Abstract
Many aspects of brain processing are intimately linked to brain rhythms. Essentially all classical brain rhythms, i.e., delta, theta, alpha, beta, and sleep waves, are highly heritable. This renders brain rhythms an interesting intermediate phenotype for cognitive and behavioral traits. One brain rhythm that has been particularly strongly linked to cognition is the gamma rhythm: it is involved in attention, short- and long-term memory, and conscious awareness. It has been described in sensory and motor cortices, association and control structures, and the hippocampus. In contrast to most other brain rhythms, the gamma frequency highly depends on stimulus and task conditions, suggesting a low heritability. However, the heritability of gamma has not been assessed. Here, we show that visually induced gamma-band synchronization in humans is strongly genetically determined. Eighty twin subjects (20 monozygotic and 20 dizygotic twin pairs) viewed a moving sinusoidal grating while their brain activity was recorded using magnetoencephalography. The stimulus induced spectrally confined gamma-band activity in sensors over visual cortex in all subjects, with individual peak frequencies ranging from 45 to 85 Hz. Gamma-band peak frequencies were highly correlated across monozygotic twins (r = 0.88), but not across dizygotic twins (r = 0.32) or unrelated subjects (r = 0.02). This implies a heritability of the gamma-band frequency of 91%. This strong genetic determination suggests that gamma-related cognitive functions are under close genetic control.
Introduction
Many cognitive functions are related to brain rhythms (Buzsaki, 2006). Both brain rhythms and cognitive functions vary among individuals, and individuals also vary in their genetic makeup. It has been challenging to establish clear links between genetic and cognitive makeup (Green et al., 2008; Koten et al., 2009). Identifying the genetic basis of brain rhythms might help in the understanding of the genetic basis of associated cognitive and behavioral traits, with brain rhythms serving as an intermediate phenotype.
Many of the classical brain rhythms are highly heritable. In the 1970s, for example, Vogel (1970) and Young et al. (1972) investigated the heritabilities of the delta, theta, alpha, and beta rhythms in twins and showed heritabilities up to 0.9. Those early and subsequent related studies used resting-state or sleep recordings (Lykken, 1982; van Beijsterveldt et al., 1996; Linkowski, 1999; Posthuma et al., 2001; Smit et al., 2006; Linkenkaer-Hansen et al., 2007) (for review, see van Beijsterveldt and van Baal, 2002), because many of the classical brain rhythms are strongest under those conditions.
By contrast, the gamma-band rhythm (30–100 Hz) is strongly associated with active brain processing (Gray et al., 1989; Fries et al., 2001; Pesaran et al., 2002; Buschman and Miller, 2007; Colgin et al., 2009; Tallon-Baudry, 2009): gamma-band activity is induced by sensory stimulation in the visual (Hoogenboom et al., 2006; Muthukumaraswamy et al., 2009), auditory (Brosch et al., 2002), and somatosensory (Bauer et al., 2006) modality. It is enhanced among neurons driven by attended stimuli (Fries et al., 2001) and predicts corresponding behavioral benefits (Taylor et al., 2005; Womelsdorf et al., 2006). It reflects the content of working memory (Pesaran et al., 2002; Howard et al., 2003), predicts the quality of long-term memory encoding (Fell et al., 2001), and correlates with conscious awareness of a stimulus (Fries et al., 2002; Wyart and Tallon-Baudry, 2008). At the same time, the amplitude and peak frequency of gamma-band synchronization is highly variable across individual human (Hoogenboom et al., 2006; Muthukumaraswamy et al., 2009, 2010) and animal (Vinck et al., 2010) subjects, similar to cognitive functioning.
In contrast to most classical frequency bands, the peak frequency of gamma-band activity is highly variable within an individual subject: gamma frequency depends on stimulus and task parameters, such as stimulus contrast (Ray and Maunsell, 2010), velocity (Swettenham et al., 2009), and size (Ray and Maunsell, 2011). This suggests that the heritability of gamma-band synchronization might be low. We therefore set out to test whether gamma-band activity is genetically determined and performed a classical twin study into the heritability of visually induced gamma-band activity by using MEG. We found that gamma-band synchronization is 91% heritable. This also suggests that cognitive functions related to gamma are under close genetic control.
Materials and Methods
Subjects.
Subjects were 80 twins, consisting of 20 monozygotic (MZ) and 20 dizygotic (DZ) pairs, who were recruited through the Netherlands Twin Registry (Boomsma et al., 2006). All subjects gave written informed consent according to institutional guidelines of the local ethics committee (Commissie Mensgebonden Onderzoek Region Arnhem-Nijmegen, The Netherlands). None had previous experience with a similar experimental setup. Only same-sex pairs were tested (MZ, 15 female pairs; DZ, 10 female pairs), with average ages (±SD) of 21.0 ± 3.0 (MZ) and 21.7 ± 2.9 (DZ). Two MZ and four DZ twins were left-handed. Zygosity was determined based on blood group polymorphisms for five twin pairs and longitudinal assessment of parental report on physical resemblance for the other pairs.
Experimental paradigm and stimuli.
The paradigm used was modified from Hoogenboom et al. (2006) and is known to induce strong gamma-band synchronization in visual cortex in human MEG. While seated in the MEG, subjects viewed a foveally presented, circular sine wave grating that contracted toward the fixation point (2.7 cycles/°; velocity, 0.75°/s; contrast, 100%) that was presented on a translucent screen 80 cm in front of their eyes by using an EIKI LCD projector. In each of a total of 180 trials, subjects had to press a response button with their right index fingers when the stimulus increased velocity (step to 1.2°/s), which could occur at any unpredictable time, between 0.75 and 3.0 s poststimulus onset. After that, subjects had a rest period of 1000 ms in which they got visual feedback about their response.
MEG recordings.
Recordings were performed with a whole-head 275-channel axial-gradiometer MEG system (CTF Systems Inc.). Horizontal and vertical EOGs were corecorded for off-line artifact rejection. All signals were low-pass filtered at 300 Hz, digitized at 1200 Hz, and subsequently stored for off-line analysis.
MEG data analysis.
Data analysis of the MEG recordings was done by using the Fieldtrip open source Matlab Toolbox (Oostenveld et al., 2011) (www.ru.nl/neuroimaging/fieldtrip).
All data were first preprocessed to remove trial parts with artifacts originating from jumps in the superconducting quantum interference devices (SQUIDS), muscle activity, or eye movements, using a semiautomatic routine after high-pass filtering with a cutoff frequency of 5 Hz. Power line fluctuations were removed by using a discrete Fourier transform. Finally, trials were extracted from the data and defined as data segments starting 1000 ms before stimulus onset until stimulus change. Only trials in which the subjects gave a correct response were taken into account.
Time frequency spectral analysis.
For all subjects and trials, we performed time frequency analyses of the MEG power of 74 sensors over visual cortex (all O-sensors, ZP01, and left and right P11–P12, P21–P23, P31–P34, P41–P43, and P51–P54). Discrete Fourier transforms were applied to 500 ms data epochs sliding in 30 ms steps, and centered at −700 ms up to +2300 ms relative to stimulus onset (25–100 Hz, 2 Hz steps, ±4 Hz smoothing), using the multitapering method with Slepian functions (Mitra and Pesaran, 1999). Average spectral power was computed as the average across trials, tapers, and the 74 MEG sensors. Spectral power was expressed as relative increase (percentage change) compared with baseline levels, i.e., epochs centered at −500 to −100 ms relative to stimulus onset.
Spectral analysis for peak frequency determination.
To determine each subject's frequency of maximum power increase, we compared the average spectra across the stimulation period with those during the baseline epoch, in the same channels as in the time frequency analysis. Spectral estimation was performed on 50% overlapping 600 ms epochs within the stimulation epoch from 600 to 3000 ms after stimulus onset (avoiding the inclusion of the response onset transients). The baseline epoch extended from 700 to 100 ms before stimulus onset. The same multitapering method was used as in the time frequency analysis. All epochs were zero-padded to 10 s. The frequency range of interest was 40 to 100 Hz (±5 Hz smoothing).
To assess each subject's peak of gamma-band activation, we computed the percentage change of power in the stimulus period relative to the baseline condition, by dividing their respective spectra, for each individual. Gamma peak frequency was subsequently defined as the frequency bin with the highest increase within this 40 to 100 Hz range, which was used as input for the genetic analysis. In this range, all subjects showed a single peak of activation. Some subjects showed a second band of activity at lower gamma frequencies (25–40 Hz). The subsequent analysis of the correlation in gamma peak frequencies required a single frequency value per subject. In subjects with two bands, we used the upper band, because it was clearly a gamma (rather than a higher beta) band, and it always showed a stronger increase versus baseline.
To compare the similarities in peak frequencies within twin pairs in both groups (MZ and DZ twins), the intraclass correlation (ICC) was computed between the gamma frequencies of the members of each twin pair (Falconer and Mackay, 1996). The corresponding regression slope was subsequently estimated by averaging the slopes of 105 regressions in which axis assignment of data points (as x or y value) was randomly permuted. To compare twin pairs to unrelated subject pairs, we computed the ICC for 20 pairs of randomly drawn unrelated subjects. This was done 100 times to estimate the average ICC for unrelated subjects.
Genetic analysis.
The genetic influences to individual differences in gamma peak frequency were estimated by fitting a genetic structural equation model (SEM) to the peak frequency data in which the observed variance of the phenotype of interest is decomposed into genetic and environmental components (Falconer and Mackay, 1996). These components include variance caused by to additive (A) and nonadditive (D; i.e., genetic dominance, possibly including epistasis) genetic factors and individual environmental effects including measurement error (E). The relative contribution of these latent factors is expressed by the path coefficients a, d, and e, which are estimated by maximum likelihood. Based on the pattern of MZ and DZ twin correlations, the influence of D was included in the model rather than a common environment factor (Falconer and Mackay, 1996). To estimate the values of a, d, and e, the gamma peak frequency data of the MZ and DZ twin pairs were analyzed by using the Mx software package for genetic linear structural equation modeling (Neale et al., 2003). Overall goodness of fit of the full (ADE) model was determined on the basis of the χ2 statistic. Subsequently, the significance of factors A and D was assessed by means of likelihood ratio tests comparing the full model with a submodel from which these factors were constrained at zero. When the fit significantly worsened, the contribution of genetic factors was considered significant. The broad heritability (h2) of the gamma peak frequency was defined as the percentage of the total variance of the phenotype that could be explained by genetic factors (A+D).
Power.
We explored the power of our experimental design to detect broad sense heritability for different values of h2 based on the current sample size, by testing ADE versus E with a 2 df test and alpha of 5%. The power to detect an h2 of 40, 60, or 80% was 39, 79, and 98%, respectively, when the contributions of additive (a2) and nonadditive (d2) effects were equal. For higher values of h2, as suggested by this study, the power was 99% or higher.
Results
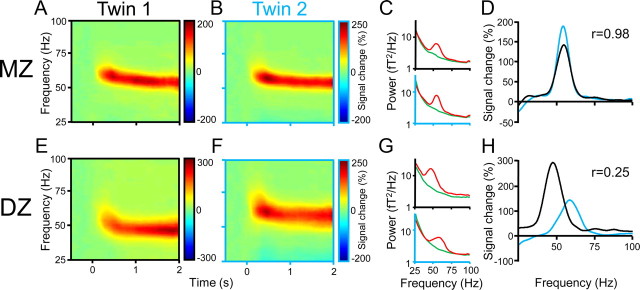
Figure 1A shows the visually induced oscillatory activity from occipital cortex of one example subject as a function of time after stimulus onset and frequency. The visual stimulus resulted in gamma-band activity that lasted for the entire stimulus duration and was confined to a frequency band from approximately 50–65 Hz. Similar gamma-band activity patterns have been described before in human subjects (Hoogenboom et al., 2006; Muthukumaraswamy et al., 2010). As also found typically, the gamma frequency was slightly higher just after stimulus onset and then settled onto a sustained level (Hoogenboom et al., 2006; Swettenham et al., 2009; Muthukumaraswamy et al., 2010). The respective data for this subject's MZ cotwin are shown in Figure 1B and reveal a strikingly similar pattern. The two subjects have spectral bands of activation that have the same temporal evolution and width and are centered on the same frequencies. For the same subjects, Figure 1C shows the raw power spectra averaged across the prestimulus baseline and the sustained activation epoch (0.8–1.8 s), and Figure 1D shows the corresponding power changes as a function of frequency. The cross-correlation of power changes across frequencies, between MZ twins, was 0.98 (95% CI, 0.97–0.98). Figure 1E–H shows the same analysis for a DZ twin pair. Figure 1, E and F, shows that visually induced oscillatory activity is dissimilar, which is confirmed by a much lower cross-correlation of 0.25 (95% CI, 0.18–0.31).
Figure 1.
Visually induced gamma-band activity in a MZ and a DZ twin pair. A, B, Time-frequency representations (TFRs) of activity in the gamma range relative to prestimulus baseline levels in two twins of a MZ pair, averaged across 74 parieto-occipital MEG sensors. Time = 0 s denotes stimulus onset. C, Spectra of average magnetic field strength during baseline (green) and stimulation (red) epochs in the same subjects. D, Percentage signal change caused by visual stimulation, superimposed for both twins (black line, twin 1; blue line, twin 2). Signal change spectra correlate significantly in this twin pair (r = 0.98; p < 0.001). E–H, Same analyses as in A–D applied to a DZ twin pair. TFRs (E, F) and signal change spectra (G, H) show strong dissimilarities.
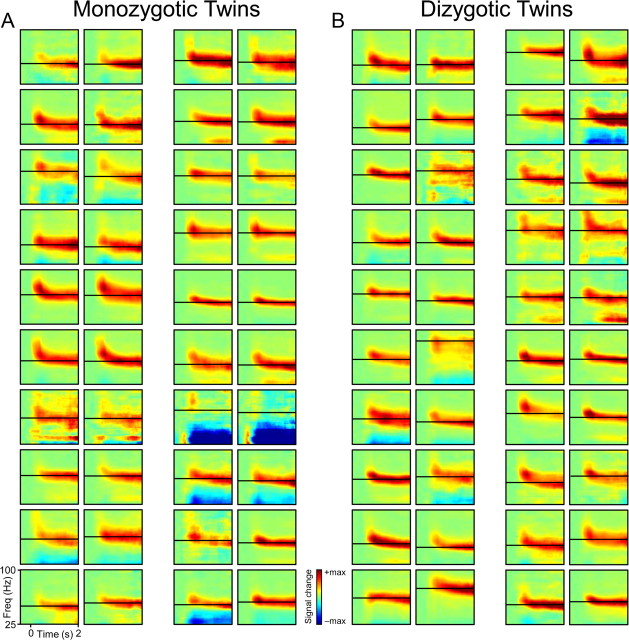
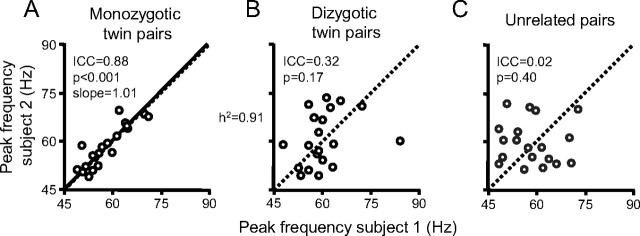
The similarity illustrated for the example MZ twin pair was consistent across the population of 20 MZ twin pairs studied (Fig. 2A). One MZ twin pair showed very weak gamma-band activation, which was again concordant for the two respective twins. Also the dissimilarity found in the example DZ twin pair was found repeatedly across the population of 20 DZ twin pairs (Fig. 2B). Both the similarity among MZ twins and the dissimilarity among DZ twins applied to several aspects of gamma-band activity, like strength, peak frequency, and width of the frequency band. For a quantitative test of gamma heritability, we focused on the peak frequency, because it is least affected by measurement conditions like the distance between the brain and the MEG sensors. Figure 3A shows the gamma peak frequencies of the MZ twin pairs, with each dot representing one twin on the x-axis and the cotwin on the y-axis (arbitrary axis assignment, see Materials and Methods). Peak frequencies ranged approximately 45–90 Hz, and there was a strong intraclass correlation (ICC) across MZ twin pairs (slope, 1.01; SD, 0.13; ICC, 0.88; 95% CI, 0.72–0.95; p < 0.001), whereas DZ twin pairs (Fig. 3B) showed low resemblance (ICC, 0.32; 95% CI, − 0.13–0.66; p = 0.17). Nonrelated subjects (Fig. 3C) had an even lower correlation of their peak frequencies (ICC, 0.02; p = 0.40), which was significantly lower than the DZ twin ICC (p < 0.001).
Figure 2.
TFRs of visually induced gamma-band activities in all 80 recorded subjects. A, MZ twins. B, DZ twins. Adjacent TFRs represent twin and cotwin of a single pair, with the upper left twins being pair 1, and the lower right twins pair 20. Color scales are normalized to each subject's maximum signal change. Horizontal black lines indicate each subject's peak frequency of gamma-band activation during the sustained activation epoch. TFRs of MZ pair 15 and DZ pair 2 are the example subjects from Figure 1.
Figure 3.
Correlation between gamma-peak frequencies. A, MZ twins. B, DZ twins. Each data point represents the peak frequency of one twin versus that of his or her cotwin (random axis assignment). Slope values are estimated by random permutations of x- and y-values. C, Example set of 20 unrelated subject pairs. The presented ICC is an average of 100 of such sets. h2, heritability estimate based on genetic structural equation modeling of the total of MZ and DZ data.
This marked difference between MZ and DZ pairs suggests a strong genetic determination of the gamma peak frequency. The relative contributions of additive genetic (A), nonadditive genetic (D), and environmental factors (E) were estimated through genetic SEM (see Materials and Methods for details). The standardized A, D, and E variance components were 0.225 and 0.681, corresponding to a broad heritability of 91% (CI, 80–96%). The standardized environmental variance component was estimated at 9% (95% CI, 4.8%–20%). Removing the influence of A and D factors from the model gave a significant worsening of the goodness of fit (χ2 = 32; df = 2; p < 0.001).
Discussion
In this study, we focused on the frequency of visually induced gamma-band activity for several reasons. The estimation of heritability of a particular quantitative trait requires that this trait be quantified in each twin individually. If the individual estimate was noisy, it would lead to an underestimation of the similarity among monozygotes and thereby to an underestimation of the actual heritability. Such noise appears particularly weak in the case of gamma-band activity in the visual system (Hoogenboom et al., 2006; Muthukumaraswamy et al., 2009, 2010). For a related reason, we focused on the gamma-band peak frequency. This parameter is immune to several factors like distance between brain and sensors, skull shape, or instrumentation noise.
The heritability found here applies strictly only to visually induced gamma. Yet, visual gamma shares central features with gamma-band activities in other areas or under different conditions (Bragin et al., 1995; Pesaran et al., 2002; Bauer et al., 2006). It is positively correlated to processing and functioning, is sustained along the corresponding process or function, and is of limited band width for a given subject and condition (for review, see Fries, 2009). Thus, gamma-band activities in different areas and conditions might well be similar in their underlying mechanisms and potentially reflect a fundamental process subserving several cognitive functions (Fries, 2009; Tiesinga and Sejnowski, 2009).
Previous twin studies on the heritability of brain rhythms focused on the classical EEG rhythms, delta, theta, alpha, and beta, and sleep rhythms (Vogel, 1970; Young et al., 1972; Lykken, 1982; van Beijsterveldt et al., 1996; Linkowski, 1999; Posthuma et al., 2001; Smit et al., 2006; Linkenkaer-Hansen et al., 2007). These were studied mainly during rest, when they are particularly pronounced (for review, see van Beijsterveldt and van Baal, 2002). By contrast, gamma-band activity in our study was induced by the visual stimulus and was most likely involved in stimulus processing and task performance (Fries, 2009). In agreement with this, the gamma peak frequency is modulated substantially by stimulus features such as contrast (Ray and Maunsell, 2010), size (Ray and Maunsell, 2011), or velocity (Swettenham et al., 2009). Such stimulus dependence might suggest a lower heritability of gamma than alpha peak frequency, because the latter is essentially fixed for a given individual (Steriade et al., 1990; Klimesch et al., 1996). By contrast, alpha frequency heritability has been found to be smaller (71–83%) than the gamma-frequency heritability observed here (≥91%) (Posthuma et al., 2001; Smit et al., 2006).
The gamma rhythm has consistently been linked positively to cognitive functions (Gray et al., 1989; Fries et al., 2001; Pesaran et al., 2002; Buschman and Miller, 2007; Tallon-Baudry, 2009) and the fMRI BOLD signal in both animals (Logothetis et al., 2001) and humans (Scheeringa et al., 2011). This has prompted particular interest in this rhythm. The strong heritability that we found for visually induced gamma-band activity warrants an investigation of the underlying genetic mechanisms, which might elucidate the causal role of gamma for cognition. Mechanisms behind gamma-band activity have so far been derived primarily from in vitro studies in combination with theoretical work (Traub et al., 2003; Kopell and Ermentrout, 2004; Bartos et al., 2007; Tiesinga and Sejnowski, 2009). Those studies highlighted the role of GABAergic inhibition. GABAergic inhibition is affected in schizophrenia (Lewis et al., 2005), which in turn is related to reduced gamma-band activity (Kwon et al., 1999). The current findings mandate future studies that will investigate in detail the interplay between GABAergic inhibition, gamma-band synchronization, and the vulnerability for conditions like schizophrenia.
Footnotes
S.V.P. and P.F. were supported by a European Young Investigator Award (to P.F.). P.F. was supported by a European Union FP7 grant. D.I.B. was supported by European Research Council Advanced Investigators Grant ERC-230374. We thank P. Uhlhaas and J. M. Schoffelen for critical reading of the manuscript; P. Gaalman, B. Daams, and S. Berends for technical support; and J. M. Vink for assistance.
References
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJ, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, Posthuma D, van Beijsterveldt TC, Hudziak JJ, Bartels M, Willemsen G. Netherlands Twin Register: from twins to twin families. Twin Res Hum Genet. 2006;9:849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol. 2002;87:2715–2725. doi: 10.1152/jn.2002.87.6.2715. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Rhythms of the brain. New York: Oxford UP; 2006. [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. Harlow, Essex, UK: Addison Wesley Longman; 1996. [Google Scholar]
- Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernández G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4:1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Schröder JH, Roelfsema PR, Singer W, Engel AK. Oscillatory neuronal synchronization in primary visual cortex as a correlate of stimulus selection. J Neurosci. 2002;22:3739–3754. doi: 10.1523/JNEUROSCI.22-09-03739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Green AE, Munafò MR, DeYoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage. 2006;29:764–773. doi: 10.1016/j.neuroimage.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schimke H, Pachinger T. Alpha frequency, reaction time, and the speed of processing information. J Clin Neurophysiol. 1996;13:511–518. doi: 10.1097/00004691-199611000-00006. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci U S A. 2004;101:15482–15487. doi: 10.1073/pnas.0406343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koten JW, Jr, Wood G, Hagoort P, Goebel R, Propping P, Willmes K, Boomsma DI. Genetic contribution to variation in cognitive function: an fMRI study in twins. Science. 2009;323:1737–1740. doi: 10.1126/science.1167371. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiat. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Smit DJ, Barkil A, van Beijsterveldt TE, Brussaard AB, Boomsma DI, van Ooyen A, de Geus EJ. Genetic contributions to long-range temporal correlations in ongoing oscillations. J Neurosci. 2007;27:13882–13889. doi: 10.1523/JNEUROSCI.3083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkowski P. EEG sleep patterns in twins. J Sleep Res. 1999;8:11–13. doi: 10.1046/j.1365-2869.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lykken DT. Presidential address, 1981. Research with twins: the concept of emergenesis. Psychophysiology. 1982;19:361–373. doi: 10.1111/j.1469-8986.1982.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76:691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD, Swettenham JB, Jones DK. Visual gamma oscillations and evoked responses: variability, repeatability and structural MRI correlates. Neuroimage. 2010;49:3349–3357. doi: 10.1016/j.neuroimage.2009.11.045. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie, Maes H. MX: statistical modeling. Richmond, VA: Department of Psychiatry, Virginia Commonwealth University; 2003. [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Neale MC, Boomsma DI, de Geus EJ. Are smarter brains running faster? Heritability of alpha peak frequency, IQ, and their interrelation. Behav Genet. 2001;31:567–579. doi: 10.1023/a:1013345411774. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Smit CM, Wright MJ, Hansell NK, Geffen GM, Martin NG. Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. Int J Psychophysiol. 2006;61:235–243. doi: 10.1016/j.ijpsycho.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinás RR, Lopes de Silva FH, Mesulam MM. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Swettenham JB, Muthukumaraswamy SD, Singh KD. Spectral properties of induced and evoked gamma oscillations in human early visual cortex to moving and stationary stimuli. J Neurophysiol. 2009;102:1241–1253. doi: 10.1152/jn.91044.2008. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C. The roles of gamma-band oscillatory synchrony in human visual cognition. Front Biosci. 2009;14:321–332. doi: 10.2741/3246. [DOI] [PubMed] [Google Scholar]
- Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area V4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Cunningham MO, Gloveli T, LeBeau FE, Bibbig A, Buhl EH, Whittington MA. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci U S A. 2003;100:11047–11052. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Lima B, Womelsdorf T, Oostenveld R, Singer W, Neuenschwander S, Fries P. Gamma-phase shifting in awake monkey visual cortex. J Neurosci. 2010;30:1250–1257. doi: 10.1523/JNEUROSCI.1623-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel F. Genetic basis of normal human electroencephalogram (EEG) Humangenetik. 1970;10:91–114. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma-band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci. 2008;28:2667–2679. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JP, Lader MH, Fenton GW. A twin study of the genetic influences on the electroencephalogram. J Med Genet. 1972;9:13–16. doi: 10.1136/jmg.9.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]