Abstract
Background
After menopause, several androgens continue to be produced primarily by the adrenal glands; these can be converted into estrogens via aromatization or into androgen metabolites. It is unclear if androgens are associated with endometrial cancer risk independently of their being precursors to estrogens or if alternative metabolic pathways influence risk.
Methods
We measured prediagnostic serum concentrations of 12 androgens and their metabolites using highly sensitive liquid chromatography–tandem mass spectrometry assays in a nested case-control study of postmenopausal women from the Women’s Health Initiative Observational Study (313 endometrial cancer case subjects, 354 matched control subjects). Estrogens were previously assayed. We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for endometrial cancer with adjustment for confounders.
Results
Compared to the lowest concentrations, the highest levels of adrenal androgens were associated with increased endometrial cancer risk: dehydroepiandrosterone (5th vs 1st quintile: OR = 1.85, 95% CI = 1.06 to 3.25), androstenedione (OR = 2.36, 95% CI = 1.34 to 4.16), and testosterone (OR = 1.91, 95% CI = 1.12 to 3.24). Downstream androgen metabolites were not associated with endometrial cancer. Although increased risks for the parent androgens were still suggested after adjustment for unconjugated estradiol, the associations attenuated, and with the exception of androstenedione, were no longer statistically significant. We also evaluated ratios of estrogens relative to their androgenic precursors; both higher unconjugated estrone:androstenedione and higher unconjugated estradiol:testosterone were associated with increased endometrial cancer risk.
Conclusions
We identified increased risks for endometrial cancer with the highest levels of adrenal androgens and high levels of estrogens relative to these androgens. As adrenal androgens can be aromatized to estrogens, this suggests androgens likely influence endometrial carcinogenesis via estrogen metabolism.
Higher estrogen levels over the life course, especially when unopposed by progesterone, are a well-known risk factor for endometrial cancer (1). We reported that many estrogens and estrogen metabolites are associated with substantially increased risk for endometrial cancer in postmenopausal women (2). However, after menopause, estrogen levels decrease markedly and endometrial cancer risk is highest. Several androgens continue to be produced primarily by the adrenal glands; these can be converted into estrogens via aromatization or into androgen metabolites. It is unclear if androgens influence endometrial cancer risk through their being precursors to estrogens or if alternative androgenic metabolic pathways can influence risk. Existing prospective studies exploring circulating androgens and endometrial cancer relied on assays with limited sensitivity (3,4). Another analysis used highly sensitive liquid chromatography–mass spectrometry (LC-MS) assays, but measured androgens in serum drawn at the time of cancer diagnosis (5).
We determined whether prediagnostic circulating androgens were associated with endometrial cancer risk using data from a nested case-control study of postmenopausal women from the Women's Health Initiative (WHI) Observational Study (OS). We measured 12 androgens and androgen metabolites in prediagnostic serum using LC–tandem mass spectrometry (LC-MS/MS).
Methods
Study Population
The WHI-OS is a prospective cohort that enrolled 93 676 postmenopausal women, aged 50 to 79 years, at 40 US centers between 1993 and 1998 (6, 7). Women who had medical conditions with a predicted survival of less than 3 years or adherence/retention issues or who were participating in a clinical trial were excluded. At baseline, women in the WHI-OS completed self-administered questionnaires and had a physical examination, which included measurement of height and weight for the calculation of body mass index (BMI).
Our analyses use data from a nested case-control study within the WHI-OS. We described the details of this study in an analysis of estrogen metabolites and endometrial cancer risk (2). To summarize, we identified women with incident invasive endometrial cancers diagnosed between study initiation and May 2012 as case subjects. Cancers were confirmed by centrally trained physician adjudicators according to Surveillance, Epidemiology, and End Results (SEER) guidelines (8). We excluded both case subjects and control subjects from selection if they had a history of cancer at baseline other than nonmelanoma skin cancer, were current users of exogenous hormones, or did not have at least 1.1 mL of baseline serum available. Control subjects were shared with a nested case-control study of ovarian cancer (2,9), which allowed our frequency matching control subjects at least 1:1 with endometrial cancer case subjects, within strata of age at blood draw (50–54, 55–59, 60–64, 65–69, 70–74, 75–79 years); year at blood draw (1993–1996, 1997–1998); race/ethnicity (white, black, Hispanic, other/unknown); and time since last menopausal hormone therapy (MHT) use (≤1, >1 year/never). Control subjects were alive and did not have hysterectomy at the time of diagnosis of their matched case or during follow-up. There were 313 endometrial cancer case subjects and 354 control subjects in this analysis. The mean time from sample collection to diagnosis was 6.9 years (SD = 3.7 years, range = 45 days–15.0 years).
Approval for conducting WHI was obtained from human subjects review at the Fred Hutchinson Cancer Research Center (WHI Clinical Coordinating Center) and all clinical centers. All participants provided written informed consent. Our project was reviewed and exempted by the Office of Human Subjects Research at the US National Cancer Institute.
Laboratory Assays
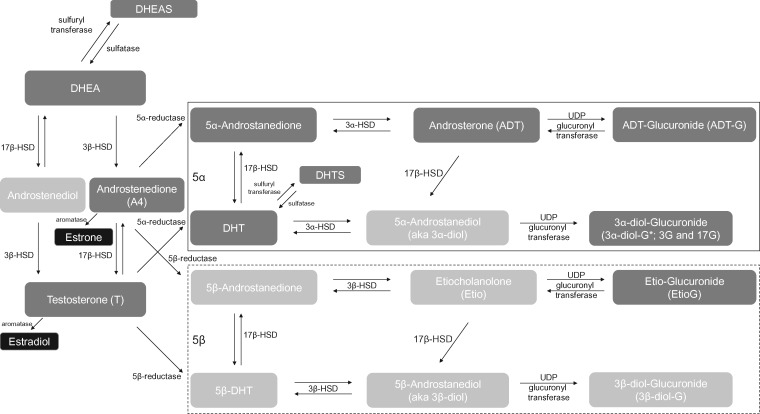
We measured prediagnostic serum concentrations of 12 androgens and androgen metabolites (Figure 1) (13). This included the principal androgens secreted by the adrenal glands: dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS), as well as androstenedione and testosterone, which are secreted by the adrenal glands and ovaries (10). We refer to these as “parent androgens” because they serve as precursors to other androgen metabolites and to the estrogens. In peripheral tissues, androstenedione and testosterone can be aromatized to form estrone or estradiol, respectively, or they can be metabolized via 5α-reductase or 5β-reductase. We previously assayed concentrations of unconjugated estrone and estradiol (2, 11). We measured seven androgen metabolites from the 5α pathway: 5α-androstane-3,17-dione (5α-androstanedione), dihydrotestosterone (DHT), dihydrotestosterone sulfate (DHTS), androsterone (ADT), ADT-glucuronide (ADT-G), and two glucuronidated isomers of 5α-androstane-3α,17β-diol (also called 5α-androstanediol or 3α-diol): 3α-diol-3G and 3α-diol-17G. Metabolites from the 5β-reduced pathway typically have less biologic activity and are found in lower concentrations than those from the 5α pathway. We assayed one of the more abundant 5β-reduced metabolites, etiocholanolone-glucuronide (Etio-G), which as a glucuronidated, excreted metabolite, is arguably a good marker of 5β-reductase activity.
Figure 1.
Overview of androgen metabolism (Figure previously published in Trabert et al. (13), used with permission). Using assays described by Trabert et al. (12), we measured the androgens and androgen metabolites in dark gray boxes; those in light gray boxes were not measured. Dehydroepiandrosterone is made in the adrenal glands and serves as a precursor for the synthesis of androgens and estrogens. The metabolic pathways at the top of the figure, framed by a solid line, are the predominant 5α pathways, which follow the initial catabolism of androstenedione and testosterone by 5α-reductase. The 5β pathways at the bottom of the figure, framed by a dotted line, are those that result from the catabolism of androstenedione and testosterone by 5β-reductase. We additionally used the sum of ADT-G + 3α-diol-3G + 3α-diol-17G as a marker of overall 5α-reduced androgenic activity (14). Estradiol and estrone were measured previously for an analysis on estrogen metabolism and endometrial cancer, see Brinton et al. (2). * = two isomers, 3α-diol-3G or 3α-diol-17G; DHEA = dehydroepiandrosterone; DHEAS = dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; DHTS = dihydrotestosterone sulfate; HSD = hydroxysteroid dehydrogenase; UDP = uridine diphosphate.
We used stable isotope dilution high-performance LC-MS/MS to quantify the androgens and androgen metabolites; we previously published these methods and provide details on the present analysis (eg, limits of quantification and coefficients of variation) in the Supplementary Material (available online) (12). Correlations between the measured hormones among control subjects were previously published (a shared control group was selected across these studies) (13). Correlations between androgens ranged from -0.08 (DHTS and 5α-androstanedione) to 0.85 (Etio-G and 3α-diol-3G). Correlations between unconjugated estradiol and the androgens ranged from 0.07 (5α-androstanedione) to 0.31 (testosterone).
Statistical Analysis
Androgens and androgen metabolites were analyzed individually and as ratios (eg, comparing adjacent metabolites from a metabolic pathway); we categorized these measures into quintiles based on the concentrations within control subjects. Additionally, we calculated a marker of 5α-reductase androgenic activity in tissues by summing ADT-G, 3α-diol-3G, and 3α-diol-17G, as described by Labrie and colleagues (14).
To estimate the overall associations between androgens and endometrial cancer, we used conditional logistic regression to generate odds ratios (ORs) and 95% confidence intervals (CIs), conditioned on matching factors (described previously) and adjusted for potential confounding factors chosen a priori: gravidity (ever, never), cigarette smoking status (never, former, current), BMI (<25, 25–29.9, ≥30 kg/m2), duration of oral contraception use (never, <5, 5 to <10, ≥10 years), and age at menarche (<12, 12–13, ≥14 years). For the overall associations, we also determined which of the effect estimates comparing the 5th vs 1st quintiles (Q5 vs Q1) were statistically significant using a Bonferroni corrected threshold of P < .003 (0.05/17 androgen measures). Tests for trend were based on the Wald statistic after modeling the median concentrations from each quintile of a given androgen as a continuous variable. We also ran the overall models with additional adjustment for unconjugated estradiol.
We evaluated differences by cancer characteristics (ie, heterogeneity), beginning with major subtype according to the “dualistic model” for endometrial cancer (15): type I (adenocarcinomas, n = 66; endometrioid adenocarcinomas, n = 194; mucinous tumors, n = 11) and type II (serous, n = 34; clear cell, n = 6; other tumors, n = 2). We also compared associations by stage at diagnosis among all subtypes (stage 2, n = 255; stage 3 and 4, n = 55; missing information, n = 3). Using a more relevant histopathologic comparison, we stratified by grade of the tumor (grades 1–2, n = 189; grades 3–4, n = 65; missing information, n = 59) among women with endometrioid adenocarcinomas or tumors with the less-specific classification of “adenocarcinomas.” Finally, we made comparisons by the time between blood collection and diagnosis (<5 years, n = 116 case subjects; ≥5 years, n = 197 case subjects). These analyses used multinomial logistic regression; all control subjects served as the reference group, and we adjusted for matching factors and the confounders. In models comparing cancer characteristics, time since menopausal hormone therapy use was defined as: never, ≤1 year, >1 year. We identified differences in associations across subgroups of cancer characteristics using χ2P values from models that treated the largest subgroup as the reference category and excluded control subjects.
We evaluated effect modification by BMI (<25, n = 235; 25–29.9, n = 194; ≥30 kg/m2, n = 236) and age at blood draw (<60 years, n = 192; ≥60 years, n = 475). Modification was assessed using the χ2P values from interaction terms between the modifiers of interest and the hormone exposures.
For sensitivity analyses, we excluded 1) potential outliers, defined as androgen concentrations greater than 5 SDs above the mean (the median number of excluded subjects per hormone was n = 14, minimum–maximum: 9–27) and 2) women who reported prior use of menopausal hormones (n = 220). These were complete-case analyses. All tests of statistical significance were two-sided and used an alpha of 0.05.
Results
We previously published details of the study population (2). In brief, we observed differences between case subjects and control subjects in line with known endometrial cancer risk factors. Compared to the control group, women with endometrial cancer had higher BMIs, earlier menarche, and later ages at menopause and less frequently reported current smoking and long-term oral contraceptive use.
Case subjects had higher median concentrations of parent androgens (DHEA, androstenedione, testosterone) compared with control subjects (P < .10; Table 1). DHEAS and several glucuronidated androgen metabolites were also elevated among case subjects, particularly ADT-G, 3α-diol-3G, and the marker of 5α-reduced androgenic activity. Concentrations of other androgens were similar between groups. Women with endometrial cancer had higher levels of parent estrogens and higher ratios of these estrogens relative to their androgenic precursors.
Table 1.
Median and interdecile ranges for concentrations (nmol/L) of measured androgens and androgen metabolites among endometrial cancer case subjects and control subjects
| Androgen or androgen metabolite | Median (10th–90th percentile) |
P * | |
|---|---|---|---|
| Cases (n = 313) | Controls (n = 354) | ||
| Parent androgens | |||
| DHEA | 5.5 (2.2–11.1) | 5.0 (2.1–10.7) | .10 |
| DHEAS | 1193.3 (512.9–3017.5) | 1161.8 (423.6–2657.9) | .10 |
| Androstenedione | 1.4 (0.9–2.4) | 1.3 (0.7–2.3) | .02 |
| Testosterone | 0.7 (0.3–1.3) | 0.6 (0.3–1.0) | .001 |
| 5α pathway | |||
| 5α-Androstanedione | 1.2 (0.8–2.4) | 1.3 (0.7–2.3) | .64 |
| DHT | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) | .34 |
| DHTS | 1.1 (0.5–2.4) | 1.1 (0.4–2.3) | .29 |
| ADT | 0.5 (0.4–0.9) | 0.5 (0.4–0.9) | .27 |
| ADT-G | 23.3 (9.1–65.7) | 19.7 (7.0–53.0) | .01 |
| 3α-diol-3G | 1.6 (0.7–4.3) | 1.4 (0.5–3.7) | .01 |
| 3α-diol-17G | 1.3 (0.5–3.4) | 1.2 (0.5–3.0) | .10 |
| Marker of androgenic activity | |||
| Sum of ADT-G + 3α-diol-3G + 3α-diol-17G | 26.4 (10.6–72.1) | 23.1 (8.4–58.3) | .01 |
| 5β pathway | |||
| Etio-G | 37.7 (14.7–87.6) | 33.6 (12.6–89.7) | .05 |
| Parent estrogens† | |||
| Unconjugated estrone | 73.7 (37.3–155.8) | 55.4 (30.1–115.9) | <.0001 |
| Unconjugated estradiol | 20.0 (6.3–55.0) | 11.6 (4.0–38.8) | <.0001 |
| Ratios | |||
| 5α-androstanedione: Androstenedione | 0.9 (0.4–2.0) | 1.0 (0.4–2.4) | .15 |
| Unconjugated estrone: Androstenedione | 49.8 (28.8–103.6) | 41.2 (23.4–97.8) | <.0001 |
| DHT: Testosterone | 0.3 (0.1–0.6) | 0.3 (0.2–0.6) | .01 |
| Unconjugated estradiol: Testosterone | 30.4 (10.6–74.8) | 18.4 (7.2–67.2) | <.0001 |
P value from Wilcoxon rank sum tests comparing distributions of a given hormone or hormone metabolite between case subjects and control subjects.
Estrogens were previously measured (concentrations in pmol/L); see Brinton et al. (2). 3α-diol-3G = 5α-androstane 3α,17β diol-3-glucuronide; 3α-diol-17G = 5α-androstane 3α,17β diol-17-glucuronide; ADT = androsterone; ADT-G = ADT-glucuronide; CI = confidence interval; DHEA = dehydroepiandrosterone; DHEAS = dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; DHTS = dihydrotestosterone sulfate; etio-G = etiocholanolone-glucuronide.
After adjustment for confounding, women with the highest parent androgen levels had almost twofold increased risk for endometrial cancer compared to women with the lowest levels (Q5 vs Q1; DHEA: OR = 1.85, 95% CI = 1.06 to 3.25; androstenedione: OR = 2.36, 95% CI = 1.34 to 4.16; and testosterone: OR = 1.91, 95% CI = 1.12 to 3.24) (Table 2; Supplementary Table 1, available online). These associations attenuated after additional adjustment for unconjugated estradiol, although increased risks were still suggested and, for androstenedione, remained statistically significant. Having higher unconjugated estrone relative to androstenedione (Q5 vs Q1: OR = 2.18, 95% CI = 1.19 to 3.98) and higher unconjugated estradiol relative to testosterone (Q5 vs Q1: OR = 2.56, 95% CI = 1.38 to 4.75) was associated with increased risk for developing endometrial cancer. However, higher DHT relative to its precursor, testosterone, reduced risk (Q5 vs Q1: OR = 0.52, 95% CI = 0.31 to 0.88). We did not identify associations with other androgen metabolites. Estradiol had the strongest risk associations with endometrial cancer (Q5 vs Q1: OR = 5.28), and this association remained when we additionally adjusted the estradiol model for ADT-G, as a proxy for background androgenic activity (Q5 vs Q1: OR = 5.50, not tabulated). The primary analyses were consistent after conducting the described sensitivity analyses.
Table 2.
Odds ratios (OR) for endometrial cancer associated with the highest quintile vs the lowest quintile (Q5 vs Q1) of circulating androgens and androgen metabolites
| Androgen or androgen metabolite | Model 1* |
Model 2† |
||
|---|---|---|---|---|
| OR (95% CI) | Ptrend‡ | OR (95% CI) | Ptrend‡ | |
| Parent androgens | ||||
| DHEA | 1.85 (1.06 to 3.25) | .07 | 1.67 (0.94 to 2.96) | .15 |
| DHEAS | 1.31 (0.76 to 2.26) | .36 | 1.20 (0.69 to 2.09) | .53 |
| Androstenedione§ | 2.36 (1.34 to 4.16) | .01 | 2.01 (1.12 to 3.60) | .06 |
| Testosterone | 1.91 (1.12 to 3.24) | .01 | 1.55 (0.89 to 2.69) | .08 |
| 5α pathway | ||||
| 5α-androstanedione | 1.13 (0.66 to 1.92) | .28 | 0.99 (0.57 to 1.71) | .54 |
| DHT | 1.04 (0.61 to 1.78) | .74 | 0.94 (0.55 to 1.62) | .93 |
| DHTS | 1.27 (0.75 to 2.17) | .51 | 1.18 (0.69 to 2.02) | .68 |
| ADT | 0.93 (0.55 to 1.57) | .48 | 0.89 (0.52 to 1.52) | .67 |
| ADT-G | 1.34 (0.75 to 2.39) | .88 | 1.19 (0.66 to 2.15) | .80 |
| 3α-diol-3G | 1.62 (0.91 to 2.91) | .77 | 1.42 (0.78 to 2.56) | .93 |
| 3α-diol-17G | 1.13 (0.65 to 1.98) | .93 | 1.04 (0.58 to 1.84) | .67 |
| Marker of androgenic activity | ||||
| Sum of ADT-G + 3α-diol-3G + 3α-diol-17G | 1.39 (0.77 to 2.52) | .99 | 1.23 (0.67 to 2.23) | .68 |
| 5β pathway | ||||
| Etio-G | 1.23 (0.72 to 2.10) | .36 | 1.13 (0.66 to 1.95) | .57 |
| Parent estrogens | ||||
| Unconjugated estrone | 2.63 (1.49 to 4.66) | .0001 | — | |
| Unconjugated estradiol | 5.28 (2.64 to 10.59) | .0001 | — | |
| Ratios | ||||
| 5α-androstanedione: Androstenedione | 0.74 (0.43 to 1.27) | .22 | 0.79 (0.46 to 1.36) | .35 |
| Unconjugated estrone: Androstenedione | 2.18 (1.19 to 3.98) | .02 | — | |
| DHT: Testosterone | 0.52 (0.31 to 0.88) | .02 | 0.59 (0.35 to 1.01) | .10 |
| Unconjugated estradiol: Testosterone§ | 2.56 (1.38 to 4.75) | .002 | — | |
The models for each androgen or androgen metabolite were run separately and adjusted for gravidity, smoking status, body mass index, duration of oral contraceptive use, and age at menarche (conditioned on matching factors). Full results for all quintiles are available as Supplementary Material (available online). 3α-diol-3G = 5α-androstane 3α,17β diol-3-glucuronide; 3α-diol-17G = 5α-androstane 3α, 17β diol-17-glucuronide; ADT = androsterone; ADT-G = ADT-glucuronide; CI = confidence interval; DHEA = dehydroepiandrosterone; DHEAS = dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; DHTS = dihydrotestosterone sulfate; etio-G = etiocholanolone-glucuronide.
Models adjusted for factors from model 1 plus unconjugated estradiol. Results not shown for models in which unconjugated estrogens were exposures.
P trend across quintiles of a given androgen or androgen metabolite.
Effect estimates comparing the 5th vs 1st quintile (from model 1) that were statistically significant using a Bonferroni correction threshold of P < .003.
The increased odds ratios we observed for the parent androgens were visible for women with both type I and type II cancers, although estimates were imprecise among the latter group (Supplementary Table 2, available online). Interestingly, we noted greater effect magnitudes for both the ratio of androstenedione to 5α-androstanedione and for 5α-androstanedione itself, associated with type II tumors. Generally, the directions and magnitudes of the associations were consistent across stage and grade as well (Supplementary Table 2, available online). Although estimates for testosterone were greater for stage 3/4 cancers than for stage 2, small sample sizes limit inference about true heterogeneity. Unconjugated parent estrogens individually, and in ratio to their androgen precursors, were strongly associated with type I cancers. Our data indicated inverse associations for increasing DHT relative to testosterone across endometrial cancer type, stage, and grade, but trends across quintiles were not statistically significant.
The associations noted in the overall analysis were consistent by time between blood draw and diagnosis, although some estimates were imprecise (Supplementary Table 3, available online). Notably, higher concentrations of androstenedione increased risk regardless of time to diagnosis.
Our data suggested stronger risks for some androgens and androgen metabolites among women younger than 60 years at baseline than among older women, but interactions by age did not reach statistical significance (Table 3; Supplementary Table 4, available online). The results indicated positive associations with androstenedione regardless of age. Associations among women aged at least 60 years explained the overall reduced risk noted for increasing DHT relative to testosterone (Q5 vs Q1: OR = 0.53, 95% CI = 0.29 to 0.98).
Table 3.
Odds ratios (OR) for endometrial cancer associated with the highest quintile vs the lowest quintile (Q5 vs Q1) of circulating androgen/androgen metabolites: modification by age at blood draw and body mass index (BMI)*
| Androgen/Metabolite | Age |
P † | BMI, kg/m2 |
P † | |||
|---|---|---|---|---|---|---|---|
| <60 years (n = 192) | ≥60 years (n = 475) | <25 (n = 235) | 25–29.9 (n = 194) | ≥30 (n = 236) | |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Parent androgens | |||||||
| DHEA | 15.14 (1.73 to 132.36) | 1.61 (0.82 to 3.15) | 5.52 (1.65 to 18.46) | 1.51 (0.52 to 4.44) | 1.02 (0.37 to 2.78) | ||
| Ptrend‡ | 0.04 | 0.30 | .29 | 0.01 | 0.79 | 0.84 | .001 |
| DHEAS | 4.33 (0.99 to 18.87) | 1.26 (0.65 to 2.44) | 3.39 (1.20 to 9.59) | 1.01 (0.36 to 2.82) | 0.51 (0.18 to 1.48) | ||
| Ptrend | 0.17 | 0.72 | .41 | 0.03 | 0.90 | 0.53 | .07 |
| Androstenedione | 4.20 (0.75 to 23.67) | 2.16 (1.14 to 4.08) | 3.01 (1.05 to 8.63) | 2.18 (0.77 to 6.21) | 2.19 (0.78 to 6.13) | ||
| Ptrend | 0.29 | 0.03 | .93 | 0.04 | 0.28 | 0.08 | .56 |
| Testosterone | 0.76 (0.24 to 2.34) | 2.37 (1.25 to 4.46) | 4.87 (1.67 to 14.24) | 0.97 (0.37 to 2.54) | 1.15 (0.42 to 3.19) | ||
| Ptrend | 0.36 | 0.001 | .17 | 0.005 | 0.87 | 0.28 | .09 |
| 5α pathway | |||||||
| 5α-androstanedione | 0.92 (0.34 to 2.50) | 1.15 (0.60 to 2.20) | 1.90 (0.67 to 5.39) | 1.31 (0.51 to 3.38) | 0.98 (0.34 to 2.83) | ||
| Ptrend | 0.72 | 0.30 | .998 | 0.27 | 0.42 | 0.28 | .03 |
| DHT | 1.39 (0.45 to 4.25) | 1.03 (0.54 to 1.95) | 3.36 (1.05 to 10.75) | 0.78 (0.31 to 1.92) | 0.56 (0.21 to 1.46) | ||
| Ptrend | 0.78 | 0.62 | .94 | 0.07 | 0.87 | 0.31 | .04 |
| DHTS | 1.27 (0.44 to 3.69) | 1.15 (0.61 to 2.16) | 2.87 (0.99 to 8.31) | 0.65 (0.24 to 1.73) | 0.93 (0.37 to 2.37) | ||
| Ptrend | 0.30 | 0.95 | .31 | 0.09 | 0.42 | 0.43 | .34 |
| ADT | 1.20 (0.43 to 3.35) | 0.77 (0.41 to 1.46) | 0.62 (0.23 to 1.63) | 0.83(0.31 to 2.17) | 0.83(0.31 to 2.19) | ||
| Ptrend | 0.66 | 0.92 | .71 | 0.51 | 0.62 | 0.88 | .73 |
| ADT-G | 3.21 (0.73 to 14.09) | 1.26 (0.64 to 2.49) | 2.06 (0.65 to 6.56) | 1.40 (0.49 to 4.01) | 0.99 (0.36 to 2.71) | ||
| Ptrend | 0.52 | 0.95 | .48 | 0.19 | 0.87 | 0.46 | .73 |
| 3α-diol-3G | 10.76 (2.01 to 57.57) | 1.29 (0.65 to 2.54) | 2.26 (0.78 to 6.62) | 0.86 (0.28 to 2.59) | 2.37 (0.78 to 7.24) | ||
| Ptrend | 0.11 | 0.69 | .40 | 0.50 | 0.81 | 0.91 | .56 |
| 3α-diol-17G | 1.55 (0.46 to 5.21) | 0.99 (0.51 to 1.93) | 1.46 (0.52 to 4.07) | 0.81 (0.29 to 2.23) | 0.63 (0.18 to 2.17) | ||
| Ptrend | 0.94 | 0.74 | .96 | 0.73 | 0.31 | 0.82 | .55 |
| Marker of androgenic activity | 3.00 (0.67 to 13.36) | 1.40 (0.71 to 2.80) | 1.70 (0.52 to 5.55) | 1.35 (0.47 to 3.90) | 1.28 (0.45 to 3.62) | ||
| Ptrend | 0.65 | 0.94 | .55 | 0.37 | 1.00 | 0.61 | .63 |
| 5β pathway | |||||||
| Etio-G | 2.70 (0.87 to 8.42) | 1.27 (0.67 to 2.40) | 2.06 (0.73 to 5.82) | 3.41 (1.14 to 10.20) | 0.59 (0.24 to 1.46) | ||
| Ptrend | 0.23 | 0.34 | .44 | 0.25 | 0.02 | 0.38 | .20 |
| Parent estrogens | |||||||
| Unconjugated estrone | 1.84 (0.57 to 5.92) | 3.02 (1.55 to 5.88) | 3.34 (1.22 to 9.16) | 1.30 (0.47 to 3.60) | 4.45 (1.17 to 16.95) | ||
| Ptrend | 0.32 | 0.0002 | .58 | 0.01 | 0.38 | 0.03 | .50 |
| Unconjugated estradiol | 4.82 (1.05 to 22.04) | 5.87 (2.67 to 12.87) | 7.26 (2.17 to 24.30) | 2.56 (0.73 to 9.05) | 5.08 (0.81 to 31.96) | ||
| Ptrend | 0.32 | <.0001 | .60 | 0.01 | 0.08 | 0.07 | .98 |
| Ratios | |||||||
| 5α-androstanedione: Androstenedione | 0.60 (0.19 to 1.90) | 0.80 (0.42 to 1.51) | 0.82 (0.30 to 2.29) | 0.86 (0.31 to 2.41) | 0.66 (0.26 to 1.66) | ||
| Ptrend | 0.42 | 0.49 | .96 | 0.41 | 0.96 | 0.68 | .07 |
| Unconjugated estrone: Androstenedione | 1.26 (0.42 to 3.77) | 3.13 (1.49 to 6.58) | 2.20 (0.77 to 6.26) | 1.25 (0.40 to 3.87) | 2.73 (0.81 to 9.23) | ||
| Ptrend | 0.80 | 0.02 | .05 | 0.19 | 0.94 | 0.35 | .99 |
| DHT: Testosterone | 1.05 (0.36 to 3.12) | 0.53 (0.29 to 0.98) | 0.53 (0.19 to 1.46) | 0.83 (0.32 to 2.20) | 0.48 (0.20 to 1.18) | ||
| Ptrend | 0.65 | 0.03 | .39 | 0.15 | 0.98 | 0.14 | .63 |
| Unconjugated estradiol: Testosterone | 3.49 (0.81 to 15.05) | 2.80 (1.39 to 5.63) | 1.41 (0.46 to 4.29) | 2.29 (0.71 to 7.37) | 1.16 (0.19 to 6.97) | ||
| Ptrend | 0.12 | 0.003 | .64 | 0.33 | 0.13 | 0.01 | .23 |
*Unconditional logistic regression models for each androgen/metabolite were used and adjusted for gravidity, smoking status, body mass index, duration of oral contraceptive use, and age at menarche and additionally adjusted for matching factors: age at baseline, year of blood draw, race/ethnicity, time since last menopausal hormone therapy use. Full results for all quintiles are available as Supplementary Material (available online). Comparisons are made to the controls within each model/stratum of the modifiers. ADT = androsterone; ADT-G = ADT-glucuronide; CI = confidence interval; DHEA = dehydroepiandrosterone; DHEAS = dehydroepiandrosterone sulfate; DHT = dihydrotestosterone; DHTS = dihydrotestosterone sulfate; etio-G = etiocholanolone-glucuronide.
χ2P values from the cross-product interaction terms between the modifiers of interest and hormone exposures.
P trend for trend across quintiles of a given hormone/metabolite.
We observed stronger risks for DHEA, DHEAS, and testosterone among women with BMI below 25 kg/m2 than among those with greater BMI (Pheterogeneity <0.10); we noted similar patterns for 5α-androstanedione, DHT, DHTS, and several glucuronidated metabolites, but null associations could not be ruled out and trends were generally not visible across quintiles (Table 3; Supplementary Table 4, available online). High levels of androstenedione conferred risk especially among women with BMI below 25 kg/m2 (Q5 vs Q1: OR = 3.01, 95% CI = 1.05 to 8.63), but positive associations were indicated for the other BMI groupings as well. Increasing unconjugated estradiol relative to testosterone elevated risks particularly among women with BMI below 30 kg/m2.
Discussion
In our population, the highest concentrations of parent androgens—DHEA, androstenedione, and testosterone—were associated with increased risk for endometrial cancer. These adrenal androgens can be aromatized to estrogens. Therefore, these associations could be driven by the influence of parent androgens on estrogen metabolism. Indeed, adjustment for unconjugated estradiol attenuated the associations and we noted that higher parent estrogen concentrations relative to their androgenic precursors increased endometrial cancer risk. Furthermore, although DHT is a potent androgen, once metabolized by 5α-reductase, it cannot be aromatized into estradiol or converted back into testosterone (see Figure 1). Our observation of reduced risk for endometrial cancer with increasing DHT relative to its precursor, testosterone, supports the idea that the potential for testosterone to be aromatized increases endometrial cancer risk. Diverting testosterone away from aromatization and into the 5α-reductase pathway may be beneficial for reducing risk. Both this ratio and the increased risk we noted with higher estradiol relative to testosterone suggest that estrogenic, rather than androgenic, pathways may be more relevant to the etiology of most endometrial cancers—which is further supported by our not identifying associations with circulating measures of androgenicity (ADT-G, the summary measure of 5α-reduced glucuronidated metabolites).
Many in vitro studies suggest that androgens have an antiproliferative effect on endometrial epithelial cells [reviewed in (1, 16)]. Ultimately, the role of androgens in the endometrium is complex and likely depends on menopausal status and the presence of cancer or precancerous conditions like hyperplasia. Aromatase is not expressed in the normal endometrium but is expressed in endometrial cancers (17). Therefore, high androgen levels may influence carcinogenesis through increased substrate availability for aromatization in peripheral tissues and the tumor itself. Although aromatase is expressed in adipose tissue, our noting stronger associations for parent androgens among lean vs obese women may signify that high androgen levels do not confer additional risk or that a threshold of risk is reached with the highest androgen levels among women already at increased risk for endometrial cancer (ie, those whose circulating and intracellular estrogen levels are likely high).
Our findings suggest estrogenic, relative to androgenic, pathways are predominant in endometrial carcinogenesis, but we cannot rule out potential androgenic influences on risk. We consistently noted increased risks associated with androstenedione. The increased risks with the parent androgens were attenuated after adjusting for unconjugated estradiol, but the directions of the effects were unchanged. These observations indicate a potential for androgens to influence carcinogenesis through not only estrogen metabolism but also androgenic pathways. Researchers report androgen receptor expression within the postmenopausal endometrium and hyperplastic tissue, but expression in endometrial cancers varies across studies; several groups noted a loss of androgen receptor expression in serous, clear cell, and high-grade endometrioid tumors (18, 19). Our observing greater effect magnitudes for both the ratio of androstenedione to 5α-androstanedione and for 5α-androstanedione itself with type II tumors suggests a potential androgenic influence on risk for these cancers. Our data otherwise indicated elevated risk with the highest parent androgen concentrations across endometrial cancer type and grade. Our serum samples were prediagnostic, so our observations do not necessarily disagree with studies evaluating protein expression in cancerous tissues, but instead suggest that high androgen levels play a role in the early development of most endometrial cancers, likely through estrogen metabolism.
In a study by Tanaka and colleagues (20), loss of 5α-reductase expression, the enzyme that converts testosterone to DHT, was associated with poorer survival among women with endometroid tumors. These researchers conclude that the observed poorer survival associated with loss of 5α-reductase and resulting lower DHT levels indicates that DHT may have inhibitory effects on proliferation (20). Some in vitro studies support this hypothesis (21). Whereas we noted that increasing DHT relative to testosterone reduced endometrial cancer risk, higher DHT itself did not reduce risk. This leads us to question if the poorer survival noted with the loss of 5α-reductase and lower DHT levels could be indicative of more testosterone being available for conversion to estradiol.
Others have reviewed the numerous studies on circulating androgens and endometrial cancer (1, 21). We compare our findings with postmenopausal endometrial cancer risks reported in a case-control study that used LC-MS and two prospective studies that used radioimmunoassays. Audet-Walsh and colleagues (5) used LC-MS and reported increased risk for endometrial cancer (n = 126 case subjects) with the highest concentrations of parent androgens and some downstream metabolites, including DHT (OR ranging from 2.43 for DHEAS to 11.83 for testosterone). Compared to our analysis, this study similarly used sensitive assays, but measured fewer androgens and did not report on ratios. Our estimates were likely attenuated relative to this study because we used serum collected before, rather than at the time of diagnosis; hormone production within a tumor could alter circulating measures.
A pooled nested case-control study (n = 124 case subjects) reported increased risk for endometrial cancer with the highest quartiles of DHEAS and androstenedione (OR = 2.90 and 2.15, respectively) (4).The highest tertile of free testosterone was associated with increased risk in a nested case-control study from the EPIC cohort (n = 192 case subjects) (3). The EPIC researchers did not find associations with the other parent androgens, including total testosterone. Their association with free testosterone may indicate that it is circulating bioavailable stores of this androgen that influence risk. We measured total testosterone and found increased risk, but our results suggest that higher estradiol may drive the testosterone association. Furthermore, Labrie and colleagues (22, 23) argue that most androgens are made and used from DHEA within peripheral tissues vs being released into circulation. Therefore, markers of androgen storage or excretion, like DHTS, ADT-G, and the summary measure of 5α-reduced androgenic activity, are better markers of potential androgenic activity in tissues than circulating testosterone (14).
Comparing odds ratios from different models across and within studies should be done cautiously because of noncollapsibility (24); we acknowledge this limitation when comparing odds ratios with and without adjustment for estradiol. However, our approach is unique. We measured 12 androgens with highly sensitive assays and used knowledge of metabolic pathways to frame analyses (ie, reported on ratios). To our knowledge, this is the largest study on the topic in terms of the number of metabolites evaluated and sample size. We minimized confounding by subclinical disease with our use of prediagnostic serum and examination of associations by time to diagnosis. These strengths allowed us to comprehensively evaluate androgen metabolism in the context of endometrial cancer risk and conclude that androgens likely primarily influence the development of this cancer through their potential to be converted into estrogens. We recognize that once endometrial cancer arises, androgens may affect the progression of this disease via other mechanisms. Further exploration in epidemiologic and tissue-based studies is needed to confirm our findings.
Funding
This work was supported in part by the Intramural Research Program of the National Cancer Institute (KAM, LAB, NW, RMP, BT). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and the US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN26820110 0004C, and HHSN271201100004C.
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD (KAM, LAB, NW, RMP, BT); Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA (KP); Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA (GLA, CC); Cancer Research Technology Program, Leidos Biomedical Research, Inc, Frederick National Laboratory for Cancer Research, Frederick, MD (XX); Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY (TER).
The authors would like to acknowledge the following WHI investigators:
Program Office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD).
Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA).
Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Jennifer Robinson (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); and Robert Brunner (University of Nevada, Reno, NV).
Women’s Health Initiative Memory Study: Mark Espeland (Wake Forest University School of Medicine, Winston-Salem, NC).
For a list of all investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
All authors declare they have no conflicts of interest.
Supplementary Material
References
- 1. Kaaks R, Lukanova A, Kurzer MS.. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 2. Brinton LA, Trabert B, Anderson GL, et al. Serum estrogens and estrogen metabolites and endometrial cancer risk among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen NE, Key TJ, Dossus L, et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr Relat Cancer. 2008;15(2):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lukanova A, Lundin E, Micheli A, et al. Circulating levels of sex steroid hormones and risk of endometrial cancer in postmenopausal women. Int J Cancer. 2004;108(3):425–432. [DOI] [PubMed] [Google Scholar]
- 5. Audet-Walsh É, Lépine J, Grégoire J, et al. Profiling of endogenous estrogens, their precursors, and metabolites in endometrial cancer patients: association with risk and relationship to clinical characteristics. J Clin Endocrinol Metabol. 2011;96(2):E330–E339. [DOI] [PubMed] [Google Scholar]
- 6. The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 7. Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9):S107–S121. [DOI] [PubMed] [Google Scholar]
- 8. Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(suppl 9):S122–S128. [DOI] [PubMed] [Google Scholar]
- 9. Trabert B, Brinton LA, Anderson GL, et al. Circulating estrogens and postmenopausal ovarian cancer risk in the Women's Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev 2016;25(4):648–656. [DOI] [PMC free article] [PubMed]
- 10. Turcu A, Smith JM, Auchus R, Rainey WE.. Adrenal androgens and androgen precursors—definition, synthesis, regulation and physiologic actions. Compr Physiol. 2014;4(4):1369–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X, Roman JM, Issaq HJ, et al. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79(20):7813–7821. [DOI] [PubMed] [Google Scholar]
- 12. Trabert B, Xu X, Falk RT, et al. Assay reproducibility of serum androgen measurements using liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2016;155(pt A):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trabert B, Michels KA, Anderson GL, et al. Circulating androgens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study [published online ahead of print Jan. 26, 2019]. Int J Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Labrie F, Bélanger A, Bélanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4–5):182–188. [DOI] [PubMed] [Google Scholar]
- 15. Murali R, Soslow RA, Weigelt B.. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15(7):e268–e278. [DOI] [PubMed] [Google Scholar]
- 16. Simitsidellis I, Saunders PTK, Gibson DA.. Androgens and endometrium: new insights and new targets. Mol Cell Endocrinol. 2018;465:48–60. [DOI] [PubMed] [Google Scholar]
- 17. Sasano H, Harada N.. Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocrine Rev. 1998;19(5):593–607. [DOI] [PubMed] [Google Scholar]
- 18. Kamal AM, Bulmer JN, DeCruze SB, et al. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br J Cancer. 2016;114(6):688.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kato J, Seto T.. Correlation of androgen receptors with histological differentiation in human endometrial carcinomas. Acta Obstet Gynecol Scand. 1985;64(3):209–212. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka S, Miki Y, Hashimoto C, et al. The role of 5alpha-reductase type 1 associated with intratumoral dihydrotestosterone concentrations in human endometrial carcinoma. Mol Cell Endocrinol. 2015;401:56–64. [DOI] [PubMed] [Google Scholar]
- 21. Ito K, Miki Y, Suzuki T, McNamara KM, Sasano H.. In situ androgen and estrogen biosynthesis in endometrial cancer: focus on androgen actions and intratumoral production. Endocr Relat Cancer. 2016;23(7):R323–R335. [DOI] [PubMed] [Google Scholar]
- 22. Labrie F, Belanger A, Cusan L, Candas B.. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. J Clin Endocrinol Metab. 1997;82(8):2403–2409. [DOI] [PubMed] [Google Scholar]
- 23. Belanger B, Belanger A, Labrie F, et al. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem. 1989;32(5):695–698. [DOI] [PubMed] [Google Scholar]
- 24. Pang M, Kaufman JS, Platt RW.. Studying noncollapsibility of the odds ratio with marginal structural and logistic regression models. Stat Methods Med Res. 2016;25(5):1925–1937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.