Abstract
Although the neurotransmitters/modulators glutamate and, more recently, glycine have been implicated in the development and maintenance of Alcohol Use Disorder (AUD) in preclinical research, human proton magnetic resonance spectroscopy (1H-MRS) studies have focused solely on the measurement of glutamate. The purpose of the present analysis was to examine the relative associations of brain glutamate and glycine levels with recent heavy drinking in 41 treatment naïve individuals with AUD using 1H-MRS. The present study is the first that we are aware of to report in vivo brain glycine levels from an investigation of addiction.
Dorsal Anterior Cingulate Cortex (dACC) glutamate and glycine concentration estimates were obtained using Two-Dimensional J-Resolved Point Resolved Spectroscopy at 3 Tesla, and past 2-week summary estimates of alcohol consumption were assessed via the Timeline Followback method. Glutamate (β= −0.44, t= −3.09, p= 0.004) and glycine (β= −0.68, t= −5.72, p< 0.001) were each significantly, inversely associated with number of heavy drinking days when considered alone. However, when both variables were simultaneously entered into a single regression model, the effect of glutamate was no longer significant (β= −0.11, t= −0.81, p= 0.42) whereas the effect of glycine remained significant (β= −0.62, t= −4.38, p< 0.001).
The present study extends the literature by demonstrating a unique, inverse association of brain glycine levels with recent heavy drinking in treatment naïve individuals with AUD. If replicated and extended, these data could lead to enhanced knowledge of how glycinergic systems change with alcohol consumption and AUD progression leading to pharmacological interventional/preventative strategies that modulate brain glycine levels.
Keywords: alcohol use disorder, proton magnetic resonance spectroscopy, glycine, glutamate, heavy drinking
Introduction
Glycine, a non-essential amino acid that is biosynthesized from serine, has key neurotransmission functions at inhibitory synapses in the spinal cord, brainstem, and cerebellum and at excitatory synapses throughout the brain, as a requisite co-agonist of glutamate at N-methyl-D-aspartate [NMDA] receptors (1–3). Glycine and glutamate are each sequestered within astrocytes with extracellular and synaptic levels tightly-regulated by the astrocytic transporters GlyT1 and GluT, respectively (4). Calcium ion flux through activated NMDA receptors (NMDAR) has been identified as critical to synaptic plasticity, a well-supported cellular mechanism of learning and memory (5). Not surprisingly, then, dysregulated NMDAR functioning is hypothesized to be central to the development of a wide variety of neuropsychiatric, neurodegenerative diseases, and addictions including Alcohol Use Disorder (AUD) (6).
Preclinical research has long demonstrated that ethanol directly inhibits NMDA-activated ion currents (7). Compensatory NMDAR adaptations that develop in response to chronic ethanol administration are believed to heavily contribute to the behavioral phenomena of ethanol tolerance, withdrawal, and relapse, including: a) increased presynaptic glutamate release and b) increased postsynaptic function of NR2A and NR2B subunit-containing NMDAR (reviewed in (8)), along with, c) decreased prefrontal glycine release during inhibitory control challenge (9), and d) restoration of normal inhibitory control via inhibition of GlyT1-mediated glycine transport (9).
Despite the critical importance of both glutamate and glycine to NMDAR function, and purportedly to the neurobehavioral phenomenology of AUD, in-vivo proton magnetic resonance spectroscopy (1H-MRS) investigations of people with AUD have focused almost exclusively on glutamate (or more commonly, the combination of glutamate and glutamine, termed “Glx”) - in large part because of the methodological challenges involved with measuring brain glycine levels using 1H-MRS at commonly-available magnetic field strengths (e.g., 3 Tesla). In line with the preclinical literature, these studies have generally found abnormal levels of cortical glutamate in individuals with AUD relative to healthy control participants (10–12).
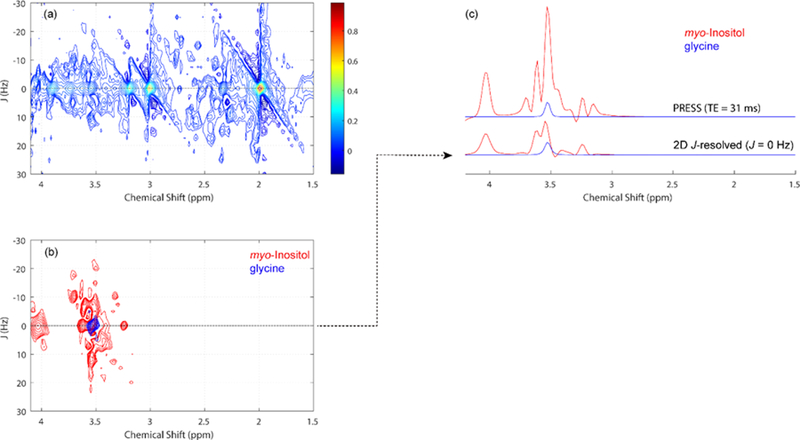
We recently published evidence of an inverse association of cortical glutamate levels with recent heavy drinking, indicating lower glutamate levels with higher levels of heavy drinking, in treatment-naïve AUD participants (13). The present investigation is a follow-up examination of the shared and unique associations between glutamate, glycine, and recent heavy drinking in AUD using a specialized Two-Dimensional (2D) 1H-MRS acquisition sequence capable of reliably measuring glycine (Figure 1) as well as glutamate (14).
Figure 1.
(a) Representative 2D J-resolved 1H MRS data recorded from a single healthy control subject. The expanded spectral region (chemical shift: 1.5 – 4.1 ppm, J: ±30 Hz) is shown for visualization purposes. (b) The individual myo-inositol and glycine 2D spectral fits as estimated using the ProFIt software, with color coding used to discriminate the two metabolites. (c) Simulated short-TE PRESS data (top spectra) and the J = 0 Hz row (bottom spectra) extracted from the individual 2D spectral fits for myo-inositol and glycine. For conventional PRESS, the 3.55 ppm glycine singlet resonance is dominated by the strongly overlapping methine resonances of myo-inositol. Due to strong J-coupling operating between the myo-inositol methine protons, 2D J-resolved 1H MRS distributes their peaks along the second dimension, largely reducing the signal contribution along the J = 0 Hz axis thus enhancing the detectability of the uncoupled glycine methylene singlet resonance. Signal integration between the chemical region 3.50 – 3.60 ppm region shows that the myo-inositol-to-glycine peak area ratio is reduced from 9.4 to 2.8 for the 2D J-resolved (J = 0 Hz) situation. The ProFit software, however, uses individual 2D MRS basis functions for all included metabolites, thus the discrimination of myo-inositol and glycine is further enhanced through the constrained chemical shift and J-coupling information supplied to the fitting algorithm.
Material and methods
Data were pooled from two studies of treatment-naïve individuals with Alcohol Use Disorder for the present investigation (n=20 (13), n=21 (15)). All participants were required to be between the ages of 21 and 40, to meet Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; (16)) criteria for alcohol dependence (including the “loss of control over drinking” and/or “inability to cut-down or stop drinking” criteria), to report consuming at least 20 standard drinks, with at least one heavy drinking day (i.e., ≥ 5/4 standard drinks in a day for men/women), in each of the two weeks preceding the study, and to not be actively seeking AUD treatment. Exclusion criteria included current DSM-IV Axis I disorder other than alcohol dependence or nicotine dependence, positive urine drug or alcohol breath screens on the day of the scan, history of severe alcohol withdrawal (seizure, delirium tremens, need for inpatient or outpatient detoxification), and current alcohol withdrawal symptoms (i.e., Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-Ar; (17)] > 3). Furthermore, participants could not be taking any psychotropic medications. Additional MRI-related exclusion criteria included presence of non-MRI safe objects in the body, claustrophobia, history of traumatic brain injury, or pregnancy. Recent heavy drinking was defined as the total number of heavy drinking days reported over the 14 days preceding MRI (as in (13)), as measured by the calendar-based Timeline Followback instrument (18).
The present study utilized the same imaging methods described in more detail in (13), including, acquisition of 2D J-PRESS data from an 18.75 cubic-centimeter (2.5cm x 2.5cm x 3.0cm) volume located within the dorsal Anterior Cingulate Cortex (dACC; i.e., the portions of Brodmann areas 24 and 32 dorsal to the genu of the corpus callosum), acquisition of T1-weighted images for segmentation and partial volume correction, spectral fitting via the ProFit algorithm, and metabolite scaling to un-suppressed water (corrected for within-voxel CSF fraction). Prior research has established the adequate model fit (M[SD] Cramer-Rao Lower Bound = 5.9 [1.3]) and test-retest reliability (intra-subject CV = 12–13%) of glycine as measured by 2D J-Resolved Point Resolved Spectroscopy (J-PRESS) at 3 Tesla in healthy volunteers (Figure 1, (14)). Bivariate associations of dACC glutamate and glycine levels with recent heavy drinking were individually characterized via bivariate scatterplots and multiple linear regression models, with heavy drinking in the previous 14 days as the dependent variable. In total, 4 regression coefficients were tested for statistical significance; α was conservatively set to p< 0.0125 (note, the observed pattern of results did not depend on adoption of this α level relative to a standard nominal α level of p<0.05 per test). To rule out coincident myo-inositol signal as an alternative explanation for potential significant associations between glycine and recent heavy drinking (see Figure 1), we added myo-inositol to the regression model as a covariate.
Results
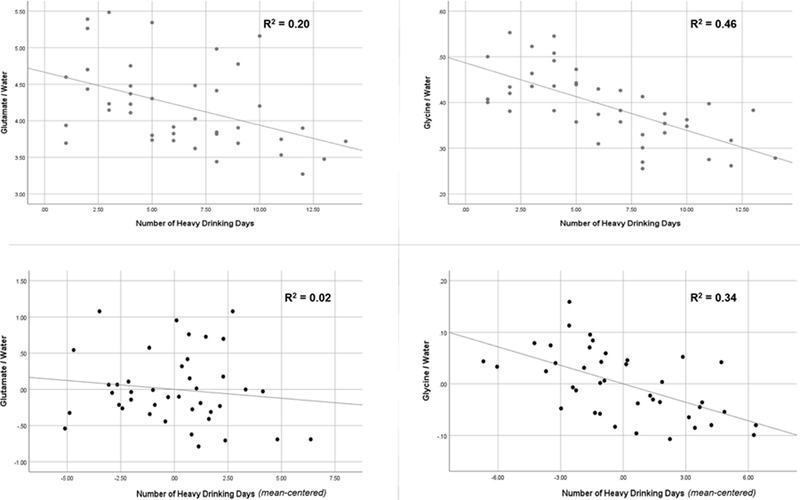
A total of 41 AUD participants were included in the present analysis (see Table 1 for demographic and alcohol use characteristics). 1H-MRS data were characterized by an average water linewidth of 6.07 FWHM (SD = 0.48) and an average NAA signal-to-noise ratio of 652.50 (SD = 105.83). Prior to evaluating glutamate and glycine levels as independent predictors in multiple regression models, age, sex, race, education, and smoking status were first evaluated as potential covariates. None were significantly associated with recent heavy drinking in regression models (all ps < 0.15) and were therefore not considered further. Glutamate and glycine were moderately correlated with one another (r = 0.54, p < 0.001). Next, glutamate and glycine were each entered into separate regression models predicting recent heavy drinking. Glutamate (β= −0.44, t= −3.09, p= 0.004) and glycine (β= −0.68, t= −5.72, p< 0.001) were each significantly, inversely associated with recent heavy drinking when considered alone (i.e., lower glutamate and glycine levels were each significantly associated with higher levels of heavy drinking). However, when both variables were simultaneously entered into a single regression model, the effect of glutamate was no longer significant (β= −0.11, t= −0.81, p= 0.42) whereas the effect of glycine remained significant (β= −0.62, t= −4.38, p< 0.001). Figure 2 displays bivariate, zero-order scatterplots (top panel) and partial regression plots (bottom panel) of these associations. Importantly, adding myo-inositol as a covariate (β= 0.04, t= 0.19, p= 0.85) to the regression model did not alter the pattern of observed findings (glycine β= −0.62, t= −4.29, p< 0.001; glutamate β= −0.14, t= −0.71, p= 0.48). Together, these results suggest that dACC glycine, and not glutamate, levels were uniquely, and significantly related to recent heavy drinking in treatment naïve individuals with AUD.
Table 1.
Demographic and alcohol use characteristics of the sample (n=41)
| Variable | No. (%) of participants / Mean | Standard Deviation |
|---|---|---|
| Gender (male) | 32 (78.0%) | |
| Smoking Status (≥10 cigarettes/day) | 39 (95.1%) | |
| Race (Caucasian) | 35 (85.4%) | |
| Age | 26.98 | 6.15 |
| Education (number of total years) | 14.24 | 2.02 |
| Alcohol Dependence Scale | 10.93 | 5.10 |
| # Heavy drinking days (past 14 days) | 6.32 | 3.55 |
| Drinks per drinking day | 7.23 | 3.35 |
| Days since last drink | 1.71 | 1.12 |
Figure 2.
Associations of glutamate and/or glycine with total number of heavy drinking days (nHDD) within the past 14 days. Top panel = scatterplot and linear fit between a) glutamate and nHDD (left) and b) glycine and nHDD (right). Bottom panel = Partial regression plot and linear fit between a) glutamate and nHDD, controlling for glycine (left) and b) between glycine and nHDD, controlling for glutamate (right).
Discussion
The primary finding from the present study was that dACC glycine levels were uniquely and significantly related to recent heavy drinking in treatment naïve AUD individuals. While initially observed to be significant, the association of glutamate levels with recent heavy drinking could be explained by the relationship (variance shared) between glutamate and glycine. The present 1H-MRS study is the first that we are aware of to report brain frontal glycine levels related to alcohol consumption in AUD individuals. It is, therefore, possible that the effects of recent drinking on brain glycine dynamics might whole, or in part, explain the previously reported abnormal glutamate levels in AUD (e.g., (10–12)).
Unlike glutamate, glycine levels can only be measured using specialized spectral-editing 1H-MRS methods (e.g., 2D J-PRESS, TE-averaged PRESS) at clinical magnetic field strengths (19). The few available studies that have reported brain glycine levels using these methods have found that they sensitively capture increases in glycine following oral glycine administration (20), as well as, theoretically-predicted elevations in first-episode psychosis patients relative to unaffected controls (21). Although suitable spectral-editing 1H-MRS methods are relatively more challenging to implement, and therefore more sparsely represented in the literature at present time, our findings suggest that implementation of such methods will be important to advancing research on the pathophysiology of AUD.
Recently, there has been growing interest in glycine, both as a co-agonist of NMDAR and as an important primary brain target of ethanol (22), which has naturally led to the development and investigation of compounds that selectively inhibit the glycine transporter, GlyT-1, in particular, derivatives of sarcosine (N-methylglycine). Because astrocytes in close proximity to GlyR and/or NMDAR express GlyT-1, inhibition of GlyT-1 has the potential to disrupt multiple potential mechanisms of the acute and chronic effects of alcohol consumption on the neurobehavioral phenomena of intoxication, alcohol reinforcement, withdrawal, and relapse (22). Before these hopes can be potentially realized, however, studies are needed to investigate the effects of GlyT-1 inhibitors on brain glycine levels, along with the effects of medication-induced changes in brain glycine levels on clinically relevant behavioral measures of AUD phenomenology.
The results of the present study should be interpreted in light of several limitations. First, the recent drinking variable relied entirely on subjective self-report; supplementation of subjective drinking data with objective biomarkers of recent alcohol consumption would strengthen confidence in our findings. Second, because assessment for the present study was limited and the sample was selected to be homogeneous on a number of key alcohol diagnostic and drinking variables, we were not able to evaluate correlations of brain glycine concentrations with additional clinically meaningful variables (e.g., alcohol craving). Third, the software used to process 2D J-PRESS data for the present study did not allow for explicit modeling of co-edited macromolecule signals, which may have disproportionately added systematic error variance to glutamate, but not glycine, estimates (19). Fourth, we did not attempt to control for participants’ cognitive state during 1H-MRS acquisition, which may have added error variance to glutamate estimates (23). Participants were instructed only to remain still during the scan. Fifth, although 2D J-PRESS substantially reduces the overlap observed between glycine and myo-inositol signals, it does not eliminate it completely (see Figure 1). Notably, follow-up analyses demonstrated that the pattern of findings obtained in the present study did not change when participants’ myo-inositol levels were covaried. Finally, a major limitation of 1H-MRS in general is that estimated metabolite levels reflect total tissue concentrations, which cannot discern between specific compartmental (e.g., neurotransmitter versus metabolic) signal contributions. This limitation makes it difficult to predict how adding glycine measurements to studies involving associations of glutamate levels with AUD phenomenology (e.g., alcohol withdrawal) should affect results. It also makes it difficult to predict whether, and in what direction, medications shown to affect glutamatergic or glycinergic neurotransmission in preclinical research will impact glutamate and/or glycine concentrations in humans (4). Expanding such preclinical research to include 1H-MRS scanning would provide an invaluable backwards-translational bridge that could greatly improve the success rate of medication development efforts.
These limitations notwithstanding, the present study extends the literature by demonstrating a unique inverse association of frontal brain glycine, but not glutamate, levels with recent heavy drinking in treatment naïve individuals with AUD. If these results are replicated and extended to behavioral/subjective measures, this could lead to an enhanced knowledge of how glycine systems might impact aspects of AUD and could lead to pharmacological interventional/preventative strategies targeted to modulate brain glycine levels to prevent or treat AUD.
Highlights.
Glutamate and glycine disturbances have been reported in alcohol use disorder
In vivo MRS research has focused exclusively on glutamate disturbances in people
Glutamate and glycine levels were each associated with recent heavy drinking
Lower metabolite concentrations correlated with relatively more heavy drinking
Only associations involving glycine were significant, controlling for glutamate levels
Acknowledgments
Funding Sources: This research was supported by the Charleston Alcohol Research Center (P50 AA010761) and a grant to Dr. Prisciandaro from ABMRF/The Alcohol Research Fund. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cummings KA, Popescu GK. Glycine-dependent activation of NMDA receptors. The Journal of general physiology 2015;145(6):513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends in biochemical sciences 2005;30(6):325–33. [DOI] [PubMed] [Google Scholar]
- 3.Legendre P The glycinergic inhibitory synapse. Cellular and molecular life sciences : CMLS 2001;58(5–6):760–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javitt DC. Excitatory Amino Acids in Schizophrenia: Both What You Have, and What You Do With Them. Biological psychiatry 2018;83(6):470–2. [DOI] [PubMed] [Google Scholar]
- 5.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends in neurosciences 1993;16(12):521–7. [DOI] [PubMed] [Google Scholar]
- 6.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature reviews Neuroscience 2007;8(6):413–26. [DOI] [PubMed] [Google Scholar]
- 7.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science (New York, NY) 1989;243(4899):1721–4. [DOI] [PubMed] [Google Scholar]
- 8.Roberto M, Varodayan FP. Synaptic targets: Chronic alcohol actions. Neuropharmacology 2017;122:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irimia C, Buczynski MW, Natividad LA, Laredo SA, Avalos N, Parsons LH. Dysregulated Glycine Signaling Contributes to Increased Impulsivity during Protracted Alcohol Abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience 2017;37(7):1853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, et al. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcoholism, clinical and experimental research 2013;37(10):1643–9. [DOI] [PubMed] [Google Scholar]
- 11.Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry 2012;71(11):1015–21. [DOI] [PubMed] [Google Scholar]
- 12.Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug and alcohol dependence 2012;125(1–2):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prisciandaro JJ, Schacht JP, Prescot AP, Renshaw PF, Brown TR, Anton RF. Associations Between Recent Heavy Drinking and Dorsal Anterior Cingulate N-Acetylaspartate and Glutamate Concentrations in Non-Treatment-Seeking Individuals with Alcohol Dependence. Alcoholism, clinical and experimental research 2016;40(3):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. Journal of magnetic resonance imaging : JMRI 2013;37(3):642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine following monitored abstinence from alcohol in treatment-naive individuals with Alcohol Use Disorder. unpublished (under review) 2019. [DOI] [PMC free article] [PubMed]
- 16.Association AP. Diagnostic and Statistical Manual of Mental Disorders 4th ed. Washington, DC: 2000. [Google Scholar]
- 17.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). British journal of addiction 1989;84(11):1353–7. [DOI] [PubMed] [Google Scholar]
- 18.Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 19.de Graaf RA. In Vivo NMR SPectroscopy: Principles and Techniques 2nd ed: John Wiley & Sons; 2007. [Google Scholar]
- 20.Kaufman MJ, Prescot AP, Ongur D, Evins AE, Barros TL, Medeiros CL, et al. Oral glycine administration increases brain glycine/creatine ratios in men: a proton magnetic resonance spectroscopy study. Psychiatry research 2009;173(2):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Kaufman MJ, Cohen BM, Jensen JE, Coyle JT, Du F, et al. In Vivo Brain Glycine and Glutamate Concentrations in Patients With First-Episode Psychosis Measured by Echo Time-Averaged Proton Magnetic Resonance Spectroscopy at 4T. Biological psychiatry 2018;83(6):484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soderpalm B, Lido HH, Ericson M. The Glycine Receptor-A Functionally Important Primary Brain Target of Ethanol. Alcoholism, clinical and experimental research 2017;41(11):1816–30. [DOI] [PubMed] [Google Scholar]
- 23.Stanley JA, Raz N. Functional Magnetic Resonance Spectroscopy: The “New” MRS for Cognitive Neuroscience and Psychiatry Research. Frontiers in Psychiatry 2018;9(76). [DOI] [PMC free article] [PubMed] [Google Scholar]