Abstract
Background
Thyroid-associated ophthalmopathy is the commonest orbital disease in adults. However, shortcomings still exist in treatments. The aim of this study was to identify the efficacy and potential mechanism of gypenosides in the treatment of thyroid-associated ophthalmopathy.
Material/Methods
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform was screened for active compounds of gypenosides, and targets were predicted using Swiss Target Prediction. The targets of thyroid-associated ophthalmopathy were obtained from Online Mendelian Inheritance in Man, Comparative Toxicogenomic Database and GeneCards Human gene database. Gene Ontology (GO), the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Reactome Pathways were determined based on the common targets. Protein-protein interaction (PPI) network was constructed to further understand of relationship among target genes, compounds and proteins. Molecular docking was performed to investigate the binding ability between gypenosides and hub genes.
Results
A total of 70 targets for gypenosides and 804 targets for thyroid-associated ophthalmopathy were obtained with 8 common targets identified. GO analysis and KEGG pathway analysis revealed that the hub genes were enriched in JAK-STAT, while Reactome pathways analysis indicated genes enriched in interleukin pathways. PPI network showed STAT1, STAT3, and STAT4 were at the center. Additionally, molecular docking indicated that STAT1 and STAT3 display good binding forces with gypenosides.
Conclusions
This study indicates that target genes mainly enriched in JAK-STAT signaling pathway, particularly in STATs, which can be combined with gypenosides. This may suggest that gypenosides have curative effect on thyroid-associated ophthalmopathy via the JAK-STAT pathway.
MeSH Keywords: Graves Ophthalmopathy, Gynostemma, Computational Biology, Molecular Docking Simulation
Background
Thyroid-associated ophthalmopathy (TAO) is the commonest orbital disease in adults [1]. Ocular lesions are caused by enlargement of the extraocular muscles and periorbital soft tissues due to activation of the surrounding connective tissues [2]. The characteristics of TAO include upper eyelid retraction, edema, periorbital erythema, eyeball protrusion, and other manifestations. About 5%–15% of patients with TAO proceed to a more serious form of the disease although most patients have a mild or a self-limiting form [3]. Patients with a more serious form may proceed to exposed keratitis, corneal ulcer, or optic neuropathy, thus affecting their appearance and vision and TAO can even lead to blindness [4].
However, there are some shortcomings with the existing treatments. Glucocorticoid therapy has anti-inflammatory and immunosuppressive effects, but requires large dosages over long periods of time that might have several hormonal complications such as hyperglycemia, elevated blood pressure, and liver failure [5]. In addition, a small number of TAO patients are not sensitive to hormonal therapy. Besides, orbital decompression surgery is invasive and needs high technical equipment with a long learning curve. Hence, it is urgent to develop new effective treatments to improve the prognosis of TAO patients.
Gypenosides, which are saponins extracted from Gynostemma pentaphyllum, are the most pharmacologically active components of this climbing plant and have been shown to have several bioactivities [6]. Gypenosides can regulate the activation of immune cells and the expression of cytokines and inhibit inflammatory response in different diseases [7,8]. Additionally, gypenosides can decrease the inflammatory response in inflammatory bowel disease by inhibiting the NF-κB and STAT3 signaling pathways [9]. Moreover, gypenosides were shown to enhance Nrf2 signaling pathway and play an antioxidant role in diabetic rats [10]. It has been reported that gypenosides not only have antiproliferation effect, but also promote apoptosis of cancer cells [11,12]. Thus, gypenosides appear to have multiple potential therapeutic targets in inflammatory infiltration, fibrotic proliferation, and oxidative stress injury. TAO is an autoimmune disease in which inflammation, fibrosis, and proliferation exist throughout the disease process. In this article, we will discuss the role and mechanism of gypenosides in TAO, and explore whether gypenosides can be a new therapeutic drug for TAO.
Material and Methods
Computational targets fishing
The molecular formulas of gypenosides were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (http://lsp.nwu.edu.cn/tcmsp.php) [13] and the effective targets were predicted using Swiss Target Prediction (http://www.swisstargetprediction.ch/) [14]. The gypenosides were collected according to the suggested screening criteria: oral bioavailability (OB) ≥30% and drug-likeness (DL) ≥0.18 [15,16]. Targets of TAO were obtained from the Online Mendelian Inheritance in Man (OMIM, https://www.omim.org/) [17], Comparative Toxicogenomic Database (CTD, http://ctd.mdibl.org/) [18], and GeneCards Human gene database (https://www.genecards.org/) [19,20]. We searched “thyroid-associated ophthalmopathy” in GeneCards and “Graves ophthalmopathy” in CTD and OMIM. The first top 500 genes of Reference Count in CTD database were collected. Common targets for gypenosides and TAO were obtained using Venny 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/index.html).
Gene Ontology (GO) analysis and pathway analyses
The Gene Ontology (GO) analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, and Reactome Pathways analysis can provide gene expression data and systematic visual information on the hub genes. Based on common targets for this study, GO, KEGG, and Reactome Pathways analysis were performed to predict the mechanisms employed by gypenosides for treating TAO using the Database for Annotation, Visualization, and Integrated Discovery (DAVID v6.8, https://david.ncifcrf.gov/) [21,22] and STRING v11.0 (https://string-db.org/).
Protein-protein interaction (PPI) network construction
To further understand the complex relationships among compounds, targets, and proteins, based on the information from the STRING protein query, protein-protein interaction (PPI) networks were constructed. We chose 0.70 and 0.10 as thresholds for gypenosides targets and common targets. Nodes disconnected with each other were not displayed. The visualization of the PPI networks was conducted by Cytoscape software 3.6.1.
Molecular docking
Based on the messages of PPI networks, hub proteins were prepared for subsequent docking. The lowest energy conformations were adopted for molecular docking via default parameters using systemsDock (http://systemsdock.unit.oist.jp/iddp/home/index) [23,24]. Docking score represents a negative logarithm of experimental dissociation/inhibition constant value (pKd/pKi) usually ranging from 0 to 10 (from weak to strong combining ability). The detailed information of working diagram is shown in Figure 1.
Figure 1.
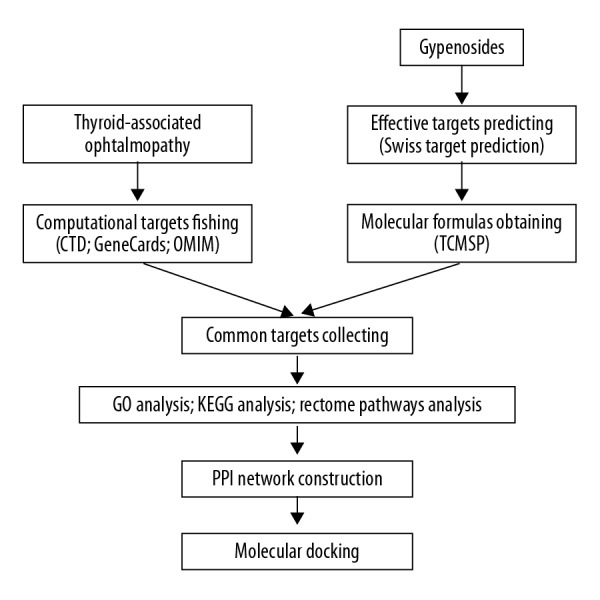
Flow diagram of work.
Results
Compounds screening and targets fishing
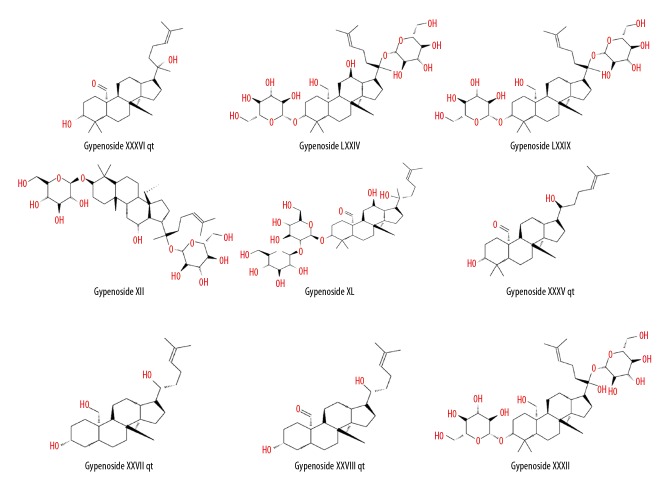
We searched “Gynostemmae Pentaphylli Herba” in the TCMSP database and screened the results with the criteria OB ≥30% and DL ≥0.18. Ultimately, 9 types of gypenosides were identified and their molecular formulas were obtained (Table 1, Figure 2). For each molecular formula, 15 targets were fished. Hence, 70 targets for gypenosides and 804 targets (105 from OMIM, 500 from CTD, and 237 from GeneCards, accessed November 17, 2018) for TAO were gathered after removing duplicates. We found 8 common targets between gypenosides and TAO; the common targets were PTGS2, AR, HSD11B1, NR3C1, STAT3, STAT1, STAT4, and VEGFA (Figure 3).
Table 1.
The characteristics of the nine types of gypenosides.
| Molecule ID | Molecule name | Molecular weight | OB (%) | DL |
|---|---|---|---|---|
| MOL009888 | Gypenoside XXXVI_qt | 458.80 | 37.85 | 0.78 |
| MOL009928 | Gypenoside LXXIV | 801.14 | 34.21 | 0.24 |
| MOL009929 | Gypenoside LXXIX | 785.14 | 37.75 | 0.25 |
| MOL009938 | Gypenoside XII | 785.14 | 36.43 | 0.25 |
| MOL009943 | Gypenoside XL | 799.12 | 30.89 | 0.21 |
| MOL009969 | Gypenoside XXXV_qt | 444.77 | 37.73 | 0.78 |
| MOL009971 | Gypenoside XXVII_qt | 418.73 | 30.21 | 0.74 |
| MOL009973 | Gypenoside XXVIII_qt | 416.71 | 32.08 | 0.74 |
| MOL009976 | Gypenoside XXXII | 787.11 | 34.24 | 0.25 |
OB – represents oral bioavailability; DL – represents drug-likeness.
Figure 2.
Molecular formulas of the 9 types of gypenosides.
Figure 3.
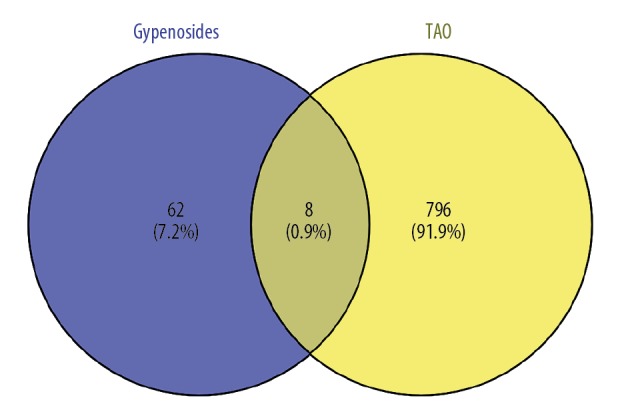
Venn diagram summarizing differentially targets of thyroid-associated ophthalmopathy and gypenosides.
GO analysis, KEGG analysis, and Reactome Pathways analysis
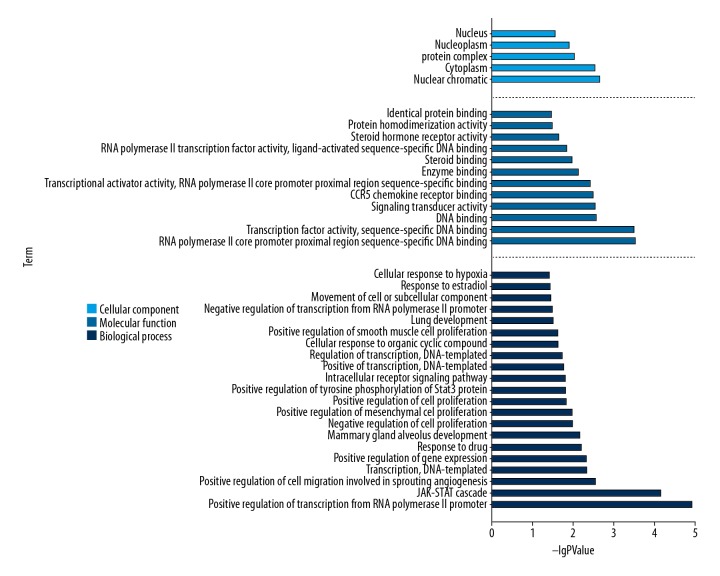
The top significant (cutoff criterion with statistic difference was P<0.05) gene ontology categories are shown in Figure 4. In biological processes (BP), the common targets were mainly enriched in positive regulation of transcription from RNA polymerase II promoter (GO: 0045944); JAK-STAT cascade (GO: 0007259); positive regulation of cell migration involved in sprouting angiogenesis (GO: 0090050); transcription, DNA-templated (GO: 0006351); positive regulation of gene expression (GO: 0010628); response to drug (GO: 0042493), etc. And in molecular functions (MF), the common targets were mainly associated with RNA polymerase II core promoter proximal region sequence-specific DNA binding (GO: 0000978); transcription factor activity, sequence-specific DNA binding (GO: 0003700); DNA binding (GO: 0003677); signal transducer activity (GO: 0004871); CCR5 chemokine receptor binding (GO: 0031730); transcriptional activator activity, RNA polymerase II core promoter proximal region sequence-specific binding (GO: 0001077); enzyme binding (GO: 0019899); steroid binding (GO: 0005496); RNA polymerase II transcription factor activity, ligand-activated sequence-specific DNA binding (GO: 0004879); steroid hormone receptor activity (GO: 0003707); protein homodimerization activity (GO: 0042803), and identical protein binding (GO: 0042802). In addition, cellular components (CC) analysis showed that the common targets were principally involving nuclear chromatin (GO: 0000790); cytoplasm (GO: 0005737); protein complex (GO: 0043234); nucleoplasm (GO: 0005654), and nucleus (GO: 0005634).
Figure 4.
Results of Gene Ontology analysis. Cellular component results indicate the key points are nuclear chromatin and cytoplasm. Biological process results indicate that gypenosides mainly positive regulation of transcription from RNA polymerase II promoter and cell migration involved in sprouting angiogenesis. Molecular function results indicate RNA polymerase II core promoter proximal region sequence-specific DNA binding and transcription factor activity, sequence-specific DNA binding are important acting sites.
Figure 5 reveals the most significant KEGG pathway of the common targets. Such common targets were enriched in pathways in cancer (hsa05200), inflammatory bowel disease (IBD) (hsa05321), pancreatic cancer (hsa05212), hepatitis B (hsa05161), JAK-STAT signaling pathway (hsa04630), microRNAs in cancer (hsa05206), VEGF signaling pathway (hsa04370), leishmaniasis (hsa05140), prolactin signaling pathway (hsa04917), chemical carcinogenesis (hsa05204), and HIF-1 signaling pathway (hsa04066). The Reactome analysis results showed common targets were enriched in interleukin-35 signaling, interleukin-21 signaling, interleukin-4 and interleukin-13 signaling, interleukin-20 family signaling, interleukin-9 signaling, and interleukin-23 signaling (Figure 6).
Figure 5.
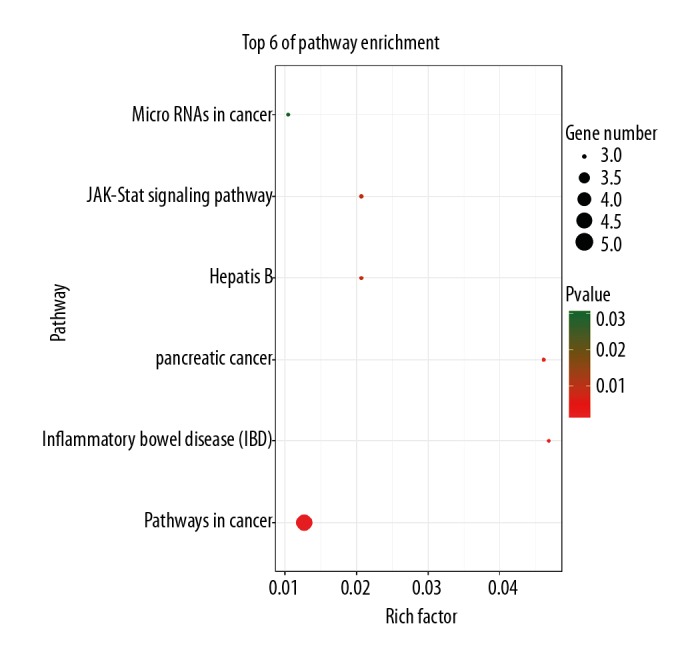
Results of Kyoto Encyclopedia of Genes and Genomes pathways analysis. The results indicate that highly correlated pathways included pathways in cancer, inflammatory bowel disease (IBD), and pancreatic cancer, particularly JAK-STAT signaling pathway. RichFactor represents the ratio of target genes in the pathway and background genes of the pathway.
Figure 6.
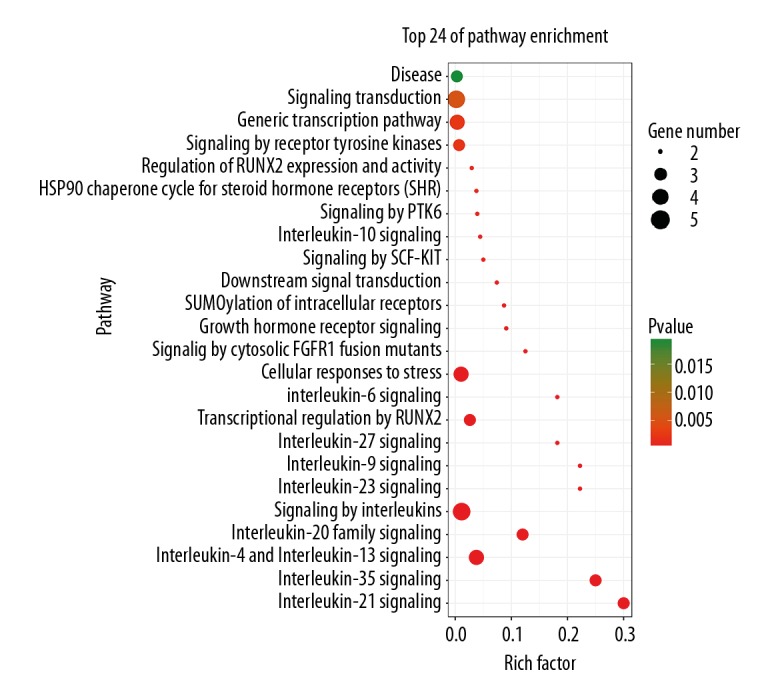
Results of Reactome Pathways analysis. The results indicate that highly correlated pathways included interleukin-35 signaling, interleukin-21 signaling, interleukin-4 and interleukin-13 signaling and interleukin-20 family signaling.
Using such a series of analyses realistically tested may provide valuable information of possible the mechanisms behind protective effects of gypenosides in TAO.
PPI network construction
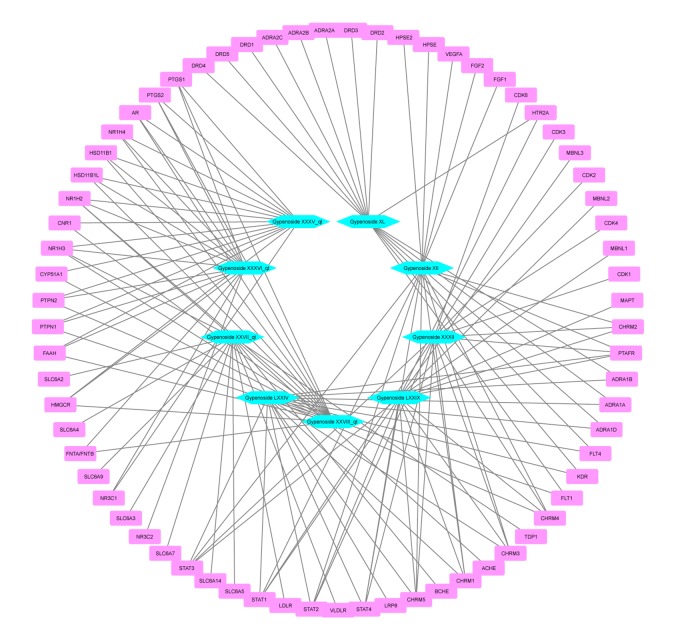
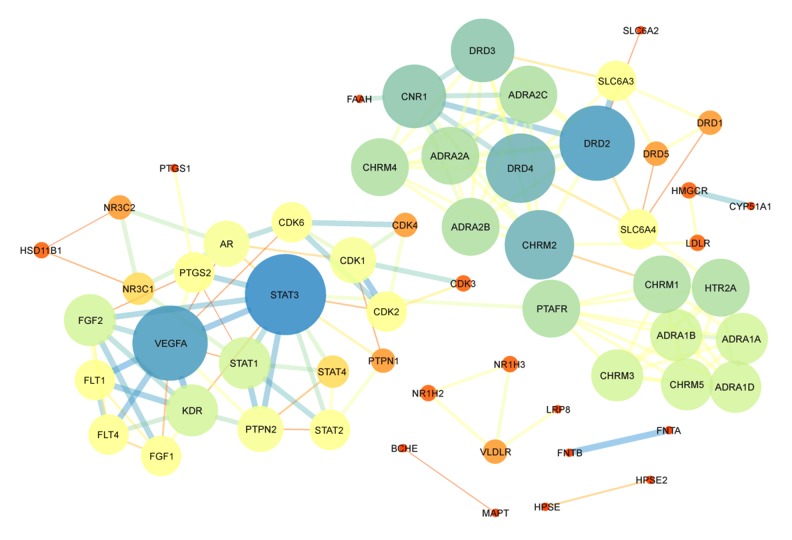
The PPI networks were obtained from the STRING database and visualized by Cytoscape 3.6.1 software. The networks of gypenosides’ active compounds and associated targets showed the relationships between compounds and targets (Figures 7, 8). The PPI networks showed STAT3, VEGFA, CNR1, DRD2, and DRD4 were at the center with high Degree scores. The construction of the PPI network was used to identify the hub genes of common targets (Figure 9). The PPI analysis indicated that STATs (STAT1, STAT3, and STAT4) were at the center of the network.
Figure 7.
Construction of the protein-protein interaction network of gypenosides’ active compounds and associated targets. Hexagons represent active compounds of gypenosides. Round rectangles represent gene symbols of targets.
Figure 8.
Construction of the protein-protein interaction network of proteins expressed by gypenosides. The 59 nodes represent 59 proteins and 148 edges represent 148 pairs of interaction among proteins. The node size and color represent Degree, while edge size and color represent combined score. The data are from STRING.
Figure 9.
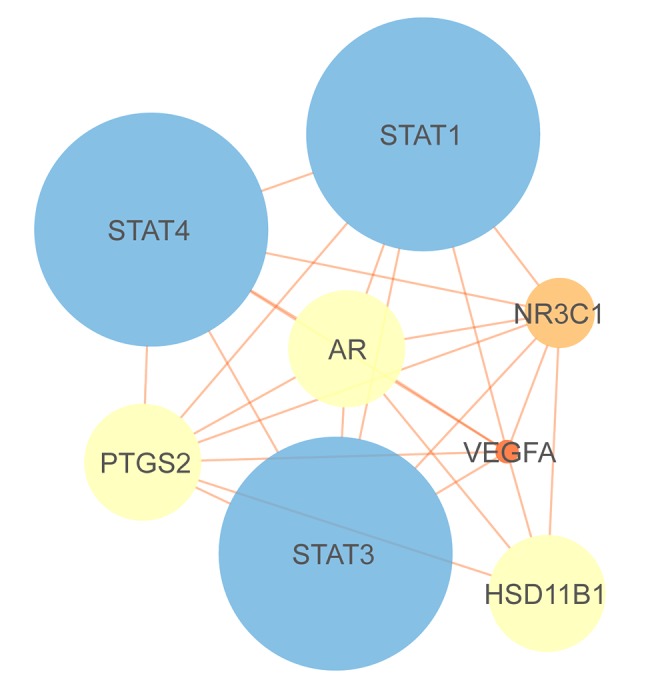
Construction of the protein-protein interaction network of proteins expressed by common targets. The 8 nodes represent 8 proteins and 25 edges represent 25 pairs of interaction among proteins. The node size and color represent Degree, while edge size and color represent combined score. The data are from STRING.
Molecular docking
Molecular docking analysis could provide the potential binding relationships between compounds and target genes. We successfully combined STAT1 and STAT3 to gypenosides with docking scores of around 5.0 (Figure 10), which indicated that STAT1 and STAT3 display good binding forces with gypenosides.
Figure 10.
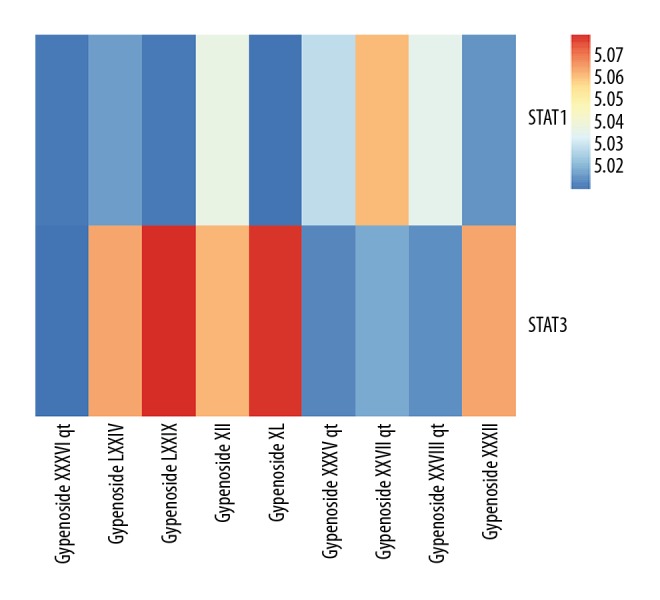
Heatmap shows docking scores of STATs combining to gypenosides. Docking score represents a negative logarithm of experimental dissociation/inhibition constant value (pKd/pKi).
Discussion
In our study, GO analysis including BP, CC, and MF, was performed to annotate the hub genes [25]. Additionally, the KEGG and Reactome pathway analyses were used to enrich the pathways of the crucial genes, which could lead to a deeper understanding of the relationships among active components, target genes, and associated pathways. The information obtained from functional and pathway enrichment analyses, PPI networks, and molecular docking analysis indicated that the common targets were significantly involved in JAK-STAT signaling pathway, pathways in cancer, and other pathways. Moreover, JAK-STAT signaling pathway showed that the STAT signaling pathway can inhibit apoptosis, and regulate cell cycle, proliferation, and differentiation (Figure 11). The core common targets in the JAK-STAT signaling pathway were STAT1 and STAT3. This might be evidence for gypenosides mediate proliferation and differentiation. Additionally, results of Reactome pathway enrichment indicated the common targets were mainly enriched in interleukin signaling pathways. This might indicate that gypenosides can treat TAO via anti-inflammatory action.
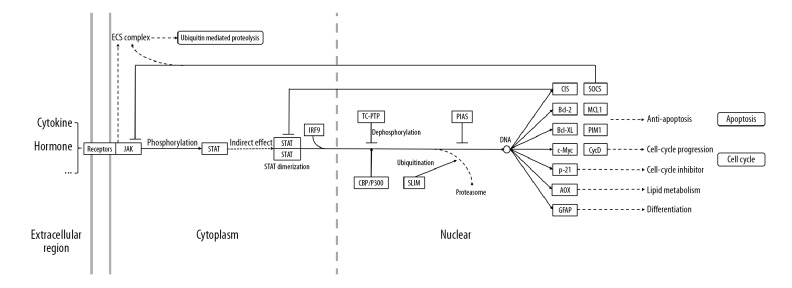
Figure 11.
Schematic drawing to illustrate JAK-STAT signaling pathway.
JAK kinase is a key family of cytoplasmic non-receptor tyrosine protein kinases and plays a pivotal role in signaling transduction pathways of many growth factors and cytokine receptors [26]. At least 7 STAT protein family members have been identified, namely STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 [27]. Receptor-binding cytokines or growth factors can induce JAK activation and phosphorylation, thus initiating the phosphorylation of STATs [28]. In our research, STAT1 and STAT3 are closely related to gypenosides and TAO. Research suggests that inhibiting the expression of STAT1 signaling pathway can inhibit the production of IP-10/CXCL 10 induced by IFN-γ in orbital fibroblasts of patients with TAO, thereby alleviating orbital inflammation [29]. The STAT3 signaling pathway might play an anti-inflammatory, antioxidant, and immunomodulatory role in chronic respiratory diseases, breast cancer, and liver inflammation [30–32]. Inflammation and oxidative stress damage to orbital tissue is the main pathogenesis of TAO [33,34]. Thus, STAT1 and STAT3 signaling pathway might be the crucial target signaling pathways of gypenosides in the treatment of TAO. It might reduce the tissue damage and remodeling caused by orbital inflammation and oxidative stress by regulating the expression of STAT1 and STAT3 signaling pathway.
As a traditional Chinese medicine, gypenosides have been studied in renal fibrosis, liver fibrosis, articular cartilage inflammation, oxidative stress, and apoptosis [35–38]. These studies suggest that gypenosides can play a good therapeutic role, and in cell experiments, although different cells and tissues have different sensitivity to gypenosides, high dose of gypenosides can increase apoptosis. However, there have been no reported adverse reactions to the doses used in animal experiments. Therefore, compared with the local and systemic complications caused by glucocorticoid therapy alone, the use of gypenosides might assist in the treatment of TAO and reduce the dosage of glucocorticoid and thus reduce the associated complications. And the use of gypenosides might be helpful for eye symptoms in patients with hormone-insensitive TAO. Although the existing studies are cell and experimental animal studies, and gypenosides has been used as a traditional Chinese medicine, the drug safety still needs to be verified by randomized controlled clinical trials.
Conclusions
Gypenosides have been proven to have anti-inflammatory and antioxidant biological functions. Through GO analysis, PPI network construction, and molecular docking, we found that gypenosides might play an anti-inflammatory and anti-oxidative role in TAO by mediating STAT1 and STAT3 signaling pathways. Molecular docking can support the application of gypenosides in the treatment of TAO, however, the findings of these in silico methods need to be further verified by in vivo and in vitro experiments.
Acknowledgement
The authors would like to thank Dr Dev Sooranna, Imperial College London, for editing the manuscript.
Footnotes
Source of support: This work was supported by National Natural Science Foundation of China (No. 81360152, No. 81260149) and the Guangxi Natural Science Foundation (No. 2018GXNSFAA281234)
Conflict of interest
None.
References
- 1.Perros PG, Krassas E. Graves orbitopathy: A perspective. Nat Rev Endocrinol. 2009;5(6):312–18. doi: 10.1038/nrendo.2009.61. [DOI] [PubMed] [Google Scholar]
- 2.Pappa A, Lawson JMM, Calder V, et al. T cells and fibroblasts in affected extraocular muscles in early and late thyroid associated ophthalmopathy. Br J Ophthalmol. 2000;84(5):517–22. doi: 10.1136/bjo.84.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menconi F, Profilo MA, Leo M, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: Rundle’s curve revisited. Thyroid. 2014;24(1):60–66. doi: 10.1089/thy.2013.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckstein AK. Thyroid associated ophthalmopathy: Evidence for CD4+T γδ T cells; De novo differentiation of RFD7+ macrophages, but not of RFD1+ dendritic cells; and loss of γδ and αβ T cell receptor expression. Br J Ophthalmol. 2004;88(6):803–8. doi: 10.1136/bjo.2003.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahli E, Gunduz K. Thyroid-associated ophthalmopathy. Turk J Ophthalmol. 2017;47(2):94–105. doi: 10.4274/tjo.80688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai WC, Wong WY, Lee MM, et al. Mechanistic study of the anti-cancer effect of Gynostemma pentaphyllum saponins in the Apc(Min/+) mouse model. Proteomics. 2016;16(10):1557–69. doi: 10.1002/pmic.201500293. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Dang Y, Wang J, et al. Gypenosides attenuate lipopolysaccharide-induced optic neuritis in rats. Acta Histochem. 2018;120(4):340–46. doi: 10.1016/j.acthis.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Yang L, Yang L, et al. Gypenoside IX suppresses p38 MAPK/Akt/NFkappaB signaling pathway activation and inflammatory responses in astrocytes stimulated by proinflammatory mediators. J Sep Sci. 2017;40(6):2137–50. doi: 10.1007/s10753-017-0654-x. [DOI] [PubMed] [Google Scholar]
- 9.Wong WY, Lee ML, Chan BD, et al. Gynostemma pentaphyllumsaponins attenuate inflammation in vitro and in vivo by inhibition of NF-κB and STAT3 signaling. Oncotarget. 2017;8(50):87401–14. doi: 10.18632/oncotarget.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao D, Zhao M, Qi X, et al. Hypoglycemic effect of Gynostemma pentaphyllum saponins by enhancing the Nrf2 signaling pathway in STZ-inducing diabetic rats. Arch Pharm Res. 2016;39(2):221–30. doi: 10.1007/s12272-014-0441-2. [DOI] [PubMed] [Google Scholar]
- 11.Cheng TC, Lu JF, Wang JS, et al. Antiproliferation effect and apoptosis mechanism of prostate cancer cell PC-3 by flavonoids and saponins prepared from Gynostemma pentaphyllum. J Agric Food Chem. 2011;59(20):11319–29. doi: 10.1021/jf2018758. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Lin W, Huang J, et al. Anti-cancer effects of Gynostemma pentaphyllum (Thunb.) Makino (Jiaogulan) Chin Med. 2016;11:43. doi: 10.1186/s13020-016-0114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ru J, Li P, Wang J, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gfeller D, Michielin O, Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29(23):3073–79. doi: 10.1093/bioinformatics/btt540. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13(6):6964–82. doi: 10.3390/ijms13066964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao W, Xu X, Wang X, et al. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145(1):1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–98. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu B, Gifford E, Wang H, et al. Analysis of the ToxCast chemical-assay space using the comparative toxicogenomic database. Chem Res Toxicol. 2015;28(11):2210–23. doi: 10.1021/acs.chemrestox.5b00369. [DOI] [PubMed] [Google Scholar]
- 19.Harel A, Inger A, Stelzer G, et al. GIFtS: Annotation landscape analysis with GeneCards. BMC Bioinformatics. 2009;10:348. doi: 10.1186/1471-2105-10-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30–1.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 21.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 22.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsin KY, Matsuoka Y, Asai Y, et al. SystemsDock: A web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016;44(W1):W507–13. doi: 10.1093/nar/gkw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8(12):e83922. doi: 10.1371/journal.pone.0083922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaudet P, Skunca N, Hu JC, Dessimoz C. Primer on the gene ontology. Methods Mol Biol. 2017;1446:25–37. doi: 10.1007/978-1-4939-3743-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xuan YT, Guo Y, Han H, et al. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA. 2001;98(16):9050–55. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee S, Biehl A, Gadina M, et al. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs. 2017;77(5):521–46. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pu WL, Bai RY, Zhou K, et al. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-κB activation. International Immunopharmacology. 2019;72:204–10. doi: 10.1016/j.intimp.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Alhusaini A, Faddaa L, Ali HM, et al. Amelioration of the protein expression of Cox2, NFκB, and STAT-3 by some antioxidants in the liver of sodium fluoride-intoxicated rats. Dose-Response. 2018;16(3) doi: 10.1177/1559325818800153. 155932581880015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang S, Dauchy RT, Hoffman AE, et al. Epigenetic inhibition of the tumor suppressor ARHI by light at night-induced circadian melatonin disruption mediates STAT3-driven paclitaxel resistance in breast cancer. J Pineal Res. 2019 doi: 10.1111/jpi.12586. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natarajan K, Meganathan V, Mitchell CT, Boggaram V. Organic dust induces inflammatory gene expression in lung epithelial cells via ROS-dependent STAT-3 activation. Am J Physiol Lung Cell Mol Physiol. 2019 doi: 10.1152/ajplung.00448.2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Yuan Y, Zhang Y, et al. Celastrol inhibits IL-1beta-induced inflammation in orbital fibroblasts through the suppression of NF-kappaB activity. Mol Med Rep. 2016;14(3):2799–806. doi: 10.3892/mmr.2016.5570. [DOI] [PubMed] [Google Scholar]
- 34.Rotondo Dottore G, Leo M, Casini G, et al. Antioxidant actions of selenium in orbital fibroblasts: A basis for the effects of selenium in Graves’ orbitopathy. Thyroid. 2017;27(2):271–78. doi: 10.1089/thy.2016.0397. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Chen X, Zong B, et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation. J Cell Mol Med. 2018;22(9):4437–48. doi: 10.1111/jcmm.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan ZH, Zhao Q. Gypenoside inhibits interleukin-1beta-induced inflammatory response in human osteoarthritis chondrocytes. J Biochem Mol Toxicol. 2017;31(9) doi: 10.1002/jbt.21926. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhang J-E, Xiao H-Q, et al. Gypenosides inhibit renal fibrosis by regulating expression of related genes in rats with unilateral ureteral obstruction. J Nephrol. 2011;24(1):112–18. doi: 10.5301/jn.2010.1944. [DOI] [PubMed] [Google Scholar]
- 38.Cui CH, Kim DJ, Jung SC, et al. Enhanced production of gypenoside LXXV using a novel ginsenoside-transforming beta-glucosidase from ginseng-cultivating soil bacteria and its anti-cancer property. J Agric Food Chem. 2017;22(5) doi: 10.3390/molecules22050844. pii: E844. [DOI] [PMC free article] [PubMed] [Google Scholar]