Abstract
Background
Emerging trials suggest fecal microbiota transplantation (FMT) is a promising treatment for ulcerative colitis; however, there is a paucity of data in Crohn disease (CD).
Objective
The objectives of this article are to determine whether single-dose FMT improves clinical and endoscopic outcomes in CD patients and to identify meaningful changes in the microbiome in response to FMT.
Methods
We performed a prospective, open-label, single-center study. Ten CD patients underwent FMT and were evaluated for clinical response (defined as decrease in Harvey-Bradshaw Index score ≥3 at one month post-FMT) and microbiome profile (16S ribosomal RNA sequencing) at one month post-FMT.
Results
Three of 10 patients responded to FMT. Two of 10 patients had significant adverse events requiring escalation of therapy. On microbiome analysis, bacterial communities of responders had increased relative abundance of bacteria commonly found in donor gut microbiota.
Conclusions
Single-dose FMT in this cohort of CD patients showed modest effect and potential for harm. Responders tended to have lower baseline alpha diversity, suggesting baseline perturbation of microbiota may be an indicator of potential responders to FMT in this patient population. Controlled trials are needed to further assess the efficacy and safety of FMT in CD and determine whether FMT is a viable option in this patient population.
Clinicaltrials.gov number: NCT02460705.
Keywords: Crohn disease, dysbiosis, fecal transplant, inflammatory bowel disease, microbiome
Key summary
- Summarize the established knowledge of this subject:
- Patients with Crohn disease (CD) are known to have intestinal dysbiosis.
- There is interest in microbial manipulation for treatment of CD, specifically by fecal microbiota transplant (FMT).
- No randomized controlled trials of FMT in CD exist but a recent meta-analysis reported 50% of patients with CD achieved clinical remission with FMT.
- Few data exist on the potential for harm of FMT in CD patients.
- What are the significant and/or new findings of this study?
- In a cohort of 10 patients with CD undergoing FMT, three showed clinical response, defined as improvement in Harvey-Bradshaw index score ≥3 points at one month post-FMT, which is lower than has previously been reported in the literature.
- There was no significant improvement in objective measures of inflammation such as fecal calprotectin, erythrocyte sedimentation rate, C-reactive protein, and Simple Endoscopic Score for CD in patients who clinically responded to FMT.
- Patients who clinically responded to FMT tended to be those with lower microbial diversity, suggesting that FMT may provide symptomatic improvement for CD patients with more perturbed microbiota at baseline.
- Two of 10 patients in this study experienced flare of their underlying disease shortly after undergoing FMT, highlighting the potential for harm with FMT and the need for controlled trials to assess the safety and efficacy of FMT in this patient population.
Introduction
The pathogenesis of Crohn disease (CD) is incompletely understood, but evidence suggests the intestinal microbiota plays a significant role.1 CD patients have significant intestinal dysbiosis,2–4 characterized by decreased diversity and bacterial load.5 Hence, there is interest in microbial restoration therapies for CD,6 particularly with fecal microbiota transplant (FMT).
While there are several promising randomized controlled trials of FMT in ulcerative colitis (UC),7,8 data in CD consist primarily of case series with variable endpoints. A recent meta-analysis identified 11 studies with 83 patients examining FMT in CD and reported 50.5% achieved clinical remission.9 However, many of these studies did not report endoscopic outcomes. Accordingly, we performed a prospective, open-label, single-center study including endoscopic outcomes and microbial analysis in patients with CD who underwent FMT.
Methods
Patient recruitment
Patients aged 18–70 years were recruited from the University of California San Francisco from July 2015 to October 2016. Patients were eligible if they had prior documentation of CD with endoscopic and histopathologic confirmation and a Harvey-Bradshaw index (HBI) score of at least 3. Patients were excluded if they were pregnant, severely immunocompromised, had significant comorbidities, were unable to give informed consent, or had concomitant Clostridium difficile infection or other enteric pathogens. Concomitant therapies with other agents were permitted to continue during the course of the study. Fifteen patients fulfilled enrollment criteria and 10 patients completed the study. All participants provided written informed consent for voluntary participation in this institutional review board-approved protocol (NCT02460705, approved July 8, 2015). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution’s human research committee.
Patient assessment
Surveys were administered within one week prior to undergoing FMT and at one month post-FMT. Surveys prior to FMT assessed clinical measures to calculate HBI including general well-being, stool frequency, abdominal pain, complications of CD, and presence of an abdominal mass. Current and prior therapies were also assessed. Surveys post-FMT assessed components of HBI and also queried escalation of therapy for CD, hospitalizations, and any new medical problems following administration of FMT. Peripheral blood was collected for analyses pre- and one month post-FMT. Stool samples were obtained to rule out enteric infection and included testing for stool culture, ova, parasites, and C. difficile toxin (by human glutamate dehydrogenase and enzyme-linked immunosorbent assay). An additional stool sample was obtained for microbiota assessment.
Study protocol and FMT procedure
Patients underwent a single FMT administered via colonoscopy. Standard bowel lavage solutions were used for purging luminal content prior to FMT. Prescreened FMT donor material was provided by a stool bank, (OpenBiome, Somerville, MA), whose donor screening process has been previously described.10 FMT material was stored at –20℃ until the day of use and then was thawed in a 30℃ water bath for 30 minutes prior to infusion. During colonoscopy, 250 cc of FMT material was instilled via the colonoscope working channel into the terminal ileum or neoterminal ileum. Three patients received antibiotic pretreatment (rifaximin 550 mg three times daily for five days) that was discontinued three days prior to FMT.
Material from eight donors was used for this study. Clinical response was defined as improvement in HBI ≥3 one month after FMT and clinical remission was defined as HBI <3. 16S ribosomal RNA (rRNA) gene sequencing was performed to evaluate changes in luminal microbiota following FMT. See the Supplemental Methods section for a detailed description of the methods used for the microbial analysis.
Clinical endpoints
Primary outcome was clinical response (defined as decrease in HBI score ≥3) one month post-FMT. Secondary endpoints included achievement of clinical remission (defined as HBI <3), improvement in Simple Endoscopic Score (SES) CD score, decrease in inflammatory markers (erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fecal calprotectin) and improvement in clinical symptoms (abdominal pain on a 0–3 point scale, stool frequency) one month post-FMT.
Follow-up
Patients were contacted at one day, one week, and two weeks post-FMT to assess immediate adverse events. Follow-up surveys assessing HBI score, adverse events, and change of concomitant medications were performed one, three, six, nine, and 12 months after undergoing FMT. Stool samples were collected for microbiome analysis one month post-FMT. 16S rRNA gene sequencing was performed to evaluate changes in luminal microbiota following FMT. Six patients underwent repeat colonoscopy one month post-FMT to assess for mucosal healing post-FMT.
Statistical analysis
Clinical data were analyzed using STATA (College Station, TX). Analyses included t test, Wilcoxon signed-rank test for paired data, and Fisher exact test for categorical data. P values of <0.05 were considered significant.
Results
Clinical outcomes
Baseline patient characteristics are presented in Table 1. Three of 10 patients achieved the primary endpoint of HBI improvement ≥3. One patient achieved clinical remission at one month post-FMT. There were no statistically significant differences in baseline characteristics of responders compared with nonresponders with the exception of disease duration (p = 0.03) (Table 1). There were no significant changes in any of the clinical parameters at one month post-FMT for responders and nonresponders, including HBI, stool frequency, pain, CRP, ESR, and fecal calprotectin (Table 2). SES CD score was not significantly different for the six patients who underwent repeat colonoscopy at one month post-FMT. One responder and two nonresponders had received pretreatment with rifaximin.
Table 1.
Baseline patient characteristics.
| Variable (mean ± SD) | All (n = 10) | Responders (n = 3) | Nonresponders (n = 7) |
|---|---|---|---|
| Age (years)a | 42 ± 15 | 47 ± 21 | 40 ± 14 |
| Male (%, n)b | 50%, 5 | 33.3%, 1 | 57.1%, 4 |
| BMI (mm/kg2)a | 23.0 ± 1.8 | 23.0 ± 0.1 | 23.0 ± 2.2 |
| Disease duration (years)a,f | 15.8 ± 14.1 | 32 ± 14.5 | 8.9 ± 6.2 |
| Current steroid use (%, n)b | 20%, 2 | 66.6%, 2 | 0%, 0 |
| Prior steroid use (%, n)b | 80%, 8 | 100%, 3 | 71.4%, 5 |
| Current biologic use (%, n)b | 50%, 5 | 33.3%, 1 | 57.3%, 4 |
| Prior biologic use (%, n)b | 70%, 7 | 66.6%, 2 | 71.4,%, 5 |
| HBIa | 8.2 ± 4.0 | 11.3 ± 4.6 | 6.9 ± 3.2 |
| SES CD scorea | 8.2 ± 6.2 | 9.3 ± 10.1 | 7.7 ± 4.8 |
| Stool frequency (number/day)a | 4.95 ± 3.40 | 6.3 ± 3.1 | 4.3 ± 3.6 |
| Pain (scale 0–3)a | 1.4 ± 0.5 | 1.7 ± 0.6 | 1.3 ± 0.5 |
| CRP (mg/l)a | 13.1 ± 21.8 | 35 ± 47.4 | 6.8 ± 7.2 |
| ESR (mm/h)a | 36.1 ± 32.1 | 65.5 ± 48.8d | 27.7 ± 24.6 |
| Fecal calprotectin (mcg/g)a | 374.6 ± 311.5c | 560.35 ± 411.04d | 312 ± 289.17e |
| Montreal classification (%, n)b | |||
| A1 (<17 years at diagnosis) | 40%, 4 | 66.6%, 2 | 28.5%, 2 |
| A2 (17–40 years at diagnosis) | 40%, 4 | 33.3%, 1 | 43%, 3 |
| A3 (>40 years at diagnosis) | 20%, 2 | 0%, 0 | 28.5%, 2 |
| L1 (terminal ileum ± limited cecal disease) | 0%,0 | 0%, 0 | 0%, 0 |
| L2 (colonic) | 70%, 7 | 33.3%, 1 | 85.7%, 6 |
| L3 (ileocolonic) | 30%, 3 | 66.6%, 2 | 14.3%, 1 |
| L4 (only upper disease) | 0%, 0 | 0%, 0 | 0%, 0 |
| B1 (nonstricturing, nonpenetrating) | 70%, 7 | 0%, 0 | 100%, 7 |
| B2 (stricturing) | 30%, 3 | 100%, 3 | 0%, 0 |
| B3 (penetrating) | 0%, 0 | 0%, 0 | 0%, 0 |
| p (perianal disease modifier) | 20%, 2 | 66.6%, 2 | 0%, 0 |
BMI: body mass index; CD: Crohn disease; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; HBI: Harvey-Bradshaw index; SES: Simple Endoscopic Score.
Baseline patient characteristics of all patients, then stratified by responders (defined as decrease in HBI score ≥3 one-month post–fecal microbiota transplantation) and nonresponders. Data are presented as mean ± SD.
Values compared by t test.
Values compared by Fisher exact test.
n = 8.
n = 2.
n = 6.
p < 0.05.
Table 2.
Clinical outcomes pre- and post-FMT.
| Respondersa |
Nonrespondersa |
|||||
|---|---|---|---|---|---|---|
| Variable | n | Pre-FMT Mean ± SD | Post-FMT Mean ± SD | n | Pre-FMT Mean ± SD | Post-FMT Mean ± SD |
| HBI (one month post-FMT) | 3 | 11.3 ± 4.6 | 8.0 ± 4.4 | 7 | 6.9 ± 3.2 | 9.7 ± 4.8 |
| Stool frequency (number/day) | 3 | 6.3 ± 3.1 | 4.7 ± 1.5 | 7 | 4.3 ± 3.6 | 7.0 ± 3.4 |
| Pain (scale 0–3) | 3 | 1.7 ± 0.6 | 1.0 ± 1.0 | 7 | 1.3 ± 0.5 | 1.4 ± 1.0 |
| CRP (mg/l) | 2 | 35.0 ± 47.4 | 27.6 ± 33.7 | 7 | 6.8 ± 7.2 | 11.0 ± 12.4 |
| ESR (mm/h) | 2 | 65.5 ± 48.8 | 67.5 ± 46 | 7 | 27.7 ± 24.7 | 32.3 ± 36.7 |
| Fecal calprotectin (mcg/g) | 2 | 560 ± 411 | 624 ± 755 | 5 | 313 ± 289 | 868 ± 776 |
| SES CD score | 3 | 9.3 ± 10.1 | 9.3 ± 9.5 | 3 | 7.7 ± 4.8 | 7.7 ± 3.8 |
CD: Crohn disease; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FMT: fecal microbiota transplantation; HBI: Harvey-Bradshaw index; SES: Simple Endoscopic Score.
Clinical outcomes of responders pre- and post-FMT and nonresponders pre- and post-FMT. Data are presented as mean ± SD.
Values compared by Wilcoxon signed-rank test.
All p values >0.05.
Microbiota analysis
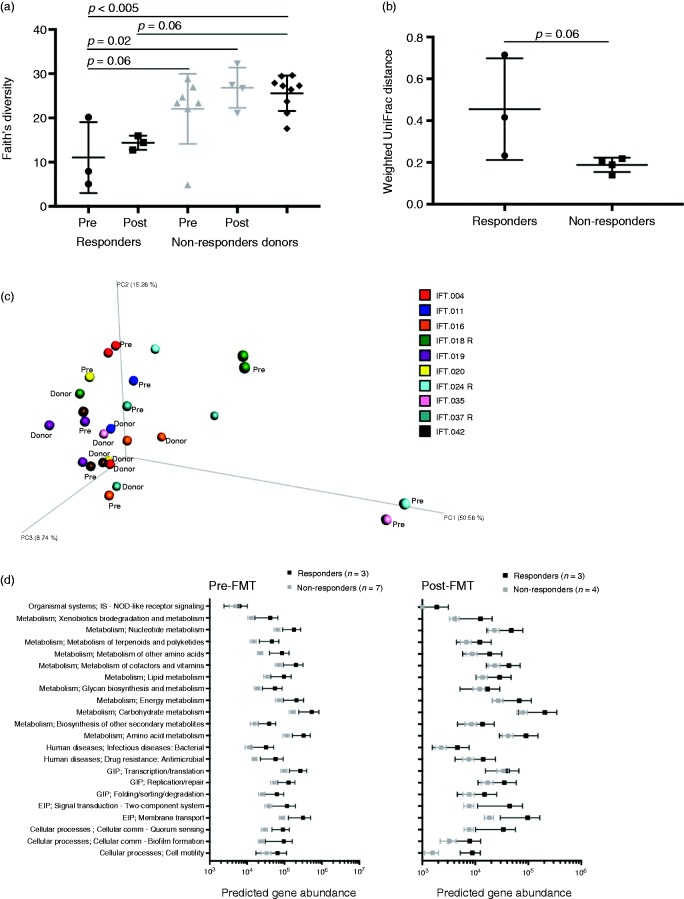
Fecal bacterial 16S rRNA biomarker sequencing revealed alpha diversity increased in two of three responders, whereas there was no difference between nonresponders and donors pre- or post-FMT (Figure 1(a)). Prior to FMT, bacterial community structure differed significantly across groups (donor, responder, nonresponder; permutational multivariate analysis of variance group-wise R2 = 0.27, p = 0.02), primarily attributable to differences between donors and responders (p = 0.003). Responders tended to have lower diversity at baseline. Following FMT, two of three responder community structures exhibited compositional change, trending greater change than nonresponders (Figure 1(b)), yet remained distinct from donors (group-wise R2 = 0.33, p = 0.002, pairwise comparison of donors to responders, p = 0.004). An accompanying analysis of unweighted UniFrac distances demonstrated a tighter clustering of post-FMT responder samples (Supplementary Figure 1(a)) than was indicated by weighted UniFrac analysis, suggesting the dynamics of less abundant taxa also could be important for responsiveness. Collinsella and several genera within Lachnospiraceae gained representatives (operational taxonomic unit, OTU) following FMT in responders (Supplementary Figure 1 (b)); however, several genera belonging to the Ruminococcaceae or Lachnospiraceae families still lacked many representatives (OTU) in post-FMT responders, and several members (OTU) of Enterobacteriaceae remained in two responders (Supplementary Figure 1(c)).
Figure 1.
Bacterial communities of clinical responders changed more following FMT than did nonresponders’ communities. (a) Bacterial alpha diversity of responder, nonresponder pre- and post-FMT, and donor groups. (b) Weighted UniFrac distances between paired pre- and post-FMT samples indicate responders changed marginally more than nonresponders following FMT. (c) Principal coordinate analysis plot showing relative (dis)similarity of individual samples using weighted UniFrac distances with participant identification denoted by color (Pre = pre-FMT sample, R = clinical responder). (d) Predicted functions (Piphillin output summarized at KEGG pathway level 2) of bacteria with significantly different relative abundances in clinically responsive vs nonresponsive patient stool samples pre- or post-FMT. EIP: environmental information processing; FMT: fecal microbiota transplantation; GIP: genetic information processing. Error bars represent SEM.
Bacterial taxon-level (OTU) differences in relative abundance were examined between responders and nonresponders pre- and post-FMT. Pre-FMT there were 46 significantly (p ≤ 0.1) different OTUs between responders and nonresponders, and 78 OTUs differed post-FMT. Enterobacteriaceae and Bifidobacterium members were more abundant in responders, whereas members of Lachnospiraceae and Ruminococcaceae had greater relative abundance in nonresponders pre-FMT. Post-FMT, a few members of Lachnospiraceae were more abundant in responders, whereas primarily Ruminococaceae (e.g. Faecalibacterium) and Bacteroides were more abundant in nonresponders. One Megasphaera OTU not detected in donor or nonresponder samples disappeared from all three responders. Piphillin11 was used to predict functional capacity of significantly different OTU with a mean difference ≥50 sequences (Supplementary Table 3). Bacteria with significantly greater relative abundance in clinical responders post-FMT did not differ in predicted function (Figure 1(d); Wilcox p > 0.05).
Adverse events
The study was halted prematurely because of a presumed CD flare in two patients within a few days of undergoing FMT. The first patient was a 37-year-old woman with colonic CD who had been diagnosed 10 years prior to enrollment in this trial. Her previous therapies included mesalamine and steroids. She was not on any therapy at the time of FMT. Within a few days of the procedure, she developed worsening abdominal pain and diarrhea. Her fecal calprotectin increased from 475 to >2000 µg/g. CRP increased from 2 to 15.5 mg/l and HBI increased from 3 to 16. The second patient was a 32-year-old woman with colonic CD. She had been diagnosed 16 years prior to enrollment in the study. She had previously failed adalimumab and infliximab (responded initially with subsequent loss of response) and was on certolizumab at the time of FMT. She had required courses of steroids on several occasions but none in the last year prior to FMT. The day following FMT, this patient experienced increased abdominal pain and diarrhea severe enough to require inpatient admission for intravenous hydration and steroids. Her HBI increased from 11 to 13 post-FMT. CRP increased from 18.3 to 33.1 mg/l. Fecal calprotectin did not increase but this is likely because she was hospitalized and received intravenous steroids before collection of this stool sample. Similarly, repeat HBI score was calculated after she received intravenous steroids for this flare of disease so the increase in HBI may be blunted by escalation of therapy (Supplementary Table 2).
Discussion
In this prospective, open-label study of FMT in CD, 30% of patients achieved the primary outcome of improvement of HBI ≥3 points one month post-FMT; however, there was no significant improvement in objective measures of inflammation such as fecal calprotectin and SES CD score. Microbiome analysis in this cohort demonstrated some potential lessons regarding baseline predictors and microbiome changes that may confer response in CD to FMT. Responders tended to be those with lower diversity, suggesting that FMT may provide symptomatic improvement for CD patients with more perturbed microbiota at baseline. Although bacterial communities of responders did not become more like donors in all cases, FMT increased the relative abundance of some bacteria observed frequently in donor microbiota and reduced those commonly associated with CD. Several of the predicted functional pathways that increased in responders post-FMT are consistent with those found to be elevated in metaproteomes of healthy individuals compared to CD patients,12 suggesting FMT restored bacterial community functionality toward a healthier state for some patients.
Our study is one of the few studies of FMT in CD to include clinical, endoscopic, and microbiome outcomes. A significant difference in our study is that our response rate is lower, with prior studies reporting up to 86.7% remission rate.13,14 However, prior studies that included endoscopic outcomes similarly showed no improvement in mucosal healing with FMT.14,15 With respect to microbiome analysis, our study was similar to a pediatric cohort that demonstrated those with the most distinct microbial communities at baseline compared to healthy donors tended to be more likely to respond to FMT.16 However, another study of FMT in refractory CD12 did not observe baseline diversity differences between responders and nonresponders.
Two patients in this cohort experienced adverse events requiring escalation of therapy within a few days of FMT that prompted early termination of the study. Donors for these patients were used in other patients without any adverse events, suggesting the adverse events were not donor specific. More data are needed to determine predictors of CD patients who may flare post-FMT and the importance of donor-host microbial interactions in such cases.
Limitations of our study include its open-label design, lack of control arm, and small sample size due to early termination of the study. At the time the study was initiated, little was known about optimal dosing of FMT for any indication other than recurrent C. difficile infection. As we learned more about the potential for antibiotics to augment engraftment,17 we opted to amend the protocol to include pretreatment antibiotics. This change introduced heterogeneity in our cohort, which is another limitation of our study. Owing to early termination of the study and the overall small number of participants, comparisons of outcomes with and without pretreatment antibiotics could not be made in this cohort, although of those who received antibiotics, two were nonresponders and one was a responder, indicating a five-day pretreatment with rifaximin was not likely to improve outcome of FMT. Furthermore, microbiome analysis in our study was limited to 16S sequencing. More in-depth sequencing with shotgun metagenomics may be required to better characterize changes post-FMT in CD patients. Finally, prior studies of FMT in UC have indicated repeated therapies may be beneficial.7,8 Our study reports outcomes after only one delivery of FMT. Given that some of our responders continued to have OTUs known to be associated with inflammation such as Enterobacteriaceae post-FMT, additional therapy could be helpful to more fully transform the colonic microbiota.
In conclusion, single-dose FMT in this cohort of CD patients showed modest effect and potential for harm. Clinical response was not reliant on luminal bacterial communities fully resembling donor communities, though some individuals developed increased relative abundance of bacteria commonly found in donor gut microbiota after FMT, which may explain their positive clinical response. Responders tended to have lower baseline alpha diversity, suggesting baseline perturbation of microbiota may be an indicator of potential responders to FMT in this patient population. The early termination of this study because of safety concerns highlights the necessity for controlled trials to assess the efficacy and safety of FMT in CD and to determine whether microbial restoration therapy with FMT is a viable option in this patient population.
Supplemental Material
Supplemental Material for Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile by Liat Gutin, Yvette Piceno, Douglas Fadrosh, Kole Lynch, Martin Zydek, Zain Kassam, Brandon LaMere, Jonathan Terdiman, Averil Ma, Ma Somsouk, Susan Lynch and Najwa El-Nachef in United European Gastroenterology Journal
Declaration of conflicting interests
Zain Kassam was an employee at OpenBiome as well as a research consultant and shareholder at Finch Therapeutics at the time of this work. The other authors have nothing to declare.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution’s human research committee.
Funding
This work was supported by an anonymous philanthropic gift.
Informed consent
All participants provided written informed consent for voluntary participation in this institutional review board-approved protocol (NCT02460705, approved July 8, 2015).
References
- 1.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol 2015; 37: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007; 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagalingam NA, Lynch SV. Role of the microbiota in inflammatory bowel diseases. Inflamm Bowel Dis 2012; 18: 968–984. [DOI] [PubMed] [Google Scholar]
- 4.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008; 134: 577–594. [DOI] [PubMed] [Google Scholar]
- 5.Damman CJ, Miller SI, Surawicz CM, et al. The microbiome and inflammatory bowel disease: Is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 2012; 107: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 6.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014; 146: 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 2015; 149: 102–109.e6. [DOI] [PubMed] [Google Scholar]
- 8.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017; 389: 1218–1228. [DOI] [PubMed] [Google Scholar]
- 9.Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J Crohns Colitis 2017; 11: 1180–1199. [DOI] [PubMed] [Google Scholar]
- 10.Vujkovic-Cvijin I, Rutishauser RL, Pao M, et al. Limited engraftment of donor microbiome via one-time fecal microbial transplantation in treated HIV-infected individuals. Gut Microbes 2017; 8: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai S, Weinmaier T, Schmidt BL, et al. Piphillin: Improved prediction of metagenomic content by direct inference from human microbiomes. PLoS One 2016; 11: e0166104–e0166104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson AR, Cantarel BL, Lamendella R, et al. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of Crohn’s disease. PLoS One 2012; 7: e49138–e49138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: Safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol 2015; 30: 51–58. [DOI] [PubMed] [Google Scholar]
- 14.Vaughn BP, Vatanen T, Allegretti JR, et al. Increased intestinal microbial diversity following fecal microbiota transplant for active Crohn’s disease. Inflamm Bowel Dis 2016; 22: 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeire S, Joossens M, Verbeke K, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis 2016; 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal A, Yeh A, Bush BR, et al. Safety, clinical response, and microbiome findings following fecal microbiota transplant in children with inflammatory bowel disease. Inflamm Bowel Dis 2018; 24: 410–421. [DOI] [PubMed] [Google Scholar]
- 17.Dethlefsen L, Huse S, Sogin ML, et al. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6: e280–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile by Liat Gutin, Yvette Piceno, Douglas Fadrosh, Kole Lynch, Martin Zydek, Zain Kassam, Brandon LaMere, Jonathan Terdiman, Averil Ma, Ma Somsouk, Susan Lynch and Najwa El-Nachef in United European Gastroenterology Journal