Abstract
Most patients who die of cancer have disseminated disease that has become resistant to multiple therapeutic modalities. Ample evidence suggests that the expression of ATP- binding cassette (ABC) transporters, especially the multidrug resistance protein 1 (MDR1, also known as P- glycoprotein or P-gp), which is encoded by ABC subfamily B member 1 (ABCB1), can confer resistance to cytotoxic and targeted chemotherapy. However, the development of MDR1 as a therapeutic target has been unsuccessful. At the time of its discovery, appropriate tools for the characterization and clinical development of MDR1 as a therapeutic target were lacking. Thirty years after the initial cloning and characterization of MDR1 and the implication of two additional ABC transporters, the multidrug resistance associated protein 1 (MRP1; encoded by ABCC1)), and ABCG2, in multidrug resistance, interest in investigating these transporters as therapeutic targets has waned. However, with the emergence of new data and advanced techniques, we propose to re- evaluate whether these transporters play a clinical role in multidrug resistance. With this Opinion article, we present recent evidence indicating that it is time to revisit the investigation into the role of ABC transporters in efficient drug delivery in various cancer types and at the blood–brain barrier.
Introduction
Despite heroic efforts to develop new anti-cancer drugs and biological therapies, and to catalog and study hundreds of potential mechanisms of resistance to these treatment modalities1, most patients with metastatic cancer will die from multidrug-resistant disease. Development of multidrug resistance (MDR)—the resistance to multiple, structurally unrelated compounds—is a frequent problem in the treatment of cancer and should be distinguished from resistance to anti-cancer drugs with precise targets and immune therapies which are not examples of MDR. There are several extensive reviews detailing the history and development of this field2–5.
Acquired MDR has been intensively studied and the basic science is well-established. Over 50 years ago, Goldstein and colleagues described a HeLa subline with actinomycin D resistance following intermittent exposure6. Some 10 years later, June Biedler selected Chinese hamster lung and fibroblast cells in actinomycin D and found the selected cells resistant to the selecting agent, as well as to vinblastine, vincristine, and daunomycin7. Keld Dano was the first to demonstrate that daunomycin was actively transported out of multidrug-resistant mouse Ehrlich ascites cells and postulated that this must be due to a membrane transporter8. This transporter, P-glycoprotein (P-gp), later found to be an ATP binding cassette (ABC) transporter, was identified in drug-resistant Chinese hamster ovary cells in 1976 by Victor Ling9. Gros and colleagues were the first to report a drug resistance gene cloned from multidrug-resistant Chinese hamster cells10 and the gene was found to confer resistance when transfected into sensitive cells11. The human gene—termed MDR1 (and later renamed ABCB1)—was reported soon after in 198612,13, thus launching the study of ABC transporters, with a family of 48 human membrane transporters involved in diverse physiologic processes14.
The second member of the ABC transporter family, MRP (subsequently renamed ABCC1), was reported by Cole and colleagues in 199215 and was found to mediate resistance to doxorubicin, etoposide, and vincristine among others16, but evidence of ubiquitous expression has meant it is unlikely to be a suitable target for anti-cancer therapy. The third ABC transporter identified, breast cancer resistance protein (BCRP and MXR; subsequently renamed ABCG2), was reported by three different groups within the span of a few months17–19. These additions increased interest in the study of ABC transporters, but added complexity to the definition of MDR. The substrates and key roles for most of these proteins have been identified—but the extent to which the transporters play a role in clinical MDR has never been clarified. Despite the clinical failure of P-gp inhibitors, recent evidence suggests that expression of ABC transporters does play a role in clinical drug resistance in some settings. We will argue that a contemporary understanding of target biology and biomarker development could identify settings where transporters involved in MDR could be considered important therapeutic targets.
ABC transporters: Structure and function
The 48 human ABC transporter genes are classified into seven subfamilies (termed ABCA through ABCG)20,21. For the sake of clarity, we will subsequently refer to transporter proteins by their subfamily names rather than by their common names. Structurally, ABC transporters are typified by a characteristic four-domain architecture consisting of two cytoplasmic nucleotide-binding domains (NBDs) that bind and hydrolyze ATP and two transmembrane domains (TMDs) that recognize and transport substrates. While the structure and function of NBDs are similar throughout families, TMDs are highly heterogeneous, allowing transporters to recognize diverse substrates and use the energy from ATP hydrolysis to translocate molecules across membranes, irrespective of the prevailing concentration gradient22. Recent work suggests that while the energy from ATP can help translocate engaged substrates, basal ATP hydrolysis drives a continuously changing conformation that may facilitate ABCB1 binding and transport of a wide range of substrates23. High resolution structures of ABCC124 and ABCG225 determined using cryo-electron microscopy have helped clarify the drug-binding domains, but have left the precise translocation mechanism unresolved.
ABC transporters regulate cellular levels of hormones, lipids, ions, xenobiotics and other small molecules by transporting molecules across cell membranes and serve a range of physiological roles, including intracellular regulation of organelles such as the mitochondrion26, lysosome27, endoplasmic reticulum28 and Golgi apparatus29. Loss of function of the transporter via germline mutation is associated with a number of heritable diseases, including cystic fibrosis (ABCC7, CFTR), pseudoxanthoma elasticum (ABCC6), Stargardt macular degeneration (ABCA4), Tangier disease (ABCA1), sitosterolemia (ABCG5 and ABCG8), and harlequin ichthyosis (ABCA12)30. These disorders present significant molecular biological and clinical challenges, as they require the development of small molecules (or gene therapy) that encourage normalization of a mutant transporter via strategies such as ribosomal read-through31, message stabilization, correction of folding defects32, correction of trafficking defects33,34, allosteric activators of activity35, modulation of protein-protein interactions36, control of post-translational regulation, regulation of protein degradation pathways37,38, or induction of compensatory mechanisms39. Prospects are improved somewhat given the notion that restoration of activity as little as 5% that of wild-type basal activity can be sufficient to partially ameliorate disorder phenotypes40,41.
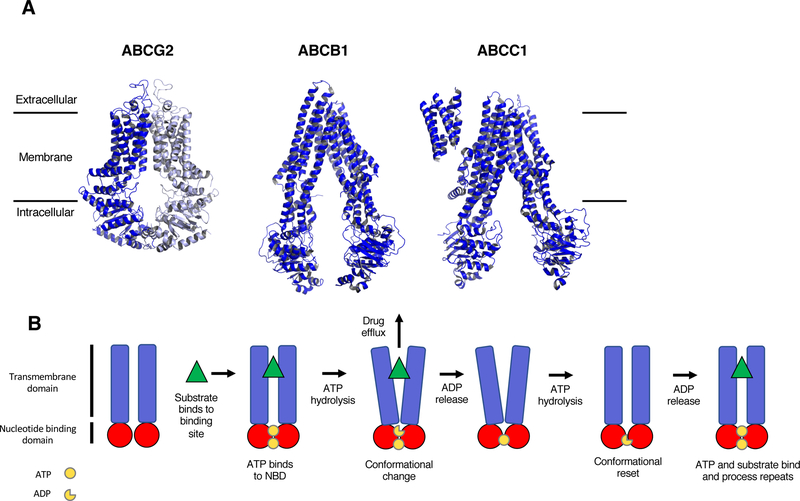
It should be noted that among the 48 ABC transporters, some have very precise substrate specificity, while others have a broad range of substrates. It is the transporters with broad substrate specificity profiles that have the potential to transport anticancer agents, and it may depend in part on level of expression as to whether transport is sufficient to confer drug resistance42. Because laboratory experiments are typically conducted in cell lines with high levels of transporter expression, it has been difficult to determine which transporters deserve the most scrutiny as mechanisms of drug resistance, and the reader is referred to several reviews compiling substrate lists of a range of transporters43–45. One detailed compilation notes that 19 of the 48 ABC transporters have been shown to efflux anticancer agents in some context43. This review will focus on the subset of ABC transporters that were first reported as multidrug efflux pumps—ABCB1, ABCC1 and ABCG2—what we have learned about their basic science, how clinical application has faltered, and what might constitute a path forward. More recent work in this massive research field will be the focus of our review due to space constraints. In Figure 1A, we present the most recent 3D structures for ABCB1, ABCC1 and ABCG2, and list common substrates of the transporters. Additionally, we provide a description of the pumping action of ABCB1 in Figure 1B.
Figure 1:
Structure and mechanism of 3 ABC transporters. A, High-resolution 3D structures of ABCG225 (PDB accession 5NJ3), ABCB123 (PDB accession 5KPI) and ABCC124 (PDB accession 5UJ9). As ABCG2 forms a homodimer, the second dimer of the full protein is shown in light blue. Common substrates and inhibitors are listed. (Although the structure for ABCC1 is that of Bos Taurus, the protein identity is 91% and the structure is likely similar to human ABCC1). B, Schematic representation of the proposed pumping action of ABCB1. The substrate binds to the binding pocket and ATP binds to the two binding sites in the NBDs. This is followed by the hydrolysis of ATP that generates a conformational change, allowing the substrate to be released from the protein. The second molecule of ATP is hydrolyzed, allowing for a conformational reset, where substrate and ATP can bind again so the process can repeat.
The physiologic roles of ABCB1, ABCC1, and ABCG2 appear to be in excretory and/or protective capacities by transporting substrates across biological membranes. In the blood-brain barrier (BBB), the blood-testis, and blood-placental barriers, expression of the pumps in the capillary endothelial cells serves to prevent entry of exogenous molecules46,47. A consequence of these protective roles is that these transporters can affect pharmacokinetic parameters of drug absorption, distribution, metabolism, excretion, and toxicity. Inhibition of ABC transporters often leads to toxicities or pharmacokinetic changes due to drug-drug interactions48 and the FDA now requires that investigational drugs be characterized with regard to their ability to interact with ABC transporters.
The ABCB1 hypothesis
Nearly 40 years of findings from cell culture and animal models implicate multidrug efflux pumps as a cause of multidrug resistance. Their potential importance in MDR is illustrated by the numerous anti-cancer agents that have been identified as substrates, including anthracyclines, taxanes, vinca alkaloids, camptothecins, epipodophyllotoxins, and tyrosine kinase inhibitors1,49. While there is considerable overlap among the substrate profiles for the various multidrug efflux pumps, there are some distinct differences. ABCC1 has been shown to transport various neutral and anionic hydrophobic compounds and products of Phase II drug metabolism, including many glutathione and glucuronide conjugates50. ABCG2 transports the anti-cancer drugs methotrexate, mitoxantrone, topotecan, irinotecan, and flavopiridol51.
In addition to studies of ABC transporter structure and function, efforts have been made to define their clinical roles in drug resistance, primarily for ABCB1, the first transporter to be discovered. These studies generally used RNA or immunohistochemical methods of detection and examined association with outcomes52. Numerous studies reported the presence of ABCB1 mRNA and protein in clinical samples—in leukemias and in kidney, colon, breast, and lung cancers—and typically ABCB1 expression portended a poor response to chemotherapy52–54. These results led to the development of clinical trials to test the ABCB1 hypothesis: that inhibitors of ABCB1 could improve response to chemotherapy and outcome via increased drug accumulation mediated by inhibition of drug transport.55,56
Failure of ABCB1 inhibitors in clinical trials
After the discovery of ABCB1, a number of “first generation” inhibitors including verapamil, quinidine, amiodarone, and cycloposorin A were identified and added to chemotherapy regimens. However, these agents were not very potent or were toxic in their own right and their ability to inhibit ABCB1 was not verified in patients5. The second-generation agents valspodar and dexverapamil were more potent ABCB1 inhibitors55, and surrogate assays were 7used to confirm that serum concentrations of the inhibitors were able to inhibit rhodamine 123 transport from ABCB1 positive circulating CD56+ cells after inhibitor administration57,58. However, neither the expression nor function of ABCB1 in tumors was verified, nor whether the inhibitors actually blocked efflux in tumor cells. Additionally, pharmacokinetic interactions with the inhibitors, such as the inhibition of cytochrome P450 by valspodar, required chemotherapy dose reductions, leading to potential underdosing of patients59. A third generation of inhibitors including dofequidar, zosuquidar, tariquidar, elacridar and biricodar were developed specifically as ABCB1 inhibitors. These were more potent and displayed less pharmacokinetic interactions, but caused some chemotherapy toxicity in combination, potentially due to inhibition of normal tissue ABCB156. After the discovery of ABCG2, elacridar and tariquidar were found to inhibit both ABCB1 and ABCG260,61 while cyclosporine A and biricodar were found to inhibit ABCB1, ABCC1 and ABCG262,63. Interestingly, inclusion of cyclosporine A to chemotherapy led to increased response in AML in one study64; however, these results could not be duplicated with other inhibitors65. Unfortunately, despite a few early successes, the majority of clinical trials with ABCB1 inhibitors, even third-generation inhibitors, did not confirm clinical benefit56.
From a clinical oncology perspective, the result was a nearly complete shutdown of study in the field after these trials were completed, and the clinical validation and development of specific inhibitors for other ABC transporters potentially involved in MDR was not pursued. Further clinical trials using ABCB1 inhibitors were vehemently discouraged66. However, some work continued on more mechanistic and functional aspects of the transporters, including work defining substrates and inhibitors; defining the role of transporters in the blood-brain barrier; and defining their roles in pharmacology. As noted above, the FDA now requires determination of the interaction of drugs with ABC transporters during development, given that transporters can be critical determinants of drug pharmacokinetics, including oral availability, drug-drug interactions, and drug toxicity. The finding in clinical trials that inhibition of ABC transporters increased chemotherapy toxicity has newer parallels in combinations with tyrosine kinase inhibitors. Recent reports of unexpected toxicity of combinations of targeted kinase inhibitors could be explained by interactions with ABC transporters67–69. Indeed, several targeted small molecules have been shown to interact with one or more ABC transporters, usually as an inhibitor70–72, but also as a substrate for transport73–75. In some cases, these interactions might be beneficial. When patients were treated with oral topotecan in combination with the tyrosine kinase inhibitor pazopanib, total topotecan exposure was 1.7-fold higher than patients treated with topotecan alone, primarily due to the fact that pazopanib inhibits ABCG2, thus increasing the absorption of orally administered topotecan76.
Unfortunately, these studies left a fundamental question unanswered: what is the role, if any, of transporter-mediated reduced drug accumulation in clinical drug resistance? Newer technologies that were not available when ABCB1 was discovered some 30 years ago—such as gene expression, gene mutation and SNP studies—have provided evidence supporting a re-examination of ABC transporters in clinical drug resistance. Additionally, genetically engineered mouse models (GEMMs) have provided new impetus for examining the role of transporters in acquired resistance to PARP inhibitors. Imaging studies and mouse models examining the role of transporters in the GI tract and at the BBB have consistently demonstrated that ABCB1 and ABCG2 are a significant impediment to anticancer agent oral absorption and brain exposure. A summary of the new evidence supporting the role of ABC transporters in drug resistance is given below.
The complex role of transporters in MDR
ABCB1 overexpression leads to resistance in some tumor subsets.
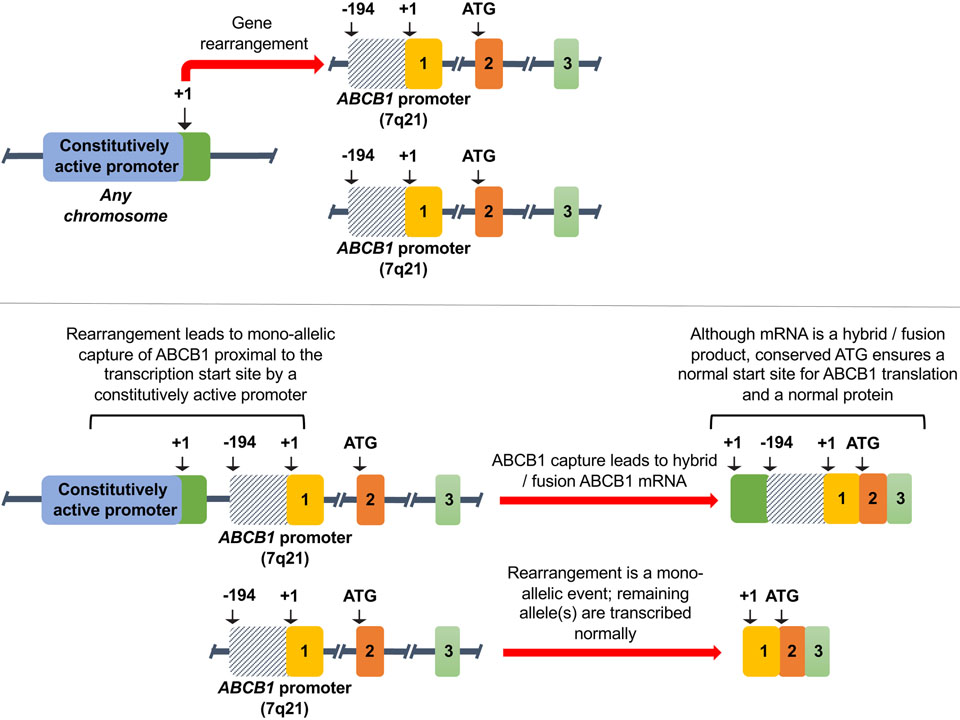
Many of the clinical trials designed to test the ABCB1 hypothesis were conducted in acute myeloid leukemia (AML). Although largely failures, we now recognize their design was optimistic and success unlikely given the focus on only ABCB1 and the lack of selection for a subset of patients with ABCB1 as a mechanism of resistance. One study reported gene expression profiling in 170 AML samples, finding that a refractory subset with ABCB1 or ABCG2 expression comprised only 13% of samples77. A recent report of whole genome sequencing of 92 patients with high-grade serous ovarian carcinoma (HGSOC) with primary and matched resistant disease concluded that CCNE1 amplification and BRCA1/2 reversions were potential mechanisms of resistance. However, the authors also found recurrent promoter fusions associated with overexpression of ABCB178. This discovery, occurring in about 8% of recurrent disease samples, suggests ABCB1 overexpression via a mechanism first reported more than a decade earlier in cell culture models and in clinical samples from patients with refractory acute lymphoblastic leukemia79,80. As shown in Figure 2, gene rearrangements can occur that place a more active promoter 5’ to the ABCB1 transcript. These events are mono-allelic, with the remaining alleles being transcribed normally. While these striking results strongly suggested the fusions were selected as part of the drug-resistant phenotype (paclitaxel and docetaxel, the mainstays of treatment of ovarian cancer, are classic ABCB1 substrates), the low occurrence rate argues that the rearrangements may occur in a particular molecular background. We previously reported that, using a Taqman low-density array, high levels of ABCB1 after drug treatment are found in only a small percentage of ovarian cancers81, suggesting any clinical trial targeting ABCB1 would be futile without identifying patients a priori with ABCB1 expressing tumors.
Figure 2:
Upregulation of ABCB1 via promoter capture. Adapted from Knutsen et al.128. Used by permission.
In a recent anecdotal observation, investigators identified unexpectedly high levels of ABCB1 in samples obtained from a patient whose anaplastic lymphoma kinase (ALK)-rearranged lung cancer had developed resistance to ceritinib, a drug used to treat lung cancers with mutations in the ALK gene, without a new ALK mutation. The investigators established cell lines from different metastatic sites and found that both the post-ceritinib treated tumor and cell lines derived from it exhibited high levels of P-gp73. The authors also found P-gp was highly expressed in 3 of 11 ALK-rearranged refractory lung cancers in which secondary ALK mutations were absent. Lung cancer studies conducted with P-gp inhibitors in the 1990s2 could not have envisioned the subset stratification that would be needed to find the patients in whom P-gp could confer drug resistance.
Expression of multiple transporters.
It is now clear that multiple ABC transporters may be expressed in a single tumor type. A study that examined expression of all 48 human ABC transporters in 281 acute myeloid leukemia samples found in a multivariate analysis, ABCB1, ABCG1, and ABCG2 linked to overall survival and the overall survival decreased with increasing number of transporter genes co-expressed82. Similar results were observed in childhood AML, where ABCA3, ABCB1, ABCC3, and ABCG2 were measured by RT-PCR, and the increasing number of co-expressed transporters conferred shorter relapse free-survival83. Finally, in a set of 11 paired samples taken from patients with AML at diagnosis and relapse, a 2-fold overexpression of at least one ABC transporter capable of transporting anthracyclines or vinca-alkaloids was found in 10 of the paired samples84. Together, these results suggest that ABCB1 alone may not be the prevalent mechanism of resistance in AML, and that coexpression of transporters may require inhibition of multiple transporters to achieve clinical benefit. This may be especially true for primitive leukemic CD34+/38- cells, the putative “stem cell” population. These cells have been shown to express high levels of ABCG2 as well as ABCB1 and ABCC1 in some patient samples85. Expression of ABCG2 and ABCB1 in this population has been found to correlate with response to chemotherapy both clinically and when measured ex vivo in leukemic blast samples86.
Support for the idea that multiple transporters might underlie the resistance phenotype in some solid tumors has also emerged, suggesting that coordinate expression of transporters in normal tissues will lead to expression of multiple transporters in cancer. Whether all, or a subset of these pumps may be involved in anticancer drug efflux remains to be determined. A study of all ABC transporters in pancreatic cancer found significant up-regulation of ABCB4, ABCB11, ABCC1, ABCC3, ABCC5, ABCC10, and ABCG2 at the RNA level in tumors relative to normal pancreas87. ABCB1, physiologically at high levels in a normal pancreas, was not upregulated beyond normal pancreatic tissue expression. Upregulation of transporter expression beyond the high levels found in normal tissue counterparts may not matter, as in drug-refractory hepatocellular carcinoma, where expression of multiple transporters has been reported, albeit in different studies88,89.
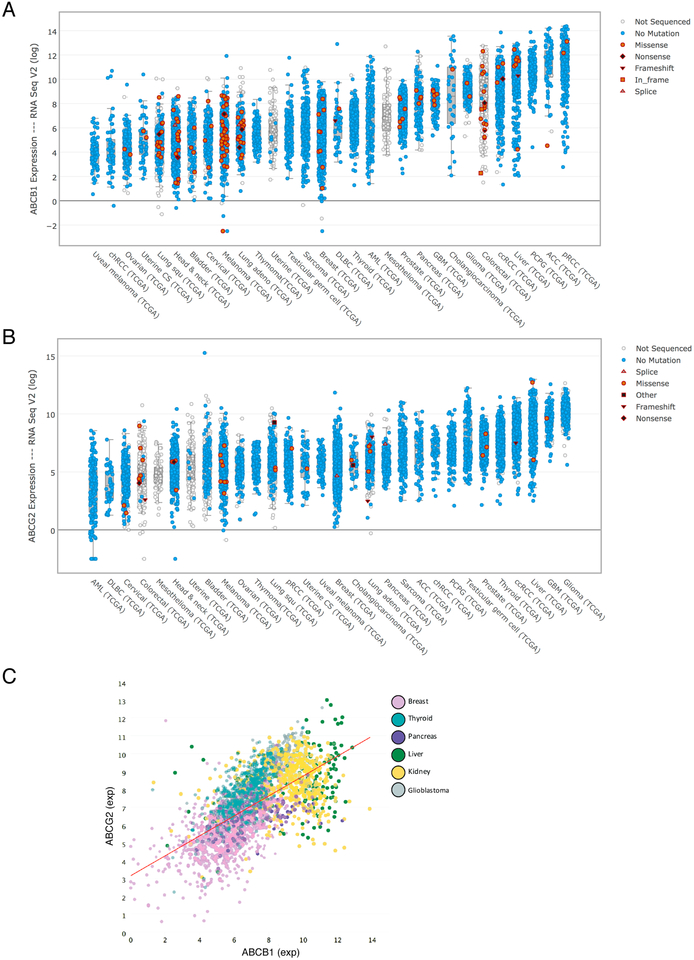
With the advent of genomic analysis, we have an unbiased approach to measuring ABC transporter mRNA expression. Figures. 3A and 3B show the range of ABCB1 and ABCG2 expression, respectively, in RNA-Seq data from The Cancer Genome Atlas (TCGA) Bioportal. Figure 3C shows the broad range of expression of both ABCB1 and ABCG2 in a variety of tumor types. Their expression ranges over 1000-fold, a far greater range of expression than was reported in studies using older technologies. Although a frequent criticism of early ABCB1 studies was that in vitro levels of ABCB1 did not reflect levels in the patient tumors90, this broad range suggests very high levels in some tumors. It is interesting that tumor types most refractory to chemotherapy are among those with the highest levels of expression. In Figure 3C, hepatocellular and kidney cancers have the highest expression of both ABCB1 and ABCG2. While tyrosine kinase inhibitors are approved for both, these cancers remain enigmatically resistant to the mainstay chemotherapeutics that are substrates for ABC transporters and form the backbone of treatment for almost every other tumor type. The ability to detect RNA levels more accurately highlights the single most important deficiency in our understanding of MDR—how do we show that ABC transporters measurably reduce anti-cancer drug accumulation in cancer cells, and in a clinically significant way? The centrality of this question is highlighted when one considers also that the quantitative RNA methods generally do not isolate cancer cells from the normal tissue stroma surrounding them. Indeed, it has been reported that in the case of breast cancer, ABCB1 expression is found primarily in tumor-associated macrophages and not in the tumor samples themselves91. Discordance between RNA and immunohistochemical data is thought to be responsible for this problem in some cases92, but antibody specificity questions have also been raised93.
Figure 3:
Expression of ABCB1 and ABCG2 in patient tumor samples. Data from the cBioPortal website (www.cbioportal.org) showing expression of ABCB1 (A) or ABCG2 (B) in various tumor types. (C) Expression of both ABCB1 and ABCG2 in breast, thyroid, pancreas, liver, kidney and glioblastoma tumors taken from the TCGA database. The data are viewed with tcgaminer, an R Shiny-based program. The data shown in this figure are based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov.
What is clear in the cases outlined above is that only a fraction of cancers express levels of an ABC transporter that might lead to drug resistance—for ABCB1, 13% of AML, 8% of ovarian cancer and 30% of the relatively rare ALK+ NSCLC—suggesting that ABCB1 should have been targeted as cancer mutations are today. And, expression of more than one transporter is common. Clinical studies without molecular characterization such as those conducted in the past could never achieve statistical significance and could be potentially confounded by inhibiting normal ABCB1 in patients who did not have ABCB1-expressing tumors.
SNP studies implicate transporters as critical factors to chemotherapy response.
One way to answer the question of whether ABCB1 matters in cancer drug resistance is to evaluate the impact of polymorphic variants on chemotherapy response or cancer outcome. Polymorphic variants of ABC transporter genes that impair substrate efflux could be associated with a higher cancer incidence, due to decreased xenobiotic efflux and impaired normal tissue protection because of decreased transporter efficacy. But they could also be associated with better outcome once a cancer is diagnosed, because of reduced chemotherapy drug efflux. However, multiple variables confound such associations—not all cancers are linked to xenobiotic exposure; not all cancer chemotherapeutics are substrates for transporters; current methods provide incomplete information on the transport activity of polymorphic variants; and clinical outcome may be impacted by coexisting polymorphic variants in multiple genes. As a result, the literature for outcomes associated with polymorphic variants of ABCB1 is contradictory94–97.
The largest SNP association study to date was based on RNA sequence data found in the TCGA database. This study examined sequence and expression data from 4616 ovarian cancer patients who had received any form of adjuvant chemotherapy. Johnatty et al. examined the correlation of three common coding SNPs tagging either C1236T (rs1128503), G2677T/A (rs2032582), or C3435T (rs1045642) of ABCB1 in patients with prior chemotherapy98 and found only a marginal association of C1236T with improved overall survival; although in 143 serous ovarian tumors ABCB1 over-expression was associated with a worse prognosis in sub-optimally debulked patients98.
Transporter expression in mouse models of acquired resistance.
Expression of ABC transporters emerged as the principal mechanism of resistance in an elegant series of studies published by the groups of Borst and Rottenberg involving a genetically engineered mouse model (GEMM) of hereditary breast cancer that arises in the mammary epithelium of mice deficient for Brca1 and tp53. Treatment of animals from this model with doxorubicin led to a robust response, but when tumors recurred, upregulation of the Abcb1a and Abcb1b (the murine orthologs of ABCB1) was found99. These resistant tumors were additionally resistant to docetaxel, another substrate of ABCB1. Transport of 99mTc-MIBI was also demonstrated in resistant tumors that overexpressed Abcb1a/b and was used to predict the efficacy of the ABCB1 inhibitor tariquidar100. Similar results were obtained when the mice were treated with the PARP inhibitor olaparib: tumors responded initially, and recurring tumors overexpressed Abcb1a/b as a mechanism of resistance101. In tumors where Abcb1a/b was upregulated, the addition of tariquidar to olaparib treatment resensitized tumors to olaparib101. In the mouse tumors, even moderate increases in ABCB1 expression—as little as five-fold compared to treatment-naïve tumors—conferred resistance that could be reversed by the ABCB1 inhibitor tariquidar102.
In a separate study using the same model, when tumors arising from this model were treated with the topoisomerase I inhibitor topotecan, some, but not all, resistant tumors expressed higher levels of Abcg2 as a mechanism of resistance, suggesting that this transporter is not as readily upregulated103. Of note, expression of Abcc1 and Abcc4, which have also been implicated in topotecan resistance, were not found to be increased103. While the ABCG2 inhibitor Ko143 was not able to completely overcome Abcg2-mediated resistance in topotecan-resistant tumors, treatment of mice with pegylated SN-38 was found to be effective104.
Similar observations have been reported in other GEMM models. In a model of BRCA2-deficient cancer generated in K14cre;Brca2F/F;p53F/F mice that yields BRCA2-deficient carcinosarcomas, ABCB1 expression was high in chemo-naive tumors, resulting in intrinsic doxorubicin and olaparib resistance reversible by tariqudar105. Interestingly, in this same model, expression of ABCB1 was associated with an epithelial-to-mesenchymal transition (EMT) phenotype105. An examination of ABCB1 expression in human triple negative breast cancer samples demonstrated that tumors with an EMT phenotype (claudin low) had high basal levels of ABCB1 compared to basal-like tumors106.
Despite these recent mouse studies implicating ABCB1 as a significant resistance mechanism in breast cancer, it is not understood why it has not been as clearly implicated in humans. This could be due to the fact that basal levels of ABCB1 are higher in rodents, leading to increased ABCB1 expression in response to drug treatment107. The use of human breast epithelial organoids may be one way to overcome this limitation associated with murine-derived tumors108. Alternatively, as relatively small increases in ABCB1 expression were linked to resistance, this increase might not have been detected in human patient samples with some techniques such as microarrays or by examining protein expression using immunohistochemistry107.
Decreased oral bioavailability due to transporters.
Although not directly involved in drug resistance, transporter expression in the GI tract is known to affect the oral bioavailability of some chemotherapy drugs that are transporter substrates. This is especially true for taxanes and topotecan, which are not given orally due to interactions with ABCB1 and ABCG2, respectively. The dual ABCB1/ABCG2 inhibitor elacridar has been combined with oral taxol109 or topotecan110 to increase oral bioavailability in patients; however, the clinical efficacy of these combinations has not been reported. The potential for transporters in the gut to cause drug resistance has been suggested for imatinib, where both ABCB1 and ABCG2 were induced in Caco-2 intestinal cells chronically exposed to the drug111. If this were to occur in the gut, it would limit oral availability of the imatinib, resulting in lower serum concentrations and resistance to the drug. Although an intriguing possibility, this has yet to be demonstrated clinically.
Transporters at the BBB limit brain penetration.
One of the stunning discoveries made during the evolution of this field was the critical importance of ABCB1 at the blood brain barrier (BBB), first shown in mouse models developed by Schinkel et al. in which deletion of Abcb1a and Abcb1b resulted in CNS toxicity from ivermectin112. After the discovery of ABCG2, mice lacking Abcb1a, Abcb1b and Abcg2 were generated, and a systematic series of investigations demonstrated the often overlapping and synergistic role of these two transporters in restricting the entrance of anticancer therapeutics to the brain in murine models in which brain concentration of chemotherapeutics is measured in wild-type versus transporter-deficient mice.
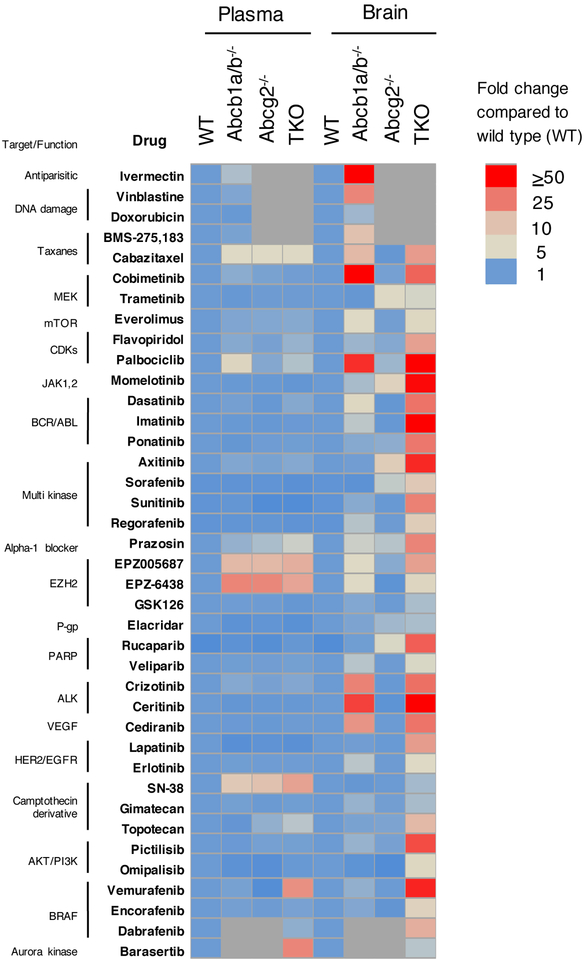
Fig. 4 presents a summary of published data for multiple agents, drawing on data from the many murine studies carried out by several groups. While the lack of either Abcb1a/b or Abcg2 typically has only minimal effects on blood levels (left 4 columns), steady-state brain levels of substrates are markedly higher when both transporters are deleted rather than either alone. As one example, mice lacking Abcb1a/b had 2.3-fold higher steady-state brain levels of vemurafenib, while Abcg2-deficient mice had no change. However, brain concentrations in mice lacking both Abcb1a/b and Abcg2 were approximately 43-fold higher than in wild-type mice113. This suggests a remarkable compensatory function for the two transporters. Similarly, a recent report on the ALK kinase inhibitor ceritinib demonstrated that brain concentrations of ceritinib are increased approximately 37-fold in the absence of Abcb1a/b, and 87-fold in the absence of both Abcb1a/b and Abcg2114. Interestingly, knockout of Abcg2 alone did not increase brain accumulation. Presumably for these drugs, Abcb1a/b is able to compensate for the absence of Abcg2. Ceritinib, approved by the FDA in 2014 for the treatment of lung cancers harboring ALK-rearrangements and vemurafenib, approved in 2011 for the treatment of melanoma, are likely restricted from the brain by ABCB1 and ABCG2 and therefore cannot control early metastatic disease in the brain. This is particularly troubling for vemurafenib, as high serum concentrations are necessary for treatment115.
Figure 4:
Effect of transporter deletion on plasma or brain levels of drugs. Fold increase in plasma and brain drug levels in mice deficient for Abcb1a/b, Abcg2 or all three (TKO) transporters is compared to wild-type mice, which are assigned a value of 1. Grey blocks denote mice not studied. Adapted from Basseville et al129 and compiled from references112–114,130–159. Used by permission.
Imaging demonstrates utility of transport inhibition at the BBB
Other in vivo models have used inhibitors to demonstrate the role of ABC transporters at the BBB. In mouse models, [11C]erlotinib was restricted from the brain by both Abcb1 and Abcg2 and deletion of both transporters led to the greatest increase in brain penetration116. In non-human primates, co-administration of [11C]erlotinib with elacridar resulted in a 3.5-fold increase in brain penetration117. Similarly, in healthy human subjects, significant increases in the brain of the ABCB1-specific substrate, (R)-[11C]verapamil, were demonstrated during ABCB1 inhibition with high doses of tariquidar. By contrast, high doses of tariquidar did not lead to increased brain levels of [11C]tariquidar, a substrate of both ABCB1 and ABCG2, owing to the ability of ABCG2 to compensate for inhibition of ABCB1 function118. More marked increases were observed when individuals carrying the ABCG2 polymorphic variant known to reduce protein level and function were tested. These studies align with the animal data and validate work in clinical medicine.
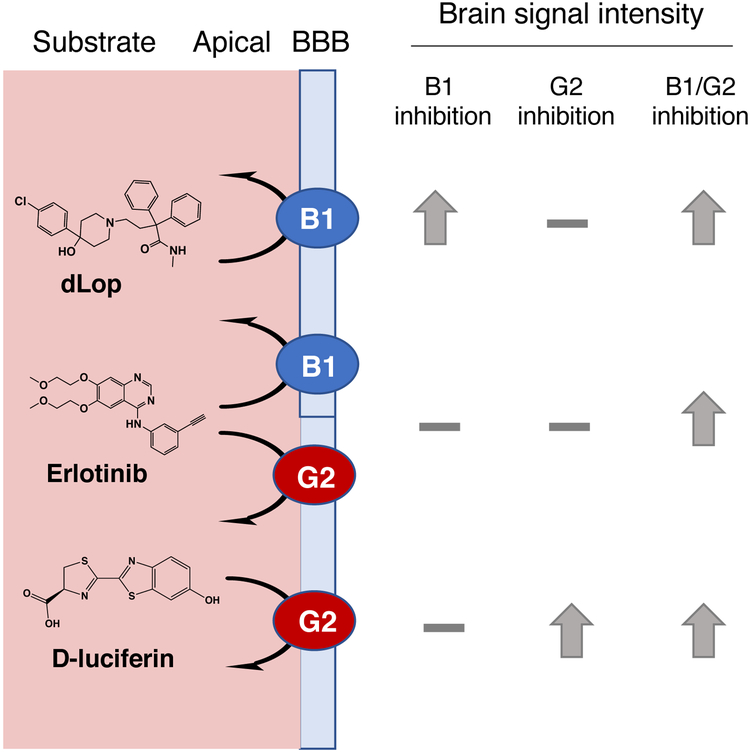
Innis and colleagues studied [N-methyl-11C] N-desmethyl-loperamide (d-loperamide, dLop) as a PET imaging agent for the central nervous system (CNS). Like loperamide, its metabolite, dLop, is also transported exclusively by ABCB1119. Mice lacking Abcb1a/b had a 3.5-fold higher brain uptake of [11C]dLop compared to wild-type mice by PET measurements; in non-human primates, inhibition of ABCB1 with DCPQ yielded a 7-fold increase in brain penetration of [11 C]dLop119. Clinical studies of [11C]dLop showed that it had low brain retention120 and co-administration of tariquidar with [11C]dLop resulted in a 2- to 4-fold increase in the human brain121.
Finally, D-luciferin, the substrate for firefly luciferase, was shown to be a substrate of ABCG2122, and was subsequently shown to be selectively transported by ABCG2 rather than ABCB1 or ABCC1123. When firefly luciferase was expressed in a mouse model in glia behind the BBB under the control of the GFAP promoter, co-administration of D-luciferin and an ABCG2 inhibitor resulted in increased bioluminescent signal in murine brains as compared to mice administered D-luciferin alone123. This is shown in Figure 5. These studies pave the way for use of radiotracers in patients to confirm the ability of inhibitors to increase brain penetration of substrate drugs, and as possible biomarkers for assessing the MDR status of patient tumors.
Figure 5:
The utility and function of PET radiotracers and other probes for imaging ABC transporter function, using the CNS as a model. Combination with inhibitors of known function, or administration to knockout mice, provides insight into function. ABCB1: des-methyl loperamide (dLop) is a specific substrate of ABCB1, producing almost no signal intensity under baseline conditions. Upon inhibition of ABCB1, high brain intensity is observed, though ABCG2 inhibition has no effect. In the instance of a dual substrate of both ABCB1 and ABCG2, such as erlotinib, specifically blocking either ABCB1 or ABCG2 results in a minimal increase in brain signal, and only dual inhibition or knockout produces an effect. An alternative imaging strategy using the specific ABCG2 substrate D-luciferin, with transgenic mice expressing firefly luciferase in astrocytes. Brain bioluminescence signal was low, and specific inhibition of ABCG2 but not ABCB1 produced elevated signal. Third-generation inhibitors such as tariquidar and elacridar are considered to primarily inhibit ABCB1, while Ko143 (which has not been used in humans) acts primarily on ABCG2. No gold-standard probe for dual inhibition of ABCG2 and ABCB1 exists. These imaging tools can act as the basis for studies of multidrug resistance in tumors, and efficacy and dose-optimization of new inhibitors.
Studies of the blood-brain barrier have addressed the ability to target ABC transporters in patients to increase drug distribution into sanctuaries such as the brain121. Some investigators have attempted to demonstrate this in human cancers using radiolabeled substrates, particularly [94Tc]-Sestamibi by either planar or PET imaging in patients with ABCB1-expressing cancers before and after administration of an ABCB1 inhibitor. Except for a small number of patients124, these studies have never shown the type of differential observed in laboratory models125,126. But the caveats mentioned above concerning patient selection and inhibitor choice apply to these studies as well.
While data from imaging is limited, it represents the one method that could be further developed to both demonstrate the impact of drug efflux and the ability to alter it, and act as a biomarker for clinical trials. Such studies have been needed for a very long time, even in the absence of a strategy to inhibit ABCB1.
Conclusions
We have asked whether sufficient evidence exists to re-open investigation of a question encumbered by the weight of a succession of negative clinical trials. We would answer the question with a qualified yes. Yes, because there are clear examples of ABCB1 limiting drug accumulation in selected cancers in patients, of ABCB1 or ABCG2 associated with poor clinical outcomes, and of gene rearrangements resulting in high levels of ABCB1 in patients whose tumors exhibit drug resistance.
Certainly, adequate and consistent drug delivery has never been documented in clinical oncology, and results of the few studies to determine this suggest highly variable drug penetration127. Whether ABC transporters play a role in variable drug delivery is at present unknown. Clinical trials examining the effectiveness of inhibitors of ABC transporters were conducted without selection of patients whose tumors had high levels of transporter expression. If personalized medicine does nothing else, it should be possible to identify patients whose cancer cells overexpress a transporter that reduces drug efficacy—and who would not be expected to benefit from therapies with agents that are substrates for transporters. So that while we may not enhance efficacy with inhibitors of drug transporters, it is entirely plausible, and indeed expected, that we should predict a lack of efficacy and we would argue this would be a valuable accomplishment.
But while we should be able to successfully predict failure, our yes remains qualified, because we do not yet have the means to reliably detect the presence or importance of subsets of cells expressing ABC transporters in the clinic, and second, we do not have definitive proof that inhibitors could increase drug accumulation in cancers in a clinical setting without unacceptable toxicity. We need a clinically validated method to detect the proteins (or a highly correlated biomarker), and we need a validated imaging assay to detect function in tumors. However, we can’t accomplish this without reigniting interest in the transporter field. If we can first answer the question of when and where drug efflux matters, we can then focus on whether clinical outcomes can be improved by adding inhibitors to chemotherapy again.
Acknowledgements
The authors appreciate the help of Sabrina Lusvarghi with figures and the editorial assistance of George Leiman. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Gottesman MM, Lavi O, Hall MD & Gillet JP Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu Rev Pharmacol Toxicol 56, 85–102 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Tamaki A, Ierano C, Szakacs G, Robey RW & Bates SE The controversial role of ABC transporters in clinical oncology. Essays Biochem 50, 209–232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharom FJ ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 9, 105–127 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Schinkel AH & Jonker JW Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55, 3–29 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Gottesman MM, Fojo T & Bates SE Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Rev Cancer 2, 48–58 (2002). [DOI] [PubMed] [Google Scholar]
- 6.GOLDSTEIN MN, SLOTNICK IJ & JOURNEY LJ In vitro studies with HeLa cell line sensitive and resistant to actinomycin D. Ann N Y Acad Sci 89, 474–483 (1960). [DOI] [PubMed] [Google Scholar]
- 7.Biedler JL & Riehm H Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res 30, 1174–1184 (1970). [PubMed] [Google Scholar]
- 8.Dano K Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta 323, 466–483 (1973). [DOI] [PubMed] [Google Scholar]; Discovered that drug resistance was due to active drug extrusion
- 9.Juliano RL & Ling V A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta 455, 152–162 (1976). [DOI] [PubMed] [Google Scholar]; First publication to identify P-gp as a 170 kDa membrane protein.
- 10.Gros P, Croop J, Roninson I, Varshavsky A & Housman DE Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 83, 337–341 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gros P, Ben Neriah Y, Croop JM & Housman DE Isolation and expression of a complementary DNA that confers multidrug resistance. Nature 323, 728–731 (1986). [DOI] [PubMed] [Google Scholar]; First report of a cloned MDR1 (ABCB1) gene from drug-resistant hamster cells
- 12.Roninson IB et al. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A 83, 4538–4542 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of the cloning of the human MDR1 (ABCB1) gene.
- 13.Ueda K et al. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun 141, 956–962 (1986). [DOI] [PubMed] [Google Scholar]
- 14.Gottesman MM & Ling V The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 580, 998–1009 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Cole SPC et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654 (1992). [DOI] [PubMed] [Google Scholar]; First paper to report the cloning of the MRP (ABCC1) gene.
- 16.Mirski SEL, Gerlach JH & Cole SPC Multidrug Resistance in a Human Small Cell Lung Cancer Cell Line Selected in Adriamycin1. Cancer Res. 47, 2594–2598 (1987). [PubMed] [Google Scholar]
- 17.Doyle LA et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95, 15665–15670 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]; First publication identifying the BCRP (ABCG2) gene.
- 18.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V & Dean M A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58, 5337–5339 (1998). [PubMed] [Google Scholar]
- 19.Miyake K et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59, 8–13 (1999). [PubMed] [Google Scholar]
- 20.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C & Gottesman MM Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5, 219–234 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE & Gottesman MM P-glycoprotein: from genomics to mechanism. Oncogene 22, 7468–7485 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Dean M, Hamon Y & Chimini G The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res 42, 1007–1017 (2001). [PubMed] [Google Scholar]
- 23.Esser L et al. Structures of the Multidrug Transporter P-glycoprotein Reveal Asymmetric ATP Binding and the Mechanism of Polyspecificity. J Biol Chem 292, 446–461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson ZL & Chen J Structural Basis of Substrate Recognition by the Multidrug Resistance Protein MRP1. Cell 168, 1075–1085.e1079 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Taylor NMI et al. Structure of the human multidrug transporter ABCG2. Nature 546, 504–509 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Burke MA & Ardehali H Mitochondrial ATP-binding cassette proteins. Transl Res 150, 73–80 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Chapuy B et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia 22, 1576–1586 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Kashiwayama Y et al. 70-kDa peroxisomal membrane protein related protein (P70R/ABCD4) localizes to endoplasmic reticulum not peroxisomes, and NH2-terminal hydrophobic property determines the subcellular localization of ABC subfamily D proteins. Experimental cell research 315, 190–205 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Tsuchida M, Emi Y, Kida Y & Sakaguchi M Human ABC transporter isoform B6 (ABCB6) localizes primarily in the Golgi apparatus. Biochem Biophys Res Commun 369, 369–375 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Tarling EJ, de Aguiar Vallim TQ & Edwards PA Role of ABC transporters in lipid transport and human disease. Trends Endocrinol Metab 24, 342–350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch EM et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 447, 87–91 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Cohen FE & Kelly JW Therapeutic approaches to protein-misfolding diseases. Nature 426, 905–909 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Robert R et al. Structural analog of sildenafil identified as a novel corrector of the F508del-CFTR trafficking defect. Molecular Pharmacol 73, 478–489 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Basseville A et al. Histone deacetylase inhibitors influence chemotherapy transport by modulating expression and trafficking of a common polymorphic variant of the ABCG2 efflux transporter. Cancer Res 72, 3642–3651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma T et al. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem 277, 37235–37241 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Hillebrand M et al. Live cell FRET microscopy: homo- and heterodimerization of two human peroxisomal ABC transporters, the adrenoleukodystrophy protein (ALDP, ABCD1) and PMP70 (ABCD3). J Biol Chem 282, 26997–27005 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Grove DE, Rosser MF, Ren HY, Naren AP & Cyr DM Mechanisms for rescue of correctable folding defects in CFTRDelta F508. Mol Biol Cell 20, 4059–4069 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalepa G, Rolfe M & Harper JW Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 5, 596–613 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Genin EC, Gondcaille C, Trompier D & Savary S Induction of the adrenoleukodystrophy-related gene (ABCD2) by thyromimetics. J Steroid Biochem Mol Biol 116, 37–43 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Kerem E Pharmacologic therapy for stop mutations: how much CFTR activity is enough? Curr Opin Pulmonary Med 10, 547–552 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Ramalho AS et al. Five percent of normal cystic fibrosis transmembrane conductance regulator mRNA ameliorates the severity of pulmonary disease in cystic fibrosis. Am J Respiratory Cell Mol Biol 27, 619–627 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Mlejnek P, Kosztyu P, Dolezel P, Bates SE & Ruzickova E Reversal of ABCB1 mediated efflux by imatinib and nilotinib in cells expressing various transporter levels. Chem Biol Interact 273, 171–179 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Ween MP, Armstrong MA, Oehler MK & Ricciardelli C The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit Rev Oncol Hematol 96, 220–256 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Fletcher JI, Williams RT, Henderson MJ, Norris MD & Haber M ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist Updat 26, 1–9 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Beretta GL, Cassinelli G, Pennati M, Zuco V & Gatti L Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem 142, 271–289 (2017). [DOI] [PubMed] [Google Scholar]
- 46.de Lange EC Potential role of ABC transporters as a detoxification system at the blood-CSF barrier. Adv Drug Deliv Rev 56, 1793–1809 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Kannan P et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther 86, 368–377 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi YH & Yu AM ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des 20, 793–807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharom FJ The P-glycoprotein multidrug transporter. Essays Biochem 50, 161–178 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Cole SP Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol 54, 95–117 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Mao Q & Unadkat JD Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J 17, 65–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein LJ et al. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst 81, 116–124 (1989). [DOI] [PubMed] [Google Scholar]
- 53.Amiri-Kordestani L, Basseville A, Kurdzeil K, Fojo A & Bates S Targeting MDR in Breast and Lung Cancer: Discriminating its Potential Importance from the Failure of Drug Resistance Reversal Studies. Drug Resist Updat (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robey RW, Massey PR, Amiri-Kordestani L & Bates SE ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem 10, 625–633 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonard GD, Fojo T & Bates SE The role of ABC transporters in clinical practice. Oncologist 8, 411–424 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Binkhathlan Z & Lavasanifar A P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets 13, 326–346 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Robey R et al. Efflux of rhodamine from CD56+ cells as a surrogate marker for reversal of P-glycoprotein-mediated drug efflux by PSC 833. Blood 93, 306–314 (1999). [PubMed] [Google Scholar]
- 58.Witherspoon SM et al. Flow cytometric assay of modulation of P-glycoprotein function in whole blood by the multidrug resistance inhibitor GG918. Clin Cancer Res 2, 7–12 (1996). [PubMed] [Google Scholar]; One of the earliest papers to suggest using efflux of rhodamine from CD56+ cells as a surrogate for P-gp inhibition.
- 59.Leonard GD, Polgar O & Bates SE ABC transporters and inhibitors: new targets, new agents. Curr Opin Investig Drugs 3, 1652–1659 (2002). [PubMed] [Google Scholar]
- 60.de Bruin M, Miyake K, Litman T, Robey R & Bates SE Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett 146, 117–126 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Robey RW et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64, 1242–1246 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Minderman H, O’Loughlin KL, Pendyala L & Baer MR VX-710 (biricodar) increases drug retention and enhances chemosensitivity in resistant cells overexpressing P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Clin Cancer Res 10, 1826–1834 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Qadir M et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res 11, 2320–2326 (2005). [DOI] [PubMed] [Google Scholar]
- 64.List AF et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood 98, 3212–3220 (2001). [DOI] [PubMed] [Google Scholar]; One of the first papers to show clinical benefit from the addition of a P-gp inhibitor to chemotherapy.
- 65.Cripe LD et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 116, 4077–4085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Libby E & Hromas R Dismounting the MDR horse. Blood 116, 4037–4038 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Dy GK & Adjei AA Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J Clin 63, 249–279 (2013). [DOI] [PubMed] [Google Scholar]
- 68.Wei XX et al. A Phase I Study of Abiraterone Acetate Combined with BEZ235, a Dual PI3K/mTOR Inhibitor, in Metastatic Castration Resistant Prostate Cancer. Oncologist 22, 503–e543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin J et al. A Phase I/II Study of the Investigational Drug Alisertib in Combination With Abiraterone and Prednisone for Patients With Metastatic Castration-Resistant Prostate Cancer Progressing on Abiraterone. Oncologist 21, 1296–1297e (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai C et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res 68, 7905–7914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mi YJ et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 70, 7981–7991 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tiwari A et al. Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol 78, 153–161 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Katayama R et al. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine 3, 54–66 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathias TJ et al. The FLT3 and PDGFR inhibitor crenolanib is a substrate of the multidrug resistance protein ABCB1 but does not inhibit transport function at pharmacologically relevant concentrations. Invest New Drugs 33, 300–309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dohse M et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos 38, 1371–1380 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerklaan BM et al. Phase I and pharmacological study of pazopanib in combination with oral topotecan in patients with advanced solid tumours. Br J Cancer 113, 706–715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilson CS et al. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood 108, 685–696 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patch AM et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 521, 489–494 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Huff LM, Wang Z, Iglesias A, Fojo T & Lee JS Aberrant transcription from an unrelated promoter can result in MDR-1 expression following drug selection in vitro and in relapsed lymphoma samples. Cancer Res 65, 11694–11703 (2005). [DOI] [PubMed] [Google Scholar]; Demonstration of P-gp upregulation via promoter capture.
- 80.Huff LM, Lee JS, Robey RW & Fojo T Characterization of gene rearrangements leading to activation of MDR-1. J Biol Chem 281, 36501–36509 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Gillet JP et al. Clinical Relevance of Multidrug Resistance Gene Expression in Ovarian Serous Carcinoma Effusions. Mol Pharm (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marzac C et al. ATP Binding Cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica 96, 1293–1301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartholomae S et al. Coexpression of Multiple ABC-Transporters is Strongly Associated with Treatment Response in Childhood Acute Myeloid Leukemia. Pediatr Blood Cancer 63, 242–247 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Patel C et al. Multidrug resistance in relapsed acute myeloid leukemia: evidence of biological heterogeneity. Cancer 119, 3076–3083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raaijmakers M et al. Breast cancer resistance protein in drug resistance of primitive CD34+38- cells in acute myeloid leukemia. Clin Cancer Res 11, 2436–2444 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Ho M, Hogge D & Ling V MDR1 and BCRP1 expression in leukemic progenitors correlates with chemotherapy response in acute myeloid leukemia. Exp Hematol 36, 433–442 (2008). [DOI] [PubMed] [Google Scholar]
- 87.Mohelnikova-Duchonova B et al. Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues. Pancreas 42, 707–716 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Namisaki T et al. Differential expression of drug uptake and efflux transporters in Japanese patients with hepatocellular carcinoma. Drug Metab Dispos 42, 2033–2040 (2014). [DOI] [PubMed] [Google Scholar]
- 89.Fujikura K et al. BSEP and MDR3: Useful Immunohistochemical Markers to Discriminate Hepatocellular Carcinomas From Intrahepatic Cholangiocarcinomas and Hepatoid Carcinomas. Am J Surg Pathol 40, 689–696 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Keizer HG et al. Correlation of multidrug resistance with decreased drug accumulation, altered subcellular drug distribution, and increased P-glycoprotein expression in cultured SW-1573 human lung tumor cells. Cancer Res 49, 2988–2993 (1989). [PubMed] [Google Scholar]
- 91.Faneyte IF, Kristel PM & van de Vijver MJ Determining MDR1/P-glycoprotein expression in breast cancer. Int J Cancer 93, 114–122 (2001). [DOI] [PubMed] [Google Scholar]
- 92.Beck WT et al. Methods to detect P-glycoprotein-associated multidrug resistance in patients’ tumors: Consensus recommendations. Cancer Res 56, 3010–3020 (1996). [PubMed] [Google Scholar]
- 93.Rao VV, Anthony DC & Piwnica-Worms D Multidrug resistance P-glycoprotein monoclonal antibody JSB-1 crossreacts with pyruvate carboxylase. J Histochem Cytochem 43, 1187–1192 (1995). [DOI] [PubMed] [Google Scholar]
- 94.Dulucq S et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 112, 2024–2027 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Zu B et al. MDR1 gene polymorphisms and imatinib response in chronic myeloid leukemia: a meta-analysis. Pharmacogenomics 15, 667–677 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Hur EH et al. C3435T polymorphism of the MDR1 gene is not associated with P-glycoprotein function of leukemic blasts and clinical outcome in patients with acute myeloid leukemia. Leuk Res 32, 1601–1604 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Kalgutkar AS et al. N-(3,4-dimethoxyphenethyl)-4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2[1H]-yl)-6,7-dimethoxyquinazolin-2-amine (CP-100,356) as a “chemical knock-out equivalent” to assess the impact of efflux transporters on oral drug absorption in the rat. J Pharm Sci 98, 4914–4927 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Johnatty SE et al. ABCB1 (MDR1) polymorphisms and ovarian cancer progression and survival: a comprehensive analysis from the Ovarian Cancer Association Consortium and The Cancer Genome Atlas. Gynecol Oncol 131, 8–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rottenberg S et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci U S A 104, 12117–12122 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Leeuwen FW, Buckle T, Kersbergen A, Rottenberg S & Gilhuijs KG Noninvasive functional imaging of P-glycoprotein-mediated doxorubicin resistance in a mouse model of hereditary breast cancer to predict response, and assign P-gp inhibitor sensitivity. Eur J Nucl Med Mol Imaging 36, 406–412 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Rottenberg S et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 105, 17079–17084 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pajic M et al. Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res 69, 6396–6404 (2009). [DOI] [PubMed] [Google Scholar]
- 103.Zander SA et al. Sensitivity and acquired resistance of BRCA1;p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res 70, 1700–1710 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Zander SA et al. EZN-2208 (PEG-SN38) overcomes ABCG2-mediated topotecan resistance in BRCA1-deficient mouse mammary tumors. PLoS One 7, e45248(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaspers JE et al. BRCA2-deficient sarcomatoid mammary tumors exhibit multidrug resistance. Cancer Res 75, 732–741 (2015). [DOI] [PubMed] [Google Scholar]
- 106.Henneman L et al. Selective resistance to the PARP inhibitor olaparib in a mouse model for BRCA1-deficient metaplastic breast cancer. Proc Natl Acad Sci U S A 112, 8409–8414 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of acquired resistance to olaparib via P-gp upregulation in a BRCA1-deficient mouse model.
- 107.Rottenberg S & Borst P Drug resistance in the mouse cancer clinic. Drug Resist Updat 15, 81–89 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Wu M et al. Dissecting genetic requirements of human breast tumorigenesis in a tissue transgenic model of human breast cancer in mice. Proc Natl Acad Sci U S A 106, 7022–7027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Malingre MM et al. Co-administration of GF120918 significantly increases the systemic exposure to oral paclitaxel in cancer patients. Br J Cancer 84, 42–47 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuppens IE et al. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res 13, 3276–3285 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Burger H & Nooter K Pharmacokinetic Resistance to Imatinib Mesylate: Role of the ABC Drug Pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the Oral Bioavailability of Imatinib. Cell Cycle 3, 1502–1505 (2004). [DOI] [PubMed] [Google Scholar]
- 112.Schinkel AH et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77, 491–502 (1994). [DOI] [PubMed] [Google Scholar]; Seminal paper showing a major protective role for P-gp at the blood-brain barrier.
- 113.Durmus S, Sparidans RW, Wagenaar E, Beijnen JH & Schinkel AH Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol Pharm 9, 3236–3245 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Kort A, Sparidans RW, Wagenaar E, Beijnen JH & Schinkel AH Brain accumulation of the EML4-ALK inhibitor ceritinib is restricted by P-glycoprotein (P-GP/ABCB1) and breast cancer resistance protein (BCRP/ABCG2). Pharmacol Res 102, 200–207 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Flaherty KT et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363, 809–819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Traxl A et al. Breast Cancer Resistance Protein and P-Glycoprotein Influence In Vivo Disposition of 11C-Erlotinib. J Nucl Med 56, 1930–1936 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Tournier N et al. Strategies to inhibit ABCB1- and ABCG2-mediated efflux transport of erlotinib at the blood-brain barrier: a PET study in non-human primates. J Nucl Med (2016). [DOI] [PubMed] [Google Scholar]
- 118.Bauer M et al. Pilot PET Study to Assess the Functional Interplay Between ABCB1 and ABCG2 at the Human Blood-Brain Barrier. Clin Pharmacol Ther 100, 131–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lazarova N et al. Synthesis and evaluation of [N-methyl-11C]N-desmethyl-loperamide as a new and improved PET radiotracer for imaging P-gp function. J Med Chem 51, 6034–6043 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seneca N et al. Human brain imaging and radiation dosimetry of 11C-N-desmethyl-loperamide, a PET radiotracer to measure the function of P-glycoprotein. J Nucl Med 50, 807–813 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kreisl WC et al. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J Nucl Med 51, 559–566 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y et al. ABCG2/BCRP expression modulates D-Luciferin based bioluminescence imaging. Cancer Res 67, 9389–9397 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Bakhsheshian J, Wei BR, Hall MD, Simpson RM & Gottesman MM In Vivo Bioluminescent Imaging of ATP-Binding Cassette Transporter-Mediated Efflux at the Blood-Brain Barrier. Methods Mol Biol 1461, 227–239 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; Direct measurement of the function of ABCG2 at the blood-brain barrier in a mouse model
- 124.Agrawal M et al. Increased 99mTc-sestamibi accumulation in normal liver and drug-resistant tumors after the administration of the glycoprotein inhibitor, XR9576. Clin Cancer Res 9, 650–656 (2003). [PubMed] [Google Scholar]
- 125.Kelly RJ et al. A Pharmacodynamic Study of Docetaxel in Combination with the P-glycoprotein Antagonist, Tariquidar (XR9576) in Patients with Lung, Ovarian, and Cervical Cancer. Clin Cancer Res 17, 569–580 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kelly RJ et al. A pharmacodynamic study of the P-glycoprotein antagonist CBT-1® in combination with paclitaxel in solid tumors. Oncologist 17, 512(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bates SE, Amiri-Kordestani L & Giaccone G Drug development: portals of discovery. Clin Cancer Res 18, 23–32 (2012). [DOI] [PubMed] [Google Scholar]
- 128.Knutsen T et al. Cytogenetic and molecular characterization of random chromosomal rearrangements activating the drug resistance gene, MDR1/P-glycoprotein, in drug-selected cell lines and patients with drug refractory ALL. Genes Chromosomes Cancer 23, 44–54 (1998). [DOI] [PubMed] [Google Scholar]
- 129.Basseville A et al. in ABC Transporters - 40 Years on (ed George M. Anthony) 195–226 (Springer International Publishing, 2016). [Google Scholar]
- 130.van Asperen J, van Tellingen O, Tijssen F, Schinkel AH & Beijnen JH Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer 79, 108–113 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marchetti S et al. “Effect of the drug transporters ABCB1, ABCC2, and ABCG2 on the disposition and brain accumulation of the taxane analog BMS-275,183”. Invest New Drugs 32, 1083–1095 (2014). [DOI] [PubMed] [Google Scholar]
- 132.Choo EF et al. Role of P-glycoprotein on the brain penetration and brain pharmacodynamic activity of the MEK inhibitor cobimetinib. Mol Pharm 11, 4199–4207 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Vaidhyanathan S, Mittapalli RK, Sarkaria JN & Elmquist WF Factors influencing the CNS distribution of a novel MEK-1/2 inhibitor: implications for combination therapy for melanoma brain metastases. Drug Metab Dispos 42, 1292–1300 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang SC et al. P-glycoprotein, CYP3A, and plasma carboxylesterase determine brain and blood disposition of the mTOR Inhibitor everolimus (Afinitor) in mice. Clin Cancer Res 20, 3133–3145 (2014). [DOI] [PubMed] [Google Scholar]
- 135.Kort A et al. Brain and Testis Accumulation of Regorafenib is Restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm Res (2015). [DOI] [PubMed] [Google Scholar]
- 136.de Vries NA et al. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs 30, 443–449 (2012). [DOI] [PubMed] [Google Scholar]
- 137.Lagas J et al. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther 9, 319–326 (2010). [DOI] [PubMed] [Google Scholar]
- 138.Tang SC et al. P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) limit sunitinib brain accumulation which can be enhanced by oral elacridar treatment. Proc Amer Assoc Can Res 51 (2010). [Google Scholar]
- 139.Parrish KE et al. Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther 355, 264–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Durmus S et al. P-glycoprotein (MDR1/ABCB1) and breast cancer resistance protein (BCRP/ABCG2) restrict brain accumulation of the JAK1/2 inhibitor, CYT387. Pharmacol Res 76, 9–16 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Lagas JS et al. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res 15, 2344–2351 (2009). [DOI] [PubMed] [Google Scholar]
- 142.Poller B et al. Differential impact of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) on axitinib brain accumulation and oral plasma pharmacokinetics. Drug Metab Dispos 39, 729–735 (2011). [DOI] [PubMed] [Google Scholar]
- 143.Zhou L et al. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos 37, 946–955 (2009). [DOI] [PubMed] [Google Scholar]
- 144.Zhang P et al. ABCB1 and ABCG2 restrict the brain penetration of a panel of novel EZH2-Inhibitors. Int J Cancer 137, 2007–2018 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Sane R, Agarwal S, Mittapalli RK & Elmquist WF Saturable active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier leads to nonlinear distribution of elacridar to the central nervous system. J Pharmacol Exp Ther 345, 111–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin F et al. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res 20, 2703–2713 (2014). [DOI] [PubMed] [Google Scholar]
- 147.Chuan Tang S et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 134, 1484–1494 (2014). [DOI] [PubMed] [Google Scholar]
- 148.Durmus S et al. Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1) restrict oral availability and brain accumulation of the PARP inhibitor rucaparib (AG-014699). Pharm Res 32, 37–46 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Wang T, Agarwal S & Elmquist WF Brain distribution of cediranib is limited by active efflux at the blood-brain barrier. J Pharmacol Exp Ther 341, 386–395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Polli J et al. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}−6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos 37, 439–442 (2009). [DOI] [PubMed] [Google Scholar]
- 151.Tang SC et al. P-glycoprotein, CYP3A, and Plasma Carboxylesterase Determine Brain Disposition and Oral Availability of the Novel Taxane Cabazitaxel (Jevtana) in Mice. Mol Pharm 12, 3714–3723 (2015). [DOI] [PubMed] [Google Scholar]
- 152.Lin F et al. Abcc4 together with abcb1 and abcg2 form a robust cooperative drug efflux system that restricts the brain entry of camptothecin analogues. Clin Cancer Res 19, 2084–2095 (2013). [DOI] [PubMed] [Google Scholar]
- 153.de Vries NA et al. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13, 6440–6449 (2007). [DOI] [PubMed] [Google Scholar]; First demonstration that P-gp and ABCG2 cooperate to limit brain penetration of substrate drugs in mice deficient in both transporters.
- 154.Salphati L, Lee LB, Pang J, Plise EG & Zhang X Role of P-glycoprotein and breast cancer resistance protein-1 in the brain penetration and brain pharmacodynamic activity of the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos 38, 1422–1426 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Vaidhyanathan S et al. Factors Influencing the Central Nervous System Distribution of a Novel Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitor GSK2126458: Implications for Overcoming Resistance with Combination Therapy for Melanoma Brain Metastases. J Pharmacol Exp Ther 356, 251–259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchetti S et al. Effect of the drug transporters ABCG2, Abcg2, ABCB1 and ABCC2 on the disposition, brain accumulation and myelotoxicity of the aurora kinase B inhibitor barasertib and its more active form barasertib-hydroxy-QPA. Invest New Drugs 31, 1125–1135 (2013). [DOI] [PubMed] [Google Scholar]
- 157.Mittapalli RK, Vaidhyanathan S, Dudek AZ & Elmquist WF Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther 344, 655–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang J et al. P-glycoprotein (MDR1/ABCB1) and Breast Cancer Resistance Protein (BCRP/ABCG2) affect brain accumulation and intestinal disposition of encorafenib in mice. Pharmacol Res (2017). [DOI] [PubMed] [Google Scholar]
- 159.Kort A et al. Brain Accumulation of Ponatinib and Its Active Metabolite, N-Desmethyl Ponatinib, Is Limited by P-Glycoprotein (P-GP/ABCB1) and Breast Cancer Resistance Protein (BCRP/ABCG2). Mol Pharm 14, 3258–3268 (2017). [DOI] [PubMed] [Google Scholar]