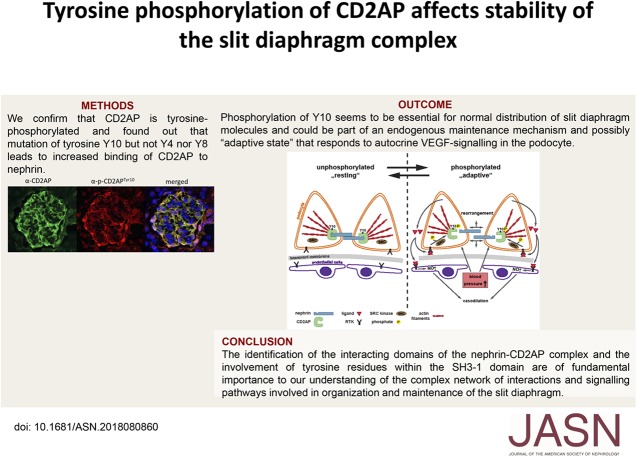
Significance Statement
The connection between the slit diaphragm and actin network of podocytic foot processes involves complex signaling between slit diaphragm proteins and multiple signaling pathways of the actin machinery. CD2AP, a slit diaphragm–associated scaffolding protein, is considered a “stabilizer” of the complex that connects the slit diaphragm protein nephrin to the cell’s cytoskeleton. In this study, the authors define CD2AP as a phosphorylation target of receptor tyrosine kinases stimulated by VEGF-A in podocytes. They demonstrate that phosphorylation of tyrosine at position Y10 of the SH3-1 domain of CD2AP can change the affinity of CD2AP to nephrin and is indispensable for CD2AP function and slit diaphragm functionality in vivo. These findings implicate CD2AP phosphorylation as a molecular target in proteinuric kidney diseases.
Keywords: podocyte, CD2AP, nephrin, Phosphorylation
Visual Abstract
Abstract
Background
CD2-associated protein (CD2AP), a slit diaphragm–associated scaffolding protein involved in survival and regulation of the cytoskeleton in podocytes, is considered a “stabilizer” of the slit diaphragm complex that connects the slit diaphragm protein nephrin to the cytoskeleton of the cell. Tyrosine phosphorylation of slit diaphragm molecules can influence their surface expression, but it is unknown whether tyrosine phosphorylation events of CD2AP are also physiologically relevant to slit diaphragm stability.
Methods
We used isoelectric focusing, western blot analysis, and immunofluorescence to investigate phosphorylation of CD2AP, and phospho-CD2AP antibodies and site-directed mutagenesis to define the specific phosphorylated tyrosine residues. We used cross-species rescue experiments in Cd2apKD zebrafish and in Drosophila cindrRNAi mutants to define the physiologic relevance of CD2AP phosphorylation of the tyrosine residues.
Results
We found that VEGF-A stimulation can induce a tyrosine phosphorylation response in CD2AP in podocytes, and that these phosphorylation events have an important effect on slit diaphragm protein localization and functionality in vivo. We demonstrated that tyrosine in position Y10 of the SH3–1 domain of CD2AP is indispensable for CD2AP function in vivo. We found that the binding affinity of nephrin to CD2AP is significantly enhanced in the absence of Y10; however, unexpectedly, this increased affinity leads not to stabilization but to functional impairment of the glomerular filtration barrier.
Conclusions
Our findings provide insight into CD2AP and its phosphorylation in the context of slit diaphragm functionality, and indicate a fine-tuned affinity balance of CD2AP and nephrin that is influenced by receptor tyrosine kinase stimulation.
CD2AP belongs to a family of scaffolding/adaptor proteins containing three SRC Homology 3 (SH3) domains followed by a prolin-rich domain and a potential actin-binding site in proximity to the C terminus. In podocytes, CD2AP is expressed at the slit diaphragm, a specialized intercellular junction between neighboring podocytes covering the outer surface of the glomerular tuft. CD2AP interacts with several proteins at the slit diaphragm. One of the major components is nephrin, a transmembrane adhesion protein of the IgG Superfamily.1 Nephrin expression levels and subcellular localization are affected in several renal and proteinuric kidney diseases such as diabetes and all forms of nephrotic syndrome. It was previously shown that nephrin is a signaling protein that is activated by phosphorylation.2–4 However, the interaction of CD2AP with nephrin appeared to be of very low stoichiometry and the precise interacting domains are still largely unresolved.5 In this manuscript, we describe a physiologically important tyrosine-phosphorylation response of CD2AP. Tyrosine phosphorylation of CD2AP was initially described by Kirsch et al.6 to promote binding to c-Cbl, but since then no further work on the role of CD2AP phosphorylation or its physiologic role has been published. During the past two decades, tyrosine phosphorylation within SH3 domains of several signaling proteins has been described.7–9 Tyrosine-phosphorylation within an SRC Homology 2 (SH2) domain or phospho-tyrosine binding (PTB) domains facilitates protein-protein interactions which initiate signal propagation. In contrast, tyrosine phosphorylation of SH3 domains is described to prevent or reduce the affinity of protein-protein interactions.10 Tyrosine phosphorylation is generally enriched in adaptor proteins and can influence protein functionality. For example, Fabian et al.11 showed that tyrosine-phosphorylation results in conformational changes of the τ peptide in contrast to serine phosphorylation which has no effect. Only a few studies of nephrin and its interaction with other slit diaphragm proteins have convincingly demonstrated protein-protein interactions.12–15 On reviewing these data, it is evident that the described binding between CD2AP and nephrin is of low stoichiometry. This led us to hypothesize that this protein-protein interaction could be dependent on the phosphorylation status of CD2AP.
Methods
Antibodies and Cytokines
Primary antibodies that were used for western blotting and immunofluorescence studies: rabbit anti-CD2AP H-290 (sc-9137), rabbit anti-GAPDH FL-335 (sc-25778), rabbit anti-FLT1 (sc-316), mouse anti-pTyr (PY20, sc-508) (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-myc 71D10 (2278S), rabbit anti-flag DYKDDDDK-tag (2368S) (Cell Signaling Technology, Beverly, MA), sheep anti-CD2AP (AF4474; Littleton, CO), and rabbit anti-Neuropilin-1 (Elabscience, Houston, TX). Phalloidin Alexa fluor 488 A12379 (Molecular Probes, Eugene, OR) was used, and secondary antibodies: Alexa Fluor 350 donkey anti-sheep and Alexa Fluor 555 donkey anti-rat (Jackson ImmunoResearch); and goat anti rabbit IgG-HRP (sc-2004), goat anti-mouse IgG-HRP (sc-2005), and goat anti-rat IgG-HRP (sc-2006) (Santa Cruz, CA). Cytokines: VEGF-A and FGF-4 were purchased from Cell Sciences (Canton, MA). Inhibitor: λ-phosphatase (6753) (Cell Signaling Technology, Beverly, MA). The Src-inhibitor PP1 and pervanadate (prepared from sodium orthovanadate and peroxide) were purchased from (Merck, Darmstadt, Germany).
Generation of Phospho-CD2AP Antibodies
The specific phospho-CD2AP antibodies were generated by standard protocol by two different companies. Polyclonal antibodies from rabbit: anti-CD2APtyr4/8/10 (sequence: MVD{pY}VE{pY}D{pY}DAV-Ahx-KKC-amide), anti-CD2APtyr119 (sequence: CKVLFE{pY}IPQNEDEL-amide), and anti-CD2APtyr273/280 (sequence: Ac-AKE{pY}CRTLFA{pY}EGTN-Ahx-C-amide). The polyclonal antibodies were generated by 21st Century Biochemicals, Marlboro, MA. mAb from rat: anti-CD2APtyr10 (sequence: VEYD{pY}DAVHDDELT), generated by Genscript (Piscataway, NJ). Test bleeds were tested by western blot and appropriate antibodies were affinity purified. All experiments were performed with affinity-purified phospho-CD2AP antibodies.
Western Blot Analysis
For analysis of whole-cell protein lysates, cultured HEK-293T cells were lysed on ice in RIPA buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.5% sodium deoxycholate, 1% Nonidet P-40, and 0.1% SDS) containing protease inhibitors (Complete mini; Roche, Mannheim, Germany). For analysis of whole-cell protein lysates from cultured podocytes, treated or untreated cells were lysed on ice in lysis buffer (RIPA buffer with 1 mM sodium orthovanadate, 50 mM NaF). To all buffers, 200 µg/L okadaic acid and 20 g/L N-Ethylmaleimide (Sigma-Aldrich, St. Louis, MO) were added. Lysates were centrifuged at 11,000 rpm, and aliquots of the supernatants (40 µg protein/lane) were separated by 10% or 14% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA). The samples were probed with primary antibodies and the antigen-antibody complexes were detected with horseradish peroxidase–labeled secondary antibodies. They were then visualized by using SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical Co.) according to the manufacturer’s protocol. For Phos-tag experiments, precast polyacrylamide gels containing Phos-tag (Wako, Richmond, VA) with zinc ion were used. The western blot was then performed according to the manufacturer’s protocol.
Podocyte Culture
Culture of conditionally immortalized murine and human podocytes was performed as described previously.21 All experiments were performed using immortalized podocytes. For propagation of murine podocytes, cells were cultured on type I collagen (BD Biosciences, Bedford, MA) at 33°C in the presence of 10 U/ml recombinant mouse γ-IFN (Cell Sciences, Canton, MA) to enhance expression of a thermosensitive T antigen. Podocytes were maintained at 37°C for 14 days without γ-IFN for inducing differentiation. The medium consists of RPMI 1640 (Biochrom, Berlin, Germany) containing 10% FBS (PAA, Pasching, Austria), 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA), and 10 U/ml recombinant mouse γ-IFN. The cells were inoculated every 3 or 4 days into new medium. Human podocytes were a gift from Moin Saleem, Bristol, UK. Human podocytes followed the same temperature shift between permissive and nonpermissive conditions as mouse podocytes, but they were cultured without γ-IFN and were not grown on collagen-coated flasks. For experiments, cells were treated for the indicated times with 20 ng/ml VEGF-A or FGF-4, with pervanadate (10 mM) for 30 minutes or with the Src-inhibitor PP1 (5 µM) for 1 hour. Dephosphorylation of CD2AP with λ-phosphatase (4000 U per 100 µg protein) was performed in cell lysates.
HEK-293T Cell Culture
Human Embryonic Kidney 293T cells (DSMZ, Braunschweig, Germany) were cultured in Dulbecco’s MEM high-glucose medium with l-Glutamine (PAA, Pasching, Austria), containing 10% FBS (PAA, Pasching, Austria) and 1% antibiotic-antimycotic solution (PAA, Pasching, Austria).
Plasmid Construction
Myc-CD2AP and Cindr tyrosine mutants were generated by Site-Directed Mutagenesis with the pcDNA3.1CD2AP-myc or pUAST-EGFP-Cindr-PC construct using PfuUltra II Fusion HS DNA Polymerase (Agilent, Santa Clara, CA).
Transfection
The day before transfection, HEK-293T cells were seeded onto plates. Cells were transfected using Fugene HD transfection reagent for 48 hours at 37°C (Promega, Mannheim, Germany) according to the manufacturer’s protocol. The cells were harvested 48 hours after transfection and used for immunoprecipitation.
Immunoprecipitation
HEK-293T cells were transfected as mentioned above. Then, the cells were washed carefully with ice-cold PBS on ice. For lysis, 900 µl RIPA buffer (50 mM TrisHCl pH 7.5, 200 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% triton, and 0.25% deoxycholate+protease inhibitors) was added to cells. The lysate was incubated for 15 minutes on ice and centrifuged at 14,000 rpm for 15 minutes at 4°C. Then, 50 µl Flag-beads (50% slurry in triton buffer) (Sigma, St. Louis) were added to the supernatant and rotated overhead at 4°C for 1 hour (up to overnight). Afterwards, the beads were centrifuged at 3000 rpm for 1 minute at 4°C and washed with RIPA buffer five times. Proteins were eluted by boiling the beads in Laemmli buffer and separated by SDS-PAGE.
Generation of an Adenoviral Expression Vector
The following primers were used to generate the attB-PCR product of the gene of interest for adenoviral expression: attB1: ACAAGTTTGTACAAAAAAGCAGGCT; attB2: ACCACTTTGTACAAGAAAGCTGGGT.
pDNOR221 vector (300 ng) (Invitrogen) containing attP sites and an equimolar amount of purified attB-PCR product were mixed with 5× BP Clonase Reaction buffer (Invitrogen) and TE buffer (pH 8.0) to a volume of 16 µl. Then, 4 µl BP Clonase enzyme mix was added to the sample, which was then incubated at 25°C for 1 hour. Two microliters of the Proteinase K solution was added to each reaction and incubated for 10 minutes at 37°C. This entry clone plasmid contained attL sites and was transformed into chemically competent Escherichia coli cells.
The entry clone plasmid was then purified and cloned into the pAd/CMV/V5-DEST vector (Invitrogen), following the same protocol as described above. This plasmid was then transformed into electro-competent cells, selected by antibiotic resistance, and purified for the expression procedure.
Transient Transfection through the Adenoviral System
HEK-293T cells (5×105) were seeded onto six-well plates containing 2 ml normal growth medium 1 day before transfection. On the day of transfection, the culture medium was replaced with 1.5 ml normal growth medium without antibiotics. At least 5 µg pAd/CMV/V5-DESTTM vector containing the DNA of interest was purified and digested with Pac I to expose the inverted terminal repeats. Lipofectamine 2000 (ThermoFisher) was then used as the DNA delivery system. The DNA–Lipofectamine 2000 complexes were added drop-wise to each well and the cells were incubated overnight at 37°C in a 5% CO2 incubator. The next day, the transfection medium was replaced with DMEM medium containing 10% FCS and 1% PAA. Forty-eight hours post-transfection, the cells were trypsinized and transferred to a 10-cm tissue culture plate containing 10 ml complete DMEM medium. The medium was changed every 2–3 days until approximately 80% cytopathic effect regions were observed. Adenovirus-containing cells were harvested and lysed by two freeze/thaw cycles and the cell debris was pelleted by centrifugation. The supernatant containing viral particles was aliquoted and stored at −80°C. All of the procedures were performed in an S2 lab.
Adenoviral Transduction of Cultured Podocytes
The cultured podocytes were plated and allowed to differentiate at 37°C. On the day of transduction, the adenoviral stock was thawed and the appropriate amount of virus was diluted into fresh complete medium. This was added onto the cells and incubated at 37°C overnight. The following day, the medium containing virus was removed and replaced with fresh, complete culture medium. The cells were harvested after VEGF-A treatment at the indicated time points and protein expression was analyzed by western blot.
Immunofluorescence
After dissection, kidneys of 8-week-old C57/Bl6 mice were flushed with PBS and immediately frozen in tissue molds containing optimal cutting temperature compound. Sections were blocked with 10% normal donkey serum, then stained with the appropriate primary antibody followed by the appropriate secondary antibody. Fluorescence was enhanced with Aqua PolyMount+DAPI. For immunofluorescence of cultured podocytes, fixing was performed with 4% PFA followed by the staining protocol mentioned above. The pictures were taken with a Leica Inverted-2 confocal microscope or with a Leica DM LB microscope and Leica Application Suite Software (Leica, Bonn, Germany). Postprocessing was done with Photoshop 7.
Analysis of the Crystal Structure of CD2AP/CD2
The structural model of the first SH3 domain of human CD2AP in complex with a CD2-derived peptide was downloaded from the Protein Data Bank (Protein Data Bank entry code 2J6O) and analyzed using COOT.16 Figures depicting protein structures were prepared using Pymol (Version 1.8.6.2; Schrödinger, LCC).
Animals
Zebrafish experiments were conducted following the methods previously described by us in the study by Hentschel et al.17 In Brief, zebrafish (L-FABP:DBP-EGFP) were mated and housed at 28.5°C in embryo-rearing medium (E3). Fertilized embryos (1–4-cell stage) were injected using a Nanoject II injection device (Drummond Scientific, Broomall, PA). For overexpression experiments, mRNA of wild-type or tyrosine-mutated CD2AP or Cindr was diluted 1:1 with injection buffer (20 mm HEPES, 200 mm KCl, and 0.01% phenol red) and injected at a final concentration of 5 ng in a total volume of 4.6 nl. The capped RNAs were synthesized using mMESSAGE mMACHINE kits (Ambion) according to the manufacturer’s protocol. Because capped RNA has a 7-methyl guanosine cap structure at the 5′ end, it mimics most eukaryotic mRNAs found in vivo.
For rescue experiments, wild-type or tyrosine-mutated CD2AP/Cindr mRNA was combined with CD2AP-morpholino, which was diluted in injection buffer, and injected at final concentrations of 5 ng mRNA and 25 μM morpholino in a total volume of 4.6 nl. Scrambled morpholino was injected as a control. Morpholino sequences were designed and ordered from GeneTools (Philomath, OR) as follows: standard control sequence, 5′-CCTCTTACCTCAGTTACAATTTATA-3′; and CD2-associated protein (Cd2ap), 5′-CATACTCCACCACCACCTCAACCAT-3′. The CD2AP-morpholino sequence was blasted to ensure that no off-target annealing occurred for sequence matches >14 nt, and we excluded crosshybridization with the cRNA constructs. Then, at 120 hours postfertilization, zebrafish larvae were anesthetized with a 1:28 dilution of 4 mg/ml tricaine (MESAB, 1% Na2HPO4, pH 7.0) and photographed at ×10 magnification. The fluorescence of GFP-labeled vitamin D–binding protein in the retinal vessel plexus of the zebrafish eye was measured and analyzed with ImageJ. Animal work was conducted according to the guidelines of the American Physiologic Society and was approved by the Animal Care and Use Committee of the Mount Desert Island Biologic Laboratory (protocol 14–06). All efforts were made to minimize the number of animals used and their suffering.
Drosophila Experiments
Cindr was targeted by crossing UAS-cindrRNAi2.21+2318 transgenic flies to the GMR-GAL4 driver line,19,20 which drove transgene expression in the eye from larval development and throughout pupal development. Similarly, we crossed UAS-CD2AP; UAS-cindrRNAi2.21+23 or UAS-CD2APY10F; UAS-cindrRNAi2.21+23 to GMR-GAL4. To generate the UAS-CD2AP and UAS-CD2APY10F fly lines, CD2AP cDNA was cloned into the pUAST-myc vector and site-directed mutagenesis utilized to modify Y10 to phenylalanine. Vectors were injected into Drosophila embryos (BestGene Inc.) to establish stable transgenic fly lines. Pupae were gathered as white prepupae and maintained at 25°C until dissection at 27 or 40 hours after puparium formation (APF) using standard protocols. Primary antibodies were rat anti-ECad2 (1:20, DSHB), mouse anti-Rst (Mab24A5.1; 1:40, DSHB), and rabbit anti-Hbs (AS14, 1:2500, 20), and secondary antibodies were obtained from Jackson ImmunoResearch. Fluorescent images were captured with a Leica Microsystems DM5500 microscope or a Leica Microsystems TCS SP5 DM confocal microscope. Images were minimally processed in Photoshop (Adobe) and tracings hand-drawn using Illustrator (Adobe). Interommatidial cells were pseudo-colored pink in Photoshop. Mispatterning of the eye was analyzed as described previously.18
Statistical Analyses
The statistical analyses of data were performed in Microsoft Excel or with GraphPad Prism software and results are presented as an average of all data points from each experiment; error bars correspond to the SEM. We used the unpaired t test to compare the results of each test group. A P value <0.05 was considered statistically significant.
Results
CD2AP Is Tyrosine Phosphorylated in Response to VEGF-A Stimulation
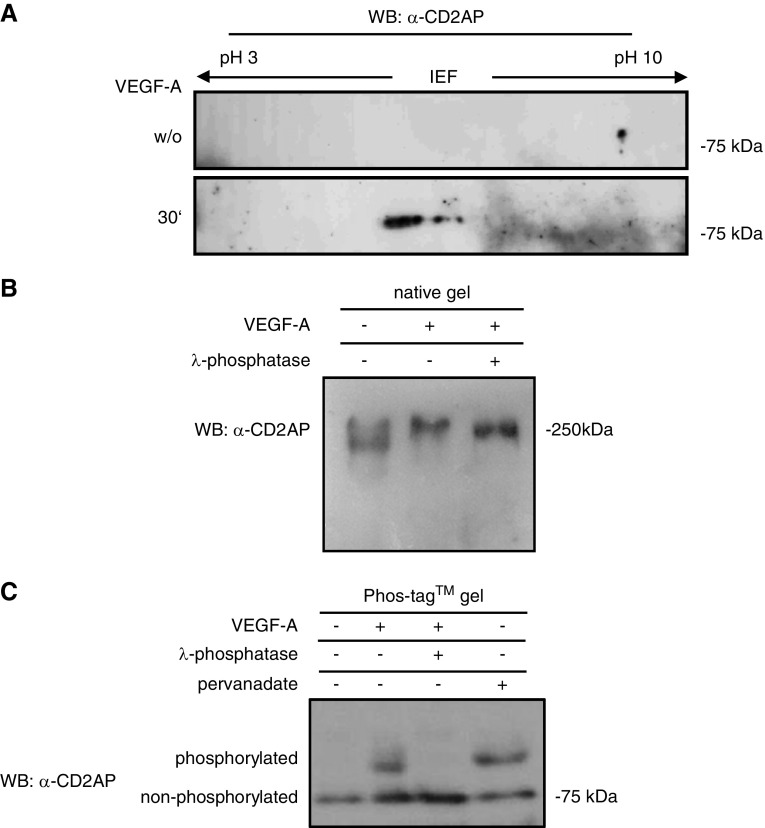
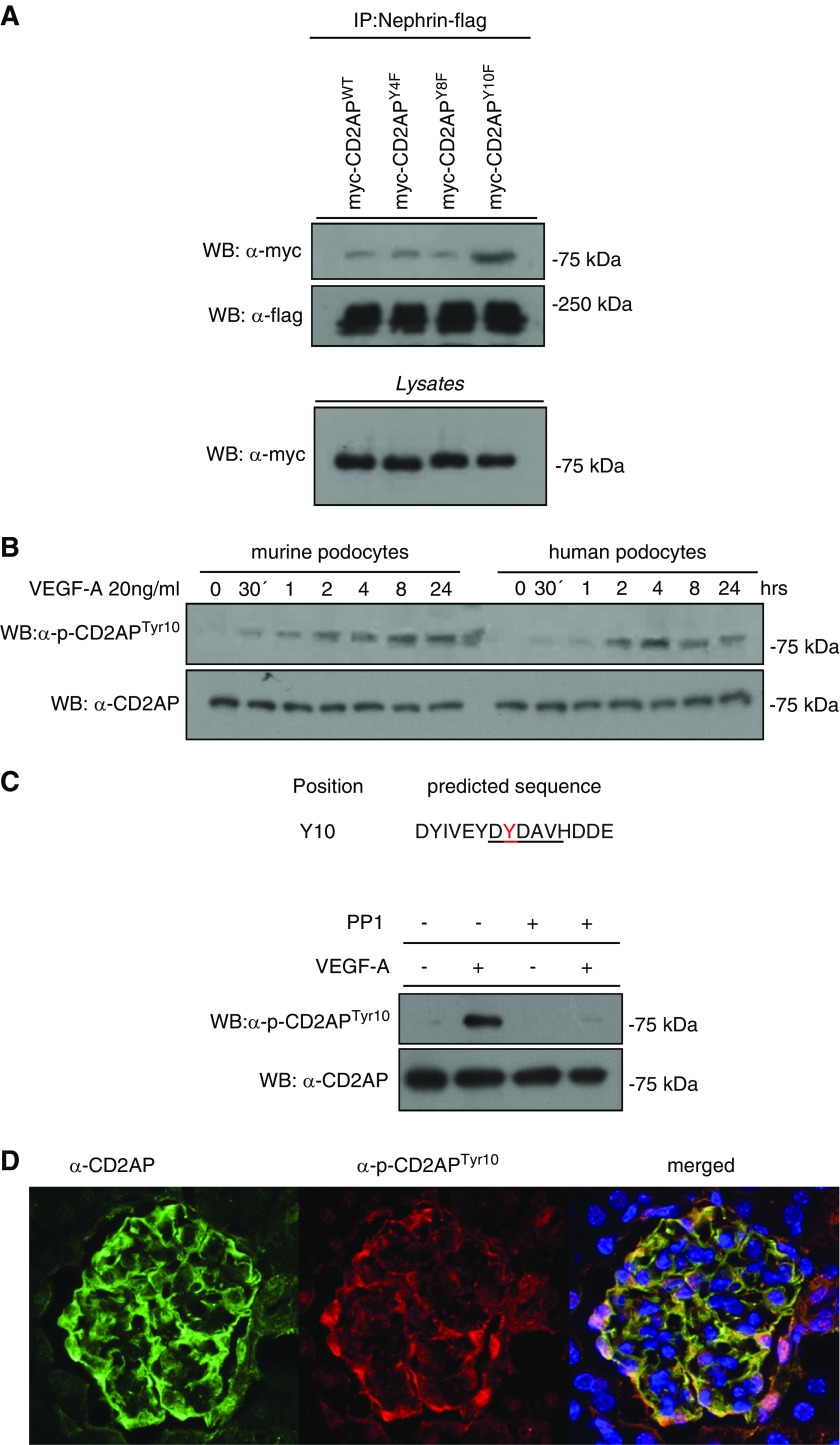
On the basis of our previous results, which show that podocyte survival is dependent on the autocrine response to VEGF-A and that podocytes express the receptor tyrosine kinases VEGFR1 and VEGFR2,21 we examined whether VEGF-A stimulation can induce a tyrosine phosphorylation response in CD2AP. Cultured differentiated murine podocytes were stimulated for 30 minutes with 20 ng/ml VEGF-A. Native cell lysates were analyzed by isoelectric focusing using a nonlinear gradient of pH 3–10 with increased resolution between pH 5 and pH 7. Western blotting of the isoelectric focusing of untreated cells with an anti-CD2AP antibody showed one spot (Figure 1A, upper panel). Upon VEGF-A stimulation, CD2AP was detected as three spots with a shift to lower pI (Figure 1A, lower panel). Interestingly, stimulation with a different receptor tyrosine kinase stimulator (FGF-4) also induced a shift of CD2AP; however, this was to a lower pI (Supplemental Figure 1A).
Figure 1.
CD2AP is tyrosine phosphorylated after VEGF-stimulation in podocytes. (A) Two-dimensional isoelectric electrophoresis using an anti-CD2AP antibody shows a shift of CD2AP after treatment with VEGF-A after 30 minutes. (B) Native gel electrophoresis using an anti-CD2AP antibody shows a weight shift after VEGF-A stimulation. Treatment with λ-phosphatase partially restores the weight shift. (C) Phos-tag gel shows phosphorylated and nonphosphorylated proteins as different bands. λ-phosphatase dephosphorylates and pervanadate phosphorylates CD2AP. Western blot was analyzed with an anti-CD2AP antibody. IEF, isoelectric focusing; WB, western blotting; w/o, without.
In addition, we analyzed by native gel electrophoresis whether CD2AP phosphorylation leads to shifts in mol wt. CD2AP of cells stimulated with VEGF-A displays a significant shift to higher mol wt. Notably, treatment of the lysate of VEGF-stimulated cells with λ-phosphatase significantly impairs this shift (Figure 1B). To confirm that CD2AP is phosphorylated, we used a Phos-tag precast gel that allows detection of non- and phosphorylated CD2AP as different bands. To increase an overall tyrosine phosphorylation, we treated differentiated podocytes with 10 mM pervanadate for 30 minutes. Upon VEGF-A and pervanadate stimulation, CD2AP was detected as phosphorylated and nonphosphorylated protein running as two different bands. Incubation with λ-phosphatase leads to dephosphorylation of CD2AP (Figure 1C). Furthermore, immunoprecipitation experiments of CD2AP underline that CD2AP is tyrosine phosphorylated (Supplemental Figure 1B). Expression of VEGFR1 and the coreceptor Neuropilin-1 in cultured murine and human podocytes was verified by western blot with lysates from two different passages (Supplemental Figure 1C).
Tyrosine Phosphorylation in the SH3–1 Domain of CD2AP Changes its Binding to Nephrin and Shows a Prolonged Phosphorylation Response after VEGFR Stimulation
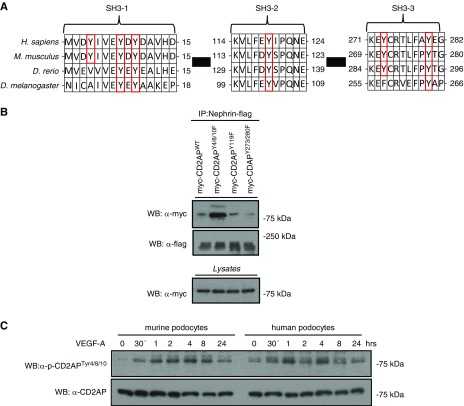
The alignment of CD2AP amino-acid sequences of several species revealed six evolutionarily conserved tyrosines within the three SH3 domains (SH3–1, SH3–2, and SH3–3). Four of these tyrosine residues, Y8, Y10, Y119, and Y280, were evolutionarily conserved from vertebrates to Drosophila (Figure 2A). In this context, we generated tyrosine (Y)-to-phenylalanine (F) mutations for all conserved SH3 domain tyrosine residues by site-directed mutagenesis, which resulted in three constructs: Y4/8/10F, Y119F, and Y273/280F (each labeled with the myc-tag sequence). Next, we transfected HEK-293T cells with these constructs and flag-tagged nephrin. Immunoprecipitation experiments showed a strongly enhanced binding affinity of the Y4/8/10F mutant to nephrin (Figure 2B). For further analysis, custom-made phospho-CD2AP antibodies, specific to phosphorylated tyrosine residues 4/8/10, 119, and 273/280, were used. Their specificity was verified by western blot with lysates of transfected HEK-293T cells (Figure 2C, Supplemental Figure 1D). Next, we tested these antibodies on lysates from murine and human podocytes after VEGF-A stimulation for >24 hours. Western blots show the presence of tyrosine phosphorylation of Y4/8/10, Y119, and Y273/280. However, phosphorylation intensity was more distinct in Y4/8/10 compared with Y119 or Y273/280 (Supplemental Figure 1D). Phosphorylation profiles for Y119 showed a significantly weaker signal, but also in a typical activation pattern, whereas Y273/280 showed no recognizable activation pattern (Supplemental Figure 1E).
Figure 2.
Phosphorylation of tyrosines Y4/8/10 has a characteristic activation profile and antagonizes interactions with nephrin. (A) Phylogenetic and sequence analysis of indicated species shows evolutionarily conserved tyrosines in each of the three SH3 domains. (B) Myc-tagged CD2AP DNA was point-mutated on indicated tyrosine sites. HEK-293T cells transfected with myc-CD2AP, myc-CD2AP tyrosine mutants, and flag-Nephrin were immunoprecipitated with a flag-antibody and western blot was performed with a Myc-antibody. (C) Differentiated podocytes were treated with 20 ng/ml VEGF-A for 24 hours. Western blot analysis of lysates was performed using a p-CD2APtyr4/8/10 antibody. CD2AP was used as loading control. D. rerio, Danio rerio; D. melanogaster, Drosophila melanogaster; H. sapiens, Homo sapiens; IP, immunoprecipitation; M. musculus, Mus musculus; WB, western blotting.
Because these two experiments suggested a functional relevance for the Y4/8/10-motif, we focused the following experiments on these phosphorylation sites. The specificity of the antibodies used was further validated by immunofluorescence and western blot analysis of specific non- and phosphorylated peptides, as well as pervanadate and λ-phosphatase treatment (Supplemental Figure 1, F–H).
CD2APptyr4/8/10 Is Detected in the Leading Edges and Focal Contacts of Murine and Human Podocytes
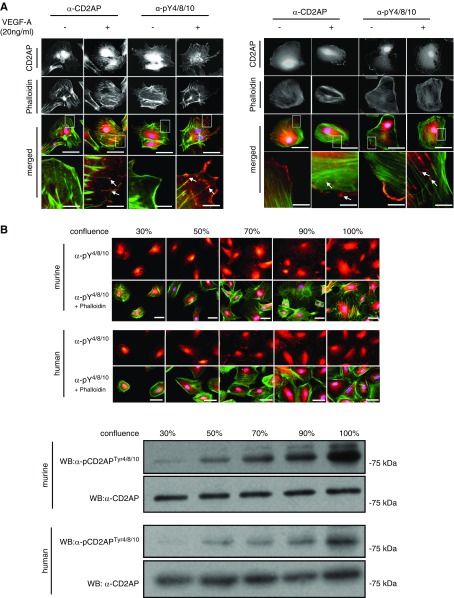
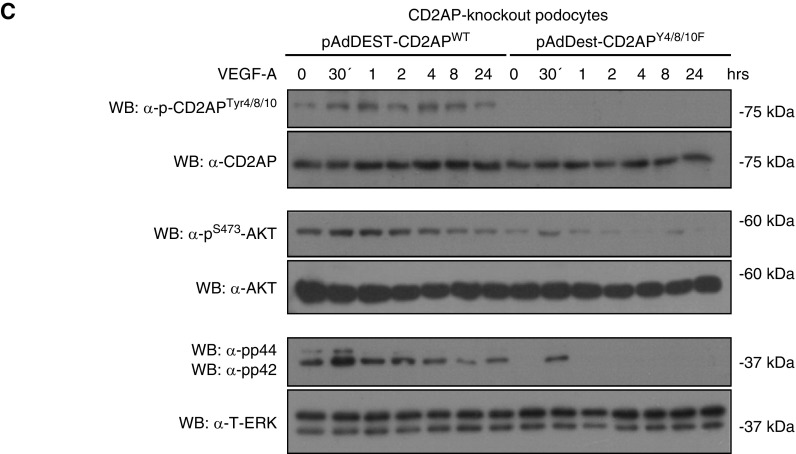
To examine whether the phosphorylation response is associated with a changed expression pattern, murine and human podocytes were differentiated and immunofluorescence staining was performed. After VEGF-A stimulation, we detected an enhanced CD2APptyr4/8/10 staining at the leading edges of podocytes and in the focal contacts (Figure 3A). Because it is established that changing cell density alters tyrosine phosphorylation of focal adhesion components, including Src, paxillin, and focal adhesion kinase,22 we performed cell confluence assays with differentiated murine and human podocytes and compared phosphorylation. To accomplish this, we stained cells from 30% to 100% confluency directly with the pCD2APtyr4/8/10 antibody, phalloidin, and DAPI. In addition, we performed western blot analysis. Interestingly, with both approaches we could detect a significantly enhanced phosphorylation of the Y4/8/19-motif with increasing confluency in murine as well as in human podocytes (Figure 3B, lower panel). To further explore functional consequences, we examined AKT and ERK responses in the absence of the specific tyrosine residues, because we previously showed that survival signaling is diminished in CD2AP−/− podocytes. We generated adenoviral vectors expressing CD2APWT or CD2APY4/8/10F. These vectors were transduced into cultured CD2AP−/− podocytes to generate phospho-competent and phospho-deficient CD2AP-expressing podocytes. Interestingly, phosphorylation of AKT and ERK is significantly diminished in podocytes expressing CD2APY4/8/10F compared with CD2APWT. These results indicate that phosphorylation of CD2AP is essential for activation of downstream survival targets in podocytes (Figure 3C).
Figure 3.
Phosphorylated CD2AP is found at the leading edges and in focal contacts, and cellular confluency enhances CD2AP phosphorylation. (A) Differentiated murine and human podocytes untreated and treated with VEGF-A were stained with CD2AP or p-CD2APtyr4/8/10 antibodies (red) and costained with phalloidin (green) and DAPI (blue). Scale bars, 20 µm. Boxed regions are enlarged (bottom panel). White arrows depict phosphorylated CD2AP at leading edges. Scale bars, 2 µm. (B) Murine and human podocytes cultured to 30%–100% confluency were stained with anti–p-CD2APtyr4/8/10, phalloidin, and DAPI. Western blot of podocyte lysates at different confluence percentages, probed with p-CD2APtyr4/8/10 and CD2AP antibodies. Scale bars, 10 µm. (C) CD2AP-knockout podocytes were transduced with adenoviral vectors expressing CD2APWT and CD2APY4/8/10F. Podocytes were treated with 20 ng/ml VEGF-A for 24 hours. Western blot analysis of lysates was performed using p-CD2APtyr4/8/10, p-AKTS273, and p-ERKThr202/Tyr204 antibodies. CD2AP, AKT, and ERK were used as loading controls. WB, western blotting.
Cross-Species Rescue Experiments in Zebrafish
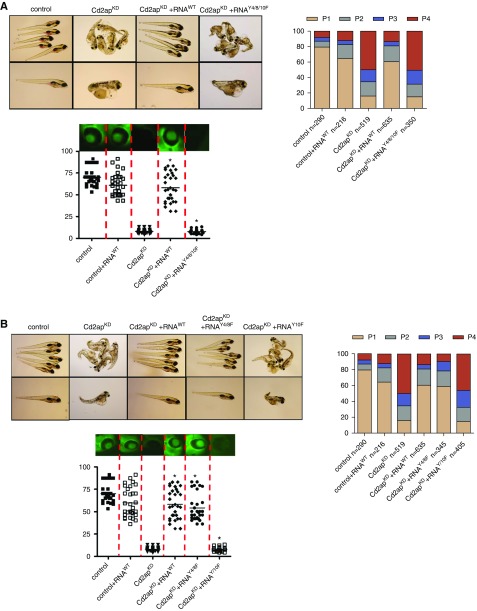
To test the biologic relevance of the Y4/8/10 motif in vivo, we performed cross-species phenotype rescue experiments. First, we analyzed the edema phenotype generated by CD2AP knockdown in zebrafish. This phenotype was scored as follows: phenotype 1, normal; phenotype 2, mild edema; phenotype 3, severe edema; and phenotype 4, very severe edema. We observed a significant amount of edema in CD2APKD zebrafish as well as a significant loss of high–mol wt protein from the circulation (proteinuria), as previously described.17 In addition, most CD2APKD larva died at 5 days postfertilization. Next, we found that overexpression of murine wild-type CD2AP-mRNA completely rescued both edema and proteinuria phenotypes (Figure 4A). In contrast, overexpression of Y4/8/10F-mutated CD2AP mRNA rescued neither the edema nor the proteinuria phenotypes and did not modify the mortality of the CD2AP-knockdown animals. These data indicate that these tyrosine residues are indispensable for normal glomerular filtration barrier function in vivo (Figure 4A). Next, we generated single tyrosine mutants and found that the mRNA from CD2AP that lacks tyrosine at position 10 was unable to rescue the phenotype, whereas mRNA from CD2AP that lacks tyrosine residues 4 and 8 led to a full rescue effect of the phenotype (Figure 4B).
Figure 4.
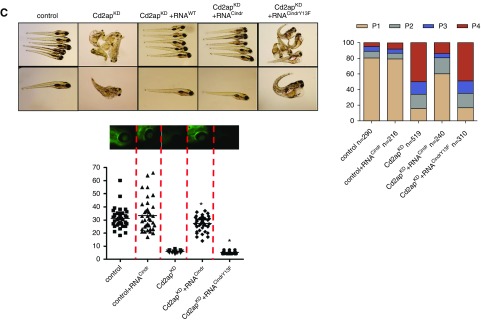
Phosphorylation of CD2AP at tyrosine 10 is physiologically relevant for a functional glomerular filtration barrier. Fertilized zebrafish eggs were injected at the 1–4-cell stage with a CD2AP-morpholino and murine CD2AP-capped mRNA. (A) Images (left) and phenotypic analyses (right) of larval zebrafish 120 hours after injection. Phenotypes were categorized into four groups: P1, no edema; P2, mild edema; P3, severe edema; and P4, very severe edema. Fluorescent images of the retinal vessel plexus (bottom panel) and bar graph of fluorescence intensity of eGFP-DBP in fish eyes (at least 30 animals for each treatment were analyzed). Error bars show the mean±SEM; *P≤0.001 by unpaired t test. CD2APtY4/8/10F mRNA could not rescue the phenotypes associated with CD2APKD, whereas coinjection of CD2APWT mRNA restored wild-type phenotypes. (B) Larval zebrafish 120 hours after injection (left) and phenotypic analyses (right). Fluorescent images and eGFP-DBP analyses of the retinal vessel plexus of at least 30 animals (bottom panel). Error bars indicate mean±SEM; *P≤0.001 by unpaired t test. Injection of CD2APY10F into CD2APKD larvae failed to restore wild-type phenotypes, unlike injection of both CD2APWT mRNA and CD2APY4/8F mRNA. (C) Larval zebrafish 120 hours after injection (left) and phenotypic analyses (right). Fluorescent images and eGFP-DBP analyses of the retinal vessel plexus of at least 30 animals (bottom panel). Error bars indicate mean±SEM; *P≤0.001 by unpaired t test. Injection of Cindr RNA into CD2APKD larvae restored wild-type phenotypes, unlike injection of CindrY13F mRNA.
Rescue Experiments in Zebrafish Using the Drosophila Ortholog Cindr
As described above, the tyrosine at position Y10 in the SH3–1 domain is evolutionarily conserved. It is also found in the CD2AP paralog CIN85 which, similar to CD2AP, has three SH3 domains. Despite significant sequence similarity between the two proteins, they have independent and even opposing functions in podocytes.24 Downregulation, as well as knockout, of CD2AP leads to upregulation of CIN85 in podocytes, and CIN85 can induce ubiquitination and endocytosis of nephrin. In addition, CIN85 overexpression in zebrafish larvae leads to proteinuria, and CIN85 overexpression cannot rescue the phenotypes of CD2AP knockdown.24 CD2AP and CIN85 are the results of a genome duplication event early in the evolutionary history of vertebrates, and a corresponding single-copy gene is found throughout all of the major groups of the invertebrates.25 In the fruit fly (Drosophila melanogaster), the CD2AP/CIN85 ortholog Cindr is found to be necessary for proper eye patterning.18 This single-copy gene arose before the evolution of podocytes and CD2AP was likely co-opted into a role for podocyte function after the gene-duplication event. Interestingly, when we performed rescue experiments in CD2AP-knockdown zebrafish with CindrWT RNA, we observed complete rescue of edema and proteinuria phenotypes (Figure 4C). In contrast, CindrY13F RNA with replacement of tyrosine at position 13 (which corresponds to Y10 in CD2AP) could not rescue edema or proteinuria caused by CD2AP knockdown (Figure 4C).
Rescue Experiments in D. melanogaster
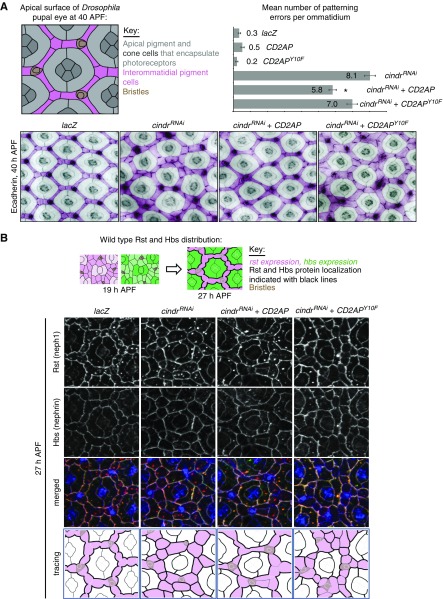
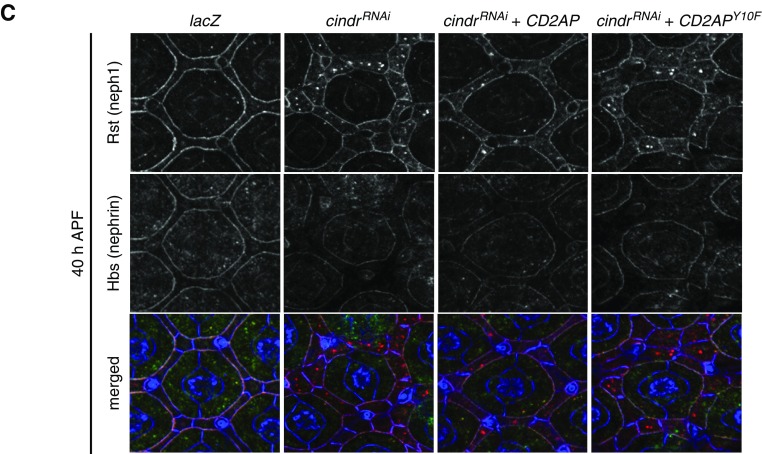
Because proteinuria in the zebrafish model was functionally rescued by expression of the Drosophila Cindr, we next used Drosophila as an in vivo model to analyze whether tyrosine mutation would affect Cindr/CD2AP function and the localization of interacting protein partners. As previously described, reducing Cindr in Drosophila severely disrupts patterning of epithelial tissues.18 The fly compound eye is characterized by the ordered arrangement of interommatidial pigment-producing cells into a lattice that separates ommatidia (Figure 5, A and B).26,27 Each ommatidium comprises a bundle of photoreceptor cells encapsulated by stereotypically shaped pigment and cone cells (Figure 5A). This striking arrangement of ommatidial and interommatidial cells is generated during pupal development. By 40 hours APF, the pattern of the eye is established. We found that expression of cindrRNAi transgenes using the GMR-GAL4 driver line severely disrupted eye patterning (Figure 5A, lower panel). When we used this model in a cross-species rescue setting, we found that simultaneous expression of mammalian CD2AP, but not CD2APY10, significantly compensated for loss of Cindr to restore eye patterning (Figure 5A, lower panel). Expression of only CD2AP or CD2APY10F in an otherwise wild-type background did not disrupt eye patterning (data not shown). Because Cindr is known to interact with the orthologs of neph1 and nephrin, we examined their localization when Cindr was reduced and the CD2AP or CD2APY10F constructs expressed. Rst (neph1) and Hbs (nephrin) are expressed in ommatidial and interommatidial cells in early pupal development but then become restricted to specific cell populations. As illustrated in Figure 5B, Rst becomes restricted to the interommatidial lattice cells and Hbs to the ommatidial pigment cells.28,29 Consequently, Rst-Hbs complexes become localized to membranes between interommatidial and ommatidial pigment cells and cleared from other cell-cell contacts (Figure 5B). Reducing Cindr does not modify rst or hbs expression but, and as previously described,30 Rst and Hbs remained equally distributed about the entire circumference of lattice cells (Figure 5B). This, in part, accounts for mispatterning of GMR>cindrRNAi retinas because correct localization of Rst-Hbs complexes is essential for the guidance of cells into appropriate positions during eye patterning.28,29 Earlier investigations suggested that Cindr secures Rst-Hbs complexes at appropriate locations while also regulating their endocytosis.30 Interestingly, expression of CD2AP largely restored correct localization of both Rst and Hbs in eyes dissected at 27 hours APF (Figure 5B). In contrast, CD2APY10F failed to modify cindrRNAi-induced Rst/Hbs mislocalization (Figure 5B). At 40 hours APF, Rst remained densely localized about the entire circumference of most interommatidial cells expressing cindrRNAi but Hbs was severely reduced, suggesting that, unless engaged in secure Rst-Hbs complexes, Hbs is readily degraded. Surprisingly, expression of CD2AP did not restore the correct density of Hbs at 40 hours APF (Figure 5C), suggesting that although CD2AP was able to substitute for loss of Cindr earlier, the protein failed to maintain this role long-term. These data indicate that phosphorylation on Y13(Y10) is indispensable for Cindr (CD2AP)-mediated cell patterning and the appropriate regulation of its binding partners Rst and Hbs (neph1 and nephrin) in vivo.
Figure 5.
CD2AP but not CD2APY10F rescues cindrRNAi eye-mispatterning. (A) Illustration of an ommatidium at 40 hours APF, with cell types labeled. Small regions of eyes expressing UAS-lacZ (abbreviated to GMR>lacZ), cindrRNAi (which severely disrupted patterning), CD2AP and cindrRNAi (which partly rescued patterning), and CD2APY10F and cindrRNAi. Quantification of the mean number of patterning defects per ommatidium: x axis plots error number for each genotype, as shown. Seventy-five ommatidia were analyzed per genotype. Error bars indicate SEM. Asterisk represents statistically significant differences between patterning defects in GMR>cindrRNAi and GMR>cindrRNAi +CD2AP eyes. GMR>cindrRNAi, CD2APY10F did not differ significantly from GMR>cindrRNAi. (B) Illustrations of rst (neph1) and hbs (nephrin) expression and localization at 19 hours APF and 27 hours APF. Immunofluorescence of ECadherin (blue), Rst (red), and Hbs (green) in a control GMR>lacZ eye at 27 hours APF and eyes expressing cindrRNAi (which disrupted Rst and Hbs localization), cindrRNAi and CD2AP (which partly rescued Rst/Hbs localization), and cindrRNAi and CD2APY10F. In tracings of each image (bottom panels), interommatidial cells are pink and density of black cell outlines represents density of Rst/Hbs detected at membranes. (C) Immunofluorescence of ECadherin (blue), Rst (red), and Hbs (green) in a control GMR>lacZ eye at 40 hours APF and eyes expressing cindrRNAi (which disrupted Rst localization; little Hbs is observed at cell membranes), cindrRNAi and CD2AP (which partly rescued Rst localization but not Hbs, which is not robustly detected at cell membranes), and cindrRNAi and CD2APY10F.
Phosphorylation of Tyrosine 10 in CD2AP via Src-Kinase Influences Nephrin Binding
To further support our in vivo data, we examined the influence of tyrosine residue 10 on the interaction of CD2AP with nephrin. We performed immunoprecipitation using single mutants for the tyrosine residues 4, 8, and 10, as described for Figure 2B. The experiments revealed enhanced binding of CD2AP with nephrin when the tyrosine residue 10 was replaced by a phenylalanine residue (Figure 6A). Immunoprecipitation experiments with CIN85, which binds to the coiled-coil region of CD2AP, and p85, which binds to the proline-rich region of CD2AP, did not show any differences between WT and CD2AP tyrosine mutants (Supplemental Figure 2A).
Figure 6.
Phosphorylation of CD2AP at Tyr10 is physiologically relevant in podocytes. (A) HEK-293T cells were transfected with myc-tagged CD2AP, mutated CD2APY4F, CD2APY8F, or CD2APY10F and cotransfected with flag-tagged Nephrin. Coimmunoprecipitation was performed with a flag-antibody and western blot with a myc-antibody. (B) Western blot of lysates of murine and human differentiated podocytes treated for 24 hours with VEGF-A, probed with anti-pCD2APtyr10 and anti-CD2AP. (C) The Src-recognition sequence is predicted to contain tyrosine 10 in CD2AP. Differentiated murine podocytes were treated or untreated with VEGF-A (20 ng/ml) and the specific Src inhibitor PP1 (5 µm, preincubated 1 hour before VEGF-A treatment). Western blot was performed with anti-pCD2APtyr10 and anti-CD2AP. (D) Cryosections of murine glomeruli stained with anti-CD2AP (green), anti-pCD2APtyr10 (red), and DAPI (blue). IP, immunoprecipitation; WB, western blotting.
Time-course experiments with VEGF-A over 24 hours with a custom-made monoclonal phospho-CD2APtyr10 antibody revealed a significantly prolonged tyrosine phosphorylation response of CD2AP (Figure 6B).
Furthermore, we identified a conserved Src binding sequence within the SH3–1 domain surrounding tyrosine residue 10. Western blot analysis revealed a strong reduction of phosphorylation of tyrosine residue 10 in the presence of the Src-kinase inhibitor PP1 (5 µm, 1 hour pretreatment), which indicates a direct involvement of Src-kinase in CD2AP phosphorylation (Figure 6C). Moreover, inhibition of Src was performed by the Src-inhibitor SU6656 (data not shown), which also revealed a strong reduction of CD2AP phosphorylation. With the caveat suggested in the literature that neither inhibits Src-kinase activity 100%, these results clearly indicate that Src-kinase is involved in this signaling cascade. Interestingly, staining of glomeruli with antibodies to CD2AP and pCD2APTyr10 revealed that the majority of the protein is phosphorylated, indicated by a complete costaining with total CD2AP (Figure 6D).
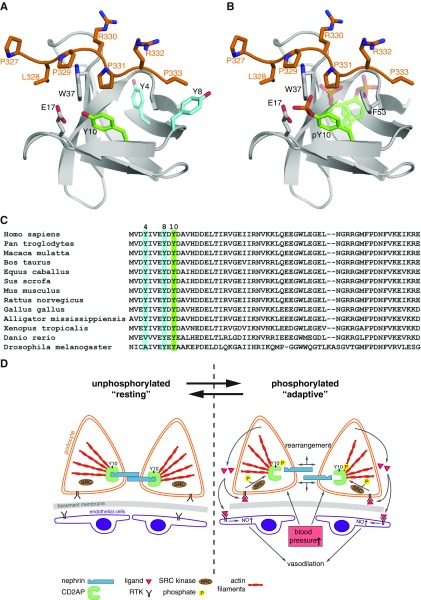
By modeling the crystal structure of the SH3–1 domain together with a peptide of CD2, we investigated the role of the phosphorylation of tyrosine residue 10 in steric binding. We found that the addition of a phosphate group to the aromatic hydroxyl group of Y10 induces an electrostatic repulsion between Y10 and E17 of SH3–1, which would lead to severe steric clashes with P331 of the peptide and F53 of SH3–1 (Figure 7, A–C).
Figure 7.
Phosphorylation of Tyr10 induces conformational changes that alter ligand binding. (A) Model of the CD2AP-SH3A (CMS-SH3–1) domain in complex with a CD2 peptide (residues 327–333). Crystal structure (Protein Data Bank entry code 2J6O) of SH3-A is shown in gray and the CD2 peptide in orange. The side chains of Y4 and Y8 are shown in blue, and the side chain of Y10 in green. (B) Addition of a phosphate group to the aromatic hydroxyl group of Y10 induces electrostatic repulsion between Y10 and E17 of SH3-A. Repositioning of the side chain of Y10 (indicated in transparent green) would lead to severe steric clashes with P331 of the peptide and F53 of SH3-A, respectively. Hence, phosphorylation of Y10 would modify interactions with proline-rich peptides and ligands including nephrin. (C) Alignment of conserved protein sequence of CD2AP/CMS from different species. Nonphosphorylated tyrosines 4 and 8 are highlighted in blue, and Y10 is highlighted in green. Y4 is not strictly conserved between all sequences shown. (D) Schematic overview of proposed CD2AP phosphorylation. RTK activation (e.g., by VEGF) leads to phosphorylation of CD2AP on tyrosine 10 via Src-kinase. This leads to steric changes and rearrangement between the connection of CD2AP to nephrin and actin filaments. Thus, autocrine VEGF can lead to an adaptive state in podocytes compensating for vasodilatation- and BP-induced dynamics. RTK, receptor tyrosine kinase.
Discussion
Defects in podocyte signaling and mutations in structural proteins of the podocyte are the basis of many inherited glomerular diseases leading to glomerulosclerosis.31 CD2AP is highly expressed in podocytes and plays an important role in the maintenance of the glomerular slit diaphragm, because the knockout in mice32 leads to a severe nephrotic syndrome and the human mutation33 results in an FSGS phenotype. On the basis of these initial observations it was proposed that CD2AP functions as a scaffold, linking the slit diaphragm protein nephrin to the actin cytoskeleton, and thus is indispensable for slit diaphragm integrity.34 We and others have previously shown that CD2AP is actively involved in the transmission of intracellular signals involved in survival regulation of the podocyte.23,35–38 Because the expression of nephrin only changes with the onset of proteinuria, CD2AP is not necessary to build a functional filtration barrier.25 These data have prompted the hypothesis that direct interactions between CD2AP and nephrin are not essential for podocyte function and that CD2AP has many more additional functional roles than originally proposed. Several groups have examined interactions between CD2AP and nephrin but the domains/motifs that mediate this interaction have not been mapped.5,32 Upon review of published data and on the basis of our own experimental experience, we suggest that CD2AP-nephrin interactions are of low stoichiometry and are strongly influenced by reversible modifications such as phosphorylation. Here, we confirm that CD2AP is tyrosine phosphorylated. We demonstrate a switch to CD2AP phosphorylation in cells cultured to confluence in vitro, suggesting that CD2AP phosphorylation is a key signal in sensing cell density to counter-balance proliferation. With our cross-species rescue approach, we defined Y10 in the SH3–1 domain of CD2AP as the biologically relevant residue. Our data indicate that Y10-phosphorylation is indispensable for proper function of the glomerular filtration barrier in zebrafish. These data correlate with our observation that CD2AP is phosphorylated in vertebrate glomeruli: immunofluorescence utilizing a pCD2APTyr10 antibody reveals that phosphorylation of Y10 represents the physiologic state. In addition, our data in Drosophila indicate that Y13 phosphorylation is essential for the correct localization of the nephrin/neph1 orthologs Hbs and Rst and correct patterning of Drosophila eye tissue. We also define Src as one potential kinase responsible for Y10 phosphorylation, which correlates with published literature reporting that Src-kinase influences the activity of slit diaphragm proteins.2 Interestingly, immunoprecipitation experiments with tyrosine mutants revealed modified binding to nephrin when CD2AP was phosphorylated. Specifically, we found that mutation of tyrosine Y10, but not Y4 or Y8, led to increased binding of CD2AP to nephrin. Molecular modeling of the published crystal structure of the SH3–1 domain indicated that the addition of a phosphate group to the aromatic hydroxyl group of Y10 leads to electrostatic repulsion between Y10 and E17 of SH3–1 and severe steric clashes that would modify interactions between CD2AP and its binding partners, including nephrin. Phosphorylation of Y4 and Y8 would not induce these molecular effects. Surprisingly, our data suggest that enhanced binding of nephrin and CD2AP in the absence of Y10 phosphorylation is functionally unfavorable for the podocytes, which is counter to current views of the importance of this interaction. In normal murine glomeruli we detected a complete overlap of p-CD2APtyr10 with total CD2AP, indicating that this reflects the “normal” state. Immunofluorescence staining of kidneys from diabetic mice with a loss of podocytes revealed a diminished staining of p-CD2APtyr10 but also a loss of total CD2AP staining (data not shown).
In summary, we provide the first evidence that identification of the interacting domains of the nephrin-CD2AP complex and the involvement of tyrosine residues within the SH3–1 domain is of fundamental importance to our understanding of the complex network of interactions and signaling pathways involved in organization and maintenance of the slit diaphragm. Phosphorylation of Y10 seems to be essential for normal distribution of slit diaphragm molecules and could be part of an endogenous maintenance mechanism and possibly “adaptive state” that responds to autocrine VEGF-signaling in the podocyte (Figure 7D). This indicates that further characterization of CD2AP phosphorylation profiles in different podocyte damage models and human biopsy specimens is necessary and might lead to functional disease profiling of the slit diaphragm complex. Further understanding of the interactions between CD2AP and nephrin molecules will enable identification of critical molecular targets that could be the subjects of future therapeutic strategies for the treatment of proteinuric kidney diseases.
Disclosures
Dr. Tossidou reports grants from German Research Council, grants from Fritz Thyssen Foundation, and grants from BMBF during the conduct of the study. Dr. Worthmann reports grants from German Research Council, grants from Fritz Thyssen Foundation, and grants from BMBF during the conduct of the study. Dr. Bolanos-Palmieri reports grants from German Research Council, grants from Fritz Thyssen Foundation, and grants from BMBF during the conduct of the study. All of the remaining authors have nothing to disclose.
Funding
This work was supported by German Research Council (Deutsche Forschungsgemeinschaft) grants SCHI 587/11-1 to Dr. Schiffer and MU379777/1-1 to Dr. Müller-Deile, as well as the Fritz Thyssen Foundation (10.16.2.026MN) to Dr. Eschenburg, Bundesministerium für Bildung und Forschung grant 01GM1518A to Dr. Schiffer, and Wesleyan Project grants to Dr. Johnson. Dr. Johnson, Dr. Drost, and Dr. de Jonge were supported by Wesleyan University start-up funds and project grants awarded to Dr. Johnson.
Supplementary Material
Acknowledgments
Dr. Schiffer, Dr. Tossidou, Dr. Haller, and Dr. Johnson designed the research studies. Dr. Schroder, Dr. Kardinal, Dr. Tossidou, Dr. Johnson, Dr. Worthmann, Dr. Bolanos-Palmieri, and Dr. Schiffer conducted experiments. Mr. Willerding, Dr. Reubold, and Dr. Eschenburg modelled the SH3 domain. Dr. Schiffer, Dr. Müller-Deile, Dr. Tossidou, and Dr. Schroder performed zebrafish experiments. Dr. Johnson and Dr. Drost performed Drosophila experiments. Dr. Johnson, Dr. Tossidou, Dr. Teng, and Dr. Schiffer analyzed data. Dr. Tossidou, Dr. Johnson, and Dr. Schiffer wrote the manuscript. Dr. Jobst-Schwan did the artwork of schematic overview.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080860/-/DCSupplemental.
Supplemental Figure 1, A–H. CD2AP is tyrosine phosphorylated in response to VEGF-A stimulation.
Supplemental Figure 2A. Absence of tyrosine 10 does not affect binding of proteins to the proline-rich or coiled-coil region of CD2AP.
References
- 1.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, et al.: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, et al.: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Martin CE, Jones N: Nephrin signaling in the podocyte: An updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol (Lausanne) 9: 302, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tryggvason K, Pikkarainen T, Patrakka J: Nck links nephrin to actin in kidney podocytes. Cell 125: 221–224, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Palmén T, Lehtonen S, Ora A, Kerjaschki D, Antignac C, Lehtonen E, et al.: Interaction of endogenous nephrin and CD2-associated protein in mouse epithelial M-1 cell line. J Am Soc Nephrol 13: 1766–1772, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Kirsch KH, Georgescu MM, Shishido T, Langdon WY, Birge RB, Hanafusa H: The adapter type protein CMS/CD2AP binds to the proto-oncogenic protein c-Cbl through a tyrosine phosphorylation-regulated Src homology 3 domain interaction. J Biol Chem 276: 4957–4963, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Smithgall TE: SH2 and SH3 domains: Potential targets for anti-cancer drug design. J Pharmacol Toxicol Methods 34: 125–132, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Songyang Z, Cantley LC: Recognition and specificity in protein tyrosine kinase-mediated signalling. Trends Biochem Sci 20: 470–475, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Ingley E: Src family kinases: Regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta 1784: 56–65, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Liu BA, Nash PD: Evolution of SH2 domains and phosphotyrosine signalling networks. Philos Trans R Soc Lond B Biol Sci 367: 2556–2573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabian H, Otvos L Jr., Szendrei GI, Lang E, Mantsch HH: Tyrosine- versus serine-phosphorylation leads to conformational changes in a synthetic tau peptide. J Biomol Struct Dyn 12: 573–579, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Verma R, Venkatareddy M, Kalinowski A, Patel SR, Salant DJ, Garg P: Shp2 associates with and enhances nephrin tyrosine phosphorylation and is necessary for foot process spreading in mouse models of podocyte injury. Mol Cell Biol 36: 596–614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertuccio C, Veron D, Aggarwal PK, Holzman L, Tufro A: Vascular endothelial growth factor receptor 2 direct interaction with nephrin links VEGF-A signals to actin in kidney podocytes. J Biol Chem 286: 39933–39944, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quack I, Woznowski M, Potthoff SA, Palmer R, Königshausen E, Sivritas S, et al.: PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J Biol Chem 286: 12959–12970, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleem MA, Ni L, Witherden I, Tryggvason K, Ruotsalainen V, Mundel P, et al.: Co-localization of nephrin, podocin, and the actin cytoskeleton: Evidence for a role in podocyte foot process formation. Am J Pathol 161: 1459–1466, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emsley P, Lohkamp B, Scott WG, Cowtan K: Features and development of coot. Acta Crystallogr D Biol Crystallogr 66: 486–501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, et al.: Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746–F1750, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Johnson RI, Cagan RL: A quantitative method to analyze Drosophila pupal eye patterning. PLoS One 4: e7008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman M: Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87: 651–660, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Müller-Deile J, Worthmann K, Saleem M, Tossidou I, Haller H, Schiffer M: The balance of autocrine VEGF-A and VEGF-C determines podocyte survival. Am J Physiol Renal Physiol 297: F1656–F1667, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Batt DB, Roberts TM: Cell density modulates protein-tyrosine phosphorylation. J Biol Chem 273: 3408–3414, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Möller CC, Wei C, et al.: CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest 121: 3965–3980, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng B, Schroder P, Müller-Deile J, Schenk H, Staggs L, Tossidou I, et al.: CIN85 deficiency prevents nephrin endocytosis and proteinuria in diabetes. Diabetes 65: 3667–3679, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, et al.: CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J Biol Chem 285: 25285–25295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cagan R: Principles of Drosophila eye differentiation. Curr Top Dev Biol 89: 115–135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cagan RL, Ready DF: The emergence of order in the Drosophila pupal retina. Dev Biol 136: 346–362, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Fischbach KF, Corbin V, Cagan RL: Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev Biol 344: 948–956, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao X, Zhang W, Krencik R, Deng H, Wang Y, Girton J, et al.: The JIL-1 kinase interacts with lamin Dm0 and regulates nuclear lamina morphology of Drosophila nurse cells. J Cell Sci 118: 5079–5087, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Johnson RI, Bao S, Cagan RL: Interactions between Drosophila IgCAM adhesion receptors and cindr, the Cd2ap/Cin85 ortholog. Dev Dyn 241: 1933–1943, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, et al.: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Löwik MM, Groenen PJ, Pronk I, Lilien MR, Goldschmeding R, Dijkman HB, et al.: Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int 72: 1198–1203, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tossidou I, Kardinal C, Peters I, Kriz W, Shaw A, Dikic I, et al.: CD2AP/CIN85 balance determines receptor tyrosine kinase signaling response in podocytes. J Biol Chem 282: 7457–7464, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Xavier S, Niranjan T, Krick S, Zhang T, Ju W, Shaw AS, et al.: TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol 20: 2127–2137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummins TD, Wu KZL, Bozatzi P, Dingwell KS, Macartney TJ, Wood NT, et al.: PAWS1 controls cytoskeletal dynamics and cell migration through association with the SH3 adaptor CD2AP. J Cell Sci 131, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asanuma K, Campbell KN, Kim K, Faul C, Mundel P: Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci U S A 104: 10134–10139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.