Abstract
Freshwater ecosystems are linked at various spatial and temporal scales by movements of biota adapted to life in water. We review the literature on movements of aquatic organisms that connect different types of freshwater habitats, focusing on linkages from streams and wetlands to downstream waters. Here, streams, wetlands, rivers, lakes, ponds, and other freshwater habitats are viewed as dynamic freshwater ecosystem mosaics (FEMs) that collectively provide the resources needed to sustain aquatic life. Based on existing evidence, it is clear that biotic linkages throughout FEMs have important consequences for biological integrity and biodiversity. All aquatic organisms move within and among FEM components, but differ in the mode, frequency, distance, and timing of their movements. These movements allow biota to recolonize habitats, avoid inbreeding, escape stressors, locate mates, and acquire resources. Cumulatively, these individual movements connect populations within and among FEMs and contribute to local and regional diversity, resilience to disturbance, and persistence of aquatic species in the face of environmental change. Thus, the biological connections established by movement of biota among streams, wetlands, and downstream waters are critical to the ecological integrity of these systems. Future research will help advance our understanding of the movements that link FEMs and their cumulative effects on downstream waters.
KEY TERMS: aquatic ecology, biotic integrity, connectivity, rivers/streams, wetlands
INTRODUCTION
Spatial and temporal connections created by flows of energy, materials, and organisms within and among habitats are needed to sustain ecosystem structure and function. In aquatic ecosystems, large rivers, lakes, and coastal waters depend on flows of physical and chemical (i.e., non-living) materials, such as water, nutrients, organic matter, and sediment, from upstream ecosystems (see Fritz et al. and Lane et al., this issue, for discussion of physical and chemical connections). These physiochemical flows support and interact with biological connections created by the active or passive movements of living aquatic and semi-aquatic organisms or their propagules, moving in diverse ways, across different spatial and temporal scales (Gounand et al., 2017). Because biological connections among aquatic habitats have important and long-lasting effects on species distributions (Dias et al., 2014), population and community dynamics (Perkin and Gido, 2012; Crook et al., 2015), biodiversity (Jeltsch et al., 2013), water quality (Vaughn, 2017), and ecosystem function (Lundberg and Moberg, 2003), they are an integral part of all aquatic ecosystems.
In this paper, we review and synthesize the literature on biological connections between small or temporary streams, wetlands, and downstream waters such as rivers, lakes, and coastal waters. This subset of freshwater ecosystem connections was the focus of the U.S. Environmental Protection Agency’s recent review and synthesis (USEPA, 2015; Alexander et al., this issue). The overall objective of the Clean Water Act (CWA) is to restore and maintain the chemical, physical, and biological integrity of the Nation’s waters. When the CWA was enacted in 1972, ecosystem integrity was a relatively new concept and aquatic ecology still a young science. Research and monitoring over the next decade made it increasingly clear that, despite gains in chemical water quality under the CWA, water quality standards that considered only chemical pollutant concentrations were insufficient to restore or maintain aquatic ecological integrity, defined as the sum of chemical, physical, and biological integrity of aquatic ecosystems (Karr and Dudley, 1981; Karr, 1993). These findings helped to motivate new research into factors affecting aquatic ecological integrity. A key factor that has emerged from this research is the importance of structural and functional connectivity of streams, wetlands, and downstream waters (Alexander et al., this issue).
This review summarizes how movements of biota link streams, riparian and floodplain wetlands, and non-floodplain wetlands to downstream waters, and why these connections are important. Together with lakes, ponds, and other freshwater habitats, streams and wetlands collectively make up dynamic freshwater ecosystem mosaics (FEMs) of watersheds—that is, the diverse collection of integrated freshwater habitats needed to sustain aquatic life and the ecological integrity of these systems (Karr and Dudley, 1981; Karr, 1995). Many aquatic species either require or facultatively use resources derived from different habitat types in these mosaics, which vary spatially and temporally in response to seasonal, decadal, or episodic changes in environmental conditions (Pickett and Cadenasso, 1995; Stanford et al., 2005; Mushet et al., 2013; Datry et al., 2016). Heterogeneous habitat mosaics depend on exchanges of different types of materials, energy, and organisms across ecosystem boundaries. These exchanges form, in effect, meta-ecosystems with potential for feedbacks between habitat diversity, biological diversity, and ecosystem function at different spatial and temporal scales (Loreau et al., 2003; Alsterberg et al., 2017).
We first present an overview of biological connectivity (Figure 1; Table 1), in terms of the pathways, modes, purposes, and taxa involved in movements and key factors that determine the degree and scales (e.g., distances, frequencies, and rates) at which these movements occur. We present examples of biological connections along stream networks and between stream channels and riparian/floodplain and non-floodplain wetlands (Table 1). We then consider how these connections affect the structure and function of downstream waters, and discuss areas of future research that will provide new insights into the role of biological connectivity in the integrity of freshwater ecosystems.
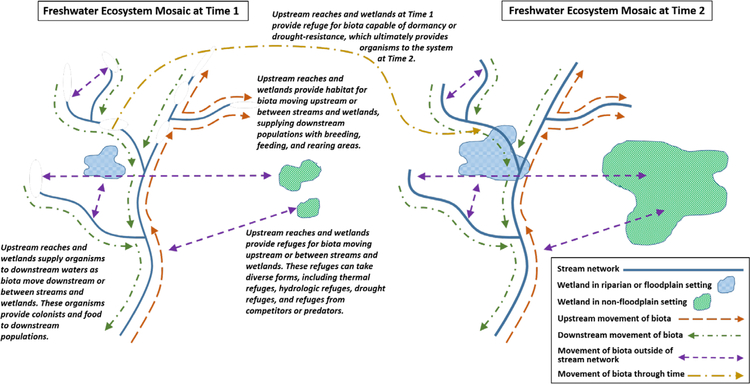
Figure 1.
Schematic illustrating how upstream reaches, riparian and floodplain wetlands, and non-floodplain wetlands influence the integrity of downstream waters via movement of aquatic and semi-aquatic biota throughout the freshwater ecosystem mosaic, in both space and time. For illustrative purposes, upstream movements are shown on the right side of the stream network; downstream movements are shown on the left side the stream network. Although not shown in this schematic, these movements can also occur vertically (i.e., to and from the hyporheic zone) and across watershed boundaries. Modified from Meyer et al., 2007.
Table 1.
Examples of biological connections along stream networks, between stream networks and riparian/floodplain wetlands, and between stream networks, riparian/floodplain wetlands, and non-floodplain wetlands. Examples are presented in terms of the pathways by which organisms move (with water, over land, in air, or “hitchhiking” on other organisms), mode of transport (active or passive), and type of organism. We present only one example per category, although in many cases numerous examples have been documented.
| PATHWAY | MODE OF TRANSPORT | EXAMPLES | ||
|---|---|---|---|---|
| Along Stream Network | Between Stream Network and Riparian/Floodplain Wetlands | Between Stream Network, Riparian/Floodplain Wetlands, and Non-Floodplain Wetlands | ||
| With water | Active |
Invertebrates: Significant net upstream movement was observed for multiple snail taxa, with individuals moving a maximum of 200 m upstream (Huryn and Denny, 1997). Fish: Intermittent streams provided rearing habitat for coho salmon, and smolts that overwintered in these streams were larger than smolts that overwintered in perennial streams (Wigington et al., 2006). Amphibians: Two stream salamander species tend to move in an upstream direction within the stream network (Grant et al., 2010). |
Invertebrates: Aquatic invertebrates crawled from headwater stream channels, along stream bottoms and across channel boundaries, to forested floodplains (Smock, 1994). Fish: During the spring flood pulse, spotted gar moved a median distance of roughly 130 m per day among diverse aquatic habitats across the floodplain (Snedden et al., 1999). |
Invertebrates: Non-flying and flight- capable invertebrates dispersed with equal frequency via pond fill-and-spill through temporary channels or overland flow (Van de Meutter et al., 2006). Fish: Fishes dispersed between lakes and seasonal wetlands via transient hydrologic connections (Hohausova et al., 2010). |
| Passive |
Microorganisms: Hydrologic connectivity between high and low elevation stream sites increased the incidence of frog infection by a chytrid fungus (Sapsford et al., 2013). Algae: Stream corridors provided the primary dispersal pathway for benthic diatoms (Dong et al., 2016). Plants: Downstream dispersal along stream networks influenced the structure of forest plant communities, even for plants not adapted for water dispersal of seeds (Honnay et al., 2001). Invertebrates: 167 aquatic invertebrate taxa were collected in springtime drift samples, at total drift densities of 0.26–26.04 m−3 day−1 (Pond et al., 2016). Fish: Eggs of pelagic-spawning prairie fishes were estimated to be transported up to 177 km downstream before hatching (Platania and Altenbach, 1998). |
Algae: Floodplain habitat contributed to four-fold increases in phytoplankton biomass and higher percentages of diatoms and green algae (Lehman et al., 2008). Plants: Dispersal of riparian plant seeds by water resulted in a 36–58% increase in the pool of plant species colonized flooded riparian areas (Jansson et al., 2005). Invertebrates: Aquatic invertebrates drifted with waterflow from headwater stream channels to forested floodplains (Smock, 1994). |
Plants: For two wetland plant species that disperse via both wind and water, water dispersal results in the transport of more seeds over longer distances (Soomers et al., 2013). Invertebrates: Genetic analyses of an invasive aquatic snail species indicate that the snail disperses readily via water throughout flooded rice fields (Van Leeuwen et al., 2013). |
|
| Over land | Active |
Invertebrates: Adults of 15 caddisfly species (out of 26 species considered) flew at least 1.5 km away (the farthest distance evaluated in the study) from permanent water (Graham et al., 2017). Amphibians: Two stream salamander species move over land between adjacent headwater streams (Grant et al., 2010). Reptiles: Reptiles moved both upstream and downstream along dry riverbeds (Sanchez- Montoya et al., 2016). Mammals: Mammals moved both upstream and downstream along dry riverbeds (Sanchez-Montoya et al., 2016). |
Reptiles: Two turtle species used a variety of aquatic and terrestrial habitats throughout a river floodplain, with 95% of population movements occurring within a riparian zone 449 m from the river (Bodie and Semlitsch, 2000). Mammals: River otters use a variety of aquatic habitats, including streams and rivers, wetlands, and open waters (Newman and Griffin, 1994). |
Amphibians: Columbia spotted frogs occupy widely distributed wetlands in summer, then migrate over land to deeper lakes to overwinter (Pilliod et al., 2002). Reptiles: American alligators use hydrologically isolated seasonal wetlands for nesting and nursery habitat and riverine systems for non-nesting habitat (Subalusky et al., 2009a). Mammals: Translocated river otters ranged from 1.2–54.0 km, from riverine habitats to isolated aquatic habitats throughout the landscape (Spinola et al., 2008). |
| In air | Active | Invertebrates: Up to roughly one-half of an adult mayfly population flew upstream along the stream network upon emergence (Hershey et al., 1993). |
Invertebrates: Stream insects and noninsect invertebrates aerially colonized temporary floodplain aquatic habitats (Tronstad et al., 2007). Birds: The Rocky Mountain population of sandhill cranes overwinters and breeds in both riverine and wetland habitats (Pacific Flyway Council and Central Flyway Council, 2016). |
Invertebrates: Numerous flight-capable insects use both streams and non- floodplain wetlands (Williams, 1996). Birds: Abundance of migratory waterbirds was positively related to the area of semipermanent wetlands within 10 km of each study wetland (Webb et al., 2010). |
| Passive |
Microorganisms: At least 30 viable taxa of protozoans have been sampled atmospherically (Schlichting, 1969). Algae: At least 150 viable taxa of algae have been sampled atmospherically (Schlichting, 1969). |
Plants: Many wetland plant species adapted to wind dispersal (37–46% of all species) are found in wetlands that rely on rainwater and groundwater inputs (Soons, 2006). |
Plants: Wind-dispersed wetland plant species make up a high percentage (4550%) of all species in more terrestrial wetland types (Soons, 2006). Invertebrates: 7 of 51 rotifer species and 5 of 25 microcrustacean species that colonized temporary pond habitats only reached those ponds via airborne dispersal (Lopes et al., 2016). |
|
| With other organisms | Passive |
Algae: Numerous diatom genera (including the nuisance species Didymosphenia geminata) were found on the fur of minks surveyed in 2 Patagonian streams (Leone et al., 2014). Plants: Via consumption, internal transport, and egestion, fish provided a means for upstream dispersal of the seeds they consume (Horn, 1997). Invertebrates: The distribution of unionid freshwater mussels was determined by distribution of the mussels’ host fishes, which they rely on for dispersal (Schwalb et al., 2013). |
Microorganisms: Up to 19 genera of protozoans were found on insects collected from ponds, including insects known to frequent streams (Stewart and Schlichting, 1966). Algae: Up to 25 genera of algae were found on insects collected from ponds, including insects known to frequent streams (Stewart and Schlichting, 1966). Plants: Common carp consume and egest viable unbranched burreed seeds, creating the potential to internally transport seeds up to 27 km (Pollux et al., 2007). |
Microorganisms: Approximately 15% of 397 wild geese screened for a chytrid fungus were found to carry the fungus on their feet (Garmyn et al., 2012). Plants: Approximately 7% of seeds ingested by ducks remained viable after passing through their digestive tracts, making it possible for ducks to internally transport wetland plant seeds up to 1,400 km (Mueller and van der Valk, 2002). Invertebrates: Waterbird movements across North America contributed significantly to gene flow among cladoceran and bryozoan populations (Figuerola et al., 2005). |
Although this review is focused on biological connections among freshwater habitats, we recognize that ecologically important biological connections also exist between aquatic habitats and other ecosystems, including terrestrial (Nakano and Murakami, 2001; Gibbons, 2003; Baxter et al., 2004; Rine et al., 2016) and marine (Schindler et al., 2005; Rine et al., 2016) systems. We also recognize that biological connections affect physical and chemical connections (and vice versa). For example, biota play critical roles in maintaining physical and chemical connections to downstream waters, through their effects on organic matter breakdown (Wallace and Webster, 1996), algal productivity and microbial activity (Feminella and Hawkins, 1995), sediment mobilization (Hassan et al., 2008; Statzner, 2012), and storage, transport, and release of nutrients and contaminants (Krümmel et al., 2003; Walters et al., 2008; Popova et al., 2016). Any comprehensive examination of overall connectivity among these systems must consider physical, chemical, and biological connections, both within FEMs and between FEMs and other ecosystems. For the purposes of this review, however, we focus solely on how the movements of biota create biological connections throughout FEMs.
DESCRIBING BIOLOGICAL CONNECTIONS
How and Why Aquatic Organisms Move
Biological connections result from the active or passive movement of living organisms or their reproductive materials (e.g., seeds, eggs, genes) through space (e.g., via dispersal or migration) or time (e.g., via dormancy). All aquatic and semi-aquatic organisms—including microbes, algae, plants, invertebrates, and vertebrates (Table 1)—move within and among components of the FEM. These movements may occur for multiple reasons, including dispersal (permanent, undirected movement away from an existing population or breeding habitat); migration (periodic, directed movements away from and returning to an existing population or habitat); persistence in time through dormancy (Auffret et al., 2015); or localized movements within and between FEM components that allow an organism to acquire resources (e.g., food, protection from predators, mates) (Smock, 1994; Lamoureux and Madison, 1999). In some cases, these localized movements may be required, such as when a species obligately uses different habitat types at different life cycle stages (Huryn and Gibbs, 1999; Gibbons et al., 2006; Subalusky et al., 2009a); in other cases, species may move facultatively within and among habitats throughout their life cycles.
Biological connections are established via multiple pathways (Figure 1; Table 1), and can be measured in several ways. Spatially, these pathways include the passive transport of aquatic and semi-aquatic biota by water, wind, or “hitchhiking” on other organisms, and the active movement of biota through water, over land (for organisms with semi-aquatic or terrestrial life stages), or through the air (for birds or insects) (Table 1). Key parameters used to quantify or describe biological connections include the distance an organism or propagule can move (or duration, when considering movement through time); the frequency with which these movements occur (e.g., once a generation vs. multiple times over its lifespan); the rate at which these movements occur (e.g., in terms of number of individuals or amount of biomass per unit time); and the timing of these movements (e.g., seasonally vs. randomly throughout year).
Even within a single species, organisms often move via more than one pathway (e.g., aquatic invertebrates with flight-capable adults; plants with seeds that can be dispersed by water, wind, and/or animals), and individuals can vary in their movement patterns (e.g., Rasmussen and Belk, 2017). For organisms that move only via water, biological connections coincide with hydrologic flowpaths (Fritz et al. and Lane et al., this issue). However, many species are capable of overland movement, via either passive transport or active movement. Overland movements establish important biological connections that can cross both ecosystem and watershed boundaries, even when surface hydrologic flowpaths are disrupted or absent (Figure 1) (Hughes et al., 2009). Species also may create biological connections within and among FEM components through time, via dormancy or drying-resistant stages (Figure 1).
The variety of movements that organisms undertake reflects the multitude of life history strategies (and their inherent trade-offs; Stearns, 1989) that species have evolved to optimize survival and reproductive fitness within FEMs. These movements allow organisms to recolonize habitats; avoid inbreeding; escape biotic or abiotic stressors; locate mates; and acquire the resources needed to survive and reproduce. They also connect populations and communities throughout the FEM and contribute to species persistence and resilience to disturbance and environmental change (Labbe and Fausch, 2000; Fagan, 2002; Bohonak and Jenkins, 2003).
Cumulatively, these movements enhance and sustain biodiversity at all levels of biological organization, from genes to ecosystems. Dispersal and migration contribute to population and species persistence through the maintenance of genetic diversity (e.g., Waples, 2010), location of mates and breeding habitats (Semlitsch, 2008); rescue of small populations threatened with local extinction (Brown and Kodric-Brown, 1977); and colonization of new habitats (e.g., Hecnar and McLoskey, 1996; Tronstad et al., 2007). The functions of dispersal (e.g., to avoid kin competition, limit inbreeding, colonize new habitat patches) are also determinants of the distance over which an organism will actually move, which has consequences for local and regional aquatic biodiversity (Duputié and Massol, 2013). Thus, these movements, and the biological connections they establish, are critical to the structure and function of aquatic ecosystems (Bornette et al., 1998; Steiger et al., 2005; Meyer et al., 2007).
Biological connectivity occurs along spatial and temporal gradients, from highly isolated habitats with relatively little or no movement of biota into or out of the system to highly connected habitats with extensive movement of biota into and out of the system, and all conditions between these extremes. Gradients of connectivity are important for maintaining ecosystem integrity, as different types and scales of movement can have unique effects. For example, mass river insect migrations into headwater streams provide food subsidies to support young-of-year fish (Uno and Power, 2015), including diadromous salmon (Bramblett et al., 2002). On the other hand, lower rates of movement between more isolated habitats can decrease the spread of pathogens (e.g., Hess, 1996) and invasive species (e.g., Bodamer and Bossenbroek, 2008) and increase regional genetic diversity through local adaptation (e.g., Fraser et al., 2011).
Key Factors That Affect Biotic Movements
Key factors affecting the movement of biota through FEMs include: (1) climate and other environmental conditions that determine the distribution, relative abundance, and quality of aquatic habitats within the FEM; (2) physical features that facilitate or impede the movement of species between aquatic habitats; and (3) traits and behaviors of the species present in the system. These factors are not independent of each other, and interact in complex ways. For example, even though passively-dispersing organisms can control the timing of their movements to some extent, passive dispersal tends to be riskier than active dispersal when there is unsuitable intervening habitat (Bonte et al. 2012). Each factor also can be altered by human activities that enhance or restrict biological connections (Crook et al., 2015). Some human activities create physical features that impede movement (e.g., by damming stream networks); other activities may create physical features that facilitate movement (e.g., by creating drainage ditches from wetlands to streams).
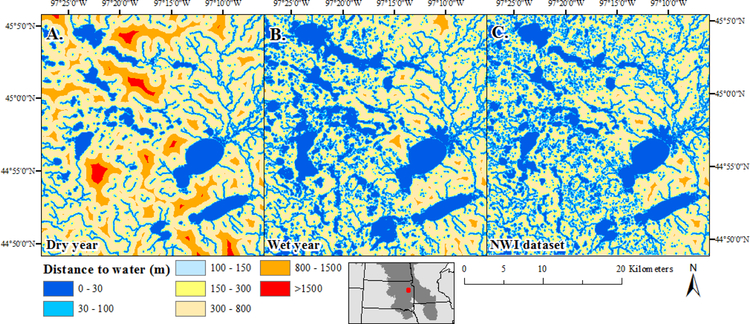
For biota that move only in water, any factors that influence water storage and flowpaths (i.e., hydrologic connections; Leibowitz et al., this issue) also will influence biological connections. Climate is a key determinant of hydrologic connectivity, as well as the relative size, density, and spatial arrangement of FEM components. In physiographic regions such as formerly-glaciated portions of North America’s Great Plains Ecoregion, seasonal or longer-term climate cycles have dramatic effects on surface water storage and flowpaths, and thus on resources available to aquatic biota (Figure 2; Vanderhoof et al., 2016). Hydrologic connections are enhanced in wet years, and distances between habitat components decrease; in dry years, these hydrologic connections are diminished and habitat components are farther apart.
Figure 2.
Euclidean distance to nearest water changes depending on how and when wetland/lake extent is defined. Euclidean distance to nearest water using: A) wetland/lake extent during a dry year (1990, DOY 130), as defined by Landsat imagery; B) wetland/lake extent during a wet year 2011, DOY 156), as defined by Landsat imagery; and C) wetland/lake extent, as defined by the National Wetlands Inventory (NWI) dataset, included here for reference.
These types of cycles result in flood pulse dynamics (Junk et al., 1989); wetland fill-and- spill dynamics (Tromp-van Meerveld and McDonnell, 2006; Shaw et al., 2013); wetland-lake- stream fill-and-merge dynamics (Leibowitz et al., 2016; Vanderhoof and Alexander, 2016; Vanderhoof et al., 2016); and high-volume stormflows in arid streams (Stanley et al., 1997; Goodrich et al., this issue). For example, drought-to-deluge climate cycles dramatically affect stream and wetland densities in the Prairie Pothole Region (Vanderhoof et al., 2016). Movements of biota throughout FEMs in response to these spatial or temporal changes in the number, extent, arrangement and quality of the component aquatic habitats are thus highly variable in both space and time (Figure 1), and reflect the strong selection pressure these dynamics exert on aquatic species (Grant, 2011; Mushet et al., 2013).
Physical barriers between different aquatic habitats, such as steep gradients, waterfalls, mountain ranges, dams, or intervening inhospitable habitats, can restrict movements needed to establish or maintain biological connectivity (e.g., Greathouse et al., 2006; Hanfling and Weetman, 2006; Hall et al., 2011). When all other factors (e.g., climate, topography, geology) are equal, large, high-quality aquatic habitats separated by shorter distances are more likely to be biologically connected, due to greater carrying capacity and lower costs associated with movement (MacArthur and Wilson, 1967; Hanski, 1999). During dry years, organisms moving between aquatic habitats must traverse greater distances via aerial or overland movement (Figure 2). Greater spatial distance between suitable habitats may increase the number and variety of intervening landscape patches through which organisms must move, decreasing the probability of traversing them successfully (Bonte et al., 2012). Mortality due to predators or natural hazards (e.g., adverse environmental conditions) generally increases with the distance an organism has to travel to reach another habitat (Bowler and Benton, 2009).
Ultimately, biological connections depend on the biota present in the system. The physical structure of the FEM determines the system’s structural connectivity; the species present (or potentially present) determine how structural connectivity is translated into actual or functional connectivity (Calabrese and Fagan, 2004; Wainwright et al., 2011). Species traits and individual behaviors, such as dispersal mode, dispersal propensity, life cycle requirements, and responses to disturbance or environmental cues, arise over time in response to abiotic and biotic selection pressures. In turn, these determine why, when, how, and how far organisms move throughout the FEM—and thus the potential for biological connectivity.
BIOLOGICAL CONNECTIVITY THROUGHOUT FRESHWATER ECOSYSTEM MOSAICS
The establishment of biological connections throughout a FEM depends on the movement of living organisms (or their propagules) between the discrete habitats that the FEM comprises. These movements can occur longitudinally, along streams networks; laterally, between stream networks, riparian/floodplain wetlands and non-floodplain wetlands; vertically, between streams and wetlands and their hyporheic zones; or through time (Ward, 1989). Although vertical and temporal movements can have important effects on aquatic ecosystems (Hairston, 1996; Stubbington, 2012; Vander Vorste et al., 2016), we focus here on longitudinal and lateral connections that directly or indirectly (e.g., through “stepping-stone” movements) affect downstream waters.
In the following sections, we summarize evidence that demonstrates how and why biota move along stream networks and between stream networks and wetlands. We provide specific examples illustrating the different pathways, modes of transport, and types of organisms involved in Table 1. In Figures 3 through 6, we illustrate biological connections with example organisms, using the visual framework laid out in Figure 1.
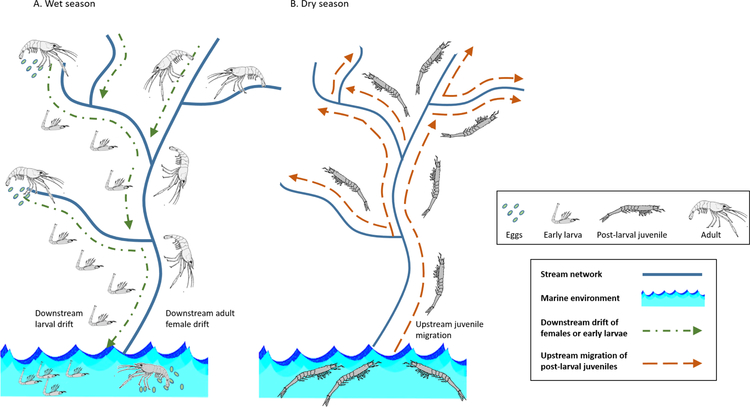
Figure 3.
Migration of freshwater shrimps (modified from Bauer, 2013). Like mayflies, caridean shrimps are found on every continent except Antarctica, move actively and passively within and between diverse habitats, and are important food sources for other aquatic organisms. Some caridean shrimps are commercially valuable, and many are amphidromous. (A) Biological connections during the wet season. In many amphidromous species, adult females spawn in streams and early-stage larvae drift downstream to develop in marine waters. In other species, adult females drift downstream to spawn in marine environments. (B) Biological connections during the dry season. Post-larval juveniles migrate back upstream to mature in freshwater habitats.
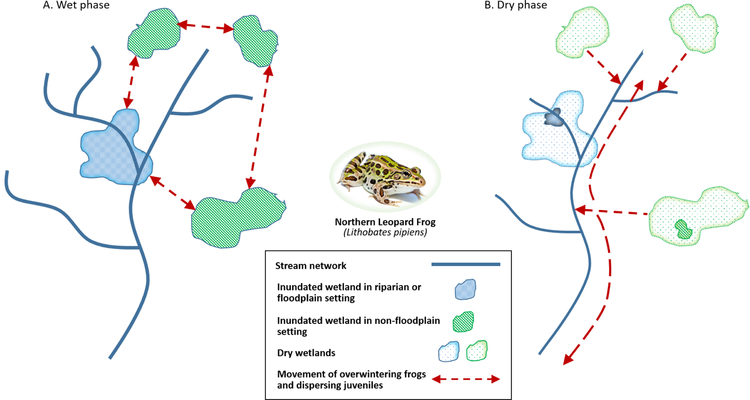
Figure 6.
Habitat complementarity in amphibians. Amphibians (frogs, salamanders, and caecilians) are globally distributed except in Antarctica and Greenland, with particularly high concentrations in neotropical regions. Adults often inhabit terrestrial habitats but require moist environments; eggs must be deposited in water and immature stages are often fully aquatic. Northeastern populations of the Northern Leopard Frog (NLF) exhibit uncharacteristically high levels of genetic diversity, which has been attributed to large stable populations inhabiting landscapes with high-densities of wetlands (A). Under drought conditions, NLFs move into streams, then disperse rapidly to recolonize wetlands when drought conditions end (B) (Mushet et al., 2013). Because NLF are not freeze-tolerant, they move into deep wetlands or flowing water to survive harsh northern winters.
Movement of Biota Along Stream Networks
Biological connections are clearly evident along stream networks, as organisms travel downstream with the flow of water. A diverse collection of organisms (e.g., microbes, algae, aquatic invertebrates, fishes) are passively transported or actively move downstream along hydrologically connected stream channels (Table 1). These movements establish biological connections between upstream habitats and downstream waters.
Many aquatic and semi-aquatic species inhabit headwater streams (Meyer et al., 2007). These species are often found throughout a range of stream sizes (Hall et al., 2001; Freeman et al., 2007) and flow durations (Schlosser, 1987; Feminella, 1996; Labbe and Fausch, 2000), and move into and out of headwater streams at different points in their life cycles (Horwitz, 1978; Ebersole et al., 2006; Meyer et al., 2007). For certain taxa, headwater streams—including intermittent and ephemeral streams—support highly diverse communities (e.g., Besemer et al., 2013) and provide critical habitat at one or more stages of their life cycles (Koizumi et al., 2016; Woelfle-Erskine et al., 2017).
The use of headwater streams as habitat is especially evident for diadromous species that migrate between headwater streams and marine environments during their life cycles, such as Pacific and Atlantic salmon, American eels, and certain neotropical shrimps (Figure 3). Many of these taxa are either obligate or facultative users of headwater streams (Erman and Hawthorne, 1976; Ebersole et al., 2006; Wigington et al., 2006; Hitt et al., 2012), but over their life cycles they travel the entire length of the river network. Thus, the presence of diadromous taxa provides robust evidence of biological connections along stream networks.
Biological connections are not reliant on diadromy, however, as nondiadromous organisms are also capable of significant movement along river networks. Many fishes require different habitats during different life stages, and move significant distances both upstream and downstream throughout their life cycles (e.g., Gorman, 1986; Labbe and Fausch, 2000; Hitt and Angermeier, 2008; Falke et al., 2010; Kanno et al., 2014). For example, Schrank and Rahel (2004) found that Bonneville cutthroat trout moved from less than 1 to more than 80 km after spawning. Many fish spawn in headwater streams, including those with intermittent flow (Erman and Hawthorne, 1976; Schrank and Rahel, 2004; Ebersole et al., 2006). For example, Wigington et al. (2006) found that intermittent streams were an important source of coho salmon smolts in Oregon, where juveniles survived dry periods in residual pools located within intermittent stream channels. Many salmonids also rear in headwater streams (Brown and Hartman, 1988; Curry et al., 1997; Bramblett et al., 2002), and these habitats can provide higher quality habitat for juvenile fish, as evidenced by increased growth, size, and overwinter survival in these habitats (Ebersole et al., 2006; Ebersole et al., 2009). Coho salmon smolts that overwintered in intermittent Oregon streams were larger than those from perennial streams (Wigington et al., 2006). Fishes also can transport other organisms (e.g., seeds, pathogens, glochidia), carrying them against flow or extending their dispersal distances (e.g., Chick et al., 2003; Senderovich et al., 2010; Schwalb et al., 2013), as they move through stream networks (Figure 4). For example, Schwalb et al. (2011) estimated that host fishes could disperse freshwater mussel larvae from 8 to 1645 km, depending on host fish species.
Figure 4.
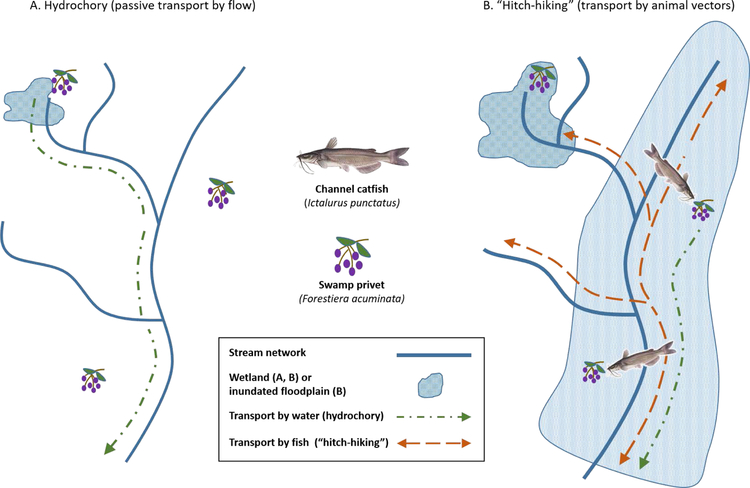
Aquatic plant dispersal via hydrochory and “hitch-hiking”. Aquatic plants can disperse passively by wind or moving water, actively by animal vectors, or both. (A) Streamflow carries propagules—here, mature fruits of swamp privets—from headwater wetlands to downstream wetlands, where seeds settle and germinate (Nilsson et al., 2010). (B) Plants can also disperse by “hitch-hiking” on animal vectors. Here, frugivorous channel catfish move into seasonally inundated floodplains to feed on mature fruits from off-channel swamp privets (Chick et al., 2003). Dispersal is accomplished by transport, excretion, and germination of viable seeds in habitats throughout the river-floodplain network.
Prairie fishes provide another clear demonstration of biological connections along the river network. Many prairie fishes release their eggs into the water column, and eggs develop as they are transported downstream with water flow (Fausch and Bestgen, 1997; Platania and Altenbach, 1998; Durham and Wilde, 2006). When unimpeded (e.g., by dams), downstream transport of these drifting eggs and larvae can be extensive (e.g., more than 350 km; Platania and Altenbach, 1998). Adult fishes, which are capable of long-distance migrations, then move upstream prior to egg release (Fausch and Bestgen, 1997; Durham and Wilde, 2006). Maintenance of prairie fish populations thus depends on these bi-directional biological connections along these river networks (Fausch and Bestgen, 1997; Durham and Wilde, 2006). Pelagic-spawning mussels create similar biological connections along stream networks, via downstream drift and upstream movement attached to host fishes (Schwalb et al., 2010).
Headwater streams also provide habitat for diverse and abundant stream invertebrates (Meyer et al., 2007) and serve as collection areas for terrestrial and riparian invertebrates that fall into them (Kawaguchi and Nakano, 2001; Eberle and Stanford, 2010). These aquatic and terrestrial invertebrates can be transported downstream with water flow (Figures 3 and 5) (Elliott, 1971; Müller, 1982; Brittain and Eikeland, 1988; Reynolds et al., 2014). Cumulatively, export of invertebrates from numerous headwater streams within a single network to downstream waters can be substantial (Wipfli and Gregovich, 2002), especially in intermittent and ephemeral streams, as terrestrial invertebrates accumulate in these channels during dry periods and are then transported downstream upon channel rewetting (Corti and Datry, 2012; Rosado et al., 2015).
Figure 5.
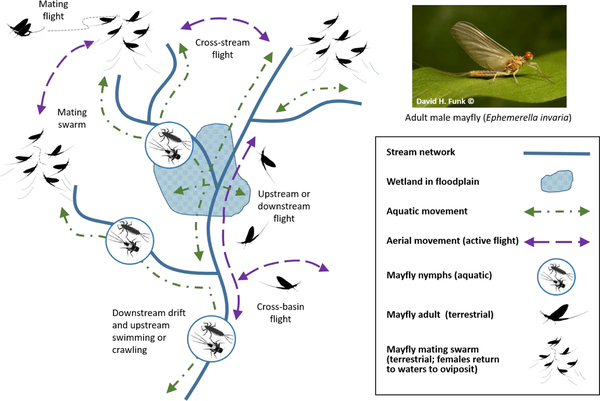
Movements associated with the aquatic and terrestrial life stages of a mayfly. Mayflies are found in freshwater and brackish habitats on all continents except Antarctica, and are important food sources for a wide range of aquatic and terrestrial organisms. Despite a reputation as weak” fliers, mayflies can disperse over very long distances (up to 700 km; Sartori and Brittain, 2015). Mayfly nymphs are relatively long-lived typically up to a year) and fully aquatic. They swim, crawl, or drift in streamflow to find food and shelter, avoid predators, escape unfavorable conditions, and colonize new underwater habitats (green dot-dashed lines). In contrast, adult mayflies are short-lived (typically 24–48 hr) and fully terrestrial. Adult mayflies move actively to disperse, reproduce, and oviposit in streams or wetlands (purple dashed lines).
To compensate for loss of individuals to downstream drift, invertebrate populations in headwater streams are maintained and replenished through processes such as high productivity and upstream dispersal (Figures 3 and 5) (Hershey et al., 1993; Humphries and Ruxton, 2002). For organisms capable of directed movement over long distances (e.g., winged adult forms of aquatic insects), these downstream-to-upstream connections can occur over significant network distances. In addition, these connections are often not dependent on hydrologic connections (Figure 5). Upstream and downstream movements along, but not necessarily within, streams (e.g., dispersal over land or aerially) further strengthen linkages between upstream habitats and downstream waters (Grant et al., 2010). For example, dry stream channels can serve as dispersal corridors for terrestrial adult forms (Bogan and Boersma, 2012; Steward et al., 2012), and stream networks can create transportation corridors for terrestrial and semi-aquatic fauna (Sánchez- Montoya et al., 2016; Goodrich et al., this issue).
Movement of Biota Between Stream Networks and Wetlands
In addition to the longitudinal connections described above, biota also create lateral connections throughout FEMs as organisms move from the river network into riparian/floodplain and non-floodplain wetlands (Figure 1). These movements occur via the same pathways as longitudinal movements (Table 1), although the relative importance of different pathways may vary. Hydrologic connections between stream networks and wetlands are typically more variable than hydrologic connections between upstream and downstream areas of stream networks, particularly in perennially flowing systems. Non-floodplain wetlands are generally more spatially distant from (and thus typically less hydrologically connected to) stream channels than riparian/floodplain wetlands, and may lack even intermittent surface water connections. As a result, movements via non-water pathways tend to increase in prevalence and importance for these lateral connections.
Research has clearly demonstrated that organisms move laterally between river networks and wetlands, thereby establishing biological connections throughout FEMs. Wetlands support diverse communities of aquatic, amphibious, and terrestrial plant and animal species, which are adapted to the periodic or episodic inundation of these habitats (Galat et al., 1998; Robinson et al., 2002; Rooney et al., 2013; Granado and Henry, 2014). Adaptations of stream-dwelling organisms to variable moisture conditions, as well as their ability to rapidly disperse and exploit temporary or seasonal hydrologic connections, provide strong evidence that biological connections exist between rivers and other aquatic habitats over relatively long time frames.
When overbank flow causes rivers to expand laterally, surface hydrologic connections between the river network and adjacent wetland habitats are created (Figures 1 and 4) (Junk et al., 1989). Aquatic biota can move into these newly flooded wetland habitats (Junk et al., 1989; Smock, 1994; Tockner et al., 2000; Robinson et al., 2002; Tronstad et al., 2007), and then eventually return to the river network when flooding recedes (Copp, 1989; Smock, 1994; Richardson et al., 2005). In unregulated rivers, floodplain inundation greatly increases the area and diversity of aquatic habitats, and these habitats often have high primary productivity (Junk et al., 1989; Tockner et al., 1999; Tockner et al., 2000; Brooks and Serfass, 2013). As a result, floodplains are important habitats for fish (Copp, 1989; Snedden et al., 1999; Bestgen et al., 2000; Schramm and Eggleton, 2006; Alford and Walker, 2013), aquatic life stages of amphibians (Richardson et al., 2005), and aquatic invertebrates (Smock et al., 1992; Smock, 1994).
There is abundant evidence that fishes move between the main river channel and wetlands when these habitats are hydrologically connected (Table 1). Many fish species disperse into riparian/floodplain wetlands to feed, reproduce, rear young, and seek refuge from harsh conditions (e.g., Copp, 1989; Matheney and Rabeni, 1995; King et al., 2003; Crook and Gillanders, 2006; Pease et al., 2006; Henning et al., 2007; Jeffres et al., 2008; Zeug et al., 2009; Burgess et al., 2013). Fishes can also carry other organisms with them as they move. For example, channel catfish that move into seasonally inundated floodplains can consume and transport viable swamp privet seeds to downstream floodplains (Figure 4; Chick et al., 2003).
Oxbow lakes can be important fish feeding and rearing habitats (Baranyi et al., 2002; Zeug et al., 2005; Shoup and Wahl, 2009; Zeug et al., 2009). For example, isotopic analysis of gizzard shad in the Brazos River, Texas, showed that isotopic signatures of both juvenile and adult fish varied between main channel and oxbow habitats (Zeug et al., 2009). The isotopic signatures of adult fish were more variable in oxbows, indicating that these individuals fed in main channel vs. oxbow habitats to varying degrees (Zeug et al., 2009). Fish also move between lacustrine wetlands (wetlands associated with lakes) and large lakes when hydrologic connections exist (Jude and Pappas, 1992; Miyazono et al., 2010).
The presence of fish in non-floodplain wetlands clearly demonstrates that these wetlands are biologically as well as hydrologically connected to other waters, even if those hydrologic connections are intermittent. For example, fish were present in 21% of 63 Carolina bays surveyed, even though many of the bays dried out during part of the year. Fish travelled up to 4 km from a Florida lake into seasonal wetlands, eventually colonizing 9 of the 25 temporary habitats samples (Hohausová et al., 2010). If non-floodplain wetlands are periodically connected to other aquatic habitats by surficial water flows, fish, other swimming organisms, and organisms transported by flowing water (e.g., invertebrates, seeds) can move into non-floodplain wetlands via hydrologic connections (Baber et al., 2002; Hulsmans et al., 2007; Herwig et al., 2010).
Stream invertebrates (e.g., insects, crayfish, mussels, cladocerans, copepods, rotifers, and gastropods) also move into wetlands during seasonal or episodic periods of hydrologic connectivity (Junk et al., 1989; Ilg et al., 2008). Even in small headwater streams, thousands of invertebrates can drift or crawl between streams and riparian wetlands per day (Smock, 1994). Many invertebrate species have evolved life history strategies to exploit these habitats, such as the ability to rapidly colonize newly flooded areas, short life cycles that allow them to complete their life cycles before floodplains dry again, and the use of aquatic refuges or dormant life stages to persist (sometimes for many years) until wetlands are re-inundated (Tronstad et al., 2007).
Biological connections are also established by organisms typically thought of as less mobile. Primary producers, including phytoplankton and aquatic and emergent plants, are capable of moving between the river network and wetlands, as seeds, plant fragments, and whole organisms are transported back and forth between these habitats via multiple pathways (Table 1, Figure 4) (e.g., Barrat-Segretain, 1996; Middleton, 2000; Soons, 2006; Angeler et al., 2010; Nilsson et al., 2010). Seeds from vegetation within the channel or from upstream wetlands can be transported with water flow and deposited on bordering or downstream riparian areas and floodplains (Gurnell, 2007; Boudell and Stromberg, 2008; Gurnell et al., 2008; Nilsson et al., 2010). Lateral expansion of the river network can dislodge viable plant fragments in riparian/floodplain wetlands, which can then be transported down the river network and re- establish in downstream waters (e.g., Truscott et al., 2006).
As the examples above illustrate, hydrologic connections establish multiple biological connections. Biological connections do not require hydrologic connections, however. Particularly for habitats that are less frequently connected via surface water flowpaths (e.g., non- floodplain wetlands), biological connections often depend on non-water mediated movements of biota. Aquatic and semi-aquatic species have evolved numerous strategies to survive and thrive in landscapes that often lack surface hydrologic connections (Bohonak and Jenkins, 2003). Although movements via crawling, flying, wind, “hitchhiking,” and dormancy can be cryptic, sporadic, or asymmetric, and thus difficult to observe directly, these connections are common (Table 1).
Many aquatic species require or facultatively use resources in more than one habitat type to complete their life cycles or to persist when preferred habitats are scarce (Figure 6) (Skagen and Knopf, 1993; Ribera, 2008; Mushet et al., 2013). Numerous flight-capable insects, including mayflies, caddisflies, diving beetles, backswimmers, whirligig beetles, water striders, water boatmen, scavenger beetles, crane flies, and nonbiting midges, use both streams and non- floodplain wetlands (Williams, 1996). In a survey of 150 aquatic insect species in the orders Coleoptera (beetles) and Hemiptera (true bugs) in perennial stream pools, cattle troughs, and seasonal ponds, Bogan et al. (2013) reported that 46 species (31%) were generalists occurring in at least two of the three habitats sampled. Many non-floodplain wetlands (e.g., western vernal pools, Carolina and Delmarva bays) support generalist invertebrate and amphibian species that also inhabit streams, lakes, or riparian/floodplain wetlands (Hudson et al., 1990; Leeper and Taylor, 1998; Zedler, 2003). Observations that non-floodplain wetlands such as prairie potholes often lack endemic biota (i.e., biota restricted to a small geographic area) suggest that these habitats are not isolated over sufficiently long time frames to allow local speciation, and thus have been or currently are biologically connected to other aquatic habitats (van der Valk and Pederson, 2003).
Seeds and invertebrates can be passively dispersed among non-floodplain wetlands by wind (Galatowitsch and van der Valk, 1996). This pathway can be particularly important in seasonal wetlands, as large numbers of transportable seeds, resting eggs, cysts, diapausing larvae, and adults can be picked up from dry-phase soils and dispersed. Some invertebrate species colonizing temporary pond habitats rely solely on airborne dispersal (Table 1; Lopes et al., 2016). Vanschoenwinkel et al. (2009) collected 850 viable dormant eggs, larvae, and adults, from 17 aquatic invertebrate taxa, in windsocks erected near temporary rock pools. Wind- dispersed wetland plant species make up a high percentage (45–50%) of all species in more terrestrial wetland types (Soons, 2006).
Active overland dispersal throughout FEMs is also common, as insects, amphibians, reptiles, birds, and mammals can move among wetlands and stream networks on the ground or in the air (Table 1) (Lamoureux and Madison, 1999; Clark, 2000; Milam and Melvin, 2001; Gibbons et al., 2006, Attum et al., 2007; Spinola et al., 2008; Subalusky et al., 2009a, Subalusky et al., 2009b). Aerial dispersal of individuals from multiple taxonomic orders and phyla is a significant source of stream invertebrate colonists in newly inundated floodplain habitats (Tronstad et al., 2007; Vanschoenwinkel et al., 2009). For example, Tronstad et al. (2007) investigated aerial insect colonization of floodplains in an unregulated coastal plain river, and reported high densities (maximum ≈ 80,000 individuals m−2) in floating trays placed in floodplain waters, as well as high densities (21,291 individuals m−2) of passively dispersing (e.g., via wind or animal vectors) microcrustaceans. Bogan et al. (2013) determined that several aquatic insect species occurring only in stream pools are either flightless or have weak dispersal abilities, whereas species occurring only in seasonal ponds are capable of frequent and long distance dispersal. These findings suggest that biota occupying non-floodplain wetlands may actually be better long-distance dispersers than biota occupying other aquatic habitats.
Overland biotic movements also create biological connections between wetlands and the river network (e.g., Newman and Griffin, 1994; Swimley et al., 1999; Bodie and Semlitsch, 2000) that are independent of hydrologic connections. Many amphibian species move between wetlands and streams throughout their life cycles (Lamoureux and Madison, 1999; Babbitt et al., 2003; Green, 2005; Petranka and Holbrook, 2006; Mushet et al., 2013), and numerous studies have demonstrated that amphibians commonly disperse in non-floodplain wetland landscapes, often in large numbers. For example, Gibbons et al. (2006) documented more than 360,000 juvenile amphibians, from 24 species, emigrating from one Carolina bay during a single breeding season; more than 95% of the biomass (about 1,330 kg) came from juveniles of the southern leopard frog, which is known to use both stream and wetland habitats (Pope et al., 2000; Mushet et al., 2013). Riverine turtles can move hundreds of meters between rivers and wetlands to find suitable foraging, mating, nesting, rearing, and overwintering habitat throughout the year (Bodie and Semlitsch, 2000; Bodie, 2001). River-dwelling mammals such as river otters also move between rivers and wetlands (Newman and Griffin, 1994; Swimley et al., 1999).
The movement of migratory water- and shorebirds (e.g., ducks, geese, cranes) provides perhaps the most extensive example of biological connections throughout FEMs. Wetlands are often critical habitats for these species, and used by large numbers of birds. For example, Webb et al. (2010) observed more than 1.6 million birds, representing 72 migratory bird species, actively using roughly 40 playas (shallow, wind-formed wetland depressions) in Nebraska during a 3-year spring migration study. Many of these migratory water- and shorebirds have been documented to use multiple aquatic habitats (e.g., streams, wetlands, estuaries) throughout their life cycles (Krapu et al., 1984; LaGrange and Dinsmore, 1989; Folk and Tacha, 1990; Adair et al., 1996; Austin and Richert, 2005; Ballard et al., 2010). Use of these different habitats is often opportunistic, and dispersal among them varies with temporal and spatial changes in habitat availability (Farmer and Parent, 1997; Haig et al., 1998; Ballard et al., 2010). Because these birds cover large distances with their migrations, they create biological connections that can link aquatic habitats over large spatial scales.
Because many organisms disperse to and from riparian/floodplain and non-floodplain wetlands as “hitchhikers” on actively dispersing fauna (Table 1), the biological connections established by one taxon can frequently be transformed into multiple potential connections. For example, seeds of aquatic and riparian plants can be actively dispersed between riverine and riparian/floodplain wetlands when they are consumed by fish (Figure 4; Pollux et al., 2007). Viable seeds and vegetative plant parts can travel great distances within the guts of or externally attached to migratory birds (Murkin and Caldwell, 2000; Amezaga et al., 2002; Figuerola and Green, 2002), which move between wetlands and river networks depending on temporally dynamic habitat availability (Murkin and Caldwell, 2000; Haukos et al., 2006). Recent evidence also suggests that invertebrates are commonly transported by birds, as well as mammals (Figuerola and Green, 2002; Figuerola et al., 2005; Allen, 2007; Frisch et al., 2007). Because migratory birds can fly such long distances during their migrations, maximum dispersal distances for hitchhiking organisms have been estimated at 1,400 km (Mueller and van der Valk, 2002). Invertebrates can also serve as the transport vector for smaller organisms, such as algae and protozoa (Table 1).
WHY BIOLOGICAL CONNECTIONS THROUGHOUT FRESHWATER ECOSYSTEM MOSAICS MATTER
The examples detailed above provide strong evidence of the movements of diverse biota along stream networks and between streams and wetlands. Taken together, these movements create the incredible diversity, variability, and complexity of biological connections in FEMs. Assessing the effects of these connections, however, is even more challenging than documenting the occurrence of movement among habitats. Despite these challenges, an increasing number of studies are explicitly addressing both the occurrence and the importance of biological connections that affect the structure and function of downstream waters.
In this section, we discuss how biotic movements affect FEM structure and function. We first consider these effects in terms of the functions by which upstream habitats can influence population and community structure in downstream waters (Leibowitz et al., this issue): as sources of organisms to downstream waters; as sinks that retain organisms and reduce their provision to downstream waters; as refuges that support persistence of populations and biodiversity in downstream waters; as lags that temporarily “store” organisms or propagules (e.g., seeds) before providing them to downstream waters; and as transformers that provide resources needed for the development of individuals to different forms (e.g., different life stages) and for the evolution of locally adapted populations. Because each of these functions exerts effects at multiple levels of biological organization, from genes to ecosystems, we also find it useful to discuss biological connections in terms of how connections among streams, wetlands, and downstream waters affect individuals, populations, and communities throughout the entire FEM.
Although we are primarily focused on upstream-to-downstream connections and resulting effects on downstream waters, it is important to note that these functions and effects often depend on bi-directional movements—that is, biological connections in a downgradient direction, and their resulting effects on downstream waters, often rely at least in part on biological connections in an upgradient direction. For example, biota must be able to reach upstream refuges under adverse conditions, and then recolonize newly habitable downstream habitats when adverse conditions abate. Ultimately, then, the ecological integrity of FEMs requires that the full complexity of biological connections, in all dimensions and directions, be considered.
Biological Connections and the Functions of Streams and Wetlands
All three of the habitat types considered here—streams (including perennially and intermittently flowing channels), riparian/floodplain wetlands, and non-floodplain wetlands— can function as sources, sinks, refuges, lags, and transformers of biota for downstream waters. As defined here, these functions are not necessarily independent and discrete, and can work synergistically. For example, growth of an organism in a headwater stream or wetland, and subsequent movement into downstream waters, could arguably be considered a source, refuge and/or transformer function of the upstream habitat.
Existing evidence clearly supports the idea that streams and wetlands commonly serve essential source and refuge functions for downstream waters. Streams and wetlands are sources of organisms and propagules, which then can serve as food or colonists in downstream waters (e.g., Thorp and Delong, 2002; Bunn et al., 2003; Hein et al., 2003; Keckeis et al., 2003; Gurnell et al., 2008). This provision of organisms occurs via multiple pathways, including active or passive movements in water, over land, aerially, or attached to other organisms (Table 1, Figure 1). The source function served by tributaries and wetlands often stems from the refuge function served by these habitats. Under adverse abiotic or biotic conditions in downstream waters, streams and wetlands can serve as refuge habitats (Meyer et al., 2004; Chester and Robson, 2011; Bogan et al., 2013; Cañedo-Argüelles et al., 2015); when biota leave these refuges and return to downstream waters, these habitats may then act as sources of individuals to downstream waters.
Streams and wetlands also function as sinks, lags, and transformers via numerous biological connections to downstream waters. For example, wetlands serve as sinks for seeds and plant fragments that remain in these habitats but do not germinate (Middleton, 2000), or for fish that are stranded when wetlands are no longer connected via surface water pathways (Nagrodski et al., 2012). Lags can occur when movement from wetlands back to the stream network is delayed (e.g., by dormancy or by temporary drying of hydrologic flowpaths) (e.g., Smock, 1994; Tronstad et al. 2007). When used as spawning or rearing habitats, streams and wetlands can be considered transformers that allow organisms to “transform” from one stage of development to another; this function is particularly evident for species that undergo ontogenetic habitat shifts between different FEM components (Huryn and Gibbs 1999, Gibbons et al. 2006). For example, American alligators in southern Georgia use seasonal wetlands for nesting and nursery areas and riverine habitats for non-nesting habitat (Subalusky et al., 2009a).
Biological Connections at the Organismal Level
At the organismal level, biological connections throughout FEMs provide individuals in downstream waters access to two key resources: food and habitat. Movement of organisms throughout FEMs creates biological connections that supply food for other organisms and that allow organisms to access suitable habitats.
Along stream networks, there is clear evidence that upstream areas provide food to downstream waters. Many fish feed on drifting insects (e.g., Nakano and Murakami, 2001; Wipfli and Gregovich, 2002), so the biological connections created by invertebrate drift provide food resources for downstream fish (Figure 5). Wipfli and Gregovich (2002) estimated that drifting insects and detritus from fishless headwater streams in Alaska supported between 100 and 2,000 young-of-year salmonids per km in a large, salmon-bearing stream. Increased invertebrate drift also has been associated with increased fish growth (Wilzbach et al., 1986; Nielsen, 1992; Rosenfeld and Raeburn, 2009), indicating that drift provides a valuable food resource, particularly when food is limiting (Boss and Richardson, 2002).
Wetlands also contribute food resources to downstream waters. Phytoplankton communities in river networks can be enhanced by conditions that promote high productivity in temporarily connected floodplain wetlands (Hein et al., 2003). This pattern holds even when little flow passes through floodplains relative to total flows through the main channel (Lehman et al., 2008). High production of algal biomass in floodplains ultimately contributes high quality food resources (e.g., in terms of labile carbon and essential fatty acids) to downstream waters (Thorp and Delong, 2002; Bunn et al., 2003; Lehman et al., 2008), which then supports downstream fisheries.
Similarly, invertebrates emerging from wetlands (Leeper and Taylor, 1998) can become important food sources for fishes and other biota in nearby streams, particularly when one considers cumulative emergence from numerous wetlands across the landscape. The biota inhabiting wetlands convert organic matter in those wetlands into biomass, which then can subsidize other aquatic and terrestrial components of the ecosystem (Semlitsch and Bodie, 1998; Brooks, 2000; Gibbons et al., 2006).
Non-floodplain wetlands such as Carolina and Delmarva bays are often immensely productive amphibian breeding habitats, and are critical for the persistence of pond-breeding amphibian populations that can move to other water bodies (Sharitz and Gibbons, 1982; see Biological Connections at the Population Level, below). Given the proximity of many Carolina and Delmarva bays to tributaries (12–19% of Carolina bays within 100 m, roughly 90% within 1.6 km; Sharitz, 2003), amphibians emigrating from these bays could transfer large amounts of energy and organic matter into rivers and streams.
In addition to food, streams and wetlands provide organisms in downstream waters access to additional habitats; under adverse conditions in downstream waters, these habitats may serve as refuges. This provision of habitat is particularly evident for fishes that can actively move into upstream habitats. For example, headwater streams and small tributaries can provide fishes refuge from flow (Wigington et al., 2006; Koizumi et al., 2013) and temperature extremes (Peterson and Rabeni, 1996; Curry et al., 1997; Baxter and Hauer, 2000; Labbe and Faush, 2000; Bradford et al., 2001). Use of these refuge habitats can result in increased food availability, growth, and average egg size (Peterson and Rabeni, 1996), demonstrating that these upstream areas can provide downstream organisms with high-quality habitats that influence individual reproductive success and survival (e.g., Ebersole et al., 2009).
The refuge function served by upstream habitats can be especially important in intermittent streams, where perennial habitats (e.g., permanent pools) can serve as refuges during drying for fish (Pires et al., 1999; Labbe and Fausch, 2000; Fritz and Dodds, 2002; May and Lee, 2004; Wigington et al., 2006) and invertebrates (Fritz and Dodds, 2004). In other cases, intermittent channels themselves may serve as refuges, by allowing species adapted to drying conditions to persist (Meyer et al., 2004).
Biological Connections at the Population Level
As discussed above, the movement of biota throughout FEMs provides organisms access to food and habitat. Ultimately, these biological connections and their effects at the organismal level have repercussions at the population level, most notably in terms of population persistence and genetic diversity. Biological connections allow stream biotic assemblages to recolonize both downstream and upstream habitats following disturbances (Fritz et al., 2002; Franssen et al., 2006; Chester and Robson, 2011). For many biota, upstream areas are a source of colonists for downstream reaches (Meyer and Wallace, 2001; Hanfling and Weetman, 2006), allowing organisms to persist and recolonize downstream areas once adverse conditions have abated (Meyer and Wallace, 2001; Meyer et al., 2004; Huryn et al., 2005; Bogan et al., 2013; Cañedo- Argüelles et al., 2015). Particularly in streams subject to alternating periods of flooding and drying, populations depend on dispersal out of intermittent reaches before drying occurs, and subsequent recolonization of these habitats once water flow resumes. Prairie stream fishes provide a good example of this, as they can quickly move upstream or downstream into newly available habitat—including previously dry, rewetted channels—during and after floods (Harrell et al., 1967; Fritz et al., 2002; Franssen et al., 2006).
The persistence of prairie stream fish populations requires biological connections along entire stream networks. Many studies have documented significant associations between impoundment of prairie streams and loss of native fishes (Winston et al., 1991; Luttrell et al., 1999; Falke and Gido, 2006; Matthews and Marsh-Matthews, 2007). Prairie stream fishes can require more than 100 km of undisrupted stream channel (i.e., channels with no impoundments or drying associated with human withdrawal) to support persistent populations (Perkin and Gido, 2011), and impoundments can disrupt both downstream transport of developing eggs and larvae and upstream and downstream movement of adult fish. Fragmentation of river networks also has consistently been related to local extinction of salmonid populations (Morita and Yamamoto, 2002; Letcher et al., 2007).
Biological connections among wetlands and downstream waters also can be important for population persistence in these downstream habitats. Riparian/floodplain wetland habitats can be significant sources of fish recruitment in streams and rivers (Brown and Hartman, 1988; Crook and Gillanders, 2006; Pease et al., 2006). For example, Crook and Gillanders (2006) analyzed otolith chemical signatures to show that floodplain lakes were estimated to be the source of 98% of the young-of-year carp for areas 140 km downstream of the floodplain lakes, illustrating that upstream habitats can have significant effects on downstream populations.
Loss of hydrologic connectivity between wetlands and stream networks eliminates feeding, breeding, rearing, and refuge habitat for the many fully aquatic species that use wetlands for these purposes. If species do not demonstrate plasticity in behavior, habitat preference, or life cycle requirements, loss of access to these wetlands can result in local extirpation (Crook et al., 2015). In the Missouri River, flow regulation and disconnection of the river from its historical floodplain has coincided with declines in many species that rely on floodplain wetlands (e.g., fish, plants, insects, mussels, reptiles, birds, and mammals) (Galat et al., 1998). Biodiversity increased when these wetlands were reconnected to the river during major flood events (Galat et al., 1998).
The importance of streams and wetlands for populations in downstream waters is not limited to biota capable to active movement. Establishment and reproduction of refuge floodplain populations can be important wetland seed sources for the river network, especially when catastrophic flooding scours streambed vegetation and seed banks (Gurnell et al., 2008). Many taxa with limited mobility can be moved over longer distances via “hitchhiking” on more mobile organisms, with resulting population-level effects across extensive spatial scales. For example, winter migration of waterbirds can be an important mechanism for spring colonization of aquatic habitats separated by hundreds or even thousands of kilometers (Frisch et al., 2007). Figuerola et al. (2005) found that, for three of four invertebrate species examined, movement of waterbirds explained a significant amount of gene flow between populations located across North America.
These population-level effects can also be examined in terms of the maintenance of genetic connectivity and diversity. Genetic connectivity results from biotic dispersal and subsequent reproduction and gene flow. This gene flow connects spatially subdivided populations (e.g., headwater vs. downstream populations, populations in spatially distant wetlands), making it more likely that populations will retain higher levels of within-population genetic diversity and enhancing both population persistence and adaptive capacity in changing environments (Lande and Shannon, 1996; Ishiyama et al., 2015). Floods that periodically connect different parts of the river network generate the potential for gene flow across time and space by mixing individuals from different locations (e.g., upstream/downstream, river channel/floodplain) and different years (e.g., eggs that might have diapaused for tens or even hundreds of years) (Jenkins and Boulton, 2003; Frisch and Threlkeld, 2005). The combination of organismal movement and different life history strategies supports gene flow for individual species, as well as overall biodiversity in FEMs (see Biological Connections at the Community Level, below). In general, genetic connectivity decreases with increasing spatial distance (Wright, 1943). In river networks, it is also strongly influenced by the hierarchical structure of the network, the direction of dispersal (upstream, downstream, or both), dispersal modes and pathways used (e.g., swimming, flying), and species’ life histories (Morrissey and de Kerckhove, 2009; Hudy et al., 2010). Species that disperse frequently or over long distances tend to have higher within-population genetic diversity (Fer and Hroudova, 2008; Mullen et al., 2010).
Individual species behavior also can profoundly affect observed genetic patterns, via out- of-network gene flow (e.g., aerial or terrestrial dispersal by insects or amphibians) (Grant et al., 2010; Alexander et al., 2011), very high levels of within-network gene flow (e.g., fish that move and reproduce throughout the network) (Chaput-Bardy et al., 2009), or use of complementary habitats (Figure 6; Mushet et al., 2013). For example, in a microsatellite analysis of northern leopard frog populations that recolonized wetland habitats after an extended drought, Mushet et al. (2013) observed high levels of genetic diversity and low population genetic structure (Fst 0.0–0.05) among populations in wetlands separated by distances up to 65 km. These results indicate that dispersing juveniles of this frog, which breeds in seasonal wetlands and overwinters in deep or flowing waters to avoid the sub-freezing temperatures, connect FEM habitats over long distances in the northern Great Plains (Figure 6).
Population-level effects of streams and wetlands on downstream waters can be closely related to where along the connectivity-isolation continuum these habitats fall. For native populations, persistence may depend on isolation, rather than connectivity (Letcher et al., 2007; Cook et al., 2010). Both natural and artificial physical barriers, which reduce connectivity and increase isolation, can protect headwater habitats and populations by isolating them from colonization by and hybridization with invasive species (Freeman et al., 2007; Fausch et al., 2009). These effects are also reflected in the genetic structure of populations, as illustrated by the fact that most genetically pure cutthroat trout populations are confined to small, high elevation streams that are naturally or anthropogenically isolated (Cook et al., 2010). However, this isolation can also adversely affect native species via reduced genetic connectivity potentially leading to reduced reproductive fitness and increased risk of local extinction. Barriers to fish movement can result in increased genetic divergence between headwater and downstream populations, as well as loss of headwater genetic diversity (Wofford et al., 2005; Hanfling and Weetman, 2006; Deiner et al., 2007; Fausch et al., 2009; Gomez-Uchida et al., 2009).
Biological Connections at the Community Level
In addition to effects at the organismal and population level, biological connections between streams, wetlands and downstream waters also affect the structure of biotic communities. Fish assemblages among connected streams tend to have more species in common (Matthews and Robinson, 1998; Hitt et al., 2003; Grenouillet et al., 2004), and measures of river network structure (e.g., link magnitude) can be significantly related to fish assemblage structure (e.g., Osborne and Wiley, 1992; Smith and Kraft, 2005). Perkin and Gido (2012) demonstrated the importance of biological connections in structuring fish communities by examining the effects of stream network fragmentation. In 12 Kansas stream networks, fragmentation by road crossings affected both alpha diversity (species richness) and beta diversity (dissimilarity): fish species richness decreased in isolated segments, whereas dissimilarity to downstream sites increased (Perkin and Gido, 2012).
Community-level effects of biological connections are also evident for invertebrates. Fritz and Dodds (2002, 2004) examined invertebrate assemblages before and after drying in intermittent prairie streams and reported that initial recovery of invertebrate richness, richness of invertebrate drift, and richness of aerially colonizing insects were negatively related to distance from upstream perennial water (i.e., upstream refuge habitats). Recovery from disturbance in these intermittent streams appears to depend on biological connections via both downstream drift of colonizers and downstream (and potentially upstream) movement of aerially dispersing, egg-depositing adults (Miller and Golladay, 1996; Dodds et al., 2004). Communities in downstream waters also are by wetlands. For example, variability in wetland habitat availability and condition both within and across years enables multiple fish species with specific habitat requirements or preferences to reproduce and rear young (Robinson et al., 2002), thereby contributing to the maintenance of fish diversity throughout river networks (Shoup and Wahl, 2009).
This pattern of movements between different habitats allowing for the persistence of different species is true for invertebrates, as well. For example, initial microinvertebrate colonizers of newly flooded riparian habitats in one arid system were washed downstream from distant upstream reaches of the river network, illustrating biological connections along the entire stream network, including ephemeral and intermittent streams (Jenkins and Boulton, 2003). In just a few days, species hatching from diapausing eggs in transported sediments greatly increased size and diversity of the downstream microinvertebrate community (Jenkins and Boulton, 2003).
Lateral biological connections between the river channel and riparian/floodplain wetlands and open waters such as oxbow lakes, are integral to the viability of many riverine species (Bunn and Arthington, 2002; Shoup and Wahl, 2009) and increase overall levels of species productivity and biodiversity in river systems (Junk et al., 1989). In a 5-year study of fish in floodplain lakes, Shoup and Wahl (2009) found that hydrology and water chemistry differed across individual oxbow lakes, which thus varied in suitability for different fish species; they concluded that the entire floodplain should be considered a single functioning unit that supports the overall biological integrity of the river. Hydrologic connectivity between channels and riparian/floodplain wetlands can significantly enhance riparian vegetation diversity (Jansson et al., 2005) and determine floodplain wetland community structure (Boschilia et al., 2008). These connections can significantly influence macroinvertebrate community structure in riparian areas, as well (Obolewski et al., 2009; Paillex et al., 2009), and can help support invertebrate diversity throughout the river system (Reckendorfer et al., 2006). For example, composition of floodplain invertebrate assemblages in the Rhône River, France, was strongly related hydrologic connectivity between floodplain habitats and the main river channel, in part due to increased voltinism (i.e., shorter life cycles) with increased hydrologic connectivity (Paillex et al., 2007). Fish assemblages in riparian wetlands along the semiarid region of the Murray River, Australia similarly showed a large decline in diversity when those wetlands were disconnected from the river through hydrologic modifications, a trend which was reversed when connections were restored (Vilizzi et al., 2013).
Biotic movement, both within non-floodplain wetland habitats and between these habitats and other FEM components, has well-documented effects on community structure and biodiversity of these mosaics, particularly for amphibians (e.g., Wellborn et al., 1996; Snodgrass et al., 2000; Julian et al., 2013). Similarity between spatially separated populations and communities—measured in terms of genetic or community structure—provides additional evidence of biological connectivity between non-floodplain wetlands and river networks (Ivey and Richards, 2001; Capers et al., 2010). For example, Capers et al. (2010) determined that aquatic habitats (small isolated wetlands to large lakes) located more closely together had more similar plant communities regardless of habitat type and local determinants of community structure (e.g., rainfall and soil type).
Isolation of non-floodplain wetlands can also contribute to the long-term genetic diversity of populations (King et al., 1996). For example, present-day Pacific vernal pool wetland communities are characterized by endemic species that have evolved within globally-distributed genera (King et al., 1996; Keeley and Zedler, 1998; Zedler, 2003). Over geologic time, passively-dispersing species colonized, then became locally adapted to, spatially isolated vernal pool landscapes. In these wetland ecosystems, relatively infrequent biological connections have resulted in the creation of new, endemic species from the rootstock of ancient, widespread lineages. Despite their relatively high spatial isolation, Pacific vernal pools are now rich reservoirs of genetic and species diversity (Zedler, 2003). The existence and periodic connectivity of such reserves are especially important at a time when changing environmental conditions are threatening biodiversity of aquatic species worldwide (Carpenter et al., 2011).
SYNTHESIS AND IMPLICATIONS
Based on existing scientific evidence, biota clearly link FEMs via movements within and among their aquatic habitat components. Even freshwater habitats that appear to be hydrologically isolated are connected by movements of biota that affect all levels of biological organization, from genes to ecosystems. For species that are only capable of moving via water (e.g., most fish, many aquatic invertebrates), biological connections largely depend on hydrologic connections. The hydrologic flowpaths used by biota do not need to be permanent, as water-dependent life stages and movements (e.g., juvenile dispersal, adult migration) are timed to coincide with intermittent flows or take advantage of ephemeral or episodic flows. Furthermore, downgradient flow does not restrict biota to downstream movement, as biota can actively with or against the direction of hydrologic flows. Even greater flexibility is present in aquatic species that also are capable of moving overland. This group includes many fully aquatic organisms (e.g., algae, invertebrates with no terrestrial life stage) that nonetheless have evolved mechanisms for terrestrial movement between aquatic habitats, via flying, walking, crawling, hopping, “hitchhiking”, drifting in wind, or some combination of these (Table 1). The diverse nature of biotic movements reflects aquatic species’ many adaptations to life in dynamic freshwater ecosystems. As a result of their remarkable diversity, biological connections are far more widespread, complex, and variable than hydrologic connections in aquatic ecosystems.
The temporal and spatial scales over which physical pathways for movement (structural connectivity) and actual movements (functional connectivity) link aquatic habitats vary with the environmental conditions and species assemblages present in them (Baguette et al., 2013). A complete discussion of the biotic and abiotic factors that influence biological connectivity in freshwater ecosystems, including the evolutionary trade-offs in species traits associated with growth, reproduction, and survival (Bonte et al., 2012; Kubisch et al., 2014), is beyond the scope of this paper. However, existing and emerging information on the effects of surface water dynamics (e.g., Figure 2) on habitat stability can provide new insights into aquatic species’ distributions (Williams, 2006), biodiversity (Marten et al., 2006; Dehling et al., 2010), range sizes (Ribera and Volger, 2000; Hjalmarsson et al., 2015), metapopulation and metacommunity dynamics (Larned et al., 2010), and dispersal (Hof et al., 2012).
The diversity of habitats and species in FEMs makes them ideal systems for investigating scales of biological connectivity, and the relationships between habitat heterogeneity, habitat stability, and biodiversity (Jeltsch et al., 2013). Future research to advance our understanding of the timing, rate, frequency, and distance of movements can build upon past work by focusing not on model species, but rather on assemblages with the range of life histories representing species’ adaptations to conditions in different FEM landscape settings. It will come as no surprise that data availability still poses the critical limitation to quantifying biological connectivity (Calabrese and Fagan, 2004; Bergsten and Zetterberg, 2013). Actual movement is challenging to measure at any scale, and is particularly difficult to observe and quantify for small or cryptic organisms and infrequent, long-distance dispersal events that have ecologically and evolutionarily significant consequences (e.g., Ishiyama et al., 2015).
While challenging to obtain, these data are needed to improve the accuracy of connectivity metrics and the performance of models to predict connectivity. Our understanding of biological connections has been advanced by explicit consideration of landscape-scale habitat structure, for example dendritic stream networks (Fagan, 2002; Grant et al., 2007) and wetland habitat modularity (Fletcher et al., 2013). Modeling methods capable of incorporating local- to macro-scale connectivity of streams, wetlands, lakes, and ponds with specific information about habitats, focal species, and species-landscape interactions (e.g., habitat area or quality, population abundance) and connectivity attributes (e.g., Euclidean distance, landscape resistance, direction of movement) are now available (Galpern et al., 2011). Recent advances in the theory and application of multi-layer networks to ecological systems are also forwarding the development of analytical methods that can evaluate biological connectivity across species, over multiple spatial and temporal scales, and in response to diverse ecological and socio-ecological processes (Kivelä et al., 2014; Pilosof et al., 2017).
For practical reasons, research on biological connections is often conducted in single systems, looking at individual species, assemblages, or ecosystem types. In reality, biological connectivity is the cumulative effects of multiple species moving, via multiple pathways and across multiple habitat types, to make use of the full range of resources occurring throughout heterogeneous FEMs (Figure 1). The movements of organisms, and the materials they transport, are essential to the functions of streams and wetlands, which in turn provide critical functions to downstream waters by serving as sources of colonists, food, and genetic diversity; as sinks for organisms; as refuges from adverse abiotic and biotic conditions; as transformers via organism growth and development and subsequent return to downstream waters; and as lags via dormancy and temporary isolation. Thus, the diverse connections among different components of FEMs, which vary in space and time, across species and even across individuals within a species, are needed to sustain aquatic life and maintain the ecological integrity of downstream waters.
ACKNOWLEDGMENTS
We thank all the authors and reviewers of the U.S. EPA report Connectivity of Streams & Wetlands to Downstream Waters: A Review & Synthesis of the Scientific Evidence for their input to that report, upon which the paper presented here is based. We also thank Micah Bennett, Joe Ebersole, Rose Kwok, Lora Smith, and three anonymous reviewers for their comments on the draft manuscript. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
LITERATURE CITED
- Adair SE, Moore JL, and Kiel WH, 1996. Wintering Diving Duck Use of Coastal Ponds: An Analysis of Alternative Hypotheses. The Journal of Wildlife Management 60:83–93. DOI: 10.2307/3802043. [DOI] [Google Scholar]
- Alexander LC, Fritz KM, Schofield KA, Autrey BC, DeMeester JE, Golden HE, Goodrich DC, Kepner WG, Lane CR, LeDuc SD, Leibowitz SG, McManus MG, Pollard AI, Kiperwas HR, Ridley CE, Vanderhoof MK, and Wigington PJ Jr., In review (this issue). Featured collection introduction: connectivity of streams and wetlands to downstream waters. Journal of the American Water Resources Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander LC, Hawthorne DJ, Palmer MA, and Lamp WO, 2011. Loss of Genetic Diversity in the North American Mayfly Ephemerella invaria Associated with Deforestation of Headwater Streams. Freshwater Biology 56:1456–1467. [Google Scholar]
- Alford JD, and Walker MR, 2013. Managing the Flood Pulse for Optimal Fisheries Production in the Atchafalaya River Basin, Louisiana (USA). River Research and Applications 29:279–296. [Google Scholar]
- Allen MR, 2007. Measuring and Modeling Dispersal of Adult Zooplankton. Oecologia 153:135–143. [DOI] [PubMed] [Google Scholar]
- Alsterberg C, Roger F, Sundbäck K, Juhanson J, Hulth S, Hallin S and Gamfeldt L, 2017. Habitat Diversity and Ecosystem Multifunctionality—The Importance of Direct and Indirect Effects. Science Advances 3:e1601475, DOI: 10.1126/sciadv.1601475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezaga JM, Santamaria L, and Green AJ, 2002. Biotic Wetland Connectivity—Supporting a New Approach for Wetland Policy. Acta Oecologica 23:213–222. [Google Scholar]
- Angeler DG, Alvarez-Cobelas M, Rojo C, and Sanchez-Carrillo S, 2010. Phytoplankton Community Similarity in a Semiarid Floodplain Under Contrasting Hydrological Connectivity Regimes. Ecological Research 25:513–520. [Google Scholar]
- Attum O, Lee YM, Roe JH, and Kingsbury BA, 2007. Upland-wetland Linkages: Relationship of Upland and Wetland Characteristics with Watersnake Abundance. Journal of Zoology 271:134–139. [Google Scholar]
- Auffret AG, Plue J, and Cousins SAO, 2015. The Spatial and Temporal Components of Functional Connectivity in Fragmented Landscapes. Ambio 44:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J and Richert A, 2005. Patterns of Habitat Use by Whooping Cranes During Migration: Summary from 1977–1999 Site Evaluation Data. Proceedings North American Crane Workshop 9:79–107. [Google Scholar]
- Babbitt KJ, Baber MJ, and Tarr TL, 2003. Patterns of Larval Amphibian Distribution Along a Wetland Hydroperiod Gradient. Canadian Journal of Zoology 81:1539–1552. [Google Scholar]
- Baber MJ, Childers DL, Babbitt KJ, and Anderson DH, 2002. Controls on Fish Distribution and Abundance in Temporary Wetlands. Canadian Journal of Fisheries and Aquatic Sciences 59:1441–1450. [Google Scholar]
- Baguette M, Blanchet S, Legrand D, Stevens VM, and Turlure C, 2013. Individual Dispersal, Landscape Connectivity and Ecological Networks. Biological Reviews 88:310–326. [DOI] [PubMed] [Google Scholar]
- Ballard B, Dale James J, Bingham R, Petrie M, and Wilson B, 2010. Coastal Pond Use by Redheads Wintering in the Laguna Madre, Texas. Wetlands 30:669–674. [Google Scholar]
- Baranyi C, Hein T, Holarek C, Keckeis S, and Schiemer F, 2002. Zooplankton Biomass and Community Structure in a Danube River Floodplain System: Effects of hydrology. Freshwater Biology 47:473–482. [Google Scholar]
- Barrat-Segretain MH, 1996. Strategies of Reproduction, Dispersion, and Competition in River Plants: A Review. Vegetatio 123:13–37. [Google Scholar]
- Bauer RT, 2013. Amphidromy in Shrimps: A Life Cycle Between Rivers and the Sea. Latin American Journal of Aquatic Research 41(4): 633–650. [Google Scholar]
- Baxter CV and Hauer FR, 2000. Geomorphology, Hyporheic Exchange, and Selection of Spawning Habitat by Bull Trout (Salvelinus confluentus). Canadian Journal of Fisheries and Aquatic Sciences 57:1470–1481. [Google Scholar]
- Baxter CV, Fausch KD, Murakami M, and Chapman PL, 2004. Fish Invasion Restructures Stream and Forest Food Webs by Interrupting Reciprocal Prey Subsidies. Ecology 85:2656–2663. [Google Scholar]
- Bergsten A and Zetterberg A, 2013. To Model the Landscape as a Network: A Practitioner’s Perspective. Landscape and Urban Planning 119:35–43. [Google Scholar]
- Besemer K, Singer G, Quince C, Bertuzzo E, Sloan W, and Battin TJ, 2013. Headwaters Are Critical Reservoirs of Microbial Diversity for Fluvial Networks. Proceedings of the Royal Society B 280:20131760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestgen AC, Chaubey I, Ward GM, and Dunn L, 2000. Flood Pulse Dynamics of an Unregulated River Floodplain in the Southeastern U.S. Coastal Plain. Ecology 81:2730–2741. [Google Scholar]
- Bodamer BL and Bossenbroek JM, 2008. Wetlands as Barriers: Effects of Vegetated Waterways on Downstream Dispersal of Zebra Mussels. Freshwater Biology 53:2051–2060. [Google Scholar]
- Bodie JR, 2001. Stream and Riparian Management for Freshwater Turtles. Journal of Environmental Management 62:443–455. [DOI] [PubMed] [Google Scholar]
- Bodie JR and Semlitsch RD, 2000. Spatial and Temporal Use of Floodplain Habitats by Lentic and Lotic Species of Aquatic Turtles. Oecologia 122:138–146. [DOI] [PubMed] [Google Scholar]
- Bogan MT and Boersma KS, 2012. Aerial Dispersal of Aquatic Invertebrates Along and Away from Arid-land Streams. Freshwater Science 31:1131–1144. [Google Scholar]
- Bogan MT, Boersma KS, and Lytle DA, 2013. Flow Intermittency Alters Longitudinal Patterns of Invertebrate Diversity and Assemblage Composition in an Arid-land Stream Network. Freshwater Biology 58:1016–1028. [Google Scholar]
- Bohonak AJ and Jenkins DG, 2003. Ecological and Evolutionary Significance of Dispersal by Freshwater Invertebrates. Ecology Letters 6:783–796. [Google Scholar]
- Bonte D, Van Dyck H, Bullock JM, Coulon A, Delgado M, Gibbs M, Lehouck V, Matthysen E, Mustin K, Saastamoinen M, and Schtickzelle N, 2012. Costs of Dispersal. Biological Reviews 87:290–312. [DOI] [PubMed] [Google Scholar]
- Bornette G, Amoros C, and Lamouroux NL, 1998. Aquatic Plant Diversity in Riverine Wetlands: The Role of Connectivity. Freshwater Biology 39:267–283. [Google Scholar]
- Boschilia SM, Oliveira EF, and Thomaz SM, 2008. Do Aquatic Macrophytes Co-occur Randomly? An Analysis of Null Models in a Tropical Floodplain. Oecologia 156:203–214. [DOI] [PubMed] [Google Scholar]
- Boss SM and Richardson JS, 2002. Effects of Food and Cover on the Growth, Survival, and Movement of Cutthroat Trout (Oncorhynchus clarki) in Coastal Streams. Canadian Journal of Fisheries and Aquatic Sciences 59:1044–1053. [Google Scholar]
- Boudell JA and Stromberg JC, 2008. Flood Pulsing and Metacommunity Dynamics in a Desert Riparian Ecosystem. Journal of Vegetation Science 19:373–380. [Google Scholar]
- Bowler DE and Benton TG, 2009. Variation in Dispersal Mortality and Dispersal Propensity Among Individuals: The Effects of Age, Sex and Resource Availability. Journal of Animal Ecology 78:1234–41. [DOI] [PubMed] [Google Scholar]
- Bradford MJ, Grout JA, and Moodie S, 2001. Ecology of Juvenile Chinook Salmon in a Small Non-natal Stream of the Yukon River Drainage and the Role of Ice Conditions on Their Distribution and Survival. Canadian Journal of Zoology 79:2043–2054. [Google Scholar]
- Bramblett RG, Bryant MD, Wright BE, and White RG, 2002. Seasonal Use of Small Tributary and Main-stem Habitats by Juvenile Steelhead, Coho Salmon, and Dolly Varden in a Southeastern Alaska Drainage Basin. Transactions of the American Fisheries Society 131:498–506. [Google Scholar]
- Brittain JE and Eikeland TJ, 1988. Invertebrate Drift: A Review. Hydrobiologia 166:77–93. [Google Scholar]
- Brooks RT, 2000. Annual and Seasonal Variation and the Effects of Hydroperiod on Benthic Macroinvertebrates of Seasonal Forest (“Vernal”) Ponds in Central Massachusetts, USA. Wetlands 20:707–715. [Google Scholar]
- Brooks RP and Serfass TL, 2013. Wetland-riparian Wildlife of the Mid-Atlantic Region: An Overview Pages 259–268 in Mid-Atlantic Freshwater Wetlands: Advances in Wetlands Science, Management, Policy, and Practice. Brooks RP and Wardrop DH, editors. Springer, New York, NY. [Google Scholar]
- Brown TG, and Hartman GF, 1988. Contribution of Seasonally Flooded Lands and Minor Tributaries to the Production of Coho Salmon in Carnation Creek, British Columbia. Transactions of the American Fisheries Society 117:546–551. [Google Scholar]
- Brown JH and Kodric-Brown A, 1977. Turnover Rates in Insular Biogeography: Effect of Immigration on Extinction. Ecology 58:445–449. [Google Scholar]
- Bunn SE and Arthington AH, 2002. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environmental Management 30:492–507. [DOI] [PubMed] [Google Scholar]
- Bunn SE, Davies PM, and Winning M, 2003. Sources of Organic Carbon Supporting the Food Web of an Arid Zone Floodplain River. Freshwater Biology 48:619–635. [Google Scholar]
- Burgess OT, Pine III WE, and Walsh SJ, 2013. Importance of Floodplain Connectivity to Fish Populations in the Apalachicola River, Florida. River Research and Applications 29:718–733. [Google Scholar]
- Calabrese JM and Fagan WF, 2004. A Comparison-Shopper’s Guide to Connectivity Metrics. Frontiers in Ecology and the Environment 2:529–536. [Google Scholar]
- Cañedo-Argüelles M, Boersma KS, Bogan MT, Olden JD, Phillipsen I, Schriever TA, and Lytle DA, 2015. Dispersal Strength Determines Meta-community Structure in a Dendritic Riverine Network. Journal of Biogeography 42:778–790. [Google Scholar]
- Capers RS, Selsky R, and Bugbee GJ, 2010. The Relative Importance of Local Conditions and Regional Processes in Structuring Aquatic Plant Communities. Freshwater Biology 55:952–966. [Google Scholar]
- Carpenter SR, Stanley EH, and Vander Zanden MJ, 2011. State of the World’s Freshwater Ecosystems: Physical, Chemical, and Biological Changes. Annual Review of Environment and Resources 36:75–99. [Google Scholar]
- Chaput-Bardy A, Fleurant C, Lemaire C, and Secondi J, 2009. Modelling the Effect of In-stream and Overland Dispersal on Gene Flow in River Networks. Ecological Modelling 220:3589–3598. [Google Scholar]
- Chester ET and Robson BJ, 2011. Drought Refuges, Spatial Scale and Recolonization by Invertebrates in Non-perennial Streams. Freshwater Biology 56:2094–2104. [Google Scholar]
- Chick JH, Cosgriff RJ, and Gittinger LS, 2003. Fish as Potential Dispersal Agents for Floodplain Plants: First Evidence in North America. Canadian Journal of Fisheries and Aquatic Sciences 60:1437–1439. [Google Scholar]
- Clark WR, 2000. Ecology of Muskrats in Prairie Wetlands Pages 287–313 in Prairie Wetland Ecology: The Contribution of the Marsh Ecology Research Program. Murkin HR, van der Valk AG, and Clark WR, editors. Iowa State University Press, Ames, IA. [Google Scholar]
- Cook N, Rahel FJ, and Hubert WA, 2010. Persistence of Colorado River Cutthroat Trout Populations in Isolated Headwater Streams of Wyoming. Transactions of the American Fisheries Society 139:1500–1510. [Google Scholar]
- Copp GH, 1989. The Habitat Diversity and Fish Reproductive Function of Floodplain Ecosystems. Environmental Biology of Fishes 26:1–27. [Google Scholar]
- Corti R and Datry T, 2012. Invertebrates and Sestonic Matter in an Advancing Wetted Front Travelling Down a Dry River Bed (Albarine, France). Freshwater Science 31:1187–1201. [Google Scholar]
- Crook DA and Gillanders BM, 2006. Use of Otolith Chemical Signatures to Estimate Carp Recruitment Sources in the Mid-Murray River, Australia. River Research and Applications 22:871–879. [Google Scholar]
- Crook DA, Lowe WH, Allendorf FW, Erös T, Finn DS, Gillanders BM, Hadwen WL, Harrod C, Hermoso V, Jennings S, Kilada RW, Nagelkerken I, Hansen MM, Page TJ, Riginos C, Fry B, and Hughes JM, 2015. Human Effects on Ecological Connectivity in Aquatic Ecosystems: Integrating Scientific Approaches to Support Management and Mitigation. Science of the Total Environment 534:52–64. [DOI] [PubMed] [Google Scholar]
- Curry RA, Brady C, Noakes DLG, and Danzmann RG, 1997. Use of Small Streams by Young Brook Trout Spawned in a Lake. Transactions of the American Fisheries Society 126:77–83. [Google Scholar]
- Datry T, Pella H, Leigh C, Bonada N, and Hugueny B, 2016. A Landscape Approach to Advance Intermittent River Ecology. Freshwater Biology 61:1200–1213. [Google Scholar]
- Dehling DM, Hof C, Brändle M, and Brandl R, 2010. Habitat Availability Does Not Explain the Species Richness Patterns of European Lentic and Lotic Freshwater Animals. Journal of Biogeography 37(10):1919–26. [Google Scholar]
- Deiner K, Garza JC, Coey R, and Girman DJ, 2007. Population Structure and Genetic Diversity of Trout (Oncorhynchus mykiss) Above and Below Natural and Man-made Barriers in the Russian River, California. Conservation Genetics 8:437–454. [Google Scholar]
- Dias MS, Oberdorff T, Hugueny B, Leprieur F, Jézéquel C, Cornu J-F, Brosse S, Grenouillet G, and Tedesco PA, 2014. Global Imprint of Historical Connectivity on Freshwater Fish Biodiversity. Ecology Letters 17:1130–1140. [DOI] [PubMed] [Google Scholar]
- Dodds WK, Gido K, Whiles MR, Fritz KM, and Matthews WJ, 2004. Life on the Edge: The Ecology of Great Plains Prairie Streams. BioScience 54:205–216. [Google Scholar]
- Dong X, Li B, He F, Gu Y, Sun M, Zhang H, Tan L, Xiao W, Liu S, and Cai Q, 2016. Flow Directionality, Mountain Barriers and Functional Traits Determine Diatom Community Structuring of High Mountain Streams. Scientific Reports 6:24711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duputié A and Massol F, 2013. An Empiricist’s Guide to Theoretical Predictions on the Evolution of Dispersal. Interface Focus 3:20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham BW and Wilde GR, 2006. Influence of Stream Discharge on Reproductive Success of a Prairie Stream Fish Assemblage. Transactions of the American Fisheries Society 135:1644–1653. [Google Scholar]
- Eberle LC and Stanford JA, 2010. Importance and Seasonal Availability of Terrestrial Invertebrates as Prey for Juvenile Salmonids in Floodplain Spring Brooks of the Kol River (Kamchatka, Russian Federation). River Research and Applications 26:682–694. [Google Scholar]
- Ebersole JL, Wigington PJ, Baker JP, Cairns MA, Church MR, Hansen BP, Miller BA, LaVigne HR, Compton BW, and Leibowitz SG, 2006. Juvenile Coho Salmon Growth and Survival Across Stream Network Seasonal Habitats. Transactions of the American Fisheries Society 135:1681–1697. [Google Scholar]
- Ebersole JL, Colvin ME, Wigington PJ, Leibowitz SG, Baker JP, Church MR, Compton JE, Miller BA, Cairns MA, Hansen BP, and LaVigne HR, 2009. Modeling Stream Network-scale Variation in Coho Salmon Overwinter Survival and Smolt Size. Transactions of the American Fisheries Society 138:564–580. [Google Scholar]
- Elliott JM, 1971. Distances Travelled by Drifting Invertebrates in a Lake District Stream. Oecologia 6:350–379. [DOI] [PubMed] [Google Scholar]
- Erman DC and Hawthorne VM, 1976. The Quantitative Importance of an Intermittent Stream in the Spawning of Rainbow Trout. Transactions of the American Fisheries Society 105:675–681. [Google Scholar]
- Fagan WF, 2002. Connectivity, Fragmentation, and Extinction Risk in Dendritic Metapopulations. Ecology 83:3243–3249. [Google Scholar]
- Falke JA and Gido KB, 2006. Effects of Reservoir Connectivity on Stream Fish Assemblages in the Great Plains. Canadian Journal of Fisheries and Aquatic Sciences 63:480–493. [Google Scholar]
- Falke JA, Bestgen KR, and Fausch KD, 2010. Streamflow Reductions and Habitat Drying Affect Growth, Survival, and Recruitment of Brassy Minnow Across a Great Plains Landscape. Transactions of the American Fisheries Society 139:1566–1583. [Google Scholar]
- Farmer AH and Parent AH, 1997. Effects of the Landscape on Shorebird Movements at Spring Migration Stopovers. The Condor 99:698–707. [Google Scholar]
- Fausch KD and Bestgen KR, 1997. Ecology of Fishes Indigenous to the Central and Southwestern Great Plains Pages 131–166 in Ecology and Conservation of Great Plains Vertebrates. Knopf FL and Samson FB, editors. Springer-Verlag, New York, NY. [Google Scholar]
- Fausch KD, Rieman BE, Dunham JB, Young MK, and Peterson DP, 2009. Invasion Versus Isolation: Trade-offs in Managing Native Salmonids with Barriers to Upstream Movement. Conservation Biology 23:859–870. [DOI] [PubMed] [Google Scholar]
- Feminella JW, 1996. Comparison of Benthic Macroinvertebrate Assemblages in Small Streams Along a Gradient of Flow Permanence. Journal of the North American Benthological Society 15:651–669. [Google Scholar]
- Feminella JW and Hawkins CP, 1995. Interactions Between Stream Herbivores and Periphyton: A Quantitative Analysis of Past Experiments. Journal of the North American Benthological Society 14:465–509. [Google Scholar]
- Fer T and Hroudova Z, 2008. Detecting Dispersal of Nuphar lutea in River Corridors Using Microsatellite Markers. Freshwater Biology 53:1409–1422. [Google Scholar]
- Figuerola J and Green AJ, 2002. Dispersal of Aquatic Organisms by Waterbirds: A Review of Past Research and Priorities for Future Studies. Freshwater Biology 47:483–494. [Google Scholar]
- Figuerola J, Green AJ, and Michot TC, 2005. Invertebrate Eggs Can Fly: Evidence of Waterfowl-mediated Gene Flow in Aquatic Invertebrates. The American Naturalist 165:274–280. [DOI] [PubMed] [Google Scholar]
- Fletcher RJ, Revell A, Reichert BE, Kitchens WM, Dixon JD, and Austin JD, 2013. Network Modularity Reveals Critical Scales for Connectivity in Ecology and Evolution. Nature Communications 4:2572. [DOI] [PubMed] [Google Scholar]
- Folk MJ and Tacha TC, 1990. Sandhill Crane Roost Site Characteristics in the North Platte River Valley. The Journal of Wildlife Management 54:480–486. [Google Scholar]
- Franssen NR, Gido KB, Guy CS, Tripe JA, Shrank SJ, Strakosh TR, Bertrand KN, Franssen CM, Pitts KL, and Paukert CP, 2006. Effects of Floods on Fish Assemblages in an Intermittent Prairie Stream. Freshwater Biology 51:2072–2086. [Google Scholar]
- Fraser DJ, Weir LK, Bernatchez L, Hansen MM, and Taylor EB, 2011. Extent and Scale of Local Adaptation in Salmonid Fishes: Review and Meta-analysis. Heredity 106:404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MC, Pringle CM, and Jackson CR, 2007. Hydrologic Connectivity and the Contribution of Stream Headwaters to Ecological Integrity at Regional Scales. Journal of the American Water Resources Association 43:5–14. [Google Scholar]
- Frisch D and Threlkeld ST, 2005. Flood-mediated Dispersal Versus Hatching: Early Recolonisation Strategies of Copepods in Floodplain Ponds. Freshwater Biology 50:323–330. [Google Scholar]
- Frisch D, Green AJ, and Figuerola J, 2007. High Dispersal Capacity of a Broad Spectrum of Aquatic Invertebrates Via Waterbirds. Aquatic Sciences 69:568–574. [Google Scholar]
- Fritz KM and Dodds WK, 2002. Macroinvertebrate Assemblage Structure Across a Tallgrass Prairie Stream Landscape. Archiv für Hydrobiologie 154:79–102. [Google Scholar]
- Fritz KM and Dodds WK, 2004. Resistance and Resilience of Macroinvertebrate Assemblages to Drying and Flood in a Tallgrass Prairie Stream System. Hydrobiologia 527:99–112. [Google Scholar]
- Fritz KM, Tripe JA, and Guy CS, 2002. Recovery of Three Fish Species to Flood and Seasonal Drying in a Tallgrass Prairie Stream. Transactions of the Kansas Academy of Science 105:209–219. [Google Scholar]
- Fritz KM, Schofield KA, Alexander LC, McManus MG, Golden HE, Lane CR, Kepner WG, LeDuc SD, DeMeester JE, and Pollard AI, In review (this issue). Physical and Chemical Connectivity of Streams and Riparian Wetlands to Downstream Waters: A Synthesis. Journal of the American Water Resources Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galat DL, Fredrickson LH, Humburg DD, Bataille KJ, Bodie JR, Dohrenwend J, Gelwicks GT, Havel JE, Helmers DL, Hooker JB, Jones JR, Knowlton MF, Kubisiak J, Mazourek J, McColpin AC, Renken RB, and Semlitsch RD, 1998. Flooding to Restore Connectivity of Regulated, Large-river Wetlands. BioScience 48:721–733. [Google Scholar]
- Galatowitsch SM and van der Valk AG, 1996. The Vegetation of Restored and Natural Prairie Wetlands. Ecological Applications 6:102–112. [Google Scholar]
- Galpern P, Manseau M, and Fall A, 2011. Patch-based Graphs of Landscape Connectivity: A Guide to Construction, Analysis and Application for Conservation. Biological Conservation 144:44–55. [Google Scholar]
- Garmyn A, Van Rooij P, Pasmans F, Hellebuyck T, Van Den Broeck W, Haesebrouch F, and Martel A, 2012. Waterfowl: Potential Environmental Reservoirs of the Chytrid Fungus Batrachochytrium dendrobatidis. PLoS ONE 7(4): e35038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons JW, 2003. Terrestrial Habitat: A Vital Component for Herpetofauna of Isolated Wetlands. Wetlands 23:630–635. [Google Scholar]
- Gibbons JW, Winne CT, Scott DE, Willson JD, Glaudas X, Andrews KM, Todd BD, Fedewa LA, Wilkinson L, Tsaliagos RN, Harper SJ, Greene JL, Tuberville TD, Metts BS, Dorcast ME, Nestor JP, Young CA, Akre T, Reed RN, Buhlmann KA, Norman J, Croshaw DA, Hagen C, and Rothermel BB, 2006. Remarkable Amphibian Biomass and Abundance in an Isolated Wetland: Implications for Wetland Conservation. Conservation Biology 20:1457–1465. [DOI] [PubMed] [Google Scholar]
- Gomez-Uchida D, Knight TW, and Ruzzante DE, 2009. Interaction of Landscape and Life History Attributes on Genetic Diversity, Neutral Divergence and Gene Flow in a Pristine Community of Salmonids. Molecular Ecology 18:4854–4869. [DOI] [PubMed] [Google Scholar]
- Goodrich DC, Kepner WG, Levick LR, and Wigington PJ Jr., In review (this issue). Southwestern intermittent and ephemeral stream connectivity. Journal of the American Water Resources Association. [Google Scholar]
- Gorman OT, 1986. Assemblage Organization of Stream Fishes: The Effect of Rivers on Adventitious Streams. The American Naturalist 128:611–616. [Google Scholar]
- Gounand I, Harvey E, Little CJ, and Altermatt F, 2017. Meta-Ecosystems 2.0: Rooting the Theory into the Field. Trends in Ecology and Evolution 10.1016/j.tree.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Graham SE, Storey R, and Smith B, 2017. Dispersal Distances of Aquatic Insects: Upstream Crawling by Benthic EPT Larvae and Flight of Adult Trichoptera Along Valley Floors. New Zealand Journal of Marine and Freshwater Research 51:146–164. [Google Scholar]
- Granado DC and Henry R, 2014. Phytoplankton Community Response to Hydrologic Variations in Oxbow Lakes with Different Levels of Connection to a Tropical River. Hydrobiologia 721:223–238. [Google Scholar]
- Grant EHC, 2011. Structural Complexity, Movement Bias, and Metapopulation Extinction Risk in Dendritic Ecological Networks. Journal of the North American Benthological Society 30:252–258. [Google Scholar]
- Grant EHC, Lowe WH, and Fagan WF, 2007. Living in the Branches: Population Dynamics and Ecological Processes in Dendritic Networks. Ecology Letters 10:165–175. [DOI] [PubMed] [Google Scholar]
- Grant EHC, Nichols JD, Lowe WH, and Fagan WF, 2010. Use of Multiple Dispersal Pathways Facilitates Amphibian Persistence in Stream Networks. Proceedings of the National Academy of Sciences of the United States of America 107:6936–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greathouse EA, Pringle CM, McDowell WH, and Holmquist JG, 2006. Indirect Upstream Effects of Dams: Consequences of Migratory Consumer Extirpation in Puerto Rico. Ecological Applications 16:339–352. [DOI] [PubMed] [Google Scholar]
- Green DM, 2005. Bufo americanus, American Toad Pages 692–704 in Amphibian Declines: The Conservation Status of United States Species. Lannoo M, editor. University of California Press, Berkeley, CA. [Google Scholar]
- Grenouillet G, Pont D, and Herisse C, 2004. Within-basin Fish Assemblage Structure: The Relative Influence of Habitat Versus Stream Spatial Position on Local Species Richness. Canadian Journal of Fisheries and Aquatic Sciences 61:93–102. [Google Scholar]
- Gurnell AM, 2007. Analogies Between Mineral Sediment and Vegetative Particle Dynamics in Fluvial Systems. Geomorphology 89:9–22. [Google Scholar]
- Gurnell A, Thompson K, Goodson J, and Moggridge H, 2008. Propagule Deposition Along River Margins: Linking Hydrology and Ecology. Journal of Ecology 96:553–565. [Google Scholar]
- Haig SM, Mehlman DW, and Oring LW, 1998. Avian Movements and Wetland Connectivity in Landscape Conservation. Conservation Biology 12:749–758. [Google Scholar]
- Hairston NG Jr., 1996. Zooplankton Egg Banks as Biotic Reservoirs in Changing Environments. Limnology and Oceanography 41:1087–1092. DOI: 10.4319/lo.1996.41.5.1087 [DOI] [Google Scholar]
- Hall RO, Likens GE, and Malcom HM, 2001. Trophic Basis of Invertebrate Production in 2 Streams at the Hubbard Brook Experimental Forest. Journal of the North American Benthological Society 20:432–447. [Google Scholar]
- Hall CJ, Jordaan A, and Frisk MG, 2011. The Historic Influence of Dams on Diadromous Fish Habitat with a Focus on River Herring and Hydrologic Longitudinal Connectivity. Landscape Ecology 26:95–107. [Google Scholar]
- Hanfling B and Weetman D, 2006. Concordant Genetic Estimators of Migration Reveal Anthropogenically Enhanced Source-sink Population Structure in the River Sculpin, Cottus gobio. Genetics 173:1487–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski I, 1999. Metapopulation Ecology. Oxford University Press., Oxford UK. [Google Scholar]
- Harrell RC, Davis BJ, and Dorris TC, 1967. Stream Order and Species Diversity of Fishes in an Intermittent Oklahoma Stream. American Midland Naturalist 78:428–436. [Google Scholar]
- Hassan MA, Gottesfeld AS, Montgomery DR, Tunnicliffe JF, Clarke GKC, Wynn G, Jones-Cox H, Poirier R, MacIsaac E, Herunter H, and Macdonald SJ, 2008. Salmon-driven Bed Load Transport and Bed Morphology in Mountain Streams. Geophysical Research Letters 35:L04405. [Google Scholar]
- Haukos DA, Miller MR, Orthmeyer DL, Takekawa JY, Fleskes JP, Casazza ML, Perry WM, and Moon JA, 2006. Spring Migration of Northern Pintails from Texas and New Mexico, USA. Waterbirds 29:127–136. [Google Scholar]
- Hecnar SJ and McLoskey RT, 1996. Regional Dynamics and the Status of Amphibians. Ecology 77:2091–2097. [Google Scholar]
- Hein T, Baranyi C, Herndl GJ, Wanek W, and Schiemer F, 2003. Allochthonous and Autochthonous Particulate Organic Matter in Floodplains of the River Danube: The Importance of Hydrological Connectivity. Freshwater Biology 48:220–232. [Google Scholar]
- Henning JA, Gresswell RE, and Fleming IA, 2007. Use of Seasonal Freshwater Wetlands by Fishes in a Temperate River Floodplain. Journal of Fish Biology 71:476–492. [Google Scholar]
- Hershey AE, Pastor J, Peterson BJ, and Kling GW, 1993. Stable Isotopes Resolve the Drift Paradox for Baetis Mayflies in an Arctic River. Ecology 74:2315–2325. [Google Scholar]
- Herwig BR, Zimmer KD, Hanson MA, Konsti ML, Younk JA, Wright RW, Vaughn SR, and Haustein MD, 2010. Factors Influencing Fish Distributions in Shallow Lakes in Prairie and Prairie-parkland Regions of Minnesota, USA. Wetlands 30:609–619. [Google Scholar]
- Hess GR, 1996. Linking Extinction to Connectivity and Habitat Destruction in Metapopulation Models. The American Naturalist 148:226–236. [Google Scholar]
- Hitt NP and Angermeier PL, 2008. Evidence for Fish Dispersal from Spatial Analysis of Stream Network Topology. Journal of the North American Benthological Society 27:304–320. [Google Scholar]
- Hitt NP, Frissell CA, Muhlfeld CC, and Allendorf FW, 2003. Spread of Hybridization Between Native Westslope Cutthroat Trout, Oncorhynchus clarki lewisi, and Nonnative Rainbow Trout, Oncorhynchus mykiss. Canadian Journal of Fisheries and Aquatic Sciences 60:1440–1451. [Google Scholar]
- Hitt NP, Eyler S, and Wofford JEB, 2012. Dam Removal Increases American Eel Abundance in Distant Headwater Streams. Transactions of the American Fisheries Society 141:1171–1179. [Google Scholar]
- Hjalmarsson AE, Bergsten J, Monaghan MT, 2015. Dispersal Is Linked to Habitat Use in 59 Species of Water Beetles (Coleoptera: Adephaga) on Madagascar. Ecography 38(7):732–9. [Google Scholar]
- Hof C, Brändle M, Dehling DM, Munguía M, Brandl R, Araújo MB, and Rahbek C, 2012. Habitat Stability Affects Dispersal and the Ability to Track Climate Change. Biology Letters February 29:rsbl20120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohausová E, Lavoy RJ, and Allen MS, 2010. Fish Dispersal in a Seasonal Wetland: Influence of Anthropogenic Structures. Marine and Freshwater Research 61:682–694. [Google Scholar]
- Honnay O, Verhaeghe W, and Hermy M, 2001. Plant Community Assembly Along Dendritic Networks of Small Forest Streams. Ecology 82:1691–1702. [Google Scholar]
- Horn MH, 1997. Evidence for Dispersal of Fig Seeds by the Fruit-eating Characid Fish Brycon guatemalensis Regan in a Costa Rican Tropical Rain Forest. Oecologia 109:259–264. [DOI] [PubMed] [Google Scholar]
- Horwitz RJ, 1978. Temporal Variability Patterns and the Distributional Patterns of Stream Fishes. Ecological Monographs 48:307–321. [Google Scholar]
- Hudson PL, Lenat DR, Caldwell BA, and Smith D, 1990. Chironomidae of the Southeastern United States: A Checklist of Species and Notes on Biology, Distribution, and Habitat Fish and Wildlife Research 7, U.S. Department of the Interior, U.S. Fish and Wildlife Service, Washington, DC. [Google Scholar]
- Hudy M, Coombs JA, Nislow KH, and Letcher BH, 2010. Dispersal and Within-stream Spatial Population Structure of Brook Trout Revealed by Pedigree Reconstruction Analysis. Transactions of the American Fisheries Society 139:1276–1287. [Google Scholar]
- Hughes JM, Schmidt DJ, and Finn DS, 2009. Genes in Streams: Using DNA to Understand the Movement of Freshwater Fauna and Their Riverine Habitat. BioScience 59:573–583. [Google Scholar]
- Hulsmans A, Moreau K, De Meester L, Riddoch BJ, and Brendonck L, 2007. Direct and Indirect Measures of Dispersal in the Fairy Shrimp Branchipodopsis wolfi Indicate a Small-scale Isolation-by-distance Pattern. Limnology and Oceanography 52:676–684. [Google Scholar]
- Humphries S and Ruxton GD, 2002. Is There Really a Drift Paradox? Journal of Animal Ecology 71:151–154. [Google Scholar]
- Huryn AD and Denny MW, 1997. A Biomechanical Hypothesis Explaining Upstream Movements by the Freshwater Snail Elimia. Functional Ecology 11:472–483. [Google Scholar]
- Huryn AD and Gibbs KE, 1999. Riparian Sedge Meadows in Maine. A Macroinvertebrate Community Structured by River-floodplain Interaction Pages 363–382 in Invertebrates in Freshwater Wetlands of North America: Ecology and Management. Batzer DP, Rader RB, and Wissinger SA, editors. John Wiley & Sons, New York, NY. [Google Scholar]
- Huryn AD, Slavik KA, Lowe RL, Parker SM, Anderson DS, and Peterson BJ, 2005. Landscape Heterogeneity and the Biodiversity of Arctic Stream Communities: A Habitat Template Analysis. Canadian Journal of Fisheries and Aquatic Sciences 62:1905–1919. [Google Scholar]
- Ilg C, Dziock F, Foeckler F, Follner K, Gerisch M, Glaeser J, Rink A, Schanowski A, Scholz M, Deichner O, and Henle K, 2008. Long-term Reactions of Plants and Macroinvertebrates to Extreme Floods in Floodplain Grasslands. Ecology 89:2392–2398. [DOI] [PubMed] [Google Scholar]
- Ishiyama N, Koizumi I, Yuta T, and Nakamura F, 2015. Differential Effects of Spatial Network Structure and Scale on Population Size and Genetic Diversity of the Ninespine Stickleback in a Remnant Wetland System. Freshwater Biology 60:733–744. [Google Scholar]
- Ivey CT and Richards JH, 2001. Genetic Diversity of Everglades Sawgrass, Cladium jamaicense (Cyperaceae). International Journal of Plant Sciences 162:817–825. [Google Scholar]
- Jansson R, Zinko U, Merritt DM, and Nilsson C, 2005. Hydrochory Increases Riparian Plant Species Richness: A Comparison Between a Free-flowing and a Regulated River. Journal of Ecology 93:1094–1103. [Google Scholar]
- Jeffres CA, Opperman JJ, and Moyle PB, 2008. Ephemeral Floodplain Habitats Provide Best Growth Conditions for Juvenile Chinook Salmon in a California River. Environmental Biology of Fishes 83:449–458. [Google Scholar]
- Jeltsch F, Bonte D, Pe’er G, Reineking B, Leimgruber P, Balkenhol N, Schröder B, Buchmann CM, Mueller T, Blaum N, Zurell D, Böhning-Gaese K, Wiegand T, Eccard JA, Hofer H, Reeg J, Eggers U, and Bauer S, 2013. Integrating Movement Ecology with Biodiversity Research – Exploring New Avenues To Address Spatiotemporal Biodiversity Dynamics. Movement Ecology 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins KM and Boulton AJ, 2003. Connectivity in a Dryland River: Short-term Aquatic Microinvertebrate Recruitment Following Floodplain Inundation. Ecology 84:2708–2723. [Google Scholar]
- Jude DJ and Pappas J, 1992. Fish Utilization of Great Lakes Coastal Wetlands. Journal of Great Lakes Research 18:651–672. [Google Scholar]
- Julian JT, Rocco G, Turner MM, and Brooks RP, 2013. Assessing Wetland-riparian Amphibian and Reptile Communities of the Mid-Atlantic Region Pages 313–337 in Mid-Atlantic Freshwater Wetlands: Advances in Wetlands Science, Management, Policy, and Practice. Brooks RP and Wardrop DH, editors. Springer, New York, NY. [Google Scholar]
- Junk WJ, Bayley PB, and Sparks RE, 1989. The Flood Pulse Concept in River-Floodplain Systems Pages 110–127 in Proceedings of the International Large River Symposium, Canadian Special Publication of Fisheries and Aquatic Sciences 106. Dodge DP, editor, Ottawa, Canada. [Google Scholar]
- Kanno Y, Letcher BH, Coombs JA, Nislow KH, and Whiteley AR, 2014. Linking Movement and Reproductive History of Brook Trout To Assess Habitat Connectivity in a Heterogeneous Stream Network. Freshwater Biology 59:142–154. [Google Scholar]
- Karr JR, 1993. Defining and Assessing Ecological Integrity: Beyond Water Quality. Environmental Toxicology and Chemistry 2:1521–31. [Google Scholar]
- Karr JR, 1995. Protecting Aquatic Ecosystems: Clean Water Is Not Enough Pages 7–13 in Biological Assessment and Criteria: Tools for Water Resource Planning and Decision Making. Davis WS and Simon TP, editors. Lewis Publishers, Boca Raton, FL. [Google Scholar]
- Karr JR and Dudley DR, 1981. Ecological Perspective on Water Quality Goals. Environmental Management 5:55–68. [Google Scholar]
- Kawaguchi Y and Nakano S, 2001. Contribution of Terrestrial Invertebrates to the Annual Resource Budget for Salmonids in Forest and Grassland Reaches of a Headwater Stream. Freshwater Biology 46:303–316. [Google Scholar]
- Keckeis S, Baranyi C, Hein T, Holarek C, Riedler P, and Schiemer F, 2003. The Significance of Zooplankton Grazing in a Floodplain System of the River Danube. Journal of Plankton Research 25:243–253. [Google Scholar]
- Keeley JE and Zedler PH, 1998. Characterization and Global Distribution of Vernal Pools Pages 1–14 in Ecology, Conservation, and Management of Vernal Pool Ecosystems—Proceedings from a 1996 Conference. Witham CW, Bauder ET, Belk D, Ferren WR Jr., and Ornduff R, editors. California Native Plant Society, Sacramento, CA. [Google Scholar]
- King JL, Simovich MA, and Brusca RC, 1996. Species Richness, Endemism and Ecology of Crustacean Assemblages in Northern California Vernal Pools. Hydrobiologia 328:85–116. [Google Scholar]
- King AJ, Humphries P, and Lake PS, 2003. Fish Recruitment on Floodplains: The Roles of Patterns of Flooding and Life History Characteristics. Canadian Journal of Fisheries and Aquatic Sciences 60:773–786. [Google Scholar]
- Kivelä M, Arenas A, Barthelemy M, Gleeson JP, Moreno Y, and Porter MA, 2014. Multilayer Networks. Journal of Complex Networks 2:203–271. [Google Scholar]
- Koizumi I, Kanazawa Y, and Tanaka Y, 2013. The Fishermen Were Right: Experimental Evidence for Tributary Refuge Hypothesis During Floods. Zoological Science 30:375–379. [DOI] [PubMed] [Google Scholar]
- Koizumi I, Tanaka Y, and Kanazawa Y, 2016. Mass Immigration of Juvenile Fishes into a Small, Once-dried Tributary Demonstrates the Importance of Remnant Tributaries as Wintering Habitats. Ichthyological Research. DOI: 10.1007/s10228-016-0564-1. [DOI] [Google Scholar]
- Krapu GL, Facey DE, Fritzell EK, and Johnson DH, 1984. Habitat Use by Migrant Sandhill Cranes in Nebraska. The Journal of Wildlife Management 48:407–417. [Google Scholar]
- Krümmel E, Macdonald R, Kimpe L, Gregory-Eaves I, Demers M, Smol J, Finney B, and Blais J, 2003. Aquatic Ecology: Delivery of Pollutants by Spawning Salmon. Nature 425:255–256. [DOI] [PubMed] [Google Scholar]
- Kubisch A, Holt RD, Poethke H-J, and Fronhofer EA, 2014. Where Am I and Why? Synthesizing Range Biology and the Eco-evolutionary Dynamics of Dispersal. Oikos 123:5–22. [Google Scholar]
- Labbe TR and Fausch KD, 2000. Dynamics of Intermittent Stream Habitat Regulate Persistence of a Threatened Fish at Multiple Scales. Ecological Applications 10:1774–1791. [Google Scholar]
- LaGrange TG and Dinsmore JJ, 1989. Habitat Use by Mallards During Spring Migration Through Central Iowa. Journal of Wildlife Management 53:1076–1081. [Google Scholar]
- Lamoureux VS and Madison DM, 1999. Overwintering Habitats of Radio-implanted Green Frogs, Rana clamitans. Journal of Herpetology 33:430–435. [Google Scholar]
- Lande R and Shannon S, 1996. The Role of Genetic Variation in Adaptation and Population Persistence in a Changing Environment. Evolution 50:434–437. [DOI] [PubMed] [Google Scholar]
- Lane CR, Leibowitz SG, Autrey BC, LeDuc SD, and Alexander LC, In review (this issue). Hydrological, Physical, and Chemical Functions and Connectivity of Non-floodplain Wetlands to Downstream Waters: A Review. Journal of the American Water Resources Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larned ST, Datry T, Arscott DB, and Tockner K, 2010. Emerging Concepts in Temporary- River Ecology. Freshwater Biology 55:717–738. [Google Scholar]
- Leeper DA and Taylor BE, 1998. Insect Emergence from a South Carolina (USA) Temporary Wetland Pond, with Emphasis on the Chironomidae (Diptera). Journal of the North American Benthological Society 17:54–72. [Google Scholar]
- Lehman PW, Sommer T, and Rivard L, 2008. The Influence of Floodplain Habitat on the Quantity and Quality of Riverine Phytoplankton Carbon Produced During the Flood Season in San Francisco Estuary. Aquatic Ecology 42:363–378. [Google Scholar]
- Leibowitz SG, Wigington PJ Jr., Schofield KA, Alexander LC, Vanderhoof MK, and Golden HE, In review (this issue). Connectivity of Streams and Wetlands to Downstream Waters: An Integrated Systems Framework. Journal of the American Water Resources Association. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SG, Mushet DM, and Newton WE, 2016. Intermittent Surface Water Connectivity: Fill and Spill vs. Fill and Merge Dynamics. Wetlands 36:323–342. [Google Scholar]
- Leone PB, Cerda J, Sala S, and Reid B, 2014. Mink (Neovison vison) as a Natural Vector in the Dispersal of the Diatom Didymosphenia geminata. Diatom Research 29:259–266. doi: 10.1080/0269249X.2014.890957. [DOI] [Google Scholar]
- Letcher BH, Nislow KH, Coombs JA, O’Donnell MJ, and Dubreuil TL, 2007. Population Response to Habitat Fragmentation in a Stream-dwelling Brook Trout Population. PLOS ONE 2:e1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes PM, Bozelli R, Bini LM, Santangelo JM, and Declerck SAJ, 2016. Contributions of Airborne Dispersal and Dormant Propagule Recruitment to the Assembly of Rotifer and Crustacean Zooplankton Communities in Temporary Ponds. Freshwater Biology 61:658–669. doi: 10.1111/fwb.12735. [DOI] [Google Scholar]
- Loreau M, Mouquet N, and Holt RD, 2003. Meta-ecosystems: A Theoretical Framework for a Spatial Ecosystem Ecology. Ecology Letters 6:673–679. doi: 10.1046/j.1461-0248.2003.00483.x. [DOI] [Google Scholar]
- Lundberg J and Moberg F, 2003. Mobile Link Organisms and Ecosystem Functioning: Implications for Ecosystem Resilience and Management. Ecosystems 6:87–98. doi: 10.1007/s10021-002-0150-4. [DOI] [Google Scholar]
- Luttrell GR, Echelle AA, Fisher WL, and Eisenhour DJ, 1999. Declining Status of Two Species of the Macrhybopsis aestivalis Complex (Teleostei: Cyprinidae) in the Arkansas River Basin and Related Effects of Reservoirs as Barriers to Dispersal. Copeia 1999:981–989. [Google Scholar]
- MacArthur RH and Wilson EO, 1967. The Theory of Island Biogeography. Princeton University Press, Princeton, NJ. [Google Scholar]
- Marten A, Braendle M, and Brandl R, 2006. Habitat Type Predicts Genetic Population Differentiation in Freshwater Invertebrates. Molecular Ecology 15(9):2643–2651. [DOI] [PubMed] [Google Scholar]
- Matheney MP and Rabeni CF, 1995. Patterns of Movement and Habitat Use by Northern Hogsuckers in an Ozark Stream. Transactions of the American Fisheries Society 124:886–897. [Google Scholar]
- Matthews WJ and Marsh-Matthews E, 2007. Extirpation of Red Shiner in Direct Tributaries of Lake Texoma (Oklahoma-Texas): A Cautionary Case History from a Fragmented River-reservoir System. Transactions of the American Fisheries Society 136:1041–1062. [Google Scholar]
- Matthews WJ and Robinson HW, 1998. Influence of Drainage Connectivity, Drainage Area and Regional Species Richness on Fishes of the Interior Highlands in Arkansas. American Midland Naturalist 139:1–19. [Google Scholar]
- May CL and Lee DC, 2004. The Relationship Among In-channel Sediment Storage, Pool Depth, and Summer Survival of Juvenile Salmonids in Oregon Coast Range Streams. North American Journal of Fisheries Management 24:761–774. [Google Scholar]
- Meyer A, Kaschek N, and Meyer EI, 2004. The Effect of Low Flow and Stream Drying on the Distribution and Relative Abundance of the Alien Amphipod, Echinogammarus berilloni (Catta, 1878) in a Karstic Stream System (Westphalia, Germany). Crustaceana 77:909–922. [Google Scholar]
- Meyer JL and Wallace JB, 2001. Lost Linkages and Lotic Ecology: Rediscovering Small Streams Pages 295–317 in Ecology: Achievement and Challenge. Press MC, Huntly NJ, and Levin S, editors. Blackwell Science, Oxford, UK. [Google Scholar]
- Meyer JL, Strayer DL, Wallace JB, Eggert SL, Helfman GS, and Leonard NE, 2007. The Contribution of Headwater Streams to Biodiversity in River Networks. Journal of the American Water Resources Association 43:86–103. [Google Scholar]
- Middleton B, 2000. Hydrochory, Seed Banks, and Regeneration Dynamics Along the Landscape Boundaries of a Forested Wetland. Plant Ecology 146:169–184. [Google Scholar]
- Milam JC and Melvin SM, 2001. Density, Habitat Use, Movements, and Conservation of Spotted Turtles (Clemmys guttuta) in Massachusetts. Journal of Herpetology 35:418–427. [Google Scholar]
- Miller AM and Golladay SW, 1996. Effects of Spates and Drying on Macroinvertebrate Assemblages of an Intermittent and Perennial Prairie Stream. Journal of the North American Benthological Society 15:670–689. [Google Scholar]
- Miyazono S, Aycock JN, Miranda LE, and Tietjen TE, 2010. Assemblage Patterns of Fish Functional Groups Relative to Habitat Connectivity and Conditions in Floodplain Lakes. Ecology of Freshwater Fish 19:578–585. [Google Scholar]
- Morita K and Yamamoto S, 2002. Effects of Habitat Fragmentation by Damming on the Persistence of Stream-dwelling Charr Populations. Conservation Biology 16:1318–1323. [Google Scholar]
- Morrissey MB and de Kerckhove DT, 2009. The Maintenance of Genetic Variation Due to Asymmetric Gene Flow in Dendritic Metapopulations. The American Naturalist 174:875–889. [DOI] [PubMed] [Google Scholar]
- Mueller MH and van der Valk AG, 2002. The Potential Role of Ducks in Wetland Seed Dispersal. Wetlands 22:170–178. [Google Scholar]
- Mullen LB, Woods HA, Schwartz MK, Sepulveda AJ, and Lowe WH, 2010. Scale-dependent Genetic Structure of the Idaho Giant Salamander (Dicamptodon aterrimus) in Stream Networks. Molecular Ecology 19:898–909. [DOI] [PubMed] [Google Scholar]
- Müller K, 1982. The Colonization Cycle of Insects. Oecologia 53:202–207. [DOI] [PubMed] [Google Scholar]
- Murkin HR and Caldwell PJ, 2000. Avian Use of Prairie Wetlands Pages 249–286 in Prairie Wetland Ecology: The Contribution of the Marsh Ecology Research Program. Murkin HR, van der Valk AG, and Clark WR, editors. Iowa State University Press, Ames, IA. [Google Scholar]
- Mushet DM, Euliss NH Jr., and Stockwell CA, 2013. Complex Spatial Dynamics Maintain Northern Leopard Frog Genetic Diversity in a Temporally Varying Landscape. Herpetological Conservation and Biology 8:163–175. [Google Scholar]
- Nagrodski A, Raby GD, Hasler CT, Taylor MK, and Cooke SJ, 2012. Fish Stranding in Freshwater Systems: Sources, Consequences, and Mitigation. Journal of Environmental Management 103:133–141. [DOI] [PubMed] [Google Scholar]
- Nakano S and Murakami M, 2001. Reciprocal Subsidies: Dynamic Interdependence Between Terrestrial and Aquatic Food Webs. Proceedings of the National Academy of Sciences 98:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DG and Griffin CR, 1994. Wetland Use by River Otters in Massachusetts. Journal of Wildlife Management 58:18–23. [Google Scholar]
- Nielsen JL, 1992. Microhabitat-specific Foraging Behavior, Diet, and Growth of Juvenile Coho Salmon. Transactions of the American Fisheries Society 121:617–634. [Google Scholar]
- Nilsson C, Brown RL, Jansson R, and Merritt DM, 2010. The Role of Hydrochory in Structuring Riparian and Wetland Vegetation. Biological Reviews 85:837–858. [DOI] [PubMed] [Google Scholar]
- Niño-García JP, Ruiz-González C, and del Giorgio PA, 2016. Interactions Between Hydrology and Water Chemistry Shape Bacterioplankton Biogeography Across Boreal Freshwater Networks. The ISME Journal 10:1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obolewski K, Glinska-Lewczuk K, and Kobus S, 2009. Effect of Hydrological Connectivity on the Molluscan Community Structure in Oxbow Lakes of the Lyna River. Oceanological and Hydrobiological Studies 38:75–88. [Google Scholar]
- Osborne LL and Wiley M, 1992. Influence of Tributary Position on the Structure of Warmwater Fish Communities. Canadian Journal of Fisheries and Aquatic Sciences 49:671–681. [Google Scholar]
- Pacific Flyway Council and Central Flyway Council, 2016. Pacific and Central Flyways Management Plan for the Rocky Mountain Population of Greater Sandhill Cranes Pacific Flyway Council and Central Flyway Council, care of the U.S. Fish and Wildlife Service’s Pacific Flyway Representative, Vancouver, Washington: 47pp. [Google Scholar]
- Paillex A, Castella E, and Carron G, 2007. Aquatic Macroinvertebrate Response Along a Gradient of Lateral Connectivity in River Floodplain Channels. Journal of the North American Benthologicl Society 26:779–796. doi: 10.1899/06-12.1. [DOI] [Google Scholar]
- Paillex A, Doledec S, Castella E, and Merigoux S, 2009. Large River Floodplain Restoration: Predicting Species Richness and Trait Responses to the Restoration of Hydrological Connectivity. Journal of Applied Ecology 46:250–258. [Google Scholar]
- Pease AA, Davis JJ, Edwards MS, and Turner TF, 2006. Habitat and Resource Use by Larval and Juvenile Fishes in an Arid-land River (Rio Grande, New Mexico). Freshwater Biology 51:475–486. [Google Scholar]
- Perkin JS and Gido KB, 2011. Stream Fragmentation Thresholds for a Reproductive Guild of Great Plains Fishes. Fisheries 36:371–383. [Google Scholar]
- Perkin JS and Gido KB, 2012. Fragmentation Alters Stream Fish Community Structure in Dendritic Ecological Networks. Ecological Applications 22:2176–2187. [DOI] [PubMed] [Google Scholar]
- Peterson JT and Rabeni CF, 1996. Natural Thermal Refugia for Temperate Warmwater Stream Fishes. North American Journal of Fisheries Management 16:738–746. [Google Scholar]
- Petranka JW and Holbrook CT, 2006. Wetland Restoration for Amphibians: Should Local Sites Be Designed To Support Metapopulations or Patchy Populations? Restoration Ecology 14:404–411. [Google Scholar]
- Pickett STA and Cadenasso ML, 1995. Landscape Ecology: Spatial Heterogeneity in Ecological Systems. Science 269:331–334. [DOI] [PubMed] [Google Scholar]
- Pilliod DS, Peterson CR, and Ritson PI, 2002. Seasonal Migration of Columbia Spotted Frogs (Rana luteiventris) Among Complementary Resources in a High Mountain Basin. Canadian Journal of Zoology 80:1849–1862. [Google Scholar]
- Pilosof S, Porter MA, Pascual M, and Kéfi S, 2017. The Multilayer Nature of Ecological Networks. Nature Ecology and Evolution 1, DOI: 10.1038/s41559-017-0101. [DOI] [PubMed] [Google Scholar]
- Pires AM, Cowx IG, and Coelho MM, 1999. Seasonal Changes in Fish Community Structure of Intermittent Streams in the Middle Reaches of the Guadiana Basin, Portugal. Journal of Fish Biology 54:235–249. [Google Scholar]
- Platania SP and Altenbach CS, 1998. Reproductive Strategies and Egg Types of Seven Rio Grande Basin Cyprinids. Copeia 1998:559–569. [Google Scholar]
- Pollux BJA, Ouborg NJ, Van Groenendael JM, and Klaassen M, 2007. Consequences of Intraspecific Seed-size Variation in Sparganium emersum for Dispersal by Fish. Functional Ecology 21:1084–1091. [Google Scholar]
- Pond GJ, Fritz KM, and Johnson BR, 2016. Macroinvertebrate and Organic Matter Export from Headwater Tributaries of a Central Appalachian Stream. Hydrobiologia 779:75–91. [Google Scholar]
- Pope SE, Fahrig L and Merriam NG, 2000. Landscape Complementation and Metapopulation Effects on Leopard Frog Populations. Ecology 81: 2498–2508. [Google Scholar]
- Popova ON, Haritonov AY, Anishchenko OV, and Gladyshev MI, 2016. Export of Biomass and Metals from Aquatic to Terrestrial Ecosystems via the Emergence of Dragonflies (Insecta: Odonata). Contemporary Problems of Ecology 9:458–473. [Google Scholar]
- Rasmussen JE and Belk MC, 2017. Individual Movement of Stream Fishes: Linking Ecological Drivers with Evolutionary Processes. Reviews in Fisheries Science and Aquaculture 25:70–83. [Google Scholar]
- Reckendorfer W, Baranyi C, Funk A, and Schiemer F, 2006. Floodplain Restoration by Reinforcing Hydrological Connectivity: Expected Effects on Aquatic Mollusc Communities. Journal of Applied Ecology 43:474–484. [Google Scholar]
- Reynolds DR, Reynolds AM, Chapman JW, 2014. Non-volant Modes of Migration in Terrestrial Arthropods. Animal Migration 2:8–28. [Google Scholar]
- Ribera I, 2008. Habitat Constraints and the Generation of Diversity in Freshwater Macroinvertebrates Pages 289–311 in Aquatic Insects: Challenges to Populations. Proceedings of the Royal Entomological Society’s 24th Symposium. Lancaster J and Briers RA, editors. CAB International Press, Oxfordshire UK. [Google Scholar]
- Ribera I and Vogler AP, 2000. Habitat Type as a Determinant of Species Range Sizes: The Example of Lotic-lentic Differences in Aquatic Coleoptera. Biological Journal of the Linnean Society 71:33–52. [Google Scholar]
- Richardson JS, Naiman RJ, Swanson FJ, and Hibbs DE, 2005. Riparian Communities Associated with Pacific Northwest Headwater Streams: Assemblages, Processes, and Uniqueness. Journal of the American Water Resources Association 41:935–947. [Google Scholar]
- Rine KM, Wipfli MS, Schoen ER, Nightengale TL, and Stricker CA, 2016. Trophic Pathways Supporting Juvenile Chinook and Coho Salmon in the Glacial Susitna River, Alaska: Patterns of Freshwater, Marine, and Terrestrial Food Resource Use Across a Seasonally Dynamic Habitat Mosaic. Canadian Journal of Fisheries and Aquatic Sciences 73:1626–1641. [Google Scholar]
- Robinson CT, Tockner K, and Ward JV, 2002. The Fauna of Dynamic Riverine Landscapes. Freshwater Biology 47:661–677. [Google Scholar]
- Rooney RC, Carli C, and Bayley S, 2013. River Connectivity Affects Submerged and Floating Aquatic Vegetation in Floodplain Wetlands. Wetlands 33:1165–1177. [Google Scholar]
- Rosado J, Morais M, and Tockner K, 2015. Mass Dispersal of Terrestrial Organisms During First Flush Events in a Temporary Stream. River Research and Applications 31(7):912–917. DOI: 10.1002/rra.2791. [DOI] [Google Scholar]
- Rosenfeld JS and Raeburn E, 2009. Effects of Habitat and Internal Prey Subsidies on Juvenile Coho Salmon Growth: Implications for Stream Productive Capacity. Ecology of Freshwater Fish 18:572–584. [Google Scholar]
- Sánchez-Montoya MM, Moleón M, Sánchez-Zapata JA, and Tockner K, 2016. Dry Riverbeds: Corridors for Terrestrial Vertebrates. Ecosphere 7(10):e01508. [Google Scholar]
- Sapsford SJ, Alford RA, and Schwarzkopf L, 2013. Elevation, Temperature, and Aquatic Connectivity All Influence the Infection Dynamics of the Amphibian Chytrid Fungus in Adult Frogs. PLoS ONE 8(12):e82425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori M and Brittain JE, 2015. Order Ephemeroptera Pages 873–891 in Freshwater Invertebrates: Ecology and General Biology, Fourth Edition. Thorp J and Rodgers DC, editors. Academic Press, New York, NY. [Google Scholar]
- Schindler DE, Leavitt PR, Brock CS, Johnson SP, and Quay PD, 2005. Marine-derived Nutrients, Commercial Fisheries, and Production of Salmon and Lake Algae in Alaska. Ecology 86:3225–3231. [Google Scholar]
- Schlichting HE Jr., 1969. The Importance of Airborne Algae and Protozoa. Journal of the Air Pollution Control Association 19:946–951. [DOI] [PubMed] [Google Scholar]
- Schlosser IJ, 1987. A Conceptual Framework for Fish Communities in Small Warmwater Streams Pages 17–24 in Community and Evolutionary Ecology of North American Stream Fishes. Matthews WJ and Heins DC, editors. University of Oklahoma Press, Norman, OK. [Google Scholar]
- Schramm HL and Eggleton MA, 2006. Applicability of the Flood-pulse Concept in a Temporal Floodplain River Ecosystem: Thermal and Temporal Components. River Research and Applications 22:543–553. [Google Scholar]
- Schrank AJ and Rahel FJ, 2004. Movement Patterns in Inland Cutthroat Trout (Oncorhynchus clarki utah): Management and Conservation Implications. Canadian Journal of Fisheries and Aquatic Sciences 61:1528–1537. [Google Scholar]
- Schwalb AN, Garvie M, and Ackerman JD, 2010. Dispersion of Freshwater Mussel Larvae in a Lowland River. Limnology and Oceanography 55:628–638. [Google Scholar]
- Schwalb AN, Cottenie K, Poos MS, and Ackerman JD, 2011. Dispersal Limitation of Unionid Mussels and Implications for Their Conservation. Freshwater Biology 56:1509–1518. doi: 10.1111/j.1365-2427.2011.02587.x. [DOI] [Google Scholar]
- Schwalb AN, Morris TJ, Mandrak NE, and Cottenie K, 2013. Distribution of Unionid Freshwater Mussels Depends on the Distribution of Host Fishes on a Regional Scale. Diversity and Distributions 19:446–454. [Google Scholar]
- Semlitsch RD, 2008. Differentiating Migration and Dispersal Processes for Pond-breeding Amphibians. The Journal of Wildlife Management 72:260–267. [Google Scholar]
- Semlitsch RD and Bodie JR, 1998. Are Small, Isolated Wetlands Expendable? Conservation Biology 12:1129–1133. [Google Scholar]
- Senderovich Y, Izhaki I, and Halpern M, 2010. Fish as Reservoirs and Vectors of Vibrio cholerae. PLoS ONE 5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharitz RR, 2003. Carolina Bay Wetlands: Unique Habitats of the Southeastern United States. Wetlands 23:550–562. [Google Scholar]
- Sharitz RR and Gibbons JW, 1982. The Ecology of Southeastern Shrub Bogs (Pocosins) and Carolina Bays: A Community Profile FWS/OBS-82/04, U.S. Department of the Interior, U.S. Fish and Wildlife Services Program, Washington, DC. [Google Scholar]
- Shaw DA, Pietroniro A, and Martz LW, 2013. Topographic Analysis for the Prairie Pothole Region of Western Canada. Hydrological Processes 27:3105–3114. [Google Scholar]
- Shoup DE and Wahl DH, 2009. Fish Diversity and Abundance in Relation to Interannual and Lake-specific Variation in Abiotic Characteristics of Floodplain Lakes of the Lower Kaskaskia River, Illinois. Transactions of the American Fisheries Society 138:1076–1092. [Google Scholar]
- Skagen SK and Knopf FL, 1993. Toward Conservation of Midcontinental Shorebird Migrations. Conservation Biology 7:533–541. [Google Scholar]
- Smith TA and Kraft CE, 2005. Stream Fish Assemblages in Relation to Landscape Position and Local Habitat Variables. Transactions of the American Fisheries Society 134:430–440. [Google Scholar]
- Smock LA, 1994. Movements of Invertebrates Between Stream Channels and Forested Floodplains. Journal of the North American Benthological Society 13:524–531. [Google Scholar]
- Smock LA, Gladden JE, Riekenberg JL, Smith LC, and Black CR, 1992. Lotic Macroinvertebrate Production in Three Dimensions: Channel Surface, Hyporheic, and Floodplain Environments. Ecology 73:876–886. [Google Scholar]
- Snedden GA, Kelso WE, and Rutherford DA, 1999. Diel and Seasonal Patterns of Spotted Gar Movement and Habitat Use in the Lower Atchafalaya River Basin, Louisiana. Transactions of the American Fisheries Society 128:144–154. [Google Scholar]
- Snodgrass JW, Komoroski MJ, Bryan AL, and Burger J, 2000. Relationships Among Isolated Wetland Size, Hydroperiod, and Amphibian Species Richness: Implications for Wetland Regulations. Conservation Biology 14:414–419. [Google Scholar]
- Soomers H, Karssenberg D, Soons MB, Verweij PA, Verhoeven JTA, and Wassen MJ, 2013. Wind and Water Dispersal of Wetland Plants Across Fragmented Landscapes. Ecosystems 16:434–451. [Google Scholar]
- Soons MB, 2006. Wind Dispersal in Freshwater Wetlands: Knowledge for Conservation and Restoration. Applied Vegetation Science 9:271–278. [Google Scholar]
- Spinola RM, Serfass TL, and Brooks RP, 2008. Survival and Post-release Movements of River Otters Translocated to Western New York. Northeastern Naturalist 15:13–24. [Google Scholar]
- Stanford JA, Lorang MS, and Hauer FR, 2005. The Shifting Habitat Mosaic of River Ecosystems. International Association of Theoretical and Applied Limnology – Proceedings 29:123–36. [Google Scholar]
- Stanley EH, Fisher SG, and Grimm NB, 1997. Ecosystem Expansion and Contraction in Streams. BioScience 47:427–435. [Google Scholar]
- Statzner B, 2012. Geomorphological Implications of Engineering Bed Sediments by Lotic Animals. Geomorphology 157–158:49–65. [Google Scholar]
- Stearns SC 1989. Trade-Offs in Life-History Evolution. Functional Ecology 3:259–268. [Google Scholar]
- Steiger J, Tabacchi E, Dufour S, Corenblit D, and Peiry JL, 2005. Hydrogeomorphic Processes Affecting Riparian Habitat Within Alluvial Channel-floodplain River Systems: A Review for the Temperate Zone. River Research and Applications 21:719–737. doi: 10.1002/rra.879. [DOI] [Google Scholar]
- Steward AL, von Schiller D, Tockner K, Marshall JC, and Bunn SE, 2012. When the River Runs Dry: Human and Ecological Values of Dry Riverbeds. Frontiers in Ecology and the Environment 10:202–209. [Google Scholar]
- Stewart KW and Schlichting HE Jr., 1966. Dispersal of Algae and Protozoa by Selected Aquatic Insects. Journal of Ecology 54:551–562. [Google Scholar]
- Stubbington R, 2012. The Hyporheic Zone as an Invertebrate Refuge: A Review of Variability in Space, Time, Taxa and Behavior. Marine and Freshwater Research 63:293–311. [Google Scholar]
- Subalusky AL, Fitzgerald LA, and Smith LL, 2009a. Ontogenetic Niche Shifts in the American Alligator Establish Functional Connectivity Between Aquatic Systems. Biological Conservation 142:1507–1514. [Google Scholar]
- Subalusky AL, Smith LL, and Fitzgerald LA, 2009b. Detection of American Alligators in Isolated, Seasonal Wetlands. Applied Herpetology 6:199–210. [Google Scholar]
- Swimley TJ, Brooks RP, and Serfass TL, 1999. Otter and Beaver Interactions in the Delaware Water Gap National Recreation Area. Journal of the Pennsylvania Academy of Science 72:97–101. [Google Scholar]
- Thorp JH and Delong AD, 2002. Dominance of Autochthonous Autotrophic Carbon in Food Webs of Heterotrophic Rivers. Oikos 96:543–550. [Google Scholar]
- Tockner K, Schiemer F, Baumgartner C, Kum G, Weigand E, Zweimüller I, and Ward JV, 1999. The Danube Restoration Project: Species Diversity Patterns Across Connectivity Gradients in the Floodplain System. Regulated Rivers: Research & Management 15:245–258. [Google Scholar]
- Tockner K, Malard F, and Ward JV, 2000. An Extension of the Flood Pulse Concept. Hydrological Processes 14:2861–2883. [Google Scholar]
- Tromp-van Meerveld HJ, and McDonnell JJ, 2006. Threshold Relations in Subsurface Stormflow: 2. The Fill and Spill Hypothesis. Water Resources Research 42:W02411. [Google Scholar]
- Tronstad LM, Tronstad BP, and Benke AC, 2007. Aerial Colonization and Growth: Rapid Invertebrate Responses to Temporary Aquatic Habitats in a River Floodplain. Journal of the North American Benthological Society 26:460–471. [Google Scholar]
- Truscott AM, Soulsby C, Palmer SCF, Newell L, and Hulme PE, 2006. The Dispersal Characteristics of the Invasive Plant Mimulus guttatus and the Ecological Significance of Increased Occurrence of High-flow Events. Journal of Ecology 94:1080–1091. [Google Scholar]
- Uno H and Power ME, 2015. Mainstem-tributary Linkages by Mayfly Migration Help Sustain Salmonids in a Warming River Network. Ecology Letters 18:1012–1020. [DOI] [PubMed] [Google Scholar]
- USEPA, 2015. Connectivity of Streams & Wetlands to Downstream Waters: A Review & Synthesis of the Scientific Evidence. U.S. Environmental Protection Agency, Office of Research and Development; EPA/600/R-14/475F. [Google Scholar]
- Van de Meutter F, Stoks R, and De Meester L, 2006. Lotic Dispersal of Lentic Macroinvertebrates. Ecography 29: 223–230. doi: 10.1111/j.2006.0906-7590.04483.x. [DOI] [Google Scholar]
- van der Valk AG and Pederson RL, 2003. The SWANCC Decision and its Implications for Prairie Potholes. Wetlands 23:590–596. [Google Scholar]
- Van Leeuwen CHA, Huig N, van der Velde G, Van Alen TA, Wagemaker CAM, Sherman CDH, Klaassen M, and Figuerola J, 2013. How Did This Snail Get Here? Several Dispersal Vectors Inferred for an Aquatic Invasive Species. Freshwater Biology 58:88–99. [Google Scholar]
- Vander Vorste R, Malard F, and Datry T, 2016. Is Drift the Primary Process Promoting the Resilience of River Invertebrate Communities? A Manipulative Field Experiment in an Intermittent Alluvial River. Freshwater Biology 61:1276–1292. [Google Scholar]
- Vanderhoof MK and Alexander LC, 2016. The Role of Lake Expansion in Altering the Wetland Landscape of the Prairie Pothole Region, United States. Wetlands 36(Suppl 2):309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoof MK, Alexander LC, and Todd JM, 2016. Temporal and Spatial Patterns of Wetland Extent Influence Variability of Surface Water Connectivity in the Prairie Pothole Region, United States. Landscape Ecology 31(4):805–824. [Google Scholar]
- Vanschoenwinkel B, Gielen S, Seaman M, and Brendonck L, 2009. Wind mediated Dispersal of Freshwater Invertebrates in a Rock Pool Metacommunity: Differences in Dispersal Capacities and Modes. Hydrobiologia 635:363–372. [Google Scholar]
- Vaughn CC, 2017. Ecosystem Services Provided by Freshwater Mussels. Hydrobiologia DOI 10.1007/s10750-017-3139-x. [DOI] [Google Scholar]
- Vilizzi L, McCarthy BJ, Scholz O, Sharpe CP, and Wood DB, 2013. Managed and Natural Inundation: Benefits for Conservation of Native Fish in a Semi-arid Wetland System. Aquatic Conservation: Marine and Freshwater Ecosystems 23:37–50. [Google Scholar]
- Wainwright J, Turnbull L, Ibrahim TG, Lexartza-Artza I, Thornton SF, and Brazier RE, 2011. Linking Environmental Regimes, Space and Time: Interpretations of Structural and Functional Connectivity. Geomorphology 126:387–404. [Google Scholar]
- Wallace JB and Webster JR, 1996. The Role of Macroinvertebrates in Stream Ecosystem Function. Annual Review of Entomology 41:115–139. [DOI] [PubMed] [Google Scholar]
- Walters DM, Fritz KM, and Otter RR, 2008. The Dark Side of Subsidies: Adult Stream Insects Export Organic Contaminants to Riparian Predators. Ecological Applications 18:1835–1841. [DOI] [PubMed] [Google Scholar]
- Waples RS, 2010. Spatial-temporal Stratifications in Natural Populations and How They Affect Understanding and Estimation of Effective Population Size. Molecular Ecology Resources 10:785–796. [DOI] [PubMed] [Google Scholar]
- Ward JV, 1989. The Four-dimensional Nature of Lotic Ecosystems. Journal of the North American Benthological Society 8(1): 2–8. [Google Scholar]
- Webb EB, Smith LM, Vrtiska MP, and LaGrange TG, 2010. Effects of Local and Landscape Variables on Wetland Bird Habitat Use During Migration Through the Rainwater Basin. Journal of Wildlife Management 74:109–119. [Google Scholar]
- Wellborn GA, Skelly DK, and Werner EE, 1996. Mechanisms Creating Community Structure Across a Freshwater Habitat Gradient. Annual Review of Ecology and Systematics 27:337–363. [Google Scholar]
- Wigington PJ, Ebersole JL, Colvin ME, Leibowitz SG, Miller B, Hansen B, LaVigne HR, White D, Baker JP, Church MR, Brooks JR, Cairns MA, and Compton JE, 2006. Coho Salmon Dependence on Intermittent Streams. Frontiers in Ecology and the Environment 4:513–518. [Google Scholar]
- Williams DD, 1996. Environmental Constraints in Temporary Fresh Waters and Their Consequences for the Insect Fauna. Journal of the North American Benthological Society 15:634–650. [Google Scholar]
- Williams DD, 2006. The Biology of Temporary Waters. Oxford University Press, Oxford UK. [Google Scholar]
- Wilzbach MA, Cummins KW, and Hall JD, 1986. Influence of Habitat Manipulations on Interactions Between Cutthroat Trout and Invertebrate Drift. Ecology 67:898–911. [Google Scholar]
- Winston MR, Taylor CM, and Pigg J, 1991. Upstream Extirpation of Four Minnow Species Due to Damming of a Prairie Stream. Transactions of the American Fisheries Society 120:98–105. [Google Scholar]
- Wipfli MS and Gregovich DP, 2002. Export of Invertebrates and Detritus from Fishless Headwater Streams in Southeastern Alaska: Implications for Downstream Salmonid Production. Freshwater Biology 47:957–969. [Google Scholar]
- Woelfle-Erskine C, Larsen LG, and Carlson SM, 2017. Abiotic Habitat Thresholds for Salmonid Over-summer Survival in Intermittent Streams. Ecosphere 8:e01645. [Google Scholar]
- Wofford JEB, Gresswell RE, and Banks MA, 2005. Influence of Barriers to Movement on Within-watershed Genetic Variation of Coastal Cutthroat Trout. Ecological Applications 15:628–637. [Google Scholar]
- Wright S, 1943. Isolation by Distance. Genetics 28:114–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedler PH, 2003. Vernal Pools and the Concept of “Isolated Wetlands”. Wetlands 23:597–607. [Google Scholar]
- Zeug SC, Peretti D, and Winemiller KO, 2009. Movement into Floodplain Habitats by Gizzard Shad (Dorosoma cepedianum) Revealed by Dietary and Stable Isotope Analyses. Environmental Biology of Fishes 84:307–314. [Google Scholar]
- Zeug SC, Winemiller KO, and Tarim S, 2005. Response of Brazos River Oxbow Fish Assemblages to Patterns of Hydrologic Connectivity and Environmental Variability. Transactions of the American Fisheries Society 134:1389–1399. [Google Scholar]