Abstract
Background
Transarterial chemoembolization (TACE) affects hepatic perfusion, and might have an impact on portal pressure in patients with hepatocellular carcinoma (HCC).
Objective
The objective of this article is to report the secondary outcome “hepatic hemodynamics” from the AVATACE trial, a prospective randomized, placebo-controlled trial on the efficacy of conventional TACE in combination with bevacizumab or placebo.
Methods
Hepatic venous pressure gradient (HVPG) was measured at baseline (prior to first TACE), within nine days (“acute effects”), two months (“intermediate effects”) and six months (“long-term effects”) after the first TACE.
Results
Of 28 patients with early-intermediate stage HCC, n = 20 (71%) had clinically significant portal hypertension (CSPH, HVPG ≥ 10 mmHg) at baseline (median, 12 (interquartile range (IQR): 9–19) mmHg). TACE had neither “acute effects” nor “intermediate effects” on HVPG. However, in 13 patients with available HVPG measurement at month 6, there was a significant increase in HVPG (median, 16 (IQR: 11–19) mmHg) compared with baseline (median, 10 (IQR: 5–12) mmHg; p = 0.007). Portal hypertension-related complications occurred exclusively in patients with CSPH (8 (40%) vs 0).
Conclusions
Repeated TACE was associated with a significant long-term increase in HVPG. This should be considered when deciding whether to continue with TACE or switch to systemic treatment, since CSPH drives the development of complications.
Keywords: Hepatic venous pressure gradient, hepatocellular carcinoma, portal hypertension, transarterial chemoembolization
Key summary
Transarterial chemoembolization (TACE) increases portal blood flow in patients with hepatocellular carcinoma.
The clinical implications of these (potentially transient) changes in hepatic hemodynamics have not yet been established.
Whereas TACE had no impact on portal pressure in short-term follow-up, we observed a significant increase in hepatic venous pressure gradient (HVPG) after six months and repeated TACE.
The increase in HVPG may be explained by architectural changes within the liver and splanchnic area as a result of TACE-induced vascular endothelial growth factor upregulation and liver fibrosis progression.
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent cancer entities worldwide, causing every second cancer-related death in men and every sixth in women.1 Approximately 90% of all cases have underlying cirrhosis and about every third patient with cirrhosis will develop HCC.2 In Western countries, around 50% of HCCs are diagnosed at an advanced stage amendable only to palliative treatment.3
Unlike in other tumor entities, prognosis in HCC is determined not only by cancer biology,4,5 but also by the severity of underlying liver disease,6,7 and thus, both factors have to be considered for treatment selection.8 While surgical and local ablative treatments are available for early stages of HCC, transarterial chemoembolization (TACE) represents the treatment of choice in patients with intermediate-stage HCC9 and may also be considered in selected patients with unresectable early-stage HCC.10 Intermediate-stage HCC patients are defined to be asymptomatic with liver-limited, multinodular, unresectable tumors and well-preserved liver function.9 TACE combines the effects of conventional embolization and cytotoxic chemotherapy and allows for increased drug delivery into the tumor with less systemic toxicity as compared with conventional systemic chemotherapy.11
Even though the tumor surrounding liver parenchyma in earlier stages of cirrhosis or in noncirrhotic HCC receives its blood supply mainly from the portal vein and not predominantly from the arterial system (like the tumor), TACE can lead to a deterioration of liver function, which may ultimately lead to liver-related deaths.11,12 Transarterial embolization seems to have a transient effect on hepatic hemodynamics (decrease in hepatic arterial blood flow, which is accompanied by an increase in portal blood flow).13–15 However, the clinical implications of these transient changes are unclear, since data on the impact of TACE on portal hypertension (PHT) and associated complications are limited. The aim of the present study was to evaluate (i) the acute, intermediate and long-term effects of repeated TACE on hepatic venous pressure gradient (HVPG) as well as (ii) the impact of HVPG on the development of PHT-related complications in HCC patients treated with TACE.
Materials and methods
Study design
This study reports the secondary endpoint “effects on hepatic hemodynamics” of the AVATACE-1 trial,16 a randomized, placebo-controlled, double-blind phase 2 trial evaluating the effect of TACE in combination with intravenous bevacizumab or placebo in patients with intermediate-stage HCC (clinicaltrials.gov identifier: NCT00280007). Briefly, patients were randomized 1:1 to receive TACE plus either bevacizumab (5 mg/kg bodyweight every 14 days) or blinded saline infusion (“TACE only” group) intravenously for 52 weeks or until death, development of extrahepatic lesions, or untreatable tumor progression. After the first TACE, the procedure was repeated twice at four-week intervals if technically feasible and if contrast enhancement of tumors was still present at follow-up radiological imaging. Additional cycles were performed if clinically indicated (contrast uptake of nodules).16
Patients underwent repeated HVPG measurements at defined time points: prior to (baseline) and after the first TACE (week 1), at month 2, and six months after the first TACE.16 This study was approved by the local ethics committee of the Medical University of Vienna (number: 253/2005; date: July 4, 2004) and conducted in accordance with the guidelines stated in the Declaration of Helsinki. All patients provided written informed consent.
Patients and definitions
Inclusion and exclusion criteria were reported in detail previously.16 Briefly, eligibility criteria included early or intermediate stage HCC (Barcelona Clinic Liver Cancer (BCLC) A or B) not suitable for local ablative or surgical treatment. Patients were not allowed to have heart failure (New York Heart Association class ≥II; reduced left ventricular ejection fraction or impaired right ventricular function). Patients were excluded if they had major surgery within the past four weeks, variceal bleeding within two weeks prior to inclusion, or a life expectancy of less than three months.16 Furthermore, patients underwent upper gastrointestinal endoscopy and prophylactic band ligation of any large esophageal varices prior to inclusion.
Patients who did not undergo repeated HVPG measurement and those in whom NSBB therapy was initiated during the study period were excluded from the analysis.
As not all patients underwent HVPG reassessment at all time points, subgroups were formed. Patients in whom HVPG measurement was performed within nine days after the first TACE procedure were included in the “acute effects” group. All patients who received an HVPG measurement at month 2 after the initial TACE were included in the “intermediate effects” group. Finally, patients in whom HVPG evaluation was available six months after the first TACE session were assigned to the “long-term effects” group. PHT-related complications (variceal bleeding, spontaneous bacterial peritonitis, new onset of ascites or hepatic encephalopathy) that occurred between baseline and the last follow-up HVPG measurement at month 6 were recorded.
TACE procedure
Super-selective, conventional TACE (cTACE) was performed with doxorubicin (Pfizer Corporation) at a dose of 75, 50 and 25 mg/m2 according to serum bilirubin levels (<1.5, 1.5–3, and >3 mg/dl) and lipiodol (Guerbet) mixed in a 1:1 ratio as previously described.16 Thereafter, embolization was performed with Bead Block microspheres (Bead Block, Biocompatibles) 100–500 μm in size until stasis in the tumor-feeding hepatic artery branches occurred. For safety reasons only one liver lobe per session was treated in case of bilobar tumor nodules.16
HVPG measurement
HVPG measurement was performed according to a standard operating procedure as previously described.17 In brief, a balloon occlusion catheter was introduced through the inferior vena cava into a large liver vein. At least three repeated measurements of free and wedged hepatic vein pressure were performed and mean values were calculated to determine the HVPG (the difference between free and wedged hepatic vein pressures).17 Clinically significant portal hypertension (CSPH) was defined as HVPG ≥10 mmHg.18
Statistics
Statistical analyses were performed using IBM SPSS Statistics 25.0 (SPSS Inc) and GraphPad Prism 6 (GraphPad Software). Continuous variables were reported as mean±SD or median (interquartile range (IQR)) and categorical values were reported as numbers (n) and proportions (%) of patients with the respective characteristic. Comparisons of proportions were performed using chi-squared test or Fisher exact test, as applicable. Unpaired continuous variables were compared using Student t test or Mann-Whitney U test, and paired analysis of different time points using Wilcoxon signed-rank test. Correlation between ΔModel for End-Stage Liver Disease (MELD) and ΔHVPG was analyzed using Spearman correlation coefficient (ρ). Overall survival was calculated as the time from first TACE until the date of death or last follow-up if still alive. Survival was calculated and plotted using the Kaplan-Meier method. Statistical differences in survival between the two treatment groups were compared by means of the log-rank test.
Results
Patient characteristics
Of a total of 32 patients included in the trial, exclusions included one patient who missed follow-up HVPG measurements and another three patients in whom nonselective beta-blocker (NSBB) treatment was initiated during follow-up. Twenty-eight patients were included in our final data set (Figure 1). Of these, 13 patients received TACE alone (“placebo group”) and 15 patients were treated with TACE plus bevacizumab. As not all patients underwent HVPG measurements at all time points, the following patient numbers were eligible for statistical analysis within the different groups: Twenty-six patients underwent HVPG measurement right before and within nine days after the first TACE procedure and were therefore included in the “acute TACE effects” group. Twenty-three patients had an HVPG measurement at baseline and after two months and were included in the “intermediate effects” group, and finally, 13 patients underwent an additional HVPG measurement six months after the first TACE session and were available for the evaluation of “long-term effects.” These different group sizes can be explained by the fact that changes in portal pressure was a secondary endpoint in the AVATACE trial and not all patients had to undergo repeated measurement to stay in the study. Reasons for missing HVPG assessments at the three different time points are detailed in Supplementary Table 2.
Figure 1.
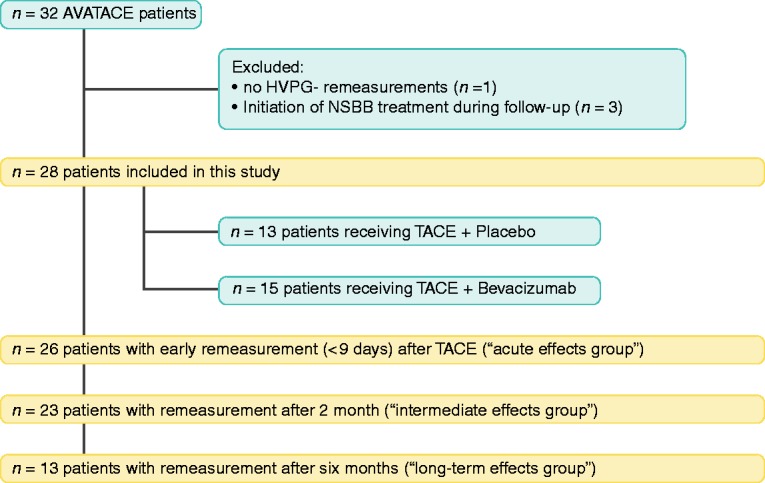
Patient flowchart.
HVPG: hepatic venous pressure gradient; NSBB, nonselective beta-blocker; TACE: transarterial chemoembolization.
Detailed patient characteristics are shown in Table 1. The majority of patients (n = 25, 89%) were male and mean age was 61 ± 8 years. Main etiologies of liver disease were viral hepatitis and alcoholic liver disease (n = 12 (43%) and n = 13 (46%), respectively). Mean Child-Pugh score was 6 ± 1 points, resulting in 22 (79%) Child-Pugh A and six (21%) Child-Pugh B patients. The mean MELD score was 10 ± 3 points. Twenty patients (71%) had CSPH and median HVPG was 12 (IQR: 9–19) mmHg. The large majority of patients had intermediate-stage HCC (BCLC B: n = 24, 86%).
Table 1.
Patient characteristics.
| All patients, n = 28 | |
|---|---|
| Sex, male (%)/female (%) | 25 (89%)/3 (11%) |
| Age, years (mean±SD) | 61 ± 8 |
| Etiology | |
| Viral hepatitis, n (%) | 12 (43%) |
| Alcohol, n (%) | 13 (46%) |
| Other, n (%) | 3 (11%) |
| Presence of ascites, n (%) | 5 (18%) |
| Varices | 9 (32%) |
| Small varices, n (%) | 4 (14%) |
| Large varices, n (%) | 5 (18%) |
| NSBB therapy | 3 (11%) |
| Propranolol, n (%) | 3 (11%) |
| INR | 1.2 ± 0.2 |
| Albumin, g/l | 37 ± 5 |
| Child-Pugh score, points (mean±SD) | 6 ± 1 |
| Child-Pugh A, n (%) | 22 (79%) |
| Child-Pugh B, n (%) | 6 (21%) |
| MELD score, points (mean±SD) | 10 ± 3 |
| PS | |
| PS 0, n (%) | 28 (100%) |
| BCLC stage | |
| A, n (%) | 4 (14%) |
| B, n (%) | 24 (86%) |
| Treatment group | |
| TACE alone, n (%) | 13 (46%) |
| TACE + bevacizumab, n (%) | 15 (54%) |
| HVPG, mmHg (median (IQR)) | 12 (9-19) |
| Level of portal hypertension | |
| No portal hypertension (HVPG < 6) | 5 (18%) |
| Subclinical portal hypertension (HVPG 6–9 mmHg) | 3 (11%) |
| Clinically significant portal hypertension (HVPG ≥ 10 mmHg) | 20 (71%) |
| Number of TACE sessions (mean±SD) | 4 ± 1 |
n: number of patients; BCLC: Barcelona Clinic Liver Cancer; PS: Performance Status; HVPG: hepatic venous pressure gradient; INR: international normalized ratio; MELD: Model for End-Stage Liver Disease; NAFLD: nonalcoholic fatty liver disease; NSBB: nonselective beta-blocker; TACE: transarterial chemoembolization.
Acute effects of TACE on HVPG
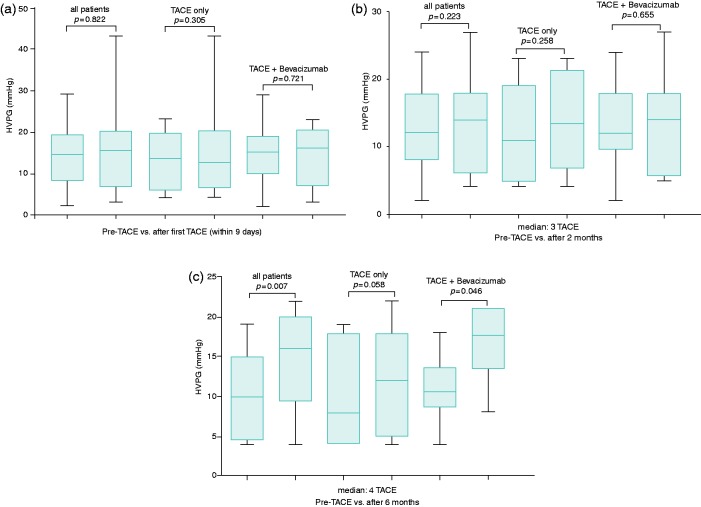
Follow-up HVPG measurement was performed within nine days after the first TACE (median: 2 days (IQR: 1–3 days)). In total, 12 patients (46%) were randomized to TACE plus placebo, while 14 patients (54%) received TACE in combination with bevacizumab (Figure 2(a)). Median HVPG after the first TACE did not change compared with baseline in the whole cohort (15 (IQR: 8–19) mmHg vs 16 (IQR: 7–20) mmHg; p = 0.822) nor in the different subgroups (TACE only: 14 (IQR: 7–20) mmHg vs 13 (IQR: 7–20) mmHg; p = 0.305; TACE + bevacizumab: 15 (10–18) mmHg vs 16 (7–20) mmHg, p = 0.721) (Figure 2(a)).
Figure 3.
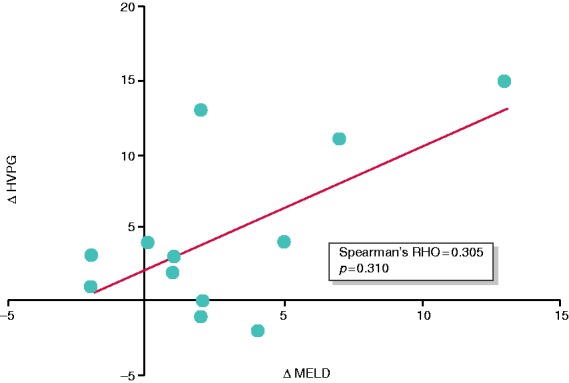
Correlation between changes in HVPG and changes in MELD score between baseline (pre-TACE) and month 6 (after repeated TACE). Abbreviations: Δ HVPG: delta hepatic venous pressure gradient; Δ MELD: delta model of end-stage liver disease.
Figure 4.
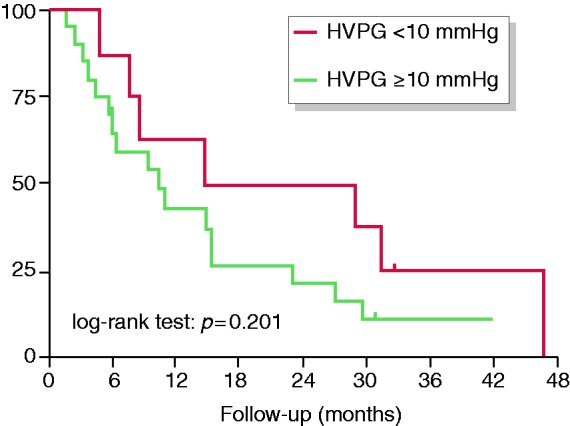
Impact of clinically significant portal hypertension at baseline on survival. Abbreviations: HVPG: hepatic venous pressure gradient.
Figure 2.
Comparison of HVPG value at baseline (before first TACE) and (a) shortly after the first TACE (“acute effects group”), (b) after two months and (c) after six months.
HVPG: hepatic venous pressure gradient; TACE: transarterial chemoembolization.
Intermediate effects of TACE on HVPG
In total, 23 patients underwent follow-up HVPG measurement two months after the first TACE (59 (IQR: 58–78) days) (Figure 2(b)). All patients had undergone three TACE sessions by then. Ten patients (43%) were treated with TACE alone and n = 13 (57%) received concomitant bevacizumab. Although there was a numerical increase in HVPG in both groups, changes were not statistically significant in the whole cohort (12 (IQR: 8–18) mmHg vs 14 (IQR: 6–18) mmHg; p = 0.223) or in the different treatment groups (TACE only: 11 (IQR: 5–19) mmHg vs 14 (7–21) mmHg); p = 0.258; TACE + bevacizumab: 12 (10–18) mmHg vs 14 (6–18) mmHg; p = 0.655) (Figure 2(b)).
Long-term effects of TACE on HVPG
Follow-up HVPG measurement after six months (median, 182 (IQR: 175–191) days) was available in 13 patients (Figure 2(c)), of whom seven were treated with TACE alone, while six patients received concomitant bevacizumab. These patients underwent 4 ± 0.5 TACE procedures. In contrast to the other time points, median HVPG significantly increased from baseline to follow-up HVPG evaluation at six months in the overall cohort (10 (IQR: 5–12) mmHg vs 16 (IQR: 11–19) mmHg, p = 0.007) and in the subgroup of patients receiving TACE + bevacizumab (11 (IQR: 10–12) mmHg vs 18 (IQR: 15–21) mmHg, p = 0.046). Additionally, there was a trend toward an increase in median HVPG in patients treated with TACE only (8 (IQR: 4–18 mmHg) vs 12 (IQR: 5–18) mmHg, p = 0.058), which did not attain statistical significance, probably because of the limited sample size/statistical power. Interestingly, a significant increase in HVPG was observed in patients with and without CSPH at baseline (Supplementary Table S1).
Supplementary Figure S1 shows overall changes and supplementary Figure S2 individual changes of HVPG over time (baseline and month 6).
Notably, there was no correlation between ΔMELD (baseline vs month 6) and ΔHVPG (Spearman ρ = 0.305, p = 0.310) (Figure 3), suggesting that the observed increase in HVPG in this cohort was not due to a significant deterioration of liver function. In line, in six patients (46%) the hepatotoxic trigger was removed, as five patients with alcoholic cirrhosis stopped drinking and one patient with hemochromatosis underwent repetitive phlebotomy. In one patient with cryptogenic cirrhosis presenting with metabolic syndrome, diabetes was well controlled. Six other patients (46%) with hepatitis C virus (HCV) cirrhosis were viremic during the study and therefore still had a hepatotoxic trigger. Finally, there was no association between radiological tumor progression and changes in HVPG at month 6 (Supplementary Table 3).
Incidence of PHT-induced complications and survival
In total, eight of 28 patients (29%) experienced PHT-related complications during the study period. Three patients (15%) developed ascites, two patients (10%) presented with variceal bleeding, four patients (20%) had an episode of hepatic encephalopathy, and two patients (10%) developed spontaneous bacterial peritonitis. Notably, all PHT-related complications occurred in patients with CSPH (n = 8 vs 0; Table 2). In the “long-term effects” subgroup, PHT-associated complications developed only in patients with CSPH who had an increase in HVPG of ≥3 mmHg ( = median change; Table 3).
Table 2.
Incidence of portal hypertension-associated complications according to hepatic venous pressure gradient (HVPG).
| HVPG < 10 mmHg (n = 8) | HVPG ≥ 10 mmHg (n = 20) | |
|---|---|---|
| Incidence of portal hypertension- associated complications | 0 (–) | 8 (40%) |
| Development of ascites | 0 (–) | 3 (15%) |
| Variceal bleeding | 0 (–) | 2 (10%) |
| Hepatic encephalopathy | 0 (–) | 4 (20%) |
| Spontaneous bacterial peritonitis | 0 (–) | 2 (10%) |
Table 3.
Comparison of the incidence of portal hypertension (PHT)-associated complications in patients with hepatic venous pressure gradient (HVPG) change above vs below the median change from baseline to follow-up evaluation at month 6.
| ΔHVPG < 3 mmHg (n = 5) | ΔHVPG ≥ 3 mmHg (n = 8) | |
|---|---|---|
| Incidence of PHT-associated complications | 0 (–) | 3 (38%) |
| Development of ascites | 0 (–) | 2 (25%) |
| Variceal bleeding | 0 (–) | 0 (–) |
| Development of hepatic encephalopathy | 0 (–) | 0 (–) |
| Development of spontaneous bacterial peritonitis | 0 (–) | 1 (13%) |
As shown in Figure 4, survival was not significantly different between patients with vs without CSPH at baseline (CSPH: 10 (95% confidence interval: 4–16) months vs no-CSPH: 15 (0–43) months, p = 0.201).
Discussion
In the present study, we evaluated changes in HVPG at different time points in patients with HCC treated with repeated TACE. While TACE had no significant short-term effects on HVPG, we observed a significant increase in HVPG after six months and repeated TACE. Importantly, all PHT-related complications occurred in patients with CSPH.19 These patients had a numerically shorter median overall survival, even though—and likely because of the limited sample size—it was not statistically significant. Furthermore, in patients with available HVPG evaluation at month six, PHT-related events occurred only in patients with CSPH and an HVPG increase of ≥3 mmHg. This finding is in line with recent publications showing that clinically evident PHT (as defined by the presence of clinical signs of PHT such as the presence of varices) was a very important poor prognostic factor in HCC patients undergoing TACE.20,21
Furthermore, previous Doppler ultrasonography studies have documented a transient increase in portal flow after transarterial embolization, which might compensate for the decrease in hepatic arterial blood flow. Portal flow peaked about one week after embolization and remained increased for at least two weeks.13–15 In our study, we neither observed an increase of HVPG within days after the first TACE nor after two months and repeated TACE, suggesting that the acute increase in portal flow after embolization as measured with ultrasound did not translate into a clinically meaningful increase in portal pressure. Our results are in line with a small pilot study22 showing that in 15 patients with HCC undergoing TACE, HVPG did not change within three days after TACE. However, no long-term effects on HVPG were investigated in this study.22
Notably, we observed an increase in HVPG after six months and repeated TACE sessions. Mechanistically, this observation could be attributed to architectural changes within the liver and splanchnic area as a result of TACE-induced vascular endothelial growth factor (VEGF) upregulation and liver fibrosis progression.
Serum VEGF levels increase after TACE and remain elevated for at least four weeks, especially after conventional TACE.23–25 VEGF is associated not only with outcome after TACE23,26 but is also involved in the pathophysiology of PHT, as it promotes vasodilation and splanchnic vascularization, aggravating hyperdynamic splanchnic circulation and eventually increasing portal pressure.27,28 Notably, anti–VEGF-targeted treatment has previously been shown to ameliorate portal hypertensive syndrome in rats.29–31 However, it did not prevent the increase in HVPG after repeated TACE in our study, since an HVPG increase was also observed in the subgroup of patients treated with TACE plus bevacizumab.
Additionally, chronic tissue injury leads to activation of quiescent stellate cells to migratory and contractile myofibroblasts. These activated stellate cells promote vasoconstriction as well as secretion of extracellular matrix proteins, which contribute to the development of liver fibrosis.32,33 Both intrahepatic vasoconstriction (functional component) and liver fibrosis (mechanical component) increase intrahepatic resistance and ultimately lead to PHT.34 Since TACE induces ischemic tissue injury not only in the tumor but to some degree also in surrounding noncancerous liver tissue,35 repeated TACE may also promote the progression of liver fibrosis. Indeed, hepatic artery ligation-induced hypoxia aggravated liver fibrosis in an experimental model of HCC, while inhibition of hypoxia-inducible-factor-1α attenuated liver fibrosis progression.36
We have to acknowledge some limitations. First, the limited sample size and the fact that not all patients underwent HVPG measurement at all time points weakens the statistical power of this analysis. However, to our knowledge, this is the first study to show the hemodynamic long-term effects of TACE on HVPG. Furthermore, we cannot prove our theories about the underlying mechanism of the observed effects, as serum samples to evaluate changes in VEGF and serial liver biopsies/liver stiffness measurements to assess progression of liver fibrosis were not available. We also cannot rule out that the observed HVPG increase may be partly attributed to the natural course of the disease. In almost half of our patients, the hepatotoxic trigger was removed, but the other half had HCV-related cirrhosis and was viremic during the study. However, a recent study evaluating the natural course of HVPG in HCV patients found a median HVPG increase of only 1.5 mmHg within 24 weeks37 compared with a median increase of 6.0 mmHg in our study. Hence, we believe that the natural course of the underlying disease only had a minor impact on our results.
In conclusion, short-term hepatic hemodynamic changes after TACE may be mild and reversible, but repeated TACE may lead to an aggravation of PHT in the long run. Importantly, only patients with CSPH developed PHT-associated complications. As portal pressure has previously been shown to play a role in the development of HCC38 and is a major driver of complications in this patient population,18 NSBB therapy of CSPH should not be withheld from patients with HCC.39 These data could provide valuable information to clinicians and are worth considering when deciding whether to continue with TACE or switch to systemic therapy. While currently available scores to guide the decision for retreatment with TACE (e.g. Assessment for Retreatment with Transarterial Chemoembolization (ART)12 or alpha fetoprotein, BCLC stage, Child-Pugh score, and treatment response (ABCR)40 score) do not include portal pressure, recent studies identified clinically evident PHT as an important prognostic factor.20,21 Therefore, we believe that HVPG measurement before TACE might provide useful information on the risk of PHT-related complications and prognosis in patients undergoing TACE. While noninvasive markers are well validated to rule PHT in or out,18 they are insufficient to monitor changes in HVPG.41 Our data suggest that monitoring HVPG during TACE may add additional important data to facilitate treatment individualization. Larger studies are warranted to confirm these results.
Supplemental Material
Supplemental Material for Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma by Bernhard Scheiner, Gregor Ulbrich, Mattias Mandorfer, Thomas Reiberger, Christian Müller, Fredrik Waneck, Michael Trauner, Claus Kölblinger, Arnulf Ferlitsch, Wolfgang Sieghart, Markus Peck-Radosavljevic and Matthias Pinter in United European Gastroenterology Journal
Acknowledgments
Author contributions include the following: Concept of the study (B.S., G.U., M.P.-R., M.P.), performance of the examinations (M.M., T.R., F.W., C.K., A.F., M.P.-R., M.P.), extraction of data (B.S., G.U., M.P.), drafting of the manuscript (B.S., M.P.), writing of the manuscript (B.S., M.P.) and revision for important intellectual content (all authors).
M.P. acts as the guarantor of the article and all authors approved the final version of the manuscript.
Declaration of conflicting interests
B.S. has received travel support from Gilead. G.U. has received travel support from Bayer. M.M. has received honoraria for consulting and/or payments for lectures from AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and W.L. Gore & Associates. T.R. has received travel support from Boehringer-Ingelheim, Gilead, Roche, MSD, and W.L. Gore & Associates; grant support from Gilead, AbbVie, MSD, Philips Healthcare, Boehringer-Ingelheim, Phenex Pharmaceuticals, and W.L. Gore & Associates; and has served as a consultant for Bayer, MSD, Gilead, AbbVie, and Boehringer-Ingelheim. C.M. has nothing to declare. F.W. has received payments for lectures from Bayer and Siemens. M.T. has received grants from MSD, honoraria for consulting from AbbVie, Gilead, Intercept, Janssen, and MSD, payments for lectures from Gilead, Intercept, MSD, and Roche, as well as travel support from AbbVie, Gilead, and Falk. C.K. has received grant support and honoraria from Bayer HealthCare, Guerbet GmbH, and BTG International Ltd. A.F. has served as a speaker and/or consultant and/or advisory board member for AbbVie, Gilead, and Intercept and owns a patent on a catheter for the measurement of hepatic venous pressure gradient. W.S. has received speaker and consulting fees and research grants from Bayer Schering Pharma. M.P.-R. has received grant support and honoraria from Bayer HealthCare and BMS and has served as a consultant for Bayer HealthCare, BMS, Lilly, ONXEO, Eisai, and Ipsen. M.P. was an advisory board member for Bayer, BMS, Eisai, and Ipsen, and has received travel support from Bayer and speaking fees from Bayer and BMS. He is also an investigator for Bayer, BMS, and Lilly.
Ethics approval
This study was approved by the local ethics committee of the Medical University of Vienna (number: 253/2005; date: July 4, 2004) and conducted in accordance with the guidelines stated in the Declaration of Helsinki.
Funding
This work was supported by the Jubiläumsfonds of the Austrian National Bank (grant 11981).
Informed consent
All patients provided written informed consent.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2.Akinyemiju T, Abera S, et al. Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinter M, Peck-Radosavljevic M. Review article: Systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther 2018; 48: 598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology 2013; 57: 2224–2234. [DOI] [PubMed] [Google Scholar]
- 5.Scheiner B, Kirstein MM, Popp S, et al. Association of platelet count and mean platelet volume with overall survival in patients with cirrhosis and unresectable hepatocellular carcinoma. Liver Cancer.. Epub ahead of print 22 June 2018. DOI:10.1159/000489833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinter M, Trauner M, Peck-Radosavljevic M, et al. Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 2016; 1: e000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabibbo G, Enea M, Attanasio M, et al. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010; 51: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 8.Adhoute X, Penaranda G, Raoul JL, et al. Barcelona clinic liver cancer nomogram and others staging/scoring systems in a French hepatocellular carcinoma cohort. World J Gastroenterol 2017; 23: 2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2018; 69: 182–236. [DOI] [PubMed] [Google Scholar]
- 10.Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019; 72: 28–36. [DOI] [PubMed] [Google Scholar]
- 11.Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev 2011; 37: 212–220. [DOI] [PubMed] [Google Scholar]
- 12.Sieghart W, Hucke F, Pinter M, et al. The ART of decision making: Retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology 2013; 57: 2261–2273. [DOI] [PubMed] [Google Scholar]
- 13.Taourel P, Dauzat M, Lafortune M, et al. Hemodynamic changes after transcatheter arterial embolization of hepatocellular carcinomas. Radiology 1994; 191: 189–192. [DOI] [PubMed] [Google Scholar]
- 14.Moriyasu F, Ban N, Nishida O, et al. Portal hemodynamics in patients with hepatocellular carcinoma. Radiology 1986; 161: 707–711. [DOI] [PubMed] [Google Scholar]
- 15.Ohnishi K, Sato S, Tsunoda T, et al. Portal venous hemodynamics in hepatocellular carcinoma. Effects of hepatic artery embolization. Gastroenterology 1987; 93: 591–596. [DOI] [PubMed] [Google Scholar]
- 16.Pinter M, Ulbrich G, Sieghart W, et al. Hepatocellular carcinoma: A phase II randomized controlled double-blind trial of transarterial chemoembolization in combination with biweekly intravenous administration of bevacizumab or a placebo. Radiology 2015; 277: 903–912. [DOI] [PubMed] [Google Scholar]
- 17.Ferlitsch A, Bota S, Paternostro R, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int 2015; 35: 2115–2120. [DOI] [PubMed] [Google Scholar]
- 18.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63: 743–752. [DOI] [PubMed] [Google Scholar]
- 19.Scheiner B, Steininger L, Semmler G, et al. Controlled attenuation parameter (CAP) does not predict hepatic decompensation in patients with advanced chronic liver disease. Liver Int 2019; 39: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim NH, Lee T, Cho YK, et al. Impact of clinically evident portal hypertension on clinical outcome of patients with hepatocellular carcinoma treated by transarterial chemoembolization. J Gastroenterol Hepatol 2018; 33: 1397–1406. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Chung JW, Lee DH, et al. Portal hypertension is associated with poor outcome of transarterial chemoembolization in patients with hepatocellular carcinoma. Eur Radiol 2018; 28: 2184–2193. [DOI] [PubMed] [Google Scholar]
- 22.Elia C, Venon WD, Stradella D, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in cirrhosis: Influence on portal hypertension. Eur J Gastroenterol Hepatol 2011; 23: 573–577. [DOI] [PubMed] [Google Scholar]
- 23.Sergio A, Cristofori C, Cardin R, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): The role of angiogenesis and invasiveness. Am J Gastroenterol 2008; 103: 914–921. [DOI] [PubMed] [Google Scholar]
- 24.Schicho A, Hellerbrand C, Kruger K, et al. Impact of different embolic agents for transarterial chemoembolization (TACE) procedures on systemic vascular endothelial growth factor (VEGF) levels. J Clin Transl Hepatol 2016; 4: 288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranieri G, Ammendola M, Marech I, et al. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J Gastroenterol 2015; 21: 6018–6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shim JH, Park JW, Kim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci 2008; 99: 2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández M, Semela D, Bruix J, et al. Angiogenesis in liver disease. J Hepatol 2009; 50: 604–620. [DOI] [PubMed] [Google Scholar]
- 28.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: From the patient to the molecule. Hepatology 2006; 43(2 Suppl 1): S121–S131. [DOI] [PubMed] [Google Scholar]
- 29.Fernández M, Mejias M, Angermayr B, et al. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol 2005; 43: 98–103. [DOI] [PubMed] [Google Scholar]
- 30.Fernández M, Mejias M, Garcia-Pras E, et al. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology 2007; 46: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 31.Reiberger T, Angermayr B, Schwabl P, et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol 2009; 51: 865–873. [DOI] [PubMed] [Google Scholar]
- 32.Friedman SL. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008; 88: 125–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev 2017; 121: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laleman W, Landeghem L, Wilmer A, et al. Portal hypertension: From pathophysiology to clinical practice. Liver Int 2005; 25: 1079–1090. [DOI] [PubMed] [Google Scholar]
- 35.Sun Z, Li G, Ai X, et al. Hepatic and biliary damage after transarterial chemoembolization for malignant hepatic tumors: Incidence, diagnosis, treatment, outcome and mechanism. Crit Rev Oncol Hematol 2011; 79: 164–174. [DOI] [PubMed] [Google Scholar]
- 36.Qu K, Yan Z, Wu Y, et al. Transarterial chemoembolization aggravated peritumoral fibrosis via hypoxia-inducible factor-1alpha dependent pathway in hepatocellular carcinoma. J Gastroenterol Hepatol 2015; 30: 925–932. [DOI] [PubMed] [Google Scholar]
- 37.Afdhal N, Everson GT, Calleja JL, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat 2017; 24: 823–831. [DOI] [PubMed] [Google Scholar]
- 38.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009; 50: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ripoll C, Genescà J, Araujo IK, et al. Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case-control study. Hepatology 2013; 58: 2079–2088. [DOI] [PubMed] [Google Scholar]
- 40.Adhoute X, Penaranda G, Naude S, et al. Retreatment with TACE: The ABCR SCORE, an aid to the decision-making process. J Hepatol 2015; 62: 855–862. [DOI] [PubMed] [Google Scholar]
- 41.Qi X, Berzigotti A, Cardenas A, et al. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol 2018; 3: 708–719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma by Bernhard Scheiner, Gregor Ulbrich, Mattias Mandorfer, Thomas Reiberger, Christian Müller, Fredrik Waneck, Michael Trauner, Claus Kölblinger, Arnulf Ferlitsch, Wolfgang Sieghart, Markus Peck-Radosavljevic and Matthias Pinter in United European Gastroenterology Journal