Abstract
Objectives
Osteoarthritis (OA) is a painful and debilitating disease and it is associated with aberrant upregulation of multiple factors, including matrix metalloproteinase 13 (MMP13), interleukin-1β (IL-1β) and nerve growth factor (NGF). In this study, we aimed to use the CRISPR/Cas9 technology, a highly efficient gene-editing tool, to study whether the ablation of OA-associated genes has OA-modifying effects.
Methods
We performed intra-articular injection of adeno-associated virus, which expressed CRISPR/Cas9 components to target each of the genes encoding MMP13, IL-1β and NGF, in a surgically induced OA mouse model. We also tested triple ablations of NGF, MMP13 and IL-1β.
Results
Loss-of-function of NGF palliates pain but worsens joint damage in the surgically induced OA model. Ablation of MMP13 or IL-1β reduces the expression of cartilage-degrading enzymes and attenuates structural deterioration. Targeting both MMP13 and IL-1β significantly mitigates the adverse effects of NGF blockade on the joints.
Conclusions
CRISPR-mediated ablation of NGF alleviates OA pain, and deletion of MMP13–1β or IL-1β attenuates structural damage in a post-traumatic OA model. Multiplex ablations of NGF, MMP13 and IL-1β provide benefits on both pain management and joint structure maintenance. Our results suggest that CRISPR-based gene editing is useful for the identification of promising drug targets and the development of feasible therapeutic strategies for OA treatment.
INTRODUCTION
Osteoarthritis (OA) is a painful joint disease affecting more than 10% of the adult population.1,2 Pathological changes of OA are complicated and involve multiple tissues, as manifested by articular cartilage (AC) destruction, joint space narrowing, synovial hyperplasia, osteophyte formation and subchondral bone sclerosis.3,4 It is recognised that OA is a highly heterogeneous disease, as patients with OA often show varied degrees of pathological features including pain, inflammation, cartilage degradation and bone spurs. Currently, OA is not curable, and clinical trials targeting OA-associated factors including matrix metalloproteinases (MMPs), inflammatory cytokines or growth factors, showed mixed results regarding the efficacy or safety of the therapeutics.5,6 Thus, it is highly important to develop new therapeutic strategies for OA treatment, which also demands a more advanced understanding of OA.
The recent development of the CRISPR/Cas9 technology has opened an avenue to easy and efficient gene editing. In this system, Cas9 proteins and the engineered single guide RNA (sgRNA) form a complex to recognise the target DNA sequence, and introduce a double-stranded break in genomic DNA, which is hazardous and therefore subjected to DNA repair.7,8 Two major DNA repair mechanisms can be used: error-proof homology-directed repair (HDR) and error-prone non-homologous end joining (NHEJ), of which the latter is more efficient but causes small insertions or deletions (indels) resulting in gene disruption.9 Thus, CRIS-PR-mediated NHEJ can be used as a highly efficient approach to achieve permanent and complete loss-of-function of disease-causing genes.
OA involves aberrant gene upregulation in joint tissues. For instance, upregulations of nerve growth factor (NGF) and interleukin-1β (IL-1β) are found in OA, and MMP13 is a dominant collagenase expressed in OA cartilage, of which all play key roles in OA pathophysiology.5,6,10 Therefore, ablation of these genes offers an attractive option to treat OA in a thorough and lasting manner. In this work, we explored CRISPR-mediated gene editing to treat OA, by targeting genes encoding NGF, IL-1β or MMP13 in a surgically induced OA mouse model. Our results demonstrated that targeting IL-1β or MMP13 reduced post-traumatic OA (PTOA) progression, and NGF ablation significantly palliated PTOA pain but accelerated joint damage. Importantly, combination of IL-1β- and MMP13-targeting in the setting of NGF targeting resulted in similar palliative effects on pain as NGF blockade alone, but minimised its side effects on joint structure.
MATERIALS AND METHODS
See online supplementary materials and methods.
RESULTS
NGF reduction through CRISPR/Cas9 significantly reduced OA pain but accelerated PTOA progression
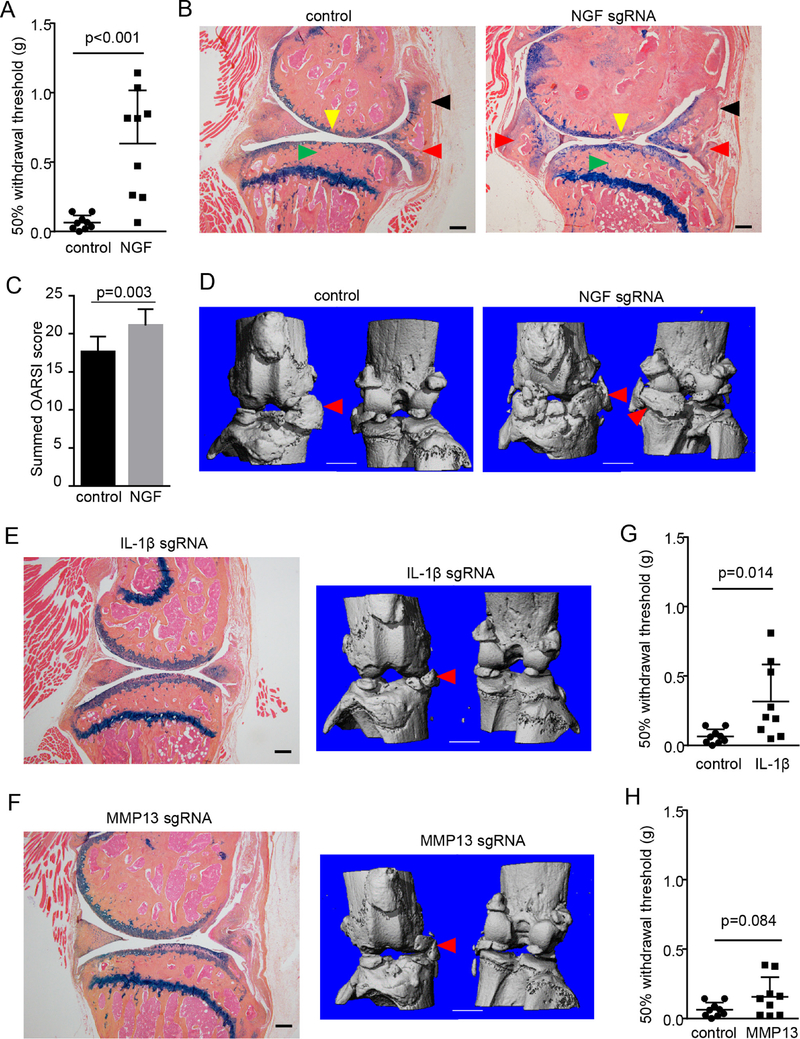
We constructed multiple adeno-associated virus (AAV) vectors for each gene based on the CRISPR/Cas9 system derived from Staphylococcus aureus.11 To identify effective guide sequences targeting the genes, we stably transfected mouse CD45− bone marrow stromal cells, which are mostly non-hematopoietic mesenchymal cells,12 with the vectors, and sequenced the targeted genomic regions. Our results showed that the AAV vectors successfully generated gene-nullifying mutations (online supplementary figures 1–3). Importantly, introduction of two vectors simultaneously caused a deletion between two targeted loci, ensuring a complete loss-of-function of the gene (online supplementary figure 1 and 2). We chose AAV serotype 5 to express the CRISPR-Cas9 system in the knee joint,13 as injection of AAV5 drove a potent, long-lasting expression of GFP in the murine joints (online supplementary figure 4). Before in vivo administration of the CRISPR-expressing AAVs, we induced PTOA in mice by partial meniscectomy. Ten days after the OA-inducing surgery, we performed intra-articular injections of two AAVs for each gene into the knee joint of the mice. Since pain is a major symptom of OA, we performed behavioural tests to determine if the pain-related behaviour was altered by CRISPR-mediated gene ablation. Our results showed that administration of AAVs targeting NGF significantly reduced pain sensitivity (figure 1A) and increased the rearing durations (online supplementary figure 5), suggesting that NGF ablation palliates OA pain and allow the mice to have more weight-bearing activities including rearing. Thus, our results confirm that NGF is a major mediator of OA pain in this animal model.
Figure 1.
OA-modifying effects by CRISPR-mediated ablation of NGF, IL-1β and MMP13. (A) Results of von Frey test on the mice receiving the PMM surgery and administration of AAV that expresses control or NGF-targeting sgRNAs. n=9. Unpaired Student’s t-test. (B) Representative histology images of osteoarthritic knee joints, which were collected 3 months after injections of control or NGF-targeting AAV. Yellow arrowheads, loss of AC; red arrowheads, osteochondrophytes; black arrowheads, synovial hyperplasia; green arrowheads, subchondral sclerosis. n=9. Scale bar, 200 μm. (C) OARSI scoring of knee joint AC destruction in the mice receiving the PMM surgery and control or NGF-targeting AAV. Both medial femoral condyle and medial tibial plateau were analysed on three-level sections of the joints and summed OARSI scores for the entire joint were presented. Unpaired Student’s t-test. n=9. (D) Representative μCT images of osteoarthritic knee joints, which were collected 3 months after injections of control or NGF-targeting AAV. Red arrowheads, osteophytes. n=9. Scale bar, 1 mm. (E,F) Representative histological and μCT results of osteoarthritic knee joints, which were collected three months after injections of IL-1β-targeting (E) or MMP13-targeting AAV (F). (G,H) Results of von Frey tests on the mice receiving the PMM surgery and administration of AAV that expresses IL-1β- (G) or MMP13-targeting AAV (H). Unpaired Student’s t-test, n=9. AAV, adeno-associated virus; AC, articular cartilage; IL-1β, interleukin-1β; MMP13, matrix metalloproteinase 13; NGF, nerve growth factor; OA, osteoarthritis; PMM, partial meniscectomy.
To evaluate the effects of the CRISPR therapy on joint structure, we also examined joint tissues through histology and μCT analyses. Three months after AAV injection, joint degeneration was evident in the mice receiving the control injection, as shown by AC degradation, synovial hyperplasia and subchondral sclerosis (figure 1B). In contrast to its impressive pain-palliative benefit, injection of NGF-targeting AAV did not demonstrate any positive effects in mitigating joint damage (figure 1B,C). The μCT results also revealed significant osteophyte outgrowth in the joints receiving NGF ablation, which is comparable to that in the control group (figure 1D). Six months after AAV injection, NGF ablation even had striking deleterious effects on joint structure in OA-inflicted joints, as demonstrated by more severe abrasion of AC, and marked enlargement and calcification of synovium (online supplementary figures 6 and 7). Collectively, our data showed that CRISPR-mediated NGF loss-of-function has significant pain-palliative efficacy but poses a risk of more severe cartilage degradation and ectopic bone formation.
Ablation of IL-1β or MMP13 ameliorates OA progression
We also analysed the joints receiving IL-1β-targeting or MMP13-targeting AAV by histology and μCT analyses. Our results demonstrated that CRISPR-mediated disruption of IL-1β or MMP13 significantly mitigated joint structure damage associated with PTOA progression 3 months after AAV injection (figure 1E,F), as they show significantly improved AC thickness. IL-1β antagonism also demonstrated significant efficacy in reducing synovial enlargement (figure 1E). We also looked at joint histology 6 months after AAV injection and found that ablation of MMP13 or IL-1β still had significant positive effects in attenuating pathological changes of the joint, by reducing AC destruction, decreasing synovial hyperplasia and lessening osteophyte growth (online supplementary figures 6 and 7). However, gene editing targeting MMP13 or IL-1β appeared to be not as effective as that targeting NGF in relieving OA pain as shown by the von Frey tests (figure 1G,H) and measurements of rearing (online supplementary figure 5), suggesting that MMP13 or IL-1β may not have a pivotal role as NGF in OA pain genesis and OA pain management requires additional measures than targeting joint catabolism.
CRISPR/Cas9-mediated gene editing extensively alters OA-associated signalling in multiple joint tissues
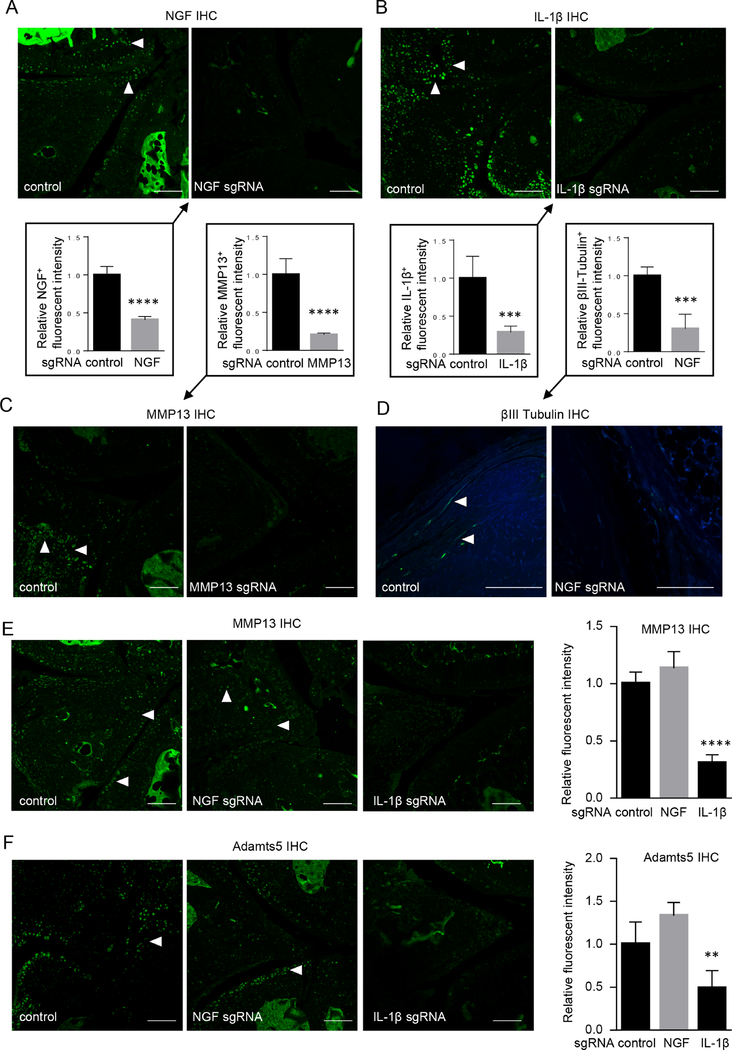
To examine the efficiency of gene ablation mediated by CRISPR/ Cas9-expressing AAVs, we performed fluorescent immunohistochemistry (IHC) studies of the target proteins and found that AAV administrations successfully decreased the expression of their respective target genes, including NGF, IL-1β and MMP13 in the knee joints (figure 2A–C, online supplementary figure 8). Our quantitative reverse transcription PCR assays also demonstrated that the mRNA expression of the targeted genes was reduced in the total joint tissues (online supplementary figure 9). Notably, the ablation of the genes involves multiple tissues inside the joint, including AC, synovium, menisci and newly formed osteophytes. This suggested that intra-articular delivery of CRIS-PR-containing AAV led to deficiency of these OA-associated genes in the entire joint, achieving well-rounded therapeutic effects. Importantly, the data from the control group showed that the expressions of NGF, IL-1β, MMP13 and Adamts5 in the synovium and meniscus were abundant (figure 2A–C, online supplementary figure 10), suggesting that aberrant upregulation of their expressions may induce PTOA pathogenesis and progression. Specifically, expressions of IL-1β and MMP13 in joint tissue such as AC, synovium and meniscus may induce catabolic responses in AC, and NGF expression could induce neurite outgrowth in the synovium and meniscus, which fosters the formation of mechanical hypersensitivity.
Figure 2.
CRISPR-mediated gene editing attenuated OA-associated downstream signalling. (A–C) Administration of gene-ablating AAV reduced the expression of the individual targets in osteoarthritic knee joints, such as NGF (A), IL-1β (B) and MMP13 (C). (D–F) NGF-targeting AAV downregulated the expression of βIII tubulin (D), MMP13 (E) and Adamts5 (F) and IL-1β-targeting AAV reduced the expression of MMP13 (E) and Adamts5 (F) in osteoarthritic knee joints. Arrowheads, IHC-positive cells. n=5. Scale bar, 50 μm. Unpaired Student’s t-test (A–D) or one-way ANOVA followed by the Tukey-Kramer test (E,F). **p<0.01, ***p<0.001, ****p<0.0001. AAV, adeno-associated virus; ANOVA, analysis of variance; IHC, immunohistochemistry; IL-1β, interleukin-1β; MMP13, matrix metalloproteinase 13; NGF, nerve growth factor; OA, osteoarthritis.
As IL-1β and NGF are pivotal factors associated with OA, we asked how the therapies targeting these genes altered the downstream signalling in the joint tissues. Consistent with the change in pain-related behaviour of the mice receiving the gene editing, neurite growth in the synovium as marked by the immunostaining of β-III tubulin, a neuronal marker, was significantly decreased in the groups receiving the administration of NGF sgRNAs (figure 2D). Interestingly, NGF deletion also caused an upregulation of MMP13 and Adamts5 (figure 2E,F), two major cartilage-degrading enzymes responsible for OA development as well as increased degradation products of Aggrecan (online supplementary figure 11). Thus, our results suggested that more severe cartilage destruction induced by NGF blockade is associated with dysregulation of catabolic enzymes in non-neuronal joint cells. Moreover, IL-1β deletion restrained the expressions of MMP13 and Adamts5 during PTOA progression, which pointed to the role of IL-1β as an inflammatory cytokine in promoting cartilage degradation. Together, we conclude that intra-articular gene editing targeting NGF and IL-1β act on multiple joint tissues, including AC, synovium and menisci, to affect downstream signalling pathways and to change the course of PTOA progression.
Multiplex gene editing of NGF, IL-1β and MMP13 provides enhanced benefits including both pain palliation and structural amelioration
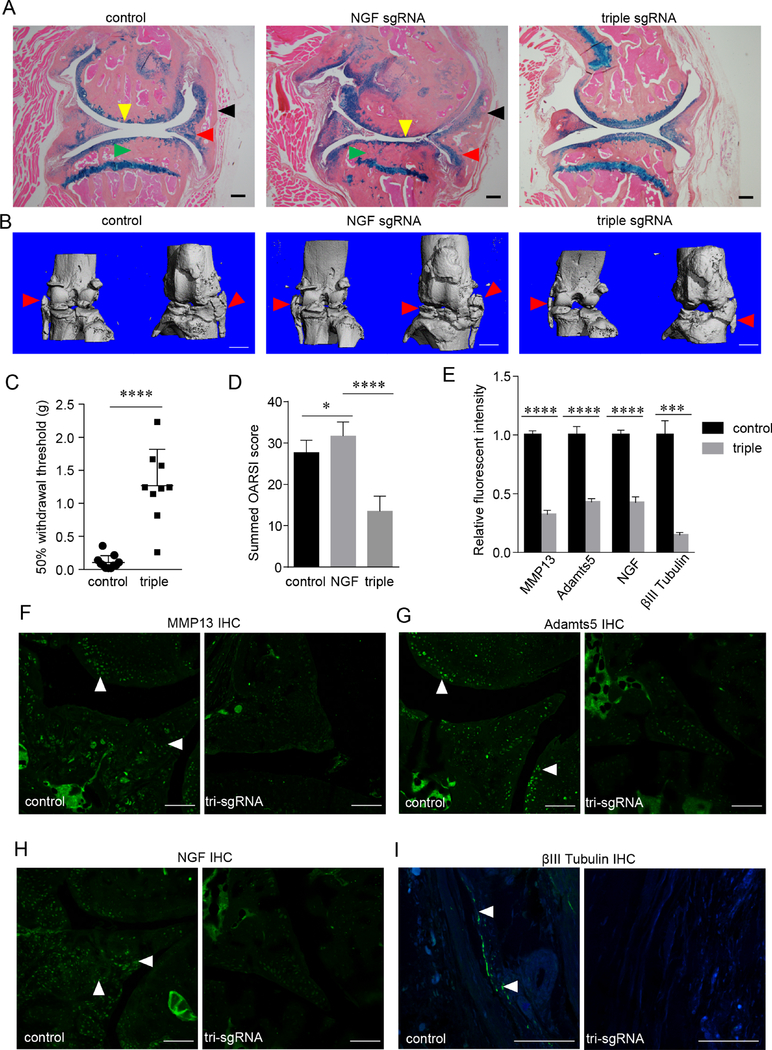
As NGF inhibition is efficacious in OA pain mitigation but shows adverse effects on joint structure, we explored multiplex inhibitions of NGF, IL-1β and MMP13, in order to find a strategy offering the pain-palliative benefit of anti-NGF therapy without adverse joint events. IL-1β and MMP13 were chosen as the supplemental targets, because they are extracellular proteins to be more accessible by currently available biologics, and their antagonism had positive effects on joint morphology as demonstrated by our study (figure 1E,F). We performed pain-related behavioural studies, through von Frey test and measurement of rearing, and found that the multiplex therapy effectively retained pain-modifying effects of NGF blockade, as demonstrated by a significantly decreased mechanical sensitivity threshold and increased rearing durations compared with the control group (figures 1A and 3C, online supplementary figure 5). Moreover, our radiographic and histological analyses revealed that multiplex gene editing did not accelerate PTOA progression as rapidly as ablation of NGF alone did (figure 3A,B, online supplementary figure 7). Specifically, histological and μCT analyses demonstrated that AC degradation, synovial hyperplasia, osteophyte formation and subchondral sclerosis in the multiplex therapy group were significantly less than those in the control or NGF-only group (figure 3A,B, online supplementary figure 7). We also used OARSI scoring to quantify AC degradation and confirmed less destructive changes in the multiplex group (figure 3D). Thus, these results suggested that inclusion of additional targets such as IL-1β and MMP13 may offset the structural adverse effects of NGF ablation while retaining its pain-palliative benefit in the mouse model.
Figure 3.
Concomitant loss-of-function of NGF, IL-1β and MMP13 relieves OA pain and mitigates OA progression. (A,B) Representative histology (A) and μCT (B) images of osteoarthritic knee joints, which were collected 6 months after injections of control, NGF-targeting or triple (NGF, IL-1β and MMP13)-targeting AAVs. Yellow arrowheads, loss of articular cartilage; red arrowheads, osteochondrophytes; black arrowheads, synovial hyperplasia; green arrowheads, subchondral sclerosis. n=9. Scale bar for histology, 200 μm. Scale bar for μCT, 1 mm. (C) Results of von Frey tests on the mice receiving the PMM surgery and control or triple-targeting AAV. n=9. Unpaired Student’s t-test. (D) OARSI scoring of AC destruction in osteoarthritic knee joints of the mice receiving control, NGF-targeting or tri-targeting AAVs. n=9. One-way ANOVA followed by the Tukey-Kramer test. (E–I) Simultaneous deletion of NGF, IL-1β and MMP13 attenuated OA-associated matrix proteases including MMP13 (F) and Adamts5 (G) and neural genes such as NGF (H) and βIII Tubulin (I), which were quantified and summarised (E). Scale bar, 50 μm. n=5, unpaired Student’s t-test. *p<0.05, ***p<0.001, ****p<0.0001. AAV, adeno-associated virus; ANOVA, analysis of variance; IL-1β, interleukin-1β; MMP13, matrix metalloproteinase 13; NGF, nerve growth factor; OA, osteoarthritis; PMM, partial meniscectomy.
Next, we performed IHC studies to examine the expression levels of downstream molecules. Our data showed that both MMP13 and Adamts5 had lower expression in the multiplex group than in the control group (figure 3E–G, online supplementary figure 12). NGF ablation through CRISPR reduced the expression of NGF in the multiplex treatment group (figure 3H). Assessment of neurite growth by immunostaining β-III tubulin demonstrated that the multiplex treatment group and the NGF-only group had similar expression of β-III tubulin in the synovium, both significantly lower than in the control group (figures 2D and 3I). Together, our results suggested that the multiplex therapy attenuates the structural adverse effects of NGF blockade, possibly through decreasing inflammation and cartilage destruction, but without a compromise on pain modification offered by NGF inhibition. Further, our study proposes a strategy to treat OA that eases pain sensitization and alleviates structural deterioration through supplementing NGF blockade with simultaneous inhibitions of catabolic and/or inflammatory factors.
DISCUSSION
In this study, we employed CRSPR/Cas9 based gene editing to treat OA, a very common, painful and debilitating disease in a surgically induced OA mouse model. Our gene editing study confirmed that NGF, IL-1β and MMP13 are promising drug targets for OA therapy, as our data demonstrated a significant improvement on joint structure or OA pain when these molecules are downregulated. Interestingly, our data of the gene editing against NGF is similar to the clinical trials that showed promising pain-palliative effects of humanised anti-NGF antibodies. However, the clinical trials exhibited an adverse structural effect presented as rapidly progressive OA, which is characterised by bone destruction,10,14 in marked contrast to subchondral sclerosis and osteophyte outgrowth observed in our study as well as in a rat model administered with anti-NGF antibodies.15 While the animal studies failed to reproduce the adverse events in the clinical trials of NGF blockade, our results suggest that antagonism of IL-1β and MMP13, though less impressive in OA pain palliation compared with that of NGF, could be a useful supplementation to NGF blockade, as it directly downregulates MMP13 and also reduces inflammation, thus maintaining AC and restraining the induction of catabolic factors including Adamts5 (figure 3E,G). Together, our study suggested that the symptoms of OA can be managed by a formula comprising blockade of NGF as well as inhibition of inflammation/cartilage degradation.
The target genes chosen in this study all undergo aberrant upregulation during OA pathogenesis, thus downregulation of their expressions could benefit OA treatment. Conveniently, the CRISPR/Cas9 technology provides an efficient approach to reduce gene expression by mutating genes through error-prone NHEJ. A single effective sgRNA generates small indels, which may lead to frameshift or missense mutation of the gene, or cause in-frame small mutations that may not completely silence the gene. To ensure a complete abolishment of the target genes, we introduced two sgRNAs that can produce deletions as long as hundreds of nucleotides in the targeted genes. Thus, the double sgRNA approach may avoid being obscured by incomplete gene ablations, to facilitate our evaluation of this explorative study. Notably, off-target effects could be a major concern for CRISPR/Cas9-mediated gene editing, and introduction of two sgRNAs would pose a higher risk of off-target effects. Although we did not observe apparent off-target effects in our study, it may be necessary to use only one sgRNA for clinical studies and to completely confirm that the off-target activity of the sgRNA is minimal or benign through a whole genome analysis. In addition, a proper delivery method, such as intra-articular rather than systemic administration, may greatly minimise the side-effects. Together, safe, effective sgRNAs as well as an appropriate delivery route should be vital for a successful CRISPR/Cas9-based gene editing to treat OA.
Blockade of NGF is the most promising strategy among current medications for OA pain, while it was also reported to be associated with the joint-related adverse events including bone destruction. In our study, CRISPR/Cas9-based ablation of the Ngf gene in the mouse joint showed enhanced ectopic bone formation. Because these joint-related adverse effects of NGF loss-of-function are in sharp contrast between humans and rodents, it is intriguing how NGF downregulation differentially induces changes in the joints of rodents and humans. An underlying mechanism could be that NGF expression in nerve is essential for the joint to retain the ability to feel pain and other neuronal activities, which protects the joint and maintains its homeostasis. Thus, neuronal expression of NGF may have effects on non-neuronal tissues/cells through nerve activities. Nonetheless, NGF may also play important functions directly on non-neuronal joint cells, which regulate their proliferation, differentiation, survival and their anabolic/catabolic activities. A more thorough investigation for the role of NGF in the entire joint would shed new lights into development of safe and effective treatment of OA pain.
Supplementary Material
Key messages.
What is already known about this subject?
Nerve growth factor (NGF), interleukin-1β (IL-1β) and matrix metalloproteinase 13 (MMP13) are upregulated and play pivotal roles in the pathogenesis of osteoarthritis (OA). Thus, these factors could be promising drug targets for OA treatment.
Positive results from clinical trials of NGF inhibition for the treatment of OA pain have been announced recently.
What does this study add?
Intra-articular delivery of CRISPR/Cas9, a highly efficient gene editing tool, causes gene-nullifying mutations of NGF, IL-1β and MMP13 in osteoarthritic murine knee joints.
CRISPR-mediated ablation of NGF palliates OA pain but worsens articular cartilage (AC) destruction and osteophyte outgrowth in the mouse OA model.
Loss-of-function of IL-1β or MMP13 attenuates AC degradation and osteophyte formation.
Multiplex targeting against NGF, IL-1β and MMP13 mitigates both OA pain and structural damage in the mouse model.
How might this impact on clinical practice or future developments?
CRISPR-based gene editing is useful for the identification of promising drug targets and the development of feasible therapeutic strategies for OA treatment.
Acknowledgements
The authors thank Ms Lily Yu for her help on processing and staining histological samples.
Funding Grants R01AR054465 and R01AR070222 of National institutes of Health; the Rush 2015 Schweppe/Armour bequest Pilot Project; the National Natural Science Foundation of China (81630066).
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Institutional Review Board of Rush University Medical Center.
REFERENCES
- 1.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol 2014;10:437–41. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 2016;12:632–44. [DOI] [PubMed] [Google Scholar]
- 4.Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 2013;9:400–10. [DOI] [PubMed] [Google Scholar]
- 6.Hellio Le Graverand-Gastineau M-P. Oa clinical trials: current targets and trials for oa. choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage 2009;17:1393–401. [DOI] [PubMed] [Google Scholar]
- 7.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jinek M, Chylinski K, Fonfara i, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012;337:816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage 2013;21:1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ran FA, Cong L, Yan WX, et al. in vivo genome editing using Staphylococcus aureus Cas9. Nature 2015;520:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Huang J, Guo R, et al. Smurf1 inhibits mesenchymal stem cell proliferation and differentiation into osteoblasts through junB degradation. J Bone Miner Res 2010;25:1246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoury M, Courties G, Fabre S, et al. Adeno-associated virus type 5-mediated intraarticular administration of tumor necrosis factor small interfering RNA improves collagen-induced arthritis. Arthritis Rheum 2010;62:765–70. [DOI] [PubMed] [Google Scholar]
- 14.Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaBranche TP, Bendele AM, Omura BC, et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis 2017;76:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.