Abstract
GABAergic inhibition in the amygdala is essential in regulating fear and anxiety. Although fast “phasic” inhibition arising through the activation of postsynaptic GABAA receptors (GABAARs) has been well described in the amygdala, much less is known about extrasynaptic GABAARs mediating persistent or tonic inhibition and regulating neuronal excitability. Here, we recorded tonic currents in the basolateral (BLA) nucleus and the lateral (LA) nucleus of the amygdala. While all BLA principal cells expressed a robust GABAergic tonic current, only 70% of LA principal cells showed a tonic current. Immunohistochemical stainings revealed that the α3 GABAAR subunit is expressed moderately in the LA and strongly throughout the BLA nucleus, where it is located mostly at extrasynaptic sites. In α3 subunit KO mice, tonic currents are significantly reduced in BLA principal cells yet not in LA principal cells. Moreover, the α3 GABAAR-selective benzodiazepine site agonist and anxiolytic compound TP003 increases tonic currents and dampens excitability markedly in wild-type BLA principal cells but fails to do so in α3KO BLA cells. Interneurons of the LA and BLA nuclei also express a tonic current, but TP003-induced potentiation is seen in only a small fraction of these cells, suggesting that primarily other GABAAR variants underlie tonic inhibition in this cell type. Together, these studies demonstrate that α3 GABAAR-mediated tonic inhibition is a central component of the inhibitory force in the amygdala and that tonically activated α3 GABAARs present an important target for anxiolytic or fear-reducing compounds.
Introduction
Neuronal circuits in the amygdala play a crucial role in the generation of fear and anxiety (Sanders and Shekhar, 1995b). Signals from the thalamus and the cerebral cortex enter the amygdala through the lateral (LA) nucleus of the amygdala, are further processed in the basolateral (BLA) nucleus of the amygdala, and finally reach the central nucleus of the amygdala, from which typical fear reactions are triggered by projections to the hypothalamus and brainstem (Aggleton, 2000). An essential component of the amygdala fear circuit is fast (or phasic) inhibition, mediated by a distinct set of synaptic GABAA receptors (GABAARs) (Sanders and Shekhar, 1991, 1995b). GABAARs are heteropentameric Cl−-permeable ion channels composed of distinct subunits derived from seven subfamilies (α1–6, β1–3, γ1–3, δ, π, θ, ε) (Whiting et al., 1999). Receptors differing in subunit composition can be distinguished by their expression patterns and their functional and pharmacological properties. In LA and BLA principal cells (PCs), fast GABAergic postsynaptic currents arise primarily through the activation of α2-containing GABAARs and, to a lesser extent, α1-containing GABAARs (Marowsky et al., 2004) (αx-containing GABAARs will be referred to as αx GABAARs from here on).
In the amygdala, little is known about the second form of GABAergic inhibition, termed tonic inhibition, and the GABAARs (Farrant and Nusser, 2005; Belelli et al., 2009) mediating it. In contrast to phasic IPSCs, tonic inhibition is characterized by a persistent low-amplitude current capable of controlling action potential firing. Hallmarks of GABAARs mediating tonic inhibition are high sensitivity to GABA and slow desensitization. Receptors containing the subunits α4δ in the dentate gyrus, α5 in CA1 pyramidal cells, and α6δ in the cerebellum are prominent examples of tonically activated GABAARs (Caraiscos et al., 2004; Farrant and Nusser, 2005). Yet, neither δ nor α5 subunits are strongly expressed in the amygdala. However, the amygdala is one of the brain structures with notably strong α3 subunit expression, along with the thalamic reticular nucleus (nRT) (Fritschy and Mohler, 1995). Targeted deletion of the Gabra3 gene (α3KO mice) produced only a mild phenotype (Winsky-Sommerer et al., 2008) with a deficit in sensorimotor information processing (Yee et al., 2005) and a powerful compensatory gain in the inhibitory postsynaptic response in the nRT (Schofield et al., 2009). Analysis of mutant mice carrying a histidine-to-arginine substitution rendering α3 GABAARs insensitive to diazepam (α3H126R) revealed a contribution to muscle relaxant but not to the sedative or anxiolytic effects of diazepam (Low et al., 2000; Crestani et al., 2001). In contrast, pharmacological studies with selective ligands suggested a possible role for α3 GABAARs in the anxiolytic action of benzodiazepine site ligands (Morris et al., 2006). Specifically, it was reported that an α3-selective inverse agonist is anxiogenic in rats (Atack et al., 2005) and that the α3-selective agonist TP003 produces anxiolytic effects in both rodent and nonhuman primate behavioral models of anxiety (Dias et al., 2005).
Given these controversial findings and the prominent expression of α3 GABAARs in the amygdala, we hypothesized that α3 GABAARs might play a role in tonic inhibition in this brain area.
Materials and Methods
Animals.
Generation of α3KO mice has been described previously in detail (Yee et al., 2005). Briefly, mice were maintained on a C57BL/6J background; hemizygous α3KO and WT male littermates were generated by crossing heterozygous mutant females with C57BL/6J males. Because the Gabra3 gene is located on the X chromosome, male mice carrying the KO allele (hemizygous) are effectively α3KO mice and were exclusively used in this study. GAD67-GFP transgenic mice were obtained from Yuchio Yanagawa (Gunma University, Maebashi City, Japan) (Tamamaki et al., 2003) and were maintained on a C57BL/6J background. C57BL/6J were obtained from Harlan Laboratories.
Electrophysiology.
Acute coronal brain slices (300–350 μm thick) were obtained from 21- to 49-d-old male mice of different genotypes, as described, using standard procedures (Marowsky et al., 2004). An upright microscope (Olympus BX51Wl) equipped with Nomarski differential interference contrast optics, an infrared videoimaging camera (VX55 Till Photonics), and a standard 100 W tungsten lamp connected to an epifluorescence system was used to visualize GFP-expressing interneurons (INs) in slices from GAD67-GFP mice. Experiments were performed at 32–34°C. During recording, slices were continuously superfused at 1–2 ml/min with artificial CSF (ACSF) containing the following (in mm): 125 NaCl, 26 NaHCO3, 1.25 NaH2PO4(H2O), 2.5 KCl, 1 MgCl2, and 2.5 CaCl2. The GABAB receptor blocker CGP 54626 (0.5 μm) was routinely added, as was kynurenic acid (2.5 mm), to block excitatory synaptic transmission. For the recordings of tonic currents and for the analysis of spontaneous IPSCs (sIPSCs), patch pipettes with tip resistances of 4–8 MΩ were filled with an internal solution containing the following (in mm): 100 CsCl, 40 HEPES, 2 MgCl2, 2 MgATP, 0.3 NaGTP, and 0.1 EGTA. Cells were voltage-clamped at −70 mV, so GABAAR-mediated currents were inward. For experiments studying the bicuculline (BIC) or TP003 effect on excitability, cells were recorded in perforated patch-clamp technique: tips were tip-filled with an internal solution containing (in mm) 130 K gluconate, 1 EGTA, 10 HEPES, 5 MgATP, 0.5 NaGTP, and 10 NaCl. The pipettes were then backfilled with the same internal solution and the pore-forming antibiotic gramicidin was added to give a final concentration of 100 μg/ml. The stock solution was prepared in methanol and contained a gramicidin concentration of 10 mg/ml (Rhee et al., 1994). After obtaining a gigaseal, the command potential was set at −60 mV, so that after perforation the cell was approximately at resting potential. When the capacitive current had stabilized (10–15 min after cell attachment), cells were taken into the current-clamp and the recording started. Evoked responses at 0.1 Hz were elicited by a patch electrode filled with ACSF. The tip was positioned in the LA or the BLA nucleus adjacent to the external capsule. If possible, the spiking threshold was established and stimulus intensity was then modified, such that action potentials were observed at a rate between 20 and 50%. BIC and TP003 were added to the external solution as indicated. Diazepam was provided by Hoffmann-La Roche. TP003 was purchased from ANAWA. All other chemicals were purchased from Sigma/Fluka or Tocris Bioscience.
Immunohistochemistry.
Distribution of the GABAAR α3, α4, α5, and δ subunits was visualized by immunoperoxidase staining of coronal sections from perfusion-fixed tissue of 4- to 12-week-old C57 Bl/6J mice. The following antibodies were used: homemade guinea pig anti-α3 and α5 subunit (Fritschy and Mohler, 1995), rabbit anti-α4 (PhosphoSolutions), and rabbit anti-δ (Millipore Bioscience Research Reagents). Specificity of all antibodies was verified by lack of staining in the corresponding KO mice (data not shown). Mice were perfused with 4% paraformaldehyde in 0.15 m sodium phosphate buffer, pH 7.4. Brains were postfixed for 3 h and incubated overnight in sodium citrate buffer, pH 4.5. After irradiation in a microwave oven, they were cryoprotected in 30% sucrose, frozen, and sectioned on a sliding microtome (40 μm). The subcellular localization of the α3 GABAAR subunit was visualized by immunofluorescence staining using gephyrin as a marker of postsynaptic sites. For a detailed description of this method, see Schneider Gasser et al. (2006).
Data analysis and statistics.
Data were recorded with a Multiclamp 700B amplifier (Molecular Devices), filtered at 3 kHz, and digitized at 20 kHz (A/D hardware, National Instruments). In all experiments, series resistance was monitored throughout the experiment by applying a hyperpolarizing pulse of 10 mV; if it changed by >20%, data were not included in the analysis. Data were acquired and analyzed with IGOR Pro software (Wave Metrics). Spontaneous events were detected with the Mini Analysis Program (Synaptosoft). For IPSC kinetics, averages of 30–50 sIPSCs per cell and condition were peak scaled and fit with the double exponential, I(t) = A1 * (exp(−t/τ1)) + A2 * (exp(−t/τ2)), with A1 and A2 as the fast and slow component amplitudes and τ1 and τ2 as their respective time constants. Tonic GABAAR-mediated current was defined as the shift in inward holding current (Ihold) after application of the GABAAR blocker BIC (Semyanov et al., 2003) and measured as described previously (Glykys et al., 2007; Krook-Magnuson et al., 2008). In short, 1 s streams of sIPSCs were sampled every 10 s. Traces were transcribed into all-point histograms, and points that fell on sIPSCs were discarded. The average values for baseline were calculated for six 1 s epochs before and for the BIC effect for six 1 s epochs after application of the blocker. A Gaussian distribution was fit to the all-points histogram, with the peak of the distribution determining the mean current for that sample. Total tonic currents were calculated from the difference of baseline and BIC mean holding current. Cell capacitance was calculated from the current transient obtained by giving a hyperpolarizing pulse of 10 mV at the beginning of each 1 s epoch. Results of several experiments are reported as average ± SEM. Simple and pairwise comparisons were performed with the appropriate two-tailed Student's t test or an ANOVA followed by Bonferroni post hoc test.
Results
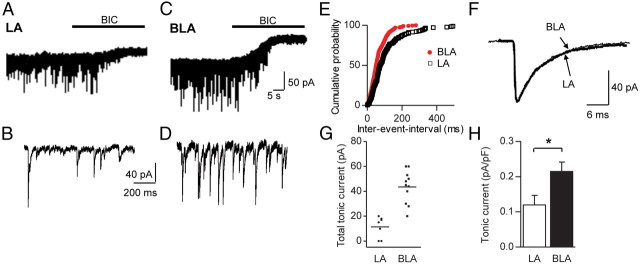
In LA and BLA PCs, application of the GABAAR antagonist BIC (25 μm) blocked sIPSCs and led to a decrease in Ihold, reflecting the presence of a tonically activated current (Fig. 1A,C). To correct for potential differences in cell size, the BIC-sensitive current was normalized to the capacitance of the respective cell (average capacitance of LA PCs 155 ± 22 pF, n = 7; BLA PCs 199 ± 16 pF, n = 11, p = 0.127, unpaired Student's t test, data not shown). Tonic currents were significantly larger in BLA than in LA PCs (Fig. 1H) (LA PCs: 0.105 ± 0.027 pA/pF, n = 5; BLA PCs: 0.215 ± 0.024 pA/pF, n = 11; p = 0.033, unpaired Student's t test). While we observed a tonic current in all BLA cells recorded, there was no shift in baseline current detectable in two of seven LA cells (Fig. 1G). Thus, when tonic current was calculated for all LA neurons, it was still present but was of smaller amplitude (0.088 ± 0.024 pA/pF, n = 7). A possible reason for the smaller tonic current in LA neurons might be a reduced activity of local GABAergic interneurons, resulting in reduced levels of ambient GABA. We therefore analyzed the frequency and amplitude of sIPSCs in LA and BLA PCs. Indeed, sIPSC frequency in LA neurons was significantly smaller than in BLA neurons (Fig. 1B,D,E; Table 1), whereas all other parameters, including sIPSC amplitude and kinetic variables, were similar (Fig. 1F; Table 1).
Figure 1.
Application of the GABAAR antagonist BIC reveals a tonic current in LA and BLA PCs. A, C, Recordings from LA and BLA PCs under control conditions [holding potential (Vh) = −70 mV, 2.5 mm kynurenic acid, 0.5 μm CGP54626]. Bath application of BIC (25 μm, black bar) leads to an outward shift in Ihold in both cells, consistent with the presence of a tonic current. Traces consist of 1 s epochs sampled every 10 s. B, D, Enlarged recordings from the traces from the LA and BLA cells above, showing sIPSCs. E, Cumulative probability plot of values of interevent intervals of LA and BLA cells. LA neurons display much higher interevent intervals, i.e., lower frequency, than their BLA counterparts. F, Average sIPSCs from LA and BLA PCs showing similar kinetics. G, Scatter plots illustrating the amplitudes of total tonic current in LA and BLA PCs with horizontal lines representing mean amplitude. Note that all BLA, but not all LA, PCs express a tonic current. H, Graph showing the comparison of tonic currents normalized to cell capacitance in LA and BLA PCs. For the average of LA PCs, only those cells displaying tonic current were considered. BLA PCs display a significantly higher tonic current than their LA counterparts. Unpaired Student's t test, *p < 0.05. Exact p values are given in the main text. Error bars represent SEM.
Table 1.
Comparison of sIPSC parameters in WT and α3KO PCs under various conditions
| Genotype | IPSC parameters |
||||
|---|---|---|---|---|---|
| Frequency (Hz) | Amplitude (pA) | Rise time (ms) | t1 (ms) | t2 (ms) | |
| α3KO | |||||
| LA PC (7) | 9.9 ± 1.4* | 32.3 ± 3.4 | 0.99 ± 0.18 | 5.08 ± 0.94 | 10.37 ± 1.13 |
| BLA PC (10) | 18.4 ± 2.5 | 37.3 ± 5.8 | 0.94 ± 0.06 | 4.19 ± 0.59 | 9.21 ± 0.59 |
| 0.5 μm TP003 (5) | 15.5 ± 3.1 | 50.6 ± 8.4 | 0.92 ± 0.05 | 5.12 ± 1.01 | 10.9 ± 2.1 |
| 1 μm diazepam (5) | 16.0 ± 1.8 | 71.0 ± 12.3* | 0.81 ± 0.07 | 7.1 ± 1.3 | 25.4 ± 2.7*** |
| WT | |||||
| LA PC (8) | 9.3 ± 1.7* | 37.2 ± 5.6 | 0.94 ± 0.04 | 5.47 ± 0.49 | 9.08 ± 1.14 |
| BLA PC (13) | 15.0 ± 1.6 | 41.5 ± 3.9 | 0.89 ± 0.05 | 5.02 ± 0.46 | 10.52 ± 0.77 |
| 0.5 μm TP003 (5) | 11.3 ± 2.1 | 43.4 ± 3.5 | 0.88 ± 0.04 | 5.40 ± 1.2 | 12.56 ± 2.2 |
| 1 μm diazepam (5) | 16.6 ± 1.6 | 62.4 ± 1.1** | 0.88 ± 0.07 | 6.8 ± 1.2 | 33.85 ± 8.3*** |
Values (mean ± SEM) significantly different from the control (solvent treated BLA principal cells of the respective genotype) are indicated with *p < 0.05, **p < 0.01, and ***p < 0.001 (Student's unpaired t test). Numbers of recorded neurons are given in parentheses. Bold indicates control values.
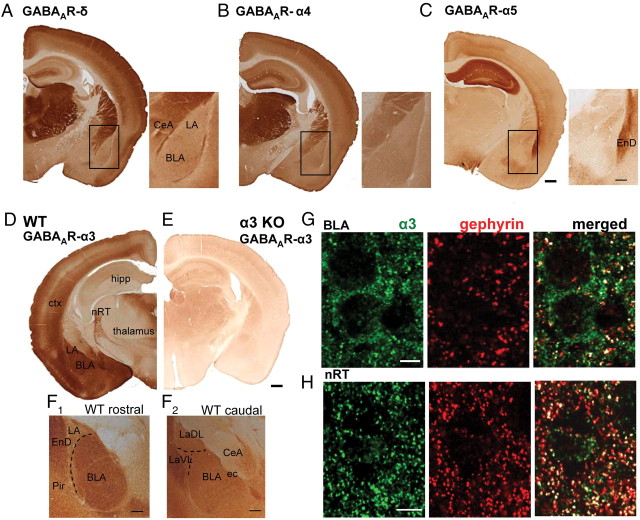
We next wanted to know whether tonic currents in LA and BLA PCs are sufficiently large to affect the excitability of these cells. Because these neurons are rarely spontaneously active in slices, we used extracellular stimulation and placed a stimulation electrode adjacent to the cortical fiber bundle, termed the external capsule, which outlines the LA and BLA nuclei. Recordings were made using the perforated patch-clamp technique with an internal solution containing the pore-forming antibiotic gramicidin, such that the internal milieu and the chloride concentration gradient across the cell membrane remained undisturbed. Application of BIC (25 μm) increased the success rate of spiking and decreased the resting membrane potential (Vm) significantly in all BLA neurons but only in three of five LA neurons (Fig. 2A–D). Thus, BIC led to a significant change only in BLA PCs [BLA PCs: Vm before (con): −66.3 ± 0.9 mV vs BIC −63.0 ± 0.6 mV (n = 6, p = 0.0012); success rate of spiking con: 0.27 ± 0.12 spikes/stimulus vs BIC 2.64 ± 0.28 spikes/stimulus (n = 6, p = 0.00428); LA PCs: Vm con: −65.8 ± 1.6 mV vs BIC −64.5 ± 1.4 mV (n = 5; p = 0.081); success rate of spiking con: 0.54 ± 0.08 spikes/stimulus vs BIC 0.71 ± 0.29 spikes/stimulus (n = 5, p = 0.518); paired Student's t test for all comparisons]. This indicated that tonic currents in the BLA nucleus, but only to a certain degree in the LA nucleus, are effective modulators of excitability in PCs. However, these results should be cautiously interpreted because BIC blocks nonselectively phasic feedforward inhibition and tonic inhibition. Tonic currents are usually mediated by extrasynaptic GABAARs containing either the α4/δ or the α5 subunit. When we studied the expression pattern of these subunits in C57BL/6J mouse by immunohistochemistry, we detected only weak immunoreactivity for these subunits (α4, δ, α5) in mouse amygdala (Fig. 3A–C). These findings are consistent with what has been reported for rat (Fritschy and Mohler, 1995; Pirker et al., 2000). However, strong immunoreactivity was observed for the α3 GABAAR subunit (Fig. 3D,F1,F2), which was completely absent in slices of a α3 GABAAR KO mice (Fig. 3E), confirming the specificity of the antibody. On rostral sections, the staining was markedly weaker in the LA nucleus compared with the BLA nucleus (Fig. 3F1). Yet this difference did not persist in more caudal slices (Fig. 3F2) in which homogenous and strong α3 subunit staining was observed. Furthermore, colabeling with the postsynaptic marker gephyrin revealed that the α3 subunit is localized preferentially extrasynaptically throughout the BLA nucleus because only few α3 clusters colocalized with gephyrin (Fig. 3G, right). This is in strong contrast to the nRT, in which most α3 clusters colocalized with gephyrin (Fig. 3H, right), consistent with functional data reporting α3-specific synaptic transmission and the absence of tonic current in nRT neurons (Cope et al., 2005).
Figure 2.
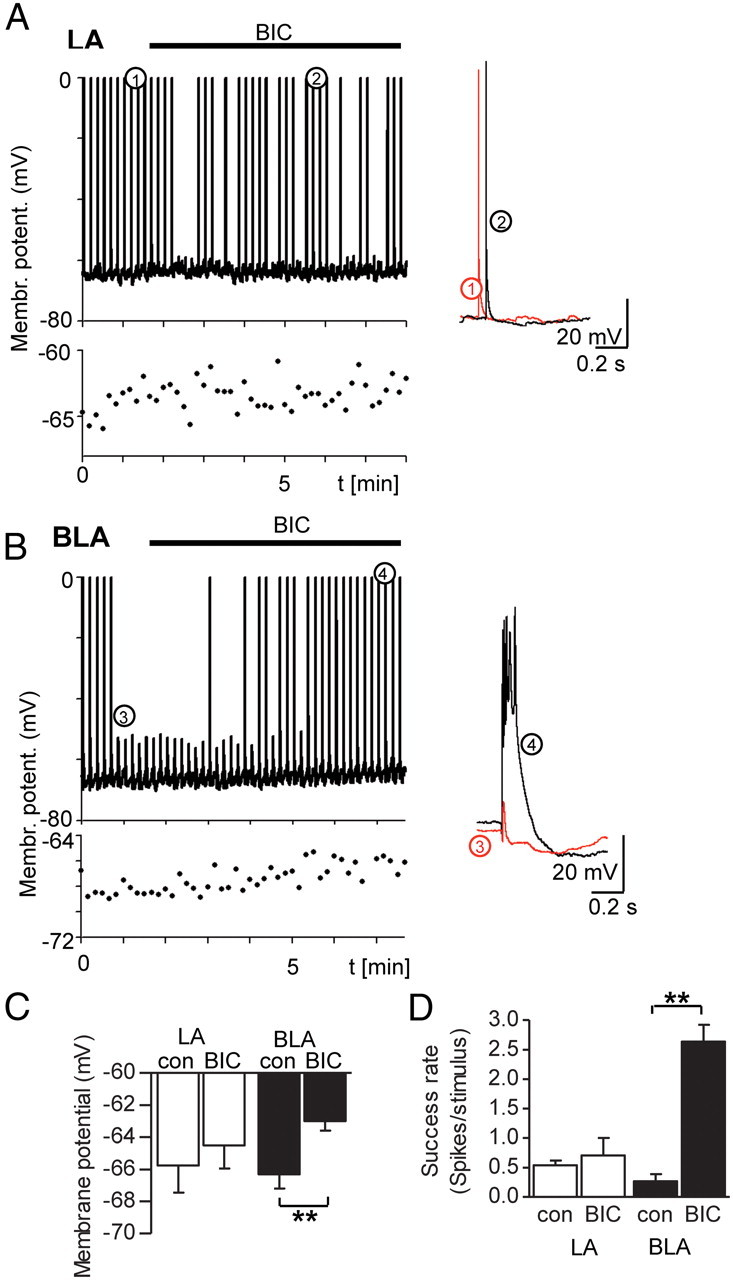
Blocking of tonic GABAergic inhibition increases excitability significantly in BLA, but not in LA, PCs. A, Trace of an LA PC, recorded in perforated patch-clamp technique with a gramicidin-containing internal solution. Below are the values for membrane potential, recorded at each stimulation event, with an enlarged millivolt scale. The time scale for the two graphs is the same. Extracellular stimulation is given at 0.1 Hz. Bath application of BIC (25 μm, black bar) leads to no significant difference in spike frequency and resting membrane potential. Events before and after BIC application, denoted by 1 and 2, are enlarged and shown superimposed to the right. B, Trace of a BLA PC, recorded under conditions identical to those of the cell in A. Below are the values for membrane potential, recorded at each stimulation event. Events before and after BIC application, denoted by 3 and 4, are enlarged and shown superimposed to the right. Application of BIC (25 μm, black bar) leads to a significant change in resting membrane potential and concomitant change in the success rate of spiking. C, Graph showing the difference for LA and BLA PCs in membrane potential before and after the application of BIC. D, Graph showing the change in the success rate of spiking before and after the application of BIC for LA and BLA PCs. **p < 0.01, paired Student's t test (C, D). Error bars represent SEM.
Figure 3.
The GABAAR α3 subunit is strongly expressed throughout LA and BLA nuclei of the amygdala and is located preferentially at extrasynaptic sites. A–C, Coronal sections from WT mice stained for the GABAAR subunits δ, α4, and α5. For each staining the amygdala is indicated by a rectangular box and is shown enlarged on the right. Scale bars: (in C) thick, 0.5 mm; (in C) thin, 0.1 mm. D, Coronal section from a WT mouse stained for the GABAAR subunit α3. Note the strong immunoreactivity in the cortex (ctx), the LA and BLA nuclei of the amygdala, and the nRT, whereas no or only weak immunoreactivity can be observed in the hippocampus (hipp) and thalamus. E, Coronal section from an α3KO mouse stained for the GABAAR subunit α3. No specific staining is observable, confirming the specificity of the antibody. Scale bar, 0.5 mm. F1, Enlargement of a more rostrally located coronal slice of a WT mouse stained for the GABAAR subunit α3, showing the LA and BLA nuclei of the amygdala and the adjacent EnD and Pir. Note the weaker staining in the LA nucleus compared with the BLA nucleus for this plane. Scale bar, 0.1 mm. F2, Enlargement of a more caudally located coronal slice from a WT mouse stained for the GABAAR subunit α3. Homogeneously strong staining for the α3 subunit is visible throughout the LaDL and LaVL nucleus, making up the LA nucleus, and the BLA nucleus. Scale bar, 0.1 mm. G, α3 GABAAR labeling (green) in BLA cells colocalizes only partially with the postsynaptic marker gephyrin (red), evident by the poor colocalization shown in white (right). This suggests that this GABAAR subunit is preferentially present at extrasynaptic sites. Scale bar, 5 μm. H, In nRT cells, α3 GABAAR labeling (green) colocalizes well with the postsynaptic marker gephyrin (red), as seen in the colocalization panel in white on the right. Scale bar, 5 μm. CeA, Central nucleus of the amygdala; ctx, cortex; ec, external capsule; EnD, endopiriform nucleus; hipp, hippocampal formation; LaDL, dorsolateral part of the LA nucleus; LaVL, ventrolateral part of the LA nucleus; Pir, piriform cortex.
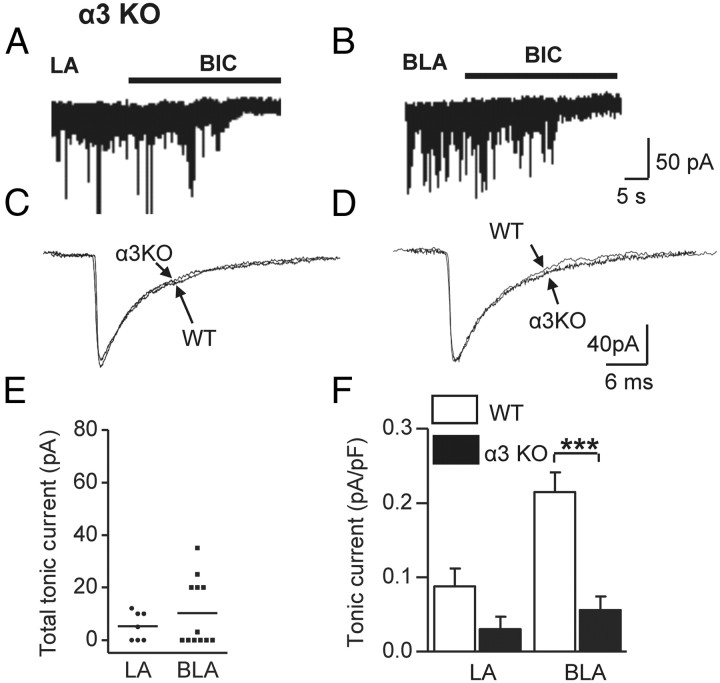
To obtain functional evidence for the involvement of the α3 subunit in tonic inhibition, we recorded tonic currents from LA and BLA PCs in α3KO slices (Fig. 4A,B) and compared them with those obtained in WT PCs. sIPSC amplitude, frequency, and kinetics were similar between WT and α3KO cells (Fig. 4C,D; Table 1); thus, we did not detect any evidence for the involvement of α3 GABAARs in synaptic transmission. Six of 12 BLA α3KO cells showed no reduction of Ihold on application of BIC; in the remaining six, the amplitude was substantially smaller than in the respective WT cells (Fig. 4E). In the LA nucleus, three of seven α3KO cells expressed no tonic current at all. Two-way ANOVA revealed that a highly significant difference between genotypes exists exclusively for the BLA nucleus (Fig. 4F) [BLA: WT 0.215 ± 0.026 pA/pF (n = 11) vs α3KO 0.056 ± 0.018 pA/pF (n = 12, t = 5.314, p < 0.001); LA: WT 0.088 ± 0.024 pA/pF vs α3KO 0.030 ± 0.017 pA/pF (both n = 7, t = 1.634, p > 0.05)]. These findings suggested that α3 GABAAR-mediated currents contribute strongly to tonic inhibition in the BLA, while their degree of contribution in the LA nucleus remains unclear.
Figure 4.
Tonic currents in BLA PCs are significantly reduced in slices from α3KO mice. A, B, Traces from representative LA and BLA cells from α3KO mice under control conditions [holding potential (Vh) = −70 mV, 2.5 mm kynurenic acid, 0.5 μm CGP54626] before and after bath application of BIC (25 μm, black bar). Only a weak outward shift in Ihold is visible in both cells. Traces consist of 1 s epochs sampled every 10 s. C, Average sIPSCs from an α3KO and a WT LA PC showing similar kinetics. D, The same as in C but for a BLA PC. E, Scatter plots illustrating the amplitude of total tonic current in α3KO cells. Note that half the BLA cells express no tonic current at all, and the residual cells show reduced amplitudes. F, Graph showing the comparison of tonic currents between WT and α3KO cells for the LA and BLA nuclei. All cells, including those without tonic current, were considered. ***p < 0.001, two-way ANOVA. Calibrations in B also apply to A. Calibrations in D also apply to C. Error bars represent SEM.
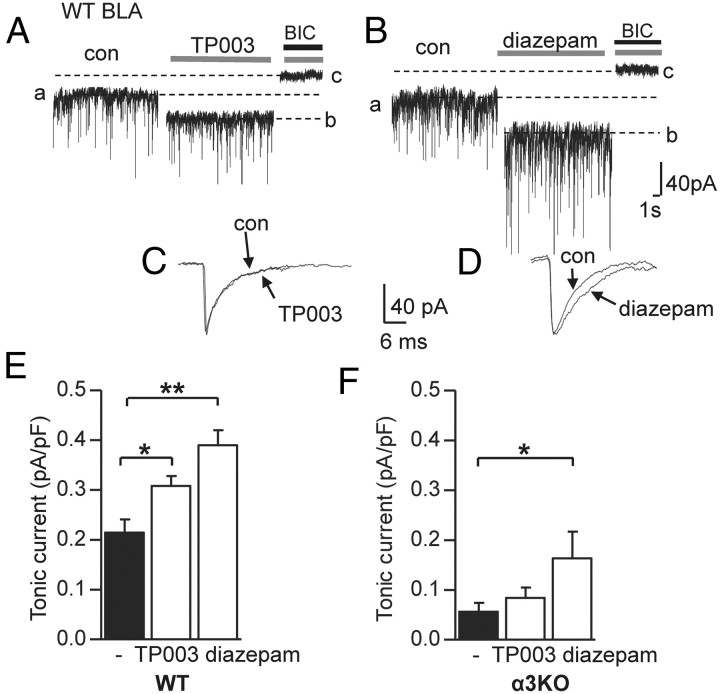
To further characterize tonic currents in the BLA nucleus, we applied the α3 GABAAR selective benzodiazepine agonist TP003 and, for comparison, the nonselective benzodiazepine diazepam, followed by BIC (Fig. 5A,B). Given that TP003-induced potentiation of GABA currents at α3 GABAARs reaches maximal values at a concentration of 10−7 m (Dias et al., 2005), 0.5 μm TP003 was used in all experiments. Both drugs significantly increased tonic current in WT BLA cells (Fig. 5E) [BIC: 0.215 ± 0.026 pA/pF (n = 11) vs TP003/BIC 0.308 ± 0.016 pA/pF (n = 6) vs diazepam/BIC 0.387 ± 0.029 pA/pF (n = 9), one-way ANOVA p = 0.0023, followed by Bonferroni post hoc test; BIC vs TP003/BIC (t = 3.62, p < 0.05); BIC vs diazepam/BIC (t = 5.92, p < 0.01); TP003/BIC vs diazepam/BIC (t = 2.59, p > 0.05)]. Diazepam also markedly increased sIPSC amplitude and decay time (Fig. 5B,D; Table 1), most likely affecting synaptically located α1 and α2 GABAARs (Marowsky et al., 2004). In contrast, TP003 had no such effect (Fig. 5A,C; Table 1), which confirms the negligible role for α3 GABAARs in synaptic transmission. To verify the selectivity of TP003 on tonically active α3 GABAAR and to investigate further the difference between TP003 and diazepam effects, the compounds, together with BIC, were also applied to BLA PCs from α3KO mice. TP003 produced no effect compared with BIC alone (Fig. 5F), underscoring the selectivity of the compound. However, diazepam-induced potentiation of tonic currents in these cells was significantly stronger than that observed with BIC alone. This provides further evidence that there are GABAARs other than α3 GABAARs carrying tonic currents in BLA PCs [α3KO: BIC 0.056 ± 0.017 pA/pF (n = 12) vs TP003/BIC 0.084 ± 0.020 pA/pF (n = 4) vs diazepam/BIC 0.172 ± 0.054 pA/pF (n = 4), one-way ANOVA p = 0.0032, followed by Bonferroni post hoc test; BIC vs TP003/BIC (t = 0.822, p > 0.05); BIC vs diazepam/BIC (t = 2.71, p < 0.05); TP003/BIC vs diazepam/BIC (t = 1.64, p > 0.05)]. Furthermore comparison of diazepam-induced potentiation in WT and α3KO cells showed that the potentiation is more than doubled in WT cells [WT: 0.387 ± 0.029 pA/pF (n = 9) vs a3KO: 0.172 ± 0.054 pA/pF (n = 4, p = 0.0079), unpaired Student's t test, comparison not shown].
Figure 5.
Tonic currents in BLA PCs are sensitive to the α3-selective compound TP003 and to the nonselective benzodiazepine diazepam. A, B, Representative traces of a BLA PC before and after bath application of TP003 (0.5 μm, gray bars) and diazepam (1 μm, gray bars), respectively, followed by bath application of BIC (25 μm, black bar). For amplitudes of tonic currents, the difference in Ihold under TP003 or diazepam (b) and BIC (c) was calculated. Note the increase in sIPSC amplitude under diazepam (b). C, Enlarged traces of average sIPSCs recorded before and after application of TP003, showing no difference. D, Enlarged traces of average sIPSC recorded before and after diazepam application. sIPSCs were peak scaled so only the diazepam-typical increase in decay time, but not in amplitude, is visible. E, Graph showing tonic current amplitudes recorded in WT BLA PCs under TP003 and diazepam (white bars) compared with BIC alone (black bar). Both compounds increase tonic current markedly. F, Graph showing the tonic current amplitudes recorded in α3KO BLA PCs under TP003 and diazepam (white bars) compared with BIC (black bar) alone. Only diazepam, but not TP003, increases tonic currents in these cells, confirming the selectivity of the compound and indicating that GABAAR variants other than α3 GABAARs also carry tonic current in BLA PCs. *p < 0.05, **p < 0.01, one-way ANOVA followed by Bonferroni post hoc test (E, F). Calibrations in B also apply to A. Calibrations in C also apply to D. Error bars represent SEM.
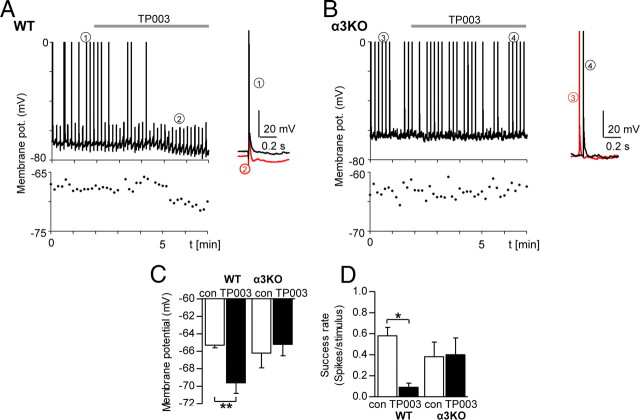
Given that BLA PCs express an α3 GABAAR-mediated tonic current that can be modulated by TP003, the compound should also be capable of influencing the excitability of these cells. To study the effect of TP003 on excitability, BLA PCs from WT and α3KO mice were again recorded using the perforated patch-clamp technique with a tip filled with a gramicidin-containing internal solution, and extracellular stimulation was given at 0.1 Hz. Application of 0.5 μm TP003 significantly reduced the Vm and the success rate of firing in five of seven BLA PCs (Fig. 6A,C,D), whereas no effect was observed in α3KO PCs (Fig. 6B–D) [WT Vm: con −65.5 ± 0.3 mV vs TP003 −69.6 ± 0.3 mV (n = 7, p = 0.0041); α3KO Vm: con −66.2 ± 1.7 mV vs TP003 −65.2 ± 1.3 mV (n = 4, p = 0.312); success rate of spiking: WT con 0.58 ± 0.08 spikes/stimulus vs TP003 0.09 ± 0.04 spikes/stimulus (n = 7, p = 0.0107); α3KO con 0.38 ± 0.14 spikes/stimulus vs TP003 0.39 ± 0.15 spikes/stimulus (n = 4, p = 0.759), paired Student's t test for all comparisons]). Thus, TP003 leads indeed to a reduced excitability in WT BLA PCs.
Figure 6.
The α3-selective compound TP003 lowers resting membrane potential and decreases the success rate of spiking in WT but not in α3KO BLA PCs. A, Graph of a WT BLA PC before and after the application of TP003 (0.5 μm, gray bar). Cells were recorded in perforated patch-clamp technique with a gramicidin-containing internal solution. Extracellular stimulation was given at 0.1 Hz. Single events denoted with 1 and 2 are enlarged and shown superimposed to the right. Below are the values for membrane potential, recorded at each stimulation event, with an enlarged mV scale. B, The same graphs as in A but for a α3KO BLA PC. C, Graph showing the comparison of membrane potential for WT and α3KO BLA PCs before and after the application of TP003. D, Graph showing the comparison of the success rate of spiking for WT and α3KO BLA PCs before and after TP003 application. *p < 0.05, **p < 0.01, paired Student's t test. Error bars represent SEM.
Because tonic inhibition in the hippocampus is thought to be cell type specific with a distinct difference between interneurons and pyramidal cells (Semyanov et al., 2003), we wondered whether there is a similar difference between amygdala PCs and interneurons. Interneurons are difficult to identify in the amygdala; hence, we used mice expressing GFP under the interneuron-specific GAD67 promoter (Tamamaki et al., 2003). Two main populations of GABAergic interneurons can be distinguished in the amygdala: local interneurons scattered throughout the LA nucleus and the BLA nucleus (McDonald, 1982) and those residing in dense clusters along the lateral and medial borders of the nuclei, termed paracapsular cells (Fig. 7A) (Marowsky et al., 2005). Application of BIC (25 μm) revealed a tonic current in both populations of interneurons (Fig. 7B,D), though with considerable differences in total amplitude (Fig. 7E). However, after normalization to capacitance to correct for different cell sizes, the relative amplitudes showed comparable values (Fig. 7F) due to LA/BLA interneurons having on average a three times higher capacitance than the extremely small paracapsular cells [tonic current: LA/BLA interneurons: 0.143 ± 0.014 pA/pF (n = 11) vs paracapsular cells 0.160 ± 0.02 pA/pF (n = 13, p = 0.509), unpaired Student's t test (Fig. 7G); capacitance: LA/BLA interneurons 141 ± 15pF vs paracapsular cells 56 ± 3pF (p = 0.000064), unpaired Student's t test, data not shown]. To investigate whether α3 GABAARs also underlie tonic current in GABAergic interneurons, we applied again the selective α3-selective benzodiazepine site agonist TP003, followed by BIC (Fig. 7H,I). TP003 led to heterogeneous results in LA/BLA interneurons: tonic current increased markedly in only one of seven cells, whereas in most of these cells no significant shift of Ihold was observed. When the average of all cells was considered, no difference was seen between the tonic currents revealed under BIC alone compared with TP003/BIC (Fig. 7J) [BIC 0.143 ± 0.014 pA/pF (n = 11) vs BIC/TP003 0.127 ± 0.02 pA/pF (n = 7, p = 0.555), unpaired Student's t test]. This indicates that α3 GABAAR-mediated tonic currents play a role only in a small fraction of these interneurons. In paracapsular cells, results were similar, with no significant potentiation of tonic currents in the presence of TP003 (Fig. 7J) [BIC 0.160 ± 0.020 pA/pF (n = 13) vs TP003/BIC 0.145 ± 0.006 pA/pF (n = 5, p = 0.664), unpaired Student's t test]. There were also no major changes seen in phasic inhibitory signaling because the frequency and amplitude of sIPSCs did not alter significantly after the application of TP003. Furthermore, waveforms of averaged sIPSC were similar (Fig. 7H,I) [sIPSC frequency: con 7.2 ± 0.9 Hz vs TP003 6.9 ± 1.4 Hz (n = 5, p = 0.778), paired Student's t test; sIPSC amplitude: con 37.5 ± 4.8 pA vs TP003 40.3 ± 6.6 pA (n = 5, p = 0.651), paired Student's t test, data not shown]. Because TP003 generated no significant current shift or change in Ihold in either population of interneurons, we concluded that primarily other GABAAR subtypes generate tonic conductance in this cell type. Consistent with this, a final comparison of the TP003-induced change of tonic currents between LA/BLA interneurons, paracapsular cells, and BLA PCs showed that TP003 leads indeed exclusively in BLA PCs to a significant increase in tonic current (Fig. 7K) [BLA PC: 143 ± 8% (n = 6); LA/BLA IN: 89 ± 17% (n = 7); paracapsular cells 91 ± 4% (n = 5), one-way ANOVA followed by Bonferroni post hoc test; BLA PCs vs LA/BLA IN (t = 3.22, p < 0.05); BLA PCs vs paracapsular cells (t = 2.872, p < 0.05); LA/BLA IN vs paracapsular cells (t = 0.0899, p > 0.05)].
Figure 7.
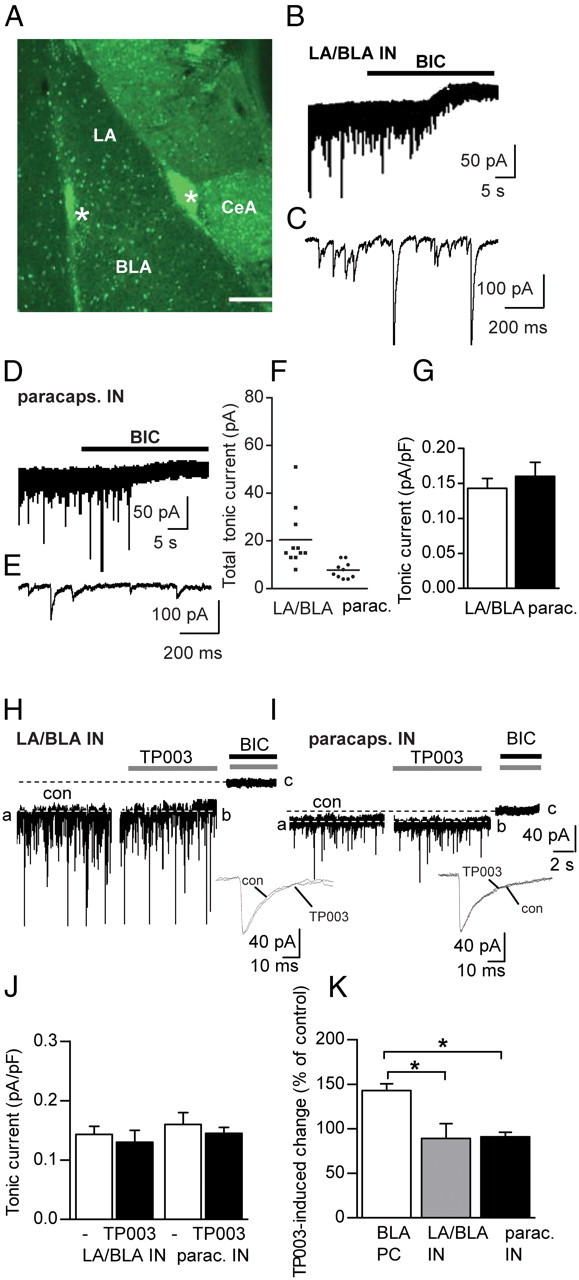
Amygdala interneurons also express a tonic conductance that is mediated primarily by GABAAR variants other than α3 GABAAR. A, Coronal slice obtained from a GAD67 GFP mouse showing the amygdala. Interneurons, identified by their GFP expression, were classified into two main populations: those residing in LA and BLA nuclei, termed LA/BLA interneurons, and those residing in dense clusters along the lateral and medial border, termed paracapsular cells, marked with asterisks. Scale bar, 200 μm. B, Representative trace of an LA/BLA interneuron before and after bath application of BIC (25 μm, black bar), revealing the presence of tonic current. Trace consists of 1 s epochs sampled every 10 s. C, Enlarged trace of B showing sIPSC. D, Representative trace of a paracapsular interneuron before and after the application of BIC (25 μm, black bar), revealing a tonic current. E, Enlarged trace of D showing sIPSC. F, Amplitudes of total tonic currents recorded in the two interneuron populations, with horizontal lines representing the mean amplitude. Note the small values for paracapsular cells. G, Comparison of tonic currents of the two amygdala interneuron populations normalized to cell capacitance, resulting in similar values for LA/BLA interneurons and paracapsular cells. H, Trace of an LA/BLA interneuron treated with TP003 (0.5 μm, gray bar), followed by BIC (25 μm, black bar). For amplitudes of TP003-modulated tonic currents, the difference in Ihold between b and c was calculated. Below are shown average sIPSC from control and TP003 trace, displaying similar kinetics. I, The same as in H for a paracapsular interneuron. J, Graph showing the comparison of the amplitudes of TP003-modulated tonic current (black bars) and those obtained under BIC alone (white bars) for LA/BLA and paracapsular interneurons. TP003 has no effect on tonic currents in amygdala interneurons. K, Graph showing the comparison of TP003 effect on BLA PCs, LA/BLA, and paracapsular interneurons, normalized to the BIC effect in the respective cell type. The α3-selective compound TP003 enhances exclusively the tonic current in BLA PCs. *p < 0.05, one-way ANOVA followed by Bonferroni post hoc test. CeA, Central nucleus of the amygdala; ec, external capsule. Calibrations in I also apply to H. Error bars represent SEM.
Discussion
Here we show, for the first time, that tonic currents are present in the majority of LA and BLA PCs and also in GABAergic interneurons located in the LA and BLA nuclei and in the paracapsular cells of the intercalated nuclei. In BLA PCs, a substantial part of tonic current is α3 GABAAR mediated. These receptors are sensitive to benzodiazepine site ligands such as diazepam and the α3-selective agonist TP003. Blocking the tonic inhibitory current of BLA PCs with BIC results in depolarization and higher excitability, whereas enhancing it with TP003 shifts the resting membrane potential to more negative values and concomitantly reduces excitability. In contrast, tonic currents in interneurons seem mediated primarily by GABAAR variants other than α3 GABAARs.
Converging immunohistochemical and electrophysiological data support our conclusion that α3 GABAARs play a central role in tonic inhibition in the BLA nucleus. In α3KO mice, BLA PCs were markedly deficient in tonic currents, with BIC-sensitive tonic currents reduced to 35% compared to WT cells. Furthermore, with the exception of the most rostral slices with weaker immunoreactivity in the LA nucleus, immunohistochemical stainings show overall strong α3 subunit immunoreactivity in this brain area. Moreover, α3 clusters preferentially do not colocalize with the synaptic marker gephyrin in this brain area and are thus most likely localized predominantly at extrasynaptic sites. Finally, the α3-selective benzodiazepine site agonist TP003 led to a substantial increase of tonic currents in WT BLA PCs but had no effect in α3KO BLA PCs. This TP003-induced increase in tonic inhibition in BLA WT cells was sufficient to dampen, or even block completely, the spiking activity of these cells.
Blocking inhibitory currents by the competitive GABAAR blocker BIC led to a more pronounced depolarization and increase in excitability in BLA compared with LA PCs, in line with the stronger tonic currents observed in BLA cells. However, BIC (25 μm) blocks phasic as well as tonic inhibitory currents, as seen in our experiments and those of others (Bai et al., 2001; Semyanov et al., 2003). Thus, the effect on membrane potential and excitability cannot be solely ascribed to the blocking of tonic currents, more so because sIPSC frequencies are high in the BLA nucleus, providing for strong phasic inhibition, whereas they are markedly lower in the LA nucleus. Other studies tried to selectively address tonic GABAergic currents by applying extremely low concentrations of GABAAR blockers such as gabazine (Cope et al., 2005) or picrotoxin (Semyanov et al., 2003). In the amygdala, however, gabazine mainly affects phasic inhibition, while no effect was observed with low concentrations of picrotoxin (1 μm) (data not shown). Thus, selective blocking of GABAergic tonic currents seemed not possible.
We observed total tonic current amplitudes between 10 pA (paracapsular cells) and 40 pA (BLA PCs), in line with another study (Olmos-Serrano et al., 2010), which reported tonic currents of 20 pA for BLA PCs. We believe, however, that our apparent tonic current amplitudes are rather an underestimation because GABA levels in slices are probably much lower than those existing in vivo (Lerma et al., 1986). Furthermore, we used GAD67 GFP mice (Tamamaki et al., 2003), which lack one allele for the conversion from glutamate to GABA. In the cortex of these mice, a 30% lower ambient GABA concentration was observed compared with WT littermates (Morishima et al., 2010).
Tonically activated α3 GABAARs
GABAARs with a benzodiazepine binding site contain an α1, α2, α3, or α5 subunit and a γ2 subunit (Sigel and Buhr, 1997). Only one of the two α subunits forms the benzodiazepine binding site with the γ2 subunit because the subunits are arranged in alternating order, α-β-α-β-γ. Among α3 GABAARs, those with two different types of α subunits seem to predominate. In crude brain extracts from mice, the total population of α3 GABAARs consists only of 27% homologous α3α3, but 56% heterologous α3α1 and 19% α3α2 [α1 GABAARs: 84% α1α1; α2 GABAARs: 46% α2α2 (Benke et al., 2004)]. Given that TP003 acts on the benzodiazepine binding site (Dias et al., 2005), the tonically active α3 GABAARs in BLA PCs most likely contain an α3 subunit at this binding site.
In general, high GABA affinity and slow desensitization are deemed crucial for the generation of tonic chloride currents. Although slowly decaying responses and slow desensitization kinetics are commonly accepted as typical for α3 GABAARs (Barberis et al., 2007), at present it is unclear to which degree these receptors are endowed with high GABA affinity. In earlier studies, Verdoorn (1994) and Gingrich et al. (1995) concluded that recombinant receptors homomeric for the α3 subunit [α3(β2γ2)] had less affinity for GABA than α1α3(β2γ2) and α1(β2γ2) receptors. Recently, Keramidas and Harrison (2010) reported opposite results, specifically, that GABA binds with higher affinity to the α3β2γ2S than to the α1β2γ2S channel. However, all these studies were conducted with recombinant receptors in heterologous cell systems, which might yield results that do not correspond to those observed in native neurons.
Although a substantial part of tonic currents in BLA PCs is α3 GABAAR mediated, our data also indicate that other GABAARs subtypes are involved. Candidates for other tonically active GABAARs are α4 and δ GABAARs because these subunits are weakly but ubiquitously expressed throughout LA and BLA nuclei (Fig. 3A,B). α5 Subunit immunoreactivity, albeit generally weak in the amygdala compared with the hippocampus, is stronger throughout the LA nucleus than the BLA nucleus (Fig. 3C), and α5 GABAARs might thus contribute substantially to tonic currents in this nucleus.
Physiological importance
The dominance of α3 GABAARs on PCs opens the possibility of exclusively modulating the excitability of this cell type, in particular cells located in the BLA nucleus. Application of TP003 should reduce the excitability in BLA PCs while it leaves those in interneurons intact, thus resulting as net outcome in a marked increase in inhibitory tone in the BLA nucleus. The BLA nucleus is considered a central site for emotional memory and regulation of anxiety (McGaugh et al., 2002), where inhibition decreases and excitation increases anxiety-like behavior (Sajdyk and Shekhar, 1997). Consequently, benzodiazepines injected directly into the BLA nucleus show an anxiolytic effect (Sanders and Shekhar, 1995a). The TP003-induced raise in inhibitory tone in the BLA nucleus should, therefore, also lead to anxiolysis. Indeed, systemic application of TP003 was reported to produce a robust anxiolytic-like effect in behavioral paradigms such as the elevated plus maze (Dias et al., 2005), which we assume is at least partly due to the increased tonic currents in BLA PCs. Furthermore, because these α3 GABAARs are diazepam sensitive, they should strongly contribute to the observed benzodiazepine-induced anxiolysis.
The role of α3 GABAARs in anxiolysis is controversial, however. Löw et al. (2000) reported that exclusively α2 GABAARs mediate the anxiolytic effects of diazepam, using mice carrying a point mutation in the α1, α2, or α3 subunit, which rendered the respective GABAAR diazepam insensitive. A possible contribution of α3 GABAARs to anxiolysis might have been overlooked, though, because of the peculiarity of α3 GABAARs, which preferentially contain two different α subunits. Given that the nonmutated α subunit prefers the position at the benzodiazepine binding site (Benke et al., 2004), the α1 (or perhaps the α2) subunit is likely to occupy the position at the benzodiazepine binding site. Consequentially, mutated α3 GABAARs are diazepam sensitive because of the presence and pharmacological dominance of the non-α3 subunit in these GABAARs. Under such circumstances, a potential involvement of α3 GABAARs in anxiolysis would have remained undetected.
Together, α3 GABAAR-mediated tonic currents contribute critically to inhibition in the BLA nucleus and to a far lesser extent in the LA nucleus and present an essential lever with which to modulate neuronal excitability. Given that the tonically activated α3 GABAARs are strongly modulated by anxiolytic compounds such as diazepam and TP003, these receptors are important elements of the inhibitory network mediating the anxiolytic effects in the amygdala.
Footnotes
This work was supported by Swiss Nation Funds. We thank Séverine Gilloz for excellent technical assistance, and Urs Gerber and Kaspar Vogt for critical reading and helpful comments.
The authors declare no competing financial interests.
References
- Aggleton J. The amygdala: a functional analysis. Ed 2. New York: Oxford UP; 2000. [Google Scholar]
- Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford K, McKernan RM, Dawson GR. Anxiogenic properties of an inverse agonist selective for alpha3 subunit-containing GABA A receptors. Br J Pharmacol. 2005;144:357–366. doi: 10.1038/sj.bjp.0706056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Barberis A, Mozrzymas JW, Ortinski PI, Vicini S. Desensitization and binding properties determine distinct alpha1beta2gamma2 and alpha3beta2gamma2 GABA(A) receptor-channel kinetic behavior. Eur J Neurosci. 2007;25:2726–2740. doi: 10.1111/j.1460-9568.2007.05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J Biol Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, et al. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring GABA(A) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- Keramidas A, Harrison NL. The activation mechanism of alpha1 beta2gamma2S and alpha3beta3gamma2S GABAA receptors. J Gen Physiol. 2010;135:59–75. doi: 10.1085/jgp.200910317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson EI, Li P, Paluszkiewicz SM, Huntsman MM. Tonically active inhibition selectively controls feedforward circuits in mouse barrel cortex. J Neurophysiol. 2008;100:932–944. doi: 10.1152/jn.01360.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martín del Río R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus: a method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE. Functional mapping of GABA A receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–1289. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- Morishima T, Uematsu M, Furukawa T, Yanagawa Y, Fukuda A, Yoshida S. GABA imaging in brain slices using immobilized enzyme-linked photoanalysis. Neurosci Res. 2010;67:347–353. doi: 10.1016/j.neures.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23:2495–2504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ebihara S, Akaike N. Gramicidin perforated patch-clamp technique reveals glycine-gated outward chloride current in dissociated nucleus solitarii neurons of the rat. J Neurophysiol. 1994;72:1103–1108. doi: 10.1152/jn.1994.72.3.1103. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Res. 1991;567:101–110. doi: 10.1016/0006-8993(91)91441-3. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry. 1995a;37:473–476. doi: 10.1016/0006-3223(94)00183-4. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995b;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Schneider Gasser EM, Straub CJ, Panzanelli P, Weinmann O, Sassoè-Pognetto M, Fritschy JM. Immunofluorescence in brain sections: simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 2006;1:1887–1897. doi: 10.1038/nprot.2006.265. [DOI] [PubMed] [Google Scholar]
- Schofield CM, Kleiman-Weiner M, Rudolph U, Huguenard JR. A gain in GABAA receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proc Natl Acad Sci U S A. 2009;106:7630–7635. doi: 10.1073/pnas.0811326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdellès B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Knapman A, Fedele DE, Schofield CM, Vyazovskiy VV, Rudolph U, Huguenard JR, Fritschy JM, Tobler I. Normal sleep homeostasis and lack of epilepsy phenotype in GABA A receptor alpha3 subunit-knockout mice. Neuroscience. 2008;154:595–605. doi: 10.1016/j.neuroscience.2008.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Keist R, von Boehmer L, Studer R, Benke D, Hagenbuch N, Dong Y, Malenka RC, Fritschy JM, Bluethmann H, Feldon J, Möhler H, Rudolph U. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci U S A. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]