Abstract
To maximize their chances of survival, animals need to rapidly and efficiently respond to aversive situations. These responses can be classified as active or passive and depend on the specific nature of threats, but also on individual fear coping styles. In this study, we show that the control of excitatory and inhibitory brain neurons by type-1 cannabinoid (CB1) receptors is a key determinant of fear coping strategies in mice. In classical fear conditioning, a switch between initially predominant passive fear responses (freezing) and active behaviors (escape attempts and risk assessment) develops over time. Constitutive genetic deletion of CB1 receptors in CB1−/− mice disrupted this pattern by favoring passive responses. This phenotype can be ascribed to endocannabinoid control of excitatory neurons, because it was reproduced in conditional mutant mice lacking CB1 receptors from cortical glutamatergic neurons. CB1 receptor deletion from GABAergic brain neurons led to the opposite phenotype, characterized by the predominance of active coping. The CB1 receptor agonist Δ9-tetrahydrocannabinol exerted a biphasic control of fear coping strategies, with lower and higher doses favoring active and passive responses, respectively. Finally, viral re-expression of CB1 receptors in the amygdala of CB1−/− mice restored the normal switch between the two coping strategies. These data strongly suggest that CB1 receptor signaling bimodally controls the spontaneous adoption of active or passive coping strategies in individuals. This primary function of the endocannabinoid system in shaping individual behavioral traits should be considered when studying the mechanisms of physiological and pathological fear.
Introduction
Humans and animals must adopt appropriate fear responses when exposed to threatening situations (LeDoux, 2000; Blanchard et al., 2001). The ability to efficiently cope with potential dangers strongly influences the consequences of aversive stimuli on the organisms (Hartley and Phelps, 2010). Human and animal studies revealed individual differences in the way of mastering imminent environmental challenges (Blanchard et al., 2001; Lázaro-Muñoz et al., 2010; Schlund et al., 2010). In rodents, defensive behaviors were clustered in either passive (or reactive) coping or active (or proactive) coping, respectively (Koolhaas et al., 1999). The predominant response to aversive stimuli of passively coping animals is immobility, whereas active “copers” tend to adopt behaviors aiming at removing the danger source (De Boer and Koolhaas, 2003; Cain and LeDoux, 2007). These different behavioral strategies rely on distinct physiological mechanisms (Koolhaas et al., 2010). However, most studies in fear conditioning only rely on the analysis of passive coping behaviors. Gozzi et al. (2010) recently reported that conditioned freezing in mice (i.e., passive coping) was inhibited to favor active coping by pharmacogenetic manipulations of amygdalar activity, suggesting that inhibition of conditioned freezing may reflect not only a quantitative attenuation of fear but also a qualitative change of fear response.
The endogenous activity of CB1 receptors is necessary for extinction of freezing in fear conditioning and of passive avoidance learning (Marsicano et al., 2002; Lafenêtre et al., 2007; Dubreucq et al., 2010), indicating that CB1 signaling controls passive fear responses. In contrast, the deletion of the CB1 gene facilitates active avoidance learning (Martin et al., 2002).
The brain circuitries controlling conditioned freezing have been extensively described. The medial prefrontal cortex (mPFC), the hippocampus, and the amygdala as well as downstream nuclei, including the hypothalamus and the periaqueducal gray (PAG), act in concert to mediate appropriate freezing responses (Laviolette et al., 2005; Myers and Davis, 2007; Resstel et al., 2009; Roozendaal et al., 2009; Sotres-Bayon and Quirk, 2010). CB1 receptors are enriched in these brain structures and they modulate conditioned freezing in a region-dependent manner (Laviolette and Grace, 2006; Resstel et al., 2009; Kamprath et al., 2011; Terzian et al., 2011; Dubreucq et al., 2012). However, the mechanisms by which CB1 receptors modulate active versus passive fear coping strategies have been poorly studied so far.
Interestingly, the behavioral functions of CB1 receptors also depend on their cell type localization (Lafenêtre et al., 2009; Bellocchio et al., 2010) and, in particular, on their ability to negatively regulate both GABAergic and glutamatergic neurotransmissions (Chevaleyre et al., 2006). CB1 receptors may thus influence fear coping strategies acting at glutamatergic and GABAergic neurons.
By submitting constitutive or cell type-specific CB1 mutant mice to classical and instrumental fear conditioning, we reveal that the endocannabinoid system (ECS) determines the fear coping strategies by exerting a specific control on glutamatergic or GABAergic neurons. In addition, CB1 expression in the amygdaloid area is sufficient to guarantee normal fear coping strategies, suggesting that the ECS drives fear coping styles at least in part by modulating the activity of neurons located in the amygdala.
Materials and Methods
Animals
Experiments were in agreement with the Committee on Animal Health and Care of INSERM and French Ministry of Agriculture and Forestry (authorization number 3306369).
Two- to four-month old male C57BL/6N (JANVIER, France) and constitutive or conditional CB1 mutant mice and their wild-type littermates were maintained on a 12 h light/dark cycle (light on, 7:00 A.M.) with food and water ad libitum. All the mutant lines were in a mixed genetic background with a predominant C57BL/6NCrl contribution (6–7 backcrossing generations). All the animals used in experiments involving mutant mice were littermates. For constitutive CB1−/− mice (Marsicano et al., 2002), the parents of the experimental animals were always heterozygous for the mutation. Conditional mutant mice were obtained as described by crossing mice carrying “floxed” CB1 alleles (CB1flox/flox; Marsicano et al., 2003) with the Cre-expressing transgenic mouse lines NEX-Cre and Dlx5/6-Cre (Goebbels et al., 2006; Monory et al., 2006) to obtain CB1flox/flox;NEX-Cre (called Glu-CB1−/−) and CB1flox/flox;Dlx5/6-Cre mice (called GABA-CB1−/−), respectively (Monory et al., 2006, 2007; Bellocchio et al., 2010). To allow a direct comparison of GABA-CB1−/− and Glu-CB1−/− mice with the same wild-type littermate mice, GABA-CB1−/− and Glu-CB1−/− and wild-type GABA-Glu-CB1+/+ littermates (called WT) derived from a double mutant line (Bellocchio et al., 2010). Briefly, GABA-CB1−/− mice were mated with Glu-CB1−/− mice to obtain a first generation of double mutant mice lacking CB1 on both GABAergic and glutamatergic neurons (GABA/Glu-CB1−/−), which were then crossed with CB1flox/floxfemales (phenotypically wild type), to avoid potential influence of the mother's genotype on the offspring phenotype of the experimental animals (Bellocchio et al., 2010). Mutant mice were genotyped at the age of 2 weeks and re-genotyped after the experiments by PCR on tail tissue as described previously (Marsicano et al., 2002; Monory et al., 2006; Bellocchio et al., 2010) using the following primer sets: CB1flox/flox: 5′-GCTGTCTCTGGTCCTCTTAAA, 5′-GGTGTCACCTCTGAAAACAGA, and 5′-CCTACCCGGTAGAATTAGCTT; Cre forward for NEX-Cre (for Glu-CB1−/−): 5′-TCTTTTTCATGTGCTCTTGG; Cre forward for Dlx5/6-Cre (for GABA-CB1−/−): 5′-AGCAATCGCACTCACAACAGA; Cre reverse for both lines: 5′-CGCGCCTGAAGATATAGAAGA. Previous extensive anatomical characterizations showed that the mutant mice used in the present study carry deletions of CB1 receptors as follows: (1) from all the cells of the body (CB1−/− mice; Marsicano et al., 2002); (2) mainly from cortical glutamatergic neurons in the dorsal telencephalon, including neurons located in neocortex, paleocortex, archicortex, hippocampal formation, and cortical portions of the amygdala (Glu-CB1−/−; Monory et al., 2006, 2007; Bellocchio et al., 2010); or (3) mainly from forebrain GABAergic neurons (GABA-CB1−/−; Monory et al., 2006, 2007; Bellocchio et al., 2010).
All experiments took place during the light phase. For fear conditioning and shock sensitivity, mice were single housed 7 d before testing. For active and passive avoidance, mice were maintained in groups (2–4 per cage).
Drugs
In the pharmacological approach, Δ9-tetrahydrocannabinol (THC, Sigma-Aldrich) was dissolved with 2% ethanol and 2% Cremophor in sterile saline solution. Naive C57BL/6N mice were weighted and received an intraperitoneal injection of vehicle, 0.3 mg/kg, 1 mg/kg, or 3 mg/kg THC 1 h before the CS re-exposure session.
Adeno-associated virus-mediated re-expression of CB1 receptors in the amygdala of constitutive mutant CB1−/− mice
Adeno-associated virus vector synthesis.
The AAV-CB1 constructions (where AAV is adeno-associated virus) were generated according to Klugmann et al. (2011). Briefly, the cDNA encoding a hemagglutinin-tagged rat CB1-receptor was cloned in an AAV expression cassette containing the 1.1 kb CMV immediate early enhancer/chicken β-actin hybrid promoter, the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE), and the bovine growth hormone polyadenylation sequence flanked by AAV2 inverted terminal repeats (pAAV-CB1). Packaging of pseudotyped AAV1/2 chimeric vectors with equal ratios of AAV1 and AAV2 capsid proteins was performed as described previously (Klugmann et al., 2005), and genomic titers were determined by quantitative real-time PCR of vector genomes using primers for WPRE (During et al., 2003).
Intra-amygdala AAV-CB1 injection.
Constitutive CB1−/− mice were deeply anesthetized with a ketamine (0.0125 mg/ml) and xylazine (0.001 mg/ml) mixture (0.2 ml/mouse, i.p). One-half microliter (0.5 μl) of either an empty viral (EV) construct or AAV-CB1 (6 × 1011 viral genomes/ml) was injected bilaterally, aiming at the amygdala (anteroposterior, −2.0 mm; mediolateral, ±2.0 mm; dorsoventral, −2.0 mm; from bregma). Vector delivery was performed at a rate of 0.1 μl/min using a mini-pump (Harvard Apparatus) with 33G injectors (Plastics One) in a stereotaxic frame (David Kopf Instruments). The injectors were left in place for an additional minute to allow vector diffusion. Mice were submitted to the fear conditioning and active avoidance procedures 5 and 6 weeks following virus administration, respectively.
Fear conditioning
Classical fear conditioning was carried out as described previously (Dubreucq et al., 2010) in a square conditioning box (Imetronic) made of gray Perspex (length, 26 cm; width, 18 cm; height, 25 cm) with a metal grid floor and located in a soundproof chamber (length, 55 cm; width, 60 cm; height, 50 cm). A video camera placed above the conditioning box allowed observation of the animals' behaviors. On the conditioning day, each mouse was placed into the conditioning chamber and left free to explore for 2 min. A single footshock (0.5 mA, 1 s, “squared” scrambler) was then co-emitted during the last second of a 20 s tone (1.5 kHz, 60 dB). Twenty-four hours after conditioning, mice were placed back in the chamber in their home cage to attenuate novelty-induced exploratory behaviors. They were then exposed to the tone [conditioned stimulus (CS)] for 8 min preceded by a 2 min pre-tone. Freezing (i.e., lack of movements except those associated with breathing) and active coping (i.e., digging, rearing and wall-sniffing/rearing) were scored (De Boer and Koolhaas, 2003; Gozzi et al., 2010) by an experimenter blind to mouse genotypes and expressed as a percentage of time. To ensure that the observed behaviors were specifically induced by the CS, the maximal freezing and active coping percentage times per minute induced by the tone were compared to pre-tone levels for each mouse, revealing a consistent and robust increase of both behaviors during CS presentation (p < 0.001 for all genotypes, paired t test, data not shown).
Active and passive avoidance
The two-way active and passive avoidance tests took place in two-compartment shuttle boxes (40 × 10 × 15 cm, Imetronic) located in dark soundproof cubicles supplied with infrared sensors and video cameras. The floor of the shuttle boxes was made of steel cabled grid releasing electric pulses (squared scrambler). Tone generators were fixed on the top of each compartment. On day 1, animals were allowed to explore both compartments for 15 min. Twenty-four hours later, mice underwent four daily sessions made of 50 active or passive avoidance trials. In the active avoidance procedure, a trial began with the tone start, which was accompanied 10 s later by the footshock (0.2 mA) until the animal changed of compartment (maximal shock duration, 15 s). Passive avoidance was conducted in the same conditions, except that changing of compartment within tone-on duration induced an acute footshock (2 s). These symmetric tasks allowed a direct comparison of the mouse groups in active or passive avoidance responses. The subsequent trial began from the compartment where the mouse was detected after a 20 s intertrial interval (ITI). In active avoidance, a correct response was assigned when the mouse transited to the other compartment before shock delivery. In passive avoidance, a correct response was assigned when the mouse remained in the original compartment during tone presentation. Avoidance performances were expressed as percentage of correct responses.
Shock sensitivity testing
Shock sensitivity was examined in each mutant line. Naive mice were introduced into the fear conditioning chamber (see above) and submitted to five footshocks (1 s) of increasing intensities (from 0.1 to 0.5 mA) every 30 s. The first shock intensity at which flinching, vocalizing, and jumping reactions appeared was taken as sensitivity threshold.
In situ hybridization of CB1 mRNA
Following the behavioral tasks, control mice (n = 10), and AAV-CB1-treated CB1−/− mice (n = 13) as well as naive GABA-CB1−/−, GLU-CB1−/−, and WT littermate mice, were killed by cervical dislocation, their brains quickly removed, frozen on dry ice, and stored at −80°C until sectioning in a cryostat (14 μm, Microm HM 500 m, Microm Microtech). The DIG-labeled riboprobes against mouse CB1 receptors were prepared as described previously (Marsicano and Lutz, 1999; Marsicano et al., 2003; Monory et al., 2006). In situ hybridization of CB1 mRNA was performed according to the standard procedure used in the laboratory (Bellocchio et al., 2010; Lourenço et al., 2010; Dubreucq et al., 2012). Signal amplification was achieved using the TSA Plus System Cyanine 3/Fluorescein kit (PerkinElmer). Blocking and wash buffers were prepared according to the manufacturer's protocol. Slides were analyzed by epifluorescence microscopy at 5× (Leica).
Only the AAV-CB1-treated CB1−/− mouse brains showing a bilateral major expression of CB1 mRNA in the basolateral amygdala (BLA), and central amygdala (CeA) nuclei were included in the final analysis (n = 8 of 13).
Statistical analysis
Total freezing and active coping time percentages during CS presentation were compared using t test (constitutive CB1 mutants) and one-way ANOVA (conditional CB1 mutants). Time course data were analyzed using two-way ANOVA for repeated measures. Correlations were assessed using the Pearson's r linear regression analysis. To facilitate the analysis of avoidance learning between genotypes of conditional mutant mice, the area under the curve (AUC) values were also calculated for each animal (trapezoid rule; Bura et al., 2007) and compared using one-way ANOVA. Bonferroni's post hoc test was applied when appropriate. Active and passive avoidance responses of wild-type mice were also compared between each sessions using 1-way ANOVA (data not shown), ensuring that both avoidance trainings induced an increase of performances.
Results
Behavioral analysis of the coping strategies adopted in fear conditioning and avoidance learning in C57BL/6N mice
In classical fear conditioning in rodent, re-exposure to a CS previously paired with an aversive stimulus induces a strong freezing response (LeDoux, 2000). As CS presentation continues, the freezing rate progressively declines due to extinction and/or habituation processes (Myers and Davis, 2007). However, CS presentation can also elicit proactive behaviors (Gozzi et al., 2010) considered as attempts to actively cope with the danger source (Koolhaas et al., 1999; Blanchard et al., 2001; De Boer and Koolhaas, 2003).
The first experiment aimed at describing the temporal expression of both freezing and active coping induced by a conditioned tone in C57BL/6N mice. Twenty-four hours following conditioning, the 8 min tone presentation provoked a behavioral pattern that can be divided into three temporal phases. In Phase 1, immediate tone re-exposure induced a strong freezing response associated with a weak active coping rate (Behavior × Time Interaction: F(7,126) = 26.22, p < 0.001; minute 1, p < 0.001; Fig. 1A). During Phase 2, simultaneous decrease of freezing and increase of active coping were observed, resulting in an equivalent expression of both response types (minutes 2–3, p > 0.05; Fig. 1A). In Phase 3, active coping eventually overcame freezing (minutes 4–8, p < 0.001; Fig. 1A). Importantly, freezing and active coping represented only a portion of the whole observation (<60%), indicating that these responses were not mutually exclusive. Thus, the coordinated decrease of freezing and increase of active behaviors seems to be the consequence of a “temporal switch” in the individual CS-induced coping strategy.
Figure 1.
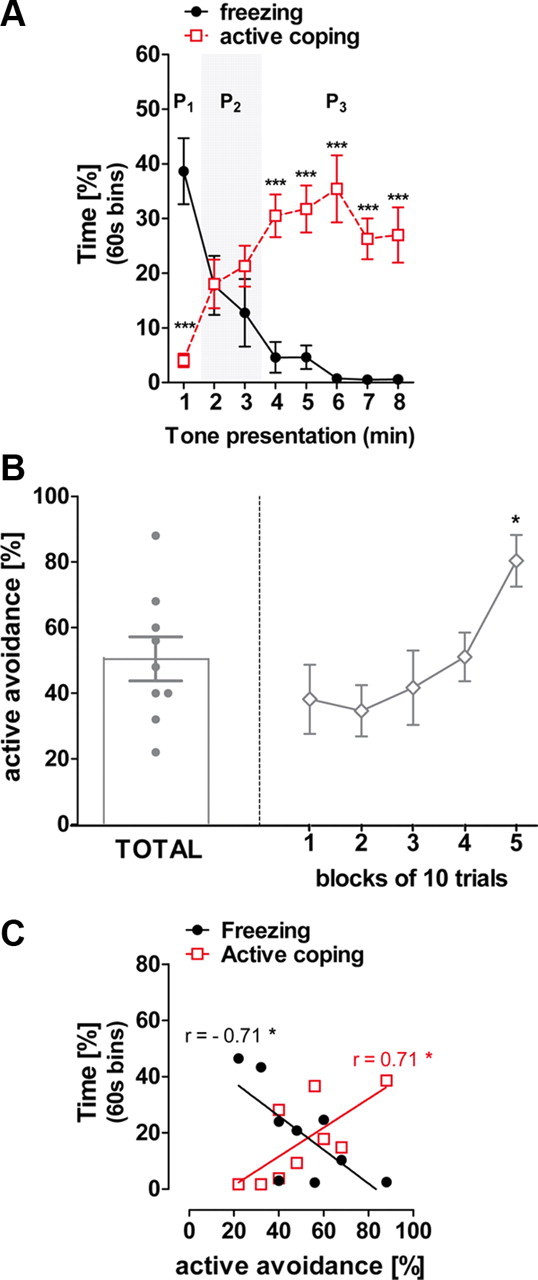
Freezing and active coping responses to tone in classical fear conditioning predict active avoidance performances in C57BL/6N mice. A, Temporal expression of freezing and active coping responses to the conditioned tone. In Phase 1 (P1) mice show a strong freezing expression associated to low active coping. In Phase 2 (P2, gray background) freezing and active coping reached an equivalent expression percentage. In Phase 3 (P3), mice displayed dominant active coping behaviors and attenuated freezing response. B, Total percentage of correct response in a single active avoidance training session (left) and within session learning curve (blocks of 10 trials, right). C, Correlation of individual freezing and active coping time percentage observed at the second minute of CS presentation against the total active avoidance performances. Data are mean ± SEM expressed as percentage. *p < 0.05; ***p < 0.001, n = 10.
Avoidance learning in rodents was proposed to depend on the individual predominant coping style (Koolhaas et al., 1999). For instance, rats showing a strong tendency to freeze in fear conditioning were those performing the poorer in active avoidance learning (Lázaro-Muñoz et al., 2010; Vicens-Costa et al., 2011). Previously fear-conditioned mice were submitted to one active avoidance session. Mice displayed 50 ± 7% of total avoidance responses, reaching ∼80% of correct responses at the end of the session (F(4,32) = 4.67, p < 0.01; Fig. 1B).
Interestingly, active avoidance responses were negatively correlated with individual freezing and positively related to active coping during Phase 2 of tone presentation, when the levels of the two behaviors reached similar levels (Fig. 1C). Thus, behavioral tendencies during fear conditioning can predict active avoidance levels.
Constitutive deletion of CB1 receptors prevents the temporal switch of coping strategies in classical fear conditioning
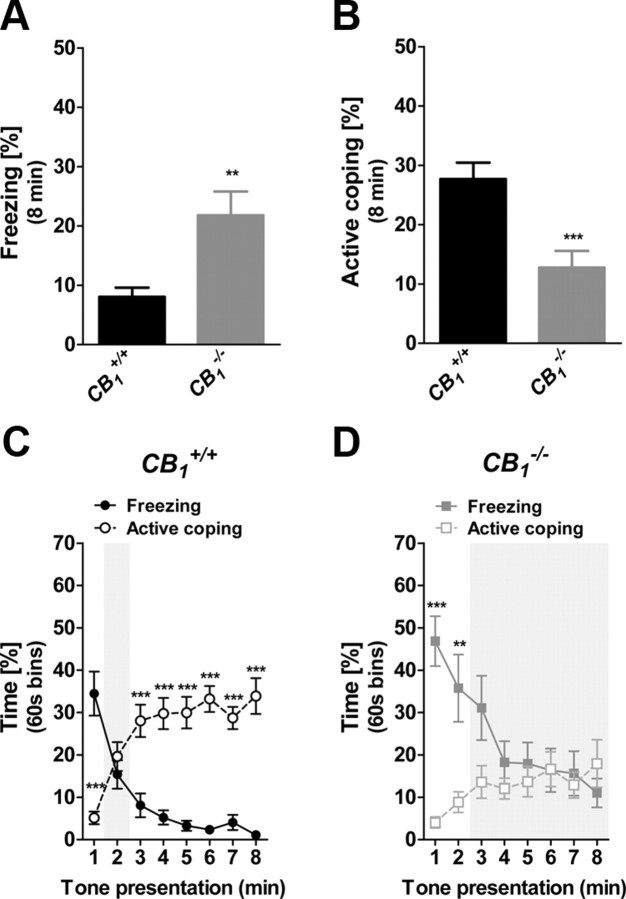
The total percentages of freezing and active behaviors during CS presentation were compared between constitutive mutant CB1−/− mice and CB1+/+ littermate controls. CS presentation induced stronger freezing (t = 3.17; p < 0.01; Fig. 2A) and weaker active coping in CB1−/− mice (t = 3.81; p < 0.001; Fig. 2B) as compared to CB1+/+ littermates. CB1+/+ mice displayed the three temporal phases leading to the switch from freezing to active coping (Behavior × Time Interaction: F(7,154) = 42.29, p < 0.001; Fig. 2C). In contrast, CB1−/− mice displayed a prolonged Phase 1, maintaining higher freezing as compared to active coping until the third/fourth minute of tone exposure (Behavior × Time Interaction: F(7,154) = 11.18, p < 0.001; minutes 1–2, p < 0.01; Fig. 2D). Interestingly, mutant mice lacked Phase 3, as freezing and active coping subsequently overlapped until the end of the session (minutes 3–8, p > 0.05; Fig. 2D), indicating that the switch between freezing and active coping did not occur. These data suggest that CB1 signaling controls the expression and the temporal relationship between passive and active responses to fear-conditioned stimuli.
Figure 2.
Constitutive deletion of CB1 receptors prevents the temporal shift between freezing and active coping in fear conditioning. A, Total freezing of CB1−/− and CB1+/+ littermates during the 8 min CS presentation. B, Total active coping of CB1−/− and CB1+/+ littermates during the 8 min CS presentation. C, Time course of freezing and active coping in CB1+/+ mice. D, Time course of freezing and active coping in CB1−/− mice. Data are mean ± SEM *p < 0.05; **p < 0.01; ***p < 0.001, n = 12 per genotype.
CB1 receptors bimodally control fear coping strategies
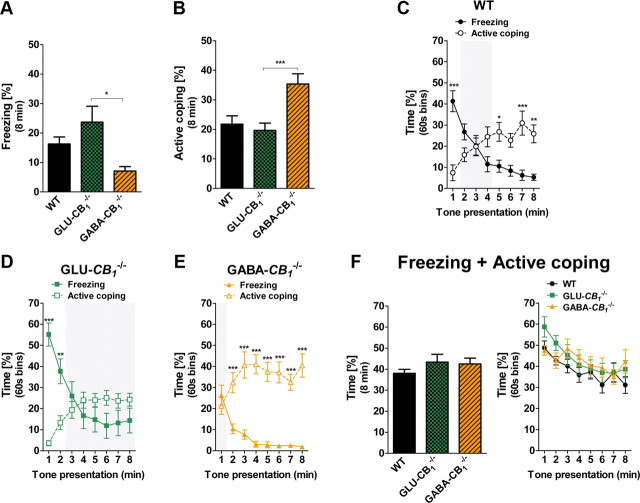
To assess whether the site of CB1 neuronal expression influences the type of fear coping strategies, we tested the Glu-CB1−/−, GABA-CB1−/−, and WT littermates mice in our fear conditioning procedure. The mouse genotype influenced the overall freezing levels (F(2,42) = 4.49; p < 0.05; Fig. 3A), with GABA-CB1−/− freezing less than Glu-CB1−/− (p < 0.05). Conversely, the CS induced stronger active coping behaviors in GABA-CB1−/− as compared to both WT and Glu-CB1−/− littermates (F(2,42) = 8.58; p < 0.001; p < 0.01 for both comparisons; Fig. 3B).
Figure 3.
Conditional deletion of CB1 receptors in GABAergic or glutamatergic neurons differentially alters the shift between freezing and active coping in fear conditioning. A, Total freezing of Glu-CB1−/− (n = 18), GABA-CB1−/− (n = 14), and WT (n = 13) littermates during the 8 min CS presentation. B, Total active coping of Glu-CB1−/−, GABA-CB1−/−, and WT littermates during the 8 min CS presentation. C, Time course of freezing and active coping of WT mice (note the presence of the three phases of behavior). D, Time course of freezing and active coping of Glu-CB1−/− mice (note the absence of Phase 3). E, Time course of freezing and active coping of GABA-CB1−/− mice (note the absence of Phase 1). F, Sum of individual freezing and active coping time scores as percentage of the total 8 min tone exposure (left) or percentage of 60 s bins (right). Data are mean ± SEM *p < 0.05; **p < 0.01; ***p < 0.001.
WT animals displayed a similar time course behavioral pattern as C57BL/6N mice, with the presence of the three temporal phases of tone-induced responses (Behavior × Time Interaction: F(7,168) = 20.45, p < 0.001; Fig. 3C). In contrast, Glu-CB1−/− mice never displayed dominant active coping responses to tone presentation, lacking the Phase 3 of the temporal behavioral expression (Behavior × Time Interaction: F(7,238) = 36.62; p < 0.001; minutes 3–8, p > 0.05; Fig. 3D). The early CS re-exposure did not elicit a dominant freezing response in GABA-CB1−/− mice (lack of Phase 1), which instead promptly adopted highly dominant active coping (Behavior × Time Interaction: F(7,182) = 12.66; p < 0.001; minute 1, p > 0.05; minutes 2–8, p < 0.001; Fig. 3E).
The state of fear has been conceived as the set of defensive behaviors elicited by a threat (Blanchard et al., 2001). In our conditions, the sum of CS-induced freezing and active coping might therefore provide an indication of the overall state of fear. Interestingly, these cumulated values did not differ between genotypes (Fig. 3F). Altogether, these data suggest that CB1 receptor expression in GABAergic or cortical glutamatergic brain neurons does not significantly impact on overall fear learning and expression, but it does determine the strategy to cope with conditioned fear stimuli.
Bimodal control of CB1 receptors on fear avoidance learning
Ubiquitous deletion of CB1 receptors in CB1−/− mice strengthened both active (genotype main effect: F(1,16) = 5.66; p < 0.05; Fig. 4A) and passive (genotype main effect: F(1,20) = 4.99; p < 0.05; Fig. 4B) avoidance learning. In conditional mutant mice, the genotype affected both active avoidance (genotype main effect: F(2,60) = 4.80; p < 0.05; Fig. 4C) and passive avoidance performances (genotype main effect: F(2,71) = 13.11; p < 0.001; Fig. 4D). AUC analyses uncovered an improved active avoidance performance in GABA-CB1−/− mice as compared to WT littermates (F(2,60) = 4.46; p < 0.05; GABA-CB1−/− vs WT, p < 0.05; Fig. 4C). Conversely, Glu-CB1−/− exhibited higher passive avoidance responses as compared to controls (F(2,71) = 12.49; p < 0.001; Glu-CB1−/− vs WT, p < 0.01; Fig. 4D).
Figure 4.
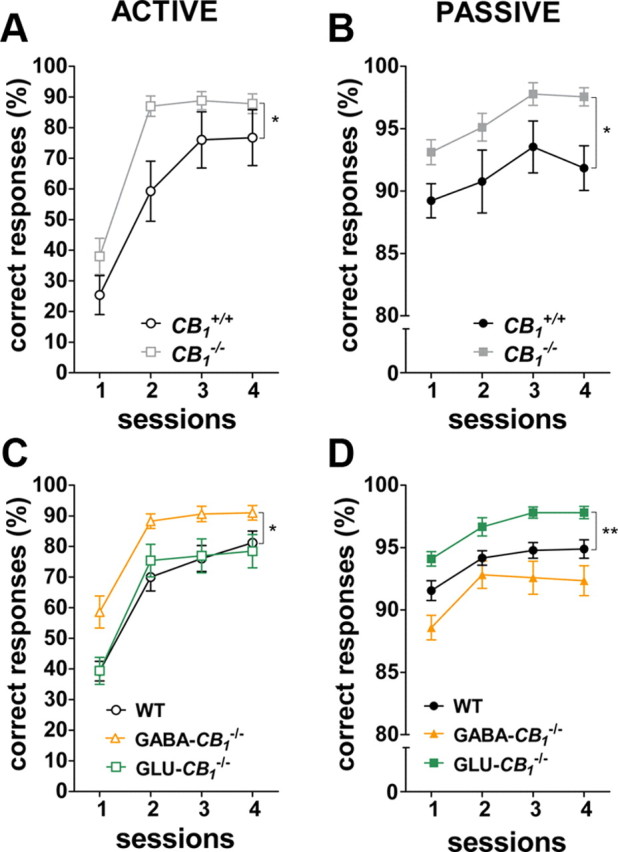
Neuronal type-specific regulation of active and passive avoidance learning by CB1 receptors. A, B, Time course of avoidance responses of CB1−/− and CB1+/+ littermates in the active [A; CB1−/− (n = 10), CB1+/+ (n = 8)] and passive [B; CB1−/− (n = 9), CB1+/+ (n = 13)] versions of the two-way avoidance paradigm. C, D, Time course of behavioral responses of conditional CB1−/− and WT in the active [C; Glu-CB1−/− (n = 17), GABA-CB1−/− (n = 16), WT (n = 30)] and passive [D; Glu-CB1−/− (n = 21), GABA-CB1−/− (n = 17), WT (n = 36)] versions of the two-way avoidance paradigm. Data are mean ± SEM. expressed as percentage of avoidance responses at each training session. *p < 0.05; **p < 0.01.
Importantly, the observed phenotypes could not be assigned to altered locomotion or pain perception, as mutants and controls displayed similar initial numbers of ITI transitions and equivalent pain responses to footshocks (data not shown). These data reveal the differential impact of CB1-dependent control of glutamatergic and GABAergic neurons on active and passive avoidance learning, respectively.
THC dose-dependently alters the coping style in classical fear conditioning
Cannabinoid receptor agonists such as THC often dose-dependently induce opposite behavioral effects (Moreira and Lutz, 2008; Bellocchio et al., 2010). We thus assessed whether an acute administration of THC at 0.3, 1, or 3 mg/kg could lead to distinct coping styles when mice were re-exposed to the CS. THC biphasically affected the total amount of freezing (F(3,40) = 13.12; p < 0.001; Fig. 5A) and the total amount of active coping (F(3,40) = 8.47; p < 0.001; Fig. 5B). Mice receiving the lowest dose of THC showed a reduced freezing response (p < 0.05; Fig. 5A) as compared to vehicle-injected mice. While the freezing time of animals treated with the intermediate dose of THC (1 mg/kg) did not differ from that of controls (p > 0.05), mice injected with 3 mg/kg THC increased overall freezing time as compared to controls (p < 0.05; Fig. 5A). However, the CS induced weaker active coping behaviors in the THC-treated (3 mg/kg) mice as compared to both vehicle-treated (p < 0.01) and THC-treated (0.3 mg/kg) mice (p < 0.001; Fig. 5B). Again, THC administered at 1 mg/kg did not alter the total amount of active coping behaviors as compared to vehicle (p > 0.05; Fig. 5B).
Figure 5.
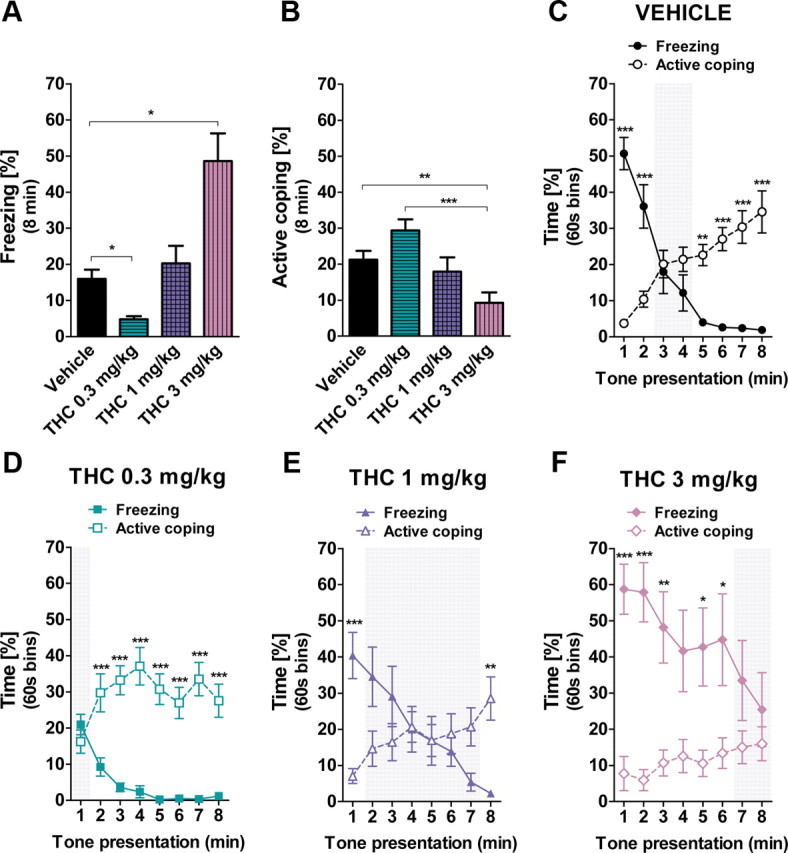
Biphasic effects of systemic THC on freezing and active coping behaviors in classical fear conditioning. A, Total freezing in C57BL/6N mice treated with vehicle (n = 11), THC 0.3 mg/kg (n = 11), 1 mg/kg (n = 11), or 3 mg/kg (n = 11) during the 8 min CS presentation. B, Total active coping of the vehicle, THC 0.3 mg/kg, THC 1 mg/kg, and THC 3 mg/kg groups during the 8 min CS presentation. C, Time course of freezing and active coping of the vehicle-injected mice (note the presence of the three phases of behavior). D, Time course of freezing and active coping of the THC 0.3 mg/kg group (note the absence of Phase 1). E, Time course of freezing and active coping of the THC 0.3 mg/kg group (note the presence of the three phases of behavior). F, Time course of freezing and active coping of the THC 3 mg/kg group (note the absence of Phase 3). Data are mean ± SEM *p < 0.05; **p < 0.01; ***p < 0.001.
Vehicle-treated animals displayed the three temporal phases of CS-induced responses (Behavior × Time Interaction: F(7,140) = 44.61, p < 0.001; Fig. 5C). Strikingly, the low dose of THC (0.3 mg/kg) prevented the initial dominant freezing response to the tone (lack of Phase 1) and favored early prevailing active coping behaviors (Behavior × Time Interaction: F(7,140) = 11.38; p < 0.001; minute 1, p > 0.05; minutes 2–8, p < 0.001; Fig. 5D). Mice submitted to the intermediate dose of THC (1 mg/kg) showed the three temporal phases of CS-induced responses, albeit the shift from dominant freezing to active coping Behaviors was slightly delayed (Behavior × Time Interaction: F(7,140) = 15.69; p < 0.001; Fig. 5E). In contrast, animals receiving the highest dose of THC (3 mg/kg) displayed a prolonged Phase 1, keeping a longer freezing response as compared to active coping until the sixth minute of CS exposure (Behavior × Time Interaction: F(7,140) = 5.44; p < 0.001; minutes 1–6, p < 0.05; Fig. 5F) and lacked the Phase 3 (minutes 7–8, p > 0.05), thereby indicating that the switch between freezing and active coping did not occur.
Overall, these data show that the exogenous activation of CB1 receptors exerts a biphasic effect on both the intensity and the prevailing type of fear-elicited behaviors along CS exposure.
Viral re-expression of CB1 receptors in the amygdala of CB1−/− mice restores the transition from passive to active coping responses
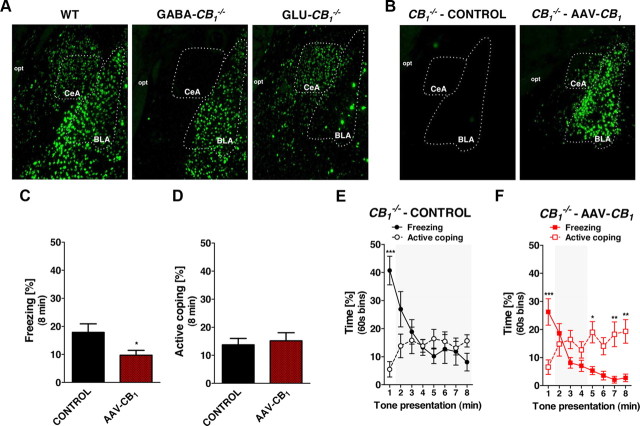
The amygdala is a key structure governing the expression of defensive responses to aversive stimuli (LeDoux, 2000; Myers and Davis, 2007; Ehrlich et al., 2009; Gozzi et al., 2010). CB1 receptor mRNA is expressed at high levels on GABAergic interneurons and at lower levels on glutamatergic neurons of the BLA, whereas it is present at low levels in GABAergic neurons of the CeA (Marsicano and Lutz, 1999; Monory et al., 2007; Bellocchio et al., 2010; Fig. 6A). As expected, CB1 mRNA expression was absent in GABAergic neurons of the amygdala of GABA-CB1−/− mice (including CeA neurons and BLA interneurons, Fig. 6A). In Glu-CB1−/− mice, CB1 mRNA was absent in glutamatergic neurons of the BLA (Fig. 6A). Notably, the control exerted by CB1 receptors signaling on both inhibitory and excitatory amygdalar neurotransmissions has been recently associated with the temporal adaptation of conditioned freezing responses (Kamprath et al., 2011) and in the control of synaptic plasticity (Marsicano et al., 2002; Azad et al., 2004). Thus, we asked whether the selective expression of CB1 receptors within the amygdala would be sufficient to induce a pattern of fear responses similar to that observed in wild-type animals. To this aim, we used the AAV-technology to re-express the CB1 receptor gene in amygdalar cells of constitutive CB1−/− mice. In situ hybridization analysis revealed that the injection of AAV-CB1 determined the expression of CB1 mRNA in a portion of the amygdala complex, including lateral, basolateral, medial, and central amygdala (Fig. 6B).
Figure 6.
AAV-mediated re-expression of CB1 receptors in the amygdala of constitutive CB1−/− mutant mice restores fear coping strategies. A, B, Representative photomicrograph (5× magnification) of CB1 mRNA expression analyzed by fluorescent in situ hybridization in the amygdala in WT, GABA-CB1−/−, and Glu-CB1−/− littermate mice (A) and in control and AAV-CB1-treated CB1−/− mice (B). Note the expression of CB1 in the central amygdaloid, CeA, and basolateral nuclei of the amygdala, BLA, in the AAV-CB1-treated mouse as compared to control. opt, Optic tract. C, Total freezing in control (n = 11) and AAV-CB1-treated (n = 8) CB1−/− littermate mice during the 8 min CS presentation. D, Total active coping in control and AAV-CB1-treated CB1−/− mice during the 8 min CS presentation. E, Time course of freezing and active coping of the control CB1−/− mice (note the absence of Phase 3). F, Time course of freezing and active coping of the AAV-CB1-treated CB1−/− mice (note the presence of the three phases of behavior). Data are mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001.
In the fear conditioning test, AAV-CB1-treated CB1−/− mice showed a reduced total freezing time during the CS presentation as compared to control CB1−/− mice injected with an empty virus (AAV-EV-treated-CB1−/− t = 2.15; p < 0.05; Fig. 6C). However, the treatment did not influence the overall active coping behavior expression time (t = 0.40; p > 0.05; Fig. 6D). As expected, the control AAV-EV-treated-CB1−/− mice did not display the temporal transition from the CS-induced freezing to active coping behaviors (Behavior × Time Interaction: F(7,126) = 10.91; p < 0.001; minute 1, p < 0.001; minutes 2–8, p > 0.05; Fig. 6E) because they lacked the Phase 3 of the fear responses (see Fig. 2). Notably, the temporal switch was restored in AAV-CB1-treated CB1−/− mice (Behavior × Time Interaction: F(7,98) = 15.08; p < 0.001; minute 1, p < 0.001; minutes 2–4, p > 0.05; minutes 5, 7–8, p < 0.01; Fig. 6F), which exhibited a behavioral pattern very similar to that of C57BL/6N and CB1+/+ mice (Figs. 1A, 2C).
Thus, re-expression of CB1 receptors in the amygdaloid complex is sufficient to rescue wild type-like fear coping strategies in constitutive CB1−/− mice.
Discussion
In natural settings, “active” or “passive” strategies represent the most efficacious responses to avoid potential dangers (Blanchard et al., 2011). The adoption of such coping styles depends on the nature of the threat, but they are also largely subserved by individual propensity toward active or passive fear responses (Koolhaas et al., 2010). The present data confirm that these individual strategies are observable in fear conditioning experiments (Cain and LeDoux, 2007; Gozzi et al., 2010) and show the following: (1) that the balanced control of excitatory and inhibitory neurons by CB1 receptors is a determinant of the individual preference toward active or passive coping with aversive situations; (2) that pharmacological CB1 receptor activation exerts a biphasic control of fear coping strategies; and (3) that CB1 receptors in the amygdala play a key role in the balance between passive and active coping strategies.
Conditioned fear responding is influenced by individual variations in defensive strategies
Extinction of conditioned freezing in wild-type mice was accompanied by an increase of active coping responses. This might just indicate that the decrease in freezing occurs when fear attenuates, with animals progressively returning to normal activities. This interpretation does not hold for several reasons. First, digging, rearing, and wall sniffing are considered defensive behaviors that rodents express when confronted with potentially threatening situations (Koolhaas et al., 1999; De Boer and Koolhaas, 2003; Cain and LeDoux, 2007; Blanchard et al., 2011). Indeed, in our conditions these responses were clearly distinguishable from the home cage behaviors of mice in the absence of any aversive CS. Second, freezing and active coping durations reached <60% of the total observation time, indicating that they did not represent the whole behavioral repertoire of the animals. Third, both freezing and active behaviors reached higher levels during tone presentation than during the pre-tone period in all animals, indicating that they were tone-induced (i.e., fear-elicited) behavioral responses. Finally, as previously reported in rats (Lázaro-Muñoz et al., 2010; Vicens-Costa et al., 2011), we were able to measure the relationship between the type of coping adopted by individual mice in fear conditioning and active avoidance performances. Altogether, these observations indicate that passive or active fear coping strategies likely represent individual behavioral traits maintained over different experimental conditions.
Freezing is considered a reliable index of fear memory in fear conditioning experiments (Maren, 2008). However, conditioned fear can be expressed through a wide variety of behavioral responses and some individuals can still adopt predominant active strategies to cope with the CS, suggesting that weak freezing does not necessarily imply a low fear state and/or lower fear memory (Maren, 2008; Gozzi et al., 2010). Indeed, “low freezer” animals in classical fear conditioning are the best performers in active avoidance (Lázaro-Muñoz et al., 2010; Vicens-Costa et al., 2011; present results), implying paradoxically that animals with “lower” levels of fear and/or memory in one test would display higher fear and/or memory in the other test. Great and obvious differences exist between pavlovian fear conditioning and instrumental avoidance learning (Hartley and Phelps, 2010). However, our observations and those of others suggest that the performances in these fear-based memory tests might be not exclusively due to the ability to process fear memory, but also to intrinsic “coping styles” (Koolhaas et al., 2010; Gozzi et al., 2010; Vicens-Costa et al., 2011).
CB1 receptor signaling determines the coping style to fear conditioned stimuli by acting upon GABAergic and glutamatergic neurons
In line with previous reports, ubiquitous CB1 receptor inactivation led to a predominant freezing response in classical fear conditioning and enhanced passive and active avoidance performances (Marsicano et al., 2002; Martin et al., 2002; Lafenêtre et al., 2007; Resstel et al., 2009; Dubreucq et al., 2010). Thus, the consequences of a total CB1 receptor deletion on the potentiation of either passive or active fear coping strategies are task-specific. This could argue against the existence of preserved individual coping styles among threat situations. However, in classical fear conditioning, the deletion of CB1 receptors in GABAergic neurons favored active coping whereas the mutation restricted to cortical glutamatergic neurons promoted passive coping. The two specific mutations induced higher performances in the active and passive versions of avoidance learning, respectively. Therefore, our data reveal that the CB1 receptor-dependent control of inhibitory and excitatory brain neuronal activity is a key determinant of fear coping strategies. Accordingly, Kamprath et al. (2009) reported that the suppression of CB1 receptors from glutamatergic neurons strengthened freezing after a fear sensitization procedure, supporting that the endogenous control of CB1 receptor signaling on excitatory neurotransmission mediates adaptation of passive fear responding. Recent studies pointed out very specific roles of CB1 receptors expressed on other restricted neuronal populations in the expression of conditioned fear responses. In particular, mice bearing a CB1 receptor deletion in the hypothalamus and mediobasal amygdala showed a dominant active coping strategy (i.e., digging behavior) in tone-cued fear conditioning (Dubreucq et al., 2012). Conversely, the specific suppression of CB1 receptors on type-1 dopamine receptor-expressing cells (Monory et al., 2007), including the medium spiny neurons of the striatum, favored freezing responses in both tone-cued and contextual fear conditioning settings (Terzian et al., 2011). Thus, the endocannabinoid control of fear responses is exerted at different brain sites. However, our data strongly suggest that CB1 receptors, by balancing inhibitory and excitatory brain neuronal activity, are one of the biological determinants of individual fear coping styles.
Exogenous THC administration exerted a biphasic effect on fear coping strategies, with low doses favoring active coping and higher doses promoting passive responses in classical fear conditioning, respectively. Such biphasic effects of cannabinoids were also reported on other behavioral dimensions, including unconditioned anxiety and food intake (Moreira and Lutz, 2008; Bellocchio et al., 2010). Interestingly, the low and high doses of THC induced opposite fear coping styles than those observed respectively in the Glu-CB1−/− and GABA-CB1−/− mice, suggesting that these biphasic effects might be mediated by cell-type specific CB1 receptors as shown previously (Puighermanal et al., 2009; Bellocchio et al., 2010; Piet et al., 2011). Overall, the bidirectional effects of acute exogenous stimulation of CB1 receptors support a balanced control of the ECS on fear coping strategies.
CB1 receptors in the amygdala are sufficient to guarantee normal fear coping strategies
The functional neuroanatomy of fear learning and processing has been extensively characterized in the last decades (LeDoux, 2000; Myers and Davis, 2007; Ehrlich et al., 2009; Hartley and Phelps, 2010). Much less, conversely, is known about the neuroanatomical and neuroendocrine substrates of individual coping styles (Koolhaas et al., 2010), rendering difficult the identification of the site(s) where the ECS controls active or passive fear responses.
The amygdaloid complex is a major integration site for learning and adaptation of passive and active responses to fear conditioned stimuli (LeDoux, 2000; Myers and Davis, 2007; Choi et al., 2010; Hartley and Phelps, 2010; Lázaro-Muñoz et al., 2010), and amygdalar CB1 receptors are necessary for a normal expression of conditioned freezing (Marsicano et al., 2002; Tan et al., 2010, 2011; Kamprath et al., 2011). The virus-mediated re-expression of CB1 receptors in CB1−/− mice shows that amygdalar CB1 receptor signaling is sufficient to mediate adaptation of conditioned freezing and, importantly, to restore a normal switch between passive and active coping.
BLA neurons contain CB1 receptors both at glutamatergic neurons and GABAergic interneurons (Marsicano and Lutz, 1999; Katona et al., 2001, 2006; Bellocchio et al., 2010). Amygdalar CB1 receptor signaling is necessary for acquisition of conditioned freezing to olfactory CS, likely through a control of the firing activity of principal neurons projecting to the prelimbic division of the mPFC (Laviolette and Grace, 2006; Tan et al., 2010, 2011). Thus, due to its main presynaptic terminal localization (Piomelli, 2003), the re-expression of the CB1 receptor might reinstate normal fear coping by acting at intra-amygdala circuits and/or at BLA-mPFC projections.
The CeA is emerging as a key subcortical structure mediating the adoption of active and passive fear conditioned responses (Choi et al., 2010; Gozzi et al., 2010; Ehrlich et al., 2009). CB1 receptors are expressed in the CeA, and their endogenous signaling is required for short-term extinction of conditioned freezing (Kamprath et al., 2011). The ECS might thus control the expression of active and passive fear strategies through a modulation of GABAergic and glutamatergic neurotransmissions within the CeA.
The amygdala is also connected to downstream structures, including the PAG and the hypothalamus, that are crucially involved in the expression of defensive responses and accompanying neuroendocrine signals (Resstel et al., 2009; Roozendaal et al., 2009). Interestingly, the local stimulation of CB1 receptors in the PAG attenuates freezing expression in contextual fear conditioning (Resstel et al., 2008; Moreira et al., 2009). Therefore, presynaptic CB1 receptors expressed on BLA-PAG projections might also participate in the behavioral consequences of our viral-mediated reinstatement of CB1 gene expression.
Conclusion
Inefficient fear coping characterizes the pathophysiology of important psychiatric diseases, including phobias and post-traumatic stress disorders. Our data highlight a complex, task-dependent regulation of the ECS on defensive strategies that is driven, at least in part, by a bimodal control of CB1 receptors on glutamatergic and GABAergic neuronal activities. Overall, this study suggests that the differential impact on active or passive fear coping strategies should be considered for designing therapeutic approaches involving the modulation of CB1 signaling.
Footnotes
This work was supported by INSERM/AVENIR, Region Aquitaine, Fyssen Foundation, CONACyT, EU-FP7 (REPROBESITY, HEALTH-F2–2008-223713), European Research Council (ENDOFOOD, ERC-2010-StG-260515), Fondation pour la Recherche Medicale, and UFA/DFH (G2R-FA-151-07). We thank the staff of the Animal and Genotyping Facilities of NeuroCentre Magendie for mouse caring and genotyping and J. Lourenço, C. T. Wotjak, S. Caillé, and the members of Marsicano laboratory for valuable suggestions.
The authors declare no competing financial interests.
References
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, Chaouloff F, Piazza PV, Marsicano G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25:205–218. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Bura SA, Castañé A, Ledent C, Valverde O, Maldonado R. Genetic and pharmacological approaches to evaluate the interaction between the cannabinoid and cholinergic systems in cognitive processes. Br J Pharmacol. 2007;150:758–765. doi: 10.1038/sj.bjp.0707152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, LeDoux JE. Escape from fear: A detailed behavioral analysis of two atypical responses reinforced by CS termination. J Exp Psychol Anim Behav Process. 2007;33:451–463. doi: 10.1037/0097-7403.33.4.451. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: Consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224:106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Dubreucq S, Kambire S, Conforzi M, Metna-Laurent M, Cannich A, Soria-Gomez E, Richard E, Marsicano G, Chaouloff F. Cannabinoid type 1 receptors located on single-minded 1–expressing neurons control emotional behaviors. Neuroscience. 2012;204:230–244. doi: 10.1016/j.neuroscience.2011.08.049. [DOI] [PubMed] [Google Scholar]
- During MJ, Young D, Baer K, Lawlor P, Klugmann M. Development and optimization of adeno-associated virus vector transfer into the central nervous system. Methods Mol Med. 2003;76:221–236. doi: 10.1385/1-59259-304-6:221. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B, Wotjak CT. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 2009;8:203–211. doi: 10.1111/j.1601-183X.2008.00463.x. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Häring M, Gaburro S, Doengi M, Lutz B, Pape HC. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology. 2011;36:652–663. doi: 10.1038/npp.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Symes CW, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Klugmann M, Goepfrich A, Friemel CM, Schneider M. AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Front Behav Neurosci. 2011;5:37. doi: 10.3389/fnbeh.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res. 2007;56:367–381. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lafenêtre P, Chaouloff F, Marsicano G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology. 2009;57:715–721. doi: 10.1016/j.neuropharm.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro-Muñoz G, LeDoux JE, Cain CK. Sidman instrumental avoidance initially depends on lateral and basal amygdala and Is constrained by central amygdala-mediated pavlovian processes. Biol Psychiatry. 2010;67:1120–1127. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lourenço J, Cannich A, Carta M, Coussen F, Mulle C, Marsicano G. Synaptic activation of kainate receptors gates presynaptic CB1 signaling at GABAergic synapses. Nat Neurosci. 2010;13:197–204. doi: 10.1038/nn.2481. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutiérrez SO, van der Stelt M, López-Rodriguez ML, Casanova E, Schütz G, Zieglgänsberger W, Di Marzo V, Behl C, Lutz B. CB1 Cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wölfel B, Dodt HU, Zieglgänsberger W, Wotjak CT, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)–tetrahydrocannabinol in mice. PloS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, Guimarães FS. Antiaversive effects of cannabinoids: is the periaqueductal gray involved? Neural Plast. 2009;2009:625469. doi: 10.1155/2009/625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Piet R, Garenne A, Farrugia F, Le Masson G, Marsicano G, Chavis P, Manzoni OJ. State-dependent, bidirectional modulation of neural network activity by endocannabinoids. J Neurosci. 2011;31:16591–16596. doi: 10.1523/JNEUROSCI.4297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Lisboa SF, Aguiar DC, Corrêa FM, Guimarães FS. Activation of CB1 cannabinoid receptors in the dorsolateral periaqueductal gray reduces the expression of contextual fear conditioning in rats. Psychopharmacology (Berl) 2008;198:4. doi: 10.1007/s00213-008-1156-1. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Moreira FA, Guimarães FS. Endocannabinoid system and fear conditioning. Vitam Horm. 2009;81:421–440. doi: 10.1016/S0083-6729(09)81016-9. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Siegle GJ, Ladouceur CD, Silk JS, Cataldo MF, Forbes EE, Dahl RE, Ryan ND. Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. Neuroimage. 2010;52:710–719. doi: 10.1016/j.neuroimage.2010.04.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala–prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20:1486–1496. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J Neurosci. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzian AL, Drago F, Wotjak CT, Micale V. The dopamine and cannabinoid interaction in the modulation of emotions and cognition: assessing the role of cannabinoid CB1 receptor in neurons expressing dopamine D1 receptors. Front Behav Neurosci. 2011;5:49. doi: 10.3389/fnbeh.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens-Costa E, Martínez-Membrives E, López-Aumatell R, Guitart-Masip M, Cañete T, Blázquez G, Tobeña A, Fernández-Teruel A. Two-way avoidance acquisition is negatively related to conditioned freezing and positively associated with startle reactions: a dissection of anxiety and fear in genetically heterogeneous rats. Physiol Behav. 2011;103:148–156. doi: 10.1016/j.physbeh.2010.12.009. [DOI] [PubMed] [Google Scholar]