Abstract
Growing and regenerating axons need to interact with the molecules in the extracellular matrix as they traverse through their environment. An important group of receptors that serve this function is the integrin superfamily of cell surface receptors, which are evolutionarily conserved αβ heterodimeric transmembrane proteins. The function of integrins is controlled by regulating the affinity for ligands (also called “integrin activation”). Previous results have shown that CNS inhibitory molecules inactivate axonal integrins, while enhancing integrin activation can promote axon growth from neurons cultured on inhibitory substrates. We tested two related molecules, kindlin-1 and kindlin-2 (Fermitin family members 1 and 2), that can activate β1, β2, and β3 integrins, for their effects on integrin signaling and integrin-mediated axon growth in rat sensory neurons. We determined that kindlin-2, but not kindlin-1, is endogenously expressed in the nervous system. Knocking down kindlin-2 levels in cultured sensory neurons impaired their ability to extend axons, but this was partially rescued by kindlin-1 expression. Overexpression of kindlin-1, but not kindlin-2, in cultured neurons increased axon growth on an inhibitory aggrecan substrate. This was found to be associated with enhanced integrin activation and signaling within the axons. Additionally, in an in vivo rat dorsal root injury model, transduction of dorsal root ganglion neurons to express kindlin-1 promoted axon regeneration across the dorsal root entry zone and into the spinal cord. These animals demonstrated improved recovery of thermal sensation following injury. Our results therefore suggest that kindlin-1 is a potential tool for improving axon regeneration after nervous system lesions.
Introduction
To regenerate in the damaged CNS, axons must be able to interact with the surrounding extracellular matrix (ECM). Integrins are αβ heterodimeric transmembrane proteins that interact with ECM molecules (Hynes, 2002; Lemons and Condic, 2008). Their functions are regulated by a conformational change in the extracellular domain from a low-affinity to a high-affinity state (also called “inside-out ” signaling or integrin activation) and “outside-in ” signaling (the signaling cascades propagated intracellularly following integrin–ligand binding). Enhancing ligand-binding affinity via integrin activation promotes neurite outgrowth from cultured CNS and PNS neurons (Ivins et al., 2000; Lein et al., 2000; Lemons and Condic, 2006; Tan et al., 2011). Crucially, integrin activation also enhances the ability of neurons to extend axons in the presence of inhibitory molecules such as amino-Nogo and the chondroitin sulfate proteoglycan (CSPG) aggrecan, which can both inactivate axonal integrins (Hu and Strittmatter, 2008; Tan et al., 2011) and are highly expressed after a CNS injury (Liu et al., 2006).
Many pathways affect integrin activation, but they mediate their effect via two families of protein, kindlin and talin, that bind to two distinct sites of the cytoplasmic domain of β integrins. Kindlins (kindlin-1, -2, -3) are evolutionarily conserved proteins containing the four point one protein, ezrin, radixin, and moesin (FERM) domain (Moser et al., 2009) that promote localization to integrin-linked adhesion sites such as focal adhesions and podosomes (Ussar et al., 2006). Kindlin-1 is found predominantly in epithelial cells, kindlin-2 is present in all tissues except blood cells, while kindlin-3 is expressed exclusively in cells of the hematopoietic system (Moser et al., 2009). Loss of kindlin results in impaired integrin activation, leading to abnormalities ranging from skin and intestinal disorders (kindlin-1), early, preimplantation embryonic lethality (kindlin-2), and defective coagulation and leukocyte adhesion deficiency (kindlin-3) (Lai-Cheong et al., 2010). Kindlin-2-deficient zebrafish display nervous system abnormalities (Dowling et al., 2008a), but otherwise little is known about kindlin function in the nervous system. Meanwhile, coexpression of kindlin with talin FERM domain produces a synergistic activation of integrin (Ma et al., 2008; Montanez et al., 2008) through a direct interaction between the proteins and β integrin cytoplasmic tails (Harburger et al., 2009).
Here, we examined the role of kindlin-1 and kindlin-2 in axon growth from cultured neurons, and the potential effects on axon regeneration using an in vivo dorsal root crush model. In addition, we investigated the effect of manipulation of kindlin on integrin functions, which are impaired by molecules that are upregulated in the damaged CNS.
Materials and Methods
Dorsal root ganglion neuron culture.
Dorsal root ganglia (DRGs) were dissected from Sprague Dawley rats (∼3 months). The ganglia were collected, dissociated with collagenase and trypsin, rinsed in calcium- and magnesium-free PBS, transfected with expression constructs encoding mCherry, kindlin-1-mCherry or kindlin-2-mCherry, and plated onto laminin (1 μg/ml) or aggrecan–laminin (25:1 μg/ml) in DMEM supplemented with insulin–transferrin–selenium (1×), penicillin–streptomycin–fungizone (1×), and 10 ng/ml nerve growth factor.
To culture DRG explants, the ganglia were removed, cut into four to six smaller pieces, and placed onto aggrecan–laminin (25:1 μg/ml) in the same medium as above.
Retinal ganglion cell culture.
Eyes from Sprague Dawley rats were removed, and the retinas were dissected out and dissociated using the papain dissociation kit (Worthington). Briefly, papain and DNase (1:10) were added and incubated at 37°C for 90 min. Then, the cells were triturated, and the resulting cell suspension was transferred to a new tube for centrifugation at 300 × g at room temperature for 5 min. The supernatant was then discarded, and the pellet was resuspended in a resuspension solution (containing EBSS, albumin ovomucoid inhibitor, and DNase). After that, the cell suspension was centrifuged through a discontinuous density gradient prepared by albumin ovomucoid inhibitor at 70 × g at room temperature for 6 min. The pellet collected was subsequently resuspended in Neurobasal A supplemented with B27, l-glutamine (2 mm), and gentamicin (1:200). Dissociated retinal ganglion cells (RGCs) were then plated onto laminin (10 μg/ml) at 100,000 cells/well and cultured for 5–7 d in Neurobasal A supplemented with B27 (1×), 2 mm l-glutamine, and gentamicin.
Hippocampal neuron culture.
Primary hippocampal neuron cultures were prepared as described by Marland et al. (2011). Briefly, hippocampal tissues were dissected out from Sprague Dawley rat embryos [embryonic day 18 (E18)] in calcium- and magnesium-free HBSS (HBSS-CMF), digested with 0.25% trypsin (Invitrogen) for 15 min at 37°C, plated onto coverslips coated with poly-d-lysine (PDL) (0.25 mg/ml) in DMEM supplemented with 10% fetal calf serum and an additional 0.8% glucose at a density of 1 × 105 cells/ml. After 4 h, the culture medium was changed to Neurobasal supplemented with B27 (2×) and GlutaMAX (1×) for 21 d (culture medium changed every 7 d).
Mixed cerebellar culture.
Primary mixed cerebellar cultures were prepared by following Furuya et al. (1998), with slight modifications. Briefly, whole cerebella were dissected out from Sprague Dawley rat embryos (E18) in ice-cold HBSS-CMF, digested with 0.1% trypsin for 10 min at 37°C, plated onto coverslips coated with PDL (0.5 mg/ml) at a density of 5 × 106 cells/ml, and cultured in DMEM/F12 supplemented with B27 (1×), N2 supplement (1×), GlutaMAX (1×), and T3 hormone (0.5 ng/ml) for 17 d (culture medium changed every 7 d).
Human embryonic kidney cells.
Human embryonic kidney (HEK) cells were plated on PDL-coated T25 tissue culture flasks in DMEM supplemented with 10% fetal calf serum. mCherry, kindlin-1-mCherry, and kindlin-2-mCherry DNA construct transfection was performed using the Neon Transfection System (Invitrogen), with the parameters of 1300 V, three pulses, and 10 ms pulse duration. Successfully transfected cells were selected using G418 (1 mg/ml), until ∼95% of cells were positive.
Transfection of DRG neurons.
Transfection of dissociated DRG neurons was performed using Neon Transfection Kit (Invitrogen; MPK1096), following the manufacturer's instructions, with the parameters of 1200 V, two pulses, and 20 ms pulse duration.
Knockdown of kindlin-2.
The expression vector pSUPER (Oligoengine) was used to produce a short hairpin RNA (shRNA) to mediate kindlin-2 knockdown. Two sequences targeting kindlin-2 (target base pairs 1536–1554 and 1690–1708 in the kindlin-2 coding sequences) were designed as follows: (1) sense, 5′-GATCCCCCAGACAGCTCTTACAACCTTTCAAGAGAAGGTTGTAAGAGCTGTCTGTTTTTA-3′; antisense, 5′-AGCTTAAAAACAGACAGCTCTTACAACCTTCTCTTGAAAGGTTGTAAGAGCTGTCTGGGG-3′; (2) sense, GATCCCCCAAACAGATAACAGCACGGTTCAAGAGACCGTGCTGTTATCTGTTTGTTTTTA-3′; antisense, AGCTTAAAAACAAACAGATAACAGCACGGTCTCTTGAACCGTGCTGTTATCTGTTTGGGG-3′. The sense and antisense oligonucleotides were annealed using an annealing buffer (100 mm potassium acetate, 30 mm HEPES-KOH at pH 7.4, and 2 mm magnesium acetate). pSUPER vector was digested using restriction endonucleases HindIII and BglII. The annealed oligonucleotides were subsequently ligated into the vector and cloned in bacteria. Transfection of DRG neurons with these shRNA-producing constructs were performed using Neon Transfection Kit (Invitrogen), with the same parameters as for plasmid transfection described above.
Postfixation immunocytochemistry.
Cell cultures on coverslips were fixed with 4% paraformaldehyde (PFA), permeabilized with 0.1% Triton X-100, blocked with goat or donkey serum, and incubated with primary antibodies at 4°C overnight. They were then washed and incubated with secondary antibodies for 1 h at room temperature before being mounted on slides. Primary antibodies were used against kindlin-1, kindlin-2 [both raised in rabbits, as described by Ussar et al. (2006)], βIII-tubulin (Sigma-Aldrich; T8660; 1:400), Purkinje cell protein 2 (Abgent; AP6356P; 1:2000), pY397 FAK (Biosource; 44-624G; 1:100), GFP (Abcam; ab6662; 1:500), and mCherry (Clontech; 632543; 1:200).
Live immunocytochemistry.
Antibody against activated integrin, 9EG7 (BD Biosciences Pharmingen; 550531; 1:50 in culture medium), was added to the cultures for 15 min at 37°C. After washing with culture medium, the cultures were fixed with 4% PFA. They were then incubated in FITC-conjugated goat anti-rat antibody for 1 h, before being mounted on slides.
Axon growth assay.
Two parameters were quantified as a measure of axon growth for the in vitro experiments as follows: (1) percentage of neurons with axons longer than the cell body diameter and (2) average of the longest axons extended by each neuron.
Quantitative immunofluorescence.
At least 20 axons per coverslip were first selected at random and imaged. An area of axon (>30 μm long) was then traced, and the fluorescence intensity of immunostaining was analyzed using the Leica Application Suite (Leica Microsystems).
In situ hybridization.
Templates for in situ hybridization probe synthesis were generated by PCR using primers for mouse kindlin-1, attached with SP6 or T7 promoter sequence: sense, 5′-ATTTAGGTGACACTATAGATGCGAGTTTAGGGATGTCAG-3′; antisense, 5′-TAATACGACTCACTATAGGATGTTCCAGCCTCGATCTTTG-3′ and 5′-TAATACGACTCACTATAGGTGATAGCCCTTTGACAGAGCA-3′, generating PCR products of 410 and 489 bp, respectively. In vitro transcription for RNA probes was achieved using SP6 or T7 RNA polymerase from the DIG RNA Labeling Kit (SP6/T7) (Roche). In situ hybridization on DRG sections was performed as described by Carulli et al. (2006).
RNA extraction from cultures.
RNA was extracted using acid guanidinium thiocyanate phenol chloroform method. Briefly, DRG neurons or HEK cell cultures detached from the culture surface using trypsin and were scraped with a cell scraper. The collected materials were centrifuged at 13,000 rpm at 4°C for 2 min. The pellet was then lysed with Solution D (containing guanidinium thiocyanate, 0.75 m sodium citrate, 10% N-lauroylsarcosine, and β-mercaptoethanol) and kept on ice for 15 min. Subsequently, 2 m Na-acetate, pH 4 (1:10 v/v; Sigma-Aldrich), and water-saturated phenol (1:10 v/v) were added, followed by chloroform/isoamyl alcohol (49:1; 1:5 v/v; Fluka). The samples were kept on ice for 15 min and centrifuged at 13,000 rpm at 4°C for 15 min. The top layer (aqueous phase) was collected and RNA was precipitated with cold isopropanol (1.25:1 v/v) at −80°C for at least an hour. After thawing, the mixture was centrifuged at 13,000 rpm at 4°C for 15 min. The pellet was collected, washed with cold 70% ethanol, left to dry, and resuspended in DEPC-treated water. RNA samples were then treated with DNase to remove any contaminating DNA remaining in the sample.
Reverse transcription-PCR.
Reverse transcription was performed using SuperScript III First-Strand cDNA Synthesis kit (Invitrogen). Primers were generated to detect the following: kindlin-1 (forward, 5′-CTACACCTTCTTTGACTTGAATCC-3′; reverse, 5′-TGCGAGTTTAGGGATGTCAG-3′) and kindlin-2 (forward, 5′-GTACCGAAGTAGACTGCAAGG-3′; reverse, 5′-GCAGACCCGATTTCTATATTGG-3′), generating PCR products of 298 and 588 bp, respectively. PCR products were subjected to agarose gel electrophoresis at 100–130 V for ∼45 min.
Western blot.
Cell lysates from DRG or HEK cell cultures were collected in RIPA buffer (Roche), supplemented with protease inhibitors and phosphatase inhibitor mixtures (Roche). The protein concentrations were subsequently determined using BCA Protein Assay Kit (Pierce). Protein extracts were subjected to SDS-PAGE and blotted onto activated polyvinylidene difluoride (Hybond-P) membranes (GE Healthcare). The membrane were blocked with 5% skimmed milk in Tris-buffered saline with Tween 20 (TBST), and then incubated with primary antibodies at 4°C overnight. This was followed by incubation in HRP-conjugated secondary antibodies at room temperature for 1 h. Protein bands were visualized using ECL-detecting reagents (GE Healthcare).
Preparation of mCherry and kindlin-1-mCherry viruses.
To subclone kindlin-1-mCherry construct into an adenoassociated viral (AAV) vector, kindlin-1-mCherry insert was first amplified through PCRs with primers linked to restriction site sequences [forward (containing EcoRI site), 5′-CCGGGAATTCACCATGGTGAGCAAGGGCGA-3′; reverse (containing XbaI site), 5′-CCGGTCTAGATCAGTCCTGACCGCCAGT-3′] using Finnzymes Phusion Hot Start High Fidelity DNA Polymerase (Finnzymes). The PCR products were subjected to agarose gel electrophoresis, and the bands of the correct size (∼2700 bp) were cut out and purified using the Wizard SV Gel and PCR Cleanup System (Promega). Subsequently, the DNA fragments were digested with EcoRI and XbaI restriction enzymes to produce sticky ends, before being separated by gel electrophoresis and purified again. The AAV2 vector was digested and purified in a similar manner. The subcloning of control mCherry construct into the AAV vector was made with the following modifications: The primers used for the PCR for DNA amplification were as follows: forward (containing EcoRI site), 5′-CCGGGAATTCACCATGGTGAGCAAGGGCGA-3′; reverse (containing XbaI site), 5′-CCGGTCTAGACTTGTACAGCTCGTCCAT-3′; which produced a DNA fragment of ∼700 bp.
mCherry and kindlin-1-mCherry inserts and AAV vector were ligated using a T4 ligase kit (Promega), and the resulting ligation products were then transformed into bacteria. A single clone containing the correct sequence was then amplified and purified using a Megaprep Kit (Invitrogen). The DNA constructs in the AAV vectors were used to produce AAV serotype 2 (AAV2) viruses at high titers, in association with The Christopher and Dana Reeve Foundation Consortium Vector Core, The Salk Institute, and Virapur.
Dorsal root injury with concurrent DRG injection of virus.
Animal surgeries were performed in accordance with the United Kingdom Animal (Scientific Procedure) Act of 1986. Adult male Lewis rats were used for these experiments. Food and water were provided ad libitum, with a 12 h light/dark exposure. Surgeries were performed with the rats anesthetized in 2% isoflurane (2 L/min), mixed with oxygen (1.5 L/min).
The virus solutions for injection were prepared by mixing either AAV2-kindlin-1-mCherry or AAV2-mCherry viruses with AAV2-fGFP viruses at a 1:1 ratio, resulting in a final viral load of 1.46 × 1010 TU/ml. Under anesthesia, rats underwent a left hemi-laminectomy from the level of C5 to T1, to expose the DRGs from C5 to C8. Then, 1 μl of the virus was injected into the C6 and C7 DRGs by using a 33 gauge steel needle (Hamilton Company) attached to a 26 gauge 10 μl syringe (Hamilton Company), driven by an infusion syringe pump (World Precision Instruments) at 0.2 μl/min. The needle was left in place for another 3 min before being removed. Immediately following the injection, a unilateral quadruple dorsal root crush (10 s, repeated three times) was performed on roots C5 to C8 on the left.
Sensory behavior analysis.
Lewis rats that had undergone concurrent DRG virus injection and dorsal root injury were tested for changes in temperature and mechanical sensations weekly, until being killed at week 6 (after surgery). For temperature sensation assessment, a portable infrared-emitting light source (Ugo Basile) was placed under the footpads of the rat forepaws. The time that elapsed between the onset of the heat stimulus and the withdrawal of the forepaw by the rat was recorded. If the rat failed to respond to the stimulus after 20 s, the test was terminated. The measurement was repeated three times for each forepaw. For pressure sensation assessment, a probe connected to an electronic anaesthesiometer (model 1601C; Life Science Instruments) was applied to the glaborous footpads of the forepaws, with gradual increases in pressure until the rat withdrew its forepaw. The maximal pressure recorded by the force transducer was then recorded after paw withdrawal. If the rat failed to respond when the force applied reached 200 g, the test was terminated. Five trials were performed for each forepaw, and the average was determined after eliminating the highest and lowest measurements. Six animals each from the kindlin-1-mCherry and mCherry groups were analyzed. Animals that failed to recover their sensation (did not withdraw their paws in response to temperature or pressure) 2 weeks after surgery were not included in the analysis. Comparisons between the groups were performed using two-way ANOVA with post hoc Bonferroni's analysis.
Immunohistochemistry: cryosections.
Rats from the dorsal root injury experiment were killed with an overdose of sodium pentobarbital, perfused transcardially with PBS followed by 4% PFA, before being dissected for a 2 cm section of spinal cord attached to the injured roots and injected DRGs. The tissue was then postfixed in PFA overnight, cryoprotected in 30% sucrose solution for 3 d, embedded in OCT (Thermo Fisher Scientific), and cut longitudinally or horizontally using a cryostat into 14 μm sections. Sections were washed in PBS, permeabilized with 0.1% Triton X-100, blocked with 10% goat serum, and incubated with primary antibodies (diluted in blocking solution) overnight at 4°C. After incubation with secondary antibodies for 1 h at room temperature, the sections were mounted on slides. Primary antibodies were used against glial fibrillary acidic protein (GFAP) (Dako; Z0334; 1:500), GFP (Abcam; ab6662; 1:500), and mCherry (Clontech; 632543; 1:200).
Axon regeneration assay.
To analyze axonal regeneration through the dorsal root injury zone and into the spinal cord, we assessed the number of axons (per section) crossing the following three regions: the crush site, the dorsal root entry zone (DREZ), and the spinal cord. The crush site was defined as a line drawn just proximal to the root crush, the DREZ as the region nearest to the root, which is demarcated by a GFAP-immunopositive boundary, and the spinal cord as any area within the spinal cord ipsilateral to the root crush that is beyond the DREZ. Six animals each from the kindlin-1-mCherry and mCherry groups were analyzed. Comparisons between the groups in each region were analyzed using Student's t test.
Results
Analysis of kindlin-1 and kindlin-2 expression in the nervous system
Previous studies of the expression of the different kindlin isoforms have been focused on tissues in which pathologies have been described, in particular the skin, intestinal epithelia, and hematopoietic-derived cells (Lai-Cheong et al., 2010). To date, no detailed examination of the expression of kindlin isoforms has been performed in the nervous system. Kindlin-2 is the only isoform that has been detected in the brain (Ussar et al., 2006). By performing RT-PCR on RNA extracted from cultured DRG neurons and whole brains, we verified the presence of kindlin-2 in these tissues (Fig. 1A). Immunocytochemistry performed on cultured DRG neurons, RGCs, hippocampal neurons, and cerebellar Purkinje neurons, meanwhile, also demonstrated that kindlin-2 was expressed by neurons in vitro (Fig. 1C). Moreover, the localization of kindlin-2 extends from the cell body all the way into the axons (Fig. 1C). In addition, non-neuronal cells surrounding the neurons in culture, such as fibroblasts, Schwann cells, and astrocytes, all exhibited expression of kindlin-2 (Fig. 1C).
Figure 1.
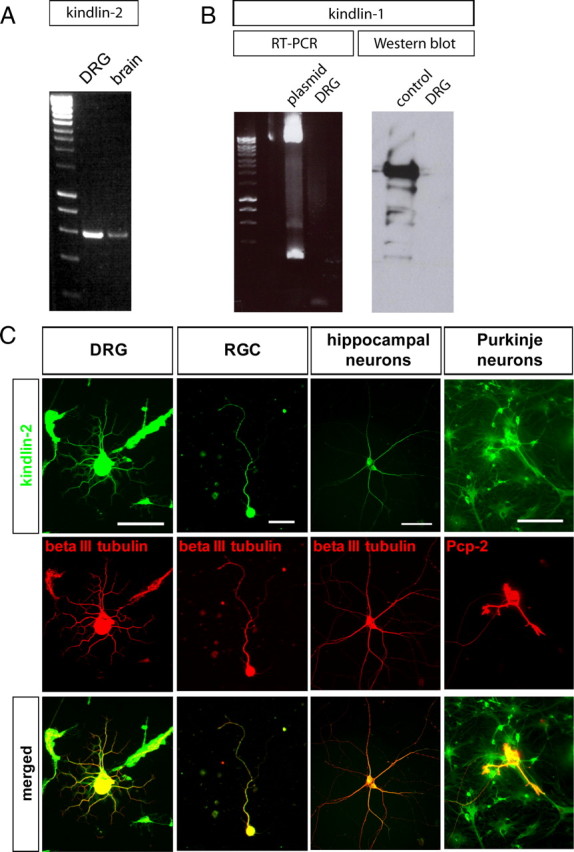
Expression of kindlin-1 and kindlin-2 in the nervous system. A, Kindlin-2 is expressed by the brain and cultured DRG neurons, as detected by Western blot. B, Kindlin-1 is not expressed by DRG neurons, as validated by RT-PCR and Western blot. Kindlin-1 plasmid was used as a positive control. C, Kindlin-2 is expressed by a variety of cultured neurons, including DRG neurons, RGCs, hippocampal neurons, and cerebellar Purkinje neurons, in addition to non-neuronal cells such as fibroblasts, Schwann cells, and astrocytes. In neurons, the expression extends all the way into the axons. Scale bars: Left to right, 100, 25, 100, 100 μm.
In contrast, RT-PCR and Western immunoblotting both reported no expression of kindlin-1 in cultured DRG neurons (Fig. 1B), thus corroborating results from an earlier study that showed the absence of kindlin-1 in the nervous system (Ussar et al., 2006).
Kindlin-1, but not kindlin-2, overexpression enhances integrin activation and signaling in DRG axons
Previous studies have shown that kindlin-1 overexpression in keratinocytes obtained from Kindler syndrome patients restores the normal cellular phenotype by enhancing integrin activation (Lai-Cheong et al., 2009). We therefore examined the effects of kindlin expression on integrin activation and signaling in cultured adult sensory neurons. Dissociated DRG neurons were transfected with either kindlin-1-mCherry or mCherry expression vectors, and cultured on laminin or aggrecan–laminin substrata for 2 d in vitro (div). The mCherry fluorescence was visible in the cell body, axons, and growth cones with both constructs (Fig. 2A). First, we determined whether expression of kindlin-1 alters the amount of β1 integrin on the surface of the axons, using immunocytochemistry in unpermeabilized cultures. No difference in the intensity of β1 immunostaining on the surface of axons was observed between those transfected with kindlin-1 and with mCherry (Fig. 2B). Next, we asked whether expression of kindlin-1 affects the integrin activation state. A monoclonal antibody that specifically detects activated integrin, 9EG7, was used to stain live unfixed axons to determine the status of integrin activation. On laminin substrate, kindlin-1-expressing axons exhibited more intense 9EG7 immunostaining compared with mCherry control axons (+26.7%; p < 0.05, Student's t test), indicating that excess kindlin-1 increases integrin activation (Fig. 2C). On aggrecan–laminin substrates, on which integrins in control DRG axons become inactivated, kindlin-1 expression induced a more marked enhancement of 9EG7 binding in DRG axons (+57.4%; p < 0.05, Student's t test) (Fig. 2C). One of the major downstream signaling events following integrin activation and ligand binding is the phosphorylation of focal adhesion kinase (FAK) at tyrosine-397 (pY397 FAK) (Parsons, 2003). Quantitative analyses of pY397 FAK immunofluorescence intensity showed that kindlin-1 expression did not alter pY397 FAK level in axons from DRG neurons cultured on laminin (in which the level of pY397 FAK is already high) (p = 0.12, Student's t test). However, when cultured in the presence of aggrecan, which lowers pFAK levels relative to laminin alone, kindlin-1 transfection increased pY397 FAK levels (+25.7%; p < 0.05, Student's t test) (Fig. 2D). Our result therefore suggests that the elevated levels of kindlin-1 can overcome the lowering of pFAK activation due to aggrecan. Overall, these experiments show that kindlin-1 overexpression can activate integrins and increase integrin signaling, overcoming the inhibitory effect of aggrecan.
Figure 2.
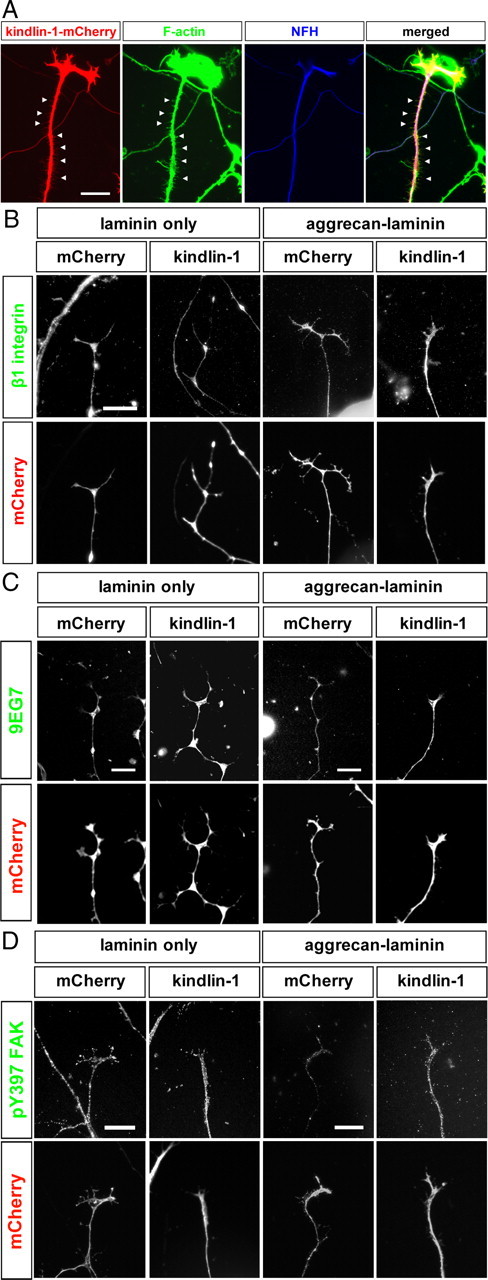
Effects of transgenic expression of kindlin-1 on integrin signaling in neurons. A, Kindlin-1 is expressed in the axon and growth cone of cultured DRG neurons, following transfection of kindlin-1-mCherry plasmid. Kindlin-1 is also present in the F-actin-positive, neurofilament-H-negative side branches arising from the axon shaft. Scale bar, 25 μm. B, Kindlin-1 expression does not affect the surface level β1 integrin on axons. Scale bar, 15 μm. C, Kindlin-1 expression enhances the intensity of 9EG7 immunostaining in axons. Scale bar, 15 μm. D, Kindlin-1 expression increases axonal pY397 FAK levels in DRG neurons cultured on a growth-inhibitory (aggrecan–laminin), but not growth-promoting (laminin) substrate. Scale bar, 15 μm.
We also examined the effect of overexpression of kindlin-2 in DRG neurons. Unlike kindlin-1, kindlin-2 is endogenously expressed in DRG neurons. The level of 9EG7 intensity in the axons remained unchanged after kindlin-2 transfection, whether the neurons were cultured on laminin (p = 0.44, Student's t test) or aggrecan–laminin (p = 0.58, Student's t test). Together, these data suggest that the status of integrin activation and signaling was not significantly affected by kindlin-2 overexpression.
Kindlin-1, but not kindlin-2, overexpression enhances DRG axon growth on aggrecan–laminin
To investigate whether kindlin-induced integrin activation might affect axon growth, we overexpressed kindlin-1 or kindlin-2 in adult rat DRG neurons. The neurons were cultured on substrates consisting of either laminin (which activates β1 integrins and promotes axon growth) or on an aggrecan–laminin mixture (which inhibits integrin activation and signaling). After 2 div, no differences in axon growth were observed between DRG neurons transfected with kindlin-2 and mCherry control (on laminin, percentage neurons with axons, p = 0.92; axon length, p = 0.23; on aggrecan–laminin, percentage neurons with axons, p = 0.26; axon length, p = 0.78; Student's t test) (Fig. 3A–C).
Figure 3.
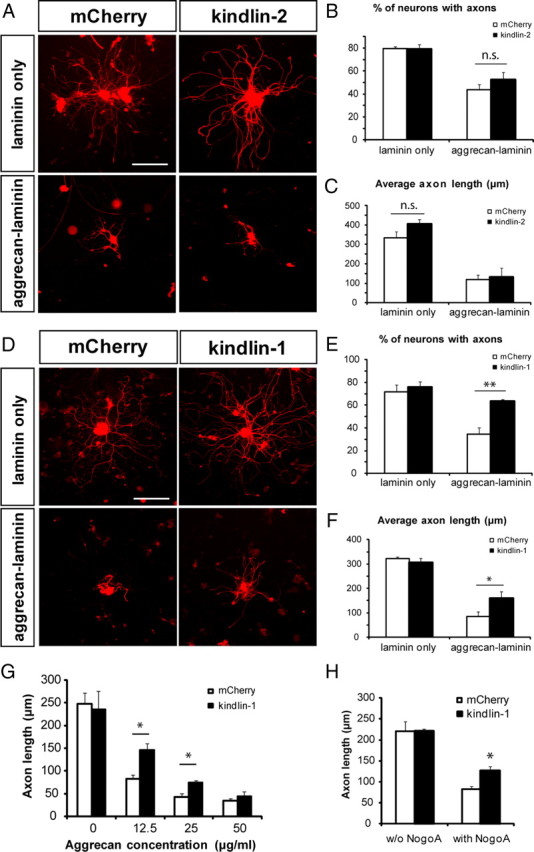
Effect of kindlin-1 and kindlin-2 overexpression on axon growth. A–C, Kindlin-2 overexpression has no effect on axon growth from cultured DRG neurons, on both growth-promoting (laminin) and growth-inhibitory (aggrecan–laminin) substrates. Scale bar, 200 μm. D–F, Kindlin-1 expression does not affect axon growth on DRG neurons cultured on laminin, but reverses the inhibition of axon growth on neurons cultured on aggrecan–laminin. Scale bar, 200 μm. G, The effect of kindlin-1 expression on axon growth on laminin substrate mixed with different concentrations of aggrecan. H, Kindlin-1 expression reverses the inhibitory effect of Nogo-A on axon growth. Data are mean ± SEM and were analyzed with Student's t test. **p < 0.01; *p < 0.05; n.s., not significant.
Because kindlin-1 increases integrin activation in cultured adult DRG neurons, we asked whether forced expression of this protein would affect axon growth. In transfected neurons, kindlin-1 visualized using the mCherry tag was present in the cell body as well as in the axons and growth cones (Fig. 2A). Many of the transfected axons had many short, actin-positive, tubulin-negative sprouts arising from the axon shafts (Fig. 2A). On laminin substrates, transfection of kindlin-1 did not enhance the already prolific axon growth from DRG neurons, either in terms of percentage of neurons with axons (p = 0.44, Student's t test) or axon length (p = 0.42, Student's t test) (Fig. 3D–F). However, when cultured on mixed aggrecan–laminin substrate on which axon growth is inhibited relative to laminin alone, neurons that expressed kindlin-1 displayed increased axon growth. The percentage of DRG neurons bearing axons was returned to almost the control (laminin-only) level (p < 0.01, Student's t test) and the average axon length was also significantly increased (p < 0.05, Student's t test) (Fig. 3D–F). These results indicate that kindlin-1 expression enables DRG neurons to overcome the inhibitory effect of aggrecan on axon growth. Additionally, we also saw a similar effect in PC12 cells transfected to express kindlin-1 (data not shown).
To investigate the effect of aggrecan concentration on the growth-promoting ability of kindlin-1 expression, we cultured DRG neurons on laminin mixed with varying concentrations of aggrecan. At a lower aggrecan concentration (12.5 μg/ml), kindlin-1 expression induced a greater increase in axon growth (p < 0.05, Student's t test), while at a higher aggrecan concentration (50 μg/ml), kindlin-1 expression did not affect axon growth (Fig. 3G). To determine the generality of kindlin-1 as an integrin activator in promoting axon growth in an integrin-mediated system, we expressed kindlin-1 in DRG neurons cultured on mixed Nogo-A–laminin substrate and found that kindlin-1 expression also enhances axon growth on this CNS myelin model (p < 0.05, Student's t test) (Fig. 3H).
Kindlin-2 is necessary for normal DRG axon growth
The finding that kindlin-1 expression promotes axon growth on aggrecan–laminin substrate, while kindlin-2 overexpression does not have any effect, was unexpected because the effects of the two molecules had previously been thought to be interchangeable. Kindlin-2 is the only form of kindlin present in the nervous system and, specifically, in neurons (Ussar et al., 2006) (Fig. 1A,B). This led us to examine whether kindlin-2 is necessary for the normal outgrowth of axons from DRG neurons.
By using an expression vector system to express shRNA targeting two different sequences in the kindlin-2 gene, the effect of knocking down kindlin-2 in DRG neurons cultured on laminin was analyzed. To demonstrate that the level of kindlin-2 was decreased as a consequence of kindlin-2 shRNA expression, quantitative immunofluorescence was performed to determine the amount of kindlin-2 protein in shRNA-expressing neurons, demonstrating a significant ∼50% fall (p < 0.05 compared with control GFP plasmid; p < 0.01 compared with scrambled shRNA; Student's t test) (Fig. 4A,B).
Figure 4.
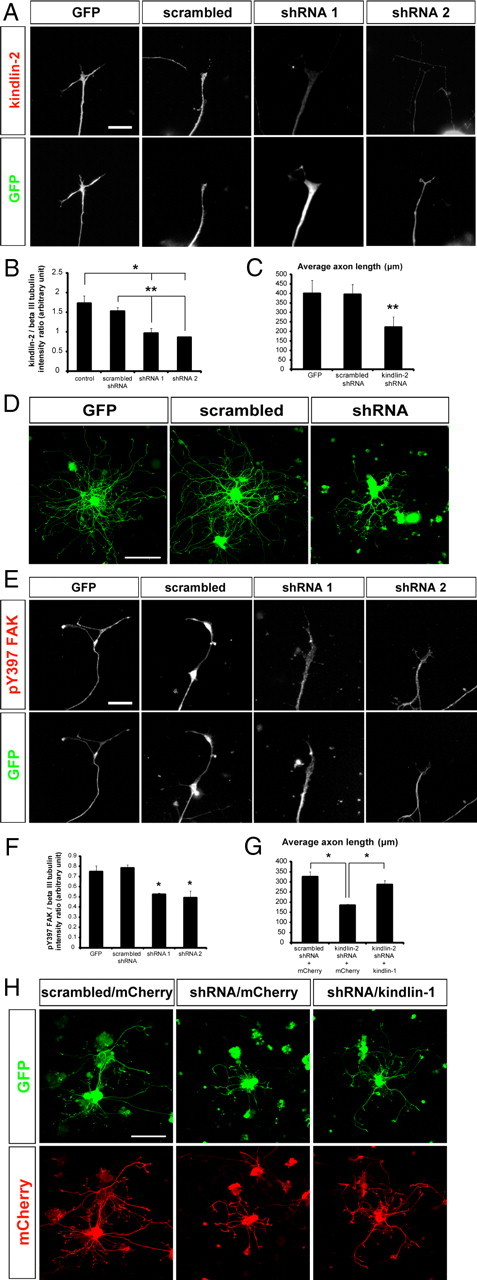
Effects of shRNA-mediated kindlin-2 knockdown on neurons. A, B, Kindlin-2 knockdown significantly reduces the level of kindlin-2 in axons. Scale bar, 15 μm. C, D, Kindlin-2 knockdown decreases axon growth from cultured DRG neurons. Scale bar, 200 μm. E, F, Kindlin-2 knockdown impairs integrin signaling in the axons, as indicated by the lower level of pY397 FAK. Scale bar, 15 μm. G, H, Transgenic expression of kindlin-1 partially reverses the inhibitory effect of kindlin-2 knockdown on axon growth. Scale bar, 200 μm. Data are mean ± SEM and were analyzed with Student's t test. **p < 0.01; *p < 0.05.
When assayed for axon growth after 3 div, neurons expressing the control GFP vector or scrambled sequence were able to extend axons normally, but those expressing kindlin-2 shRNA exhibited greatly reduced axon length (p < 0.01, Student's t test) (Fig. 4C,D). This suggests that kindlin-2 is involved in the process of axon growth from normal DRG neurons. Assessment of integrin function by pY397 FAK immunostaining revealed a 30.0–37.1% decrease (p < 0.05, Student's t test) (Fig. 4E,F), without a change in the level of total FAK (data not shown), demonstrating that outside-in integrin signaling was decreased in shRNA-transfected neurons compared with GFP or scrambled controls. This is consistent with the idea that kindlin-2 is part of the mechanism for integrin activation (Montanez et al., 2008; Ussar et al., 2008). Next, to determine whether the inhibition of axon growth due to kindlin-2 downregulation could be rescued by kindlin-1 expression, kindlin-2 shRNA and kindlin-1 expression constructs were cotransfected into DRG neurons. Kindlin-2 shRNA-mediated inhibition on axon growth was overcome by the concurrent expression of kindlin-1, albeit not back to control levels (p < 0.05, Student's t test), suggesting that kindlin-1 can partially substitute for kindlin-2 in promoting axon growth in kindlin-2-deficient neurons (Fig. 4G,H).
Kindlin-1 expression in vivo promotes axon regeneration and recovery of sensory functions after dorsal root crush
Encouraged by the positive effects of kindlin-1 expression on axon growth in cultured sensory neurons, we asked whether there is a similar effect on the regeneration of severed axons into the spinal cord. To enable expression of kindlin-1 in neurons in vivo, an AAV2-kindlin-1 vector was produced. Kindlin-1-mCherry and mCherry DNAs were subcloned into an AAV vector under a CMV promoter, and subsequently packaged into a serotype 2 AAV as described in Materials and Methods. AAV2 vectors were chosen because they preferentially transduce neurons, give stable long-term gene expression, and are without significant toxicity (McCown et al., 1996), as had been previously used for the successful transduction of DRG neurons for in vivo axon regeneration experiments (Andrews et al., 2009).
Male adult Lewis rats were subjected to unilateral (left) quadruple dorsal root crush at the C5–C8 levels, with concurrent unilateral injection of AAV2-kindlin-1-mCherry or AAV2-mCherry viruses [mixed with AAV2-GFP, at a ratio of 1:1 (v/v)] into the C5–C6 DRGs, as in our previous experiment (Andrews et al., 2009). The animals were allowed 6 weeks for recovery and regeneration, during which time they underwent a weekly assessment of temperature and pressure sensation, before being killed for histological analysis.
Transduction of AAV2-kindlin-1-mCherry and AAV2-mCherry into DRGs resulted in visible mCherry expression in some neurons in the ganglia (Fig. 5A). However, beyond the ganglia, the immunostaining was poor with a high background, and we were unable to reliably assay mCherry in axons. Because of this, we further verified kindlin-1 mRNA expression in DRG neurons by in situ hybridization. This confirmed the presence of kindlin-1 transcripts in DRGs transduced with AAV2-kindlin-1-mCherry (Fig. 5B). In addition, we harvested uninjured DRG neurons injected with AAV2-kindlin-1-mCherry or AAV2-mCherry viruses and cultured them on mixed aggrecan–laminin (25:1 μg/ml) substrate as before, and found that the kindlin-1-expressing explants extended longer axons compared with mCherry control (776.79 vs 444.21 μm; p < 0.05, Student's t test) (Fig. 5C).
Figure 5.
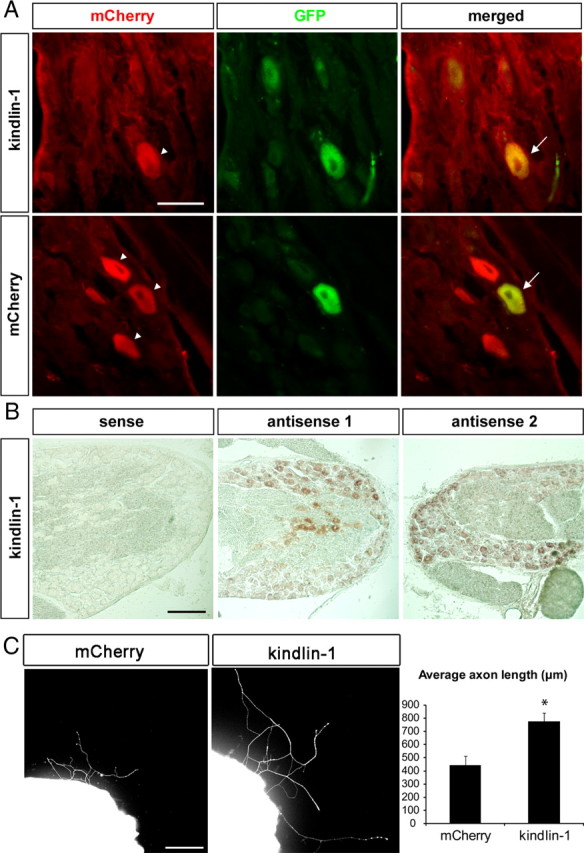
Kindlin-1 expression following AAV2-kindlin-1-mCherry injection. A, DRG neurons express GFP and kindlin-1-mCherry following coinjection of AAV2 viruses. The arrowheads denote neurons expressing mCherry or kindlin-1, as detected by anti-mCherry antibody, while the arrows indicate neurons coexpressing the proteins with GFP. Scale bar, 50 μm. B, In situ hybridization confirms the expression of kindlin-1 transcript following AAV2-kindlin-1-mCherry injection. Scale bar, 50 μm. C, DRG explants expressing kindlin-1 extended longer axons on mixed aggrecan–laminin substrate compared with mCherry control. Scale bar, 50 μm. Data are mean ± SEM and were analyzed with Student's t test. *p < 0.05.
The regeneration of sensory axons was quantified by counting GFP-labeled axons crossing the crush site, the DREZ, and in the dorsal horn and dorsal column of the spinal cord, as in our previous studies (Andrews et al., 2009). At the crush site, no difference in the numbers of axons was observed between kindlin-1 and mCherry control groups (p = 0.28, Student's t test) (Fig. 6A). However, at the boundary of the DREZ, significantly more regenerating axons were found crossing the region demarcated by GFAP-expressing astrocytes in the kindlin-1 group, compared with control (p < 0.01, Student's t test) (Fig. 6B,C). Furthermore, kindlin-1 expression markedly enhanced the number of regenerating axons within the spinal cord, into both the dorsal horn (gray matter) and dorsal column (white matter) (p < 0.05 for both, Student's t test) (Fig. 6D–G). These results indicate that transduction of DRG neurons with kindlin-1 promotes regeneration of sensory axons into the CNS environment after dorsal root crush.
Figure 6.
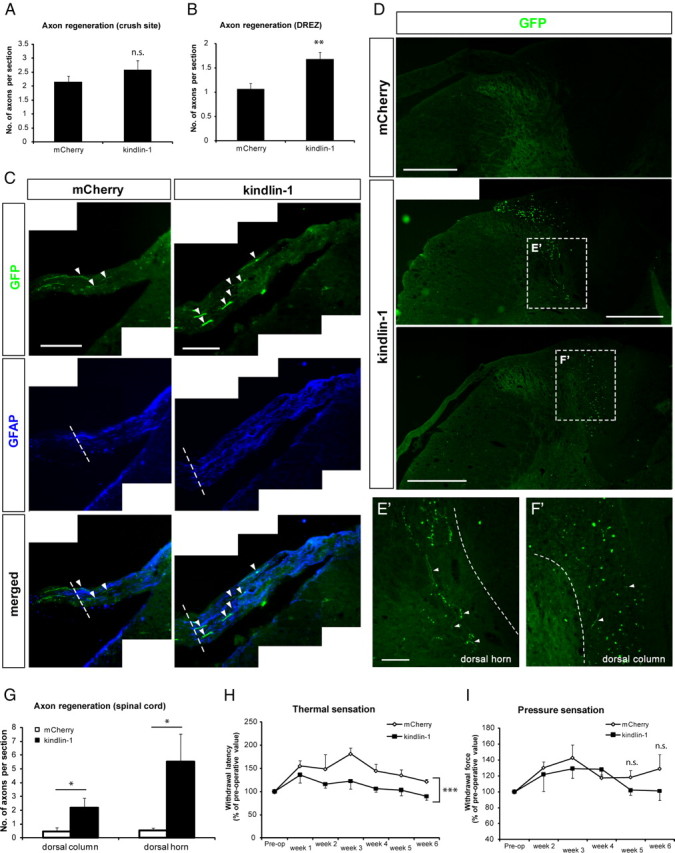
Kindlin-1 expression by DRG neurons promotes axon regeneration across DREZ and into spinal cord. A, More axons regenerate across the DREZ into the GFAP-expressing region in the kindlin-1 animals compared with control. Scale bar, 100 μm. B, C, Quantification of axon regeneration, showing that kindlin-1 enhances axon regeneration across DREZ (C) but not at the injury site (B). D–G, Axon regeneration into both the dorsal horn (E′) and dorsal column (F′) increases following kindlin-1 expression. The axons in the dorsal columns (F′) appear as dots in transverse section picture, which can be seen to be axons running in the caudo-rostral plane when focusing up and down. Scale bars: D, 500 μm; E′, F′, 100 μm. H, I, Quantification of behavioral results indicate a significant improvement in the recovery of thermal (H), but not pressure (I) sensation. Data are mean ± SEM and were analyzed with either Student's t test (B, C, G) or two-way ANOVA with post hoc Bonferroni's analysis (H, I). ***p < 0.001; **p < 0.01; *p < 0.05; n.s., not significant.
The sensory functions of the injured rats were assessed with a hot-plate test and a pressure test, for temperature and pressure sensations, respectively (Galtrey and Fawcett, 2007). Neither tests revealed any evidence of hyperalgesia, indicating that neither the dorsal root crush nor the DRG virus injection had induced any sensory hypersensitivity in the animals (Fig. 6H,I). The hot-plate test data for temperature sensation showed that, after dorsal root crush, the level of sensation decreased (i.e., longer withdrawal latency) immediately after injury, but gradually recovered to near-normal levels over the course of the experiment. Animals in the kindlin-1 group demonstrated a faster recovery of temperature sensation than those injected with mCherry virus (p = 0.0001; F = 17.79; two-way ANOVA with post hoc Bonferroni's analysis) (Fig. 6H). The level of pressure sensation similarly decreased (i.e., increased withdrawal force) immediately following dorsal root crush. However, the recovery of pressure sensation was much slower, such that meaningful values were only obtained from 2 weeks after surgery onward. No difference was recorded in the recovery of pressure sensation between animals in the two groups up to 4 weeks after surgery (p = 0.1874; F = 1.796; two-way ANOVA with post hoc Bonferroni's analysis) (Fig. 6I). At 5 and 6 weeks, however, kindlin-1, but not mCherry, animals appeared to have recovered more pressure sensation, although this difference was not statistically significant (at 5 weeks, p = 0.17; at 6 weeks, p = 0.22; Student's t test) (Fig. 6I).
Discussion
Kindlin-2, but not kindlin-1, is expressed by neurons
Kindlin-1 is absent in the nervous system. Kindlin-2, meanwhile, is ubiquitously expressed in all tissues, but studies on its functions have so far been primarily limited to embryonic development, muscle formation, and carcinogenesis (Ussar et al., 2006; Lai-Cheong et al., 2010), with only one study reporting nervous system abnormalities in kindlin-2-deficient zebrafish (Dowling et al., 2008a). Although kindlin-2 mRNA has been detected in the brain, the precise expression location has not been explored (Ussar et al., 2006). Here, we examined kindlin expression in neurons and glia in the PNS and CNS. In DRG cultures treated with antimitotic drugs to remove non-neuronal cells, RT-PCR demonstrated kindlin-2 transcript in the neurons. In addition, immunocytochemistry on cultured DRG neurons, retinal ganglion cells, hippocampal neurons, and cerebellar Purkinje neurons all demonstrated that kindlin-2 is expressed by neurons, as well as by non-neuronal cells including fibroblasts, Schwann cells, and astrocytes. In these in vitro studies, kindlin-2 protein was detected in all parts of the neuron, including the cell body, dendrites, and axons, reaching their distal tips. Endogenously expressed kindlin-2 is therefore present in growth cones where it can associate with integrins to affect axon growth. We also searched for kindlin-1 in neurons and glia, confirming that it is absent in the nervous system (Ussar et al., 2006).
Kindlin-1 expression enhances integrin activation and signaling
Kindlins have been shown to participate in integrin activation in collaboration with talin (which is also expressed by DRG neurons). Expression of kindlin-1 in DRG neurons increased the proportion of axonal integrin in the activated conformation, and also increased integrin signaling as demonstrated by the phosphorylation of FAK. These effects were greater when the axons were cultured on a substrate that contained a mixture of the CSPG aggrecan (representing inhibitory CSPGs upregulated at sites of CNS damage) and the integrin ligand laminin. Aggrecan inhibits integrin-mediated axon growth through inactivation of integrins, so the ability of kindlin-1 overexpression to overcome this inhibition is of significance for axon regeneration in the damaged CNS. Meanwhile, kindlin-2 overexpression did not affect integrin activation or signaling.
Kindlin is necessary for axon growth
We observed that shRNA-mediated knockdown of kindlin-2 greatly impaired axon growth from DRG neurons. Similar effects on other integrin-mediated processes had been observed following depletion of kindlin-2 [e.g., in mouse myoblasts, which failed to spread or expand normally during myocyte elongation; or in mouse ESCs, which adhered poorly to laminin and fibronectin (Dowling et al., 2008b; Montanez et al., 2008)]. The reduction of pY397 FAK levels in kindlin-2 shRNA-transfected axons is also consistent with data showing that kindlin-2 knockdown in murine ESCs or αIIbβ3-expressing CHO cells suppressed activation of integrin (Montanez et al., 2008; Ussar et al., 2008). Interestingly, we were able to replace the function of endogenous kindlin-2 through transfection of kindlin-1.
Overexpression of kindlin-1 and -2 also had different effects on axon growth, consistent with their effects on integrin activation and signaling. Kindlin-1 expression enhanced axon growth, and enabled the axons to overcome the inhibitory effect of aggrecan on mixed aggrecan–laminin substrate. Kindlin-2 overexpression, however, failed to promote axon growth on both laminin and aggrecan–laminin substrates.
Differential effects of kindlin-1 and -2
Our results show that kindlin-1 and -2 are not completely equivalent in DRG neurons. This is somewhat unexpected, since the molecules were thought to be interchangeable, and we show that kindlin-1 can partially compensate for the function of kindlin-2 when it is knocked down. Previous experiments have reported an increase of integrin activation following coexpression of kindlin-2 and talin head into αIIbβ3 integrin-expressing CHO cells (Shi et al., 2007; Ma et al., 2008; Montanez et al., 2008). However, work in which kindlin-2 had been overexpressed without coexpression of the talin head did not produce consistent results, with some groups reporting a small, but statistically significant elevation in integrin activation after kindlin-2 transfection, while others saw no effect (Dowling et al., 2008a; Montanez et al., 2008). In our work, it may be that the basal content of kindlin-2 in DRG neurons is high enough to give maximal integrin activation and signaling in axons, such that introduction of additional kindlin-2 has no further impact.
In contrast, we found that kindlin-1 expression in DRG neurons promoted integrin activation, signaling, and axon growth when cultured on aggrecan–laminin. In addition to promoting axon growth, kindlin-1-transfected neurons had many short, actin-containing branches arising from the axons, mirroring the localization of endogenous kindlin-1 around the cell periphery, cell–cell junctions, and focal adhesions (Siegel et al., 2003; Herz et al., 2006; Ussar et al., 2006; Lai-Cheong et al., 2008). The fact that kindlin-1 expression only enhances axon growth in the presence of inhibitory aggrecan is consistent with the idea that aggrecan impairs integrin signaling, which can be restored by integrin activation (Tan et al., 2011). This is important in axon regeneration, since CSPG upregulation usually follows a CNS injury (Jaworski et al., 1999; McKeon et al., 1999; Asher et al., 2000; Afshari et al., 2010).
The reason for the different effects produced by kindlin-1 and kindlin-2 transfection is unclear. Structurally, the amino acid sequences share 62% homology, including the conserved phosphotyrosine (PTB) site, through which they interact with the cytoplasmic tail of β integrin (Ussar et al., 2006; Moser et al., 2009; Lai-Cheong et al., 2010). Presumably, there is a difference between the two isoforms, in three-dimensional conformation, interaction, or function. Patients lacking functional kindlin-1 suffer from Kindler syndrome, despite having normal kindlin-2 expression (Kindler, 1954; Ussar et al., 2006). In keratinocytes, both kindlin-1 and kindlin-2 colocalize with focal adhesions; but upon calcium-induced differentiation, the colocalization of kindlin-1, but not kindlin-2, is lost (Ussar et al., 2006). Collectively, these results suggest that kindlin-1 and kindlin-2 serve specific, nonredundant functions.
Promotion of axon regeneration and sensory recovery following kindlin-1 expression in vivo
Having demonstrated that kindlin-1 expression enables axons to regenerate in the presence of an inhibitory CSPG, we asked whether it would also be possible to promote axon regeneration in vivo. We therefore used an AAV2-kindlin-1 construct to transduce DRG neurons in adult rats with crush lesion of the cervical dorsal root. We found that animals in the kindlin-1 group exhibited a higher number of axons crossing the DREZ and markedly more growing into the spinal cord, compared with those in the control group. In addition, kindlin-1-treated animals showed faster sensory recovery. These results are consistent with several earlier reports that integrin activation could enhance axon growth from neurons in vitro, even when cultured in the presence of inhibitory molecules such as amino-Nogo or aggrecan (Ivins et al., 2000; Lein et al., 2000; Hu and Strittmatter, 2008; Tan et al., 2011), and also with our demonstration that expression of a tenascin-binding integrin can promote axon regeneration (Andrews et al., 2009). CNS injuries cause an upregulation of myelin-related inhibitors (e.g., Nogo, oligodendrocyte myelin glycoprotein, myelin-associated glycoprotein) (McKerracher et al., 1994; Mukhopadhyay et al., 1994; GrandPré et al., 2000, 2002; Prinjha et al., 2000; Kottis et al., 2002; Wang et al., 2002) and CSPGs (e.g., aggrecan, brevican, phosphacan, versican), which impair regenerative responses (Jaworski et al., 1999; McKeon et al., 1999; Asher et al., 2000; Afshari et al., 2010). Our experiment suggests that integrin activation might be a general method to overcome these inhibitory effects and promote axon regeneration in vivo.
As well as ensuring activation of integrins, it is also critical that axons express compatible integrins that recognize matrix molecules around them. We previously sought to ensure this by expressing α9 integrin, which recognizes tenascin-C upregulated in CNS injuries, into DRG neurons following dorsal root crush and a dorsal column crush lesion. This strategy was very successful with PC12 cells and DRG in vitro but virus-mediated transduction of neurons in vivo only modestly improved axon regeneration, to a lesser extent than in the present experiments (Andrews et al., 2009). This result, together with those discussed above, suggest that Nogo-A and CSPGs in the injury site would have inactivated the α9 integrin transduced into sensory axons in our previous experiment (Hu and Strittmatter, 2008; Andrews et al., 2009; Tan et al., 2011). In the present experiments, the integrins expressed by DRG neurons, although activated, were not well matched to the molecules in the environment surrounding their cut axons. A combination of the two strategies (i.e., expression of an appropriate integrin as well as ensuring its proper function by forced activation) may therefore be necessary for successful induction of really prolific CNS axon regeneration using an integrin strategy.
Most studies have highlighted the role of kindlin-1 in integrin activation (Ussar et al., 2008; Harburger et al., 2009; Plow et al., 2009), but it is by no means the only mechanism through which kindlin-1 acts. Kindlin-1 also interacts with focal adhesion-associated proteins such as α-actinin, FAK, migfilin, and ILK, to support lamellipodium formation and regulate cell shape and migration (Tu et al., 2003; Has et al., 2009; Lai-Cheong et al., 2010). Nevertheless, it is probable that the effects we saw were mostly due to integrin activation, because the enhanced axon growth in vitro following kindlin-1 expression was accompanied by an increase in integrin activation and integrin signaling, although we cannot rule out other effects.
In conclusion, our results demonstrated that kindlin is necessary for normal integrin-dependent axon growth from sensory neurons. Overexpression of kindlin-1, but not kindlin-2, promoted axon growth in the presence of inhibitory aggrecan and produced promising enhancement of axon regeneration and sensory recovery in the spinal cord. Kindlin may therefore be a useful component of an integrin-based approach to the treatment of nervous system injuries.
Footnotes
This work was supported by grants from the Medical Research Council, The Henry Smith Charity, The John and Lucille van Geest Foundation, the European Union Framework 6 Network of Excellence NeuroNE, the European Union Framework 7 Programmes Spinal Cord Repair and Plasticise, and the National Institute for Health Research Cambridge Biomedical Research Centre. We also thank Fardad Afshari for his assistance.
The authors declare no competing financial interests.
References
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 2010;58:857–869. doi: 10.1002/glia.20970. [DOI] [PubMed] [Google Scholar]
- Andrews MR, Czvitkovich S, Dassie E, Vogelaar CF, Faissner A, Blits B, Gage FH, ffrench-Constant C, Fawcett JW. α9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci. 2009;29:5546–5557. doi: 10.1523/JNEUROSCI.0759-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Gibbs E, Russell M, Goldman D, Minarcik J, Golden JA, Feldman EL. Kindlin-2 is an essential component of intercalated discs and is required for vertebrate cardiac structure and function. Circ Res. 2008a;102:423–431. doi: 10.1161/CIRCRESAHA.107.161489. [DOI] [PubMed] [Google Scholar]
- Dowling JJ, Vreede AP, Kim S, Golden J, Feldman EL. Kindlin-2 is required for myocyte elongation and is essential for myogenesis. BMC Cell Biol. 2008b;9:36. doi: 10.1186/1471-2121-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Makino A, Hirabayashi Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res Brain Res Protoc. 1998;3:192–198. doi: 10.1016/s1385-299x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J Peripher Nerv Syst. 2007;12:11–27. doi: 10.1111/j.1529-8027.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- GrandPré T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- GrandPré T, Li S, Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Harburger DS, Bouaouina M, Calderwood DA. Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects. J Biol Chem. 2009;284:11485–11497. doi: 10.1074/jbc.M809233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, Herz C, Zimina E, Qu HY, He Y, Zhang ZG, Wen TT, Gache Y, Aumailley M, Bruckner-Tuderman L. Kindlin-1 Is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am J Pathol. 2009;175:1442–1452. doi: 10.2353/ajpath.2009.090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz C, Aumailley M, Schulte C, Schlötzer-Schrehardt U, Bruckner-Tuderman L, Has C. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J Biol Chem. 2006;281:36082–36090. doi: 10.1074/jbc.M606259200. [DOI] [PubMed] [Google Scholar]
- Hu F, Strittmatter SM. The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci. 2008;28:1262–1269. doi: 10.1523/JNEUROSCI.1068-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ivins JK, Yurchenco PD, Lander AD. Regulation of neurite outgrowth by integrin activation. J Neurosci. 2000;20:6551–6560. doi: 10.1523/JNEUROSCI.20-17-06551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Kelly GM, Hockfield S. Intracranial injury acutely induces the expression of the secreted isoform of the CNS-specific hyaluronan-binding protein BEHAB/brevican. Exp Neurol. 1999;157:327–337. doi: 10.1006/exnr.1999.7062. [DOI] [PubMed] [Google Scholar]
- Kindler T. Congenital poikiloderma with traumatic bulla formation and progressive cutaneous atrophy. Br J Dermatol. 1954;66:104–111. doi: 10.1111/j.1365-2133.1954.tb12598.x. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, Braun PE. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Lai-Cheong JE, Ussar S, Arita K, Hart IR, McGrath JA. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J Invest Dermatol. 2008;128:2156–2165. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Parsons M, Tanaka A, Ussar S, South AP, Gomathy S, Mee JB, Barbaroux JB, Techanukul T, Almaani N, Clements SE, Hart IR, McGrath JA. Loss-of-function FERMT1 mutations in kindler syndrome implicate a role for fermitin family homolog-1 in integrin activation. Am J Pathol. 2009;175:1431–1441. doi: 10.2353/ajpath.2009.081154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Cheong JE, Parsons M, McGrath JA. The role of kindlins in cell biology and relevance to human disease. Int J Biochem Cell Biol. 2010;42:595–603. doi: 10.1016/j.biocel.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Lein P, Gallagher PJ, Amodeo J, Howie H, Roth JA. Manganese induces neurite outgrowth in PC12 cells via upregulation of alpha(v) integrins. Brain Res. 2000;885:220–230. doi: 10.1016/s0006-8993(00)02943-7. [DOI] [PubMed] [Google Scholar]
- Lemons ML, Condic ML. Combined integrin activation and intracellular cAMP cause Rho GTPase dependent growth cone collapse on laminin-1. Exp Neurol. 2006;202:324–335. doi: 10.1016/j.expneurol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Lemons ML, Condic ML. Integrin signaling is integral to regeneration. Exp Neurol. 2008;209:343–352. doi: 10.1016/j.expneurol.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO, Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–446. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marland JR, Pan D, Buttery PC. Rac GTPase-activating protein (Rac GAP) α1-Chimaerin undergoes proteasomal degradation and is stabilized by diacylglycerol signaling in neurons. J Biol Chem. 2011;286:199–207. doi: 10.1074/jbc.M110.166728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, Li J, Breese GR, Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Montanez E, Ussar S, Schifferer M, Bösl M, Zent R, Moser M, Fässler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Legate KR, Zent R, Fässler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Plow EF, Qin J, Byzova T. Kindling the flame of integrin activation and function with kindlins. Curr Opin Hematol. 2009;16:323–328. doi: 10.1097/MOH.0b013e32832ea389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282:20455–20466. doi: 10.1074/jbc.M611680200. [DOI] [PubMed] [Google Scholar]
- Siegel DH, Ashton GH, Penagos HG, Lee JV, Feiler HS, Wilhelmsen KC, South AP, Smith FJ, Prescott AR, Wessagowit V, Oyama N, Akiyama M, Al Aboud D, Al Aboud K, Al Githami A, Al Hawsawi K, Al Ismaily A, Al-Suwaid R, Atherton DJ, Caputo R, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin-extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am J Hum Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- Ussar S, Wang HV, Linder S, Fässler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Exp Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Ussar S, Moser M, Widmaier M, Rognoni E, Harrer C, Genzel-Boroviczeny O, Fässler R. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]


