Abstract
Binge-like patterns of excessive drinking during young adulthood increase the propensity for alcohol use disorders (AUDs) later in adult life; however, the mechanisms that drive this are not completely understood. Previous studies showed that the δ-opioid peptide receptor (DOP-R) is dynamically regulated by exposure to ethanol and that the DOP-R plays a role in ethanol-mediated behaviors. The aim of this study was to determine the role of the DOP-R in high ethanol consumption from young adulthood through to late adulthood by measuring DOP-R-mediated [35S]GTPγS binding in brain membranes and DOP-R-mediated analgesia using a rat model of high ethanol consumption in Long Evans rats. We show that DOP-R activity in the dorsal striatum and DOP-R-mediated analgesia changes during development, being highest during early adulthood and reduced in late adulthood. Intermittent access to ethanol but not continuous ethanol or water from young adulthood leads to an increase in DOP-R activity in the dorsal striatum and DOP-R-mediated analgesia into late adulthood. Multiple microinfusions of naltrindole into the dorsal striatum or multiple systemic administration of naltrindole reduces ethanol consumption, and following termination of treatment, DOP-R activity in the dorsal striatum is attenuated. These findings suggest that DOP-R activity in the dorsal striatum plays a role in high levels of ethanol consumption and suggest that targeting the DOP-R is an alternative strategy for the treatment of AUDs.
Introduction
Despite advances in the development of medications for alcohol use disorders (AUDs), there is still a need for new effective treatments. Naltrexone, the U.S. Food and Drug Administration-approved opioid treatment for AUDs, is primarily a μ-opioid peptide receptor (MOP-R) antagonist with modest activity at κ- (KOP-R) and δ-opioid peptide receptors (DOP-R). Naltrexone reduces ethanol consumption in animals (Altshuler et al., 1980; Stromberg et al., 1998) and in humans (Volpicelli et al., 1992; O'Malley et al., 2002; Anton et al., 2006); however, not all patients respond to or tolerate naltrexone treatment due to adverse effects (Hollister et al., 1981; Crowley et al., 1985; Mitchell et al., 1987; Volpicelli et al., 1992). Genetic variations in MOP-Rs may also contribute to naltrexone's reduced efficacy (Oslin et al., 2006).
Binge-like excessive drinking during adolescence and young adulthood increases the risk of developing alcohol dependence later in adulthood (Clapper and Lipsitt, 1992; Bonomo et al., 2004; Wells et al., 2004). Therefore, an important step in the development of new treatments for AUDs is to identify receptors and/or neural pathways that are altered by ethanol consumption during development. A large body of evidence suggests the MOP-R plays a role in ethanol-mediated behaviors (Reid and Hunter, 1984; Gardell et al., 1996; Stromberg et al., 1998; Lê et al., 1999; Roberts et al., 2000; Hall et al., 2001; Becker et al., 2002; Ciccocioppo et al., 2002). Similarly, the DOP-R is dynamically regulated by ethanol exposure (Charness et al., 1993; Winkler et al., 1998; Méndez et al., 2004), is implicated in ethanol reward (Froehlich et al., 1991; Borg and Taylor, 1997; Froehlich et al., 1998; Matsuzawa et al., 1999a,b; Shippenberg et al., 2008), and plays a role in ethanol self-administration. DOP-R antagonists have been shown to have mixed effects on ethanol consumption and seeking in rats; some studies have shown they reduce ethanol consumption and seeking (Krishnan-Sarin et al., 1995a,b; Franck et al., 1998; June et al., 1999; Hyytiä and Kiianmaa, 2001; Ciccocioppo et al., 2002; Nielsen et al., 2012), while other studies have not shown this effect (Stromberg et al., 1998; Ingman et al., 2003; Margolis et al., 2008). Activation of the enkephalinergic system is proposed to be important for the maintenance of high ethanol intake (Froehlich et al., 1991); however, the precise role of DOP-Rs in ethanol consumption is unclear. It has been shown that DOP-R agonists can both increase and decrease ethanol intake (Margolis et al., 2008; Barson et al., 2009, 2010; van Rijn and Whistler, 2009; van Rijn et al., 2010). The differences can be explained by the length of ethanol exposure, route of drug administration, and variation in animal drinking models and rodent strains. To understand how DOP-R activity changes through development and with ethanol exposure, we used a rat model of high ethanol consumption (Simms et al., 2008) to measure DOP-R activity by measuring DOP-R-mediated analgesia and [35S]GTPγS binding in brain regions in rats consuming ethanol from young to late adulthood.
Materials and Methods
Drugs and chemicals
Ethanol (95% v/v) was purchased from Gold Shield. [35S]-GTPγS (250 μCi; 9.25 MBq) was supplied from PerkinElmer. Naltrindole hydrochloride, (3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aa-octahydroquinolino[2,3,3-g]isoquinoline dihydrobromide (TAN67), (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80), [d-Ala2, N-MePhe4, Gly5-ol]-enkephalin (DAMGO), GTPγS, GDP, HEPES, dl-dithiothreitol, tricine, dimethyl sulfoxide (DMSO), magnesium chloride (MgCl2), EDTA, and saponin were purchased from Sigma-Aldrich. PBS was purchased from Fisher Scientific. Complete Mini Protease Inhibitor Cocktail tablets were purchased from Roche. Wheat germ agglutinin scintillation proximity assay (SPA) beads were purchased from GE Healthcare. Isoflurane was purchased from Baxter Healthcare. Guide cannulae, dummies, and injectors were purchased from Plastics One.
Animals and housing
Male Long-Evans rats [postnatal day 49 (P49); 175–200 g] were purchased from Harlan and housed individually in a temperature controlled environment (22 ± 2°C) with a 12 h reverse light/dark cycle. Rats were given at least 1 week to acclimatize with food and water available ad libitum. Ethanol-exposed and water-control rats were randomly assigned from one cohort of animals. All procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the humane care and use of laboratory animals.
Two-bottle choice drinking procedures
Intermittent access to 20% ethanol.
The intermittent-access 20% ethanol two-bottle choice drinking procedure was used, which does not require sucrose fading (Simms et al., 2008). In brief, ethanol-naive rats (n = 12) were given access to bottles of ethanol (20% v/v) and water for 24-h-long sessions on alternate days (three 24-h-long sessions each week) with water available only on days between ethanol exposures. All fluids were presented in 100 ml graduated glass cylinders with stainless-steel drinking spouts inserted through two grommets in front of the cage. The placement of the ethanol bottle was alternated before the start of each drinking session to control for side preferences. Bottles were weighed 30 min and 24 h after the fluids were presented, and measurements were taken to the nearest gram. The weight of each rat was measured daily to calculate the grams per kilogram ethanol intake.
Continuous access to 20% ethanol.
A separate group of ethanol-naive young adult rats (P56; n = 10) was given continuous access to one bottle containing a 20% (v/v) ethanol solution and one bottle of water. No initiation procedures were used before the first ethanol drinking session. The placement of the ethanol bottle was alternated daily to control for side preferences.
DOP-R ligands
Naltrindole is as a potent and highly selective nonpeptide DOP-R antagonist (Portoghese et al., 1988) that crosses the blood–brain barrier (Weber et al., 1991). Initial studies performed in smooth muscle preparations showed that naltrindole potently inhibited the DOP-R agonists, DADLE ([D-Ala2, D-Leu5]-Enkephalin) and DPDPE ([D-Pen2,5]-Enkephalin), by over 100-fold compared to inhibition of the MOP-R agonists DAMGO and morphine, and the KOP-R agonist U50488 H (trans-(±)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide) (Portoghese et al., 1988). Furthermore, naltrindole (20 mg/kg, s.c.) selectively antagonized analgesia in mice elicited by the DOP-R agonist, DSLET (D-Ser2-Leu5-Enkephalin-Thr6), but not analgesia elicited by morphine or U50488H (Portoghese et al., 1988). Naltrindole has since been used extensively both in vitro and in vivo due to its high selectivity for the DOP-R (Krishnan-Sarin et al., 1995a; Saitoh et al., 2004, 2005; Perrine et al., 2006; Hallett and Brotchie, 2007).
SNC80 and TAN67 were used since these small molecule compounds are reported to be very selective for the DOP-R (Bilsky et al., 1995; Kamei et al., 1995; Calderon et al., 1997; Tseng et al., 1997) compared to DOP-R peptide agonists, such as DPDPE, which are less selective for the DOP-R with activity at the MOP-R in the micromolar range (Scherrer et al., 2004). SNC80 has been shown to preferentially compete against sites labeled by [3H]naltrindole rather than against those labeled by [3H]DAMGO or the KOP-R agonist [3H]U69593 in mouse whole-brain assays (Bilsky et al., 1995). Our previous studies showed that SNC80-mediated [35S]GTPγS stimulation was potently inhibited by naltrindole (IC50, 31 ± 3.9 nmol/L), but not by naltrexone (IC50, >10 μmol/L), in striatal membranes of long-term high-ethanol-consuming rats (Nielsen et al., 2008), indicating that both naltrindole and SNC80 act on a common receptor. TAN67 elicits DOP-R-mediated analgesia in mice such that TAN67-mediated analgesia is attenuated by pretreatment with the DOP-R antagonist 7-benzylidenenaltrexone, but not by the MOP-R antagonist β-funaltrexamine or the KOP-R antagonist nor-binaltorphimine (Kamei et al., 1995; Tseng et al., 1997).
DOP-R-mediated coupling using [35S]GTPγS binding
Rat brains were collected from separate groups of rats for binding experiments: naive rats (P56, P84 and P154), ethanol-consuming rats given access to intermittent 20% ethanol from P56 (P84, P154), ethanol-consuming rats given access to continuous 20% ethanol from P56 (P154), and ethanol consuming rats administered naltrindole (3 mg/kg, i.p.) or vehicle daily for 28 days from P56 (P112). Following decapitation, rat brains were removed, and the following brain regions were dissected: dorsal striatum, nucleus accumbens, brainstem, cerebral cortex, hippocampus, cerebellum, spinal cord, amygdala, ventral tegmental area, and the remaining (pooled) midbrain. Following removal, brain regions were quickly frozen using liquid nitrogen and stored at −80°C until used. Rat brain regions were quickly thawed and suspended in a homogenization buffer (50 mm Tris-HCl, 1 mm EDTA, 3 mm MgCl2, pH 7.4; 1 g brain tissue/20 ml buffer). The tissues from each brain region were individually homogenized on ice (900 rpm; 15 strokes), centrifuged (1000 × g, 10 min, 4°C followed by 20000 × g, 15 min, 4°C), and resuspended in assay buffer (100 mm HEPES · NaOH, 5 mm MgCl2, 100 mm NaCl, 10 μg/ml saponin, and one mammalian protease inhibitor tablet per 25 ml, pH 7.5) before being snap-frozen in liquid nitrogen and stored at −80°C until required. Binding assays (n = 3, each in triplicate) were performed in 96-well plates on ice with each reaction containing [35S]GTPγS (50 pm), cell membrane (10 μg of protein), GDP (30 μm), and SPA beads (0.5 mg) with assay buffer (as above) and the opioid ligands. Single drug dose–response curves (0.1 nm to 100 μm) of [35S]GTPγS-stimulated binding were performed with the DOP-R agonists TAN67 and SNC80 and the MOP-R agonist DAMGO in each rat brain region. SNC80 was initially dissolved in DMSO to produce a 10 mm stock concentration that was then diluted with assay buffer for the [35S]GTPγS assay. Assay plates were shaken for 45 min at 25°C and centrifuged (1500 rpm, 5 min, 25°C) before [35S]GTPγS-stimulated binding was assessed using NXT TopCounter. [35S]GTPγS-stimulated binding is expressed as a percentage increase over basal [35S]GTPγS binding.
Antinociceptive testing
The tail-flick latency test (D'Amour and Smith, 1941) was used to quantify DOP-R-mediated antinociception in rats using TAN67 (60 mg/kg, i.p.) (Kamei et al., 1995; Suzuki et al., 1995). Before drug (or vehicle) administration, baseline antinociceptive testing was performed, with the thermal stimulus applied to the lower third of the ventral surface of the rat's tail at 5 min intervals until three latency values of were within ±1 s (mean latency, 2.4 s; range, 2.0–3.0 s). To determine the time of the maximal antinociceptive effect, testing was performed immediately before each drug treatment and then 5, 15, 30, 45, 60, and 90 min and 2, 3, 4, 5, and 6 h after drug treatment, or until baseline levels of antinociception were achieved. A maximum of 9 s was used to minimize tissue damage to the rat's tails. The tail-flick latency values were converted to a percentage of the maximum possible effect (%MPE) (Brady and Holtzman, 1982): %MPE = (postdrug latency − predrug latency)/(maximum latency [9 s] − predrug latency) × 100%.
DOP-R-mediated antinociception with high ethanol consumption.
Groups of adult rats (P56; n = 6) were tested for TAN67-mediated [60 mg/kg, i.p., or vehicle (distilled water), 1 ml/kg, i.p.] tail-flick antinociception at 30 min after dosing (i.e., time of predetermined maximal antinociceptive effect), before access to either intermittent 20% ethanol or water only. TAN67-mediated (60 mg/kg or vehicle, i.p.) antinociception was reassessed in the same groups of rats consuming 20% intermittent ethanol or water at ages P84 and P154. Antinociceptive testing was performed at the end of the 2-d-long period of access to water only to limit any direct effects of ethanol on pain perception. Separate groups of naive young adult rats (P56; n = 4) were also administered either naltrindole (3 mg/kg, i.p.), naltrexone (1 mg/kg, i.p.), or vehicle 15 min before TAN67-mediated antinociception (60 mg/kg, i.p.) was assessed. All drug solutions were freshly prepared immediately before each injection.
Intrastriatal administrations of DOP-R ligands.
Groups of rats (n = 14) given access to intermittent 20% ethanol from age P56 and maintained at a stable level of ethanol consumption for 4 weeks were anesthetized continuously with isoflurane. Bilateral guide cannulae (C235G, 26 gauge) were stereotaxically implanted into the dorsal striatum (1.0 mm anterior; 3.2 mm mediolateral, 3.7 mm ventral to bregma) (Wang et al., 2007). Cannulae were held in place by dental cement and anchored with four surrounding skull-implanted screws. Following recovery, rats were further given intermittent access to ethanol (20% v/v) and water for 2 weeks to produce maintained stable levels of ethanol consumption. Rats were then given bilateral intrastriatal injections of naltrindole (0.5, 1, or 2 μg) or vehicle (PBS). Compounds or vehicle were given once a week in a Latin square design over 4 weeks such that rats received each compound in a counterbalanced manner. Following the round of naltrindole administrations, rats were then similarly administered bilateral intrastriatal injections of SNC80 (0.05, 0.5, or 5 ng) or vehicle (PBS) as one injection each week over the next 4 weeks. Compounds (or vehicle) were injected via internal cannulae extending 0.5 mm beyond the tip of the guide cannulae in the dorsal striatum 15 min before access to ethanol (20%) and water. The volume infused with a Hamilton 10 μl syringe (1701) was 1 μl per side at a rate of 0.5 μl/min, with the injectors kept in position in the cannulae for an additional 2 min (Wang et al., 2007). All drug solutions were freshly prepared immediately before each injection. Naltrindole and SNC80 were initially dissolved in distilled water and DMSO, respectively, in 10 mm solutions that were then diluted with PBS to achieve final solutions for injection in the doses, as above. Vehicle solutions for injection in naltrindole- and SNC80-treated rats were similarly prepared using distilled water or DMSO, respectively, diluted with PBS. Ethanol and water consumption were measured after 30 min and 24 h of ethanol access. Injection times and doses of naltrindole (0.5, 1, and 2 μg) and SNC80 (0.05, 0.5, and 5 ng) were based on previous studies (Ross and Smith, 1997; Olive and Maidment, 1998; Hyytiä and Kiianmaa, 2001; Wang et al., 2007). SNC80 was used in this experiment because TAN67 was discontinued from the original source, and TAN67 also produced convulsant effects in the previous antinociceptive studies. At the end of the experiments, rat brains were collected, and 75-μm-thick brain slices were cut, mounted, and stained to verify coordinates of the implanted cannulae. Only data from subjects with cannulae located in the region of interest were included in the analysis. Data from subjects with cannulae located outside of the region of interest were analyzed separately as diffusion controls (n = 6).
Multiple intrastriatal administrations of naltrindole.
A separate group of naive rats were anesthetized continuously with isoflurane. Bilateral guide cannulae (C235G, 26 gauge) were stereotaxically implanted into the dorsal striatum (1.0 mm anterior, 3.2 mm mediolateral, and 3.7 mm ventral to bregma) (Wang et al., 2007). Cannulae were held in place by dental cement and anchored with four surrounding skull-implanted screws. Following recovery, rats were given bilateral intrastriatal injections of naltrindole (2 μg; n = 8), vehicle (PBS; n = 7), or a sham injection (n = 7) 15 min before the first exposure to 20% ethanol and water, and then 15 min before access to 20% ethanol and water on the following ethanol days for seven ethanol exposures using the intermittent access to 20% ethanol procedure. After seven exposures to intermittent 20% ethanol, the administrations of naltrindole or vehicle were terminated, and the rats were maintained on the intermittent access schedule for an additional five exposures. After this time, the group of rats that received vehicle (PBS) and sham injections previously were then given intrastriatal injections of naltrindole (2 μg; n = 7) and vehicle (PBS; n = 5), respectively, 15 min before access to ethanol (20% v/v) and water on the ethanol days for three ethanol exposures using the intermittent access to 20% ethanol procedure. After three exposures to intermittent 20% ethanol, the administrations of naltrindole or vehicle were terminated, and the rats were maintained on the intermittent access schedule to measure posttreatment baseline drinking levels.
Multiple administrations of systemic naltrindole.
Naltrindole (3 mg/kg, i.p., n = 7, or vehicle, distilled water, 1 ml/kg, i.p., n = 7) was administered once a day to groups of adult rats (P56), 30 min before the start of the drinking session on the first ethanol exposure day using the intermittent-access 20% ethanol two-bottle drinking procedure. The daily injections continued for 28 consecutive days (12 ethanol exposures). All drug solutions were freshly prepared immediately before each injection. Bottles were weighed 24 h following the start of the drinking session, and measurements were taken to the nearest gram. After 4 weeks of intermittent 20% ethanol access, the daily administration of either naltrindole or vehicle was terminated, and the rats were maintained on the intermittent-access schedule for an additional 28 d before rat brain membranes were collected. Injection times and doses of naltrindole (3 mg/kg) were based on previous studies (Saitoh et al., 2005; Nielsen et al., 2008). This dose of naltrindole (3 mg/kg) produces selective DOP-R-mediated anxiogenic activity in rats (Saitoh et al., 2005).
Effect of naltrindole on blood ethanol concentrations
Groups of adult naive rats (range, 337–381 g) were administered naltrindole (3 mg/kg, i.p.; n = 9) or vehicle only (distilled water; 1ml/kg, i.p.; n = 8) 30 min before an injection of ethanol (1.5 g/kg, i.p.). Whole blood was collected from the lateral tail vein at 0.5, 1, 2 and 4 h after ethanol dosing. Blood samples were centrifuged for 13 min at 8000 rpm, and sera were separated and stored at −80°C until the assay was performed. Sera samples (10 μl) were precipitated in 3.5% perchloric acid (50 μl final volume). Ethanol was quantified by the alcohol dehydrogenase assay (Ling et al., 2003). Triplicate samples (7 μl) of supernatant from each sample were incubated with 0.5 m Tris-HCl buffer, pH 8.8, containing 5.5 μg/ml of alcohol dehydrogenase and 1.5 mm β-nicotinamide adenine dinucleotide (400 μl total volume) and incubated for 40 min at room temperature. Levels of the reduced β-NADH product were quantified by spectrophotometric absorbance at 340 nm, and the corresponding ethanol concentration was determined by a standard calibration curve.
Statistics
Statistical analyses were performed using GraphPad Prism. Data from ex vivo GTPγS binding assays were analyzed by nonlinear regression using a sigmoidal curve with variable slope to determine EC50 values. Data from ex vivo functional binding assays and tail-flick analgesia studies were analyzed using one-way ANOVA with Newman–Keuls post hoc analysis and Student's t test where appropriate, with an overall significance criterion of p < 0.05. Heavy drinking rat behavioral data were analyzed using a repeated-measures one-way ANOVA with Newman–Keuls post hoc analysis and Student's t test where appropriate, with an overall significance criterion of p < 0.05. Multiple dosing studies were analyzed by a two-factor ANOVA (treatment by time) with Bonferroni's post hoc test and Student's t test where appropriate, with an overall significance criterion of p < 0.05. Blood ethanol concentrations (BECs) were analyzed by a two-factor ANOVA (treatment by time) with Bonferroni's post hoc test.
Results
High ethanol intake using intermittent access to 20% ethanol
Young adult rats (P56) given intermittent access to 20% (v/v) ethanol displayed a rapid onset and escalation of ethanol intake. Ethanol consumption increased within 2 weeks of intermittent access to 20% ethanol and was maintained until late adulthood (P154; Fig. 1A). Our previous studies (Simms et al., 2008) reported that Long Evans rats given intermittent access to 20% ethanol produce BECs in the range of 10 to 100 mg/dl over 30 min of access, with 30% of rats with levels above 93 mg/dl.
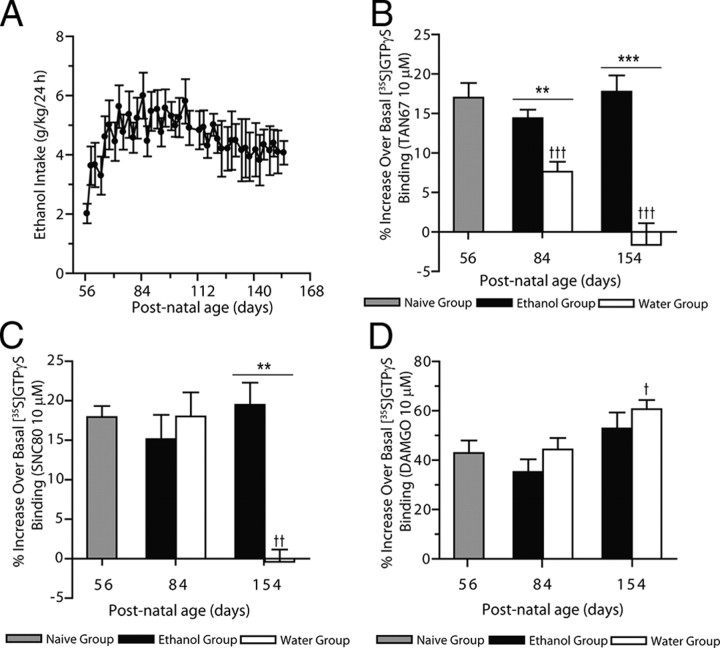
Figure 1.
Intermittent ethanol consumption prevents the developmental decrease in DOP-R-mediated [35S]GTPγS binding in the striatum. A, Rats given intermittent access to 20% ethanol from young adulthood (P56) consume high amounts of ethanol for long periods (n = 8). The values are expressed as the mean ± SEM of ethanol consumed (g/kg/24 h). B, DOP-R-mediated [35S]GTPγS binding by TAN67 in the striatum is high in young adult rats (gray bar) and reduced in adult and aged adult rats (white bars) consuming water only. High DOP-R-mediated [35S]GTPγS binding by TAN67 in the striatum in young adult rats (gray bar) is maintained in adult and aged adult rats consuming high amounts of ethanol since P56 (black bars). The values are expressed as the mean ± SEM percentage of basal [35S]GTPγS binding by TAN67 (10 μm; n = 3, each in triplicate). C, DOP-R-mediated [35S]GTPγS binding by SNC80 in the striatum is high in young adult rats (gray bar) and adult rats (P84; white bar) and reduced in aged adult rats (P154; white bar) consuming water only. High DOP-R-mediated [35S]GTPγS binding by SNC80 in the striatum in young adult rats (gray bar) is maintained in adult and aged adult rats consuming high amounts of ethanol since P56 (black bars). The values are expressed as the mean ± SEM percentage of basal [35S]GTPγS binding by SNC80 (10 μm; n = 3, each in triplicate). D, MOP-R-mediated [35S]GTPγS binding by DAMGO in the striatum is high in aged adult rats (P154; white bar) consuming water only compared to young adult rats (gray bar). MOP-R-mediated [35S]GTPγS binding by DAMGO in the striatum of high-ethanol-consuming adult and aged adult rats (black bar) is not different from MOP-R-mediated [35S]GTPγS binding by DAMGO in the striatum of young adult rats (gray bar) or aged-matched rats consuming water only (white bars). The values are expressed as the mean ± SEM percentage increase over basal [35S]GTPγS binding by DAMGO (10 μm; n = 3, each in triplicate). †p < 0.05; ††p < 0.01; †††p < 0.001 [compared to young adult (P56) rats]; **p < 0.01; ***p < 0.001 (comparison between aged-matched rats consuming either ethanol and water or water only); one-way ANOVA with repeated measures, with the Newman–Keuls post hoc analysis or Student's t test).
Developmental decrease in DOP-R-mediated [35S]GTPγS binding in the dorsal striatum
DOP-R-mediated [35S]GTPγS stimulation by TAN67 in dorsal striatal membranes prepared from young adult rats was higher in potency (Table 1) and efficacy (F(2,30) = 19.42, p < 0.0001; Fig. 1B) compared to that in adult rats and aged adult rats. Similarly, DOP-R-mediated [35S]GTPγS stimulation by the DOP-R agonist SNC80 in dorsal striatal membranes prepared from young adult rats was higher in potency compared to that in adult and aged rats (Table 1). DOP-R-mediated [35S]GTPγS stimulation by the DOP-R agonist SNC80 was also higher in efficacy in dorsal striatal membranes prepared from young adult rats compared to those from aged rats (P154; F(2,23) = 9.88, p < 0.001; Fig. 1C). In contrast, MOP-R-mediated [35S]GTPγS stimulation by DAMGO in dorsal striatal membranes prepared from young adult rats and adult rats was lower in potency (Table 1) and efficacy (F(2,21) = 4.54, p < 0.05; Fig. 1D) compared to aged adult rats.
Table 1.
Opioid receptor-mediated [35S]GTPγS binding by TAN67, SNC80, and DAMGO in the dorsal striatum of rats given intermittent access to 20% ethanol or water only from young adulthood into late adulthood
| Treatment | Postnatal age (days) | Mean (±SEM) EC50 values for [35S]GTPγS stimulation |
||
|---|---|---|---|---|
| TAN67 | SNC80 | DAMGO | ||
| Naive | P56 | 0.05 ± 0.002 | 0.13 ± 0.04 | 0.66 ± 0.03 |
| Water only from P56 | P84 | 2.1 ± 0.2*** | 0.32 ± 0.03** | 0.62 ± 0.03 |
| P154 | >10 | >10 | 0.30 ± 0.02*** | |
| 20% Ethanol from P56 | P84 | 0.05 ± 0.003††† | 0.14 ± 0.05†† | 2.7 ± 0.1***,††† |
| P154 | 0.05 ± 0.004 | 0.14 ± 0.03 | 1.3 ± 0.2**,††† | |
Data were analyzed using one-way ANOVA with Newman–Keuls post hoc analysis and Student's t test (n = 3 per group, each in triplicate).
** p < 0.01;
***p< 0.001 (compared to naive P56 rats).
††p < 0.01;
†††p < 0.001 (compared to aged-matched rats consuming water only).
Intermittent ethanol consumption prevents the developmental decrease in DOP-R-mediated [35S]GTPγS binding in the dorsal striatum
DOP-R-mediated [35S]GTPγS stimulation by TAN67 in dorsal striatal membranes prepared from ethanol-naive young adult rats was not different in potency or efficacy compared to adult and aged adult rats consuming high amounts of ethanol since young adulthood (Fig. 1B; Table 1). DOP-R-mediated [35S]GTPγS stimulation by TAN67 in dorsal striatal membranes prepared from adult (P84) and aged adult (P154) rats consuming 20% intermittent ethanol from young adulthood was higher in potency and efficacy compared to DOP-R-mediated [35S]GTPγS stimulation by TAN67 in dorsal striatal membranes prepared from adult and aged rats consuming water only from young adulthood (Fig. 1B; Table 1). DOP-R-mediated [35S]GTPγS stimulation by SNC80 was not different in potency in dorsal striatal membranes prepared from ethanol-naive young adult rats compared to adult and aged rats consuming high amounts of ethanol since young adulthood (Table 1). DOP-R-mediated [35S]GTPγS stimulation by SNC80 in dorsal striatal membranes prepared from adult (P84) and aged adult rats (P154) consuming 20% intermittent ethanol from young adulthood was higher in potency compared to that in adult and aged adult rats consuming water only from young adulthood (Table 1). DOP-R-mediated [35S]GTPγS stimulation by SNC80 in dorsal striatal membranes prepared from aged adult rats (P154) consuming 20% intermittent ethanol from young adulthood was higher in efficacy compared to that in aged adult rats consuming water only from young adulthood (Fig. 1C). In contrast, MOP-R-mediated [35S]GTPγS stimulation by DAMGO in dorsal striatal membranes prepared from ethanol-naive young adult rats was higher in potency, albeit not efficacy, compared to adult rats, and higher in potency and efficacy compared to aged adult rats consuming 20% intermittent ethanol since young adulthood (Fig. 1D; Table 1). MOP-R-mediated [35S]GTPγS stimulation by DAMGO in dorsal striatal membranes prepared from adult (P84) and aged adult (P154) rats consuming 20% intermittent ethanol from young adulthood (P56) was lower in potency, albeit not efficacy, compared to adult and aged adult rats consuming water only (Table 1; Fig. 1D).
Developmental decrease in DOP-R-mediated analgesia
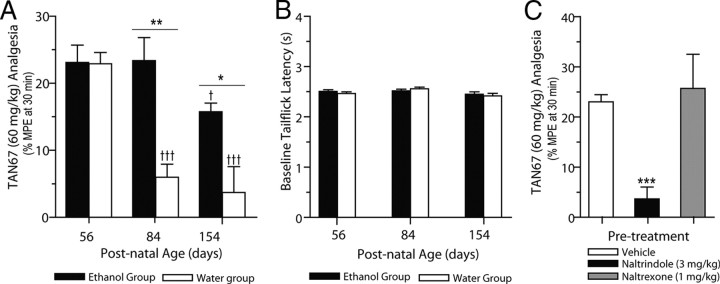
The DOP-R agonist TAN67 (60 mg/kg, i.p.) produced analgesia in naive young adult rats (P56), with a peak analgesic effect observed at 30 min after dosing (Fig. 2A) and a duration of action of ≈3 h. In contrast, TAN67-mediated (60 mg/kg, i.p.) analgesia was reduced in adult rats (P84; p < 0.001) and aged (P154; p < 0.001) rats (F(2,12) = 18.54, p < 0.001; Fig. 2A). Vehicle-treated rats did not produce any significant levels of analgesia (P56, 1.1 ± 1.0% MPE; P84, −1.9 ± 2.4% MPE; P154, −1.7 ± 1.5% MPE). TAN67-mediated (60 mg/kg, i.p.) analgesia in ethanol-naive young adult rats was attenuated by pretreatment with the DOP-R antagonist naltrindole (3 mg/kg, i.p.; p < 0.001), but not by naltrexone (1 mg/kg, i.p.; p > 0.05; Fig. 2C). Our previous studies have shown that naltrexone (1 mg/kg, i.p.) significantly attenuates morphine-mediated (5 mg/kg, i.p.) analgesia in ethanol-naive young adult rats (Nielsen et al., 2008).
Figure 2.
Intermittent ethanol consumption prevents the developmental decrease in DOP-R-mediated analgesia. A, DOP-R-mediated tail-flick analgesia by TAN67 (60 mg/kg, i.p.) is high in young adult rats (P56) and is reduced in adult and aged adult rats consuming water only (white bars). DOP-R-mediated analgesia by TAN67 (60 mg/kg, i.p.) is maintained in adult and aged adult rats consuming high amounts of ethanol since P56 (black bars). Data are expressed as the mean ± SEM %MPE at 30 min after dosing (n = 6 per group). B, Baseline levels of tail-flick analgesia are not changed with age and are not different between rats consuming either 20% intermittent ethanol (black bars) or water only (white bars). Data are expressed as the mean ± SEM tail-flick latency (seconds). C, TAN67-mediated (60 mg/kg, i.p.) DOP-R analgesia in young adult rats (P56) is reduced by pretreatment with naltrindole (3 mg/kg, i.p.), but not by pretreatment with naltrexone (1 mg/kg, i.p.). †††p < 0.001 [compared to young adult rats (P56)]; *p < 0.05; **p < 0.01 (comparison between aged-matched rats consuming intermittent 20% ethanol or water only); ***p < 0.001 (compared to vehicle-treated rats); one-way ANOVA with repeated measures, with the Newman–Keuls post hoc analysis or Student's t test.
Intermittent ethanol consumption prevents the developmental decrease in DOP-R-mediated analgesia
TAN67-mediated (60 mg/kg, i.p.) analgesia was not reduced in adult rats (P84) and aged (P154) rats given intermittent access to 20% ethanol from age P56 (p > 0.05; Fig. 2A). TAN67-mediated (60 mg/kg, i.p.) analgesia in adult (P84; p < 0.01) and aged (P154; p < 0.05) rats that were given intermittent access to 20% ethanol from age P56 was greater than TAN67-mediated (60 mg/kg, i.p.) analgesia in the aged-matched rats consuming water only (Fig. 2A). TAN67-mediated (60 mg/kg, i.p.) analgesia in aged rats (P154) that were given intermittent access to 20% ethanol from age P56 was reduced compared to TAN67-mediated (60 mg/kg, i.p.) analgesia in young adult rats (P56; p < 0.05), but not different from TAN67-mediated (60 mg/kg, i.p.) analgesia in adult rats (P84; p > 0.05) given intermittent access to 20% ethanol (Fig. 2A). TAN67-mediated (60 mg/kg, i.p.) analgesia in the group of young adult rats (P56) before access to 20% intermittent ethanol was not different (p > 0.05) from TAN67-mediated (60 mg/kg, i.p.) analgesia in the group of young adult rats (P56) before access to water only. Vehicle-treated rats in the ethanol group did not produce any significant levels of analgesia (P56, 1.2 ± 1.1% MPE; P84, −1.9 ± 2.2% MPE; P154, 2.8 ± 0.2% MPE). There were no age-related differences in baseline levels of analgesia and no differences in baseline levels of analgesia or hyperalgesic responses between age-matched groups of rats given intermittent 20% ethanol or water only (p > 0.05; Fig. 2B).
Continuous ethanol consumption does not maintain DOP-R-mediated [35S]GTPγS binding in the dorsal striatum
Continuous access to 20% ethanol produced a delayed escalation of ethanol consumption that was not maintained out for long periods compared to rats given access to intermittent 20% ethanol (Fig. 3A). Young adult rats (P56) increased their ethanol consumption within 2 weeks of continuous access to 20% ethanol, although this level of ethanol consumption was not maintained for long periods.
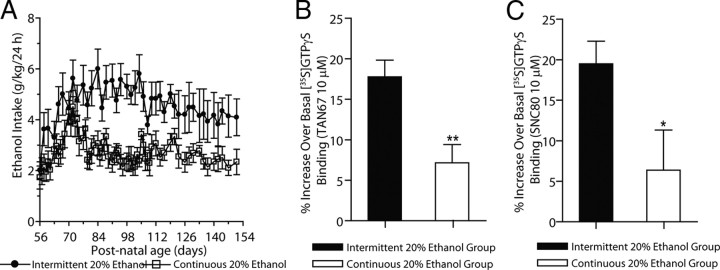
Figure 3.
Continuous ethanol consumption does not maintain DOP-R-mediated [35S]GTPγS binding in the striatum. A, Rats given intermittent access to 20% ethanol from young adulthood (P56) consume large amounts of ethanol for long periods. Rats given continuous access to 20% ethanol from young adulthood (P56) do not consume large amounts of ethanol. The values are expressed as the mean ± SEM of ethanol consumed (grams per kilogram per 24 h). B, C, DOP-R-mediated [35S]GTPγS binding by TAN67 (B) and SNC80 (C) in the dorsal striatum is higher in P154 rats given intermittent access to 20% ethanol since P56 (black bars) compared to P154 rats given access continuous 20% ethanol since P56 (white bars). The values are expressed as the mean ± SEM percentage increase over basal [35S]GTPγS binding by TAN67 and SNC80 (10 μm; n = 3, each in triplicate). *p < 0.05; **p < 0.01 (comparison between intermittent and continuous 20% ethanol groups; Student's t test).
The cumulative amounts of ethanol consumed in rats given either intermittent (three 24-h-long ethanol exposures per week) or continuous (seven 24-h-long exposures per week) access to 20% ethanol from P56 to P154 are 55.4 ± 5.9 g (mean ± SEM) and 127.8 ± 3.6 g, respectively. DOP-R-mediated [35S]GTPγS-stimulated binding in dorsal striatal membranes of adult rats at age P154 given long-term continuous access to 20% ethanol since P56 (TAN67, EC50, 1.2 ± 0.4 μm; SNC80, EC50, 811 ± 71 nm) was significantly lower compared to that of age-matched controls with intermittent access to 20% ethanol (TAN67, EC50, 57 ± 3.5 nm; p < 0.05; SNC80, EC50, 139 ± 31 nm; p < 0.001; Fig. 3B,C).
Intermittent ethanol consumption prevents the developmental decrease in DOP-R-mediated [35S]GTPγS binding specifically in the dorsal striatum
Opioid receptor-mediated [35S]GTPγS stimulation was measured in various brain regions of adult rats (P154) that were consuming either 20% intermittent ethanol or water only since P56. DOP-R-mediated [35S]GTPγS stimulation by TAN67 and SNC80 was high in the dorsal striatum of ethanol-consuming rats compared to water-consuming rats (Table 2). In contrast, DOP-R-mediated [35S]GTPγS stimulation by TAN67 and SNC80 was absent in ventral striatal structures, such as the nucleus accumbens, of both ethanol-consuming and water-only-consuming rats (Table 2). DOP-R-mediated [35S]GTPγS stimulation by TAN67 in the rat spinal cord was moderate, albeit higher in ethanol-consuming rats compared to water-consuming rats (Table 2). In comparison, moderate DOP-R-mediated [35S]GTPγS stimulation in the rat cerebral cortex and the brainstem of high-ethanol-consuming rats was not different from that of water-consuming rats (Table 2). Furthermore, DOP-R-mediated [35S]GTPγS stimulation was absent in the VTA, amygdala, hippocampus, cerebellum, and midbrain of both high-ethanol- and water-only consuming rats (Table 2). In contrast, MOP-R-mediated [35S]GTPγS stimulation by DAMGO was reduced in the dorsal striatum, cerebral cortex, brainstem, spinal cord, and the amygdala, but not in the nucleus accumbens, ventral tegmental area, and midbrain of high-ethanol-consuming rats compared to water-consuming rats (Table 2).
Table 2.
Opioid receptor-mediated [35S]GTPγS binding by TAN67, SNC80, and DAMGO in brain regions of P154 rats consuming either intermittent 20% ethanol or water only for long periods from age P56
| Brain region | Mean (±SEM) EC50 values (μm) for opioid-mediated [35S]GTPγS stimulation |
|||||
|---|---|---|---|---|---|---|
| TAN67 |
SNC80 |
DAMGO |
||||
| 20% ethanol | Water only | 20% ethanol | Water only | 20% ethanol | Water only | |
| Dorsal striatum | 0.03 ± 0.01 | >10 | 18 ± 2.6 | >10 | 0.84 ± 0.02*** | 0.37 ± 0.02 |
| Nucleus accumbens | >10 | >10 | >10 | >10 | 0.32 ± 0.02 | 0.32 ± 0.03 |
| Spinal cord | 3.8 ± 0.4* | 5.0 ± 0.4 | >10 | >10 | 0.56 ± 0.04*** | 0.30 ± 0.02 |
| Cerebral cortex | 3.7 ± 0.6 | 3.5 ± 0.8 | 1.3 ± 0.7 | 2.3 ± 0.8 | 3.0 ± 0.4*** | 0.50 ± 0.08 |
| Brainstem | 5.5 ± 0.3 | 5.9 ± 0.2 | >10 | >10 | 0.07 ± 0.004*** | 0.05 ± 0.002 |
| Midbraina | >10 | >10 | >10 | >10 | 1.4 ± 0.5 | 1.8 ± 0.4 |
| Ventral tegmental area | >10 | >10 | >10 | >10 | 0.28 ± 0.03 | 0.28 ± 0.02 |
| Amygdala | >10 | >10 | >10 | >10 | >10 | 1.4 ± 0.3 |
| Cerebellum | >10 | >10 | >10 | >10 | 0.32 ± 0.08 | 0.18 ± 0.09 |
| Hippocampus | >10 | >10 | >10 | >10 | >10 | >10 |
Data were analyzed using Student's t test (n = 3 per group, each in triplicate).
*p < 0.05;
***p < 0.001, compared water consuming rats.
aRemaining midbrain tissue after removal of VTA and amygdala.
Inhibition of DOP-Rs in the dorsal striatum selectively reduces high ethanol consumption
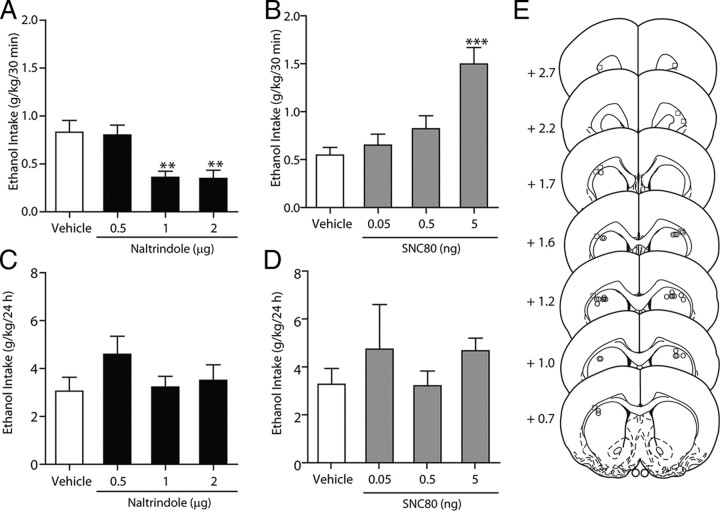
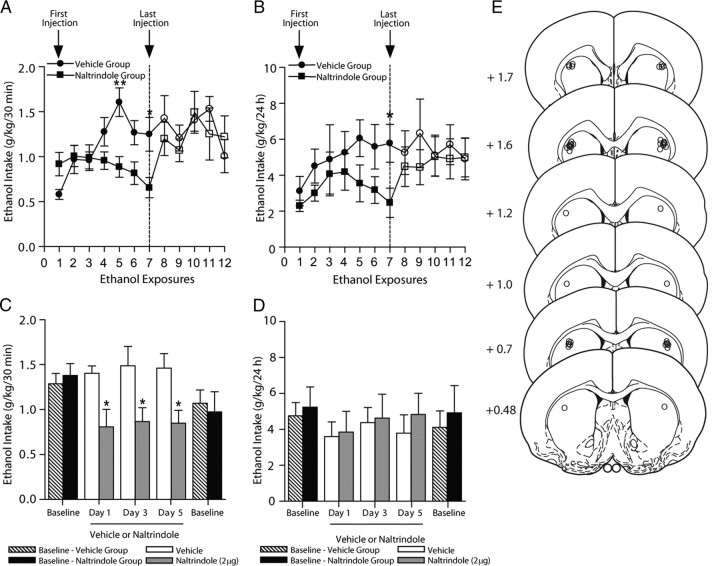
The effects of naltrindole given via microinfusions directly into the dorsal striatum were examined in ethanol-consuming rats using the intermittent-access voluntary 20% ethanol two-bottle choice drinking procedure. In rats that were maintained on a stable level of ethanol consumption for at least 6 weeks (18 drinking sessions), intrastriatal naltrindole reduced ethanol consumption (Fig. 4A, cannula placements; Fig. 4E). There was an overall main effect on ethanol consumption after 30 min of ethanol access (F(3,55) = 6.54; p < 0.01). Post hoc analysis revealed that in intrastriatal naltrindole reduced ethanol consumption at doses of 1 μg (p < 0.01) and 2 μg (p < 0.05), compared to vehicle (PBS). Following 24 h of ethanol access, intrastriatal naltrindole did not produce any effects on ethanol consumption (p > 0.05; Fig. 4C). Further post hoc analysis revealed no effect of any doses of naltrindole on ethanol consumption after 24 h. There was no overall effect on water consumption by naltrindole treatment after 30 min (p > 0.05) or 24 h (p > 0.05) of access compared to vehicle-treated rats and no overall effect on preference for ethanol by naltrindole treatment after 30 min (p > 0.05) or 24 h (p > 0.05; Table 3). There was no overall effect on rat body weight with naltrindole treatment (p > 0.05). In rats with cannulae implanted in a separate brain region adjacent to the dorsal striatum, the external capsule (Fig. 4E, cannula placements), the microinjection of naltrindole did not produce any effects on consumption of 20% ethanol after 30 min (p > 0.05) or 24 h (p > 0.05) of access compared to vehicle-treated rats (data not shown).
Figure 4.
Inhibition of DOP-Rs in the dorsal striatum selectively reduces high ethanol consumption. A, C, Intrastriatal administration of naltrindole selectively reduces ethanol intake in high-ethanol-consuming rats using the intermittent-access voluntary 20% ethanol two-bottle choice drinking procedure after 30 min (A) but not 24 h (C) of access. B, D, Intrastriatal administration of SNC80 increases ethanol intake in ethanol consuming rats using the intermittent-access voluntary 20% ethanol two-bottle choice drinking procedure after 30 min (B) but not 24 h (D) of access. The values are expressed as the mean ± SEM ethanol consumed (grams per kilogram per 30 min and 24 h). E, Schematic representations of the injection cannulae placements in coronal sections of the dorsal striatum of rats included in the data analysis (circles; n = 14) and cannulae placements in coronal sections of a separate brain region adjacent to the dorsal striatum, the external capsule, of rats not included in data analyses (squares; n = 6) [adapted from Paxinos and Watson (1997)]. Brain slices are 75 μm thick. Numbers indicate the distance anterior to the bregma in millimeters. **p < 0.01; ***p < 0.001 (compared to vehicle; one-way ANOVA with repeated measures; with the Newman–Keuls post hoc analysis). n = 14.
Table 3.
Water consumption and preference for ethanol in ethanol-consuming rats given intermittent access to 20% ethanol and water for 30 min and 24 h following intrastriatal administration of naltrindole and SNC80
| Drug treatment | Dose | Water consumption (mean ± SEM, in ml) |
Preference for ethanol (mean ± SEM, % preference) |
||
|---|---|---|---|---|---|
| 30 min | 24 h | 30 min | 24 h | ||
| Naltrindole (μg) | Vehicle | 2.8 ± 0.6 | 27.2 ± 1.9 | 35.5 ± 8.2 | 23.5 ± 4.0 |
| 0.5 | 2.6 ± 0.6 | 23.9 ± 2.6 | 32.4 ± 7.5 | 34.5 ± 5.2 | |
| 1 | 2.6 ± 0.5 | 24.9 ± 2.2 | 26.5 ± 7.3 | 28.6 ± 4.8 | |
| 2 | 2.4 ± 0.5 | 25.3 ± 2.5 | 23.7 ± 6.8 | 29.2 ± 5.7 | |
| SNC80 (ng) | Vehicle | 3.7 ± 0.7 | 24.1 ± 2.5 | 34.7 ± 5.4 | 32.0 ± 6.4 |
| 0.05 | 4.5 ± 0.7 | 26.5 ± 2.4 | 31.9 ± 5.8 | 29.3 ± 6.3 | |
| 0.5 | 4.6 ± 0.6 | 27.1 ± 2.3 | 25.3 ± 5.1 | 26.6 ± 5.2 | |
| 5 | 5.5 ± 0.8 | 29.4 ± 1.9* | 47.3 ± 4.2 | 32.9 ± 3.5 | |
Data were analyzed using one-way ANOVA with Newman–Keuls post hoc analysis (n = 14 per group).
*p < 0.05 (compared to vehicle-treated rats).
Activation of the DOP-Rs in the dorsal striatum increases ethanol consumption
The effects of SNC80 given via infusions directly into the dorsal striatum were also examined in ethanol-consuming rats using the intermittent-access voluntary 20% ethanol two-bottle choice drinking procedure. In the same group of rats that were maintained on a stable level of ethanol consumption for at least 6 weeks (>18 drinking sessions), SNC80 increased ethanol consumption (Fig. 4B). There was an overall main effect on ethanol consumption after 30 min of ethanol access (F(3,55) = 17.64; p < 0.00001). Post hoc analysis revealed that intrastriatal SNC80 (5 ng) increased ethanol consumption compared to vehicle (PBS; p < 0.001). Following 24 h of ethanol access, intrastriatal SNC80 did not produced any effects on ethanol consumption (p > 0.05; Fig. 4D), with post hoc analysis revealing no effect of any doses of SNC80 on ethanol consumption. The levels of ethanol consumption in vehicle-treated rats in the SNC80 treatment group (Fig. 4A,C) were not different (p > 0.05) from the levels of ethanol consumption in vehicle-treated rats in the naltrindole treatment group after either 30 min or 24 h of access to ethanol (Fig. 4B,D). There was no overall effect on water consumption by SNC80 after 30 min of access compared with vehicle-treated rats (p > 0.05), although there was an overall effect on water consumption by SNC80 after 24 h of access (F(3,55) = 3.11, p < 0.05; Table 3). Post hoc analysis revealed that intrastriatal SNC80 (5 ng) increased water consumption after 24 h of access to 20% ethanol (p < 0.05; Table 3). There was no overall effect, albeit a strong trend, on preference for ethanol by SNC80 after 30 min of access (F(3,55) = 2.61; p = 0.065), and no overall effect after 24 h of access (p > 0.05; Table 3). There was no overall effect on rat body weight with SNC80 treatment (p > 0.05).
Microinfusions of naltrindole into the dorsal striatum prevents the escalation of high ethanol consumption
Using the intermittent access to 20% ethanol procedure, we examined the effect of microinfusion of naltrindole into the dorsal striatum on ethanol intake. Naltrindole (2 μg) was infused into the dorsal striatum before the first ethanol exposure and then before ethanol access for the next six ethanol exposures. In rats administered naltrindole before the first ethanol exposure, the initial drinking levels in rats following the first ethanol exposure were not significantly different from vehicle-treated rats (p > 0.05; Fig. 5A,B). Naltrindole significantly reduced ethanol intake at ethanol exposures 5 and 7 compared to vehicle-treated rats (Fig. 5A,B, cannulae placements; Fig. 5E). After 30 min of access, there was an overall effect on ethanol consumption by naltrindole with treatment (F(1,91) = 11.05; p < 0.01), time (F(1,91) = 2.74; p < 0.05), and interaction of treatment by time (F(1,91) = 4.19; p < 0.001). Post hoc analyses showed reduced ethanol intake at ethanol exposures 5 (p < 0.01) and 7 (p < 0.05; Fig. 5A). After 30 min of access, there was an overall effect on preference for ethanol (F(1,91) = 9.57; p < 0.01) but no effect on water consumption (p > 0.05). After 24 h of access, there was an overall effect on ethanol consumption by naltrindole with treatment (F(1,91) = 10.05; p < 0.05), and post hoc analyses showed reduced ethanol intake at ethanol exposure 7 (p < 0.05; Fig. 5B). After 24 h of access, there was an overall effect of preference for ethanol (F(1,91) = 6.20; p < 0.05) but no effect on water consumption (p > 0.05). When the naltrindole treatment was terminated, the reduced levels of ethanol consumption following 30 min and 24 h of access were not maintained compared to post-vehicle treatment baseline drinking levels (Fig. 5A,B). There were no overall effects on ethanol consumption for the next five ethanol exposures between the vehicle-treated and naltrindole-treated groups following cessation of the microinfusions after 30 min (p > 0.05) or 24 h (p > 0.05) of access to ethanol. Similarly, there were no overall effects during the postinfusion period for five ethanol exposures on preference for ethanol after 30 min (p > 0.05) or 24 h (p > 0.05) of access, and also no overall effects on water consumption after 30 min (p > 0.05) or 24 h (p > 0.05) of access. There was no overall effect between treatment groups on rat body weight (p > 0.05). Following cessation of naltrindole administration (exposure 8), ethanol intake after 30 min of access was higher compared to ethanol intake levels after the last ethanol exposure during naltrindole treatment (exposure 7; p < 0.05) and was not different from ethanol intake levels of rats after the first ethanol exposure following cessation of vehicle treatment (exposure 8; p > 0.05). Following cessation of vehicle administration (exposure 8), ethanol intake after 30 min of access was not different compared to ethanol intake levels after the last ethanol exposure during vehicle treatment (exposure 7; p > 0.05). Following cessation of naltrindole administration (exposure 8), ethanol intake after 24 h of access was not different compared to ethanol intake levels after the last ethanol exposure during naltrindole treatment (exposure 7; p > 0.05), and not different from ethanol intake levels of post-vehicle-treated rats (exposure 8; p > 0.05). Following cessation of vehicle administration (exposure 8), ethanol intake after 24 h of access was not different compared to ethanol intake levels after the last ethanol exposure during vehicle treatment (exposure 7; p > 0.05).
Figure 5.
Repeated microinfusions of naltrindole into the dorsal striatum prevents the escalation of high ethanol consumption. Rats were microinfused with naltrindole (2 μg; n = 8; closed squares) or vehicle (n = 7; closed circles) into the dorsal striatum, 15 min before the drinking session on the ethanol days for seven ethanol exposures using the intermittent access to 20% ethanol procedure starting on the day of the first ethanol exposure. A, B, Naltrindole significantly reduced ethanol consumption after 30 min (drinking sessions 5 and 7; A) and 24 h (drinking session 7; B) of ethanol access. The administration of either naltrindole or vehicle was terminated after seven ethanol exposures. Following cessation of microinfusions, ethanol consumption in the naltrindole-treated group (open squares) was not different from that of the vehicle-treated group (open circles) for the next five ethanol exposures after 30 min (A) or 24 h (B) of ethanol access. C, D, Rats maintained on high levels of ethanol intake were microinfused with naltrindole (2 μg; n = 7; gray bars) or vehicle (n = 5; white bars) into the dorsal striatum 15 min before the drinking session on the ethanol days for three ethanol exposures using the intermittent access to 20% ethanol procedure. Repeated intrastriatal administration of naltrindole (gray bars) selectively reduces ethanol intake compared to vehicle-treated rats (white bars) over three ethanol exposures in high-ethanol-consuming rats using the intermittent-access voluntary 20% ethanol two-bottle choice drinking procedure after 30 min (C) but not 24 h (D) of access. The administration of either naltrindole or vehicle was terminated after three ethanol exposures. Following cessation of microinfusions, ethanol consumption in the naltrindole-treated group (black bars) was not different from that in the vehicle-treated group (striped bars) after 30 min (C) or 24 h (D) of ethanol access. The values are expressed as the mean ± SEM ethanol consumed (grams per kilogram per 30 min and 24 h). *p < 0.05; **p < 0.01 (comparisons between treatment groups; two-way ANOVA with Bonferroni's post hoc test). E, Schematic representations of the injection cannulae placements in coronal sections of the dorsal striatum of rats included in the data analysis (circles) [adapted from Paxinos and Watson (1997)]. Brain slices are 75 μm thick. Numbers indicate the distance anterior to the bregma in millimeters.
Repeated microinfusions of naltrindole into the dorsal striatum of high drinking rats reduces ethanol consumption
Microinfusions of naltrindole (2 μg) into the dorsal striatum before ethanol exposure on days 1, 3, and 5 reduced ethanol intake compared to vehicle-treated rats (Fig. 5C, cannula placements; Fig. 5E). After 30 min of access, there was an overall effect of naltrindole on ethanol consumption by naltrindole on each of the three ethanol exposure (F(1,30) = 19.38; p < 0.0001). Post hoc analyses showed reduced ethanol intake at ethanol exposures 1–3 (p < 0.05; Fig. 5C). After 30 min of access, there was no overall effect on preference for ethanol (p > 0.05) or on water consumption (p > 0.05). After 24 h of access, there was no overall effect on ethanol consumption by naltrindole with treatment (p > 0.05), preference for ethanol (p > 0.05), or water intake (p > 0.05) (Fig. 5D). There was no overall effect between treatment groups on rat body weight (p > 0.05). Baseline drinking levels of the groups of rats before the microinfusions of either naltrindole or vehicle were not different (p > 0.05; Fig. 5C,D). When the naltrindole treatment was terminated, the levels of ethanol consumption after 30 min and 24 h of access were not different compared to ethanol intake levels after the last ethanol exposure during naltrindole treatment (exposure 3; p > 0.05) and also not different from post-vehicle treatment baseline drinking levels (p > 0.05; Fig. 5C,D).
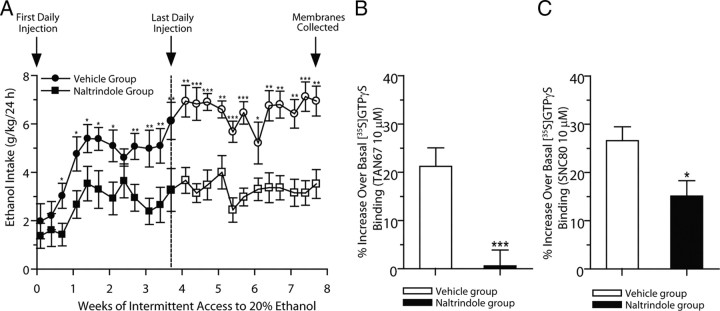
Long-term systemic treatment with naltrindole reduces escalation of ethanol consumption and induces a long-lasting reduction in ethanol consumption when the drug is no longer present
Administration of naltrindole (3 mg/kg, i.p.) for 28 d significantly reduced the escalation and maintenance of ethanol intake compared to vehicle in high-ethanol-consuming rats using the intermittent-access 20% ethanol two-bottle choice drinking procedure. Initial drinking levels in rats administered naltrindole following the first ethanol exposure were not significantly different from those of vehicle-treated rats (p > 0.05; Fig. 6A). Naltrindole reduced the escalation of ethanol intake and over time continued to significantly reduce ethanol intake for up to 4 weeks compared to the vehicle-treated group. There was an overall effect on ethanol consumption with treatment (F(1,288) = 213.7; p < 0.001), time (F(23,288) = 8.78; p < 0.001), and treatment by time interaction (F(23,288) = 1.92; p < 0.01). Post hoc analyses showed reduced ethanol intake at ethanol exposures 3–7 (p < 0.05) and ethanol exposures 9–12 (p < 0.01; Fig. 6A). When the naltrindole treatment was terminated after 28 d (12 ethanol exposures), the reduced drinking levels were maintained compared to post-vehicle treatment baseline drinking levels (exposures 13, 16, 20–22, and 24, p < 0.01; exposures 14, 15, 17, 18, and 23, p < 0.001; Fig. 6A). There was an overall effect on preference for ethanol with treatment (F(1,288) = 148.6; p < 0.0001), time (F(23,288) = 21.90; p < 0.0001), and treatment by time interaction (F(23,288) = 2.74; p < 0.0001). On the ethanol exposure days, water consumption was higher in the naltrindole group with treatment (F(1,288) = 127.2; p < 0.0001) and time (F(23,288) = 8.14; p < 0.0001), but not for the treatment by time interaction (p > 0.05). However, there was no effect with treatment on total fluid consumption (i.e., ethanol and water; p > 0.05) and no effect on water intake on alternate water-only days (p > 0.05). There was also no overall effect of treatment on rat body weight (p > 0.05). Following cessation of naltrindole administration (exposure 13), ethanol intake was not different following subsequent ethanol exposures, compared to the last ethanol exposure during naltrindole treatment (exposure 12; p > 0.05; Fig. 6A). Cessation of naltrindole treatment did not result in a rapid escalation of ethanol consumption, compared to the profiles of initial escalations in drinking observed in untreated or vehicle-treated rats. Naltrindole treatment reduced drinking for up to 28 d after the treatment period was terminated. Therefore, naltrindole treatment did not cause any significant rebound increase in drinking after the treatment period was terminated.
Figure 6.
Long-term systemic treatment with naltrindole reduces escalation of ethanol intake and induces a long-lasting reduction in ethanol intake and DOP-R-mediated [35S]GTPγS binding when the drug is no longer present. A, Rats were administered either naltrindole (3 mg/kg, i.p.; n = 7; closed squares) or vehicle (n = 7; closed circles) on each of 28 consecutive days, 30 min before the drinking session. Naltrindole significantly reduced ethanol consumption after 24 h of ethanol access at each drinking session after the exposure to 20% ethanol. The administration of either naltrindole or vehicle was terminated after 28 d (12 ethanol exposures). Ethanol consumption in the naltrindole-treated (open squares) but not the vehicle-treated group (open circles) remained lower for an additional 28 d. The values are expressed as the mean ± SEM ethanol consumed (grams per kilogram per 24 h). *p < 0.05; **p < 0.01; *** p < 0.001 (two-way ANOVA with Student's t test, comparisons between treatment groups). B, C, Membrane samples collected from rats at 28 d after the last daily administration of naltrindole or vehicle were analyzed for DOP-R [35S]GTPγS stimulation in the dorsal striatum. DOP-R-mediated dorsal striatal [35S]GTPγS binding using TAN67 (B) and SNC80 (C) in naltrindole-pretreated rats was reduced compared to high DOP-R [35S]GTPγS stimulation in vehicle-pretreated rats. The values are expressed as the mean ± SEM percentage increase over basal [35S]GTPγS binding by TAN67 and SNC80 (10 μm; n = 3, each in triplicate). *p < 0.05; **p < 0.01 (Student's t test, comparisons between treatment groups).
DOP-R-stimulated [35S]GTPγS binding in the dorsal striatum is reduced in rats pretreated with naltrindole
In rat brain membranes collected 28 d after the last multiple administration of naltrindole or vehicle treatment, DOP-R [35S]GTPγS stimulation by TAN67 in the dorsal striatum was higher in vehicle-pretreated rats in efficacy (p < 0.001) and potency (EC50, 36 ± 4.3 nm) compared to naltrindole-pretreated rats (EC50, >10 μm; Fig. 6B). Similarly, DOP-R [35S]GTPγS stimulation by SNC80 in the dorsal striatum was higher in vehicle-pretreated rats in efficacy (p < 0.05) and potency (EC50, 23 ± 3.2 nm) compared to naltrindole-pretreated rats (EC50, 857 ± 61 nm; p < 0.001; Fig. 6C).
Naltrindole does not affect blood ethanol concentrations
BECs in response to the challenge dose of ethanol (1.5 g/kg, i.p.) did not differ between rats pretreated with naltrindole (3 mg/kg, i.p.) or vehicle only (distilled water, 1 ml/kg, i.p.) at each time point (p > 0.05; Table 4).
Table 4.
Lack of effect of naltrindole on rat blood ethanol concentrations
| Time after ethanol injection (h) | Blood ethanol concentration (mean ± SEM, mg/dl) |
|
|---|---|---|
| Vehicle (n = 8) | Naltrindole (3 mg/kg; n = 9) | |
| 0.5 | 214 ± 28.2 | 164 ± 7.9 |
| 1 | 160 ± 12.3 | 151 ± 14.6 |
| 2 | 158 ± 10.7 | 144 ± 14.1 |
| 4 | 54.0 ± 5.9 | 58.6 ± 11.6 |
Rats were administered naltrindole (3 mg/kg, i.p.) or vehicle (distilled water, 1 ml/kg, i.p.) 30 min before ethanol (1.5 g/kg, i.p.). Blood was collected from the lateral tail vein over a 4 h period after the ethanol dosing period and ethanol was quantified using the alcohol dehydrogenase assay.
Discussion
We show that the DOP-R in the dorsal striatum plays a role in ethanol consumption. Our findings demonstrate that DOP-R activity in the dorsal striatum and DOP-R-mediated analgesia are highest in young adult rats and decrease with age. Intermittent, high ethanol consumption prevents the developmental decrease in DOP-R function, an effect that is maintained into late adulthood. Furthermore, we show that intermittent high ethanol consumption, but not continuous ethanol or water consumption, from young adulthood results in an increase in DOP-R activity in the dorsal striatum. Previous studies showed that long-term ethanol-consuming rats have increased striatal DOP-R binding (Lucchi et al., 1984, 1985), although other studies show no changes (Turchan et al., 1999; Saland et al., 2004). This suggests that long-term intermittent high ethanol consumption leads to changes in DOP-R activity. In contrast, we show that MOP-R activity in the dorsal striatum is increased with age but is reduced in the dorsal striatum and other brain regions with long-term high ethanol consumption, as reported previously (Lucchi et al., 1984, 1985; Saland et al., 2005).
Developmental decrease in DOP-R activity
Our previous studies showed that DOP-R agonist (SNC80)-mediated [35S]GTPγS stimulation was potently inhibited by naltrindole (IC50, 31 ± 3.9 nmol/L), but not by naltrexone (IC50 > 10 μmol/L), in striatal membranes of long-term high-ethanol-consuming rats (Nielsen et al., 2008), indicating that both naltrindole and SNC80 are activating the DOP-R. DOP-R activity in the dorsal striatum and DOP-R-mediated analgesia is highest in young adulthood and decreases with age. The developmental decrease in TAN67-mediated analgesia is comparable to age-related reductions in spinally administered DPDPE-mediated analgesia (Crisp et al., 1994a,b; Rahman and Dickenson, 1999). Although binding studies revealed no age-related changes in receptor density or affinity in the spinal cord (Hoskins et al., 1998; Rahman et al., 1998), our studies show a developmental decrease in DOP-R activity in the dorsal striatum using the [35S]GTPγS assay, suggesting that G-protein coupling may be developmentally decreased. Studies in adult rats show relatively low DOP-R-mediated [35S]GTPγS binding in the spinal cord (Cichewicz et al., 2004; Narita et al., 2007), caudate nucleus, and nucleus accumbens (Saland et al., 2004), consistent with our findings of low DOP-R coupling in the dorsal striatum of aged rats.
Maintained DOP-R-mediated analgesia with high ethanol consumption
We also show that high and intermittent ethanol consumption from young adulthood maintains DOP-R-mediated thermal analgesia into late adulthood. This increase in DOP-R-mediated analgesia does not appear to be due to relief of any hyperalgesia in ethanol-consuming rats (Gatch and Lal, 1999). Although the DOP-R reportedly mediates mechanical over thermal analgesia in mice (Scherrer et al., 2009), our studies in rats show that long-term, high ethanol consumption maintains functional DOP-Rs to alleviate thermal nociception. However, DOP-R agonists reportedly have higher analgesic activity in states of disrupted physiological conditions (Kamei et al., 1994, 1997). Stressors, morphine, and ethanol administration change the distribution of DOP-R from the cytoplasmic compartment to the plasma membrane and increase DOP-R activity (Commons, 2003; Méndez et al., 2004, 2008; Hack et al., 2005; Méndez and Morales-Mulia, 2006). As it is difficult to directly demonstrate the redistribution of DOP-R using immunohistochemistry due to nonspecific activity of DOP-R antibodies (Scherrer et al., 2009), we measured the ex vivo activity of the DOP-R using [35S]GTPγS binding that directly measures coupling to G-proteins in membranes prepared from rat brains.
There have been studies demonstrating that there are links between ethanol consumption and analgesia. Ethanol stimulates the activity of the endogenous opioids, β-endorphins and enkephalins, which target opioid receptors to increase basal dopamine release in the mesolimbic pathway (Herz, 1997). Central administrations of these endogenous opioids also produce opioid-receptor-mediated thermal analgesia (Takemori and Portoghese, 1993; Tseng et al., 1995; Chen et al., 2007b). DOP-R-mediated analgesia in high-ethanol-consuming rats may be due to the modest increase in DOP-R activity in the spinal cord of ethanol-consuming rats, but may also be related to DOP-R activity in the dorsal striatum, as nigrostriatal structures are shown to play a role in nociception. Striatal dopamine is reportedly critical for mechanisms of opioid-mediated antinociception (Sawynok and Reid, 1987). Injection of 6-hydroxydopamine into the caudate–putamen inhibits morphine-mediated thermal analgesia (Nakamura et al., 1973), and administrations of morphine into the rat globus pallidus produce thermal analgesia.
Role of the DOP-R in the dorsal striatum with ethanol consumption
Rat brain ethanol concentrations and ethanol retention times are greater in the rat striatum following systemic ethanol administration (Chen et al., 2007a), which may contribute to higher DOP-R activity in the striatum. As the dorsal striatum is implicated to contain major components of homeostatic pathways controlling ethanol consumption (Wang et al., 2007; Logrip et al., 2008), our studies suggest that DOP-R inhibition could modulate ethanol reward pathways in the dorsal striatum. Furthermore, naltrindole does not affect the clearance of ethanol, suggesting a central, rather than a peripheral or metabolic, effect of DOP-R inhibition on ethanol intake. The changes in ethanol consumption after short but not long access periods by intrastriatal administration of naltrindole and SNC80 in high-ethanol-consuming rats suggest that these effects are temporary. The reductions in ethanol consumption by repeated intrastriatal naltrindole infusions in ethanol-consuming rats are not maintained following cessation of treatment. In comparison, multiple systemic administrations of naltrindole starting from the day of the first ethanol exposure reduce ethanol consumption for 28 d, and following cessation of treatment, there is no escalation in ethanol consumption for another 28 d, suggesting long-lasting reductions on ethanol consumption by systemic naltrindole. Furthermore, rats systemically pretreated with naltrindole had attenuated DOP-R-mediated coupling in the dorsal striatum measured at 28 d after the last daily injection. Together, these results suggest that inhibiting DOP-Rs during the initiation of ethanol consumption in young adulthood, when DOP-R activity is high, produces longer-lasting reductions in ethanol consumption and DOP-R activity in the dorsal striatum. However, the long-lasting reductions in ethanol consumption following cessation of multiple systemic, but not intrastriatal, administrations of naltrindole suggest that additional brain regions may be involved in these long-lasting effects of chronic systemic naltrindole, which remains to be investigated.
The DOP-R has been reported to play roles in anxiety and emotional states (Filliol et al., 2000; Saitoh et al., 2004, 2005; Perrine et al., 2006). Naltrindole (3–5 mg/kg, s.c.) produces anxiogenic activity in rats (Saitoh et al., 2004, 2005; Perrine et al., 2006). Furthermore, pretreatment with SNC80 antagonized the anxiogenic effects of naltrindole (Saitoh et al., 2005), and conversely, naltrindole blocked the anxiolytic effects of SNC80 (Saitoh et al., 2004; Perrine et al., 2006) in rats. We cannot rule out that changes in DOP-R activity by ethanol consumption may lead to changes in anxiogenic activity in rats that may then contribute to high ethanol consumption, which remains to be investigated.
Disparities in results of studies on the role of DOP-Rs in ethanol consumption and seeking may be attributed to brain-region-specific differences in DOP-R activity. Our data show that both systemic and intrastriatal administration of naltrindole reduce ethanol intake comparable to reduced ethanol consumption and seeking resulting from DOP-R antagonists given systemically (Krishnan-Sarin et al., 1995a) or into the nucleus accumbens (Hyytiä and Kiianmaa, 2001). Similarly, our data show that intrastriatal administration of SNC80 increases ethanol intake, comparable to increased ethanol intake following DOP-R agonists or enkephalinase inhibitors given systemically (Froehlich et al., 1991; van Rijn et al., 2010) or into the nucleus accumbens (Barson et al., 2009). Previous studies have shown that these disparities relate to subtypes of the DOP-R (DOP-R1 and DOP-R2) that have differential roles on ethanol consumption and seeking (van Rijn and Whistler, 2009). As TAN67 is more selective for DOP-R1 (Kamei et al., 1995; Tseng et al., 1997) than SNC80 (Baker and Meert, 2002; Pacheco et al., 2005; Rawls et al., 2005; Saitoh et al., 2005), The reduced TAN67-mediated, but not SNC80-mediated, DOP-R activity at P84 in ethanol-naive rats may reflect reduced DOP-R1 over DOP-R2 activity at this age.
As the intermittent ethanol consumption procedure involves day-on/day-off access to ethanol compared to the continuous-access ethanol procedure, we hypothesize that intermittent access to ethanol leads to an increase in DOP-R activity in the dorsal striatum. Compared to rats given continuous access to ethanol, rats given intermittent access to ethanol consumed greater daily amounts of ethanol on ethanol exposure days but consumed lower cumulative amounts of ethanol. This suggests that the pattern of drinking using the intermittent ethanol consumption procedure, rather than total amount of ethanol consumed, is important for the increase in DOP-R activity in the dorsal striatum.
We have shown that high intermittent ethanol intake in young adulthood increases DOP-R activity into late adulthood and that DOP-R activity in the dorsal striatum plays a role in high ethanol consumption. Together, this suggests that the modulating activity at the DOP-R may be useful to treat young adult drinkers to prevent AUDs later in adult life.
Footnotes
This work was supported by funding from the State of California for medical research through the University of California, San Francisco (S.E.B) and Department of Defense Grant W81XWH-07-1-0075 (S.E.B). S.E.B. and C.K.N. were supported in part by the Sidney Baer Trust and the Essel Foundation as National Alliance for Research on Schizophrenia and Depression Young Investigators. We thank Joan Holgate, Tiffany Ho, Brian Medina, and Haley Pierson for excellent technical support. The experiments contained herein comply with the current laws of the United States of America. All procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the humane care and use of laboratory animals.
The authors declare no competing financial interests.
References
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther. 2002;302:1253–1264. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Leibowitz SF, Hoebel BG. Opioids in the nucleus accumbens stimulate ethanol intake. Physiol Behav. 2009;98:453–459. doi: 10.1016/j.physbeh.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Kraus J, Loh HH, Schroeder H, Höllt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Calderon SN, Wang T, Bernstein RN, Davis P, Hruby VJ, McNutt RW, Rothman RB, Rice KC, Porreca F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: a cohort study over seven years. Addiction. 2004;99:1520–1528. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Borg PJ, Taylor DA. Involvement of mu- and delta-opioid receptors in the effects of systemic and locally perfused morphine on extracellular levels of dopamine, DOPAC and HVA in the nucleus accumbens of the halothane-anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:582–588. doi: 10.1007/pl00004987. [DOI] [PubMed] [Google Scholar]
- Brady LS, Holtzman SG. Analgesic effects of intraventricular morphine and enkephalins in nondependent and morphine-dependent rats. J Pharmacol Exp Ther. 1982;222:190–197. [PubMed] [Google Scholar]
- Calderon SN, Rice KC, Rothman RB, Porreca F, Flippen-Anderson JL, Kayakiri H, Xu H, Becketts K, Smith LE, Bilsky EJ, Davis P, Horvath R. Probes for narcotic receptor mediated phenomena. 23. Synthesis, opioid receptor binding, and bioassay of the highly selective delta agonist (+)-4-[(alpha R)-alpha-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]- N,N-diethylbenzamide (SNC 80) and related novel nonpeptide delta opioid receptor ligands. J Med Chem. 1997;40:695–704. doi: 10.1021/jm960319n. [DOI] [PubMed] [Google Scholar]
- Charness ME, Hu G, Edwards RH, Querimit LA. Ethanol increases delta-opioid receptor gene expression in neuronal cell lines. Mol Pharmacol. 1993;44:1119–1127. [PubMed] [Google Scholar]
- Chen JC, Lin CC, Ng CC, Chiu TF, Shyr MH. Uneven distribution of ethanol in rat brain following acute administration, with the highest level in the striatum. J Stud Alcohol Drugs. 2007a;68:649–653. doi: 10.15288/jsad.2007.68.649. [DOI] [PubMed] [Google Scholar]
- Chen W, Song B, Lao L, Pérez OA, Kim W, Marvizón JC. Comparing analgesia and mu-opioid receptor internalization produced by intrathecal enkephalin: requirement for peptidase inhibition. Neuropharmacology. 2007b;53:664–676. doi: 10.1016/j.neuropharm.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Cox ML, Welch SP, Selley DE, Sim-Selley LJ. Mu and delta opioid-stimulated [35S]GTP gamma S binding in brain and spinal cord of polyarthritic rats. Eur J Pharmacol. 2004;504:33–38. doi: 10.1016/j.ejphar.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Clapper RL, Lipsitt LP. Young heavy drinkers and their drinking experiences: predictors of later alcohol use. Int J Addict. 1992;27:1211–1221. doi: 10.3109/10826089209047345. [DOI] [PubMed] [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Hoskins DL, Dayal B, Chinrock KM, Uram M. Effects of aging on spinal opioid-induced antinociception. Neurobiol Aging. 1994a;15:169–174. doi: 10.1016/0197-4580(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Crisp T, Stafinsky JL, Hoskins DL, Perni VC, Uram M, Gordon TL. Age-related changes in the spinal antinociceptive effects of DAGO, DPDPE and beta-endorphin in the rat. Brain Res. 1994b;643:282–286. doi: 10.1016/0006-8993(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Wagner JE, Zerbe G, Macdonald M. Naltrexone-induced dysphoria in former opioid addicts. Am J Psychiatry. 1985;142:1081–1084. doi: 10.1176/ajp.142.9.1081. [DOI] [PubMed] [Google Scholar]
- D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–79. [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Franck J, Lindholm S, Raaschou P. Modulation of volitional ethanol intake in the rat by central delta-opioid receptors. Alcohol Clin Exp Res. 1998;22:1185–1189. [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Badia-Elder NE, Zink RW, McCullough DE, Portoghese PS. Contribution of the opioid system to alcohol aversion and alcohol drinking behavior. J Pharmacol Exp Ther. 1998;287:284–292. [PubMed] [Google Scholar]
- Gardell LR, Hubbell CL, Reid LD. Naltrexone persistently reduces rats' intake of a palatable alcoholic beverage. Alcohol Clin Exp Res. 1996;20:584–588. doi: 10.1111/j.1530-0277.1996.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Lal H. Effects of ethanol and ethanol withdrawal on nociception in rats. Alcohol Clin Exp Res. 1999;23:328–333. [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall FS, Sora I, Uhl GR. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Brotchie JM. Striatal delta opioid receptor binding in experimental models of Parkinson's disease and dyskinesia. Mov Disord. 2007;22:28–40. doi: 10.1002/mds.21163. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Johnson K, Boukhabza D, Gillespie HK. Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend. 1981;8:37–41. doi: 10.1016/0376-8716(81)90084-3. [DOI] [PubMed] [Google Scholar]
- Hoskins DL, Gordon TL, Crisp T. The effects of aging on mu and delta opioid receptors in the spinal cord of Fischer-344 rats. Brain Res. 1998;791:299–302. doi: 10.1016/s0006-8993(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Hyytiä P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Ingman K, Salvadori S, Lazarus L, Korpi ER, Honkanen A. Selective delta-opioid receptor antagonist N,N(CH3)2-Dmt-Tic-OH does not reduce ethanol intake in alcohol-preferring AA rats. Addict Biol. 2003;8:173–179. doi: 10.1080/1355621031000117400. [DOI] [PubMed] [Google Scholar]
- June HL, McCane SR, Zink RW, Portoghese PS, Li TK, Froehlich JC. The delta 2-opioid receptor antagonist naltriben reduces motivated responding for ethanol. Psychopharmacology (Berl) 1999;147:81–89. doi: 10.1007/s002130051145. [DOI] [PubMed] [Google Scholar]
- Kamei J, Iwamoto Y, Misawa M, Nagase H, Kasuya Y. Streptozotocin-induced diabetes selectively enhances antinociception mediated by delta 1- but not delta 2-opioid receptors. Life Sci. 1994;55:PL121–PL126. doi: 10.1016/0024-3205(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Kamei J, Saitoh A, Ohsawa M, Suzuki T, Misawa M, Nagase H, Kasuya Y. Antinociceptive effects of the selective non-peptidic delta-opioid receptor agonist TAN-67 in diabetic mice. Eur J Pharmacol. 1995;276:131–135. doi: 10.1016/0014-2999(95)00026-h. [DOI] [PubMed] [Google Scholar]
- Kamei J, Kawai K, Mizusuna A, Saitoh A, Morita K, Narita M, Tseng LF, Nagase H. Supraspinal delta 1-opioid receptor-mediated antinociceptive properties of (−)-TAN-67 in diabetic mice. Eur J Pharmacol. 1997;322:27–30. doi: 10.1016/s0014-2999(97)00085-x. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Jing SL, Kurtz DL, Zweifel M, Portoghese PS, Li TK, Froehlich JC. The delta opioid receptor antagonist naltrindole attenuates both alcohol and saccharin intake in rats selectively bred for alcohol preference. Psychopharmacology (Berl) 1995a;120:177–185. doi: 10.1007/BF02246191. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Portoghese PS, Li TK, Froehlich JC. The delta 2-opioid receptor antagonist naltriben selectively attenuates alcohol intake in rats bred for alcohol preference. Pharmacol Biochem Behav. 1995b;52:153–159. doi: 10.1016/0091-3057(95)00080-g. [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Ling W, Rawson RA, Compton M. Clinical treatment of opioid addiction and dependence. Methods Mol Med. 2003;84:285–295. doi: 10.1385/1-59259-379-8:285. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D. Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake. FASEB J. 2008;22:2393–2404. doi: 10.1096/fj.07-099135. [DOI] [PubMed] [Google Scholar]
- Lucchi L, Rius RA, Uzumaki H, Govoni S, Trabucchi M. Chronic ethanol changes opiate receptor function in rat striatum. Brain Res. 1984;293:368–371. doi: 10.1016/0006-8993(84)91245-9. [DOI] [PubMed] [Google Scholar]
- Lucchi L, Rius RA, Govoni S, Trabucchi M. Chronic ethanol induces changes in opiate receptor function and in met-enkephalin release. Alcohol. 1985;2:193–195. doi: 10.1016/0741-8329(85)90044-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Different roles of mu-, delta- and kappa-opioid receptors in ethanol-associated place preference in rats exposed to conditioned fear stress. Eur J Pharmacol. 1999a;368:9–16. doi: 10.1016/s0014-2999(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Roles of 5-HT3 and opioid receptors in the ethanol-induced place preference in rats exposed to conditioned fear stress. Life Sci. 1999b;64:PL241–PL249. doi: 10.1016/s0024-3205(99)00144-7. [DOI] [PubMed] [Google Scholar]
- Méndez M, Morales-Mulia M. Ethanol exposure differentially alters pro-enkephalin mRNA expression in regions of the mesocorticolimbic system. Psychopharmacology (Berl) 2006;189:117–124. doi: 10.1007/s00213-006-0503-3. [DOI] [PubMed] [Google Scholar]
- Méndez M, Morales-Mulia M, Leriche M. [3H]DPDPE binding to delta opioid receptors in the rat mesocorticolimbic and nigrostriatal pathways is transiently increased by acute ethanol administration. Brain Res. 2004;1028:180–190. doi: 10.1016/j.brainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Méndez M, Morales-Mulia M, Perez-Luna JM. Ethanol-induced changes in proenkephalin mRNA expression in the rat nigrostriatal pathway. J Mol Neurosci. 2008;34:225–234. doi: 10.1007/s12031-008-9039-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Morley JE, Levine AS, Hatsukami D, Gannon M, Pfohl D. High-dose naltrexone therapy and dietary counseling for obesity. Biol Psychiatry. 1987;22:35–42. doi: 10.1016/0006-3223(87)90127-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kuntzman R, Maggio A, Conney AH. Decrease in morphine's analgesic action and increase in its cataleptic action by 6-hydroxydopamine injected bilaterally into caudate and putamen areas; partial restoration by L-DOPA plus decarboxylase inhibition. Neuropharmacology. 1973;12:1153–1160. doi: 10.1016/0028-3908(73)90072-5. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyoshi K, Narita M, Suzuki T. Functional reduction in mu-opioidergic system in the spinal cord under a neuropathic pain-like state following chronic ethanol consumption in the rat. Neuroscience. 2007;144:777–782. doi: 10.1016/j.neuroscience.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Pierson HB, Li R, Saini SK, Ananthan S, Bartlett SE. A novel delta opioid receptor antagonist, SoRI-9409, produces a selective and long-lasting decrease in ethanol consumption in heavy-drinking rats. Biol Psychiatry. 2008;64:974–981. doi: 10.1016/j.biopsych.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, Bartlett SE. The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addict Biol. 2012;17:224–234. doi: 10.1111/j.1369-1600.2010.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Maidment NT. Opioid regulation of pallidal enkephalin release: bimodal effects of locally administered mu and delta opioid agonists in freely moving rats. J Pharmacol Exp Ther. 1998;285:1310–1316. [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini WH, O'Brien CP. Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addict Biol. 2006;11:397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- Pacheco DF, Reis GM, Francischi JN, Castro MS, Perez AC, Duarte ID. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci. 2005;78:54–60. doi: 10.1016/j.lfs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. European journal of pharmacology. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Rahman W, Dickenson AH. Electrophysiological studies on the postnatal development of the spinal antinociceptive effects of the delta opioid receptor agonist DPDPE in the rat. British J Pharmacol. 1999;126:1115–1122. doi: 10.1038/sj.bjp.0702418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W, Dashwood MR, Fitzgerald M, Aynsley-Green A, Dickenson AH. Postnatal development of multiple opioid receptors in the spinal cord and development of spinal morphine analgesia. Brain Res Dev Brain Res. 1998;108:239–254. doi: 10.1016/s0165-3806(98)00054-6. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Hewson JM, Inan S, Cowan A. Brain delta2 opioid receptors mediate SNC-80-evoked hypothermia in rats. Brain Res. 2005;1049:61–69. doi: 10.1016/j.brainres.2005.04.074. [DOI] [PubMed] [Google Scholar]
- Reid LD, Hunter GA. Morphine and naloxone modulate intake of ethanol. Alcohol. 1984;1:33–37. doi: 10.1016/0741-8329(84)90033-8. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain. 1997;73:151–157. doi: 10.1016/S0304-3959(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M, Valenzuela CF, Savage DD. Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcohol Clin Exp Res. 2004;28:98–104. doi: 10.1097/01.ALC.0000108658.00243.BF. [DOI] [PubMed] [Google Scholar]
- Saland LC, Hastings CM, Abeyta A, Chavez JB. Chronic ethanol modulates delta and mu-opioid receptor expression in rat CNS: immunohistochemical analysis with quantitiative confocal microscopy. Neurosci Lett. 2005;381:163–168. doi: 10.1016/j.neulet.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Effect of 6-hydroxydopamine-induced lesions to ascending and descending noradrenergic pathways on morphine analgesia. Brain Res. 1987;419:156–165. doi: 10.1016/0006-8993(87)90579-8. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O'Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tsuji M, Mori T, Misawa M, Endoh T, Nagase H. Effects of a highly selective nonpeptide delta opioid receptor agonist, TAN-67, on morphine-induced antinociception in mice. Life Sci. 1995;57:155–168. doi: 10.1016/0024-3205(95)00256-6. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Portoghese PS. Enkephalin antinociception in mice is mediated by delta 1- and delta 2-opioid receptors in the brain and spinal cord, respectively. Eur J Pharmacol. 1993;242:145–150. doi: 10.1016/0014-2999(93)90074-r. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Tsai JH, Collins KA, Portoghese PS. Spinal delta 2-, but not delta 1-, mu-, or kappa-opioid receptors are involved in the tail-flick inhibition induced by beta-endorphin from nucleus raphe obscurus in the pentobarbital-anesthetized rat. Eur J Pharmacol. 1995;277:251–256. doi: 10.1016/0014-2999(95)00084-x. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Narita M, Mizoguchi H, Kawai K, Mizusuna A, Kamei J, Suzuki T, Nagase H. Delta-1 opioid receptor-mediated antinociceptive properties of a nonpeptidic delta opioid receptor agonist, (-)TAN-67, in the mouse spinal cord. J Pharmacol Exp Ther. 1997;280:600–605. [PubMed] [Google Scholar]
- Turchan J, Przewłocka B, Toth G, Lason W, Borsodi A, Przewłocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SJ, Greene DL, Sharma SD, Yamamura HI, Kramer TH, Burks TF, Hruby VJ, Hersh LB, Davis TP. Distribution and analgesia of [3H][D-Pen2, D-Pen5]enkephalin and two halogenated analogs after intravenous administration. J Pharmacol Exp Ther. 1991;259:1109–1117. [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99:1529–1541. doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Winkler A, Búzás B, Siems WE, Heder G, Cox BM. Effect of ethanol drinking on the gene expression of opioid receptors, enkephalinase, and angiotensin-converting enzyme in two inbred mice strains. Alcohol Clin Exp Res. 1998;22:1262–1271. [PubMed] [Google Scholar]