Abstract
The medial temporal lobes (MTL) are known to play a crucial role in memory processes. Anatomical findings from animal studies suggest partially segregated MTL pathways converge in the hippocampus, with a posterior stream including parahippocampal and medial lateral entorhinal cortex and an anterior stream including perirhinal and lateral entorhinal cortex. These streams may operate on spatial and nonspatial information, respectively. In humans, such a functional dissociation has been suggested between parahippocampal and perirhinal cortex. Data from rodents and nonhuman primates suggest a similar dissociation between medial and lateral entorhinal cortex, which are reciprocally connected to parahippocampal and perirhinal cortex, but evidence for functional subregions within entorhinal cortex in humans is lacking. We addressed this issue using high-resolution fMRI with improved spatial normalization. Volunteers (n = 28) performed a working memory paradigm involving the retrieval of spatial (scenes) and nonspatial (faces) information after distraction. A clear dissociation between MTL subcircuits emerged. A perirhinal–lateral entorhinal pathway was more involved in the retrieval of faces after distraction, whereas a parahippocampal–medial entorhinal pathway was more involved in the retrieval of scenes after distraction. A cluster in posterior hippocampus showed a deactivation for the retrieval of faces after distraction. Our data thus provide direct evidence for a functional specialization within human entorhinal cortex and thereby strongly support MTL models that emphasize the importance of partially segregated parallel processing streams.
Introduction
The medial temporal lobes (MTL) are pivotal in episodic memory, yet the functional contributions of MTL subregions [hippocampus (HC), entorhinal cortex (EC), perirhinal cortex (PRC), and parahippocampal cortex (PHC)] remain controversial (Eichenbaum et al., 2007). Animal studies suggest partially segregated parallel MTL subcircuits with distinct neocortical connections: HC receives information from ventral and dorsal visual regions via an anterior [PRC, lateral EC (LEC)] and a posterior pathway [PHC, medial EC (MEC)], respectively (Suzuki and Amaral, 1994a,b; Burwell, 2000; Lavenex and Amaral, 2000; Eichenbaum et al., 2007; van Strien et al., 2009). Current functional models suggest that these streams process distinct information content (Davachi, 2006; Eichenbaum et al., 2007), and human MTL lesion studies revealed content-specific memory impairments (Bohbot et al., 2000; Bird et al., 2007; Peters et al., 2007a; Taylor et al., 2007).
Human PRC and PHC may be differentially involved in nonspatial (object-related) versus spatial processing (Epstein and Kanwisher, 1998; Davachi et al., 2003; Eichenbaum et al., 2007; Ekstrom and Bookheimer, 2007; Awipi and Davachi, 2008; Litman et al., 2009; Duarte et al., 2011; Staresina et al., 2011), sometimes referred to as item/context processing (Davachi, 2006; Eichenbaum et al., 2007), domain-specificity (Davachi, 2006), or domain-sensitivity, given the relative nature of these functional preferences (Litman et al., 2009). In rodents, functional dissociations for spatial and nonspatial processing have been reported between MEC and LEC (Hargreaves et al., 2005; Deshmukh and Knierim, 2011), and even their hippocampal projection targets in proximal and distal CA1 (Henriksen et al., 2010). Although spatial representations in human EC may resemble those in rodents (Doeller et al., 2010), direct evidence for domain-sensitive functional subregions in human EC is lacking.
The HC at the top of the MTL hierarchy (Lavenex and Amaral, 2000) may support domain-general relational (Davachi et al., 2003; Eichenbaum et al., 2007; Staresina et al., 2011), multi-attribute (Wixted and Squire, 2011), or spatial processing (Bird and Burgess, 2008). One MTL account emphasizes content-specific representations in HC (spatial) and PRC (object-related), possibly underlying MTL involvement in memory and higher-order perception (Barense et al., 2005, 2007; Lee et al., 2005a,b; Graham et al., 2006, 2010), and even within HC, posterior and anterior subregions may support spatial and object-related processing (Pihlajamäki et al., 2004; Lee et al., 2008; Graham et al., 2010).
To examine functional specialization in MTL subcircuits, we exploited the phenomenon that retrieval-related MTL activity is elevated after distraction disrupts rehearsal (Sakai et al., 2002). Using a modified working memory paradigm (Sakai et al., 2002), in which an abstract cue triggered retrieval of a face or scene after distraction, we tested whether nonspatial (faces) versus spatial (scenes) retrieval after distraction differentially recruits the PRC–LEC versus the PHC–MEC subcircuits, and explored the role of HC in this interaction. Since, during memory retrieval, original encoding-related cortical activity may be reinstated (Nyberg et al., 2000; Wheeler et al., 2000; Sakai et al., 2002; Kahn et al., 2004; Johnson and Rugg, 2007; Danker and Anderson, 2010), we additionally examined neocortical activations for reinstatement-related patterns.
Materials and Methods
Participants.
Data from 28 volunteers (16 male; mean age, 25.2 years; age range, 21–31 years) are included in the analysis. Data from two more subjects were excluded (because of incidental findings in the T1 structural image and because of an uncorrected impairment in visual perception). All remaining participants were right-handed, had normal or corrected-to-normal vision and reported no history of psychiatric or neurological disorders. They gave informed written consent before their participation and received monetary reimbursement (10€/h). The study procedure was approved by the local ethics committee (Hamburg Board of Physicians).
General procedure.
All participants completed a short behavioral training session before MR scanning. Immediately after the scanning session, which lasted ∼70 min, participants filled out a number of questionnaires that are not part of the present report. After a short pause, all participants completed a surprise recognition memory test that is also not relevant for the present report.
fMRI task.
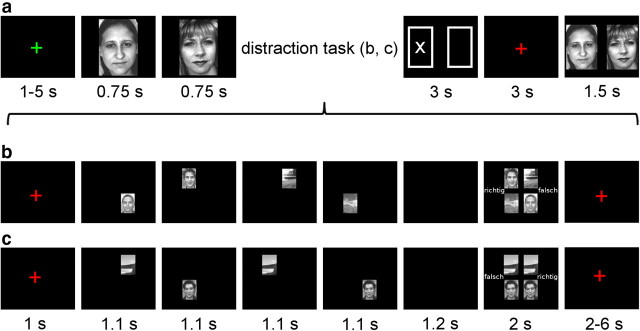
The fMRI task was adapted from a previous study (Sakai et al., 2002). Participants underwent six sessions of 28 trials each. Each trial consisted of a working memory task (Fig. 1a) with a nested distraction task (Fig. 1b,c). For the memory task, stimuli consisted of a total of 168 grayscale photographs of neutral faces (Endl et al., 1998) and outdoor scenes (various internet sources), respectively. For the distraction task, an additional set of face and scene photographs were used (five each, taken from the same sources). During each trial, after a variable intertrial interval of 1 to 5 s (drawn randomly from a uniform distribution), participants first viewed a set of either two faces or two scenes shown consecutively for 750 ms with a 50 ms gap, which they were asked to memorize in the order they appeared. Next, they solved either a distraction task (dist) or a no-distraction task (nodist): four pictures (two faces, two scenes) flashed in random order at four different screen locations for 1100 ms with a 200 ms gap and then, after a 1200 ms delay, finally appeared together for 2000 ms. Participants indicated via button press whether the resulting final picture array was correct (i.e., the same pictures were shown in the same positions as before) or incorrect (i.e., one picture was replaced by or switched locations with another picture). These response options (correct and incorrect) were presented on the left and right side of the screen in pseudorandom order.
Figure 1.
Experimental paradigm. a–c, The task involved a memory task (a) with a nested distraction task (b, c). Subjects encoded either two faces or two scenes (a; here only the face condition is shown). There was a blank screen for 50 ms between the encoding stimuli. Immediately after encoding, in 50% of all trials, subjects performed a distraction task (b) during which they had to memorize the identity and position of two faces and two scenes shown briefly in four quadrants of the screen with 200 ms of blank screen between individual distraction stimuli. These were then shown together and participants had to decide whether the same pictures reappeared in the same positions (“richtig,” correct) or whether one picture had switched position or was replaced by another stimulus (“falsch,” incorrect). The no-distraction task (c) was performed in the remaining 50% of all trials and was identical to the distraction task with the exception that two identical faces and two identical scenes were shown. Additionally, the no-distraction task was always correct and therefore placed minimal demands on working memory. The response assignment for correct and incorrect was randomized between left and right buttons across trials for both distraction and no-distraction tasks. Following the distraction or no-distraction task, a cue was shown (a) indicating whether the first or second item from the memory set was to be retrieved (3 s). Then a fixation cross appeared (3 s), after which the two items from the memory set were presented together (position randomized) and subjects indicated the cued item via button press.
In the distraction condition, four different pictures [two faces (randomly: both female, both male, or one female, one male), two scenes], drawn from a pool of four different faces and four different scenes, were shown. In contrast, the no-distraction condition always consisted of the same face and scene, each presented twice. Additionally, participants were informed that the no-distraction task would always be correct, while the distraction task would either be correct or incorrect, so as to ensure that the no-distraction condition would place little or no demands on visual working memory resources.
After a variable delay of 2 to 6 s (drawn randomly from a uniform distribution), an abstract cue (two boxes representing the two memory set stimuli, with the target item marked by a cross) was presented for 3 s (Fig. 1a), indicating whether participants were to retrieve the first or second picture from the initial memory set. Six seconds after cue presentation, both pictures from the initial memory set were shown again in random order for 1.5 s and participants were asked to indicate the cued picture via button press. All pictures in the memory task were trial-unique, and there was no overlap between the stimuli in the distraction and memory task. Face memory sets were compiled such that, for a given memory set of two faces, only two male or two female faces were included.
Directly before scanning, participants completed a training session consisting of 20 distraction-only trials and eight combined memory and distraction trials as described above. The experiment was programmed using the software package Presentation (Neurobehavioral Systems).
fMRI data acquisition.
fMRI data were acquired using a 3T Siemens TIM-TRIO scanner with a 32-channel head coil. During each of the six sessions, 307 volumes were acquired, using a T2*-weighted EPI sequence (37 slices, 2 × 2 × 2 mm, no gap, TR = 2.37 s, TE = 30 ms) that was previously established to identify differential functional responses of amygdala subregions (Gamer et al., 2010). The first five volumes of each session were discarded to allow for stabilization of the BOLD signal. The field of view was aligned to the longitudinal axis of the HC and covered the temporal and occipital lobes as well as parts of the parietal and inferior prefrontal cortex. Additionally, a T1-weighted MPRAGE structural image was acquired for each participant (240 slices, 1 × 1 × 1 mm). Participants viewed the experiment via a head-coil-mounted mirror and responses were logged with an MRI-compatible button box.
Behavioral data analysis.
Using Matlab (MathWorks) and SPSS, we analyzed the proportion of correct responses (accuracy) as well as mean reaction times on the distraction and memory task for correct responses via 2 × 2 repeated-measures ANOVAs with the factors material (faces/scenes) and distraction (dist/nodist).
Anatomical regions of interest.
We created hand-drawn, unilateral anatomical ROIs using established procedures (Pruessner et al., 2000, 2002) based on the average high-resolution T1 image. Using MRIcron (Rorden et al., 2007), we segmented the MTL into PRC, EC, and PHC as well as HC, starting in anterior MTL at the first appearance of the collateral sulcus and ending in posterior MTL at the disappearance of the posterior HC. The border between anterior parahippocampal gyrus (PRC, EC) and posterior parahippocampal gyrus (PHC) was determined according to the disappearance of the gyrus intralimbicus.
fMRI data analysis.
All analyses were performed using SPM-08-4010 (Wellcome Department of Cognitive Neurology, University College London). For each subject, functional volumes were spatially realigned to the mean functional scan using a six-parameter affine transformation and unwarped to account for movement-related effects. A first-level general linear model was then created on the unsmoothed non-normalized functional images. Scans from all six sessions were concatenated and session-specific constants were included in the model. The high-pass filter was adapted accordingly. For trials on which the memory task was solved correctly, four experimental conditions were modeled: faces/dist, faces/nodist, scenes/dist, and scenes/nodist. For each condition, regressors were created by convolving the event-train of event onsets with the canonical hemodynamic response function, modeling each trial phase: (1) the onset of the memory set, (2) the distraction task, (3) the cue period, and (4) the test phase. Error trials were modeled separately for faces and scenes. An autoregressive model, AR(1) (adapted to account for the concatenated sessions at the first level, see above), was used to model serial autocorrelations in the data. Contrast images for the memory set and cue periods were created for each of the four conditions. We also created differential contrast images for memory encoding (faces vs scenes for the memory set phase).
High-resolution T1 anatomical images were then segmented into gray matter, white matter, and CSF using the segmentation routine implemented in SPM08. These segmented images were used to create a study-specific structural template and single-subject flow fields using the DARTEL toolbox (Ashburner, 2007) for SPM08. Single-subject contrast images were normalized to Montreal Neurological Institute space (MNI space) via the template and the single-subject flow fields. During this normalization step, images were resampled to an isotropic voxel size of 1 mm3 and smoothed with a narrow Gaussian kernel of 3 mm full-width at half maximum to optimize the detection of small activations within MTL subregions.
Two analyses were then performed on the smoothed and normalized contrast images at the second level. First, encoding-related activity was analyzed using a one-sample t test (faces vs scenes) on the memory set contrast. Second, cue-related activity was analyzed using a 2 (faces/scenes) × 2 (dist/nodist) full factorial model as implemented in SPM08 (i.e., faces/dist, faces/nodist, scenes/dist, scenes/nodist). We tested the differential involvement of the PRC–LEC and PHC–MEC pathways as well as HC in the retrieval of faces and scenes after distraction using interaction contrasts ([1 −1 −1 1] for faces, [−1 1 1 −1] for scenes) within the full factorial model. Interaction effects were then further analyzed by extracting single-subject beta weights from the interaction peak coordinates and submitting them to further statistical analyses using SPSS.
Correction for multiple comparisons.
For all analyses, the threshold was set to p < 0.05, FWE-corrected for multiple comparisons. For main effects, p values were corrected across the whole acquired volume. Since we had specific a priori hypotheses regarding the differential contributions of the PRC–LEC and PHC–MEC pathways, interaction effects were assessed using small volume correction across unilateral masks of anatomically defined regions of interest (see Anatomical regions of interest, above). Interaction effects within the HC were treated similarly for exploratory purposes. Interaction effects during retrieval outside of the MTL [e.g., fusiform face area (FFA)] were corrected using activation peaks from the encoding period (first presentation of the stimuli; Fig. 1a).
Results
Behavioral results
The task consisted of a memory task with a nested distraction task. We first report behavioral results for the distraction task followed by behavioral results for the main task of interest (memory).
First, we examined whether performance in the distraction task differed between conditions. We analyzed distraction task accuracy [mean (SD) accuracy in percentages: faces/dist, 79.4 (8.7); faces/nodist, 99.1 (1.3); scenes/dist, 79.5 (7.4); scenes/nodist, 98.6 (2.3)] as well as reaction times [RTs; mean (SD) RT in milliseconds: faces/dist, 1324 (102); faces/nodist 890 (91); scenes/dist, 1315 (103); scenes/nodist, 887 (91)]. ANOVA with the factors material (faces/scenes) and distraction (dist/nodist) confirmed significant differences between the distraction and no-distraction task. Responses in the distraction task were less accurate (mean accuracy: dist = 79.5%, nodist = 98.8%; F(1,27) = 230.628, p < 0.001) and slower (mean RT: dist = 1320 ms, nodist = 889 ms; F(1,27) = 1148.792, p < 0.001) than responses in the no-distraction task. No other main effects or interactions were significant (all p ≥ 0.378).
We next examined whether performance in the memory task differed as a function of distraction. We analyzed memory accuracy [mean (SD) accuracy in percentages: faces/dist, 90.8 (8.0); faces/nodist, 91.4 (9.5); scenes/dist, 87.8 (9.9); scenes/nodist, 91.6 (8.7)] as well as RTs [mean (SD) RT in milliseconds: faces/dist, 990 (61); faces/nodist, 965 (70); scenes/dist, 973 (55); scenes/nodist, 966 (71)]. ANOVA with the factors material (faces/scenes) and distraction (dist/nodist) again confirmed significant behavioral effects of distraction. Accuracy on the memory task was reduced after distraction (mean accuracy: dist = 89.3%, nodist = 91.5%; F(1,27) = 7.714, p = 0.010) and RTs increased (mean RT: dist = 982 ms, nodist = 966 ms; F(1,27) = 7.632, p = 0.010). No other main effects or interactions were significant (all p ≥ 0.083).
Imaging results
All reported imaging results refer to activity during the retrieval period (cue period), during which only an abstract cue was shown (Fig. 1a). This cue indicated to subjects whether the first or second item from the memory set was to be retrieved from memory. Importantly, stimulation during the cue period was identical for face and scene trials.
Note our general analysis procedure. We first report main effects of material and distraction before moving on to the critical material × distraction interaction analyses (including post hoc analyses). Here we first report PHC and PRC effects, followed by EC effects (LEC and MEC) and HC effects. We also applied an additional small volume correction procedure using functional ROIs based on activity peaks both from the literature and from the encoding period (first presentation of the stimuli; Fig. 1a).
Main effects of material
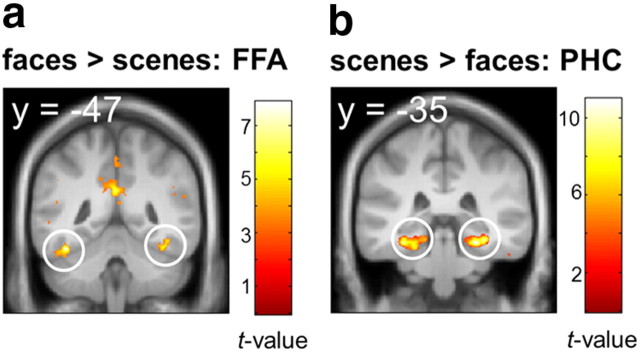
Reliable main effects of material emerged during the cue period, such that cueing faces > scenes elicited activity in bilateral FFA [peak MNI coordinates (x, y, z): left: −40, −47, −21, t(27) = 7.90, pFWE < 0.001; right: 40, −49, −19, t(27) = 7.20, pFWE < 0.001; Fig. 2a] and cueing of scenes > faces elicited activity in bilateral PHC (right: 26, −35, −16, t(27) = 10.96, pFWE < 0.001; left: −33, −33, −17, t(27) = 10.43, pFWE < 0.001; Fig. 2b) encompassing the parahippocampal place area (Epstein and Kanwisher, 1998), suggesting that the cue elicited reinstatement of material-specific representations. No suprathreshold clusters were observed in PRC, EC, or HC at p < 0.001 uncorrected.
Figure 2.
Main effects of material during cue presentation. a, b, Retrieval of faces (a) activated bilateral FFA (left: −40, −47, −21; right: 40, −49, −19) while retrieval of scenes (b) activated bilateral PHC (right: 26, −35, −16; left: −33, −33, −17). Display threshold p < 0.001 uncorrected, k > 5 voxels. Maps are projected onto the mean DARTEL-normalized T1.
Main effects of distraction
Retrieval after distraction versus no-distraction was associated with the activation of an extensive bilateral frontoparietal network, but no suprathreshold clusters were observed in MTL at p < 0.001 uncorrected. Even at a liberal threshold of p < 0.01 uncorrected, no main effect of distraction was observed within the HC. Therefore, main effects of distraction are of no further interest here.
Material × distraction interactions
We next tested the hypothesis that MTL activity during retrieval after distraction is domain-sensitive such that (1) the PRC–LEC pathway is more involved in recovery of faces and (2) the PHC–MEC pathway is more involved in recovery of scenes (material × distraction interactions during cue processing) after distraction. These results are complemented with an analysis of HC.
PRC and PHC
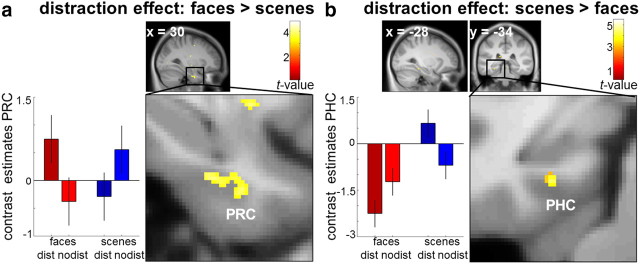
Bilateral PRC showed a greater distraction effect for faces than scenes (right PRC: 28, −11, −30, t(27) = 4.18, pFWE = 0.027; 30, −8, −33, t(27) = 3.99, pFWE = 0.048; plotted in Fig. 3a; left PRC: −26, 0, −40, t(27) = 4.00, pFWE = 0.047). Single-subject beta weights were extracted from the peak at 30, −8, −33. Repeated-measures ANOVA with the factors material and distraction did not reveal any additional significant main effect of material or distraction (all p ≥ 0.609). Paired, two-sided t tests on the beta weights revealed significantly stronger activation for faces after distraction compared with no-distraction (t(27) = 2.972, p = 0.002) and a significant, opposite effect for scenes (t(27) = 2.817, p = 0.009). In contrast, left PHC showed a greater distraction effect for scenes (−28, −34, −15; t(27) = 4.70, pFWE = 0.004; Fig. 3b). Follow-up analyses revealed a significant main effect of material with stronger activation during scene retrieval than face retrieval (F(27) = 25.297, p < 0.001), but no main effect of distraction (p = 0.528). Post hoc t tests confirmed stronger activation for scenes after distraction compared with no-distraction (t(27) = 3.722, p = 0.001) and the reverse effect for faces (t(27) = 2.811, p = 0.009). To confirm the functional dissociation between PHC and PRC, we submitted these data to a three-way repeated-measures ANOVA (Nieuwenhuis et al., 2011) with the factors region (PHC vs PRC), material (faces vs scenes), and distraction (distraction vs no-distraction). This revealed a highly significant three-way interaction (F(27) = 34.164, p < 0.001), confirming that the nature of the material × distraction interactions differed significantly between regions.
Figure 3.
Interaction of material and distraction during cue presentation. a, b, PRC (30, −8, −33) showed a stronger distraction effect for faces than scenes (a) while the reverse pattern emerged in PHC (−28, −34, −15; b). Display thresholds p < 0.001 uncorrected, k > 5 voxels. Error bars denote SEM. Maps are projected onto the mean, contrast-enhanced DARTEL-normalized T1.
EC
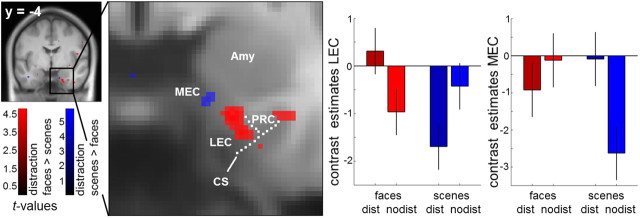
A corresponding functional dissociation emerged within the EC. LEC showed a greater distraction effect for faces than scenes (20, −4, −34; t(27) = 4.60, pFWE = 0.004), whereas the reverse effect was seen in a directly adjacent cluster in MEC (15, −5, −30; t(27) = 4.03, pFWE = 0.025; Fig. 4). Follow-up analyses revealed an additional main effect of material in LEC, i.e., stronger activation for cueing faces than scenes (F(27) = 6.080, p = 0.020), but no main effect of distraction (p = 0.990). Post hoc t tests revealed greater LEC activity for face retrieval after distraction versus no-distraction (t(27) = 2.525, p = 0.018), and the reverse effect for scenes (t(27) = 2.555, p = 0.017). For MEC, we observed an additional main effect of material, again with stronger activation for cueing faces (F(27) = 4.868, p = 0.036), and no main effect of distraction (p = 0.178). However, MEC activity was greater for scene retrieval after distraction versus no-distraction (t(27) = 4.047, p < 0.001), but showed no significant difference for faces (t = 0.862, p = 0.396). A confirmative three-way repeated-measures ANOVA (Nieuwenhuis et al., 2011) with the factors region (LEC vs MEC), material, and distraction revealed again a highly significant three-way interaction (F(27) = 26.492, p < 0.001), confirming that the nature of the material × distraction interactions differed significantly between LEC and MEC.
Figure 4.
Differential activation within EC during cue presentation. LEC (20, −4, −34) showed a stronger distraction effect for faces versus scenes, whereas a directly adjacent cluster in MEC (15, −5, −30) showed a stronger distraction effect for scenes versus faces. The dashed lines mark the location of the collateral sulcus (CS) and the putative border between EC and PRC, the midpoint of the medial bank of the collateral sulcus (Pruessner et al., 2002). Amy, Amygdala. Display threshold p < 0.001 uncorrected, k > 5 voxels. Error bars denote SEM. Maps are projected onto the mean, contrast-enhanced DARTEL-normalized T1.
Additionally, a cluster in more posterior left LEC (extending into PRC) showed a greater distraction effect for faces than scenes (−28, −22, −27; t(27) = 4.43, pFWE = 0.007), whereas bilateral posterior MEC showed scene-sensitive activity during retrieval after distraction, albeit only at an uncorrected threshold (p < 0.001, left: −18, −20, −22, t(27) = 3.32, pFWE = 0.177; right: 18, −18, −22, t(27) = 3.12, pFWE = 0.283).
HC
A posterior HC cluster showed a stronger distraction effect for scenes than faces (22, −40, −2; t(27) = 4.26, pFWE = 0.025). Follow-up analysis revealed no additional main effect of material or distraction (all p ≥ 0.309). However, the interaction effect was mainly driven by a significant deactivation for faces after distraction compared with no-distraction (t(27) = 3.128, p < 0.001). In contrast, activity for scene retrieval was only nonsignificantly greater than without distraction (t(27) = 1.846, p = 0.076).
Small volume correction based on functional ROIs
To confirm the results obtained using anatomy-based small volume correction (across anatomical ROIs, as described above), we also applied functionally based small volume correction (using spheres centered at activation peaks from the literature). We again examined material (faces/scenes) × distraction (dist/nodist) interaction contrasts. For the EC, we used an 8 mm sphere centered at 21, −9, −30 (Doeller et al., 2010). Within this sphere, both a face-selective lateral cluster (20, −4, −33; t(27) = 4.83, pFWE = 0.002) and a directly adjacent scene-selective medial cluster (15, −5, −30; t(27) = 4.03, pFWE = 0.031) emerged. For PRC, we used an 8 mm sphere centered at 33, −6, −30 and for PHC an 8 mm sphere centered at ±27, −36, −9 (Staresina et al., 2011). Within the PRC sphere, three adjacent face-selective peaks emerged (28, −11, −30, t(27) = 4.18, pFWE = 0.020; 30, −8, −33, t(27) = 3.99, pFWE = 0.035; 32, −3, −33, t(27) = 3.94, pFWE = 0.040), whereas within the PHC sphere, a single scene-selective cluster emerged (−28, −34, −15; t(27) = 4.70, pFWE = 0.004). Therefore, regardless of whether ROIs were selected based on anatomical or functional grounds, we observed differential involvement of the PRC–LEC and PHC–MEC pathways.
Reactivation of encoding-related areas during retrieval after distraction
We also examined whether face- and scene-sensitive clusters during retrieval after distraction correspond to regions that are material-sensitive during the encoding phase (i.e., during the initial encoding of the two memory set items at the beginning of each trial; Fig. 1a). The scene-sensitive PHC cluster from the cue period (−28, −34, −15) survived correction (pFWE = 0.004) across an 8 mm sphere centered at −29, −41, −16, a peak that showed significantly greater activity for scene encoding than face encoding at p < 0.00001, uncorrected. Likewise, a face-selective FFA cluster from the cue period (45, −39, −22) survived correction (pFWE = 0.041) across an 8 mm sphere centered at 46, −46, −21, a peak that showed significantly greater activity for face encoding than scene encoding at p < 0.00001, uncorrected. Follow-up analyses for this FFA region revealed an additional main effect of material, with stronger activation during face retrieval (F(27) = 8.124, p = 0.008), as well as a main effect of distraction (F(27) = 4.962, p = 0.034). The latter appears to be carried by a strong difference between faces after distraction and no-distraction (t(27) = 4.010, p < 0.001), while scenes after distraction and no-distraction did not differ significantly (t(27) = 0.705, p = 0.487).
Discussion
We examined the involvement of MTL pathways (PRC–LEC/PHC–MEC) in the retrieval of nonspatial (faces) and spatial (scenes) information after distraction from active rehearsal. In line with anatomical connectivity data, PRC and PHC showed a clear dissociation for nonspatial and spatial retrieval after distraction. Importantly, our data reveal a corresponding functional dissociation between LEC and MEC in humans, complementing rodent findings (Hargreaves et al., 2005; Deshmukh and Knierim, 2011). Additionally, FFA and PHC showed main effects for face and scene retrieval.
Perirhinal and parahippocampal cortex
Previous studies suggested differential roles of PRC and PHC in nonspatial and spatial encoding (Awipi and Davachi, 2008; Staresina et al., 2011) or item and context encoding (Davachi et al., 2003; Ranganath et al., 2004). MTL responses during memory retrieval may also be domain-specific (Peters et al., 2007b), but here evidence is less conclusive (Ekstrom and Bookheimer, 2007; Duarte et al., 2011). Our results provide further evidence that PRC and PHC are involved in nonspatial and spatial retrieval, respectively. However, this does not rule out the possibility that PRC and PHC may still interact, e.g., via the dense projections from PHC to PRC (but less vice versa) (Suzuki and Amaral, 1994b). Furthermore, material sensitivity along the anterior–posterior MTL axis might be gradual rather than categorical (Litman et al., 2009).
Functional subregions within EC
We observed a similar dissociation between lateral and medial EC subregions, consistent with anatomical data in nonhuman primates and rodents showing reciprocal connections between PRC and LEC and between PHC and MEC (Suzuki and Amaral, 1994a; van Strien et al., 2009) and corresponding functional dissociations between LEC and MEC in rodents (Hargreaves et al., 2005; Deshmukh and Knierim, 2011). Yet, these functional dissociations between EC subregions have previously not been described in humans, although there is some evidence for spatial grid-cells in human EC (Doeller et al., 2010).
EC localization in our study corresponds well with previous human imaging studies (Doeller et al., 2010). However, we describe directly adjacent EC subregions with strikingly different response profiles. Nevertheless, they likely contain signals from multiple of at least eight distinct human EC subregions that have been identified (Insausti et al., 1995). Furthermore, with respect to EC, the nonspatial versus spatial distinction may be oversimplified, as, for example, in rodents, caudal MEC may contribute to nonspatial recollection (Sauvage et al., 2010) and LEC may be involved in object-related spatial processing (Deshmukh and Knierim, 2011).
Input into EC originates in PRC and PHC (Suzuki and Amaral, 1994a), but also in polysensory association cortices (Lavenex and Amaral, 2000). EC lesions in monkeys produce less severe visual recognition impairments than PRC lesions (Meunier et al., 1993) and direct projections from PRC/PHC to HC exist (Suzuki and Amaral, 1990). Thus, characterizing EC as a mere relay station to HC may be oversimplified. Rather, EC may be in a position to further integrate PRC/PHC representations, potentially incorporating hippocampal feedback (Lavenex and Amaral, 2000; Deshmukh and Knierim, 2011). Our observation that EC subregions are modulated not by material alone, but show more complex material × distraction interaction effects, supports this view.
Implications for functional MTL models
Our data conform well with MTL models describing a PRC–LEC subcircuit processing nonspatial and a PHC–MEC subcircuit processing spatial representations (Eichenbaum et al., 2007; see also Davachi, 2006). Functional MTL accounts have prominently focused on psychologically distinct processes underlying recognition memory, i.e., recollection and familiarity: PRC is thought to support familiarity and a PHC–HC network is thought to support recollection (Eichenbaum et al., 2007; Yonelinas et al., 2010). However, Squire and colleagues propose a more unified account of MTL memory processes (Squire et al., 2007; Wixted and Squire, 2011), in which HC as well as PRC may support both processes, and, in HC patient studies, report memory and learning deficits across processes (Jeneson et al., 2010) and stimulus content (Shrager et al., 2007; Kim et al., 2011). In a recent neuroanatomy-based approach (Wixted and Squire, 2011), they suggested that MTL subregions may process different attributes of memory (see also Squire et al., 2007). Since we examined retrieval triggered by an abstract cue rather than recognition, our study does not address the familiarity/recollection debate directly. However, process- and content-based MTL accounts are not incompatible, as PRC may support familiarity via object-related processing while PHC may support recollection by processing (spatial) context (Brown and Aggleton, 2001; Ranganath et al., 2004; Montaldi et al., 2006; Eichenbaum et al., 2007). A similar account may apply to the item/context or item/source distinction (Davachi et al., 2003, 2006; Diana et al., 2007). Process- and content-based MTL accounts may be further integrated by jointly controlling both factors (Bird et al., 2007; Taylor et al., 2007).
We did not observe domain-general HC effects (Davachi et al., 2003; Eichenbaum et al., 2007; Staresina et al., 2011) after distraction. One reason may be that participants were required to retrieve perceptual rather than associative information, which previously yielded domain-sensitive PRC/PHC signals but no domain-general HC effects (Peters et al., 2007b). In contrast, a posterior HC cluster showed a stronger distraction effect for scenes than faces, which may fit with the literature on spatial processing in (posterior) HC (Pihlajamäki et al., 2004; Barense et al., 2005, 2007; Lee et al., 2005a,b, 2008; Graham et al., 2006, 2010; Awipi and Davachi, 2008; Bird and Burgess, 2008). However, as this interaction was primarily driven by a deactivation for faces, we are reluctant to draw firm conclusions.
Some controversy surrounds a potential MTL involvement in perception (Squire et al., 2006; Baxter, 2009; Suzuki, 2009). Evidence from human lesion (Barense et al., 2005, 2007; Lee et al., 2005a, 2005b; Graham et al., 2006; but see Levy et al., 2005; Shrager et al., 2006; Kim et al., 2011) and imaging studies (Lee et al., 2008; Barense et al., 2010) in line with lesion studies in nonhuman primates and rodents (for review, see Murray et al., 2007; Baxter, 2009; Graham et al., 2010) support an account in which PRC implements feature integration of complex visual stimuli while HC implements spatial processing, underlying both higher-order perception and memory (Graham et al., 2010). It has been argued that perceptual and memory processes in animals are often confounded and that MTL lesions in humans often encompass extra-MTL areas (Squire et al., 2006; Suzuki, 2009); however, a recent study showing impaired perception in MTL patients provided exact volumetric assessments of lesions and fMRI responses (Lee and Rudebeck, 2010). Nevertheless, as perceptual input during retrieval was held constant, our findings likely reflect distinct memory processes, rather than perceptual processes. Our data show clear PRC–LEC involvement in nonspatial retrieval. However, in contrast to PHC–MEC, we observed no HC involvement in spatial retrieval.
Domain sensitivity and the role of distraction
Our data suggest domain-sensitive MTL retrieval signals are elevated after distraction, compatible with the idea that they facilitate cortical reinstatement of the retained (visual) material after disrupted rehearsal, a mechanism that has been proposed for a similar effect using verbal material (Sakai et al., 2002). The material main effects during retrieval in FFA (faces) and PHC (scenes) may reflect such domain-sensitive reinstatement (for review, see Danker and Anderson, 2010). In line with this account, FFA and PHC activation during retrieval after distraction partly overlapped with encoding-related domain-sensitive activations.
Distraction modulated PRC and PHC and was instrumental in revealing differences between EC subregions. Elevated (material-specific) MTL signals after distraction may reflect higher retrieval demands (Sakai et al., 2002) in contrast to the no-distraction condition, when information is still maintained in visual working memory. Additionally, a higher degree of visual interference before retrieval, which may impair object recognition memory in rodents with MTL lesions including PRC (Bartko et al., 2010; McTighe et al., 2010; see also Cowell et al., 2006), may have required the MTL to resolve a higher level of feature ambiguity after distraction. Distraction might therefore boost retrieval-related MTL signals, facilitating their detection.
One might have expected elevated activity for faces in PRC–LEC and scenes in PHC–MEC that is additionally elevated after distraction. While all regions show stronger activation for their hypothesized material after distraction, interactions were often somewhat more complex (Figs. 3, 4), which may have several reasons. First, we did not directly present images from different categories (Litman et al., 2009) but used an abstract cue. Second, neighboring MTL regions may inhibit each other through interconnections (Suzuki and Amaral, 1994b; Burwell, 2000; Lavenex and Amaral, 2000), and higher PHC–MEC activation for scenes after distraction might thus lead to PRC–LEC deactivation, and vice versa.
Conclusions
We show that retrieval of spatial and nonspatial content after distraction involves the PHC–MEC and PRC–LEC pathways within the MTL, mirroring rodent findings of functional specialization in these circuits (Hargreaves et al., 2005; Deshmukh and Knierim, 2011). These domain-sensitive signals might play a role in cortical reinstatement. In line with primate neuroanatomy (Suzuki and Amaral, 1994a,b), our findings provide strong evidence for a functional organization of the human MTL according to memory content (Davachi, 2006; Eichenbaum et al., 2007).
Footnotes
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (PE-1627/2-1).
The authors declare no competing financial interests.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. J Exp Psychol Learn Mem Cogn. 2008;34:769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. J Neurosci. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RN, Lee AC, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: effects of viewpoint. Hippocampus. 2010;20:389–401. doi: 10.1002/hipo.20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Heightened susceptibility to interference in an animal model of amnesia: impairment in encoding, storage, retrieval—or all three? Neuropsychologia. 2010;48:2987–2997. doi: 10.1016/j.neuropsychologia.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. 2009;61:667–677. doi: 10.1016/j.neuron.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bird CM, Shallice T, Cipolotti L. Fractionation of memory in medial temporal lobe amnesia. Neuropsychologia. 2007;45:1160–1171. doi: 10.1016/j.neuropsychologia.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Allen JJ, Nadel L. Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann N Y Acad Sci. 2000;911:355–368. doi: 10.1111/j.1749-6632.2000.tb06737.x. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. J Neurosci. 2006;26:12186–12197. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull. 2010;136:87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh SS, Knierim JJ. Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. doi: 10.3389/fnbeh.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463:657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Res. 2011;1373:110–123. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Bookheimer SY. Spatial and temporal episodic memory retrieval recruit dissociable functional networks in the human brain. Learn Mem. 2007;14:645–654. doi: 10.1101/lm.575107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endl W, Walla P, Lindinger G, Lalouschek W, Barth FG, Deecke L, Lang W. Early cortical activation indicates preparation for retrieval of memory for faces: an event-related potential study. Neurosci Lett. 1998;240:58–60. doi: 10.1016/s0304-3940(97)00920-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci U S A. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Scahill VL, Hornberger M, Barense MD, Lee AC, Bussey TJ, Saksida LM. Abnormal categorization and perceptual learning in patients with hippocampal damage. J Neurosci. 2006;26:7547–7554. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser MB, Moser EI. Spatial representation along the proximodistal axis of CA1. Neuron. 2010;68:127–137. doi: 10.1016/j.neuron.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Tuñón T, Sobreviela T, Insausti AM, Gonzalo LM. The human entorhinal cortex: a cytoarchitectonic analysis. J Comp Neurol. 1995;355:171–198. doi: 10.1002/cne.903550203. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Kirwan CB, Hopkins RO, Wixted JT, Squire LR. Recognition memory and the hippocampus: a test of the hippocampal contribution to recollection and familiarity. Learn Mem. 2010;17:63–70. doi: 10.1101/lm.1546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Jeneson A, van der Horst AS, Frascino JC, Hopkins RO, Squire LR. Memory, visual discrimination performance, and the human hippocampus. J Neurosci. 2011;31:2624–2629. doi: 10.1523/JNEUROSCI.5954-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee AC, Rudebeck SR. Human medial temporal lobe damage can disrupt the perception of single objects. J Neurosci. 2010;30:6588–6594. doi: 10.1523/JNEUROSCI.0116-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005a;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee AC, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, Hodges JR, Graham KS. Perceptual deficits in amnesia: challenging the medial temporal lobe “mnemonic” view. Neuropsychologia. 2005b;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 2008;18:683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Levy DA, Shrager Y, Squire LR. Intact visual discrimination of complex and feature-ambiguous stimuli in the absence of perirhinal cortex. Learn Mem. 2005;12:61–66. doi: 10.1101/lm.84405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, Davachi L. Category-specificity in the human medial temporal lobe cortex. Hippocampus. 2009;19:308–319. doi: 10.1002/hipo.20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM. Paradoxical false memory for objects after brain damage. Science. 2010;330:1408–1410. doi: 10.1126/science.1194780. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proc Natl Acad Sci U S A. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Koch B, Schwarz M, Daum I. Domain-specific impairment of source memory following a right posterior medial temporal lobe lesion. Hippocampus. 2007a;17:505–509. doi: 10.1002/hipo.20297. [DOI] [PubMed] [Google Scholar]
- Peters J, Suchan B, Köster O, Daum I. Domain-specific retrieval of source information in the medial temporal lobe. Eur J Neurosci. 2007b;26:1333–1343. doi: 10.1111/j.1460-9568.2007.05752.x. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M, Tanila H, Könönen M, Hänninen T, Hämäläinen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Parahippocampal reactivation signal at retrieval after interruption of rehearsal. J Neurosci. 2002;22:6315–6320. doi: 10.1523/JNEUROSCI.22-15-06315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Beer Z, Ekovich M, Ho L, Eichenbaum H. The caudal medial entorhinal cortex: a selective role in recollection-based recognition memory. J Neurosci. 2010;30:15695–15699. doi: 10.1523/JNEUROSCI.4301-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci. 2006;26:2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Bayley PJ, Bontempi B, Hopkins RO, Squire LR. Spatial memory and the human hippocampus. Proc Natl Acad Sci U S A. 2007;104:2961–2966. doi: 10.1073/pnas.0611233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Shrager Y, Levy DA. Lack of evidence for a role of medial temporal lobe structures in visual perception. Learn Mem. 2006;13:106–107. doi: 10.1101/lm.178406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31:8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA. Perception and the medial temporal lobe: evaluating the current evidence. Neuron. 2009;61:657–666. doi: 10.1016/j.neuron.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neurosci Lett. 1990;115:43–48. doi: 10.1016/0304-3940(90)90515-b. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J Neurosci. 1994a;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994b;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Taylor KJ, Henson RN, Graham KS. Recognition memory for faces and scenes in amnesia: dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2428–2438. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci U S A. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. The medial temporal lobe and the attributes of memory. Trends Cogn Sci. 2011;15:210–217. doi: 10.1016/j.tics.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Aly M, Wang WC, Koen JD. Recollection and familiarity: examining controversial assumptions and new directions. Hippocampus. 2010;20:1178–1194. doi: 10.1002/hipo.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]