Abstract
Objectives
This study investigated effects of reduced serum condition and vascular endothelial growth factor (VEGF) on angiogenic potential of adipose stromal cells (ASCs) in vitro.
Materials and methods
Adipose stromal cells were cultured in three different types of medium: (i) F12/DMEM (FD) supplemented with 10% FBS from passage 0 (P0) to P6; (ii) FD supplemented with 2% FBS at P6; and (iii) FD supplemented with 2% FBS plus 50 ng/ml of VEGF at P6. Morphological changes and growth rate of ASCs were recorded. Changes in stemness, angiogenic and endogenic genes’ expressions were analysed using Real‐Time PCR.
Results
Adipose stromal cells changed from fibroblast‐like shape when cultured in 10% FBS medium to polygonal when cultured in 2% FBS plus VEGF‐supplemented medium. Their growth rate was lower in 2% FBS medium, but increased with addition of VEGF. Real‐Time PCR showed that ASCs maintained most of their stemness and angiogenic genes’ expression in 10% FBS at P1, P5 and P6, but this increased significantly in 2% FBS at P6. Endogenic genes expression such as PECAM‐1, VE chaderin and VEGFR‐2 decreased after serial passage in 10% FBS, but increased significantly at P6 in 2% FBS. Addition of VEGF did not cause any significant change in gene expression level.
Conclusion
Adipose stromal cells had greater angiogenic potential when cultured in reduced serum conditions. VEGF did not enhance their angiogenic potential in 2% FBS‐supplemented medium.
Introduction
Human mesenchymal stem cells (MSCs) are of interest due to their ability to differentiate into mesodermal cell types including adipocytes, osteocytes and chondrocytes 1 and transplantation of MSCs derived from bone marrow has improved prognosis for patients with chronic ischaemic heart disease following surgical revascularization 2, 3. Recently, angiogenic potential of stem cells has been studied actively in the fields of tissue engineering and regenerative medicine 4, 5, 6, 7. Stem cells have been recognized to have potential as angiogenic therapy for treatment of cardiovascular disease particularly for patients with ischaemic heart disease where it is important to repair and regenerate healthy blood vessels to improve perfusion into ischaemic tissues.
Trans‐differentiation of MSCs particularly derived from human adipose stroma, into other cell lineages such as cardiogenic 8, angiogenic 9 and neurogenic 10, has been reported by a number of investigators. Furthermore, human adipose stromal cells (ASCs) have become a more attractive option for cell‐based therapy compared to bone marrow‐derived stem cells (BMSCs) due to their abundance and for being robust in preparation 11. ASCs also have similar cell and molecular characteristics to those of BMSCs 12, and ASCs have been shown to be able to retain stemness properties in serial passage up to P10 13.
A potential use of ASCs in soft tissue reconstruction has been reported, for cosmetic surgery 14, however, to maintain transplanted soft tissue, adequate blood supply is essential to prevent progressive resorption of grafted tissue 15, 16. Although angiogenic potential of ASCs has been reported 17, 18, understanding effects of growth factors and different culture conditions, including low‐serum and serum‐free conditions, on ASC angiogenic differentiation ability is not well established. Use of serum‐free medium has been found to reduce germline potential, indicated by lower spermatogonial stem cell frequency 19, which may affect self‐renewal and differentiation ability of stem cells. Use of growth factors such as vascular endothelial growth factor (VEGF) to encourage angiogenesis has been demonstrated to also stimulate angiogenic potential of stem cells 20. VEGF is the main regulator for physiological angiogenesis during embryogenesis, skeletal growth and reproductive functions 21, 22. Several reports have shown that angiogenic potential of ASCs can be stimulated by serum and by co‐culture with endothelial cells 23 as well as reduced serum and hypoxic conditions, to stimulate VEGF secretion 18. Use of human serum in xeno‐free culture medium has been tested on differentiational ability of ASCs 24, however, the studies did not provide extensive understanding of changes in differentiation gene expression level of angiogenic and stemness genes in cells after differentiation. As the effect of growth factors such as VEGF 18 and basic fibroblast growth factor (bFGF) 25 on angiogenic potential has been well documented, their effects in conditions such as low serum need to be further investigated, in terms of changes in angiogenic genes’ expression. Moreover, stemness genes’ expression of differentiated cells needs to be further determined to obtain clearer understanding of the process.
As VEGF is more important for angiogenic differentiation than bFGF, we have chosen to evaluate the effect of reduced serum in culture medium supplemented with VEGF alone, on angiogenic potential of ASCs, using a quantitative gene expression approach. Thus, we have aimed to evaluate the effect of reduced serum conditions in the presence of VEGF, on stemness and angiogenic genes’ expression level in serial passage ASCs. This study is a preliminary step in determining the most suitable culture conditions in which to increase ASC ability to undergo angiogenic differentiation, in preparing cells for therapeutic purposes. In this investigation, real‐time PCR was used to study changes in angiogenic, endogenic and stemness genes’ expression levels of ASCs in reduced serum conditions, and in the presence of VEGF as angiogenic inducer. Evaluation of these genes’ expression levels may help in further understanding mechanism of angiogenic differentiation of ASCs.
Materials and methods
Isolation and expansion of ASCs
Human adipose tissues (n = 6) were collected after intra‐operative lipectomy suction, at Subang Jaya Medical Centre, and adipose tissue was obtained from patients (aged 40–60 years) after receiving informed consent. All procedures performed in this study were reviewed and approved (approval number: 02‐01‐02‐SF290) by the Research and Ethical Board of Universiti Kebangsaan Malaysia. Adipose tissues were digested in 0.3% type I collagenase solution (Sigma‐Aldrich, St. Louis, MO, USA) for 1 h at 37 °C. Digested samples were centrifuged at 500 g for 10 min to obtain the adipose stromal cells. ASCs were then suspended in medium consisting of equal volumes of Ham's F12 and Dulbecco's modified Eagle's medium (DMEM/F12; Gibco‐Invitrogen, Grand Island, NY, USA), supplemented with 10% foetal bovine serum (FBS; Gibco‐Invitrogen), 1% glutamax (Gibco‐Invitrogen), 1% antibiotic‐antimycotic (Gibco‐Invitrogen) and 50 μg/ml vitamin C (Sigma‐Aldrich). Cells were then cultured in T25 flasks (Falcon; BD Biosciences, San Jose, CA, USA) and maintained at 37 °C with 5% CO2. Culture medium was changed every 3 days. When the primary cultured ASCs (P0) reached 80–90% confluence, each specimen was trypsinized with 0.125% trypsin‐ethylene diamine tetra acetic acid (EDTA) (Gibco‐Invitrogen) and expanded for the next passage by 1:4, under the same culture conditions. Seeding density was 5000 cells/cm2 for each of the passage. ASCs were cultured in medium supplemented with 10% FBS up to passage 6 (P6) and total number of cells, growth rate and viability were determined using the trypan blue exclusion technique; they were counted using a haemocytometer. Cell morphology was monitored using inverted light microscopy. Endogenous expression levels of stemness, angiogenic and endogenic genes of the ASCs at P1, P5 and P6 were quantified using real‐time PCR.
Effect of reduced serum and VEGF supplementation on ASCs
Adipose stromal cells were divided into 3 groups at passage 6: (i) ASCs cultured in medium supplemented with 2% FBS plus 50 ng/ml VEGF; (ii) ASCs cultured in medium supplemented with 2% FBS without VEGF; and (iii) ASCs culture in medium supplemented with 10% FBS as the control group. Culture seeding density was 5000 cells/cm2 for each group and medium was changed every 3 days. Alterations in cell morphology were monitored using an inverted microscope and the total number of cells was counted at the end of each passage, to determine their growth rate. Stemness, angiogenic and endogenic genes expression levels of ASCs at P6 in these three groups were quantified using real‐time PCR.
Total RNA extraction
Total RNA was extracted from the ASCs at P1, P5 and P6 (n = 6) in medium supplemented with 10% FBS; ASCs cultured to P6 (n = 6) in medium supplemented with 2% FBS plus 50 ng/ml of VEGF; and ASCs cultured to P6 (n = 6) in medium supplemented with 2% FBS without VEGF. Total RNA was extracted using TRI reagent (Molecular Research Center, Cincinnati, OH, USA) in accordance with manufacturer's instructions. Polyacryl carrier (Molecular Research Center) was added to precipitate total RNA which was then washed in 75% ethanol and air‐dried. The total RNA was then dissolved in RNAse‐ and DNase‐free distilled water (Invitrogen, Grand Island, NY, USA) and immediately stored at −80 °C. Yield and purity of total RNA were determined using a spectrophotometer (Bio‐Rad, Hercules, CA, USA).
Complementary DNA synthesis
Complementary DNA (cDNA) was synthesized from 100 ng of total RNA using SuperScript III reverse transcriptase (Invitrogen). The procedure was carried out according to the manufacturer's recommendations and protocol conditions were as follows: 10 min at 23 °C for primer annealing; 60 min at 50 °C for reverse transcription; and 5 min at 85 °C for reaction termination.
Real‐time PCR analysis
Real‐time PCR was performed using cDNA as template to evaluate stemness gene (ABCG‐2, Oct‐4, TERT, BST‐1, FGF4, SOX‐2, Rex‐1, Fzd9 and Nanog‐3), angiogenic gene (Hepatocyte Growth Factor (HGF), Placenta Growth Factor (PGF), Vascular Endothelial Growth Factor (VEGF), Angiopoetin‐1, basic Fibroblast Growth Factor (bFGF)) and endogenic gene (CD34, Ve‐cadherin, PECAM‐1, eNOS, vWF, and VEGFR‐2) expression level in ASCs cultured to P1, P5 and P6 (n = 6) in medium supplemented with 10% FBS; ASCs cultured at P6 (n = 6) in medium supplemented with 2% FBS plus 50 ng/ml of VEGF and ASCs cultured at P6 (n = 6) in medium supplemented with 2% FBS without VEGF. Real‐time PCR was carried out using specific gene primer sequences (Tables 1, 2, 3), designed using Primer 3 software (http://frodo.wi.mit.edu/primer3/), based on published GeneBank database sequences. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) was used as reference gene. The real‐time PCR reaction was carried out using BioRad iCycler (Bio‐Rad) with SYBR green as indicator. Each reaction mixture consisted of iQ SYBR Supermix, forward and reverse primers (5 μm each), deionized water and 1 μl of cDNA template. Reaction conditions were: cycle 1, 95 °C for 3 min (1X), cycle 2, step 1 95 °C for 10 s and step 2 61 °C for 30 °s (40×). Specificity of the PCR product was confirmed with melting curve analysis and 2% agarose gel electrophoresis. Expression level of each gene was normalized to GAPDH.
Table 1.
Stemness gene primer sequences and PCR product sizes
| Gene | Accession number | Sequence (5‐3′) sense and antisense primers | Product size, base pair (bp) |
|---|---|---|---|
| Glyceradehyde‐3‐phosphate dehydrogenase (GAPDH) | NM_002046 |
tcc ctg agc tga acg gga ag gga gga gtg ggt gtc gct gt |
217 |
| Octamer‐binding protein3/4 (Oct‐4) | NM_002701 |
aag gat gtg gtc cga gtg tg gaa gtg agg gct ccc ata gc |
180 |
| SRY‐related HMG‐box 2 (Sox2) | NM_003106 |
tta cct ctt cct ccc act cca ggt agt gct ggg aca tgt gaa |
132 |
| Fibroblast growth factor 4 (FGF4) | NM_002007 |
gat gag tgc acg ttc aag ga ggt tcc cct tct tgg tct tc |
118 |
| Reduced expression‐1 (Rex‐1) | NM_174900 |
aaa ggt ttt cga agc aag ctc ctg cga gct gtt tag gat ctg |
185 |
| Nanog 3 | NM_024865 |
ctg tga ttt gtg ggc ctg aa tgt ttg cct ttg gga ctg gt |
153 |
| Frizzled 9 (FZD9) | NM_003508 |
cag aga agc tgg aga agc tca agg cgt tcg tag aca tag caa |
100 |
| ATP‐binding cassette, sub‐family G, member 2 (ABCG2) | NM_004827 |
agc tgc aag gaa aga tcc aa tgc cca tca caa cat cat ct |
119 |
| Bone marrow stromal cell antigen 1 (BST‐1) | NM_004334 |
cga tta cca atc ctg ccc ta ttt gat ggg ata ggc tcc tg |
154 |
Table 2.
Angiogenic gene primer sequences and PCR product sizes
| Gene | Accession number | Sequence (5‐3′) sense and antisense primers | Product size, base pair (bp) |
|---|---|---|---|
| Vascular endothelial growth factor (VEGF) | NM_001033756 |
ccc act gag gag tcc aag at aaa tgc ttt ctc cga tct ga |
173 |
| Hepatocyte growth factor (HGF) | NM_001010932 |
ctg gtt ccc ctt caa tag ca ctc cag ggc tga cat ttg at |
168 |
| Placenta growth factor (PGF) | NM_002632 |
gtt cag ccc atc ctg tgt ct ctt cat ctt ctc ccg cag ag |
199 |
| Angiopoietin 1 (Ang‐1) | NM_001146 |
gaa ggg aac cga gcc tat tc gct ctg ttt tcc tgc tgt cc |
108 |
| Basic fibroblast growth factor (bFGF) | NM_002006 |
ccg tta cct ggc tat gaa gg act gcc cag ttc gtt tca cc |
158 |
Table 3.
Endogenic gene primer sequences and PCR product sizes
| Gene | Accession number | Sequence (5‐3′) sense and antisense primers | Product size, base pair (bp) |
|---|---|---|---|
| Endothelial cell adhesion molecule 1 (PECAM‐1) | NM_000442 |
tca aat gat cct gcg gta ttc cca cca cct tac ttg aca gga |
169 |
| CD 34 | NM_001773 |
tgc aac atc tcc cac taa acc tct tat ttt gct cca ggc aga |
128 |
| Vascular endothelial cadherin (VE Cadherin) | NM_001795 |
cca cga aac gtg aag ttc aaa gca tcc cat tgt ctg aga tga |
163 |
| Endothelial nitric oxide synthase (eNOS) | NM_000603 |
ttt gcc ctt atg gat gtg aag cgc atc aaa gaa agc tca gtc |
139 |
| Von willebrand factor (vWF) | NM_000552 |
gac ctt gct gaa gag tca tcg gcc agt cag ctt gaa att ctg |
184 |
| Vascular endothelial growth factor receptor 2 (VEGFR2) | NM_002253 |
gca atc cct gtg gat ctg aa act cca tgc cct tag cca ct |
193 |
Immunocytochemical staining of ASCs
Adipose stromal cells at P6 were cultured on glass coverslips. At 60–80% confluence, cells were fixed in 4% paraformaldehyde overnight. Samples were then treated with 3% hydrogen peroxide (Sigma‐Aldrich) for 5 min, followed by 1% bovine serum albumin (Sigma‐Aldrich) incubation for 1 h at room temperature to reduce non‐specific antibody binding. Samples were rinsed in Tris buffered saline (TBS; Dako, Glostrup, DA, Denmark) before being incubated in mouse anti‐human PECAM‐1 (1:100 dilution; Dako) and mouse anti‐human vWF (1:400 dilution; Dako) monoclonal antibody, for 30 min at room temperature. Samples were rinsed in TBS to wash out any unbound antibody, before being incubated in secondary, anti‐mouse antibody, labelled with horseradish peroxidase (HRP) polymer (Dako), for 30 min at room temperature. Then, samples were rinsed again in TBS, and freshly prepared 3,3′‐diaminobenzidine was applied for 10 min to develop the brownish coloured final reaction product per positive sample. All slides were rinsed in TBS and counterstained with haematoxylin (Sigma‐Aldrich), before being mounted with DPX (Sigma‐Aldrich).
Statistical analysis
Numerical data were expressed as mean ± standard error of mean (SEM). Differences in quantitative RT‐PCR (n = 6) data between tested groups were analysed for significance using Student's t‐test. P‐value <0.05 was considered to be significant.
Results
Morphology and growth rate of ASCs
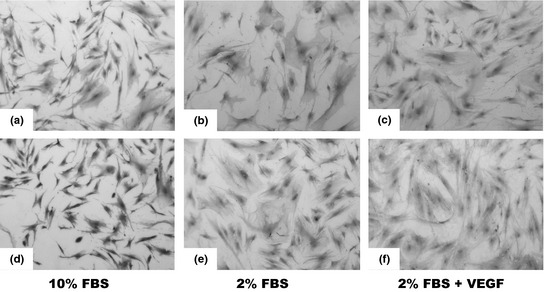
Adipose stromal cells at P1 had fibroblast‐like morphology when cultured in medium supplemented with 10% FBS (Fig. 1a). Serially passaging the cells to P5, cultured in medium supplemented with 10% FBS revealed no change in morphology and cells maintained the fibroblastic features, however, cell density was lower after 3 days in culture (Fig. 1b). When ASCs were cultured in medium supplemented with reduced serum (2% FBS) at P6, they had flatten morphology with relatively larger cytoplasmic volume (Fig. 1c). Addition of VEGF into the medium also caused increase in cytoplasmic volume relative to other features, and higher cell density compared to those in culture supplemented with 2% FBS alone (Fig. 1d).
Figure 1.
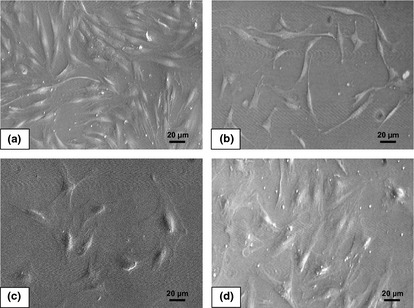
Morphology of adipose stromal cells (ASCs). (a) ASCs at P1 cultured in medium with 10% FBS show fibroblastic and spindle‐like morphology (50×). (b) ASCs at P5 cultured in medium with 10% FBS show similar features to those of ASCs at P1, but have lower cell density on the third‐day culture (50×). (c) ASCs at P6 cultured in medium with 2% FBS are relatively larger and have flatten morphology (50×). (d) ASCs at P6 cultured in medium with 2% FBS plus vascular endothelial growth factor with flatten morphology, but with some elongation features (×50).
Adipose stromal cells at P1, P5 and P6 cultured in medium supplemented with 10% FBS needed 3 and 4 days, respectively, to reach confluence, whereas ASCs at P6 cultured in both medium supplemented with 2% FBS and medium supplemented with 2% FBS plus VEGF needed 16 and 14 days, respectively, to reach confluence. Growth kinetics (Fig. 2) of ASCs cultured in medium supplemented with 10% FBS at P1 (704.8 ± 30.3 cell/cm2/day), P5 (599.8 ± 33.8 cell/cm2/day) and P6 (521.9 ± 30.6 cell/cm2/day) revealed significantly higher growth rate compared to ASCs at P6 cultured in medium supplemented with 2% FBS (62.5 ± 10.5 cell/cm2/day) and ASCs at P6 cultured in medium supplemented with 2% FBS plus VEGF (101 ± 50.2). ASCs cultured in medium supplemented with VEGF had significantly higher growth rate (P < 0.05) compared to ASCs cultured in medium supplemented with only 2% FBS.
Figure 2.
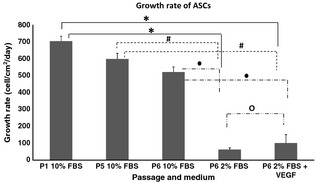
Growth rate of adipose stromal cells (ASCs) (n = 6). ASCs at P1 cultured in medium with 10% FBS have the highest growth rate (704.8 ± 30.3 cell/cm2/day) and at P5; ASC growth rate shows a slight decrease (599.8 ± 33.8 cell/cm2/day). Growth rate of ASCs decreased significantly by P6 (P < 0.05) when cultured in medium with 2% FBS (62.5 ± 10.5 cell/cm2/day) and in medium with 2% FBS added plus vascular endothelial growth factor (VEGF). There was significant increase in growth rate when VEGF was added to the medium. Significant difference between groups (P < 0.05 and n = 6) is labelled as follows: *refers to P < 0.05 relative to ASCs at P1 in 10% FBS; #refers to P < 0.05 relative to ASCs at P5 in 10% FBS; ●refers to P < 0.05 relative to ASCs at P6 in 10% FBS; and ○refers to P < 0.05 relative to ASCs at P6 in 2% FBS.
Stemness genes’ expression levels of ASCs
Expression level of ABCG2 (Fig. 3a) showed no significant changes between ASCs at P1 and P5 cultured in medium supplemented with 10% FBS. ABCG2 was expressed significantly higher (P < 0.05), 4‐fold, in ASCs at P6 cultured in medium supplemented with only 2% FBS and in medium supplemented with 2% FBS plus VEGF (3‐fold) compared to both ASCs groups at P1, P5 and P6. BST‐1 (Fig. 3b) had a similar pattern to that of ABCG2; its expression increased significantly (P < 0.05) by P6 when cultured in medium supplemented with only 2% FBS (6‐fold) and in medium supplemented with 2% FBS plus VEGF (6‐fold) compared to ASCs at P1, P5 and P6 cultured in medium supplemented with 10% FBS. FGF4 (Fig. 3c) also had a similar pattern to that of ABCG2 and BST1, increased significantly (P < 0.05) at P6 when cultured in medium supplemented with only 2% FBS (50‐fold) and in medium supplemented with 2% FBS plus VEGF (90‐fold). FZD9 (Fig. 3d) expression level increased significantly (P < 0.05) in the cells by P6 in medium supplemented with only 2% FBS (3‐fold) and in medium supplemented with 2% FBS plus VEGF (3‐fold) compared to ASCs at P1 and P6 cultured in 10% FBS. However, only ASCs at P6 cultured in medium supplemented with 2% FBS plus VEGF showed significantly higher (P < 0.05) FZD9 expression compared to P5 cultures (by 1.5‐folds). Sox2 (Fig. 3e) expression increased significantly (P < 0.05) at P6 cultured in medium supplemented with only 2% FBS (4‐fold) and in medium supplemented with 2% FBS plus VEGF (3‐fold) compared to P1 cultures. However, only ASCs at P6 cultured in medium supplemented with only 2% FBS (2‐fold) showed significant change compared to ASCs at P5 and P6 cultured in 10% FBS. Oct4 (Fig. 3f) expression increased significantly in cells cultured in both media (2% FBS only and 2% FBS supplemented with VEGF) at P6 compared to ASCs cultured at P1 and P6 in 10% FBS. Rex1 (Fig. 3g) had an increasing pattern of expression at P6 when cultured in medium supplemented with only 2% FBS and in medium supplemented with 2% FBS plus VEGF, but no significant changes were recorded. Nanog3 expression (Fig. 3h) increased significantly by P6 when ASCs were cultured in medium supplemented with 2% FBS (9‐fold) and medium supplemented with 2% FBS plus VEGF (4‐fold) compared to ASCs at P1, P5 and P6 cultured in medium supplemented with 10% FBS.
Figure 3.
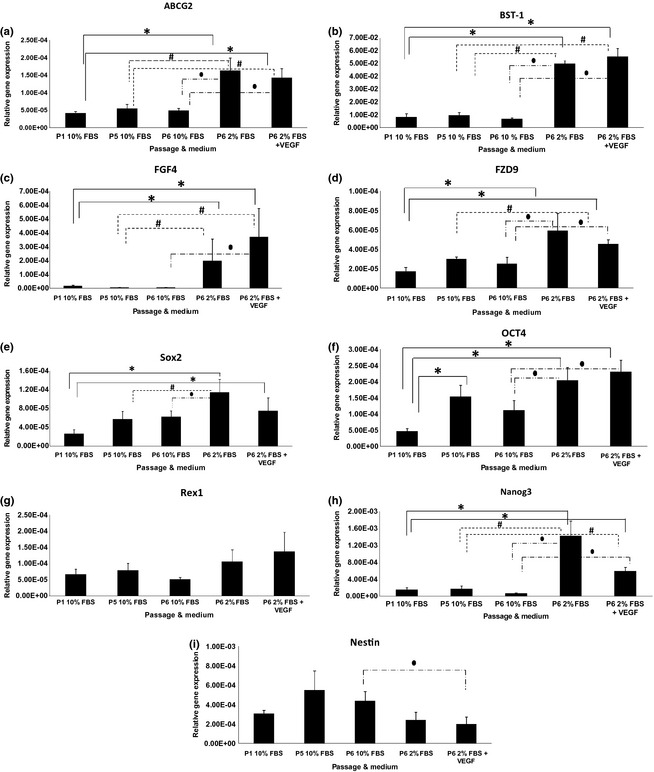
Stemness genes’ expression level (relative to GAPDH expression level) of human adipose stromal stem cells (ASCs) in various media. Significant difference between groups (P < 0.05 and n = 6) is labelled as follows: *refers to P < 0.05 relative to ASCs at P1 in 10% FBS; #refers to P < 0.05 relative to ASCs at P5 in 10% FBS; and ●refers to P < 0.05 relative to ASCs at P6 in 10% FBS.
Angiogenic genes’ expression levels of ASCs
Hepatocyte Growth Factor (Fig. 4a) expression in ASCs did not show any significant changes in any of the treatment groups. PGF (Fig. 4b) expression in ASCs cultured in 2% FBS supplemented with VEGF decreased significantly (P < 0.05) compared to ASCs cultured in 10% FBS at both P1 and P6. VEGF (Fig. 4c) expression increased significantly (P < 0.05), approximately 3‐fold, in ASCs at P6 cultured in both media (2% FBS only and 2% FBS supplemented with VEGF) compared to ASCs at P1, P5 and P6 cultured in medium supplemented with 10% FBS. However, significant reduction of VEGF expression was observed in ASCs at P6 cultured in medium supplemented with 2% FBS plus VEGF compared to ASCs cultured in medium supplemented with only 2% FBS. Ang‐1 expression (Fig. 4d) increased significantly (P < 0.05), 2‐fold, in ASCs at P6 when cultured in medium supplemented with 2% FBS compared to ASCs at P5 and P6 cultured in medium supplemented with 10% FBS. Ang‐1 expression also increased significantly (P < 0.05), 2‐fold, in ASCs at P6 cultured in medium supplemented with 2% FBS plus VEGF compared to ASCs at P1 and P6 cultured in medium supplemented with 10% FBS. BFGF expression (Fig. 4e) increased significantly (P < 0.05), 3‐fold, in ASCs at P6 cultured in medium supplemented with 2% serum compared to ASCs at P1, P5 and P6 cultured in medium supplemented in 10% FBS. BFGF expression also increased significantly (P < 0.05), 2‐fold, in ASCs at P6 cultured in medium supplemented with 2% serum plus VEGF compared to ASCs at P1, P5 and P6. However, BFGF expression was reduced when ASCs at P6 cultured in medium supplemented with 2% FBS plus VEGF compared to ASCs cultured in medium supplemented with only 2% FBS.
Figure 4.
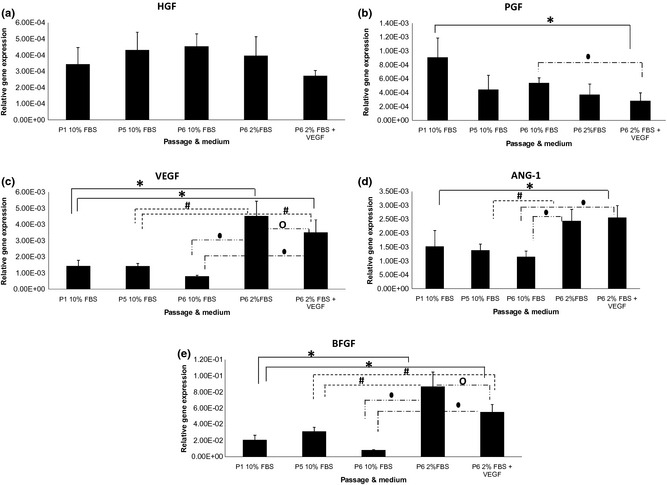
Angiogenic genes’ expression level (relative to GAPDH) of human adipose stromal stem cells (ASCs) in various media. Significant difference between groups (P < 0.05 and n = 6) is labelled as follows: *refers to P < 0.05 relative to ASCs at P1 in 10% FBS; #refers to P < 0.05 relative to ASCs at P5 in 10% FBS; ●refers to P < 0.05 relative to ASCs at P6 in 10% FBS; and ○refers to P < 0.05 relative to ASCs at P6 in 2% FBS.
Endogenic genes’ expression levels of ASCs
CD34 expression (Fig. 5a) increased significantly (P < 0.05) in ASCs at P6 cultured in medium supplemented with 2% FBS and medium supplemented with 2% serum plus VEGF compared with ASCs at P1, P5 and P6 cultured in medium supplemented in 10% FBS. VE Cadherin expression (Fig. 5b) decreased significantly (P < 0.05), 25‐fold, when ASCs at P5 were compared to P1. VE Cadherin expression increased significantly (P < 0.05), 17‐fold, when ASCs at P6 were cultured in medium supplemented with 2% serum and medium supplemented with 2% serum plus VEGF compared to ASCs at P5 in 10% FBS. However, only VE Cadherin in ASCs at P6 cultured in 2% FBS supplemented with VEGF increased significantly (P < 0.05) compared to ASCs at P6 in 10% FBS. PECAM‐1 expression (Fig. 5c) decreased significantly (P < 0.05), 2‐fold, when ASCs at P5 compared with culture at P1. However, PECAM‐1 expression increased significantly (P < 0.05), 20‐fold, when ASCs at P6 were cultured in medium supplemented with only 2% FBS compared to P1 and P5 cultures. No significant changes were observed in PECAM‐1 expression of ASCs at P6 when compared between 2% FBS and 2% FBS plus VEGF. eNOS gene (Fig. 5d) did not show any significant difference in its expression in any of the treatment groups but vWF gene (Fig. 5e) expression increased significantly (P < 0.05) in ASCs at P6 cultured in both media (2% FBS only and 2% FBS supplemented with VEGF) compared to ASCs at P6 in 10% FBS. VEGFR‐2 (Fig. 5f) was significantly lower (P < 0.05), in ASCs at P5 compared to P1 cultured in the same medium. However, VEGFR‐2 expression of ASCs increased significantly (P < 0.05) at P6 when cultured in medium supplemented with 2% serum only (17‐fold) compared to P5 and P6 in 10% FBS. VEGFR expression in 2% serum plus VEGF increased significantly (P < 0.05) when compared to P6 in 10% FBS.
Figure 5.
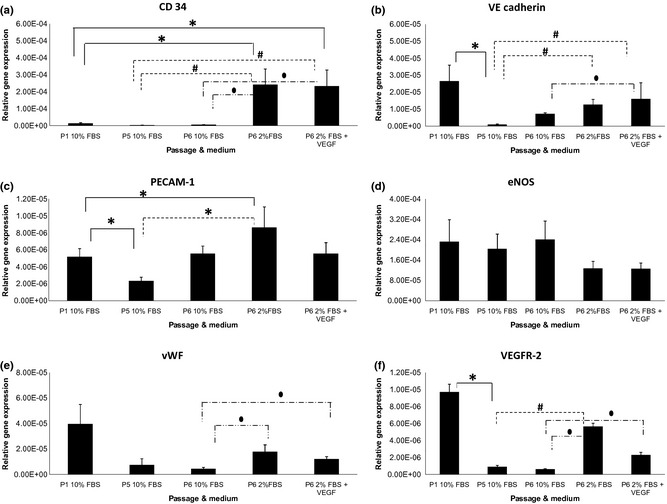
Endogenic genes’ expression level (relative to GAPDH) of human adipose stromal stem cells (ASCs) in various media. Significant difference between groups (P < 0.05 and n = 6) is labelled as follows: *refers to P < 0.05 relative to ASCs at P1 in 10% FBS; #refers to P < 0.05 relative to ASCs at P5 in 10% FBS; and ●refers to P < 0.05 relative to ASCs at P6 in 10% FBS.
Immunostaining of ASCs
Adipose stromal cells cultured in 10% FBS‐supplemented medium did not show positive staining with anti‐PECAM‐1 and only stained lightly with anti‐vWF (Fig. 6). In comparison, ASCs cultured in 2% FBS or 2% FBS plus VEGF were stained positively for both anti‐PECAM‐1 and anti‐vWF. ASCs cultured in medium supplemented with 2%FBS only showed stronger positive staining with anti‐PECAM‐1 and anti‐vWF compared to groups with added with VEGF (Fig. 6).
Figure 6.
Immunostaining of PECAM‐1 (a–c) and vWF (d–f) on cultured human adipose stromal stem cells (ASCs). ASCs cultured in 10%FBS‐supplemented medium did not stain with anti‐PECAM‐1 and only stained lightly with anti‐vWF. ASCs cultured in 2% FBS or 2% FBS plus vascular endothelial growth factor (VEGF) stained positive with both anti‐PECAM‐1 and anti‐vWF. (Magnification = ×100).
Discussion
Angiogenesis is a unique mechanism involved in development and expansion of blood vessels, as well as the lymphatic vasculature of the body and angiogenesis is important for repair and maintenance of perfusion in ischaemic diseases. The mechanism is particularly important for treatment of ischaemic heart disease, although it could be viewed as a harmful process, in cases of tumour development. Use of stem cells in treatment of cardiovascular diseases has been applied clinically to patients in the late stages of the disease although recently, treatment of cardiovascular disease has shifted towards use of stem cells in development of new blood vessels. Thus, in the present study, we explored angiogenic potential of ASCs in different media by evaluating expression levels of stemness, angiogenic and endogenic genes using real‐time PCR.
The study described here has focused on ASCs’ potential to undergo pro‐angiogenetic changes when grown in three types of culture media: (i) medium supplemented with 10% FBS; (ii) medium supplemented with 2% FBS; and (iii) medium supplemented with 2% FBS plus 50 ng/ml VEGF. Morphologically, ASCs at P1, P5 and P6 cultured in medium supplemented with 10% FBS had fibroblast‐like phenotypic features, being elongated and spindle‐like in form. This is a basic feature of stem cells in culture 26. However, at P6, when serum was reduced to 2%, ASCs assumed an apparently larger and flatten morphology. Reduction in serum concentration caused proliferation rate of ASCs to reduce, hence reduction in the total cell number at counting and growth rate. However, there was significant increase in ASC growth rate when VEGF was added to medium compared to ASCs cultured in medium with 2% serum alone.
Analysis of gene expression level using real‐time PCR showed that ASCs expressed all stemness genes including pluripotent stem‐cell markers, Sox2, Oct4, Rex1 and Nanog3 up to P6 in medium supplemented with 10% FBS; they maintained steady expression of stemness genes except of Oct4, which increased in expression at P5. However, the growth kinetic and differentiation ability of ASC were altered when cultured at later passages 13, 27, 28. While telomerase reverse transcriptase (TERT) was not expressed or at least not expressed at a level to be detected by real‐time PCR, in various cultures, Bernardo et al. have reported that telomerase activity and TERT were not detected in bone marrow MSCs and their telomeres shortened during culture 29. However, Katz et al. showed that ASCs did not express telomerase 30. We found that on the contrary, most stemness gene expression levels increased when ASCs were cultured in medium with reduced serum (2% FBS) at P6 compared to ASCs in medium supplemented with 10% FBS at P1, P5 and P6. However, addition of VEGF did not cause any significant changes in terms of this stemness gene expression. These data suggest that reduced serum may induce endogenous mechanisms of ASCs to maintain their stemness and self‐renewing properties.
Data from real‐time PCR showed that angiogenic genes’ expression in the ASCs at P1, P5 and P6 did not have any difference in expression when cultured in medium supplemented with 10% serum. However, most angiogenic genes’ expression increased when ASCs at P6 were cultured in medium with reduced serum (2% FBS). Gene expression levels of vascular endothelial growth factor (VEGF), angiopoetin‐1 (Ang‐1) and basic fibroblast growth factor (BFGF) increased significantly, while both hepatocyte growth factor (HGF) and placenta growth factor (PGF) had steady expressions even in reduced serum medium. These angiogenic factors play important roles in development of angiogenesis. Angiogenesis is triggered by tissue‐derived signals such as VEGF, which is controlled by ephrin‐B2. Ephrin‐B2 is a transmembrane ligand for Eph receptor tyrosine kinases, which induce sprouting behaviour and motility in angiogenic endothelium 31. Ang‐1 decreases endothelial cell permeability and increases vascular stabilization through recruitment of pericytes and smooth muscle cells to expanding blood vessels during angiogenesis 32. BFGF induces endothelial cell proliferation and migration as well as the release of proteolytic enzymes during angiogenesis 33. Increase of these factors indicates that ASCs cultured with reduced serum have angiogenic potential superior to ASCs cultured in higher serum concentrations. However, addition of VEGF to medium did not cause a great deal of difference in expression of angiogenic genes of ASCs; expression of VEGF and bFGF mRNA were significantly low. This could be due to the exogenous source of VEGF that reduced the endogenous expression levels of both VEGF and bFGF for cell proliferation.
Most endogenic gene expression levels reduced after serial passage in 10% FBS (from P1 to P5), but when ASCs at P6 were cultured in reduced serum concentration (2% FBS only), endogenic markers such as CD34 (endothelial cell marker), platelet/endothelial cell adhesion molecule 1 (PECAM‐1), vascular endothelial cadherin (VE Cadherin), von Willebrand factor (vWF) and VEGFR‐2 increased significantly. During angiogenesis, PECAM‐1 is involved in endothelial cell migration and attachment. It is also involved in organization of endothelial cells into vascular tubes 34. Vascular endothelial cadherin (VE Cadherin) is important in development of angiogenesis during embryonic development. It induces cell proliferation and apoptosis, as well as VEGF synthesis 35. Thus, increase in PECAM‐1 and VE‐Cadherin expression in ASCs cultured in reduced serum, increased endogenic potential of ASCs. Increased level of VEGFR‐2, a receptor for VEGF, may indicate that ASCs at P6 cultured in 2% serum have differentiated or had characteristics of endothelial cells. This is supported by evidence showing that VEGFR‐2 is one of the markers in early development of endothelial and blood cells 36. Furthermore, endothelial nitric oxide synthase (eNOS) and vWF expression levels increased in culture with 2% FBS. Overall, addition of VEGF to culture medium did not show any significant difference in expression of endogenic genes, in ASCs.
Increase in stemness, angiogenic and endogenic genes’ expression when ASCs were cultured in medium with reduced concentration of serum could induce adaptation to low growth factor‐culture conditions by increasing endogenous production of mitotic agents. Low serum concentration, which leads to reduction in nutrient and growth factors needed by the cells, could potentially cause stressful events in the culture. Gimble et al. reported that ASCs increased production of paracrine growth factors in stressful environments 37. Rehman et al. demonstrated that VEGF expression and other angiogenic factors such as HGF and bFGF significantly increased when ASCs were cultured in 5% FBS without growth factors, in hypoxic conditions, for 72 h 18. Gimble et al. also reported that ASCs had the ability to increase growth factor and cytokines production and to promote new blood vessels from surrounding blood vessels of ischaemic tissue. Reduction in serum could also increase differentiation ability of ASCs 37. Serum increased proliferation of stem cells as it contains many cytokines and growth factors, such as platelet‐derived growth factor (PDGF) and epidermal growth factor (EGF). Other growth factors such as transforming growth factor beta (TGFβ) and fibroblast growth factor‐2 (FGF‐2) increased ASC growth rate, but not their differentiation ability 38.
Addition of VEGF to medium with 2% serum did not increase angiogenic potential of the ASCs. However, they still maintained their stemness characteristics and angiogenic potential, as these genes were expressed. Growth kinetics of the ASCs did not show any significant increase with addition of VEGF compared to the 10% FBS culture. Similar results have been demonstrated where proliferation of ASCs cultured in EGM‐2 medium supplemented with VEGF did not increase significantly 39, although the reason for this is still unclear. Perhaps VEGF alone may not be able to induce angiogenic differentiation of ASCs and can only do so in the presence of other growth factors. Brindle et al. have shown that VEGF alone is not sufficient to induce formation of stable blood vessels in tissue 40. Other investigators have used different combinations of growth factors in induction medium. Apart from VEGF, Miranville et al. used Matrigel to demonstrate that addition of other growth factors such as bFGF, epidermal growth factor (EGF), insulin‐like growth factor (IGF‐1) and platelet‐derived growth factor (PDGF) is able to increase angiogenic potential of the stem cells 41. Use of VEGF and bFGF in combination has successfully induced endothelial differentiation 42 and use of endothelial growth media (EGM‐2) supplemented with 2% FBS, VEGF and other growth factors such as IGF and EGF is also able to induce angiogenesis 6. Although VEGF may be a potent angiogenic factor at the beginning of angiogenesis, other angiogenic factors are needed for cells to proceed to subsequent stages of angiogenesis.
In conclusion, angiogenic potential of ASCs could be induced in medium with reduced concentration of serum after serial passaging. However, addition of VEGF did not cause any significant increase in angiogenic or endogenic genes’ expression of ASCs cultured in 2% FBS‐supplemented medium. Thus, enhancement of angiogenic potential of ASCs should be investigated in the future by combining VEGF with other growth factors.
Acknowledgements
We would like to thank the Ministry of Science, Technology and Innovation of Malaysia: eScienceFund 02‐01‐02‐SF0290 and Ministry of Higher Education of Malaysia: HIR‐MOHE project UM.C/HIR/MOHE/ENG/44.
References
- 1. Lindner U, Kramer J, Rohwedel J, Schlenke P (2010) Mesenchymal stem or stromal cells: toward a better understanding of their biology. Transfus. Med. Hemother. 37, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao Q, Ye X (2011) Additive value of adult bone‐marrow‐derived cell transplantation to conventional revascularisation in chronic ischemic heart disease: a systemic review and meta‐analysis. Expert. Opin. Biol. Ther. 11, 1569–1579. [DOI] [PubMed] [Google Scholar]
- 3. Brunskill SJ, Hyde CJ, Doree CJ, Watt SM, Martin‐Rendon E (2009) Route of delivery and baseline left ventricular ejection fraction, key factors of bone‐marrow derived cell therapy for ischaemic heart disease. Eur. J. Heart Fail. 11, 887–896. [DOI] [PubMed] [Google Scholar]
- 4. Al Sabti H (2007) Therapeutic angiogenesis in cardiovascular disease. J. Cardiothorac. Surg. 2, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Annex BH (2002) Therapeutic angiogenesis: a treatment for the new millennium or passing fad? Cardiol. Rounds 6, 1–6. [Google Scholar]
- 6. DiMuzio P, Tulenko T (2007) Tissue engineering application to vascular bypass graft development: the use of adipose‐derived stem cells. J. Vasc. Surg. 45, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayati AR, Nur Fariha MM, Tan GC, Tan AE, Chua K (2011) Potential of human deciduas stem cells for angiogenesis and neurogenesis. Arch. Med. Res. 42, 291–300. [DOI] [PubMed] [Google Scholar]
- 8. Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P (2004) Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem. Biophys. Res. Commun. 314, 420–427. [DOI] [PubMed] [Google Scholar]
- 9. Verseijden F, Jahr H, Posthumus‐van S, Ten Hagen TL, Hovius SER, Seynhaeve ALB et al (2009) Angiogenic capacity of human adipose‐derived stromal cells during angiogenic differentiation: an in vitro study. Tissue Eng. Part A. 15, 445–452. [DOI] [PubMed] [Google Scholar]
- 10. Dhar S, Yoon ES, Kachgal S, Evans GRD (2007) Long‐term maintenance of neuronally differentiated human adipose derived stem cells. Tissue Eng. 13, 2625–2632. [DOI] [PubMed] [Google Scholar]
- 11. Helder MN, Knippenberg M, Klein‐Nilend J, Wuisman PIJM (2007) Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 13, 1799–1808. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez AM, Elabd C, Amri Ez‐Z, Ailhaud G, Dani C (2005) The human adipose tissue is a source of multipotent stem cells. Biochimie 87, 125–128. [DOI] [PubMed] [Google Scholar]
- 13. Wan Safwani WKZ, Makpol S, Sathapan S, Chua KH (2011) The changes of stemness biomarkers expression in human adipose‐derived stem cells during long‐term manipulation. Biotechnol. Appl. Biochem. 58, 261–270. [DOI] [PubMed] [Google Scholar]
- 14. Alhadlaq A, Tang M, Mao JJ (2005) Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimensions: implications in soft tissue augmentation and reconstruction. Tissue Eng. 11, 556–566. [DOI] [PubMed] [Google Scholar]
- 15. Patrick CW (2011) Tissue engineering strategies for adipose tissue repair. Anat. Rec. 263, 361–366. [DOI] [PubMed] [Google Scholar]
- 16. Patrick CW, Zheng B, Johnston C, Reece GP (2002) Long‐term implantation of preadipocytes‐seeded PLGA scaffolds. Tissue Eng. 8, 283–293. [DOI] [PubMed] [Google Scholar]
- 17. Planat‐Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R et al (2004) Plasticity of human adipose lineage cells towards endothelial cells: physiological and therapeutic perspectives. Circulation 109, 656–663. [DOI] [PubMed] [Google Scholar]
- 18. Rehman J, Traktuev D, Li J, Merfeld‐Clauss S, Temm‐Grove CJ, Bovenkerk JE et al (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298. [DOI] [PubMed] [Google Scholar]
- 19. Kanatsu‐Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T (2011) Serum‐ and feeder‐free culture of mouse germline stem cells. Biol. Reprod. 84, 97–105. [DOI] [PubMed] [Google Scholar]
- 20. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A et al (2003) VEGF guide angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Neufeld G, Cohen T, Genrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9–22. [PubMed] [Google Scholar]
- 22. Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat. Med. 9, 669–676. [DOI] [PubMed] [Google Scholar]
- 23. Merfeld‐Claus S, Gollahalli N, March KL, Traktuev DO (2010) Adipose tissue progenitor cells directly interact with endothelial cells to induce vascular network formation. Tissue Eng. Part A. 16, 2953–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajala K, Lindroos B, Hussein SM, Lappalainen RS, Pekkanen‐Mattila M, Inzunza J et al (2010) A defined and xeno‐free culture method enabling the establishment of clinical‐grade human embryonic, induced pluripotent and adipose stem cells. PLoS One 5, e10246. doi: 10.1371/journal.pone.0010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kilroy GE, Foster SJ, Wu X, Ruiz J, Sherwood S, Heifetz A et al (2007) Cytokine profile of human adipose‐derived stem cells: expression of angiogenic, hematopoietic, and pro‐inflammatory factors. J. Cell. Physiol. 212, 702–709. [DOI] [PubMed] [Google Scholar]
- 26. Boquest AC, Shahdadfar A, Brinchmann JE, Collas P (2005) Isolation of stromal stem cells from human adipose tissue. Methods Mol. Biol. 325, 35–46. [DOI] [PubMed] [Google Scholar]
- 27. Wenger SL, Senft JM, Sargent LM, Bamezai R, Bairawa N, Grant SG (2004) Comparison of established cell lines at different passages by karyotype and comparative genomic hybridization. Biosci. Rep. 24, 631–639. [DOI] [PubMed] [Google Scholar]
- 28. Wan Safwani WKZ, Makpol S, Sathapan S, Chua KH (2012) The impact of long‐term in vitro expansion on the senescence‐associated markers of human adipose‐derived stem cells. Appl. Biochem. Biotechnol. 166, 2101–2113. [DOI] [PubMed] [Google Scholar]
- 29. Bernardo MA, Zaffaroni N, Novara F, Cometa AM, Avanzini MA, Moretta A (2007) Human bone marrow–derived mesenchymal stem cells do not undergo transformation after long‐term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 67, 9142–9149. [DOI] [PubMed] [Google Scholar]
- 30. Katz AJ, Tholpady A, Tholpady SA, Shang H, Ogle RC (2005) Cell surface and transcriptional characterization of human adipose‐derived adherent stromal (hADAS) cells. Stem Cells 23, 412–423. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A (2010) Ephrin‐B2 controls VEGF‐induced angiogenesis and lymphagiogenesis. Nature 465, 483–486. [DOI] [PubMed] [Google Scholar]
- 32. Fangiani E, Lorentz P, Kopfstein L, Christofori G (2011) Angiopoietin‐1 and ‐2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 71, 5717–5727. [DOI] [PubMed] [Google Scholar]
- 33. Chhokar V, Tucker AL (2003) Angiogenesis: basic mechanisms and clinical applications. Semin. Cardiothorac. Vasc. Anesth. 7, 253–280. [Google Scholar]
- 34. Cao G, O'Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A et al (2002) Involvement of human PECAM‐1 in angiogenesis and in vitro endothelial cell migration. Cell Physiol. 282, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 35. Vestweber D (2007) VE‐Cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 28, 223–232. [DOI] [PubMed] [Google Scholar]
- 36. Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H (1998) Progressive lineage analysis by cell sorting and culture identifies FLK + VE‐Cadherin + cells at a diverging point of endothelial and hemopoietic lineages. Development 125, 1747–1757. [DOI] [PubMed] [Google Scholar]
- 37. Gimble JM, Katz AD, Bunnell BA (2007) Adipose‐derived stem cells for regenerative medicine. Circ. Res. 100, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tapp H, Hanley EN Jr, Patt JE, Gruber HE (2009) Adipose‐derived stem cells: characterization and current application in orthopaedic tissue repair. Exp. Biol. Med. 234, 1–9. [DOI] [PubMed] [Google Scholar]
- 39. Suga H, Shigeura T, Inoue K, Kato H, Aoi N, Murase S et al (2007) Rapid expansion of human adipose‐derived stromal cells preserving multipotency. Cytotherapy 9, 738–745. [DOI] [PubMed] [Google Scholar]
- 40. Brindle NP, McCarthy MJ, Bell PR (1999) Angiogenic revascularization in ischaemic disease: molecular techniques hold promise, though they are still some way off. BMJ 318, 1500–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A (2004) Improvement of postnatal neovascularization by human adipose tissue‐derived stem cells. Circulation 110, 349–355. [DOI] [PubMed] [Google Scholar]
- 42. Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC (2005) Human adipose tissue derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem. Biophys. Res. Commun. 332, 370–379. [DOI] [PubMed] [Google Scholar]


