Abstract
Mercury generates free radicals and subsequently increases oxidative stress, which leads to renal injury. Tribulus terrestris (TT) has good anti-inflammatory and antioxidant properties. Hydroalcoholic extract of different dose of TT was evaluated against mercuric chloride-induced nephrotoxicity. Rats (n = 6) were treated with TT at doses of 100, 200, and 300 mg/kg. Drugs were administered orally for 7 days. Single dose of mercuric chloride (5 mg/kg, intraperitoneal) on the 5th day caused significant elevation of blood urea nitrogen, serum creatinine, malondialdehyde, liver fatty acid binding protein, kidney injury molecule-1, and kidney mercury level and fall in glutathione, superoxide dismutase, glutathione peroxidase, and histopathological changes in disease control as compared to normal control group (P < 0.001). Dose of TT 200 and 300 mg/kg significantly (P < 0.001) prevented the renal injury, and mercury accumulation in kidney tissues significantly decreases in higher dose, i.e., 300 mg/kg as compared to control group. Our result indicates that the treatment of TT exerted significant protection against renal damage induced by mercuric chloride possibly due to its antioxidant and anti-inflammatory properties and by decreasing the renal accumulation of mercury.
Key words: Antioxidant, kidney injury molecule-1, liver fatty acid binding protein, mercuric chloride, nephrotoxicity, Tribulus terrestris
INTRODUCTION
Mercury is a toxic heavy metal and has been shown to induce various diseases associated with the nervous system, kidneys, liver, and other organs. It has several resources to come in contact with consumers such as environmental and occupational. Environmental mercury is present in each tropical level of the food chain, while occupational exposure to mercury has been strengthened by industrial discharge, extensive agriculture practices, vaccine preservatives, dental amalgams, and by many other sources.[1]
The primary target organ for inorganic mercury is the kidney; hence, it is known as nephrotoxic agent.[2,3] Because of the high bonding affinity between mercury and sulfur, mercury binds to metallothioneins and small molecular weight thiols such as cysteine[4] and glutathione (GSH).[5]
Herbal medicines demonstrate good compatibility with the human body and have stood the test of time because of their safety, efficacy, cultural acceptability, and few side effects. Among those, Tribulus terrestris L. (TT, Zygophyllaceae) is an herb with a wide distribution in subtropical regions. TT is used for kidney disorders by removing the gravel from the urine and stone in the bladder.[6,7,8,9,10]
It has already been proven that TT plant extract has antioxidative, apoptosis inhibitory, and vasodilator properties, as well as protecting renal epithelial cells against damages due to oxalates and ethylene glycol consumption.[7,8,9,10] The aim of this study was to investigate the protective effects of TT extracts against acute kidney injury (AKI) induced by mercuric chloride.
MATERIALS AND METHODS
Experimental animals
The study was conducted at the Department of Pharmacology, All India Institute of Medical sciences, New Delhi, India, in August 2016 (document number 944/IAEC/16). The animals were housed in clean polypropylene cages, maintained on a 12-h light/dark cycle under controlled temperature (25°C ± 2°C) and humidity (55% ± 5%). Animals were given continuous access to food and water.
Chemicals
The hydroalcoholic extract of TT was purchased from Sunpure Pvt. Ltd., New Delhi. Mercuric chloride purchased from Sisco Research Laboratories (SRL) Pvt. Ltd., Mumbai, India. The rat kidney injury molecule (KIM)-1 and liver fatty acid binding protein (L-FABP) ELISA kits were purchased from Sincere Biotech Company, China. All other chemicals were purchased locally and of highest purity grade.
Experimental procedure
Wistar rats (n = 6/group) were randomly divided into six groups as indicated below.
Group 1 (normal control): Normal saline (1 ml/kg/day; p.o.) for 7 days
Group 2 (mercuric chloride control): Normal saline (1 ml/kg/day; p.o.) was given for 7 days, and on the 5th day of treatment, a single injection of mercuric chloride at the dose of 5 mg/kg intraperitoneal (i.p.) was given
Group 3–5 (TT 100, 200, and 300 mg/kg/day extract + mercuric chloride): The TT 100, 200, and 300 mg/kg/day; p.o. were administered to Wistar rats for 7 days, and on the 5th day, a single injection of mercuric chloride at the dose of 5 mg/kg i.p. was given
Group 6 (TT per se): The TT a dose of 300 mg/kg/day p.o. at the same ratio of each drug (100 mg) was administered to rats for 7 days.
Estimation of renal function test
The blood urea nitrogen (BUN) and serum creatinine levels had been measured using commercially accessible kits as per the manufacturer's instructions. Absorbance was measured by a ultraviolet spectrophotometer at 510 nm for creatinine and 340 nm for BUN.
Measurement of oxidative stress markers
Kidney tissue homogenate (10%) was prepared as explained above, and the parameters such as malondialdehyde (MDA), superoxide dismutase (SOD), GSH, and glutathione peroxidase (GPx) were estimated as per the methods described in literature.
Measurement of acute kidney injury and histopathological assessment
These two measurements are done by according to our previous publication.[11]
Measurement of mercury in kidney tissue by inductive coupled plasma atomic emission spectroscopy
Five hundred milligrams of the tissue was placed in a digestion vessel unit, and 3 ml of nitric acid and 1 ml of hydrogen peroxide were added. The samples were placed in a digester (Sineo Company) and scheme 2 was selected where the temperature was increased incrementally up to 160°C. Finally, the digested samples were assessed using inductively coupled plasma atomic emission spectroscopy.
Statistical analysis
All the data were represented as mean ± standard error of the mean. The data were analyzed using Wilcoxon rank -sum test with direct comparison of respective groups. The P < 0.05 was considered statistically significant. The data were analyzed using the standard statistical software for GraphPad Prism version 5.03 (San Diego, CA, USA).
RESULTS
Effect on renal function test
Mercuric chloride-induced Wistar male rats exhibited significant (P < 0.001) elevation of serum creatinine and BUN when compared with normal Wistar male rats. Treatment of TT at dose of 100/200 mg/kg reduced the elevated level of serum creatinine and BUN as compared to mercuric chloride-induced Wistar male rats in a dose-dependent manner [Figure 1a and b]. However, the dose of TT at 300 mg/kg each significantly (P < 0.001) decreased the serum creatinine levels as compared to mercuric chloride control group.
Figure 1.

Effect of Tribulus terrestris on kidney function tests serum creatinine (a) and blood urea nitrogen (b) in different experiment group in mercuric chloride-induced nephrotoxicity. Each bar represents mean ± standard error of the mean, (n = 6). Mercuric chloride control: Mercuric chloride at 5 mg/kg TT. 100/200/300 + mercuric chloride: Mercuric chloride at 5 mg/kg in combination of TT at 100/200/300 mg/kg each, respectively. TT, at 300 mg/kg of drug as per se group. a*** P < 0.005 a*** P < 0.001, a*** P < 0.01 versus normal control, b** P <0.05, b** P <0.01, b** P < 0.001 vs. control and b*** P < 0.05, b*** P < 0.01, b*** P < 0.001 versus control. BUN: Blood urea nitrogen, TT: Tribulus terrestris
Effect on oxidative stress
Wistar male rats from mercuric chloride-induced group presented with significant increase in tissue MDA level [Figure 2a], decrease in SOD enzyme activities [Figure 2b], GSH content [Figure 2c], and GPx content [Figure 2d]. Earlier treatment with TT, Boerhavia diffusa, and Terminalia chebula significantly suppressed renal MDA level (P < 0.001) and attenuated the depletion of antioxidant defense system and significantly attenuated the decrease in of SOD activity (P < 0.001), GSH levels (P < 0.001) and GPx activity (P < 0.001) comparison to mercuric chloride-treated group. However, the highest dose of TT at 300 mg/kg each significantly (P < 0.001) reduced the oxidative stress markers as compared to mercuric chloride control group.
Figure 2.
Effect of Tribulus terrestris on (a) MDA level (b) SOD activity (c) GSH content (d) GPx level in different experiment group in mercuric chloride (5 mg/kg, i.p., once) induced nephrotoxicity. Each bar represent mean ± SEM., (n = 6). Mercuric chloride Control: Mercuric chloride at 5 mg/kg. TT. 100/200/300 + Mercuric chloride: Mercuric chloride at 5 mg/kg in TT at 100/200/300 mg/kg, respectively. TT, at 300 mg/kg of drug as per se group. a*** P < 0.005 a*** P < 0.001, a*** P < 0.0.01 vs. Normal control, b** P < 0.05, b** P < 0.01, b** P < 0.001 vs. control and b*** P < 0.05, b*** P < 0.01, b*** P < 0.001 vs. control
Effect on acute kidney injury
KIM-1 was measured to estimate the AKI in the proximal tubule of kidney. Figures 3 and 4 represent the KIM-1 and L-FABP data. The KIM-1 and L-FABP levels were significantly (P < 0.001) increased in mercuric chloride-treated group as compared to normal control group. The administration of TT at a dose of 100/200 mg/kg was unable to reduce significantly the levels of KIM-1 and L-FABP as compared to mercuric chloride control group. However, the administration of TT at a dose of 300 mg/kg significantly reduced the levels of KIM-1 and L-FABP.
Figure 3.

Effect of Tribulus terrestris on Kim-1 level level in different experiment group in mercuric chloride (5 mg/kg, i.p., once) induced nephrotoxicity. Each bar represent mean ± SEM., (n=6). Mercuric chloride Control: Mercuric chloride at 5 mg/kg. TT. 100/200/300 + Mercuric chloride: Mercuric chloride at 5 mg/kg in TT at 100/200/300 mg/kg each, respectively. TT, at 300 mg/kg of drug as per se group. a*** P < 0.005 a*** P < 0.001, a*** P < 0.0.01 vs. Normal control, b** P < 0.05, b** P < 0.01, b** P < 0.001 vs. control and b*** P < 0.05, b*** P < 0.01, b*** P < 0.001 vs. control
Figure 4.

Effect of Tribulus terrestris on L-FABP level in different experiment group in mercuric chloride (5 mg/kg, i.p., once) induced nephrotoxicity. Each bar represent mean ± SEM., (n = 6). Mercuric chloride Control: Mercuric chloride at 5 mg/kg. TT. 100/200/300 + Mercuric chloride: Mercuric chloride at 5 mg/kg in combination of TT at 100/200/300 mg/kg each, respectively. TT, at 300 mg/kg of drug as per se group. a*** P < 0.005 a*** P < 0.001, a*** P < 0.0.01 vs. Normal control, b** P < 0.05, b** P < 0.01, b** P < 0.001 vs. control and b*** P < 0.05, b*** P < 0.01, b*** P < 0.001 vs. control
Effect on histopathological structural changes
The dose of TT 100/200/300 mg/kg, respectively, showed 50%–75% intact/viable tubular epithelium and remaining were necrotized representing grade 1 protection [Figure 5].
Figure 5.
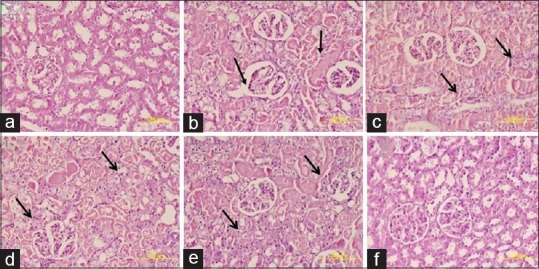
Effect of Tribulus terrestris on histopathological change in proximal and glomerulus tubular cell: (a) Group 1 kidney shows regular morphology with well-developed tubular and glomerulus epithelial cell. (b) Group 2 kidney exhibits diffuse acute proximal tubular necrosis, glomerular atrophy and degeneration of renal tubular cells. (c and d) Kidney section from Groups 3 and 4 shows mild inflammation and proximal tubule degeneration. (e) In kidney section from Group 5, there was no inflammation and tubules appeared nearly normal. (f) Group 6 exhibits normal structure of proximal tubular cells and cortex
Effect on mercury accumulation
Based on the results in Table 1 shows the data for mercury accumulation in rat kidney tissue. Mercury was found to be significantly accumulated in mercuric chloride control rats as compared to normal control. However, TT (300 mg/kg) significantly decreased the mercury concentration in kidney tissue as compared to mercuric chloride control group.
Table 1.
The effect of Tribulus terrestris on mercury accumulation in the kidneys of male Wistar rats
| Name of group | Mercury concentration (μg/g) |
|---|---|
| Normal control | 0.09 |
| Mercuric chloride control | 27.21±0.22*a |
| TT, 200 mg/kg/day + mercuric chloride | 19.1±0.21*b |
| TT 200 mg/kg/day | 0.13 |
The animals in Groups 2 and 3 were given 8 mg/kg intraperitoneally of mercuric chloride on the 7th day of treatment. The animals in Group 1 received vehicle (normal saline [1 ml/kg/day]; postoperative) on the 7th day of treatment. The animals in Groups 3 and 4 were given TT, BD, and TC once a day for 7 days whereas the animals in Groups 1 and 2 were given vehicle. The data are expressed as the mean±SEM and n=6 for each treatment group. *aP<0.05 versus normal control, *bP<0.05 versus mercuric chloride control. TT: Tribulus terrestris, BD: Boerhaavia diffusa, TC: Terminalia chebula, SEM: Standard error of the mean
DISCUSSION
Mercuric chloride is an unsafe environmental and industrial toxin which initiates pernicious consequences for different body tissues of people and animals. There are many sources of mercury exposure, for example, inward breath of mercury vapors, ingestion of contaminated food/water, gold mining, the creation of lights/batteries, through therapeutic medicines and dental amalgam fillings.[12] Kidneys are the principle focuses for inorganic mercury and may prompt AKI.[13] The objectives of mercury-actuated nephrotoxicity are thiol-containing enzymes and antioxidants such as GSH.
In the present study, we determined the effect of TT on mercuric chloride-induced nephrotoxicity in Wistar rats by measuring renal function, acute kidney damage markers, histopathological features, and levels of certain antioxidants and activity of enzymes involved in the biosynthesis of antioxidants. Overall, our results suggested that the administration of TT had nephroprotective effects in rats administered 5 mg/kg i.p. of mercuric chloride after 7 days of treatment with TT.
Clinically, it is well established that serum creatinine and BUN levels are indicators of renal function.[14] The normal reference values for BUN and creatinine earlier reported in our laboratory ranged from 20 to 30 mg/dl and 0.4 to 0.6 mg/dl, respectively. In our results, the values of BUN and creatinine indicative of severe kidney dysfunction ranged from 60 to 80 mg/dl and 2 to 3 mg/dl, respectively.[15]
Our results indicate that the administration of TT at 200 mg/kg each for 7 days significantly decreased the mercuric chloride-induced increase BUN and serum creatinine levels. The administration of lower doses (100 mg/kg i.p.) of TT did not significantly decrease mercuric chloride-induced nephrotoxicity based on serum creatinine, SOD, GSH, and GPx.
We hypothesized that the nephroprotective efficacy of TT could be due to their attenuation of renal oxidative stress. It is well established that increased oxidative stress induces lipid peroxidation and depletion of antioxidants like GSH and decreases the levels and activity of the enzymes SOD and GPx, which play a role in oxidative defense.[16] Tissue MDA and GSH levels and the activity of GPx and SOD have been reported to be indicators of oxidative stress.[17] MDA levels have been reported to be positively correlated with the magnitude of cellular toxicity.[18] In addition, GSH, GPx, and SOD play a critical role in maintaining the oxidative/antioxidant balance in cells.[19]
In this study, the administration of a single dose of 5 mg/kg i.p. of mercuric chloride significantly decreased the levels of GSH, GPx, and SOD and increased the levels of MDA. Our findings are consistent with previous studies indicating that the reactive oxygen species (ROS) are involved in the pathogenesis of mercuric chloride-induced nephrotoxicity.[20] Our results indicated that the administration of 100 or 200 mg/kg i.p. of TT significantly attenuated the renal oxidative stress induced by mercuric chloride as evidenced by the decrease in the levels of creatinine and BUN and increase in the level of GSH, SOD, and GPx. Consistent with our results, it has been reported that TT significantly decreases apoptosis in mouse kidney.[21] TT has been reported to decrease ROS and increase the removal of gravel from the urine and calculi from the bladder of mice.[22] TT significantly enhances mitochondrial function and inhibits the release of cytochrome c. Based on the results of this study and others,[20,22] we hypothesize that the nephroprotective effects of the TT may be due to antioxidant and anti-inflammatory efficacy.
In this study, a single administration of 5 mg/kg i.p. of mercuric chloride on the 5th day of treatment significantly increased the accumulation of mercury in animals as compared to animals given vehicle only. The findings of this study are consistent with literature showing that kidney accumulates and retains mercury to a greater degree than other organs.[23,24] The administration of 200 mg/kg i.p. each of TT decreases (not significantly) the accumulation of mercury compared to mercuric chloride control group. However, it is possible that increasing the length of treatment could produce a significant decrease in mercury accumulation although this remains to be determined.
A number of studies indicate that increase in the level of the proteins KIM-1 and L-FAB represent a biomarker for renal damage.[25] Furthermore, KIM-1 and L-FAB have predictive value for AKI in patients undergoing cardiac surgery.[26,27] In this study, mercuric chloride as previously reported[28] significantly increased the expression of KIM-1 and L-FAB in the glomeruli and renal tubules of Wistar rats. The treatment of TT at dose of 200 mg/kg significantly decreased the expression of KIM-1 and L-FABP. As previously reported,[28,29] a single injection of 5 mg/kg i.p. of mercuric chloride produced a number of renal morphological changes.[29]
CONCLUSION
Our data suggest that the antioxidant efficacy of the TT treatment plays a significant role attenuating the nephrotoxic effects of mercuric chloride. Provided our results can be extended to humans, they suggest that the combination of TT could be used in the pharmacotherapy of nephrotoxicity due to mercuric chloride and possibly other heavy metals.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012;45:344–52. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13:385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, et al. The role of metallothionein in oxidative stress. Int J Mol Sci. 2013;14:6044–66. doi: 10.3390/ijms14036044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovářová J, Svobodová Z. Can thiol compounds be used as biomarkers of aquatic ecosystem contamination by cadmium? Interdiscip Toxicol. 2009;2:177–83. doi: 10.2478/v10102-009-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhatre S, Nesari T, Somani G, Kanchan D, Sathaye S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn Rev. 2014;8:45–51. doi: 10.4103/0973-7847.125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HH, Ahn EK, Hong SS, Oh JS. Anti-inflammatory effect of tribulusamide D isolated from Tribulus terrestris in lipopolysaccharide-stimulated RAW264.7 macrophages. Mol Med Rep. 2017;16:4421–8. doi: 10.3892/mmr.2017.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushik J, Tandon S, Gupta V, Nayyar J, Singla SK, Tandon C. Response surface methodology based extraction of Tribulus terrestris leads to an upsurge of antilithiatic potential by inhibition of calcium oxalate crystallization processes. PLoS One. 2017;12:e0183218. doi: 10.1371/journal.pone.0183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammoda HM, Ghazy NM, Harraz FM, Radwan MM, ElSohly MA, Abdallah II. Chemical constituents from Tribulus terrestris and screening of their antioxidant activity. Phytochemistry. 2013;92:153–9. doi: 10.1016/j.phytochem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Jiang YH, Yang CH, Li W, Wu S, Meng XQ, Li DN. Aqueous extracts of Tribulus terrestris protects against oxidized low-density lipoprotein-induced endothelial dysfunction. Chin J Integr Med. 2016;22:193–200. doi: 10.1007/s11655-015-2321-0. [DOI] [PubMed] [Google Scholar]
- 11.Sharma US, Yadav HN, Khare VM, Singh S, Gupta YK, Tiwari AK. Combination of Tribulus terrestris, Boerhaavia diffusa and Terminalia chebula reverses cisplatin-induced nephrotoxicity in Wistar rats. Pharmacologia. 2019;10:1–11. [doi: 10.5567/pharmacologia. 2019] [Google Scholar]
- 12.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, et al. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–63. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 13.Gowda S, Desai PB, Kulkarni SS, Hull VV, Math AA, Vernekar SN. Markers of renal function tests. N Am J Med Sci. 2010;2:170–3. [PMC free article] [PubMed] [Google Scholar]
- 14.Ighodaroab OM, Akinloyeb OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex J Med. 2018;54:287–93. [Google Scholar]
- 15.Karwasra R, Kalra P, Nag TC, Gupta YK, Singh S, Panwar A. Safety assessment and attenuation of cisplatin induced nephrotoxicity by tuberous roots of Boerhaavia diffusa. Regul Toxicol Pharmacol. 2016;81:341–52. doi: 10.1016/j.yrtph.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Kuyumcu F, Aycan A. Evaluation of oxidative stress levels and antioxidant enzyme activities in burst fractures. Med Sci Monit. 2018;24:225–34. doi: 10.12659/MSM.908312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorente L, Martín MM, Abreu-González P, Domínguez-Rodriguez A, Labarta L, Díaz C, et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit Care. 2013;17:R290. doi: 10.1186/cc13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghanbari A, Zare G, Khazaei M, Moradi M, Raoofi A. Tribulus terrestris hydroalcoholic extract effect on cisplatin-induced apoptosis in mice kidney. Int J Morphol. 2016;34:713–8. [Google Scholar]
- 20.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawardekar SB, Patel TC. Evaluation of the effect of Boerhavia diffusa on gentamicin-induced nephrotoxicity in rats. J Ayurveda Integr Med. 2015;6:95–103. doi: 10.4103/0975-9476.146545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prathapan A, Vineetha VP, Raghu KG. Protective effect of Boerhaavia diffusa L. Against mitochondrial dysfunction in angiotensin II induced hypertrophy in H9c2 cardiomyoblast cells. PLoS One. 2014;9:e96220. doi: 10.1371/journal.pone.0096220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aldahmash BA, El-Nagar DM, Ibrahim KE. Reno-protective effects of propolis on gentamicin-induced acute renal toxicity in Swiss albino mice. Nefrologia. 2016;36:643–52. doi: 10.1016/j.nefro.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–72. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 25.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeoye BO, Asenuga ER, Oyagbemi AA, Omobowale TO, Adedapo AA. The protective effect of the ethanol leaf extract of Andrographis paniculata on cisplatin-induced acute kidney injury in rats through nrf2/KIM-1 signalling pathway. Drug Res (Stuttg) 2018;68:23–32. doi: 10.1055/s-0043-118179. [DOI] [PubMed] [Google Scholar]
- 27.Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins. Indian J Pharmacol. 2013;45:354–8. doi: 10.4103/0253-7613.115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kader AM, Qutaym AA, Saeedan AS, Hamad AM, Alkharfy KM. Nephroprotective and hepatoprotective effects of Tribulus terrestris Linn growing in Saudi Arabia. J Pharm Pharmacognosy Res. 2016;4:144–52. [Google Scholar]
- 29.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]



