Abstract
Selective retrieval of a specific target memory often leads to the forgetting of related but irrelevant memories. Current cognitive theory states that such retrieval-induced forgetting arises due to inhibition of competing memory traces. To date, however, direct neural evidence for this claim has not been forthcoming. Studies on selective attention suggest that cortical inhibition is mediated by increased brain oscillatory activity in the alpha/beta frequency band. The present study, testing 18 human subjects, investigated whether these mechanisms can be generalized to selective memory retrieval in which competing memories interfere with the retrieval of a target memory. Our experiment was designed so that each cue used to search memory was associated with a target memory and a competitor memory stored in separate brain hemispheres. Retrieval-induced forgetting was observed in a condition in which the competitor memory interfered with target retrieval. Increased oscillatory alpha/beta power was observed over the hemisphere housing the sensory representation of the competitor memory trace and predicted the amount of retrieval-induced forgetting in the subsequent memory test. These results provide the first direct evidence for inhibition of competing memories during episodic memory retrieval and suggest that competitive retrieval is governed by inhibitory mechanisms similar to those employed in selective attention.
Introduction
Remembering a previously experienced episode is often challenged by interference from currently irrelevant memory representations. Executive control mechanisms aid selective remembering in such ambiguous retrieval situations by actively inhibiting competing memories (for review, see Levy and Anderson, 2002). The effect of inhibition can be observed on a later test, where previously interfering information shows impaired recall, a phenomenon termed retrieval-induced forgetting (RIF; Anderson et al., 1994). Previous neuroimaging studies indicate that prefrontal control mechanisms play a crucial role in producing RIF (Johansson et al., 2007; Kuhl et al., 2007; Wimber et al., 2009; Hanslmayr et al., 2010; Staudigl et al., 2010). However, no study has yet been able to directly track the inhibition of competing memories due to a spatiotemporal overlap between target-related and competitor-related neural activity. We applied a visual half-field manipulation in an episodic memory task (Gratton et al., 1997) to disentangle target from competitor-related activity during selective memory retrieval. Our results provide the first direct evidence for the inhibition of competing memories.
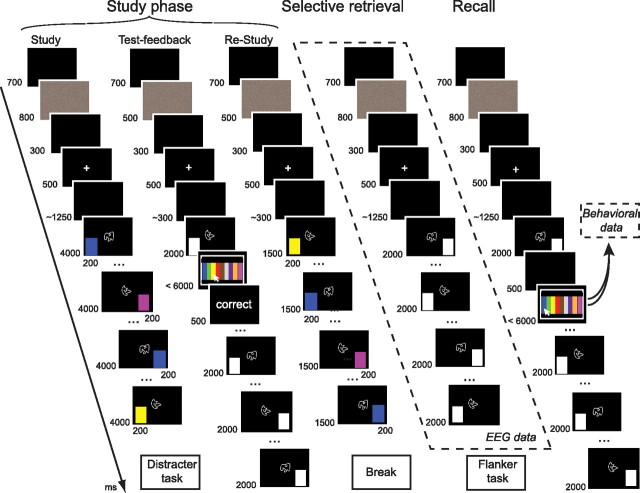
Participants encoded cue-associate (shape–color) pairs, attending to the cue at central fixation. Cues were presented twice, first together with an associate in one visual field (e.g., left of fixation) and later in the sequence of trials with a second associate in the opposite visual field (e.g., right of fixation; Fig. 1). The two associates either had the same color (non-interference condition) or two different colors (interference condition). During a subsequent selective retrieval phase, some of the cues were presented and participants were asked to retrieve the target associate studied in one of the visual fields (Fig. 1). The other color associated with the cue, studied in the opposite visual field, was not addressed and served as the competitor associate. In a final recall task, memory for all cue-associate pairs was assessed. RIF was expected for competitors from the interference condition encoded with cues that underwent selective retrieval, as compared to baseline items (cue-associate pairs for which the cue was not presented during selective retrieval).
Figure 1.
Experimental design exemplifying trial procedures for all experimental phases and possible succession of trials from the non-interference (one-color) and interference (two-color) conditions.
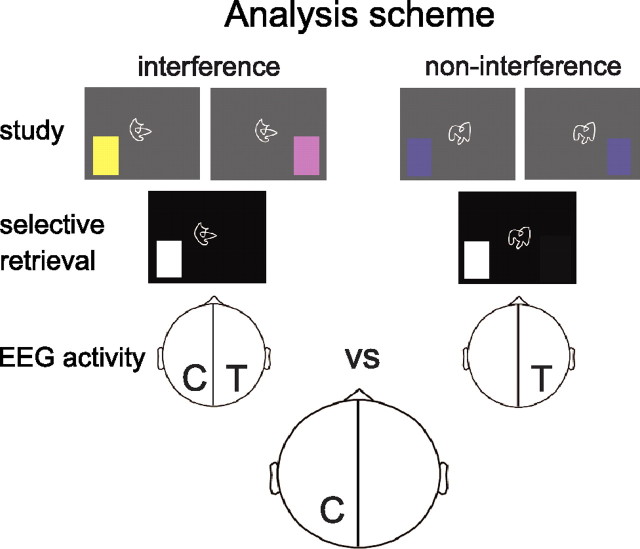
EEG analyses contrasted interference and non-interference conditions during selective retrieval. Importantly, conditions were identical in terms of sensory stimulation and differed only in whether or not the competitor associate, encoded contralateral to the target associate, was causing interference (Fig. 2). Studies in the selective visual attention domain have shown an increase of alpha/beta power (10–20 Hz) over the brain hemisphere where a competing item is processed (Worden et al., 2000; Kelly et al., 2006; Sauseng et al., 2009b; Händel et al., 2011). Additionally, multimodal imaging studies demonstrated that periods of high alpha/beta activity index reduced excitability of the cortex (Romei et al., 2008; Sauseng et al., 2009a) and decreased metabolic demands (Hanslmayr et al., 2011b; Scheeringa et al., 2011). Therefore, increased alpha/beta power over the brain hemisphere housing competing memories during selective retrieval would signify inhibition (Klimesch et al., 2007; Jensen and Mazaheri, 2010; Hanslmayr et al., 2011a). Such a finding would be indicative of a generic, cross-domain mechanism mediating inhibition in both selective visual attention and episodic memory retrieval.
Figure 2.
Schematic illustration of obtaining the lateralized EEG correlates of inhibition. EEG activity related to visual stimulation and basic retrieval processing of the target (‘T’) is identical in the interference and non-interference conditions, whereas inhibition of the competitor (‘C’) in the hemisphere ipsilateral to the retrieval cue is unique to the interference condition (left).
Materials and Methods
Participants
Eighteen participants (11 female) with a mean age of 25 years (range: 18–38) and normal or corrected-to-normal vision completed the experiment and were included in the data analysis. All participants were right-handed, reported no history of neurological disease, and gave written informed consent. The study was approved by the Ethical Review Board at Lund University.
Stimulus material
Two-hundred and sixteen abstract line drawings (Groh-Bordin et al., 2007) were selected and counterbalanced across sessions (108 each), subjects, and conditions. The abstract shapes served as memory cues and were paired with nine different colors (associates), corresponding to basic color categories (blue, green, yellow, red, brown, gray, violet, orange, and pink; Taft and Sivik, 1997). Association between shapes and colors was randomized for the first block of the session and subsequently counterbalanced across blocks and shapes so that each color appeared equally often in each experimental condition.
Experimental setup
Participants were seated in front of a 17 inch monitor (65 cm distance) with a resolution of 1280 × 1024 pixels. During the experiment, the abstract shapes were presented at a central position with a size of 6 × 5 cm (visual angle: 5.3 × 4.4°), together with rectangular boxes, that covered the lower half of the screen (3.1 cm/3° to the left or right from fixation). The boxes were 9.3 × 13.7 cm in size (visual angle: 8.1 × 11.9°) and were filled with color during the study phase.
Procedure
Participants were tested in two sessions on two separate days, 4–15 d apart (M = 8). Each session contained 18 blocks, with each block including the whole procedure as described below.
During all phases of the experiment, each trial began with a polychromatic mask to avoid afterimages from the preceding stimulation (800 ms in the study, practice, and final recall phase and 500 ms in the test–feedback and re-study phases). This was followed by a blank screen (300 ms), a fixation cross (500 ms), and a stimulation-free baseline interval (1000–1500 ms in the study, practice, and final recall phase and 200–400 ms in the test–feedback and re-study phases). Throughout the experiment, participants were instructed to fixate the center of the screen as indicated by the fixation cross or the centrally presented shape and to avoid any eye movements or blinks except during the polychromatic mask. In all phases except for the recall phase, order of presentation was randomized, with the constraint that the same shape never occurred twice in a row.
Study phase.
During study, participants were presented with six different shapes, each combined with instances of all colors. Shapes were presented twice, first together with a color in one visual field and later with a color presented in the opposite visual field. Half of the shapes were presented with the same color at left and right positions, which comprised the non-interference condition. The other half was presented with two different colors, one color at the left position and another color at the right position, which comprised the interference condition. Shapes were presented for 4000 ms. The color box was flashed for 200 ms at the onset of the shape to avoid saccades to the color (Gratton et al., 1997). Participants were instructed to memorize the whole percept including shape, color, and box position in the current trial (left or right). After initial study, participants were instructed to count backwards in steps of three from a random three digit number for 15 s. Following this distracter task, participants were sequentially presented with all abstract shapes together with a white box to the left or right of fixation and instructed to covertly recall the color that was previously presented at that position. After 2000 ms of cue presentation, all nine possible colors appeared on the screen and participants were instructed to select the correct color by mouse click within 6000 ms. After a response, participants received feedback on their performance on a screen stating “correct” or “wrong” (500 ms). To ensure high memory performance levels, a re-study phase followed test–feedback recall, which was identical to the initial study block except for that the shapes were only presented for 1500 ms.
Selective retrieval phase.
During the selective retrieval phase, two of the shapes from each interference and non-interference condition were presented. The shapes were displayed for 2000 ms together with one white box serving as an unambiguous retrieval cue for the color that was previously presented at that position (target associate). As in previous studies (Hanslmayr et al., 2010; Staudigl et al., 2010), each cue–target pair was retrieved twice. However, only one position was cued, addressing memory for the target color initially presented in the right visual field (RVF) or in the left visual field (LVF). For each condition, one of the shapes was presented with a white box in the RVF and the other was presented with a box in the LVF. As in prior studies (Hanslmayr et al., 2010; Staudigl et al., 2010), participants were instructed to covertly retrieve the appropriate target color and to try to visualize the initial shape–color combination as it had appeared in the cued visual field during study. Trials were separated by 700 ms stimulation-free intertrial-intervals. After selective retrieval, a distracter task was carried out (Eriksen flanker task, duration: 70 s; Eriksen and Eriksen, 1974; Corballis and Gratton, 2003).
Final recall phase.
In the final recall phase, all initially studied color–shape associations were tested. Cue-associate pairs for which the shape was not presented during the retrieval practice phase served as baseline items. Associates that were not retrieved, but were associated with the same shape as the retrieved (target) items, served as competitor items. The test was identical to the recall test during the study phase, with the difference that the order was constrained such that competitor items from both interference and non-interference conditions, together with a corresponding set of baseline items, were tested before the target items. This was done to avoid the possibility that output interference in the final recall test would confound the effects of memory inhibition (Anderson et al., 1994; Johansson et al., 2007). In addition, presentation of the nine possible colors was delayed for 500 ms after offset of the retrieval cue.
EEG recording and preprocessing
EEG was recorded from 32 Ag/AgCl scalp electrodes with a 500 Hz sampling rate and amplified from DC to 200 Hz on a Neuroscan NuAmps system, referenced to the left mastoid and re-referenced offline to a common average reference. Data were corrected for eye blinks and vertical eye movements using two additional channels assessing activity in the vertical and horizontal electrooculogram, applying the algorithm implemented in Neuroscan Edit 4.4. Continuous EEG was epoched from −500 ms before stimulus onset to 2000 ms poststimulus. All epochs containing remaining artifacts as identified by careful visual inspection were excluded from further analyses. A high-pass filter (0.05 Hz, −3 dB, 12 dB/octave roll-off) was applied off-line before further analyses.
Data analysis
Behavioral data analyses.
To test for RIF, recall performance in the final recall test for the competitor items was compared with recall performance for the baseline items from the interference and the non-interference condition. This was done by means of a 2 × 2 × 2 repeated-measures ANOVA with the factors CONDITION (interference vs non-interference), ITEM (competitor vs baseline), and HEMISPHERE (left vs right). The last factor was included to test possible hemisphere-specific behavioral effects. The behavioral analysis included only items that were successfully encoded in the study phase as indicated by correct performance in the recall–feedback test after initial study. RIF effects were expected to be reflected in a CONDITION × ITEM interaction, characterized by lower recall performance for competitors when compared to baseline items in the interference condition. Significant effects were followed-up by planned comparisons employing uncorrected paired-samples Student's t tests (two-tailed).
EEG analysis methods.
Analyses focused on the EEG data recorded during the selective retrieval phase, where the interference and the non-interference retrieval conditions were contrasted. Note that both conditions were identical in terms of sensory stimulation and differed only by whether or not the competitor item studied contralaterally to the target item was interfering (Fig. 2). EEG analysis was carried out using self-written MATLAB (MathWorks) codes applying a Gabor transformation to derive a time–frequency representation from the EEG signal (e.g., Hanslmayr et al., 2010). To avoid filter artifacts at the edges of the epochs, the data were filtered in a slightly larger time interval, but analyses were restricted to the 2500 ms epochs. The data were filtered in a frequency range of 2–30 Hz with a filter parameter for time–frequency resolution (gamma) set to 1, resulting in a circular spatial aspect ratio of the Gaussian function supporting the Gabor transformation. To quantify event-related power changes, poststimulus power change was calculated in relation to a prestimulus baseline period (−500 to 0 ms; cf. Pfurtscheller and Aranibar, 1977). All statistical analyses of EEG power were conducted using nonparametric Wilcoxon signed-rank tests (two-tailed).
Visual field-specific EEG analysis and selection of time windows.
To identify the time–frequency windows in which hemisphere-specific effects emerged, we subtracted the EEG activity of the non-interference condition from the interference condition for trials for which the white boxes, functioning as retrieval cues, were presented in the same visual field during the selective retrieval phase (see Fig. 3). Interference and non-interference conditions should both entail retrieval processes, but only the interference condition would contain activity that is related to the inhibition of competitor items in the hemisphere ipsilateral to the retrieval cue. Thus, the strategy to subtract EEG activity of the non-interference condition from the interference condition should reveal the neural activity that is specific to the effects of retrieval competition and inhibition. The comparison included only trials for which the cue–competitor combinations were successfully retrieved during the study phase (Storm et al., 2006). Visual field-specific power increases at ipsilateral electrodes were most pronounced in a frequency band between 11.5–14 Hz for LVF trials and between 17 and 20 Hz for RVF trials (data not shown). To directly compare between visual fields, these slightly different frequency bands were summarized for the analyses reported below by investigating changes in the higher alpha–lower beta (11.5–20 Hz) frequency band for both LVF and RVF trials. To make sure that the observed activity is indeed lateralized between the hemispheres and did not result from a general pattern across hemispheres, significant power differences in the alpha/beta frequency band between left (P3/PO3) and right (P4/PO4) electrode pools were identified by means of continuous Wilcoxon signed-rank tests (10 ms time windows) for both LVF and RVF trials. To test whether the obtained laterality differences were driven by power differences between non-interference and interference conditions at electrode sites over the ipsilateral competitor-related or the contralateral target-related hemisphere, we additionally calculated Wilcoxon signed-rank tests for 10 ms time windows separately for the left and right electrode pools.
Figure 3.
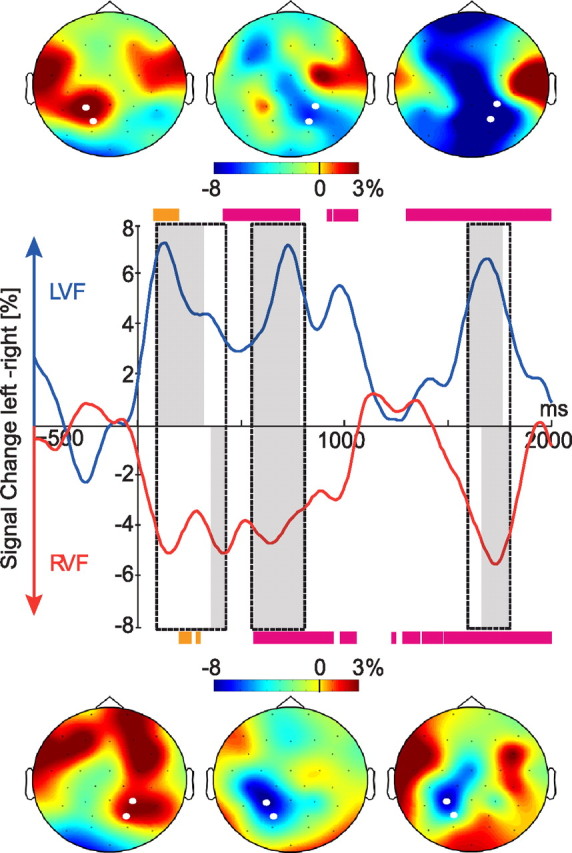
Visual field-specific lateralization effects. Middle row, Laterality index for LVF and RVF trials. Gray-shaded areas indicate significant laterality differences in alpha/beta power between left and right electrode pools specific for each LVF and RVF trials. Dotted boxes show the time windows selected for further statistical analyses to capture effects in both LVF and RVF trials. Colored bars at the top and bottom of the plot indicate significant differences between interference and non-interference conditions at the electrode pool ipsilateral (orange) and contralateral (magenta) to the retrieval cue. Top and bottom rows, Topographic maps showing the alpha/beta power distribution over the scalp in the time windows indicated by the gray-shaded areas in the middle row for LVF (top row) and RVF (bottom row) trials. Left and right electrode pools are depicted in white.
Competitor-related and target-related lateralization analysis.
Based on the visual field-specific analysis, we isolated competitor-related and target-related effects on alpha/beta power (11.5–20 Hz) independent of the specific visual field in which the respective memory items were presented. Due to the similarity in lateralization and only slight differences in timing, the visual field-specific time windows were integrated into three time windows that were used for further analyses, capturing effects in both LVF and RVF trials starting from the earliest onset of laterality effects to the latest offset in either LVF or RVF trials. To increase sensitivity, we summarized EEG power within the competitor and target hemispheres for the LVF and RVF trials (by flipping the topography for the RVF condition; cf. Spitzer and Blankenburg, 2011). As a result, for both LVF and RVF trials the activity in the competitor hemisphere was artificially represented at the left electrodes, while activity in the target hemisphere was now reflected at right electrodes. We compared EEG power between the interference and the non-interference conditions at right (P4/PO4) and left posterior electrode pools (P3/PO3). We also investigated the lateralization of these effects by comparing the differences between the interference and the non-interference condition between the two posterior electrode pools.
Lateralization effects modulated by memory status of competitor and target.
Central to the idea of memory inhibition is the assumption that competitors should only be inhibited when they are strong enough to interfere during memory retrieval (Anderson et al., 1994; Bäuml, 1998; Norman et al., 2007). That is, memory inhibition engaged to handle retrieval competition is driven by the strength of the competitor memory rather than by the strength of the target memory (Anderson, 2003; Storm et al., 2006). EEG effects related to interference and memory inhibition should therefore be observable for trials where competitors have a high potential to interfere, regardless of the strength of the target memory. To test for this assumption, we contrasted EEG power for trials within the interference condition for which encoding had been successful for both the target and the competitor (fully encoded), only the competitor (competitor encoded), or only the target (target encoded). We expected a similar increase in early alpha/beta power for the fully-encoded and competitor-encoded trials when compared to target-encoded trials. The comparison only included participants (n = 10) that had a reasonable high number of trials for which competitors or targets were not learned (>10, M = 20). The analysis focused on alpha/beta power (11.5–20 Hz) over target-related right and competitor-related left posterior electrode pools in the first time window obtained in the preceding analysis (90–430 ms).
Effects nonspecific to hemisphere.
To investigate the effects of selective memory retrieval on neural oscillations that occurred independently of hemisphere, we compared EEG power between the interference and the non-interference condition, collapsing LVF and RVF trials without flipping the EEG channels along the midline. This was done to replicate previous findings showing that retrieval in an interference condition leads to higher theta power (5–8 Hz) than retrieval in a non-interference condition (Hanslmayr et al., 2010). As in the comparison for the alpha/beta oscillations, we only included trials for which the cue–competitor combinations were successfully retrieved during the study phase. Time windows and electrodes were selected based on visual inspection of the dataset (see Fig. 5).
Figure 5.
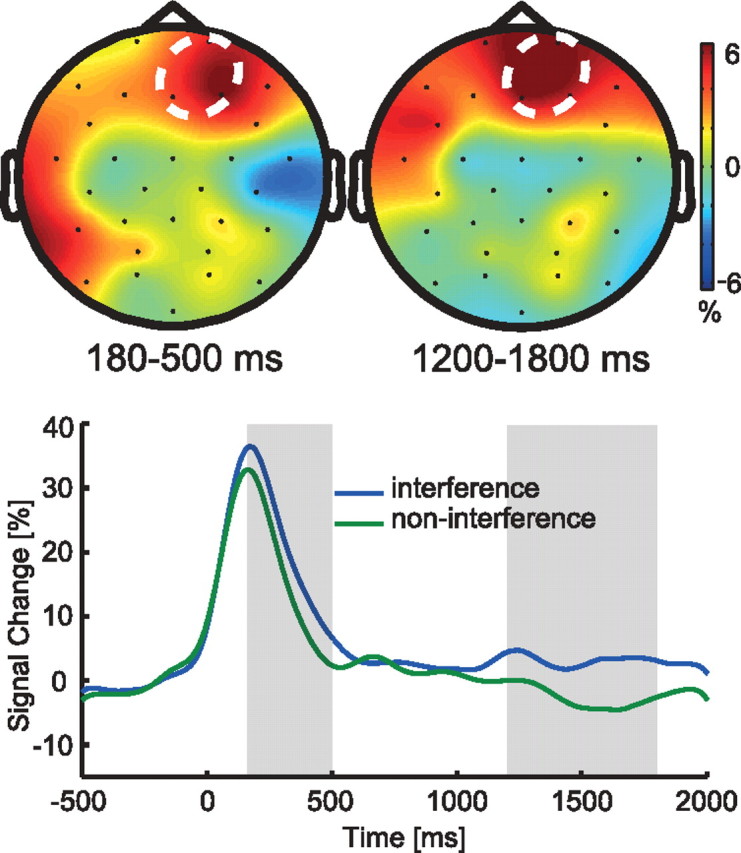
Difference in theta power between interference and non-interference conditions. Top row, Topographical maps for the time windows selected for statistical analysis. White circles indicate electrodes selected for analysis (FZ, FP2, F4). Bottom row, Theta power for interference and non-interference conditions at the electrode pool indicated in the upper row. Gray shaded areas correspond to the time windows depicted in the upper row.
In a second step, analogous to the alpha/beta effects, we expected an increase of theta power in the early (180–500 ms) time window with higher interference in trials where the competitor had been successfully encoded, regardless of encoding status of the target. Based on inspection of the data set, we compared EEG power in a narrow frequency band around 6.5 (± 0.2) Hz at a right frontocentral electrode pool (FC6, C4).
Brain behavior and cross-frequency correlations.
To investigate the functional significance of the early alpha/beta increase, we tested whether EEG power in the 90–430 ms time window correlated with the forgetting of competitors and facilitation of the targets. With respect to previous findings (Hanslmayr et al., 2010), we also correlated early (180–500 ms) and late (1200–1800 ms) theta power with forgetting and facilitation. A forgetting index was obtained by subtracting recall scores for competitor items from baseline recall rates and dividing the result by baseline recall for each subject (Kuhl et al., 2007). Positive scores indicate retrieval-induced forgetting corrected for baseline memory performance. Similarly, a facilitation index was obtained by subtracting baseline recall rates from competitor recall rates and dividing the result by baseline recall rates. Given the suggested link between inhibition and interference detection in RIF (Anderson, 2003), we correlated early alpha/beta power with theta power in the interference condition. Correlation analyses were conducted by calculating nonparametric Spearman's rank correlation coefficients (rs).
Results
Behavioral results
The behavioral data from the final recall test are reported in Table 1. The ANOVA revealed a significant CONDITION (interference vs non-interference) by ITEM (competitor vs baseline) interaction (F(1,17) = 4.57, p < 0.05). This interaction was due to lower recall performance for competitor compared to baseline items in the interference condition (t(17) = 2.157, p < 0.05). No such effect was evident in the non-interference condition (t(17) = 1.657, p > 0.10). This pattern demonstrates that our manipulation was effective in causing RIF and selectively triggered inhibitory mechanisms in the interference condition. A main effect of CONDITION emerged (F(1,17) = 32.947, p < 0.001) due to better recall in the non-interference condition. Notably, there was no CONDITION by ITEM by HEMISPHERE (left vs right) interaction (F(1,17) = 0.268, p > 0.6), indicating that RIF did not differ between the left and right hemisphere.
Table 1.
Recall rates for baseline and competitor items by hemisphere
| Hemisphere | Interference condition |
Non-interference condition |
||
|---|---|---|---|---|
| Baseline | Competitor | Baseline | Competitor | |
| Right | 0.93 ± 0.01 | 0.91 ± 0.02 | 0.97 ± 0.01 | 0.99 ± 0.00 |
| Left | 0.96 ± 0.01 | 0.92 ± 0.02 | 0.98 ± 0.01 | 0.99 ± 0.00 |
| Both | 0.94 ± 0.01 | 0.91 ± 0.01 | 0.97 ± 0.01 | 0.99 ± 0.00 |
Note. Recall scores (M ± SEM) in the final test phase for learned baseline and competitor items by condition and hemisphere.
To investigate whether retrieval practice also had facilitatory effects on memory performance, a similar three-way ANOVA was conducted with the factors CONDITION (interference vs non-interference), ITEM (target vs baseline), and HEMISPHERE. However, no significant main effect for ITEM or any interaction with this factor emerged (F(1,17) = 1.769, p > 0.2), showing that retrieval practice did not lead to higher recall rates of practiced when compared to unpracticed items, neither within the interference condition (M = 0.91, SEM = 0.02 vs M = 0.93, SEM = 0.01; t(17) = 1.190, p > 0.25) nor within the non-interference condition (M = 0.98, SEM = 0.004 vs M = 0.97, SEM = 0.01; t(17) = 1.277, p > 0.2). These null results are most likely due to ceiling effects given the high overall recall rates and will therefore not be discussed further.
EEG results
Visual field-specific effects
Results of the visual field-specific analysis are shown in Figure 3 (gray-shaded areas), depicting stretches of significant (p < 0.05) differences between electrode pools. Magnitude of the differences is visualized as lateralization indices obtained by subtracting alpha/beta power at the right electrode pool from the one at the left electrode pool (see Fig. 3, middle row). For trials in which the retrieval cue was presented in the LVF, positive values on this lateralization index indicate higher power over the ipsilateral left hemisphere housing the representation of the competitor item. For RVF cues, higher power over the ipsilateral competitor-related hemisphere is indicated by negative values. The statistical analysis revealed three time windows in which lateralization effects were significant for each LVF and RVF trial. The three time windows showing significant laterality effects differed slightly between LVF and RVF trials (LVF: 90–330 ms, 560–790 ms, and 1600–1770 ms; RVF: 360–430 ms, 570–810 ms, and 1670–1800 ms). In these three time windows, the power over target-related contralateral electrode sites was decreased compared to competitor-related electrodes ipsilateral to the cue (Fig. 3, p values <0.05). Early in the epoch (LVF: 80–200 ms; RVF: 200–260 and 280–300 ms) the laterality differences were due to higher alpha/beta power in the interference condition over the ipsilateral competitor-related hemisphere (see orange color bars in Fig. 3; p values <0.05). Later in the epoch (LVF onset: 420 ms; RVF onset: 570 ms), laterality differences were due to an underlying decrease in alpha/beta power for the interference condition over the contralateral target-related hemisphere (color bars in magenta, Fig. 3; p values <0.05).
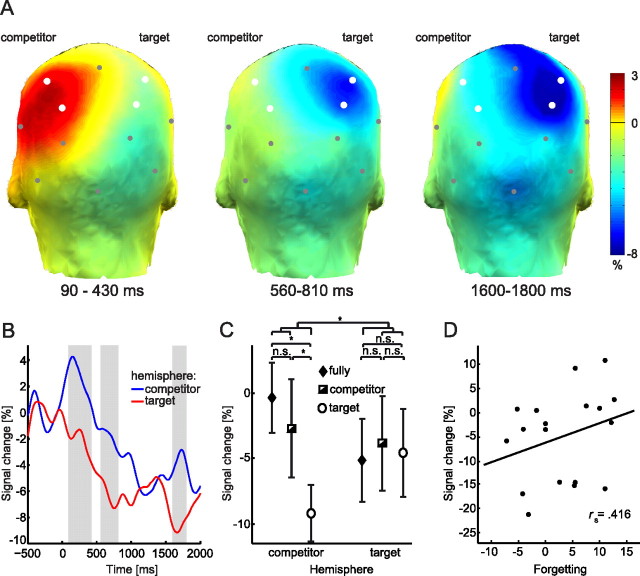
Competitor-related and target-related effects
Flipping the EEG channels along the midline for the RVF condition and pooling trials across LVF and RVF conditions allowed the assessment of competitor-related activity at left hemispheric posterior electrodes and of target-related activity at right hemispheric posterior electrodes. The time course of the difference in alpha/beta power between the interference and non-interference conditions for the competitor and target-related hemispheres is plotted in Figure 4B. The first effect between 90 and 430 ms after stimulus onset was driven by higher levels of alpha/beta power in the interference condition than in the non-interference condition over the competitor-related hemisphere (Z = 2.373, p < 0.05; Fig. 4A, left). Over the target-related hemisphere, alpha/beta power was numerically decreased in the interference condition when compared to the non-interference condition in this time window, but no significant difference between the two conditions emerged (Z = 1.285, p > 0.15). The alpha/beta power increase for the interference versus non-interference condition was lateralized, as reflected by significantly stronger alpha/beta power over the competitor-related hemisphere than over the target-related hemisphere (Z = 2.461, p < 0.05). A second effect was obtained 560–810 ms after stimulus onset. When compared to the non-interference condition, alpha/beta power in the interference condition was significantly decreased over the target-related hemisphere (Z = 3.245, p < 0.005; Fig. 4A, middle). No difference between the two conditions was obtained over the competitor-related hemisphere (Z = 1.372, p > 0.15). The effect was lateralized; the difference between interference and non-interference conditions was significantly greater in the target-related hemisphere than in the competitor-related hemisphere (Z = 3.070, p < 0.005). A third effect emerged 1600–1800 ms after stimulus onset. Alpha/beta power was significantly decreased for the interference condition when compared to the non-interference condition over both hemispheres (Z values >2.678, p values <0.01; Fig. 4A, right). However, the effect was again lateralized, with a higher alpha/beta power decrease over the target-related hemisphere than over the competitor-related hemisphere (Z = 2.286, p < 0.05).
Figure 4.
Competitor-related and target-related alpha/beta power at posterior electrodes sites and its correlation with later forgetting. A, The competitor-related power differences between conditions (interference − non-interference) are shown over the left hemisphere. Target-related activity is depicted over the right hemisphere. Electrodes selected for statistical analyses are depicted in white. B, Alpha/beta power differences between conditions (interference − non-interference) at the selected electrode pools over competitor and target hemisphere. Gray-shaded areas correspond to the time windows shown in A. C, Mean (SEM) alpha/beta power between 90 and 430 ms for trials from the interference condition depending on whether both target and competitor (“fully encoded”), only the competitor (“competitor encoded”), or only the target (“target encoded”) were successfully learned during the study phase. Asterisk (*) indicates significant comparisons (p < 0.05); n.s., not significant; n = 10. D, Correlation between forgetting [(baseline recall − competitor recall)/baseline recall)] and alpha/beta power in the interference condition over the competitor hemisphere (n = 18).
Lateralization effects modulated by interference of the competitor
Both visual field-specific analyses and summarized competitor-related and target-related analyses suggest that competitive retrieval in the interference condition is characterized by an early increase in alpha/beta power over the competitor-related brain hemisphere. We further investigated this assumption by taking into account that inhibition associated with RIF is dependent on the degree of interference arising from the competitor rather than on the strength of the target item (Anderson et al., 1994; Bäuml, 1998; Anderson, 2003; Norman et al., 2007). Thus, we tested whether early alpha/beta power (90–430 ms) is modulated by the memory status of the competing memory trace. Only competitor items that are encoded in memory should interfere with the retrieval of the target. The results of this analysis revealed higher alpha/beta power for fully encoded trials over the competitor-related hemisphere when compared to target-encoded trials (Z = 2.701, p < 0.01; see Fig. 4C); no significant difference between the two conditions was obtained over the target-related hemisphere (Z = 0.459, p > 0.6; see Fig. 4C). We also obtained a significant increase of alpha/beta power between 90 and 430 ms over the competitor hemisphere for competitor-encoded trials relative to target-encoded trials (Z = 2.497, p < 0.05). No effect was obtained over the target hemisphere (Z = 0.255, p > 0.75). There was no difference between fully encoded trials and competitor-encoded trials over either the competitor hemisphere (Z = 0.968, p > 0.3) or the target hemisphere (Z = 0.663, p > 0.5). The mean increase of alpha/beta power for fully-encoded and competitor-encoded trials when compared to target-encoded trials was significantly greater over the competitor hemisphere than over the target hemisphere (Z = 2.293, p < 0.05).
Interference effects on theta power, nonspecific to hemisphere
We examined theta power differences between the interference and non-interference conditions, as two previous studies indicated that enhanced frontal theta power indexes interference in selective memory retrieval (Hanslmayr et al., 2010; Staudigl et al., 2010). The results of this analysis are plotted in Figure 5. Replicating the prior studies, theta power was higher in the interference than in the non-interference condition at frontal electrode sites (FZ, FP2,and F4; see Fig. 5). This difference reached significance in two time windows, 180–500 ms (Z = 2.112, p < 0.05) and 1200–1800 ms (Z = 1.982, p < 0.05).
We also tested whether theta power as the presumed indicator of interference detection was modulated by memory strength of the competitor. When compared to target encoded trials (MSignal change = 7.4%, SEM = 5.4), theta power was increased for both fully encoded (MSignal change = 19.0%, SEM = 5.1; Z = 1.988, p < 0.05) and for competitor encoded trials (MSignal change = 17.6%, SEM = 4.2; Z = 2.09, p < 0.05). There was no difference between fully-encoded and competitor-encoded trials (Z = 0.357, p > 0.7).
Correlation between alpha/beta power, forgetting, facilitation, and theta power
We tested whether the lateralized effects of alpha/beta power and the frontal theta effects predicted the amount of RIF. The early alpha/beta power increase in the interference condition over the competitor-related hemisphere between 90 and 430 ms correlated positively with later forgetting (rs = 0.416, p < 0.05, n = 18, one-tailed; Fig. 4D). No correlation was observed for the non-interference condition over the competitor hemisphere (rs = 0.143, p > 0.25, n = 18, one-tailed), and no correlation was present for the interference condition over the target hemisphere (rs = 0.011, p > 0.45, n = 18, one-tailed). Theta power did not correlate with forgetting in either the early or late time windows (rs < 0.154, p > 0.25, n = 18, one-tailed). Facilitation correlated with neither forgetting (rs = −0.165, p > 0.5, n = 18, one-tailed) nor EEG power (all rs > −0.305, p > 0.1, n = 18, one-tailed). Across frequencies, we observed a nonsignificant trend for a positive correlation between early alpha/beta and theta power in the interference condition (rs = 0.333, p < 0.1, n = 18, one-tailed).
Discussion
A prominent theory of forgetting states that retrieval competition in episodic memory is resolved by inhibitory mechanisms that weaken competing memory representations (Ciranni and Shimamura, 1999; Levy and Anderson, 2002; Anderson, 2003; Spitzer et al., 2009). Our study is the first to provide direct neural evidence of inhibition directed at interfering episodic memory representations. Adapting a visual half-field approach to study the mechanisms underlying retrieval competition, we separated competitor-related activity from target-related activity during selective memory retrieval. As predicted, our novel paradigm induced reliable RIF for memories that competed with the selective retrieval of desired information. In parallel, the EEG data show an increase of posterior alpha/beta power over the hemisphere that stored the competing memories. Importantly, this oscillatory pattern was reliably associated with the degree of interference arising from the competing memory representation (Anderson et al., 1994; Bäuml, 1998; Norman et al., 2007). The lateralized increase of alpha/beta power was specific to retrieval situations where the selection of a target had to be achieved against the interference of a competitor. Furthermore, the effect was dependent on the strength of the competing representation, as defined by whether the competitor was encoded or not but independent of the strength of the target memory representation (Anderson, 2003). It is widely agreed that an increase in oscillatory alpha/beta power reflects cortical inhibition (Klimesch et al., 2007; Jensen and Mazaheri, 2010; Hanslmayr et al., 2011a). Higher alpha/beta oscillatory power is assumed to result from the rhythmic activity of inhibitory interneurons (Klimesch et al., 2007; Jensen and Mazaheri, 2010); it correlates negatively with the BOLD signal in task-relevant brain areas (Hanslmayr et al., 2011b; Scheeringa et al., 2011) and signifies the filtering and attenuation of information in perception, attention, and working memory (Worden et al., 2000; Kelly et al., 2006; Hanslmayr et al., 2007; Sauseng et al., 2009b; Händel et al., 2011). The present results extend this pattern to the episodic memory domain and provide the first direct evidence that competing visual memories are indeed inhibited during selective memory retrieval. Further corroborating this claim, an increase of alpha/beta power over the competitor hemisphere predicted later forgetting. Interestingly, the competitor-related alpha/beta power increase emerged in an early time window and preceded the decrease of alpha/beta power in the target-related hemisphere. This time course indicates that inhibition is rapidly exerted to attenuate the irrelevant competing memory to facilitate retrieval of the relevant target memory.
Two recent fMRI study applied multivoxel pattern classifier algorithms to investigate competition and interference resolution in episodic memory (Kuhl et al., 2011; Öztekin and Badre, 2011). Reduced performance of the classifier in identifying target memory representations (Kuhl et al., 2011) and the degree of reactivation of competing information (Öztekin and Badre, 2011) indicated higher interference and predicted the degree of forgetting. However, these studies were unable to show how interference resolution modulates the representations of competing memories. In accordance with theories on RIF (Anderson, 2003), our data give direct evidence that interference resolution can be achieved by the fast and efficient inhibition of competing memories.
The lateralization effects of increased alpha/beta power over competitor-related areas in the present study closely mirror the findings from several prior studies in the selective visual attention domain (Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006; Rihs et al., 2007; Sauseng et al., 2009b; Händel et al., 2011). As foreshadowed by theories on memory inhibition and RIF (Anderson and Spellman, 1995; Levy and Anderson, 2002; Anderson, 2003), our results suggest that the neural mechanisms regulating information selection in visual attention may generalize to the episodic memory domain. Alpha/beta oscillations are generated by frontoparietal executive control networks (Capotosto et al., 2009; Sauseng et al., 2009b; Thut et al., 2011). In line with previous neuroimaging studies on RIF (Johansson et al., 2007; Kuhl et al., 2007; Wimber et al., 2009), our findings indicate that memory inhibition is a top-down controlled process (Anderson and Spellman, 1995).
The pattern of decreased alpha/beta power over target-related areas gives further evidence for cross-domain mechanisms of interference resolution. Decreased alpha power is considered to reflect the higher excitability of cell assemblies in sensory areas, allowing facilitated processing of target information in selective attention and working memory (e.g., Sauseng et al., 2005; Thut et al., 2006; Lundqvist et al., 2011) and indicating material-specific sensory reactivation of long-term memories (Khader and Rösler, 2011). A larger decrease in this frequency range has been observed in high load and interference conditions (Khader and Rösler, 2011; Lundqvist et al., 2011). Parallel effects are evident in our data and presumably indicate the effortful reactivation of neural populations housing sensory features of episodic memories.
The similarity between the current alpha/beta effect and those observed in studies of selective attention raises the question of whether the effect in the present study likewise reflects an attentional mechanism. Several facets of our data speak against such an interpretation. The lateralization effects between the interference and non-interference conditions were obtained despite identical sensory stimulation. The effects thus varied purely as a function of the location where the interfering competitor memory had been encoded. This makes it difficult to explain a lateralized increase of alpha/beta power with selective attention mechanisms without recurring to the memory domain. It could be argued that differences in visual stimulation and perceptual processing between interference and non-interference conditions during encoding could have influenced selective attention during the selective retrieval phase. However, the stability of the alpha/beta increase across several comparisons, showing that alpha/beta power only covaried with memory strength of the competitor under identical encoding situations, rules out such an explanation. Alternatively, the early alpha/beta increase signifies the engagement of selective attention processes that act to facilitate target retrieval, for example, by attenuating irrelevant visual input. However, such an account would predict that the EEG effect is modulated by the encoding status of the target and that it correlates with facilitated target recall. Neither was the case. Importantly, the early alpha/beta power increase correlated exclusively with later forgetting (not facilitation). Together, these findings are difficult to explain by spatial attention accounts.
The frequency range capturing the lateralization effects in the present study (11.5–20 Hz) overlapped with what has traditionally been regarded as the beta frequency band (Engel and Fries, 2010). It has been suggested that beta frequencies are specifically engaged in sensorimotor processing (Pfurtscheller et al., 1996) or in signaling the current cognitive state (Engel and Fries, 2010). Oscillations in a similar frequency range as that in our study have been labeled and interpreted as upper alpha activity in studies on selective attention and motivated forgetting (e.g., Worden et al., 2000; Rihs et al., 2007; Bäuml et al., 2008). Alpha and beta activity co-occur during episodic memory retrieval and selective attention (Zanto and Gazzaley, 2009) and emerge simultaneously in realistic neural network models (Lundqvist et al., 2011). Also, beta oscillatory activity correlates with higher working memory functioning due to more effective inhibition of distracting information (Zanto and Gazzaley, 2009) and predicts a decrease in neural activity in task-active brain regions (Hanslmayr et al., 2011b; Scheeringa et al., 2011). These findings show that the alpha and beta frequency bands are highly related and might serve similar neural and cognitive functions.
A number of previous studies have shown that frontal theta power varies as a function of interference in response conflict tasks (Hanslmayr et al., 2008; Cavanagh et al., 2009) and more specifically during episodic memory retrieval (Khader and Rösler, 2011). For instance, higher levels of theta power are obtained during competitive retrieval when compared to noncompetitive memory retrieval (Hanslmayr et al., 2010). Our observation of increased oscillatory theta power over frontal electrode sites closely replicates prior findings (Hanslmayr et al., 2010; Staudigl et al., 2010). Timing differences between the present and prior studies could be due to differences in stimulus material (verbal vs pictorial), experimental design (blocked vs alternating trial succession), and EEG baseline conditions. The selective increase in theta power indicates that the interference condition indeed triggered higher levels of competition than the non-interference condition. Similarly, the behavioral results indicate that competition was specifically elicited in the interference condition as reflected in the lower recall rates of the competitor compared with baseline items. Finally, our data show that early theta and alpha/beta power are similarly triggered by the interference potential of the competitor, and a trend for a cross-frequency correlation suggests that effects in the two frequency bands may be interrelated.
Conclusions
The present experiment sheds new light onto how retrieval competition is resolved in episodic memory. Using lateralized presentation of visual memories, we were able to disentangle competitor-related from target-related neural activity. Our results provide direct neural evidence that retrieval competition is resolved by inhibition of sensory brain areas processing the interfering memory representations. Moreover, our results demonstrate that inhibition occurs quickly (starting within 100 ms after cue presentation), suggesting that inhibition in the episodic memory system is highly efficient. These findings closely mirror results from studies in the selective visual attention domain and imply that similar processes regulate retrieval competition in episodic memory.
Footnotes
This work was supported by grants from the Crafoord Foundation (20090712; to G. T. W.), the Swedish Research Council (VR 60229601; to M. J.), and the German Research Council (DFG HA 5622/1-1; to S.H.). We thank C. Turnstedt, J. Klipvik, and S. Nilsson for assistance with data collection, H. Zimmer for providing the stimulus material, and M. Wimber, B. Spitzer, D. B. Terhune, and T. Staudigl for valuable comments on an earlier version of the article.
References
- Anderson MC. Rethinking interference theory: executive control and the mechanisms of forgetting. J Mem Lang. 2003;49:415–445. [Google Scholar]
- Anderson MC, Spellman BA. On the status of inhibitory mechanisms in cognition: memory retrieval as a model case. Psychol Rev. 1995;102:68–100. doi: 10.1037/0033-295x.102.1.68. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Bäuml KH. Strong items get suppressed, weak items do not: the role of item strength in output interference. Psychon Bull Rev. 1998;5:459–463. [Google Scholar]
- Bäuml KH, Hanslmayr S, Pastötter B, Klimesch W. Oscillatory correlates of intentional updating in episodic memory. Neuroimage. 2008;41:596–604. doi: 10.1016/j.neuroimage.2008.02.053. [DOI] [PubMed] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29:5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Cohen MX, Allen JJ. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J Neurosci. 2009;29:98–105. doi: 10.1523/JNEUROSCI.4137-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciranni MA, Shimamura AP. Retrieval-induced forgetting in episodic memory. J Exp Psychol Learn Mem Cogn. 1999;25:1403–1414. doi: 10.1037//0278-7393.25.6.1403. [DOI] [PubMed] [Google Scholar]
- Corballis PM, Gratton G. Independent control of processing strategies for different locations in the visual field. Biol Psychol. 2003;64:191–209. doi: 10.1016/s0301-0511(03)00109-1. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations - signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- Gratton G, Corballis PM, Jain S. Hemispheric organization of visual memories. J Cogn Neurosci. 1997;9:92–104. doi: 10.1162/jocn.1997.9.1.92. [DOI] [PubMed] [Google Scholar]
- Groh-Bordin C, Busch NA, Herrmann CS, Zimmer HD. Event-related potential repetition effects at encoding predict memory performance at test. Neuroreport. 2007;18:1905–1909. doi: 10.1097/WNR.0b013e3282f2a61d. [DOI] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J Cogn Neurosci. 2011;23:2494–2502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bäuml KH. Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage. 2007;37:1465–1473. doi: 10.1016/j.neuroimage.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Pastötter B, Bäuml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the stroop task. J Cogn Neurosci. 2008;20:215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Aslan A, Bäuml KH. Theta oscillations predict the detrimental effects of memory retrieval. Cogn Affect Behav Neurosci. 2010;10:329–338. doi: 10.3758/CABN.10.3.329. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Gross J, Klimesch W, Shapiro KL. The role of alpha oscillations in temporal attention. Brain Res Brain Res Rev. 2011a;67:331–343. doi: 10.1016/j.brainresrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Volberg G, Wimber M, Raabe M, Greenlee MW, Bäuml KH. The relationship between brain oscillations and BOLD signal during memory formation: A combined EEG-fMRI study. J Neurosci. 2011b;31:15674–15680. doi: 10.1523/JNEUROSCI.3140-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Aslan A, Bäuml KH, Gäbel A, Mecklinger A. When remembering causes forgetting: Electrophysiological correlates of retrieval-induced forgetting. Cereb Cortex. 2007;17:1335–1341. doi: 10.1093/cercor/bhl044. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Khader PH, Rösler F. EEG power changes reflect distinct mechanisms during long-term memory retrieval. Psychophysiology. 2011;48:362–369. doi: 10.1111/j.1469-8986.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci U S A. 2011;108:5903–5908. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Lansner A. Theta and gamma power increases and alpha/beta power decreases with memory load in an attractor network model. J Cogn Neurosci. 2011;23:3008–3020. doi: 10.1162/jocn_a_00029. [DOI] [PubMed] [Google Scholar]
- Norman KA, Newman EL, Detre G. A neural network model of retrieval-induced forgetting. Psychol Rev. 2007;114:887–953. doi: 10.1037/0033-295X.114.4.887. [DOI] [PubMed] [Google Scholar]
- Öztekin I, Badre D. Distributed patterns of brain activity that lead to forgetting. Front Hum Neurosci. 2011;5:86. doi: 10.3389/fnhum.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol. 1977;42:817–826. doi: 10.1016/0013-4694(77)90235-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Jr, Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 1996;98:281–293. doi: 10.1016/0013-4694(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25:603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–2018. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Doppelmayr M, Pecherstorfer T, Freunberger R, Hanslmayr S. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum Brain Mapp. 2005;26:148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC. Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia. 2009a;47:284–288. doi: 10.1016/j.neuropsychologia.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol. 2009b;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MC. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Spitzer B, Blankenburg F. Stimulus-dependent EEG activity reflects internal updating of tactile working memory in humans. Proc Natl Acad Sci U S A. 2011;108:8444–8449. doi: 10.1073/pnas.1104189108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer B, Hanslmayr S, Opitz B, Mecklinger A, Bäuml KH. Oscillatory correlates of retrieval-induced forgetting in recognition memory. J Cogn Neurosci. 2009;21:976–990. doi: 10.1162/jocn.2009.21072. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bäuml KH. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J Neurosci. 2010;30:11356–11362. doi: 10.1523/JNEUROSCI.0637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm BC, Bjork EL, Bjork RA, Nestojko JF. Is retrieval success a necessary condition for retrieval-induced forgetting? Psychon Bull Rev. 2006;13:1023–1027. doi: 10.3758/bf03213919. [DOI] [PubMed] [Google Scholar]
- Taft C, Sivik L. Salient color terms in four languages. Scand J Psychol. 1997;38:29–34. [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J. Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol. 2011;21:1176–1185. doi: 10.1016/j.cub.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimber M, Rutschmann RM, Greenlee MW, Bäuml KH. Retrieval from episodic memory: Neural mechanisms of interference resolution. J Cogn Neurosci. 2009;21:538–549. doi: 10.1162/jocn.2009.21043. [DOI] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]