM, Lane-Cordova AD, et al. (2017). Effect of acute aerobic exercise and histamine receptor blockade on arterial stiffness in African Americans and Caucasians. J Appl Physiol (1985), 122, 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris AA, Patel RS, Binongo JN, et al. (2013). Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc, 2, e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranadive SM, Yan H, Lane AD, et al. (2016). Aerobic exercise training and arterial changes in African Americans versus Caucasians. Med Sci Sports Exerc, 48, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffernan KS, Jae SY, & Fernhall B (2007). Racial differences in arterial stiffness after exercise in young men. Am J Hypertens, 20, 840–845. [DOI] [PubMed] [Google Scholar]
- 22.Guo J, Fujiyoshi A, Masaki K, et al. (2017). The role of initial and longitudinal change in blood pressure on progression of arterial stiffness among multiethnic middle-aged men. J Hypertens, 35, 111–117. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, Yan W, Ge D, et al. (2008). Relationships of cardio-vascular phenotypes with healthy weight, at risk of overweight, and overweight in US youths. Pediatrics, 121, 115–122. [DOI] [PubMed] [Google Scholar]
- 24.Zhu H, Yan W, Ge D, et al. (2007). Cardiovascular characteristics in American youth with prehypertension. Am J Hypertens, 20, 1051–1057. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Chen W, Yun M, et al. (2014). Sex and race (black-white) differences in the relationship of childhood risk factors to adulthood arterial stiffness: the Bogalusa Heart Study. Am J Med Sci, 348, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goel A, Maroules CD, Mitchell GF, et al. (2017). Ethnic difference in Proximal aortic stiffness: an observation from the Dallas heart study. JACC Cardiovasc Imaging, 10, 54–61. [DOI] [PubMed] [Google Scholar]
- 27.Carnethon MR, Pu J, Howard G, et al. (2017). Cardiovascular health in African Americans: a Scientific statement from the American heart association. Circulation, 136, e393–e423. [DOI] [PubMed] [Google Scholar]
- 28.Loehr LR, Rosamond WD, Chang PP, Folsom AR, & Chambless LE (2008). Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol, 101, 1016–1022. [DOI] [PubMed] [Google Scholar]
- 29.Chan PS, Nichol G, Krumholz HM, et al. , American Heart Association National Registry of Cardiopulmonary Resuscitation, I. (2009). Racial differences in survival after in-hospital cardiac arrest. JAMA, 302, 1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlachopoulos C, Aznaouridis K, & Stefanadis C (2010). Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol, 55, 1318–1327. [DOI] [PubMed] [Google Scholar]
- 31.Townsend RR, Wilkinson IB, Schiffrin EL, et al. (2015). Recommendations for Improving and Standardizing vascular research on arterial stiffness: a Scientific statement from the American heart association. Hypertension, 66, 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Shlomo Y, Spears M, Boustred C, et al. (2014). Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol, 63, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjeldsen SE, Aksnes TA, & Ruilope LM (2014). Clinical implications of the 2013 ESH/ESC hypertension guidelines: targets, choice of therapy, and blood pressure monitoring. Drugs R, 14, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton PK, Carey RM, Aronow WS, et al. (2017). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of high blood pressure in adults: a Report of the American College of Cardiology/American heart association Task Force on clinical Practice guidelines. Hypertension, 71, e13–e15. [DOI] [PubMed] [Google Scholar]
- 35.Ruan L, Chen W, Srinivasan SR, et al. (2009). Relation of plasma homocysteine to arterial stiffness in black and white young adults (from the Bogalusa Heart Study). Am J Cardiol, 103, 985–988. [DOI] [PubMed] [Google Scholar]
- 36.Yun M, Li S, Sun D, et al. (2015). Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa Heart Study. J Hypertens, 33, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]