Abstract
Background
The recurrence of hepatocellular carcinoma (HCC) is the strongest survival-limiting factor after liver transplantation (LT) in patients with HCC. In the face of donor organ shortage, it is necessary to identify factors associated with HCC recurrence in order to maximize the utility of the available grafts.
Objective
To study the phenomenon of HCC recurrence after LT at a European transplantation centre over the past 20 years.
Methods
Data from 304 HCC patients who underwent LT were prospectively recorded. Clinical and pathological factors were assessed for their association with recurrence.
Results
Fifty-one patients (16.8%) had HCC recurrence after LT. Patients exceeding the Milan criteria developed HCC recurrence more frequently. The time point of recurrence did not affect survival after recurrence. Furthermore, there was no difference in survival between patients with intra- and extrahepatic recurrence. However, patients with recurrence due to needle tract seeding had a significantly better outcome than patients with other sites of recurrence.
Conclusion
Our data support a restrictive use of patient selection criteria to help identify patients who have an increased risk of HCC recurrence after LT, and highlight the need to improve patient selection before LT in order to minimize the rate of HCC recurrence.
Keywords: Liver cancer, hepatocellular carcinoma, recurrence, treatment, survival
Key summary
- Summary of the established knowledge on this subject:
- Liver transplantation is a potentially curative treatment for patients with hepatocellular carcinoma (HCC).
- HCC recurrence after liver transplantation is the strongest survival-limiting factor in this group of patients.
- In many countries, patients are selected based on the so-called Milan criteria. However, whether these criteria should be expanded, so as to offer liver transplantation to a larger group of HCC patients, is under discussion.
- What are the significant and/or new findings of this study?
- In our analysis, which spanned a period of 20 years, patients exceeding the Milan criteria developed HCC recurrence more frequently.
- The time point (early vs. late) and the site of HCC recurrence (intra- vs. extrahepatic) did not affect survival after recurrence.
- Patients with recurrence due to needle tract seeding after liver biopsy had a significantly better outcome than patients with other sites of recurrence.
Introduction
Liver transplantation (LT) can be a cure for certain patients with hepatocellular carcinoma (HCC). Due to the shortage of donor organs, patients need to be carefully selected for LT. HCC recurrence after LT is a recognized and not uncommon clinical challenge (reviewed in Welker et al.1). In fact, it is the strongest survival-limiting factor after LT, diminishing its curative intention (as demonstrated by our recent report on the long-term survival of HCC patients treated with LT at our centre2). In order to maximize the utility of available grafts, criteria for patient selection aim to reduce the risk of recurrence. Currently, the so-called Milan criteria (single tumour ≤5 cm or at least three tumours each ≤3 cm, and an absence of macrovascular invasion and extrahepatic spread), which have been shown to incur a low rate of HCC recurrence,3 are endorsed as patient selection criteria by the major international guidelines.4–6 However, the Milan criteria are regarded as too restrictive by some as they can exclude HCC patients for who LT might be a likely cure. Therefore, in an attempt to make LT available to a larger group of HCC patients, expanded criteria such as the University of California San Francisco UCSF (single tumour ≤6.5 cm or at least three tumours, each ≤4.5 cm, with a total tumour diameter of ≤8 cm)7 and up-to-seven criteria (the sum of the largest tumour diameter and the number of tumour nodules not to exceed seven)8 have been proposed, and have been shown to produce outcomes similar to the Milan criteria. However, these criteria have usually been evaluated over relatively short time spans, and data on the long-term impact of criteria expansion are scarce.
The risk of HCC recurrence appears to be not only a function of tumour size and number. Other factors, which have been identified as being associated with recurrence, are alpha-fetoprotein (AFP) levels above various thresholds,9–12 microvascular invasion,9,13–15 the neutrophil to lymphocyte ratio9,15 and poor tumour differentiation.9,13–15 A score incorporating pre-LT AFP levels in addition to the Milan criteria has been shown to be superior to the Milan criteria alone in predicting HCC recurrence,10,12 and the Model Of Recurrence After Liver transplant (MORAL) score has even included the neutrophil to lymphocyte ratio.9 However, these and other molecular markers still await further evaluation, and therefore do not guide clinical decision-making today.
In this study, we evaluated the phenomenon of HCC recurrence in patients who had received an LT at our centre over the past 20 years. We aimed to identify factors associated with the development of HCC recurrence as well as determinants of post-recurrence survival.
Patients and methods
Patients
Between February 1998 and June 2017, LT was performed on 304 patients with HCC at our centre (including 34 patients with an incidental diagnosis of HCC in the explant). Bridging therapies and the management of patients exceeding the Milan criteria are outlined in Foerster et al. and Otto et al. (2006 and 2013).2,16,17
Patients were followed up until 31 March 2018. All patient data analysed in this study were retrieved from our prospectively maintained database. Analysis of patient data is regulated for hospitals in the federal state of Rhineland-Palatinate by § 37 Landeskrankenhausgesetz LKG (state hospital code). Under its provisions, hospitals are permitted to conduct retrospective and prospective data collection and analysis. Therefore, no approval by an ethical committee was required for the present study. All patients gave their written informed consent for treatment, use of samples and data utilization in clinical studies. The study was conducted according to the ethical guidelines of the Declaration of Helsinki 1975 and good clinical practice guidelines.
All explanted organs were sent for pathological review. The reported grading was based on the best available information: either on the histology from the explant if sufficient vital tissue was present (n = 256), or, if this was not the case, on the histology gained via biopsy or resection of the HCC before LT. The donor risk index (DRI)18 was determined for all LT where the data were available, except for domino LT and combined liver–kidney transplantations (n = 265).
The most frequently employed immunosuppressive regimens were the combinations of a calcineurin inhibitor (tacrolimus or cyclosporine) or a mechanistic target of rapamycin inhibitor (sirolimus or everolimus) with mycophenolate mofetil (MMF) (target trough levels are detailed in Foerster et al.2).
All patients were followed up after LT at our outpatient clinic on a regular basis (at least every 3–6 months). Radiological follow-up imaging via computed tomography or magnetic resonance imaging was performed every 6 months during the first 2 years, and then annually until 5 years after LT. After 5 years, patients underwent abdominal ultrasound and chest X-ray examination every 6–12 months.
Information on the cause of death was available for 133 of 142 deceased patients, and accordingly used in our cause of death analysis.
Statistical analysis
Continuous variables are presented as median and interquartile range, and discrete variables as numbers and percentages. Variables were evaluated using the chi-square or the Mann–Whitney U-test, and p-values < 0.05 were considered statistically significant. Survival was analysed using Kaplan–Meier curves and the log-rank test. Cox proportional hazard models were employed for multivariate testing of variables which had p-values < 0.01 in the univariate analyses. All statistical analyses were performed using IBM SPSS Statistics Version 23 (Chicago, IL). Graphs were plotted in SPSS or Microsoft Excel for Mac 2011 (Redmond, WA).
Results
Survival after liver transplantation
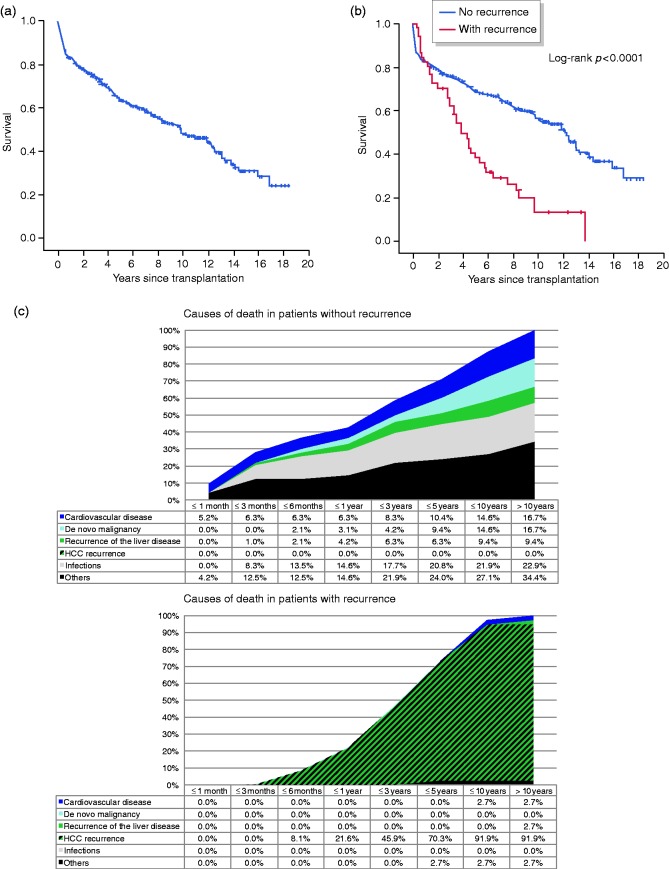
The 1-, 3-, 5-, 10- and 15-year survival rates were 82.8, 73.3, 63.0, 48.3 and 30.1%, respectively. The median survival was 9.6 years (Figure 1(a)). Of 304 patients, 51 (16.8%) developed HCC recurrence by the end of observation (31 March 2018). The overall survival was significantly different between patients with and without recurrence (Figure 1(b); log-rank p < 0.0001). With regard to HCC recurrence, the median survival was 4.2 vs. 12.2 years. At 5 and 10 years, the survival rates were 38.6 vs. 68.6% and 13.5 vs. 56.3%, respectively (Table 1). The cause of death analysis revealed significant differences between patients with and without recurrence for all causes except recurrence of the underlying liver disease, with HCC recurrence being the most frequent cause in patients with recurrence (Figure 1(c); Supplementary Table 1).
Figure 1.
(a) Graph showing overall survival of hepatocellular carcinoma patients who underwent liver transplantation at our centre between February 1998 and June 2017. (b) Kaplan–Meier curve comparing survival of hepatocellular carcinoma patients stratified according to hepatocellular carcinoma recurrence. The calculated value of the corresponding log-rank test is shown. (c) Graphs showing the cumulative relative causes of death of transplanted hepatocellular carcinoma patients over time, stratified according to the presence or absence of recurrence. Between the two groups, differences were significant for all causes except recurrence of the underlying liver disease (Supplementary Table 1).
HCC: hepatocellular carcinoma.
Table 1.
Survival rates of transplanted patients with hepatocellular carcinoma stratified according to the absence or presence of recurrence.
| Year | Patients |
||
|---|---|---|---|
| All | Without recurrence | With recurrence | |
| 1 | 82.8 | 82.8 | 82.4 |
| 3 | 73.3 | 75.6 | 62.2 |
| 5 | 63.0 | 68.6 | 38.6 |
| 10 | 48.3 | 56.3 | 13.5 |
| 15 | 30.1 | 35.8 | 0 |
Numbers represent percentages.
Comparison between patients with and without HCC recurrence
At the end of the observation period, 60% of patients without recurrence and 20% of those with recurrence were still alive (Table 2; p < 0.001). Overall, patients with recurrence were significantly more often female (33 vs. 21%; p = 0.047) and had more often been transplanted before the introduction of the model for end-stage liver disease (MELD)-based allocation system (i.e. before 16 December 2006; 57 vs. 35%; p = 0.004). Regarding transplantation criteria (as per pathology review of the explant), the proportions of patients outside the Milan criteria, outside the Milan but inside the UCSF criteria or outside the Milan but inside the up-to-seven criteria were significantly higher among patients with recurrence (57 vs. 23%, 49 vs. 22% and 45 vs. 13%, respectively; p < 0.001 for all three comparisons). Patients with HCC recurrence had a significantly higher T (tumour) stage (p < 0.001), poorer tumour grading (p = 0.015), higher number of tumour nodules (p < 0.001), greater nodule size (>5 cm; p = 0.032) and more frequent microvascular invasion (p < 0.001). While the AFP levels before LT or transarterial chemoembolization (TACE) did not differ, the frequency of patients with an AFP level > 400 ng/mL was significantly higher among patients with HCC recurrence (12 vs. 2%; p = 0.002).
Table 2.
Comparison of baseline characteristics of patients with or without hepatocellular carcinoma recurrence after receiving a liver transplant at the University Medical Center Mainz between 1998 and 2017 (n = 304).
| Patients |
p-value | ||
|---|---|---|---|
| Without recurrence (n = 253) | With recurrence (n = 51) | ||
| Alive | 151 (60%) | 11 (22%) | <0.001 |
| Time to last follow-up/death (years) | 5.1 (1.8–10.2) | 3.5 (1.4–6.3) | 0.096 |
| Recurrence-free survival (years) | 5.1 (1.8–10.2) | 1.8 (0.7–3.7) | <0.001 |
| Time on the waiting list (days) | 201.0 (114.0–358.0) | 199.0 (55.0–314.0) | 0.132 |
| Time from HCC diagnosis until LT (days)a | 299.5 (198.5–472.5) | 303.0 (198.0–412.0) | 0.994 |
| DRI | 1.68 (1.40–2.00) | 1.66 (1.35–2.05) | 0.796 |
| Age | 59.9 (55.0–65.3) | 59.9 (53.7–64.1) | 0.759 |
| Male gender | 201 (79%) | 34 (67%) | 0.047 |
| LT before 16 December 2006 (pre-MELD era) | 89 (35%) | 29 (57%) | 0.004 |
| Cold ischaemia time (minutes) | 570.0 (495.0–684.0) | 556.0 (452.0–671.0) | 0.447 |
| Outside Milan criteria (explant) | 59 (23%) | 29 (57%) | <0.001 |
| Outside Milan but inside UCSF criteria (explant) | 53 (22%) | 25 (49%) | <0.001 |
| Outside Milan but inside up-to-seven criteria (explant) | 32 (13%) | 23 (45%) | <0.001 |
| T stage (explant)b | |||
| T0c | 42 (17%) | 6 (12%) | <0.001 |
| T1 | 101 (40%) | 10 (20%) | |
| T2 | 96 (38%) | 20 (39%) | |
| T3 | 13 (5%) | 14 (27%) | |
| T4 | 1 (0%) | 1 (2%) | |
| Grading | |||
| G1 | 46 (18%) | 7 (14%) | 0.015 |
| G2 | 144 (57%) | 28 (55%) | |
| G3 | 35 (14%) | 15 (29%) | |
| Gx | 28 (11%) | 1 (2%) | |
| Number of tumour nodules | |||
| 0 | 42 (17%) | 5 (10%) | < 0.001 |
| 1 | 115 (45%) | 12 (24%) | |
| 2 | 35 (14%) | 4 (8%) | |
| 3 | 20 (8%) | 5 (10%) | |
| multifocal | 41 (16%) | 25 (49%) | |
| Nodule size > 5 cm | 27 (11%) | 11 (22%) | 0.032 |
| Microvascular invasion | 27 (11%) | 16 (31%) | <0.001 |
| AFP (ng/mL) before LT | 8.8 (5.0–19.0) | 7.9 (3.7–37.0) | 0.834 |
| AFP (ng/mL) before TACE | 13.3 (5.8–59.7) | 24.0 (4.8–269.0) | 0.641 |
| AFP before LT > 400 ng/mL | 6 (2%) | 6 (12%) | 0.002 |
| Absence of vital tumour tissue in the explant | 43 (17%) | 5 (10%) | 0.199 |
| Pre-treatment | |||
| With TACE | 185 (73%) | 42 (82%) | 0.167 |
| With resection | 19 (8%) | 8 (16%) | 0.061 |
| Other than resection or TACE | 60 (24%) | 8 (16%) | 0.209 |
| Number of TACE | |||
| 0 | 67 (26%) | 9 (18%) | 0.559 |
| 1–3 | 68 (27%) | 14 (27%) | |
| 4–6 | 76 (30%) | 19 (35%) | |
| 7 or more | 42 (17%) | 9 (20%) | |
| Modality of pre-LT HCC diagnosis | |||
| Incidentaloma | 33 (13%) | 1 (2%) | 0.037 |
| Radiological evidence | 51 (20%) | 8 (16%) | |
| Histology | 169 (67%) | 42 (82%) | |
| Histological confirmation by liver biopsy | 150 (59%) | 34 (67%) | 0.325 |
| Aetiology | |||
| Alcohol | 121 (48%) | 20 (39%) | 0.261 |
| Hepatitis B | 41 (16%) | 10 (20%) | 0.553 |
| Hepatitis B+D | 11 (4%) | 2 (4%) | 0.891 |
| Hepatitis C | 90 (36%) | 19 (37%) | 0.819 |
| Child–Pugh class | |||
| Child A | 105 (42%) | 26 (54%) | 0.201 |
| Child B | 61 (25%) | 12 (25%) | |
| Child C | 82 (33%) | 10 (21%) | |
| MELD score | 12.0 (9.0–18.0) | 9.0 (7.0–13.1) | 0.002 |
Continuous variables are expressed as median with interquartile ranges in brackets, categorical variables as n and frequencies (%). p-values < 0.05 are considered to be statistically significant and are highlighted in bold.
AFP: alpha-fetoprotein; DRI: donor risk index; HCC: hepatocellular carcinoma; LT: liver transplantation; MELD: model for end-stage liver disease; TACE: transarterial chemoembolization; UCSF: [University of California San Francisco].
Excluding patients with incidentalomas.
Based on the TNM classification of malignant tumours as defined by the Union for International Cancer Control.
No vital tumour tissue present in the explant.
The factors absence of vital tumour tissue in the explant, age, blood group, cold ischaemia time, DRI, time from HCC diagnosis until LT and time on the waiting list did not differ significantly between the two groups (Table 2 and Supplementary Table 2). Among the different aetiologies of the underlying liver disease, the kinds of pre-treatment and the prescribed immunosuppressive regimens post-LT, only subtle differences could be observed. In the group of patients with HCC recurrence, nonalcoholic steatohepatitis (NASH) was significantly more frequent (6 vs. 1%; p = 0.028), there was a trend towards more frequent pre-treatment with resection (16 vs. 8%; p = 0.061) and MMF was significantly less frequently prescribed (45 vs. 60%; p = 0.042). While there was no difference regarding the use of liver biopsy, the rate of histological confirmation of HCC before LT (i.e. the combination of patients undergoing hepatic resection or liver biopsy before LT) was significantly higher in the group of patients with HCC recurrence (82 vs. 67%; p = 0.037).
In the univariate Cox regression analysis, factors significantly associated with recurrence were an LT before 16 December 2006 (pre-MELD era), female gender, an HCC exceeding the Milan criteria, an HCC exceeding the Milan criteria but meeting the UCSF or up-to-seven criteria, a high T stage, poor differentiation, a high number of tumour nodules, a nodule size > 5 cm, microvascular invasion, NASH as aetiology of the underlying liver disease and increasing AFP levels before LT (Table 3). At multivariate testing, T stage and AFP levels before LT reached significance.
Table 3.
Proportional hazards of recurrence-free survival (only factors with a p-value < 0.05 are shown).
| HR | Lower 95% CI | Upper 95% CI | p-value | HR | Lower 95% CI | Upper 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|
| LT before 16 December 2006 (pre-MELD era) | 0.559 | 0.319 | 0.980 | 0.042 | ||||
| Female gender | 1.903 | 1.062 | 3.412 | 0.031 | ||||
| Outside Milan criteria (explant) | 3.451 | 1.982 | 6.009 | <0.001 | ||||
| Outside Milan but inside UCSF criteria (explant) | 2.891 | 1.669 | 5.007 | <0.001 | ||||
| Outside Milan but inside up-to-seven criteria (explant) | 4.148 | 2.387 | 7.208 | <0.001 | ||||
| T stage (explant; T0 = reference) | <0.001 | 0.047 | ||||||
| T1 | 0.776 | 0.282 | 2.135 | 0.623 | 0.711 | 0.269 | 1.879 | 0.491 |
| T2 | 1.554 | 0.624 | 3.871 | 0.344 | 1.018 | 0.402 | 2.573 | 0.970 |
| T3 | 5.164 | 1.981 | 13.463 | 0.001 | 2.541 | 0.914 | 7.061 | 0.074 |
| T4 | 82.839 | 7.499 | 915.062 | <0.001 | 0.000 | 0.000 | 0.994 | |
| Grading (G1 = reference) | 0.006 | |||||||
| G2 | 1.139 | 0.497 | 2.609 | 0.759 | ||||
| G3 | 2.910 | 1.185 | 7.147 | 0.020 | ||||
| Gx | 0.270 | 0.033 | 2.195 | 0.221 | ||||
| Number of tumour nodules (0 = reference) | <0.001 | |||||||
| 1 | 0.957 | 0.337 | 2.716 | 0.934 | ||||
| 2 | 1.161 | 0.311 | 4.324 | 0.824 | ||||
| 3 | 1.954 | 0.565 | 6.756 | 0.290 | ||||
| >3 | 4.807 | 1.838 | 12.569 | 0.001 | ||||
| Nodule size > 5 cm | 1.998 | 1.025 | 3.897 | 0.042 | ||||
| Microvascular invasion | 3.249 | 1.796 | 5.878 | <0.001 | ||||
| NASH | 4.575 | 1.411 | 14.834 | 0.011 | ||||
| AFP (ng/mL) before LT | 1.001 | 1.001 | 1.002 | <0.001 | 1.001 | 1.000 | 1.002 | 0.007 |
Multivariate analysis was performed on variables with a p-value < 0.01 in the univariate analysis.
AFP: alpha-fetoprotein; CI: confidence interval; HR: hazard ratio; LT: liver transplantation; MELD: model for end-stage liver disease; NASH: nonalcoholic steatohepatitis; UCSF: [University of California San Francisco]; p-values<0.05 are considered to be statistically significant and are highlighted in bold.
Comparison between patients with early and late HCC recurrence
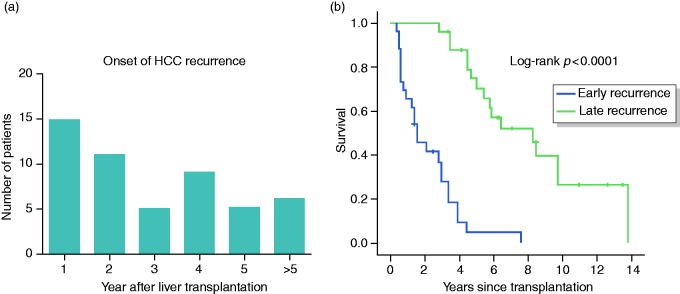
Of the 51 patients with HCC recurrence, 15 (29%) developed their recurrence in the first, 11 (22%) in the second, 5 (10%) in the third, 9 (18%) in the fourth and 5 (10%) in the fifth year after LT (Figure 2(a)). In six patients (12%), the onset was > 5 years after LT and was regarded as very late recurrence. The latest onset of HCC recurrence was 12.4 years after LT (which was a case of extrahepatic manifestation and considered to be due to needle tract seeding). Patients with recurrence were divided in two groups depending on the onset of recurrence. Those who had been diagnosed within 2 years after LT (n = 26) were regarded as early and the other recurrences were regarded as late (n = 25). Survival differed significantly between the two groups (Figure 2(b); log-rank p < 0.0001). At the end of the observation period, 8% of patients with early and 36% of patients with late recurrence were still alive (Supplementary Table 3; p = 0.014). The time to last follow-up/death and recurrence-free survival, also when considering intra- or extrahepatic recurrence, were significantly different between the two groups (p < 0.001). However, survival after recurrence did not differ (p = 0.167).
Figure 2.
(a) Number of patients with the diagnosis of hepatocellular carcinoma recurrence n years after liver transplantation. (b) Kaplan–Meier curve comparing survival of hepatocellular carcinoma patients with hepatocellular carcinoma recurrence stratified according to the onset of hepatocellular carcinoma recurrence (early: ≤2 years after liver transplantation, late: >2 years after liver transplantation). The calculated value of the corresponding log-rank test is shown.
HCC: hepatocellular carcinoma.
Regarding transplantation criteria, the frequency of patients meeting the Milan criteria trended to be higher in patients with late recurrence (56 vs. 31%; p = 0.069). For this group, the smaller share of patients exceeding the Milan but meeting the expanded transplantation criteria even reached significance (exceeding Milan but meeting UCSF: 32 vs. 65%; p = 0.017; exceeding Milan but meeting up-to-seven: 28 vs. 62%; p = 0.016). Furthermore, the frequencies of T3- and T4-stage HCCs, poor differentiation and multifocal disease were significantly higher in patients with early recurrence (T3 and T4: 46 vs. 12%; p = 0.007; G3 grading: 46 vs. 12%; p = 0.041; multifocal disease: 65 vs. 32%; p = 0.017). The share of patients with nodules > 5 cm in size only tended to be higher in the early recurrence group (31 vs. 12%; p = 0.103). Nevertheless, patients with early recurrence had significantly more frequent microvascular invasion (50 vs. 12%; p = 0.003) and higher AFP levels, both before TACE and before LT (AFP before LT: 12.2 vs. 5.6 ng/mL; p = 0.011). In addition, the rate of histological confirmation by liver biopsy was significantly higher in patients with late HCC recurrence (84 vs. 50%; p = 0.010).
Sites of HCC recurrence and survival
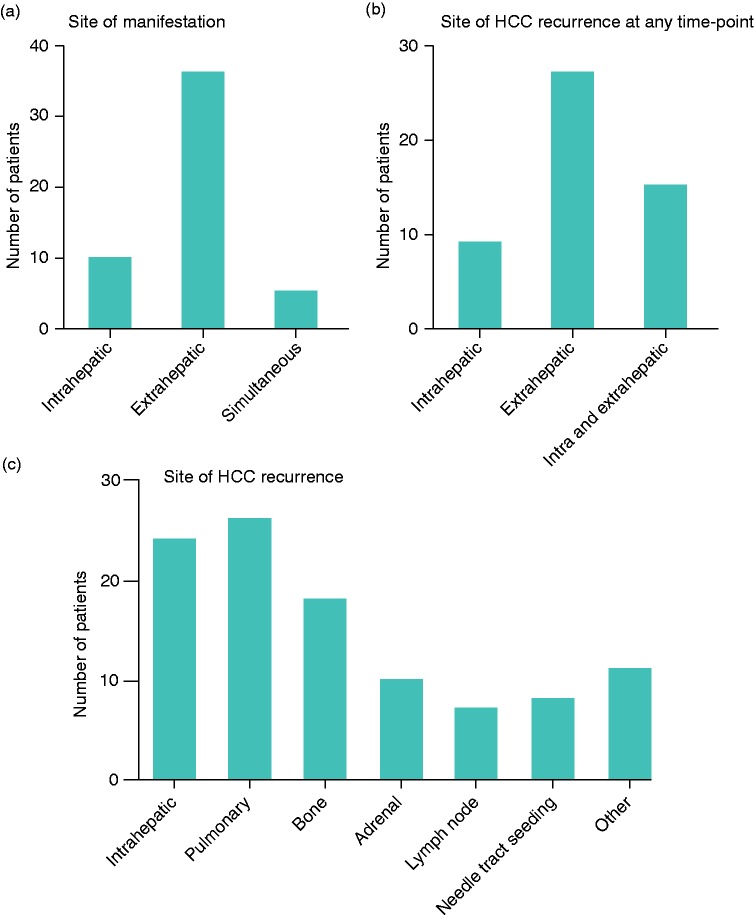
Considering the sites of HCC recurrence, the first manifestation of recurrence was intrahepatic in 10 (20%), extrahepatic in 36 (71%), and simultaneously intra- and extrahepatic in 5 patients (10%; Figure 3(a) and Supplementary Table 5). When studying the sites of recurrence at any time point, 9 patients (18%) had only intrahepatic, 27 (53%) only extrahepatic, and 15 (29%) both intra- and extrahepatic recurrence (Figure 3(b) and Supplementary Table 5). In total, 24 patients (47%) had intrahepatic, 26 (51%) pulmonary, 18 (35%) bone, 10 (20%) adrenal and 7 (14%) lymph node recurrence (Figure 3(c) and Supplementary Table 5). In eight patients (16%), recurrence was considered to be due to needle tract seeding.
Figure 3.
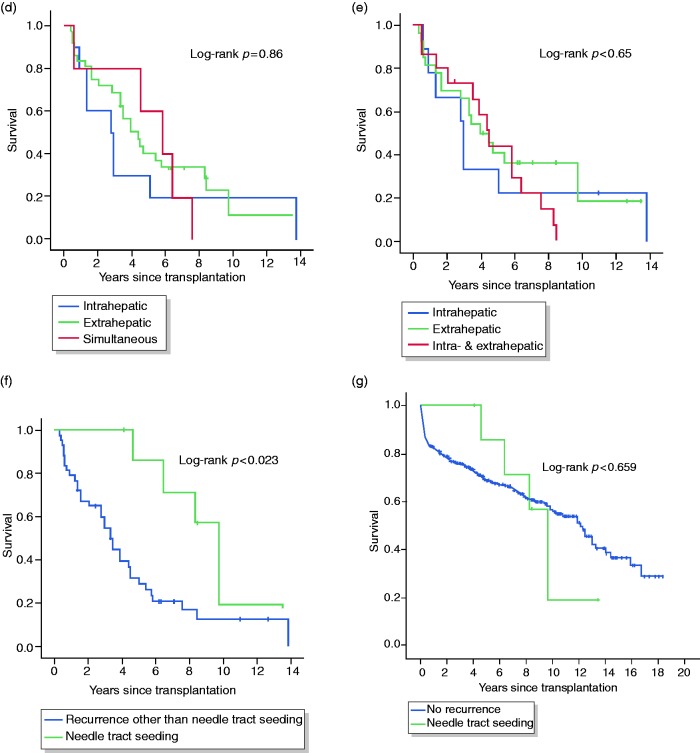
(a)–(c) Number of patients with intra- vs. extrahepatic vs. simultaneous hepatocellular carcinoma recurrence as first manifestation (a) or at any time point (b), and with distinct sites of hepatocellular carcinoma recurrence (c). (d)–(g) Kaplan–Meier curves comparing survival of hepatocellular carcinoma patients with hepatocellular carcinoma recurrence stratified according to the site of recurrence as first manifestation (d) or at any time point (e), comparing patients with hepatocellular carcinoma recurrence with and without needle tract seeding (f), and comparing patients with needle tract seeding with patients without hepatocellular carcinoma recurrence (g). The calculated value of the corresponding log-rank test is shown.
HCC: hepatocellular carcinoma.
Among patients with HCC recurrence, the site of recurrence did not significantly influence survival, except for patients with needle tract seeding who had significantly better survival (Figure 3(d) to (f) and Supplementary Table 5; log-rank p for needle tract seeding = 0.023). For this subgroup of patients, survival was not different from patients who had remained recurrence-free (Figure 3(g); log-rank p = 0.659). In addition, all of the eight patients with needle tract seeding had their recurrence > 2 years after LT, which was the only significant difference when comparing early and late recurrence with regard to the site of HCC recurrence (Supplementary Table 6; p = 0.002).
Discussion
After our recent report2 on the long-term survival of liver transplanted HCC patients at our institution, we present an in-depth analysis of HCC recurrence in our cohort in order to further explore the phenomenon of HCC recurrence after LT. Overall, the rate of recurrence of 16.8% and the detected frequencies of recurrence sites appear to be in line with previous reports.19
The goal of LT is to provide a cure for patients with HCC and HCC recurrence jeopardizes this objective. This is underscored by our data, in which the median survival of patients without recurrence was almost three times as long as their counterparts. Here, our cause of death analysis confirmed that HCC recurrence is the major contributor to mortality in the affected patients.
In order to refine the present patient selection criteria, factors that increase the risk of recurrence need to be identified. It is clear that tumour-related factors are highly relevant for recurrence. Naturally, the individual tumour burden, tumour biology and corresponding surrogate parameters such as number of nodules, nodule size, tumour volume, T stage, grading and AFP levels influence the risk of recurrence. These factors have been evaluated in multiple previous studies (among others7–15,20,21) and were also found in our cohort.
Regarding the effect of treatments before LT on the outcomes after LT, our data are in accordance with a recent study that reported similar survival and recurrence rates for patients who had or had not received pre-treatment with locoregional therapies.22 That resection tended to have been more frequently utilized as a pre-treatment in patients with HCC can potentially be explained by either a more aggressive tumour biology of the affected patients (patients who were first treated with hepatic resection typically received an LT as a salvage treatment after recurrence) or by tumour cell seeding due to manipulation during surgery.
Because of the length of our observation period, we were able to include cases of very late recurrence (>5 years after LT). Recurrence became apparent mostly during the first 2 years but also as late as >12 years after LT. It is noteworthy that survival after recurrence was similar regardless of its onset.
Regarding the site of recurrence, it is an important finding that intra- or extrahepatic recurrence, or the combination of both, did not result in different outcomes. The only recurrence site that stood out was needle tract seeding after liver biopsy. While needle tract seeding is, in the long run, a potentially deadly complication after liver biopsy, its rate is fortunately low (8 out of 304 patients in total = 2.6%, and of 184 who had a liver biopsy = 4.3%). It is also reassuring that recurrence from needle tract seeding occurs > 2 years after LT and is associated with significantly better survival than the other recurrence sites. Therefore, our data do not suggest that the practice of liver biopsy should be abandoned altogether, but that it should be used sensibly when imaging techniques produce inconclusive results.
Since the introduction of the Milan criteria, it has been debated whether the criteria are too restrictive and should therefore be expanded. A large international multicenter study showed that the risk of recurrence and death increases with increasing nodule size and number of nodules.8 The question is whether the transplantation criteria can be expanded beyond the Milan criteria without increasing the risk of recurrence and reducing post-LT survival to an unacceptable extent. While the studies that established expanded criteria such as the UCSF and up-to-seven criteria have argued that their proposed expansion would not negatively affect post-LT outcomes,7,8 our data support restrictive use of the transplantation criteria. In light of these data, it is generally accepted that some patients exceeding the Milan criteria will have a low risk of recurrence, while others meeting the Milan criteria will have a high risk. Therefore, further parameters including biomarkers are needed to discriminate between these patients. Patients with an increased risk of recurrence could be subjected to more frequent clinical monitoring and, for example, receive cross-sectional imaging on a routine basis for 5 years after LT.
More recent studies on criteria expansion have demonstrated a benefit from incorporating pre-LT AFP levels,23–25 but the long-term effect on HCC recurrence is unknown. Taking into account the phenomenon of very late recurrence, it appears necessary to evaluate expanded criteria over long time spans.
The major limitations of our study are its monocentric design and its lack of molecular read-outs, such as gene expression data. Therefore, our data set is not adequate for the refinement of the transplantation criteria. Future studies should be multicentric and include modern assays, such as RNA and genome sequencing. However, our data can be generally compared with transplantation programmes that adhere to the Milan criteria, which is used in the majority of programmes in the Western world. In turn, our data are not suitable for comparison with transplantation programmes that follow extended transplantation criteria (such as the UCSF or up-to-seven criteria).
Identifying patients that are best suited for LT and the minimization of HCC recurrence after LT remain important clinical challenges. Parameters that are routinely available after LT can be utilized during follow-up to focus on patients with an increased risk of HCC recurrence. For patient selection before LT, better prognostic parameters are needed to include patients outside the Milan criteria but with a low risk of HCC recurrence. For now, our 20-year experience with LT in HCC patients lends support to a restrictive use of transplantation criteria, especially in times of donor organ shortage.
Supplementary Material
Acknowledgements
We thank all staff members of the Interdisciplinary Transplantation Center (ITx) Mainz who contributed to this work.
Author contributions
FF, TZ and PRG conceived the study. MHL maintained the patient database. FF, JV, JUM, AW, MAW, GO, TZ and PRG collected and analysed data, and wrote the manuscript.
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
Analysis of patient data is regulated for hospitals in the federal state of Rhineland-Palatinate by § 37 LKG (state hospital code). Under its provisions, hospitals are permitted to conduct retrospective and prospective data collection and analysis. Therefore, no approval by an ethical committee was required for the present study.
Informed consent
All patients gave their written informed consent for treatment, use of samples and data utilization in clinical studies.
References
- 1.Welker MW, Bechstein WO, Zeuzem S, et al. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int 2013; 26: 109–118. [DOI] [PubMed] [Google Scholar]
- 2.Foerster F, Mittler J, Darstein F, et al. Recipient liver function before liver transplantation influences post-transplantation survival in patients with HCC. Eur J Intern Med 2018; 55: 57–65. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach J, Kulik LM, Finn R, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67: 358–380. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 2014; 3: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 7.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: Validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007; 7: 2587–2596. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: A retrospective, exploratory analysis. Lancet Oncol 2009; 10: 35–43. [DOI] [PubMed] [Google Scholar]
- 9.Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: A new MORAL to the story. Ann Surg 2017; 265: 557–564. [DOI] [PubMed] [Google Scholar]
- 10.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: A model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012; 143: 986–994 e983. quiz e914–985. [DOI] [PubMed] [Google Scholar]
- 11.DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011; 253: 166–172. [DOI] [PubMed] [Google Scholar]
- 12.Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017; 66: 552–559. [DOI] [PubMed] [Google Scholar]
- 13.Kornberg A, Kupper B, Tannapfel A, et al. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: Clinical patterns and outcome variables. Eur J Surg Oncol 2010; 36: 275–280. [DOI] [PubMed] [Google Scholar]
- 14.Sotiropoulos GC, Molmenti EP, Losch C, et al. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res 2007; 12: 527–534. [PubMed] [Google Scholar]
- 15.Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients. J Am Coll Surg 2015; 220: 416–427. [DOI] [PubMed] [Google Scholar]
- 16.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl 2006; 12: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 17.Otto G, Schuchmann M, Hoppe-Lotichius M, et al. How to decide about liver transplantation in patients with hepatocellular carcinoma: Size and number of lesions or response to TACE?. J Hepatol 2013; 59: 279–284. [DOI] [PubMed] [Google Scholar]
- 18.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant 2006; 6: 783–790. [DOI] [PubMed] [Google Scholar]
- 19.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017; 14: 203–217. [DOI] [PubMed] [Google Scholar]
- 20.Welling TH, Eddinger K, Carrier K, et al. Multicenter study of staging and therapeutic predictors of hepatocellular carcinoma recurrence following transplantation. Liver Transpl 2018; 24: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman MA, Ghobrial RM, Tong MJ, et al. Recurrence of hepatocellular carcinoma following liver transplantation: A review of preoperative and postoperative prognostic indicators. Arch Surg 2008; 143: 182–188. discussion 188. [DOI] [PubMed] [Google Scholar]
- 22.Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of pretransplant bridging locoregional therapy for patients with hepatocellular carcinoma within Milan criteria undergoing liver transplantation: Analysis of 3601 patients from the US Multicenter HCC Transplant Consortium. Ann Surg 2017; 266: 525–535. [DOI] [PubMed] [Google Scholar]
- 23.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018; 154: 128–139. [DOI] [PubMed] [Google Scholar]
- 24.Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015; 62: 158–165. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2016; 65: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.