Abstract
Background
With the number of annual global travellers reaching 1.2 billion, many individuals encounter greater levels of air pollution when they travel abroad to megacities around the world. This study’s objective was to determine if visits to cities abroad with greater levels of air pollution adversely impact cardiopulmonary health.
Methods
A total of 34 non-smoking healthy adult participants who travelled abroad to selected cities from the New York City (NYC) metropolitan area were pre-trained to measure lung function, blood pressure and heart rate (HR)/HR variability (HRV) and record symptoms before, during and after travelling abroad. Outdoor particulate matter (PM)2.5 concentrations were obtained from central monitors in each city. Associations between PM exposure concentrations and cardiopulmonary health endpoints were analysed using a mixed effects statistical design.
Results
East and South Asian cities had significantly higher PM2.5 concentrations compared with pre-travel NYC PM2.5 levels, with maximum concentrations reaching 503 μg/m3. PM exposure–related associations for lung function were statistically significant and strongest between evening Forced Expiratory Volume in the first second (FEV1) and same-day morning PM2.5 concentrations; a 10-μg/m3 increase in outdoor PM2.5 was associated with a mean decrease of 7 mL. Travel to a highly polluted city (PM2.5 > 100 μg/m3) was associated with a 209-ml reduction in evening FEV1 compared with a low polluted city (PM2.5 < 35 μg/m3). In general, participants who travelled to East and South Asian cities experienced increased respiratory symptoms/scores and changes in HR and HRV.
Conclusions
Exposure to increased levels of PM2.5 in cities abroad caused small but statistically significant acute changes in cardiopulmonary function and respiratory symptoms in healthy young adults. These data suggest that travel-related exposure to increased PM2.5 adversely impacts cardiopulmonary health, which may be particularly important for travellers with pre-existing respiratory or cardiac disease.
Keywords: air pollution, particulate matter, travel, non-communicable diseases, cardiopulmonary health, climate change
Introduction
International tourist arrivals, which reached 1.24 billion in 2016, are expected to increase worldwide by 3.3% per year, reaching 1.8 billion travellers by 2030.1 While Europe remains the most popular destination, travel to Asia and Africa has increased significantly.1 This large and growing travel population affects travel-related health risk numbers, and therefore, well-being and health have become a part of travel concerns.
Megacities—metropolitan areas with populations exceeding 10 million inhabitants—2 are unique microenvironments that have high population densities, causing high energy demand, resulting in the burning of fossil fuels either at power-plants or for transportation needs.2–4 According to the World Health Organization (WHO) air quality databases, air pollution levels are rising in many of the world’s largest cities. The highest pollution levels were recorded in low- and middle-income countries in WHO’s Eastern Mediterranean and South-East Asia regions, with annual mean levels often exceeding 5–10× WHO guidelines.5 Megacities in Asia, including New Delhi, Mumbai, Dhaka, Shanghai and Beijing, rank among the most polluted cities, in terms of annual mean particulate matter (PM) concentrations. Residents and travellers to these and similar destinations may, therefore, be at increased risk from exposure to high levels of ambient air pollution, especially during certain agricultural seasons and weather conditions that result in major pollution episodes, as seen in New Delhi in late 2016 and 2017.
The health effects associated with exposure to air pollution is well documented, and early large-scale epidemiological studies, as well as more recent toxicity studies, have shown that exposure to air pollution, particularly PM pollution, is significantly associated with adverse health effects. These documented associations include human mortality, ischemic heart disease, asthma exacerbations, systemic inflammation and oxidative stress and a range of other health impacts, particularly among vulnerable populations.6–12
Travelling incurs many risks and, given the adverse health outcomes that air pollution can cause,6,8–11,13 it is reasonable to investigate the relative risk of air pollution exposure while travelling abroad in comparison with other risk factors,14,15 such as infectious diseases, which also contribute to travel-related adverse health risks. International travellers arriving unprepared in polluted cities, especially during an air pollution episode, may lack the necessary adaptation, precautionary measures or advice on how to minimize associated health risks.
Other than our brief Research Letter published elsewhere,16 to date, we are unaware of evidence in the scientific literature of studies that have looked at respiratory and/or cardiovascular health impacts when individuals travel abroad to cities having significantly higher air pollution levels than their home city. With the increase in worldwide travel, individuals are exposed to widely varying environmental pollution levels, inherent to each city, within a matter of hours. These relatively sudden air pollution exposure changes, especially in the case of PM, could have an adverse effect on the cardiopulmonary system. In this study, we followed individuals who travelled abroad and then returned to the US, in order to test the hypothesis that exposure to increased levels of inhaled PM2.5 adversely impacts the cardiopulmonary system in individuals who visit highly polluted megacities.
Methods
Study design
This study enrolled 42 non-smoking participants who travelled from the New York City (NYC) metropolitan area to cities abroad, categorized into four regions (Europe, South Asia, East Asia and Africa). Study participants were recruited using flyers, online social media posts, emails and personal introductions. The participants measured cardiopulmonary endpoints and recorded respiratory symptom data before, during and after travel—1 week before travel until departure, first week upon arrival in the city abroad and for the first week once returned to the home city. Each participant was required to provide at least 5 days of morning and evening measurements at each location to be included in the analyses. Inclusion requirements were travelling and staying in a city abroad for at least a week, > 21 years old and a non-smoker (status was self-reported). Details on regions and exclusion criteria are presented in the online supplementary section. New York University School of Medicine Institutional Review Board (NYUSOM IRB) approval was obtained for the study (Study number: s-14-02151).
Cardiopulmonary health measurements and symptoms
Participants were pre-trained to measure and record their own health data in the morning and evening each day, for 1 week in each study location (i.e. pre-travel NYC, city abroad and post-travel NYC). Lung function was measured with a Koko PeakPro6 spirometer17 (Ferraris, Louisville, CO), according to American Thoracic Society (ATS) guidelines.18 Participants performed three consecutive measurements each morning and evening. The highest value of the three measurements was used for analysis. Systolic/diastolic blood pressure (SBP/DBP, respectively) and heart rate (HR)/HR variability (HRV) were measured using a wrist BP monitor (Omron, 3 series—BP629) and a Polar H7 HR sensor, respectively, as previously described.19 The RR interval (Interval between successive heartbeats) files were further processed using KUBIOS software.20
Participants recorded symptoms occurring throughout the day during the study period: shortness of breath, cough, chest tightness, wheeze, difficulty in breathing, throat irritation, cough with phlegm, nasal irritation, nasal congestion, rhinorrhea, eye irritation, headache, nausea and light headedness. A self-reported ranking was marked for each symptom, with 0 being none, 1—slight, 2—moderate, 3—strong, 4—very strong and 5—had to use medication.
PM measurements
Central monitor ambient PM2.5 data were downloaded from government and other air-monitoring organizations (country dependent) for each participant’s location. In the NYC metropolitan area, the closest United States Environmental Protection Agency (USEPA) central monitor to participants’ homes, and when abroad, US embassy (preferred) or local government central monitors in the respective city, were used to obtain hourly values and calculate morning and evening means for the respective study periods. Further information on central monitor sources and locations are given in Table S4 available as Supplementary data at JTM online.
Statistical analyses
Mixed effects models were applied to repeated measures longitudinal data (of exposure and health metrics) to study the associations between health outcomes and PM2.5 concentrations. Health–exposure relationships were modelled using a participant-specific random intercept term (participant as a random effect), along with covariates (PM concentrations, age and height) and fixed terms (pollution level/region/city). The model slope represented the fraction of the health endpoint of interest that was associated with a unit change in PM concentration.
The linear mixed model equation is in the form
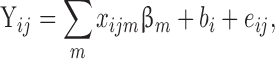 |
where yij is the health outcome of interest for the ith participant on the jth day for i = 1.., 34, j = 1,…nij. Fixed effect variables are x’s (e.g. region), and PM2.5 concentrations were included as covariates. Random effect (participant) is represented by bi, and eij represents the error component/natural variation. βm is the parameter estimate for ‘m’ number of fixed effects/covariates. For all statistical tests, P < 0.05 was considered statistically significant (Minitab Version 18 software). The equation terms may vary depending on the specific test and statistical software used.
Results
Cohort characteristics
The study population was made up of healthy, young adults, comprising mostly university students based in the NYC metropolitan area and New Jersey. A total of 42 participants were initially enrolled, of which 4 participants did not provide sufficient exposure or health data (i.e. less than 5 days of data provided at each location) and were excluded. Additionally, four other participants who travelled to Mexico City, Lima and Costa Rica (initially enrolled in the pilot phase) were excluded from the analyses due to the potential confounding factors, such as higher elevation and/or confounding activities, such as primarily hiking during holiday, beach going or frequent late-night activity with alcohol consumption. A total of 34 participants were included in the statistical analyses (23 female and 11 male): 10 East Asian, 6 South Asian and 18 Caucasian participants. Study participants did not use protective masks during their stay abroad. The cities and the participant number travelled to each city, and descriptive statistics of the study population are given in the Supplementary section (Tables S1 and S2 available as Supplementary data at JTM online).
Environmental measurements
PM2.5 concentrations varied largely between baseline and travel abroad cities. In general, mean PM2.5 concentrations of the cities abroad were significantly higher than pre-travel NYC concentrations (Figure 1). Descriptive statistics of outdoor PM2.5 concentrations are given in Table S3 available as Supplementary data at JTM online.
Figure 1.
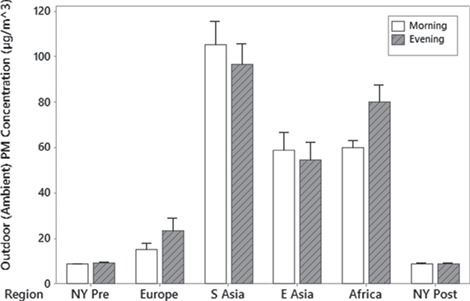
Mean outdoor (Ambient) PM2.5 concentrations by region. Error bars are one standard error from the mean. Pre = before travel, Post = after returning to resident city.
Significant differences in PM2.5 exposure levels were observed when analysed by region. East Asia (n = 12 participants) and South Asia (n = 7 participants) cities had higher mean outdoor PM2.5 concentrations than Europe and NYC. Cities in South Asia had the highest mean [±standard deviation (SD)] PM2.5 values as a group (105 ± 85 μg/m3 and 97 ± 74 μg/m3 for morning and evening, respectively). The highest mean PM2.5 concentration for Europe was 24 μg/m3 (outdoor evening measurements).
Health endpoint changes and PM exposure
Pulmonary function
PM-related changes in lung function were analysed by the observed decrease in FEV1 or Peak Expiratory Flow (PEF) per 10 μg/m3 PM: (i) overall; (ii) by cities grouped into low, medium or high pollution categories; and (iii) by region.
Overall, an increase in travel-related PM concentration was associated with statistically significant decrements in lung function. In particular, evening pulmonary function was affected by PM concentrations earlier in the day. A 10-μg/m3 increase in same-day morning central monitor PM2.5 was associated with a 7-ml decrease [95% confidence interval (CI): −11, −3ml] in evening FEV1, controlling for height, age and sex. PEF decrements were also observed: a 10-μg/m3 increase in outdoor morning PM2.5 concentration was associated with a 1-L/min decrease in evening PEF.
Lung function and PM2.5 associations were also analysed by using ambient PM2.5 concentrations to assign participants to low, medium and highly polluted ‘cities’ categories: Low (0–35 μg/m3), Medium (36–100 μg/m3) and High (> 100 μg/m3). Based upon the predictive mixed effects model, travel from a ‘Low’-polluted city to a ‘High’-polluted city was shown to result in a statistically significant mean decrement of 209 ml in evening FEV1, associated with the outdoor PM2.5 (Table 1). Main effects plots showing the fitted (predicted) means of evening FEV1 for low, medium and highly polluted cities are given in Figure 2.
Table 1.
Difference of predicted FEV1 means between the pollution categories from the mixed effects model after post-analysis using a Tukey method pairwise comparison test
| Difference—Category | Difference of means (Litres) | Standard Error (SE) of difference | Degrees of Freedom (DF) | Simultaneous 95% CI | T value | Adjusted P value |
|---|---|---|---|---|---|---|
| Medium—Low | −0.042 | 0.040 | 323 | (−0.136, 0.053) | −1.03 | 0.557 |
| High—Medium | −0.168 | 0.058 | 325 | (−0.305, −0.031) | −2.88 | 0.012 |
| High—Low | −0.209 | 0.042 | 323 | (−0.309, −0.109) | −4.94 | 0.000 |
Figure 2.
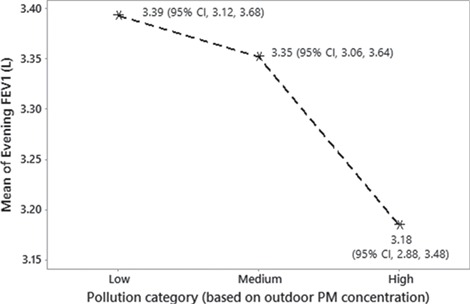
Main effects plot of fitted means for evening FEV1 with an outdoor pollution category assigned to each city: Low (0–35 μg/m3), Medium (36–100 μg/m3) and High (>100 μg/m3).
The travel abroad ‘region’ was also a significant predictor of FEV1 change and was mainly driven by the decrements in participants travelling to South Asia. Interestingly, travel to East Asia was associated with a positive change in FEV1 (+60 ml). However, when analysed by city separately, ‘city’-dependent significant PM-associated decrements in FEV1 were observed for Beijing and Xian in East Asia, despite the small number of participants travelling to each city.
We also assessed whether recovery from FEV1 decrements occurred upon return to NYC, in participants who were followed for 1 week after return The mixed effects model showed that, in participants who had decrements, travel back to NYC was associated with a mean 80-ml increase in FEV1 (95% CI, 122 ml, 40 ml).
BP, HR and HRV effects
SBP and DBP were not significantly affected by travel-related changes in PM2.5 concentration. Evening HR, however, was positively correlated (P < 0.05) with increasing evening outdoor PM2.5 concentration. A 10-μg/m3 increase of outdoor evening PM2.5 was associated with an increase of 0.2 (beats/min) in evening HR (P < 0.05).
The mixed effect model also demonstrated that an increase in PM2.5 was associated with a reduction in HRV. Evening SD of normal–normal intervals (SDNN) and percentage of successive RR intervals that differ by more than 50 ms (PNN50) were significantly correlated with evening outdoor PM2.5 concentration, and an increase of 10 μg/m3 was associated with a change of −0.6 ms (95% CI, −1.04, −0.24) and − 0.7% (95% CI, −1.09, −0.25), respectively, for evening SDNN and PNN50.
Respiratory symptoms
Significant travel-related changes in symptoms were observed, and means of the total symptoms score (sum of all individual symptom scores) varied between the baseline and travel abroad periods, depending upon the region to which the participants travelled (Figure 3). A general linear model ANOVA demonstrated that travelling from a ‘Low’-polluted city to a ‘High’-polluted city was associated with an increase of 3.9 points (95% CI, 2.724, 5.072) of the total symptom score, adjusting for age and sex.
Figure 3.
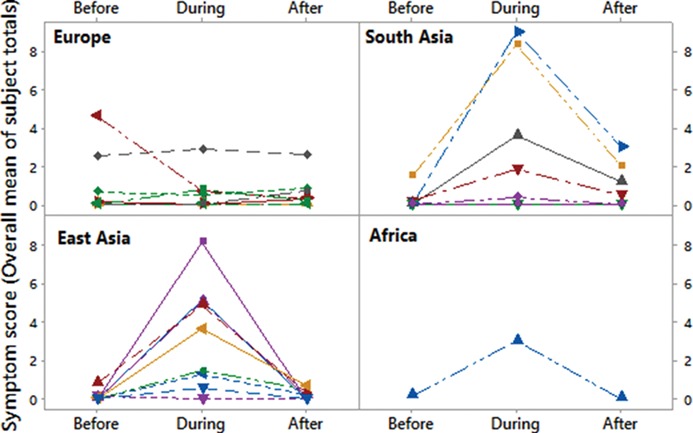
Mean of total symptom score before, during and after travel abroad. The mean value in each location is the mean of all participants’ total symptom score. Total score = sum of all individual scores for each respiratory symptom analysed.
However, several individuals did not show a linear increase in symptom score with increasing PM2.5 while abroad or showed no symptoms at all, even at high abroad PM2.5 concentrations that caused symptoms in other participants (Figures S1 and S2 available as Supplementary data at JTM online).
A mixed effects analysis demonstrated that participants who travelled to South and East Asia had significantly higher mean adverse respiratory symptom scores, as compared with pre-travel NYC and travellers to Europe (P < 0.05). Many of the participants who experienced a travel-related increase in symptoms showed a recovery upon return to NYC, particularly for travellers returning from South and East Asia (Figure 3).
The symptoms experienced during the time spent abroad were predominantly upper respiratory tract related. Cough, throat irritation, nasal congestion and rhinorrhea had the highest travel abroad scores among all individual symptoms analysed, with the symptom intensity (represented by the score) varying between regions and the pollution category of the city (low, medium or high) (Figure 4; Figure S3 available as Supplementary data at JTM online). Overall, study participants who travelled to Europe had lower mean symptom scores, as compared with participants who travelled to South or East Asia. The highest mean symptom score was observed for throat irritation in individuals who travelled to East Asian cities (Figure S3 available as Supplementary data at JTM online).
Figure 4.
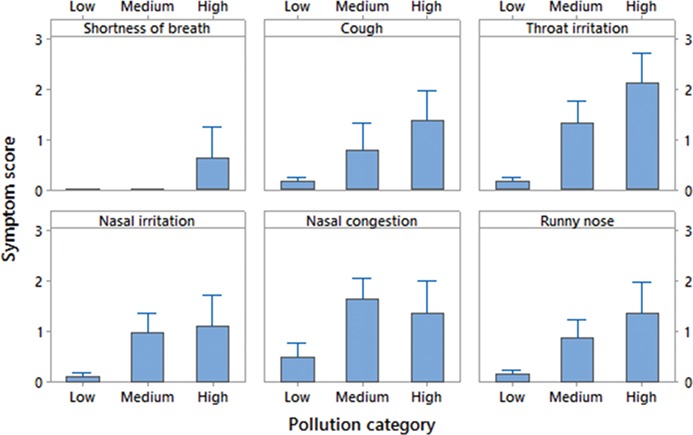
Respiratory symptom and their average (of total) scores abroad, by pollution category (low, medium and high). Africa is not shown in the graph because only one participant travelled to the region, and calculation of overall symptoms with n = 1 introduces a bias when comparing.
Discussion
This study provides the first documentation that travelling to polluted cities abroad can adversely impact health by resulting in acute measurable changes and symptoms. Travel-related exposure to higher levels of PM2.5 in cities abroad (compared with NYC) was associated with adverse health effects, including a reduction in lung function, an increase in HR, a reduced HRV and an increase in respiratory symptoms. Thus, PM-associated health effects should be considered as a key factor in considering travellers’ health.
Studies on impacts of PM exposure, especially fine PM, on lung and cardiac function in healthy, young adults are rare but have reported adverse changes in respiratory and cardiac health endpoints/biomarkers following drastic changes in PM exposure.21–23 Most studies have examined at risk populations such as children, adults with existing respiratory disease (i.e. asthmatics and Chronic Obstructive Pulmonary Disease (COPD) patients) or elderly cohorts.
A reduction in lung function (mainly FEV1) was significantly associated with an increase in PM2.5 concentration, after adjusting for age, sex and height. The change in FEV1 associated with PM exposure in this study is consistent with previous studies (mostly groups with impaired respiratory function), which have reported similar or lower changes in FEV1 per unit change in PM concentrations. Panis et al.24 found that in healthy adults with an age range (16–71) larger than the present study (22–39), an increase of 10 μg/m3 PM10 was associated with a 12.8 ml lower FEV1. In the landmark Hyde Park study,25 reductions of up to 6% in FEV1 were reported in mild to moderate asthmatics, a potentially susceptible subpopulation, exposed up to 76.1 μg/m3 of PM2.5. In COPD patients, a meta-analysis study showed that a 10-μg/m3 increase in ambient PM10 was significantly associated with a FEV1 reduction of 3.38 ml.26 Therefore, the observed changes in lung function associated with increased PM exposures in this study is in overall agreement with previous studies.
While previous human chamber studies that exposed volunteers to fine PM have shown no, or very small, decrement in lung function,27 it is important to consider that due to IRB and ethical considerations, chamber studies typically have had limitations to how high PM concentrations can be employed. However, participants in this study were observationally exposed to much higher PM2.5 levels in polluted cities abroad and for longer hours/days, and therefore, their dose could be much higher than in chamber studies.
In addition to pulmonary effects, changes in the cardiovascular system were observed. Previous studies have shown similar PM-related effects on HR. An early study in Germany (n = 2681 adults) showed that an increase of 75 μg/m3 of suspended PM was associated with a 1.12 beats/min increase in HR.28 Similarly, another study showed that increased PM10 (×100) during high air pollution days was significantly associated with a 5.89 beats/min increase in evening HR.29
In regard to potential PM-related effects on BP, our mixed effects analyses did not find statistically significant associations with SBP or DBP. However, when analysed using a general linear model, travelling to a medium-level polluted city (36–100 μg/m3) was associated with a 4.3 mmHg increase in morning DBP. Brook and Rajagopalan30 reviewed similar results, where increased exposure to PM was associated with elevated BP, although some studies showed no or very small associations between BP and PM exposure.31
In addition to effects on HR and BP, time domain parameters of HRV were significantly correlated with PM2.5 exposure in this study. SDNN and PNN50 had an inverse relationship with a PM increase, such that a 10-μg/m3 increase in evening outdoor PM2.5 was associated with −0.6 ms and − 0.7 ms changes, respectively. Increases in PM have been shown to have comparable negative impacts on SDNN in previous studies.29,32 SDNN is the estimate of overall HRV, and a PM-associated reduction in HRV signifies that a change in the balance of the sympathetic and parasympathetic nervous system can occur when travelling to highly polluted cities.
Travel to polluted cities abroad was also associated with an increase in respiratory symptoms. Studies on acute respiratory symptoms associated with exposure to elevated levels of PM are relatively rare. The few studies available have shown similar respiratory symptom changes associated with exposure to elevated levels of air pollution in adults.33,34
Although the PM-related changes reported in this study were relatively small when considered on a per 10 μg/m3 basis, exposures were much higher in the polluted cities abroad. PM concentrations in highly polluted cities can reach 500 μg/m3 or higher, and thus international travellers, as a vulnerable population, may experience reductions in lung function that can be categorized as adverse according to ATS guidelines.35 For healthy persons, the ATS guidelines grade FEV1 decrements as mild, moderate or severe for reductions of 3–10%, 10–20% or larger than 20%, respectively. However, while a reversible change in lung function alone may not be considered adverse or biologically important, the ATS guideline on the adverse effects of air pollution36 indicates that loss of lung function in combination with the presence of symptoms, as seen in this study, constitutes an adverse health effect.
In this study, the incidence and severity of respiratory symptoms were significantly correlated with the travel abroad PM concentrations, pollution category and travel region. Some study participants who travelled to East and South Asia had to use medication to manage respiratory symptoms they experienced during their stay abroad, which demonstrates quality of life can be significantly impacted in travellers who experience such situations. The PM2.5 concentrations in these cities were over 300 μg/m3. Interestingly, individuals who travelled to East Asia reported higher symptom scores, as compared with those who travelled to South Asia, given similar PM concentrations (data not shown). This difference in symptom scores suggests that PM composition may play a role in response.
However, other factors may have influenced symptom scores, as shown in the results of mixed effect model analyses. Study participants did show inter-individual variability in responding to PM2.5 (i.e. some did not show any increase in symptoms when exposed to similar PM2.5 levels as symptomatic responders who travelled to the same cities or regions). Symptom score–PM correlations also suggest there might be a threshold effect above ~40 μg/m3. The observation of this mass-based threshold, however, is confounded by (i) region-dependent differences in composition and (ii) some study participants not showing symptoms at higher PM concentrations.
Despite the strengths of the underlying study design (and repeated measures from each participant, providing a total of over 700 data points), there were limitations. The travel abroad city may have been a better measure than region, as used in this study, because variations in concentrations and composition can exist even within the same region (e.g. Beijing vs Seoul). Within a city, however, PM composition can be assumed to be more homogeneous. Having balanced numbers of participants travelling to study cities abroad would have improved the statistical power, thereby enabling an inter-comparison of effects by travel cities.
Another study limitation was that morning and evening measurements were not taken at a fixed time. In addition, although each participant was trained individually and evaluated for accuracy in equipment use prior to travel, some participants required more days to learn the optimum technique for lung function measurements. Therefore, whenever possible, training was started and measurements were collected 2–3 days prior to the first day of the study period. However, due to various reasons, some participants failed to collect the required number of days of baseline measurements and therefore were not included in the analyses.
Unlike in many other studies, participants were not required to follow any strict activity procedures, and participants may have practised ‘prudent avoidance’, remaining indoors when outdoor pollution levels were high. Importantly, despite the widespread use of face masks during pollution episodes in urban centres in Asia, none of the participants reported using masks when they were abroad. Other factors that may have influenced exposure levels include accommodation type (air-conditioned hotel rooms vs homes without air conditioner), possible exposure to passive smoking (although participants were non-smokers) and temperature and relative humidity of the cities abroad. We did not consider temperature and relative humidity in the statistical models, which factors, at extreme levels, could influence traveller-reported symptoms and HR. Health of participants could have also been impacted by other air pollutants, such as Ozone or NOxs; however, previous epidemiological studies have shown that PM exposure is the main driver for pulmonary health impacts.
This study used a repeated measures study design, with mixed effects models applied to study the potential associations between measured health endpoints and PM exposure concentrations. Mixed models have been used successfully, in longitudinal health–exposure studies, to demonstrate the impact of PM on cardiopulmonary health.22,24,26,37,38 During the exploratory and mixed effect model analyses, multiple statistical comparisons were performed using the health and PM data, and given the chosen statistical significance of P < 0.05, it is possible that some of the positive associations occurred by chance. However, this is unlikely for this study, since evening FEV1 was significantly associated with PM in both the exploratory as well as repeated measures analyses.
Conclusions
Findings from this study collectively support the hypothesis that exposure to inhaled PM air pollution can adversely impact cardiopulmonary health in individuals who visit polluted cities abroad. PM concentrations in most-travelled abroad cities were several times higher than pre-travel NYC/NJ baseline levels. Elevated PM2.5 levels at the travel cities were significantly associated with changes in the health endpoints examined in this study, with the strongest statistically significant associations found between evening lung function measurements and the same day’s morning PM2.5 concentrations. Such changes were not observed for morning lung function. Respiratory symptoms were also affected by PM2.5 and primarily related to irritation of the upper respiratory tract, associated with symptoms such as cough, throat irritation and nasal congestion. In addition to its effects on the respiratory tract, PM2.5 exposures abroad also impacted cardiac metrics, with small but statistically significant changes in BP, HR and HRV.
The import of the results of this study lies especially in the outcomes being considered collectively. The ATS recommends that reversible loss of lung function in combination with the presence of symptoms should be considered as adverse.36 Although the changes in each outcome (respiratory and cardiac) were small in this healthy young population, our data collectively suggest that exposure to rapidly changing levels of air pollution while visiting abroad during air pollution episodes can have more serious implications among susceptible populations. Individuals who travel abroad should be advised of the potential health effects of air pollution in the cities they are visiting, especially if a city is experiencing, or predicted to experience, high PM levels during the travel period, particularly taking into consideration the patient’s health condition. While the focus of this study was on international travel, we believe our findings can be applicable to domestic travel in large countries such as India, China and Brazil, where pollution concentrations and profiles can vary drastically among regions within the country. Travel medicine doctors should be aware of these issues and, prior to international visits, inform patients of the potential for adverse cardiopulmonary effects when travelling to polluted cities abroad. Recommendations may include avoiding travel to highly polluted cities (e.g. during late fall burning of agricultural fields or wintertime inversion conditions), carrying preemptive asthma medication and the use of suitable particle masks (with physician guidance).
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Core Center (ES000260) and Training (ES007324 to T.G.) grants, the Air and Waste Management Association Scholarship 2017 (to R.V.) and the New York University College of Global Public Health grant (to T.G.).
Author contributions
M.J.R.V. and T.G. planned the project. M.J.R.V. conducted study participant trainings, acquired data and performed the statistical analyses. M.J.R.V., T.G. and G.D.T. advised on the study design and analysis and edited the manuscript. L.C.C., E.S. and Y.Y. contributed to the experimental methods. C.C.L. contributed to equipment calibrations and reviewed statistical analyses. All authors approved the work and final version of the manuscript.
Supplementary Material
Acknowledgements
We wish to thank all study participants for enroling and providing data for the study, Drs Dan Costa and Rick Peltier, Chris Sanford and Jing-Shiang Hwang for their advice and input in developing the methods and John Adragna for his help with processing central monitor data.
Conflict of Interest: None declared.
References
- 1. UN World Tourism Organization UNWTO Tourism Highlights 2017 Edition. World Tourism Organization, Madrid, Spain, 2017, 978-92-844-1902-9. [Google Scholar]
- 2. Molina MJ, Molina LT. Megacities and atmospheric pollution. J Air Waste Manag Assoc 2004; 54:644–80. [DOI] [PubMed] [Google Scholar]
- 3. Gurjar BR, Jain A, Sharma A et al. Human health risks in megacities due to air pollution. Atmos Environ 2010; 44:4606–13. [Google Scholar]
- 4. Carmichael GR, Ferm M, Thongboonchoo N et al. Measurements of sulfur dioxide, ozone and ammonia concentrations in Asia, Africa, and South America using passive samplers. Atmos Environ 2003; 37:1293–308. [Google Scholar]
- 5. World Health Organization WHO Urban Ambient Air Pollution Database. WHO, Geneva, Switzerland, 2016. [Google Scholar]
- 6. Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J Suppl 2003; 40:76s–80s. [DOI] [PubMed] [Google Scholar]
- 7. Peters A, Liu E, Verrier RL et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology 2000; 11:11–7. [DOI] [PubMed] [Google Scholar]
- 8. Pinault L, Tjepkema M, Crouse DL et al. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health 2016; 15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pope CA 3rd, Burnett RT, Thun MJ et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002; 287:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 2006; 56:709–42. [DOI] [PubMed] [Google Scholar]
- 11. Thurston GD, Ahn J, Cromar KR et al. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP diet and health cohort. Environ Health Perspect 2016; 124:484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schraufnagel DE, Balmes JR, Cowl CT et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies' Environmental Committee, part 1: the damaging effects of air pollution. Chest 2019; 155:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dockery DW, Pope CA 3rd, Xu X et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med 1993; 329:1753–9. [DOI] [PubMed] [Google Scholar]
- 14. Vilcassim MJR, Gordon T, Sanford CA. Does air pollution contribute to travelers’ illness and deaths?—evidence from a case report and need for further studies. J Travel Med 2018; 25:tay002–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guindi MN, Flaherty GT, Byrne M. Every breath you take: how does air pollution affect the international traveller? J Travel Med 2018; 25:1, tay021. [DOI] [PubMed] [Google Scholar]
- 16. Vilcassim MJR, Thurston GD, Chen LC, Lim CC, Gordon T. Exposure to greater air pollution when traveling abroad is associated with decreased lung function. Am J Respir Crit Care Med 2019; E-pub 2019/03/14. (Article in Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brouwer AF, Roorda RJ, Brand PL. Comparison between peak expiratory flow and FEV(1) measurements on a home spirometer and on a pneumotachograph in children with asthma. Pediatr Pulmonol 2007; 42:813–8. [DOI] [PubMed] [Google Scholar]
- 18. Miller MR, Hankinson J, Brusasco V et al. Standardisation of spirometry. Eur Respir J 2005; 26:319–38. [DOI] [PubMed] [Google Scholar]
- 19. Zhou S, Behrooz L, Weitzman M et al. Secondhand hookah smoke: an occupational hazard for hookah bar employees. Tob Control 2017; 26:40–5. [DOI] [PubMed] [Google Scholar]
- 20. Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV—heart rate variability analysis software. Comput Methods Programs Biomed 2014; 113:210–20. [DOI] [PubMed] [Google Scholar]
- 21. Kipen H, Rich D, Huang W et al. Measurement of inflammation and oxidative stress following drastic changes in air pollution during the Beijing Olympics: a panel study approach. Ann N Y Acad Sci 2010; 1203:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu S, Deng F, Wei H et al. Association of cardiopulmonary health effects with source-appointed ambient fine particulate in Beijing, China: a combined analysis from the Healthy Volunteer Natural Relocation (HVNR) study. Environ Sci Technol 2014; 48:3438–48. [DOI] [PubMed] [Google Scholar]
- 23. Wu S, Deng F, Niu J, Huang Q, Liu Y, Guo X. Association of heart rate variability in taxi drivers with marked changes in particulate air pollution in Beijing in 2008. Environ Health Perspect 2010; 118:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Int Panis L, Provost EB, Cox B et al. Short-term air pollution exposure decreases lung function: a repeated measures study in healthy adults. Environ Health 2017; 16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCreanor J, Cullinan P, Nieuwenhuijsen MJ et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N Engl J Med 2007; 357:2348–58. [DOI] [PubMed] [Google Scholar]
- 26. Bloemsma LD, Hoek G, Smit LAM. Panel studies of air pollution in patients with COPD: systematic review and meta-analysis. Environ Res 2016; 151:458–68. [DOI] [PubMed] [Google Scholar]
- 27. Samet JM, Graff D, Berntsen J, Ghio AJ, Huang YC, Devlin RB. A comparison of studies on the effects of controlled exposure to fine, coarse and ultrafine ambient particulate matter from a single location. Inhal Toxicol 2007; 19:29–32. [DOI] [PubMed] [Google Scholar]
- 28. Peters A, Perz S, Doring A, Stieber J, Koenig W, Wichmann HE. Increases in heart rate during an air pollution episode. Am J Epidemiol 1999; 150:1094–8. [DOI] [PubMed] [Google Scholar]
- 29. Pope CA 3rd, Verrier RL, Lovett EG et al. Heart rate variability associated with particulate air pollution. Am Heart J 1999; 138:890–9. [DOI] [PubMed] [Google Scholar]
- 30. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens 2009; 3:332–50. [DOI] [PubMed] [Google Scholar]
- 31. Auchincloss AH, Diez Roux AV, Dvonch JT et al. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-ethnic Study of Atherosclerosis (MESA). Environ Health Perspect 2008; 116:486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gold DR, Litonjua A, Schwartz J et al. Ambient pollution and heart rate variability. Circulation 2000; 101:1267–73. [DOI] [PubMed] [Google Scholar]
- 33. Wu S, Ni Y, Li H et al. Short-term exposure to high ambient air pollution increases airway inflammation and respiratory symptoms in chronic obstructive pulmonary disease patients in Beijing, China. Environ Int 2016; 94:76–82. [DOI] [PubMed] [Google Scholar]
- 34. Schindler C, Keidel D, Gerbase MW et al. Improvements in PM10 exposure and reduced rates of respiratory symptoms in a cohort of Swiss adults (SAPALDIA). Am J Respir Crit Care Med 2009; 179:579–87. [DOI] [PubMed] [Google Scholar]
- 35. Ametican Thoracic Society What constitutes an adverse health effect of air pollution? Am J Respir Crit Care Med 2000; 161:665–73. [DOI] [PubMed] [Google Scholar]
- 36. Thurston GD, Kipen H, Annesi-Maesano I et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J 2017; 49 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spira-Cohen A, Chen LC, Kendall M, Lall R, Thurston GD. Personal exposures to traffic-related air pollution and acute respiratory health among Bronx schoolchildren with asthma. Environ Health Perspect 2011; 119:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mirowsky J, Gordon T. Noninvasive effects measurements for air pollution human studies: methods, analysis, and implications. J Expo Sci Environ Epidemiol 2015; 25:354–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


