Abstract
Repulsive Eph forward signaling from limb-derived ephrins guides the axons of lateral motor column (LMC) motor neurons. LMC axons also express ephrinAs, while their EphA receptors are expressed in the limb mesenchyme. In vitro studies have suggested that reverse signaling from limb-derived EphA4 to axonal ephrinAs might result in attraction of LMC axons. However, genetic evidence for this function is lacking. Here we use the Dunn chamber turning assay to show that EphA proteins are chemoattractants and elicit fast turning responses in LMC neurons in vitro. Moreover, ectopic expression of EphA4 in chick hindlimb changes the limb trajectory of LMC axons. Nervous system-specific deletion of EphA4 in mice resulted in fewer LMC axon projection errors than the ubiquitous deletion of EphA4. Additionally, a signaling-incompetent EphA4 mutant partially rescued guidance errors in the hindlimb, suggesting that limb-derived EphA4 contributes to the establishment of LMC projections. In summary, we provide evidence for a role of EphA:ephrinA attractive reverse signaling in motor axon guidance and in vivo evidence of in-parallel forward Eph and reverse ephrin signaling function in the same neuronal population.
Introduction
The establishment of a precise map of neuronal connections during development depends on the navigational decisions made by growing axons with the help of guidance cues in their environment (Dickson, 2002). Among the prominent molecular players implicated in axonal pathfinding are Eph receptor tyrosine kinases and their ephrin ligands, whose interactions can lead to bidirectional signaling. Activation of Eph receptors results in “forward” signaling events within the Eph-expressing cell, while membrane-attached ephrin ligands trigger “reverse” signaling responses within the ephrin-expressing cell (Egea and Klein, 2007). Eph and ephrin coexpression on the same neurons as well as in vitro experiments suggest that forward and reverse signaling can function in parallel and direct axonal pathway selection. However, a genetic in vivo test of such dual function remains elusive.
Motor axon projections to the hindlimb represent a convenient and well established system to study ephrin:Eph signaling. Limb-innervating motor neurons are situated in the spinal cord within the lateral motor column (LMC). The laterally positioned (LMCL) neurons innervate dorsal limb muscles, while the medially positioned (LMCM) neurons innervate ventral limb muscles (Landmesser, 1978). The canonical model of LMC axon guidance involves EphA4-expressing LMCL axons being repelled from ephrinAs in the ventral limb. Deletion of EphA4 in mice leads to ventral rerouting of LMCL axons (Helmbacher et al., 2000), a phenotype attributed to the lack of repulsion from ephrinAs. Conversely, ectopic expression of EphA4 in chick LMCM causes misprojections of LMCM axons into the dorsal nerve, confirming that EphA4 forward signaling in motor axons is important for the dorsal trajectory selection (Eberhart et al., 2002; Kania and Jessell, 2003).
In addition to its expression in LMCL axons, EphA4 is present in dorsal limb mesenchyme, while ephrinAs are also detected in LMC neurons (Iwamasa et al., 1999; Eberhart et al., 2000; Marquardt et al., 2005). In vitro, EphAs and ephrinAs on LMCL axons do not interact in cis, and can signal independently (Marquardt et al., 2005). Since ephrinA reverse signaling leads to increased axon growth (Marquardt et al., 2005; Kao and Kania, 2011), the presence of EphA4 in the limb mesenchyme suggests that attractive reverse signaling from EphA4 in the dorsal limb to axonal ephrinAs could contribute to LMCL axon guidance. However, a role of mesenchymal EphA4 in dorsal pathway selection in vivo remains to be demonstrated. Here we show that EphA proteins act as chemoattractants for LMCL axons in an in vitro turning assay and upon ectopic overexpression in vivo. The role of endogenous mesenchymal EphA4 as an attractive cue for LMC axons can be uncovered when EphA4 forward signaling in the axons is eliminated. Our results indicate that attractive EphA:ephrinA reverse signaling contributes to limb motor axon pathfinding.
Materials and Methods
Animals.
Hb9-GFP, Prx1-cre, Nestin-cre, PGK-cre, EphA4lx, EphA4EGFP, and EphA4 knock-out mice have been described previously (Lallemand et al., 1998; Tronche et al., 1999; Kullander et al., 2001; Logan et al., 2002; Wichterle et al., 2002; Grunwald et al., 2004; Herrmann et al., 2010). All the mutants were maintained in a comparable mixed 129/Svev × C57BL/6 background. Fertilized chick eggs (Couvoir Simetin) were stored for a maximum of 1 week at 18°C, incubated at 38°C, and staged according to standard protocols (Hamburger and Hamilton, 1951). Embryos used were of either sex.
Dissociated motor neuron cultures and turning assay.
Dissociated motor neuron cultures were prepared, and turning assay was performed as described previously (Dudanova et al., 2010). EphA-Fc chimeric proteins (R&D Systems) and human IgG Fc-fragment (Jackson Immunoresearch) were preclustered with anti-human Fc antibodies (Jackson Immunoresearch) at a 5:1 ratio for 1 h at room temperature (RT). Phosphatidylinositol-specific phospholipase C (PI-PLC; 1 U/ml, Sigma) was added for 1 h before the assay.
Chick in ovo electroporation.
Chick limb electroporations were performed as described previously (Luria et al., 2008).
Retrograde neuronal fills.
Retrograde fills in chick embryos were performed as described previously (Kao and Kania, 2011). E12.5 mouse embryos were eviscerated and kept in DMEM/F-12 medium (Invitrogen) aerated with 5% CO2/95% O2. A 6% lysine-fixable tetramethylrhodamine-dextran (molecular weight 3000, Invitrogen) solution in PBS with 0.4% Triton X-100 was injected into the ventral shank and allowed to diffuse for 5–6 h at RT. Embryos with labeling in the dorsal nerve were excluded from analysis.
Biochemistry.
SDS-PAGE was performed as described previously (Dudanova et al., 2010). The following primary antibodies were used: mouse monoclonal anti-EphA4 (anti-Sek, BD Biosciences), 1:1000; and mouse monoclonal anti-tubulin (clone DM 1A, Sigma), 1:50,000.
Immunostaining.
Ephrin/Eph-Fc overlays were performed as described previously (Kao and Kania, 2011). For immunostaining, embryos were fixed in 4% paraformaldehyde in 0.1 m phosphate buffer for 1–1.5 h at 4°C and cryoprotected in 30% sucrose, and 15–25 μm transverse cryosections were made. The sections were dried, rehydrated in PBS, and incubated in blocking solution (4% goat serum, 4% donkey serum, 2% bovine serum albumin in PBS) with 0.3% Triton X-100 overnight at 4°C. Primary antibodies were applied in blocking solution with 0.1% Triton X-100 for 2–4 h at RT, followed by Cy2-, Cy3-, or Cy5-conjugated secondary antibodies (Jackson Immunoresearch) for 2 h at RT. Immunostaining of motor neuron cultures and HeLa cells was performed as described previously (Dudanova et al., 2010). The following primary antibodies were used: rabbit anti-EphA4 (S20, Santa Cruz Biotechnology), 1:300; mouse anti-neurofilament 160 (clone NN18, Sigma), 1:500; mouse anti-Islet1 (39.4D5, Developmental Studies Hybridoma Bank), 1:50; rabbit anti-Lim1 (gift from A. Huber-Brösamle, Helmholtz Center Munich, Munich, Germany), 1:800; rabbit anti-GFP (Invitrogen), 1:2000; and rat anti-HA (Roche), 1:2000. Embryo sections with retrograde tracings were examined with a Leica SP2 confocal microscope. All other immunostainings were examined with an Axioplan epifluorescent microscope (Zeiss) and were documented with MetaMorph software.
Statistical analysis.
Data are shown as the mean ± SEM, and the t test was used to analyze statistical significance.
Results
EphA:ephrinA reverse signaling mediates attractive turning responses in LMCL axons
To investigate whether EphAs can act as chemoattractants in an acute turning assay, dissociated primary lumbar LMC neurons from E12.5 Hb9-GFP transgenic mouse embryos (Wichterle et al., 2002) were exposed to a linear gradient of preclustered EphA-Fc fusion proteins in the Dunn chamber (Yam et al., 2009; Dudanova et al., 2010). No turning was observed in a control Fc gradient (average turning angle β = 0.7 ± 2.5°) (Fig. 1A–C,F). Application of EphA4-Fc resulted in attractive turning of motor axons (β = 9.4 ± 2.8° for 1 μg/ml EphA4-Fc and 12.5 ± 3.1° for 10 μg/ml EphA4-Fc) (Fig. 1A–C,G,H). In addition, EphA7, another EphA protein enriched in the dorsal limb (Araujo et al., 1998), induced attractive turning with similar strength (β = 9.7 ± 3.4° for 1 μg/ml EphA4-Fc and 13.4 ± 2.7° for 10 μg/ml EphA4-Fc) (Fig. 1A–C,I,J). The average speed of axon growth was comparable in all conditions (Fig. 1D).
Figure 1.
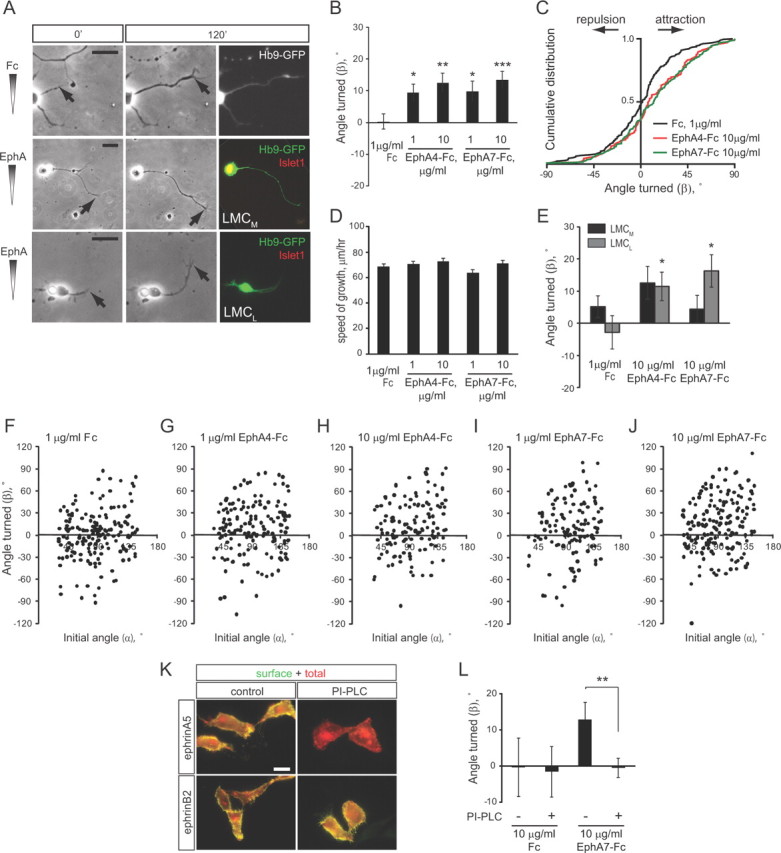
EphAs induce attractive turning of LMC axons. A, Representative examples of Hb9-GFP+ LMC neurons in indicated gradients at the beginning of the assay (0′) and after 2 h (120′). All images were aligned so that the gradient increases up the y-axis. Arrows point to the axon tip. Neurons were stained for Islet1 after the assay (note yellow nucleus in LMCM neuron). Scale bars, 20 μm. B, C, Graphs showing means ± SEM (B) and cumulative distributions (C) of turning angles (β) of LMC axons in the indicated gradients. The numbers of axons analyzed are as follows: Fc, 175 axons/8 cultures; 1 μg/ml EphA4-Fc, 160 axons/11 cultures; 10 μg/ml EphA4-Fc, 134 axons/8 cultures; 1 μg/ml EphA7-Fc, 123 axons/8 cultures; 10 μg/ml EphA7-Fc, 177 axons/8 cultures. *p < 0.05, **p < 0.01, ***p < 0.001, all conditions are compared with Fc. D, Quantification of the speed of axon growth in the indicated gradients. None of the differences are significant. E, Turning responses of LMCM and LMCL neurons in the indicated gradients. Numbers of axons analyzed: Fc, 93 LMCM and 34 LMCL axons/8 cultures; 10 μg/ml EphA4-Fc, 58 LMCM and 35 LMCL axons/8 cultures; 10 μg/ml EphA7-Fc, 67 LMCM and 45 LMCL axons/8 cultures. *p < 0.05, compared with the same population in Fc gradient. All the values are included in the quantification in B and C. F–J, Scatter plots of the angle turned (β) versus initial angle (α) for the indicated conditions. K, Examples of HeLa cells transfected with HA- and mCherry-tagged ephrin constructs and immunostained for HA without permeabilization to detect the surface population of ephrins. mCherry autofluorescence shows the total ephrin protein. Treatment with PI-PLC completely removes the surface population of GPI-anchored ephrinA5, but not transmembrane ephrinB2. Scale bar, 20 μm. L, Quantification of LMC turning responses with and without PI-PLC treatment. Numbers of axons analyzed: Fc, 19 axons/1 culture; Fc with PI-PLC, 33 axons/2 cultures; EphA7-Fc, 47 axons/3 cultures; EphA7-Fc with PI-PLC, 89 axons/4 cultures. **p < 0.01.
To determine the divisional identity of the LMC neurons that responded to EphA-Fc gradients, we monitored the expression of the LMCM marker Islet1 (Tsuchida et al., 1994). The average turning angles of LMCL, but not LMCM axons, were significantly different between control and EphA gradients (Fig. 1A,E). The higher turning angles observed for LMCM neurons in response to EphA4 were not significantly different from control, and are likely due to the ability of EphA4 to bind ephrinBs, which are expressed in LMCM neurons (Luria et al., 2008) and can mediate attractive reverse signaling (Kao and Kania, 2011). These data demonstrate that EphA proteins induce rapid attractive turning of LMCL axons, consistent with the growth of these axons toward the EphA4-expressing limb mesenchyme in vivo.
To test whether the turning response to EphAs is mediated by axonal ephrinAs, we treated the cultures with PI-PLC, which removes glycosyl phosphatidylinositol (GPI)-anchored proteins from cell surface (Marquardt et al., 2005) (Fig. 1K). The addition of PI-PLC completely abolished turning in an EphA7-Fc gradient (Fig. 1L), suggesting that the attractive response to EphAs is mediated by ephrinA reverse signaling.
Ectopic expression of EphA4 in limb mesenchyme is sufficient to redirect LMCL projections
We reasoned that if LMCL axons are attracted to EphA4 in the dorsal limb, then expression of EphA4 in the ventral limb should attract some LMCL axons into the ventral nerve. To test this idea, EphA4::GFP or GFP control plasmids were introduced into chick hindlimbs at Hamburger-Hamilton (HH) stage 18/19 by in ovo electroporation (Fig. 2A), leading to ectopic patches of EphA4::GFP or GFP-only expression in the ventral limb (Fig. 2B,B′,C,C′,D). To detect misprojecting LMCL neurons, we injected horseradish peroxidase (HRP) into the ventral shank of HH stage 28/29 embryos, and monitored LMC expression of the LMCL marker Lim1 (Tsuchida et al., 1994). Significantly more LMCL neurons innervated the ventral limb in embryos overexpressing EphA4::GFP (14 ± 4% of all HRP+ neurons) than in controls (4 ± 3%) (Fig. 2F–H). The distribution of free net ephrinAs appeared unchanged upon EphA4::GFP overexpression (Fig. 2B″,C″,E); therefore, LMCL axon misrouting was not due to EphA4::GFP masking repulsive ephrinAs in the ventral mesenchyme. Thus, the redirection of LMCL axons by ectopic EphA4 is likely mediated by attractive ephrinA reverse signaling.
Figure 2.
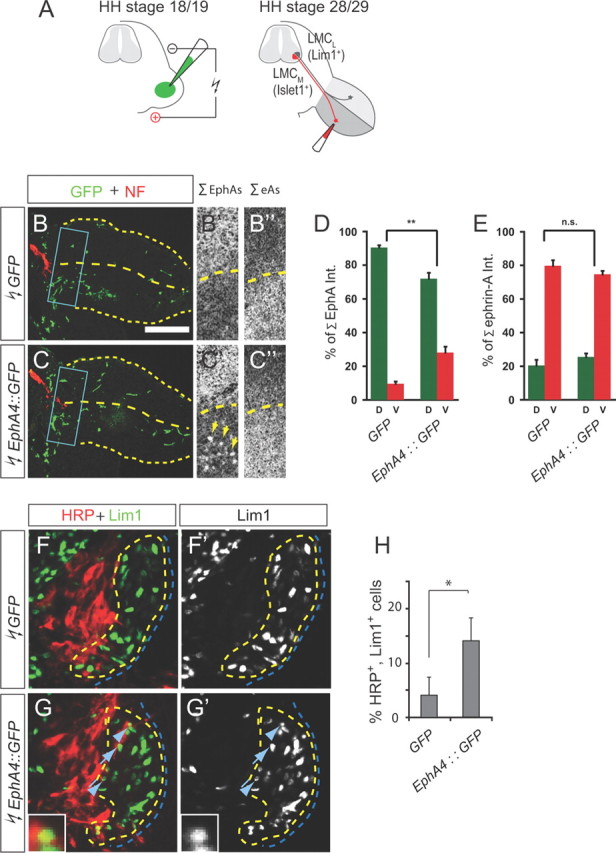
Ectopic expression of EphA4 in the ventral limb reroutes LMCL axons. A, Scheme of chick in ovo electroporation and retrograde tracings. B, C, Detection of GFP (green) and neurofilament (NF, red) in the hindlimb of chick HH stage 23/24 embryos electroporated with GFP (B) or EphA4::GFP (C). Stippled boxes indicate the region of bifurcation of dorsal and ventral nerves, shown at a higher magnification in the middle and right panels. B′, C′, detection of free EphAs by ephrinA5-Fc overlay. Arrows in C′ point to patches of ectopic EphA4. B″, C″, detection of free ephrinAs by EphA4-Fc overlay. Scale bars: B, C, 300 μm; B′, B″, C′, C″, 150 μm. D, E, Quantification of expression of free EphAs and free ephrinAs in GFP- or EphA4::GFP-electroporated embryos. n = 4 embryos. **p < 0.01. n.s., Not significant. F, G, Detection of HRP and Lim1 in the LMC of chick embryos electroporated with GFP or EphA4::GFP and injected with HRP into the ventral shank. Examples of Lim1+ LMCL neurons labeled with HRP are indicated by arrows and arrowheads. Cell marked by arrowhead is shown at higher magnification (G, G′, insets). Scale bars: F, F′, G, G′, 35 μm; G, G′, insets, 8 μm. H, Quantification of HRP-labeled LMCL neurons as a percentage of all HRP-labeled LMC neurons. Numbers of embryos analyzed: n = 4 for GFP and EphA4::GFP. Minimum number of HRP+ neurons counted per embryo: 109. *p < 0.05.
The contribution of mesenchymal EphA4 to LMC axon guidance
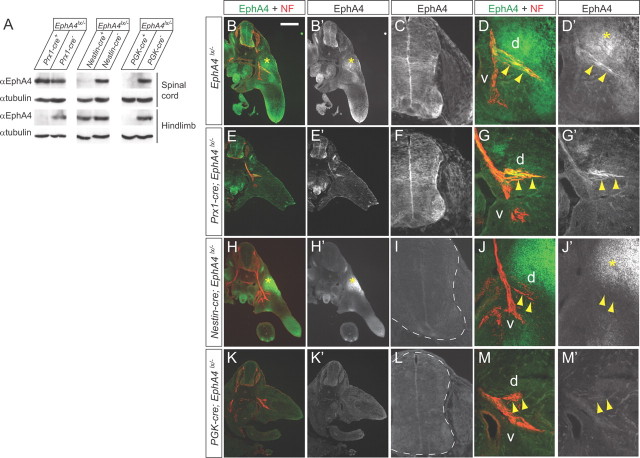
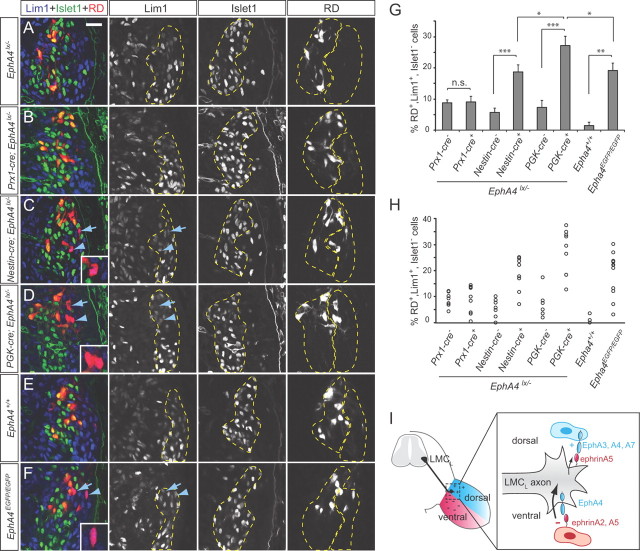
If mesenchymal EphA4 contributes to LMCL axon guidance, we expected that (1) genetic deletion of EphA4 in the hindlimb should produce LMCL pathfinding errors, and (2) ablating EphA4 only in motor neurons should result in a weaker phenotype compared with a general EphA4 knockout. To test these predictions, we generated limb-specific and nervous system-specific EphA4 knockouts by crossing the conditional EphA4lx line (Herrmann et al., 2010) with Prx1-cre and Nestin-cre transgenic mice (Tronche et al., 1999; Logan et al., 2002). In addition, EphA4lx/lx mice were crossed with a ubiquitous deleter line, PGK-cre, to obtain EphA4-null mutants. Control EphA4lx/lx embryos had unaltered amounts of EphA4 protein and did not display guidance errors in the hindlimb (Herrmann et al., 2010; and data not shown). In all experiments with conditional knockouts, cre+;EphA4lx/− embryos were compared with cre−;EphA4lx/− littermates.
Western blot and immunohistochemical analyses confirmed the absence of EphA4 protein in the hindlimbs of Prx1-cre;EphA4lx/− embryos, and in the spinal cords and dorsal limb nerves of Nestin-cre;EphA4lx/− mutants (Fig. 3A–J). In PGK-cre;EphA4lx/− embryos, no EphA4 protein was present in any tissue (Fig. 3A,K–M). To assess LMCL projection fidelity in the mutants, we monitored Islet1 and Lim1 expression in LMC neurons labeled by rhodamine dextran (RD) injections into the ventral limb. In EphA4lx/− control embryos, the number of RD-labeled LMCL neurons was low (5–8% of all RD+ LMC neurons) (Fig. 4A,G,H). Some LMCL misprojections in these controls could be due to the reduced amount of EphA4 protein in heterozygous EphA4lx/− embryos compared with EphA4lx/lx and wild-type embryos (compare to EphA4+/+ controls; data not shown). In Prx1-cre;EphA4lx/− embryos, the numbers of ventrally projecting LMCL neurons were not different from control littermates (9.1 ± 1.8%) (Fig. 4B,G,H), indicating that further removal of EphA4 from the hindlimb does not have a strong impact on axon guidance. In contrast, in Nestin-cre;EphA4lx/− mutants, the fraction of RD-labeled LMCL neurons was 18.7 ± 2.3% and significantly higher than in controls (Fig. 4C,G,H), consistent with a critical role of axonal EphA4 in LMCL axon pathfinding (Helmbacher et al., 2000; Eberhart et al., 2002; Luria et al., 2008). Interestingly, the LMCL error rate in these embryos was significantly lower than in PGK-cre;EphA4lx/− embryos (27.2 ± 2.9%) (Fig. 4D,G,H), indicating that when EphA4 is absent from motor neurons only, some LMCL axons are guided correctly by limb-derived EphA4. These results reveal a significant role for mesenchymal EphA4 in attracting motor axons.
Figure 3.
EphA4 expression in conditional EphA4 mutants. A, Representative Western blots of spinal cord and hindlimb lysates from E12.5 embryos showing complete removal of EphA4 protein from the hindlimb in Prx1-cre;EphA4lx/− embryos, from the spinal cord in Nestin-cre;EphA4lx/− embryos, and from both tissues in PGK-cre;EphA4lx/− embryos. α-Tubulin is shown as loading control. B–M, Cross sections of E11.5 embryos of the indicated genotypes labeled with anti-EphA4 and anti-NF antibodies. B, E, H, K, overviews; C, F, I, L, staining in the spinal cord; D, G, J, M, staining in the hindlimb. Dashed lines indicate the contours of the spinal cord. d, Dorsal nerve branch; v, ventral nerve branch. Asterisks in B′, D′, H′, and J′ mark the domain of EphA4 expression in the dorsal limb. Arrowheads point to the dorsal nerve branch. Scale bar: (in B), overviews, 400 μm; higher-power magnifications, 100 μm.
Figure 4.
Guidance errors in EphA4 mutants. A–F, Single confocal plane images of the spinal cord of the indicated genotypes after ventral RD injections and staining for Islet1 and Lim1. Arrows and arrowheads point to Islet1−, Lim1+ LMCL neurons labeled with RD. Examples indicated by arrowheads are shown in the insets at higher magnification. Dashed lines indicate LMCM and LMCL domains. Scale bar: (in A) A–F, 50 μm. G, Quantification of RD-labeled LMCL cells as a percentage of all RD-labeled LMC cells. Numbers of embryos analyzed: Prx1-cre−;EphA4lx/−, n = 8; Prx1-cre+;EphA4lx/−, n = 9; Nestin-cre−;EphA4lx/−, n = 7; Nestin-cre+;EphA4lx/−, n = 8; PGK-cre−;EphA4lx/−, n = 6; PGK-cre+;EphA4lx/−, n = 9; EphA4+/+, n = 3; EphA4EGFP/EGFP, n = 12. Minimum number of RD+ cells counted per embryo: 106. *p < 0.05, **p < 0.01, ***p < 0.001. H, Scatter plots of individual values from each embryo. I, Model of LMCL axon guidance by EphA forward and ephrinA reverse signaling. EphrinAs expressed ventrally act as repellants, triggering forward EphA4 signaling in the axon (thick arrow), while EphAs expressed dorsally act as weak attractants, eliciting reverse ephrinA signaling (thin arrow).
Forward signaling-deficient EphA4 partially rescues LMC guidance errors
To further explore the contribution of EphA4:ephrinA reverse signaling to LMC axon guidance in vivo, we took advantage of the EphA4EGFP mouse line (Grunwald et al., 2004), in which the intracellular domains of EphA4 are replaced by EGFP, making the receptor signaling incompetent, while it retains its ability to bind ephrins and trigger reverse signaling (Grunwald et al., 2004; Filosa et al., 2009). The expression levels of EphA4-EGFP protein were similar to the wild-type isoform (Grunwald et al., 2004; and data not shown). Retrograde tracings revealed a high number of LMCL axons in the ventral limbs of EphA4EGFP/EGFP mutants (19.1 ± 2.4% of all RD+ neurons) compared with EphA4+/+ littermates (1.5 ± 1.1%) (Fig. 4E–H), confirming an important role of forward signaling in this pathway choice. However, the fraction of misguided LMCL neurons was significantly lower than in PGK-cre;EphA4lx/− knockouts (27.2 ± 2.9%) (Fig. 4D,G,H), indicating that intact EphA4 extracellular domain is sufficient to rescue some of the LMCL guidance errors, most likely by activating ephrinA reverse signaling.
Discussion
Our genetic experiments demonstrate a contribution of both forward and Eph:ephrin reverse signaling to the establishment of limb innervation. EphA4 forward signaling appears to be the “dominant” guidance system for the dorsal/ventral pathway choice, because genetic ablation of EphA4 expression in motor neurons had a stronger impact on axon pathfinding than its deletion from the limb. The effects of EphA4 ablation in the hindlimb may, however, be partially compensated by other EphA proteins. While EphA4 is the main EphA receptor on LMCL axons, dorsal limb mesenchyme also expresses EphA7 (Araujo et al., 1998; Krawchuk and Kania, 2008) and EphA3 (Iwamasa et al., 1999). Therefore, partial removal of chemoattractive cues from the dorsal limb of Prx1-cre;EphA4lx/− mice would not have a large impact on the LMC axon projection fidelity when EphA4 forward signaling in motor axons is intact (Fig. 4I). However, with EphA4 forward repulsion eliminated, LMC axons might rely on attractive cues to a greater extent, and the deletion of one limb-derived EphA would have a more pronounced effect on pathway selection. Indeed, we observed a significant difference in the number of LMCL misprojections between the full EphA4 knockout, on the one side, and the nervous system knockout and forward signaling-deficient mutant, on the other. In fact, this difference was unexpectedly large, considering the remaining expression of other EphAs in the hindlimb. Our new findings on the function of ephrinA reverse signaling in motor axons extend previous observations in other systems where the role of reverse signaling has been demonstrated genetically. For instance, reverse signaling is required for the guidance of retinotectal (Rashid et al., 2005), vomeronasal (Knöll et al., 2001), and olfactory sensory projections (Cutforth et al., 2003), and mediates axon–axon interactions in spinal sensory–motor circuits (Wang et al., 2011). Moreover, a recent study by Bonanomi et al. (2012) provided independent and complementary evidence for a requirement for ephrinA reverse signaling in LMC axon guidance. In contrast to our findings (Dudanova et al., 2010, and this study), Bonanomi et al. (2012) reported that EphAs and GDNF alone lack chemoattractive activity and are only able to attract LMC motor axons when added in combination. This discrepancy may be due to methodological differences. While Bonanomi et al. (2012) analyzed a mixed population of all lumbar LMC axons, we used only the lower half of the LMC, which is affected in vivo, and distinguished between LMCL and LMCM turning responses.
Together with the work of Bonanomi et al. (2012), our present findings firmly establish parallel reverse and forward signaling as an important mode of Eph/ephrin function. Several questions remain unanswered. Do EphA4 receptors in limb mesenchymal cells become activated upon engagement with ephrinAs on motor axons, and do these cells respond to EphA4 forward signaling? And what prevents motor axons from adhering to EphA4-positive mesenchymal cells, a process that could interfere with their further growth? One possibility is that EphA ectodomains are proteolytically cleaved (Inoue et al., 2009; Oricchio et al., 2011). Cleavage would eventually terminate EphA forward signaling in mesenchymal cells and allow motor axons to continue growing into the limb.
Footnotes
This study was supported by the Max Planck Society, the Deutsche Forschungsgemeinschaft (Grant SFB870), the German-Israeli Foundation, and Canadian Institutes of Health Research (Grant MOP-77556). We thank L. Loschek for preliminary experiments in the early stage of the project; T. Marquardt, A. Huber-Brösamle, and E. Bianchi for help with establishing techniques; J. Lindner and M. Liang for technical assistance; D. Marinescu and S. Krinner for mouse genotyping; A. Huber-Brösamle for the Lim1 antibody; and G. Gatto for discussions.
References
- Araujo M, Piedra ME, Herrera MT, Ros MA, Nieto MA. The expression and regulation of chick EphA7 suggests roles in limb patterning and innervation. Development. 1998;125:4195–4204. doi: 10.1242/dev.125.21.4195. [DOI] [PubMed] [Google Scholar]
- Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, Pierchala BA, Pfaff SL. Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals. Cell. 2012;148:568–582. doi: 10.1016/j.cell.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutforth T, Moring L, Mendelsohn M, Nemes A, Shah NM, Kim MM, Frisén J, Axel R. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114:311–322. doi: 10.1016/s0092-8674(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Gatto G, Klein R. GDNF acts as a chemoattractant to support ephrinA-induced repulsion of limb motor axons. Curr Biol. 2010;20:2150–2156. doi: 10.1016/j.cub.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Swartz M, Koblar SA, Pasquale EB, Tanaka H, Krull CE. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in pathfinding. Dev Neurosci. 2000;22:237–250. doi: 10.1159/000017446. [DOI] [PubMed] [Google Scholar]
- Eberhart J, Swartz ME, Koblar SA, Pasquale EB, Krull CE. EphA4 constitutes a population-specific guidance cue for motor neurons. Dev Biol. 2002;247:89–101. doi: 10.1006/dbio.2002.0695. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Filosa A, Paixão S, Honsek SD, Carmona MA, Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R, Klopstock T, Kullander K, Rose CR, Pasquale EB, Klein R. Neuron-glia communication via EphA4/ephrin-A3 modulates LTP through glial glutamate transport. Nat Neurosci. 2009;12:1285–1292. doi: 10.1038/nn.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IC, Korte M, Adelmann G, Plueck A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T, Klein R. Hippocampal plasticity requires postsynaptic ephrinBs. Nat Neurosci. 2004;7:33–40. doi: 10.1038/nn1164. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Helmbacher F, Schneider-Maunoury S, Topilko P, Tiret L, Charnay P. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development. 2000;127:3313–3324. doi: 10.1242/dev.127.15.3313. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Pence MA, Shapera EA, Shah RR, Geoffroy CG, Zheng B. Generation of an EphA4 conditional allele in mice. Genesis. 2010;48:101–105. doi: 10.1002/dvg.20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue E, Deguchi-Tawarada M, Togawa A, Matsui C, Arita K, Katahira-Tayama S, Sato T, Yamauchi E, Oda Y, Takai Y. Synaptic activity prompts gamma-secretase-mediated cleavage of EphA4 and dendritic spine formation. J Cell Biol. 2009;185:551–564. doi: 10.1083/jcb.200809151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamasa H, Ohta K, Yamada T, Ushijima K, Terasaki H, Tanaka H. Expression of Eph receptor tyrosine kinases and their ligands in chick embryonic motor neurons and hindlimb muscles. Dev Growth Differ. 1999;41:685–698. doi: 10.1046/j.1440-169x.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- Kania A, Jessell TM. Topographic motor projections in the limb imposed by LIM homeodomain protein regulation of ephrin-A:EphA interactions. Neuron. 2003;38:581–596. doi: 10.1016/s0896-6273(03)00292-7. [DOI] [PubMed] [Google Scholar]
- Kao TJ, Kania A. Ephrin-mediated cis-attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Knöll B, Zarbalis K, Wurst W, Drescher U. A role for the EphA family in the topographic targeting of vomeronasal axons. Development. 2001;128:895–906. doi: 10.1242/dev.128.6.895. [DOI] [PubMed] [Google Scholar]
- Krawchuk D, Kania A. Identification of genes controlled by LMX1B in the developing mouse limb bud. Dev Dyn. 2008;237:1183–1192. doi: 10.1002/dvdy.21514. [DOI] [PubMed] [Google Scholar]
- Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–112. doi: 10.1023/a:1008868325009. [DOI] [PubMed] [Google Scholar]
- Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- Luria V, Krawchuk D, Jessell TM, Laufer E, Kania A. Specification of motor axon trajectory by ephrin-B:EphB signaling: symmetrical control of axonal patterning in the developing limb. Neuron. 2008;60:1039–1053. doi: 10.1016/j.neuron.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Oricchio E, Nanjangud G, Wolfe AL, Schatz JH, Mavrakis KJ, Jiang M, Liu X, Bruno J, Heguy A, Olshen AB, Socci ND, Teruya-Feldstein J, Weis-Garcia F, Tam W, Shaknovich R, Melnick A, Himanen JP, Chaganti RS, Wendel HG. The eph-receptor a7 is a soluble tumor suppressor for follicular lymphoma. Cell. 2011;147:554–564. doi: 10.1016/j.cell.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid T, Upton AL, Blentic A, Ciossek T, Knöll B, Thompson ID, Drescher U. Opposing gradients of ephrin-As and EphA7 in the superior colliculus are essential for topographic mapping in the mammalian visual system. Neuron. 2005;47:57–69. doi: 10.1016/j.neuron.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wang L, Klein R, Zheng B, Marquardt T. Anatomical coupling of sensory and motor nerve trajectory via axon tracking. Neuron. 2011;71:263–277. doi: 10.1016/j.neuron.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]