Abstract
Learning and memory are supported by anatomically and functionally distinct systems. Recent research suggests that stress may alter the contributions of multiple memory systems to learning, yet the underlying mechanism in the human brain remains completely unknown. Using event-related functional magnetic resonance imaging, we asked in the present experiment whether stress may modulate the engagement of hippocampus-based “declarative” and striatum-based “procedural” memory systems during classification learning in humans and what brain mechanisms are involved in this effect. We found that stress reduced declarative knowledge about the learning task and changed the used learning strategy from a single-cue-based declarative strategy to a multicue-based procedural strategy, whereas learning performance per se remained unaffected by stress. Neuroimaging revealed that hippocampal activity correlated positively with task performance in the control condition, whereas striatal activity correlated with performance in the stress condition. After stress, hippocampal activity was reduced and even negatively correlated with learning performance. These findings show for the first time that stress alters the engagement of multiple memory systems in the human brain. Stress impaired the hippocampus-dependent system and allowed the striatum to control behavior. The shift toward “procedural” learning after stress appears to rescue task performance, whereas attempts to engage the “declarative” system disrupt performance.
Introduction
Our memory is composed of multiple anatomically and functionally distinct systems (Squire and Zola-Morgan, 1996). Two of these systems have received particular attention: (1) a hippocampus-dependent, declarative memory system that supports the acquisition of flexible knowledge, and (2) a rather rigid, striatum-dependent, procedural memory system (Mishkin and Petri, 1984; Squire and Zola-Morgan, 1991; Knowlton et al., 1996a; Packard and Knowlton, 2002). These systems process information in parallel and simultaneously (White and McDonald, 2002). However, they are not independent but may interact in a cooperative (Voermans et al., 2004) or competitive manner (Poldrack et al., 2001; Schroeder et al., 2002). The possibility of competition between memory systems raises the question how their engagement is modulated to optimize behavior.
Stress and glucocorticoid hormones (cortisol in humans) released during stressful experiences influence a wide range of cognitive functions, including learning and memory processes (de Quervain et al., 2000; Roozendaal et al., 2009; Schwabe et al., 2011). Accumulating evidence suggests that stress may also modulate the engagement of multiple memory systems. In navigation tasks, stress favors the use of striatum-dependent “response” strategies, at the expense of hippocampus-dependent “place” strategies (Kim et al., 2001; Schwabe et al., 2007, 2010b). The neural mechanism underlying this stress-induced modulation of multiple memory systems, however, remains elusive. Moreover, it is still unknown whether stress affects the engagement of hippocampal and striatal memory systems beyond navigation tasks.
Declarative and procedural memory processes have also been studied in probabilistic classification learning (PCL) tasks, which require online learning of probabilistic cue–outcome associations based on trial-by-trial feedback (Knowlton et al., 1994, 1996a; Poldrack et al., 2001). Converging evidence from neuropsychological studies in patients with amnesia or basal ganglia dysfunctions and neuroimaging studies in healthy subjects suggests that both the hippocampus and the striatum may contribute to PCL tasks (Knowlton et al., 1996a,b; Moody et al., 2004; Foerde et al., 2006). In line with the idea of competing memory systems, hippocampal and striatal activity correlate negatively during classification learning (Poldrack et al., 2001).
In the present experiment, we asked whether stress may modulate the engagement of hippocampus-based declarative and striatum-based procedural memory systems in classification learning and, if so, what the underlying brain processes are. Therefore, we exposed healthy participants to stress or a control condition before they learned a PCL task in the scanner. Participants may learn PCL tasks by using single cues (“simple strategy”) or by using a combination of multiple cues (“complex strategy”; Gluck et al., 2002; Thomas and LaBar, 2008). Because evidence shows that simple strategies are supported by the hippocampus, whereas complex strategies require an intact striatum (Shohamy et al., 2004), we used mathematical models to identify the strategy a participant was using (Gluck et al., 2002; Lagnado et al., 2006). Moreover, we tested participants' declarative task knowledge at the end of the experiment. We predicted that stress would (1) shift PCL from hippocampal to striatal control, (2) promote the use of multicue-based strategies, and (3) reduce declarative task knowledge.
Materials and Methods
Participants.
Sixty healthy, right-handed subjects with normal or corrected-to-normal vision participated in this experiment (30 men, 30 women; age: mean, 23.82 years; SEM, 0.31 years). Participation was limited to those between 18 and 30 years of age, without medication intake, and with no reported history of any psychiatric or neurological disorders. Furthermore, we excluded smokers and women using hormonal contraceptives from participation because previous studies showed that smoking and hormonal contraceptive intake alter the cortisol response to stress (Kirschbaum et al., 1999; Rohleder and Kirschbaum, 2006). The data of one woman of the control group had to be excluded because of technical problems during scanning. All participants provided written informed consent in accordance with procedures approved by the ethics committee of the German Psychological Association.
Stress induction.
To control for diurnal variations of the stress hormone cortisol, all testing took place between 12.30 and 6.30 P.M. After their arrival at the laboratory, participants were randomly assigned to the stress or control condition (15 men and 15 women per group). In the stress condition, participants underwent the socially evaluated cold pressor test (SECPT) as described in detail previously (Schwabe et al., 2008). Briefly, participants were asked to immerse their right hand up to and including the wrist for 3 min in ice water (0–2°C). During hand immersion, they were videotaped and monitored by a rather cold and nonreinforcing experimenter. Participants in the control condition submerged their right hand up to and including the wrist for 3 min into warm water (35–37°C). They were neither monitored by an unsociable experimenter nor videotaped.
To assess the effectiveness of the stress induction by the SECPT, blood pressure, salivary cortisol, and subjective feeling were measured at different time points across the experiment. Blood pressure was measured with a Dinamap system (Critikon) before, during, and immediately after the stress/control condition. Saliva samples were collected with Salivette (Sarstedt) collection devices before and immediately after the stress/condition as well as 25 and 90 min after the stress/control condition (i.e., immediately before and immediately after the learning task). Saliva samples were stored at −20°C until analysis. Free cortisol concentrations were measured using an immunoassay (IBL International). Interassay and intraassay coefficients of variance were <10%. To measure the subjective stressfulness of the treatment, participants indicated immediately after the stress/control condition on a scale from 0 (“not at all”) to 100 (“very much”) how stressful, painful, and unpleasant the experience was.
Probabilistic classification learning task.
Twenty-five minutes after the stress/control condition, participants completed a PCL task, known as the “weather prediction task” (Knowlton et al., 1994, 1996a; Poldrack et al., 2001), in the scanner. This interval between stress and learning was chosen because of the delayed secretion of the stress hormone cortisol, which reaches peak levels at ∼25 min poststress (Schwabe et al., 2008). Before the task started, participants were instructed that they would see different cards and that they should learn to predict the weather based on the presented cards. Between one and three (out of four) cards appeared on each trial, yielding 14 different cue patterns. These cue patterns were associated with two possible outcomes (sun and rain) in a probabilistic manner such that a particular cue was associated with the outcome “sun” with a probability of 75.6, 57.5, 42.5, or 24.4% across 100 trials; these probabilities were same as in previous studies using this task (Knowlton et al., 1994, 1996a; Foerde et al., 2006). A response was counted as correct if it matched the outcome associated most strongly with the referring cue pattern.
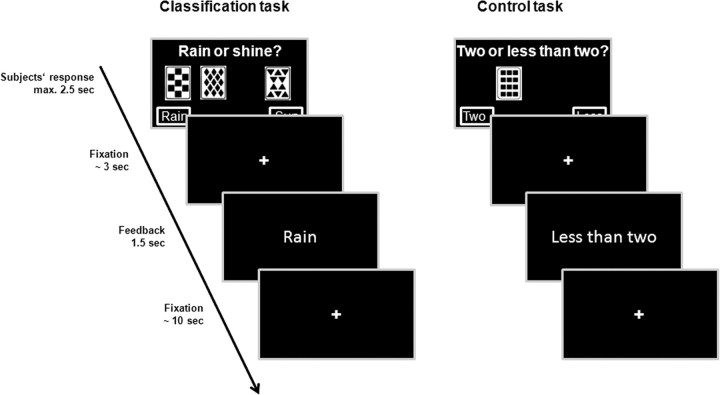
Participants completed 100 PCL trials. On each trial, they saw 1 of the 14 cue patterns and had 2.5 s to respond “sun” with a right button press or “rain” with a left button press. After a short fixation period (1.5–6 s), they received feedback about the actual weather by presenting the word “sun” or “rain” for 1.5 s. The interval between feedback offset and the onset of the next trial varied between 8 and 12 s (Fig. 1).
Figure 1.
PCL task and control task. In the PCL task, participants were presented one, two, or three cards per trial and asked to predict the weather (“rain” or “sunshine”) based on these cards. Feedback about the correct outcome was given after each trial. The control task had similar motor and perceptual characteristics as the PCL task but no learning demands; here, participants were asked to indicate if two less than two cards were presented.
Explicit task knowledge test.
After finishing the PCL task, participants completed (outside the scanner) a questionnaire containing 10 items that assessed explicit task knowledge. For example, participants were asked how many cards were presented per trial or which card was most strongly associated with the outcome “sun.” One point was given for each correct answer (i.e., participants could reach up to 10 points in the explicit knowledge test).
Learning strategy analysis.
The used learning strategy was assessed with a mathematical model in which the actual responses of a participant across the whole task were compared with ideal responses if a participant was reliably using a particular strategy (for details, Gluck et al., 2002; Lagnado et al., 2006). We constructed ideal data defined as the pattern of results that was expected across the 100 trials if a participant was reliably using a certain strategy. A least-means-squared estimate indicated the fit between the ideal data (for each strategy) and the participants' actual responses. This estimate resulted in a score between 0 and 1, with 0.0 indicating a perfect fit between the ideal data and participants' actual responses. Comparing across all strategies examined, the strategy associated with the lowest score was defined as the best-fit for that participant. If the best-fit score was >0.1, participants' strategy was classified as “nonidentifiable” (Gluck et al., 2002). For the sake of simplicity and in line with previous studies (Thomas and LaBar, 2008), we divided the four strategies that participants may use to solve the PCL task (one cue, singleton, multimax, and multimatch; Lagnado et al., 2006) into “simple” (including one cue and singleton) and “complex” (including multimatch and multimax) strategies. More details of the strategy analysis have been previously described (Gluck et al., 2002; Lagnado et al., 2006).
Visual–motor control task.
In a visual–motor control task, participants were presented between one and three (identical) cards and asked to indicate by left or right button press whether <2 or ≥2 cards are shown (Fera et al., 2005). Same as in the PCL task, 14 different cue patterns were used, one pattern was presented per trial, and participants had 2.5 s to respond, Also, like the PCL task, the correct answer was presented (“Less than two” or “Two or more”) for 1.5 s after a 1.5–6 s fixation period, and the next trial started 8–12 s after feedback offset. Thus, the procedure of the control task was exactly the same as in the PCL task, except that participants had not to learn the probabilistic association between the cue patterns and outcomes. Participants also completed 100 trials of the control task. PCL and control trials alternated randomly.
MRI acquisition.
Imaging was performed with a 3 T Philips Achieva scanner. For each participant, a high-resolution T1-weighted anatomical scan was acquired (slice thickness, 1 mm; 220 sagittal slices). For functional imaging, 810 T2-weighted echoplanar images were acquired parallel to the anterior commissure-posterior commissure plane (30 slices; slice thickness, 3 mm; repetition time, 2.0 s; echo time, 30 ms; 64 × 64 matrix; 2 × 2 mm pixel size; 200 mm FOV). The first three images were discarded to allow T1 equilibration.
Data analysis.
Behavioral data were analyzed by means of χ2 tests, t tests for independent samples, and mixed-design ANOVAs followed by appropriate post hoc tests. All reported p values are two-tailed.
Preprocessing and analysis of the event-related fMRI data were performed using SPM8 (Wellcome Trust Center for Neuroimaging, University College London). Functional data were corrected for slice timing and head motion. Structural images were segmented into gray matter, white matter, and CSF. Gray matter images were normalized to the MNI template image. Normalized gray matter images were used for normalization of the structural and functional images. Finally, data were spatially smoothed using an 8 mm full-width half-maximum Gaussian kernel.
PCL trials and control trials were modeled using the canonical hemodynamic response function. Furthermore, we included the fixation and the button press as well as the six movement regressors counting information about motion correction into our model. The data were filtered in the temporal domain using a nonlinear high-pass filter with a 128 s cutoff. Contrast images were generated for PCL trials minus control trials. These difference contrasts were taken to a second-level group two-sample t test, allowing a direct comparison between the stress and control group. In addition, on the second level, we also conducted whole-brain correlation analyses (simple regression) for each group, where we correlated the difference in brain activity between PCL and control trials with classification performance (expressed as percentage correct responses).
We used explorative whole-brain analyses as well as region of interest (ROI) analyses. A priori ROIs were the hippocampus, the caudate nucleus, the putamen, and the orbitofrontal cortex because these structures were implicated in probabilistic classification learning in earlier studies (Poldrack et al., 2001; Moody et al., 2004; Shohamy et al., 2004; Foerde et al., 2006). The referring masks were taken from the Harvard-Oxford subcortical and cortical atlases. For the explorative whole brain analyses, the significance threshold was set to p < 0.05 on voxel level, corrected for multiple testing [familywise error (FWE) correction], and a minimum cluster size of five voxels. ROI analyses were performed using the small volume correction options of SPM8 (p < 0.05).
Results
Subjective, autonomic, and endocrine responses to stress
Changes in subjective feeling, blood pressure, and concentrations of the glucocorticoid stress hormone cortisol verified the successful stress induction by the SECPT. As expected, participants in the stress condition rated the treatment as significantly more stressful, painful, and unpleasant than controls (all t(58) > 7.25, all p < 0.001; Table 1). Systolic and diastolic blood pressure increased significantly in response to the SECPT but not in response to the control condition (time point of measurement × group interactions for systolic and diastolic blood pressure: both F(1,116) > 23.18, both p < 0.001; Table 1). Similarly, salivary cortisol concentrations increased after the exposure to the SECPT but not after the control condition (F(3,171) = 11.53, p < 0.001). Cortisol levels peaked 25 min after the treatment, when the scanning session started (Table 1). Men and women did not differ in their physiological or subjective responses to stress (all p > 0.15).
Table 1.
Subjective, autonomic, and endocrine responses to stress
| Control | Stress | |
|---|---|---|
| Subjective assessments | ||
| Stressful | 1.00 ± 0.56 | 57.00 ± 5.41** |
| Painful | 0.33 ± 0.33 | 59.00 ± 4.97** |
| Unpleasant | 6.00 ± 3.82 | 42.33 ± 5.65** |
| Systolic blood pressure | ||
| Before treatment | 117.59 ± 2.66 | 122.08 ± 2.89 |
| During treatment | 116.50 ± 2.56 | 138.05 ± 2.70** |
| After treatment | 113.01 ± 2.53 | 115.54 ± 2.72 |
| Diastolic blood pressure | ||
| Before treatment | 68.51 ± 1.76 | 69.94 ± 2.57 |
| During treatment | 67.01 ± 2.10 | 81.96 ± 1.69** |
| After treatment | 66.26 ± 1.62 | 65.49 ± 1.37 |
| Salivary cortisol | ||
| Before treatment | 11.17 ± 1.42 | 9.28 ± 0.78 |
| 1 min after treatment | 10.36 ± 1.26 | 8.42 ± 0.63 |
| 25 min after treatment | 7.90 ± 0.88 | 14.52 ± 1.84** |
| 90 min after treatment | 6.12 ± 0.93 | 8.05 ± 0.83 |
Subjective assessments were given on a scale from 0 to 100.
**Control group versus stress group, p <0.01, Bonferroni corrected. Data represent mean ± SEM.
Probabilistic classification learning after stress: behavioral data
During fMRI scanning at 3 T, ∼25 min after the stress exposure, participants alternated between a PCL task, known as the “weather prediction task” (Knowlton et al., 1994, 1996a; Poldrack et al., 2001), and a visual–motor control task (Fera et al., 2005; Fig. 1). In the PCL task, participants learned to predict “the weather” based on cards presented on a screen. In the control task, participants were presented between one, two, or three cards and asked to indicate whether two or less than two cards are shown. This task had the same motor and perceptual characteristics as the PCL task but no learning demands.
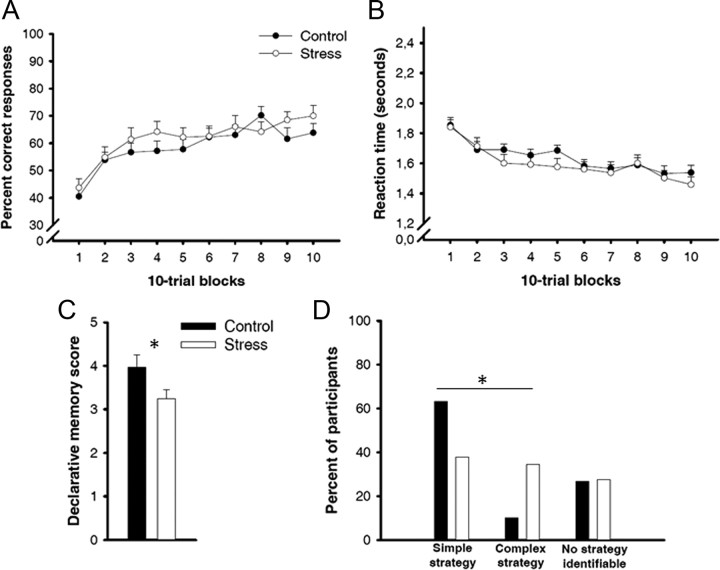
Learning curves in the PCL task were comparable between stress and control groups (main effect group and group × training block interaction: both F < 1, both p > 0.32). Both groups gradually improved their classification performance across training (main effect training block: F(9,513) = 12.47, p < 0.001), from ∼42% correct responses at the beginning to ∼67% correct responses at the end of training (Fig. 2A). At the same time, reaction times decreased gradually and to a comparable extent in both groups (main effect training block: F(9,513) = 19.36, p < 0.001; main effect group and group × training block interaction: both F < 1, both p > 0.45; Fig. 2B). Interestingly, we obtained significant correlations between PCL performance and salivary cortisol levels across both groups and for all time points of measurement (before treatment: r = 0.31; 1 min after treatment: r = 0.26; 25 min after treatment: r = 0.33; 90 min after treatment: r = 0.28; all p < 0.05). There was, however, no significant correlation between the stress-induced increase in cortisol from baseline to 25 min post-treatment and performance; nor were there any correlations between blood pressure or subjective stress ratings and performance (all p > 0.15).
Figure 2.
Behavioral results in the PCL task. A, B, Percentage correct responses increased (A) and reaction times decreased (B) similarly in the stress and control groups across training, indicating successful classification learning in both groups. C, Although stress did not affect participants' learning curves in the PCL task, it reduced declarative knowledge about the task. D, Furthermore, stress changed the strategies used during classification learning: stressed participants, compared with nonstressed controls, significantly more often used complex strategies associated with the procedural system and significantly less often used simple strategies associated with the declarative system. Asterisks indicate significant differences between the stress and control groups (p < 0.05). Data represent mean ± SEM.
Although stress had no effect on the learning curves in the PCL task, it affected the declarative knowledge about the PCL task. Participants exposed to the stressor before learning remembered significantly fewer details of the PCL task than controls (t(57) = 2.04, p = 0.046; Fig. 2C). Furthermore, mathematical analyses that compared participants' actual responses with ideal data if a participant was reliably using a particular learning strategy (for details, see Materials and Methods) revealed that stress had a significant influence on the strategy used during classification learning (χ2(1) = 5.88, p = 0.015). The use of simple, single-cue-based strategies associated with the hippocampal system (Shohamy et al., 2004) decreased from 63 to 37% and the use of complex, multicue-based strategies associated with the striatal system (Shohamy et al., 2004) increased from 10 to 35% in stressed participants relative to controls (Fig. 2D). The use of multicue-based strategies was associated with salivary cortisol levels 25 min (r = 0.29, p = 0.029) and 90 min (r = 0.27, p = 0.043) after the treatment as well as the systolic blood pressure during the SECPT/control condition (r = 0.31, p = 0.016).
Thus, our behavioral data suggest that stress did not affect the acquisition of the PCL task per se but that it changed the nature of classification learning from flexible, declarative learning to inflexible, procedural learning. Performance in the control task was in all participants very high (average correct, 94.2%) and not influenced by stress (p = 0.64). Moreover, there were no differences between men and women with respect to learning performance, explicit task knowledge, or strategy use (all p > 0.20).
Probabilistic classification learning after stress: imaging data
Corroborating earlier studies on the neural correlates of probabilistic classification learning (Poldrack et al., 2001; Moody et al., 2004; Fera et al., 2005; Foerde et al., 2006), our imaging data showed that, compared with the control task, the engagement in the PCL task was associated with increased activation in the caudate nucleus, the putamen, the hippocampus, the parahippocampal cortex, the orbitofrontal cortex, the cingulate cortex, and the inferior frontal cortex (Table 2). In support of the idea that simple, single-cue-based strategies are associated with the declarative system, whereas complex, multicue-based strategies are related to the procedural system (Shohamy et al., 2004), the use of single-cue strategies correlated with activity of the hippocampus [(−20, −38, 2), Z = 2.84, pcorr = 0.05, FWE corrected] and the use of multicue strategies with activity of the putamen [(−30, −16, 2), Z = 3.50, pcorr = 0.05, FWE corrected] and the caudate nucleus [(16, 12, 18), Z = 3.26, pcorr = 0.07, FWE corrected] during the PCL task (Table 3).
Table 2.
Significant activations for the contrast PCL-control for the entire sample and for the group comparisons
| Cluster | MNI coordinates (mm) |
Tmax | pcorr | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Entire sample | ||||||
| Left caudate nucleus | 314 | −10 | 14 | 0 | 10.11 | <0.001 |
| Right caudate nucleus | 320 | 8 | 10 | 2 | 10.70 | <0.001 |
| Left putamen | 59 | −14 | 8 | −6 | 7.45 | <0.001 |
| Right putamen | 124 | 16 | 14 | −4 | 6.65 | <0.001 |
| Left hippocampus | 36 | −22 | −32 | −6 | 4.60 | 0.003 |
| Right hippocampus | 18 | 22 | −36 | 0 | 3.35 | 0.056 |
| Left parahippocampal cortex | 16 | −14 | −34 | −10 | 3.77 | 0.012 |
| Right parahippocampal cortex | 12 | 16 | −34 | −8 | 3.29 | 0.036 |
| Left orbitofrontal cortex | 374 | −32 | 24 | −6 | 10.81 | <0.001 |
| Right orbitofrontal cortex | 336 | 30 | 24 | −8 | 10.84 | <0.001 |
| Left cingulate cortex | 2503 | −4 | 28 | 36 | 12.53 | <0.001 |
| Right cingulate cortex | 2503 | 6 | 20 | 44 | 15.28 | <0.001 |
| Left inferior frontal lobe | 2400 | −30 | 22 | −4 | 12.66 | <0.001 |
| Right inferior frontal lobe | 2400 | 30 | 22 | −4 | 12.68 | <0.001 |
| Control group greater than stress group | ||||||
| Right hippocampus | 9 | 22 | −34 | 4 | 2.93 | 0.045 |
| Stress group greater than control group | ||||||
| No suprathreshold activations | ||||||
The significance threshold was set at pcorr < 0.05 (FWE corrected according to SPM8; for ROIs: small volume correction).
Table 3.
Correlations between activity in the PCL task (vs control task) and the engagement of single-cue versus multicue strategies
| Cluster | MNI coordinates (mm) |
Tmax | pcorr | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlation | ||||||
| Left hippocampus | 31 | −20 | −38 | 2 | 3.00 | 0.050 |
| Negative correlation | ||||||
| Right caudate nucleus | 74 | 16 | 12 | 18 | 3.50 | 0.070 |
| Left putamen | 71 | −30 | −16 | 2 | 3.80 | 0.050 |
| Right putamen | 55 | 22 | 14 | −10 | 3.70 | 0.066 |
Multicue strategies were coded as 0 and single-cue strategies as 1. Thus, positive correlations indicate areas that correlate with the use of a single-cue strategy and negative correlations indicate areas that correlate with the use of a multicue strategy. The significance threshold was set at pcorr < 0.05 (FWE corrected according to SPM8; for ROIs: small volume correction).
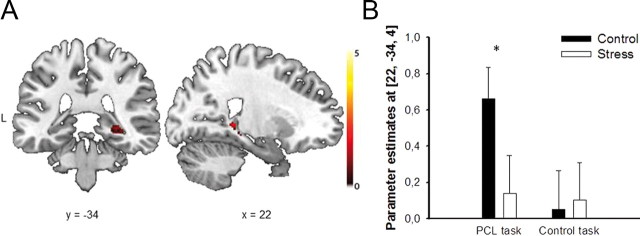
There was a large overlap between the brain areas that were recruited during the PCL task in the stress and control groups. Both groups showed significant activations of the striatum during classification learning. However, in contrast to the control group, participants in the stress group showed no significant activation of medial temporal lobe structures during classification learning (Tables 4, 5). In particular, stressed participants, compared with control participants, showed significantly reduced activation (i.e., less positive evoked activity) in the right hippocampus during the PCL task [(22, −34, 4), Z = 2.93, pcorr = 0.045, FWE corrected; Fig. 3, Table 2]. Notably, stress reduced hippocampal activity in the PCL task but not in the visual–motor control task (pcorr > 0.90). In the opposite contrast control-PCL, there were no significant group differences in brain activity.
Table 4.
Significant activations for the contrast PCL-control in the control group
| Cluster | MNI coordinates (mm) |
pcorr | ||||
|---|---|---|---|---|---|---|
| x | y | z | Tmax | |||
| Left caudate nucleus | 303 | −8 | 10 | −2 | 6.84 | <0.001 |
| Right caudate nucleus | 303 | 8 | 14 | 0 | 7.31 | <0.001 |
| Left putamen | 39 | −14 | 8 | −6 | 4.98 | 0.006 |
| Right putamen | 103 | 14 | 10 | −6 | 4.76 | 0.010 |
| Left hippocampus | 17 | −20 | −34 | −6 | 4.50 | 0.012 |
| Right hippocampus | 20 | 22 | −34 | 2 | 5.42 | <0.001 |
| Left parahippocampal cortex | 16 | −16 | −32 | −10 | 3.43 | 0.047 |
| Left orbitofrontal cortex | 344 | −32 | 26 | −4 | 7.43 | <0.001 |
| Right orbitofrontal cortex | 119 | 34 | 24 | −6 | 7.87 | <0.001 |
| Left cingulate cortex | 690 | −2 | 26 | 34 | 8.46 | <0.001 |
| Right cingulate cortex | 690 | 8 | 18 | 46 | 11.47 | <0.001 |
| Right inferior frontal lobe | 215 | 34 | 18 | −2 | 10.25 | <0.001 |
The significance threshold was set at pcorr < 0.05 (FWE-corrected according to SPM8; for ROIs: small volume correction).
Table 5.
Significant activations for the contrast PCL-control in the stress group
| Cluster | MNI coordinates (mm) |
Tmax | pcorr | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Left caudate nucleus | 280 | −10 | 14 | 0 | 7.66 | <0.001 |
| Right caudate nucleus | 280 | 8 | 10 | 2 | 8.85 | <0.001 |
| Left putamen | 52 | −14 | 8 | −6 | 5.31 | 0.003 |
| Right putamen | 96 | 16 | 14 | −4 | 4.83 | 0.008 |
| Left orbitofrontal cortex | 168 | −30 | 26 | −6 | 8.16 | <0.001 |
| Right orbitofrontal cortex | 103 | 30 | 24 | −8 | 7.45 | <0.001 |
| Left cingulate cortex | 624 | −5 | 25 | 38 | 9.97 | <0.001 |
| Right cingulate cortex | 624 | 4 | 22 | 44 | 12.94 | <0.001 |
| Left inferior frontal lobe | 39 | −46 | 22 | 24 | 7.53 | 0.002 |
| Right inferior frontal lobe | 456 | 52 | 22 | 32 | 8.76 | <0.001 |
| Left insular cortex | 155 | −30 | 22 | −2 | 10.74 | <0.001 |
| Right angular gyrus | 80 | 34 | −60 | 50 | 7.49 | 0.003 |
| Right precuneus | 30 | 8 | −66 | 44 | 7.70 | 0.002 |
The significance threshold was set at pcorr < 0.05 (FWE corrected according to SPM8; for ROIs: small volume correction).
Figure 3.
Stress effect on hippocampal activity during classification learning. A, During the PCL task, the right hippocampus was significantly less activated in the stress group than in the control group (pcorr < 0.05, FWE corrected). Coronal and sagittal sections are shown, superimposed on a T1-template image. L, left. B, Parameter estimates of the peak voxel for the control and stress groups in the PCL and control task, respectively. Data represent mean ± SEM (*p < 0.05).
There were no significant correlations between brain activity and blood pressure or subjective stress ratings. However, we obtained correlations between activity in the caudate nucleus and salivary cortisol levels at baseline (x = 8, y = 6, z = 10; Z = 3.24, p = 0.069, FWE corrected), 1 min after the treatment (x = 8, y = 6, z = 10; Z = 3.12, p = 0.096, FWE corrected), 25 min after the treatment (x = −6, y = 12, z = 2; Z = 3.08, p = 0.099, FWE corrected), and 90 min after the treatment (x = 10, y = 14, z = 10; Z = 3.49, p = 0.045, FWE corrected; all p ≤ 0.001, uncorrected). The cortisol increase in response to stress (i.e., the increase from baseline to 25 min post-treatment) did not correlate with caudate activity, thus suggesting that cortisol concentrations per se are associated with caudate activity but not the stress-induced increase in cortisol.
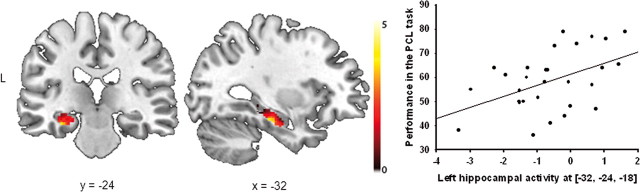
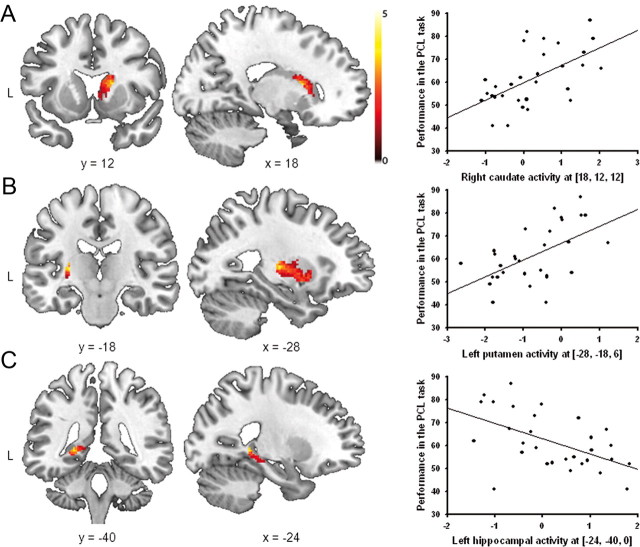
To assess whether stress changes the contribution of declarative and procedural memory systems to classification learning, we correlated the brain activity in the contrast PCL task minus control task in the stress and control groups with performance in the PCL task (expressed as percentage correct responses across the whole task). In line with previous studies implicating medial temporal lobe structures in classification learning in healthy controls (Reber et al., 1996; Foerde et al., 2006), these analyses showed that activity in the left hippocampus correlated positively with PCL performance in nonstressed controls [(−32, −24, −18), Z = 3.60, pcorr = 0.029, FWE corrected, cluster size: 95 voxels; Fig. 4], whereas there were no significant correlations between performance and striatal activity (all pcorr > 0.50, all Z < 2.45) or any other region that was significantly activated during PCL. In stressed participants, however, task performance correlated significantly with activity in the right caudate nucleus [(18, 12, 12), Z = 3.79, pcorr = 0.015, FWE corrected, cluster size: 32 voxels] and the left putamen [(−28, −18, 6), Z = 3.68, pcorr = 0.032, FWE corrected, cluster size: 13 voxels; Fig. 5A,B]. Moreover, and in sharp contrast to the control group, activity in the left hippocampus correlated negatively with task performance in the stress group [(−28, −18, 6), Z = 3.51, pcorr = 0.04, FWE corrected, cluster size: 10 voxels; Fig. 5C]; there were no further correlations between task performance and any other areas activated during PCL.
Figure 4.
Correlations between performance (across all PCL trials) and brain activity (PCL minus control task) in the control group. Performance in the PCL task was significantly correlated with activity in the left hippocampus. Coronal and sagittal sections are shown, superimposed on a T1-template image. Each data point represents a single participant. The scatter plot illustrates the association between brain activity and performance. The analysis, however, was conducted at the whole-brain level. L, left.
Figure 5.
Correlations between performance (across all PCL trials) and brain activity (PCL minus control task) in the stress group. A, B, Under stress, there was a positive correlation between the performance in the PCL task and activity in the right caudate and the left putamen. C, Activity in the left hippocampus, however, correlated negatively with classification learning in stressed participants. Coronal and sagittal sections are shown, superimposed on a T1-template image. Each data point represents a single participant. The scatter plots illustrate the association between brain activity and performance. The analyses, however, were conducted at the whole-brain level. L, left.
These group differences support the notion that stress modulates the contribution of hippocampus-dependent (“declarative”) and striatum-dependent (“procedural”) systems to classification learning in a manner that favors procedural over declarative learning.
Discussion
Probabilistic classification learning may be controlled by declarative or procedural memory systems (Poldrack et al., 2001). Our findings show that stress before learning modulates the engagement of these systems and their contribution to task performance. Stress did not affect classification accuracy. However, it changed the nature of learning. Participants exposed to stress before learning remembered significantly fewer details about the task and used significantly more often a complex, procedural learning strategy, suggesting that stress favored the procedural system, at the expense of the declarative system. This conclusion is supported by our fMRI data. In line with previous findings (Foerde et al., 2006), successful PCL was correlated with hippocampal activity in nonstressed controls. In stressed participants, however, the success in the PCL task correlated with striatal activity.
How can this stress-induced shift in the contribution of declarative and procedural memory systems to classification learning be explained? A likely explanation takes the sensitivity of the hippocampus to stress and glucocorticoid stress hormones into account. The hippocampus expresses both mineralocorticoid receptors and glucocorticoid receptors, the two receptor types that mediate glucocorticoid actions in the brain, at a very high density (de Kloet et al., 2005). Stress or glucocorticoid administration before learning reduces hippocampal long-term potentiation (Diamond et al., 2007), suppresses learning-related increases in hippocampal spine density (Diamond et al., 2006), and impairs hippocampus-dependent memory processes (McEwen, 1999; de Quervain et al., 2000, 2003; Schwabe et al., 2009). Thus, we propose that stress impaired the hippocampus-dependent system and hence allowed the striatum-dependent system to control classification learning. In line with this view, the activity of the hippocampus was significantly reduced in stressed participants during classification learning. Because previous data suggested that stress or administration of glucocorticoids may reduce the BOLD signal in the hippocampus (Pruessner et al., 2008; Lovallo et al., 2010), it is important to note at this point that stress did not affect hippocampal activity in the visual–motor control task. Thus, stress appeared to reduce specifically PCL-related activity in the hippocampus and not hippocampal activity (i.e., the BOLD signal in the hippocampus) in general.
Although stress did not alter striatal activation during PCL, glucocorticoid levels per se (i.e., regardless of whether participants were exposed to the stressor or not) were associated with enhanced PCL performance and increased striatal activity. These data are in line with recent findings suggesting that glucocorticoid injections into the dorsal striatum enhance memory in a striatum-dependent task in rodents (Quirarte et al., 2009). Because neither stress nor the stress-induced increase in cortisol was associated with striatal activity, it is rather unlikely that changes in striatal activity alone can account for the shift from declarative to procedural learning after stress. It is, however, tempting to speculate that glucocorticoid-related increases in striatal activity, in the face of reduced hippocampal activity after stress, may contribute to the stress-induced shift toward more procedural learning.
Moreover, the finding that stress did not affect striatal activity might suggest that it is not the absolute strength of one system that determines which system can control learning but the relative strength of one system compared with the other. Under control conditions, both the hippocampus and the striatum were active during PCL but the latter had no (direct) influence on performance. Stress disrupted the hippocampal system and thus may have increased the relative strength of the striatal system, allowing it to control behavior.
The influence of stress on the engagement of striatal and hippocampal memory systems may also have been mediated by a third structure (e.g., the amygdala). There is ample evidence that the major stress hormones (i.e., glucocorticoids and noradrenaline) interact in the (basolateral) amygdala, which then modulates memory processes in other brain areas, such as the hippocampus (Roozendaal et al., 2006, 2009). Moreover, the finding that stress favors caudate-based over hippocampus-based navigation learning (Kim et al., 2001) can be mimicked by intraamygdala injections of anxiogenic drugs (Wingard and Packard, 2008). Thus, although we did not find stress-related changes in amygdala activity in the contrast PCL-control, a modulatory role of the amygdala on the engagement of memory systems seems likely, possibly in combination with direct stress hormone effects on the hippocampus.
Most interestingly, whereas activity in the hippocampus was positively correlated with classification performance in the control group, it correlated negatively with performance in the stress group. This finding is well in line with recent findings on the influence of stress on navigation tasks in rodents. Learning these tasks can be supported by hippocampus-dependent “place” strategies or striatum-dependent “response” strategies. Control animals usually show a strong preference for place strategies. Stress or glucocorticoid injections, however, lead to a relative increase in the engagement of response strategies (Kim et al., 2001; Schwabe et al., 2010b). Those animals that keep using the place strategy are significantly impaired in performance, both compared with nonstressed controls that use the place strategy and compared with stressed animals that switch to the response strategy (Schwabe et al., 2010b). Together with these findings, the present data suggest that switching from the hippocampus-dependent system to the striatum-dependent system after stress rescues task performance. The continued engagement of the (dysfunctional) hippocampus-dependent system after stress, however, impedes learning.
Our results are also in line with previous studies showing reduced hippocampal activity after stress (de Quervain et al., 2003; Pruessner et al., 2008) and a bias toward more striatum-dependent learning in navigation tasks (Kim et al., 2001; Schwabe et al., 2007, 2010b). The present findings, however, extend these earlier findings in several important ways. First, we did not focus on a single (hippocampus-dependent) system but on the engagement of multiple (hippocampal and nonhippocampal) memory systems and show that, after stress, the striatum-based system may replace the hippocampus-based system and thus rescue task performance. Second, while previous studies used navigation tasks to study memory systems and their modulation by stress (Kim et al., 2001; Schwabe et al., 2007, 2010b), the present study used a nonspatial task with different processing demands and shows for the first time that stress modulates competing memory systems also in classification learning. Third, and most importantly, previous evidence for the stress-induced modulation of multiple memory systems in humans was largely behavioral (i.e., assumptions about the involved brain areas remained rather speculative). Here, we provide for the first time direct evidence that stress modulates the engagement of multiple memory systems in the human brain.
The fact that stressed participants were not impaired in classification accuracy corroborates the idea that healthy individuals can make use of multiple parallel (striatum-based and hippocampus-based) memory systems, which are equally able to support performance in PCL tasks (Reber et al., 1996; Poldrack et al., 2001; Foerde et al., 2006). However, by shifting classification learning from hippocampal to striatal control, stress altered the nature of learning. Stress before learning reduced the use of single-cue strategies, indications of hippocampus-based learning (Shohamy et al., 2004), as well as the level of subsequent declarative knowledge about the task. Declarative task knowledge appears to be a prerequisite for the flexible use of the acquired knowledge. For example, amnesic patients may learn a PCL task at a same rate as healthy controls. Yet, they lack declarative knowledge about the task and are unable to use their (nondeclarative) knowledge in a novel situation (Reber et al., 1996). Similar deficits in the flexibility of knowledge in the absence of hippocampus-dependent memory are obtained in rats with fornix lesions (Eichenbaum et al., 1990). Thus, the stress-induced bias toward procedural learning may lead to less flexible knowledge that cannot easily be transferred to novel situations.
Previous evidence suggests that distraction can bias the engagement of declarative and procedural systems in classification learning as well (Foerde et al., 2006). In the present experiment, participants were exposed to the stressor 25 min before the beginning of the PCL task. Thus, although stress and distraction effects are not mutually exclusive, it is rather unlikely that stress acted as a distractor during learning. Nevertheless, the consequences of distraction and stress on classification learning may be similar in that both disrupt the declarative system. Performance of a secondary task during classification learning occupies working memory resources required for the elaborative encoding and taxing retrieval associated with declarative memory, making declarative learning difficult (Foerde et al., 2006). We suggest that stress interferes with declarative learning via glucocorticoid effects on hippocampal (and possibly prefrontal cortical) functioning, thus modulating the competition between memory systems toward the striatum-dependent procedural system. Moreover, together with the previous findings on the influence of distraction on classification learning, the present data suggest that there are at least two forces that shift learning from hippocampal to striatal control: one that increases the load of the declarative system during learning (e.g., dual-tasking and possibly also stressful experiences) and another that is due to events that occurred before learning (e.g., glucocorticoid elevations after a stressful encounter).
This is, to the best of our knowledge, the first study to demonstrate a stress-induced shift in the neural systems controlling learning and memory in humans. More specifically, we show for the first time that stress modulates the contribution of multiple memory systems to classification learning in a manner that favors striatum-dependent procedural learning over hippocampus-dependent declarative learning. Procedural learning requires no conscious processing (Squire, 2004); it involves only little cognitive load and might therefore allow focusing on coping with the stressful situation. Furthermore, the engagement of the declarative system after stress is associated with impaired task performance. However, although the shift from hippocampus-dependent to striatum-dependent learning after stress appears to be generally adaptive, it may come at the expense of acquiring flexible knowledge. The shift from declarative to nondeclarative memory after stress might have important implications for stress-related disorders, such as depression, addiction, or post-traumatic stress disorder, that are characterized by dysfunctional learning and memory processes (Schwabe et al., 2010a). Moreover, if the present findings translate also to retrieval situations, they might be relevant for another dramatic phenomenon termed “forgotten baby syndrome,” which occurs when parents forget their babies in the car and which has been related to the predominance of nondeclarative memory under stress (Halonen et al., 2011).
Footnotes
This work was supported by a grant from the German Research Foundation (DFG; SCHW1357/5-1). We thank Julia Bonk and Carsten Siebert for help with data collection, Christian J. Merz for help with fMRI analyses, Tobias Otto for technical assistance, and Silke Lissek and Martin Tegenthoff for their help with fMRI scanning.
References
- de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Henke K, Aerni A, Treyer V, McGaugh JL, Berthold T, Nitsch RM, Buck A, Roozendaal B, Hock C. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–12302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Woodson JC, Conrad CD, Bachstetter AD, Mervis RF. Influence of predator stress on the consolidation versus retrieval of the long-term spatial memory and hippocampal spinogenesis. Hippocampus. 2006;16:571–576. doi: 10.1002/hipo.20188. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast. 2007;2007:60803. doi: 10.1155/2007/60803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RG. Hippocampal representation in place learning. J Neurosci. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, Lee S, Zoltick B, Meeter M, Myers CE, Gluck MA, Weinberger DR, Mattay VS. Neural mechanisms underlying probabilistic category learning in normal aging. J Neurosci. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Knowlton BJ, Poldrack RA. Modulation of competing memory systems by distraction. Proc Natl Acad Sci U S A. 2006;103:11778–11783. doi: 10.1073/pnas.0602659103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: individual variability in strategies for probabilistic category learning. Learn Mem. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JD, Zoladz PR, Diamond DM. Neurobiology of forgotten baby syndrome. Poster presented at the 41st meeting of the Society for Neuroscience; November 2011; Washington DC. 2011. [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Mem. 1994;1:106–120. [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996a;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Paulsen JS, Swerdlow NR, Swenson M. Dissociations within nondeclarative memory in Huntington's disease. Neuropsychology. 1996b;10:538–548. [Google Scholar]
- Lagnado DA, Newell BR, Kahan S, Shanks DR. Insight and strategy in multiple-cue learning. J Exp Psychol Gen. 2006;135:162–183. doi: 10.1037/0096-3445.135.2.162. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology. 2010;35:15–20. doi: 10.1016/j.psyneuen.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Petri HL. Memories and habits. Some implications for the analysis of learning and retention. In: Squire LR, Butters N, editors. Neuropsychology of learning and memory. New York: Guilford; 1984. pp. 287–296. [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Paré-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, de la Teja IS, Casillas M, Serafín N, Prado-Alcalá RA, Roozendaal B. Corticosterone infused into the dorsal striatum selectively enhances memory consolidation of cued water-maze training. Learn Mem. 2009;16:586–589. doi: 10.1101/lm.1493609. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Knowlton BJ, Squire LR. Dissociable properties of memory systems: differences in the flexibility of declarative and nondeclarative knowledge. Behav Neurosci. 1996;110:861–871. doi: 10.1037//0735-7044.110.5.861. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Kirschbaum C. The hypothalamic-pituitary-adrenal (HPA) axis in habitual smokers. Int J Psychophysiol. 2006;59:236–243. doi: 10.1016/j.ijpsycho.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Wingard JC, Packard MG. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus. 2002;12:280–284. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Oitzl MS, Philippsen C, Richter S, Bohringer A, Wippich W, Schachinger H. Stress modulates the use of spatial and stimulus-response learning strategies in humans. Learn Mem. 2007;14:109–116. doi: 10.1101/lm.435807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Böhringer A, Wolf OT. Stress disrupts context-dependent memory. Learn Mem. 2009;16:110–113. doi: 10.1101/lm.1257509. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, Oitzl MS. Memory formation under stress: quantity and quality. Neurosci Biobehav Rev. 2010a;34:584–591. doi: 10.1016/j.neubiorev.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Schächinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as switch between memory systems. J Cogn Neurosci. 2010b;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: an update and integration. Neurosci Biobehav Rev. 2011 doi: 10.1016/j.neubiorev.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Grossman S, Sage J, Gluck MA, Poldrack RA. Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology. Brain. 2004;127:851–859. doi: 10.1093/brain/awh100. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci U S A. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LA, LaBar KS. Fear relevancy, strategy use, and probabilistic learning of cue-outcome associations. Learn Mem. 2008;15:777–784. doi: 10.1101/lm.1048808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voermans NC, Petersson KM, Daudey L, Weber B, Van Spaendonck KP, Kremer HP, Fernández G. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wingard JC, Packard MG. The amygdala and emotional modulation of competition between cognitive and habit memory. Behav Brain Res. 2008;193:126–131. doi: 10.1016/j.bbr.2008.05.002. [DOI] [PubMed] [Google Scholar]