Abstract
Background
Very few data regarding the use of infliximab in children with very early-onset inflammatory bowel disease (VEO-IBD) have been reported.
Objective
We aimed to assess the efficacy and the safety of infliximab in children with VEO-IBD compared with older children.
Methods
Children treated with infliximab were identified within the Italian IBD registry. The primary outcome was the rate of clinical remission at weeks 14 and 54. Secondary outcomes included the proportion of partial clinical response, treatment duration, and incidence of adverse events.
Results
Forty-two children with VEO-IBD were compared with 130 children with IBD. Despite significantly higher infliximab withdrawals in VEO-IBD patients during induction (42.9% vs 7.7% p < 0.01), remission rates at week 14 were similar (28.6% vs 43.8%, p = 0.10). At week 54 fewer VEO-IBD children were in remission (15.8% vs 54.3%, p < 0.01). The treatment duration was shorter in VEO-IBD (median 12.0 vs 18.4 months, p < 0.01). During the induction phase, adverse events were more common in the VEO-IBD group (p < 0.01).
Conclusion
Compared with older children, VEO-IBD patients have higher rates of infliximab failures, lower remission rates at one year, and more often experience adverse events during induction.
Keywords: Children, inflammatory bowel disease, infliximab, very early onset
Key summary
Current knowledge
Infliximab is effective in inducing and maintaining remission in children with inflammatory bowel disease (IBD).
Children with very early-onset inflammatory bowel disease (VEO-IBD) generally have a more aggressive course and are often refractory to standard treatments.
Key findings of this study
Children with VEO-IBD treated with infliximab have higher discontinuation rates before weeks 14 and 54 compared to older children and have shorter periods of treatment.
Rates were similar between VEO-IBD and older patients for those achieving remission at week 14 but were lower at week 54.
During the induction phase, children with VEO-IBD experienced adverse events more frequently than older patients.
Introduction
Up to 30% of patients with inflammatory bowel diseases (IBD), encompassing Crohn’s disease (CD), ulcerative colitis (UC) and inflammatory bowel disease unclassified (IBD-U), are diagnosed before the age of 18 years.1 Patients diagnosed before age 6 years are defined as very early-onset IBD (VEO-IBD)2 and account for about 10% of all paediatric IBD.3
Compared with older children, VEO-IBD patients have particular characteristics such as a higher incidence of IBD-U, more extensive inflammation, predominant colonic involvement, a stronger genetic predisposition and often a more severe course of the disease.4
Infliximab, a chimeric monoclonal antibody against the soluble and the membrane tumour necrosis factor (TNF)-α, is an established treatment for IBD,5–7 and real-life experiences have shown that remission rates are as high as 64% and 40% in CD and UC children, respectively.8
Based on the results of two controlled clinical trials that involved children older than age 7 years,9,10 infliximab has been approved by the United States Food and Drug Administration and by the European Medical Agency in children age 6 years or older, thus excluding children with VEO-IBD for whom infliximab is an off-label therapy.
To date, few data exist on the efficacy and safety of infliximab in VEO-IBD: previous studies suggest that patients with VEO-IBD may have a decreased response rate to standard doses of infliximab, an increased loss of response during maintenance and a more frequent need for drug optimisation.11,12
Our study aimed to assess the efficacy and the safety of infliximab in children with VEO-IBD compared with older children. The primary outcome was the rate of clinical remission at 14 and 54 weeks of treatment. Secondary outcomes included the rate of partial clinical response, the evaluation of clinical scores and inflammatory markers at 14 and 54 weeks, the duration of treatment with infliximab, the causes of infliximab withdrawal, and the incidence of adverse events.
Materials and methods
We performed a retrospective study within the IBD National Registry of the Italian Society for Pediatric Gastroenterology Hepatology and Nutrition (SIGENP), whose methodology has been described elsewhere in detail.3 The institutional review board and the ethics committee of each hospital approved the data collection in the registry (for the coordinating centre: Ethics Committee of the Sapienza University of Rome, Prot n 688/17, ethical approval 20 July 2017). A written and signed informed consent was obtained from the parents of the patients to join the registry. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Participants in this study were patients aged 0–17 years previously diagnosed with CD, UC or IBD-U, according to clinical, radiological or endoscopic findings as suggested by the Porto criteria,13 treated with infliximab starting from 1 January 2009 to the retrieval date of 1 March 2018, and with at least 12 months of follow-up. The study end date was identified as the date of the most recent clinic visit before the retrieval date.
To exclude the most frequent causes of monogenic IBD (among all: chronic granulomatous disease, Wiskott-Aldrich syndrome, and X-linked inhibitor of apoptosis (XIAP) deficiency), children with VEO-IBD should have been investigated at least with an immunological work-up including complete blood count, lymphocyte subsets (CD3+, CD4+, CD8+, CD16+/56+, CD19+), immunoglobulins class G, A, and M levels, and neutrophil oxidative burst assay with dihydrorhodamine flow cytometric assay, and should not have had a history of severe infections or haemophagocytic lymphohistiocytosis (HLH).
Genetic studies, including targeted gene panels sequencing or whole-exome sequencing, were not required for inclusion.
The decision to start, escalate or stop infliximab was at the discretion of the treating physician according to the international guidelines and to the clinical status of the patient.
As indicated by the manufacturers' instructions, infliximab was administered at a dose of 5 mg/kg at weeks 0, 2 and 6 (induction phase) and then every eight weeks (maintenance phase).
Patients with VEO-IBD treated with infliximab within their seventh year of age were defined as the case population. Patients with the diagnosis of IBD treated with infliximab from the age of 7 to 17 years were considered as the comparison group.
Demographic and clinical data including age at diagnosis, localisation and behaviour of the disease according to the Paris classification14 and therapies previous to infliximab were collected. Baseline details on infliximab treatment included age at infliximab start, initial dose and concomitant therapies. Data on infliximab optimisation, such as dose escalation (from 5 mg/kg to 10 mg/kg) or interval shortening (from eight to four weeks) and need for adjunctive therapies both during induction or maintenance were noted.
The Pediatric Crohn's Disease Activity Index (PCDAI) for CD and Pediatric Ulcerative Colitis Activity Index (PUCAI) for UC and IBD-U, erythrocyte sedimentation rate (ERS), C-reactive protein (CRP), and faecal calprotectin at the time of infliximab start, after 14 weeks (end of induction phase) and after 54 weeks (maintenance phase) were recorded.
Adverse events during treatment and reasons for infliximab withdrawal, including the need for surgery at any time during follow-up, were also collected.
Outcome measures
Efficacy of infliximab treatment was evaluated at week 14, corresponding to the end of the induction phase and the date of the first maintenance dose, and at week 54, corresponding to one year of treatment. The disease was considered to be in remission if PCDAI or PUCAI were <10; a change of at least 20 points from baseline defined partial response. Infliximab failure was defined as the absence of clinical response at the end of the induction period (primary failure) and as the loss of efficacy during the maintenance period after an initial response (secondary failure).
Statistical analysis
R Statistics for Windows, version 3.5.1, was used to perform statistical analysis.
Continuous data are presented as medians with interquartile ranges (IQRs), and categorical data are presented as absolute numbers and percentages.
The Mann-Whitney U test was used to compare continuous data between children with VEO-IBD and older patients; the Fisher exact test was used for categorical variables.
All statistical tests were two tailed. A p value < 0.05 was considered significant.
Results
Baseline characteristics
Within the registry, 1724 patients were identified, and 174 children treated with infliximab were considered for inclusion. Two patients were excluded because they had a monogenic disease (one with a UC phenotype diagnosed with Loeys Dietz syndrome and one with chronic granulomatous disease who developed a CD phenotype at age 6 years) while 172 patients were enrolled in the study according to the inclusion criteria: forty-two children had VEO-IBD and were treated with infliximab before age 7 years while 130 children received infliximab between age 7 and 17 years.
All VEO-IBD patients had an immunological work-up and 24 (57.1%) had undergone genetic studies.
Baseline characteristics of patients are reported in Table 1.
Table 1.
Baseline characteristics of patients.
| VEO-IBD 42 patients | IBD 130 patients | p values | |
|---|---|---|---|
| Male sex, n (%) | 22 (52.4) | 66 (50.8) | 0.86 |
| First-degree familiarity, n (%) | 4 (9.1) | 14 (10.6) | 1.0 |
| Age at diagnosis (years), median (IQR) | 3.4 (2.1–4.6) | 12.2 (10.2–13.8) | <0.01 |
| Age at infliximab start (years), median (IQR) | 5.0 (3.0–5.6) | 13.7 (11.6–15.2) | <0.01 |
| Type of IBD, n (%) | |||
| CD | 9 (21.4) | 77 (59.2) | <0.01 |
| UC | 28 (66.7) | 51 (39.2) | <0.01 |
| IBD-U | 5 (11.9) | 2 (1.5) | 0.01 |
| Location for CD, n (%) | |||
| L1 | 1 (11.1) | 4 (5.2) | 0.43 |
| L2 | 3 (33.3) | 14 (18.2) | 0.37 |
| L3 | 5 (55.6) | 53 (68.8) | 0.46 |
| L4a | 0 | 25 (32.5) | 0.05 |
| L4b | 0 | 13 (16.9) | 0.34 |
| Behavior for CD, n (%) | |||
| B1 | 8 (88.9) | 66 (85.7) | 1.00 |
| B2 | 0 | 9 (11.7) | 0.59 |
| B3 | 1 (11.1) | 5 (6.5) | 0.50 |
| p | 4 (44.4) | 28 (36.4) | 0.72 |
| Location of UC, n (%) | |||
| E1 | 1 (3.6) | 1 (2.0) | 1.00 |
| E2 | 3 (10.7) | 9 (17.6) | 0.52 |
| E3 | 6 (21.4) | 3 (5.9) | 0.06 |
| E4 | 18 (64.3) | 38 (74.5) | 0.44 |
| Extraintestinal manifestations n (%) | 1 (2.4) | 27 (20.7) | <0.01 |
| Arthritis | 0 | 12 (9.2) | 0.04 |
| Sclerosing cholangitis | 1 (2.4) | 5 (3.8) | 1.00 |
| Psoriasis | 0 | 8 (6.1) | 0.20 |
| Previous medications, n (%) | |||
| 5-Aminosalicylate | 20 (47.6) | 45 (34.6) | 0.14 |
| Corticosteroids | 33 (78.6) | 88 (67.7) | 0.24 |
| Thiopurines | 27 (64.2) | 74 (56.9) | 0.47 |
| Methotrexate | 2 (4.8) | 9 (6.9) | 1.00 |
| Enteral nutrition | 3 (7.1) | 32 (24.6) | 0.01 |
| Antibiotics | 7 (16.7) | 11 (8.4) | 0.15 |
| Cyclosporine | 6 (14.3) | 0 | <0.01 |
| Thalidomide | 2 (4.8) | 4 (3.1) | 0.63 |
| Tacrolimus | 1 (2.4) | 0 | 0.24 |
| Adalimumab | 1 (2.4) | 4 (3.1) | 1.00 |
| Etanercept | 0 | 1 (0.8) | 1.00 |
| Surgery | 1 (2.4)a | 0 | 0.24 |
| Scores and inflammatory markers median (IQR) | |||
| PCDAI | 35.0 (32.5–47.5) | 30.0 (20.0–35.0) | 0.11 |
| PUCAI | 45.0 (37.5–60.0) | 45 (35.0–65.0) | 0.88 |
| CRP | 0.5 (0.3–2.8) | 0.8 (0.2–2.2) | 0.83 |
| ESR | 34.5 (17.5–51.3) | 37.0 (22.0–64.3) | 0.22 |
| Faecal calprotectin | 550.0 (241.5–800.0) | 800.0 (238.0–1443.0) | 0.44 |
| PCDAI > 30, n (%) | 5 (55.6) | 39 (50.6) | 1.00 |
| PUCAI > 65 | 7 (25.0) | 13 (25.5) | 1.00 |
| Concomitant drugs, n (%) | 31 (73.8) | 69 (53.1) | 0.02 |
| Steroids | 19 (45.2) | 28 (21.5) | <0.01 |
| Thiopurines | 14 (33.3) | 31 (23.8) | 0.23 |
| Methotrexate | 4 (12.5) | 5 (3.8) | 0.22 |
| 5-Aminosalicylate | 1 (2.4) | 5 (3.8) | 1.00 |
| Enteral nutrition | 1 (2.4) | 4 (3.1) | 1.00 |
CD: Crohn's disease; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IBD: inflammatory bowel disease; IBD-U: inflammatory bowel disease unclassified; IQR: interquartile range; PCDAI: Pediatric Crohn's Disease Activity Index; PUCAI: Pediatric Ulcerative Colitis Activity Index; UC: ulcerative colitis; VEO-IBD: very early-onset inflammatory bowel disease.
A child with CD received an ileal stoma.
Most of the patients with VEO-IBD had a diagnosis of UC and IBD-U, compared with older children (28 (66.7%) and five (11.9%) vs 51 (39.2%) and two (1.5%), respectively, p < 0.01), whereas the diagnosis of CD was significantly less frequent in VEO-IBD (9 (21.4%) vs 77 (59.2%), p < 0.01).
Extraintestinal manifestations were more common in the IBD group than in VEO-IBD patients. No significant difference in the clinical scores and inflammatory markers was found between VEO-IBD children and older patients at the start of infliximab treatment.
About 14% of patients in the younger group were treated with cyclosporine before infliximab vs no patient in the older group (p < 0.01), while previous treatment with enteral nutrition was reported more frequently after age 7 years (p = 0.01).
Children in both groups started infliximab at the standard dose of 5 mg/kg. All patients had scheduled dosing. Compared with older patients, children with VEO-IBD received more frequently a combination therapy with other drugs (31 (73.8%) vs 69 (53.1%), p = 0.02), in particular steroids (19 (45.2%) vs 28 (21.5%), p < 0.01) and immunosuppressors (18 (42.9%) vs 36 (27.7%), p = 0.08).
Efficacy during the induction phase
A significantly higher proportion of VEO-IBD patients stopped infliximab and changed therapy before reaching week 14, compared with older children (18 (42.9%) vs 10 (7.7%), p < 0.01).
At week 14, 12 (28.6%) patients with VEO-IBD (two CD, nine UC, one IBD-U) and 57 (43.8%) older children (42 CD, 13 UC, two IBD-U) achieved remission (p = 0.10). Five (11.9%) and eight (6.1%) children in the two groups had a partial response (p = 0.31).
Eight (19.0%) patients in the younger group and 10 (7.7%) in the older one required a dose escalation during the induction phase (p = 0.04).
No significant differences were found in the clinical scores and inflammatory markers at week 14 (Table 2).
Table 2.
Clinical scores and inflammatory markers at week 14 and week 54.
| VEO-IBD 42 patients | IBD 130 patients | p values | |
|---|---|---|---|
| Time 14 weeks, number of patients (%) | 24 (57.1) | 120 (92.3) | <0.01 |
| PCDAI | 0 (0–8.8) | 10.0 (0–15.0) | 0.17 |
| PUCAI | 20.0 (5.0–40.0) | 15.0 (5.0–30.0) | 0.98 |
| CRP (mg/dl) | 0.3 (0–0.5) | 0.2 (0.1–0.6) | 0.64 |
| ESR (mm/h) | 24.0 (13.0–53.0) | 18.0 (10.0–31.0) | 0.21 |
| Faecal calprotectin (mg/kg) | 125.4 (69.3–425.0) | 156.0 (44.5–491.0) | 0.99 |
| Time 54 weeks, number of patients (%) | 8 (19.0) | 73 (56.1) | <0.01 |
| PCDAI | /a | 3 (0–10.0) | / |
| PUCAI | 12.5 (0–22.5) | 10.0 (0–11.3) | 0.57 |
| CRP (mg/dl) | 0.5 (0.3–0.9) | 0.2 (0.1–0.3) | 0.06 |
| ESR (mm/h) | 24.0 (13.5–39.5) | 15.0 (7.5–32.0) | 0.41 |
| Faecal calprotectin (mg/kg) | 179.5 (139.8–219.3) | 42.0 (8.5–260.0) | 0.44 |
CD: Crohn's disease; CRP: C-reactive protein; ERS: erythrocyte sedimentation rate; IBD: inflammatory bowel disease; PCDAI: Pediatric Crohn's Disease Activity Index; PUCAI: Pediatric Ulcerative Colitis Activity Index; VEO-IBD: very early-onset inflammatory bowel disease.
Data are presented as medians and interquartile range.
Only one patient with CD continued infliximab to week 54.
Efficacy during the maintenance phase
Nineteen (45.2%) patients with VEO-IBD and 105 (80.8%) treated after age 7 years continued after week 14 (p < 0.01). Of these, eight (42.1%) patients in the younger group and 73 (69.5%) in the older group continued infliximab up to week 54 (p = 0.03). At that time, three (15.8%) children of the former (one CD, two UC) and 57 (54.3%) of the latter group (42 CD, 13 UC, two IBD-U) were in remission (p < 0.01); two (10.5%) and six (5.7%) children in the two groups had a partial response (p = 0.35).
Four (21.1%) children with VEO-IBD and 40 (38.1%) older children required a dose escalation during the maintenance period (p = 0.20): One (5.5%) vs 12 (11.4%) children had a dose increase, one (5.5%) vs 17 (16.2%) children had an interval reduction between doses, and one (5.5%) vs 11 (10.5%) children had both a dose increase and an interval reduction.
For the patients who reached week 54, clinical scores and inflammatory markers were similar between the two populations (Table 2).
Duration of infliximab treatment
Duration of infliximab maintenance was significantly shorter in patients with VEO-IBD (median 12.0 (IQR 8.2–12.3) months) compared with older children (median 18.4 (IQR 9.9–26.3) months) (p < 0.01).
The overall duration of infliximab treatment according to age is shown in Figure 1.
Figure 1.
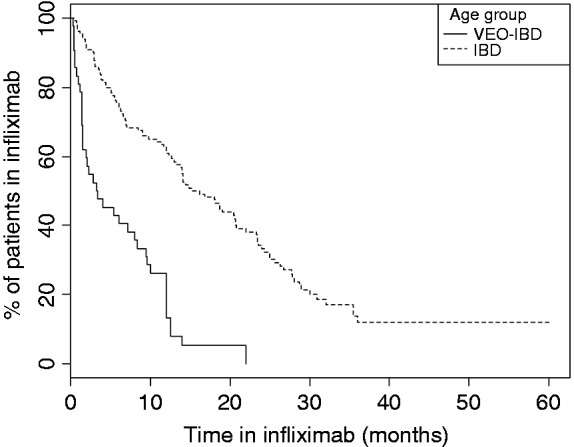
Kaplan–Meier curve representing the time to infliximab discontinuation in patients with very early-onset inflammatory bowel disease (VEO-IBD) and IBD (log-rank p < 0.01).
Reasons for withdrawal
Discontinuation rate of infliximab before starting the maintenance phase was higher in VEO-IBD patients compared with older patients (23 (54.8%) vs 25 (19.2%) children, respectively (p < 0.01)) both for primary failure (14 (33.3%) vs 17 (13.1%) children, p < 0.01) and adverse events (nine (21.4%) vs eight (6.2%) p < 0.01). Six (14.3%) patients in the younger group underwent surgery during or immediately after the induction phase compared with five (3.8%) patients in the older group (p = 0.03).
Twelve of 19 (63.2%) children with VEO-IBD and 68 of 105 (64.8%) older children stopped infliximab at any time during the maintenance phase (p = 1.00). A secondary failure occurred in nine (47.4.0%) younger children and in 41 (39.0%) older patients (p = 0.61): Four (21.1%) and seven (6.6%) children in the two groups, respectively, required surgery.
One (5.3%) child with VEO-IBD and 11 (10.5%) older children stopped infliximab because of an adverse event (p = 1.00), while remission was the cause of drug withdrawal in two (10.5%) children in the younger group and 16 (15.2%) controls (p = 0.73).
Safety
As shown above, the incidence of adverse events was more frequent in VEO-IBD patients within the first 14 weeks of treatment whereas it was similar during the maintenance period.
The overall proportion of patients who experienced an adverse event during the entire period of treatment and the type of adverse event are reported in Table 3.
Table 3.
Adverse events.
| VEO-IBD 42 patients | IBD 130 patients | p values | |
|---|---|---|---|
| Patients, n (%) | 10 (23.8) | 21 (16.2) | 0.26 |
| Type of adverse event, n (%) | |||
| Infection | 1 (2.3) | 3 (2.3) | 1.00 |
| Allergic reaction | 8 (19.0) | 16 (12.3) | 0.31 |
| Psoriasis | 1 (2.3) | 1 (0.8) | 0.43 |
| Flu-like syndrome | 0 | 1 (0.8) | 1.00 |
IBD: inflammatory bowel disease; VEO-IBD: very early-onset inflammatory bowel disease.
Adverse events led to drug withdrawal in 10 among 10 (100%) children with VEO-IBD and in 19 (90.5%) among 21 older children (p = 1.00).
Discussion
Our study describes the largest cohort of children with VEO-IBD treated with infliximab, and it is the first to directly compare the efficacy and safety of infliximab in children with VEO-IBD and older children.
Children with VEO-IBD had higher rates of infliximab failure during both the induction and the maintenance period despite similar levels of disease severity and inflammatory markers at the time of infliximab start and the more frequent association with steroids and with immunomodulators.
More children with VEO-IBD required a dose intensification during induction similarly to the findings reported by deBruyn and colleagues, who showed in real-world experience that children younger than age 10 years at diagnosis had increased odds of requiring infliximab optimisation (odds ratio 6.5% confidence interval 2.0–21.1), although age at infliximab start had no influence.12
Overall, our findings are less favourable than those from the Children's Hospital of Philadelphia (CHOP) cohort,11 among which 66% of young children showed a response to the induction of therapy and 36% continued maintenance therapy at one year. This difference could be explained by our tighter definition of remission and by the different distribution of disease phenotype in the two cohorts. Indeed, contrary to the CHOP cohort, the diagnosis of UC was more frequent in our VEO-IBD patients, and infliximab has been reported to be less effective in children with UC than CD (remission rate at one year 55.8% and 28.6%, respectively, in the two registration trials).9,10 Data on the safety profile of infliximab were similar in the CHOP cohort and ours.
It could be hypothesised that the different efficacy of infliximab in children treated before age 7 years is due to the peculiarities of VEO-IBD and, in particular, to the genetics and the role of mechanisms of inflammation in addition to the TNF pathway.
We tried to limit this possibility by excluding patients with a monogenic VEO-IBD or with overt immunological abnormalities.
On the other hand, the variability of infliximab response among different age groups may be related to pharmacokinetic factors dependent also on anthropometric parameters.15 Indeed, weight and body surface can influence infliximab clearance and serum concentrations, which correlate with the possibility of clinical and endoscopic remission16–18 and the loss of efficacy during maintenance.19
The dosing of infliximab is weight based, but the correlation between infliximab clearance and body weight is not linear.20 Thus patients with lower body weight (<40 kg) are expected to have about 40% lower drug exposure: In other words, to achieve the desired trough drug levels patients with low weight, meaning young children, may require higher drug doses.
In a study by Hämäläinen and colleagues, young children treated with the induction dose of 5 mg/kg had significantly lower levels of infliximab by week 2 even if the difference was less marked by week 6.21 In the CHOP cohort, only eight among 22 patients whose trough levels were obtained had detectable drug levels with no anti-drug antibodies.
On this basis, it could be supposed that young children with IBD should be treated with higher doses or with more frequent administrations compared with older children to improve the response to infliximab.
The strength of our study is its national collaborative nature, the relatively high number of patients with VEO-IBD that have been enrolled and in the direct comparison between the cohort of young and the cohort of older children.
However, several limitations should be considered starting with the retrospective design of the study. The distribution of IBD phenotype was not homogenous in our two groups, UC being more frequent in VEO-IBD children, and CD in older children, similarly, however, to what has been reported in other large studies.22,23
To limit this bias, we performed subanalyses according to the type of IBD and to specific disease characteristics (i.e. perianal disease for CD patients) and the findings remained consistent with the main analysis (data not shown). However, the small numbers of patients in each subgroup limited the significance of the subanalyses, and a difference in the responses of the three IBD phenotypes cannot be excluded entirely.
All VEO-IBD patients had an immunological work-up, but only about 60% of VEO-IBD had genetic investigations; however, none of these children had a history of severe infections or HLH, haematological or immunological abnormalities or extraintestinal manifestations that have been reported to be suggestive for a monogenic disorder.24
Moreover, we did not have data regarding infliximab trough levels, and so we were not able to evaluate whether children with VEO-IBD had lower levels of infliximab compared with older patients. Thus we cannot prove that the differences in clinical response were due to different pharmacokinetic profiles.
Finally, endoscopic data were not available for the majority of the patients, and faecal calprotectin levels were incomplete, limiting the possibility to evaluate mucosal healing.
In conclusion, our study showed higher rates of failure and lower efficacy of infliximab treatment in children with VEO-IBD. Larger prospective studies, including the evaluation of trough drug levels, are required to understand the pharmacokinetics of infliximab in children with VEO-IBD and to develop new treatment regimens to optimise infliximab's efficacy and safety in this group of patients.
Acknowledgements
The authors would like to thank Prof Gabriele Stocco (University of Trieste) and Dr Samuele Naviglio (IRCCS ‘Burlo Garofolo’, Trieste).
Declaration of conflicting interests
The authors declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Ethics approval
The institutional review board and the ethics committee of each hospital approved the data collection in the registry (for the coordinating centre: Ethics Committee of the Sapienza University of Rome, Prot n 688/17, ethical approval 20 July 2017). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Written and signed informed consent was obtained from the parents of the patients to join the registry.
References
- 1.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn's disease: A population-based cohort study. Gastroenterology 2008; 135: 1106–1113. [DOI] [PubMed] [Google Scholar]
- 2.Uhlig HH, Schwerd T, Koletzko S, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology 2014; 147: 990–1007.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloi M, Lionetti P, Barabino A, et al. Phenotype and disease course of early-onset pediatric inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 597–605. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early- compared to later-onset pediatric Crohn's disease. Am J Gastroenterol 2008; 103: 2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014; 8: 1179–1207. [DOI] [PubMed] [Google Scholar]
- 6.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 1: Ambulatory care – An evidence-based guideline from European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018; 67: 257–291. [DOI] [PubMed] [Google Scholar]
- 7.Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, part 2: Acute severe colitis – An evidence-based consensus guideline from the European Crohn's and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018; 67: 292–310. [DOI] [PubMed] [Google Scholar]
- 8.Merrick VM, Mortier K, Williams LJ, et al. Real-life anti-tumor necrosis factor experience in more than 500 patients: High co-immunosuppression rates but low rates of quantifying treatment response. J Pediatr Gastroenterol Nutr 2018; 66: 274–280. [DOI] [PubMed] [Google Scholar]
- 9.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology 2007; 132: 863–873. quiz 1165–1166. [DOI] [PubMed] [Google Scholar]
- 10.Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol 2012; 10: 391–399.e1. [DOI] [PubMed] [Google Scholar]
- 11.Kelsen JR, Grossman AB, Pauly-Hubbard H, et al. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr 2014; 59: 758–762. [DOI] [PubMed] [Google Scholar]
- 12.deBruyn JC, Jacobson K, El-Matary W, et al. Long-term outcomes of infliximab use for pediatric Crohn disease: A Canadian multicenter clinical practice experience. J Pediatr Gastroenterol Nutr 2018; 66: 268–273. [DOI] [PubMed] [Google Scholar]
- 13.Levine A, Koletzko S, Turner D, et al. ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014; 58: 795–806. [DOI] [PubMed] [Google Scholar]
- 14.Levine A, Griffiths A, Markowitz J, et al. Paediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm Bowel Dis 2011; 17: 1314–1321. [DOI] [PubMed] [Google Scholar]
- 15.Steenholdt C, Bendtzen K, Brynskov J, et al. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis 2016; 22: 1999–2015. [DOI] [PubMed] [Google Scholar]
- 16.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: A retrospective analysis of the ACCENT I trial. Gut 2014; 63: 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naviglio S, Lacorte D, Lucafò M, et al. Causes of treatment failure in children with inflammatory bowel disease treated with infliximab: A pharmacokinetic analysis. J Pediatr Gastroenterol Nutr 2019; 68: 37–44. [DOI] [PubMed] [Google Scholar]
- 18.Singh N, Rosenthal CJ, Melmed GY, et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 1708–1713. [DOI] [PubMed] [Google Scholar]
- 19.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 2006; 4: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 20.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: A population pharmacokinetic study. Inflamm Bowel Dis 2014; 20: 2247–2259. [DOI] [PubMed] [Google Scholar]
- 21.Hämäläinen A, Sipponen T and Kolho KL. Serum infliximab concentrations in pediatric inflammatory bowel disease. Scand J Gastroenterol 2013; 48: 35–41. [DOI] [PubMed] [Google Scholar]
- 22.Benchimol EI, Mack DR, Nguyen GC, et al. Incidence, outcomes, and health services burden of very early onset inflammatory bowel disease. Gastroenterology 2014; 147: 803–813.e7. quiz 813.e14–813.e15. [DOI] [PubMed] [Google Scholar]
- 23.Benchimol EI, Bernstein CN, Bitton A, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: Distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol 2017; 112: 1120–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kammermeier J, Dziubak R, Pescarin M, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of 2 years. J Crohns Colitis 2017; 11: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


