Abstract
Exercise has been shown to positively augment adult hippocampal neurogenesis; however, the cellular and molecular pathways mediating this effect remain largely unknown. Previous studies have suggested that microglia may have the ability to differentially instruct neurogenesis in the adult brain. Here, we used transgenic Csf1r-GFP mice to investigate whether hippocampal microglia directly influence the activation of neural precursor cells. Our results revealed that an exercise-induced increase in neural precursor cell activity was mediated via endogenous microglia and abolished when these cells were selectively removed from hippocampal cultures. Conversely, microglia from the hippocampi of animals that had exercised were able to activate latent neural precursor cells when added to neurosphere preparations from sedentary mice. We also investigated the role of CX3CL1, a chemokine that is known to provide a more neuroprotective microglial phenotype. Intraparenchymal infusion of a blocking antibody against the CX3CL1 receptor, CX3CR1, but not control IgG, dramatically reduced the neurosphere formation frequency in mice that had exercised. While an increase in soluble CX3CL1 was observed following running, reduced levels of this chemokine were found in the aged brain. Lower levels of CX3CL1 with advancing age correlated with the natural decline in neural precursor cell activity, a state that could be partially alleviated through removal of microglia. These findings provide the first direct evidence that endogenous microglia can exert a dual and opposing influence on neural precursor cell activity within the hippocampus, and that signaling through the CX3CL1–CX3CR1 axis critically contributes toward this process.
Introduction
Throughout adulthood, continuous birth of new neurons persists in the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricle (Reynolds and Weiss, 1992; Richards et al., 1992). While SVZ neurogenesis is important for the maintenance of olfactory functions (Imayoshi et al., 2008; Mouret et al., 2009), in the SGZ this process is thought to be critical for the ongoing hippocampal plasticity that is required for learning and memory (Deng et al., 2010). Although the production of new neurons declines progressively with age, there is now evidence that the adult SGZ retains a population of latent neural precursor cells (NPCs) that can be activated, thereby stimulating neurogenesis (Walker et al., 2008).
A variety of experience-based paradigms have been used to experimentally induce neurogenesis within the hippocampus (Gould and Tanapat, 1999), including environmental enrichment and voluntary exercise. Rodents with access to a running wheel exhibit significantly enhanced cell proliferation and neurogenesis within the SGZ, as well as improved performance in spatial memory and learning tasks (van Praag et al., 1999a,b). Importantly, voluntary exercise also counteracts the decline in NPC activity that normally occurs with aging (Blackmore et al., 2009; Jinno, 2011) and slows the associated cognitive impairment (van Praag et al., 2005; Sahay et al., 2011).
The effect of exercise on cell proliferation is not restricted to NPCs. Previous research has shown that a 10 d running paradigm increases proliferation of both cortical and hippocampal microglia (Ehninger and Kempermann, 2003; Olah et al., 2009). Microglia are versatile modulators of neurogenesis, and their influence on NPC activity is dependent on their activation status (Butovsky et al., 2006; Ziv et al., 2006; Cacci et al., 2008; Choi et al., 2008). Proinflammatory microglia are generally associated with reduced neurogenesis, as in this state they produce reactive oxygen species and nitric oxide, and release proinflammatory cytokines (Monje et al., 2003; Nakanishi et al., 2007). Conversely, neuroprotective microglia stimulate neurogenesis through the release of anti-inflammatory cytokines and growth factors (Aarum et al., 2003; Morgan et al., 2004; Battista et al., 2006; Butovsky et al., 2006; Walton et al., 2006; Ziv et al., 2006; Deierborg et al., 2010). However, the cellular and molecular pathways that mediate the positive effects of voluntary exercise are largely unknown, and whether exercise-induced microglial proliferation and/or altered activation status contribute to increased NPC activity also remains unclear (Vukovic et al., 2011). In addition, most studies that have investigated the role of microglia in adult hippocampal neurogenesis have been primarily correlative and a direct effect of microglia on NPCs is yet to be demonstrated. In this study, we therefore sought to address this issue by using transgenic models to investigate whether microglia have a direct regulating effect on the activity of adult hippocampal NPCs in response to voluntary exercise and aging.
Materials and Methods
Animals
We took advantage of Csf1r-GFP (MacGreen) transgenic mice to elucidate the role of microglia in NPC activity. In these mice, the coding sequence for the reporter gene green fluorescent protein (GFP) is expressed under the restricted control of the macrophage-specific Csf1r gene (Sasmono et al., 2003); Csf1r encodes the receptor for macrophage colony-stimulating factor 1, one of the earliest genes expressed in the macrophage lineage. In Cx3cr1gfp mice, GFP is knocked in at the mutant Cx3cr1 locus (Jung et al., 2000), resulting in GFP expression under Cx3cr1 promoter control and deficiency in this chemokine receptor when using Cx3cr1gfp/gfp knock-in mice. Csf1r-GFP and Cx3cr1gfp mice were backcrossed for >10 generations onto a C57BL/6J background. Mice used in this study were adult females, all of which were 6–8 weeks of age at the start of the exercise paradigm unless otherwise specified. Animals were housed in pairs, either with or without access to the running wheel; these groups are termed runner and nonrunner mice, respectively, throughout this study. All experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, with approval from the University of Queensland Animal Ethics Committee. Animals were maintained on a 12 h light/dark cycle with food and water provided ad libitum.
Immunostaining
GFP (Vector Laboratories) and Iba1 (ionized calcium binding adaptor molecule 1; Wako Chemical) antibodies were used to confirm that the GFP-positive (GFPpos) cells in the Csf1r-GFP mice were in fact microglia. For this, Csf1r-GFP mice (8 weeks old; n = 2) were deeply anesthetized with sodium pentobarbitone (150 mg/kg; Virbac) and transcardially perfused with 10 ml of PBS, pH 7.5, followed by 30 ml of 10% neutral buffered formalin, pH 7.5. The brains were postfixed in formalin, cryoprotected in 30% sucrose, and sectioned at 40 μm thickness using a sledge vibratome. All staining was done on free-floating sections (one in six series) through the entire hippocampus. Sections were washed three times for 10 min at room temperature (RT) and then incubated for 1 h in a blocking solution (1% BSA, 0.2% Triton X-100 in PBS). Sections were then left overnight at 4°C immersed in diluent containing monoclonal mouse anti-GFP (1:200) and rabbit anti-mouse Iba1 (1:250). The following day, sections were washed and incubated for 1 h at RT with goat anti-mouse Alexa Fluor 488 (1:500; Invitrogen) and goat anti-rabbit Alexa Fluor 546 (1:500; Invitrogen). Sections were washed three times in PBS at RT (5 min per wash), with the nuclear stain DAPI (1:1000; Invitrogen) included in the final wash. Sections were then immediately mounted in Dako fluorescent mounting medium.
Bromodeoxyuridine staining.
To quantify hippocampal precursor proliferation and neurogenesis in young and aged wild-type and Cx3cr1gfp/gfp mice, immunostaining for 5-bromo-2′-deoxyuridine (BrdU) and doublecortin (DCX) was performed. Adult (∼8-week-old) wild-type (n = 3) and Cx3cr1gfp/gfp (n = 4) animals were given a single intraperitoneal injection of the thymidine analog BrdU (100 mg/kg; Sigma-Aldrich) 2 h before being killed. To visualize proliferating cells in aged (20-month-old) animals, wild-type and Cx3cr1gfp/gfp animals were injected with BrdU once daily for 5 consecutive days at consistent time points and killed 48 h after the final BrdU injection. Brain tissue was then processed and immunostained as described above; however, an additional acid treatment step was included, where sections were treated in 1N HCl at 45°C for 20 min, washed in 0.1 m boric acid, pH 7.4, and then incubated overnight at 4°C in a rat anti-BrdU antibody (1:500; AbCam) and a primary rabbit anti-DCX antibody (1:500; AbCam) diluted in PBS, 10% normal goat serum, and 0.2% Triton X-100. The secondary antibodies goat anti-rat Alexa Fluor 568 (1:1000; Invitrogen) and goat anti-rabbit Alexa Fluor 647 (1:1000; Invitrogen) were then applied as appropriate. Similar procedures were used to investigate microglia proliferation under the various experimental conditions, for which colocalization of BrdU with GFP or Iba1 staining was assessed.
Imaging and quantification.
Images were taken at 20× magnification using a Zeiss Axio Imager microscope and AxiocamMRm/3 camera together with AxioVision Software (Zeiss, version 4.8.2). To determine the number of BrdU-positive (BrdUpos) and DCX-positive (DCXpos) cells within each section, Z-stacks were taken at 1.75 μm intervals throughout the entire 40 μm section.
Hippocampal cell preparations
Mice were killed by cervical dislocation, their brains immediately removed, and the hippocampus dissected. Adult hippocampal tissue was digested by incubation in a mixture containing 0.1% papain (Worthington Biochemical Corporation) and 0.1% DNaseI (Roche Australia) in HBSS (Thermo Scientific) for 16 min at 37°C, triturating twice during the incubation period. Next, the tissue was centrifuged at 100 × g for 5 min, after which the pellet was resuspended and washed twice in 2 ml of neurosphere growth medium: mouse NeuroCult NSC basal medium containing mouse NeuroCult NSC proliferation supplements (Stem Cell Technologies), 2% BSA (Invitrogen), and 2 μg/ml heparin (Sigma). The medium also included 20 ng/ml purified mouse receptor-grade epidermal-like growth factor (BD Biosciences) and 10 ng/ml recombinant bovine fibroblast growth factor-2 (Roche). The solution was then mechanically triturated until smooth and filtered through a 40 μm cell sieve (Falcon; BD Biosciences) to obtain a single cell suspension, which was further processed using fluorescence-activated cell sorting (FACS). Snapshots of GFPpos hippocampal microglia, counterstained for 5 min at RT with DRAQ5 nuclear dye (1:1000; Abcam) were obtained using AMNIS ImageStream 100.
Fluorescence-activated cell sorting and culturing procedures
To deplete microglia from hippocampal cells cultures, dissociated cells were sorted using a FACSVantage SE DiVa sorter (BD Biosciences). Six to eight hippocampi were used per preparation to obtain a single-cell suspension; GFPpos cells (i.e., microglia) were removed from cell cultures (i.e., microglia-depleted cultures), whereas the control preparations were simply passed through the cell sorter. The cells were sorted into 24-well plates (30,000 events/well) containing 2 ml of neurosphere medium.
Hippocampal cell suspensions were depleted of microglia and plated at 20,000 events/well. Next 10,000 GFPpos cells derived from either nonrunner or runner mice were added to the wells containing microglia-depleted suspensions. To test the effect of CX3CL1, recombinant mouse CX3CL1 (R&D Systems) was added to the cultures to produce concentrations ranging from 10 to 400 ng/ml. The cells were grown in neurosphere growth medium and incubated at 37°C in a humidified 5% CO2 incubator for 14 d. After the 2 week incubation, neurospheres were measured under a bright-field microscope using an eyepiece graticule. Neurospheres with a diameter of ≥40 μm were counted. Where appropriate, neurosphere numbers were normalized against the matching control and thus expressed as the percentage change for individual experiments.
BrdU-positive cell counts using FACS.
Animals were provided with access to the running wheel for a period of 2 weeks, during which time BrdU was administered in the drinking water. Whole hippocampi were analyzed by FACS as previously described (Catts et al., 2008; Colditz et al., 2010). Briefly, the hippocampus was dissociated and a single-cell suspension prepared, as described above. The cell preparation was then fixed with ethanol, and cells were stained using the nuclear marker 7-aminoactinomycin D (Invitrogen) to obtain the total number of cells in the preparation. To obtain the number of proliferating cells, phycoerythrin-conjugated anti-BrdU antibody (BD PharMingen) staining was performed according to the manufacturer's instructions. The cell suspension was then analyzed using an LSRII flow cytometer (BD Biosciences).
MHCII staining.
MHCII-positive cells were quantified, analyzed, and sorted using FACS. In brief, cells were incubated with unconjugated rat anti-CD16/32 (1:100; BD PharMingen) for 5 min at RT, followed by incubation with anti-I-A/I-E antibody (1:200) for 15 min at RT. The cells were washed once in fresh medium before analysis and/or FACS.
Intraparenchymal infusion of CX3CR1 blocking antibody
Adult Csf1r-GFP mice were anesthetized via intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), after which an osmotic minipump cannula (Alzet) was inserted directly into the hilus region of the hippocampus (stereotaxic coordinates relative to bregma: anterior/posterior, −1.8 mm; medial/lateral, −1.0 mm; dorsal/ventral, −1.7 mm). Experimental mice received 5 μg of α-CX3CR1 (Torrey Pines Biolabs; n = 8) (Bachstetter et al., 2011) or control IgG (Sigma-Aldrich; n = 6 animals) per day (flow rate of 0.11 μl/h) over a 2 week period, during which time the animals had access to a running wheel. After completion of the infusion period, the mice were killed, hippocampi were dissected, and cell preparations from two animals were pooled for each experimental repeat. Neurospheres were cultured in either the presence or absence of GFPpos microglia, which were sorted using FACS, as detailed above.
ELISA
A mouse CX3CL1 ELISA Quantikine (MCX310, R&D Systems) was used, according to the manufacturer's instructions, to determine soluble CX3CL1 concentrations in the hippocampus and serum. Dissected hippocampi were snap frozen in cooled isopentane and stored at −80°C. On the day of the assay, frozen hippocampal tissue was thawed on ice. Next, to create a single-cell suspension, the tissue was triturated 15 times in Tris-buffered saline, pH 7.4, containing protease inhibitor mixture (Sigma-Aldrich) and EDTA. Supernatant containing soluble CX3CL1 was collected after centrifugation at 1000 × g for 10 min at 4°C.
To measure serum CX3CL1 levels, blood was collected transcardially at the time mice were killed and was allowed to clot at 4°C for 4 h, after which the sample was centrifuged at 2000 × g for 20 min at 4°C. Serum was isolated, and a protease inhibitor mixture was added (10% total serum volume; Sigma). Serum samples were then stored at −20°C until the ELISA was performed. All ELISA samples were run in duplicate.
Absorbance was determined using a model microplate reader at wavelengths of 450 and 540 nm as per the manufacturer's instructions. A standard curve was developed using Graphpad Prism (version 5.0c) and linear-regression analysis applied to determine the concentration of CX3CL1 present. Total protein concentration was determined using a BCA protein assay kit (Pierce Biotechnology) according to the manufacturer's instructions. Absorbance was measured with a PolarSTAR Optima spectrophotometer (BMG Labtech).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5.0c). Data were analyzed using an unpaired two-tailed Student's t test or a one-way ANOVA with Newman–Keuls post hoc test, as appropriate. Values are expressed as the mean ± SEM with significance determined at p < 0.05.
Results
Microglia regulate neural precursor activation following running
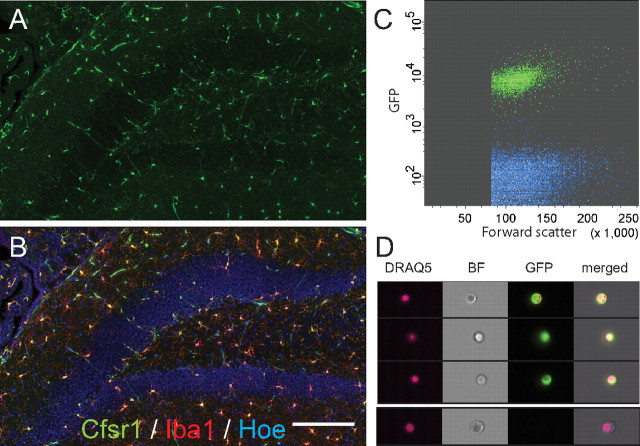
To selectively isolate microglia, we took advantage of Csf1r-GFP transgenic mice. In cross sections of adult brain tissue, GFPpos cells displayed the typical ramified morphology that is characteristic of brain microglia (Fig. 1A). Double immunofluorescence staining with the marker Iba1 confirmed all GFPpos cells to be microglia, including those observed in the hippocampus (Fig. 1A,B). We next used FACS to isolate microglia from hippocampal cell preparations. GFPpos cells formed a discrete population within hippocampal cell suspensions from Csf1r-GFP mice (Fig. 1C). The GFPpos cells accounted for ∼10% of the total cell population. Sample snapshots of nucleated (DRAQ5pos) GFPpos cells, taken during flow cytometric analysis, are shown in Figure 1D. No neurosphere formation was observed when the GFPpos microglia were sorted and cultured in neurosphere medium (data not shown).
Figure 1.
Characterization of hippocampal transgene expression and neurosphere formation frequency in Csf1r-GFP mice. A, Photomicrograph showing the adult hippocampus of a Csf1r-GFP mouse. Note that GFPpos cells display the typical ramified morphology characteristic of brain microglia. B, Double-immunofluorescence staining for Iba1 (red) and GFP (green) confirmed that the GFPpos cells were indeed microglia. Cell nuclei are shown in blue. C, GFPpos cells formed a discrete population of cells within hippocampal cell suspensions from Csf1r-GFP mice, constituting ∼10% of all cells. D, Photomicrographs of single Draq5pos/GFPpos cells and Draq5pos/GFPneg cells from dissociated Csf1r-GFP hippocampus, taken during flow cytometry. Note the morphological homogeneity of sorted microglia. Scale bar, 160 μm.
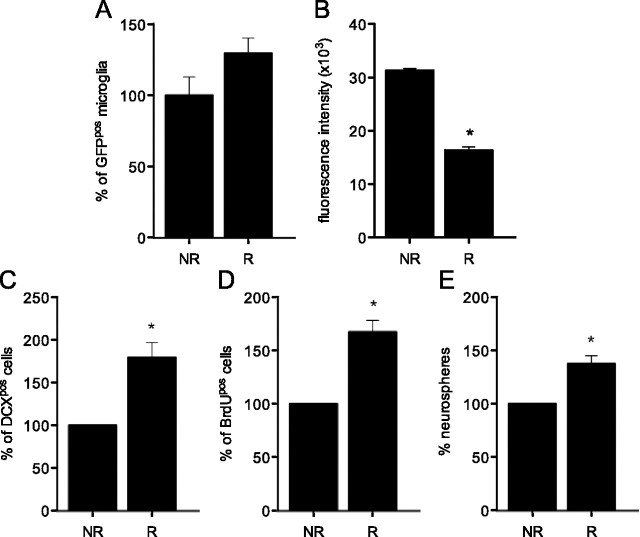
Following running, the overall number of GFPpos microglia was not significantly different between runner and nonrunner mice, although a trend toward higher numbers of GFPpos cells was observed in runner animals (Fig. 2A). The median intensity of GFP fluorescence within the population of hippocampal microglia was reduced by 47% in runner animals, indicating reduced expression of Csf1r in response to voluntary exercise (Fig. 2B). As anticipated, DCXpos and BrdUpos cell numbers were significantly increased, by 67 ± 10% and 74 ± 10%, respectively, in the hippocampus of runners (p = 0.004 and p = 0.017, respectively) (Fig. 2C,D). Furthermore, when hippocampal cells were sorted and cultured for a period of 14 d, a significant increase (37.3 ± 7%; p = 0.001) in the number of neurospheres was observed in cultures derived from the hippocampi of runners compared with nonrunner controls (Fig. 2E).
Figure 2.
Effects of voluntary exercise on microglial proliferation and neural precursor cell activity. A, The total number of GFPpos hippocampal microglia was not significantly increased as a result of exercise (n = 10). B, Flow cytometry also revealed a significant decrease in the median intensity of GFP fluorescence following a 2 week running period, suggesting reduced Csf1r-driven transgene expression under this experimental condition (*p < 0.05; n = 3). C, Running (R) significantly increased the number of DCXpos cells in the dentate gyrus of the adult mouse hippocampus compared with nonrunner (NR) controls (*p < 0.05, n = 5 per experimental condition). D, E, Similarly, significantly greater numbers of BrdUpos cells (D) and neurospheres (E) were observed following voluntary running (*p < 0.05, n = 3–5 per experimental condition). R, Runner mice; NR, Nonrunner mice.
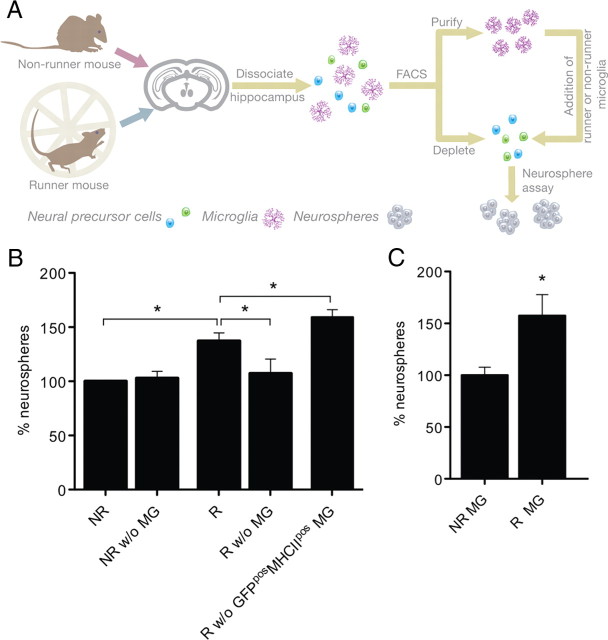
To address whether microglia play a role in the increase in the number of neurospheres obtained following running, we selectively depleted GFPpos microglia from hippocampal neurosphere preparations of runner and nonrunner mice. Conversely, we also isolated GFPpos microglia from runner and nonrunner hippocampi and added these to microglia-depleted neurosphere cultures of naive control mice. A schematic of these experiments is shown in Figure 3A.
Figure 3.
Microglia mediate the beneficial effects of exercise on NPC activity. A, Schematic diagram of the experimental paradigm used to assess the role of hippocampal microglia in NPC activation following running. In brief, hippocampi from experimental Csf1r-GFP mice were dissociated, after which GFPpos microglia were either sorted as a purified cell population or depleted from neurosphere preparations through FACS. B, Hippocampal cell preparations from nonrunner mice (NR) and runner (R) mice were split in half such that microglia MG could be removed from part of the obtained suspension. NR w/o MG, Nonrunner without microglia; R w/o MG, runner without microglia. Depletion of microglia from nonrunner hippocampal cultures did not affect the neurosphere-forming frequency compared with baseline (p > 0.05; n = 5 per experimental condition). Note, however, that depletion of microglia from hippocampal neurosphere cultures completely annulled the positive effect of voluntary exercise on NPC activity (*p < 0.05; n = 5 per experimental condition). Removal of MHCIIpos microglia from cell preparations of runner hippocampi resulted in a further increase in the frequency of neurosphere formation (*p < 0.05; n = 6 per experimental condition), suggesting that MHCIIpos microglia negatively control NPC activity. C, Hippocampal cell preparations were depleted of microglia and cocultured with microglia derived from nonrunners and runners. NR MG, nonrunner microglia; R MG, runner microglia. Addition of microglia from runner mice resulted in a significantly greater number of neurospheres in hippocampal cell cultures from sedentary mice that were depleted of endogenous microglia relative to controls in which microglia from nonrunner mice were added (*p < 0.05; n = 7 per experimental condition).
Depletion of GFPpos microglia from hippocampal cell suspensions of nonrunner mice did not alter precursor activation, based on the observation that the frequency of neurosphere formation was not significantly different from that in the control. However, removal of GFPpos microglia from hippocampal neurosphere preparations of runner mice abolished the effect of voluntary exercise on the neurosphere formation frequency, causing a significant 30% reduction (n = 5, p < 0.05) in neurosphere numbers compared with the cultures in which microglia were retained (Fig. 3B). These data suggest that voluntary exercise changes the function of microglia, which in turn contributes to the activation of NPCs.
As a previous study had reported a positive correlation between neurogenesis and MHCII expression under environmentally enriched conditions (Ziv et al., 2006), we next depleted GFPposMHCIIpos microglia from hippocampal cell cultures of both nonrunner and runner mice via FACS. Selective removal of these cells resulted in a significant further increase (p < 0.05) in neurosphere number in runner mice (Fig. 3B), but not in nonrunner animals. Thus, GFPposMHCIIpos cells appear to negatively regulate, rather than stimulate, neural precursor activation following running. Additional analysis showed that the proportion of MHCIIpos microglia was reduced by 37% in animals with access to the running wheel compared with nonrunner mice. This result suggests that subpopulations of microglia exert differential effects on NPC activation.
To further investigate this possibility, we selectively isolated GFPpos microglia from the hippocampus of either runner or nonrunner mice and added them to hippocampal cell preparations of nonrunner control mice that were depleted of microglia. In line with our previous findings, the addition of microglia from runner mice increased the neurosphere formation frequency by 57 ± 20% (n = 7 per group, p = 0.02) (Fig. 3C). This highlights the beneficial influence of voluntary exercise on microglia and reveals a novel role for these cells in mediating exercise-induced activation of NPCs.
Is CX3CL1 involved in regulating microglia-dependent precursor activation following running?
Having established a critical role for microglia in mediating the beneficial effects of exercise on NPC activation, we next sought to unravel the underlying molecular mechanism that regulates microglia function in relation to neural precursor activation. Previous reports have highlighted that signaling through the CX3CR1–CX3CL1 axis results in more neuroprotective microglia (Cardona et al., 2006), with positive modulation of hippocampal neurogenesis (Bachstetter et al., 2011). Using double-immunofluorescent staining procedures, we found that the total number of BrdUpos (i.e., proliferating) microglia was increased in the hippocampus of runner wild-type mice (runner: 15.50 ± 3.78 cells; nonrunner: 4.00 ± 0.91 cells; n = 4 per group; p = 0.03), which is similar to the observations of Olah et al. (2009). However, as these cells represent only a very small proportion of the total number of microglia, the overall number was not significantly changed (Fig. 2A). The number of BrdUpos microglia in runner and nonrunner Cx3cr1gfp/gfp mice [hereafter referred to as knock-out (KO) mice] were not significantly different from each other (nonrunner KO: 14.00 ± 1.08; runner KO: 16.25 ± 1.65 cells; n = 4 per group; p = 0.30) and similar to the number of double-positive cells seen in wild-type runner mice.
In line with Bachstetter et al. (2011), we confirmed reduced hippocampal neurogenesis in 2-month-old KO mice. An ∼25% decrease in the density of BrdUpos cells (p = 0.032) (Fig. 4A) and DCXpos cells (p = 0.036) (Fig. 4B) was observed in the SGZ of the KO animals compared with their wild-type counterparts. Similarly, aged (20-month-old) KO animals had significantly fewer BrdUpos cells (∼65% less) than wild-type animals (p = 0.049) (Fig. 4C) in the SGZ and the granule cell layer. The total number of DCXpos cells was also reduced to ∼60% of that observed in the wild-type mice (p = 0.037) (Fig. 4D). The number of BrdUposDCXpos cells within the SGZ of aged KO animals was also significantly reduced compared with that in wild-type controls (0.43 ± 0.07 vs 0.17 ± 0.03 cells/mm; n = 4 per group; p = 0.01). Thus, deficiency in CX3CR1 results in impaired hippocampal neurogenesis in both young and aged mice.
Figure 4.
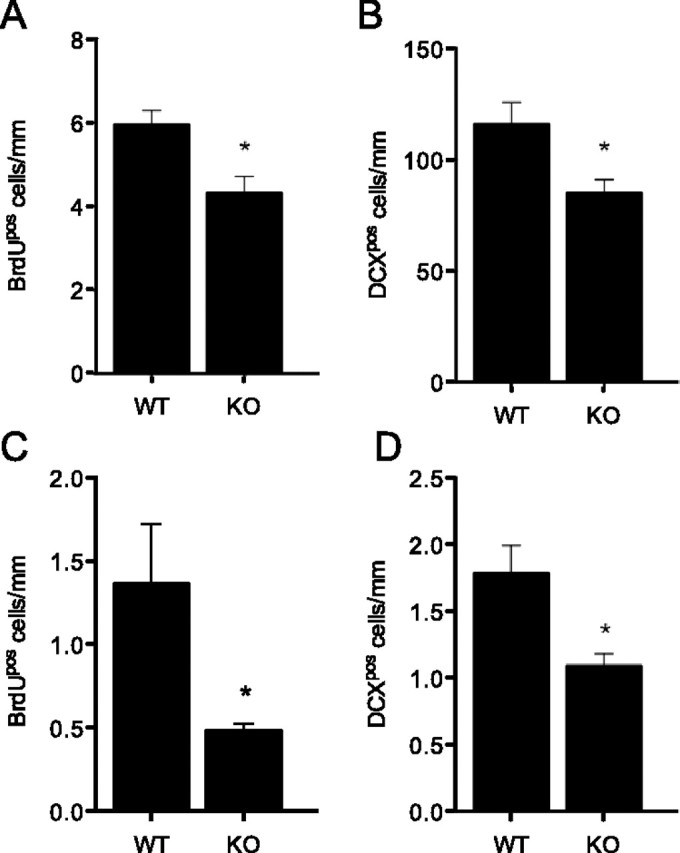
CX3CR1 deficiency negatively affects adult hippocampal neurogenesis. A–D, Cx3cr1gfp/gfp (KO) hippocampi had significantly lower BrdUpos and DCXpos cell numbers compared with their wild-type (WT) counterparts in young (2-month-old) (A, B) and aged (20-month-old) (C, D) animals (*p < 0.05; n = 3–4 per experimental condition).
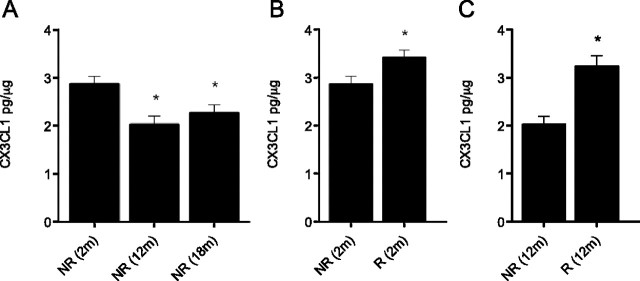
To examine a putative role for CX3CL1–CX3CR1 signaling in neural precursor activation following exercise, we next measured the levels of soluble CX3CL1 in the hippocampus of both young and aged animals. CX3CL1 levels were significantly reduced in 12- and 18-month-old nonrunner mice (by 22% and 20%, respectively) compared with young (2-month-old) animals (p < 0.05) (Fig. 5A). This reduction in soluble CX3CL1 levels within the hippocampus thus correlated with the age-related reduction in NPC activity. We therefore reasoned that if soluble CX3CL1 levels were responsible for mediating, at least in part, the exercise-dependent effect of microglia on NPC activation, it should be possible to detect an increase in soluble CX3CL1 levels after voluntary exercise. Indeed, following a running period of 14 d, the levels of soluble CX3CL1 were significantly increased in the hippocampus of the runners when compared with nonrunner controls (p = 0.039) (Fig. 5B). This increase in soluble CX3CL1 levels was specific to the hippocampus, as no concomitant increase in serum levels was observed (data not shown). To examine whether voluntary exercise could also counter the decline in soluble CX3CL1 levels observed within the aging hippocampus, 12-month-old wild-type mice were allowed access to a running wheel. After 14 d, a nonsignificant trend toward increased levels of soluble CX3CL1 was observed in runners compared with nonrunner controls (data not shown). However, following an extended running period of 28 d, a significant increase in soluble CX3CL1 was observed (p = 0.004) (Fig. 5C), with levels being restored to those observed in young (2-month-old) mice. Thus, running increases soluble CX3CL1 levels in the hippocampus.
Figure 5.
Effect of age and voluntary exercise on CX3CL1/fractalkine levels in the adult mouse hippocampus. A, Under sedentary conditions, a significant decrease in the levels of soluble (i.e., cleaved) CX3CL1 was observed in 12- and 18-month-old mice compared with their younger (2-month-old) counterparts (*p < 0.05; n = 4 per experimental condition). B, Voluntary wheel running resulted in a small but significant increase in hippocampal CX3CL1 levels in young mice (*p < 0.05; n = 6 per experimental condition). C, Exercise also increased hippocampal CX3CL1 levels in aged mice. Voluntary wheel running for a period of 28 d significantly increased CX3CL1 levels in 12-month-old mice (*p < 0.05; n = 5 per experimental condition), restoring these to the levels normally observed in young, nonrunner hippocampus (compare with A). R, Runner; NR, nonrunner.
We next employed a CX3CR1 blocking antibody to disrupt local CX3CL1 signaling in the hippocampus. Intraparenchymal infusion of α-CX3CR1 blocking antibody over a 2 week running period reduced neurosphere numbers by 60% compared with IgG infusion (p < 0.05) (Fig. 6A). The importance of microglia in mediating the beneficial effects of voluntary exercise was again confirmed through their depletion, after which a reduction in neurosphere numbers was observed. However, there was no difference in neurosphere numbers between α-CX3CR1- and IgG-infused animals following depletion of microglia (14 ± 3 vs 18 ± 3 neurospheres; p > 0.05), indicating that the negative effect of blocking the CX3CL1–CX3CR1 signaling pathway was microglia dependent. No difference in the number of GFPpos microglia was observed between the experimental conditions (5.87 ± 1.15% vs 5.91 ± 1.37%).
Figure 6.
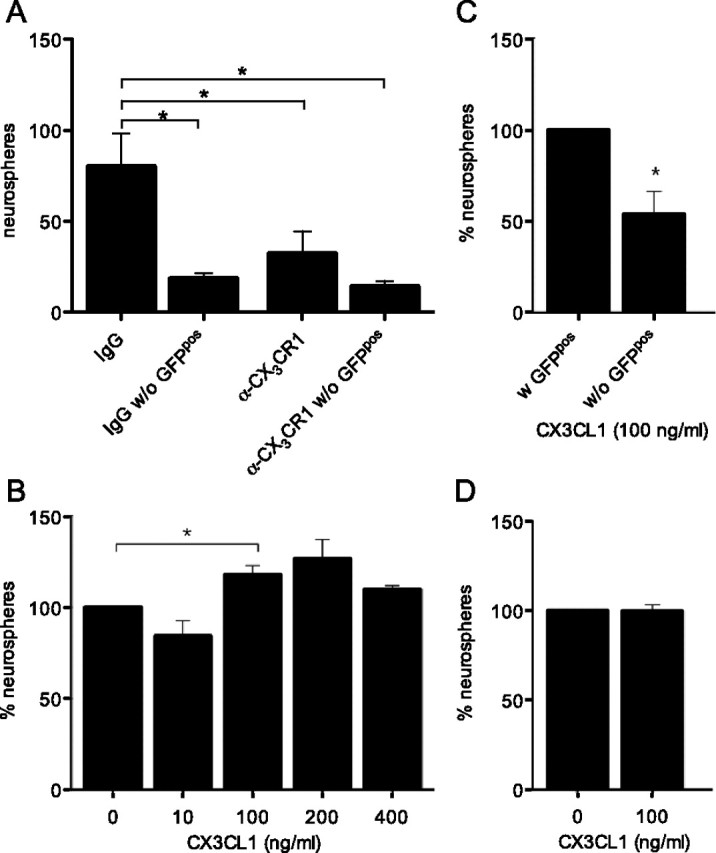
Signaling through the CX3CL1–CX3CR1 axis mediates neural precursor activation. A, Intraparenchymal infusion of a CX3CR1 blocking antibody into the hippocampus abrogated the effect of running on the frequency of neurosphere formation to a similar extent as microglia depletion (*p < 0.05; n = 3–4 per experimental condition). B, Addition of CX3CL1 to crude hippocampal cell preparations resulted in a dose-dependent increase in neurosphere numbers, with the optimal dose being 100 ng/ml (*p < 0.05; n = 3 per experimental condition). C, Microglia are critical for mediating the CX3CL1 effect as their depletion abolished the response. An approximate twofold reduction in the neurosphere-forming frequency was observed in cultures from which microglia were depleted (*p < 0.05; n = 5 per experimental condition), despite the presence of 100 ng/ml CX3CL1. D, No benefits of CX3CL1 addition on neural precursor activity were observed in hippocampal neurosphere preparations from Cx3cr1gfp/gfp mice, indicating that the effect was specifically mediated via CX3CR1.
We next sought to confirm in vitro that CX3CL1 was indeed one of the factors mediating the effect exerted by hippocampal microglia on NPC activity following running, hypothesizing that the presence of this chemokine within the culture medium in such a scenario would increase neurosphere formation. Indeed, the addition of recombinant CX3CL1 to hippocampal neurosphere cultures increased the activation of neural precursors in a dose-dependent manner (Fig. 6B). A concentration of 100 ng/ml CX3CL1 was selected as the optimal dose with which a significant increase (18.1 ± 4.9%, p = 0.003) in the number of neurospheres was observed over baseline values. The positive effect of CX3CL1 addition on NPC activity was abolished when microglial cells were depleted from hippocampal neurosphere cultures; in the absence of microglia, neurosphere numbers were reduced by 54.10 ± 12% (p = 0.006) (Fig. 6C). The addition of CX3CL1 to neurosphere cultures from hippocampi of CX3CR1-deficient mice did not change the neurosphere-forming frequency, confirming that the observed effect was indeed mediated via signaling through CX3CR1 (Fig. 6D). These data thus indicate that the effect of CX3CL1 signaling on NPCs is mediated through CX3CR1pos microglia.
Microglia suppress neural precursor cell activity in the aged brain
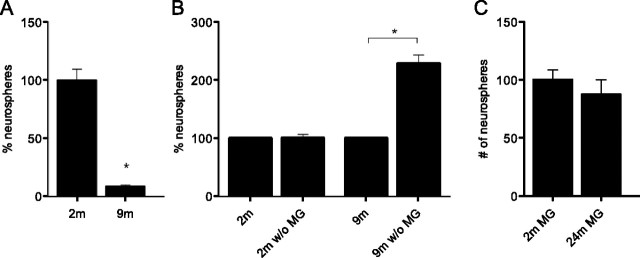
Finally, as a reduction in NPC activation is a naturally occurring event associated with aging (Walker et al., 2008), we also investigated whether changed microglial function contributes to this phenomenon. We first reconfirmed the age-related decline in neurosphere formation frequency in aged (9-month-old) mice compared with young (2-month-old) wild-type mice. The number of neurospheres declined significantly (by 91%) between 2 and 9 months of age (Fig. 7A). To assess whether an altered, perhaps more proinflammatory, activation profile of microglia within the aging brain (Frank et al., 2006; Henry et al., 2009) contributes to the decline in NPC activation, we depleted microglia from hippocampal neurosphere cultures of both young and aged mice (Fig. 7B). As in our earlier experiments, the depletion of microglia from hippocampi of young animals had no significant effect on neurosphere number. However, the depletion of microglia from aged hippocampi resulted in a doubling of neurosphere numbers in 9-month-old animals (p = 0.001; Fig. 7B). These findings indicate that microglia in the aged brain contribute to suppression of NPC activity. Addition of aged (24-month-old) nonrunner microglia to hippocampal neurosphere preparations of young mice did not result in a significant decline in neurosphere numbers (2-month-old mice: 100 ± 8 neurospheres; 24-month-old mice: 87 ± 12 neurospheres; n = 6; p = 0.48) (Fig. 7C), which may reflect the high intrinsic growth potential of NPCs at this age.
Figure 7.
Microglia in the aged hippocampus negatively control neural precursor cell activity. A, The frequency of neurosphere formation, which is a direct reflection of NPC activity, significantly declines with age between 2 and 9 months of age (*p < 0.05; n = 3 per experimental condition). B, Depletion of microglia from the hippocampi of young (2-month-old) sedentary mice did not affect the neurosphere frequency. Note, however, that microglia depletion at more advanced ages significantly increased neurosphere numbers (*p < 0.05; n = 3 per experimental condition). C, Addition of microglia from aged hippocampus to microglia-depleted hippocampal cell cultures of young mice did not significantly impact on the neurosphere frequency compared with control cultures in which hippocampal microglia from 2-month-old mice were added (p > 0.05; n = 6 per experimental condition); MG, microglia; w/o MG, without microglia.
Discussion
In the present study, we aimed to better understand the role of microglia in NPC activity under physiological conditions that are known to influence neurogenesis: exercise and aging. We took advantage of Csf1r-GFP transgenic mice to develop a novel ex vivo model system in which microglia could be selectively depleted or, alternatively, isolated by flow cytometry and subsequently added to neurosphere cultures (Fig. 3A). In doing so, we were able to show that endogenous hippocampal microglia influence NPC activity following a 2 week voluntary running paradigm. Conversely, the addition of hippocampal microglia isolated from mice allowed to exercise voluntarily for 2 weeks resulted in activation of the NPC population and an increase in the neurosphere-forming frequency in preparations from sedentary mice. Depletion of microglia from neurosphere preparations of aged sedentary mice partially alleviated the reduction in NPC activity that is naturally observed with aging. We were also able to show that voluntary exercise increased soluble CX3CL1 protein levels within the hippocampus, whereas intraparenchymal infusion of a CX3CR1 blocking antibody but not control IgG eliminated the microglia-mediated increase in neurosphere formation frequency that is normally observed following running. Additional in vitro experiments supported the view that signaling through the CX3CL1–CX3CR1 axis critically contributes to modifying the microglia phenotype toward one that increases NPC activity in support of neurogenesis.
Role of microglia in neural precursor activation
There is now a substantial body of evidence to suggest that NPC activity and neurogenesis within the adult hippocampus are influenced by a wide variety of physiological factors, including exercise, aging, depression, or inflammation (for review, see Vukovic et al., 2011). It has been speculated that altered immune and/or microglial activation status in some of the aforementioned parameters may influence NPC activity. However, direct evidence in support of this has been limited. The extent to which microglia can influence hippocampal neurogenesis has largely remained ambiguous due to the lack of an appropriate model system. PU1−/− mice, which do not have the monocytic cell lineage and thus lack microglia (McKercher et al., 1996; Henkel et al., 1999; Dakic et al., 2005), would appear to be the most likely candidates for assessment of the influence of microglia; however, these animals are embryonically lethal (18 d postcoitum) and were therefore unsuitable for our study. Previous in vivo studies attempting to elucidate the role of microglia and their activation status in adult neurogenesis have been mostly correlative, examining, for example, microglia numbers under various experimental conditions but not directly demonstrating a specific role for these cells in the regulation of NPC activity and neurogenesis (Ekdahl et al., 2003; Monje et al., 2003; Ziv et al., 2006; Olah et al., 2009). Additional in vitro studies have yielded generalized insights regarding how microglia could differentially influence adult neurogenesis (Walton et al., 2006; Deierborg et al., 2010), with Walton et al. (2006) being the first to suggest that a soluble factor found in the microglia-conditioned medium could rescue the ability of extensively passaged SVZ neural precursors to differentiate into immature neurons. Other studies have assessed neurogenesis in response to artificial stimulation with lipopolysaccharide or cytokines (Aarum et al., 2003; Deierborg et al., 2010). However, most of these investigations used neocortical microglia preparations derived from newborn pups and maintained in culture for extended periods of time. As microglial phenotypes appear to differ across brain regions and ages (Lawson et al., 1990; Ren et al., 1999; Kim et al., 2000; Butovsky et al., 2006; Njie et al., 2012), any conclusions drawn from these studies would be difficult to extrapolate to adult hippocampal neurogenesis.
Our approach, using Csf1r-GFP transgenic mice in combination with FACS, offered us the unique advantage of being able to assess the role of hippocampal microglia in adult neurogenesis under more physiological conditions that are known to influence NPC activity in situ. Through depletion of microglia from runner hippocampi or their addition to neurosphere preparations from sedentary mice, we have been able to provide the first direct evidence that the positive effect of voluntary exercise on NPC activity within the hippocampus is dependent on microglia. In line with the prevailing scientific view that microglia are more proinflammatory in the aging brain, and to some degree dysfunctional (Conde and Streit, 2006; Njie et al., 2012), we were able to demonstrate that removal of microglia from preparations of 9- and 20-month-old mice increased the neurosphere formation frequency. Thus, hippocampal microglia directly and differentially influence NPC activity in situ in two physiological paradigms: exercise and aging.
Dual and seemingly opposing roles of microglia in adult neurogenesis have been reported in previous studies. For instance, the decline in NPC activity within the aging hippocampus occurs in parallel with an increase in the overall density of microglia and signs of activation (Ogura et al., 1994; Mouton et al., 2002; Sandhir et al., 2008). Conversely, increases in the number of presumably beneficial microglia within the dentate gyrus, as a result of exposure to enriched environments or physical activity, have been correlated with elevated hippocampal neurogenesis (Ziv et al., 2006; Choi et al., 2008), although this observation has been disputed (Long et al., 1998). Others have reported an increase in the relatively small proportion of proliferating microglia following running (Ehninger and Kempermann, 2003; Steiner et al., 2004; Choi et al., 2008; Olah et al., 2009). Our own observations align with those reports that showed no significant change in the overall microglial density but an increase in proliferating microglia.
There was a change, however, in the proportion of MHCIIpos microglia, the number of which decreased following running in our study, whereas others have reported no change (Olah et al., 2009). Intriguingly, we were able to show that depletion of this relatively small subpopulation of microglia resulted in a modest but significant increase in the frequency of neurosphere formation in runner mice. This novel finding suggests that MHCIIpos microglia, which have mostly been studied under inflammatory conditions, control running-induced neurogenesis in a negative rather than positive fashion, as was previously suggested by Ziv et al. (2006). Such a regulatory role for MHCIIpos microglia is also in line with a cytokine profile that is known to inhibit neurogenesis (Henry et al., 2009). As the microglia of older mice typically show increased MHCII expression, it remains to be elucidated whether the inhibitory effects of microglia from the aged brain can be neutralized by depletion of MHCIIpos microglia.
Microglia-mediated neural precursor activation is dependent on CX3CL1 signaling
As previous studies have indicated a key role for CX3CR1 in regulating microglial activation status (Mizuno et al., 2003; Cardona et al., 2006), we assessed whether signaling through this chemokine receptor could be responsible for the influence of microglia on NPC activity. The chemokine CX3CL1, which is the CX3CR1 ligand, is highly expressed in CNS neurons (Harrison et al., 1998), including mature neurons of the dentate gyrus (Kim et al., 2011), and is normally produced in a membrane-bound form from which the chemokine domain can be released through proteolytic cleavage (Bazan et al., 1997). The CX3CL1–CX3CR1 axis therefore represents a pathway for direct communication between neural cells and microglia. CX3CR1 deficiency deregulates microglia function and reportedly results in excessive release of proinflammatory factors in various models of neurological disease (Mizuno et al., 2003; Cardona et al., 2006). Recent studies have revealed that CX3CL1 levels are reduced in the aged rat hippocampus (Wynne et al., 2010; Bachstetter et al., 2011), which correlates with the naturally occurring decline in neurogenesis. In the present study, these findings have also been shown to be true in the mouse hippocampus. In addition, we have demonstrated that CX3CR1 deficiency results in decreased levels of basal neurogenesis in both young and aged mice.
Until now, the question remained as to whether the impact of CX3CR1 deficiency on adult neurogenesis was indeed specifically mediated via microglia. In direct support of a putative role for CX3CL1 in regulating NPC activity, we found that voluntary running increased soluble CX3CL1 within the hippocampus of both young and aged mice. Although it cannot be excluded that some membrane-bound CX3CL1 protein was included in the total amount of soluble CX3CL1 obtained during tissue preparation, all samples were processed simultaneously and in a similar fashion. Thus, the main conclusions we have drawn regarding control versus test conditions remain valid. Importantly, exercise effectively countered the naturally occurring age-related decline in CX3CL1 levels within the hippocampus of aged mice, restoring them to those seen in young animals. These findings are supported by a recent study by Barrientos et al. (2011), who suggested a potential beneficial link between running and altered neuroinflammatory responses. In our study, direct evidence for a role of CX3CL1 in regulating NPC activity was demonstrated via intraparenchymal infusion of a CX3CR1 blocking antibody into the hippocampus, which largely abolished the positive effect that microglia normally exert on these cells during running. Furthermore, addition of CX3CL1 to neurosphere preparations from sedentary wild-type but not CX3CR1-deficient mice resulted in a dose-dependent increase in the neurosphere-forming frequency. Thus, through the use of knock-out mice, blocking antibodies, and depletion studies, we have demonstrated that exercise-induced precursor activation is indeed mediated via signaling through CX3CR1, which is present on the microglial surface.
In summary, we have demonstrated a direct association between the beneficial effects of exercise in relation to NPC activation and increased levels of soluble CX3CL1 within the hippocampus, which in turn appears to modulate microglia activation status. Manipulation of the CX3CL1–CX3CR1 axis therefore provides a putative therapeutic avenue to counter the decline in NPC activity and neurogenesis within the aging brain.
Footnotes
This study was funded by a National Health and Medical Research Council program grant (to P.F.B.) and supported by the Estate of Dr. Clem Jones AO. A Smart Futures Fellowship from The Queensland Government supported J.V. We thank Virginia Nink, John Wilson, and Geoff Osborne for help with flow cytometry; George Rigley for technical assistance; and the staff of The University of Queensland Biological Resources facility for breeding and maintaining the animals used in this study. We also thank Ashley Cooper and Rowan Tweedale for editorial assistance, and Dee McGrath for assistance with illustrations.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol Aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Blackmore DG, Golmohammadi MG, Large B, Waters MJ, Rietze RL. Exercise increases neural stem cell number in a growth hormone-dependent manner, augmenting the regenerative response in aged mice. Stem Cells. 2009;27:2044–2052. doi: 10.1002/stem.120. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-γ differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cacci E, Ajmone-Cat MA, Anelli T, Biagioni S, Minghetti L. In vitro neuronal and glial differentiation from embryonic or adult neural precursor cells are differently affected by chronic or acute activation of microglia. Glia. 2008;56:412–425. doi: 10.1002/glia.20616. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Catts VS, Al-Menhali N, Burne TH, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur J Neurosci. 2008;28:883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Veeraraghavalu K, Lazarov O, Marler S, Ransohoff RM, Ramirez JM, Sisodia SS. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59:568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz MJ, Catts VS, Al-menhali N, Osborne GW, Bartlett PF, Coulson EJ. p75 neurotrophin receptor regulates basal and fluoxetine-stimulated hippocampal neurogenesis. Exp Brain Res. 2010;200:161–167. doi: 10.1007/s00221-009-1947-6. [DOI] [PubMed] [Google Scholar]
- Conde JR, Streit WJ. Microglia in the aging brain. J Neuropathol Exp Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU. 1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201:1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P. Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience. 2010;171:1386–1396. doi: 10.1016/j.neuroscience.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Regional effects of wheel running and environmental enrichment on cell genesis and microglia proliferation in the adult murine neocortex. Cereb Cortex. 2003;13:845–851. doi: 10.1093/cercor/13.8.845. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychol. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel GW, McKercher SR, Leenen PJ, Maki RA. Commitment to the monocytic lineage occurs in the absence of the transcription factor PU. 1. Blood. 1999;93:2849–2858. [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jinno S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol. 2011;519:451–466. doi: 10.1002/cne.22527. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, Ludwig A, Lira SA, Jung S. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–e167. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Calhoun ME, Jucker M, Hengemihle JM, Ingram DK, Mouton PR. Stereological analysis of astrocyte and microglia in aging mouse hippocampus. Neurobiol Aging. 1998;19:497–503. doi: 10.1016/s0197-4580(98)00088-8. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU. 1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90:89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- Mouret A, Lepousez G, Gras J, Gabellec MM, Lledo PM. Turnover of newborn olfactory bulb neurons optimizes olfaction. J Neurosci. 2009;29:12302–12314. doi: 10.1523/JNEUROSCI.3383-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25:649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2012;33:195.e1–e12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Olah M, Ping G, De Haas AH, Brouwer N, Meerlo P, Van Der Zee EA, Biber K, Boddeke HW. Enhanced hippocampal neurogenesis in the absence of microglia T cell interaction and microglia activation in the murine running wheel model. Glia. 2009;57:1046–1061. doi: 10.1002/glia.20828. [DOI] [PubMed] [Google Scholar]
- Ren L, Lubrich B, Biber K, Gebicke-Haerter PJ. Differential expression of inflammatory mediators in rat microglia cultured from different brain regions. Brain Res Mol Brain Res. 1999;65:198–205. doi: 10.1016/s0169-328x(99)00016-9. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood. 2003;101:1155–1163. doi: 10.1182/blood-2002-02-0569. [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999b;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukovic J, Blackmore DG, Jhaveri D, Bartlett PF. Activation of neural precursors in the adult neurogenic niches. Neurochem Int. 2011;59:341–346. doi: 10.1016/j.neuint.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24:1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]