Abstract
The dorsal premotor cortex (PMd) uses prior sensory information for motor preparation. Here, we used a conditioning-and-map approach in 11 healthy male humans (mean age 27 years) to further clarify the role of PMd in anticipatory motor control. We transiently disrupted neuronal processing in PMd, using either continuous theta burst stimulation (cTBS) at 80% (inhibitory cTBS) or 30% (sham cTBS) of active motor threshold. The conditioning effects of cTBS on preparatory brain activity were assessed with functional MRI, while participants lifted a light or heavy weight in response to a go-cue (S2). An additional pre-cue (S1) correctly predicted the weight in 75% of the trials. Participants were asked to use this prior information to prepare for the lift. In the sham condition, grip force showed a consistent undershoot, if the S1 incorrectly prompted the preparation of a light lift. Likewise, an S1 that falsely announced a heavy weight produced a consistent overshoot in grip force. In trials with incorrect S1, preparatory activity in left PMd during the S1–S2 delay period predicted grip force undershoot but not overshoot. Real cTBS selectively abolished this undershoot in grip force. Furthermore, preparatory S1–S2 activity in left PMd no longer predicted the individual undershoot after real cTBS. Our results provide converging evidence for a causal involvement of PMd in anticipatory downscaling but not upscaling of grip force, suggesting an inhibitory role of PMd in anticipatory grip force control during object lifting.
Introduction
Prior sensory information is readily implemented in the preparation and anticipatory guidance of our actions. For instance, people match the applied force to the expected weight of an object when grasping and lifting an object (Johansson and Westling, 1988, Flanagan et al., 2001, Cole and Rotella, 2002). Previous research suggests that the dorsal premotor cortex (PMd) codes predictive aspects of sensory information in the context of manual motor control. The PMd is involved in selecting hand movements based on sensorimotor mapping rules (Picard and Strick, 1996, Passingham et al., 1998, Kurata et al., 2000, Toni et al., 2002, Amiez et al., 2006, van Eimeren et al., 2006). In this process, the left PMd plays a dominant role when action selection is based on an arbitrary (nonspatial) mapping rule (Schluter et al., 1998). Specifically, the PMd processes sensory information that is relevant to a pending action and uses it for movement preparation (Boussaoud, 2001, Astafiev et al., 2003, Hoshi and Tanji, 2006, Schubotz, 2007, Grafton et al., 2008).
This presumed role of the PMd has been substantiated by studies in which low-frequency repetitive transcranial magnetic stimulation (rTMS) was used to transiently suppress cortical excitability in the left PMd (Chouinard et al., 2005, Christensen et al., 2007, Nowak et al., 2009). Low-frequency rTMS of the left PMd disrupted the predictive scaling of forces based on arbitrary color cues in a grip-and-lift task (Chouinard et al., 2005, Nowak et al., 2009) and altered the impact of an incorrect predictive cue on subsequent visuomotor mapping (Ward et al., 2010).
Motivated by this work, we combined rTMS and functional magnetic resonance imaging (fMRI) to further clarify the functional relevance of the left PMd in implementing prior sensory information into the scaling of grip force. We used continuous theta burst stimulation (cTBS) to mildly and transiently disrupt neural processing in the stimulated left rostral PMd (Huang et al., 2005). In addition, participants underwent fMRI to map the lasting effects of cTBS on preparatory brain activity. During fMRI, subjects performed a grip-and-lift task in which an arbitrary visual pre-cue (S1) correctly (75%) or incorrectly (25%) predicted whether subjects had to lift a heavy or light weight. A second visual cue (S2) that always correctly predicted the object weight triggered subjects to perform the grip-and-lift task. Subjects were asked to prepare for the task based on the information given by the S1 cue. When S1 and S2 cues were incongruent, subjects had to readjust the prepared grip according to the S2 information.
The study was designed to test several hypotheses: (1) At the behavioral level, we reasoned that the prior knowledge about the weight provided by S1 would interfere with optimal anticipatory grip force control, if S1 and S2 were incongruent. We expected a relative overshoot or undershoot of grip force, when subjects wrongly prepared to lift a heavy or light weight, respectively.
(2) We postulated that the regional BOLD signal in PMd should reflect anticipatory coding of predictive information given by the S1 pre-cue (Chouinard et al., 2005, Christensen et al., 2007, Schubotz, 2007, Ward et al., 2010). Specifically, we hypothesized that the level of sustained BOLD activity in PMd during the S1–S2 period should indicate the relative strength of grip force anticipation triggered by the S1 cue, and, thus, should predict the magnitude of inappropriate grip force scaling in trials where S1 and S2 cues were different.
(3) Regarding the disruptive effects of cTBS, we predicted that real cTBS would impair the anticipatory force scaling in the stimulated left PMd, resulting in a reduction of relative grip force overshoot and undershoot in trials with different S1 and S2 cues. Likewise, the preparatory activity of the stimulated PMd should no longer predict the grip force behavior after real cTBS of left PMd. Finally, we expected that the disruptive effect of cTBS on anticipatory force control in rostral PMd might be compensated by changes in preparatory activity in the rostral part of the supplementary motor area (SMA), which is also involved in conditional response selection based on arbitrary sensorimotor associations (Kurata et al., 2000).
Materials and Methods
Participants
Eleven healthy male humans (age 27 ± 6.5 years; mean ±SD) without a neurological or psychiatric history participated. Subjects were recruited from the student population of the University of Kiel and were naive to the purpose of the study. Participants were consistently right-handed according to the Edinburgh handedness inventory (Oldfield, 1971). The experimental procedures were approved by the local ethics committee of the Christian Albrechts University. Written informed consent was obtained before the study.
Experimental design
We used fMRI to assess the conditioning effects of inhibitory cTBS on regional neural activity during motor preparation (Fig. 1). Each participant underwent two experimental sessions in a counterbalanced order. The experimental sessions were identical apart from the cTBS protocol, which used either a biologically real or a very low sham intensity of stimulation. At least 7 d separated the two sessions to exclude carry-over effects of TBS conditioning.
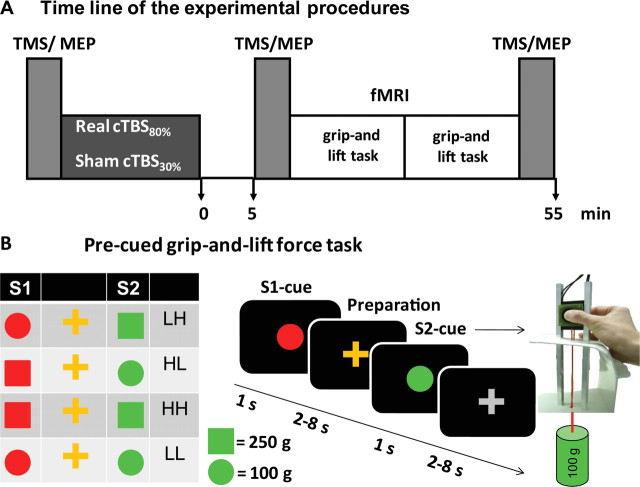
Figure 1.
Experimental design. A, Time line of the experimental procedures. See Materials and Methods for further details. TMS/MEP = Measurements of MEP with single-pulse TMS of left primary motor hand area. B, Visually guided grip-and-lift force task. During fMRI, subjects were presented with a S1 pre-cue (red color) and a S2 go-cue (green color) with a variable delay between S1 and S2. The cues were projected on the screen for 1 s, thereafter an orange or gray cross was projected during a jittered period of 2–8 s. The shape of the stimulus indicated the weight to be lifted. A circle or a square predicted a light (100 g) or a heavy (250 g) weight. In 75% of the trials, the preparatory S1-cue correctly predicted the S2 cue. Depending on the combination of S1 and S2 cues, there were two trial types with correct pre-cue (HH, LL) and two trial types with incorrect pre-cue (LH, HL).
Figure 1A illustrates the order of the experimental procedures. At the beginning of each experimental session, participants were intensively trained on the experimental grip-and-lift task, first outside and then inside the MR scanner, for ∼20 min. The experimental task required participants to grasp and lift a manipulandum with their right dominant hand (for details, see below). After participants were familiarized with the motor task, cortical excitability of the left primary motor hand area (M1HAND) was probed with single-pulse TMS of left M1HAND. We then applied either “real” or “sham” cTBS to the left rostral PMd. For real TBS (intervention condition), stimulus intensity was set at 80% of active motor threshold (AMT), whereas intensity was reduced to 30% of individual AMT during sham TBS (control condition). Otherwise, the cTBS protocols were identical.
After cTBS conditioning, participants rested for 5 min without moving their hands or feet. We introduced this resting period because previous studies showed that short periods of voluntary motor activity shortly before or after cTBS can modulate the conditioning effects of cTBS on cortical excitability (Huang et al., 2005, Gentner et al., 2008). After the 5-min resting period, single-pulse TMS was again applied to left M1HAND and motor-evoked potentials (MEPs) were recorded from right first dorsal interosseus (FDI) muscle to capture acute TBS-induced changes in corticospinal excitability (post-cTBS1 measurement). Participants were then taken to the MR scanner and fMRI started ∼15 min after the end of cTBS. Participants performed a grip-and-lift task with their right hand during fMRI. The experimental session was completed by measuring cortical excitability with single-pulse TMS over the left M1HAND (post-cTBS2 measurement) in the TMS laboratory. The post-cTBS2 measurement started ∼55 min after the end of TBS.
Pre-cued grasp-and-lift task
During fMRI, participants performed a grasp-and-lift task that required precision grips with the right dominant hand (Fig. 1B). An MRI-compatible custom-made force transducer (Dasch Instruments) was used for the grasp-and-lift task. The transducer had two flat vertical grip surfaces (40 × 40 mm) spaced 28 mm apart that measured the isometric pinch force exerted between the pads of the thumb and index finger (spring excursion <0.5 mm) and the load force. The grip surfaces were covered with thin felt.
Participants lay supine in the MR scanner with the left arm extended in a comfortable posture. The right arm was extended comfortably so that the right hand rested with a semiprone posture on a custom-made platform that supported the force transducer. The force transducer was placed between the fingertip of the right thumb and index finger and could therefore be gripped and lifted without any elbow- or shoulder-joint movement. The lateral edges of the force transducer fitted smoothly in grooves of two vertically orientated aluminum bars, so that the device could easily move up and down without tilting. A weight of 100 or 250 g was fixed with a string to the lower end of the transducer. The weights could be changed between trials without the participant being aware of the change in weight.
We used an event-related fMRI paradigm. A single trial lasted on average 12 s and started with the presentation of a symbolic preparatory S1 cue that was presented in the center of a screen 15 cm above subjects' visual field for 1 s. One of two S1 pre-cues was pseudorandomly presented from trial to trial (Fig. 1B). A red circle instructed participants to prepare for grasping and lifting a light weight (100 g), while a red square prompted subjects to prepare for grasping and lifting a heavy weight (250 g) (Fig. 1B). An orange cross appeared in the center of the visual field after the S1 cue. Participants were asked to fixate the cross and prepare for the grasp-and-lift task. The cross was presented for 2–8 s, and stimulus duration was pseudorandomly varied from trial to trial, resulting in a variable preparatory S1–S2 period.
At the end of the preparatory period, a symbolic target cue was centrally presented for 1 s. Again, there were two S2 cues: a green circle instructed participants to grasp and lift the light weight, whereas a green square prompted subjects to grasp and lift the heavy weight (Fig. 1B). Participants were asked to lift the manipulandum 2–4 cm up and then put the manipulandum back on the platform. After the S2 cue, a gray fixation cross was presented in the center of the screen for a variable period that was jittered between 2 and 8 s in steps of 1 s.
Participants were instructed to actively prepare for lifting the weight that was indicated by the S1 pre-cue and to grip and lift the device as fast and as accurately as possible in response to the S2 target cue. In 75% of the trials the preparatory S1-cue correctly predicted the S2 cue. Participants were explicitly informed about the predictive value of the S1 cue. Participants were informed that S1 would correctly predict the forthcoming weight in the majority of trials, and were instructed to lift the weight swiftly and as accurately as possible also in incorrectly pre-cued trials.
The experimental task resulted in four experimental conditions of interest. There were two conditions in which the S1 pre-cue and S2 target cue had the same shape. In these trials, the S1 pre-cue correctly predicted the weight that had to be lifted. Participants prepared for a heavy lift and then lifted the heavy weight [referred to as heavy–heavy (HH) condition[, or they prepared for a light lift and lifted the light weight [referred to as light–light (LL) condition]. In the remaining two conditions, the S1 and S2 pre-cue differed in shape, and, thus, the S1 cue was incorrect. Here, the S1 pre-cue either triggered the preparation for a light lift, but the S2 target cue indicated a heavy lift [referred to as light–heavy (LH) condition] or the S1 pre-cue indicated a heavy lift, but subjects were then instructed to lift the light weight [referred to as heavy–light (HL) condition]. There was also a fifth experimental condition that served as low-level control condition. In these “control trials,” a blue diamond was presented as S1 and S2 cue in the center of the screen (Fig. 1B). These cues were of no behavioral relevance. Participants had only to lay still and watch these cues without preparing for or performing any grip or lift.
Functional magnetic resonance imaging
The fMRI measurements were split in two consecutive runs. Each fMRI run included 40 grasp-and-lift trials (10 trials per experimental condition) and 15 control trials that were intermingled in a pseudorandom order. A single run lasted for 11 min. There was always an examiner (B.F.L.v.N. or C.K.) in the MR-room who changed the weights (100 and 250 g) from trial to trial. A cue that was only visible to the examiner indicated which weight was to be lifted in the next trial.
MRI was performed on a 3.0 T Philips Achieva MR scanner with an eight-channel array head coil (Philips). Participants wore headphones for noise protection, and foam pads restricted head motion. We used a T2*-weighted gradient echoplanar imaging (EPI) sequence with an echo time of 35 ms. The field of view covered the whole brain (230 × 230 mm) and a pixel size 2.9 × 2.9 mm was used. Each EPI volume was obtained within 3000 ms (repetition time) and comprised 36 axial slices with a voxel size of 2.9 × 2.9 × 3.0 mm and interslice gaps of 0.3 mm. We also obtained a whole-brain structural MRI dataset using a three-dimensional T1-weighted FLASH sequence (repetition time 7.7 ms, axial field of view: 230 mm, 160 contiguous slices, voxel size 1 × 1 × 1 mm).
Measurement of corticospinal excitability with TMS of left M1HAND
Motor cortical excitability was assessed with single-pulse TMS over the left M1HAND using a biphasic pulse configuration and a figure-of-eight shaped MC-B70 coil with an outer diameter of 70 mm, connected to a MagPro-100 stimulator (MagVenture). TMS was applied while participants were comfortably seated in an armchair with the head stabilized by a neck rest. Both arms were supported by a cushion to facilitate complete relaxation of the arm and hand muscles. Subjects were instructed to relax but to keep their eyes open and fixate on a wall two meters in front of them.
The coil was positioned tangentially to the skull over the left M1HAND with the handle pointing backwards and laterally at an angle of ∼45° to the sagittal plane. At this coil orientation, the second phase of the biphasic TMS pulse induces an electrical current in the brain tissue with a posterior–lateral to anterior–medial direction approximately perpendicular to the central sulcus, which is optimal for evoking a motor response in the contralateral hand (Mills et al., 1992).
We defined the scalp site where a single TMS pulse at slightly suprathreshold intensity consistently yielded maximal MEP in the right contralateral FDI muscle. This “motor hot spot” was used as stimulation site for all TMS measurements and was used as anchor point to define the site for TBS of the left PMd. To individually adjust the stimulus intensity, we determined the resting and active MT. We first determined the resting MT in the relaxed FDI muscle, which was defined as the minimum stimulus intensity that produced an MEP of >50 μV in five of 10 consecutive trials. We then measured the active MT defined as the lowest stimulus intensity at which MEPs were elicited in five of 10 consecutive trials during tonic contraction of the FDI muscle at ∼10% of maximum force level, using a criterion for the MEP of 100–250 μV peak-to-peak amplitude. MTs were determined by gradually decreasing and increasing the stimulus intensity in steps of 1% of maximum stimulator output.
MEPs were recorded with surface electromyography (EMG). Ag–AgCl disc surface electrodes were attached over the right FDI muscle using a belly-tendon montage. Changes in corticospinal excitability were assessed over the left M1HAND with single-pulse TMS, using a stimulus intensity that elicited MEPs with ∼1 mV peak-to-peak amplitude in the right FDI muscle. The stimulus intensity was determined at baseline in the real and sham TBS sessions and then kept constant across the entire experimental session. The reference electrode was placed at the wrist. EMG activity was continuously monitored using visual (oscilloscope) and auditory (speakers) feedback to ensure complete relaxation at rest and a constant level of EMG activity during tonic contraction. The raw EMG signals were amplified by 1000 (D360, Digitimer Ltd), filtered between 20 and 1000 Hz, and digitized at 5000 Hz per channel (CED Power1401, 16-bit-ADC; Cambridge Electronic Design). The administration of TMS pulses as well as EMG data recording, storage, and analyses was performed with Signal software (Cambridge Electronic Design).
Continuous theta burst stimulation of left dorsal premotor cortex
We used cTBS for conditioning of left PMd because cTBS produces a lasting suppression of regional excitability in the stimulated cortex (Huang et al., 2005). The cTBS protocol involved repeated administration of short high-frequency bursts. Each burst consisted of three pulses given at an interstimulus interval (ISI) of 20 ms (corresponding to a rate of 50 Hz). These high-frequency triple-pulse bursts were repeated every 200 ms. Theta burst stimulation was given to the left PMd as a continuous train lasting for 40 s. The site for PMd stimulation was defined in relation to the motor hot spot with the coil being placed 2 cm anterior and 1 cm medial to the left M1HAND. This coil positioning procedure used the functionally localized M1HAND as anchor point and was adopted from Schluter et al. (1998), who used this coil positioning procedure to interfere with processing in left PMd during the selection of visually cued movements. In addition, this coil location closely corresponds to the probabilistic location of the rostral PMd (Picard and Strick, 2001). The intensity of real cTBS was set at 80% of the individual AMT (cTBS80%); for sham cTBS we used an intensity of 30% of the individual AMT (cTBS30%). The latter intensity was predicted to be ineffective in terms of inducing action potentials in the PMd. We opted for low-intensity cTBS rather than using a sham coil because we wished to induce somatosensory stimulation of the scalp during sham cTBS (Helmich et al., 2006).
Data analysis
Analysis of grip and lift force data.
The nonmetallic custom-made force transducer (Dasch Instruments) was connected to the computer-based SC/ZOOM data acquisition and analysis system (Department of Physiology, Umeå University, Sweden) via a fiber optic connector. Grip and lift forces were sampled at a rate of 200 Hz.
We focused our analysis on the initial changes in grip and load force until the first peak to assess initial preparatory scaling of grip force (see Fig. 3). Four force measures were determined for each trial: (1) peak grip force (GF), (2) peak load force (LF), (3) peak rate of grip force (GFR), and (4) peak rate of load force (LFR). We also calculated the reaction time (RT), which was defined as the time between the onset of the S2 target cue and the onset of increase in GF. Mean values of each measure were calculated for each of the four experimental conditions of interest (i.e., HH, LL, LH, and LH).
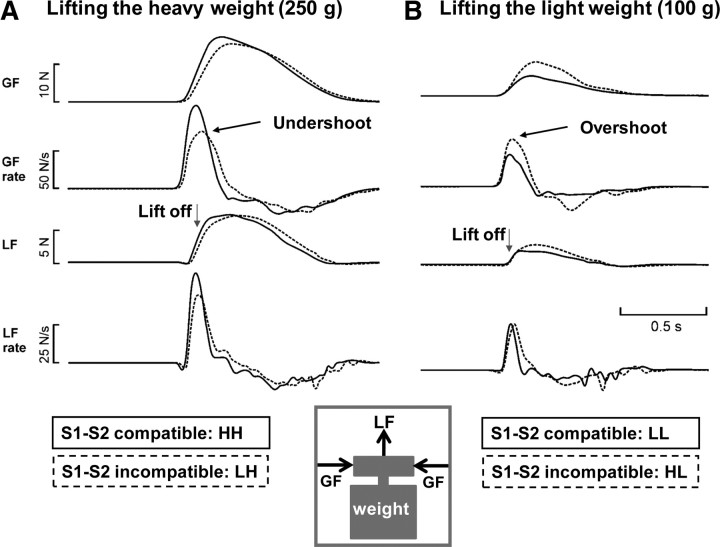
Figure 3.
Impact of the validity of the pre-cue on the grip force curves. Mean GF, GF rate, LF, and LF rate for the four different trial types of a representative subject are shown. Normal lines indicate the correctly pre-cued trials; the dotted lines represent the mean data of the incorrectly pre-cued trials. A depicts the mean data of trials requiring subjects to lift a heavy weight, while B shows the mean data of trials requiring a light lift. Trial types according to S1–S2 sequence: HH, LL, LH, HL.
In a first step, we explored the patterns of normal task performance without perturbation of left PMd using only the data recorded after sham TBS30%. We computed separate two-factorial repeated measures of ANOVA with the factors Weight (2 levels: 100 g vs 250 g) and S1 Validity (2 levels: correct vs incorrect S1 pre-cue) using GF, LF, GFR, LFR, and RT as dependent variables. We also performed an additional three-factorial ANOVA that included the factor Type of Intervention (2 levels: real cTBS80% vs sham cTBS30%) to assess the conditioning effects of real cTBS80% on grip force control.
We were particularly interested in the behavioral consequences of an incorrect pre-cue on grasping and lifting. To this end, we calculated the ratio between the GF in incorrectly pre-cued and correctly pre-cued trials for each weight (GFHL/GFLL and GFLH/GFHH). The same ratio was also calculated for the other grip and lift force measures (i.e., GFR, LF, and LFR). Using these ratios as dependent variables, two-factorial ANOVAs tested whether the absolute Weight (2 levels: 100 g vs 250 g) or the Type of Intervention (2 levels: real cTBS80% vs sham cTBS30%) influenced the effect of the incorrect pre-cue on task performance.
Previous grip force studies have consistently shown that the somatosensory information acquired by a recent lift influences the predictive scaling of forces for a subsequent lift (Johansson and Westling, 1988, Gordon et al., 1993, Chouinard et al., 2005). Therefore, we performed supplementary two-factorial ANOVAs with the factors Weight (heavy vs light weights) and Compatibility with Previous Lift (same weight vs different weight) for the real cTBS and sham cTBS session.
Prompted by the reviewers comment, we performed an additional analysis that assessed the impact of the last trial on grip force control. In agreement with previous work, applied grip force was influenced by the weight of the previous trial. When the previous trial required a heavy lift, subjects showed a relative overshoot in peak grip force when lifting a light weight. Conversely, there was a relative undershoot in peak grip force, when a heavy lift followed a light lift. Critically, this effect was not influenced by the type of TMS being significant after sham cTBS: F(10) = 38.49; p < 0.001 and after real cTBS F(10) = 22.19; p = 0.001).
Greenhouse-Geisser (GG) correction for nonsphericity was applied if necessary. Conditional on significant F-values, ANOVAS were followed by post hoc two-sided paired-sample t tests. Statistical threshold was set at p ≤ 0.05. Group data are given as mean ± SD if not specified otherwise.
Analysis of motor-evoked potentials.
Peak-to-peak amplitudes (mV) of the MEP recorded from the right FDI muscle were measured trial-by-trial, and mean MEP amplitudes were calculated for each block of measurements (NuCursor software, Sobell Department of Motor Neuroscience and Movement Disorders, Institute of Neurology, Queen Square, London, UK). Repeated-measures ANOVAS were used to test for lasting effects of cTBS over left PMd on excitability of ipsilateral left M1HAND. The ANOVA model included the factors Type of Intervention (2 levels: real cTBS80% vs sham cTBS30%) and Block of Measurement (3 levels: baseline, measurements starting 5 and 55 min after TBS conditioning). ANOVAS were followed by post hoc two-sided paired-sample t tests conditional of significant F-values. For all analyses, a significance level of p < 0.05 was applied after nonsphericity (GG) correction.
Analysis of the fMRI data.
The fMRI data were processed and analyzed using statistical parametric mapping (SPM) software (Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm). The first two scans of each session were discarded to allow for steady-state magnetization. The remaining images were realigned to the first image and spatially normalized to MNI stereotactic space using a standard EPI template as implemented in SPM. The normalized images were spatially smoothed with a Gaussian kernel of 9 mm full-width at half-maximum.
At the individual level, we constructed a general linear model that comprised both experimental sessions and took into account the factorial design. The presentation of the S1 onset pre-cue, the variable interval between S1 and S2 (i.e., preparatory period), and the onset of the S2 pre-cue were modeled separately for each of the five trial types (i.e., HH, LL, LH, HL, and control trials) using delta functions convolved with a hemodynamic response function (HRF). Based on this model we computed t-statistical maps that expressed regional changes in BOLD signal for experimental contrasts of interest for each voxel in the brain.
Second level analysis tested for experimental modulations of the regional BOLD signal during the preparatory S1–S2 period. The data for the second stage of analysis comprised pooled parameter estimates for each contrast of interest across all subjects in a random-effects analysis. Contrast images for each subject were entered into a one sample t test for each contrast of interest to identify brain regions that increased their neuronal activity (as indexed by the BOLD signal) in the preparatory period between the S1 pre-cue and the S2 target cue. Another t test was computed to identify brain regions where the real cTBS80% protocol increased or decreased regional preparatory activity relative to the sham TBS30% condition. We also used a one-sample t test for main-effects of brain activity during the different conditions. A paired t test was calculated to test for weight-specific brain activities during the preparatory phase for preparing to lift a heavy or light weight. The mean difference in grip force between the correctly and incorrectly pre-cued trials for each weight were included in the analysis as covariate of interest to test whether interindividual variations in preparatory brain activity during the S1–S2 period correlated with interindividual differences in the behavioral impact of the incorrect pre-cue on task performance.
In addition to rostral PMd, it has been shown that the rostral part of the SMA is also involved in conditional response selection based on arbitrary sensorimotor associations.(Sakai et al., 1999, Kurata et al., 2000) This raises the possibility that activity in rostral SMA might compensate for the disruptive effects of real cTBS80% on anticipatory force control in the stimulated left rostral PMd. Therefore, we used simple regression analysis to test whether the effects of real cTBS80% on anticipatory grip force control were less pronounced in subjects in whom real cTBS80% changed preparatory S1–S2 activity in rostral SMA.
All t tests performed within SPM were one tailed and included all voxels within the brain. The height threshold for the resulting statistical parametric maps (t-score maps) was set at an uncorrected p value of p < 0.01. All SPMs were transformed to the unit normal Z-distribution to create a statistical parametric map (SPM). P values were corrected at the cluster level applying an uncorrected extent threshold of p < 0.01. A cluster that failed to meet the significance criterion but consisted of >50 contiguous voxels is reported as statistical trend if the peak voxel in the cluster exceeded an uncorrected p < 0.001.
Since cTBS targeted the left rostral PMd and our experiment was specifically designed to explore the role of left PMd in the implementation of advance sensory information in movement preparation, the left rostral PMd was defined as a priori region of interest (ROI). The rostral SMA was defined as second ROI since we expected the rostral SMA to compensate for the lesion effect induced by real cTBS80% of left PMd.
Within the two ROIs, statistical threshold was set to p < 0.001 (uncorrected). The spherical ROIs (14 mm radius) were placed into the left PMd and left rostral PMd, centered on MNI stereotactic coordinates (x = −24, y = −3, z = 54 for PMd and x = −9, y = 9, z = 51 for SMA) that correspond to published activation peaks during a visuomotor response selection task (van Eimeren et al., 2006).
For left rostral PMd and rostral SMA, correction for multiple comparison was only performed for all voxels within the ROI. For all remaining voxels, statistical results were corrected across the whole brain.
All statistical parametric maps are superimposed onto a T2-weighted structural MRI template provided by MRIcro (http://www.cabiatl.com/mricro/mricro/index.html). The voxels of the activation maps are color-coded according to their Z values and for illustrative purposes thresholded at an uncorrected p value of p < 0.01.
Results
Changes in corticospinal excitability in left M1HAND
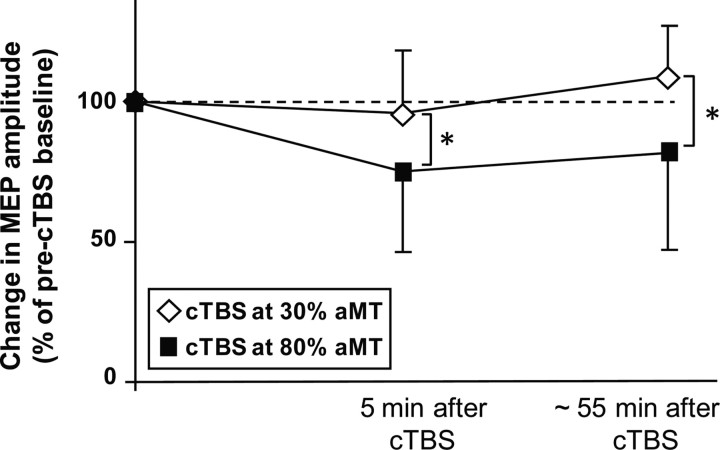
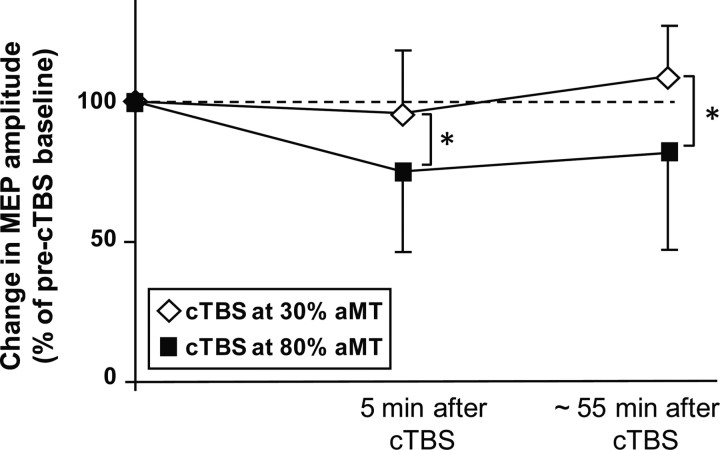
Real cTBS80% induced a sustained decrease in mean MEP amplitude in the right FDI muscle, which was not found after sham cTBS30% (Fig. 2). A differential effect of the two rTMS protocols on corticospinal excitability in ipsilateral M1HAND was confirmed by the ANOVA, showing an interaction between Type of Intervention and Block of Measurement (F(2,20) = 9.62, p = 0.001). Post hoc paired t test revealed significant decrease of the MEP amplitude 5 min (t(10) = 5.123, p < 0.001) and 55 min after the end of real cTBS80% (t(10) = 4.64, p = 0.001), but no consistent changes after sham cTBS30%.
Figure 2.
Conditioning effects of cTBS to left PMd on corticospinal excitability in left M1HAND. Group data of relative changes in mean peak-to-peak amplitude of the MEPs normalized to the mean amplitude before the intervention. The filled squares give the MEP amplitudes after real cTBS80% of left PMd. The open diamonds represent the MEP amplitudes after sham cTBS30% of left PMd. The first post-cTBS measurement was performed 5 min after the end of cTBS before fMRI. The second post-cTBS measurement was performed after the end of the fMRI session (i.e., ∼55 min after the end of cTBS).
There were no differences in resting MT, active MT, or mean MEP amplitude at baseline between the two experimental sessions. This lasting decrease in corticospinal excitability in ipsilateral M1HAND was comparable to the inhibitory after effects that have been reported in previous 1 Hz rTMS studies (Gerschlager et al., 2001, Chouinard et al., 2003, Suppa et al., 2008). This inhibitory effect of real cTBS80% on M1HAND excitability did not correlate with the cTBS-induced changes in grip force undershoot, nor did it correlate with cTBS-induced changes in weight-specific preparatory activity. This may be due to the fact that corticospinal excitability was assessed at rest, whereas the behavioral and fMRI measures were obtained during an active motor context during a pre-cued grip-and-lift task.
Grip force control
All participants found the tasks easy to perform. Error rate was very low with <2 error trials per fMRI session and are not considered further. The validity of the pre-cue had consistent effects on task performance. Compared with correct pre-cues, incorrect pre-cues resulted in longer mean reaction times (F(1,10) = 14.9; p = 0.003). Neither the absolute weight that had to be lifted nor the intensity of cTBS influenced mean RT. There were no significant interactions among the experimental factors that influenced mean RT.
Table 1 summarizes the group data for the grip force measures of interest. The weight that had to be lifted in a given trial had a consistent effect on all four force measures with higher force and force rate levels when subjects grasped and lifted the heavy weight (GF, GFR, LF, LFR; p < 0.001). The measurements during the control session in which we applied sham TBS at very low intensity revealed that the validity of the S1 pre-cue affected the force generation. Incorrect pre-cues produced opposite effects on force measures during heavy or light lifts: gripping and lifting a light weight was associated with a higher GF, GFR, and LFR in incorrectly pre-cued trials (HL) than correctly pre-cued (LL) grip-and-lift trials (Fig. 3B). In other words, the force profiles showed relative overshoot in force production when subjects had anticipated a heavy weight but had to lift a light weight in the HL condition. Conversely, participants consistently applied lower GF, GFR, and LFR (i.e., undershoot) when gripping and lifting a heavy weight after the S1 pre-cue had wrongly announced a light weight (Fig. 3A). Hence, force profiles displayed a relative undershoot when subjects wrongly anticipated to lift a light weight in the LH condition. A repeated-measures ANOVA confirmed the relative undershoot (LH trials) and overshoot (HL trials) in force output. There was a significant interaction between the validity of the S1 pre-cue and the actual weight that had to be lifted for peak grip force (GFsham; F(1,10) = 21.9; p = 0.001), peak grip force rate (GFRsham; F(1,10) = 26.7; p < 0.001), and peak lift force rate (LFRsham; F(1,10) = 5.8; p = 0.037).
Table 1.
Group values for each variable
| LLTBS30% (SD) | HLTBS30% (SD) | HHTBS30% (SD) | LHTBS30% (SD) | LLTBS80% (SD) | HLTBS80% (SD) | HHTBS80% (SD) | LHTBS80% (SD) | |
|---|---|---|---|---|---|---|---|---|
| RT (s) | 0.55 (0.16) | 0.57 (0.19) | 0.53 (0.15) | 0.57 (0.16) | 0.56 (0.09) | 0.59 (0.12) | 0.56 (0.1) | 0.59 (0.11) |
| GF (N) | 5.23 (2.93) | 6.16 (3.34) | 11.25 (4.56) | 10.86 (4.35) | 6.19 (2.84) | 7.07 (3.23) | 11.87 (2.78) | 12.39 (3.17) |
| GF rate (N/s) | 39.51 (30.34) | 45.89 (31.06) | 68.24 (28.73) | 63.76 (34.04) | 46.73 (30.18) | 54.70 (32) | 72.15 (30.71) | 74.27 (26.92) |
| LF (N) | 2.11 (1.3) | 2.15 (1.21) | 5.22 (1.74) | 5.15 (1.58) | 2.04 (0.69) | 2.06 (0.62) | 5.27 (0.72) | 5.27 (0.86) |
| LF rate (N/s) | 25.16 (17.43) | 26.17 (15.91) | 44.98 (22.79) | 41.76 (18.51) | 27.13 (14.09) | 27.42 (13.32) | 46.10 (20.06) | 46.10 (16.99) |
Group values of each behavioral variable for the four experimental conditions separately for the two experimental conditions. N, Newton; s, second.
The same pattern emerged when comparing the ratios between incorrectly and correctly pre-cued trials (i.e., comparing the HL:LL ratio and LH:HH ratio). Paired t tests revealed highly significant differences between the two ratios for GFsham, GFRsham, and LFRsham (p < 0.001). Individual HL:LL ratios were mainly >1 reflecting the overshoot triggered by the incorrect “HEAVY” pre-cue. Conversely, individual LH:HH ratios were consistently <1 reflecting the undershoot induced by the incorrect “LIGHT” pre-cue. With respect to the relative magnitude, the undershoot as indexed by the LH:HH ratio (Fig. 4; black bars) was less pronounced compared with the overshoot as indexed by the HL:LL ratio (Fig. 4, white bars). Paired t tests revealed highly significant differences between the two ratios for GFsham, GFRsham, and LFRsham (p < 0.001).
Figure 4.
Effect of the validity of the pre-cue on grip and lift force. The white columns give the mean overshoot in force production caused by an incorrect S1 pre-cue indicating a heavy weight. The overshoot corresponds to the ratio between HL and HH trials. The black columns give the mean undershoot in force production caused by an incorrect S1 pre-cue indicating a light weight. The undershoot corresponds to the ratio between LH and LL trials. Trial types according to S1–S2 sequence: HH, LL, LH, HL. Error bars indicate SD. Paired t test, *p < 0.001.
We tested whether the magnitude of the effect of S1–S2 discrepancy, as reported in Figure 4, on force responses is entirely accountable for by S1, or whether the S1–S2 discrepancy contributes to the execution of the force response planned by the same S1 weight cue. Paired t tests revealed highly significant differences (p < 0.001) by directly comparing the gripforces (GF, GFR, LF, and LFR) from HL versus HH and LH versus LL, indicating that the force response was not entirely planned based on the information provided by S1. The data rather indicate that the predictive information provided by S1 interfered with the information provided by S2, causing a response conflict.
A two-factorial repeated-measures ANOVA with the factor Weight (heavy vs light weight) and Compatibility with Previous Lift (same weight vs different weight) was performed to test whether the weight of the previous lift influenced predictive grip force control. In agreement with previous work (Johansson and Westling, 1988, Gordon et al., 1993, Chouinard et al., 2005), the ANOVA showed that grip force was influenced by the weight of the previous trial. When the previous trial required a heavy lift, subjects showed a relative overshoot in grip force when lifting a light weight. Conversely, subjects showed a relative undershoot in grip force, when a heavy lift followed a light lift.
Critically, this effect was not influenced by the type of TMS being significant after sham cTBS: F(10) = 38.49; p < 0.001 as well as after real cTBS F(10) = 22.19; p = 0.001). Post hoc paired t tests revealed for the sham cTBS session a significant decrease in GF for heavy weights when the previous lift was a light weight (mean ± SD 11.89 ± 4.0 N for previous heavy weight and 11.28 ± 3.9 N for previous light weight; t(10) = 3.49; p = 0.006) and a significant increase in GF for light weights when the previous lift was a heavy weight (mean ±SD 5.39 ± 3.0 N for previous light weight and 6.0 ± 3.0 N for previous heavy weight; t(10) = 5.35; p < 0.006). Post hoc paired t tests revealed for the real cTBS session a significant decrease in GF for heavy weights when the previous lift was a light weight (mean ±SD 11.62 ± 3.5 N for previous heavy weight and 10.82 ± 3.5 N for previous light eight; t(10) = 3.38; p = 0.007) and a significant increase in GF for light weights when the previous lift was a heavy weight (mean ±SD 4.73 ± 2.0 N for previous light weight and 5.8 ± 2.6 N for previous heavy weight; t(10) = 3.62; p < 0.005).
Effect of premotor cTBS on anticipatory grip force control
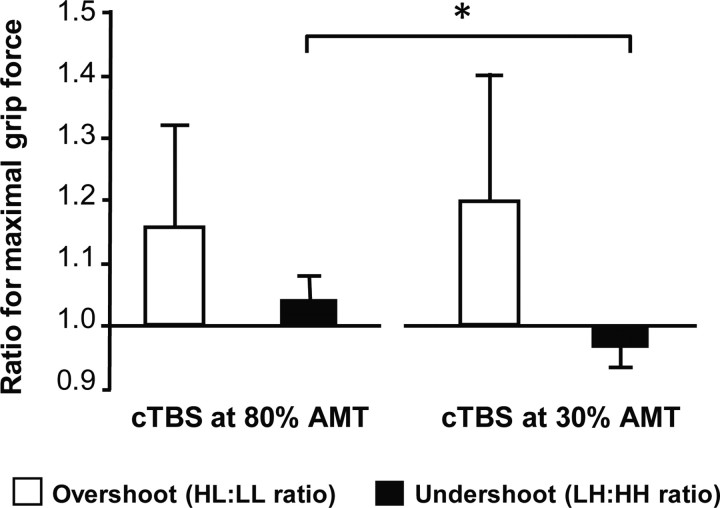
We also tested whether real cTBS80% changed the pattern of task performance as opposed to performance after sham cTBS at 30% of AMT. A two-factorial repeated-measures ANOVA including the factors Force Ratio (2 levels; HL/LL and LH/HH) and Type of Intervention (2 levels, cTBS80% and sham cTBS30%) revealed a significant interaction between ratio and intervention only for GF (F(1,10) = 4.79; p = 0.05), but not for the other three variables.
Figure 5 plots the relative overshoot (HL:LL ratio) and undershoot (LH:HH ratio) of mean GF separately for the sessions in which real cTBS80% or sham cTBS30% was given to left PMd. While the relative overshoot was comparable between the two experimental sessions, there was a difference in the relative undershoot. Real cTBS80% of left PMd abolished the relative undershoot in LH trials when the pre-cue had incorrectly announced a light weight (t(10) = 2.51, p = 0.031). In contrast, the relative overshoot in HL trials was not changed by real cTBS80% when an incorrect pre-cue had wrongly announced a heavy weight (p > 0.3). The individual changes in the magnitude of undershoot in LH trials did not correlate with the individual changes in corticospinal excitability after real cTBS80% (r = −0.12; p = 0.72).
Figure 5.
Effect of real cTBS80% of left PMd on maximal grip force. The white columns give the mean overshoot in force production (i.e., the ratio between HL and HH trials) caused by an incorrect S1 pre-cue indicating a heavy weight. The black columns give the mean undershoot in force production (i.e., the ratio between LH and LL trials) caused by an incorrect S1 pre-cue indicating a light weight. Compared with sham cTBS30%, real cTBS80% abolished the undershoot in maximal grip force caused by an incorrect lightweight cue. The asterisk denotes a significant difference of the pairwise comparison at p < 0.05. Error bars indicate SD.
Preparatory brain activity triggered by the pre-cue
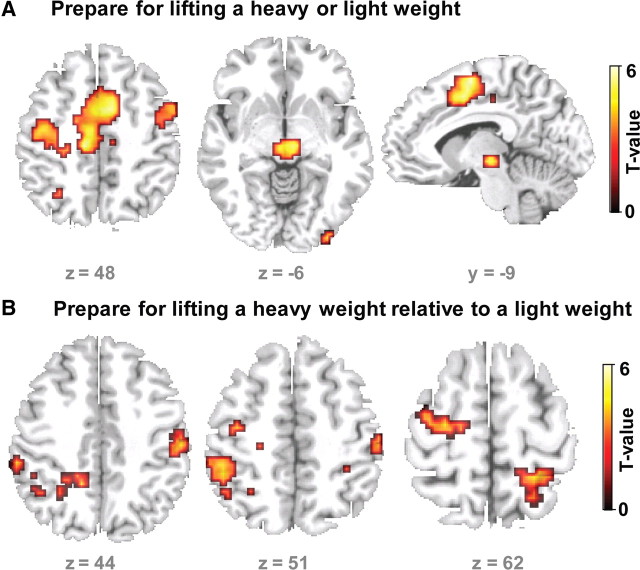
Analysis of the fMRI data acquired in the control session (i.e., after sham cTBS30%) revealed sustained increases in the preparatory period between the S1 pre-cue and S2 target cue in a large cluster covering a bilateral set of dorsal and mesial premotor areas. Increased BOLD signal levels were found in the SMA, caudal cingulate motor area, left and right PMd (Fig. 6A). Regional peak activation in left PMd was located in the caudal portion of PMd (at x, y, z = −27, − 21, 60; Z = 3.83), the rostral PMd (at x, y, z = −12, −9, 54; Z = 2.92; Psvc = 0.002), and the rostral SMA (at x, y, z = −9, − 21, 48; Z = 3.91; Psvc<0.001). The subthalamic region was also activated bilaterally when participants prepared for the grip-and-lift task (Fig. 6A).
Figure 6.
Regional increases in BOLD signal during preparation (S1–S2 interval). A, Main effect of preparation regardless of the weight indicated by the S1 pre-cue. The sagittal, coronal, and axial slices show the regions that showed an increase in BOLD signal during the preparation of a lift (heavy and light). B, Relative increases in BOLD signal during the preparation for lifting a heavy weight relative to preparing for lifting a light weight. The statistical parametric maps are based on the fMRI data recorded after sham cTBS30% of left PMd.
Distinct clusters in left and right PMd, left supramarginal gyrus, as well as left and right medial intraparietal sulcus (IPS) showed significant greater activation when participants prepared for lifting the heavy weight as opposed to preparing for lifting the light weight (Fig. 6B). The relative increase in preparatory activity for the heavy weight was significant in the left PMd (peak at x, y, z = −27, − 15, 60; Z = 3.04, Psvc = 0.001). No cluster in the brain showed increased preparatory activity for the lightweight relative to the heavyweight cue, even when applying a liberal threshold of p < 0.01 (uncorrected).
Preparatory activity in left PMd predicts undershoot in grip force
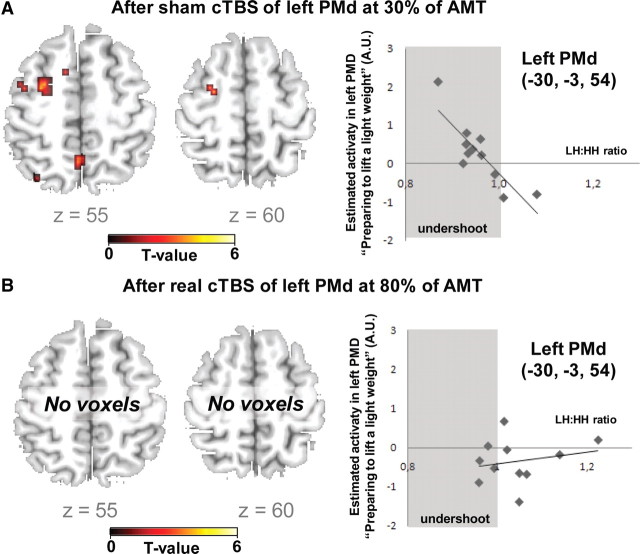
We hypothesized that in the absence of real cTBS80%, the level of preparatory activity in the left PMd would predict the behavioral impact of the incorrect S1 pre-cue on force generation. To test this hypothesis, we performed a second-level regression analysis based on the preparatory activity during the control fMRI session (i.e., after sham cTBS30%). In each subject, we calculated the mean LH:HH ratio of peak grip force during the control session, which indicates the relative undershoot of grip force when lifting the heavy weight after having prepared to lift the light weight. Using the LH:HH ratio as covariate of interest, we found that the magnitude of preparatory activity (of light weights) in left rostral PMd correlated with the relative undershoot in maximal grip force in the LH condition (peak correlation at x, y, z = −30, −3, 54; Z = 3.73, Psvc = 0.025). The greater the preparatory activity in left PMd, the greater was the relative undershoot in peak grip force when lifting the heavy weight after preparing to lift the light weight (Fig. 7A).
Figure 7.
Linear relationship between regional activation during motor preparation and the relative undershoot in maximal grip force in trails where an incorrect S1 pre-cue announced a light weight after sham cTBS30% (A) or real cTBS80% (B) of left PMd. A, In the control session without effective cTBS, preparatory activity during the S1–S2 period predicted the individual undershoot in maximal grip force. The higher the preparatory activity in the left PMd, the larger was the undershoot in trials with an incorrect S1 pre-cue indicating a light weight. B, This linear relationship was abolished after real cTBS80% of left PMd. The left panels show axial slices of the statistical parametric map for the linear relationship between preparatory activity and force undershoot. The corresponding scatter plots for the peak voxel in left PMd are presented on the right (x, y, z = −30, −3, 54). The parameter estimates of preparatory BOLD signal changes are plotted along the y-axis. The maximal grip force ratios (LH/LL trials) are displayed along the x-axis. The gray color marks the area with negative LH/HH force ratio (i.e., undershoot). The regression line gives the estimated linear relation.
We performed the same type of regression analysis using the individual HL:LL ratio of peak grip force. The HL:LL ratio reflects the relative overshoot in grip force when lifting the light weight after having prepared to lift the heavy weight. In this analysis, interindividual variations of preparatory activity in the left PMd did not correlate with interindividual differences in the amount of overshoot. In contrast, preparatory S1–S2 activity in the caudal SMA showed a statistical trend toward a positive correlation with the individual overshoot in the HL condition (peak correlation at x, y, z = −9, −18, 54; Z = 3.92, Puncorrected < 0.001). The greater the preparatory activity in left caudal SMA, the greater the overshoot in peak grip force when lifting the light weight after having prepared for lifting the heavy weight. This statistical relationship did not survive whole brain correction for multiple comparisons.
Preparatory brain activity after inhibitory theta burst stimulation of left PMd
The level of preparatory neuronal activity in left PMd was not altered by the real cTBS80% protocol compared with control cTBS30%. However, real cTBS80% of left PMd abolished the relationship between the preparatory activity in the stimulated left PMd and the behavioral effect of incorrect pre-cueing on force generation (Fig. 7B). In the control session with low-intensity cTBS30%, interindividual variations of preparatory activity in left PMd predicted the behavioral effect of an incorrect LIGHT pre-cue on the undershoot in grip force (r = 0.85; p = 0.001), whereas preparatory activity in the stimulated left rostral PMd no longer predicted individual variations in the relative undershoot in LH trials after real cTBS80% (r = 0.24; p = 0.48).
We examined whether interindividual variations in the effects of real cTBS80% on anticipatory grip force control (i.e., the reduction in grip force undershoot in LH trials) were associated with interindividual variations in cTBS80%-induced changes in preparatory S1–S2 activity. Specifically, we were interested to test whether the interindividual differences in cTBS80%-induced change of GF undershoot (i.e., change in LH:HH ratio) were correlated with cTBS80%-induced changes in weight-specific preparatory activation (i.e., preparatory S1–S2 activity for light lifts relative to heavy lifts). Given its involvement in conditional response selection based on arbitrary sensory cues (Sakai et al., 1999, Kurata et al., 2000), we reasoned that real cTBS80% of left rostral PMd might trigger a compensatory increase in preparatory activity for lightweight lifts in rostral SMA, and that this compensatory recruitment might vary across subjects.
Confirming our hypothesis, a cluster in left rostral SMA (peak correlation at x, y, z = −6, 18, 54; Z = 3.32, Psvc < 0.001 and x, y, z = −15, 12, 57; Z = 2.99; Psvc = 0.001) showed a linear relationship between the cTBS effect on weight-specific preparatory activity and undershoot in LH trials (r = 0.868; p = 0.001; Fig. 8): In subjects showing a relative increase in preparatory S1–S2 activity for light lifts (relative to heavy lifts) after real cTBS80%, real cTBS80% did not affect the undershoot in response to an incorrect S1 pre-cue (Fig. 8). Conversely, real cTBS80% induced a clear reduction in grip force undershoot in those subjects showing no increase in preparatory S1–S2 activity for light lifts (relative to heavy lifts) (Fig. 8). Additional clusters showing the same linear relationship were located in the left globus pallidus internus (peak at stereotactic coordinates x, y, z = −12, 3, 6; Z = 3.78 Punc < 0.001) and right medial prefrontal cortex (peak at x, y, z = 9, 57, 33; Z = 3.95; Punc < 0.001).
Figure 8.
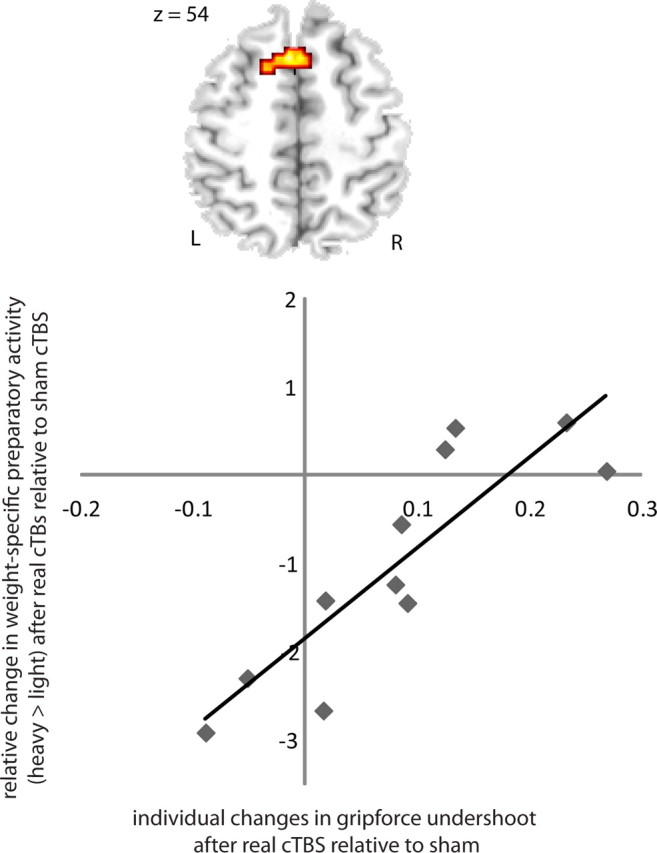
Relationship between cTBS-induced change in force undershoot and weight-specific preparatory activity in left rostral SMA. Subjects in whom fMRI revealed a relative increase in preparatory S1–S2 activity for light lifts (relative to heavy lifts) after real cTBS80%, showed no or little change in grip force undershoot (LH/HH ratio) after real cTBS80%. Conversely, real cTBS80% induced a clear reduction in grip force undershoot in those subjects who showed an increase in preparatory S1–S2 activity for light lifts (relative to heavy lifts) after real cTBS80%. The axial slice (top) illustrates the cluster in left rostral SMA showing a linear relation between cTBS-induced change in force undershoot and weight-specific preparatory activity. The corresponding scatter plot for the peak voxel in left rostral SMA is illustrated in the bottom (x, y, z = −6, 18, 54). The regression line gives the estimated linear relation.
We also tested for a linear relationship between the individual decreases in MEP amplitude after real cTBS80% and preparatory activity during the task. The interindividual variation in MEP suppression did not correlate with individual changes in BOLD signal in the left rostral PMd during the preparatory period (r = 0.036; p = 0.917).
Discussion
The experiments yielded three main findings. First, cTBS80% of left PMd selectively impairs anticipatory downscaling, abolishing the grip force undershoot but not overshoot in trials with incorrect S1. Second, in the absence of cTBS80%, individual variations in preparatory activity of left PMd as triggered by a “lightweight” cue predicted the relative grip force undershoot in LH trials. Third, this association between preparatory activity in left PMd and individual variations in grip force undershoot was cancelled by cTBS80% of left PMd. Together, these experiments offer the first demonstration that human left PMd contributes to anticipatory downscaling of grip force based on arbitrary visual cues.
Anticipatory grip force control based on arbitrary visual cues
Grip-and-lift tasks involving small objects have been used intensively to study the role of prediction on sensorimotor control (Flanagan et al., 2006, Johansson and Flanagan, 2009). Here, we used a novel S1–S2 paradigm in which predictive grip force control was informed by prior visual information based on arbitrary cues. In contrast to previous work (Chouinard et al., 2005), anticipatory force scaling was challenged by introducing a conflict between two “predictive” visual cues, an incorrect S1 pre-cue and a correct S2 go-cue, rather than by causing a conflict between an incorrect visual cue and somatosensory feedback during the task signaling the prediction error. Because the S2 go-cue was always correct, task performance always created somatosensory feedback that was concordant with the predictive visual information provided by this cue. However, including a S1 cue did not prevent the subjects to take the previous lift into account for the scaling of gripforce, therefore we propose that there are two scaling mechanisms that are reflected in the behavioral data (proprioceptive and visual), but cTBS80% only influenced the visually cued interference and not the proprioceptive interference. These results are in line with previous data showing that the proprioceptive information gained during the previous can be disturbed by inhibiting the primary cortex and that the visuomotor information is stored in the PMd (Chouinard et al., 2005).
Although the S2 go-cue always provided the correct information about object weight, the incorrect S1 cue still interfered with anticipatory force control causing an undershoot (in case of an incorrect lightweight pre-cue) or an overshoot (in case of an incorrect “heavyweight” pre-cue). This finding indicates that subjects actually used the S1 pre-cue for motor preparation. It further shows that the correct predictive information provided by the S2 go-cue was not sufficient to rapidly discard the inappropriate preparatory set evoked by the incorrect S1 stimulus.
The longer reaction times after an incorrect S1 stimulus indicates that grip initiation was delayed to allow for partial reprogramming of grip force (Loh et al., 2010). Without a delay in reaction time, the undershoot (after an incorrect lightweight pre-cue) and the overshoot (after an incorrect heavyweight pre-cue) might have been even higher. Interestingly, the relative overshoot caused by an incorrect heavyweight pre-cue in HL-trials was more pronounced in magnitude as opposed to the relative undershoot produced by incorrect lightweight pre-cues in LH-trials. This might reflect a general bias of the motor system to apply too much rather than too little grip force to avoid dropping the object.
The variable delay between the S1 pre-cue and the S2 go-cue enabled us to dissociate preparatory activity in left PMd from event-related activity evoked by the visual cues, or by task performance itself. We reasoned that if left PMd codes the predictive information revealed by the S1 pre-cue, preparatory activity in left PMd should predict the behavioral consequences of an incorrect S1 pre-cue on force scaling. In fact, preparatory activity of left PMd predicted interindividual variations in grip force undershoot following an incorrect lightweight pre-cue. This was, however, not the case when an incorrect heavyweight pre-cue caused an overshoot in force scaling. Here it was the SMA rather than the PMd showing a correlation between preparatory activity and interindividual variations in grip force overshoot in HL-trials. The results suggest that the left PMd is primarily concerned with predictive downscaling of grip force, while other premotor areas such as the SMA might preferentially support predictive upscaling of grip force in humans (Vaillancourt et al., 2007, Haller et al., 2009).
Causal involvement of PMd in anticipatory force scaling
The transient dysfunction of left PMd (as induced by cTBS80%) impaired the ability to implement prior information given by the lightweight pre-cue into motor preparation. cTBS80% of left PMd abolished predictive grip force undershoot in trials with incorrect S1 without having any consistent effect on anticipatory upscaling of grip force in response to a heavyweight pre-cue. Furthermore, cTBS80% abolished the relationship between preparatory PMd activity and individual variations in grip force undershoot. We infer that preparatory activity in PMd tunes the motor system toward low grip forces and thus prevents inappropriately high force levels.
Two previous rTMS studies showed that inhibitory rTMS applied over the left PMd impairs the ability to use arbitrary visual information for anticipatory force scaling in a grip-and-lift task (Chouinard et al., 2005, Nowak et al., 2009). Specifically, after 1 Hz rTMS (Chouinard et al., 2005) or cTBS (Nowak et al., 2009), healthy subjects no longer used the weight information provided by the color of the go-cue, but scaled their forces to the weight of the previous lift (Chouinard et al., 2005). In this study, the grip force task critically differed from the task used by Chouinard et al. (2005) and Nowak et al. (2009) in that the visual go-cue was always correct. This ensured that subjects used a visual mode of anticipatory force control. In trials with incorrect pre-cues, anticipatory grip force control had to integrate the diverging visual information provided by the S1 and S2 cue. Our task enabled us to assess the specific involvement of PMd in anticipatory upscaling and downscaling of grip force based on arbitrary visual cues. Extending previous work (Chouinard et al., 2005, Nowak et al., 2009), we show that a transient disruption of the PMd selectively impaired downscaling of force if a visual mode of anticipatory control is reinforced by the paradigm.
The preparatory S1–S2 period did not only require to prepare for the grip and lift, but also to withhold the grip until the appearance of the S2 go-cue. Application of the GABA antagonist bicuculline in monkeys to the PMd reduces the ability to withhold reaching movements of the contralateral limb in a visually guided reaching task (Sawaguchi et al., 1996). This raises the possibility that in the present study, regional PMd activity during the S1–S2 period was not only related to motor preparation but also to preventing a premature grip. This inhibitory activity should be larger in trials in which subjects prepared for a heavy lift. In fact, we found a higher level of S1–S2 activity in left PMd when subjects prepared for lifting a heavy weight as opposed to a light weight. We hypothesize that in left PMd, inhibitory activity preventing a premature motor response prevailed in the S1–S2 period following a heavyweight pre-cue, whereas preparatory activity coding the anticipated force dominated the S1–S2 period following a lightweight pre-cue. This would explain why S1–S2 activity in PMd only predicted the relative force undershoot in LH-trials with an incorrect lightweight pre-cue, but not overshoot in HL-trials with an incorrect heavyweight pre-cue.
A recent electrophysiological study in two monkeys supports the notion that inhibitory processes in PMd might prevail during motor preparation (Kaufman et al., 2010). Extracellular recordings were obtained from chronically implanted multielectrode arrays in contralateral PMd while monkeys performed a visuospatially instructed delayed reach task. Recordings revealed a significant rise in overall firing rate of inhibitory interneurons, but not pyramidal cells during the delay period. This raises the possibility that in our fMRI measurements, the BOLD signal increase in PMd during the S1–S2 period was mainly driven by a net increase in inhibitory activity.
We propose that one important role of the PMd is to prevent excessive motor activity during motor preparation and execution. Hence, when preparing for a heavy lift, PMd activity during the S1–S2 period is more concerned with preventing a premature grip. Conversely, the PMd is more engaged in preparatory downscaling of the grip force level when preparing for a light lift.
A similar effect, albeit not reaching significance, was also found for lift force control in the present study. Furthermore, several previous neuroimaging studies reported that the higher the premotor activity, the less the force was that had to be applied across a range of manual tasks, including a power grip task (Ward and Frackowiak, 2003), index finger abduction(van Duinen et al., 2008), static precision grip (Kuhtz-Buschbeck et al., 2001), and dynamic precision grip (Ehrsson et al., 2001). Therefore, it can be assumed that the involvement of PMd in downscaling is not specific to the present paradigm, but generalizes across all manual skills requiring a fine and flexible tuning of the motor output.
In everyday life, we do not integrate two conflicting sources of predictive weight information when grasping and lifting an object. The ability to effectively rescale the motor output based on visual cues is more relevant to visually guided activities required by human-machine systems, for instance the manipulation of tools during minimally invasive surgery or performing a landing with an airplane.
In contrast to predictive force scaling based on arbitrary visual cues, cTBS80% over left PMd did not influence anticipatory force scaling based on the weight of the previous grip. Despite of the presence of a visual S1 pre-cue, the motor system still implemented the somatosensory information about the weight of the previous lift in anticipatory grip force control. This mechanism was not modified by premotor cTBS, suggesting that the stimulated left PMd does not play a crucial role in anticipatory grip force control based on the somatosensory information obtained during the previous lift. This notion is in good agreement with a previous TMS study (Chouinard et al., 2005) in which inhibitory 1 Hz rTMS of left M1HAND but not 1 Hz rTMS of PMd impaired predictive scaling of forces based on information acquired during a previous lift. Together, these findings suggest two complementary mechanisms of anticipatory grip force scaling based on arbitrary visual or somatosensory inputs with a selective involvement of the PMd in the former and the M1HAND in the latter.
Redistribution of preparatory activity within premotor areas
The magnitude of the disruptive effect of cTBS80% over left PMd on predictive downscaling of grip force correlated with a shift in preparatory S1–S2 activity in left SMA: cTBS80% did not affect predictive downscaling, when SMA increased its preparatory activity for lightweight lifts (relative to heavyweight lifts) after cTBS80%. Conversely, cTBS80% disrupted predictive downscaling, when SMA was unchanged after cTBS80%.
The putative role of the rostral SMA in motor control makes this region a plausible candidate for functional compensation: the rostral SMA is critical to conditional response selection based on learned rules (Sakai et al., 1999, Kurata et al., 2000, Donohue et al., 2008). It shows sustained activity during tasks requiring delayed rule-based responses and is engaged in producing appropriate and withholding inappropriate motor responses according to these rules (Mostofsky and Simmonds, 2008), including the reprogramming of actions based on changes in conditional response rules (Neubert et al., 2010). We therefore propose that in this study, a relative increase in preparatory activity of rostral SMA effectively compensated for the lesion effect induced in left PMd. This finding speaks against a strict functional segregation between lateral and medial premotor areas in predictive motor control. It suggests a gradual functional differentiation that enables the motor system to maintain functional integrity in the presence of a focal lesion by redistributing neural activity between medial and lateral premotor areas.
Our results further show that the ability to recruit the rostral SMA varied from subject to subject, resulting in a variable behavioral deficit. This observation highlights the potential of a combined neuroimaging-rTMS approach to identify individual differences in functional reorganization at behavioral level.
Footnotes
B.F.L.v.N. and B.R.B. were supported by De Nederlandse Organisatie voor Wetenschappelijk Onderzoek (VIDI Research Grant #016.076.352). H.R.S. was supported by a structural grant to NeuroImageNord (Bundesministerium für Bildung und Forschung Grant no. 01GO 0511) and a grant of excellence by the LundbeckFonden on the Control of Action (ContAct, Grant no. R59 A5399).
The authors declare no competing financial interests.
References
- Amiez C, Kostopoulos P, Champod AS, Petrides M. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J Neurosci. 2006;26:2724–2731. doi: 10.1523/JNEUROSCI.4739-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussaoud D. Attention versus intention in the primate premotor cortex. Neuroimage. 2001;14:S40–45. doi: 10.1006/nimg.2001.0816. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Van Der Werf YD, Leonard G, Paus T. Modulating neural networks with transcranial magnetic stimulation applied over the dorsal premotor and primary motor cortices. J Neurophysiol. 2003;90:1071–1083. doi: 10.1152/jn.01105.2002. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci. 2005;25:2277–2284. doi: 10.1523/JNEUROSCI.4649-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MS, Lundbye-Jensen J, Geertsen SS, Petersen TH, Paulson OB, Nielsen JB. Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat Neurosci. 2007;10:417–419. doi: 10.1038/nn1873. [DOI] [PubMed] [Google Scholar]
- Cole KJ, Rotella DL. Old age impairs the use of arbitrary visual cues for predictive control of fingertip forces during grasp. Exp Brain Res. 2002;143:35–41. doi: 10.1007/s00221-001-0965-9. [DOI] [PubMed] [Google Scholar]
- Donohue SE, Wendelken C, Bunge SA. Neural correlates of preparation for action selection as a function of specific task demands. J Cogn Neurosci. 2008;20:694–706. doi: 10.1162/jocn.2008.20042. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, King S, Wolpert DM, Johansson RS. Sensorimotor prediction and memory in object manipulation. Can J Exp Psychol. 2001;55:87–95. doi: 10.1037/h0087355. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Bowman MC, Johansson RS. Control strategies in object manipulation tasks. Curr Opin Neurobiol. 2006;16:650–659. doi: 10.1016/j.conb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–2053. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol. 1993;69:1789–1796. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Schmitt P, Van Horn J, Diedrichsen J. Neural substrates of visuomotor learning based on improved feedback control and prediction. Neuroimage. 2008;39:1383–1395. doi: 10.1016/j.neuroimage.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Chapuis D, Gassert R, Burdet E, Klarhöfer M. Supplementary motor area and anterior intraparietal area integrate fine-graded timing and force control during precision grip. Eur J Neurosci. 2009;30:2401–2406. doi: 10.1111/j.1460-9568.2009.07003.x. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Siebner HR, Bakker M, Münchau A, Bloem BR. Repetitive transcranial magnetic stimulation to improve mood and motor function in Parkinson's disease. J Neurol Sci. 2006;248:84–96. doi: 10.1016/j.jns.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential involvement of neurons in the dorsal and ventral premotor cortex during processing of visual signals for action planning. J Neurophysiol. 2006;95:3596–3616. doi: 10.1152/jn.01126.2005. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci. 2009;10:345–359. doi: 10.1038/nrn2621. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71:59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Kaufman MT, Churchland MM, Santhanam G, Yu BM, Afshar A, Ryu SI, Shenoy KV. Roles of monkey premotor neuron classes in movement preparation and execution. J Neurophysiol. 2010;104:799–810. doi: 10.1152/jn.00231.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci. 2001;14:382–390. doi: 10.1046/j.0953-816x.2001.01639.x. [DOI] [PubMed] [Google Scholar]
- Kurata K, Tsuji T, Naraki S, Seino M, Abe Y. Activation of the dorsal premotor cortex and pre-supplementary motor area of humans during an auditory conditional motor task. J Neurophysiol. 2000;84:1667–1672. doi: 10.1152/jn.2000.84.3.1667. [DOI] [PubMed] [Google Scholar]
- Loh MN, Kirsch L, Rothwell JC, Lemon RN, Davare M. Information about the weight of grasped objects from vision and internal models interacts within the primary motor cortex. J Neurosci. 2010;30:6984–6990. doi: 10.1523/JNEUROSCI.6207-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 1992;85:17–21. doi: 10.1016/0168-5597(92)90096-t. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107:13240–13245. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DA, Berner J, Herrnberger B, Kammer T, Grön G, Schönfeldt-Lecuona C. Continuous theta-burst stimulation over the dorsal premotor cortex interferes with associative learning during object lifting. Cortex. 2009;45:473–482. doi: 10.1016/j.cortex.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Passingham RE, Toni I, Schluter N, Rushworth MF. How do visual instructions influence the motor system? Novartis Found Symp. 1998;218:129–141. doi: 10.1002/9780470515563.ch8. discussion 141–146. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Imaging the premotor areas. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Pütz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci. 1999;19:RC1. doi: 10.1523/JNEUROSCI.19-10-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaguchi T, Yamane I, Kubota K. Application of the GABA antagonist bicuculline to the premotor cortex reduces the ability to withhold reaching movements by well-trained monkeys in visually guided reaching task. J Neurophysiol. 1996;75:2150–2156. doi: 10.1152/jn.1996.75.5.2150. [DOI] [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends Cogn Sci. 2007;11:211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Suppa A, Bologna M, Gilio F, Lorenzano C, Rothwell JC, Berardelli A. Preconditioning repetitive transcranial magnetic stimulation of premotor cortex can reduce but not enhance short-term facilitation of primary motor cortex. J Neurophysiol. 2008;99:564–570. doi: 10.1152/jn.00753.2007. [DOI] [PubMed] [Google Scholar]
- Toni I, Rowe J, Stephan KE, Passingham RE. Changes of cortico-striatal effective connectivity during visuomotor learning. Cereb Cortex. 2002;12:1040–1047. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. doi: 10.1016/j.neuroimage.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits NM, Zijdewind I. Relation between muscle and brain activity during isometric contractions of the first dorsal interosseus muscle. Hum Brain Mapp. 2008;29:281–299. doi: 10.1002/hbm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Münchau A, Büchel C, Weiller C, Siebner HR. Implementation of visuospatial cues in response selection. Neuroimage. 2006;29:286–294. doi: 10.1016/j.neuroimage.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Bestmann S, Hartwigsen G, Weiss MM, Christensen LO, Frackowiak RS, Rothwell JC, Siebner HR. Low-frequency transcranial magnetic stimulation over left dorsal premotor cortex improves the dynamic control of visuospatially cued actions. J Neurosci. 2010;30:9216–9223. doi: 10.1523/JNEUROSCI.4499-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]