Abstract
Abnormalities of synaptic transmission and plasticity in the hippocampus represent an integral part of the altered programming triggered by early life stress. Prenatally restraint stressed (PRS) rats develop long-lasting biochemical and behavioral changes, which are the expression of an anxious/depressive-like phenotype. We report here that PRS rats showed a selective impairment of depolarization- or kainate-stimulated glutamate and [3H]d-aspartate release in the ventral hippocampus, a region encoding memories related to stress and emotions. GABA release was unaffected in PRS rats. As a consequence of reduced glutamate release, PRS rats were also highly resistant to kainate-induced seizures. Abnormalities of glutamate release were associated with large reductions in the levels of synaptic vesicle-related proteins, such as VAMP (synaptobrevin), syntaxin-1, synaptophysin, synapsin Ia/b and IIa, munc-18, and Rab3A in the ventral hippocampus of PRS rats. Anxiety-like behavior in male PRS (and control) rats was inversely related to the extent of depolarization-evoked glutamate release in the ventral hippocampus. A causal relationship between anxiety-like behavior and reduction in glutamate release was demonstrated using a mixture of the mGlu2/3 receptor antagonist, LY341495, and the GABAB receptor antagonist, CGP52432, which was shown to amplify depolarization-evoked [3H]d-aspartate release in the ventral hippocampus. Bilateral microinfusion of CGP52432 plus LY341495 in the ventral hippocampus abolished anxiety-like behavior in PRS rats. These findings indicate that an impairment of glutamate release in the ventral hippocampus is a key component of the neuroplastic program induced by PRS, and that strategies aimed at enhancing glutamate release in the ventral hippocampus correct the “anxious phenotype” caused by early life stress.
Introduction
The effects of stress on the brain have long been associated with the onset and exacerbation of several neuropsychiatric disorders such as depression, anxiety, drug addiction, and epilepsy (McEwen, 2012). Alterations in glutamate neurotransmission are believed to play a role in the pathophysiology of such disorders (Ongür, 2008; Chen et al., 2010). Exposure to stress and treatment with glucocorticoids alter glutamatergic neurotransmission and neuroplasticity in brain regions related to depression and anxiety, such as the hippocampus, amygdala, and prefrontal cortex (Mozhui et al., 2010; reviewed by Popoli et al., 2012). Musazzi et al. (2010) have shown that acute stress led to an accumulation of presynaptic SNARE complexes in cortical synaptic membranes, thus raising the interesting possibility that stress directly affects the presynaptic machinery of glutamate release. The study of glutamate release in response to chronic stress is still at its infancy (Moghaddam, 2002; Yamamoto and Reagan, 2006), and there are no data on how early life stress affects glutamate release in the adult life. The latter issue is particularly relevant because early life stress causes long-lasting changes in neuroplasticity that result in an increased vulnerability to stress-related disorders in adult life (Meaney et al., 2007; Darnaudéry and Maccari, 2008; Lupien et al., 2009). Prenatal restraint stressed (PRS) rats represent a model that recapitulates some of the features of depression and anxiety (Maccari et al., 1995; Dugovic et al., 1999; Darnaudéry et al., 2006; Maccari and Morley-Fletcher, 2007; Zuena et al., 2008; Van Waes et al., 2009; Laloux et al., 2012; Mairesse et al., 2012a). Interestingly, male PRS rats show a prominent anxious-like phenotype, whereas female PRS rats are more prone to develop a depressive-like phenotype (Zuena et al., 2008; Morley-Fletcher et al., 2011; Van Waes et al., 2011). PRS rats show also a reduced number of proteins involved in signal transduction and neuroplasticity regulation as revealed by a recent mass spectrometry analysis (Mairesse et al., 2012b). Most of these changes are reversed by chronic antidepressant treatment (Morley-Fletcher et al., 2003, 2004, 2011; Mairesse et al., 2012a). Hence, PRS rats represent an animal model of stress-related disorders that meets the criterion of construct validity because it replicates environmental factors implicated in the etiology of depression and anxiety (Krishnan and Nestler, 2008, 2010). In addition, most of the abnormalities in synaptic transmission and plasticity in the hippocampus of PRS rats are seen in the ventral hippocampus (Zuena et al., 2008; Morley-Fletcher et al., 2011), the specific portion of the hippocampus that encodes memories related to stress and emotions (Fanselow and Dong, 2010). We report here that male PRS rats show a selective impairment of glutamate release in the ventral hippocampus associated with anxiety and a reduced expression of the SNARE proteins and vesicle-associated proteins, as well as the mammalian uncoordinated-18 Munc-18, and the glutamate terminal-specific monomeric GTP-binding protein, Rab3a. Pharmacological enhancement of glutamate release in the ventral hippocampus corrected the anxious-like phenotype of PRS rats.
Materials and Methods
Animals
Forty nulliparous female Sprague Dawley rats (20 for control and 20 for PRS groups), weighing ∼250 g, were purchased from a commercial breeder (Charles River). Animals were housed at constant temperature (22 ± 2°C) and under a regular 12 h light/dark cycle (lights on at 8.00 A.M.). Pregnant females were randomly assigned to stressed or control groups. (n = 12 per group).
Stress protocol
Animals were subjected to PRS according to our standard protocol (Maccari et al., 1995; Morley-Fletcher et al., 2003). From day 11 of pregnancy until delivery, pregnant female rats were subjected to three stress sessions daily (45 min each), during which they were placed in transparent plastic cylinders and exposed to bright light. Only male offspring from litters containing 10–14 pups with a comparable number of males and females were used for the experiments. A maximum of one or two male pups were taken from each litter for each measure to remove any litter effects (Becker and Kowall, 1977; Chapman and Stern, 1979). All experiments followed the rules of the European Communities Council Directive 86/609/EEC. The local ethical committee approved the prenatal stress procedure. We used the same sets of animals (3 month olds) for anxiety and glutamate release (see correlation); in microinfusion experiments we used the same sets of animals (3 month olds) for the two tests of anxiety. For the other experiments we used separate sets of animals (2 month olds).
Assessment of glutamate and GABA release in superfused synaptosomal preparations
Purified synaptosomes isolated from the ventral and the dorsal hippocampus (dissected as described by Robertson et al., 2005), the perirhinal cortex, the prefrontal cortex, the amygdala, and the striatum were prepared as described by Dunkley et al. (1986), with minor modifications. Briefly, the tissue was homogenized in 10 volumes of 0.32 m sucrose, buffered to pH 7.4 with TRIS (final concentration 0.01 m) using a glass Teflon tissue grinder (clearance 0.25 mm). The homogenate was centrifuged at 1000 × g for 5 min, to remove nuclei and debris; the supernatant was gently stratified on a discontinuous Percoll gradient (6, 10, and 20% v/v in Tris-buffered sucrose) and centrifuged at 33,500 × g for 5 min. The layer between 10 and 20% Percoll (synaptosomal fraction) was collected and washed by centrifugation. The synaptosomal pellet was then resuspended in physiological medium (standard medium) with the following composition (in mm): 140 NaCl, 3 KCl, 1.2 MgSO4, 1.2 CaCl2, 1.2 NaH2PO4, 5 NaHCO3, 10 mm HEPES, and 10 glucose, pH 7.2–7.4. Synaptosomal protein levels were determined according to Bradford (1976). Synaptosomes were incubated for 15 min a 37°C in a rotary water bath in the absence (experiments of endogenous glutamate and GABA release) or presence of [2,3-3H]d-aspartate (20–50 nm; sp. act. 11.3 Ci/mmol, PerkinElmer).
Identical portions of the synaptosomal suspensions were layered on microporous filters at the bottom of parallel chambers in a Superfusion System (Raiteri and Raiteri 2000; Ugo Basile) maintained at 37°C and superfused at 0.5 ml/min with standard physiological solution.
When studying the release of neurotransmitter evoked by kainic acid (Tocris Bioscience) or depolarizing concentrations of K+, synaptosomes were transiently (90 s) exposed, at t = 39 min, to 10 μm kainic acid or to 20 (amygdala) or 12 (all other regions) mm K+ (substituted for NaCl in the superfusate). Superfusion was always performed with media containing 50 μm amino-oxyacetic acid (Sigma) to inhibit GABA metabolism. Three superfusate fractions were collected according to the following scheme: two 3 min fractions (basal release), one before (t = 36–39 min, b1) and one after (t = 45–48 min, b3) a 6 min fraction (t = 39–45 min; evoked release, b2). Fractions collected and superfused synaptosomes were counted for radioactivity or for endogenous amino acid content. Endogenous glutamate and GABA were measured by HPLC analysis after precolumn derivatization with o-phthalaldehyde and separation on a C18 reverse-phase chromatographic column (10 × 4.6 mm, 3 μm; at 30°C; Chrompack) coupled to a fluorimetric detector (excitation wavelength, 350 nm; emission wavelength, 450 nm). Buffers and the gradient program were as follows: solvent A, 0.1 m sodium acetate, pH 5.8/methanol, 80:20; solvent B, 0.1 m sodium acetate, pH 5.8/methanol, 20:80; solvent C, 0.1 m sodium acetate, pH 6.0/methanol, 80:20; gradient program, 100% C for 4 min from the initiation of the program; 90% A and 10% B in 1 min; isocratic flow, 2 min; 78% A and 22% B in 2 min; isocratic flow, 6 min; 66% A and 34% B in 3 min; 42% A and 58% B in 1 min; 100% B in 1 min; isocratic flow, 2 min; 100% C in 3 min; flow rate, 0.9 ml min−1. Homoserine was used as the internal standard. Synaptosomal protein contents were determined according to Bradford (1976). The amount of endogenous glutamate and GABA from synaptosomes in superfusate fractions was expressed as picomoles per milligram of protein (pmol mg−1 protein). Radioactivity in each superfusate fraction was quantified by liquid scintillation. The amount of radioactivity released into each superfusate fraction was expressed as a percentage of the total synaptosomal tritium content at the start of the fraction collected (fractional efflux). The depolarization-induced overflow was estimated by subtracting the neurotransmitter content into the first and the third fractions collected (basal release, b1 and b3) from that in the 6 min fraction collected during and after the depolarization pulse (evoked release, b2).
Assessment of [3H]d-aspartate release in hippocampal slice preparations
Slices (0.4 mm thick) from the dorsal or ventral hippocampus were prepared using a Mcllwain tissue chopper (Mickle Laboratory Engineering) and then placed in a superfusion medium with the following composition (in mm): 125 NaCl, 3 KCl, 1.2 MgSO4, 1.2 CaCl2, 1 NaH2PO4, 22 NaHCO3, and 10 glucose (aeration with 95% O2 and 5% CO2), pH 7.2–7.4, at 2–4°C. Slices were rinsed by changing the physiological solution every 20 min. Slices were labeled with 90 nm [3H]d-aspartate (20 min at 37°C) in standard medium in an atmosphere of 95% O2 and 5% CO2. After washing with tracer-free medium, slices were transferred to parallel superfusion chambers (one slice/chamber) and superfused (1 ml/min at 37°C). After 60 min of superfusion to equilibrate the system, six 5 min samples were collected. Slices were exposed to 30 mm K+ in the absence or presence of 3-[[(3,4-dichlorophenyl)methyl]amino]propyl]diethoxymethyl) phosphinic acid (CGP52432; Tocris Bioscience) and (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495; Tocris Bioscience) for 5 min, starting from t = 70 min of superfusion. Drugs were added from t = 30 min of superfusion until the end of the experiments. Samples collected and solubilized slices (Soluene; Canberra Packard) were counted for radioactivity. The amount of radioactivity released into each superfusate fraction was expressed as fractional efflux (see above). Drug effects were expressed as “induced overflow” and were estimated by subtracting the neurotransmitter content into the second and the fifth fractions collected from that in the third and in the fourth fractions collected.
In vivo studies
Kainate-induced motor seizures and electroencephalography/electromyography recording
We assessed kainate-induced limbic motor seizures in separate groups of control and PRS rats (n = 6 per group). Kainate-induced seizures represent an established experimental animal model of temporal lobe epilepsy in humans (Sharma et al., 2007; Joëls, 2009). Animals underwent 2 weeks of habituation to electroencephalogram (EEG)/electromyogram (EMG) recording before behavioral assessment for motor seizures. Rats were anesthetized with ketamine/xylazine (75/10 mg/kg, i.m.). Electrodes for EEG recordings were chronically implanted using a stereotaxic apparatus. Three stainless steel screw electrodes were threaded through the skull bilaterally over the frontal and parietal cortex to record the EEG. One electrode threaded through the midline of the frontal bone was used as ground. Teflon-coated multistranded stainless steel wires with 2 mm exposed at the tips (Goodfellow Sarl) were placed in the dorsal neck muscles to record the EMG. EEG and EMG leads were attached to a connector (MS363; Plastics One) and fixed to the skull with dental acrylic.
Recording.
For registration, the electrodes were connected to a preamplifier (8213; Pinnacle Technology) through the plastic connector. This preamplifier avoids the registration of electrical interferences. The preamplifier was connected to a rotating swivel allowing free animal movements, and the swivel was connected to the EEG/EMG Data Conditioning and Acquisition System (8206; Pinnacle Technology), which was USB linked to a computer. Signal acquisition was performed using the Sirena acquisition suite (Pinnacle Technology). The EEG and EMG were recorded at a frequency of 400 Hz. Both EEGs were lowpass filtered at 40 Hz. EMG signals were highpass filtered at 10 Hz and subjected to a 100 Hz lowpass cutoff. After surgery, rats were individually housed in Plexiglas cages (30 cm diameter, 40 cm high), and left undisturbed for a postsurgery recovery period of 2 weeks. During the second week of recovery, rats were habituated to the EEGs/EMG recording procedure for the following 2 weeks. Habituation consisted of two recording sessions of 8 h and one session of 24 h. At the end of the habituation period, the day of the experiment, EEGs/EMG recordings started 1 h after the light switch-on and continued for the next 8 h. Two hours after the beginning of the registration, kainate (Tocris Bioscience) was injected intraperitoneally at doses (7 mg/kg) in the same range as those reported by previous studies (Sperk et al., 1983; Berg et al., 1993), and were proven to cause full limbic motor seizures in control rats. The presence of characteristic spikes and/or spike clusters activity was correlated to each stage of behavioral seizure, after kainate injection.
Kainate-induced seizures.
Motor seizures were observed for 4 h following systemic kainate injection, and manually scored according to Racine (1972), as follows: 0, absence of seizures; 1, staring spells, immobilization, and hypoactivity; 2, paroxysmal wet dog shake and head nodding; 3, motor seizures associated with masticatory movements and tail arching; 4, rearing with forelimb jerks and salivation; 5, generalized convulsions with loss of postural control and intense myoclonic jerks lasting at least 1 h; and 6, “full status epilepticus” and death.
Assessment of anxiety-like behavior
We assessed anxiety-like behavior in control and PRS rats by using the elevated plus maze (EPM) and the light–dark tests. All animals used for ex vivo measurements of neurotransmitter release and immunoblot analysis of protein expression had been tested for anxiety-like behavior at least 1 week earlier. The EPM test was performed essentially as described by Pellow et al., 1985. The test was performed between 13:00 and 16:00 h, lasted for 5 min, and began with the placement of the rat in the center of the maze with the head facing a closed arm. The time spent in open and closed arms was recorded on-line by a video camera and the percentage of time spent in open arms was calculated. We also measured the number of entries into the open and closed arms, the number of crossings through the center, the number of episodes of head dips over the size of the maze, the number of episodes of rearing, and the latency to enter the open and the closed arms.
The light and dark box setup consisted of two compartments: one light compartment (45 × 32 × 32 cm, 50 lux; light box) and one dark compartment (30 × 32 × 32 cm, 5 lux). The compartments were connected via a small opening (10 × 15 cm) enabling transition between the two boxes. Rats were placed in the light compartment and the time spent in each compartment and the latency to the first entry into the light compartment during the 5 min test, were assessed on-line via a video camera located above the box. Behavior was automatically analyzed using video tracking software (View Point).
Microinfusions of CGP52432 and LY341495 in the ventral hippocampus
All control and PRS rats used for these experiments had been tested for anxiety at the light–dark box 1 week before surgery. Rats were injected intraperitoneally with an anesthetic solution containing ketamine (100 mg/kg), xylazine (8 mg/kg), and acepromazine (1 mg/kg), placed into a David Kopf stereotaxic apparatus with the incisor bar 5.0 mm above the interaural line, and bilaterally implanted with permanent cranial guide cannulae (22 gauge; Plastic One) into the ventral hippocampus (anteroposterior + 5.5; mediolateral ± 4.5; dorsoventral −5.5 mm from bregma and skull; Paxinos and Watson, 2007). Cannulae were fixed with dental acrylic cement directly anchored to the skull. After surgery, obturators were inserted into the guide cannulae, rats were returned to their home cage and were left undisturbed for a 7–10 d recovery period. Twelve control and 12 PRS rats were selected for microinfusion experiments and behavioral analysis. LY341495 and CGP52432 were dissolved in PBS (1.05 mm KH2PO4, 2 Na2HPO4, 3 mm H2O, 154 mm NaCl, pH 7.4) to obtain final concentrations of 100 pg/μl LY341495 and 1 ng/μl CGP52432. After 2 d of habituation to microinjection procedures, two groups of control and two groups of PRS rats received bilateral injections of either PBS alone (vehicle) or PBS containing CGP52432 and LY341495. The internal injection cannulae were connected to lengths of polyethylene tubing that in turn were connected to 10.0 μl Hamilton syringes. Injections were made bilaterally in a volume of 1 μl/side over a period of 2 min. After 1 min, the injection cannulae were withdrawn, the obturators replaced, and rats were returned to their home cage for 15 min before the start of behavioral assessments. All rats underwent two consecutive tests of 5 min in the light–dark box and the EPM, as described above. The two tests were performed one immediately after the other. This behavioral protocol may confound data of the second test (the EPM) because the stress associated with the execution of the first test (the light–dark box) might differentially affect the performance in the EPM in the four group of rats (control rats injected with vehicle, PRS rats injected with vehicle, control rats injected with CGP52432 plus LY341495, and PRS rats injected with CGP52432 plus LY341495. Despite these potential limitations, the execution of two consecutive tests was necessary to avoid the bias of reinjecting the mixture of drugs in the same animals without having knowledge of the neuroadaptive changes that intrahippocampal injection of CGP52432 and LY341495 may cause.
The correct position of the guide cannula in the ventral hippocampus was confirmed in all rats by injection of 1 μl of methylene blue (5%, dissolved in saline).
Western blot analysis
Two groups of control and PRS rats (n = 6 per group) were killed by decapitation and the ventral and dorsal portions of the hippocampus were rapidly dissected (Robertson et al., 2005). To isolate synaptosomes, tissue was manually homogenized with a potter in 10 vol of HEPES-buffered sucrose (0.32 m sucrose, 4 mm HEPES, pH 7.4). All procedures were performed at 4°C. Homogenates were centrifuged at 1000 × g for 10 min and resulting supernatants were centrifuged at 10,000 × g for 15 min. The pellet obtained from the second centrifugation was resuspended in 10 vol of HEPES-buffered sucrose and then spun again at 10,000 × g for 15 min. This pellet contained the crude synaptosomal fraction. To validate the purity of this synaptosomal fraction we used anti-histon H3, anti-β-tubulin, anti-synapsin Ia/b in immunoblot analysis. BCA assay was used to determine protein concentration. Synaptosomes lysates were resuspended in Laemmli reducing buffer and 20 μg of each sample were first separated by electrophoresis on Criterion TGX 4–15% precast SDS-polyacrylamide gels (Bio-Rad) and later transferred to nitrocellulose membranes (Bio-Rad). Transfer was performed at 4°C in a buffer containing 35 mm TRIS, 192 mm glycine, and 20% methanol. We used the following primary antibodies: rabbit polyclonal anti-synapsin Ia/b (sc-20780, 1:4000), rabbit polyclonal anti-synapsin IIa (sc-25538, 1:4000), rabbit polyclonal anti-synaptophysin (sc-9116, 1:80,000), rabbit polyclonal anti-VAMP (synaptobrevin, sc-13992, 1:2000), rabbit polyclonal anti-syntaxin-1 (sc-13994, 1:5000), and mouse monoclonal anti-SNAP-25 (sc-136267, 1:10000) (all purchased from Santa Cruz Biotechnology); mouse monoclonal anti-rab3a (107111, 1:2000), mouse monoclonal anti-Munc-18 (116011, 1:2000), mouse monoclonal anti-VGLUT-1 (135511, 1:2000), rabbit polyclonal anti-GluK3 (180203, 1:1000), and rabbit polyclonal GluK5 (180103, 1:1000) (all purchased from Synaptic System); rabbit polyclonal anti-Gluk1 (AGC-008, 1:1000) and rabbit polyclonal anti-GluK2 (AGC-009, 1:1000) (both purchased from Alomone Labs); rabbit polyclonal anti-GluK4 (ab67404, 1:1000) (purchased from Abcam); rabbit polyclonal anti-GLAST (GLAST11-A, 1:1000), rabbit polyclonal anti-GLT-1 (GLT11-A, 1:1000), and rabbit polyclonal anti-EAAC-1 (EAAC11-A, 1:1000) (all purchased from Alpha Diagnostic International); and mouse monoclonal anti-β-actin (A5316, 1:80,000) (purchased from Sigma). Secondary anti-mouse or anti-rabbit antibodies (purchased from GE Healthcare) were used a dilution at 1:10,000.
Densitometric analysis was performed with Quantity One software (Bio-Rad) associated to a GS-800 scanner. The ratio of individual proteins to β-actin was then determined and these values were compared for statistical significance.
Statistical analysis
Data of release experiments, immunoblot analysis, and behavioral data with light–dark box and EPM (excluding data obtained in microinfused animals) were analyzed by Student's t test (PRS vs control rats). Data of kainate-induced seizures were analyzed by two-way ANOVA for repeated measures followed by the Neumann–Keuls post hoc. Behavioral data obtained after microinfusions with vehicle or CGP52432 + LY341495 were analyzed by two-way ANOVA (group × treatment) followed by the Neumann–Keuls post hoc. A p value <0.05 was considered as statistically significant.
Results
PRS selectively reduced depolarization-evoked release of glutamate in superfused synaptosomes isolated from the ventral hippocampus
To study the effects of PRS on neurotransmitter release we used superfused synaptosomes prepared from adult male PRS rats and their age-matched controls. Our superfusion method eliminates the components in neurotransmitter release mediated by the inverse operation of membrane transporters and the influence of endogenous ligands acting at presynaptic receptors, thus allowing a reliable estimation of how the intrinsic release machinery responds to depolarization or other stimuli (Raiteri et al., 1974; Raiteri and Raiteri, 2000). Depolarization-evoked release in this system is exocytotic and entirely depended on extracellular Ca2+ (Bonanno et al., 2005). Synaptosomes prepared from the ventral and dorsal hippocampus, perirhinal cortex, prefrontal cortex, striatum, and amygdala from control or PRS rats were preloaded with d-[3H]-aspartate (a nonmetabolizable analog of glutamate), and then challenged with depolarizing concentrations of K+. Both uptake and basal (nonevoked) release of d-[3H]-aspartate in all brain regions did not differ between control and PRS rats. In contrast, depolarization-evoked d-[3H]-aspartate release was selectively and substantially reduced in synaptosomes prepared from the ventral hippocampus of PRS rats (t = 10.70; df = 10; p < 0.01). No difference in depolarization-evoked d-[3H]-aspartate release between control and PRS rats was seen in all other brain regions (Fig. 1A). In a different set of experiments, synaptosomes from ventral and dorsal hippocampus and perirhinal cortex were challenged with depolarizing concentrations of K+, and the superfusate was used for measurements of endogenous glutamate and GABA release. Again, depolarization-evoked glutamate release was largely reduced in the ventral hippocampus of PRS rats (t = 4.70; df = 10; p < 0.01), with no changes in the dorsal hippocampus or perirhinal cortex (Fig. 1B). Neither basal nor evoked GABA release differed between control and PRS rats in any brain region (Fig. 1C).
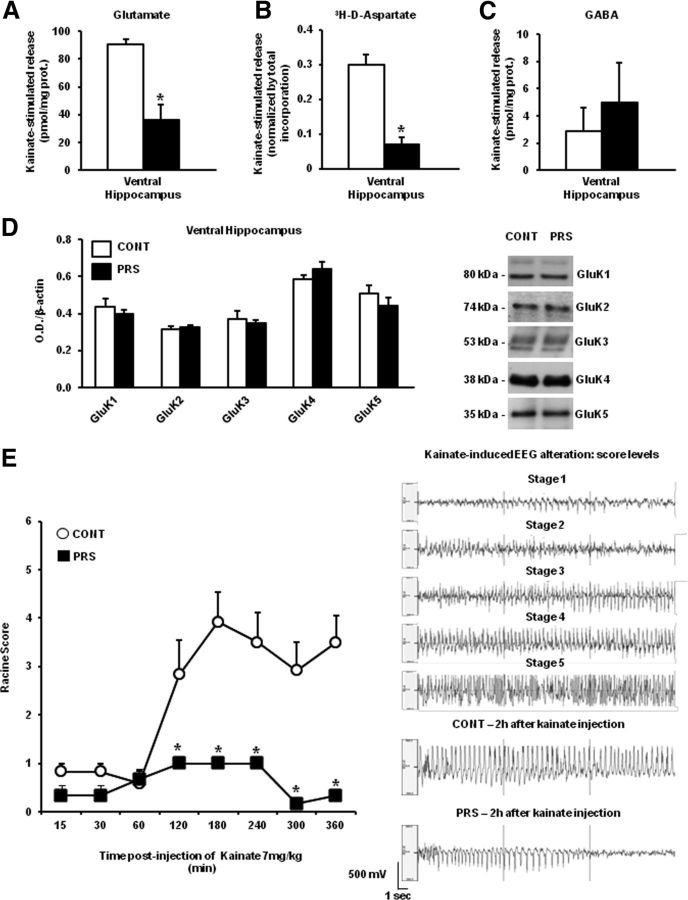
Figure 1.
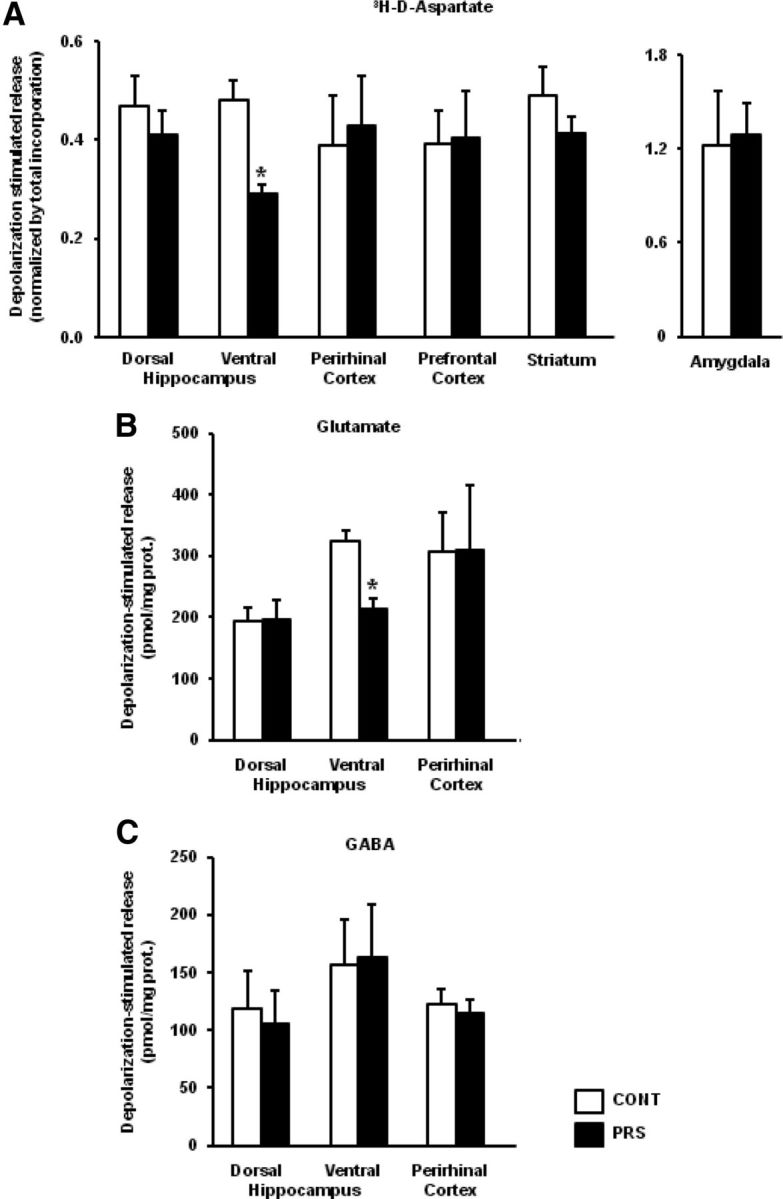
PRS causes a selective impairment of depolarization-evoked glutamate release in synaptosomes from the ventral hippocampus. Superfused synaptosomal preparations from the dorsal and ventral hippocampus, perirhinal cortex, prefrontal cortex, striatum, or amygdala of control (CONT) or PRS rats (one control and one PRS animal in each experiment) were stratified at the bottom of superfusion chambers (three superfusion chambers for each synaptosomal preparation) and superfused as described (see Materials and Methods). The total [3H]d-ASP content in the synaptosomal fraction at the start of the superfusion period amounted, respectively, to control dorsal hippocampus: 221.34 ± 14.75 nCi; PRS dorsal hippocampus: 226.44 ± 13.58 nCi, not significant (n.s.); control ventral hippocampus: 273.21 ± 37.02 nCi; PRS ventral hippocampus: 232.45 ± 21.32 nCi, n.s.; control perirhinal cortex: 341.14 ± 44.81 nCi; PRS perirhinal cortex: 298.31 ± 46.23 nCi, n.s.; control prefrontal cortex: 209.30 ± 18.33 nCi; PRS prefrontal cortex: 218.82 ± 23.44 nCi, n.s.; control striatum: 238.55 ± 15.56 nCi; PRS striatum: 246.07 ± 30.47 nCi, n.s.; control amygdala: 191.13 ± 10.09 nCi; PRS amygdala: 176.89 ± 16.89 nCi, n.s. Data are expressed as nCi/chamber and correspond to the amount of radioactive tracer taken up by each synaptosomal preparation. At t = 39 min of superfusion, synaptosomes were challenged with 20 (amygdala) or 12 (all other regions) mm K+. Synaptosomes were used for measurements of d-[3H]-aspartate (A), glutamate (B), or GABA (C) release. Data are expressed as K+-induced overflow. Glutamate and GABA overflow is expressed as pmol/mg prot. The evoked release of d-[3H]-aspartate is expressed as the percentage of the total tritium content in synaptosomes. High K+ depolarization-induced overflow is expressed as stimulated release over basal release. Values are means ± SEM of six experiments run in triplicate (3 superfusion chambers for each experimental condition). *p < 0.01 versus the respective controls.
PRS reduced both kainate-evoked glutamate release in the ventral hippocampus and behavioral responses to kainate
To examine whether the difference between control and PRS rats was stimulus specific, we also challenged synaptosomes with kainic acid. At least in our superfusion system, kainate (10 μm) substantially enhanced both glutamate and GABA release. Again, a substantial reduction in kainate-stimulated glutamate (t = 2.28; df = 10; p < 0.05) and d-[3H]-aspartate (t = 25.89; df = 10; p < 0.01) release was found in the ventral hippocampus of PRS rats (Fig. 2A,B). In contrast, kainate-stimulated GABA released was unchanged in synaptosomes prepared from the ventral hippocampus of PRS rats (Fig. 2C). To exclude that the reduction in kainate-stimulated glutamate release in the ventral hippocampus was due to changes in the expression of kainate receptors, we measured the levels of kainate receptor subunits (GluK1–5) by immunoblotting. GluK1–5 protein levels did not differ between PRS rats and control rats (Fig. 2D). As a behavioral counterpart of the study of kainate on glutamate release, we examined kainate-induced motor seizures in control and PRS rats. Systemic injection of kainate (7 mg/kg, i.p.) in control rats induced secondarily generalized partial limbic motor seizures characterized by motor arrest, wet dog shake, head nodding, masticatory movements, and rearing with forepaw clonus. Some control rats developed generalized tonic–clinic seizures and status epilepticus. Interestingly, kainate-induced seizures were largely reduced in PRS rats (group × time F(1,8) = 11.31, p < 0.01) (Fig. 2E). The average seizure severity score of PRS rats at 120–240 min following kainate injection was around “1” of the Racine scale. In contrast, the average score of control rats was between 3 and 4 at 120 min following kainate injection. None of PRS rats showed generalized seizures and status epilepticus in response to kainate (Fig. 2E). PRS and control rats did not differ in the temporal latency to the induction of motor seizures. Representative EEG traces corresponding to a score of 1 to 5 of the Racine scale are shown in Figure 2E. Typical EEG recording of control and PRS rats at 2 h following kainate injection are also shown (Fig. 2E).
Figure 2.
Reduced kainate-stimulated glutamate release in the ventral hippocampus and kainate-induced limbic motor seizures in PRS rats. Kainate-induced release of glutamate, d-[3H]-aspartate, and GABA in superfused synaptosomes prepared from the ventral hippocampus of control (CONT) or PRS rats are shown in A, B, and C, respectively. Data are expressed as reported in Figure 1, as kainate-induced overflow. Values are means ± SEM of six experiments run in triplicate (3 superfusion chambers for each experimental condition). *p < 0.05 or p < 0.01 versus the respective controls. Immunoblot analysis of GluK1–5 kainate receptor subunits in the ventral hippocampus of control and PRS rats is shown in D. Values are means ± SEM of six determinations. Behavioral score of kainate-induced seizures in control and PRS rats is shown in E. Kainate was injected at the dose of 7 mg/kg, intraperitoneally. Values are means ± SEM of six determinations. *p < 0.01 versus the respective data obtained in control rats. Representative EEG traces corresponding to different stages of kainate-induced seizures and representative traces obtained in control and PRS at 2 h following kainate injection.
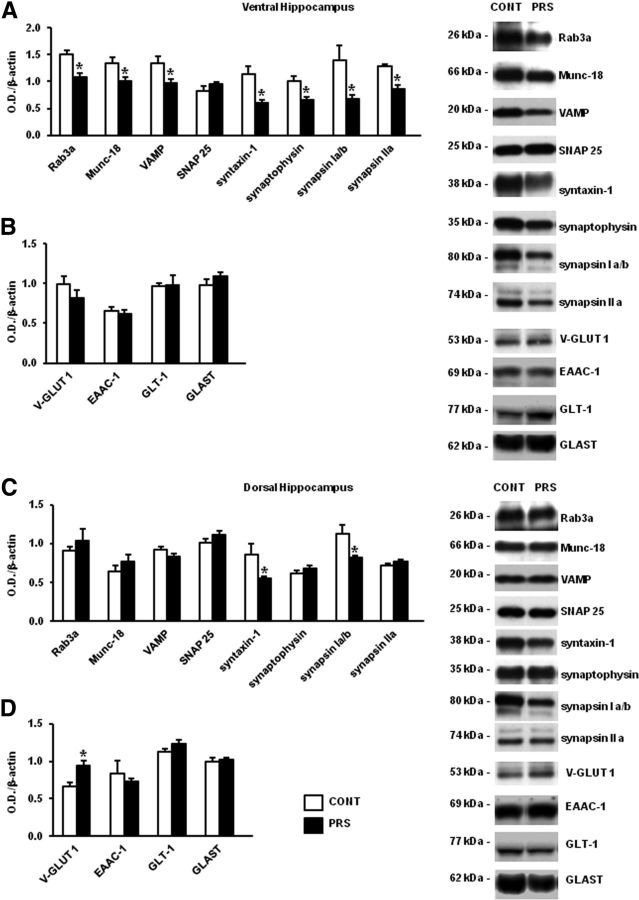
The reduction of evoked glutamate release was associated with lower expression of synaptic vesicle-related proteins in the ventral hippocampus of PRS rats
We measured the levels of synaptic vesicle-associated proteins and membrane glutamate transporters in purified synaptosomal membranes prepared from the ventral and dorsal hippocampus of control and PRS rats. Substantial reductions in the levels of Rab3A (t = 4.76, df = 10; p < 0.01), Munc-18 (t = 2.78; df = 10; p < 0.05), VAMP (synaptobrevin) (t = 2.91; df = 10; p < 0.05), syntaxin-1 (t = 3.30; df = 10; p < 0.01), synaptophysin (t = 3.41; df = 10; p < 0.01), synapsin Ia/b (t = 2.65; df = 10; p < 0.05), and synapsin IIa (t = 5.41; df = 10; p < 0.01) were found in the ventral hippocampus of PRS rats (Fig. 3A), whereas levels of SNAP25, and the glutamate transporters, v-Glut1, GLAST EACC-1, and GLT-1 did not change (Fig. 3B). Levels of synapsin Ia/Ib were lowered by as much as 60% and levels of syntaxin by ∼50% in the ventral hippocampus of PRS rats. We only found reductions in the levels of synapsin Ia/Ib (t = 2.70; df = 10; p < 0.05) and syntaxin (t = 3.30; df = 10; p < 0.01), and an increase in the levels of v-Glut1 (t = 2.90; df = 10; p < 0.05) in the dorsal hippocampus of PRS rats (Fig. 3C,D). Levels of all other proteins did not change in the dorsal hippocampus.
Figure 3.
PRS reduced expression of synaptic vesicle-associated proteins in the ventral hippocampus. Immunoblot analysis of SNAREs, vesicle-associated proteins, and glutamate transporters in synaptosomal fractions collected from the ventral (A, B) and dorsal (C, D) hippocampus of adult PRS and control (CONT) male rats. Values are means ± SEM of six determinations. *p < 0.05 or p < 0.01 versus the respective controls.
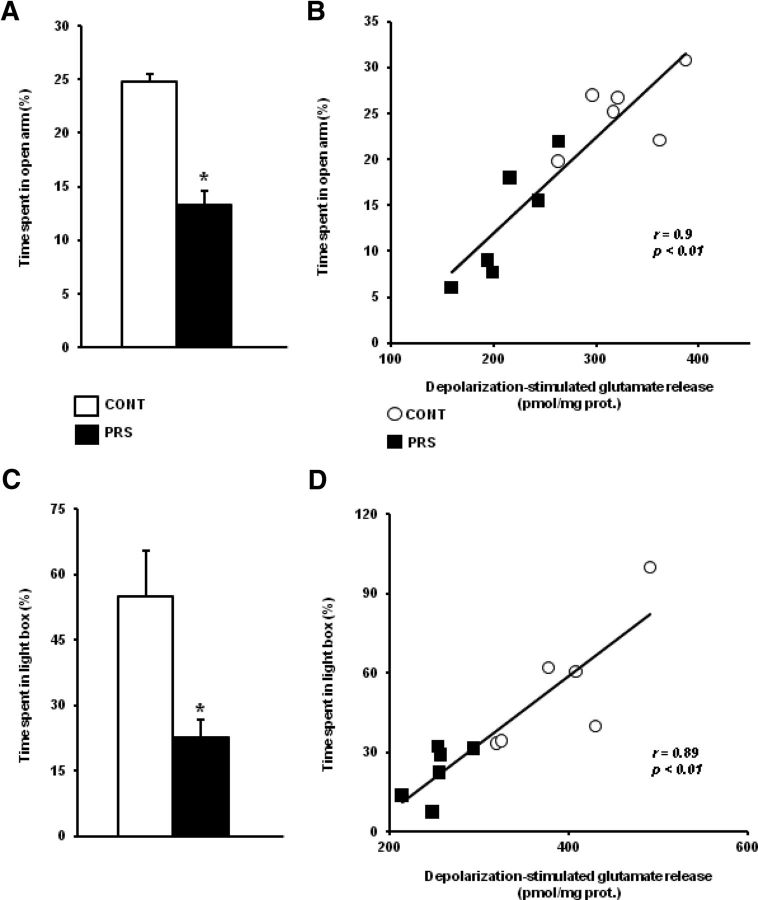
The reduction in depolarization-evoked glutamate release in the ventral hippocampus correlated positively with anxiety-like behavior in PRS rats
PRS rats show anxiety-like behavior (Vallée et al., 1997; Zuena et al., 2008; Morley-Fletcher et al., 2011), and the ventral portion of the hippocampus is involved in emotion and anxiety (Fanselow and Dong, 2010). Hence, we examined the correlation between depolarization-evoked glutamate release in the ventral hippocampus and anxiety-like behavior in control and PRS rats. Both control and PRS rats used for measurements of glutamate release in synaptosomes (see above) had been tested for anxiety-like behavior in the EPM. PRS rats spent less time in the open arm of the EPM, as expected (t = 3.40; df = 10; p < 0.01) (Fig. 4A). We found a positive correlation between the time spent by animals in the open arm of the EPM and the extent of depolarization-stimulated glutamate release in the ventral hippocampus (r = 0.90; p < 0.01), indicating that anxiety-like behavior was inversely related to the evoked release of glutamate (Fig. 4B). We extended the study to additional groups of control and PRS rats tested for anxiety-like behavior in the light–dark box. PRS rats spent less time in the light compartment of the light–dark box (t = 2.90; df = 10; p < 0.05) (Fig. 4C). There was a positive correlation between the time spent by control and PRS rats in the light compartment and the extent of depolarization-stimulated glutamate release in the ventral hippocampus (r = 0.89; p < 0.01; Fig. 4D), confirming the inverse correlation between anxiety-like behavior and glutamate release.
Figure 4.
Negative correlation between depolarization-evoked release in the ventral hippocampus and anxiety-like behavior. Anxiety-like behavior in the EPM and light–dark box is shown in A and C, where the time spent in the open arm of the EPM and in the light compartment of the light–dark box is shown. Different groups of rats were used for behavioral analysis in the EPM and light–dark box. Data are means ± SEM of six determinations. *p < 0.01 or p < 0.05 versus the respective controls. Correlation analysis between the time spent in the open arm of the EPM or in the light compartment of the light–dark box and depolarization-evoked glutamate release in synaptosomes prepared from the ventral hippocampus of control (CONT) and PRS rats is shown in B and D, respectively.
Pharmacological enhancement of glutamate release in the ventral hippocampus corrects anxiety-like behavior in PRS rats
To examine whether the reduction of glutamate release in the ventral hippocampus was causally related to anxiety-like behavior in PRS rats we used a mixture of drugs that block presynaptic type-2/3 metabotropic glutamate (mGlu2/3) receptors and GABAB receptors. These receptors are known to negatively regulate glutamate release in the hippocampus and other brain regions (reviewed by Chalifoux and Carter, 2011; Nicoletti et al., 2011). We combined the selective GABAB receptor antagonist, CGP52432 (Lanza et al., 1993) with the preferential mGlu2/3 receptor antagonist, LY341495 (Schoepp et al., 1999). To examine whether this mixture was able to enhance glutamate release we could not use isolated synaptosomal preparations because the method of superfused synaptosomes eliminates the influence of endogenously activated presynaptic receptors on neurotransmitter release (see above). Thus, we measured d-[3H]-aspartate release in preloaded hippocampal slice preparations. We used saturating concentrations of CGP52432 and LY341495 (10 and 1 μm, respectively). At these concentrations, LY341495 is still a preferential blocker of mGlu2/3 receptors with respect to other mGlu receptor subtypes (Schoepp et al., 1999). The addition of CGP52432 and LY341495 enhanced high-K+ (30 mm) evoked d-[3H]-aspartate release in slices prepared from the ventral hippocampus of both control and PRS rats (39 ± 14 and 29 ± 8% above values obtained with 30 mm K+ alone, respectively; n = 5), without affecting basal d-[3H]-aspartate release. Interestingly, the mixture of CGP52432 and LY341495 had no effect on depolarization-evoked d-[3H]-aspartate release in slices from the dorsal hippocampus of control and PRS rats (data not shown). We therefore decided to study anxiety-like behavior in control and PRS rats after bilateral microinfusion of CGP53432 plus LY341495 in the ventral hippocampus. Based on previous studies (Jackson and Kuehl, 2002; Barker et al., 2006; Li and Pan, 2007;Dong et al., 2012) we first tested three doses of CGP53432 (1 ng, 10 ng, or 50 ng) always combined with 100 pg of LY341495. The mixture containing 10 ng or 50 ng of CGP53432 increased rearing and wet dog shake, whereas the mixture containing 1 ng of CGP53432 did not cause changes in motor activity or spontaneous motor behavior in control rats. Thus, we decided to use 1 ng of CGP53432 combined with 100 pg of LY341495 for the study of anxiety-like behavior in control and PRS rats. All animals used for this study had been tested for anxiety-like behavior in the light–dark box 14–17 d before microinfusion experiments (Fig. 5A). Following microinfusion with vehicle or CGP52432 plus LY341495 all animals were consecutively tested in the light–dark box and in the EPM. This behavioral protocol is unusual because data of the second test (the EPM) might have been confounded by the effects of the first test (the light–dark box). However, we adopted this strategy to examine the effect of CGP52432 and LY341495 in two different tests of anxiety without the need to reinject the drugs in the ventral hippocampus. PRS rats infused with vehicle in the ventral hippocampus spent less time in the light compartment of the light–dark box, as expected. This paradigm of anxiety-like behavior was corrected by the mixture of CGP53432 and LY341495. Intrahippocampal infusion of CGP52432 and LY341495 had no effect on control unstressed rats (group × treatment, F(1,20) = 5.40; p < 0.05; n = 6 per group; Fig. 5B). PRS rats treated with vehicle showed also an increased latency to enter the light compartment of the box, which, again, was corrected by treatment with CGP53432 and LY341495 (group × treatment, F(1,20) = 33.54; p < 0.01; Table 1). In contrast, the number of entries into the light and dark compartment did not differ among the four groups of rats (Table 1). The “curative” effect of CGP53432 and LY341495 on anxiety-like behavior of PRS rats was supported by EPM data. PRS rats treated with vehicle, but not PRS rats treated with CGP53432 plus LY341495, spent less time in the open arm of the EPM (group × treatment, F(1,20) = 18.16; p < 0.01; n = 6 per group; Fig. 5C). PRS rats treated with vehicle also showed a reduced number of entries into the open arm, a reduced number of episodes of head dips (which is a surrogate indicator for anxiety like-behavior), an increased latency to enter the open arm, and a reduced latency to enter the closed arm. Most of these alterations were corrected by treatment with CGP53432 plus LY341495 (number of entries into the open arm: group× treatment, F(1,20) = 7.79; p < 0.05; episodes of head dips: F(1,20) = 25.64; p < 0.01; latency to enter the open arm: F(1,20) = 5.05; p < 0.05; latency to enter the closed arm: F(1,20) = 24.20; p < 0.05) (Table 2). The four groups of rats did not differ with respect to the number of entries into the closed arm, the number of crossings through the central area of the EPM, and the episodes of rearing (Table 2), excluding nonspecific effects of the treatments on motor behavior.
Figure 5.
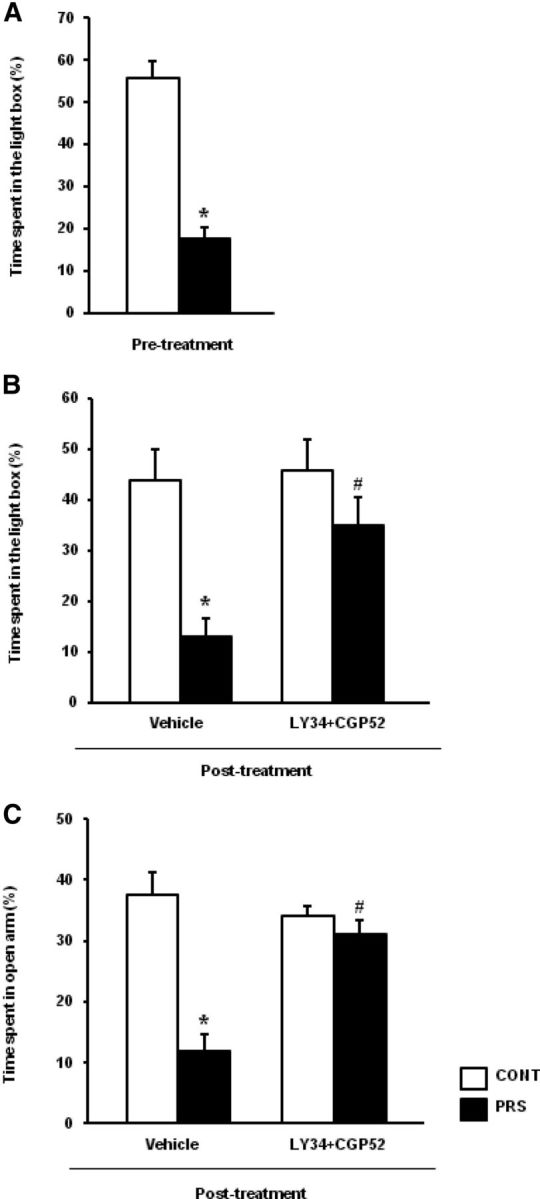
Pharmacological enhancement of glutamate release corrects anxiety-like behavior in PRS rats. All rats were tested in the light–dark box 1 week before surgery (i.e., 14–17 d before drug microinfusions in the ventral hippocampus). The time spent in the light compartment of the light–dark box in this pretest performed 14–17 d before is shown in A. Values are means ± SEM of 12 control (CONT) and 12 PRS rats. *p < 0.01 versus control rats. Behavioral data obtained in unstressed and PRS rats following microinfusion of vehicle or CGP52432 plus LY341495 in the ventral hippocampus are shown in B and C. Animals were first tested in the light–dark box and immediately after in the EPM. The effects of the first test experience might confound the interpretation of data of the second test (the EPM). However, the mixture of CGP52432 and LY341495 reduced anxiety-like behavior in PRS rats in both tests. The time spent in the open arm of the EPM and in the light compartment of the light–dark box in control and PRS rats bilaterally infused with 1 μl PBS containing 1 ng of CGP52432 and 100 pg of LY341495 or PBS alone (vehicle) in the ventral hippocampus are shown in B and C, respectively. Values are means ± SEM of six rats per group. p < 0.01 or p < 0.05 versus the respective control values (*) or versus the respective values treated with vehicle (#).
Table 1.
Number of entries and latency to enter the two compartments of the light–dark box in control and PRS rats bilaterally infused with vehicle or CGP53432 plus LY341495 into the ventral hippocampus
| Number of entries | Latency to enter (s) | |
|---|---|---|
| CONT/vehicle | ||
| Light compartment | 5.5 ± 0.6 | 46 ± 9.0 |
| Dark compartment | 5.9 ± 0.6 | 28 ± 9.5 |
| PRS/vehicle | ||
| Light compartment | 5.2 ± 1.0 | 228 ± 31* |
| Dark compartment | 4.7 ± 0.9 | 9.9 ± 1.8 |
| CONT/ CGP + LY | ||
| Light compartment | 5.2 ± 0.9 | 49 ± 7.3 |
| Dark compartment | 5.5 ± 0.8 | 22 ± 3.2 |
| PRS/CGP + LY | ||
| Light compartment | 6.3 ± 0.4 | 35 ± 3.8 |
| Dark compartment | 6.8 ± 0.4 | 18 ± 3.2 |
Table 2.
EPM data of control and PRS rats bilaterally infused with vehicle or CGP53432 plus LY341495 into the ventral hippocampus
| CONT/vehicle | PRS/vehicle | CONT/CGP + LY | PRS/CGP + LY | |
|---|---|---|---|---|
| Number of entries into the open arm | 5.5 ± 0.6 | 2.3 ± 0.5a | 5.0 ± 0.4 | 4.3 ± 0.2 |
| Number of entries into the closed arm | 8.2 ± 0.5 | 7.0 ± 0.6 | 11 ± 1.7 | 9.2 ± 0.7 |
| Number of crossings through the center | 13 ± 0.8 | 11 ± 1.7 | 13 ± 1.2 | 12 ± 1.0 |
| Episodes of head dips | 11 ± 1.5 | 4.8 ± 1.4b | 14 ± 1.4 | 9.7 ± 1.3c |
| Rearing episodes | 10 ± 1.5 | 8.8 ± 0.5 | 8.5 ± 1.6 | 10 ± 0.9 |
| Latency to enter the open arm | 6.2 ± 1.4 | 71 ± 25d | 5.9 ± 1.4 | 14 ± 2.9 |
| Latency to enter the closed arm | 37 ± 4.2 | 1.9 ± 0.5e | 57 ± 15 | 12 ± 4.9 |
Data were obtained from the same control (CONT) and PRS rats of Figure 5B; see legend for details on treatments with CGP53432 and LY341495. Values are means ± SEM of six rats per group.
ap < 0.01 versus all other groups;
bp < 0.01 versus CONT/vehicle and CONT/CGP + LY, and p < 0.05 versus PRS/CGP + LY;
cp < 0.05 versus CONT/CGP + LY and PRS/vehicle;
dp < 0.01 versus all other groups; and
ep < 0.05 versus CONT/vehicle and p < 0.01 versus Cont/CGP + LY. CONT, controls.
Discussion
We have shown for the first time that prenatal restraint stress, which is a model that recapitulates some of the features of depression and anxiety, causes a selective impairment of glutamate release in the ventral hippocampus, a brain region that specifically encodes memories related to stress and emotions (Fanselow and Dong, 2010). The reduced glutamate release in PRS rats was not due to an impaired glutamate synthesis in presynaptic terminals because it was also seen in synaptosomes preloaded with d-[3H]-aspartate. Martisova et al. (2012) found a reduced expression of vesicular glutamate transporters in the hippocampus of rats subjected to maternal separation, which is another model of early life stress. In contrast, VGLUT1 expression was unchanged in the ventral hippocampus of PRS rats, thus excluding a reduced glutamate transport into synaptic vesicles. Our data strongly suggest that PRS causes a long-lasting dysfunction in the intrinsic machinery controlling exocytotic glutamate release in the ventral hippocampus. Regulated neurotransmitter release depends on Ca2+ sensors, C2 domain proteins that associate with phospholipids, the three proteins of the SNARE complex (VAMP, SNAP25, syntaxin), and other proteins regulating the trafficking of synaptic vesicles, such as synaptophysin, synapsins, munc-18, and Rab3A (for review, see Han et al., 2010; Epp et al., 2011; Hussain and Davanger, 2011; Walter et al., 2011). Synaptophysin acts as a regulator of the SNARE complex (Hinz et al., 2001), and is also considered as a marker protein of presynaptic nerve endings (Thome et al., 2001; Grillo et al., 2005). Synapsins are involved in the clustering of synaptic vesicles to the reserve pool near the release sites in presynaptic terminals (Valtorta et al., 1992; Greengard et al., 1993). Munc-18 is a molecular chaperone of syntaxin-1, which is involved in mechanisms of SNARE-mediated membrane fusion and docking of large dense-core vesicles to the plasma membrane (Han et al., 2010). Rab3A, a member of a large family of monomeric GTP-binding proteins, regulates the trafficking of synaptic vesicles and cooperates with synapsin II in promoting the latest steps of neurotransmitter release (Sakane et al., 2006; Coleman and Bykhovskaia, 2010). PRS caused large reductions in the levels of all these proteins (except SNAP25) in the ventral hippocampus, and only reductions in the levels of syntaxin and synapsin Ia/b in the dorsal hippocampus. This profile of expression of vesicle-associated proteins fits nicely with the finding that glutamate release was reduced in the ventral hippocampus, but not in the dorsal hippocampus of PRS rats.
A potential consequence of the reduced glutamate release in the ventral hippocampus is that PRS rats become refractory to paroxysmal activity sustained by an enhanced release of glutamate. In release experiments, we used kainate as an alternative to high concentrations of K+. Kainate acting at presynaptic receptors is known to either stimulate or depress glutamate and GABA release depending on the concentrations and the hippocampal subregions (Ferkany et al., 1982; Poli et al., 1985; Chittajallu et al., 1996; Schmitz et al., 2001; Rodríguez-Moreno and Sihra, 2004). In our synaptosomal preparations, kainate caused a large release of glutamate, which was blunted in the ventral hippocampus of PRS rats. PRS rats were highly resistant to kainate-induced limbic motor seizures, which model temporal lobe epilepsy in humans (Ben-Ari and Cossart, 2000; Coulter et al., 2002). All PRS rats treated with kainate showed only mild motor signs, and none of them developed the typical secondarily generalized limbic motor seizures, which were instead seen in control rats. However, the relationship between early life stress and kainate-induced seizures is uncertain because a single episode of restraint stress on gestational day 18 enhanced kainate-induced seizures in adult gonadectomized offspring (Frye and Bayon, 1999), whereas treatment with β-methasone on gestational day 15 reduced the susceptibility to fluorothyl-induced clonic seizures, but not to kainate-induced seizures, at postnatal day 15 (Velíšek, 2011).
PRS had profound effects on glutamate release, but it failed to affect GABA release in the ventral hippocampus. The lack of changes in VGLUT1 expression and d-[3H]-aspartate uptake excluded that the number of glutamatergic nerve terminals was reduced in the ventral hippocampus of PRS rats. Reductions in munc-18 and Rab3A might provide some specificity for glutamate versus GABA release. Accordingly, munc-18 regulates the size of the readily releasable vesicle pool in glutamatergic but not GABAergic terminals (Augustin et al., 1999), and Rab3A is preferentially, albeit not exclusively, expressed in glutamatergic terminals (Geppert et al., 1994). Our data suggest that PRS causes an imbalance between excitatory and inhibitory neurotransmission in the ventral hippocampus, an effect that might perturb cognitive functions related to stress and emotions (for review, see Bannerman et al., 2004; Engin and Treit, 2007; Fanselow and Dong, 2010). Presynaptic alterations in the glutamate/GABA balance have been associated with anxiety, depressive-like behavior, and memory impairment (Tordera et al., 2007; Garcia-Garcia et al., 2009; Chen et al., 2010). Thus, the imbalance between excitatory and inhibitory neurotransmission in the ventral hippocampus might contribute to explain the anxious/depressive-like phenotype of PRS rats (Vallée et al., 1997; Zuena et al., 2008; Morley-Fletcher et al., 2011; see also present data).
Another important aspect of our study is the regional specificity in the reduction of glutamate release seen in PRS rats. Previous studies have shown that stressors of various types can have profound effects on glutamatergic transmission not only in the hippocampus but also in the prefrontal cortex, striatum, and amygdala (Fumagalli et al., 2009; Mozhui et al., 2010; Uchida et al., 2011; Farley et al., 2012; for review, see Popoli et al., 2012). For example, Fumagalli et al. (2009) have found that PRS rats challenged with a swim test in adulthood showed an attenuated phosphorylation of the NR1 subunit of NMDA receptors in the prefrontal cortex, but not in the hippocampus. In our PRS rats, glutamate release was reduced in the ventral hippocampus, but not in the dorsal hippocampus, prefrontal cortex, perirhinal cortex, striatum, or amygdala. The specificity for ventral versus dorsal hippocampus is in agreement with previous data showing that group-I mGlu receptor signaling is selectively blunted in the ventral hippocampus of PRS rats (Zuena et al., 2008). Also, the lack of changes in glutamate release in the dorsal hippocampus is in agreement with the evidence that PRS rats do not show abnormalities in spatial memory unless they are >10 months of age (Vallée et al., 1999), when changes in the expression of postsynaptic mGlu receptors are prominent (Van Waes et al., 2009).
To examine whether a causal relationship exists between reduction of glutamate release in the ventral hippocampus and anxiety-like behavior in PRS rats, we performed microinfusion studies with a mixture of mGlu2/3 and GABAB receptor antagonists, which was proven to selectively enhance glutamate release in the ventral hippocampus. All animals selected for this experiment had been tested for anxiety-like behavior ∼2 weeks before intrahippocampal microinfusions. Following infusions with vehicle or CGP52432 and LY341495, we designed a behavioral protocol based on two consecutive tests in the light–dark box (first) and the EPM (immediately after). This is unusual because repetitive tests for the assessment of anxiety-like behavior are generally performed with at least 1 week of interval to avoid the influence of the previous test experience (Vǒikar et al., 2004; Cryan and Holmes, 2005; Paylor et al., 2006; Ballaz et al., 2007) and treatment-dependent fluctuations in behavior that may occur between two consecutive tests (Izídio et al., 2005; Ramos, 2008). We adopted the strategy of two consecutive behavioral tests to avoid the need to reinject CGP52432 and LY341495 in the ventral hippocampus without having knowledge of the neuroplastic changes induced by these drugs in the hippocampus. This may confound the interpretation of the EPM data (but not the interpretation of the light–dark box data) following injection of CGP52432 and LY341495 in unstressed and PRS rats. Taking into account these possible limitations, our data suggest a causal relationship between reduction of glutamate release in the ventral hippocampus and anxiety-like behavior in PRS rats. The doses of CGP52432 and LY341495 we have used (1 and 100 pg, respectively), did not cause nonspecific changes in motor activity in the light–dark box and EPM, and did not affect anxiety-like behavior in unstressed control rats. Thus, pharmacological enhancement of glutamate release in the ventral hippocampus could specifically reverse anxiety-like behavior in PRS rats.
The mechanisms by which PRS causes a dysfunction in glutamate release in the ventral hippocampus is unknown. PRS rats are characterized by a hyper-reactivity of the hypothalamic—pituitary–adrenal axis, which results into a prolonged corticosterone response to stress (Maccari et al., 1995), and this might have a causal role in the dysfunction of glutamate release in the ventral hippocampus (Popoli et al., 2012). The hypothesis that high levels of corticosterone cause a long-lasting reduction in glutamate release in PRS rats warrants further investigation. We cannot exclude that changes in glutamatergic neurotransmission occurring in other brain regions contribute to the anxiety-like phenotype of PRS rats. Our data suggest that an impairment of glutamate release in the ventral hippocampus may lie at the core of the neuroplastic program induced by PRS, and strongly correlates with the development of anxiety-like behavior in these rats.
In conclusion, these findings support the “glutamatergic hypothesis” of depression and anxiety (Maeng and Zarate, 2007; Matrisciano et al., 2007; Hashimoto, 2009; Popoli et al., 2012), and suggest to extend the study of the balance between excitatory and inhibitory neurotransmission in the ventral hippocampus in other putative animal models of anxiety to develop new therapeutical strategies for stress-related disorders.
Footnotes
This study was supported by North University of Lille-Lille1 and the Sapienza University of Rome (Frame Agreement signed between the two universities on 15/02/2007) and by Centre National de la Recherche Scientifique in the framework of the European Research Team (GDRE 691) “Early Programming of Modern Diseases” (Coordinators S. Maccari and Dr. A. Moles). J. Marrocco was supported by the Ministry of French Education. J. Mairesse received funding from Fondation Recherche Médicale.
The authors declare no competing financial interests.
References
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Ballaz SJ, Akil H, Watson SJ. Previous experience affects subsequent anxiety-like responses in rats bred for novelty seeking. Behav Neurosci. 2007;121:1113–1118. doi: 10.1037/0735-7044.121.5.1113. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barker GR, Bashir ZI, Brown MW, Warburton EC. A temporally distinct role for group I and group II metabotropic glutamate receptors in object recognition memory. Learn Mem. 2006;13:178–186. doi: 10.1101/lm.77806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Kowall M. Crucial role of the postnatal maternal environment in the expression of prenatal stress effects in the male rats. J Comp Physiol Psychol. 1977;91:1432–1446. doi: 10.1037/h0077401. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Berg M, Bruhn T, Johansen FF, Diemer NH. Kainic acid-induced seizures and brain damage in the rat: different effects of NMDA- and AMPA receptor antagonists. Pharmacol Toxicol. 1993;73:262–268. doi: 10.1111/j.1600-0773.1993.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, Raiteri M, Racagni G, Popoli M. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of synaptic function. Curr Opin Neurobiol. 2011;21:339–344. doi: 10.1016/j.conb.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RH, Stern JM. Failure of severe maternal stress or ACTH during pregnancy to affect emotionality of male rat offspring: implications of litter effects for prenatal studies. Dev Psychobiol. 1979;12:255–267. doi: 10.1002/dev.420120309. [DOI] [PubMed] [Google Scholar]
- Chen G, Henter ID, Manji HK. Presynaptic glutamatergic dysfunction in bipolar disorder. Biol Psychiatry. 2010;67:1007–1009. doi: 10.1016/j.biopsych.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Coleman WL, Bykhovskaia M. Cooperative regulation of neurotransmitter release by Rab3a and synapsin II. Mol Cell Neurosci. 2010;44:190–200. doi: 10.1016/j.mcn.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA, McIntyre DC, Löscher W. Animal models of limbic epilepsies: what can they tell us? Brain Pathol. 2002;12:240–256. doi: 10.1111/j.1750-3639.2002.tb00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modeling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Perez-Martin M, Bélizaire G, Maccari S, Garcia-Segura LM. Insulin-like growth factor 1 reduces age-related disorders induced by prenatal stress in female rats. Neurobiol Aging. 2006;27:119–127. doi: 10.1016/j.neurobiolaging.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dong E, Wellman LL, Yang L, Sanford LD. Effects of microinjections of Group II metabotropic glutamate agents into the amygdala on sleep. Brain Res. 2012;1452:85–95. doi: 10.1016/j.brainres.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C, Maccari S, Weibel L, Turek FW, Van Reeth O. High corticosterone levels in prenatally stressed rats predict persistent paradoxical sleep alterations. J Neurosci. 1999;19:8656–8664. doi: 10.1523/JNEUROSCI.19-19-08656.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Epp N, Rethmeier R, Krämer L, Ungermann C. Membrane dynamics and fusion at late endosomes and vacuoles–Rab regulation, multisubunit tethering complexes and SNAREs. Eur J Cell Biol. 2011;90:779–785. doi: 10.1016/j.ejcb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley S, Dumas S, El Mestikawy S, Giros B. Increased expression of the Vesicular Glutamate Transporter-1 (VGLUT1) in the prefrontal cortex correlates with differential vulnerability to chronic stress in various mouse strains: effects of fluoxetine and MK-801. Neuropharmacology. 2012;62:503–517. doi: 10.1016/j.neuropharm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Ferkany JW, Zaczek R, Coyle JT. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature. 1982;298:757–779. doi: 10.1038/298757a0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Prenatal stress reduces the effectiveness of the neurosteroid 3 alpha,5 alpha-THP to block kainic-acid-induced seizures. Dev Psychobiol. 1999;34:227–234. [PubMed] [Google Scholar]
- Fumagalli F, Pasini M, Frasca A, Drago F, Racagni G, Riva MA. Prenatal stress alters glutamatergic system responsiveness in adult rat prefrontal cortex. J Neurochem. 2009;109:1733–1744. doi: 10.1111/j.1471-4159.2009.06088.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Elizalde N, Matrov D, Harro J, Wojcik SM, Venzala E, Ramírez MJ, Del Rio J, Tordera RM. Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol Psychiatry. 2009;66:275–282. doi: 10.1016/j.biopsych.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Südhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Grillo CA, Piroli GG, Wood GE, Reznikov LR, McEwen BS, Reagan LP. Immunocytochemical analysis of synaptic proteins provides new insights into diabetes-mediated plasticity in the rat hippocampus. Neuroscience. 2005;136:477–486. doi: 10.1016/j.neuroscience.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Han GA, Malintan NT, Collins BM, Meunier FA, Sugita S. Munc18–1 as a key regulator of neurosecretion. J Neurochem. 2010;115:1–10. doi: 10.1111/j.1471-4159.2010.06900.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hinz B, Becher A, Mitter D, Schulze K, Heinemann U, Draguhn A, Ahnert-Hilger G. Activity-dependent changes of the presynaptic synaptophysin-synaptobrevin complex in adult rat brain. Eur J Cell Biol. 2001;80:615–619. doi: 10.1078/0171-9335-00196. [DOI] [PubMed] [Google Scholar]
- Hussain S, Davanger S. The discovery of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex and the molecular regulation of synaptic vesicle transmitter release: the 2010 Kavli Prize in neuroscience. Neuroscience. 2011;190:12–20. doi: 10.1016/j.neuroscience.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Izídio GS, Lopes DM, Spricigo L, Jr, Ramos A. Common variations in the pretest environment influence genotypic comparisons in models of anxiety. Genes Brain Behav. 2005;4:412–419. doi: 10.1111/j.1601-183X.2005.00121.x. [DOI] [PubMed] [Google Scholar]
- Jackson GL, Kuehl D. The GABA(B) antagonist CGP 52432 attenuates the stimulatory effect of the GABA(B) agonist SKF 97541 on luteinizing hormone secretion in the male sheep. Exp Biol Med. 2002;227:315–320. doi: 10.1177/153537020222700503. [DOI] [PubMed] [Google Scholar]
- Joëls M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009;50:586–597. doi: 10.1111/j.1528-1167.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloux C, Mairesse J, Van Camp G, Giovine A, Branchi I, Bouret S, Morley-Fletcher S, Bergonzelli G, Malagodi M, Gradini R, Nicoletti F, Darnaudéry M, Maccari S. Anxiety-like behavior and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinology. 2012;37:1646–1658. doi: 10.1016/j.psyneuen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Lanza M, Fassio A, Gemignani A, Bonanno G, Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993;237:191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioral and neurobiological alterations. Psychoneuroendocrinology. 2007;32(Suppl 1):S10–S15.19. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Silletti V, Laloux C, Zuena AR, Giovine A, Consolazione M, van Camp G, Malagodi M, Gaetani S, Cianci S, Catalani A, Mennuni G, Mazzetta A, van Reeth O, Gabriel C, Mocaër E, Nicoletti F, Morley-Fletcher S, Maccari S. Chronic agomelatine treatment corrects the abnormalities in the circadian rhythm of motor activity and sleep/wake cycle induced by prenatal restraint stress in adult rats. Int J Neuropsychopharmacol. 2012a;6:1–16. doi: 10.1017/S1461145711001970. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Vercoutter-Edouart AS, Marrocco J, Zuena AR, Giovine A, Nicoletti F, Michalski JC, Maccari S, Morley-Fletcher S. Proteomic characterization in the hippocampus of prenatally stressed rats. J Proteomics. 2012b;75:1764–1770. doi: 10.1016/j.jprot.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, Ramírez MJ. Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology. 2012;62:1944–1953. doi: 10.1016/j.neuropharm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Panaccione I, Zusso M, Giusti P, Tatarelli R, Iacovelli L, Mathé AA, Gruber SH, Nicoletti F, Girardi P. Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication? Mol Psychiatry. 2007;12:704–706. doi: 10.1038/sj.mp.4002005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Koehl M, Casolini P, Van Reeth O, Maccari S. Prenatal stress in rats predicts immobility behavior in the forced swim test. Effects of a chronic treatment with tianeptine. Brain Res. 2003;989:246–251. doi: 10.1016/s0006-8993(03)03293-1. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudéry M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S. Chronic treatment with imipramine reverses immobility behavior, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology. 2004;47:841–847. doi: 10.1016/j.neuropharm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Mairesse J, Soumier A, Banasr M, Fagioli F, Gabriel C, Mocaer E, Daszuta A, McEwen B, Nicoletti F, Maccari S. Chronic agomelatine treatment corrects behavioral, cellular, and biochemical abnormalities induced by prenatal stress in rats. Psychopharmacology. 2011;217:301–313. doi: 10.1007/s00213-011-2280-x. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M. Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One. 2010;5:e8566. doi: 10.1371/journal.pone.0008566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat brain in stereotaxic coordinates. Ed 6. London: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Poli A, Contestabile A, Migani P, Rossi L, Rondelli C, Virgili M, Bissoli R, Barnabei O. Kainic acid differentially affects the synaptosomal release of endogenous and exogenous amino acidic neurotransmitters. J Neurochem. 1985;45:1677–1686. doi: 10.1111/j.1471-4159.1985.tb10522.x. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M. Synaptosomes still viable after 25 years of superfusion. Neurochem Res. 2000;25:1265–1274. doi: 10.1023/a:1007648229795. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Angelini F, Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur J Pharmacol. 1974;25:411–414. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Beattie JE, Reid IC, Balfour DJ. Regulation of corticosteroid receptors in the rat brain: the role of serotonin and stress. Eur J Neurosci. 2005;21:1511–1520. doi: 10.1111/j.1460-9568.2005.03990.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A, Sihra TS. Presynaptic kainate receptor facilitation of glutamate release involves protein kinase A in the rat hippocampus. J Physiol. 2004;557:733–745. doi: 10.1113/jphysiol.2004.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, Yoshida T, Miyoshi J, Kamiya H, Takai Y, Sasaki T. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci U S A. 2006;103:10029–10034. doi: 10.1073/pnas.0600304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2001;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–999. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Kainic acid induced seizures: neurochemical and histopathological changes. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA. Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry. 2001;50:809–812. doi: 10.1016/s0006-3223(01)01229-x. [DOI] [PubMed] [Google Scholar]
- Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behavior and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Otsuki K, Yamagata H, Hobara T, Suzuki T, Miyata N, Watanabe Y. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. 2011;69:359–372. doi: 10.1016/j.neuron.2010.12.023. [DOI] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. J Neurosci. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age-related glucocorticoid secretion and cognitive performance: a longitudinal study in the rat. Eur J Neurosci. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Valtorta F, Benfenati F, Greengard P. Structure and function of the synapsins. J Biol Chem. 1992;267:7195–7198. [PubMed] [Google Scholar]
- Van Waes V, Enache M, Zuena A, Mairesse J, Nicoletti F, Vinner E, Lhermitte M, Maccari S, Darnaudéry M. Ethanol attenuates spatial memory deficits and increases mGlu1a receptor expression in the hippocampus of rats exposed to prenatal stress. Alcohol Clin Exp Res. 2009;33:1346–1354. doi: 10.1111/j.1530-0277.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- Van Waes V, Darnaudéry M, Marrocco J, Gruber SH, Talavera E, Mairesse J, Van Camp G, Casolla B, Nicoletti F, Mathé AA, Maccari S, Morley-Fletcher S. Impact of early life stress on alcohol consumption and on the short- and long-term responses to alcohol in adolescent female rats. Behav Brain Res. 2011;221:43–49. doi: 10.1016/j.bbr.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Velíšek L. Prenatal corticosteroid exposure alters early developmental seizures and behavior. Epilepsy Res. 2011;95:9–19. doi: 10.1016/j.eplepsyres.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vǒikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing o in C57BL/6J and 129S2/SV mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- Walter AM, Groffen AJ, Sørensen JB, Verhage M. Multiple Ca2+ sensors in secretion: teammates, competitors or autocrats? Trends Neurosci. 2011;34:487–497. doi: 10.1016/j.tins.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Reagan LP. The glutamatergic system in neuronal plasticity and vulnerability in mood disorders. Neuropsychiatr Dis Treat. 2006;2:7–14. [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alem à GS, Morley-Fletcher S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]