Abstract
The organophosphorus chemical warfare agents were initially synthesized in the 1930’s and are some of the most toxic compounds ever discovered. The standard means of decontamination are either harsh chemical hydrolysis or high temperature incineration. Given the continued use of chemical warfare agents there are ongoing efforts to develop gentle environmentally friendly means of decontamination and medical counter measures to chemical warfare agent intoxication. Enzymatic decontamination offers the benefits of extreme specificity and mild conditions, allowing their use for both environmental and medical applications. The most promising enzyme for decontamination of the organophosphorus chemical warfare agents is the enzyme phosphotriesterase from Pseudomonas diminuta. However, the catalytic activity of the wild-type enzyme with the chemical warfare agents falls far below that seen with its best substrates, and its stereochemical preference is for the less toxic enantiomer of the chiral phosphorus center found in most chemical warfare agents. Rational design efforts have succeeded in the dramatic improvement of the stereochemical preference of PTE for the more toxic enantiomers. Directed evolution experiments, including site-saturation mutagenesis, targeted error-prone PCR, computational design, and quantitative library analysis, have systematically improved the catalytic activity against the chemical warfare nerve agents. These efforts have resulted in greater than 4-orders of magnitude improvement in catalytic activity and have led to the identification of variants that are highly effective at detoxifying both G-type and V-type nerve agents. The best of these variants have the ability to prevent intoxication when delivered as a post-exposure treatment for VX and as a pre-exposure treatment for G-agent intoxication with observed protective factors up to 60-fold. Combining the best variant, H257Y/L303T, with a PCB polymer coating has enabled the development of a long lasting circulating prophylactic treatment that is highly effective against sarin.
Keywords: organophosphate, chemical warfare agents, nerve agent, phosphotriesterase, organophosphate hydrolase, enzyme evolution
Graphical Abstract

1.1. BACKGROUND
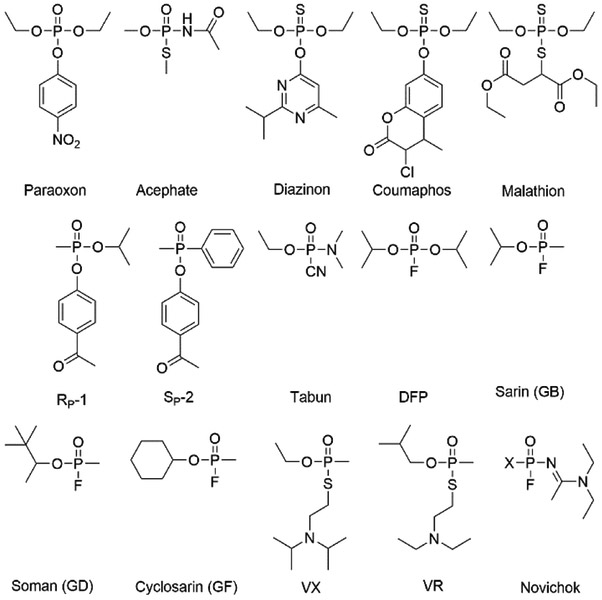
The organophosphate nerve agents are among the most toxic compounds known. The neurotoxic effects of the organophosphates are due to their ability to act as irreversible inhibitors of the enzyme acetylcholine esterase, which is required to terminate nerve stimulation at synapses.1, 2 Organophosphate nerve agents were originally discovered in the search for agricultural insecticides, and their impact on modern agriculture is immense with thousands of tons being applied annually for crop protection.8,4 Unfortunately, the most toxic organophosphates have been utilized as chemical warfare agents.4 The insecticides generally contain either a diethyl or dimethyl phosphorus center, as exemplified by paraoxon, and have LD50 values on the order of 1 mg/kg (Scheme 1).5 The more modern insecticides, like malathion or diazinon, use a thiophosphate core, which reduces the toxicity to mammals from ten to 100-fold.6, 7 By contrast, the first organophosphate chemical warfare agent, tabun (GA), which was discovered in Germany in 1937, has an LD50 of 0.12 mg/kg.4, 8
Scheme 1.
Structures of organophosphorus insecticides and chemical warfare nerve agents.
German scientists followed up the discovery of tabun in 1937 with the discoveries of sarin (GB) in 1938 and soman (GD) in 1944, which collectively became known as the G-type agents.4 Unlike the achiral insecticides, the chemical warfare agents are chiral phosphonates and the toxicity of the SP-enantiomer is significantly greater than the RP-enantiomer.8 The more toxic enantiomer of GB has a subcutaneous LD50 of 41 μg/kg, while GD has a subcutaneous LD50 of 38 μg/kg. The final major member of the G-agent series, cyclosarin (GF), was discovered at the end of World War II and has an LD50 of 46 μg/kg.4, 9
In the 1950’s, England and the United States developed a new series of organophosphate chemical warfare agents known as the V-type agents.4 The initial member of this series, amiton, like the G-agents, was originally intended as an insecticide, but its toxicity was considered too high for commercial applications. The V-agents use a thiol containing leaving group and have substantially more toxicity than the G-agents, as well as being more persistent in the environment.10 The most well-known V-agent, VX, was developed as a chemical warfare agent by the British during the 1950s.4 VX has an intravenous LD50 of 13 μg/kg.8 Independently, the Russians developed a similar compound, VR, which has a subcutaneous LD50 of 11 μg/kg.11, 12 The final class of chemical warfare agent was developed by the Russian military during the 1970’s.12 These compounds, termed Novichok agents, combine the fluorine leaving group of the G-agents, with a nitrogen containing substituent. While toxicological data are not publicly available for these compounds, they are anecdotally said to be either similar in toxicity to VR, or substantially more toxic.12, 13
1.2. MODERN USE AND DECONTAMINATION OF CHEMICAL WARFARE AGENTS
Since the signing of the Chemical Weapons Convention in 1993, the manufacture, stockpiling and use of chemical weapons has largely been curtailed by major countries.14 However, multiple instances in recent history have highlighted the continued need to find the means of effectively neutralizing these highly toxic compounds. The use of sarin has been documented in Ghouta and Khan Shaykhun, Syria in 2013 and 2017, respectively.15, 16 The attack on Ghouta resulted in the deaths of approximately 1400 civilians. VX was used in an assassination in Kuala Lampur, Malaysia in 2017,17, 18 and a Novichok agent was used in an attempted assassination in Salisbury, United Kingdom in 2018, which resulted in the death of an unintended civilian.19 The high toxicity of organophosphorus nerve agents has also made them of interest to terrorist and extremist organizations. The Aum Shinrikyo group in Japan was able to utilize sarin and VX in terrorist activities. The most serious of these attacks resulted in the deaths of 19 people.20, 21
1.3. DECONTAMINATION OF AND MEDICAL TREATMENT OF ORGANOPHOSPHATE NERVE AGENTS
The high toxicity, ease of synthesis, and indiscriminate nature of organophosphate nerve agents have led a search for effective means of decontamination. Unfortunately, there are currently no non-destructive and environmentally sensitive methods for the decontamination of chemical warfare agents. In response to the use of a chemical warfare agent in Salisbury, UK in 2018, all potentially contaminated items were removed and incinerated.19 Other widely used methods currently employed to decontaminate chemical warfare agents generally involve high concentrations of base, concentrated sodium hypochlorite, or high temperature incineration, all of which are destructive and release potentially hazardous by-products.22 Decontamination of living victims is currently limited to attempting to wash the agent off the skin using either water or dilute bleach.23 Medical counter measures are currently limited to injection of atropine to counter the effects of the nerve agent, benzodiazepines to control seizures, and oximes such as 2-PAM, which have a limited ability to reactivate acetylcholine esterase.23,24 There are no currently available medical means to detoxify chemical warfare agents once in the body of a victim.
Enzymatic action has the potential to provide the means of non-destructive and environmentally sensitive decontamination that would be able to treat both large areas and living victims, as well as potentially being adapted as a medical prophylactic or treatment for organophosphate intoxication. Numerous enzymes have been identified, which are able to degrade organophosphate nerve agents (reviewed in Goldsmith 201825 and Bigley 201326). The best studied enzyme is the Pseudomonas diminuta phosphotriesterase (PTE), now known as Brevundimonas diminuta. PTE was first isolated in 1974 from mixed soil and sewage bacterial cultures under enrichment for parathion hydrolysis.27 The gene for the protein was found to be located on a plasmid. An identical protein was identified from a Flavobacterium sp. in 1973, also located on a plasmid, from soil contaminated with diazinon.28 While the proteins were identical, the plasmids were found to be unique.29 Much later, a close homolog (>90% sequence identity) was found on a transposable element in Agrobacterium radiobacter.30 The best substrate for PTE is the insecticide paraoxon, where the cleavage of the phosphorus oxygen bond eliminates the neurotoxicity of the organophosphate (Figure 1A).31
Figure 1:
(A) Hydrolysis of paraoxon catalyzed by PTE. (B) Fisher diagram of a chiral substrate for PTE binding in the active site of PTE. Large group binding pocket shown as magenta circle, small group binding pocket represented by blue circle. (C) Substrate binding pockets of PTE with substrate analog diisopropyl methyl phosphonate. Large group pocket (His254, His257, Leu271, and Met17) is shown in magenta. Small group pocket (Gly60, Ile106, Thr303, Ser308) is shown in blue, Leaving group pocket (Trp131, Phe132, Phe306, Tyr309) is shown in white. Figure prepared from PDB:1EZ2 using UCSF Chimera.83
2.1. STRUCTURE, MECHANISM AND SUBSTRATE PROFILE OF PTE
PTE was found to be a metal dependent hydrolase with zinc as the native metal, although the zinc can be substituted with nickel, manganese, cobalt, or cadmium.32, 33 NMR and EPR studies showed that the enzyme contained a coupled binuclear metal center.34, 35 The crystal structure with an intact metal center was solved in 1995 and inhibitor bound structures followed in 1996.36, 37 The structure of PTE was found to be dimeric with a (α/β)8-barrel protein fold, where the eight core helixes are wrapped around the eight core β-strands (Figure 2A). The metal binding site is found at the C-terminal end of the central core with ligating residues coming from the ends of the β-strands. The α-metal is more buried and is ligated by His55 and His57 and a carboxylate group from Asp301 (Figure 2B). The more solvent exposed β-metal is ligated by His201 and His230. Additionally, the metals are bridged by a carboxylated side chain from Lys169 and a hydroxide. The bridging hydroxide is hydrogen bonded to the free oxygen of Asp301, which also hydrogen bonds with His254. In the apo-structure there is an additional water molecule ligated to the β-metal. In the substrate analog bound form, the ligand is found with the phosphoryl oxygen ligated to the β-metal in the place of the terminal water. In the structure solved using the substrate analog diisopropyl methyl phosphonate, a tight coordination to the β-metal is observed with a bond distance of 2.54 Å (Figure 2C).38 Additional EPR experiments were utilized to demonstrate that substrate interaction with the β-metal was in fact catalytically relevant.39
Figure 2:
Crystal structure of PTE. (A) (α/β)8 fold of the PTE monomer with central β-strands colored orange. Substrate binding residues and metals are shown as sticks. Active site loop-7 is shown in blue. (B) Metal center of PTE. Green disk shows the plane of the metal center as defined by the coordinating nitrogen atoms form His57, His201, and His230. Also shown is His254, which is proposed to shuttle protons out of the active site. (C) Substrate analog diisopropyl methyl phosphonate bound to the metal center of PTE. Figure was constructed from PDB:1EZ2 using USCF Chimera.83
The enzymatic mechanism of PTE has been reviewed recently and will only be covered briefly here.26 It has been demonstrated that hydrolysis of organophosphates by PTE proceeds via a direct nucleophilic attack of a hydroxide on the phosphorus center resulting in inversion of stereochemistry.40 The bridging hydroxide in the metal center was identified as the attacking nucleophile by examination of the pH dependence of the binuclear coupling in the manganese substituted enzyme.41 Isotope effects and linear free energy analysis determined that the enzyme catalyzed reaction goes through a late transition state with near complete bond cleavage to the leaving group.42-44 The catalytic mechanism of PTE is shown in Scheme 2 45 In this mechanism the binding of substrate displaces the terminal water bound to the β-metal. The phosphoryl oxygen coordinates to the metal, polarizing the phosphorus center and allowing nucleophilic attack to occur by the bridging hydroxide. The leaving group departs as the unprotonated species, and the proton from the hydroxide is abstracted by the carboxyl group of Asp301, which in turn passes the proton to His254 and on to bulk solvent via a proton shuttle. The single coordination of the substrate to the β-metal and the bidentate binding of the phosphodiester product have been observed by both EPR spectroscopy and X-ray crystallography.38, 46
Scheme 2:
Catalytic mechanism of PTE. Proton from reaction is shuttled to solvent via His254.
The best substrates for PTE are insecticides with electron withdrawing aromatic leaving groups such as paraoxon (Scheme 1)33 However, the substrate profile of PTE is quite broad. PTE can hydrolyze phosphorothiol insecticides such as acephate.47 Some phosphotriesterase enzymes are noted for a lack of activity against thiophosphates,48 but PTE will readily hydrolyze thiophosphate insecticides with both aromatic alcohol leaving groups, such as diazinon and coumaphos,32 and thiol leaving groups, such as malathion.49 PTE will also quite readily hydrolyze both phosphonates and phosphinates, such as compounds 1 and 2.50, 51 While typical substrates for PTE contain alcohol or thiol leaving groups, PTE will also catalyze the cleavage of the phosphorus carbon bond of tabun, as well as the cleavage of phosphorus halide bonds in diisopropyl fluorophosphate (DFP).47, 52 PTE is quite notable because of its ability to hydrolyze all of the known chemical warfare agents including GA, GB, GD, GF, VX, VR, and the Novichok agents.52-57 The enzymatic efficiency of PTE approaches the limits of diffusion for substrates such as paraoxon.58 Unfortunately, the activity with the chemical warfare agents falls orders of magnitude lower (Table 1). Against GA, the wild-type enzyme has an enzymatic efficiency of 7.6 × 105 M−1 s−1, which is 2-orders of magnitude lower than for the hydrolysis of paroxon.52 The activity falls to 1.5 × 104 M−1 s−1 for GD, and for VX and VR, the activity falls to 3 × 102 M−1 s−1 and 4.3 × 100 M−1 s−1, respectively.51, 53, 54
Table 1:
Kinetic constants for wild-type PTE with insecticides and nerve agent substrates.
| Compound |
kcat (s−1) |
Km (mM) |
kcat/Km (M−1 s−1) |
Ref |
|---|---|---|---|---|
| paraoxon | 7800 | 0.20 | 4.0 × 107 | 33 |
| acephate | 1.5 | 86 | 1.7 × 101 | 47 |
| diazinon | 200 | 0.45 | 4.5 × 105 | 32 |
| coumaphos | 700 | 0.39 | 1.8 × 106 | 32 |
| malathion | 2.5 | 0.41 | 6.2 × 103 | 49 |
| RP-1 | 100 | 0.17 | 5.8 × 105 | 51 |
| SP-1 | 40 | 1.5 | 2.7 × 104 | 51 |
| RP-2 | nd | nd | 1.1 × 103 | 50 |
| SP-2 | nd | nd | 3.2 × 106 | 50 |
| tabun | 77 | 0.10 | 7.6 × 105 | 52 |
| DFP | 290 | 0.57 | 5.1 × 105 | 47 |
| sarin (GB) | 430 | 1.8 | 2.4 × 105 | 56 |
| soman (GD) | 12 | 0.80 | 1.5 × 104 | 56 |
| cyclosarin (GF) | 210 | 0.90 | 2.3 × 105 | 56 |
| VX | 0.9 | 2.9 | 3 × 102 | 54 |
| SP-VR | nd | nd | 4.3 × 10° | 53 |
Originally, the substrate-binding site was probed using a series of chiral phosphate substrates with differing substituents attached to the phosphorus center.59, 60 This series is represented schematically in Figure 1B, where the two ester groups varied from methyl to phenyl alcohols. It was found that the wild-type enzyme preferred substrates that placed the larger of the groups to the right in the Fisher projection (SP- for phosphates, RP- for phosphonates). The substrate binding site of PTE can be subdivided into three binding pockets that interact with the leaving group (Trp131, Phe132, Phe306, Tyr309), the large group (His254, His257, Ile-271, Met317) and the small group (Gly60, Ile106, Leu303, Ser308), depending on how mutations altered the activity Figure 1C).37, 59
3.1. EVOLVING THE STEREOSELECTIVITY OF PTE BY RATIONAL DESIGN
Unfortunately, wild-type PTE prefers the less toxic enantiomer of the chemical warfare agents.51, 53, 54 Rational redesign of the substrate binding pocket of PTE was carried out using a series of phosphate, phosphonate and phosphinate substrates.61 Systematic modulation of the relative bulk of amino acid side chains in the large and small binding pockets were found to dramatically alter the stereochemical preference of PTE (Figure 3). Decreasing the size of the small group pocket by introduction of the mutation G60A, resulted in a dramatic increase in the enantioselectivity. Increasing the size of the small binding pocket with the mutation I106G, decreases the selectivity, and combining this mutation with F132G resulted in a reversal of the stereoselectivity. Simultaneously increasing the size of the small group pocket, while decreasing the size of the large group pocket resulted in a dramatic reversal of the enantiomeric selectivity observed in the wild-type enzyme. It was found that the stereoselectivity of PTE could be modulated over 8-orders of magnitude using rational design.61, 62
Figure 3:
Enhancement relaxation and reversal of enantioselectivity of PTE by rational design of substrate binding pocket. A = G60A, B = S308,. C = wild-type, D = I106G, E = I106G/F132G, F = I106T/F132A/H254G/ H257W, G = I106G/H257Y and H = I106G/F132G/H257Y. Figure reconstructed from reference 61.
3.2. ADAPTING PTE FOR G-AGENT DECONTAMINATION
A series of substrate analogs that incorporated the authentic chiral centers found in the chemical warfare agents with a p-nitrophenol or p-acetophenol leaving group was constructed to allow screening of PTE variants (Scheme 3).51, 63, 64 In enzyme evolution, site-saturation mutagenesis is a technique where small libraries are constructed that allow for all 20 amino acids at a site of interest to enable rapid identification of the optimal amino acid at that site. Site-saturation mutagenesis was carried out targeting the substrate binding residues of PTE. Screening against the (SPSC)-p-nitrophenyl analog of GD (SPSC-4) rapidly led to the identification of the H254G/H257W/L303T (GWT) variant of PTE, which is 1000-fold enhanced for this enantiomer relative to the wild-type enzyme (Figure 4).63 Screening against a p-acetophenyl analog also identified the variant H257Y/L303T (YT), which preferred the more toxic enantiomer and demonstrated broad substrate specificity.51 Further screening of site-saturation libraries enabled the identification of variants that were enhanced up to 3530-fold (GWT-d3 = GWT+A80V/K185R/I274N/S61T) for the SPSC-chiral center of GD (SPSc-4) and 1860-fold (GWT-f1 = GWT+M317L/K185R/I274N) for the (SP)-chiral center of GF (SP-5).56 Further recombination of variants as a partial six-site library enhanced the activity for the GF chiral center to 12,100-fold (GWT-f4 = GWT+A80V/K185R/ I274N/I106C/F132I/L271I). Further enhancement was achieved by screening of error-prone libraries against the GF chiral center to able the identification of a variant that was 15,500-fold (GWT-f5 = GWTf4 + R67H) enhanced for the hydrolysis of (SP)-5.56
Scheme 3.
Chiral center analogs used to evolve PTE for G-agent decontamination. X = NO2 or COCH3.
Figure 4:
Optimization of PTE for chemical warfare chiral centers. (a) Optimization for the GB chiral center. (b) Optimization for the GD chiral center. (c) Optimization for the GF chiral center. Variant identities are: QF = H254Q /H257F, QFRN = QF+K185R/I274N, GWT = H254G/H257W/ L303T, GWTd2 = GWT+A80V/K185R/I274N, GWTd3 = GWTd2+S61T, GWTf1 = GWT+M317L/K185R/I274N, GWTf4 = GWTd2 + I106C/F132I/L271I, GWTf5 = GWTf4 + R67H. Data from reference 56.
The variants of PTE which showed promise against the G-agent chiral center analogs were tested for activity with the authentic nerve agents.51, 56 The variant G60A, which has enhanced stereoselectivity for the (RP)-stereocenters, was found to prefer the (RP)-enantiomers of GB and GD by approximately an order of magnitude (Table 2).51 The GWT variant was found to have reversed stereoselectivity for the G-agent with an order of magnitude preference for the (SP)-enantiomer of GB. The variant was found to have reasonable activity against the (SP)-enantiomers of both GB and GD with kcat/Km of 1.5 × 104 M−1 s−1 and 2.2 × 104 M−1 s−1, respectively. Unfortunately, the further enhanced variants for the chiral center analogs did not perform well with the authentic nerve agents.56 In general, the additional mutations had little effect on the activity with the authentic nerve agents. However, the variant GWT-d2 (GWT + A80V/K185R/I274N) demonstrated approximately 5-fold better activity against GF than the GWT variant, and the GWT-f5 variant demonstrated 10-fold better activity against GB. Surprisingly, the broad specificity variant H257Y /L303T was found to have very high activity with all of the G-agents and to prefer the more toxic (SP)-enantiomers.51 With (SP)-GB, the YT variant was shown to have a catalytic activity of 3 × 105 M−1 s−1 with a kcat of 520 s−1 and a Km of 260 μM.51, 56 Similarly, against GD the kcat was 240 s−1 and the kcat/Km was ~1 × 105 M−1 s−1. Against GF a kcat of 130 s−1 and a catalytic efficiency of 8 × 105 M−1 s−1 were found.
Table 2.
Kinetic constants for variants of PTE with G-agents.*
| GB kcat/Km (M−1 s−1) | GD kcat/Km (M−1 s−1) | GB kcat/Km (M−1 s−1) | ||||
|---|---|---|---|---|---|---|
| Variant | RP | SP | RP | SP | RP | SP |
| wild-type | 9 × 104 | 9 × 104 | 2.6 × 103 | nd | 2.3 × 106 | nd |
| G60A | 1.2 × 105 | 2.7 × 104 | 2.5 × 104 | 2.2 × 103 | nd | nd |
| H254G/H257W/L303T | 9.7 × 102 | 1.5 × 104 | nd | 2.2 × 103 | nd | nd |
| H257Y/L303T | 3.7 × 104 | 3 × 105 | 6.8 × 103 | 8.9 × 104 | nd | 8 × 105 |
| PTE_28 | nd | nd | nd | 6.5 × 104 | nd | 3.2 × 106 |
| PTE_27.14 | nd | nd | nd | 5 × 105 | nd | 2.7 × 106 |
| PTE_27.16 | nd | nd | nd | 2.0 × 106 | nd | 2.3 × 106 |
An effort to computationally explore the sequence space of the PTE active site utilized a stabilized PTE variant (dPTE2 = T54M/K77D/S111E/R118E/L182R/A203D/A214D/S222D/ S238D/S264A/I274L/M293A/K294D/Q342D/A347E/G348T/T350D/T352D) and computationally predicted which sets of active site residues could be tolerated without dramatic loss of stability.65, 66 Initially, 49 variants were selected for purification based on the computational results, and a second round of computation carried out based on the variants which performed best in experiments. These experiments resulted in the identification of three variants, which showed improvement for GD and GF hydrolysis. PTE_28 (dPTE2 + I106C/H254G/H257W/L303T) demonstrated enzymatic efficiency of 6.5 × 104 M−1 s−1 for (SP)-GD and 3.2 × 106 M−1 s−1 for (SP)-GF. The two additional variants (PTE_27.14 = dPTE2 + H254G/H257W/L303T and PTE_27.16 = PTE_27.14 + F306I) displayed similar activity for (SP)-GF, but better activity against (SP)-GD with catalytic efficiencies of 5 × 105 M−1 s−1 and 2 × 106 M−1 s−1, respectively.
3.3. ADAPTION OF PTE FOR V-AGENT DECONTAMINATION
Unfortunately, the activity of wild-type PTE against the V-agents falls several orders of magnitude below that with the G-agent, and the V-agents are substantially more toxic.8, 11, 53, 54 Initial efforts to adapt PTE for V-agent decontamination utilized rational design of the active site. Targeting the active site residues His254 and His257 resulted in a variant with an apparent enhancement of approximately 10-fold over wild-type PTE, however, the kinetic constants and stereospecificity of these variants were not determined.67 Two different groups have utilized site-saturation mutagenesis of active site residues to enhance the active site of PTE for V-agent hydrolysis. Schofield and co-workers targeted all 12 active site residues sequentially, and screened against the insecticides malathion and demeton-S.49 Using this approach, they were able to identify a variant that showed an apparent 26-fold enhanced activity for VX-hydrolysis, but kinetic constants and stereospecificity were not determined. Raushel and coworkers developed a new substrate analog diethyl-VX which contains the authentic leaving group found in VX for library screening.54 Construction and screening of two-site saturation mutagenesis libraries identified the variant VRN-VQFL (A80V/K185R/I274N-F132V/H254Q/H257F/S308L), which was 233-fold enhanced for VX hydrolysis. VRN-VQFL shows only a 3-fold selectivity for the two enantiomers of VX with a catalytic efficiency of 1.1 × 105 M−1 s−1 for the faster enantiomer, although the preferred enantiomer was not identified (Table 3).
Table 3:
Kinetic constants for variants of PTE with V-agents.
| VX kcat/Km | VR kcat/Km | Ref | |||
|---|---|---|---|---|---|
| Variant | RP | SP | RP | SP | |
| Wild-type | 8.4 × 101 | 8.4 × 101 | 1.1 × 102 | 4.3 × 10° | 53 |
| VRN-VQFL | 1.1 × 105 | 4.3 × 104 | 2.4 × 103 | nd | 53 |
| L7ep3a* | 8.3 × 105 | 2.2 × 105 | 2.2 × 103 | 2.1 × 102 | 53 |
| L7ep3aG* | 2.0 × 104 | 6.2 × 103 | 6.9 × 102 | 2.6 × 103 | 53 |
| G5-C23 | 8.3 × 104 | 1.1 × 105 | 5.1 × 104 | 1.3 × 104 | 68 |
| G5-A53 | 3.3 × 103 | 2.9 × 104 | 1.1 × 104 | 5.8 × 104 | 68 |
| IV-A1 | nd | 4.2 × 104 | nd | 8.8 × 104 | 69 |
| d1-IVA1 | nd | 5.8 × 104 | nd | 2.0 × 105 | 70 |
| 10-1D11 | nd | 8.5 × 105 | nd | 4.5 × 104 | 70 |
| BHR-9 | 2.3 × 103 | NO | 7.23 × 103 | NO | 71 |
| BHR-19 | 6.1 × 104 | 6.1 × 104 | 1.25 × 105 | NO | 71 |
| BHR-23 | 4.2 × 104 | 7.7 × 105 | 3.0 × 104 | NO | 71 |
| BHR-39 | 2.9 × 102 | 2.6 × 104 | 8.2 × 103 | 1.3 × 103 | 71 |
| BHR-73 | 2.2 × 103 | 2.5 × 104 | 7.3 × 103 | 4.0 × 104 | 71 |
| BHR-73-MNW | 2.0 × 103 | 1.6 × 104 | 1.1 × 104 | 5.8 × 104 | 71 |
| BHR-75 | 7 × 102 | 1.1 × 104 | 1.1 × 102 | 1.3 × 104 | 71 |
Chiral preference inferred from chiral center analogs. NO = not observed. nd = not determined.
Further work on V-agent hydrolysis has involved more technically challenging techniques for enzyme evolution. Raushel and coworkers targeted one of the active site loops of PTE (loop-7) using targeted error-prone PCR (Figure 2).54 Using this technique, mutation rates for a particular segment of the gene can be much higher than can typically be obtained using error-prone PCR. Targeting loop-7 led to the discovery of the variant L7ep3a (I106C/F132V/H254Q/H257F/A270V/L272M/I274N/S308L), which has a catalytic efficiency of 8.3 × 105 M−1 s−1 for VX and is 9,900-fold enhanced over wild-type PTE.53 The L7ep3a variant was found to also hydrolyze VR with a maximal efficiency of 2.2 × 103 M−1 s−1, which is 20-fold improved over wild-type enzyme. It is thought that L7ep3a prefers the (RP)-enantiomer, based on work with chiral center analogs, although with a catalytic efficiency of 2.1 × 102 M−1 s−1 for the slower enantiomer, this variant is 49-fold improved over the wild-type PTE for (SP)-VR.53
Further development of substrate analogs to target VX and VR were undertaken resulting in the synthesis of dimethyl-VX (DMVX) as an alternate VX analog and O-methyl VR (OMVR) as a chiral VR analog.53 Using these analogs and the L7ep3a variant for the starting point of further rational design led to the discovery of the L7ep3a I106G variant, which has a preference for the (SP)-enantiomer of the VR-chiral center. With a catalytic efficiency of 2.6 × 103 M−1 s−1for VR and a preference for the more toxic enantiomer, this variant is ~600-fold improved for (SP)-VR.
Tawfik and coworkers were able to combine recombination experiments, where libraries of previously identified mutants are assembled in random order, with computational design of new mutations to discover new variants for the hydrolysis of the V-agents.68 Benefiting from the ability to screen libraries against authentic V-agents in an acetylcholine esterase inactivation assay, they were able to identify the variant G5-C23 (K77A/A80V/F132E/T173N/G208D/H254G/I274N), which has an enzymatic efficiency of 8.3 × 104 M−1 s−1 for (SP)-VX, as well as a 20-fold preference for the (SP)-enantiomer. In the same study, Tawfik and coworkers identified the variant G5-A53 (K77A/A80V/I106A/F132E/T173Q/G208D/A203F/H254G/I274N), which has a catalytic efficiency of 5.8 × 104 M−1 s−1 for (SP)-VR and is 5800-fold improved over wild-type.
Further shuffling of mutations and computational design using Rosetta software of potentially positive variants allowed the further enhancement of activity for VR. In the computational design phase, the potential transition state for the hydrolysis of VR was docked into the active site of the crystal structure of PTE to identify residues potentially in close contact with the substrate.69 Libraries were constructed with the predicted beneficial mutations and screened with VX or VR. The best-identified variant was IV-A1 (G5-A53 + C59M/T173N/F203A/D233G/Δ266/P342S), which had a Kcat/Km of 8.8 × 104 M−1 s−1. Later work focused on the computational stabilization of the protein structure, and recombining the IV-A1 mutant with a series of stabilizing mutations resulted in the variant d1-IVA1 (IV-A1 +Y309W/R118E/A203D/S222D/S238D/M293V/S342P/G348T/T352E), which has a Kcat/Km of 2 × 105 M−1 s−1 for (SP)-VR and is 17,000-fold improved over wild-type PTE.70 The addition of the stabilizing mutations along with the introduction of three additional computationally predicted mutations resulted in the variant 10-1D11 (G5-C23 + A80M/S276M/A270S/L271M/Y309W/R118E/A203D/S222D/S238D/M293V/G348T/T352E), which was found to have a kcat/Km of 8.5 × 10s M−1 s−1 for (SP)-VX, which is 10,000-fold better than the wild-type enzyme.70 Additional attempts to computationally redesign the PTE active site failed to yield improved variants.66
In recent work by Raushel and coworkers, a library was constructed of the active site mutations found in all of the reported variants enhanced for V-agent hydrolysis.71 The total library of 28,800 variants was screened to a 1.1-fold coverage against a panel of V-agent analogs. A total of 241-variants of interest were identified and data for all variants were subjected to quantitative analysis of the effects of the individual mutations. This work demonstrated a high degree of synergism that exists in the PTE system, which complicates the accumulation of small incremental increases in activity. Seventy-four variants of interest were purified and characterized with authentic V-agents. The best variant for VX was found to be BHR-23 (A80V/F132V/K185R/H254R/I274S), which has a Kcat/Km of 7.7 × 105 for (SP)-VX and an 18-fold preference for the (SP)-enantiomer. The best variant identified for (SP)-VR was BHR-73 (A80V/I106A/F132E/K185R/H254G/I274N), which has a Kcat/Km of 4.0 × 104 M−1 s−1 for (SP)-VR and is 9000-fold enhanced over wild-type pTE. Recombination of this variant with additional mutations identified in the work of Tawfik and coworkers as beneficial for VR hydrolysis, resulted in the variant BHR-73-MNW (BHR-73 +C59M/T173N/Y309W), which has a Kcat/Km of 5.8 × 104 M−1 s−1 and is 13,400-fold enhanced over the wild-type enzyme.68, 69 This variant demonstrates little selectivity with VR, however, many other variants identified possessed strong activity and stereoselectivity for both VX and VR allowing them to potentially be used to enzymatically purify isolated enantiomers of the V-type nerve agents.71 For example, the variant BHR-75 (A80V/I106A/F132E/K185R/H254Q/H257F/I274Q) hydrolyzes the (SP)-enantiomer with an efficiency of 1.3 × 104 M−1 s−1 and (RP)-VR with an efficiency of 1.1 × 103 M−1 s−1, giving a 120-fold selectivity. Similarly, BHR-19 (A80V/F132C/K185R/H254R/I274Q/S308L) has an enzymatic efficiency of 1.3 × 10s M−1 s−1 for (RP)-VR with more than 500-fold selectivity for this enantiomer, which will allow it to function in the facile isolation of the (SP)-enantiomer. With VX the variant BHR-9 (A80V/I106C/K185R/H254R/I274Q/S308L) is more than 100-fold selective for the (RP)-enantiomer, while BHR-39 has an 89-fold selectivity for the (SP)-enantiomer.
3.4. DEVELOPMENT OF PTE AS A MEDICAL COUNTER MEASURE
The use of pTE for environmental detection and decontamination of organophosphates and the development of other enzymes for medical treatment of organophosphate intoxication have been reviewed elsewhere.25, 72-74 Wild-type pTE has been shown to be able to protect test animals against intoxication by insecticides as well as the chemical warfare agent tabun.52, 75 Unfortunately, the higher toxicity of other chemical warfare agents results in wild-type PTE not providing sufficient protection against intoxication.76-78 The development of PTE variants with high activity for the toxic enantiomer of the G-agents and V-agents has renewed interest in PTE as an antidote for organophosphate intoxication.
The two main approaches to the medical application to the prevention of organophosphate toxicity are post-exposure antidote applications and pre-exposure prophylactic treatment. Because the majority of acetylcholine esterase is inhibited prior to the appearance of symptoms the prophylactic treatment is preferred, though more challenging, and requires long circulation times to be effective. Intramuscular injection would be the easiest route to achieve long duration treatment, however, organophosphates circulate quickly throughout the body upon exposure, so in order to afford protection against intoxication, PTE must circulate in the blood.79 Unfortunately, intramuscular injection of PTE fails to deliver PTE into the blood stream.79 Wales and coworkers were able to show that unmodified PTE has a mean retention time in the blood of approximately 1 h after intravenous injection.80 Modification of wild-type PTE by high molecular weight polyethylene glycol has been demonstrated to increase the mean retention time of PTE in the blood up to 47 h.80 However, they also showed that intravenous injection of PTE induced an immune response.
Worek and coworkers were able to demonstrate that the VX optimized variant of PTE G5-C23 can be used as a post exposure antidote to VX intoxication.79 The injection of 2-5 mg/kg body weight was able to prevent death and dramatically reduce symptoms of intoxication in guinea pigs when injected intravenously up to 15 min after exposure to an otherwise lethal dose of VX. They were further able to demonstrate that intraosseous injection of PTE could also prevent death when given as a post exposure treatment, however, the intraosseous administration was less effective at delaying the signs of intoxication than intravenous injection.81
The H257Y/L303T variant (YT-PTE) was found to be effective at preventing G-agent intoxication when given as a pretreatment (Douglas Cerisoli, personal communication). When injected 20 min prior to exposure to GA, 1 mg/kg body weight of YT-PTE was able to prevent all toxicity in guinea pigs. At 1 mg/kg a protective factor of 60 was found when animals were injected with GB. A dose of 5 mg/kg afforded a 5.5-fold protection against GD, and at 1 mg/kg a protective factor of 9.2 was observed when GF was used as the toxic challenge. Despite these encouraging results, in order for PTE to be used as a prophylactic treatment, the enzyme will need to stay in circulations for multiple days. Recent work by Jiang and coworkers found a poly(carboxybetaine) (PBC) polymer layer can be applied to PTE by covalent attachment to lysine residues on the surface of the enzyme.82 The application of the PBC polymer increased the circulation time 60-fold over the native enzyme, and dramatically reduced the measured immune response to intravenous injection of PTE. When the PBC polymer was applied to the YT-variant of PTE, injection of 5 mg/kg body weight afforded full protection against multiple 2 X LD50 doses of sarin, which were given daily until 7-days post treatment. This is the first demonstration of a truly prophylactic treatment against chemical warfare agents.
4. CONCLUSIONS
Much has been accomplished over the last few years in the development of PTE for the decontamination of chemical warfare agents. Variants are now available to specifically target the toxic (SP)-enantiomers of the chiral phosphorus centers of the chemical warfare agents. The broad-spectrum variant YT-PTE has been demonstrated to effectively decontaminate all of the G-agents, and when coupled to the PCB polymer coating provide long lasting protection against intoxication by G-agents. Multiple variants are now available to decontaminate the V-agents with the G5-C23 variant having been demonstrated as an effective post-exposure antidote to VX intoxication. Additionally, multiple variants with strong stereoselectivity for both (SP)- and (RP)-enantiomers have been identified, which will have applications in biotechnology and should simplify the development of more advanced variants. With these advances the scourge of organophosphate chemical warfare agents may well be in sight.
Supplementary Material
Highlights.
PTE has been evolved for chemical warfare agent decontamination.
High activity and broad specificity against G-agents has been attained.
High activity and stereospecificity against VX and VR has been accomplished.
In vivo protection against both G-agents and VX has been demonstrated.
PTE has been adapted as a long circulations prophylactic against G-agents.
Acknowledgments:
This work was supported in part by the National Institutes of Health (GM 116894) and the Robert A. Welch Foundation (A-840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- [1].Maxwell DM, Brecht KM, Koplovitz I, and Sweeney RE (2006) Acetylcholinesterase inhibition: does it explain the toxicity of organophosphorus compounds?, Arch. Toxicol 80, 756–760. [DOI] [PubMed] [Google Scholar]
- [2].Costanzi S, Machado JH, and Mitchell M (2018) Nerve Agents: What They Are, How They Work, How to Counter Them, ACS Chem. Neurosci 9, 873–885. [DOI] [PubMed] [Google Scholar]
- [3].Atwood D, and Paisley-Jones C Pesticides Industry Sales and Usage 2008-2012 Market Estimates, U.S. Environmental Protection Agency, Biological and Economic Analysis Division, Office of Pesticide Program, Office of Chemical Safety and Pollution Prevention, Washington, DC. [Google Scholar]
- [4].Tucker JB (2006) War of Nerves Chemical Warfare from World War I to Al-Qaeda, Anchor Books, New York. [Google Scholar]
- [5].Houze P, Berthin T, Raphalen JH, Hutin A, and Baud JF (2018) High Dose of Pralidoxime Reverses Paraoxon-Induced Respiratory Toxicity in Mice, Turk. J. Anaesthesiol. Reanim 46, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jokanovic M (2001) Biotransformation of organophosphorus compounds, Toxicology 166, 139–160. [DOI] [PubMed] [Google Scholar]
- [7].Krstic DZ, Colovic M, Kralj MB, Franko M, Krinulovic K, Trebse P, and Vasic V (2008) Inhibition of AChE by malathion and some structurally similar compounds, J. Enzyme Inhib. Med. Chem 23, 562–573. [DOI] [PubMed] [Google Scholar]
- [8].Benschop HP, and De Jong LPA (1988) Nerve agent stereoisomers: analysis, isolation and toxicology, Acc. Chem. Res 21, 368–374. [Google Scholar]
- [9].Koplovitz I, Gresham VC, Dochterman LW, Kaminskis A, and Stewart JR (1992) Evaluation of the toxicity, pathology, and treatment of cyclohexylmethylphosphonofluoridate (CMPF) poisoning in rhesus monkeys, Arch. Toxicol 66, 622–628. [DOI] [PubMed] [Google Scholar]
- [10].Munro N (1994) Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: implications for public protection, Environ. Health Perspect 102, 18–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koplovitz I, Shutz M, Schulz S, and Railer R (2001) Toxicity and Treatment of Russian V-agent (VR) Intoxication in Guinea Pigs, In 2001 ECBC Scientific Conference on Chemical and Biological Defence Research, Drug Assessment Divsion US Army Medical Research Institute of Chemica Defence, Hunt Valley, MD. [Google Scholar]
- [12].Mirzayanov VS (2009) State secrets : an insider's chronicle of the Russian chemical weapons program, Outskirts Press, Inc., Denver, Colorado. [Google Scholar]
- [13].Nepovimova E, and Kuca K (2018) Chemical warfare agent NOVICHOK - mini-review of available data, Food Chem. Toxicol 121, 343–350. [DOI] [PubMed] [Google Scholar]
- [14].Uzumcu A (2014) The Chemical Weapons Convention--disarmament, science and technology, Anal. Bioanal. Chem 406, 5071–5073. [DOI] [PubMed] [Google Scholar]
- [15].OPCW. (29 June 2017) Report of the OPCW Fact-Finding Mission in Syria Regarding an Alleged Incident in Khan Shaykhun, Syrian Arab Republic April 2017, Technical Secretariat, Cs-2017-0407(E). [Google Scholar]
- [16].Rosman Y, Eisenkraft A, Milk N, Shiyovich A, Ophir N, Shrot S, Kreiss Y, and Kassirer M (2014) Lessons Learned From the Syrian Sarin Attack: Evaluation of a Clinical Syndrome Through Social MediaLessons Learned From the Syrian Sarin Attack, Ann. Intern. Med 160, 644–648. [DOI] [PubMed] [Google Scholar]
- [17].BBC. (24 February 2017) Kim Jong-nam killing: 'VX nerve agent' found on his face, In BBC News, BBC. [Google Scholar]
- [18].Yusof MN (27 November 2017) Statment by H.E. Ambassador Ahmad Nazri Yusof Perminant Representative of Malaysia to the OPCW, pp CS-2017-0787(E), OPCW. [Google Scholar]
- [19].Wilson P (18 April 2018) Statement by HE Ambassador Peter Wilson Perminant Representative of the United Kingdom of Great Britian and Northern Ireland to the OPCW, pp CS-2018-1020(E), OPCW. [Google Scholar]
- [20].OPCW. (2001) The Sarin Gas Attack in Japan and the Related Forensic Investigation, www.opcw.org/media-center/news/2001/06/sarin-gas-attack-japan-and-related-forensic-investigation, retreive 2/November/2019.
- [21].Tsuchihashi H, Katagi M, Nishikawa M, and Tatsuno M (1998) Identification of metabolites of nerve agent VX in serum collected from a victim, J. Anal. Toxicol 22, 383–388. [DOI] [PubMed] [Google Scholar]
- [22].Munro NB, Talmage SS, Griffin GD, Waters LC, Watson AP, King JF, and Hauschild V (1999) The sources, fate, and toxicity of chemical warfare agent degradation products, Environ. Health Perspect 107, 933–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leikin JB, Thomas RG, Walter FG, Klein R, and Meislin HW (2002) A review of nerve agent exposure for the critical care physician, Crit. Care Med 30, 2346–2354. [DOI] [PubMed] [Google Scholar]
- [24].Reddy SD, and Reddy DS (2015) Midazolam as an anticonvulsant antidote for organophosphate intoxication-A pharmacotherapeutic appraisal, Epilepsia 56, 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goldsmith M, and Ashani Y (2018) Catalytic bioscavengers as countermeasures against organophosphate nerve agents, Chem. Biol. Interact 292, 50–64. [DOI] [PubMed] [Google Scholar]
- [26].Bigley AN, and Raushel FM (2013) Catalytic mechanisms for phosphotriesterases, Biochim. Biophys. Acta 1834, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Munnecke DM, and Hsieh DP (1974) Microbial decontamination of parathion and p-nitrophenol in aqueous media, Appl. Microbiol 28, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sethunathan N, and Yoshida T (1973) A Flavobacterium sp. that degrades diazinon and parathion, Can. J. Microbiol 19, 873–875. [DOI] [PubMed] [Google Scholar]
- [29].Harper LL, McDaniel CS, Miller CE, and Wild JR (1988) Dissimilar plasmids isolated from Pseudomonas diminuta MG and a Flavobacterium sp. (ATCC 27551) contain identical opd genes, Appl. Environ. Microbiol 54, 2586–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horne I, Sutherland TD, Harcourt RL, Russell RJ, and Oakeshott JG (2002) Identification of an opd (organophosphate degradation) gene in an Agrobacterium isolate, Appl. Environ. Microbiol 68, 3371–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dumas DP, Durst HD, Landis WG, Raushel FM, and Wild JR (1990) Inactivation of organophosphorus nerve agents by the phosphotriesterase from Pseudomonas diminuta, Arch. Biochem. Biophys 277, 155–159. [DOI] [PubMed] [Google Scholar]
- [32].Dumas DP, Caldwell SR, Wild JR, and Raushel FM (1989) Purification and properties of the phosphotriesterase from Pseudomonas diminuta, J. Biol. Chem 264, 19659–19665. [PubMed] [Google Scholar]
- [33].Omburo GA, Kuo JM, Mullins LS, and Raushel FM (1992) Characterization of the zinc binding site of bacterial phosphotriesterase, J. Biol. Chem 267, 13278–13283. [PubMed] [Google Scholar]
- [34].Chae MY, Omburo GA, Lindahl PA, and Raushel FM (1993) Antiferromagnetic Coupling in the Binuclear Metal Cluster of Manganese-Substituted Phosphotriesterase, J. Am. Chem. Soc 115, 12173–12174. [Google Scholar]
- [35].Omburo GA, Mullins LS, and Raushel FM (1993) Structural characterization of the divalent cation sites of bacterial phosphotriesterase by 113Cd NMR spectroscopy, Biochemistry 32, 9148–9155. [DOI] [PubMed] [Google Scholar]
- [36].Benning MM, Kuo JM, Raushel FM, and Holden HM (1995) Three-dimensional structure of the binuclear metal center of phosphotriesterase, Biochemistry 34, 7973–7978. [DOI] [PubMed] [Google Scholar]
- [37].Vanhooke JL, Benning MM, Raushel FM, and Holden HM (1996) Three-Dimensional Structure of the Zinc-Containing Phosphotriesterase with the Bound Substrate Analog Diethyl 4- Methylbenzylphosphonate, Biochemistry 35, 6020–6025. [DOI] [PubMed] [Google Scholar]
- [38].Benning MM, Hong SB, Raushel FM, and Holden HM (2000) The binding of substrate analogs to phosphotriesterase, J. Biol. Chem 275, 30556–30560. [DOI] [PubMed] [Google Scholar]
- [39].Samples CR, Raushel FM, and DeRose VJ (2007) Activation of the binuclear metal center through formation of phosphotriesterase-inhibitor complexes, Biochemistry 46, 3435–3442. [DOI] [PubMed] [Google Scholar]
- [40].Lewis VE, Donarski WJ, Wild JR, and Raushel FM (1988) Mechanism and stereochemical course at phosphorus of the reaction catalyzed by a bacterial phosphotriesterase, Biochemistry 27, 1591–1597. [DOI] [PubMed] [Google Scholar]
- [41].Samples CR, Howard T, Raushel FM, and DeRose VJ (2005) Protonation of the binuclear metal center within the active site of phosphotriesterase, Biochemistry 44, 11005–11013. [DOI] [PubMed] [Google Scholar]
- [42].Caldwell SR, Raushel FM, Weiss PM, and Cleland WW (1991) Transition-state structures for enzymatic and alkaline phosphotriester hydrolysis, Biochemistry 30, 7444–7450. [DOI] [PubMed] [Google Scholar]
- [43].Donarski WJ, Dumas DP, Heitmeyer DP, Lewis VE, and Raushel FM (1989) Structure-activity relationships in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta, Biochemistry 28, 4650–4655. [DOI] [PubMed] [Google Scholar]
- [44].Hong SB, and Raushel FM (1996) Metal-substrate interactions facilitate the catalytic activity of the bacterial phosphotriesterase, Biochemistry 35, 10904–10912. [DOI] [PubMed] [Google Scholar]
- [45].Aubert SD, Li Y, and Raushel FM (2004) Mechanism for the hydrolysis of organophosphates by the bacterial phosphotriesterase, Biochemistry 43, 5707–5715. [DOI] [PubMed] [Google Scholar]
- [46].Kim J, Tsai PC, Chen SL, Himo F, Almo SC, and Raushel FM (2008) Structure of diethyl phosphate bound to the binuclear metal center of phosphotriesterase, Biochemistry 47, 9497–9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Watkins LM, Mahoney HJ, McCulloch JK, and Raushel FM (1997) Augmented hydrolysis of diisopropyl fluorophosphate in engineered mutants of phosphotriesterase, J. Biol. Chem 272, 25596–25601. [DOI] [PubMed] [Google Scholar]
- [48].Porzio E, Merone L, Mandrich L, Rossi M, and Manco G (2007) A new phosphotriesterase from Sulfolobus acidocaldarius and its comparison with the homologue from Sulfolobus solfataricus, Biochimie 89, 625–636. [DOI] [PubMed] [Google Scholar]
- [49].Schofield DA, and Dinovo AA (2010) Generation of a mutagenized organophosphorus hydrolase for the biodegradation of the organophosphate pesticides malathion and demeton-S, J. Appl. Microbiol 109, 548–557. [DOI] [PubMed] [Google Scholar]
- [50].Li Y, Aubert SD, Maes EG, and Raushel FM (2004) Enzymatic resolution of chiral phosphinate esters, J. Am. Chem. Soc, 126, 8888–8889. [DOI] [PubMed] [Google Scholar]
- [51].Tsai PC, Bigley A, Li Y, Ghanem E, Cadieux CL, Kasten SA, Reeves TE, Cerasoli DM, and Raushel FM (2010) Stereoselective hydrolysis of organophosphate nerve agents by the bacterial phosphotriesterase, Biochemistry 49, 7978–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Raveh L, Segall Y, Leader H, Rothschild N, Levanon D, Henis Y, and Ashani Y (1992) Protection against tabun toxicity in mice by prophylaxis with an enzyme hydrolyzing organophosphate esters, Biochem. Pharmacol 44, 397–400. [DOI] [PubMed] [Google Scholar]
- [53].Bigley AN, Mabanglo MF, Harvey SP, and Raushel FM (2015) Variants of Phosphotriesterase for the Enhanced Detoxification of the Chemical Warfare Agent VR, Biochemistry 54, 5502–5512. [DOI] [PubMed] [Google Scholar]
- [54].Bigley AN, Xu C, Henderson TJ, Harvey SP, and Raushel FM (2013) Enzymatic neutralization of the chemical warfare agent VX: evolution of phosphotriesterase for phosphorothiolate hydrolysis, J. Am. Chem. Soc, 135, 10426–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tsai PC, Fan Y, Kim J, Yang L, Almo SC, Gao YQ, and Raushel FM (2010) Structural determinants for the stereoselective hydrolysis of chiral substrates by phosphotriesterase, Biochemistry 49, 7988–7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tsai PC, Fox N, Bigley AN, Harvey SP, Barondeau DP, and Raushel FM (2012) Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents, Biochemistry 51, 6463–6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].P Harvey S, Guelta MA, Berg FJ, Bigley AN, and Raushel FM (2016) Kinetics and Stereochemistry of the Hydrolysis of Novel Chemical Nerve Agents by a Series of Phosphotriesterase Mutants. , Journal of Chemical and Biological Defense 1, 18–32. [Google Scholar]
- [58].Caldwell SR, Newcomb JR, Schlecht KA, and Raushel FM (1991) Limits of diffusion in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta, Biochemistry 30, 7438–7444. [DOI] [PubMed] [Google Scholar]
- [59].Chen-Goodspeed M, Sogorb MA, Wu F, Hong SB, and Raushel FM (2001) Structural determinants of the substrate and stereochemical specificity of phosphotriesterase, Biochemistry 40, 1325–1331. [DOI] [PubMed] [Google Scholar]
- [60].Hong SB, and Raushel FM (1999) Stereochemical constraints on the substrate specificity of phosphotriesterase, Biochemistry 38, 1159–1165. [DOI] [PubMed] [Google Scholar]
- [61].Nowlan C, Li Y, Hermann JC, Evans T, Carpenter J, Ghanem E, Shoichet BK, and Raushel FM (2006) Resolution of chiral phosphate, phosphonate, and phosphinate esters by an enantioselective enzyme library, J. Am. Chem. Soc, 128, 15892–15902. [DOI] [PubMed] [Google Scholar]
- [62].Chen-Goodspeed M, Sogorb MA, Wu F, and Raushel FM (2001) Enhancement, relaxation, and reversal of the stereoselectivity for phosphotriesterase by rational evolution of active site residues, Biochemistry 40, 1332–1339. [DOI] [PubMed] [Google Scholar]
- [63].Hill CM, Li WS, Thoden JB, Holden HM, and Raushel FM (2003) Enhanced degradation of chemical warfare agents through molecular engineering of the phosphotriesterase active site, J. Am. Chem. Soc, 125, 8990–8991. [DOI] [PubMed] [Google Scholar]
- [64].Li WS, Lum KT, Chen-Goodspeed M, Sogorb MA, and Raushel FM (2001) Stereoselective detoxification of chiral sarin and soman analogues by phosphotriesterase, Bioorg. Med. Chem 9, 2083–2091. [DOI] [PubMed] [Google Scholar]
- [65].Goldenzweig A, Goldsmith M, Hill SE, Gertman O, Laurino P, Ashani Y, Dym O, Unger T, Albeck S, Prilusky J, Lieberman RL, Aharoni A, Silman I, Sussman JL, Tawfik DS, and Fleishman SJ (2016) Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability, Mol. Cell 63, 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Khersonsky O, Lipsh R, Avizemer Z, Ashani Y, Goldsmith M, Leader H, Dym O, Rogotner S, Trudeau DL, Prilusky J, Amengual-Rigo P, Guallar V, Tawfik DS, and Fleishman SJ (2018) Automated Design of Efficient and Functionally Diverse Enzyme Repertoires, Mol. Cell 72, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Reeves TE, Wales ME, Grimsley JK, Li P, Cerasoli DM, and Wild JR (2008) Balancing the stability and the catalytic specificities of OP hydrolases with enhanced V-agent activities, PEDS 21, 405–412. [DOI] [PubMed] [Google Scholar]
- [68].Cherny I, Greisen P Jr., Ashani Y, Khare SD, Oberdorfer G, Leader H, Baker D, and Tawfik DS (2013) Engineering V-type nerve agents detoxifying enzymes using computationally focused libraries, ACS Chem. Biol 8, 2394–2403. [DOI] [PubMed] [Google Scholar]
- [69].Goldsmith M, Eckstein S, Ashani Y, Greisen P Jr., Leader H, Sussman JL, Aggarwal N, Ovchinnikov S, Tawfik DS, Baker D, Thiermann H, and Worek F (2016) Catalytic efficiencies of directly evolved phosphotriesterase variants with structurally different organophosphorus compounds in vitro, Arch. Toxicol 90, 2711–2724. [DOI] [PubMed] [Google Scholar]
- [70].Goldsmith M, Aggarwal N, Ashani Y, Jubran H, Greisen PJ, Ovchinnikov S, Leader H, Baker D, Sussman JL, Goldenzweig A, Fleishman SJ, and Tawfik DS (2017) Overcoming an optimization plateau in the directed evolution of highly efficient nerve agent bioscavengers, PEDS 30, 333–345. [DOI] [PubMed] [Google Scholar]
- [71].Bigley AN, Desormeaux E, Xiang DF, Bae SY, Harvey SP, and Raushel FM (2019) Overcoming the Challenges of Enzyme Evolution to Adapt Phosphotriesterase for V-agent Decontamination, Biochemistry 58, 2039–2053. [DOI] [PubMed] [Google Scholar]
- [72].Ghanem E, and Raushel FM (2005) Detoxification of organophosphate nerve agents by bacterial phosphotriesterase, Toxicol. Appl. Pharmacol 207, 459–470. [DOI] [PubMed] [Google Scholar]
- [73].Van Dyk JS, and Pletschke B (2011) Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment, Chemosphere 82, 291–307. [DOI] [PubMed] [Google Scholar]
- [74].Trovaslet-Leroy M, Musilova L, Renault F, Brazzolotto X, Misik J, Novotny L, Froment MT, Gillon E, Loiodice M, Verdier L, Masson P, Rochu D, Jun D, and Nachon F (2011) Organophosphate hydrolases as catalytic bioscavengers of organophosphorus nerve agents, Toxicol. Lett 206, 14–23. [DOI] [PubMed] [Google Scholar]
- [75].Tuovinen K, Kaliste-Korhonen E, Raushel FM, and Hanninen O (1994) Phosphotriesterase--a promising candidate for use in detoxification of organophosphates, Fundam. Appl. Toxicol 23, 578–584. [DOI] [PubMed] [Google Scholar]
- [76].Tuovinen K, Kaliste-Korhonen E, Raushel FM, and Hanninen O (1996) Eptastigmine-phosphotriesterase combination in DFP intoxication, Toxicol. Appl. Pharmacol 140, 364–369. [DOI] [PubMed] [Google Scholar]
- [77].Tuovinen K, Kaliste-Korhonen E, Raushel FM, and Hanninen O (1996) Phosphotriesterase, pralidoxime-2-chloride (2-PAM) and eptastigmine treatments and their combinations in DFP intoxication, Toxicol. Appl. Pharmacol 141, 555–560. [DOI] [PubMed] [Google Scholar]
- [78].Tuovinen K, Kaliste-Korhonen E, Raushel FM, and Hanninen O (1999) Success of pyridostigmine, physostigmine, eptastigmine and phosphotriesterase treatments in acute sarin intoxication, Toxicology 134, 169–178. [DOI] [PubMed] [Google Scholar]
- [79].Worek F, Seeger T, Reiter G, Goldsmith M, Ashani Y, Leader H, Sussman JL, Aggarwal N, Thiermann H, and Tawfik DS (2014) Post-exposure treatment of VX poisoned guinea pigs with the engineered phosphotriesterase mutant C23: a proof-of-concept study, Toxicol. Lett 231, 45–54. [DOI] [PubMed] [Google Scholar]
- [80].Novikov BN, Grimsley JK, Kern RJ, Wild JR, and Wales ME (2010) Improved pharmacokinetics and immunogenicity profile of organophosphorus hydrolase by chemical modification with polyethylene glycol, J. Control Release 146, 318–325. [DOI] [PubMed] [Google Scholar]
- [81].Reiter G, Muller S, Hill I, Weatherby K, Thiermann H, Worek F, and Mikler J (2015) In vitro and in vivo toxicological studies of V nerve agents: molecular and stereoselective aspects, Toxicol. Lett 232, 438–448. [DOI] [PubMed] [Google Scholar]
- [82].Zhang P, Liu EJ, Tsao C, Kasten SA, Boeri MV, Dao TL, DeBus SJ, Cadieux CL, Baker CA, Otto TC, Cerasoli DM, Chen Y, Jain P, Sun F, Li W, Hung HC, Yuan Z, Ma J, Bigley AN, Raushel FM, and Jiang S (2019) Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents, Sci. Trans.l Med 11, doi: 10.1126/scitranslmed.aau7091. [DOI] [PubMed] [Google Scholar]
- [83].Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, and Ferrin TE (2004) UCSF Chimera--a visualization system for exploratory research and analysis, J. Comput. Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.