Abstract
Reproduction is controlled in the brain by a neural network that drives the secretion of gonadotropin-releasing hormone (GnRH). Various permissive homeostatic signals must be integrated to achieve ovulation in mammals. However, the neural events controlling the timely activation of GnRH neurons are not completely understood. Here we show that kisspeptin, a potent activator of GnRH neuronal activity, directly communicates with neurons that synthesize the gaseous transmitter nitric oxide (NO) in the preoptic region to coordinate the progression of the ovarian cycle. Using a transgenic Gpr54-null IRES-LacZ knock-in mouse model, we demonstrate that neurons containing neuronal NO synthase (nNOS), which are morphologically associated with kisspeptin fibers, express the kisspeptin receptor GPR54 in the preoptic region, but not in the tuberal region of the hypothalamus. The activation of kisspeptin signaling in preoptic neurons promotes the activation of nNOS through its phosphorylation on serine 1412 via the AKT pathway and mimics the positive feedback effects of estrogens. Finally, we show that while NO release restrains the reproductive axis at stages of the ovarian cycle during which estrogens exert their inhibitory feedback, it is required for the kisspeptin-dependent preovulatory activation of GnRH neurons. Thus, interactions between kisspeptin and nNOS neurons may play a central role in regulating the hypothalamic–pituitary–gonadal axis in vivo.
Introduction
The survival of a mammalian species depends on the ability of its individuals to quickly, effectively, and reliably transmit homeostatic signals to the hypothalamic neuronal population that releases gonadotropin-releasing hormone (GnRH) and controls reproduction. This requires the mediation of neural networks that sense moment-to-moment changes in physiological inputs throughout life (Wintermantel et al., 2006; Mayer et al., 2010; Donato et al., 2011; Mayer and Boehm, 2011). We hypothesized that interactions between hypothalamic neurons expressing kisspeptin and those expressing neuronal nitric oxide synthase (nNOS) could be of particular importance in regulating the neuroendocrine output of GnRH neurons during the ovarian cycle, as the mutation of either of these causes infertility in mice (Gyurko et al., 2002; d'Anglemont de Tassigny et al., 2007b; Lapatto et al., 2007).
In rodents, GnRH neurons are predominantly located in the preoptic region of the ventral forebrain. They project to the median eminence of the hypothalamus, where GnRH is released into portal blood vessels for delivery to the anterior pituitary. In the pituitary, GnRH elicits the secretion of luteinizing hormone (LH) and follicle-stimulating hormone, which stimulate gametogenesis and gonadal hormone secretion and thus support reproductive function. In recent years, kisspeptin and its receptor GPR54 (also known as KISS1R) have emerged as key players in the regulation of GnRH/LH release (Oakley et al., 2009; d'Anglemont de Tassigny and Colledge, 2010; Navarro and Tena-Sempere, 2011). Kisspeptin-expressing neurons, which are found primarily in the hypothalamus, directly innervate and stimulate the electrical activity of GnRH neurons, which express the kisspeptin receptor GPR54 (Clarkson and Herbison, 2009). Mice with a targeted deletion of Gpr54 are sterile (Funes et al., 2003; Seminara et al., 2003; Dungan et al., 2007; Kauffman et al., 2007; Lapatto et al., 2007), and kisspeptin–GPR54 signaling appears essential for the GnRH neuronal activation that initiates ovulation (Clarkson et al., 2008). In addition to acting directly on GnRH neurons, an increasing body of evidence suggests that kisspeptin also operates on unidentified neurons to modulate the strength of synaptic afferents and regulate GnRH secretion (Pielecka-Fortuna et al., 2008). Nitric oxide (NO), a gaseous neurotransmitter known for its important role in regulating neuronal transmission (Garthwaite, 2008) and the neuroendocrine control of reproduction (Bellefontaine et al., 2011), has recently been implicated in the regulation of GnRH neuronal activity (Clasadonte et al., 2008). This NO is produced in the vicinity of GnRH-containing perikarya by neurons expressing nNOS (Clasadonte et al., 2008), an enzyme whose activity has been shown to be tightly regulated by estrogens during the ovarian cycle in rats (d'Anglemont de Tassigny et al., 2007a; Parkash et al., 2010).
We investigated whether kisspeptin-positive neurons projected onto nNOS neurons, and whether kisspeptin signaling in these cells could contribute to the hypothalamic control of reproduction. Using genetic mouse models, we demonstrate that kisspeptin neurons directly act on NO-synthesizing neurons to promote preovulatory nNOS activation and that these interactions modulate LH secretion.
Materials and Methods
Animals
Experiments were performed on adult (2–3 months old) female C57BL/6J (Charles River Laboratories) and nNOS-null mice (Huang et al., 1993) (Jax mice; Jackson Laboratory), Gpr54-null mice (Seminara et al., 2003), Kiss1-null mice (d'Anglemont de Tassigny et al., 2007b), and their wild-type littermates. Genotypes were determined by PCR as reported previously (Huang et al., 1993; Seminara et al., 2003; d'Anglemont de Tassigny et al., 2007b). Mice were housed under a 12 h:12 h lighting schedule with ad libitum access to food and water. For most of the experiments, vaginal smears were taken daily to identify the specific day of the estrous cycle. All experiments were performed in accordance with the guidelines for animal use specified by the European Communities Council Directive of November 24th, 1986 (86/609/EEC) regarding mammalian research and were approved by the University of Lille 2 Animal Use Committee, or following the United Kingdom Home Office Project License and were approved by the Cambridge Animal Ethics Committee.
Drugs
Synthetic kisspeptin-10 [rodent metastin (45–54) amide; YY-10-NH2] was purchased from GeneCust. The NOS inhibitor N-G-nitro-l-arginine methyl ester, HCl (l-NAME), the PI3-kinase inhibitor LY294002 (440202), the MEK inhibitor U0126 (662005), and DMSO (317275) were purchased from Calbiochem. 17β-Estradiol 3-benzoate (E8515), 17β-estradiol (E8875), progesterone (P0130), and sesame oil (S3547) were purchased from Sigma.
Antibodies
Immunofluorescence.
The sheep polyclonal anti-nNOS antibody (1:3000) was a generous gift from Dr. P. C. Emson (Laboratory for Molecular Biology, Cambridge, UK) (Herbison et al., 1996). The rabbit polyclonal anti-kisspeptin antibody (1:5000; AC-566) was a generous gift from Dr. Alain Caraty (Institut National de la Recherche Agronomique, Tours, France) (Franceschini et al., 2006). The rabbit polyclonal anti-GnRH (1:3000) was a generous gift from Prof. G. Tramu (Université Bordeaux I, Talence, France) (Beauvillain and Tramu, 1980). The rabbit polyclonal antiphosphorylated nNOS (Ser1412) antibody (71-8600; 1:500) was purchased from Affinity BioReagents. The Alexa Fluor 568-conjugated donkey anti-sheep secondary antibody (A-21099; 1:500) used for nNOS immunolabeling, the Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (A21206; 1:500) used for GnRH and P-nNOS immunolabeling, and the streptavidin-conjugated Alexa Fluor 488 (S32354; 1:500) used for kisspeptin detection were purchased from Invitrogen. A biotinylated donkey anti-rabbit secondary antibody (711-065-152; 1:500) was purchased from Jackson ImmunoResearch.
Western Blotting.
The rabbit polyclonal anti-nNOS (sc-8309; 1:500) and goat polyclonal anti-actin (sc-1616; 1:1000) antibodies were purchased from Santa Cruz Biotechnology. The rabbit polyclonal anti-phospho-nNOS antibody (Ser1412; PA1-032; 1:1000) was purchased from Affinity BioReagents. The rabbit monoclonal anti-phospho-Akt (Ser473; #4060S; 1:1000), rabbit monoclonal Akt (#4691S; 1:1000), rabbit polyclonal anti-phospho-p44/42 MAPK (p-Erk1/2; Thr202/Tyr204; #9101L; 1:1000), and rabbit polyclonal anti-p44/42 MAPK (Erk1/2; #9102L; 1:1000) antibodies were purchased from Cell Signaling Technology. HRP-conjugated secondary antibodies (1:10,000) were purchased from Sigma.
Immunohistofluorescence
Animals were deeply anesthetized with chloral hydrate (400 mg/kg, i.p.) and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. Brains were removed and immersed in the same fixative for 2 h at 4°C and stored in PB until slicing. Free-floating coronal sections (60 μm thick) containing the preoptic region were cut on a Vibratome (VT1000S; Leica), collected in ice-cold PB, incubated in blocking buffer [PBS containing 5% normal donkey serum (D9663; Sigma) and 0.3% Triton X-100 (Sigma)] for 1 h at room temperature (RT) and then incubated with sheep polyclonal anti-nNOS (1:1000) and rabbit polyclonal anti-kisspeptin (1:5000), or rabbit polyclonal anti-P-nNOS (Ser1412; 1:500) primary antibodies prepared in blocking solution for 48 h at 4°C. Sections were rinsed thoroughly four to five times in PBS and exposed to secondary antibodies, first with Alexa Fluor 568-conjugated donkey anti-sheep and biotinylated donkey anti-rabbit antibodies in blocking solution for 1 h at RT and then with streptavidin-conjugate Alexa Fluor 488. Kisspeptin immunoreactivity was localized with a biotinylated donkey antirabbit IgG (1:500; Vector Laboratories). Tyramide signal amplification was accomplished by placing the sections in an avidin-biotin solution (Vectastain) for 1 h, followed by incubation in tyramide signal-amplification solution for 20 min, according to the manufacturer's instruction (TSA-Indirect kit; New England Nuclear Life Science). Deposited biotin was detected with Alexa 488-conjugated streptavidin (1:500; Invitrogen). After washing, sections were mounted on glass slides and coverslipped with Permafluor medium (434990; Immunon). Double-immunofluorescent images were acquired using an Axio Imager.Z1 ApoTome microscope (AxioCam MRm camera, AxioVision 4.6 software system; Zeiss). For illustration purposes, photomontages of the preoptic region were prepared with the help of Photoshop CS4 software (Adobe) using digitalized images acquired with a 20× objective (NA, 0.5). Single-labeled nNOS neurons and dual-labeled P-nNOS/nNOS were quantified in the upper focal plane of sections (60 μm thick) in different parts of the preoptic region using digitalized images. For confocal observation and analyses, an inverted laser scanning Axio observer microscope (LSM 710; Zeiss) with an EC Plan NeoFluor 100×/1.4 NA oil-immersion objective (Zeiss) was used (Imaging Core Facility, University of Lille 2, France).
X-gal histochemistry coupled with GnRH/nNOS double-immunocytochemistry
Free-floating, 40-μm-thick sections were cut on a cryostat from adult Gpr54-null mouse brains processed as described (see Immunohistofluorescence, above). A series of sections containing the preoptic and tuberal region of the hypothalamus was washed thoroughly with 0.1 m PBS, pH 7.4, and placed in 2% 5-bromo-4-chloro-3-indoyl-β-d-galactosidase (X-gal) solution [2 mm MgCl2, 4 mm K3Fe(CN)6, 4 mm K4Fe(CN)6, and 4 mg/ml X-gal] overnight at RT. After PBS washes and preincubation in blocking buffer (PBS containing 5% donkey serum and 0.05% Triton X-100) for 1 h at RT, sections were incubated with rabbit anti-GnRH (1:3000) and sheep anti-nNOS (1:3000) primary antibodies diluted in blocking buffer at 4°C overnight. After PBS rinses, sections were incubated with Alexa Fluor 488-conjugated donkey anti-rabbit (1:500) and Alexa Fluor 568-conjugated donkey anti-sheep (1:500) antibodies diluted in blocking buffer for 2 h at RT. Sections were rinsed thoroughly, mounted on glass slides, and coverslipped with Permafluor medium (434990; Immunon).
Sections were examined using an Axio Imager.Z1 ApoTome microscope (Zeiss) equipped with a motorized stage and an AxioCam MRm camera (Zeiss). Specific filter cubes were used for the visualization of GnRH immunoreactivity in green (excitation filter: 475/40 nm; dichroic mirror: 500 nm; barrier filter: 530/50 nm) and nNOS immunoreactivity in red (excitation filter: 550/25 nm; dichroic mirror: 570 nm; barrier filter: 605/70 nm); X-gal staining was visualized simultaneously by switching to brightfield. GnRH or nNOS cells were considered X-gal-positive if the X-gal reaction product was found within the cytoplasm of the soma or proximal dendrite. X-gal/GnRH double-labeled cells, X-gal/nNOS double-labeled cells, X-gal-labeled unidentified cells, and single-labeled GnRH or nNOS cells were counted. Analysis was performed by counting all single-labeled and double-labeled cells in two sections from each animal corresponding to plates 17–21 of the Swanson brain atlas (Swanson, 2004), focusing on the medial septal nucleus, the organum vasculosum of the lamina terminalis, the median preoptic nucleus, the anteroventral preoptic nucleus (in which nNOS neurons are known to interact morphologically with GnRH neurons), and the arcuate nucleus of the hypothalamus [a kisspeptin neuron-containing structure (Oakley et al., 2009; Navarro and Tena-Sempere, 2011) adjacent to the median eminence, to which GnRH neurons project]. Values from each mouse were used to determine mean counts and data are represented as means ± SEM.
For illustration purposes, photomontages of different parts of the preoptic region containing GnRH/nNOS-immunoreactive neurons along with X-gal staining in brightfield were prepared with the help of Photoshop CS4 software (Adobe), using digitalized images acquired with a 20× objective (NA, 0.5) (Zeiss).
Protein extraction
The preoptic region was dissected from each animal and protein extracted for Western blotting as described previously (d'Anglemont de Tassigny et al., 2007a; Parkash et al., 2010). Briefly, mice were killed by decapitation following treatment. After the rapid removal of the brain, the meninges and optic chiasm were removed and the preoptic region was dissected under a binocular magnifying glass with the Wecker scissors (Moria). The external limits for this dissection were as follows: laterally, the external border of the medial preoptic area; and dorsally, the internal border of the anterior commissures. Anteroposteriorly, the dissected region was comprised between the atlas levels 16 and 20 (Swanson, 2004). The tuberal region of the hypothalamus was similarly dissected; the external limits for this dissection were as follows: laterally, the external border of the lateral hypothalamic area; and dorsally, the roof of the third ventricle. Anteroposteriorly, the dissected region was comprised between atlas levels 26 and 31 (Swanson, 2004). After dissection, each fragment was placed in a microcentrifuge tube, snap frozen in liquid nitrogen, and stored at −80°C.
Protein extracts were prepared from each preoptic region sample in 200 μl of lysis buffer (25 mm Tris, pH 7.4, β-glycerophosphate, 1.5 mm EGTA, 0.5 mm EDTA, 1 mm sodium pyrophosphate, 1 mm sodium orthovanadate, 10 μg/ml leupeptin and pepstatin A, 10 μg/ml apoprotinin, 100 μg/ml PMSF, and 1% Triton X-100) by homogenization of the fragments through 22 and 26 gauge needles in succession. Tissue lysates were cleared by centrifugation at 14,000 rpm for 15 min at 4°C. Protein content was determined using the Bradford method (Bio-Rad) and equal amounts of protein were mixed with 4× sample buffer (Invitrogen). Samples were boiled for 5 min and stored at −80°C until use.
Western blot analyses
Samples were reboiled for 5 min after thawing and electrophoresed for 75 min at 150 V in 7% Tris-acetate, or for 50 min at 200 V in precast 4–12% MES SDS-polyacrylamide gels according to the protocol supplied with the NuPAGE system (Invitrogen). After size fractionation, the proteins were transferred onto 0.2 μm pore-size polyvinylidene difluoride membranes (LC2002; Invitrogen) in the blot module of the NuPAGE sytem (Invitrogen) for 75 min at RT. Membranes were blocked for 1 h in blocking buffer [TBS with 0.05% Tween 20 (TBST) and 5% nonfat milk] at RT, and incubated overnight at 4°C with the appropriate primary antibody diluted in blocking buffer. Membranes were washed four times with TBST the following day before being exposed to HRP-conjugated secondary antibodies diluted in blocking buffer for 1 h at RT. Immunoreactions were visualized using the ECL detection kit (NEL101; PerkinElmer). When stripping and reprobing was required, membranes were incubated in a stripping solution (62.5 mm Tris-HCl, 2% SDS, pH 6.7, and 100 mm β-mercaptoethanol) for 30 min with gentle rocking at 65°C. An HRP-conjugated secondary antibody was used to verify that all former immunoreactivity had been successfully stripped away. Immunoblots were scanned using a desktop scanner (Epson Expression 1680 PRO) and Adobe Photoshop, and band intensities were determined using ImageJ software (NIH).
Kisspeptin-10 treatment
Diestrous mice received a single intraperitoneal injection of 100 μl of PBS containing 1 nmol Kisspeptin-10 (Kp10) or PBS only. After 30 min, mice were either killed by decapitation and the hypothalamic preoptic and tuberal regions dissected and processed for Western blotting or deeply anesthetized and processed for immunofluorescence analyses.
Mouse treatment with PI3 kinase, MAPK, and NOS inhibitors
Ovariectomized mice received a single injection of LY294002 (100 mg/kg, i.p.) or U0126 (30 mg/kg, i.p.) or DMSO. After 30 min, animals received a single intraperitoneal injection of 100 μl PBS containing 1 nmol of Kp10 or PBS only. After an additional 30 min, mice were killed by decapitation and the preoptic region of each brain dissected from each brain and processed for Western blotting. For l-NAME treatment, diestrous or ovariectomized mice first received a single injection of the NOS inhibitor (50 mg/kg, i.p.) or PBS; 3 h later, they were given a second injection of either Kp10 or PBS, and killed after 30 min by decapitation. Trunk blood was collected in tubes containing EDTA (0.2 m) and centrifuged at 6500 rpm for 15 min at 4°C; the supernatant obtained (plasma) was stored at −80°C until ELISA for LH.
Estradiol treatment in ovariectomized mice
Gpr54-null mice and their wild-type littermates were bilaterally ovariectomized (day 0) under anesthesia with an intraperitoneal injection of 60 mg/kg ketamine and 10 mg/kg xylazine. On the 15th day after ovariectomy (Day 15) at 9:00 A.M., mice received either a single subcutaneous injection of estradiol benzoate (E2; 1 μg/20 g body weight in sesame oil) or vehicle alone. Mice were killed by decapitation on Day 17 between 6:30 and 7:30 P.M., and the hypothalamic preoptic region was dissected and processed for Western blotting. This protocol has previously been used to assess the effect of the preovulatory rise in estrogen levels on the phosphorylation of nNOS protein in the preoptic region (Parkash et al., 2010).
Steroid-induced LH surge protocol
Mice were bilaterally ovariectomized as described in the previous paragraph, and implanted subcutaneously with SILASTIC capsules containing E2 (1 μg/20 g body weight). The SILASTIC capsules were prepared as follows: crystalline E2 was dissolved in absolute ethanol, mixed with SILASTIC medical adhesive (Type A; Dow Corning) at a concentration of 0.1 mg/ml adhesive and injected into SILASTIC tubing (internal diameter, 1 mm; external diameter, 2.125 mm; Dow Corning) (Bronson, 1981; Clarkson et al., 2008). Six days after ovariectomy, mice received a single subcutaneous injection of 17β-estradiol 3-benzoate (1 μg/20 g of body weight in sesame oil) at 9:00 A.M. The following day, animals received another injection of progesterone (500 μg/20 g body weight, s.c., in sesame oil) at 9:00 A.M. Between 7:30 and 8:30 P.M (lights off at 8:00 P.M.) on the same day, mice were anesthetized with an overdose of chloral hydrate (400 mg/kg; i.p.) and trunk blood was collected for LH assay.
Luteinizing hormone assay
Plasma LH was measured using Rodent LH ELISA kit (ERKR7010-A; Endocrine Technologies) with a sensitivity of 0.3 ng/ml and 7% intraassay and 10% interassay coefficients of variation.
Ovarian histology
Dissected ovaries were fixed in 4% paraformaldehyde, washed in PBS, wax embedded in paraffin, sectioned at 5 μm intervals, and stained with hematoxylin and eosin.
Estrous cyclicity
To examine the possible effects of mutations on estrous cyclicity, vaginal lavages from adult (>2 months old) female nNOS-null mice and their wild-type littermates were performed everyday in the morning (10:00 A.M. to 1:00 P.M.) using 0.9% saline. The smears were observed under the microscope and the phase identified as diestrus (L) if they predominantly contained leukocytes, as proestrus (N) if they predominantly contained basal and cornified nucleated cells, and as estrus (C) if they predominantly contained cornified epithelial cells. An estrous cycle was considered normal when the vaginal lavage had leukocytes for 2 d followed by 1 d of nucleated and 1–2 d of cornified cells.
Statistics
Differences between two groups were analyzed by Student's t test. Differences between several groups were analyzed using one-way ANOVA, followed by the Student–Newman–Keuls multiple-comparison test for unequal replication. The level of significance was set at p < 0.05.
Results
Subsets of NO-synthesizing neurons are abundantly surrounded by kisspeptin-immunoreactive fibers in the preoptic region
To determine whether kisspeptin neurons morphologically interact with nNOS neurons in the preoptic region, we performed dual immunofluorescent-labeling studies using well characterized antibodies to kisspeptin (Fig. 1A) (Clarkson et al., 2009) and to nNOS (Herbison et al., 1996). Fluorescence microscopic examinations were conducted in forebrain areas in which nNOS and GnRH neurons are known to interact morphologically and functionally in mice (n = 4 mice) (Clasadonte et al., 2008). They clearly establish the existence of distinct populations of NO-synthesizing neurons that are morphologically associated with kisspeptin-immunoreactive fibers (Figs. 1B, 2). Notably, in the median preoptic nucleus (MePO) and the organum vasculosum of the lamina terminalis (OVLT), kisspeptin-immunoreactive fibers were found to be abundantly apposed to nNOS-containing cell bodies (Fig. 2C,E), whereas in the medial septum (MS) and the anteroventral preoptic nucleus (AVP), virtually no apposition was seen between kisspeptin-immunoreactive axons and the cell bodies of nNOS neurons (Fig. 2B,F).
Figure 1.
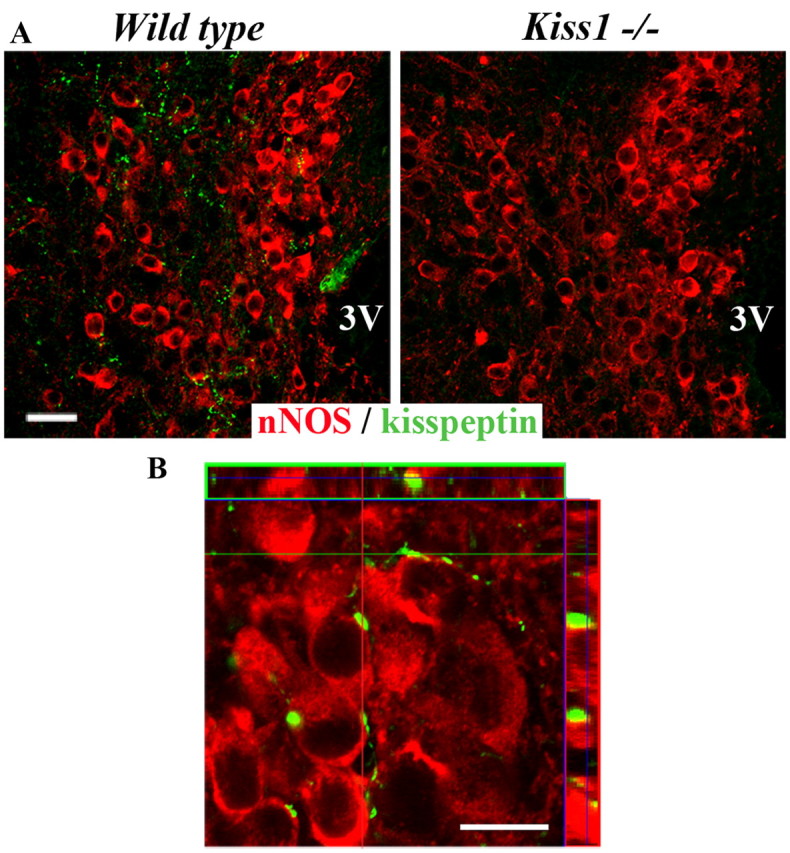
Morphological apposition between kisspeptin fibers and hypothalamic nNOS neurons in the hypothalamic preoptic region. A, Kisspeptin immunoreactivity (green) localized in fibers apposed to nNOS neurons (red) is present in wild-type mice but not in Kiss1-null littermates. B, Confocal image obtained using a 100× objective showing the close apposition between kisspeptin-immunoreactive fibers (green) and nNOS-immunoreactive neuronal cell bodies (red). The illustration is an overlay and maximum projection of the z-stack files of a color-combined image of four consecutive single confocal planes separated from each other by 400 nm. All images were acquired at the level of the OVLT in the basal forebrain. 3V, Third ventricle. Scale bars: A, 50 μm; B, 10 μm.
Figure 2.
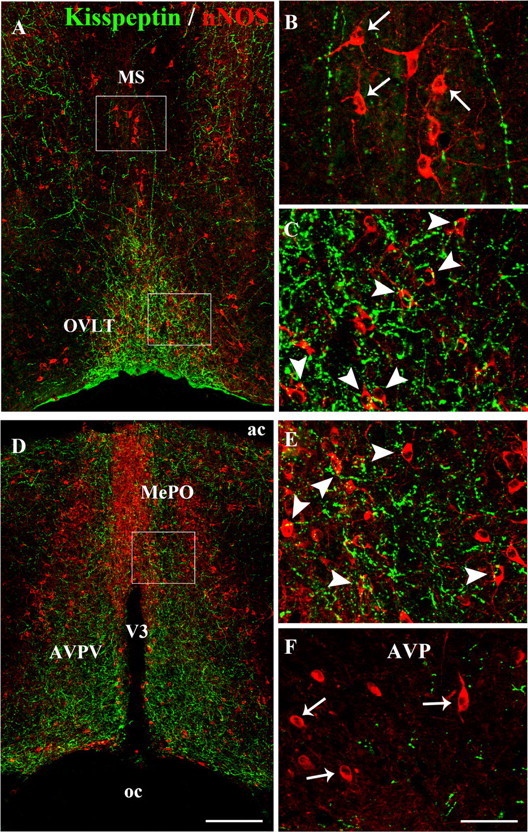
Kisspeptin neurons project onto nNOS neurons in the preoptic region. A, Low-magnification photomontage of kisspeptin (green) and nNOS (red) immunofluorescence in the hypothalamic preoptic region at the level of the OVLT and the MS. B, C, High-magnification images of the boxed areas in A, showing kisspeptin-immunoreactive fibers (green) and nNOS-immunoreactive cell bodies (red) in the MS (B) and the OVLT (C). Note that kisspeptin fibers are abundantly apposed to nNOS neurons in the OVLT (C, arrowheads), but not in the MS (B, arrows). D, Low-magnification photomontage of kisspeptin (green) and nNOS (red) immunofluorescence in the hypothalamic preoptic region at the level of the MePO and the anteroventral periventricular nucleus (AVPV). E, F, High-magnification images of the boxed area in D, showing kisspeptin-immunoreactive fibers (green) and nNOS-immunoreactive cell bodies (red) in the MePO (E) and the AVP (F). Note that abundant kisspeptin fibers are apposed to nNOS neurons in the MePO (E, arrowheads), but not in the AVP (F, arrows). V3, Third ventricle; ac, anterior commissure; oc, optic chiasm. Scale bars: A, D, 200 μm; B, C, E, F, 50 μm.
NO-synthesizing neurons in the OVLT and MePO express GPR54
Using a transgenic GPR54-LacZ knock-in mouse model, we next sought to test the intriguing possibility that nNOS neurons in the preoptic region could express GPR54. X-gal histochemistry coupled with double-immunofluorescence labeling revealed that in the OVLT and MePO, LacZ and, by extension, GPR54, was not only contained in GnRH neurons as previously described (Messager et al., 2005; Herbison et al., 2010), but also in nNOS neurons (Fig. 3). Quantitative analyses showed that 25 ± 5% and 41 ± 4% of X-gal-positive cells in the OVLT and 23 ± 2% and 57 ± 6% of X-gal-positive cells in the MePO were GnRH and nNOS neurons, respectively (n = 4 female mice). In contrast, X-gal staining in the MS was restricted to GnRH neurons (9 ± 2% of X-gal-positive cells) and a population of nNOS-immunonegative cells (Fig. 3A,B); in the AVP, GnRH neurons were the only X-gal-positive cells (Fig. 3E,H). Together with our data showing that kisspeptin-containing fibers are closely apposed to nNOS neurons in the OVLT–MePO, these results strongly suggest that kisspeptin, the ligand for GPR54, may act directly on these neurons.
Figure 3.
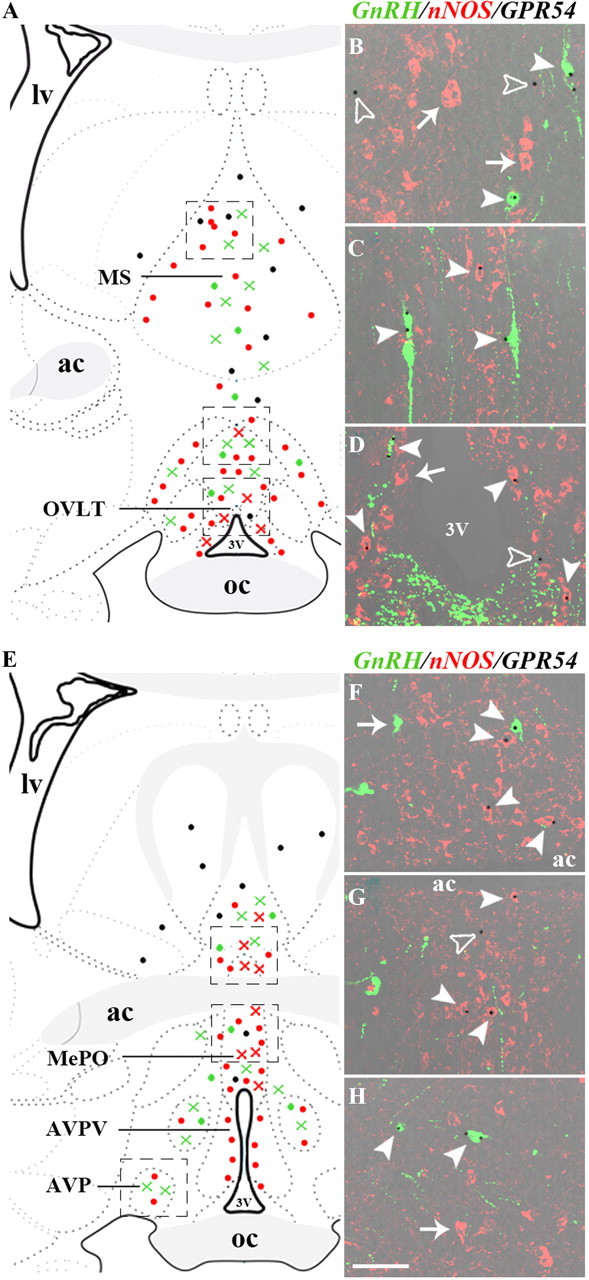
GPR54 is expressed both in nNOS and GnRH neurons in the preoptic region. A, E, Schematic brain maps demonstrating the distribution of GnRH (green) and nNOS (red) neurons expressing (crosses) or not expressing (circles) GPR54 in the preoptic region. Black circles indicate GPR54-expressing cells of unidentified phenotype. B–D, F–H, X-gal histochemistry (black dots) revealing GPR54 expression both in GnRH (green, solid arrowheads) and nNOS (red, solid arrowheads) neurons in the OVLT (D) and MePO (F, G), but not in the MS (B) or AVP (H), where GPR54 is expressed in GnRH neurons and cells of unidentified phenotype (empty arrowheads). AVPV, Anteroventral periventricular nucleus; lv, lateral ventricle; ac, anterior commissure; V3, third ventricle; oc, optic chiasm. Scale bar, 50 μm.
NO-synthesizing neurons are surrounded by kisspeptin-immunoreactive fibers in the arcuate nucleus of the hypothalamus, but do not express the GPR54 kisspeptin receptor
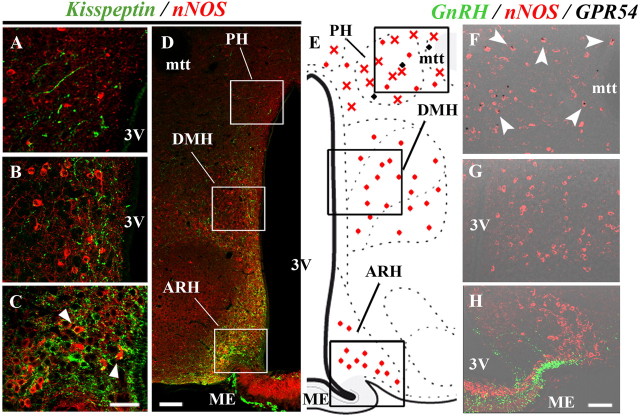
In the mouse brain, nNOS neurons are also found in the tuberal region of the hypothalamus and, in particular, within the arcuate nucleus of the hypothalamus (ARH) (Sica et al., 2009), a kisspeptin neuron-containing structure (Oakley et al., 2009; Navarro and Tena-Sempere, 2011) that is adjacent to the median eminence, to which GnRH neurons project. To determine whether kisspeptin neurons also interact morphologically and functionally with nNOS neurons in this hypothalamic region, we performed additional neuroanatomical studies. While only a few kisspeptin-immunoreactive fibers were found in apposition to nNOS neurons in the posterior hypothalamus (PH; Fig. 4A,D) and dorsomedial hypothalamus (DMH; Fig. 4B,D), numerous kisspeptin fibers were apposed to nNOS neurons in the ARH (Fig. 4C,D). Intriguingly, no GPR54 expression was seen in the ARH (Fig. 4E,H) or in the DMH (Fig. 4E,G), as revealed by X-gal staining in GPR54-LacZ knock-in mice. In contrast, X-gal staining was found to be abundant in the PH and was mostly seen in nNOS-immunoreactive neurons (Fig. 4E,F).
Figure 4.
Kisspeptin-neurons project onto nNOS neurons that do not express GPR54 in the ARH. A–D, Immunofluorescence labeling showing abundant kisspeptin fibers (green) apposed to nNOS neurons (red) in the ARH (C, arrowheads), whereas Kisspeptin-nNOS morphological interactions are less conspicuous in the DMH and the PH (A, B). E, Schematic brain map demonstrating the distribution of nNOS neurons (red) expressing (crosses) or not expressing (circles) GPR54 in the tuberal region of the hypothalamus. Black circles indicate GPR54-expressing cells of unidentified phenotype. F–H, X-gal histochemistry (black dots) revealing GPR54 expression in nNOS neurons (red, arrowheads) in the PH (F), but not in the DMH (G) or in the ARH (H), in which no GPR54 expression is detected. Note that in H, GnRH neuroendocrine axons (green) residing in the median eminence (ME) lie ventral to arcuate nNOS neurons. mtt, Mammillothalamic tract; 3V, third ventricle. Scale bars: A–C, 40 μm; D, 100 μm; F–H, 50 μm.
Kisspeptin promotes the phosphorylation of nNOS on its Ser1412 activation site in the preoptic region, but not in the tuberal region of the hypothalamus
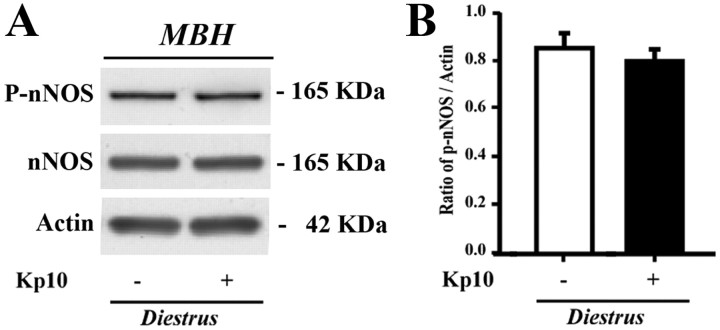
The catalytic activity of nNOS is modulated during the ovarian cycle by posttranslational modifications of the enzyme, such as phosphorylation of the Ser1412 residue (Parkash et al., 2010), which increases its catalytic activity (Rameau et al., 2007). Ser1412 phosphorylation of nNOS is maximal in the preoptic region on the afternoon of proestrus, when estrogens exert their positive feedback effects on the GnRH system (Parkash et al., 2010). To determine whether kisspeptin neurons could play a role in the regulation of these posttranslational modifications, we investigated the effects of an intraperitoneal injection of kisspeptin-10 (1 nmol, 30 min) on nNOS phosphorylation, using a phospho-specific antibody directed against Ser1412-phosphorylated nNOS (P-nNOS). In diestrous mice, in which circulating estrogen levels are low, immunofluorescence analysis demonstrated a striking increase in the proportion of nNOS neurons immunoreactive for P-nNOS following kisspeptin-10 injection (36.8 ± 6.0% in vehicle-injected vs 74.3 ± 4.0% in kisspeptin-10-injected mice, n = 4; Student's t test, p < 0.01); this effect was restricted to the OVLT–MePO region (Fig. 5A). Indeed, no induction of nNOS phosphorylation was seen in nNOS neurons in the MS or the AVP (Fig. 6). Western blot analyses revealed that P-nNOS expression was easily detected as a single band of 165 kDa in protein extracts from the mouse preoptic region (Fig. 5B), and confirmed the potent stimulatory effect of kisspeptin-10 on nNOS phosphorylation in diestrous mice as seen using immunohistochemistry (Fig. 5A). Notably, P-nNOS levels induced by kisspeptin-10 treatment in diestrous mice were comparable to those seen in proestrus (Fig. 5B). In contrast, similar analyses performed in the tuberal region of the hypothalamus clearly showed that kisspeptin-10 failed to stimulate nNOS phosphorylation in this hypothalamic region in diestrous mice (Fig. 7).
Figure 5.
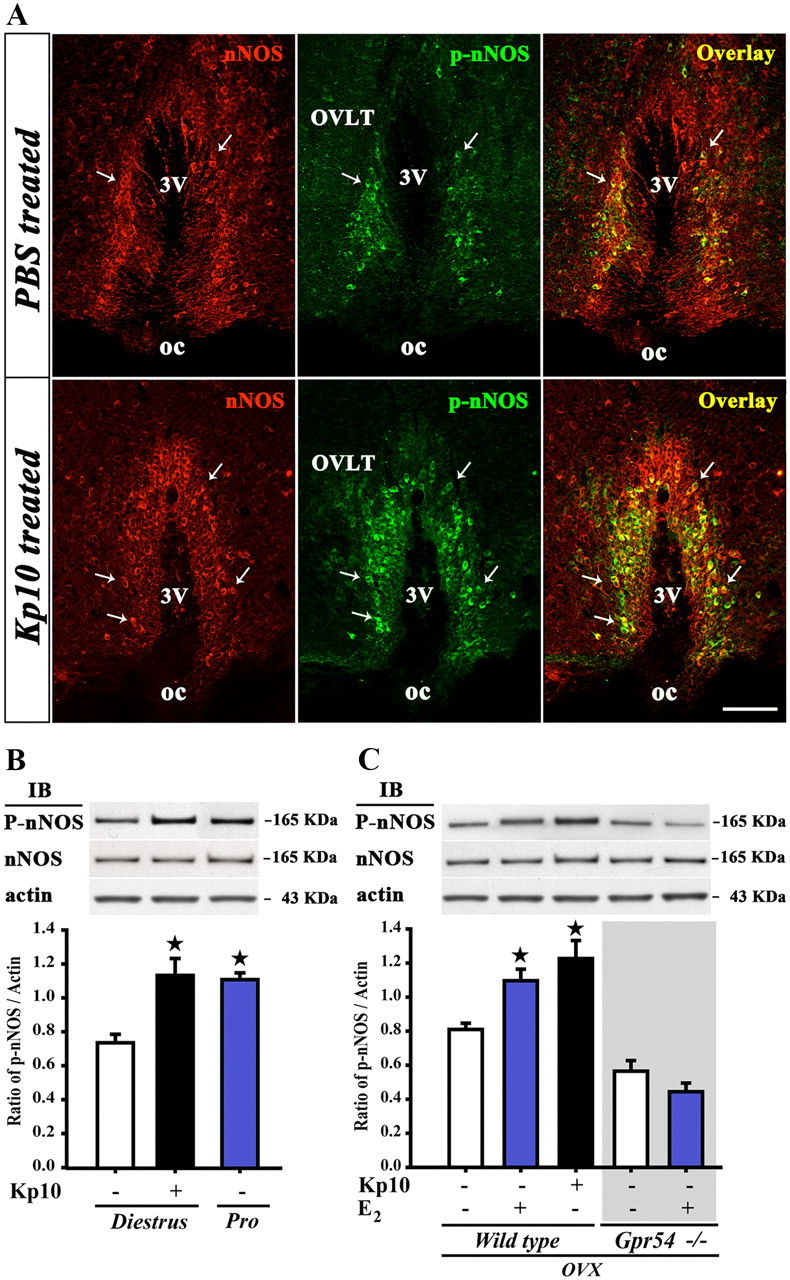
Kisspeptin and estradiol promote phosphorylation of nNOS via GPR54 activation in neurons of the preoptic region. A, nNOS (red) and P-nNOS (green) immunoreactivity in forebrain coronal sections passing through the OVLT of vehicle (PBS)- and kisspeptin (Kp10)-treated mice. Arrows show double-labeled neurons. oc, Optic chiasm; 3V, third ventricle. Scale bar, 100 μm. B, C, Immunoblotting (IB) of hypothalamic preoptic area protein extracts reveals that kisspeptin mimics both the effects of the ovarian cycle (B) and of estradiol (C) in promoting the phosphorylation of nNOS. Estradiol requires GPR54 signaling to exert its effects on nNOS phosphorylation (C). Bar graphs illustrate the mean ratio of the signal obtained for P-nNOS to that of actin in blots from five independent experiments (n = 5 animals). Error bars indicate SEM. *p < 0.05; experimental groups versus untreated controls. OVX, Ovariectomized animals.
Figure 6.
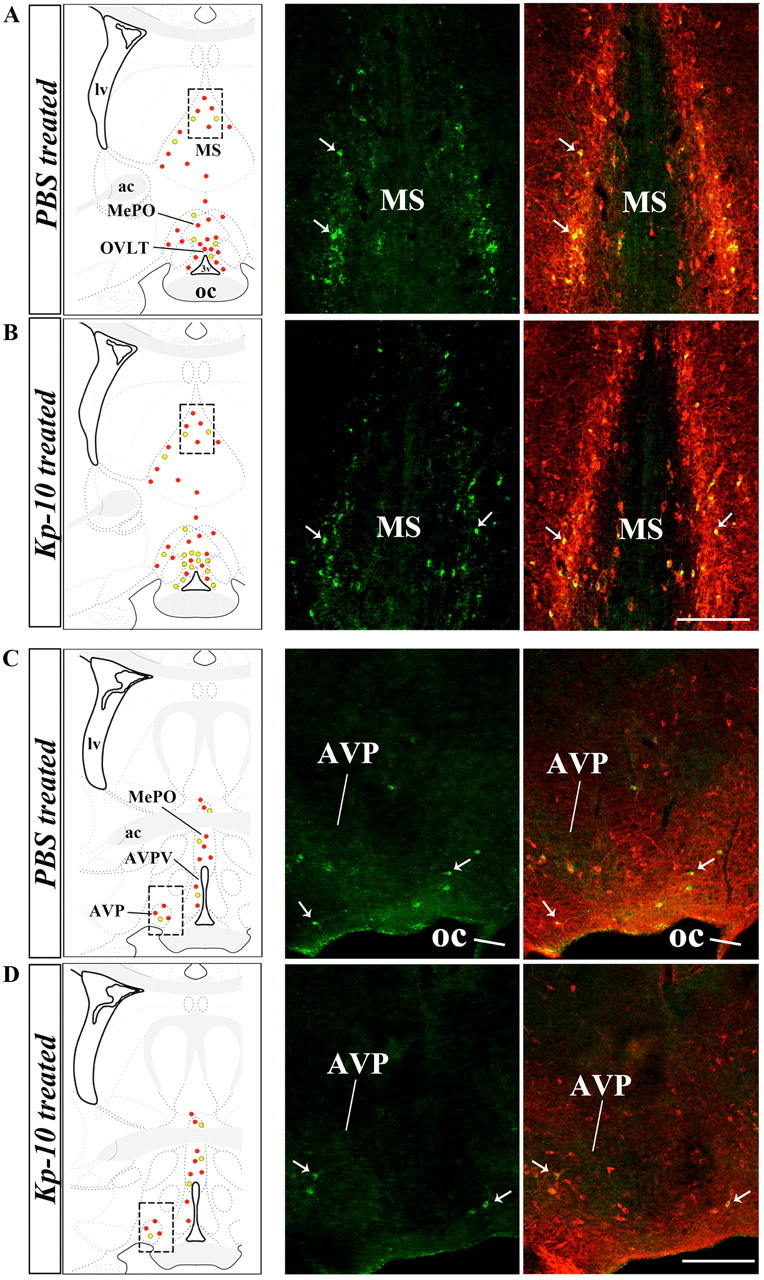
Kisspeptin promotes the phosphorylation of nNOS in the OVLT, but not in the MS or the AVP. A–D, Left, Schematic brain maps demonstrating the distribution of P-nNOS-immunoreactivity in nNOS neurons of the OVLT, MePO, MS, AVP, and anteroventral periventricular nucleus (AVPV) after kisspeptin (Kp-10) or vehicle (PBS) treatment (B, D and A, C, respectively). Red dots, Single-labeled nNOS neurons; yellow dots: P-nNOS/nNOS double-labeled neurons. Right, Representative fluorescent photomicrographs showing P-nNOS (green) and nNOS (red) immunoreactivity in the MS (A, B) and AVP (C, D) after the aforementioned treatments. oc, Optic chiasm; lv, lateral ventricle, ac, anterior commissure. Scale bar, 200 μm.
Figure 7.
Kisspeptin fails to promote the phosphorylation of nNOS in the tuberal region of the hypothalamus. A, Immunoblotting demonstrating that kisspeptin-treatment in diestrous mice has no effect on nNOS phosphorylation in the hypothalamic tuberal region. B, Bar graph illustrating the mean ratio of the signal obtained for P-nNOS to that of actin in blots from five independent experiments (n = 5 animals). MBH, Mediobasal hypothalamus. Error bars indicate the SEM.
Estradiol-promoted phosphorylation of nNOS in preoptic neurons requires kisspeptin-GPR54 signaling
To further explore the role of estrogens and kisspeptin in triggering nNOS phosphorylation in preoptic neurons, we performed experiments in ovariectomized (OVX) mice. As shown in Figure 5C, kisspeptin-10 (1 nmol, 30 min, i.p.) was as potent at inducing Ser1412 phosphorylation of nNOS in OVX mice, when compared with controls, as a subcutaneous injection of sesame oil containing 17β-estradiol 3-benzoate, which mimics the preovulatory increase in plasma estrogens that occurs at proestrus (n = 5 animals per group). To examine whether kisspeptin signaling is necessary for the estrogen-mediated activation of nNOS, we then tested the ability of estradiol to promote nNOS phosphorylation in Gpr54-null mice (Seminara et al., 2003). Western blot analyses clearly showed that estradiol-treatment did not induce any change in nNOS phosphorylation in Gpr54-null mice (p > 0.05; Fig. 5C). Together, our results strongly suggest that kisspeptin neurons act directly on NO-synthesizing neurons to promote preovulatory nNOS activation in the preoptic region.
Kisspeptin promotes nNOS phosphorylation via the activation of the PI3K/AKT signaling pathway
To gain further insight into the downstream pathways used by kisspeptin-GPR54 signaling to promote nNOS phosphorylation in preoptic neurons, we investigated whether kisspeptin activation of its cognate receptor is coupled with the activation of the phosphatidylinositol-3 kinase (PI3K)/AKT signaling pathway, which is thought to mediate the phosphorylation of Ser1412 in nNOS (Rameau et al., 2007) and has been shown to be stimulated by GPR54 signaling (Stathatos et al., 2005; Novaira et al., 2009). We also explored the putative activation of the ERK1/2 signaling pathway, which has been shown to be stimulated by the activation of GPR54 (Castellano et al., 2006). Immunoblot analysis revealed that kisspeptin-10 (1 nmol, 30 min) stimulated the phosphorylation of nNOS (Fig. 8A) and AKT (Fig. 8B), but not ERK1/2 (Fig. 8C) in wild-type diestrous mice, and that these effects of kisspeptin were absent in Gpr54-null littermates (n = 4–5 per group; Fig. 8A,B). To determine whether AKT activation is actually required for kisspeptin to exert its stimulatory effects on nNOS phosphorylation, we treated wild-type OVX mice with the PI3K inhibitor LY294002 (100 mg/kg, i.p.) for 30 min before exposing them to kisspeptin-10. As shown in Figure 8, D and E, the inhibition of PI3K activity abrogated the ability of kisspeptin to induce the phosphorylation both of nNOS and of AKT. In contrast, the treatment of mice with the MAPK inhibitor U0126 (30 mg/kg, i.p.) affected neither nNOS nor AKT phosphorylation (Fig. 8D,E), whereas it significantly inhibited the basal phosphorylation of ERK1/2 (Fig. 8F). Thus, the activation of the PI3K/AKT signaling pathway is likely to underlie the phosphorylation of nNOS induced by kisspeptin in neurons of the preoptic region.
Figure 8.
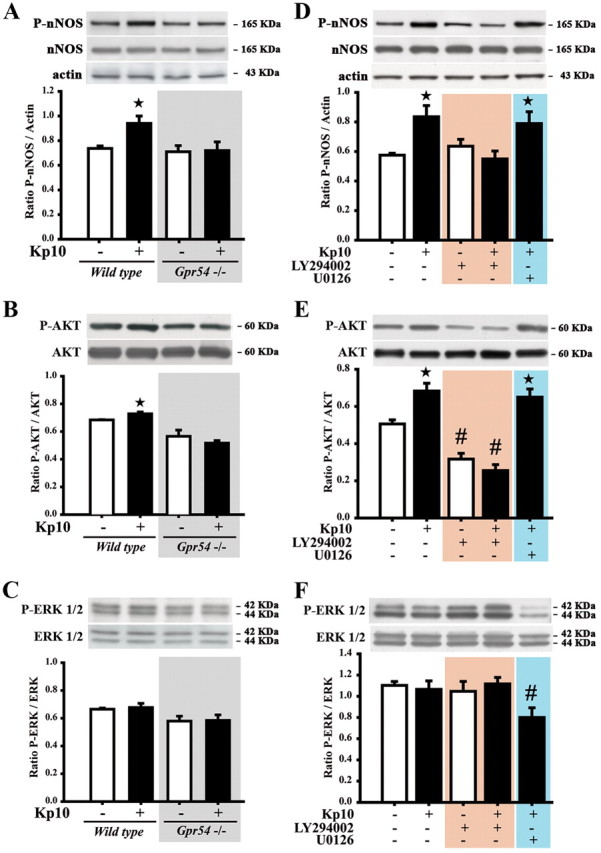
The activation of the GPR54 and PI3K-AKT pathways are necessary for the kisspeptin-induced increase in nNOS phosphorylation. A–F, Representative immunoblots showing that kisspeptin promotes nNOS (A, D) and AKT (B, E), but not ERK (C, F) phosphorylation in a GPR54-dependent manner (A, B) in diestrous (A–C) and ovariectomized (D–F) mice; the effects of kisspeptin are inhibited by the PI3K inhibitor LY294002 but not the MEK inhibitor U0126 (D–F). Bar graphs illustrate the mean ratio of the signal obtained for P-nNOS to that of actin (D), the ratio of P-AKT to that of AKT (E), and of P-ERK and that of ERK (F) from four independent experiments. Error bars indicate SEM. * and #, p < 0.05, experimental groups versus untreated controls.
NO release imposes a tonic brake on LH secretion during the estrogen-mediated negative feedback phase
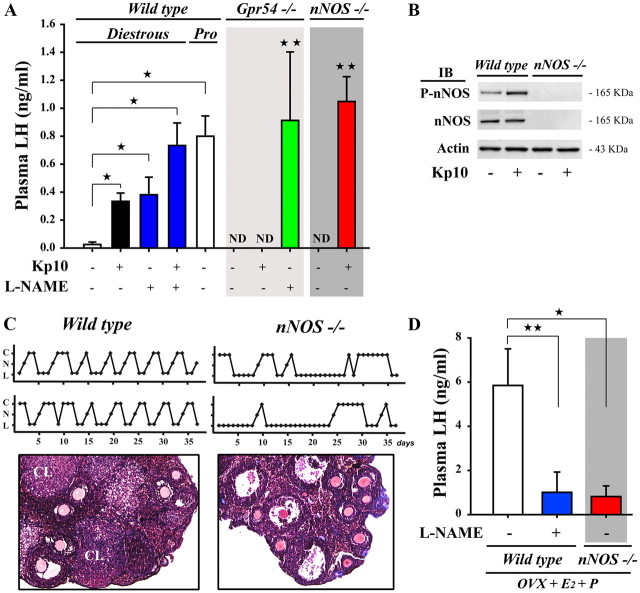
Having shown that kisspeptin and nNOS neurons morphologically and functionally interact in specific regions of the ventral forebrain in which a subset of GnRH neurons reside, we next sought to determine whether this kisspeptin–nNOS neuronal interaction could play a role in the control of LH release. In diestrous mice, treatment with kisspeptin-10 (n = 10) resulted in a robust and significant increase in plasma LH when compared with vehicle-treated animals (n = 9; Fig. 9A). Inhibiting NOS activity via the intraperitoneal administration of the NOS inhibitor l-NAME (50 mg/kg, i.p.) resulted in a comparable increase in LH release (n = 6; Fig. 9A). Kisspeptin-10 treatment of l-NAME-treated animals (n = 10) triggered the release of LH at levels comparable to those seen in proestrus (n = 4; Fig. 9A). Correspondingly, the treatment of Gpr54-null mice with l-NAME (n = 4) and of nNos-null mice with kisspeptin-10 (n = 4) resulted in the release of similar proestrus-like levels of LH (Fig. 9A). Altogether, these data reveal that NO exerts a tonic inhibitory effect on GnRH/LH release at stages of the ovarian cycle in which gonadal steroids exert their negative feedback effects.
Figure 9.
Kisspeptin-nNOS interactions are required for the neuroendocrine control of reproduction. A, Basal nNOS activity maintains the tonic inhibition on the GnRH system during the estrogen-mediated negative feedback phase, as assessed by LH release in wild-type and Gpr54- and nNOS-null mice in the presence or absence on the NOS inhibitor l-NAME. Values represent means ± SEM. *p < 0.05, **p < 0.005; experimental groups versus untreated controls. B, Western blot analysis showing the absence of nNOS protein expression in nNOS-null mice. C, Top, nNOS-null mice do not exhibit normal estrous cyclicity as do wild-type mice, but instead enter prolonged periods of diestrus. nNOS-null mice sometimes display isolated complete cycles; however, these are often followed by prolonged periods of acyclicity. C, Cornified (estrus); N, nucleated (proestrus); L, lymphocytic (diestrus). Bottom, Histology of ovaries from nNOS-null mice reveals a greater number of atretic follicles and the relative paucity or absence of corpora lutea (CL) when compared with the ovaries of wild-type littermates in which follicular development is normal. D, nNOS activity is required for the onset of the gonadal steroid-evoked LH surge in ovariectomized animals. IB, Immunoblot; ND, not determined.
Neuronal NOS activity is essential for the LH surge
Daily inspection of vaginal cytology in nNos-null mice (n = 7), in which no residual nNOS expression was seen in the preoptic region (Fig. 9B), revealed an absence of normal estrous cyclicity (Fig. 9C, top). nNos-null mice exhibited an alternation of persistent periods of vaginal cornification and a persistent diestrous state with the sporadic occurrence of complete 4–5 d ovarian cycles, whereas wild-type littermates showed regular estrous cyclicity. In contrast to ovaries from wild-type mice (n = 4), which contained large Graafian follicles and corpora lutea, histological inspection of adult nNos-null mouse ovaries (n = 4) revealed the presence of primary and secondary follicles and occasionally an early Graafian follicle, as well as numerous large follicular cysts, but few or no corpora lutea (Fig. 9C, bottom). These findings show that nNos-null mice display ovarian cyclicity deficits and may present impaired ovulation, as previously suggested (Klein et al., 1998). The ability of the hypothalamic–pituitary axis to respond to positive feedback by gonadal steroids was tested in mutant mice. While OVX wild-type mice (n = 7) exhibited an LH surge in response to gonadal steroid treatment, only one of five nNos-null littermates exhibited an LH surge (p < 0.05; Fig. 9D). Concordantly, l-NAME treatment to inhibit NOS activity (n = 10) mimicked the effects of the null mutation on the gonadal steroid-induced LH surge in wild-type mice (Fig. 9D, p = 0.003).
Discussion
We report here that NO-synthesizing neurons are important components of the neural circuit used by estrogens and kisspeptin neurons to trigger the onset of the preovulatory GnRH/LH surge in mice. The use of a genetic strategy to visualize the GPR54-expressing cells targeted by kisspeptin demonstrates that, in addition to GnRH neurons themselves, discrete populations of nNOS neurons abundantly surrounded by kisspeptin fibers also express the kisspeptin receptor GPR54 in the preoptic region. We found that kisspeptin selectively promotes the phosphorylation of nNOS at the Ser1412 activation site by activating the PI3K/AKT signaling pathway in these neurons. A null mutation of Gpr54 prevents both kisspeptin and estradiol effects on nNOS phosphorylation in ovariectomized mice, showing that estrogens require kisspeptin–GPR54 signaling to influence nNOS activity during the ovarian cycle, an effect that is maximal on the day of proestrus when positive feedback comes into play (d'Anglemont de Tassigny et al., 2007a; Parkash et al., 2010; present study). Correspondingly, mice harboring a null mutation of the nNos gene exhibit a markedly impaired ability to generate an LH surge in response to gonadal steroids, and a conspicuous ovulation deficiency. Together, these observations provide a number of new insights that expand our current understanding of kisspeptin- and NO-mediated regulation of the hypothalamic–pituitary–gonadal axis in adult mammals.
NO, which travels readily across cellular membranes and enters presynaptic and postsynaptic sites, is capable of coordinating neuronal inputs in a restricted brain volume defined by its half-life and diffusion constant (Gally et al., 1990). Since NO cannot be stored in synaptic vesicles, unlike other neurotransmitters, mechanisms that regulate its synthesis in time and space are crucial in determining its biological effect (Garthwaite, 2008). In the mouse (Clasadonte et al., 2008; present study), as in the rat (Grossman et al., 1994; Herbison et al., 1996), GnRH neurons are surrounded by NO-synthesizing neurons in the basal forebrain, one of the major sites of NOS expression in the CNS (Bredt et al., 1991; Yamada et al., 1996; Edelmann et al., 2007). The production of neuronal NO in the vicinity of GnRH neurons is tightly regulated by estrogens (d'Anglemont de Tassigny et al., 2007a; Parkash et al., 2010) and has been shown to directly modulate GnRH neuronal activity (Clasadonte et al., 2008). The present findings reveal, intriguingly, that NO has a dual effect on GnRH/LH release during the ovarian cycle. Pharmacological and genetic inhibition of nNOS activity significantly increase basal and kisspeptin-stimulated LH release, respectively, in diestrous mice, suggesting that during the negative-feedback phase of estrogen action, a constitutive basal level of NO release maintains the tonic inhibition of GnRH neurons, keeping LH levels at their nadir. These results are in agreement with data collected from ewes that suggest a role for nitric oxide in the preoptic region in the suppression of GnRH/LH secretion during estrogen-mediated negative feedback in seasonal anoestrus (Stefanovic et al., 2000; McManus et al., 2007), but stand out from those of a previous study performed in rats, in which the pharmacological inhibition of nNOS failed to alter basal or kisspeptin-evoked LH release (Navarro et al., 2005). However, sex differences may account for this discrepancy, as the Navarro study was performed using males, and a recent human study has demonstrated that kisspeptin-stimulated LH release is sexually dimorphic (Jayasena et al., 2011). In contrast, we also show that the blockade of NO synthesis blunts the LH surge in steroid-primed mice, known to involve the activation of kisspeptin–GPR54 signaling (Clarkson et al., 2008) initiated by the kisspeptin neuronal population residing in the anteroventral periventricular nucleus (Clarkson et al., 2008; Gottsch et al., 2009; Mayer et al., 2010). Together, these results suggest that NO- and kisspeptin-synthesizing neurons interact in synergy to coordinate the progression of the ovarian cycle, and thus reconcile the recent evidence that kisspeptin signaling plays a key role in the neuroendocrine control of reproduction (Funes et al., 2003; Seminara et al., 2003; d'Anglemont de Tassigny et al., 2007b; Dungan et al., 2007; Kauffman et al., 2007; Lapatto et al., 2007; Clarkson et al., 2008; Mayer et al., 2010; Mayer and Boehm, 2011), with the originally postulated role for NO in controlling GnRH secretion (Rettori et al., 1993; Mahachoklertwattana et al., 1994), the onset of the preovulatory GnRH/LH surge (Bonavera et al., 1993; Aguan et al., 1996; d'Anglemont de Tassigny et al., 2007a), and fertility (Gyurko et al., 2002). These findings also raise the exciting possibility that the estrogen-evoked kisspeptin-mediated activation of nNOS neurons in proestrus serves as an intermediate synchronizing switch for the GnRH system that enables the transition between pulsatile and peak release of GnRH (Christian and Moenter, 2010). NO could operate such a switch by acting either directly on GnRH neurons (Clasadonte et al., 2008) or on its trans-synaptic inputs (Pielecka-Fortuna et al., 2008; Zhang et al., 2009; Pielecka-Fortuna and Moenter, 2010), or both.
A recent study has demonstrated, provocatively, that kisspeptin neurons and GPR54-expressing cells are dispensable for reproductive function when ablated during a certain window early in development (Mayer and Boehm, 2011), suggesting that the GnRH system can be turned on independently of kisspeptin neurons and kisspeptin/GPR54 signaling. Accordingly, another recent study has demonstrated that an agonist of the NMDA-type glutamate receptor, which triggers puberty, ovulation, and GnRH secretion in both rodents (Urbanski and Ojeda, 1990; Brann and Mahesh, 1991) and primates (Plant et al., 1989; Claypool et al., 2000), is capable of activating the reproductive axis in both Kiss- and Gpr54-null mice, and that these effects may involve nNOS neurons (d'Anglemont de Tassigny et al., 2010). Since most nNOS neurons of the preoptic region express the NMDA receptor (d'Anglemont de Tassigny et al., 2007a) and estrogen receptor α (Scordalakes et al., 2002; Sato et al., 2005), both of which are critical for the estrogen positive-feedback effect on GnRH neurons (Brann and Mahesh, 1991; Wintermantel et al., 2006) and nNOS activity (d'Anglemont de Tassigny et al., 2007a, 2009), it is tempting to speculate that nNOS neurons may be involved in the cellular mechanisms underlying compensation in animals in which neurons mediating kisspeptin/GPR54 signaling are absent (Mayer and Boehm, 2011). Estrogens may indeed regulate nNOS activity via several redundant pathways that are not mutually exclusive. For instance, in parallel with promoting the phosphorylation-dependent activation of nNOS (Parkash et al., 2010), estrogens have been shown to stimulate nNOS activity by modulating the physical and functional coupling of nNOS to NMDA-type glutamate receptors in vivo (d'Anglemont de Tassigny et al., 2007a; Parkash et al., 2010), a phenomenon also observed in vitro in primary hypothalamic neurons in the absence of kisspeptin signaling (d'Anglemont de Tassigny et al., 2009).
Intriguingly, our immunofluorescence experiments demonstrated that the systemic administration of kisspeptin selectively induces nNOS phosphorylation in an area proximal to the OVLT, a brain area devoid of a blood–brain barrier (Broadwell and Brightman, 1976; Ciofi et al., 2009) and containing numerous kisspeptin fibers and nNOS neurons (present paper), and to which GnRH neurons have recently been shown to extend dendrites (Herde et al., 2011; Prevot, 2011). Even though the peripheral administration of kisspeptin-10 is effective in promoting GnRH/LH release, our data suggest that this variant of kisspeptin does not readily cross the blood–brain barrier and thus fails to target all hypothalamic GPR54-expressing neurons in our experimental paradigm. This important caveat notwithstanding, our studies raise the interesting possibility that the OVLT may be a privileged site for interactions between kisspeptin, nNOS, and GnRH neurons in the neuroendocrine brain.
In addition to modulating GnRH neuronal activity within the preoptic region, accumulating evidence suggests that NO is involved in the control of GnRH release within the median eminence, the termination field of GnRH neuroendocrine neurons (Rettori et al., 1993; Knauf et al., 2001; de Seranno et al., 2004, 2010), and another brain site that lies outside of the blood–brain barrier (Ciofi et al., 2009; Mullier et al., 2010). Because kisspeptin has also been shown to stimulate GnRH release from hypothalamic explants containing both the median eminence and the ARH (d'Anglemont de Tassigny et al., 2008), and because arcuate nNOS neurons reside adjacent to the median eminence (Sica et al., 2009), one could argue that a part of the effects observed on LH secretion after the systemic administration of kisspeptin and l-NAME in wild-type and nNos-null animals could be due to an alteration of nNOS activity in these arcuate neurons. However, by showing both that kisspeptin fails to modulate nNOS phosphorylation in the tuberal region of the hypothalamus and, in agreement with a previous study (Herbison et al., 2010), that nNOS neurons of the ARH do not express any detectable GPR54 promoter activity, our data clearly suggest that this population of nNOS neurons is unable to respond directly to kisspeptin. Yet the possibility remains that the kisspeptin neurons intimately associated with nNOS neurons in the ARH communicate through other signaling pathways, such as those involving dynorphin and neurokinin B, two transmitters coexpressed with kisspeptin (Ciofi et al., 2006, 2007; Goodman et al., 2007; Hrabovszky et al., 2010) and that have been postulated to drive short feedback loops within the tuberal region of the hypothalamus to control the rhythmic discharge of kisspeptin and induce the release of GnRH from fibers in the median eminence (Navarro et al., 2009; Wakabayashi et al., 2010).
Together, the results presented here identify preoptic nNOS neurons as an integral component of the neural network controlling ovarian cyclicity and ovulation and indicate that the production of NO may play an important and hitherto unappreciated role in the kisspeptin-dependent preovulatory activation of GnRH neurons. NO could potentially be used by kisspeptin neurons to facilitate synchronous activity among GnRH neurons that were previously operating independently and ultimately to maximize the release of this neurohormone into the blood.
Footnotes
This research was supported by an Equipe FRM 2005 Grant (to V.P.), ANR-07-NEURO-026-03 (to V.P.), ANR-09-BLAN-0267 (to V.P.), and BBSRC Grant BB/F01936X (to W.H.C.). N.K.H. was a Ph.D. student supported by Inserm U837. J.P. was a postdoctoral fellow supported by the Indo-French Centre for the Promotion of Advanced Research. N.B. received a doctoral fellowship from the University of Lille 2 and the Région Nord-Pas de Calais. We thank Julien Devassine for the maintenance of transgenic mouse lines, Meryem Tardivel and Marie-Hélène Gevaert for technical assistance. We thank Dr. A Caraty for his generous gift of antibodies to kisspeptin.
References
- Aguan K, Mahesh VB, Ping L, Bhat G, Brann DW. Evidence for a physiological role for nitric oxide in the regulation of the LH surge: effect of central administration of antisense oligonucleotides to nitric oxide synthase. Neuroendocrinology. 1996;64:449–455. doi: 10.1159/000127151. [DOI] [PubMed] [Google Scholar]
- Beauvillain JC, Tramu G. Immunocytochemical demonstration of LH-RH, somatostatin, and ACTH-like peptide in osmium-postfixed, resin-embedded median eminence. J Histochem Cytochem. 1980;28:1014–1017. doi: 10.1177/28.9.6157712. [DOI] [PubMed] [Google Scholar]
- Bellefontaine N, Hanchate NK, Parkash J, Campagne C, de Seranno S, Clasadonte J, d'Anglemont de Tassigny X, Prevot V. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology. 2011;93:74–89. doi: 10.1159/000324147. [DOI] [PubMed] [Google Scholar]
- Bonavera JJ, Sahu A, Kalra PS, Kalra SP. Evidence that nitric oxide may mediate the ovarian steroid-induced luteinizing hormone surge: involvement of excitatory amino acids. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology. 1991;128:1541–1547. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976;166:257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. doi: 10.1210/endo-108-2-506. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Navarro VM, Fernández-Fernández R, Castaño JP, Malagón MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–577. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience. 2007;146:630–642. doi: 10.1016/j.neuroscience.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prévot V, Levine JE. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150:5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21:673–682. doi: 10.1111/j.1365-2826.2009.01892.x. [DOI] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology. 2008;149:587–596. doi: 10.1210/en.2007-1260. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl D,L-aspartate induces the release of luteinizing hormone-releasing hormone in the prepubertal and pubertal female rhesus monkey as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology (Bethesda) 2010;25:207–217. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buée-Scherrer V, Prevot V. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci. 2007a;27:6103–6114. doi: 10.1523/JNEUROSCI.5595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A. 2007b;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Campagne C, Steculorum S, Prevot V. Estradiol induces physical association of neuronal nitric oxide synthase with NMDA receptor and promotes nitric oxide formation via estrogen receptor activation in primary neuronal cultures. J Neurochem. 2009;109:214–224. doi: 10.1111/j.1471-4159.2009.05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;30:8581–8590. doi: 10.1523/JNEUROSCI.5486-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Seranno S, Estrella C, Loyens A, Cornea A, Ojeda SR, Beauvillain JC, Prevot V. Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. J Neurosci. 2004;24:10353–10363. doi: 10.1523/JNEUROSCI.3228-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Seranno S, d'Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology. 2010;151:1760–1772. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S, Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin's effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–1363. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- Gally JA, Montague PR, Reeke GN, Jr, Edelman GM. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990;87:3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29:9390–9395. doi: 10.1523/JNEUROSCI.0763-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AB, Rossmanith WG, Kabigting EB, Cadd G, Clifton D, Steiner RA. The distribution of hypothalamic nitric oxide synthase mRNA in relation to gonadotrophin-releasing hormone neurons. J Endocrinol. 1994;140:R5–R8. doi: 10.1677/joe.0.140r005. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143:2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152:3832–3841. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci. 2010;31:1984–1998. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Jayasena CN, Nijher GMK, Comminos AN, Abbara A, Januszewki A, Vaal ML, Sriskandarajah L, Murphy KG, Farzad Z, Ghaeti MA, Bloom SR, Dhillo WS. The effects of kisspepetin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96:E1963–E1972. doi: 10.1210/jc.2011-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF. The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci. 2007;27:8826–8835. doi: 10.1523/JNEUROSCI.2099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Carnovale D, Burnett AL, Wallach EE, Zacur HA, Crone JK, Dawson VL, Nelson RJ, Dawson TM. Impaired ovulation in mice with targeted deletion of the neuronal isoform of nitric oxide synthase. Mol Med. 1998;4:658–664. [PMC free article] [PubMed] [Google Scholar]
- Knauf C, Prevot V, Stefano GB, Mortreux G, Beauvillain JC, Croix D. Evidence for a spontaneous nitric oxide release from the rat median eminence: influence on gonadotropin-releasing hormone release. Endocrinology. 2001;142:2343–2350. doi: 10.1210/endo.142.6.8073. [DOI] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- Mahachoklertwattana P, Black SM, Kaplan SL, Bristow JD, Grumbach MM. Nitric oxide synthesized by gonadotropin-releasing hormone neurons is a mediator of N-methyl-d-aspartate (NMDA)-induced GnRH secretion. Endocrinology. 1994;135:1709–1712. doi: 10.1210/endo.135.4.7523101. [DOI] [PubMed] [Google Scholar]
- Mayer C, Boehm U. Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci. 2011;14:704–710. doi: 10.1038/nn.2818. [DOI] [PubMed] [Google Scholar]
- Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci U S A. 2010;107:22693–22698. doi: 10.1073/pnas.1012406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus CJ, Valent M, Hardy SL, Goodman RL. Does nitric oxide act in the ventromedial preoptic area to mediate oestrogen negative feedback in the seasonally anoestrous ewe? Reproduction. 2007;134:137–145. doi: 10.1530/REP-06-0333. [DOI] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Tena-Sempere M. Kisspeptins and the neuroendocrine control of reproduction. Front Biosci (Schol Ed) 2011;3:267–275. doi: 10.2741/s150. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernández-Fernández R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311:126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkash J, d'Anglemont de Tassigny X, Bellefontaine N, Campagne C, Mazure D, Buée-Scherrer V, Prevot V. Phosphorylation of N-methyl-d-aspartic acid receptor-associated neuronal nitric oxide synthase depends on estrogens and modulates hypothalamic nitric oxide production during the ovarian cycle. Endocrinology. 2010;151:2723–2735. doi: 10.1210/en.2010-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Moenter SM. Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology. 2010;151:291–300. doi: 10.1210/en.2009-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci U S A. 1989;86:2506–2510. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot V. GnRH neurons directly listen to the periphery. Endocrinology. 2011;152:3589–3591. doi: 10.1210/en.2011-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci U S A. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sica M, Martini M, Viglietti-Panzica C, Panzica G. Estrous cycle influences the expression of neuronal nitric oxide synthase in the hypothalamus and limbic system of female mice. BMC Neurosci. 2009;10:78. doi: 10.1186/1471-2202-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathatos N, Bourdeau I, Espinosa AV, Saji M, Vasko VV, Burman KD, Stratakis CA, Ringel MD. KiSS-1/G protein-coupled receptor 54 metastasis suppressor pathway increases myocyte-enriched calcineurin interacting protein 1 expression and chronically inhibits calcineurin activity. J Clin Endocrinol Metab. 2005;90:5432–5440. doi: 10.1210/jc.2005-0963. [DOI] [PubMed] [Google Scholar]
- Stefanovic I, Adrian B, Jansen HT, Lehman MN, Goodman RL. The ability of estradiol to induce Fos expression in a subset of estrogen receptor-alpha-containing neurons in the preoptic area of the ewe depends on reproductive status. Endocrinology. 2000;141:190–196. doi: 10.1210/endo.141.1.7286. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Structure of the rat brain. Amsterdam: Elsevier; 2004. [Google Scholar]
- Urbanski HF, Ojeda SR. A role for N-methyl-d-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Emson P, Hökfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Gamma-aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology. 2009;150:2388–2394. doi: 10.1210/en.2008-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]