Abstract
Neural cell adhesion molecule (NCAM) is the predominant carrier of the unusual glycan polysialic acid (PSA). Deficits in PSA and/or NCAM expression cause impairments in hippocampal long-term potentiation and depression (LTP and LTD) and are associated with schizophrenia and aging. In this study, we show that impaired LTP in adult NCAM-deficient (NCAM−/−) mice is restored by increasing the activity of the NMDA subtype of glutamate receptor (GluN) through either reducing the extracellular Mg2+ concentration or applying d-cycloserine (DCS), a partial agonist of the GluN glycine binding site. Pharmacological inhibition of the GluN2A subtype reduced LTP to the same level in NCAM−/− and wild-type (NCAM+/+) littermate mice and abolished the rescue by DCS in NCAM−/− mice, suggesting that the effects of DCS are mainly mediated by GluN2A. The insufficient contribution of GluN to LTD in NCAM−/− mice was also compensated for by DCS. Furthermore, impaired contextual and cued fear conditioning levels were restored in NCAM−/− mice by administration of DCS before conditioning. In 12-month-old NCAM−/−, but not NCAM+/+ mice, there was a decline in LTP compared with 3-month-old mice that could be rescued by DCS. In 24-month-old mice of both genotypes, there was a reduction in LTP that could be fully restored by DCS in NCAM+/+ mice but only partially restored in NCAM−/− mice. Thus, several deficiencies of NCAM−/− mice can be ameliorated by enhancing GluN2A-mediated neurotransmission with DCS.
Introduction
The NMDA subtype of glutamate receptor (GluN) plays an essential role in inducing long-term potentiation and depression (LTP and LTD), in cellular models of learning and memory, and in the acquisition of learning tasks (Morris et al., 1986; Morris, 1989; Malenka and Nicoll, 1999; Lynch, 2004; Collingridge et al., 2010). Diminished GluN activity causes deficits in synaptic plasticity and cognitive function in aged animals and animal models of age-related neurodegenerative disorders (Barnes et al., 1997; Shankar et al., 1998; Potier et al., 2000; Clayton et al., 2002; Mothet et al., 2006; Battaglia et al., 2007; Chen et al., 2008). In addition, there is strong evidence supporting a role for GluN dysfunction in patients with schizophrenia (Gao et al., 2000; Ibrahim et al., 2000; Dracheva et al., 2001; Newell et al., 2005). Importantly, agents acting on the glycine binding site of GluN ameliorate the negative symptoms of schizophrenia, particularly when added to antipsychotics (Javitt, 2006). Furthermore, the partial GluN agonist d-cycloserine (DCS) (Hood et al., 1989) augments the effects of fear extinction/exposure therapy in both rodents and humans (Norberg et al., 2008), enhances declarative learning and blood-oxygen level-dependent activity in the hippocampus of healthy humans (Onur et al., 2010), cognition in Alzheimer's disease patients (Tsai et al., 1999), and improves object recognition, working memory, and episodic-like memory in mice (Zlomuzica et al., 2007; Bado et al., 2011).
Recent studies have revealed the importance of GluN modulation by cell adhesion molecules such as the neural cell adhesion molecule (NCAM) (Hammond et al., 2006; Kochlamazashvili et al., 2010). NCAM, which belongs to the Ig superfamily of cell adhesion molecules, is involved in diverse functions during neurodevelopment (Maness and Schachner, 2007), and it is functionally modified by long homopolymers of α2,8-linked sialic acid residues known as polysialic acid (PSA) (Rutishauser, 2008). Several studies have shown that PSA-NCAM expression levels are reduced in the hippocampus of patients with schizophrenia (Barbeau et al., 1995), in the aging human brain (Ni Dhúill et al., 1999; Cox et al., 2009), and in the aging rodent hippocampus (Fox et al., 1995; Seki and Arai, 1995; Abrous et al., 1997; Seki, 2002). NCAM is known to target GluNs to the postsynaptic density (PSD), to co-redistribute with GluNs within PSDs in response to activity, and to modulate GluN activity through PSA (Schuster et al., 1998; Fux et al., 2003; Dityatev et al., 2004; Hammond et al., 2006; Sytnyk et al., 2006; Kochlamazashvili et al., 2010). GluN-dependent forms of LTP and LTD in the hippocampal CA1 region and hippocampus/amygdala-dependent learning are impaired in brains deficient in either NCAM or PSA (Becker et al., 1996; Muller et al., 1996; Eckhardt et al., 2000; Stork et al., 2000; Bukalo et al., 2004; Dityatev et al., 2004; Senkov et al., 2006; Kochlamazashvili et al., 2010).
Given that impaired cognition and synaptic plasticity correlate with the dysregulation of GluNs and PSA-NCAM in aged and schizophrenic brains, we examined the functions of GluNs in LTP/LTD and contextual and cued fear memory in NCAM−/− mice. In this study, we demonstrate that DCS normalizes synaptic plasticity and hippocampus/amygdala-dependent learning in NCAM−/− mice through the modulation of GluNs.
Materials and Methods
Animals
NCAM-deficient (NCAM−/−) mice (Cremer et al., 1994) and their wild-type (NCAM+/+) littermates were inbred into the C57BL/6J genetic background for >10 generations. Adult, age-matched (3-, 12-, and 24-month-old) NCAM−/− and NCAM+/+ littermate mice of both genders from heterozygous breeding pairs were used in the electrophysiological experiments. The animals were singly derived from the internal breeding stock of the Zentrum für Molekulare Neurobiologie Hamburg-Eppendorf (ZMNH).
For the behavioral experiments, we used 3- to 5-month-old NCAM−/− males and their NCAM+/+ littermates. At least 1 week before starting the experiments, the mice were transferred from the animal facility of ZMNH to a small vivarium at the Department of Neurophysiology and Pathophysiology (UKE) where they were housed individually with food and water ad libitum on a reversed 12/12 light/dark cycle (light on at 11:00 P.M.). All behavioral experiments were performed in the afternoon during the dark phase of the cycle when mice are active under constant temperature (22 ± 1°C) and humidity (55 ± 5%). All treatments and behavioral procedures were approved by the Committee on Animal Health and Care of the local governmental body in Hamburg.
Electrophysiological recordings in hippocampal slices
Each mouse was anesthetized with CO2 and decapitated. The brain was removed from the skull, cut into equal hemispheres along the midline and sagittally sliced with a Leica VT 1000M vibratome (Leica) in ice-cold artificial CSF (ACSF) containing (in mm) 250 sucrose, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, and 1.5 MgSO4, pH 7.3. Transverse hippocampal slices were kept at room temperature in carbogen-bubbled ACSF (95% O2/5% CO2) containing 125 mm NaCl instead of 250 mm sucrose for at least 2 h before the start of recording. Recordings were performed in the same solution in a submerged chamber that was continuously superfused at room temperature with carbogen-bubbled ACSF (2–3 ml/min) that included varying concentrations of Ca2+ and Mg2+ as indicated in Results, below. Recordings of field EPSPs (fEPSPs) were performed in the stratum radiatum of the CA1b subfield with a glass pipette filled with ACSF. The resistance of the pipette was 1–2 MΩ. Stimulation pulses were applied to Schaffer collaterals via a monopolar stimulating glass electrode with resistance of <1 MΩ placed ∼300 μm closer to the CA3 subfield than the recording electrode. Basal synaptic transmission was monitored at 0.05 Hz. The inter-theta-burst-stimulation (TBS) interval was 20 s, and 4 TBSs were applied to induce LTP. The TBSs consisted of eight bursts delivered at 5 Hz. Each burst consisted of four pulses delivered at 100 Hz. The duration of each pulse was 0.2 ms, and the stimulation strength was set to provide baseline fEPSPs with amplitudes of ∼50% of the subthreshold maximum.
To detect the DCS-dependent modulation of GluN-mediated transmission, we analyzed the mean amplitude of TBS-elicited fEPSPs 80–180 ms after the beginning of each theta-burst (see Fig. 6A), i.e., at the time when the AMPA receptor-mediated component has mostly declined and the residual slow component is predominantly mediated by GluN2A receptors. This predominance was confirmed by a strong reduction in the residual slow component by a GluN2A antagonist (see Fig. 6B). The mean amplitude of the slow component is expressed as a percentage of the basal level of AMPA receptor-mediated transmission, which was measured as the peak amplitude of the first fast fEPSP elicited by the first TBS. For ANOVA analysis, we used the mean amplitude of the slow component of fEPSPs elicited by eight theta-bursts of the fourth TBS because this parameter was more prominently modulated by DCS.
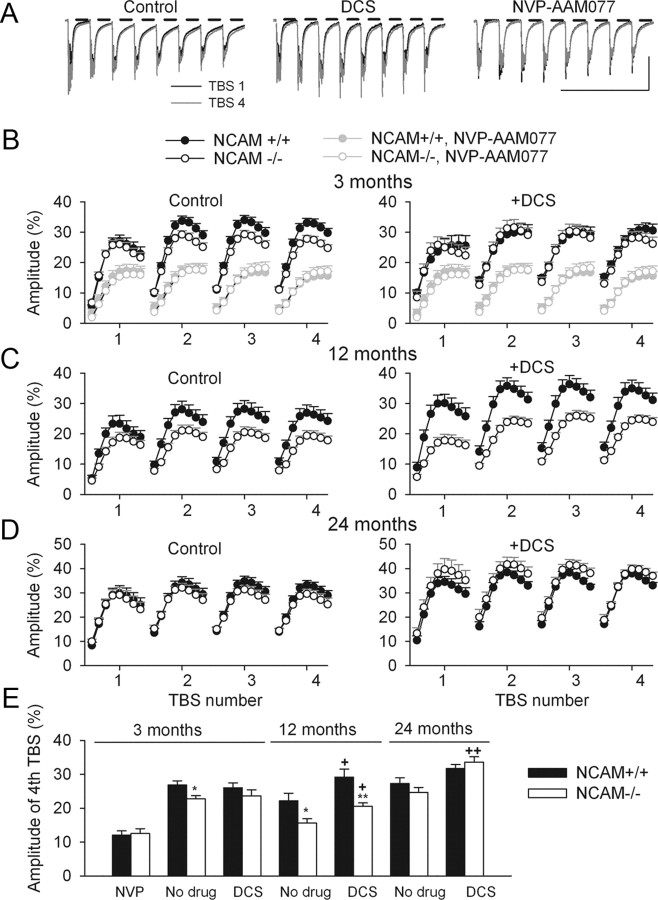
Figure 6.
Modulation of the late component of TBS-elicited fEPSPs by the GluN2A modulators DCS and NVP-AAM077. A, Examples of fEPSPs elicited by the first (black) and fourth (gray) TBS in 3-month-old NCAM+/+ mice, which were recorded in ACSF (Control) and after treatment with DCS or NVP-AAM077. Horizontal bars indicate the time intervals in which the mean amplitudes of the slow components following each theta-burst were measured. An increase in the amplitude of the slow components at the fourth TBS as compared with the first TBS is visible in controls and DCS-treated slices but not in NVP-AAM077-treated slices. Calibration: 1 mV and 500 ms. B–D, Modulation of the mean amplitude of slow fEPSP components during 4 TBSs in 3- (B), 12- (C), and 24-month-old (D) mice in control conditions (left) and after DCS application (right). A strong reduction of the slow component by NVP-AAM077 is illustrated in B. The data are presented as the ±SEM for fEPSPs collected during the LTP experiments shown in Figure 1 and 5. E, ±SEM values of slow fEPSPs elicited by the fourth TBS. *p < 0.05, **p < 0.01, significant differences between genotypes; +p < 0.05, ++p < 0.01, significant increases by DCS within a genotype (SNK test after two-way ANOVA for each age). The numbers of tested slices/mice for the NCAM+/+ and NCAM−/− genotypes are given in the legends to Figures 1 and 5.
An l-type voltage-dependent Ca2+ channel (l-VDCC)-dependent form of potentiation was induced by applying 25 mm tetraethylammonium (TEA), which is a K+ channel blocker (Aniksztejn and Ben-Ari, 1991; Huang and Malenka, 1993), for 7 min in the presence of the GluN antagonist APV (50 μm).
LTD was induced by two trains of low-frequency stimulation (LFS) applied at 1 Hz for 10 min with a 10 min interval between them. Stimulation strengths during baseline recordings, between the two trains of LFS, and after induction of LTD were set to 30–40% of the maximal fEPSPs. The stimulation strength was set to 60–70% when 1 Hz LFS trains were delivered. This protocol has been shown to induce input-specific GluN-dependent LTD in adult rodents (Kerr and Abraham, 1995; Eckhardt et al., 2000).
The data were recorded at a sampling rate of 5 kHz and then filtered (0–2.5 kHz) and analyzed using an EPC10 amplifier and Pulse software (Heka Elektronik). SigmaPlot (Systat Software) was used for statistical evaluation by two-tailed independent Student's t test or one-, two-, or three-way ANOVA (as indicated in the text) followed by the Student–Newman–Keuls (SNK) post hoc test for multiple comparisons. Differences were taken as statistically significant if p < 0.05.
Enzymatic and pharmacological treatment of hippocampal slices
To remove PSA before electrophysiological recordings of LTP, acute hippocampal slices were incubated for 2 h at 35°C in 2 ml of carbogen-bubbled ACSF supplemented with recombinant endoNF (truncated by 245 aa; 9.6 μg/ml) (Stummeyer et al., 2005). Slices incubated without any enzyme or with an inactive truncated form of endoNF (endoNF−) (9.6 μg/ml) (Stummeyer et al., 2005) served as controls. To verify the removal of PSA by endoNF and the absence of changes in the expression of PSA due to the inactive endoNF−, all treated slices were fixed overnight in 4% formaldehyde in PBS, pH 7.3, cut into 30-μm-thick subslices and immunostained for PSA using a monoclonal antibody (clone 735) (Eckhardt et al., 2000). The selected concentration of endoNF completely removed PSA in all recorded slices as shown previously (Kochlamazashvili et al., 2010).
The following compounds were used in our experiments: [(R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydro-quinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077, 0.25 μm, generously supplied by Dr. Yves Auberson from the Novartis Institutes for BioMedical Research, Basel, Switzerland), a GluN antagonist with preferential inhibition of GluN2A-containing receptors; R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenyl-methyl)-1-piperidinepropranolol (Ro 25-6981 hydrochloride, 0.5 μm; Tocris Bioscience), a GluN antagonist with specific inhibition of GluN2B-containing receptors; dl-2-amino-5-phosphonovaleric acid (APV, 50 μm; Tocris Bioscience), a broad-spectrum GluN antagonist; (R)-4-amino-3-isoxazolidone (d-cycloserine, DCS, 40 μm; Sigma), a coagonist of GluNs with a preferential effect on GluN2A-containing GluNs in the presence of ambient glycine and d-serine; tetraethylammonium (TEA), a K+ channel blocker (25 mm; Sigma); and 4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB 203580, 10 μm; Sigma), an inhibitor of p38 MAP kinase.
Fear conditioning
Training.
To assess both contextual and cued fear memories, an auditory fear conditioning paradigm was used (Senkov et al., 2006). Fear conditioning was performed as follows. (1) After 2 d of handling and habituation in a neutral context (home cage) for 5 min each day, the mice were injected intraperitoneally with DCS in a 0.9% NaCl solution or with vehicle control 15 min before training (Ledgerwood et al., 2004; Richardson et al., 2004). (2) Subsequently, the mice were placed into a conditioning chamber (context CC+) and allowed to freely explore this chamber for 180 s. The freezing level during this time was taken as the baseline (see Fig. 4C, “B”). (3) In the next step, the mice were subjected to two pairings of a 20 s conditioned stimulus (CS+) presentation (delivered to half of the mice as a patterned tone, i.e., a series of 50-ms-long 75 dB tones of 2.5 kHz at 1 Hz, and to the other half of the mice as a continuous tone of the same frequency) separated by 60 s and coterminated with a footshock (0.7 mA, 1 s) as the unconditioned stimulus. (4) Sixty seconds after the last shock, the mice were returned to their home cages.
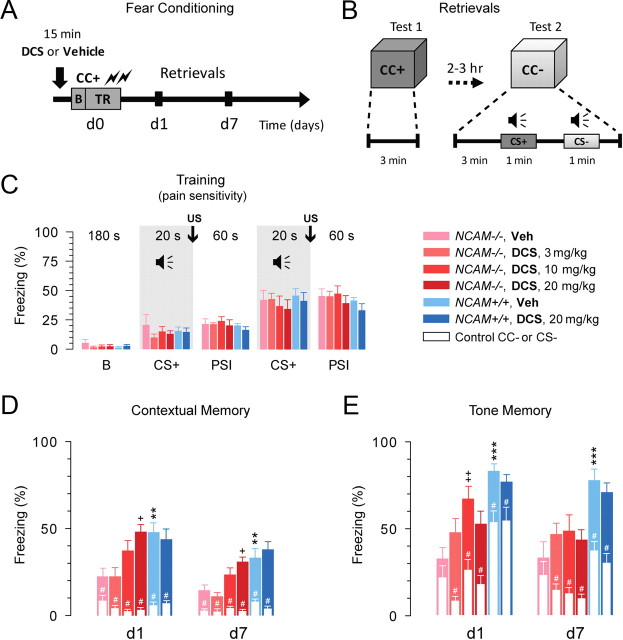
Figure 4.
Systemic administration of DCS restores contextual and tone memories in NCAM−/− mice. A, A schematic of the experiment; intraperitoneal injection of either drug or vehicle 15 min before the beginning of the training session; TR, training in the conditioned context CC+; B, baseline; d1 and d7, days 1 and 7 after training. B, A schematic of the retrieval tests at days 1 and 7; CC+ and CC−, conditioned and neutral contexts; CS+ and CS−, conditioned and neutral auditory stimuli. C, Levels of freezing before (baseline “B”, 180 s) and during the fear conditioning procedure. CS+: pairing of the conditioned tone (CS+, 20 s) with two footshocks (US), and PSI: poststimulus interval (60 s). Two-way repeated-measures ANOVA did not detect any differences between the six injected groups before or during training (p = 0.83). However, freezing increased significantly above the baseline after the first and second footshocks (p < 0.001). D, Contextual memory tests: levels of freezing on the two posttraining days d1 and d7 in the conditioned context (CC+, colorful bars) and in the control neutral context (CC−, white bars). The latter was assessed 3 min before tone presentation as shown in panel (B). All tests are taken from the same mice. E, Tone memory tests: levels of freezing during presentation of the conditioned tone (CS+, colorful bars) and the control tone (CS−, white bars) on the two posttraining days d1 and d7. The DCS dosages were 0 (vehicle, Veh), 3, 10, and 20 mg/kg of body weight; white color represents responses to the neutral context CC− and the auditory stimulus CS-. Two-way repeated-measures ANOVA revealed a difference in freezing in the CC+ between the six injected groups (p < 0.001). Freezing responses to both cued stimuli CS+ (p < 0.01) and CS− (p < 0.001) were also different between groups. +p < 0.05, ++p < 0.01, significant rescue by DCS (SNK test); **p < 0.01, ***p < 0.001, significant differences between genotypes (SNK test). #p < 0.05, significant differences between CC+ versus CC− or CS+ versus CS− (Wilcoxon test). The number of tested mice for each group: 11 (NCAM+/+, Veh), 8 (NCAM+/+, DCS 20 mg/kg), 7 (NCAM−/−, Veh), 7 (NCAM−/−, DCS 3 mg/kg), 8 (NCAM−/−, DCS 10 mg/kg), 8 (NCAM−/−, DCS 20 mg/kg).
Tests.
The following similar tests were performed on days 1 and 7 after the induction of fear conditioning in the same cohort of mice. (1) Each mouse was placed into the CC+ conditioning chamber for 3 min to assess the retention of contextual memory and then returned to its home cage. (2) Approximately 2–3 h later, the same mouse was placed into a new, neutral context (CC−, a tone cage) and allowed to freely explore this cage for 180 s (this allowed us to monitor freezing in the CC− and to discriminate between the CC+ and CC− as a measure of the level of generalization). (3) The tone previously paired with footshock (either patterned or continuous, see Training, above) was presented for 60 s to assess tone memory; 120 s later, a 60 s presentation of the unpaired CS− tone was given (either patterned or continuous), the unpaired tones were counterbalanced with the paired ones during the tests; e.g., if CS+ was the patterned tone, CS− was the continuous tone, and vice versa. (4) Sixty seconds after the last tone presentation, the mice were returned to their home cages.
Apparatus.
Two fear conditioning chambers with two distinct CC+ contexts were used for conditioning: an aluminum chamber that was precleaned with 75% ethanol and a Plexiglas chamber that was precleaned with 1% acetic acid in water. There were two tone cages associated with the neutral CC− contexts: a tone cage with removable wall papers with drawn rectangular cells, which was flavored with vanilla bakery oil, and another cage containing removable wall papers with drawn black spots, which was flavored with lemon bakery oil. Both cages were cleaned with water before placing a mouse inside. One of two distinct CC+ stimuli and one of two distinct CC− stimuli were presented to the mouse to minimize any dependency of the results on a particular choice of CC+ or CC−. Patterned and continuous tones were used as the CS+ and CS− because mice have been shown to discriminate well between these stimuli in a fear conditioning paradigm (Tang et al., 2003; Senkov et al., 2006).
All experiments were performed in a double-walled sound-attenuated isolation cubicle (for details, see Senkov et al., 2006). The delivery of tones and shocks was controlled by customized software (Signal 1.88, CED) using a PC computer (Pentium-II, 340 MHz, 128 MB RAM) through an AD/DA converter (Micro 1401 board, CED).
The behavior of the mice during conditioning and testing was recorded on a Pentium-4 computer (CPU 3 GHz, 2 GB RAM) using a video frame-grabber (Pixelsmart) that digitized streaming video at three frames per second and stored it on the hard-drive as a set of 8-bit gray images. These images were then converted into “avi” format movies by the freely available Java-based program ImageJ (http://rsb.info.nih.gov/ij). All recorded movies were then analyzed using Microsoft Windows Media Player 9 by a trained observer blind to the genotype and treatment of the mouse. Freezing time was taken as a measure of fear-related memory and quantified as described previously (Tang et al., 2001). Briefly, freezing was defined as the animal remaining motionless, except for respiratory movements, in a tense posture characterized by horizontal positioning of the head, a stretched state of the body, and stiffening of the tail.
DCS administration
DCS was freshly dissolved before use in a 0.9% isotonic NaCl solution and injected intraperitoneally at three different concentrations (3, 10, or 20 mg/kg) 15 min before the training procedure, which is a time that is consistent with the normal half-life of DCS in the mouse circulatory system (Conzelman and Jones, 1956). A control group of animals was injected with 0.9% NaCl solution (vehicle group) in the same manner as the DCS group.
Because fear conditioning is sensitive to stress, especially stress that occurs before training, special care was taken to minimize stress during the intraperitoneal injection. Thus, all mice were lightly sedated with a mixture of CO2 and room air for the period of the injection. Recovery from the anesthesia was fast (2–3 min), and there were no side effects on the fear conditioning experiments (Senkov et al., 2006).
Statistical analysis
To analyze the effects of the injected compounds on contextual memory, we used two-way ANOVA (Systat, Systat Software) with “Trial” as a within-group factor; where significant differences were found, post hoc SNK tests were used. Additionally, statistical comparisons between freezing responses to CS+ versus CS− and in CC+ versus CC− on a given day within each group were performed using the nonparametric Wilcoxon signed-rank test. The significant differences between groups detected by the Wilcoxon and paired t tests were the same, and we therefore refer only to p values provided by the Wilcoxon test in the text.
Results
GluN- but not l-VDCC-dependent LTP is impaired in NCAM−/− mice
Analysis of the synaptic plasticity induced by multiple trains of theta-burst stimulation (TBS) applied to Schaffer collaterals/commissural fibers at standard extracellular concentrations of Ca2+ (2.0 mm) and Mg2+ (1.5 mm) revealed impaired CA1 LTP in NCAM−/− mice (125.6 ± 2.8%) compared with NCAM+/+ mice (146.2 ± 2.6%) (Fig. 1A,F). This is in line with previous findings (Muller et al., 1996; Kochlamazashvili et al., 2010). LTP induction by TBS involves the activity of both GluNs and l-VDCCs (Huber et al., 1995; Morgan and Teyler, 2001), and both of these channels are implicated in NCAM-mediated function (Williams et al., 1994). Therefore, we investigated whether signaling via l-VDCCs may be impaired in NCAM−/− mice. A GluN-independent, l-VDCC-dependent form of LTP was induced by applying the K+ channel antagonist tetraethylammonium (TEA) in the presence of the GluN antagonist APV (Huber et al., 1995). The levels of LTP were similar in both genotypes 1 h (135.0 ± 7.2% and 148.6 ± 9.1% in NCAM−/− and NCAM+/+ mice, respectively; Fig. 1B,F) and 2 h (144.9 ± 12.7% in 5 slices from 3 NCAM−/− mice vs 143.5 ± 6.6% in 6 slices from 3 NCAM+/+ mice, data not shown) after LTP induction. These results support the view that the constitutive ablation of NCAM leads to impaired signaling via GluNs, but not via l-VDCCs.
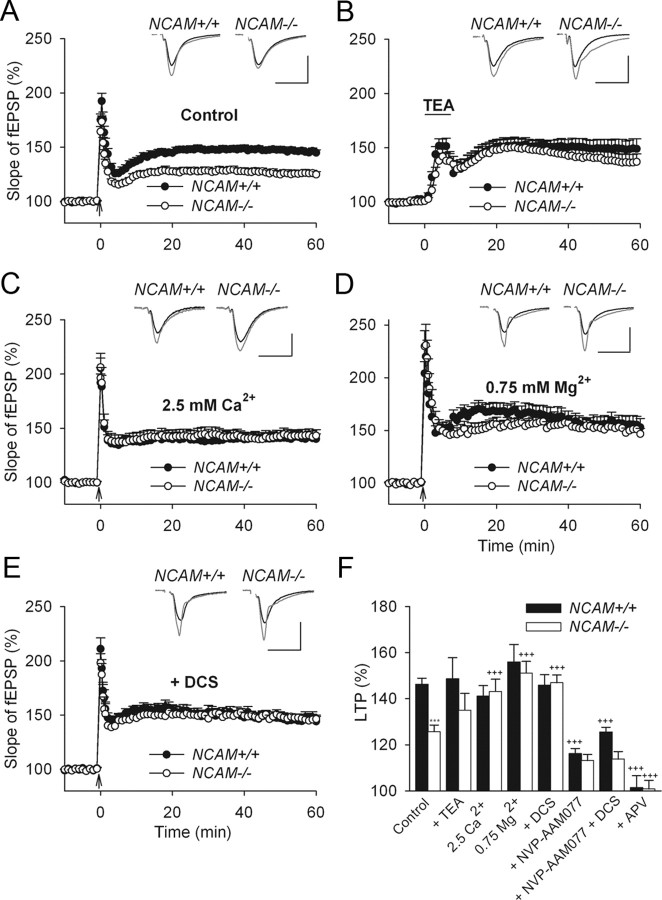
Figure 1.
NMDA receptor-dependent rather than l-type Ca2+ channel-dependent LTP is impaired in NCAM−/− mice. A, TBS-induced LTP is impaired in slices from NCAM−/− mice. B, Normal levels of l-type Ca2+ channel-dependent LTP are induced in NCAM−/− mice by TEA (25 mm, for 7 min, indicated by the horizontal bar) in the presence of the GluN antagonist APV. C, Increasing the extracellular Ca2+ concentration from 2.0 to 2.5 mm rescues LTP in NCAM−/− slices to the level of NCAM+/+ slices. D, Decreasing the extracellular Mg2+ concentration to 0.75 mm (instead of 1.5 mm) results in equal levels of LTP in the CA1 region of slices from NCAM−/− and NCAM+/+ mice. E, Restoration of impaired LTP in NCAM−/− mice by application of the GluN agonist DCS. A–E, Each value is an average of three consecutive time points. The mean slope of fEPSPs recorded 0–10 min before LTP induction is taken as 100%, and the arrows indicate the delivery of TBS. The data are presented as the mean + SEM. Each trace is the average of 30 fEPSPs recorded 10 min before or 50–60 min after LTP induction. Calibration: 1 mV and 20 ms. F, Means + SEMs of LTP levels recorded 50–60 min after LTP induction in the presence of GluN modulators. In addition to the effects depicted in panels (A–E), the graph shows that LTP is reduced to the same levels in both genotypes by the GluN2A antagonist NVP-AAM077, and it is fully blocked by the subtype-nonspecific GluN antagonist APV. Two-way ANOVA of all TBS-induced LTP values revealed effects of genotype (p = 0.023), treatment (p < 0.001), and a genotype × treatment interaction (p = 0.011). ***p < 0.001, significant difference between untreated NCAM+/+ and NCAM−/− slices; +++p ≤ 0.001, significant difference between untreated control and pharmacologically treated slices of the same genotype (SNK test). The numbers of tested NCAM+/+ and NCAM−/− slices/mice are, respectively: 24/19 and 20/17 (control), 13/6 and 12/5 (TEA), 8/5 and 8/4 (2.5 mm Ca2+), 9/6 and 8/4 (0.75 mm Mg2+), 11/4 and 12/5 (DCS), 10/8 and 7/6 (NVP-AAM077), 7/3 and 6/3 (NVP-AAM077 + DCS), and 6/3 and 7/3 (APV).
In our recent study, we observed a deficit in synaptic transmission mediated by GluN2A in NCAM−/− mice (Kochlamazashvili et al., 2010). We hypothesized that increasing the Ca2+ influx through GluNs by elevating extracellular Ca2+ levels would improve LTP induction in NCAM−/− mice, as has been previously reported in CaMKII/cre conditional NCAM-deficient mice (Bukalo et al., 2004). Indeed, elevating extracellular Ca2+ from 2.0 to 2.5 mm increased LTP in NCAM−/− mice to 143.1 ± 5.3%, a level not different from that recorded in NCAM+/+ mice (141.0 ± 4.5%, Fig. 1C,F). In addition, reducing the extracellular Mg2+ concentration from 1.5 to 0.75 mm, which is known to relieve the Mg2+ blockade of GluN receptors, also increased LTP in NCAM−/− mice (151.1 ± 5.0%) to the levels found in NCAM+/+ mice (155.9 ± 7.6%) (Fig. 1D,F). Neither Ca2+ elevation nor Mg2+ reduction changed the levels of LTP in NCAM+/+ mice, suggesting that normal Ca2+/Mg2+ levels are sufficient to induce GluN-mediated signaling in NCAM+/+ mice but not in NCAM−/− mice.
To target GluNs more specifically, we used DCS as a partial agonist of the GluN glycine binding site. DCS is known to rescue deficits in GluN-dependent LTP in 23- to 27-month-old wild-type rats (Billard and Rouaud, 2007) and injured mice (Yaka et al., 2007). Two-way ANOVA of LTP levels in untreated and DCS-treated NCAM+/+ and NCAM−/− mice revealed significant effects of genotype (p = 0.006) and DCS (p = 0.003) and a significant interaction between genotype and DCS (p = 0.002). DCS did not affect LTP in slices from 3-month-old NCAM+/+ mice (145.8 ± 4.5%) as compared with untreated slices (Fig. 1E,F), but it restored LTP in NCAM−/− mice of the same age to the levels observed in NCAM+/+ mice (146.9 ± 3.3%) (Fig. 1E,F).
To test whether the inhibition of GluN2A in NCAM+/+ mice would result in LTP impairment similar to that observed in NCAM−/− mice, we induced LTP in the presence of NVP-AAM077, an antagonist with a higher preference for GluN2A than GluN2B receptors (Bartlett et al., 2007). We used a concentration that inhibits ∼80% of the current mediated by GluN2A receptors (Bartlett et al., 2007). A three-way ANOVA of LTP levels in NCAM+/+ and NCAM−/− slices in the presence of NVP-AAM077 or DCS revealed significant effects of genotype (p = 0.002), DCS (p = 0.004), and NVP-AAM077 (p < 0.001) and a significant interaction between genotype, DCS and NVP-AAM077 treatments (p = 0.005). Application of NVP-AAM077 strongly reduced LTP in NCAM+/+ mice (116.1 ± 2.2%, Fig. 1F) to the level detected in untreated slices from NCAM−/− mice (Fig. 1A). Remarkably, inhibiting GluN2A in NCAM−/− mice did not result in any further significant reduction in LTP (113.1 ± 2.6%, Fig. 1F). As has been reported previously (Köhr et al., 2003; Liu et al., 2004; Li et al., 2006), these data support the view that GluN2A activation is required for LTP induction in adult mice. Furthermore, our data also indicate that a deficit in GluN2A-dependent synaptic transmission contributes to the LTP impairment in NCAM−/− mice.
Previous reports indicated that DCS provides greater potentiation of GluN2A- than GluN2B-mediated currents (Sheinin et al., 2001) and that ambient concentrations of glycine and d-serine are sufficient to saturate the glycine binding site of GluN2B but not GluN2A receptors (Kutsuwada et al., 1992; Priestley et al., 1995). Therefore, we asked whether DCS facilitates LTP in the mutant predominantly via GluN2A receptors. Indeed, NVP-AAM077 fully abolished the potentiating effects of DCS on LTP in NCAM−/− mice (Fig. 1F). In agreement with our data showing that NCAM+/+ mice have higher levels of synaptic transmission via GluN2A receptors than NCAM−/− mice (Kochlamazashvili et al., 2010), slices from NCAM+/+ mice treated with DCS plus NVP-AAM077 exhibited significantly elevated LTP (125.1 ± 2.0%, Fig. 1F) compared with slices from NCAM−/− mice (113.7 ± 3.2%, Fig. 1F). These data suggest that the residual (∼20%) activity of GluN2A receptors in the presence of DCS and NVP-AAM077 is higher in NCAM+/+ than in NCAM−/− mice and that in combination with other mechanisms activated by TBS, this activity facilitates LTP induction. The application of a subunit nonspecific antagonist of GluNs, APV, fully blocked LTP in both NCAM+/+ and NCAM−/− mice (Fig. 1F), confirming that this form of LTP is GluN-dependent in both genotypes.
Deficits in LTP induced by PSA ablation can be rescued with DCS
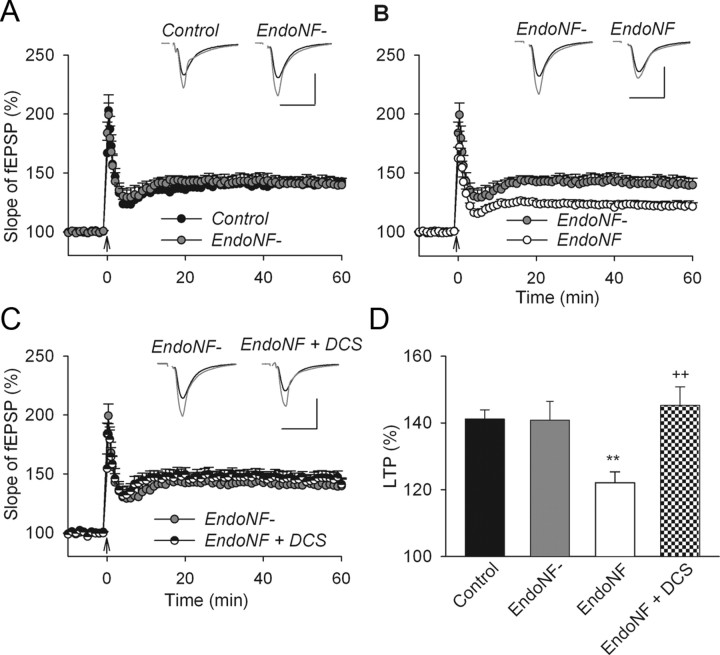
Because some pathological conditions (e.g., schizophrenia) are associated with a deficit in PSA rather than NCAM (Barbeau et al., 1995; Seki and Arai, 1999; Cox et al., 2009), we tested whether impaired LTP in PSA-deficient slices could be compensated for by GluN modulation. For this experiment, we incubated hippocampal slices from NCAM+/+ mice for 2 h with endoNF. This treatment removes the PSA that is diffusely expressed in the stratum radiatum of CA1 and CA3 and prominently expressed in mossy fibers in untreated slices or slices treated with the inactive mutated form of endoNF (endoNF−) (Kochlamazashvili et al., 2010). Control recordings from sham-incubated slices (endoNF and endoNF− treated slices were incubated for 2 h in a small chamber) showed levels of LTP (141.2 ± 2.7%; Fig. 2A) that were unchanged when compared with non-incubated NCAM+/+ slices (146.2 ± 2.6%; Fig. 1A). Furthermore, inactive endoNF− did not inhibit LTP (140.8 ± 5.6%; Fig. 2A,D). Removal of PSA by active endoNF impaired LTP (122.1 ± 3.2%; Fig. 2B,D), as previously reported for endoneuraminidase N (Becker et al., 1996; Muller et al., 1996). The level of LTP after endoNF treatment was close to the level of LTP in untreated NCAM−/− mice. This finding is in line with previous LTP recordings that demonstrated an overlap of the effects produced by constitutive ablation of NCAM and acute enzymatic removal of PSA (Muller et al., 1996). Modulation of GluNs with DCS (Fig. 2C,D) increased LTP levels in endoNF− treated slices from 122.1 ± 3.2% to 145.2 ± 5.5%. These LTP levels are equivalent to those observed after treatment with inactive endoNF− (Fig. 2C,D). Thus, these data confirm the results from the experiments in NCAM−/− mice and demonstrate that impaired LTP in both NCAM- and PSA-deficient slices can be rescued by enhancing GluN2A-mediated signaling with DCS.
Figure 2.
Restoration of impaired LTP in endoNF treated wild-type hippocampal slices via facilitation of GluN receptors. A, LTP levels are normal in slices treated with the inactive form of endoNF (endoNF−) compared with sham-treated controls. B, In contrast, LTP is impaired in slices treated with the active form of endoNF. C, Restoration of LTP in endoNF treated slices to normal levels by application of the GluN agonist DCS. A–C, The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. D, Means + SEMs of LTP levels recorded in wild-type slices 50–60 min after TBS application. One-way ANOVA revealed a significant difference between groups (p = 0.002). **p < 0.01, significant difference between endoNF treated slices and those either sham or endoNF− treated, ++p < 0.01, significant effects of DCS on endoNF-treated slices (SNK test). The number of tested slices/mice for each group is as follows: 8/8 (control), 6/5 (endoNF−), 9/9 (endoNF) and 8/5 (endoNF + DCS).
Impaired LTD can be restored in NCAM−/− mice with DCS
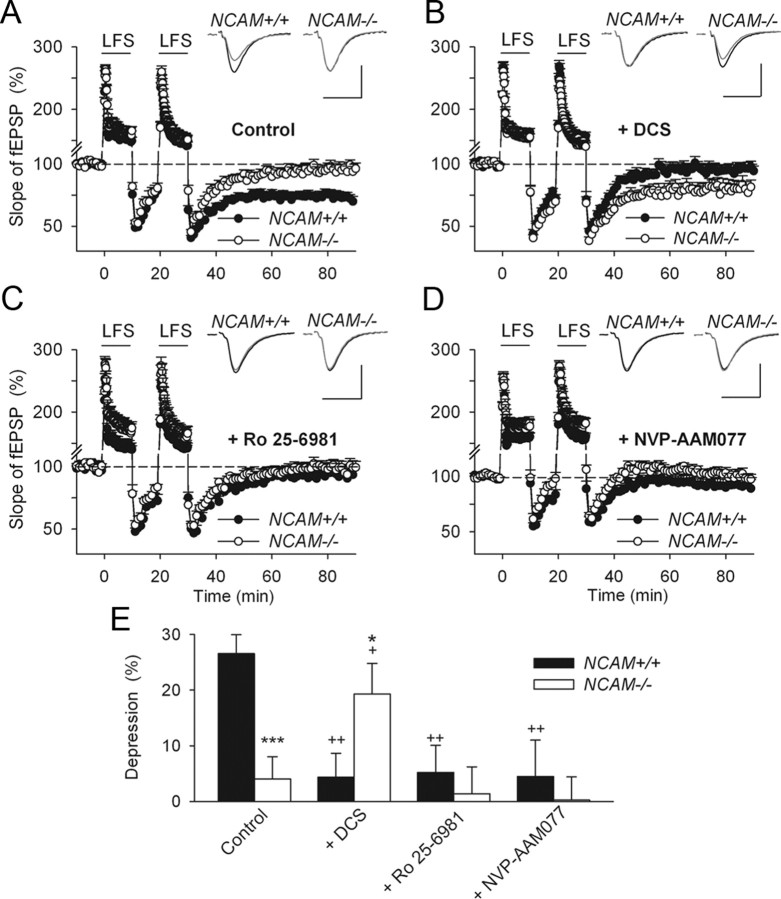
Next, we tested whether abnormalities in GluN-mediated signaling also underlie the impairment of LTD in NCAM−/− mice. To induce robust GluN-dependent LTD in slices from adult mice, we used two trains of low-frequency stimulation (Eckhardt et al., 2000; Evers et al., 2002). This protocol reliably induced LTD of 26.5 ± 3.4% in NCAM+/+ mice (Fig. 3A,E). In contrast, LTD was completely abolished in NCAM−/− mice (4.0 ± 3.9%; Fig. 3A,E), as reported for CaMKII/cre conditional NCAM-deficient mice (Bukalo et al., 2004) and for PSA-deficient brain slices (Muller et al., 1996). Applying DCS restored LTD to 19.2 ± 5.4% in NCAM−/− mice, whereas it fully blocked LTD in NCAM+/+ mice (Fig. 3B,E). Blockade of either GluN2B or GluN2A receptors by perfusion of slices with Ro 25-6981 or NVP-AAM077, respectively, inhibited LTD in both genotypes (Fig. 3C–E). These data are consistent with a model in which signaling through both GluN2A and GluN2B receptors is required to induce LTD in response to two trains of low-frequency stimulation in adult mice, and they support the view that LTD can be disrupted by either excessive or deficient levels of GluN-mediated synaptic transmission (Hansel et al., 1996, 1997). Thus, deficits in transmission via GluN2A-containing receptors impair LTD in NCAM−/− mice, and this impairment can be rescued by DCS. In NCAM+/+ mice, DCS presumably increases GluN-mediated synaptic transmission above the level at which LTD can be induced.
Figure 3.
Modulation of NMDA receptors restores impaired LTD in slices from NCAM−/− mice. A, Two trains of LFS (shown by the horizontal bar) induce LTD in untreated slices from NCAM+/+ mice but not NCAM−/− mice. B, Restoration of LTD in NCAM−/− mice and impairment of LTD in NCAM+/+ mice by application of the GluN agonist DCS. C, D, Inhibition of LTD in NCAM+/+ by the GluN2B selective antagonist Ro 25-6981 (C) and the preferentially GluN2A blocking NVP-AAM077 (D). A–D, Each value is an average of three consecutive time points. The mean slope of the fEPSPs recorded 0–10 min before LTD induction is taken as 100%. The data are presented as the ±SEM. Each trace is the average of 30 fEPSPs recorded 10 min before or 50–60 min after LTD induction. Calibration: 0.5 mV and 20 ms. E, Means + SEMs of LTD levels recorded 50–60 min after LTD induction in the presence of GluN modulators. Two-way ANOVA detected an effect of treatment (p = 0.02) and an interaction between treatment and genotype (p = 0.001). *p < 0.05 and ***p < 0.001, significant difference between NCAM+/+ and NCAM−/− mice treated with the same drug, +p < 0.05, ++p < 0.01, significant difference between control and pharmacologically treated mice of the same genotype (SNK test). The numbers of tested NCAM+/+ and NCAM−/− slices/mice for each group are as follows: 24/21 and 13/13 (control), 13/11 and 12/8 (DCS), 8/7 and 9/6 (Ro 25-6981) and 12/10 and 8/5 (NVP-AAM077).
Modulating NMDA receptors restores learning in the fear conditioning paradigm in NCAM−/− mice
NCAM−/− mice are impaired in both contextual and cued (tone) memory in fear conditioning paradigms (Stork et al., 2000; Senkov et al., 2006). To verify the hypothesis that these deficits in learning are related to a decrease in GluN2A receptor signaling, we attempted to rescue these learning deficits by enhancing GluN activity. DCS has been shown to improve performance on hippocampus-dependent spatial memory tasks and associative conditioning in aged animals (Baxter et al., 1994; Thompson and Disterhoft, 1997; Aura et al., 1998; Aura and Riekkinen, 2000) and to rescue LTP and LTD in NCAM−/− mice (present study). Therefore, we systemically injected NCAM+/+ and NCAM−/− mice with DCS or vehicle 15 min before fear conditioning training (Fig. 4A) and tested their retrieval of long-term fear memory [which by definition persists for one day or longer (Rodrigues et al., 2004)], on the first and seventh days after training (Fig. 4A,B). It is noteworthy that the tests performed on the first day after training led to a new association of the conditioned tone and the context with a lack of footshock. Therefore, the freezing measured on the seventh day was a function of both fear conditioning and memory extinction.
NCAM−/− mice injected with vehicle showed a twofold decrease in percentage of freezing after training on either test day compared with vehicle-injected NCAM+/+ mice (Fig. 4D). However, after DCS administration, these mice exhibited a dose-dependent increase in freezing in the conditioned context (CC+), showing contextual memory comparable to that in NCAM+/+ mice injected with vehicle. The normal freezing responses in NCAM−/− mice injected with DCS before, between, and immediately after the application of two footshocks (Fig. 4C) suggest that DCS changed neither pain sensitivity nor levels of anxiety during the acquisition of fear conditioning. To test whether DCS affects learning and memory in NCAM+/+ mice, DCS was injected at the dose most effective in NCAM−/− mice. This treatment did not produce any effects on contextual or tone memory (Fig. 4D,E).
The generalization of fear conditioning is an important aspect of aversive learning. It is manifested as a loss of stimulus specificity and as the emotional sensitization of associative components of memory, and leads to generalized anxiety (Laxmi et al., 2003; Ciocchi et al., 2010). To rule out the possibility that the effects of DCS were due to nonspecific increases in freezing, we assessed the ability of mice to discriminate a conditioned tone (CS+) or context (CC+) from an unconditioned neutral tone (CS−) or context (CC−). As shown in Figure 4D (white bars), NCAM−/− mice injected with DCS (10 and 20 mg/kg body weight) froze in the CC− context significantly less than vehicle-injected NCAM−/− mice on the first day after training. Thus, context discrimination was greatly improved in NCAM−/− mice by DCS (10 and 20 mg/kg body weight), especially on the first test day, reaching the control levels seen in vehicle-injected NCAM+/+ mice.
The impairment of cued memory and the reduced discrimination between CS+ and CS− in NCAM−/− mice were also restored by DCS (Fig. 4E). In particular, a dose of 10 mg/kg body weight normalized CS+ responses on the first test day, and all test doses of DCS significantly improved discrimination between tones. Thus, DCS restored LTP, LTD, and the formation of contextual and cued fear memories in young adult NCAM−/− mice.
Further decline of LTP in aged NCAM−/− mice
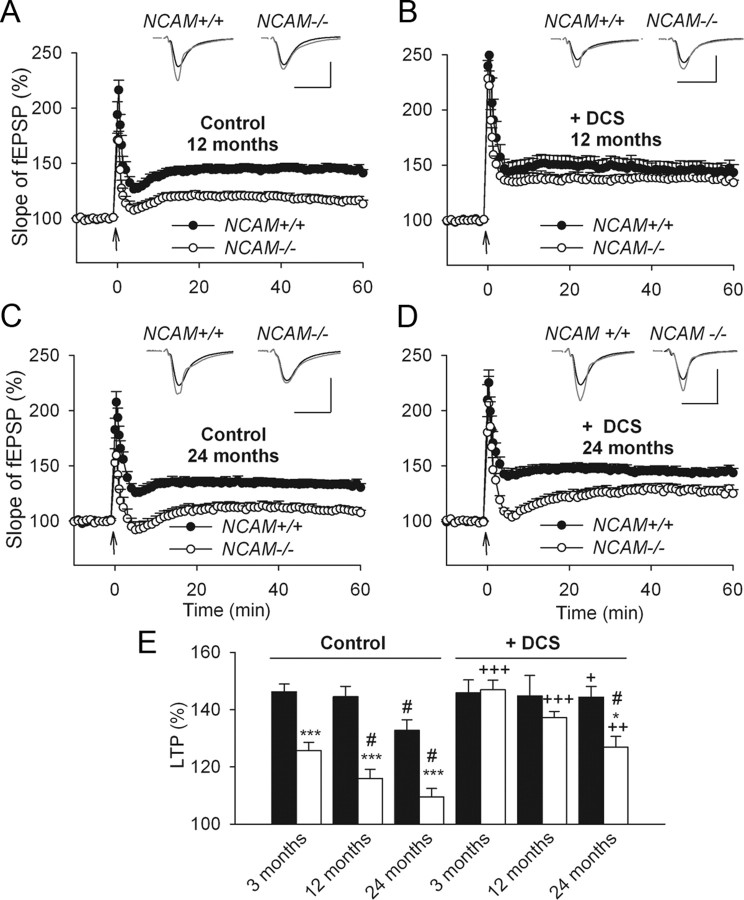
Previous studies have shown declines in NCAM and PSA-NCAM expression with aging (Fox et al., 1995; Kaur et al., 2008) and in Alzheimer's disease (Yew et al., 1999). Therefore, we examined the age-dependent contribution of NCAM to synaptic plasticity in 12- and 24-month-old mice. A two-way ANOVA of the LTP levels of untreated slices revealed significant effects of age (p < 0.001) and genotype (p < 0.001). There was a decline in LTP in 24-month-old NCAM+/+ mice (132.7 ± 3.6%), but not in 12-month-old NCAM+/+ mice (144.4 ± 3.6%), compared with 3-month-old NCAM+/+ mice (146.2 ± 2.6%; Figs. 1A; 5A,C,E). In contrast, 12-month-old NCAM−/− mice demonstrated an age-dependent reduction in the magnitude of LTP (115.9 ± 3.1%) compared with 3-month-old NCAM−/− mice (125.6 ± 2.8% Figs. 1A; 5A,E). The level of LTP in 24-month-old NCAM−/− mice was further reduced (109.5 ± 2.9% Fig. 5C,E) compared with 3-month-old and 12-month-old NCAM−/− mice.
Figure 5.
Age-dependent decline of LTP in NCAM-deficient mice. A, Normal levels of LTP in slices from 12-month-old, as compared with 3-month-old, NCAM+/+ mice. In contrast, LTP is significantly diminished in slices from 12-month-old NCAM−/− mice. B, DCS effectively restores impaired LTP in slices from 12-month-old NCAM−/− mice. C, LTP levels are reduced in slices from 24-month-old NCAM+/+ mice and lower yet in NCAM−/− mice as compared with 12-month-old mutants. D, DCS effectively restores impaired LTP in slices from 24-month-old NCAM+/+ mice. Although LTP is significantly increased by DCS in slices from 24-month-old NCAM−/− mice, it is not restored to the level seen in NCAM+/+ controls. A–D, The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. E, Means + SEMs of LTP levels recorded 50–60 min after TBS at different ages in control conditions and after treatment with the GluN agonist DCS. DCS effectively restores impaired LTP in slices from 24-month-old NCAM+/+ mice. Although LTP is significantly increased by DCS in slices from 24-month-old NCAM−/− mice, it is not restored to the level seen in NCAM+/+ controls. Three-way ANOVA revealed effects of genotype, age and treatment and an interaction between treatment and genotype (all p ≤ 0.001). *p < 0.05, ***p < 0.001, significant differences between NCAM+/+ and NCAM−/− mice treated in the same way; #p < 0.05, significant effect of aging in the same genotype (SNK test); +p ≤ 0.05, ++p < 0.01, +++p < 0.001, significant differences between untreated control and DCS-treated slices of the same genotype (t test). The numbers of tested NCAM+/+ and NCAM−/− slices/mice for each group are as follows: 24/19 and 20/17 (3-month-old, control), 11/4 and 12/5 (3-month-old, DCS), 9/3 and 9/3 (12-month-old, control), 9/4 and 7/4 (12-month-old, DCS), 8/4 and 6/3 (24-month-old, control), and 9/5 and 6/3 (24-month-old, DCS).
LTP levels were rescued by DCS in 12-month-old NCAM−/− mice (137.1 ± 2.1%) to levels nearly matching those of the age-matched NCAM+/+ controls (144.7 ± 7.1%; Fig. 5B,E). Although DCS application did not affect LTP in 3- and 12-month-old NCAM+/+ mice, it did augment LTP from 132.7 ± 3.6% to 144.3 ± 3.7% in 24-month-old NCAM+/+ mice, resulting in LTP levels similar to those seen in 12- and 3-month-old NCAM+/+ mice (Fig. 5C–E). Although LTP levels in 24-month-old NCAM−/− mice were increased by DCS from 109.5 ± 2.9% to 126.8 ± 3.7% (Fig. 5C–E), they were not fully restored to the levels seen in age-matched wild-type mice. These data demonstrate that the age-dependent decline in LTP is accelerated in NCAM−/− mice and that NCAM deficiency can be fully rescued by DCS only early in life.
To provide additional support for the view that the effects of DCS at different ages are mediated by GluN receptors, we investigated the effects of NVP-AAM077 and DCS on the sum of the late components of TBS-elicited fEPSPs (Fig. 6A, shown by 8 horizontal bars). In slices from 3-month-old mice, a two-way ANOVA revealed a strong inhibitory effect of NVP-AAM077 on the late component (p < 0.001), suggesting that it is predominantly mediated by GluN2A-containing receptors (Fig. 6A,B). A three-way ANOVA of the late component in untreated and DCS-treated slices from 3-, 12-, and 24-month-old NCAM+/+ and NCAM−/− mice revealed significant effects of genotype, DCS, and age (p < 0.001) as well as significant interactions between genotype and DCS (p = 0.002) and age and DCS (p = 0.025). DCS abrogated the difference between genotypes in 3-month-old mice (Fig. 6B,E), increased the amplitude of the late component (particularly that elicited by the fourth TBS) in slices from 12-month-old NCAM−/− mice to the level seen in untreated NCAM+/+ slices of the same age (Fig. 6C,E), and increased the amplitude of the late component in 24-month-old NCAM−/− mice to a level above control levels (Fig. 6D,E). Variability in the amplitude of the slow component between the age groups studied might be related to differential contributions of inhibitory synaptic activity to the measured signal. In summary, the NVP-AAM077-sensitive component of TBS-elicited fEPSP is modulated by DCS in parallel with the potentiating effects of DCS on LTP.
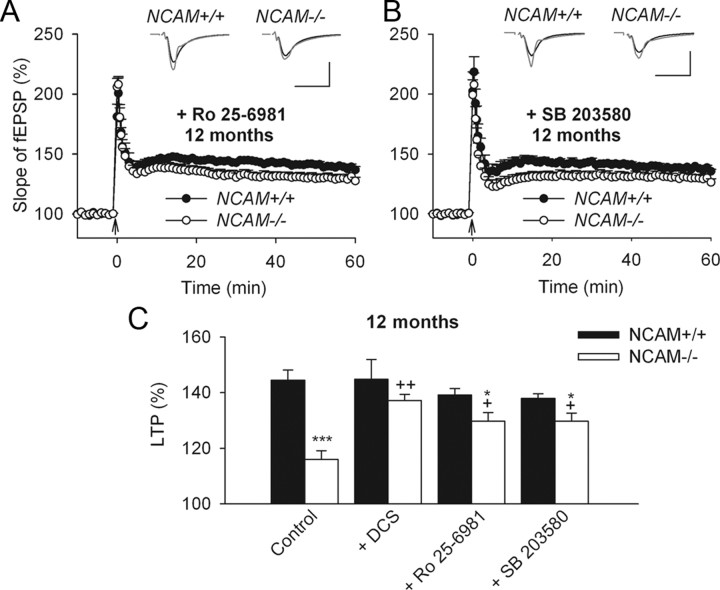
Our previous data demonstrated an increase in synaptic transmission through GluN2B receptors and downstream activation of p38 MAP kinase in 3-month-old NCAM−/− mice (Kochlamazashvili et al., 2010). Furthermore, we restored LTP at this age by applying either the GluN2B receptor antagonist Ro 25-6981 or the inhibitor of p38 MAP kinase activity SB 203580. We investigated whether these drugs could normalize LTP in older NCAM−/− mice. Although application of either Ro 25-6981 or SB 203580 enhanced LTP induction in 12-month-old NCAM−/− mice to 129.6 ± 3.1% or 129.7 ± 2.8%, respectively, the restoration was only partial because higher LTP levels were detected in NCAM+/+ mice (Ro 25-6981: 139.2 ± 4.5% and SB 203580: 138.0 ± 4.2%) (Fig. 7). There were no significant effects of Ro 25-6981 or SB 203580 on LTP in NCAM+/+ mice. Thus, DCS restored LTP in 12-month-old mice more potently than either Ro 25-6981 or SB 203580. The partial restoration of LTP by Ro 25-6981 or SB 203580 in 12-month-old animals suggest that LTP is dependent on both the balance between GluN2A versus GluN2B signaling and the absolute level of GluN2A-mediated signaling; if GluN2A signaling is too low, then LTP is reduced even when GluN2B signaling is suppressed.
Figure 7.
Partial restoration of impaired LTP in hippocampal slices from 12-month-old NCAM−/− mice through inhibition of GluN2B-containing receptor signaling. A, B, Partial rescue of impaired LTP in slices from 12-month-old NCAM−/− mice by application of the GluN2B selective antagonist Ro 25-6981 (A) or the p38 inhibitor SB 203580 (B). The presentation of traces and LTP profiles are as in Figure 1. Calibration: 1 mV and 20 ms. C, Means + SEMs of LTP levels recorded 50–60 min after TBS in control conditions and with different pharmacological treatments for 12-month-old mice. Two-way ANOVA revealed effects of genotype (p < 0.001) and a genotype × treatment interaction (p = 0.021). *p < 0.05, ***p < 0.001, significant differences between NCAM+/+ and NCAM−/− mice treated in the same way; +p < 0.05, ++p < 0.01, significant differences between untreated control and pharmacologically treated slices of the same genotype (SNK test). The numbers of tested 12-month-old NCAM+/+ and NCAM−/− slices/mice for each group are as follows: 9/3 and 9/3 (control), 9/4 and 7/4 (DCS), 6/3 and 8/4 (Ro 25-6981) and 7/4 and 8/4 (SB 203580).
Discussion
In the present study, we demonstrated that NCAM deficiency impairs synaptic plasticity and learning in young adult mice and leads to age-associated declines in LTP. Modulating NMDA receptors with DCS, which has been used to treat schizophrenia and age-associated pathologies, can reverse these deficits.
Our data suggest that the abnormal LTP in NCAM−/− mice is at least partially due to reduced synaptic GluN2A-mediated signaling during LTP induction for the following reasons. (1) Reducing extracellular Mg2+, which relieves the Mg2+-mediated GluN blockade, restores LTP in NCAM−/− mice. (2) Normal TEA-LTP suggests that signaling through GluNs is selectively impaired in the absence of NCAM, whereas signaling through l-type Ca2+ channels is not affected. (3) The NVP-AAM077-sensitive components of NMDA receptor-mediated responses and LTP are smaller in NCAM−/− mice than in NCAM+/+ mice (Kochlamazashvili et al., 2010) (present study). (4) Facilitation of GluN2A receptors by DCS restores levels of LTP in NCAM/PSA-deficient slices to levels seen in slices from wild-type mice. Our present data corroborate previous findings of reduced expression of synaptic GluNs in NCAM−/− versus NCAM+/+ mice (Sytnyk et al., 2006) and of LTP restoration in NCAM/PSA-deficient hippocampal slices by brain-derived neurotrophic factor (BDNF) (Muller et al., 2000), which induces GluN1 phosphorylation through tyrosine kinase receptors (Lin et al., 1998; Slack et al., 2004), thereby increasing GluN conductance and GluN-mediated synaptic currents (Levine et al., 1998; Xu et al., 2006). Consistent with our data, GluN2A receptors are the predominant GluNs at the postsynaptic density in mature neurons (Petralia et al., 2005), where they are responsible for LTP induction at CA3-CA1 synapses (Köhr et al., 2003; Liu et al., 2004; Li et al., 2006).
Our pharmacological analyses support the view that the activities of both GluN2A and GluN2B receptors induce LTD in wild-type adult mice in response to low-frequency stimulation. The same conclusion has been reached regarding the induction of LTD in adult rats (Fox et al., 2006). The impairment of LTD in NCAM−/− mice is noteworthy because an excess of signaling via extrasynaptic GluN2B in NCAM−/− mice promotes the induction of LTD, as found in rats (Massey et al., 2004). Because levels of basal excitatory synaptic transmission are normal in NCAM−/− mice, it is unlikely that a GluN2B-mediated increase in the basal activity of p38 MAPK (Kochlamazashvili et al., 2010) could depress basal transmission such that LTD would be occluded. Therefore, the impaired LTD in NCAM−/− mice is most likely due to a deficit in signaling via GluN2A-containing receptors, which is restored by DCS, as also has been reported for aged rats (Billard and Rouaud, 2007). In contrast, application of DCS in NCAM+/+ mice increased GluN signaling to levels above the threshold for LTD induction, thus impairing LTD induction. Notably, LTD (induced by a single episode of low-frequency stimulation in adult rats) is not affected by DCS (Billard and Rouaud, 2007), suggesting that the modulating effects of DCS on LTD are species- and/or protocol-dependent.
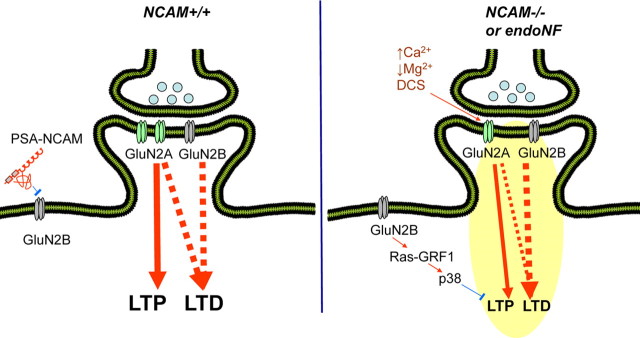
The data presented here and our previous finding that NCAM and/or PSA deficiency increase the responses mediated by the GluN2B subunit at the expense of the GluN2A subunit (Kochlamazashvili et al., 2010) suggest that NCAM and/or PSA regulate the balance in signaling between the two major subtypes of GluNs, and this balance determines the level of synaptic plasticity. In our current model, NCAM-associated PSA inhibits extrasynaptic GluN2B receptor activation at low glutamate concentrations in wild-type animals (Fig. 8), presumably via competition with glutamate for the ligand binding site (Hammond et al., 2006). As such, signaling via synaptic GluN2A predominantly contributes to LTP induction (Massey et al., 2004). For successful LTD induction in adult mice, activation of both GluN2A and GluN2B is required, whereas LTP induction depends on only GluN2A (Kochlamazashvili et al., 2010) (present study). In the absence of NCAM, extrasynaptic GluN2B receptors become more active, resulting in p38 MAPK activation (Kochlamazashvili et al., 2010), which together with the reduced signaling via GluN2A, impairs LTP. This model is also supported by a recent study (Li et al., 2011) showing that GluN2B and p38 MAPK mediate LTP disruption via soluble Aβ. Blockade of GluN2B (with Ro 25-6981) or its downstream effector p38 MAPK was able to rescue LTP in this model, as was previously shown for NCAM−/− mice by our group (Kochlamazashvili et al., 2010).
Figure 8.
Model for PSA-NCAM-mediated modulation of signaling via NMDA receptors. Endogenous and exogenous molecules are shown in black and brown, respectively. Stimulatory and inhibitory relationships are shown in red and blue, respectively. In NCAM+/+ mice, PSA-NCAM inhibits extrasynaptic GluN2B-containing receptors. LTP is induced through activation of GluN2A, whereas LTD requires the activity of both GluN2A- and GluN2B-containing receptors. In NCAM−/− mice, signal enhancement occurs via extrasynaptic GluN2B-containing receptors, whereas signal reduction occurs via GluN2A-containing receptors, which leads to impaired LTP and LTD. This model is supported by experiments from the present study with elevated extracellular Ca2+ and reduced extracellular Mg2+ or with DCS application and also by previous experiments applying Ro 25-6981, the glutamate scavenger GPT, PSA, or SB 203580 (Kochlamazashvili et al., 2010). All these treatments change the signaling balance between GluN2A- and GluN2B-mediated pathways in favor of the GluN2A-mediated pathway and restore LTP in NCAM−/− mice. Similarly, LTP is restored in endoNF treated slices from NCAM+/+ mice by DCS, Ro 25-6981, and SB 203580 and by genetic ablation of Ras-GRF1. Furthermore, fear memory is restored by DCS and Ro 25-6981 in NCAM−/− mice, whereas LTD is rescued by DCS.
Consistent with the in vitro data showing that LTP and LTD are rescued by DCS, systemic injection of DCS restores contextual and tone memory in NCAM−/− mice. The considerable evidence indicating that NCAM and associated PSA are crucially involved in hippocampus-dependent spatial and place (contextual) learning in the Morris water maze and fear conditioning paradigms includes the following: learning-associated changes in the expression of NCAM and PSA (Murphy et al., 1996; Murphy and Regan, 1999; Sandi et al., 2003; Venero et al., 2006), impaired memory in constitutively NCAM-deficient mice (Cremer et al., 1994; Stork et al., 2000; Senkov et al., 2006), and impaired memory in conditionally NCAM-deficient mice (in which the NCAM gene is inactivated by cre-recombinase under the control of the CaMKII promoter) (Bukalo et al., 2004; Bisaz and Sandi, 2012). Furthermore, temporal perturbations of NCAM or associated PSA at different phases of learning and memory lead to the same memory deficits in spatial navigation (Becker et al., 1996; Venero et al., 2006) and contextual fear conditioning (Senkov et al., 2006). Additionally, PSA deficiency throughout adulthood (achieved by eliminating polysialyltransferase ST8SiaIV/PST-1, an enzyme responsible for the postnatal attachment of PSA to NCAM) results in impaired contextual fear conditioning (Senkov et al., 2006) and impaired spatial and reversal learning in the Morris water maze (Markram et al., 2007). In contrast, a gain of PSA function by intrahippocampal injection of a PSA-mimetic peptide improves long-term spatial memory in wild-type mice (Florian et al., 2006). Because DCS has been shown to potently reverse spatial memory deficits, enhance decreased GluNs activity and hippocampal synaptic plasticity in adult and aged rodents (Quartermain et al., 1994; Aura et al., 1998; Billard and Rouaud, 2007), it will be important to analyze its potential rescue effect in aged NCAM-deficient mice. In addition, it will be interesting for follow-up studies to probe the effects of DCS on reversal learning, spatial and working memory in adult and aged NCAM−/− mice.
Our present data show that NCAM regulates both synaptic plasticity and hippocampus-dependent learning via modulation of GluNs, consistent with our previous finding that intrahippocampal injection of Ro 25-6981, which suppresses GluN2B-mediated transmission, normalizes contextual fear conditioning. Analogously with synaptic plasticity, a balance between GluN subunit activation is required for acquisition of contextual fear memory. Changes in the GluN2B/GluN2A ratio at the protein and/or functional level may influence the formation of different memories. Behavioral experiments manipulating GluN2B subunit activity have revealed that GluN2B selectively mediates trace, but not contextual fear conditioning, whereas GluN2A is required for both types of learning (Gao et al., 2010). Similarly to NCAM−/− mice, mice deficient in GluN2A exhibit deficits in hippocampal LTP and impaired fear memory (Kiyama et al., 1998).
Upon further exploration of the functional significance of NCAM for GluN function, we evaluated NMDA receptor-dependent LTP in the absence of NCAM as a function of age because it is known that NCAM polysialylation is reduced in both aged rodent and human brains. We observed the progressive decline in LTP in 3-, 12 and 24-month-old NCAM−/− mice, whereas in wild-type mice, a reduction in LTP was detected only after 24 months. DCS fully rescued LTP in 3- and 12-month-old NCAM−/− mice but only partially restored LTP in 24-month-old mutants. In 24-month-old NCAM +/+ mice, LTP was fully restored by DCS (the present study), as has been reported for aged rats (Billard and Rouaud, 2007). These data are in line with remarkable positive effects of DCS on cognitive processing in the elderly (Mohr et al., 1995; Tsai et al., 1999). Thus, modulating the GluN1 glycine binding site may effectively treat age-associated memory impairments, which are recognized as the early stage of age-related pathologies and are characterized as reversible functional alterations in neurotransmission. However, if NCAM expression is impaired with further aging and neurodegeneration, this manipulation might be insufficient to fully restore synaptic and cognitive function. It has been hypothesized that physiological changes associated with normal aging occur earlier in people with schizophrenia compared with the general population (Kirkpatrick et al., 2008). Similarly, we found that the age-dependent LTP decline is accelerated in NCAM−/− mice compared with NCAM+/+ mice. Furthermore, a progressive decline in PSA-NCAM with age is observed in schizophrenia (Barbeau et al., 1995; Seki and Arai, 1999; Cox et al., 2009) and accompanies structural changes in the brain. Similarly to schizophrenic and elderly patients (Convit et al., 2001), brain size and weight are decreased in NCAM−/− mice (Wood et al., 1998; Tereshchenko et al., 2011). Overall, DCS ameliorates impairments related to schizophrenia (Javitt, 2006), other age-related brain pathologies (Mohr et al., 1995; Tsai et al., 1999), and deficits found in adult and aged NCAM−/− mice. Thus, NCAM/PSA is linked to these pathological conditions. Finally, and in agreement with our previous findings (Kochlamazashvili et al., 2010), our present observations suggest that therapeutically normalizing the balance between GluN2A- and GluN2B-containing receptors may help to treat synaptic and cognitive deficits associated with NCAM dysregulation.
Footnotes
M.S. and A.D. were supported by the Deutsche Forschungsgemeinschaft (Di 702/4-1,2,3 to A.D). A.D. was supported by the Italian Institute of Technology and by a grant from the Government of the Russian Federation. NVP-AAM077 was a kind gift from Dr. Yves Auberson at the Novartis Institute for BioMedical Research, Basel, Switzerland. M.S. is the New Jersey Professor of Spinal Cord Research and a consultant at Shantou University Medical College. We thank Harold Cremer for the NCAM-deficient mice, Achim Dahlmann for genotyping, Eva Kronberg for animal care, and Thomas Coate and Jonathan Cohen for manuscript editing.
References
- Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudéry M, Le Moal M, Mayo W. Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res. 1997;744:285–292. doi: 10.1016/S0006-8993(96)01115-8. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L, Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991;349:67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- Aura J, Riekkinen P., Jr Pre-training blocks the improving effect of tetrahydroaminoacridine and d-cycloserine on spatial navigation performance in aged rats. Eur J Pharmacol. 2000;390:313–318. doi: 10.1016/s0014-2999(00)00054-6. [DOI] [PubMed] [Google Scholar]
- Aura J, Riekkinen M, Riekkinen P., Jr Tetrahydroaminoacridine and d-cycloserine stimulate acquisition of water maze spatial navigation in aged rats. Eur J Pharmacol. 1998;342:15–20. doi: 10.1016/s0014-2999(97)01512-4. [DOI] [PubMed] [Google Scholar]
- Bado P, Madeira C, Vargas-Lopes C, Moulin TC, Wasilewska-Sampaio AP, Maretti L, de Oliveira RV, Amaral OB, Panizzutti R. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology. 2011;218:461–470. doi: 10.1007/s00213-011-2330-4. [DOI] [PubMed] [Google Scholar]
- Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci U S A. 1995;92:2785–2789. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-d-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–452. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer's disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Lanthorn TH, Frick KM, Golski S, Wan RQ, Olton DS. D-cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol Aging. 1994;15:207–213. doi: 10.1016/0197-4580(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Becker CG, Artola A, Gerardy-Schahn R, Becker T, Welzl H, Schachner M. The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J Neurosci Res. 1996;45:143–152. doi: 10.1002/(SICI)1097-4547(19960715)45:2<143::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by d-cycloserine. Eur J Neurosci. 2007;25:2260–2268. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- Bisaz R, Sandi C. Vulnerability of conditional NCAM-deficient mice to develop stress-induced behavioral alterations. Stress. 2012 doi: 10.3109/10253890.2011.608226. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT, Schweizer M, Dityatev A, Schachner M. Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci. 2004;24:1565–1577. doi: 10.1523/JNEUROSCI.3298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen P, Tan H, Ma D, Dou F, Feng J, Yan Z. Regulation of the NMDA receptor-mediated synaptic response by acetylcholinesterase inhibitors and its impairment in an animal model of Alzheimer's disease. Neurobiol Aging. 2008;29:1795–1804. doi: 10.1016/j.neurobiolaging.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R. Volumetric analysis of the pre-frontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Conzelman GM, Jr, Jones RK. On the physiologic disposition of cycloserine in experimental animals. Am Rev Tuberc. 1956;74:802–806. doi: 10.1164/artpd.1956.74.5.802. [DOI] [PubMed] [Google Scholar]
- Cox ET, Brennaman LH, Gable KL, Hamer RM, Glantz LA, Lamantia AS, Lieberman JA, Gilmore JH, Maness PF, Jarskog LF. Developmental regulation of neural cell adhesion molecule in human prefrontal cortex. Neuroscience. 2009;162:96–105. doi: 10.1016/j.neuroscience.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, Barthels D, Rajewsky K, Wille W. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dracheva S, Marras SA, Elhakem SL, Kramer FR, Davis KL, Haroutunian V. N-methyl-d-aspartic acid receptor expression in the dorsolateral prefrontal cortex of elderly patients with schizophrenia. Am J Psychiatry. 2001;158:1400–1410. doi: 10.1176/appi.ajp.158.9.1400. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers MR, Salmen B, Bukalo O, Rollenhagen A, Bösl MR, Morellini F, Bartsch U, Dityatev A, Schachner M. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci. 2002;22:7177–7194. doi: 10.1523/JNEUROSCI.22-16-07177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Foltz J, Norreel JC, Rougon G, Roullet P. Post-training intrahippocampal injection of synthetic poly-alpha-2,8-sialic acid-neural cell adhesion molecule mimetic peptide improves spatial long-term performance in mice. Learn Mem. 2006;13:335–341. doi: 10.1101/lm.187506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Russell KI, Wang YT, Christie BR. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus. 2006;16:907–915. doi: 10.1002/hipo.20230. [DOI] [PubMed] [Google Scholar]
- Fox GB, Kennedy N, Regan CM. Polysialylated neural cell adhesion molecule expression by neurons and astroglial processes in the rat dentate gyrus declines dramatically with increasing age. Int J Dev Neurosci. 1995;13:663–672. doi: 10.1016/0736-5748(95)00067-4. [DOI] [PubMed] [Google Scholar]
- Fux CM, Krug M, Dityatev A, Schuster T, Schachner M. NCAM180 and glutamate receptor subtypes in potentiated spine synapses: an immunogold electron microscopic study. Mol Cell Neurosci. 2003;24:939–950. doi: 10.1016/j.mcn.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Gao C, Gill MB, Tronson NC, Guedea AL, Guzmán YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J. Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation. Hippocampus. 2010;20:1072–1082. doi: 10.1002/hipo.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-d-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Hammond MS, Sims C, Parameshwaran K, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid inhibits NR2B-containing N-methyl-d-aspartate receptors and prevents glutamate-induced cell death. J Biol Chem. 2006;281:34859–34869. doi: 10.1074/jbc.M602568200. [DOI] [PubMed] [Google Scholar]
- Hansel C, Artola A, Singer W. Different threshold levels of postsynaptic [Ca2+]i have to be reached to induce LTP and LTD in neocortical pyramidal cells. J Physiol Paris. 1996;90:317–319. doi: 10.1016/s0928-4257(97)87906-5. [DOI] [PubMed] [Google Scholar]
- Hansel C, Artola A, Singer W. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. Eur J Neurosci. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-d-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Huang YY, Malenka RC. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: the role of voltage-dependent Ca2+ channels in the induction of LTP. J Neurosci. 1993;13:568–576. doi: 10.1523/JNEUROSCI.13-02-00568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Mauk MD, Kelly PT. Distinct LTP induction mechanisms: contribution of NMDA receptors and voltage-dependent calcium channels. J Neurophysiol. 1995;73:270–279. doi: 10.1152/jn.1995.73.1.270. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Hogg AJ, Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry. 2000;157:1811–1823. doi: 10.1176/appi.ajp.157.11.1811. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Is the glycine site half saturated or half unsaturated? Effects of glutamatergic drugs in schizophrenia patients. Curr Opin Psychiatry. 2006;19:151–157. doi: 10.1097/01.yco.0000214340.14131.bd. [DOI] [PubMed] [Google Scholar]
- Kaur M, Sharma S, Kaur G. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology. 2008;9:441–454. doi: 10.1007/s10522-008-9168-0. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Abraham WC. Cooperative interactions among afferents govern the induction of homosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci U S A. 1995;92:11637–11641. doi: 10.1073/pnas.92.25.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci. 2010;30:4171–4183. doi: 10.1523/JNEUROSCI.5806-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhr G, Jensen V, Koester HJ, Mihaljevic AL, Utvik JK, Kvello A, Ottersen OP, Seeburg PH, Sprengel R, Hvalby Ø. Intracellular domains of NMDA receptor subtypes are determinants for long-term potentiation induction. J Neurosci. 2003;23:10791–10799. doi: 10.1523/JNEUROSCI.23-34-10791.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Laxmi TR, Stork O, Pape HC. Generalisation of conditioned fear and its behavioural expression in mice. Behav Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tian X, Hartley DM, Feig LA. Distinct roles for Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) and Ras-GRF2 in the induction of long-term potentiation and long-term depression. J Neurosci. 2006;26:1721–1729. doi: 10.1523/JNEUROSCI.3990-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble A{beta} Oligomers Inhibit Long-Term Potentiation through a Mechanism Involving Excessive Activation of Extrasynaptic NR2B-Containing NMDA Receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation–a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Markram K, Gerardy-Schahn R, Sandi C. Selective learning and memory impairments in mice deficient for polysialylated NCAM in adulthood. Neuroscience. 2007;144:788–796. doi: 10.1016/j.neuroscience.2006.10.024. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E, Knott V, Sampson M, Wesnes K, Herting R, Mendis T. Cognitive and quantified electroencephalographic correlates of cycloserine treatment in Alzheimer's disease. Clin Neuropharmacol. 1995;18:28–38. doi: 10.1097/00002826-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Teyler TJ. Electrical stimuli patterned after the theta-rhythm induce multiple forms of LTP. J Neurophysiol. 2001;86:1289–1296. doi: 10.1152/jn.2001.86.3.1289. [DOI] [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-d-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Rouaud E, Sinet PM, Potier B, Jouvenceau A, Dutar P, Videau C, Epelbaum J, Billard JM. A critical role for the glial-derived neuromodulator d-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell. 2006;5:267–274. doi: 10.1111/j.1474-9726.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Muller D, Djebbara-Hannas Z, Jourdain P, Vutskits L, Durbec P, Rougon G, Kiss JZ. Brain-derived neurotrophic factor restores long-term potentiation in polysialic acid-neural cell adhesion molecule-deficient hippocampus. Proc Natl Acad Sci U S A. 2000;97:4315–4320. doi: 10.1073/pnas.070022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, Regan CM. Sequential training in separate paradigms impairs second task consolidation and learning-associated modulations of hippocampal NCAM polysialylation. Neurobiol Learn Mem. 1999;72:28–38. doi: 10.1006/nlme.1998.3894. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, O'Connell AW, Regan CM. Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J Neurochem. 1996;67:1268–1274. doi: 10.1046/j.1471-4159.1996.67031268.x. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Ionotropic glutamate receptor binding in the posterior cingulate cortex in schizophrenia patients. Neuroreport. 2005;16:1363–1367. doi: 10.1097/01.wnr.0000174056.11403.71. [DOI] [PubMed] [Google Scholar]
- Ni Dhúill CM, Fox GB, Pittock SJ, O'Connell AW, Murphy KJ, Regan CM. Polysialylated neural cell adhesion molecule expression in the dentate gyrus of the human hippocampal formation from infancy to old age. J Neurosci Res. 1999;55:99–106. doi: 10.1002/(SICI)1097-4547(19990101)55:1<99::AID-JNR11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, Otte DM, Shah NJ, Maier W, Kendrick KM, Fink GR, Hurlemann R. The N-methyl-d-aspartate receptor co-agonist d-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67:1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier B, Poindessous-Jazat F, Dutar P, Billard JM. NMDA receptor activation in the aged rat hippocampus. Exp Gerontol. 2000;35:1185–1199. doi: 10.1016/s0531-5565(00)00122-4. [DOI] [PubMed] [Google Scholar]
- Priestley T, Laughton P, Myers J, Le Bourdellés B, Kerby J, Whiting PJ. Pharmacological properties of recombinant human N-methyl-d-aspartate receptors comprising NR1a/NR2A and NR1a/NR2B subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with d-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by d-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Kruyt ND, Murphy KJ, Regan CM. Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol Psychiatry. 2003;54:599–607. doi: 10.1016/s0006-3223(03)00182-3. [DOI] [PubMed] [Google Scholar]
- Schuster T, Krug M, Hassan H, Schachner M. Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation. J Neurobiol. 1998;37:359–372. doi: 10.1002/(sici)1097-4695(19981115)37:3<359::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Seki T. Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res. 2002;70:327–334. doi: 10.1002/jnr.10387. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Temporal and spacial relationships between PSA-NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. J Comp Neurol. 1999;410:503–513. doi: 10.1002/(sici)1096-9861(19990802)410:3<503::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Senkov O, Sun M, Weinhold B, Gerardy-Schahn R, Schachner M, Dityatev A. Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J Neurosci. 2006;26:10888–109898. doi: 10.1523/JNEUROSCI.0878-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist d-cycloserine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Wolfer D, Schuster T, Mantei N, Stork S, Hoyer D, Lipp H, Obata K, Schachner M. Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. Eur J Neurosci. 2000;12:3291–3306. doi: 10.1046/j.1460-9568.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- Stummeyer K, Dickmanns A, Mühlenhoff M, Gerardy-Schahn R, Ficner R. Crystal structure of the polysialic acid-degrading endosialidase of bacteriophage K1F. Nat Struct Mol Biol. 2005;12:90–96. doi: 10.1038/nsmb874. [DOI] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns'ka I, Nikonenko AG, Schachner M. NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J Cell Biol. 2006;174:1071–1085. doi: 10.1083/jcb.200604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wotjak CT, Wagner S, Williams G, Schachner M, Dityatev A. Potentiated amygdaloid auditory-evoked potentials and freezing behavior after fear conditioning in mice. Brain Res. 2001;919:232–241. doi: 10.1016/s0006-8993(01)03020-7. [DOI] [PubMed] [Google Scholar]
- Tang J, Wagner S, Schachner M, Dityatev A, Wotjak CT. Potentiation of amygdaloid and hippocampal auditory-evoked potentials in a discriminatory fear-conditioning task in mice as a function of tone pattern and context. Eur J Neurosci. 2003;18:639–650. doi: 10.1046/j.1460-9568.2003.02758.x. [DOI] [PubMed] [Google Scholar]
- Tereshchenko Y, Morellini F, Dityatev A, Schachner M, Irintchev A. Neural cell adhesion molecule ablation in mice causes hippocampal dysplasia and loss of septal cholinergic neurons. J Comp Neurol. 2011;519:2475–2492. doi: 10.1002/cne.22636. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by d-cycloserine in rabbits. Behav Neurosci. 1997;111:1303–1312. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer's disease with short-term d-cycloserine treatment. Am J Psychiatry. 1999;156:467–469. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- Venero C, Herrero AI, Touyarot K, Cambon K, López-Fernández MA, Berezin V, Bock E, Sandi C. Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur J Neurosci. 2006;23:1585–1595. doi: 10.1111/j.1460-9568.2006.04663.x. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Walsh FS, Doherty P. The production of arachidonic acid can account for calcium channel activation in the second messenger pathway underlying neurite outgrowth stimulated by NCAM, N- cadherin, and L1. J Neurochem. 1994;62:1231–1234. doi: 10.1046/j.1471-4159.1994.62031231.x. [DOI] [PubMed] [Google Scholar]
- Wood GK, Tomasiewicz H, Rutishauser U, Magnuson T, Quirion R, Rochford J, Srivastava LK. NCAM-180 knockout mice display increased lateral ventricle size and reduced prepulse inhibition of startle. Neuroreport. 1998;9:461–466. doi: 10.1097/00001756-199802160-00019. [DOI] [PubMed] [Google Scholar]
- Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, Matzner H, Schumann J, Trembovler V, Tsenter J, Shohami E. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 2007;21:2033–2041. doi: 10.1096/fj.06-7856com. [DOI] [PubMed] [Google Scholar]
- Yew DT, Li WP, Webb SE, Lai HW, Zhang L. Neurotransmitters, peptides, and neural cell adhesion molecules in the cortices of normal elderly humans and Alzheimer patients: a comparison. Exp Gerontol. 1999;34:117–133. doi: 10.1016/s0531-5565(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, De Souza Silva MA, Huston JP, Dere E. NMDA receptor modulation by d-cycloserine promotes episodic-like memory in mice. Psychopharmacology. 2007;193:503–509. doi: 10.1007/s00213-007-0816-x. [DOI] [PubMed] [Google Scholar]