Abstract
The direct effects of the nucleoside transporter inhibitor dilazep on the cell cycle of mesangial cells have not before been investigated. The purpose of this study was to elucidate whether dilazep can inhibit the proliferation of mesangial cells and how it interferes with the cell cycle of these cells. DNA histograms were used and BrdUrd uptake rate was measured by flow cytometry. There was no significant difference in the cell numbers among the untreated group and the 10−5M, 10−6M or 10−7M dilazep‐treated groups at 24 h of incubation. However, at 48 and 72 h, the cell numbers in the dilazep‐treated groups were significantly lower compared with that of the untreated group (P0.005). The DNA histograms of cultured rat mesangial cells at 12, 24, and 48 h of incubation with 10−5 M dilazep showed that the ratio of the S phase population in the dilazep‐treated group decreased by 2.2% at 12 h, by 9.6% at 24 h, and by 18.9% at 48 h compared with the untreated group. The ratio of the G0/G1 phase population in the dilazep‐treated group significantly increased: 6.8% at 12h (P 0.05), 13.9% at 24 h (P 0.001), and 76.5% at 48 h (P 0.001) compared with the untreated group. A flow cytometric measurement of bivariate DNA/BrdUrd distribution demonstrated that the DNA synthesis rate in the S phase decreased after 6 h (P 0.005) and 12 h (P 0.05) of incubation compared with the untreated group. These results suggest that dilazep inhibits the proliferation of cultured rat mesangial cells by suppressing the G1/S transition by prolonging G2/M and through decreasing the DNA synthesis rate
Introduction
Dilazep, an antiplatelet agent, is generally used as an antithrombotic drug in clinical practice ( Inage et al. 1985 , Maruhama et al. 1985 , Tajiri et al. 1989 , Nakazawa et al. 1993 , Yamamoto et al. 1995 ). Dilazep is also known to exert cytoprotective and antioxidant effects in some ischaemic heart models ( Tanabe et al. 1992 , Hogue et al. 1996 , Hara & Abiko 1996). In Japan, dilazep is commonly used clinically for patients with chronic glomerulonephritis and is recognized as preventing proteinuria and the progression of glomerulosclerosis ( Inage et al. 1985 , Maruhama et al. 1985 , Tajiri et al. 1989 , Yoshida et al. 1994 , Koide et al. 1995 ). However, only a few mechanisms have been proposed for the effects of dilazep on diseases of the kidney. These include (a) suppression of the decrease in anionic charge in the glomerular basement membrane ( Yamamoto et al. 1995 ), and (b) suppression of platelet adhesion and coagulation and the maintenance of the glomerular filtration rate as a result of increased renal blood flow ( Nagase et al. 1985 , Yasunaga et al. 1988 ). The former can improve proteinuria and the latter should suppress mesangial cell proliferation by inhibiting platelet‐derived growth factor released from platelets ( Iida et al. 1988 , Silver, Jaffer & Abboud 1989, Floege et al. 1992 ). Mesangial cell proliferation is believed to play an important role in pathogenesis of some glomerular diseases and might lead to glomerulosclerosis. Therefore, a relationship between dilazep and the suppression of mesangial cell growth is plausible as an explanation for the therapeutic effects, and thus needed to be studied more in order to develop better protocols to treat chronic glomerulonephritis. However, the direct effects of dilazep on mesangial cell proliferation have not been clearly demonstrated previously.
The purpose of this study was to evaluate whether dilazep can directly inhibit the proliferation of mesangial cells and, if so, how it affects the cell cycle of mesangial cells. Flow cytometric techniques were thus used as a sophisticated method to assess cell cycle progression.
MATERIALS and METHODS
The experimental protocols used in this study were approved by the Ethics Committee for Animal Experimentation at Yamaguchi University School of Medicine, and carried out according to the Guidelines for Animal Experimentation at Yamaguchi University School of Medicine, and The Law (No. 105) and Notification (No. 6) of the Japanese Government.
Preparation of mesangial cells
Rat mesangial cells were isolated by the methods described by Kawata et al. (1998) and Harper et al. (1984) . Briefly, 6‐week‐old male Wistar rats (150–210 g; Charles River, Kanagawa, Japan) were anaesthetized with diethylether and killed with a guillotine. The kidneys were dissected, and renal cortical tissues were minced with scissors under sterile conditions. The minced renal cortical tissues were passed through a series of sieves of 0.3, 0.15, and 0.075 mm pore size (Iida Seisakusho, Osaka, Japan). Trapped glomeruli were rinsed in calciummagnesium‐free Hanks' balanced salt solution, pH 7.4. and incubated with 0.2% trypsin for 20 min at 37^C. After being washed twice in Hanks' balanced salt solution, glomeruli were resuspended in RPMI 1640 (Cosmo Bio, Tokyo, Japan) with 100 U/ml penicillin, 100 μg/ml streptomycin, and 20% bovine fetal calf serum (FCS; Upstate Biotechnology, Lake Placid, NY, USA), plated at a density of 5 × 103 glomeruli per 60 mm plastic dish, and incubated at 37^C in a humidified atmosphere of 5% CO2−95% air. After 2–3 weeks, outgrowing cells were passed to subcultures and maintained in RPMI 1640 with 20% FCS. Cells were identified as mesangial cells by their growth pattern, morphological features and positive staining for Thy 1.1, α‐actin, and myosin and negative staining for cytokeratin and factor VIII. Experiments were carried out using cells from the 10th through 15th passages. All dishes used were made by Falcon (Becton Dickinson Labware, Lincoln Park, NJ, USA). Rat mesangial cells were plated at a density of 5 × 104 cells per dish in 35 mm dishes, at a density of 1 × 104 cells per dish in 60 mm culture dishes and at a density of 5 × 105 cells per dish in 100 mm culture dishes. Thereafter, the cells were incubated in the RPMI 1640 medium with 10% FCS for 36 h. When the cells were growing exponentially, the medium was changed to fresh medium with or without dilazep at a final concentration of 10−4−10−7 M in addition 10% FCS. The cells in the 35 mm dishes were used for direct cell counting. and those in the 60 mm and 100 mm dishes were used for flow cytometry. There were no significant differences in cell size or cell shape of the mesangial cells or in the staining of cells for Thy 1.1, α‐actin and myosin between the non‐treated and dilazep‐treated groups as assessed by phase‐contrast light microscopy, as previously reported ( Kawata et al. 1998 ).
Cell counting
After the RPMI 1640 medium with 10% FCS was removed from the culture dishes, 0.3 ml 0.25% EDTA and 2.7 ml 0.2% trypsin were added to each dish for several minutes. After confirming the removal of all cells, the samples were mildly agitated to obtain a homogeneous suspension. One millilitre of an aliquot was used to count the cell numbers with a Coulter Counter (Coulter Electronics, Hialeah, FL, USA).
Flow cytometry
DNA histogram
To investigate the effects of the drug on cell cycle progression, the DNA content of mesangial cells treated with dilazep was measured various times and compared the DNA histograms with those of the untreated group. For the measurement of DNA content, after incubation for 36 h with RPMI 1640 supplemented with 10% FCS, the exponentially growing mesangial cells were incubated with or without dilazep in a medium with 10% FCS, and the cells were then collected at 6, 12, 24, and 48 h. After being washed with phosphate‐buffered saline (PBS), the cells were trypsinized and resuspended in 1 ml PBS and fixed in 4 ml 100% ethanol at 4^C. DNA staining was achieved by resuspending the cells in PBS containing propidium iodide (50 μg/ml) and 0.1% RNase A (Sigma, St Louis, MO, USA) and 30 min later these cell suspensions were applied to a flowcytometer (FACScan, Becton Dickinson, Sunnyvale, CA, USA) and DNA histograms were obtained. The histograms were analysed by computer (Hewlett‐Packard Model 310) using software (CellFITTM SOFTWARE, Becton Dickinson) equipped with FACScan.
Bivariate DNA/BrdUrd distribution
For measurement of the DNA synthesis rates of the S phase cells treated with dilazep, cells were incubated with 10 μM BrdUrd (Sigma) for 30 min at each time point after exposure to the dilazep. After being washed with PBS, cells were trypsinized and resuspended in 1 ml PBS and fixed in 4 ml of 100% ethanol at 4^C. BrdUrd incorporation was detected with a minor modification of the method described by Dolbeare et al. (1983) . For DNA denaturation, the fixed cells were treated with 1 ml 4 N HCl at room temperature for 30 min. Then the cells were washed with 9 ml boraxborate buffer (pH 8.9) and centrifuged at 260g. After removing the supernatant, the cells were washed twice with PBS. BrdUrd and DNA staining were carried out by a standard two‐step procedure. Anti‐BrdUrd monoclonal antibody (Becton Dickinson) and fluorescein isothiocyanate (FITC)‐conjugated goat F(ab′)2 anti‐mouse IgG (Caltag Laboratories, CA, USA) as a secondary antibody were used as primary and secondary antibodies, respectively, at a dilution of 1 : 50 in 1 ml PBS containing 0.5% Tween‐20, 0.5% bovine serum albumin and 10% normal goat serum, for 30 min at room temperature. DNA staining was achieved with 0.5 ml propidium iodide (30 μg/ml) after treatment with 0.1% RNase A to remove double‐stranded RNA. The samples were filtered with nylon mesh to exclude aggregated cells and were measured by flow cytometry (FACScan, Becton Dickinson). At least 1 × 104 cells were measured for each sample. The mean FITC‐fluorescence intensity of BrdUrd incorporation of S phase cells was measured by gate analysis with CellFITTM SOFTWARE (Becton Dickinson).
Statistical analysis
The results are expressed as mean ± SEM and statistical significance was assessed by an analysis of variance followed by Fisher's multiple comparisons tests. Values of P 0.05 were considered significant.
Results
Effects of dilazep on mesangial cells
In the 10−4 M dilazep group, a considerable number of cultured rat mesangial cells floated and were stained with trypan blue. Therefore, 10−4 M dilazep was not used in the experiments because of the cytocidal effect at this concentration. For the other concentrations of dilazep (10−5 M, 10−6 M and 10−7 M), the trypan blue exclusion test showed that more than 90% of mesangial cells were viable throughout the experiments. The effects of dilazep on the exponentially growing mesangial cells are shown in Fig. 1. The cell proliferation of the untreated group under 10% FCS showed an almost exponential growth pattern during the experiment. Mesangial cell growth was in an exponential pattern between 0–48 h, however, from 48–72 h some inhibition to cell growth was recognized without contact inhibition. There was no significant difference in cell numbers between the untreated group and the 10−5 M, 10−6 M and 10−7 M dilazep groups at 24 h. However, at 48 and 72 h of incubation, the cell numbers of the 10−5 M, 10−6 M and 10−7 M dilazep groups were significantly suppressed, in a dose‐dependent manner, compared with those of the untreated group (P 0.005).
Figure 1(a).
The effect of dilazep on growing rat mesangial cells. Cell proliferation of the untreated group with 10% FCS was nearly exponential. There was no significant difference in cell numbers between the untreated group and the 10−5 M, 10−6 M and 10−7 M dilazep groups at 24 h. However, at 48 and 72 h, the cell numbers of the 10−5 M, 10−6 M, and 10−7 M dilazep groups were significantly suppressed, in a dose‐dependent manner, compared with the untreated group. Number of experiments = 9. Data are mean ± SEM. *P 0.0001, **P 0.005. (b) The effect of dilazep on the cell proliferation of cultured rat mesangial cells at given concentrations. Rat mesangial cell crowth was exponential between 0 and 48 h. In addition, cultured rat mesangial cell growth was suppressed from 24 to 72 h in a dose‐dependent manner. However, from 48 to 72 h some inhibition to cell growth was seen without contact inhibition.
DNA histograms
To clarify the inhibitory mechanisms of dilazep for mesangial cell proliferation, the effects of dilazep on cell cycle progression were examined by analysing the change in DNA histogram patterns. Figure 2 shows a set of representative DNA histograms of cells treated with 10−5 M dilazep at 0, 12, 24 and 48 h and Table 1 summarizes cells at each phase of the cell cycle for mesangial cells with or without dilazep. The data shows that the ratio of the G0/G1 phase population in the dilazep‐treated group was significantly increased at 12 h (6.8%, P 0.05), at 24 h (13.9%, P 0.001) and at 48 h (17.4%, P 0.001), and that the ratio of the S phase cells decreased significantly 2.2% at 12 h, 9.6% at 24 h, and 13.1% at 48 h (P 0.001) compared to the untreated group.
Figure 2(D).
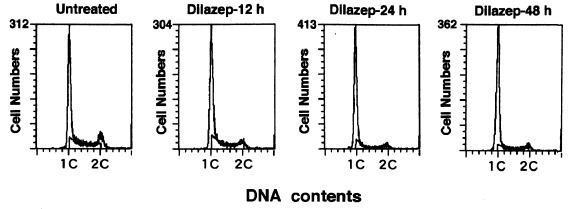
NA histograms of rat mesangial cells incubated for 12, 24, and 48 h with or without 10−5 M dilazep. After pre‐incubation for 36 h with 10% FCS to induce exponential growth, the dilazep‐treated groups were incubated with 10−5 M dilazep for 12, 24 and 48 h, with the untreated group used as a control. Dilazep‐12 h, 24 h and 48 h indicates a dilazep group treated with 105 M dilazep for 12, 24, and 48 h, respectively. The S regions as calculated by CelIFITTM SOFTWARE are shown in each histogram
Table 1.
Proportion of each cell cycle phase of cultured rat mesangial cells at a time of 12, 24, and 48 h of incubation in untreated and dilazep‐treated groups

BrdUrd incorporation rate at the S phase in cultured mesangial cells
For a further analysis of the effects of dilazep on the cell cycle of mesangial cells, BrdUr incorporation, which indicates DNA synthesis rate, was measured simultaneously with DNA content by flow cytometry for the cells treated with or without 10−5 M dilazep. Figure 3 shows representative bivariate DNA/BrdUrd distributions of the cells pulse‐labelled with BrdUrd at 12 h. This demonstrates that the DNA synthesis rate in the S phase was decreased in the dilazep‐treated group compared with the untreated group at 12 h.
Figure 3(R).

epresentative bivariate DNA/BrdUrd distribution of cultured rat mesangial cells with or without 10−5 M dilazep. The bivariate DNA/BrdUrd distribution was measured in untreated or dilazep‐treated rat mesangial cells after pulse labelling with 10 μM BrdUrd for 30 min at 12 h of incubation with 10−5 M dilazep. Dot plots show the BrdUrd content (y axis) and DNA content (x axis). After treatment with dilazep, the mean fluorescence intensity at the S phase, which is proportional to the DNA synthesis rate, was substantially lower in the 10−5 M dilazep‐treated group than in the untreated group.
Figure 4 summarizes the mean FITC‐fluorescence intensity (the mean BrdUrd content incorporated) in the S phase cells with or without dilazep at 6 and 12 h. The fluorescences were significantly decreased at both 6 h (P 0.005) and 12 h (P 0.05) in the dilazep‐treated group compared with the untreated group. However, there was no statistical difference in the mean fluorescence intensifies between 6 and 12 h‐untreated groups.
Figure 4(T).

he mean fluorescence intensifies during the S phase of cultured rat mesangial cells at 6 and 12 h of the incubation with 10−5 M dilazep. The mean fluorescence intensity in the S phase of cultured rat mesangial cells treated with 105 m dilazep was significantly lower than that of the untreated group at both 6 and 12 h of incubation with dilazep. There was no difference in the mean fluorescence intensifies between the 6 and 12 h‐untreated groups. **P 0.0005, *P 0.001, n = 6. Data are mean ±SEM.
Discussion
In this study, dilazep was demonstrated to inhibit directly the proliferation of mesangial cells in vitro. As shown in Fig. 1(b), mesangial cell growth was exponential between 0 and 48 h, however, and from 48 to 72 h some inhibition of cell growth was recognized without contact inhibition (since it was observed that growing mesangial cells had not reached the margin of the dish at 72 h). In the analysis of DNA histograms shown in Fig. 2 and Table 1, the S phase ratio of the dilazep‐treated group significantly decreased in a time‐dependent manner compared with the untreated mesangial cells growing exponentially. In contrast, the G1 phase ratios of the dilazep‐treated group significantly increased in a time‐dependent manner compared with the untreated group. These results indicate that dilazep inhibits DNA synthesis of the cell population through the inhibition of the G1/S transition and/or elongation of the duration of the G1 phase. In addition, G2/M peaks were observed in the histograms at 12 and 24 h as shown in Fig. 2, suggesting that the duration of G2/M becomes longer. Furthermore, the BrdUrd incorporation analysis showed that the mean fluorescence intensities during the S phase of cultured rat mesangial cells were significantly decreased in the dilazep‐treated group compared with the untreated group ( Fig. 4), indicating that dilazep inhibits DNA synthesis in rat mesangial S phase cells even with 10% FCS in vitro.
These results taken together show the length of G1, S and G2/M became longer with dilazep under these experimental conditions, suggesting that dilazep may inhibit the transit time in all the cell cycle phases before to 24 h. However, the results of the present cell growth experiment at 24 h showed no significant inhibitory effects of dilazep on cell growth at 24 h, but significant inhibitory effects of dilazep were observed on cell growth at 48 h, suggesting that the inhibitory effects of dilazep on cell growth take much longer than the inhibitory effects on the transit time of the cell cycle. Based upon the results in Table 1, a decrease in cell numbers of the dilazep‐treated group was not observed at 24 h compared with that of the 24‐h control. The inhibition of G1/S transition and DNA synthesis was observed within 12 h with dilazep ( Table 1 and 3(R), 4(T)), however, the inhibition of the S phase was not observed ( Table 1), suggesting that the delay in the inhibition of the S phase might contribute to the lack of change in cell numbers at 24 h in the dilazep‐treated group.
Dilazep is known in general as an agent that inhibits nucleoside transporters such as nitrobenzylthioinosine and dipyridamole ( Young & Jarvis 1983, Cass 1995, Griffiths et al. 1997 ). Furthermore, the effects of dilazep on rat mesangial cell proliferation seen in the present study were significant but mild, and it seems that dilazep might have weaker effect of cell growth suppression than anticancer drugs. Tamoxifen, an anticancer drug, was also reported to have the inhibitory effect on nucleoside transport and has an antiproliferation effect through the inhibition of DNA synthesis by blocking the DNA salvage pathway ( Cai & Lee 1996). It is also reported that dilazep has an inhibitory effect on nucleoside uptake in baby hamster kidney cells ( Plagemann & Woffendin 1988, Baer et al. 1991 ), indicating the possibility that dilazep might suppress DNA synthesis by inhibiting a nucleoside transporter. In the analysis of the DNA synthesis rate by BrdUrd incorporation ( 3(R), 4(T)), the DNA synthesis rate of the dilazep‐treated group was suppressed. These results are in agreement with those reports mentioned above.
Nueleosides should be necessary for RNA synthesis. Dilazep may affect the RNA synthesis of cultured rat mesangial cells. Murakami et al. (1995) reported that the inhibition of RNA synthesis by the anticancer drug. 5‐azacytidine, slowed the progression through the S phase of HL60 cells, suggesting that inhibition of RNA synthesis might be also involved in the inhibition of rat cultured mesangial cells with dilazep.
It has been reported that dilazep also has an adenosine potentiation effect by inhibiting the uptake of extracellular adenosine induced in an anaerobic condition such as myocardial ischaemia and adenosine has been shown to stimulate DNA synthesis in endothelial cells ( Ethier & Dobson 1997). However, it has also been reported that adenosine triphosphate accelerated mesangial cell growth through an activation of mitogen‐activated protein kinase and an increase in [3H]thymidine incorporation in a dose‐dependent manner, whereas adenosine had no such effects ( Ishikawa et al. 1994 ). There is, thus, the possibility that adenosine induced by dilazep, under anaerobic conditions, modulates mesangial cell proliferation. However, under the present experimental conditions, 10% FCS medium under aerobic conditions was used, in which less adenosine might be induced ( Belle, Goossens & Wynant 1987, Ishibashi, Hara & Abiko 1987). Thus, the effects of adenosine on mesangial cells may be negligible in these experiments.
In conclusion, taken together with the data of DNA histograms and the BrdUrd incorporation experiments in the study, direct inhibitory effects of dilazep were observed on cultured rat mesangial cell proliferation via the suppression of G1/S transition and DNA synthesis in the S phase cells. Further studies are necessary to clarify the mechanisms of dilazep inhibition on the cell cycle and cell growth in cultured rat mesangial cells and to develop better protocols to treat chronic glomerulonephritis.
Acknowledgements
The authors would like to thank Miss Rie Ishihara for her excellent technical assistance.
References
- Baer, HP , Serigese, V , Ogbunude, POJ , Moorji, A. 1991. Miofiazine derivatives: their potential as in vivo nucleoside transport inhibitors and components of antiparasitic combination treatment In: Imai S. Nakazawa M, eds. Role of Adenosine and Adenosine Nucleosides in the Biological System . Amsterdam, Elsevier Science Publishers 161, [Google Scholar]
- Belle, VH , Goossens, F , Wynant, J. 1987. Formation and release of purine cataborites during hypoperfusion, anoxia, and ischemia. Am. J. Physiol., 252, H886. [DOI] [PubMed] [Google Scholar]
- Cai, J & Lee, CW. 1996. Tamoxifen inhibits nitrobenzylthioinosine‐sensitive equilibrative uridine transport in human MCF‐7 breast cancer cells. Biochem. J., 320, 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass, CE. 1995. Nucleoside transport In: Georgopapadakou NH., ed. Drug Transport in Antimicrobial and Anticancer Chemotherapy . New York, Marcel Dekker 403. [Google Scholar]
- Dolbeare, F , Gratzner, H , Pallavicini, MG , Gray, JW. 1983. Flow cytometric measurement of total RNA content and incorporated bromodeoxyuridine. Proc. Natl. Acad. Sci. USA., 80, 5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier, MF & Dobson, JG Jr 1997. Adenosine stimulation of DNA synthesis in human endothelial cells. Am. J. Physiol., 202, H1470. [DOI] [PubMed] [Google Scholar]
- Floege, J , Bupns, MW , Alpers, C et al. 1992. Glomerular cell proliferation and PDGF expression precede glomerulonephritis in the remnant kidney model. Kidney Internl., 41, 279. [DOI] [PubMed] [Google Scholar]
- Griffiths, M , Beaumout, N , Yao, SYM et al. 1997. Cloning of a human nucleoside transporter implicated in the cellular uptake of adenosine and chemotherapeutic drugs. Nature Med., 3, 89. [DOI] [PubMed] [Google Scholar]
- Hara, A & Abiko, Y. 1996. Protective effects of dilazep and its novel derivative, K‐7259, on mechanical and metabolic derangement induced by hydrogen peroxide in the isolated perfused rat heart. J. Pharmacol. Exp. Ther., 227, 565. [PubMed] [Google Scholar]
- Harper, PA , Robinson, JM , Hoover, RL , Wright, TC , Kapnosky, MJ. 1984. Improved methods for culturing rat glomerular cells. Kidney Int., 26, 875. [DOI] [PubMed] [Google Scholar]
- Hogue, N , Hogue, ANE , Hashizume, H , Ichihara, K , Abiko, Y. 1996. K‐7259, a novel dilazep derivative, and d‐propranolol attenuate H202‐induced cell damage. J. Pharmacol. Exp. Ther., 277, 207. [PubMed] [Google Scholar]
- Iida, H , Seifert, R , Alpers, CE et al. 1988. Platelet‐derived growth factor (PDGF) and PDGF receptor are induced mesangial proliferative nephritis in the rat. Proc. Natl. Acad. Sci. USA., 88, 6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inage, H , Koyama, A , Narita, M , Tojo, S. 1985. The effects of the antiplatelet agent, dipyridamole and dilazep dihydrochloride on the in vivo platelet function and proteinuria. Jpn. J. Nephrol., 27, 1261. [PubMed] [Google Scholar]
- Ishibashi, T , Hara, A , Abiko, Y. 1987. Relationship between coronary flow and adenosine release during severe and mild hypoxia in the isolated perfused rat heart with special reference to time‐course change. Heart Vessels, 3, 113. [DOI] [PubMed] [Google Scholar]
- Ishikawa, S , Kawasumi, M , Kusaka, I , Komatsu, N , Iwao, N , Saito, T. 1994. Extracellular ATP promotes cellular growth of glomerular mesangial cells mediated via phospholipase C. Biochem. Biophys. Res. Comn., 272, 234. [DOI] [PubMed] [Google Scholar]
- Kawata, Y , Fujii, Z , Sakumura, T , Kitano, M , Suzuki, N , Matsuzaki, M. 1998. High pressure conditions promote the proliferation of rat cultured mesangia in vitro. Blochem. Biophys. Acta., 1401, 195. [DOI] [PubMed] [Google Scholar]
- Koide, H , Totsuka, Y , Sugisaki, T et al. 1995. Clinical effect of the antiplatelet drug, dilazep dihydrochloride, in patients at the microalbuminuric stage of diabetic nephropathy a multi‐center study. Jpn J. Nephrol., 37, 644. [PubMed] [Google Scholar]
- Maruhama, Y , Sasaki, M , Mukaida, H et al. 1985. Clinical effect of dilazep (Commelian kowa) on early diabetic nephropathy. Jpn Pharmacol. 7her., 18, 4689. [Google Scholar]
- Murakami, T , Li, X , Gong, J , Bhatia, U , Tragan0s, F , Darzynkiewics, Z. 1995. Induction of apoptosis by 5‐azacytidine: drug concentration‐dependent differences in cell cycle specificity. Cancer Res., 55, 3093. [PubMed] [Google Scholar]
- Nagase, M , Kobayashi, S , Sakakibara, K , Honda, N. 1985. Amelioration of albuminuria induced by dilazep administration in aminonueleoside nephrosis of rat. Jpn J. Nephrol., 27, 385. [PubMed] [Google Scholar]
- Nakazawa, K , Moriya, T , Kasai, S et al. 1993. Dilazep prevents glomerulosclerosis in accelated Masugi nephritis in the rat. Jpn J. Nephrol., 35, 329. [PubMed] [Google Scholar]
- Plagemann, PGW & Woffendin, C. 1988. Spicces differences in sensitivity of nucleoside transport in erythrocytes and cultured cells to inhibition by nitrobenzylthioinosine, dipyridamole, dilazep and lidoflazine. Bioochem. Biophys. Acta., 969, 1. [DOI] [PubMed] [Google Scholar]
- Silver, BJ , Jaffer, FE , Abboud, HE. 1989. Platelet‐derived growth factor synthesis in mesangial cells. Induction by multiple peptide mitogens. Proc. Natl. Acad. Sci. USA., 186, 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajiri, Y , Umeda, F , Kunisaki, M et al. 1989. Effect of dilazep hydrochloride on urinary albumin excretion in patients with diabetic nephropathy. Therapeut. Res., 10, 5335. [Google Scholar]
- Tanabe, S , Tamaki, T , Wada, Y , Sumiyoshi, A , Funahara, Y. 1992. Cytoprotective effect of dilazep on hydrogen peroxide perturbed vascular endothelial cells. Southeast Asian J. Trop. Med. Public Health , 23, 120. [PubMed] [Google Scholar]
- Yamamoto, M , Fukui, M , Kuramoto, T , Kabuki, K , Tomino, Y. 1995. Effects of the antiplatelet drug, dilazep dihydrochloride, on anionic site and extracellular matrix (ECM) components in glomerular basement membrane of STZ‐induced diabetic rats. J. Clin. Lab Anal., 9, 380. [DOI] [PubMed] [Google Scholar]
- Yasunaga, K , Mase, K , Nakajima, K , Yamamoto, S , Nagakura, M. 1988. Potential effectiveness of minidose aspirin combined with dilazep in antiplatelet therapy. Jpn. J. Clin. Pharmicol. Ther., 19, 453. [Google Scholar]
- Yoshida, H , Kanatsu, K , Muso, E et al. 1994. Effects of an antiplatelet drug (dilazep) in lgA nephropathy: comparison of clinical effects with renal biopsy findings. Jpn. J. Nephrol., 36, 339. [PubMed] [Google Scholar]
- Young, JD & Jarvis, SM. 1983. Nucleoside transport in animal cells. Biosci. Res., 3, 309. [DOI] [PubMed] [Google Scholar]



