Abstract
Mechanisms whereby deep brain stimulation (DBS) of the subthalamic nucleus (STN) or internal globus pallidus (GPi) reduces dyskinesias remain largely unknown. Using vacuous chewing movements (VCMs) induced by chronic haloperidol as a model of tardive dyskinesia (TD) in rats, we confirmed the antidyskinetic effects of DBS applied to the STN or entopeduncular nucleus (EPN, the rodent homolog of the GPi). We conducted a series of experiments to investigate the role of serotonin (5-HT) in these effects. We found that neurotoxic lesions of the dorsal raphe nuclei (DRN) significantly decreased HAL-induced VCMs. Acute 8-OH-DPAT administration, under conditions known to suppress raphe neuronal firing, also reduced VCMs. Immediate early gene mapping using zif268 in situ hybridization revealed that STN-DBS inhibited activity of DRN and MRN neurons. Microdialysis experiments indicated that STN-DBS decreased 5-HT release in the dorsolateral caudate–putamen, an area implicated in the etiology of HAL-induced VCMs. DBS applied to the EPN also suppressed VCMs but did not alter 5-HT release or raphe neuron activation. While these findings suggested a role for decreased 5-HT release in the mechanisms of STN DBS, further microdialysis experiments showed that when the 5-HT lowering effects of STN DBS were prevented by pretreatment with fluoxetine or fenfluramine, the ability of DBS to suppress VCMs remained unaltered. These results suggest that EPN- and STN-DBS have different effects on the 5-HT system. While decreasing 5-HT function is sufficient to suppress HAL-induced VCMs, 5-HT decrease is not necessary for the beneficial motor effects of DBS in this model.
Introduction
Deep brain stimulation (DBS) is a clinical procedure whereby electrical current is passed through surgically implanted electrodes in the brain. Over the last decade, DBS applied to the subthalamic nucleus (STN) or internal globus pallidus (GPi) has been successfully used to treat several movement disorders (Lyons, 2011; Troster, 2009), including treatment-resistant tardive dyskinesia (TD). TD is a disorder induced by chronic therapy with classical antipsychotic drugs (Damier et al., 2007; Capelle et al., 2010), which is characterized by abnormal, involuntary movements of the head, neck, face and jaw, which can persist for a prolonged time after medication withdrawal (Casey, 1985, 2004). While the GPi is the most common DBS target in cases of TD, the STN has also been shown to alleviate motor symptoms associated with chronic antipsychotic use (Zhang et al., 2006; Sun et al., 2007).
In rats, chronic HAL treatment leads to a syndrome of vacuous chewing movements (VCMs), which are considered analogous to human TD (Turrone et al., 2002). We have previously shown that DBS of the STN or entopeduncular nucleus (EPN, the rodent homolog of the GPi) effectively suppresses HAL-induced VCMs (Creed et al., 2011). However, despite its widespread clinical application in movement disorders, the mechanisms underlying the antidyskinetic effects of DBS remain largely unknown. We have shown that STN- and EPN-DBS influence activity in a variety of brain regions at sites distal from the stimulation target (Creed et al., 2012), an observation that has also been made in the clinic (Su et al., 2001; Schulte et al., 2006; Damier et al., 2007). DBS also effects several neurotransmitter systems (Lee et al., 2006; Covey and Garris, 2009; Navailles et al., 2010; Feuerstein et al., 2011; Sgambato-Faure and Cenci, 2012).
Several lines of evidence implicate the serotonin (5-HT) system in the symptoms of TD and in the effects of STN-DBS. 5-HT provides extensive innervation to the basal ganglia where it modulates dopamine neurotransmission (Alex and Pehek, 2007; Di Matteo et al., 2008). The lower incidence of TD associated with atypical antipsychotics has been ascribed to their 5-HT2 receptor antagonism (Meltzer and Nash, 1991; Kapur et al., 1999; Stahl, 1999), while antagonism of 5-HT2C receptors suppresses VCMs in rats (Kostrzewa et al., 2007; Creed-Carson et al., 2011). Moreover, symptoms of TD can be exacerbated by concomitant treatment with selective serotonin reuptake inhibitors (SSRIs), which increase synaptic 5-HT levels (Ketai, 1993; D'Souza et al., 1994; Dubovsky and Thomas, 1996; Sandler, 1996; Lauterbach and Shillcutt, 1997; Caley, 1998). While the effect of EPN-DBS on 5-HT activity has not been examined, STN-DBS has been shown to inhibit firing of serotonergic neurons in the dorsal raphe (Temel et al., 2007; Hartung et al., 2011), to decrease 5-HT release in the hippocampus and prefrontal cortex (Navailles et al., 2010), and to counter the motor effects of 5-HT agonists (Hameleers et al., 2007).
The objective of the present study was to examine the possible contribution of the serotonin system to the motor effects of DBS in the HAL-induced VCM model of TD.
Materials and Methods
Drug treatment.
Male Sprague Dawley rats initially weighing 200–250 g (Charles River) received either haloperidol decanoate (HAL, Sandoz Canada; 21 mg/kg i.m., N = 68) or sesame oil vehicle (N = 22) once every 3 weeks for 12 weeks (a total of four injections). The decanoate formulation is known to result in constant plasma levels in the 1.1–1.5 mg/kg range and reliably induces progressively increasing levels of VCMs (Turrone et al., 2003).
Vacuous chewing movement assessments.
VCM assessments were conducted once a week in a quiet room, beginning 1 week before the first HAL injection. For each VCM assessment the rat was placed on a flat round surface (26 cm in diameter, 50 cm high) and allowed to acclimate for 2 min. Over the following 2 min a trained observer blind to treatment group recorded the number of VCMs, which were defined as jaw movements in the vertical plane not directed at specific objects accompanied or not by tongue protrusions. Discrete bursts of jaw tremors were counted as one VCM.
VCM assessment in the presence of serotonergic drugs.
Each subject was tested after administration of saline or the drug of interest in a randomized cross-over design. Separate groups of rats were administered fluoxetine hydrochloride (Tocris Bioscience) 10 mg/kg, i.p.; (+)-fenfluramine hydrochloride (Sigma-Aldrich) 1 mg/kg, i.p. 1 h before VCM assessments. 2,5-dimethoxy-4-iodoamphetamine (DOI, Sigma-Aldrich) at a dose of 0.1 mg/kg i.p. was administered 30 min before VCM assessments. In a separate group of experiments, 8-hydroxy-DPAT-hydrobromide (8-OH-DPAT, Tocris Bioscience) dissolved in saline was administered via tail-vein injection at a dose of 5 μg/kg. VCMs were observed for 4 min after 8-OH-DPAT administration.
Surgery.
After 12 weeks of HAL treatment, rats were anesthetized with ketamine/xylazine (100/7.5 mg/kg i.p.) and had polymide-insulated stainless steel monopolar electrodes (250 μm in diameter with 0.6 mm of surface exposed) bilaterally implanted into the STN (AP −3.8 mm; ML +2.5 mm; DV −8.0) or EPN (AP −3.6 mm; ML +2.6 mm; DV −7.8 mm) (Paxinos and Watson, 1986). Anodes were connected to a bone screw over the somatosensory cortex. Sham surgery controls were anesthetized and had holes drilled into the skull but were not implanted with electrodes.
DBS protocol.
Starting 1 week after surgery DBS was applied using a portable stimulator (St Jude Medical, Model 6510) set to deliver 100 μA current at 90 μs pulse width. Stimulation frequency was set to 130 Hz. These settings were used to approximate a charge density similar to that used in humans, and optimized to achieve optimal anti-dyskinetic effects without inducing other motor side effects, as described previously (Hamani et al., 2010, 2012; Creed et al., 2011, 2012).
Open field tests.
After completion of VCM assessments, locomotor activity was assessed in an open field. Rats were placed in automated activity chambers (Med Associates) for a 2 min acclimation period before the onset of DBS (for animals implanted with electrodes). Horizontal activity was then recorded for 15 min during 130 Hz DBS, following which rats were returned to the home cage. Animals without electrodes were also monitored in the activity chamber for 15 min.
In situ hybridization.
Hybridization was performed using 35S-UTP labeled riboprobes prepared by in vitro transcription using consensus promoter sequences for T7 RNA polymerase and cDNA sequences complementary to bases 660–1043 (according to GenBank # NM_012551). Using the NCBI BLAST Tool, the sequences were checked for homology with the rat genome and found to be specific for their respective transcripts. Probes were diluted to a concentration of 200,000 cpm/μl in hybridization solution containing the following: 50% formamide, 35% Denhardt's solution, 10% dextran sulfate, 0.1× SSC, salmon sperm DNA (300 μg/ml), yeast tRNA (100 μg/ml), and DTT (40 μm). Slides were incubated in plastic mailers overnight at 60°C. After hybridization, sections were rinsed in 4× SSC at 60°C, treated in RNase A (20 μg/ml) solution at 45°C for 40 min, washed with agitation in decreasing concentrations of SSC containing 5 g/L sodium thiosulfate, dipped in water, dehydrated in 70% ethanol, and air-dried. The slides were exposed to Kodak BioMax film for 6 d at 4°C along with calibrated radioactivity standards. Probe specificity was confirmed by testing labeled sense and scrambled probes, both of which produced no measurable signal on film.
Densitometric analyses were performed with MCID 7.0 software (InterFocus). Before sampling, illumination level was adjusted for every film to ensure consistent background readings across all films. Standard curves obtained from calibrated radioactivity standards were used to convert raw optical density values to radioactivity levels in microcuries per gram of tissue (μCi/g). Basal ganglia regions were analyzed in eight sections per animal, with brain regions defined according to the atlas of Paxinos and Watson (1986). Readings were first averaged across all sampling windows in a section and then across all sections to produce a final density value for each region for each animal.
Microdialysis.
For microdialysis experiments, a guide cannula was implanted into the dorsolateral caudate–putamen (CPu; AP +1.4 mm; ML +2.4 mm; DV −3.0 mm) during electrode insertion surgery. After 1 week of recovery, a microdialysis probe (MAB4.15.4 −4 mm membrane, Scientific Products) was inserted into the target and perfused with Ringer's solution at a constant flow rate of 0.7 μl/min (microinjection pump, Hamilton). Following an equilibration period of 5 h, dialysate samples were collected every 12 min. Baseline levels were defined as an average of the first five samples collected. DBS was then delivered for 1 h during collection, followed by 96 min of recovery. In some experiments, fluoxetine or fenfluramine were administered 1 h before DBS onset. Samples were analyzed in real time using an Antec Leyden LC110 Alexys HPLC system coupled to a Decade-II electrochemical detection cell (ATS Scientific).
5,7-dihydroxytryptamine lesions.
A previously described procedure (Fletcher, 1995) was used with minor modifications. Rats were injected intraperitoneally with 10 mg/kg desipramine-HCl (σ) 30 min before being anesthetized with isoflurane. A 30 gauge needle was lowered into the intended raphe site and 3 μg of 5,7-dihydroxytryptamine creatine sulfate (5,7-DHT, Sigma) dissolved in 1% ascorbic acid was injected. Dose refers to the free base. For combined raphe lesions, the needle was lowered to the MRN (AP −8.0 mm; ML 0 mm; DV −8.0 mm) and 2 μl of 5,7-DHT was infused over 4 min. The needle was left in place for a further 2 min, then raised to the DRN (AP −8.0 mm; ML 0 mm; DV −6.0 mm), where the procedure was repeated. For specific lesions of the DRN or MRN, the needle was lowered to the appropriate site and 0.75 μl of 5,7-DHT was infused over 2 min; the needle was left in situ for a further 2 min.
Confirmation of serotonin lesions.
Serotonin-depleting lesions were confirmed using HPLC to determine concentrations of 5-HT and its metabolite 5-HIAA. In addition, 11C-DASB binding to the 5-HT transporter was used to assess 5-HT terminal integrity in smaller areas of the same brains. Rats were injected with an overdose of pentobarbital before decapitation and brain extraction. Brains were bisected sagittally off-center from the midline. Dorsolateral CPu and hippocampus were dissected from the smaller hemisphere, flash-frozen, and stored at −80°C HPLC, whereas the larger hemisphere was flash-frozen intact for C11-DASB autoradiography.
C11-DASB autoradiography.
Coronal cryostat sections (20 μm) were cut and thaw-mounted onto Superfrost slides (Fisher Scientific). Slides were preincubated at 4°C for 20 min in buffer containing 0.05 m Tris, 0.05 m NaCl, and 5 mm KCl, pH 7.4, before being transferred to room temperature buffer containing 4 nm C11-DASB freshly synthesized at the CAMH PET Centre for 10 min. Nonspecific binding was determined by displacement with 10 μm paroxetine. Slides were rinsed for five 60 s intervals in 4°C buffer, dipped in milliQ water for 10 s, dried with cool air, and immediately exposed to Kodak Biomax film for 24 h.
Densitometric analysis was performed using MCID software (InterFocus). A standard curve relating optical density to known quantities of [11C]DASB (μCi/g) was created. For any subject, the final binding value for any given brain region represented an average of multiple readings on five brain sections. Brain regions were defined according to the atlas of Paxinos and Watson (1986). Binding values were averaged for each region and then for each subject, and group means were compared independently for each of the regions sampled.
HPLC analyses.
Following behavioral tests, lesions were confirmed using HPLC (SP8000 with 25cm × 2.6 mm Spherisorb ODS2 column, Spectra Physics) coupled to an ESA Coulochem II electrochemical detector to measure concentration of 5-HT and its principal metabolite, 5-hydroxindoleacetic acid (5-HIIA) according to a previously published method (Wilson et al., 1994). Briefly, samples were injected into a six port Rheodyne valve fitted with a 100 μl loop. The mobile phase was composed of 0.1 m sodium acetate buffer, pH 3.9, containing 130 mg/L octane sulfonate and 3% methanol, with a flow rate of 1 ml/min. Tissue was weighed and sonicated in 1.0 ml of ice-cold H2O, then spiked with 100 μl of 1.0 N percholoric acid containing 2 μm sodium bisulfite before resonification. Samples were centrifuged at 35,000 × g for 30 min, the supernatant was removed, filtered (0.45 μm), and injected into the column. For quantification, every third sample was spiked with a mixture containing standard amounts of each of the compounds being measured.
Verification of electrode placement.
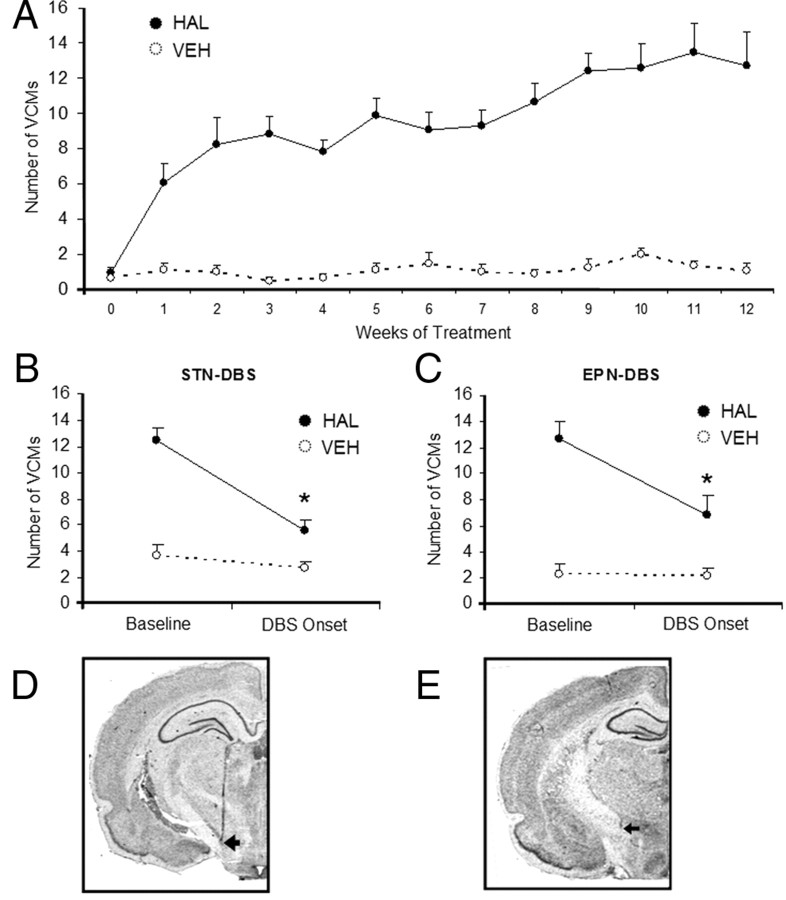
Following behavioral tests, rats were deeply anesthetized with sodium pentobarbital and brains were removed, sectioned, postfixed in 10% formalin vapor, and stained with cresyl violet to visualize electrode tracts. Only rats with electrode tips in the STN (Fig. 1D) or EPN (Fig. 1E) were included in the analyses.
Figure 1.
DBS suppresses HAL-induced VCMs. Chronic haloperidol treatment led to the induction of vacuous chewing movements, which stabilized by ∼9 weeks of treatment (N = 125 HAL; N = 80 VEH) (A). All differences between HAL- and VEH-treated groups are significant from the first week of treatment onward. Indication of statistical significance was omitted for clarity. DBS applied to the STN (B) or the EPN (C) reduced VCMs immediately after DBS onset. Representative pictographs showing placement of electrodes in the STN and EPN are shown in D and E, respectively. *p < 0.01.
Statistical analyses.
All analyses were performed using SPSS version 15. VCM results were analyzed using repeated-measures ANOVA followed by Tukey's post hoc tests where appropriate. Data from 8-OH-DPAT experiments were analyzed using paired t tests. Raphe lesion data were analyzed with one-way repeated-measures ANOVA. Microdialysis data were analyzed using repeated-measures ANOVA with treatment and target as independent factors.
Results
Effect of DBS on VCMs
As expected, HAL induced VCMs over the course of 12 weeks, the levels of which stabilized by ∼8 weeks of treatment (Fig. 1A). DBS applied to either the STN or EPN significantly decreased VCMs (Fig. 1B,C).
Effect of 5,7-DHT lesions on VCMs
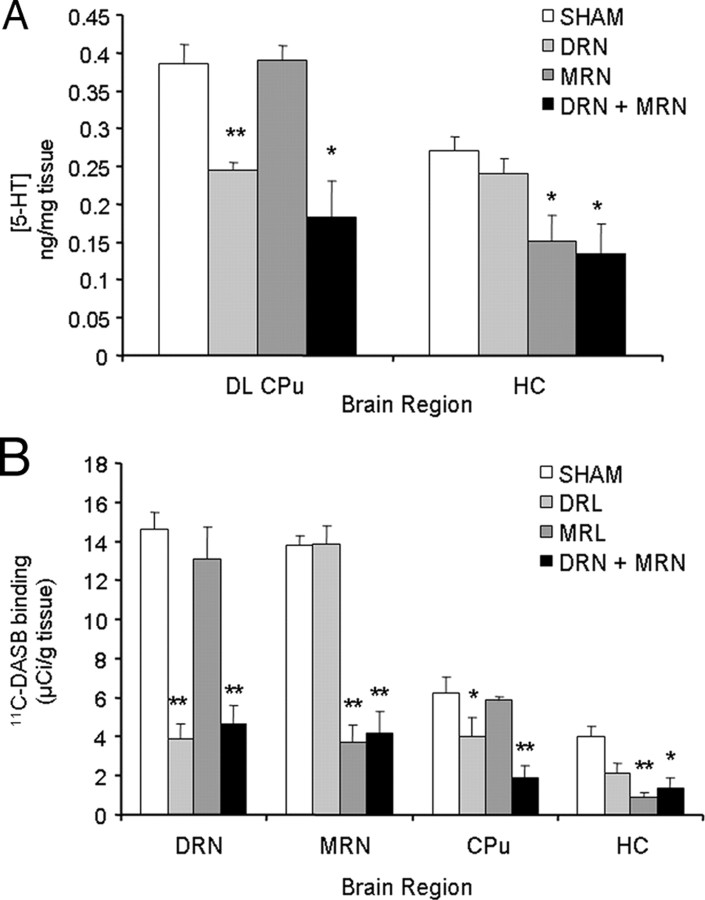
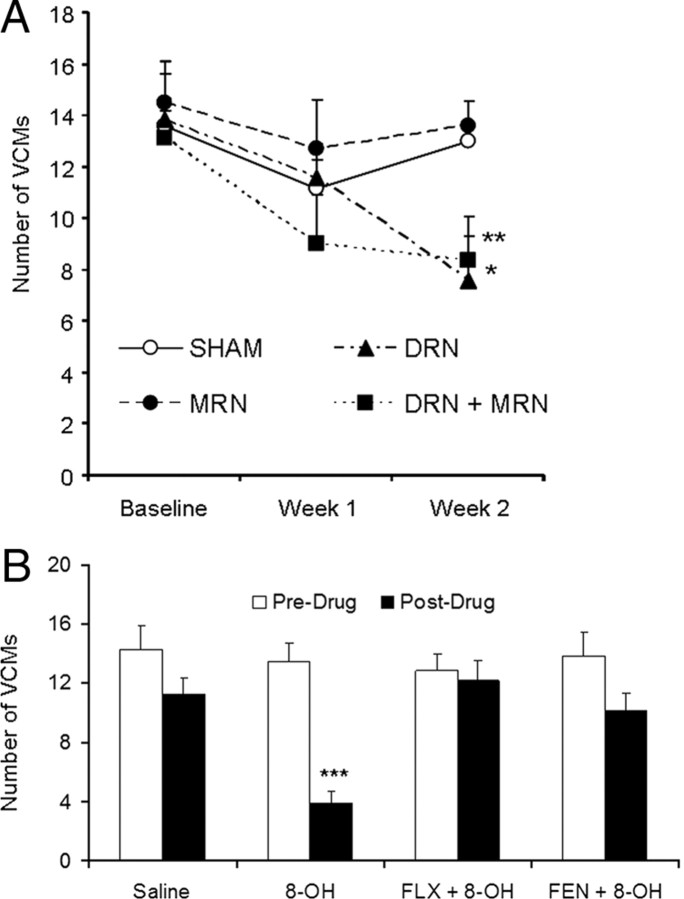
To determine the effects of decreasing brain 5-HT on HAL-induced VCMs, we performed 5,–7-DHT lesions in HAL-treated rats. VCMs were assessed before surgery and subsequently 1 and 2 weeks after surgery. Repeated-measures ANOVA indicated a significant effect of time on HAL-induced VCMs (F = 8.75, p = 0.002), which was driven by a linear decrease in VCMs over time (p = 0.001). Two weeks after surgery, VCMs were significantly decreased relative to baseline in the DRN-lesioned animals (−45%, p = 0.002) and animals that received combined DRN+MRN lesions (−37%, p = 0.02). VCMs were not different in MRN-lesioned animals (−6.4%, p = 0.719) or in subjects with SHAM lesions (−8.8%, p = 0.171). There was no effect of lesion on VCMs in rats that had not received HAL (data not shown). Lesions were confirmed using HPLC to measure striatal and hippocampal 5-HT content (Fig. 2A) and by [11C]DASB autoradiography (Fig. 2B). Relative to sham-lesioned animals, dorsolateral CPu 5-HT levels were significantly lower in rats with lesions to the DRN (−367%, p < 0.0001) and combined DRN+MRN lesions (−52.5%, p = 0.003). Levels of 5-HT in the hippocampus were significantly decreased in MRN-lesioned (−44.4%, p = 0.011) and dual-lesioned rats (−50.5%, p = 0.008). In terms of [11C]DASB autoradiography, binding was decreased in the DRN and dorsolateral CPu of DRN-lesioned animals (DL CPu: − 51.2%, p = 0.022, DRN: − 73.1%, p < 0.0001), while decreased binding in the HC and MRN was observed in MRN-lesioned animals (HC: − 77.7%, p < 0.0001, MRN: − 72.9%, p < 0.0001). Rats with dual MRN+DRN lesions exhibited decreased binding in all regions examined (DL CPu: − 69.6%, p = 0.001, HC: − 66.1%, p = 0.004, DRN: − 68.2%, p < 0.0001, MRN: − 69.6%, p < 0.0001).
Figure 2.
Confirmation of 5,7-DHT lesions. 5,7-DHT lesions were confirmed my measuring 5-HT concentration (A) and by 5-HT transporter binding using 11C-DASB autoradiography (B). Rats with lesions of the DRN exhibited decreased [5-HT] in the DL CPu, whereas MRN-lesioned animals had lower [5-HT] in the hippocampus. Animals with dual lesions of the DRN and MRN had decreased [5-HT] in both regions measured (A). The same pattern was observed with serotonin transporter binding. Animals with DRN lesions exhibited decreased binding in the DRN and CPu, whereas decreased binding was observed in the MRN and HC in MRN-lesioned animals. Animals with dual lesions had lower binding in all regions examined. *p < 0.01, **p < 0.001.
Effect of 8-OH-DPAT on VCMs
To further determine whether decreased 5-HT neuronal activity could affect HAL-induced VCMs, we administered 8-OH-DPAT at a dose previously shown to inhibit serotonergic raphe neuron firing for 8 min after systemic injection (Blier et al., 1989). Acute treatment with 8-OH-DPAT significantly decreased HAL-induced VCMs by 71.1% (p < 0.001), whereas saline injections had no effect on VCMs (p = 0.174) (Fig. 3B). There was no significant effect of 8-OH-DPAT when rats were pretreated with fluoxetine (p = 0.670) or fenfluramine (p = 0.120) pretreatment, so that no significant decreases in VCMs were observed (Fig. 3B).
Figure 3.
Reducing brain 5-HT content or activity suppresses HAL-induced VCMs. Animals that received 5,7-DHT lesions of the DRN alone or in combination with the MRN exhibited lower levels of VCMs 2 weeks after surgery. Sham lesions, or lesions of MRN only, did not affect VCM levels (A). Acute administration of 8-OH-DPAT suppressed HAL-induced VCMs (B), but had no effect when rats were pretreated with fluoxetine (FLX) or fenfluramine (FEN). N = 6–8/group. *p < 0.05; **p < 0.01.
Effect of DBS on immediate early gene induction in the raphe nucleus
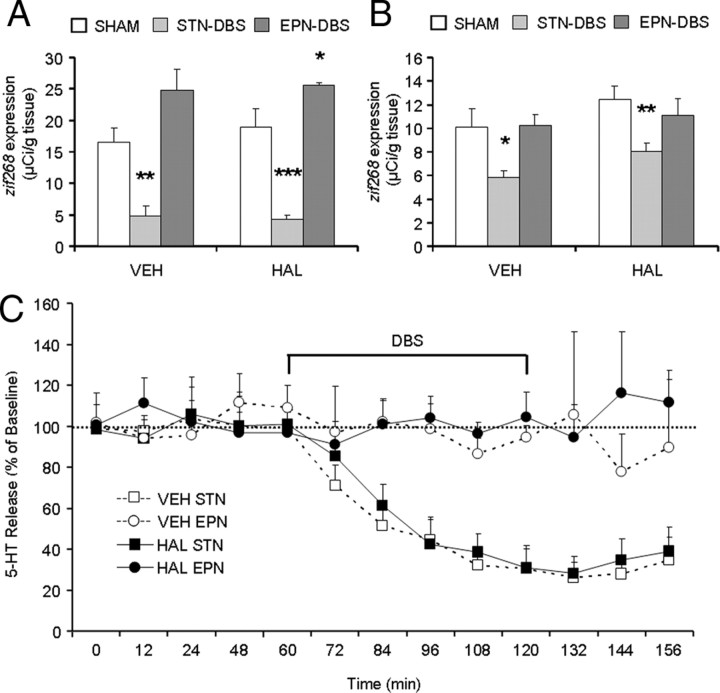
To determine whether DBS modifies activity of raphe neurons, we measured expression of the immediate early gene zif268. While zif268 expression tended to be higher in HAL-treated rats relative to vehicle controls, this effect did not reach significance in either the DRN (p = 0.287) or MRN (p = 0.144). In contrast, DBS applied to the STN significantly decreased zif268 expression in the DRN (VEH: − 70.9%, p = 0.001; HAL: − 77.9%, p < 0.0003) and MRN (VEH: − 42.5%, p = 0.0245; HAL: − 35.3%, p = 0.0056). There was no significant effect of EPN-DBS on zif268 expression in either VEH- or HAL-treated rats in either the MRN or DRN (Fig. 4A,B).
Figure 4.
Effect of DBS on 5-HT function. STN-DBS decreased expression of the immediate early gene zif268 in both HAL- and VEH-treated rats in the DRN (A) and MRN (B), whereas EPN-DBS had no effect. Similarly, STN-DBS significantly decreased 5-HT release in the DL CPu in both HAL- and VEH-treated rats (C). This decrease occurred immediately after DBS onset and persisted for up to 36 min after DBS offset. DBS application is indicated from 60 min to 120 min. Indication of statistical significance is omitted for clarity. EPN-DBS had no effect on 5-HT levels. N = 7–8/group. *p < 0.05.
Effect of DBS on striatal 5-HT release
We performed in vivo microdialysis to determine whether DBS affects 5-HT release in the CPu. Overall, there was a significant effect of DBS target (F = 97.47, p < 0.001) but not of HAL-treatment (F = 2.31, p = 0.143) on 5-HT release in the DL CPu. STN-DBS induced a significant reduction of extracellular 5-HT levels in the DL CPu in both VEH- and HAL-treated rats, whereas EPN-DBS did not alter 5-HT release. The decreased 5-HT release induced by STN-DBS was progressive, and started immediately after DBS onset (VEH: − 29.0%, p = 0.050; HAL: − 40.7%, p = 0.017), reaching maximal levels in the sample measured immediately after DBS offset (VEH: − 74.1%, p < 0.0001; HAL: − 70.5%, p < 0.001). 5-HT levels remained decreased 60 min after the stimulation period (VEH: − 74.1%, p = 0.0002; HAL: − 59.9% p = 0.0002). In both HAL- and VEH-treated animals undergoing EPN-DBS, 5-HT levels were not significantly different from baseline at any time point (Fig. 4C).
Effect of serotonergic drug pretreatment on VCM-suppressing effects of DBS
To determine whether the decrease in 5-HT release induced by DBS is necessary for the antidyskinetic effects of DBS, we pharmacologically elevated brain levels of 5-HT by pretreatment with fluoxetine or fenfluramine before the application of DBS. Given the high concentration of 5-HT2 receptors in the DL CPu and their implication in the motor effects of classical antipsychotics (Meltzer and Nash, 1991; Creed-Carson et al., 2011), we also examined the effect of pretreatment with the nonspecific 5-HT2 agonist DOI on the VCM suppressing effects of DBS.
Fluoxetine
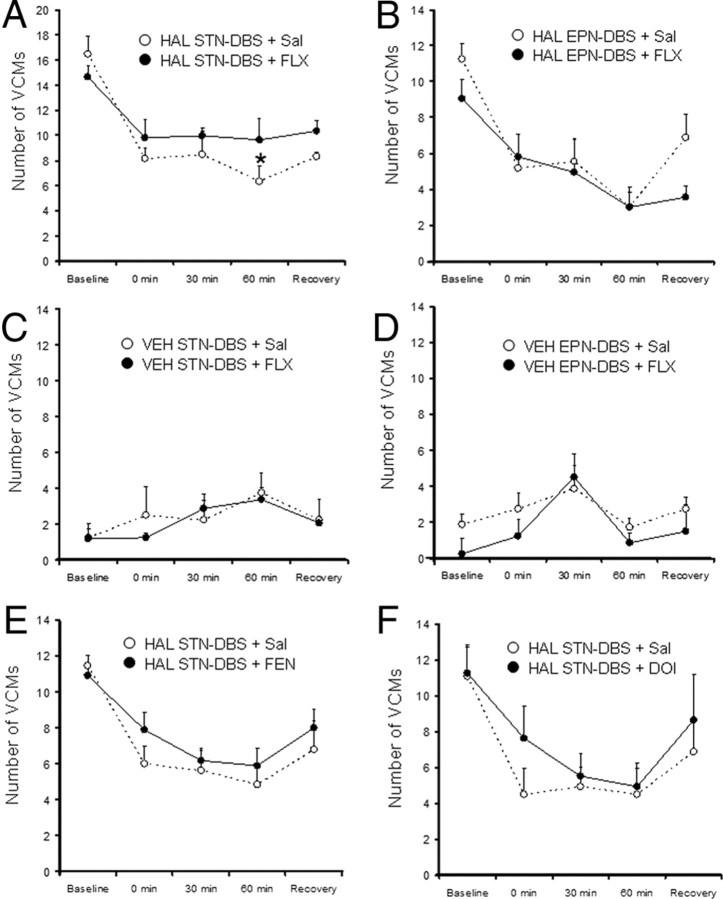
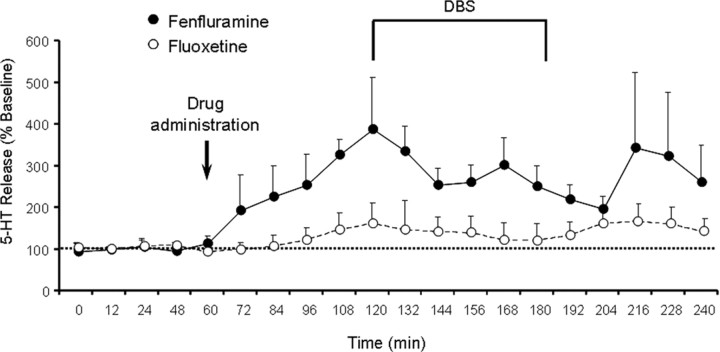
There was a significant effect of HAL treatment (F = 137.51, p < 0.001) on VCMs, with HAL-treated animals exhibiting higher levels of VCMs than VEH-treated rats. There was also a significant effect of time (F = 25.56, p < 0.001) and a significant interaction between HAL treatment and time (F = 39.43, p < 0.001). This interaction was likely due to a decrease in VCMs among HAL-treated rats during DBS, which was not observed in VEH-treated animals. While there was no significant main effect of fluoxetine (F = 0.35, p = 0.554), there was a significant interaction between fluoxetine and time (F = 4.206, p = 0.005) and between fluoxetine, time, and haloperidol (F = 3.81, p = 0.009). Pairwise comparisons revealed that among HAL-treated animals undergoing STN-DBS, fluoxetine pretreatment attenuated the decrease in VCMs induced by DBS. However, this fluoxetine effect only achieved statistical significance at the 60 min time point (Fig. 5A). There were no significant effects of fluoxetine in VEH-treated animals undergoing STN-DBS (Fig. 5C) or in any animals receiving EPN-DBS (Fig. 5B,D). Fluoxetine had no effect on total distance traveled in the open field (Fig. 6). At the dosing parameters used, fluoxetine induced a 60% increase in 5-HT peak release and 5-HT levels remained elevated for the duration of DBS. The mean increase across the five time points measured was 32.9% relative to baseline (Fig. 7).
Figure 5.
Effect serotonergic pharmacomanipulation of DBS efficacy. DBS suppressed VCMs for the 60 min of DBS application in all HAL-treated groups (A, B, E, F). After 60 min of STN-DBS, VCM levels were higher after fluoxetine pretreatment than after saline pretreatment (A). There were no differences between saline and fluoxetine pretreatment on VCMs during STN-DBS in VEH-treated rats (C). Fluoxetine pretreatment did not alter the efficacy of EPN-DBS (B, D). VCM levels were greater during STN-DBS when rats had been pretreated with fenfluramine (E) and DOI (F), although these differences did not achieve statistical significance. N = 8/group (HAL-treated groups) and N = 6/group (VEH-treated groups).
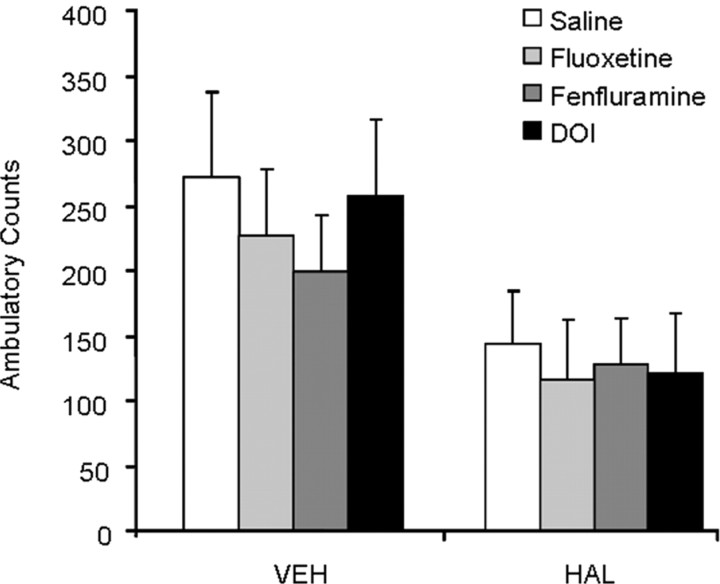
Figure 6.
Effect of serotonergic pharmacomanipulation on open field activity. HAL-treated animals exhibited lower levels of spontaneous ambulatory activity. None of the serotonergic drugs administered before DBS influenced activity in the open field arena.
Figure 7.
Effect of serotonergic manipulations of 5-HT release. Pretreatment with either fluoxetine (160% relative to baseline) or fenfluramine (386% relative to baseline) elevated 5-HT release. 5-HT release was significantly higher than baseline at the 72 min time point in fenfluramine-treated rats and at the 108 min time point after fluoxetine treatment. Indication of statistical significance was omitted for clarity. Levels remained elevated relative to baseline (fluoxetine: 132.9%, fenfluramine: 279.6%) for the duration of DBS and up to 60 min after DBS offset.
Fenfluramine
There was a significant effect of time (F = 12.086, p < 0.0001) on VCM levels, driven by decreased VCMs during DBS. There was no significant effect of fenfluramine pretreatment (F = 1.425, p = 0.250) or interaction between fenfluramine and time (F = 0.810, p = 0.541) (Fig. 5E). Fenfluramine had no effect on total distance traveled in the open field (Fig. 6). At the dosing parameters used, fenfluramine induced a 286% increase in 5-HT release and 5-HT levels remained elevated for the duration of DBS. The mean increase across the five time points measured was 279.6% relative to baseline (Fig. 7).
DOI
There was a significant effect of time (F = 18.04, p < 0.0001) on VCM levels, driven by decreased VCMs during DBS. There was no significant effect of DOI pretreatment (F = 0.59, p = 0.455) or interaction between DOI and time (F = 0.69, p = 0.612) (Fig. 5F). DOI had no effect on total distance traveled in the open field (Fig. 6).
Discussion
Despite its widespread clinical application for the treatment of movement disorders, the mechanisms underlying the therapeutic effects of DBS remain unclear. Altered 5-HT function has been implicated in the pathology of TD, and several effects of DBS on the 5-HT system have been described in preclinical studies. Here we demonstrate that reducing 5-HT neurotransmission, either acutely with 8-OH-DPAT or chronically with 5–7-DHT induced depletion of 5-HT, is sufficient to suppress HAL-induced VCMs. However, pharmacological and microdialysis studies showed that decreased brain serotonin function does not play a role in mediating the VCM-suppressing effects of DBS.
The 5-HT system has been implicated in the pathophysiology of TD by several lines of evidence. Briefly, atypical APDs are associated with much lower incidences of TD, which has been ascribed to their 5-HT antagonism or inverse agonism (Meltzer and Nash, 1991; Kapur et al., 1999; Stahl, 1999). Moreover, SSRI treatment has been shown to exacerbate or precipitate TD in patients concomitantly administered antipsychotics. In clinical and preclinical studies, 5-HT receptor antagonism can reduce VCMs (Kostrzewa et al., 2007; Creed-Carson et al., 2011). To determine whether reduced 5-HT activity is sufficient to suppress HAL-induced VCMs, we administered the 5-HT1A inhibitory autoreceptor agonist, 8-OH-DPAT. We observed VCMs during a window in which the dose and concentration of 8-OH-DPAT used has previously been shown to completely inhibit the firing of serotonergic neurons (Blier et al., 1989). When we examined VCMs during this period of quiescent 5-HT neurons, we found that VCMs were significantly reduced relative to baseline (Fig. 3B). Further linking the effects of 8-OH-DPAT to inhibition of 5-HT transmission, the effects of 8-OH-DPAT were abolished by pretreatment with either fenfluramine or fluoxetine. To further test the idea that decreased brain 5-HT may be sufficient to suppress VCMs, we performed selective serotonergic lesions of the raphe nuclei. We report that while MRN lesions had no effect, DRN or combined DRN+MRN lesions significantly reduced VCMs. This suggests that reducing 5-HT in brain areas innervated by the DRN, which include the basal ganglia, is sufficient to suppress HAL-induced VCMs.
It has been previously shown that STN-DBS inhibits firing of serotonergic neurons in the dorsal raphe (Temel et al., 2007; Hartung et al., 2011) and decreases 5-HT release in the hippocampus and prefrontal cortex (Navailles et al., 2010). However, to our knowledge, altered 5-HT release in brain areas implicated in movement control has not been documented. We examined 5-HT release in the CPu because it is an area of the basal ganglia that receives extensive serotonergic innervation and because it is a putative site of action of HAL. Here we show that STN-DBS decreases 5-HT in the DL CPu in both HAL- and VEH-treated rats, whereas EPN-DBS had no effect. The decrease in 5-HT induced by STN-DBS is likely due to decreased activation of raphe neurons, which we assessed by mapping expression of the immediate early gene zif268. In this experiment, STN- but not EPN-DBS decreased activity of DRN and MRN neurons, while EPN-DBS had no effect. Based on the inhibition of the raphe neurons, we can speculate that 5-HT release was decreased in other areas of the basal ganglia as well, since the DRN provides serotonergic innervation to this collection of nuclei (Di Matteo et al., 2008). 5-HT terminals from the DRN synapse onto dopaminergic and GABAergic neurons in the GP, STN, and SN as well as the CPu (Di Giovanni et al., 2006; Di Matteo et al., 2008), and modulation of activity of any of these output nuclei or basal ganglia nodes could significantly impact on net basal ganglia output.
Having established that decreasing brain 5-HT content or reducing the firing of dorsal raphe neurons is sufficient to suppress VCMs independently of DBS, and having found that STN-DBS decreases activity of 5-HT neurons and decreases 5-HT release in the CPu, we wanted to determine whether this decrease in 5-HT release and function was a necessary component of the mechanism underlying the VCM-suppressing effects of DBS.
We administered fluoxetine to elevate concentration of extracellular serotonin 1 h before DBS, to determine whether the VCM-suppressing effects of DBS would persist without the decrease in brain serotonin. We found that despite preventing the DBS-induced decrease in brain 5-HT pharmacologically, DBS still suppressed VCMs. This was particularly true in the case of EPN-DBS, where pretreatment with fluoxetine had no effect on VCM levels during DBS. However, in the case of the STN, VCM levels were slightly but significantly higher after pretreatment with fluoxetine than with saline. To further investigate whether this decrease in 5-HT really does contribute to the VCM-suppressing effects of STN-DBS, we used fenfluramine to achieve higher increases in 5-HT levels. In a further attempt to modulate 5-HT, we administered the 5-HT2 antagonist, DOI, which was chosen because of the high concentration of 5-HT2 receptors in the CPu, a target site of action of HAL (Di Giovanni et al., 2006). 5-TH2 receptors were also of interest because of their abundant postsynaptic distribution in the basal ganglia and because they mediate the modulatory effects of 5-HT on dopamine (Alex and Pehek, 2007; Di Matteo et al., 2008; Di Giovanni et al., 2010). There was a slight but nonsignificant effect of both fenfluramine and DOI on the VCM-suppressing effects of DBS. Together, these results suggest that preventing the DBS-induced decrease in 5-HT does not interfere with the VCM-suppressing effects of STN- or EPN-DBS.
The effect of STN-DBS on the 5-HT system has been studied in preclinical models, in the context of both motor and cognitive effects (Navailles and De Deurwaerdère, 2012); however, to our knowledge, this is the first study to compare the effects of STN- and EPN-DBS on the 5-HT system. Moreover, this is the first study to examine the basal ganglia, and to attempt to elucidate the contribution of decreased 5-HT release to the motor effects of DBS. Clinically, both the STN and GPi have been used effectively to relieve symptoms of treatment-resistant TD and tardive dystonia (Damier et al., 2007; Sun et al., 2007). Our observation that EPN-DBS suppresses VCMs in this model despite its lack of effect on the 5-HT system further strengthens our conclusion that decreasing 5-HT is not a necessary component of the DBS therapeutic mechanism.
The current findings that preventing 5-HT decreases or that agonism of postsynaptic 5-HT receptors does not interfere with the motor effects of DBS may have implications for the treatment of psychiatric side effects that may accompany DBS (Tan et al., 2011). Our results suggest that treatment with SSRIs or other drugs that elevate brain 5-HT will not likely have adverse effects on motor outcome of DBS.
We have shown that decreasing brain 5-HT content with 5,7-DHT lesions or decreasing activity of serotonergic neurons with the inhibitory autoreceptor agonist 8-OH-DPAT is sufficient to suppress VCMs induced by chronic HAL treatment. We have also demonstrated that STN-DBS decreases 5-HT release in the DL CPu and inhibits neurons in the dorsal and medial raphe nucleus, while EPN-DBS has no effect on 5-HT release or raphe neuron activation. Based on our observation that both STN- and EPN-DBS attenuate VCMs with equal efficacy, despite their differential effects on the 5-HT system, we conclude that decreases in brain 5-HT are not a necessary component of the DBS therapeutic mechanism in a model of TD.
Footnotes
This study was supported in part by funds from the Ontario Mental Health Foundation and Canadian Institutes for Health Research (CIHR) (Canada). M.C.C. is the recipient of a CIHR Doctoral Research award. We thank Mustansir Diwan, Roger Raymond, and John Chambers for excellent technical help.
References
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Steinberg S, Chaput Y, de Montigny C. Electrophysiological assessment of putative antagonists of 5-hydroxytryptamine receptors: a single-cell study in the rat dorsal raphe nucleus. Can J Physiol Pharmacol. 1989;67:98–105. doi: 10.1139/y89-017. [DOI] [PubMed] [Google Scholar]
- Caley CF. Extrapyramidal reactions from concurrent SSRI and atypical antipsychotic use. Can J Psychiatry. 1998;43:307–308. [PubMed] [Google Scholar]
- Capelle HH, Blahak C, Schrader C, Baezner H, Kinfe TM, Herzog J, Dengler R, Krauss JK. Chronic deep brain stimulation in patients with tardive dystonia without a history of major psychosis. Mov Disord. 2010;25:1477–1481. doi: 10.1002/mds.23123. [DOI] [PubMed] [Google Scholar]
- Casey DE. Spontaneous and tardive dyskinesias: clinical and laboratory studies. J Clin Psychiatry. 1985;46:42–47. [PubMed] [Google Scholar]
- Casey DE. Pathophysiology of antipsychotic drug-induced movement disorders. J Clin Psychiatry. 2004;65(Suppl 9):25–28. [PubMed] [Google Scholar]
- Covey DP, Garris PA. Using fast-scan cyclic voltammetry to evaluate striatal dopamine release elicited by subthalamic nucleus stimulation. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3306–3309. doi: 10.1109/IEMBS.2009.5333768. [DOI] [PubMed] [Google Scholar]
- Creed M, Hamani C, Nobrega JN. Deep brain stimulation of the subthalamic or entopeduncular nucleus attenuates vacuous chewing movements in a rodent model of tardive dyskinesia. Eur Neuropsychopharmacol. 2011;21:393–400. doi: 10.1016/j.euroneuro.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Creed MC, Hamani C, Nobrega JN. Early gene mapping after deep brain stimulation in a rat model of tardive dyskinesia: comparison with transient local inactivation. Eur Neuropsychopharmacol. 2012;22:506–517. doi: 10.1016/j.euroneuro.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Creed-Carson M, Oraha A, Nobrega JN. Effects of 5-HT(2A) and 5-HT(2C) receptor antagonists on acute and chronic dyskinetic effects induced by haloperidol in rats. Behav Brain Res. 2011;219:273–279. doi: 10.1016/j.bbr.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Damier P, Thobois S, Witjas T, Cuny E, Derost P, Raoul S, Mertens P, Peragut JC, Lemaire JJ, Burbaud P, Nguyen JM, Llorca PM, Rascol O. Bilateral deep brain stimulation of the globus pallidus to treat tardive dyskinesia. Arch Gen Psychiatry. 2007;64:170–176. doi: 10.1001/archpsyc.64.2.170. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E. Serotonin involvement in the basal ganglia pathophysiology: could the 5-HT2C receptor be a new target for therapeutic strategies? Curr Med Chem. 2006;13:3069–3081. doi: 10.2174/092986706778521805. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Esposito E, Di Matteo V. Role of serotonin in central dopamine dysfunction. CNS Neurosci Ther. 2010;16:179–194. doi: 10.1111/j.1755-5949.2010.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Bennett A, Abi-Dargham A, Krystal JH. Precipitation of a psychoneuromotor syndrome by fluoxetine in a haloperidol-treated schizophrenic patient. J Clin Psychopharmacol. 1994;14:361–363. [PubMed] [Google Scholar]
- Dubovsky SL, Thomas M. Tardive dyskinesia associated with fluoxetine. Psychiatr Serv. 1996;47:991–993. doi: 10.1176/ps.47.9.991. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ, Kammerer M, Lücking CH, Moser A. Selective GABA release as a mechanistic basis of high-frequency stimulation used for the treatment of neuropsychiatric diseases. Naunyn Schmiedebergs Arch Pharmacol. 2011;384:1–20. doi: 10.1007/s00210-011-0644-8. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ. Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain Res. 1995;675:45–54. doi: 10.1016/0006-8993(95)00037-q. [DOI] [PubMed] [Google Scholar]
- Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F, Raymond R, Lozano AM, Fletcher PJ, Nobrega JN. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry. 2010;67:117–124. doi: 10.1016/j.biopsych.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Hamani C, Machado DC, Hipólide DC, Dubiela FP, Suchecki D, Macedo CE, Tescarollo F, Martins U, Covolan L, Nobrega JN. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–35. doi: 10.1016/j.biopsych.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameleers R, Blokland A, Steinbusch HW, Visser-Vandewalle V, Temel Y. Hypomobility after DOI administration can be reversed by subthalamic nucleus deep brain stimulation. Behav Brain Res. 2007;185:65–67. doi: 10.1016/j.bbr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Hartung H, Tan SK, Steinbusch HM, Temel Y, Sharp T. High-frequency stimulation of the subthalamic nucleus inhibits the firing of juxtacellular labelled 5-HT-containing neurones. Neuroscience. 2011;186:135–145. doi: 10.1016/j.neuroscience.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Ketai R. Interaction between fluoxetine and neuroleptics. Am J Psychiatry. 1993;150:836–837. doi: 10.1176/ajp.150.5.836b. [DOI] [PubMed] [Google Scholar]
- Kostrzewa RM, Huang NY, Kostrzewa JP, Nowak P, Brus R. Modeling tardive dyskinesia: predictive 5-HT2C receptor antagonist treatment. Neurotox Res. 2007;11:41–50. doi: 10.1007/BF03033481. [DOI] [PubMed] [Google Scholar]
- Lauterbach EC, Shillcutt SD. Dyskinesia with fluoxetine: tardive or late-onset persistent, acute norfluoxetine dyskinesia? J Clin Psychiatry. 1997;58:85–86. [PubMed] [Google Scholar]
- Lee KH, Blaha CD, Harris BT, Cooper S, Hitti FL, Leiter JC, Roberts DW, Kim U. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006;23:1005–1014. doi: 10.1111/j.1460-9568.2006.04638.x. [DOI] [PubMed] [Google Scholar]
- Lyons MK. Deep brain stimulation: current and future clinical applications. Mayo Clin Proc. 2011;86:662–672. doi: 10.4065/mcp.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Nash JF. Effects of antipsychotic drugs on serotonin receptors. Pharmacol Rev. 1991;43:587–604. [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdère P. Contribution of serotonergic transmission to the motor and cognitive effects of high-frequency stimulation of the subthalamic nucleus or levodopa in Parkinson's disease. Mol Neurobiol. 2012;45:173–185. doi: 10.1007/s12035-011-8230-0. [DOI] [PubMed] [Google Scholar]
- Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdère P. High-frequency stimulation of the subthalamic nucleus and l-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson's disease. J Neurosci. 2010;30:2356–2364. doi: 10.1523/JNEUROSCI.5031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- Sandler NH. Tardive dyskinesia associated with fluoxetine. J Clin Psychiatry. 1996;57:91. [PubMed] [Google Scholar]
- Schulte T, Brecht S, Herdegen T, Illert M, Mehdorn HM, Hamel W. Induction of immediate early gene expression by high-frequency stimulation of the subthalamic nucleus in rats. Neuroscience. 2006;138:1377–1385. doi: 10.1016/j.neuroscience.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Sgambato-Faure V, Cenci MA. Glutamatergic mechanisms in the dyskinesias induced by pharmacological dopamine replacement and deep brain stimulation for the treatment of Parkinson's disease. Prog Neurobiol. 2012;96:69–86. doi: 10.1016/j.pneurobio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Introduction: what makes an antipsychotic atypical? J Clin Psychiatry. 1999;60(Suppl 10):3–4. [PubMed] [Google Scholar]
- Su PC, Ma Y, Fukuda M, Mentis MJ, Tseng HM, Yen RF, Liu HM, Moeller JR, Eidelberg D. Metabolic changes following subthalamotomy for advanced Parkinson's disease. Ann Neurol. 2001;50:514–520. doi: 10.1002/ana.1232. [DOI] [PubMed] [Google Scholar]
- Sun B, Chen S, Zhan S, Le W, Krahl SE. Subthalamic nucleus stimulation for primary dystonia and tardive dystonia. Acta Neurochir Suppl. 2007;97:207–214. doi: 10.1007/978-3-211-33081-4_23. [DOI] [PubMed] [Google Scholar]
- Tan SK, Hartung H, Sharp T, Temel Y. Serotonin-dependent depression in Parkinson's disease: a role for the subthalamic nucleus? Neuropharmacology. 2011;61:387–399. doi: 10.1016/j.neuropharm.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troster AI. Neuropsychology of deep brain stimulation in neurology and psychiatry. Front Biosci. 2009;14:1857–1879. doi: 10.2741/3347. [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Nobrega JN. The vacuous chewing movement (VCM) model of tardive dyskinesia revisited: is there a relationship to dopamine D(2) receptor occupancy? Neurosci Biobehav Rev. 2002;26:361–380. doi: 10.1016/s0149-7634(02)00008-8. [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, Nobrega JN. Differential effects of within-day continuous vs. transient dopamine D2 receptor occupancy in the development of vacuous chewing movements (VCMs) in rats. Neuropsychopharmacology. 2003;28:1433–1439. doi: 10.1038/sj.npp.1300233. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Nobrega JN, Carroll ME, Niznik HB, Shannak K, Lac ST, Pristupa ZB, Dixon LM, Kish SJ. Heterogeneous subregional binding patterns of 3H-WIN 35,428 and 3H-GBR 12,935 are differentially regulated by chronic cocaine self-administration. J Neurosci. 1994;14:2966–2979. doi: 10.1523/JNEUROSCI.14-05-02966.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Zhang K, Wang ZC, Ge M, Ma Y. Deep brain stimulation in the treatment of secondary dystonia. Chin Med J. 2006;119:2069–2074. [PubMed] [Google Scholar]