Abstract
Patient: Female, 55
Final Diagnosis: Gallbladder adenocarcinoma
Symptoms: Abdominal pain
Medication: —
Clinical Procedure: Cholecystectomy • pancreatic necrosectomy
Specialty: Surgery
Objective:
Rare co-existance of disease or pathology
Background:
Gallstones are a common cause of acute pancreatitis. The proposed mechanism by which choledocholithiasis induces pancreatitis is mechanical obstruction of the ampulla leading to the reflux of bile into the pancreatic duct or edema resulting from a gallstone’s passage. To our knowledge, there are no previously reported cases of gallbladder adenocarcinoma as a potential cause of acute pancreatitis. Herein, we describe a patient who presented with acute necrotizing pancreatitis, without other associated risk factors, who was found to have a fragmented friable polypoid gallbladder adenocarcinoma.
Case Report:
A 55-year old Hispanic female with prediabetes presented to the Emergency Department with severe epigastric abdominal pain radiating to her back. The patient’s clinical presentation, laboratory tests and computed tomography imaging were suggestive of acute necrotizing pancreatitis and a gallbladder lesion concerning for neoplasm. After clinical resolution of her pancreatitis, the patient was brought to the operating room for a cholecystectomy. Final pathology revealed a stage T1aN0 gallbladder adenocarcinoma.
Conclusions:
We have presented a patient with acute necrotizing pancreatitis in the absence of alcohol abuse, gallstones, biliary sludge, hypertriglyceridemia, hypercalcemia, or hereditary predisposition. Without evidence of other etiologies, we hypothesize that the friable tumor fragments of the gallbladder adenocarcinoma might be the underlying cause of pancreatitis in this patient.
MeSH Keywords: Gallbladder Neoplasms; Pancreatitis; Pancreatitis, Acute Necrotizing
Background
A variety of conditions are known to cause acute pancreatitis, the most common etiologies and their associated incidence rates include chronic alcohol abuse (35%) and cholelithiasis (40%) [1]. Other more uncommon causes include metabolic derangements, autoimmune disease, drug exposure, anatomic abnormalities, and endoscopic retrograde cholangiopancreatography [2]. However, many cases are idiopathic in nature. The possible presence of biliary sludge or microlithiasis has been demonstrated in as many as 75% of these idiopathic cases [1]. The proposed mechanisms by which gallstones and biliary sludge induce pancreatitis include mechanical ampulla obstruction leading to the reflux of bile into the pancreatic duct or edema resulting from a stone’s passage [3].
Typically, indolent until later in its disease course, gallbladder adenocarcinoma is often detected in association with biliary colic and symptomatic cholelithiasis. While gallstones are only detected in approximately 20% of adults, as many as 70% to 90% of patients with gallbladder cancer have gallstones [3]. The correlation between gallstones and gallbladder cancer is unknown but may be related to the cumulative inflammatory effects of chronic cholelithiasis leading to increased risk of developing malignancy. It is postulated that chronic inflammation seen with an inflammatory process releases cytokines, chemokines, reactive oxygen species, growth factors, and prostaglandins that contribute to the activation of oncogenes and inactivation of tumor suppressor genes. This in time leads to cell transformation, cell proliferation, inhibition of apoptosis, evasion of immune response, and angiogenesis that eventual becomes a malignancy [4].
Only a few case reports can be found in the literature of acute pancreatitis associated with early-stage gallbladder cancer. Some patients with cholelithiasis and gallbladder adenocarcinoma present with gallstone ileus or choledocholithiasis [3], but it is felt that these complications are associated with the cholelithiasis and not the neoplasm itself. In addition, gallbladder cancer has been associated with acute pancreatitis in patients with pancreaticobiliary maljunction, but the underlying cause in these patients was likely the anatomic abnormality of the main pancreatic duct [5]. A case report out of Japan described gallbladder cancer associated with hemobilia causing acute pancreatitis [5]. We describe a patient who presented with acute necrotizing pancreatitis, without cholelithiasis or other risk factors, and was found to have a fragmented polypoid gallbladder adenocarcinoma.
Case Report
A 55-year-old Hispanic female with prediabetes presented to the Emergency Department with severe epigastric abdominal pain radiating to her back. This was associated with anorexia, nausea, non-bloody vomiting, and subjective fevers and chills. She had previously reported similar pain; however, it was not as severe. She denied aggravating or alleviating factors. Her surgical history was negative. She denied any prior or current alcohol, drug, and/or tobacco use. She denied blood per rectum or melena. She denied a family history of pancreatic or biliary cancers or pancreatitis. Her physical examination revealed tenderness to palpation in the epigastrium and right upper quadrant with a positive Murphy’s sign. Laboratory values showed an elevated white blood cell (WBC) count of 18.1 thou/cm; total bilirubin of 1.3 U/L; aspartate aminotransferase of 196 U/L; alanine aminotransferase of 152 U/L; alkaline phosphatase of 105 U/L; and lipase of 16426 U/L. A right upper quadrant ultrasound revealed a solid polypoid mass in the gallbladder, no cholelithiasis, no intra or extra hepatic duct dilation and a diffuse fatty liver. Additionally, a computer tomography (CT) scan showed an intramural polypoid lesion of the gallbladder and acute pancreatitis (Figure 1). There was no evidence of a pancreatic mass on this CT imaging. Other abnormalities, such as annular pancreas, partial agenesis or malrotation, were not identified. The patient was hospitalized for acute pancreatitis of unclear etiology and a gallbladder lesion concerning for neoplasm [6].
Figure 1.
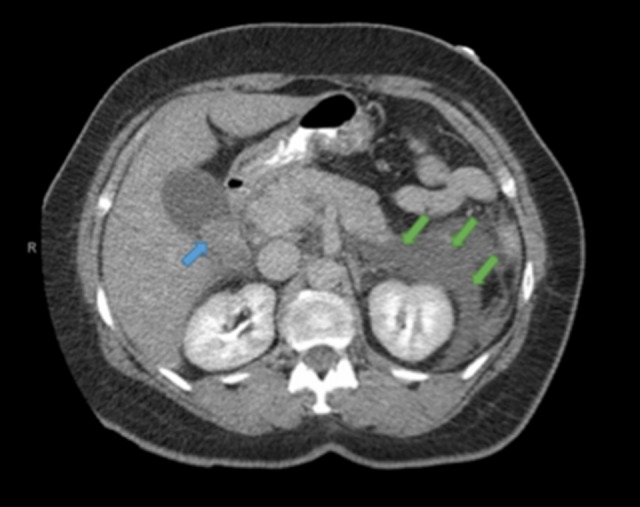
Computed tomography scan of abdomen and pelvis demonstrating an intramural polypoid lesion of the gallbladder (blue arrow) and peripancreatic edema (green arrows) consistent with acute pancreatitis.
The patient’s hospital course was complicated by sepsis secondary to necrotizing pancreatitis. Initially, the patient improved from the pancreatitis with bowel rest and aggressive fluid resuscitation; however, she worsened clinically on hospital day 6 with worsening pain, fever, and leukocytosis. Repeat CT imaging demonstrated progression of the pancreatitis with necrosis of the pancreatic body/tail, non-occlusive thrombus of the superior mesenteric/portal vein, pleural effusions, and moderate ascites (Figure 2). With aggressive fluid resuscitation and broad-spectrum antibiotics, the patient improved clinically but due to concerns for gallbladder cancer and need for pancreatic debridement, she was taken to the operating room on hospital day 16 for an open procedure [6].
Figure 2.
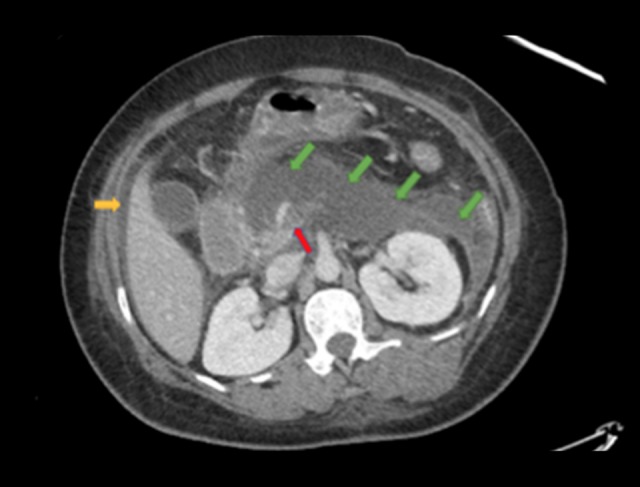
Computed tomography scan abdomen and pelvis demonstrating progression of pancreatitis now with necrosis of the body/tail (green arrows, note decreased enhancement in this region), a non-occlusive thrombus of SMV/portal vein (red arrow), moderate ascites (yellow arrow), and pleural effusions (not seen in this representative cut).
At the time of surgery there was no evidence of transmural tumor and, if malignant, it was felt to represent early disease. The patient underwent a cholecystectomy, pancreatic debridement, and necrosectomy, as well as a feeding jejunostomy tube placement due to the extensive pancreatic damage. The gall-bladder specimen was opened on the back table and revealed a polypoid lesion with multiple fragments of free-floating tumor within the gallbladder lumen, note the friable nature of the gallbladder neoplasm shown in Figure 3. There were no images taken of the gross specimen or of the free-floating fragments. There were no intraluminal gallstones, biliary sludge, or clots identified. Final pathology revealed a T1a gallbladder intramucosal adenocarcinoma with extensive high-grade dysplasia, lamina propria invasion, but sparing the submucosa and muscularis propria (T1a) (Figure 4). The cystic duct margin and radial/circumferential margin were negative for tumor [6].
Figure 3.
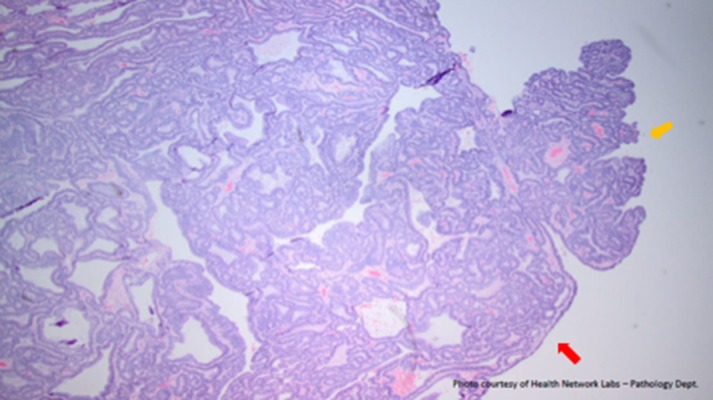
Pathology slide of the gallbladder polyp suggesting its friable nature (yellow arrow). Note the fragment that is breaking off of the larger polyp (red arrow). This is a representation of the friability of the gallbladder tumor and the friable fragments that were free floating.
Figure 4.
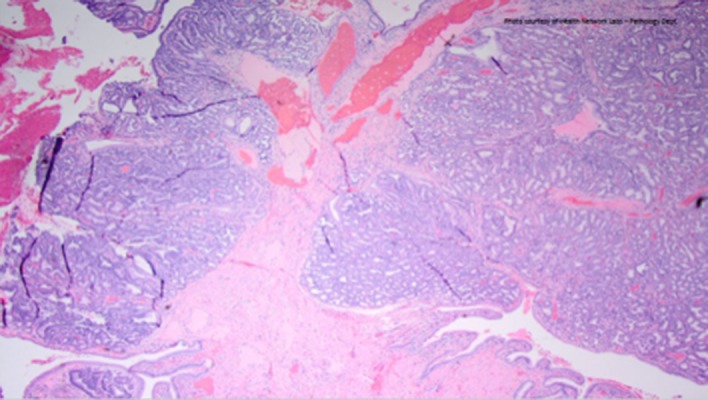
Final pathology revealed a T1a gallbladder intramucosal adenocarcinoma with extensive high-grade dysplasia, lamina propria invasion, but sparing the submucosa and muscularis propria.
Her postoperative clinical course was prolonged and complicated by multiple readmissions, a repeat pancreatic debridement, percutaneous drainage of a peripancreatic fluid collection, and prolonged parenteral nutrition. Ultimately, she recovered and 18 months after her initial surgery she was symptom-free with a good performance status and tolerating a regular diet. A recent CT scan showed complete resolution of the necrotizing pancreatitis and no signs of recurrent gallbladder cancer [6].
Discussion
Our patient presented with acute pancreatitis in the absence of common etiologies such as alcohol abuse, gallstones, biliary sludge, coagulation, hypertriglyceridemia, hypercalcemia, infectious etiology, pancreatitis associated medications, or hereditary predisposition. The suspected diagnosis of acute pancreatitis was based on compatible clinical features and supported by elevations in serum amylase and/or lipase levels. In the absence of explanatory causes, we hypothesize that the friable fragments from the gallbladder neoplasm may have caused mechanical obstruction of the pancreatic duct leading to pancreatitis. This is a similar obstructive process that occurs with gallstone pancreatitis.
The consideration of malignancy as a potential etiology of unexplained acute pancreatitis would be appropriate in patients at risk (aged >40 years) and/or patients with worrisome associated features (weight loss, new-onset diabetes mellitus). Although gallbladder cancer is the most common malignancy of the biliary tract [7], epidemiological studies have been generally limited because of its rarity, allowing only small sample sizes to be studied. Risk factors include age, female sex, and geography/ethnicity with high incidence rates seen in Latin America and Asia [8]. Carcinogenic risk factors include chronic infections, obesity, chronic inflammation (as seen in primary sclerosing cholangitis), chemical exposures (such as tobacco or some forms of heavy metals), gallbladder polyps (harbored in almost 5% of adults), and gallstones (present in the majority of patients with gallbladder cancer). Patients with gallbladder cancer tend to present late in the course of their disease (making them unlikely to benefit from surgical resection) with complaints of vague abdominal pain and nonspecific symptoms such as anorexia and weight loss [8].
Our case report was limited by not performing isolated biliary duct imaging to determine whether biliary tract obstruction or other biliary abnormalities were present. Pre-operative endoscopic retrograde cholangiopancreatography was not obtained due to the risk of exacerbating her already severe necrotizing pancreatitis. As such, another explanation for her pancreatitis could have been pancreatic divisum or other biliary tract abnormality. However, the patient is now over 24 months out from her necrotizing pancreatitis and cholecystectomy and has not demonstrated any signs or symptoms of recurrent pancreatitis or biliary pathology to suggest such diagnoses. Related literature searches revealed hemobilia associated with gallbladder cancer as a cause of acute pancreatitis with gall-bladder cancer [5,9]. This was also less likely in our case due to the lack of not only sludge and gallstones, but also coagulated blood when the specimen was opened. Additionally, the patient was not taking medications commonly associated with causing pancreatitis, and she had no known family history of pancreatitis; however, she was not tested for auto-immune pancreatitis. Further testing could be considered to rule out other rare causes of pancreatitis such as infections, genetic mutations, and autoimmune etiologies. Although this would be difficult to further pursue if no other clinical symptoms are associated with the pancreatitis. Furthermore, if the patient does have recurrent pancreatitis, a more likely explanation is that it may be idiopathic in nature as this has been reported in 10–25% of cases [10].
Conclusions
It has been reported that gallbladder cancers can present with symptoms of biliary colic and cholecystitis without associated cholelithiasis [11]. In those reports the authors suggest that the obstructive process occurring in the cystic duct is due to dislodgment of gallbladder tumors from their origin or bleeding leading to clot formation [11]. While there have been no documented reports associating gallbladder cancer fragments with acute pancreatitis, and absence of other clear sources for our patient’s pancreatitis, suggests that this case report presents a unique presentation of acute pancreatitis associated with a friable gallbladder adenocarcinoma. The patient’s clinical stage of T1aN0 suggests a good overall prognosis, with no further treatment needed after cholecystectomy. For tumors invading beyond the muscular layer (≥T2) in addition to cholecystectomy, an oncological resection including limited hepatic resection and portal lymphadenectomy is the optimal surgical approach [1]. Additionally, adjuvant chemotherapy and/or radiation is an option for high-risk patients [1].
Acknowledgments
The authors are grateful to Jacqueline A. Grove for assistance with manuscript preparation and editing.
References:
- 1.Müller BG, De Aretxabala X, González Domingo M. A review of recent data in the treatment of gallbladder cancer: What we know, what we do, and what should be done. Am Soc Clin Educ Book. 2014:e165–70. doi: 10.14694/EdBook_AM.2014.34.e165. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels A. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai TJ, Lai KH, Hsu PI, et al. Gallbladder cancer manifesting as recurrent common bile duct stone and duodenal ulcer bleeding. J Chin Med Assoc. 2009;72:434–37. doi: 10.1016/S1726-4901(09)70401-0. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zhang J, Ma H. Chronic inflammation and gallbladder cancer. Cancer Lett. 2014;345(2):242–48. doi: 10.1016/j.canlet.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K, Doi Y, Imai K, et al. Resectable gallbladder cancer presenting with acute pancreatitis caused by hemobilia. Clin J Gastroenterol. 2011;4(5):336–39. doi: 10.1007/s12328-011-0251-8. [DOI] [PubMed] [Google Scholar]
- 6.Appelbaum R, Alvarado F, Blackham A, Brodsky J. Acute necrotizing pancreatitis: A unique presentation of early-stage gallbladder adenocarcinoma.. ACS Keystone Conference; Nov 2, 2018; Hershey, Pennsylvania. Poster Presentation. [Google Scholar]
- 7.Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. Cancer J Clin. 2001;51:349–64. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 8.Hundal R, Shaffer EA. Gallbladder cancer: Epidemiology and outcome. Clin Epidemiol. 2014;6:99–109. doi: 10.2147/CLEP.S37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubota H, Kageoka M, Iwasaki H, et al. A patient with undifferentiated carcinoma of gallbladder presenting with hemobilia. J Gastroenterol. 2000;35:63–68. doi: 10.1007/pl00009979. [DOI] [PubMed] [Google Scholar]
- 10.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371(9607):143–52. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 11.Wan X, Zhang H, Chen C, et al. Clinicopathological features of gallbladder papillary adenocarcinoma. Medicine (Baltimore) 2014;93:e131. doi: 10.1097/MD.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]


