Abstract
Background
Very little is known about the impact of the wash-out period on the pharmacokinetics of a second-line biologic.
Objective
The objective of this article is to explore the impact of two different wash-out periods on the pharmacokinetics of vedolizumab and infliximab.
Methods
Patients switching from infliximab to vedolizumab were retrospectively identified. The population was divided into two groups according to wash-out period: <6 weeks or >6 weeks. Vedolizumab and infliximab trough levels (TLs) were determined and correlated with clinical and biological outcomes.
Results
A total of 71 inflammatory bowel disease patients were included. At week 6, in patients previously treated with infliximab, median vedolizumab TLs were 21.9 µg/ml and 24.9 µg/ml for the <6 weeks and >6 weeks wash-out period, respectively (p = 0.31), whereas median residual infliximab TLs were 0.5 µg/ml and 0 µg/ml (p = 0.034). The rate of treatment discontinuation was similar (p = 0.64), and the infectious events were six and two for the <6 weeks and >6 weeks wash-out period, respectively (p = 0.12) by week 30.
Conclusions
This study suggests clinicians may not need to be concerned about the impact of wash-out period on the pharmacokinetics of the second-line biologic when switching infliximab to vedolizumab. More data are required on the impact of wash-out period on safety.
Keywords: Induction, inflammatory bowel disease, infliximab, pharmacokinetics, trough level, vedolizumab, wash-out
Key summary
Summarise the established knowledge on this subject
The wash-out period is defined as the time frame between the discontinuation of one biologic and the initiation of a second one.
In practice, a wash-out period can be difficult to observe in case of primary nonresponse or loss of response to a biologic.
Very few data have been reported so far on impact of different wash-out periods on pharmacokinetics of biologics, safety and efficacy.
What are the significant and/or new findings of this study?
The pharmacokinetics of vedolizumab do not seem to be influenced by the presence of residual infliximab.
No differences in terms of efficacy have been observed, suggesting that wash-out period may not be taken into account prior switching from infliximab to vedolizumab.
No significant differences in terms of safety have been observed but a higher number of infections in case of a shorter wash-out period suggests that other studies dedicated to safety are needed.
Introduction
Infliximab (IFX) and vedolizumab (VDZ) are monoclonal antibodies (mAbs) indicated for the treatment of inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC). Primary nonresponse, secondary loss of response, or adverse events often require a rapid therapeutic intervention with a switch to a second-line biologic.1 The wash-out period is defined as the time between the discontinuation of one biologic and the initiation of a second biologic. This wash-out period is arbitrarily based on the half-life of the biologic, namely the time needed to eliminate 50% of the biologic from the bloodstream.
The mechanisms of elimination of mAbs are partly understood. The Brambell receptor FcRn represents a pivotal component to prevent immunoglobulin G (IgG) catabolism,2–4 which protects IgG from degradation and thus modulates the half-life of biologics.5,6 Concomitantly, high levels of IgG saturate FcRn expressed by the endothelial and immune cells of the reticuloendothelial system and block this salvage pathway.2 However, it should be noted that only high levels of Igs, such as intravenous Ig therapy, have been reported to enable full FcRn saturation.
In the management of moderate-to-severe IBD, patients who fail or develop adverse events to IFX may benefit from VDZ treatment and vice versa. IFX is a chimeric monoclonal IgG1 targeting soluble and membrane tumour necrosis factor alpha (TNFα), while VDZ is a humanised monoclonal IgG1 targeting α4β7 integrin, expressed by gut-specific memory T lymphocytes, blocking its interaction with mucosal vascular addressin cell adhesion molecule 1 (MadCam-1) expressed on endothelial cells. Obviously, the mechanisms of action and pharmacodynamic properties of these biologics are different, but also differences in their pharmacokinetics exist. The serum half-time of VDZ is around 25 days, whereas the serum half-time of IFX is around 14 days.7,8 VDZ pharmacokinetics is more affected by target-mediated mechanisms, corresponding to the nonlinear elimination in the two-compartment pharmacokinetic model, than anti-TNFα, which are mainly eliminated by Fc-receptor-mediated mechanisms, corresponding to a linear clearance.7 Moreover, VDZ seems to be less immunogenic than IFX, as suggested by the low occurrence of antibodies to VDZ observed in the pivotal trials.9–11
Very few data have been reported on the concomitant exposure of these two mAbs. Shortening the wash-out period between two mAbs could affect the pharmacokinetics of the subsequent mAbs, and may affect its efficacy and safety. To our knowledge, only one study in the field of rheumatology focused on the impact of the wash-out period on efficacy and safety.12 Similarly, a recent study explored the safety, efficacy and pharmacokinetics of VDZ in IBD patients after recent anti-TNFα exposure.13 The objectives of this study were to explore the impact of different wash-out periods on the pharmacokinetics, efficacy and safety both of VDZ and IFX.
Patients and methods
Study design
This study was conducted in three Belgian centres: University Hospitals Leuven (Leuven), Erasme Hospital (Brussels) and AZ Groeninge (Kortrijk); and had a retrospective design based on a prospective collection of blood samples in the different Biobanks. Each patient gave a written informed consent for blood collection that conformed to the ethical guidelines of the Declaration of Helsinki. The study was approved by the ethics committee of Erasme Hospital as central ethics committee (P2017/518, approved 31 January 2018).
Study population
Patients older than ≥18 years treated with VDZ or IFX were included if switched from IFX or VDZ. Patients treated with IFX received either IFX or biosimilar CT-P13, but the IFX abbreviation is used for both of them. Patients had to be IFX naive if switched to IFX, or similarly VDZ naive if switched to VDZ. Any patient receiving another biologic drug or small molecule in between was excluded.
The study population was divided into two groups according to their first- and second-line biologic: IFX → VDZ or VDZ→ IFX. Subsequently, both groups were divided according to the wash-out period: <6 weeks or >6 weeks. The cut-off of 6 weeks was chosen to have homogenous distribution of population in the two subgroups and based on previously published data.13 Three patients were excluded from analysis in group VDZ→ IFX because of a first infusion of IFX at 10 mg/kg.
Data collection and definitions
A case report form was retrospectively completed allowing available data collection on clinical and biological parameters. A minimal follow-up of 30 weeks was observed after the initiation of their second biologic. For CD patients, Harvey-Bradshaw index score (HBI) and C-reactive protein (CRP) were recorded. In UC patients, partial Mayo score was collected. For CD, clinical response and remission were defined as HBI reduction ≥3 points and HBI ≤4. Biological remission was defined as normalisation of CRP ≤5 mg/l in case of elevated CRP at baseline. For UC, clinical response and remission were defined as a partial Mayo score reduction of ≥3 points, and partial Mayo score of ≤2 and any individual subscore ≤1 point, respectively.
Primary nonresponse was defined as lack of response to VDZ or IFX within 10 weeks after the first infusion, whereas loss of response was defined as a loss of drug efficacy after an initial response.
Blood samples
Blood samples were prospectively collected prior to every infusion of VDZ and IFX from baseline toward week 30. Blood samples were centrifuged and stored at –20℃ until further processing.
Laboratory methods
Trough levels (TLs) of IFX and VDZ were determined using the IFX and VDZ enzyme-linked immunoassay, respectively (apDia bvba). IFX and VDZ TLs are expressed as micrograms per millilitre (µg/ml). During the induction of second-line biologic treatment (weeks 0–2–6), a double measurement both of VDZ and IFX was performed to measure the residual first-line biologics concomitantly to the second-line biologic. After the induction period, only TLs of the second-line biologic were measured.
Statistical analysis
Mann-Whitney and Kruskal-Wallis tests were used to compare continuous variables. Results were expressed as median with interquartile range (IQR). Pearson χ2 test was used to compare categorical variables. Significant difference between outcomes was set for p value lower than 0.05. All data were analysed using SPSS Statistics 23 (SPSS, Chicago, IL, USA).
Results
Study population
The overall study population included 71 patients, 40 CD and 31 UC patients. Fifty-eight patients were switched from IFX to VDZ, and 13 patients from VDZ to IFX. These two groups were subsequently divided into two wash-out periods (Figure 1). Stratifying the population according to the switch direction, no differences were observed for demographic data, previous use of biologics or treatment at baseline (Table 1). The reasons for switching IFX to VDZ were primary nonresponse (n = 4), loss of response (n = 37), adverse events to IFX (n = 15), quiescent disease with flare disease after stopping first line (n = 1) and patient decision (n = 1); reasons for switching VDZ to IFX were primary nonresponse (n = 7) and loss of response (n = 6).
Figure 1.
Flowchart of the overall population.
IBD: inflammatory bowel disease; IFX: infliximab/CT-P13; VDZ: vedolizumab.
Table 1.
Demographic and phenotypic characteristics for the overall population. Demographic and phenotypic data at baseline are presented for the overall population and based on the direction of the switch.
| Overall cohort (n = 71) | Switch out VDZ → IFX (n = 13) | Switch out IFX → VDZ (n = 58) | p value | |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Females | 37 (52.1) | 5 (38.5) | 32 (55.2) | 0.36 |
| Males | 34 (47.9) | 8 (61.5) | 26 (44.8) | 0.36 |
| Age, median (IQR) | 38 (30–49) | 44 (32–60) | 37 (30–47) | 0.17 |
| Disease features, n (%) | ||||
| Crohn disease | 40 (56.3) | 7 (53.8) | 33 (54.8) | 0.5 |
| Ulcerative colitis | 31 (43.7) | 6 (46.2) | 25 (45.2) | 0.5 |
| CD | ||||
| A1 (<17 years) | 7 (17.5) | – | 7 (21.2) | 0.22 |
| A2 (17–40 years) | 32 (80) | 6 (87.5) | 26 (78.8) | 0.57 |
| A3 (>40 years) | 1 (2.5) | 1 (12.5) | – | 0.17 |
| Location | ||||
| L1 | 10 (25) | 3 (42.8) | 7 (21.2) | 0.23 |
| L2 | 7 (17.5) | 2 (28.6) | 5 (15.2) | 0.37 |
| L3 | 23 (57.5) | 2 (28.6) | 20 (60.7) | 0.9 |
| + L4 | 2 (5) | 1 | 1 | – |
| Behaviour | ||||
| B1 | 13 (36.7) | 3 (37.5) | 10 (30.3) | 0.43 |
| B2 | 8 (16.7) | 1 (12.5) | 7 (21.2) | 0.55 |
| B3 | 18 (43.3) | 3 (50) | 15 (45.4) | 0.86 |
| Unknown | 1 (0.5) | – | 1 | – |
| Ano-perineal disease | 21 (52.5) | 5 (50) | 16 (48.5) | 0.44 |
| Previous history of CD surgery | 22 (60) | 6 (75) | 16 (48.5) | 0.61 |
| Ulcerative colitis | ||||
| E1 | 2 (6.4) | _ | 2 (8) | 0.64 |
| E2 | 15 (48.4) | 5(70) | 10 (40) | 0.07 |
| E3 | 14 (45.2) | 1 (30) | 13 (52) | 0.13 |
| Smoking status, n (%) | ||||
| Yes | 10 (14.1) | 2 (15.4) | 8 (13.8) | 0.61 |
| No | 42 (59.1) | 9 (69.2) | 33 (56.9) | 0.36 |
| Previous | 17 (23.9) | 2 (15.4) | 15 (25.8) | 0.32 |
| Unknown | 2 (2.8) | – | 2 (3.5) | – |
| Previous biotherapy, n (%) | ||||
| None | 43 (69) | 7 (53.8) | 36 (62.1) | 0.4 |
| Anti-TNF | 28 (31) | 6 (47.2) | 22 (37.9) | 0.4 |
| Adalimumab | 15 | 4 | 11 | – |
| Infliximab | 13 | 2 | 11 | – |
| Golimumab | 7 | 1 | 6 | |
| Concomitant medications at baseline, n (%) | ||||
| Steroids | 25 (35.2) | 6 (46.2) | 19 (57.6) | 0.27 |
| Immunosuppressors | 21 (29.6) | 3 (23.1) | 18 (54.5) | 0.42 |
| Methotrexate | 3 | 1 | 2 | – |
| Azathioprine | 18 | 2 | 16 | – |
| Wash-out period | ||||
| <6 weeks | 34 (47.9) | 6 (46.2) | 28 (48.3) | 0.69 |
| >6 weeks | 37 (52.1) | 7 (53.8) | 30 (51.7) | 0.69 |
CD: Crohn disease; IQR: interquartile range; TNF: tumour necrosis factor.
Impact of wash-out on pharmacokinetics during induction
Switching from IFX to VDZ
Fifty-eight patients were switched from IFX to VDZ. At the first infusion of VDZ (week 0, baseline), higher residual IFX TLs were observed with shorter wash-out periods (Figure 2). Median residual IFX TL were 7.85 µg/ml (IQR (3.35–19.15), n = 16) and 0.3 µg/ml (IQR (0–1.55) n = 21) for wash-out period <6 weeks and >6 weeks, respectively, p < 0.001 (Figure 2).
Figure 2.
Residual IFX TLs at first infusion of vedolizumab (baseline) according to wash-out periods. Median residual IFX TL at 7.85 µg/ml (IQR (3.35–19.15), n = 16) for wash-out period <6 weeks and at 0.3 µg/ml (IQR (0–1.55) n = 21) for wash-out period >6 weeks. IFX: infliximab/CT-P13; IQR: interquartile range; TL: trough level; WO: wash-out.
At week 2, median VDZ TLs were not different according to wash-out periods, p = 0.25 (Figure 3(a)). Looking at residual IFX TLs, median residual IFX TLs were 4.2 µg/ml (IQR (0.8–7.8), n = 19) and 0 µg/ml (IQR (0–0.5) n = 23) for wash-out period <6 weeks and >6 weeks, respectively, p < 0.001 (Figure 3(b)).
Figure 3.
(a) VDZ TLs at week 2 according to wash-out periods. Median VDZ TLs at 22.8 µg/ml (IQR (17.1–33.2), n = 25) and 26.9 µg/ml (IQR (20.2–32.8), n = 29) for wash-out period < 6 weeks and >6 weeks, respectively. (b) Residual IFX TLs at second infusion of VDZ according to wash-out periods. Median residual IFX TL at 4.2 µg/ml (IQR (0.8–7.8), n = 19) and 0 µg/ml (IQR (0–0.5) n = 23) for wash-out period <6 weeks and >6 weeks, respectively. IFX: infliximab/CT-P13; IQR: interquartile range; TL: trough level; VDZ: vedolizumab; WO: wash-out.
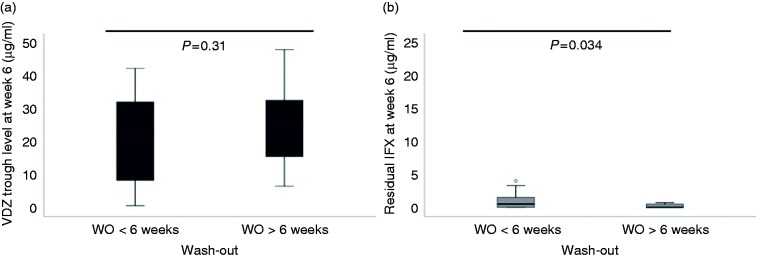
Likewise, at week 6, median VDZ TLs were similar whatever the wash-out (p = 0.31) (Figure 4(a)), whereas median residual IFX TLs remained different according to wash-out: 0.5 µg/ml (IQR (0–1.5), n = 19) and 0 µg/ml (IQR (0–0.5) n = 19) for wash-out period <6 weeks and >6 weeks, respectively, p = 0.034 (Figure 4(b)).
Figure 4.
(a) VDZ TLs at week 2 according to wash-out periods. Median VDZ TLs at 22.8 µg/ml (IQR (17.1–33.2), n = 25) and 26.9 µg/ml (IQR (20.2–32.8), n = 29) for wash-out period <6 weeks and >6 weeks, respectively. (b) Residual IFX TLs at third infusion of VDZ (week 6) according to wash-out periods. Median residual IFX TL at 0.5 µg/ml (IQR (0–1.5), n = 19) and 0 µg/ml (IQR (0–0.5) n = 19) for wash-out period <6 weeks and >6 weeks, respectively. IFX: infliximab/CT-P13; IQR: interquartile range; TL: trough level; VDZ: vedolizumab; WO: wash-out.
Switching from VDZ to IFX
Ten patients who were switched from VDZ to IFX were analysed. At baseline, median residual VDZ TLs were 12.6 µg/ml (IQR (4.2–27.2) n = 4) and 3 µg/ml (IQR (0.9–12.1) n = 5) for wash-out period <6 weeks and >6 weeks, respectively, p = 0.28.
At week 2, median IFX TLs were measured at 34.3 µg/ml (IQR (15.6–34.7), n = 4) and 19.1 µg/ml (IQR (16.1–31.3), n = 6) for the wash-out period <6 weeks and >6 weeks, respectively, p = 0.76. Median residual VDZ TLs were 9.7 µg/ml (IQR (1.5–21.2) n = 4) and 1.4 µg/ml (IQR (0–5.25) n = 5) for wash-out period <6 weeks and >6 weeks, respectively, p = 0.28.
At week 6, median IFX TLs were measured at 19.1 µg/ml (IQR (6.8–22.8), n = 4) and 11.1 µg/ml (IQR (5.6–21.2), n = 6) for the wash-out period <6 weeks and >6 weeks, respectively, p = 0.47. Median residual VDZ TLs were 3.8 µg/ml (IQR (0.5–19.2) n = 4) and 0 µg/ml (IQR (0–2.4), n = 5) for the wash-out period <6 weeks and >6 weeks, respectively, p = 0.2.
Pharmacokinetic levels at induction according to biologics use as first line or second line
IFX/CT-P13
When IFX was used as first line, median IFX TL was 20.2 µg/ml (IQR (14.4–29.9), n = 29) and 15.1 µg/ml (IQR (6.9–22.7 µg/ml), n = 22) at week 2 and 6, respectively. When IFX was used as second line, median IFX TL was 19 µg/ml (IQR (15.5–32.7), n = 12) and 15.7 µg/ml (IQR (7.7–20.7), n = 12) at weeks 2 and 6, respectively. No significant difference was observed at week 2 (p = 0.9) and week 6 (p = 0.9).
VDZ
When VDZ was used as first line, median VDZ TL was 21.8 µg/ml (IQR (19–30.5), n = 9) and 15.8 µg/ml (IQR (14–30), n = 11) weeks 2 and 6, respectively. When VDZ was used as second line, median VDZ TL was 23.7 µg/ml (IQR (19.5–33.2), n = 42) and 24.2 µg/ml (IQR (13.7–32.6), n = 33) at weeks 2 and 6, respectively. No significant difference was observed at week 2 (p = 0.67) and week 6 (p = 0.34).
Impact of wash-out on clinical outcomes
Thirty weeks after the initiation of the second biologic, 20.6% (wash-out period group <6 weeks) and 16.2% (wash-out period group >6 weeks) of patients discontinued therapy (log rank p = 0.64). Reasons for treatment discontinuation were intense arthralgia (n = 1), loss of response (n = 9), pregnancy (n = 1) and loss of follow-up (n = 2).
At baseline, nine CD patients and eight UC patients had a quiescent disease based on clinical scores because they were switched because of adverse events. These patients were excluded from the analysis. Considering HBI and CRP evolution in CD patients, patients from the <6 weeks wash-out period group (n = 7) had an initial median HBI higher than patients from the >6 weeks wash-out period group (n = 12), p = 0.03. The evolution of HBI was not different between the two groups at timepoints of follow-up excepted at week 30 (p = 0.04) (Supplementary Figure 1(a)). The evolution of CRP levels was not different between the two groups by week 30 (Supplementary Figure 1(b)). Considering partial Mayo score in UC patients (n = 9 for wash-out <6 weeks and n = 4 for wash-out <6 weeks), a progressive reduction of partial Mayo score was observed from baseline to week 30. No difference in partial Mayo score evolution was observed regardless of wash-out (Supplementary Figure 2).
Impact of wash-out on safety
The 71 patients included in our analysis represented 41 patient-years (PYs). Rate of infectious events in the overall population was 19.5 per 100 PY. The group with wash-out period <6 weeks represented 20 PYs while the group with a wash-out period >6 weeks represented 21 PYs. The rate of infectious events for the wash-out period <6 weeks and wash-out period >6 weeks was 30 and 9.5 per 100 PYs, respectively (p = 0.12). Shingles infection was reported in patients with the short wash-out period. Looking into noninfectious events, rate of adverse events for the wash-out period <6 weeks and wash-out period >6 weeks was 20 and 43 per 100 PY, respectively (p = 0.18). No death was reported. All infectious and noninfectious adverse events are summarised in Table 2.
Table 2.
Reported infectious and noninfectious adverse events according to wash-out periods.
| Wash-out <6 weeks 20 PYs | Wash-out >6 weeks 21 PYs |
|---|---|
| Infectious events | Infectious events |
| • Stomatitis | • Pulmonary infection |
| • Gastroenteritis | • Sinusitis |
| • Pulmonary infection | |
| • Urinary infection | |
| • Atypical cough | |
| • Shingles (opportunistic infection) | |
| Noninfectious adverse events | Noninfectious adverse events |
| • Alopecia | • Fatigue (n = 2) |
| • Headache | • Vomitus |
| • Arthralgia | • Skin rash (n = 2) |
| • Breast carcinoma | • Arthralgia |
| • Alopecia | |
| • Appendectomy | |
| • Pulmonary neoplasia |
PYs: patient-years.
Discussion
In routine practice, wash-out periods can be difficult to observe, especially in case of nonresponse or loss of response to biologics. The objective of this study was to analyse the impact of wash-out periods on the pharmacokinetics of the second biologic, and secondly to explore whether wash-out periods may affect safety and therapeutic efficacy. We found that the pharmacokinetic profile of IFX and VDZ at induction was similar regardless whichever the class used first. Moreover, the presence of residual drug level did not appear to affect the pharmacokinetics of the second-line biologic. Finally, the wash-out period also did not affect the rate of biological, clinical response or adverse events.
We observed that VDZ pharmacokinetics did not appear to be influenced by residual IFX TLs. This observation confirmed results previously shown despite a slightly different design.13 The same observation was made when patients were switched in the opposite direction. However, in the switch VDZ→IFX, IFX TLs tended to be higher when residual VDZ TLs were high. These results need to be interpreted with caution because of small group size but are encouraging for future, more detailed investigations of this trend.
The impact of wash-out periods on efficacy and safety is not well documented.12,13 The concern relies on the presence of two mAbs that would increase the risks of infections. At 30 weeks of follow-up, no difference in terms of efficacy was detected in the present study, which may suggest that a direct switch between IFX and VDZ can be efficacious and safe as previously shown.13 However, while the rate of infectious events was not significantly different according to the wash-out period, a higher number of infectious events was recorded when the wash-out period was short. This trend represents a potential safety issue among patients with short wash-out (<6 weeks) and has to be further evaluated in more details. This observation needs to be analysed with caution because of the short follow-up, retrospective design and cohort size. In addition, the reported infectious adverse events were not serious infections. The ARRIVE trial, including 1046 patients with rheumatoid arthritis, compared a “direct-switch” between anti-TNFα and a conventional switch after a wash-out period >8 weeks.12 Over six months of follow-up, no safety issue was observed and the authors suggested that the use of direct switching in routine was an option in patients who do not respond to, lose response to, or are unable to tolerate the anti-TNFα agent. Except for this trial in rheumatology, cohort studies on wash-out periods are still limited and the concomitant use of two biologics has been described only in case reports for refractory patients with rheumatologic diseases, IBD14–19or IBD with extraintestinal manifestations.20–22 Specific studies dedicated to evaluate the safety of two concomitant biologics are pending. In this context, a prospective study based on the use of triple combination (VDZ, adalimumab and methotrexate) has been launched in United States and Canada with study completion expected in 2021 (NCT02764762).
This study has limitations. First, the design of this study was retrospective with missing data. Second, the overall cohort includes only 71 patients and the distributions are unequal between the IFX →VDZ switch-out and the opposite VDZ→IFX switch-out groups.
This work represents the first study evaluating two different wash-out periods on the pharmacokinetics both of IFX and VDZ in real-world IBD experience. The wash-out period and residual levels of the IFX do not appear to affect the pharmacokinetics of VDZ. This study suggests that clinicians may not need to be concerned about the impact of wash-out period on the pharmacokinetics of second-line biologics when switching from infliximab to VDZ. More data are required on the impact of wash-out period on safety.
Supplemental Material
Supplemental Material for Impact of first-line infliximab on the pharmacokinetics of second-line vedolizumab in inflammatory bowel diseases by Claire Liefferinckx, Bram Verstockt, Ann Gils, Sophie Tops, Wouter Van Moerkercke, Severine Vermeire and Denis Franchimont in United European Gastroenterology Journal
Acknowledgement
The authors thank Sofie Himpe, who helped to collect data at AZ Groeninge.
Declaration of conflicting interests
C. Liefferinckx is supported by FNRS (Belgian National Fund of Scientific Research).
B. Verstockt is a doctoral fellow and has received research grants from the Belgium Week of Gastroenterology, the Belgian IBD Research and Development (BIRD), the European Crohn’s and Colitis Organization (ECCO) and the IBD Patient’s Association Flanders (CCV VZW). He has also received financial support for research from Pfizer; lecture fees from Abbvie, Ferring, Takeda Pharmaceuticals, Janssen and R Biopharm; and consultancy fees from Janssen.
A. Gils has received financial support for research from Pfizer, MSD and Takeda; lecture fees from MSD, Janssen Biologicals, Pfizer, Takeda, Abbvie and Novartis; consultancy fees from Takeda; and holds a license agreement with R-biopharm, apDia and Merck.
S. Tops has nothing to declare.
W. Van Moerkercke has nothing to declare.
S. Vermeire is a senior clinical investigator for Research Foundation Flanders (Fonds voor Wetenschappelijk onderzoek Vlaanderen (FWO)), Belgium, and has received financial support for research from MSD, Abbvie, Janssen, Pfizer, J&J and UCB Pharma; lecture fees from Abbott, Abbvie, Merck Sharpe & Dohme, Ferring Pharmaceuticals and UCB Pharma; and consultancy fees from Pfizer, Ferring Pharmaceuticals, Shire Pharmaceuticals Group, Merck Sharpe & Dohme, Pfizer, J&J, Genentech/Roche, Celgene, Gilead, Galapagos, Shire, Arena, Prodigest, Second Genome and AstraZeneca Pharmaceuticals.
D. Franchimont is research director of FNRS, and has received educational grants from Abbvie, Takeda, MSD, and has received honoraria fees for lectures or consultancy from Ferring, Falk, Chiesi, Abbvie, MSD, Centocor, Pfizer, Amgen, Janssen, Mundiphatma, Takeda and Hospira.
Funding
This work was supported by funding support from the Conseil medical de l’Hopital Erasme and Research Foundation Flanders (Fonds voor Wetenschappelijk onderzoek Vlaanderen (FWO)) (TBM grant T003716N).
Ethics approval
The study was approved by the ethics committee of Erasme Hospital as central ethics committee (P2017/518, approved 31 January 2018, and conformed to the ethical guidelines of the Declaration of Helsinki.
Informed consent
Each patient included in this study provided written informed consent for blood collection.
References
- 1.Dalal SR, Cohen RD. What to do when biologic agents are not working in inflammatory bowel disease patients. Gastroenterol Hepatol (N Y) 2015; 11: 657–665. [PMC free article] [PubMed] [Google Scholar]
- 2.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 2006; 11: 81–88. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008; 84: 548–558. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Solal JF, Cassard L, Fridman WH, et al. Fc gamma receptors. Immunol Lett 2004; 92: 199–205. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Ishii-Watabe A, Tada M, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol 2010; 184: 1968–1976. [DOI] [PubMed] [Google Scholar]
- 6.Billiet T, Dreesen E, Cleynen I, et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol 2016; 111: 1438–1445. [DOI] [PubMed] [Google Scholar]
- 7.Rosario M, Dirks NL, Gastonguay MR, et al. Population pharmacokinetics-pharmacodynamics of vedolizumab in patients with ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther 2015; 42: 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemperly A, Vande Casteele N. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet 2018; 57: 929–942. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 10.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013; 369: 699–710. [DOI] [PubMed] [Google Scholar]
- 11.Bian S, Dreesen E, Tang HT, et al. Antibodies toward vedolizumab appear from the first infusion onward and disappear over time. Inflamm Bowel Dis 2017; 23: 2202–2208. [DOI] [PubMed] [Google Scholar]
- 12.Schiff M, Pritchard C, Huffstutter JE, et al. The 6-month safety and efficacy of abatacept in patients with rheumatoid arthritis who underwent a washout after anti-tumour necrosis factor therapy or were directly switched to abatacept: The ARRIVE trial. Ann Rheum Dis 2009; 68: 1708–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Horin S, Ungar B, Kopylov U, et al. Safety, efficacy and pharmacokinetics of vedolizumab in patients with simultaneous exposure to an anti-tumour necrosis factor. Aliment Pharmacol Ther 2018; 47: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 14.Genovese MC, Cohen S, Moreland L, et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum 2004; 50: 1412–1419. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald MW, Shergy WJ, Kaine JL, et al. Evaluation of the safety of rituximab in combination with a tumor necrosis factor inhibitor and methotrexate in patients with active rheumatoid arthritis: Results from a randomized controlled trial. Arthritis Rheum 2011; 63: 622–632. [DOI] [PubMed] [Google Scholar]
- 16.Weinblatt M, Schiff M, Goldman A, et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: A randomised clinical trial. Ann Rheum Dis 2007; 66: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirten R, Longman RS, Bosworth BP, et al. Vedolizumab and infliximab combination therapy in the treatment of Crohn’s disease. Am J Gastroenterol 2015; 110: 1737–1738. [DOI] [PubMed] [Google Scholar]
- 18.Yzet C, Dupas JL, Fumery M. Ustekinumab and anti-TNF combination therapy in patients with inflammatory bowel disease. Am J Gastroenterol 2016; 111: 748–749. [DOI] [PubMed] [Google Scholar]
- 19.Sands BE, Kozarek R, Spainhour J, et al. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn’s disease not in remission while receiving infliximab. Inflamm Bowel Dis 2007; 13: 2–11. [DOI] [PubMed] [Google Scholar]
- 20.Bethge J, Meffert S, Ellrichmann M, et al. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol 2017; 4: e000127–e000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buer LCT, Høivik ML, Warren DJ, et al. Combining anti-TNF-alpha and vedolizumab in the treatment of inflammatory bowel disease: A case series. Inflamm Bowel Dis 2018; 24: 997–1004. [DOI] [PubMed] [Google Scholar]
- 22.Roblin X, Paul S, Ben-Horin S. Co-treatment with golimumab and vedolizumab to treat severe UC and associated spondyloarthropathy. J Crohns Colitis 2018; 12: 379–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Impact of first-line infliximab on the pharmacokinetics of second-line vedolizumab in inflammatory bowel diseases by Claire Liefferinckx, Bram Verstockt, Ann Gils, Sophie Tops, Wouter Van Moerkercke, Severine Vermeire and Denis Franchimont in United European Gastroenterology Journal