Significance Statement
Mitochondrial dynamics encompass cycles of fission and fusion, shifting toward fission during cell stress, resulting in mitochondrial fragmentation (which requires cleavage of outer and inner membranes) and apoptosis. Studies have suggested that Bif-1 (a protein implicated in apoptosis and mitophagy) and prohibitin-2 (which forms complexes in the inner membrane with prohibitin-1) are involved in regulation of mitochondrial dynamics. The authors demonstrate that upon cell stress, Bif-1 translocates to mitochondria and binds prohibitin-2, resulting in the disruption of prohibitin complexes and proteolytic inactivation of the inner membrane fusion protein OPA1. In mice, Bif-1 binds prohibitin-2 during renal ischemia-reperfusion injury; Bif-1-deficiency protects against OPA1 proteolysis, mitochondrial fragmentation, and apoptosis. These findings thus identify Bif-1 as an important regulator of the mitochondrial inner membrane during cell stress via interaction with prohibitin-2.
Keywords: renal ischemia, mitochondria, apoptosis
Abstract
Background
Mitochondria are dynamic organelles that undergo fission and fusion. During cell stress, mitochondrial dynamics shift to fission, leading to mitochondrial fragmentation, membrane leakage, and apoptosis. Mitochondrial fragmentation requires the cleavage of both outer and inner membranes, but the mechanism of inner membrane cleavage is unclear. Bif-1 and prohibitin-2 may regulate mitochondrial dynamics.
Methods
We used azide-induced ATP depletion to incite cell stress in mouse embryonic fibroblasts and renal proximal tubular cells, and renal ischemia-reperfusion to induce stress in mice. We also used knockout cells and mice to determine the role of Bif-1, and used multiple techniques to analyze the molecular interaction between Bif-1 and prohibitin-2.
Results
Upon cell stress, Bif-1 translocated to mitochondria to bind prohibitin-2, resulting in the disruption of prohibitin complex and proteolytic inactivation of the inner membrane fusion protein OPA1. Bif-1-deficiency inhibited prohibitin complex disruption, OPA1 proteolysis, mitochondrial fragmentation, and apoptosis. Domain deletion analysis indicated that Bif-1 interacted with prohibitin-2 via its C-terminus. Notably, mutation of Bif-1 at its C-terminal tryptophan-344 not only prevented Bif-1/prohibitin-2 interaction but also reduced prohibitin complex disruption, OPA1 proteolysis, mitochondrial fragmentation, and apoptosis, supporting a pathogenic role of Bif-1/prohibitin-2 interaction. In mice, Bif-1 bound prohibitin-2 during renal ischemia/reperfusion injury, and Bif-1-deficiency protected against OPA1 proteolysis, mitochondrial fragmentation, apoptosis and kidney injury.
Conclusions
These findings suggest that during cell stress, Bif-1 regulates mitochondrial inner membrane by interacting with prohibitin-2 to disrupt prohibitin complexes and induce OPA1 proteolysis and inactivation.
Mitochondria are a class of highly dynamic organelles that constantly undergo fission and fusion to maintain homeostasis.1–5 The dynamic nature is important to the viability of mitochondria and their bioenergetic and signaling functions within a cell. Disruption of mitochondrial dynamics is disrupted in multiple disease models leading to mitochondrial dysfunction and damage, followed by cell injury and death; conversely, preservation of mitochondrial dynamics results in the prevention of mitochondrial injury, cell death, and tissue damage in the heart, brain, and kidneys, supporting a pathogenic role of disturbance of mitochondrial dynamics.6–14
Mitochondrial dynamics involves the regulation at both mitochondrial outer and inner membranes. The fusion of mitochondrial outer membrane (MOM) depends on the functional interaction of mitofusins (Mfn1 and Mfn2), whereas fission is initiated by the dynamin-related protein Drp1, which is recruited from cytosol to MOM to form constriction rings.1–3 On the other hand, OPA1 is a key protein for the fusion of mitochondrial inner membrane (MIM).15 In cells, OPA1 exists in short and long forms. Although long OPA1 is required for MIM fusion, its proteolytic processing into short forms facilitates MIM cleavage or fission.15
Upon cell stress, mitochondrial dynamics is shifted to fission, resulting in mitochondrial fragmentation, which may contribute to mitochondrial damage and cell death.16 Mitochondrial fragmentation requires the cleavage of both MOM and MIM. MOM cleavage is a combined result of fission activation by Drp1 and fusion cessation from the binding of mitofusins by regulatory proteins such as Bak,16,17 whereas MIM cleavage depends largely on the excessive proteolysis and loss of long OPA1 and consequent arrest of fusion.15 Several proteases have been implicated in OPA1 processing in various conditions, but recent studies indicate that OMA1, a zinc metalloprotease on MIM, is mainly responsible for OPA1 proteolysis and inactivation during cell stress and apoptosis.18–21 It has been puzzling as to how OMA1 is released or activated for OPA1 proteolysis. In this regard, an intriguing scenario is that OMA1 is normally sequestered in the ring complex formed by the prohibitin proteins PHB1 and PHB2; during cell stress, the prohibitin complex may be disrupted to release OMA1.22 This possibility is supported by the observation of a large ring structure formed by prohibitins and the fact that PHB2 deficiency leads to OPA1 proteolysis, aberrant mitochondrial morphology, and cellular susceptibility to apoptosis.23,24 However, disruption of prohibitin complexes during cell stress has yet to be demonstrated and, mechanistically, the trigger of the disruption is completely unknown. In this study, we have identified Bif-1 as the trigger of the disruption of prohibitin complexes for OPA1 proteolysis and MIM cleavage using cell stress and renal ischemia-reperfusion models.
Bif-1, also known as SH3GLB1 or endophilin B1, was originally discovered as a Bax interacting protein.25,26 Functionally, Bif-1 has been implicated in Bax activation in apoptosis,27 and autophagy and mitophagy regulation.28,29 In addition, Bif-1 deficiency resulted in an aberrant mitochondrial morphology suggesting the involvement of Bif-1 in the regulation of mitochondrial dynamics,30 although the underlying mechanism is unclear. In this study, we demonstrate a role of Bif-1 in the regulation of mitochondrial dynamics at MIM. We show that Bif-1 translocates to mitochondria and interacts with PHB2 during cell stress, leading to the disruption of prohibitin complexes and OPA1 proteolysis, contributing to MIM cleavage, mitochondrial fragmentation, and apoptosis.
Methods
Bif-1 Knockout Mice and Renal Ischemia-Reperfusion Injury
All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Charlie Norwood VA Medical Center (approval no. 17–04–099). Bif-1 knockout (KO) mice were generated in previous work28 and backcrossed with C57BL/6 mice to generate heterozygous (Bif-1+/−) breeders, which were mated to produce wild-type (WT) (Bif-1+/+) and homozygous KO (Bif-1−/−) mice. The mice (male, aged 8–10 weeks) were subjected to 30 minutes of bilateral renal ischemia followed by brief (approximately 15 minutes) reperfusion for mitochondrial analysis or 2 days of reperfusion for examination of renal function and histology, as before.8 Control mice were subjected to sham operation without renal pedicle clamping. Blood samples were collected to measure serum creatinine to indicate the loss of renal function. Kidneys were harvested for histopathologic, electron microscopic, TUNEL, and biochemical analyses. In histology, tubular damage was quantified by the percentage of renal tubules showing tubular lysis, loss of brush border, tubular dilation, and cast formation. In electron microscopy, the length of mitochondria in tubular cells was measured with ImageJ software (National Institutes of Health). Mitochondria longer than 2 µm were considered filamentous. The cells with <1% filamentous mitochondria were considered ones with mitochondrial fragmentation. In TUNEL assay, 16 microscopic fields were randomly selected from each tissue section to determine the average number of TUNEL-positive cells. For coimmunoprecipitation (co-IP) analysis of Bif-1/PHB2 interaction, mouse kidneys were harvested for homogenization in isolation buffer (0.27M sucrose, 1mM EGRA, 5mM Tris-HCl, pH 7.4) containing protease inhibitor cocktails. Kidney homogenates were centrifuged at 600×g for 10 minutes at 4°C to collect the supernatant for further centrifugation at 10,000×g for 5 minutes. The resultant pellet containing crude mitochondria was dissolved in lysis buffer (240107–51; Agilent) for immunoprecipitation with anti-PHB2 or anti-Bif-1 antibodies, followed by immunoblot analysis.
Cells
HEK293 and HeLa cells from American Type Culture Collection (Manassas, VA) were maintained in minimal essential medium with 10% FBS, 1% glutamine, 1% NEAA, and 1% antibiotics. The rat kidney proximal tubule cell (RPTC) line was originally obtained from Dr. Ulrich Hopfer (Case Western Reserve University, Cleveland, OH),31 cultured in Ham F-12/DME medium with 10% FBS and 17.5 mM glucose, and plated in collagen-coated dishes to grow overnight for experiment. Bif-1-null and (WT) mouse embryonic fibroblasts (MEFs) were as described previously.27 Bif-1-knockdown RPTC and HeLa cell lines were generated by stable transfection with Bif-1 shRNA. To reconstitute Bif-1 or its W344A mutant into Bif-1-null MEF, the pPACKH1 Lentivector Expression Systems (System Biosciences, Mountain View, CA) was used according to manufacturer’s manual. Briefly, Bif-1 and W344A mutant were subcloned into the pCDH-CMV-MCS-EF1-copGFP expression lentivector. The expression plasmid and pPACKH1 packaging plasmid mixture were added to 293TN cells for packaging. The culture medium with pseudoviral particles was collected at 48–72 hours and concentrated by centrifugation. The concentrated medium was used to infect Bif-1-null MEFs two to three times with TransDux (System Biosciences) at an infection efficacy >90%, as indicated by GFP reporter expression.
Reagents and Antibodies
Digitonin and dithiobis (succinimidyl propionate) (DSP crosslinker) were purchased from ICN Biomedicals Inc. (Aurora, OH) and Pierce (Rockford, IL), respectively. Other reagents and chemicals including azide and cisplatin were purchased from Sigma (St. Louis, MO). Antibodies were from the following sources: rabbit polyclonal anti-PHB2 obtained from previous work32 and Proteintech (Rosemont, IL); mouse monoclonal anti-Bif-1 from IMGENEX (San Diego, CA); goat polyclonal anti-Bif-1 from Abcam (Cambridge, MA); mouse monoclonal anti-cytochrome c (7H8.2C12 and 6H2.B4), anti-Drp1, and anti-OPA1 from BD Pharmingen (San Diego, CA); mouse monoclonal anti-Bax (1D1) from NeoMarkers (Fremont, CA); rabbit monoclonal anti-active caspase 3 (5A1E), rabbit monoclonal anti-COX IV (3E11), rabbit polyclonal anti-PARP, anti-PHB1, and anti-phospho (serine-637)-Drp1 from Cell Signaling Technology (Danvers, MA); rabbit polyclonal anti-Bax and anti-HSP60 from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal anti-Bax (NT) and anti-Bak from Upstate (Lake Placid, NY); rabbit polyclonal anti-Fis1 from ALEXIS Biochemicals (San Diego, CA); chicken polyclonal anti-Mfn1 from Novus Biologicals (Littleton, CO); rabbit polyclonal anti-Mfn2, anti-Myc, and anti-GAPDH, and mouse monoclonal anti-β-actin from Sigma; rabbit polyclonal anti-cyclophilin B from Abcam; all secondary antibodies were from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA).
Plasmids and Transfection
Bif-1 and Mutants
Bif-1 sequence was amplified from the cDNA of RPTC cells and cloned into pcDNA3.1/Myc-His via KpnI and BamHI sites. Bif-1 deletion mutants [A: Bif-1 (27–365), B: Bif-1 (262–365), C: Bif-1 (1–261)] were PCR-amplified from pcDNA3.1/Myc-His-Bif-1 and cloned into pcDNA3.1/Myc-His via KpnI and BamHI sites. The W3448A-Bif-1 mutant was generated from pcDNA3.1/Myc-His-Bif-1 using the QuikChange site-directed mutagenesis kit (Stratagene) with the following primers: sense 5′- GTTGGAATGGATTCAGACGCGCTAATGGGGGAAAGGGG-3′ and antisense 5′-CCCCTTTCCCCCATTAGCGCGTCTGAATCCATTCCAAC-3′. For lentiviral expression, Bif-1 and W344A-Bif-1 sequences were subcloned into pCDH-CMV-MCS-EF1-copGFP vector via NheI and NotI sites.
PHB2
PHB2 sequence was PCR-amplified from pLenti6/V5-D-TOPO-Phb2-WT donor plasmid32 and subcloned into pcDNA3.1/Myc-His via EcoRI and XhoI sites. All primers were synthesized by Integrated DNA Technologies (Coralville, IA). The sequences of prepared plasmids were verified by DNA sequencing. pDsRed2-Mito (MitoRed) was purchased from BD Clontech (Palo Alto, CA). Cells were transfected at 60%–80% confluence with approximately 1–2 µg plasmid DNA using lipofectamine LTX with Plus Reagent (Invitrogen). Treatment was initiated approximately 36 hours post-transfection.
Cell Injury Models
Cells were treated with azide to induce cellular stress and apoptosis by protocols modified from previous studies.8,17,33 Cells were incubated with azide (10 mM for RPTC, 20 mM for MEFs) in glucose-free Krebs–Ringer bicarbonate solution for indicated time to induce ATP depletion. The cells were then returned to full cultured medium for recovery. In this model, mitochondrial events of apoptosis including mitochondrial fragmentation, Bax accumulation, and cytochrome c (Cyt c) release occur during azide-induced ATP depletion.8,17 However, caspase activation and apoptotic morphology develops only after the azide-treated cells are returned to normal culture medium for recovery, because caspase activation via the apoptosome requires ATP.34 Therefore, mitochondrial changes were examined immediately after azide incubation, whereas apoptosis was evaluated after 2–3 hours of recovery in culture medium.
Apoptosis
Morphologically, cells were stained with Hoechst33342 and examined by phase contrast and fluorescence microscopy. Apoptotic cells were identified by characteristic morphology including cellular condensation, formation of apoptotic bodies, and condensation and fragmentation of the nucleus. For each sample, several random fields of cells (≥100 cells per dish) were evaluated to determine the percentage of apoptotic cells. Biochemically, the proteolytic processing of caspase 3 into active fragments and cleavage of PARP were examined by immunoblotting.
Mitochondrial Morphology
Cells were transfected with MitoRed to fluorescently label mitochondria for fluorescence microscopy as previously.8,17 Briefly, cells grown on glass coverslips at 60%–80% confluence were transfected with pDsRed2-Mito (BD Clontech). After treatment, mitochondrial morphology in individual cells was evaluated. Fragmented mitochondria were short, punctate, or rounded, whereas filamentous mitochondria showed a long thread-like (HeLa) or tubular (RPTC, MEF) morphology. In each sample, random fields of cells (≥100 cells per condition) were evaluated. For quantification, the cells with different mitochondrial morphologies were counted to determine the percentage of cells with fragmented mitochondria.
Cellular Fractionation with 0.05% Digitonin
Cells were fractionated with 0.05% digitonin in an isotonic sucrose buffer (250 mM sucrose, 10 mM HEPES-NaOH, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and 0.5 mM PMSF, pH 7.2) as described previously.8,17 At low concentrations, digitonin selectively permeabilizes the plasma membrane but not the mitochondrial membrane. After digitonin incubation, the soluble part was collected as released cytosol. The digitonin insoluble part contained the membrane-bound organellar fraction enriched with mitochondria.
Mitochondrial Isolation and Treatment with Alkaline, Digitonin, or Proteinase K
HEK293 cells were transiently transfected with Myc- Bif-1 and subjected to 2 hours of azide treatment on the next day before harvest by centrifugation. Cell pellets were washed with ice-cold PBS and suspended in 200 µl homogenization buffer (10 mM HEPES-KOH, pH 7.5, 0.22 M mannitol, 0.07 M sucrose). Cells were homogenized by passing through a syringe with 27-gauge needle five times, followed by centrifugation at 500×g for 5 minutes at 4°C to collect the supernatant that was further centrifuged at 8000×g for 5 minutes at 4°C to obtain mitochondria.
For alkaline treatment, isolated mitochondria (0.25 mg/ml) were incubated in sodium carbonate solution (0.1 M Na2CO3, pH 11) on ice for 30 minutes as before.35 Samples were then centrifuged at 100,000×g for 20 minutes to obtain supernatant and the membrane pellet. Proteins in the supernatant was precipitated with 10% TCA on ice for 30 minutes, centrifuged at 15,000×g, and washed completely with cold acetone. SDS/urea lysis buffer was added to dissolve the pellets for immunoblot analysis.
For digitonin treatment, mitochondria were treated with 0.5 mg/ml digitonin on ice for 30 minutes. Every 5 minutes, the sample was vortexed for 1 minute. The sample was then centrifuged at 10,000×g for 10 minutes to separate digitonin-soluble proteins from the mitoplast. Proteins in the supernatant was concentrated by Millipore Y-10 filter (Millipore), whereas the mitoplast pellet was washed and lysed with SDS/urea lysis buffer for immunoblot analysis.
For proteinase K protection assay, mitochondria were resuspended and incubated on ice in homogenization buffer containing proteinase K with or without 0.5% Triton X-100 for 30 minutes. PMSF (4 mM) was then added to quench proteinase K activity. Proteins were precipitated with 10% TCA for 30 minutes on ice, followed by centrifugation at 15,000×g for 5 minutes. The pellet was completely washed with ice-cold acetone and dissolved in SDS/urea lysis buffer for immunoblot analysis.
Bax Insertion and Oligomerization
Bax Insertion
Exposure to an alkaline buffer strips off the loosely attached proteins, but not the membrane-inserted proteins, from mitochondria. Alkaline exposure was performed as described previously.35 Briefly, cells were permeabilized with 0.05% digitonin to release cytosolic fraction. The membrane-bound organellar fraction containing mitochondria was collected, washed once with PBS, and then incubated on ice in 0.1 M Na2CO3 at pH 11.5 for 30 minutes. After 1 hour of centrifugation at 100,000×g, the pellet was collected for immunoblot analysis to reveal the inserted Bax that was resistant to alkaline stripping.
Bax Oligomerization
Bax oligomerization was analyzed after chemical crosslinking as described previously.35 Briefly, cells were permeabilized with 0.05% digitonin to release cytosol and collect the mitochondrial-enriched organellar fraction by centrifugation, which was subjected to 30 minutes of chemical crosslinking with 1 mM DSP at room temperature. The crosslinked samples were resolved on nonreducing SDS-PAGE for immunoblot analysis.
BN-PAGE and Size-Exclusion Chromatography
BN-PAGE
Cells were fractionated with digitonin to collect the organellar fraction enriched with mitochondria, which was then subjected to chemical crosslinking with 1 mM DSP to stabilize protein interactions. BN-PAGE was performed on ice or at 4°C using NativePAGE Novex 3%–12% Bis-Tris gel (Invitrogen) with a protocol modified from our previous work.36 Briefly, the samples were resuspended in the membrane lysis buffer (10% glycerol and 0.5 M aminocaproic acid in 20 mM Bis/Tris, pH 7.0) containing 1.0% digitonin, followed by centrifugation at 15,000×g to collect the supernatant. The collected samples were then loaded on NativePAGE gels and overlaid with Coomassie Blue solution for electrophoresis. After that, the proteins were transferred to PVDF membranes for immunoblot analysis to detect prohibitins in various protein complexes. The sizes of the protein complexes were established on the basis of the mobility of the marker proteins from Invitrogen (IgM Hexamer: 1236 kD, IgM Pentamer: 1048 kD, Apoferritin band 1–720 kD, Apoferritin band 2–480 kD) and the Precision Plus Protein Standards from Bio-Rad (250–100 kD).
Size-Exclusion Chromatography
Samples were prepared as described above for BN-PAGE and loaded to AKTA Purifier-FPLC System (GE/Amersham, Pittsburgh, PA) with TSKgel G3000SWxl gel filtration column (Tosoh Bioscience, King of Prussia, PA). Fractions were eluted with 100 mM phosphate buffer (pH 7.0) at a flow rate of 0.25 ml/min, collected and monitored for the absorption at 280 nm. The eluted proteins in individual fractions were precipitated with trichloroacetic acid and subjected to SDS-PAGE for immunoblot analysis. The chromatography column was calibrated with Sigma Gel Filtration Protein Standards: thyroglobulin (669 kD), apoferritin (440 kD), β-amylase (200 kD), alcohol dehydrogenase (150 kD), BSA (66 kD), and carbonic anhydrase (29 kD).
Yeast Two-Hybrid Screening
Matchmaker GAL4 Two-Hybrid System 3 and Human Kidney Matchmaker cDNA Library from Clontech were used for yeast two-hybrid analysis. Briefly, Bif-1 was subcloned into pGBKT7 vector to generate the bait construct, which was cotransformed into yeast (strain AH109) with the cDNA library. Medium-stringency (SD/-His/-Leu/-Trp) and high-stringency (SD/-Ade/-His/-Leu/-Trp/X-α-Gal) media were used for selecting AH109 transformants subsequently. Positive transformants were picked up for sequencing cDNA inserts by using T7 sequencing primer. To pinpoint the interacting proteins, cDNA sequences were compared with those in GenBank.
Immunoanalysis
Immunoblotting, immunoprecipitation, and immunofluorescence were conducted by standard methods. For anti-Myc IP, the c-Myc Tag IP/Co-IP Assay Systems from Pierce was used. Briefly, cell lysate was collected in the immunoprecipitation buffer with 1% (w/v) CHAPS. The lysate of 500 µg protein was subjected to IP by incubation with 5 µg anti-Myc antibody-conjugated beads (Pierce). After centrifugation and washes, the precipitated proteins were collected for indicated analysis.
Statistical Analyses
Quantitative data were analyzed by t test for the comparison between two groups and by ANOVA followed by Tukey post-test for comparison between multiple groups. The data were expressed as means±SD; P<0.05 was considered to reflect significant differences. Qualitative data including cell images and immunoblots are representatives of at least three experiments.
Results
Intrinsic Pathway of Apoptosis Is Blocked in Bif-1–Deficient Cells
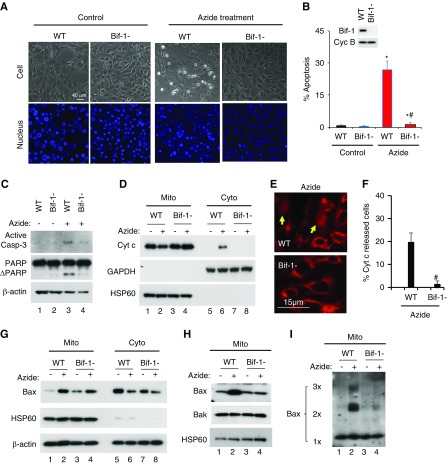
We initially compared apoptosis of WT and Bif-1-null MEFs after ATP depletion by azide, a mitochondrial respiration inhibitor. Although many WT MEFs developed apoptotic morphology, very few Bif-1-null cells did (Figure 1A). Cell counting showed that azide induced 26% apoptosis WT MEFs, whereas only 2% in Bif-1-null cells (Figure 1B). Consistently, azide induced caspase 3 activation and PARP cleavage in WT MEFs, which were suppressed in Bif-1-null cells (Figure 1C). The release of apoptogenic factors from mitochondria, such as Cyt c, is a key feature of the intrinsic pathway of apoptosis.37 In cellular fractionation assay (Figure 1D), azide induced Cyt c release from mitochondria (lane 2) into cytosol (lane 6) in WT MEFs, but not in Bif-1-null cells (lanes 4 and 8). This observation was confirmed by immunofluorescence staining, which showed that 20% of WT MEFs released Cyt c during azide treatment, whereas only 2% Bif-1-null cells did (Figure 1, E and F).
Figure 1.
Azide-induced intrinsic pathway of apoptosis is blocked in Bif-1-null MEF. (A–C) Apoptosis. WT and Bif-1-null MEFs were left untreated (Control) or treated with 20 mM azide in glucose-free buffer for 3 hours, followed by 2 hours of recovery in normal culture medium. The cells were then stained with Hoechst33342 to record cellular and nuclear morphology (A) and counted to determine the percentage of cells with apoptotic morphology (B), or were lysed for immunoblot analysis of active caspase 3, PARP, and cyclophilin B (loading control) (C). Insert in (B) is an immunoblot to confirm Bif-1 expression in WT MEFs and not in Bif-1-null cells. (D–F) Cyt c release from mitochondria. WT and Bif-1-null MEFs were treated with 20 mM azide in glucose-free buffer for 3 hours or left untreated. The cells were then fractionated into membrane-bound fraction with mitochondria (Mito) and cytosolic fraction (Cyto) for immunoblot analysis of indicated proteins (D), or fixed for immunofluorescence of Cyt c to record representative images (E) and count to determine the percentage of cells with Cyt c released into cytosol (F). Arrows in (E) point to the cells with Cyt c release. (G–I) Bax accumulation, insertion, and oligomerization in mitochondria. WT and Bif-1-null MEFs were treated with 20 mM azide in glucose-free buffer for 3 hours or left untreated. The cells were then fractionated into membrane-bound fraction with mitochondria (Mito) and cytosolic fraction (Cyto) for immunoblot analysis of indicated proteins (G). In addition, the Mito fraction was subjected to alkaline treatment to remove loosely attached proteins for immunoblot analysis of membrane-inserted Bax (H) or subjected to chemical crosslinking with 1 mM DSP for immunoblot analysis of Bax oligomerization (I). Immunoblots and images (A, C–E, G–I) are representatives of at least three independent experiments. The data (B and F) represent mean±SD (n=3; 300–600 cells evaluated per condition); *P<0.05 versus control; #P<0.05 versus WT/azide.
In the intrinsic pathway of apoptosis, Bax and Bak form a “gateway” to MOM permeabilization for the release of apoptogenic factors.38 Bax activation consists of the sequential events of Bax translocation, insertion, and oligomerization in mitochondria.39,40 Under control conditions, Bax was mainly detected in cell cytosol (Figure 1G: lanes 5 and 7); however, after azide treatment, Bax accumulated in the mitochondrial fraction in both WT and Bif-1-null MEFs (lanes 2 and 4), indicating that Bif-1 is not a key to Bax translocation. To analyze Bax insertion, mitochondrial fraction was isolated and exposed to an alkaline buffer to strip off the loosely attached proteins (but not membrane-inserted proteins) before immunoblot analysis. Without treatment, both WT and Bif-1-null MEFs had low levels of Bax inserted into mitochondria (Figure 1H: lanes 1 and 3); after azide treatment, WT MEFs (lane 2), but not Bif-1-null cells (lane 4), showed a drastic increase in Bax insertion. As expected, Bak, a Bax homolog and integral MOM protein, did not change subcellular localization during azide treatment (Figure 1H). We further examined Bax oligomerization in mitochondrial fraction (Figure 1I), showing that azide induced the formation of Bax oligomers in WT MEFs (lane 2), which was markedly suppressed in Bif-1-null cells (lane 4).
To confirm the results of MEFs, we generated Bif-1 knockdown cell lines from rat RPTC (Supplemental Figure 1A). Azide induced 39.2% apoptosis in scrambled sequence-transfected RPTC, whereas only 11% in Bif-1 knockdown cells (Supplemental Figure 1, B and C). Bif-1 knockdown also suppressed apoptosis-associated caspase 3 activation and PARP cleavage (Supplemental Figure 1D). Moreover, Cyt c release in this model was suppressed in Bif-1 knockdown cells (Supplemental Figure 2, A–C). Bif-1 knockdown did not abrogate Bax translocation (Supplemental Figure 2C), but it did suppress Bax insertion (Supplemental Figure 2D) and oligomerization (Supplemental Figure 2E) in mitochondria during apoptotic treatment. Together, these results suggest that Bif-1 promotes Bax insertion and oligomerization in mitochondria for activation of the intrinsic pathway of apoptosis.
Bif-1 Inserts into MOM for Mitochondrial Fragmentation in Apoptosis
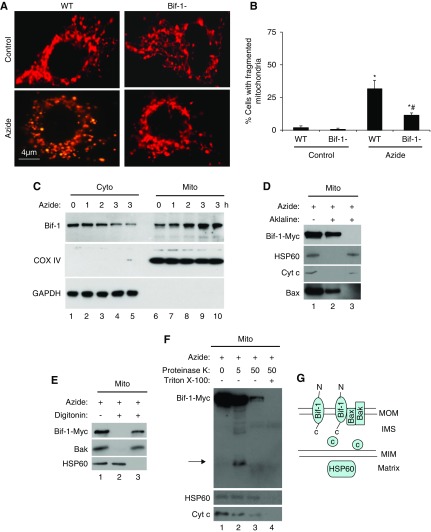
How does Bif-1 promote Bax insertion and oligomerization in mitochondria? It is generally believed that Bif-1 may directly bind and activate Bax during cell stress, resulting in the activation of the intrinsic pathway of apoptosis.27 However, we failed in detecting the interaction between endogenous Bif-1 and Bax in either control or stressed cells (not shown). Alternatively, Bif-1 may indirectly affect Bax by changing mitochondrial morphology or dynamics. In support of this possibility, Bif-1 knockdown induced an aberrant mitochondrial morphology.30 Moreover, we showed that fragmented mitochondria were sensitized to Bax insertion and oligomerization.35 We therefore hypothesized that Bif-1 might promote Bax integration in mitochondria during cell stress by inducing mitochondrial fragmentation. To test this hypothesis, we first monitored mitochondrial morphology in WT and Bif-1-null MEFs after transfection with MitoRed to label mitochondria. As shown previously,8,17 mitochondria became fragmented during azide treatment (Figure 2A). Notably, azide induced mitochondrial fragmentation in 30% WT MEFs, but only in 10% Bif-1-null cells (Figure 2B), indicating the regulation of mitochondrial dynamics by Bif-1.
Figure 2.
Azide-induced mitochondrial fragmentation in apoptosis is attenuated in Bif-1-null MEF. (A and B) Lower mitochondrial fragmentation in Bif-1-null MEFs. WT and Bif-1-null MEFs were transfected with MitoRed to fluorescently label mitochondria, then incubated with 20 mM azide in glucose-free medium for 3 hours or left untreated. Representative images of mitochondrial morphology were recorded (A) and for quantification, the cells with fragmented mitochondria were counted to determine the percentage (B). The data in (B) represent mean±SD (n=3; >100 cells evaluated per condition); * P<0.05 versus control; #P<0.05 versus WT/azide. (C) Bif-1 translocation to mitochondria. WT MEFs were treated with 20 mM azide for 0–3 hours. The cells were then fractionated into membrane-bound fraction with mitochondria (Mito) and cytosolic fraction (Cyto) for immunoblot analysis of Bif-1. Cox IV and GAPDH were probed as cytosolic and mitochondrial markers. (D) Mitochondrial Bif-1 is resistant to alkaline stripping. HEK293 cells were transfected with Bif-1-Myc and treated with 20 mM azide for 2 hours to isolate mitochondria, which were incubated with alkaline (0.1 M Na2CO3, pH 11) buffer followed by centrifugation to collect pellet and supernatant fractions for immunoblot analysis of Bif-1-Myc, HSP60, Cyt c, and Bax. Whole mitochondria without alkaline incubation was analyzed as a control. The majority of Bif-1 remained on mitochondrial membranes after alkaline incubation. (E) Digitonin extracts Bif-1 from mitochondria. Isolated mitochondria were incubated with 0.5 mg/ml digitonin and then centrifuged to collect the soluble fraction (lane 3) and the pellet (lane 2) containing mitoplast. Nontreated mitochondria were used as a control (lane 1). Like Bak, Bif-1-Myc was solubilized by digitonin, whereas the matrix protein HSP60 remained in mitoplast. (F) Proteinase K protection assay. Isolated mitochondria were treated with 5 or 50 μg/ml proteinase K for 30 minutes on ice in the absence or presence of Triton X-100 (0.5%), and then centrifuged to collect pellet for immunoblot analysis. Without Triton X-100, 5 μg/ml proteinase K partially digested Bif-1, releasing a fragment that was detected by anti-Myc antibody (lane 2), whereas 50 μg/ml proteinase K induced more digestion, likely because of compromising the integrity of outer membrane (lane 3). In the presence of Triton X-100 (lane 4), all three proteins (Bif-1, HSP60, Cyt c) were degraded as a result of complete exposure to proteinase K. Arrow: Bif-1 fragment released after proteinase K digestion. (G) Schematic diagram of Bif-1 submitochondrial localization. Marker proteins from different compartments of mitochondria are also shown.
To understand mitochondrial regulation by Bif-1, we first examined Bif-1 accumulation during cell stress. Cellular fractionation assay showed a time-dependent increase of Bif-1 in mitochondria and decrease in cytosol during azide treatment, indicating the translocation of Bif-1 from the cytosol to mitochondria (Figure 2C). To analyze the submitochondrial localization of Bif-1, we transfected human embryonic kidney (HEK293) cells with Bif-1-Myc and then treated the cells with azide for mitochondrial isolation. The isolated mitochondria were first subjected to “alkaline stripping” assay that solubilizes nonmembrane integral proteins (Figure 2D). Without alkaline treatment, mitochondria showed the presence of Bif-1-Myc, the matrix protein HSP60, intermembrane space protein Cyt c, and Bax, which inserts into MOM during apoptosis (Figure 2D: lane 1).39,40 After alkaline incubation, Bif-1-Myc and Bax remained with the membrane fraction (Figure 2D: lane 2), whereas HSP60 and Cyt c were released into the supernatant (Figure 2D: lane 3), suggesting that Bif-1 may integrate into mitochondrial membrane as Bax. To determine whether Bif-1 is located in mitochondrial outer or inner membrane, we treated mitochondria with 0.5 mg/ml digitonin to extract outer membrane proteins. As shown in Figure 2E, Bif-1-Myc and the outer membrane integral protein Bak were solubilized by digitonin (lane 3), whereas HSP60 remained with the pellet of mitoplasts (lane 2), suggesting that Bif-1 may integrate into the outer membrane during cell stress and apoptosis. Finally, we performed proteinase K protection assay (Figure 2F), where mitochondria were treated with increasing concentrations of proteinase K in the absence (lanes 1–3) or presence (lane 4) of Triton X-100. In the absence of Triton X-100, incubation with 5 µg/ml proteinase K resulted in a partial degradation of Bif-1-Myc and the appearance of a fragment in mitochondria that was recognized by anti-Myc antibody (Figure 2F: lane 2, arrow). Because Myc sequence was fused to Bif-1’s C terminus in Bif-1-Myc, generation of the fragment with Myc sequence (reactive to anti-Myc) suggests the exposure of Bif-1 C terminus inside of mitochondria. At 50 µg/ml proteinase K, most Bif-1-Myc and Cyt c were degraded, but HSP60 remained (lane 3), suggesting the loss of MOM integrity. With Triton X-100, Bif-1 as well as HSP60 and Cyt c were completely degraded (lane 4), owing to the exposure of all solubilized proteins to proteinase K. Together, these results support a model that Bif-1, after translocation to mitochondria during cell stress, becomes an integral outer membrane protein with its C terminus exposed to the intermembrane space (Figure 2G).
Bif-1 Interacts with PHB2 via C Terminus during Apoptosis
How does Bif-1 alter mitochondrial dynamics resulting in mitochondrial fragmentation in apoptosis? With this question, we initially focused on several well documented regulators of mitochondrial morphology. Azide induced a marginal accumulation of Drp1 in mitochondria (Supplemental Figure 3A: lanes 2 and 4) and a marked dephosphorylation of Drp1 at serine-637 (Supplemental Figure 3B: lanes 2 and 4), indicative of the activation of this key mitochondrial fission protein.41–43 Of note, Drp1 was also activated in Bif-1-null cells (Supplemental Figure 3, A and B). Bif-1 deficiency did not significantly change the expression of mitofusins (Mfn1 and Mfn2), Fis1, and Bak in mitochondria (Supplemental Figure 3C).
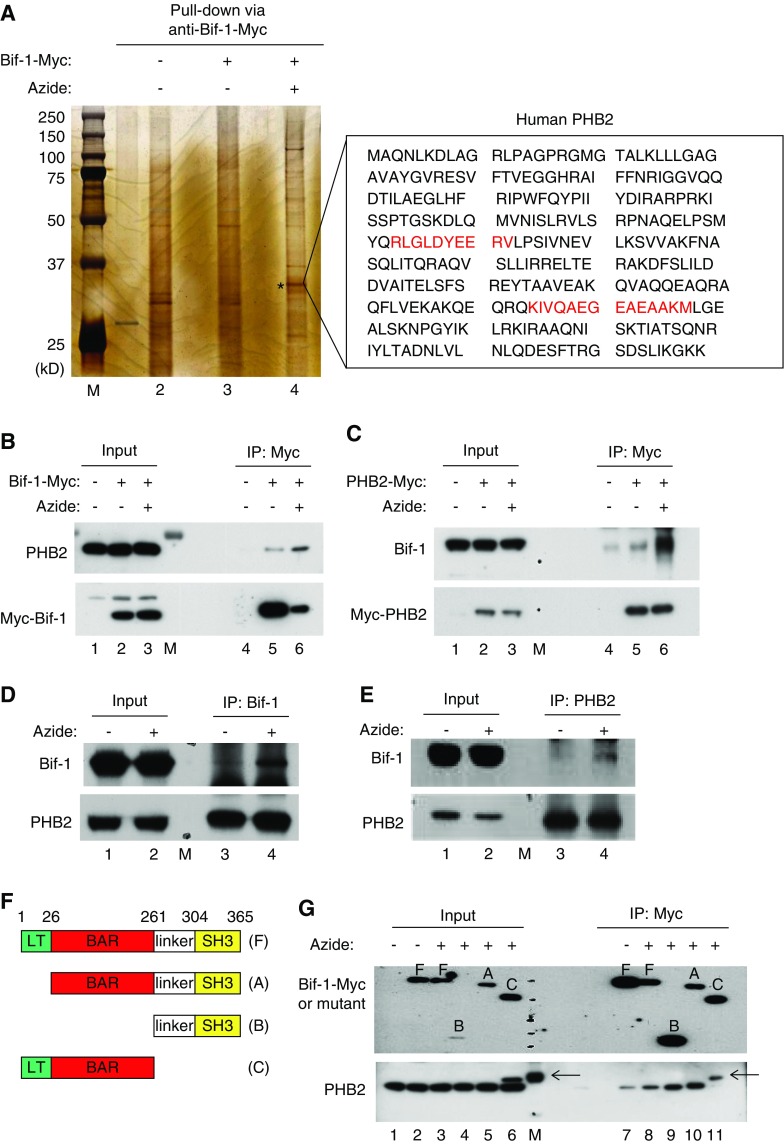
On the basis of those negative results, we decided to analyze Bif-1–interacting proteins. We first conducted yeast two-hybrid screening of human kidney cDNA library using Bif-1 as bait. The screening revealed that Bif-1 may self-interact, a finding consistent with the ability of Bif-1 to form homo-oligomers.44 In addition, the screening suggested that Bif-1 may interact with seven other proteins, including prohibitin-2 (PHB2), an MIM protein.24 In the second experiment, we performed affinity pulldown/mass spectrometry analysis to identify Bif-1–interacting proteins. HEK293 cells were transfected with Bif-1-Myc and then subjected to azide treatment, followed by immunoprecipitation with anti-Myc antibody. The immunoprecipitates were gel-resolved for silver staining (Figure 3A). Compared with control (Figure 3A: lane 3), a protein band of approximately 33 kD was consistently pulled down by anti–Bif-1-Myc from the lysate of azide-treated cells (Figure 3A: lane 4, asterisks). Mass spectrometry identified this protein as PHB2 (Figure 3A: two sequences identified by mass spectrometry are highlighted in red). Thus, both yeast two-hybrid screening and affinity pulldown mass spectrometry suggest that Bif-1 may interact with PHB2, a highly conserved protein with a predominant localization and function in the inner membrane of mitochondria.23,24,45,46
Figure 3.
Bif-1 interacts with PHB2 via C terminus during apoptosis. (A) Identification of PHB2 as a Bif-1–interacting protein by affinity pulldown and mass spectrometry. HEK293 cells were transfected with Bif-1-Myc or empty plasmid, followed by 3 hours of 10 mM azide treatment or control incubation. Whole cell lysate was then collected with CHAPS buffer for immunoprecipitation using anti-Myc antibody-conjugated beads. The resultant immunoprecipitates were resolved by SDS-PAGE for silver staining. Azide treatment consistently induced a protein band at approximately 33 kD in the Bif-1-Myc immunoprecipitate (lane 4: *). The protein band was exercised for mass spectrometry, which identified two peptide sequences of PHB2 (red text). (B and C) Co-IP of overexpressed Bif-1 and PHB2. HEK293 cells were transfected with Bif-1-Myc (B), PHB2-Myc (C), or empty plasmid. The cells were then subjected to azide treatment or control incubation. Whole cell lysate was harvested with CHAPS buffer for IP using anti-Myc–conjugated beads. The immunoprecipitates, along with inputs, were examined by SDS-PAGE and immunoblot analysis. Bif-1/PHB2 co-IP is markedly higher in azide-treated cell lysate than that of control cell lysate (lane 6 versus 5), indicating an enhanced Bif-1/PHB2 interaction during azide treatment. (D and E) Co-IP of endogenous Bif-1 and PHB2 in azide-treated cells. Cells were subjected to azide treatment or control incubation to collect whole cell lysate with CHAPS buffer for IP using anti-Bif-1 or anti-PHB2. The immunoprecipitates, along with inputs, were examined by SDS-PAGE and immunoblot analysis. Bif-1/PHB2 co-IP was induced by azide treatment (lane 4 versus 3), indicating an enhanced Bif-1/PHB2 interaction. (F) Schematic diagram of full length Bif-1 (F) and deletion mutants (A–C). (G) Mapping of Bif-1 domains that mediate Bif-1/PHB2 interaction. HEK293 cells were transfected with Myc-tagged Bif-1 or its deletion mutants, followed by azide treatment or control incubation. Whole cell lysate was harvested with CHAPS buffer for IP using anti-Myc-conjugated beads. The immunoprecipitates, along with inputs, were examined by SDS-PAGE and immunoblot analysis with anti-Myc and anti-PHB2 antibodies. The results show PHB2 co-IP with full-length Bif-1 (F) and N-terminal deletion Bif-1 mutants (A and B), but not with C-terminal deletion mutant (C). Note: The arrows indicate protein bands in lanes 6, 11, and M (molecular weight markers) that are clearly distinguished from PHB2, but their identities are currently unclear.
We then verified Bif-1/PHB2 interaction by co-IP. In untreated, Bif-1-Myc–transfected HEK293 cells, anti-Myc pulled down large amounts of Bif-1-Myc that were associated with barely detectable amounts of PHB2 (Figure 3B: lane 5). After azide treatment, anti-Myc pulled down significantly less Bif-1-Myc, which was nonetheless associated with more PHB2 (Figure 3B: lane 6 versus 5), suggesting a marked increase of Bif-1/PHB2 interaction during azide treatment. Consistently, reverse co-IP demonstrated enhanced Bif-1/PHB2 interaction after azide treatment (Figure 3C: lane 6 versus 5). Azide treatment also led to increased co-IP between endogenous Bif-1 and PHB2 proteins (Figure 3, D and E: lanes 4 versus 3).
To determine the structural domain(s) in Bif-1 that mediates its interaction with PHB2, we generated Bif-1 mutants with a deletion of the N-terminal lipid transferase (LT) sequence, LT and the Bin-Amphiphysin-Rvs (BAR) domain, or the C-terminal linker sequence with the SH3 domain (Figure 3F). Full-length Bif-1 (Figure 3F) and its deletion mutants (Figure 3A–C) were individually expressed in HEK cells for azide treatment and co-IP analysis. Like full-length Bif-1 (Figure 3F), the LT deletion mutant (Figure 3A) and LT-BAR deletion mutant (Figure 3B) co-immunoprecipitated with PHB2 (Figure 3G: lanes 8, 9, and 10); however the C-terminal linker-SH3 deletion mutant (Figure 3C) did not (Figure 3G: lane 11). The results suggest that Bif-1 may interact with PHB2 via its C-terminal domains.
Bif-1 Contributes to Prohibitin Complex Disruption and OPA1 Proteolysis in Apoptosis
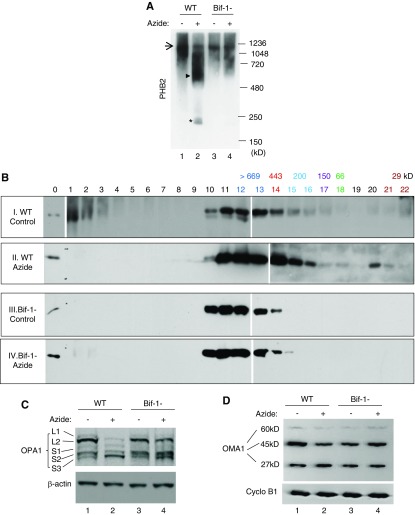
What is the cell biologic function of Bif-1/PHB2 interaction in mitochondrial damage and apoptosis? In mitochondria, PHB2 and its closely related prohibitin family member PHB1 form multimeric ring complexes, which may regulate the inner membrane fusion protein OPA1 by sequestering its processing protease OMA1.22–24,45 We therefore hypothesized that Bif-1 interaction with PHB2 may disturb the prohibitin complexes to release OMA1, causing OPA1 proteolysis and mitochondrial fragmentation. To test this possibility, we compared the changes of prohibitin complexes in WT and Bif-1-null MEFs during azide treatment. We initially analyzed the prohibitin complexes by blue-native gel electrophoresis (BN-PAGE) after chemical crosslinking to stabilize the molecular interactions as previously described (Figure 4A).24 In untreated WT cells, a large prohibitin complex was detected at approximately 1,200,000 D (Figure 4A: lane 1, arrow) and after azide treatment, this large complex was reduced, accompanied by the appearance of a smear between 480 and 720 kD (Figure 4A: lane 2, arrowhead) and a smaller protein complex of approximately 200 kD (Figure 4A: lane 2, asterisk). The smaller complex contained both PHB1 and PHB2 (not shown), suggesting that the complex may be a multimer of prohibitin proteins. In Bif-1-null cells, azide-induced breakdown of the 1.2 mD prohibitin complex was largely attenuated, showing less smear and no 200 kD band (Figure 4A: lanes 3 and 4). Similarly, Bif-1-null cells were resistant to apoptosis and prohibitin complex breakdown induced by CCCP, a mitochondrial uncoupler used in previous studies to investigate mitochondrial dynamics under cell stress (Supplemental Figure 4).
Figure 4.
Disruption of prohibitin complexes and proteolysis of OPA1 during apoptosis are attenuated in Bif-1-null MEF. (A) Blue native gel analysis of prohibitin complexes. WT and Bif-1-null MEFs were treated with azide for 3 hours in glucose-free buffer or left untreated. The cells were then fractionated to collect the mitochondria-enriched fraction for chemical crosslinking with 1 mM DSP. After resuspension, the samples were resolved by BN-PAGE for immunoblot analysis of PHB2. Arrow indicates a large prohibitin complex of approximately 1.2 mD; arrowhead indicates a smear of intermediate prohibitin complexes; * indicates a small prohibitin complex of approximately 200 kD. The results show that azide treatment induces a disruption of the large prohibitin complex into intermediate and small size complexes in WT MEFs, and the complex disruption is suppressed in Bif-1-null cells. (B) Size exclusion chromatography analysis of prohibitin complexes. Samples were prepared as (A) and loaded onto AKTA purifier FPLC for elution with 100 mM phosphate buffer (pH 7.0). Twenty two fractions were collected and concentrated for immunoblot analysis of PHB2. Lane “0”: HEK293 cell lysate. Sigma Gel Filtration Protein Standards were used for calibration. The molecular weight of each protein in the Standards and its elution fraction numbers are written in the same color. (C and D) OPA1 and OMA1 proteolysis. WT and Bif-1-null MEFs were treated with azide for 3 hours or left untreated. Whole cell lysate was collected for immunoblot analysis of OPA1, OMA1, and control proteins.
We further verified the changes of prohibitin complexes by size exclusion chromatography (Figure 4B). In the lysate of control WT cells, PHB2 started to elute from fraction 10, peaked at fractions 11–13, then decreased at fraction 14 with minimal signals in fractions 15 and 16 (Figure 4B: panel I). In the lysate of azide-treated WT cells, PHB2 elution started from fraction 10, peaked at fractions 11–14, and continued to fractions 15 and 16, which had an apparent molecular weight of approximately 200 kD (Figure 4B: panel II). In addition, some PHB2 eluted at fraction 20 apparently as monomers. The results suggest that azide treatment led to a shift of PHB2 from very large (>669 kD) complexes to medium and small complexes and monomers. Notably, the shift of PHB2 complexes during azide treatment was attenuated in Bif-1-null cells (Figure 4B: panels III and IV). Together, the blue-native gel and size exclusion chromatography analyses demonstrate Bif-1–mediated disruption of prohibitin complexes during cell stress and apoptosis.
One mechanism whereby prohibitins regulate mitochondria is through OPA1 and its processing protease OMA1.23 It has been postulated that OMA1 is normally sequestered by prohibitin ring complexes and, under cell stress, the ring complexes are disrupted to release OMA1 to cleave and inactivate OPA1.22 Does Bif-1 function upstream of prohibitins to control OPA1 processing during cell stress? To address this, we compared OPA1 processing in WT and Bif-1-null MEFs during azide treatment. Consistent with previous studies,18,19,21,47 at least five isoforms of OPA1 were detected in the mitochondrial fraction of WT cells under control conditions (Figure 4C: lane 1). After azide treatment, the two long isoforms of OPA1 disappeared, which was accompanied by increases in the short isoforms, especially the shortest OPA1-S3 (Figure 4C: lane 2). Importantly, azide-induced OPA1 proteolysis was abrogated in Bif-1-null cells (Figure 4C: lane 4). We further analyzed the autocatalytic proteolysis or degradation of OMA1, which is associated with OMA1 activation.21 Immunoblots of OMA1 showed three bands of approximately 60, 45, and 27 kD, which may represent the proform, mature form, and degradation intermediate form of OMA1.21 Azide treatment induced a decrease in 45 kD OMA1 and a marginal increase in 27 kD OMA1 in WT cells (Figure 4D: lane 2 versus lane 1), which were inhibited in Bif-1-null cells (Figure 4D: lane 4), suggesting that Bif-1 contributes to OMA1 activation. Collectively, these results support a role of Bif-1 in OMA1-mediated proteolytic processing and inactivation of OPA1 during cell stress and apoptosis.
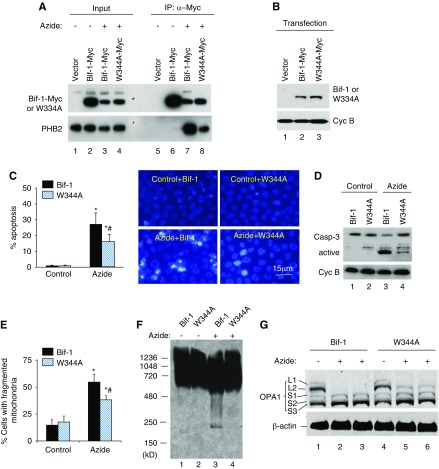
W344A Mutation in Bif-1 Reduces Bif-1/PHB2 Interaction, OPA1 Proteolysis, Mitochondrial Fragmentation, and Apoptosis
To determine whether Bif-1 regulates mitochondria by binding PHB2, we generated a Bif-1 mutant with diminished PHB2-interacting ability. A point mutation was introduced into Bif-1 at tryptophan-344 (W344A), a key residue in the protein binding pocket of SH3 domains according to previous work.48 After expression in HEK293 cells, the W344A mutant showed a much lower interaction with PHB2 than WT Bif-1 during azide treatment (Figure 5A: lane 8 versus lane 7). In quantification, the W344A mutant had 27% PHB2 interaction as compared with wild type Bif-1 (100%). We then reconstituted Bif-1-null cells with WT Bif-1 or W344A mutant (Figure 5B) and compared the reconstituted cells for their responses to azide treatment. As shown in Figure 5C, azide induced 28% apoptosis in Bif-1–reconstituted cells, whereas 16% in W344A mutant-reconstituted cells. Consistently, W344A cells showed lower caspase activation (Figure 5D). Notably, W344A cells also showed lower mitochondrial fragmentation during azide treatment (Figure 5E). Blue native gel analysis further confirmed that in Bif-1–reconstituted cells, azide induced a shift of PHB2 from large complexes to smaller complexes (Figure 5F: lane 3 versus 1), which was suppressed in W344A mutant-reconstituted cells (lane 4 versus lane 3). Moreover, azide induced OPA1 proteolysis in WT Bif-1–reconstituted cells as indicated by a complete loss of the long OPA1 isoforms (Figure 5G: lane 2 and 3). However, the same azide treatment only induced a partial OPA1 proteolysis in W344A mutant-reconstituted cells and, as a result, significant amounts of OPA1-L2 were preserved (Figure 5G: lanes 5 and 6). Collectively, these results suggest that the molecular interaction between Bif-1 and PHB2 is pivotal to the role of Bif-1 in prohibitin complex disruption, OPA1 proteolysis, and mitochondrial fragmentation during apoptosis. Of note, W334A mutation did not completely eliminate Bif-1/PHB2 interaction (Figure 5A). This might contribute to the remaining mitochondrial fragmentation and apoptosis observed in W344A mutant-reconstituted cells. In addition, other factors besides Bif-1 may work with PHB2 in mitochondrial fragmentation and apoptosis.
Figure 5.
W344A mutation in Bif-1 reduces its ability of PHB2 interaction, OPA1 proteolysis, mitochondrial fragmentation, and apoptosis. (A) PHB2 interaction. HEK293 cells were transfected with Bif-1-Myc, Myc-W344A-Bif-1, or empty vector. The cells were then left untreated or treated with azide for 3 hours to collect the membrane-bound mitochondrial fraction, which was further subjected to chemical cross-linking followed by immunoprecipitation with anti-Myc. The immunoprecipitates, along with inputs, were resolved by SDS-PAGE for immunoblot analysis with anti-Myc and anti-PHB2 antibodies. The results show a much weaker co-IP of W344A-Bif-1 with PHB2 than WT Bif-1 (lane 8 versus 7), suggesting that W344A mutation diminishes Bif-1/PHB2 interaction. (B) Reconstitution of Bif-1 or W344A mutant into Bif-1-null MEFs. Bif-1-null MEFs were reconstituted with Bif-1 or W344A mutant using the lentivector system. Whole cell lysate was then collected for immunoblot analysis to confirm the expression of Bif-1 and W344A-Bif-1. (C) Apoptosis in Bif-1 or W344A-Bif-1 reconstituted MEFs. Bif-1 or W344A-Bif-1 reconstituted MEFs were left untreated or treated with azide for 3 hours followed by 2 hours of recovery in culture medium. The cells were then stained with Hoechst33342 for morphologic examination. The cells with apoptotic morphology were counted to determine the percentage of apoptosis. Representative images of nuclear staining are also presented. (D) Caspase 3 activation in Bif-1 or W344A-Bif-1 reconstituted MEFs. Cells were treated as (C) to collect cell lysate for immunoblot analysis of caspase 3 and cyclophilin B (loading control). Caspase 3 activation was indicated by the appearance of active/cleaved fragments. (E) Mitochondrial fragmentation in Bif-1 or W344A-Bif-1 reconstituted MEFs. Cells were transfected with MitoRed to fluorescently label mitochondria and then treated with azide or left untreated. The morphology of mitochondria in individual cells was examined by fluorescence microscopy to determine the percentage of cells with fragmented mitochondria. (F) Blue native gel analysis of prohibitin complexes in Bif-1 or W344A-Bif-1 reconstituted MEFs. Cells were treated with azide for 3 hours or left untreated. The cells were then fractionated to collect the mitochondria-enriched fraction for chemical crosslinking with 1 mM DSP, followed by BN-PAGE for immunoblot analysis of PHB2. (G) OPA1 proteolysis in Bif-1 or W344A-Bif-1 reconstituted MEFs. Cells were treated with azide for 3 hours or left untreated. Whole cell lysate was collected for immunoblot analysis of OPA1 and β-actin. The data in (C and D) represent mean±SD (n=3); *P<0.05 versus control; #P<0.05 versus Bif-1/azide.
Bif-1 Binds Prohibitin-2 and Contributes to OPA1 Proteolysis and Mitochondrial Fragmentation in vivo during Renal Ischemia/Reperfusion Injury
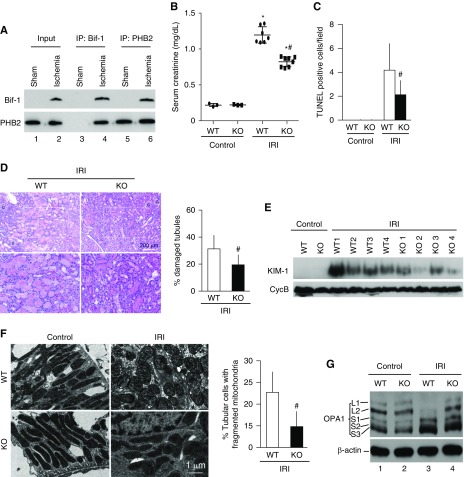
Finally, we verified Bif-1 regulation in vivo during renal ischemia/reperfusion injury (IRI), a disease condition characterized by mitochondrial fragmentation and damage followed by tubular cell death and loss of kidney function.8,49–54 We first examined Bif-1/PHB2 interaction during renal IRI in isolated mitochondria by co-IP analysis. Consistent with our cell culture results (Figure 2C), renal IRI induced a significant increase of Bif-1 in mitochondrial fraction (Figure 6A: lane 2 versus 1). co-IP of Bif-1 and PHB2 was undetectable in sham operated control tissues (lanes 3 and 5), but it became evident after renal IRI (lanes 4 and 6), indicating Bif-1/PHB2 interaction. The specificity of the antibodies used for co-IP in this studies was verified by comparing with nonimmune IgG. As shown in Supplemental Figure 5, specific protein bands were pulled down by IP antibodies but not by nonimmune IgG.
Figure 6.
Bif-1 binds prohibitin-2 and contributes to OPA1 proteolysis and mitochondrial fragmentation in mouse renal IRI. (A) Bif-1 and PHB2 interacts in mitochondrial fraction during renal IRI. Mice were subjected to 30 minutes of bilateral renal ischemia with brief (approximately 15 minutes) reperfusion. Kidneys were collected to isolate mitochondrial fraction for co-IP of Bif-1 and PHB2. (B–E) Partial resistance of Bif-1 knockout (KO) mice to renal IRI. Bif-1 KO and wild-type (WT) littermate mice were subjected to 30 minutes of bilateral renal ischemia with 48 hours of reperfusion to collect blood serum for creatinine measurement to indicate renal functional loss (B), and to collect kidney tissues for hematoxylin and eosin staining analysis of tubular damage (C), TUNEL assay of apoptosis (D) and immunoblot analysis of KIM1. (F) Lower mitochondrial fragmentation in Bif-1 KO mice during renal IRI. Bif-1KO and WT littermate mice were subjected to 30 minutes of bilateral renal ischemia with brief (approximately 15 minutes) reperfusion. Kidney tissues were fixed for electron microscopy to record representative mitochondrial morphology and determine percentage of the cells with mostly fragmented mitochondria. (G) Less OPA1 proteolysis in Bif-1 KO mice during renal IRI. Bif-1 KO and WT littermate mice were subjected to 30 minutes of bilateral renal ischemia with brief (approximately 15 minutes) reperfusion to collect kidney cortical tissues for immunoblot analysis of OPA1 and β-actin. The quantitative data in (B–E) represent mean±SD (n=3); *P<0.05 versus control; #P<0.05 versus WT/IRI.
To further determine the role of Bif-1 in vivo, we examined renal IRI in Bif-1 KO and WT mice. Bif-1 KO and WT mice had low serum creatinine under control conditions and, upon renal IRI, serum creatinine increased in both genotypes of mice. However, the increase in KO mice (0.8 mg/dl) was significantly lower than in WT mice (1.2 mg/dl), indicating less kidney injury in KO mice (Figure 6B). Consistently, histologic analysis showed less kidney tubule damage in KO mice after renal IRI (Figure 6, C and D). TUNEL assay and immunostaining of active caspase 3 showed less apoptosis in KO mice (Figure 6D, Supplemental Figure 6). Immunoblotting of KIM-1, an AKI biomarker, also further verified less IRI in Bif-1 KO mice than WT (Figure 6E). We further evaluated mitochondrial fragmentation in kidney tubular cells by electron microscopy, as previously shown.8,49 As shown in Figure 6F, renal IRI induced mitochondrial fragmentation in 23% tubular cells in WT mice compared with 14% in KO mice. Renal IRI also induced OPA1 proteolysis in WT kidney tissues, resulting in the loss of OPA1 long forms and the accumulation of short forms (Figure 6G: lane 1 versus 3). Notably, OPA1 proteolysis was largely attenuated in Bif-1 KO mice. Together, these results indicate that Bif-1 may interact with PHB-2 to regulate OPA1 and mitochondrial dynamics during cell stress in vivo in diseases.
Discussion
During cell stress and apoptosis, mitochondria often become fragmented. Suppression of mitochondrial fragmentation, either by inhibiting fission or by promoting fusion, protects against apoptosis in cultured cells and in tissues under disease conditions, supporting a role of mitochondrial fragmentation in cell injury and death.1–3,6–11,16,55,56 Structurally, mitochondrial fragmentation requires the cleavage of both mitochondrial outer and inner membranes (MOM and MIM). MOM cleavage involves the activation of Drp1 and consequent fission; moreover, fusion may be arrested because of the inactivation of mitofusins via protein interaction.17,57 However, much less is known about MIM cleavage. Our study suggests that, by binding PHB2, Bif-1 plays a critical role in the proteolysis and inactivation of OPA1, contributing to MIM cleavage in cell stress and apoptosis.
OPA1 is a key mediator of MIM fusion in mammalian cells.15 As a result of alternative splicing and proteolytic processing, OPA1 exists in long and short isoforms, and interestingly, both isoforms are required for MIM fusion.47 During apoptosis, long OPA1 isoforms are proteolytically cleaved and lost, resulting in the blockade of MIM fusion.47,58 A key protease responsible for OPA1 proteolysis during cell stress and apoptosis is OMA1, a zinc metalloprotease on MIM.18–21 However, it is unclear how OMA1 is activated for OPA1 proteolysis under these conditions. In this regard, another key regulator of OPA1 is PHB2, loss of which leads to OPA1 proteolysis, mitochondrial defects, and apoptosis or increased cellular sensitivity to apoptosis.23,59 PHB2 and its homolog partner PHB1 form multimeric ring structures that are crucial to their functions in MIM, including molecular scaffolding and protein stabilization.24,45 On the basis of these findings, it was postulated that OMA1 is normally sequestered by prohibitin ring complexes in MIM and upon mitochondrial stress, the prohibitin complexes are disrupted to release OMA1 for OPA1 proteolysis.22 This scenario, although highly intriguing, has not been tested or verified experimentally. In this study, we have provided the evidence of prohibitin complex disruption during cell stress and apoptosis, and importantly, we have identified Bif-1 as a binding partner of PHB2 and the trigger of the PHB2/OMA1/OPA1 cascade for inner membrane cleavage. With these results, we propose that upon cell stress, Bif-1 translocates to mitochondria and interacts with PHB2, resulting in the disruption of the prohibitin ring complexes and the release of OMA1. OMA1 then gains access to OPA1 for proteolysis, leading to the loss of long isoforms of OPA1 and cessation of MIM fusion, which contributes to mitochondrial fragmentation (Supplemental Figure 7).
Bif-1 was originally identified as a Bax-interacting protein contributing to Bax activation in apoptosis.25–27 Thus, the effects of Bif-1 on mitochondria and apoptosis are often attributed to its interaction with Bax. However, we failed to detect the interaction between endogenous Bif-1 and Bax under control and stress conditions (not shown). Instead, we have unveiled Bif-1/PHB2 interaction and its role in mitochondrial fragmentation and apoptosis during cell stress. Especially, we showed that W344A mutation in Bif-1 diminished Bif-1/PHB2 binding, and concomitantly, reduced mitochondrial fragmentation and apoptosis (Figure 5, C and D), supporting a critical role of Bif-1/PHB2 interaction in mitochondrial regulation. It is important to note that the W344A mutant contains a point mutation in the C-terminal SH3 domain of Bif-1, which is unlikely to affect Bif-1/Bax interaction because the latter depends on the N-terminal sequence of Bif-1 25–27. Indeed, W344A did not affect Bif-1/Bax interaction and Bax activation during azide treatment in our study (data not shown). These observations indicate that the W344A mutant is competent in binding and activating Bax, and the effects of W344A mutation on mitochondrial morphology and apoptosis are not caused by the lack of Bax interaction or activation. Consistently, previous work demonstrated that mitochondrial fragmentation during cell stress is independent of Bax.17 Together, these observations indicate that Bif-1 regulates mitochondrial morphology or dynamics independent of Bax.
How does Bif-1 participate in mitochondrial fragmentation? We have unveiled the molecular interaction between Bif-1 and PHB2, which is markedly enhanced during both in vitro and in vivo conditions of cell stress (Figures 3 and 6). Considering the subcellular localization of PHB2 and its regulation of OPA1, we hypothesized that Bif-1 might regulate MIM via PHB2/OPA1. Indeed, our subsequent experiments demonstrated the disruption of prohibitin complexes and proteolysis of OPA1 in apoptosis and, notably, Bif-1 is essential to the initiation of these molecular events at MIM (Figures 4–6). By analyzing the interaction of Bif-1 deletion mutants with PHB2, we show that Bif-1 interacts with PHB2 via its C terminus, which consists of a linker sequence and an SH3 domain. We have further identified Bif-1(W344A) with a point mutation in the SH3 domain that significantly reduced Bif-1/PHB2 interaction, supporting a role of the SH3 in mediating this molecular interaction.
co-IP of Bif-1-Myc and PHB2 was detected in mitochondrial fraction (Figures 5A and 6A), supporting the possibility of Bif-1/PHB2 interaction within mitochondria. Our further fractionation studies indicate that upon stress, Bif-1 may integrate into the outer membrane of mitochondria, with its C terminus exposed to the intermembrane space (Figure 2, D–G). If located on the outer membrane, how can Bif-1 interact with PHB2 that is on the inner membrane? To understand this, it is important to recognize the presence of “mitochondrial contact sites,” where the mitochondrial outer and inner membranes are jointed or bridged within molecular distance via protein complexes. At the contact sites, the outer and inner membranes are within molecular distance, providing a condition for interaction between the proteins located at these two membranes.60 Thus, we speculate that Bif-1 may interact with PHB2 at the contact sites between mitochondrial outer and inner membranes.
Finally, the release of Cyt c from mitochondria in apoptosis was thought to be a pure event of MOM. However, recent research has demonstrated the involvement of MIM and associate cristae structure. There are at least two ways for MIM control of Cyt c release. First, the majority of Cyt c is not freely floating in the intermembrane space of mitochondria; instead it is sequestered in the cristae structure formed by MIM. OPA1 (MIM fusion protein) is a gatekeeper of the cristae structure; in other words, when OPA1 is proteolyzed into short forms, Cyt c will be released from the cristae into mitochondrial intermembrane space for release into cytosol during apoptosis.61,62 Second, mitochondrial fragmentation, which requires the cleavage of both MOM and MIM, sensitizes mitochondria to Bax insertion and oligomerization for MOM permeabilization and Cyt c release.35 In this study, Bif-1 deficiency led to the suppression of mitochondrial fragmentation, making mitochondria resistant to Bax insertion and MOM permeabilization.
In conclusion, our study has identified Bif-1 as an important regulator of MIM during cell stress. Bif-1 translocates into mitochondria to interact with PHB2, leading to the disruption of PHB complexes and the release of OMA1. OMA1 then cuts and inactivates OPA1 to block the fusion of MIM, contributing to MIM cleavage, mitochondrial fragmentation, Cyt c release, and apoptosis.
Disclosures
None.
Supplementary Material
Acknowledgments
Dr. Cho, Dr. Xiao, Dr. S. Wang, and Dr. Dong designed experiments. Dr. Cho, Dr. Xiao, Dr. S. Wang, Dr. Gao, Dr. Rafikov, and Dr. Kirken performed experiments. Dr. Cho, Dr. Xiao, Dr. S. Wang, Dr. Black, Dr. Huang, Dr. Ding, Dr. Yoon, Dr. H.-G. Wang, and Dr. Dong analyzed results. Dr. Cho, Dr. Xiao, Dr. S. Wang, and Dr. Dong wrote the manuscript.
The study was supported in part by grants Department of Veterans Affairs (BX000319) and the National Institutes of Health (DK58831, DK87843, CA82197). Dr. Dong is a recipient of Senior Research Career Scientist award of Department of Veterans Affairs.
We thank Dr. Richard Morrison at University of Washington (Seattle, WA) for transferring Bif-1 KO mice. We also thank Dr. Andrey Shaw at Washington University (St. Louis, MO) and Dr. Yashpal Kanwar at Northwestern University (Chicago, IL) for advice on immunogold electron microscopy.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111117/-/DCSupplemental.
Supplemental Figure 1. Bif-1 knockdown suppresses azide-induced apoptosis in RPTC cells.
Supplemental Figure 2. Bif-1 knockdown suppresses Cyt c release and Bax activation in mitochondria during azide treatment of RPTC cells.
Supplemental Figure 3. Effects of Bif-1 deficiency on mitochondrial dynamics proteins.
Supplemental Figure 4. Bif-1-null cells are resistant to CCCP-induced apoptosis and prohibitin complex disruption.
Supplemental Figure 5. Verification of the specificity of the antibodies used for immunoprecipitation.
Supplemental Figure 6. Bif-1 knockout mice have less TUNEL and cleaved caspase 3 staining in kidney tissues during IRI.
Supplemental Figure 7. Schematic diagram of the Bif-1/PHB2/OMA1/OPA1 pathway in MIM cleavage.
References
- 1.Chan DC: Fusion and fission: Interlinked Processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Nunnari J, Suomalainen A: Mitochondria: In sickness and in health. Cell 148: 1145–1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youle RJ, van der Bliek AM: Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhargava P, Schnellmann RG: Mitochondrial energetics in the kidney. Nat Rev Nephrol 13: 629–646, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhan M, Brooks C, Liu F, Sun L, Dong Z: Mitochondrial dynamics: Regulatory mechanisms and emerging role in renal Pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayanga BA, Badal SS, Wang Y, Galvan DL, Chang BH, Schumacker PT, et al.: Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol 27: 2733–2747, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, et al.: Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in Podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks C, Wei Q, Cho SG, Dong Z: Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119: 1275–1285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ: Inhibiting mitochondrial fission Protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X: Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J Clin Invest 123: 5371–5388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhan M, Usman IM, Sun L, Kanwar YS: Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry HM, Huang L, Wilson RJ, Bajwa A, Sesaki H, Yan Z, et al.: Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol 29: 194–206, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Q, Sun H, Song S, Liu Y, Liu P, Livingston MJ, et al.: MicroRNA-668 represses MTP18 to Preserve mitochondrial dynamics in ischemic acute kidney injury. J Clin Invest 128: 5448–5464, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gall JM, Wang Z, Bonegio RG, Havasi A, Liesa M, Vemula P, et al.: Conditional knockout of Proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J Am Soc Nephrol 26: 1092–1102, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacVicar T, Langer T: OPA1 Processing in cell death and disease - the long and short of it. J Cell Sci 129: 2297–2306, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Suen DF, Norris KL, Youle RJ: Mitochondrial dynamics and apoptosis. Genes Dev 22: 1577–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, et al.: Bak regulates mitochondrial morphology and Pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A 104: 11649–11654, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, et al.: Regulation of OPA1 Processing and mitochondrial fusion by m-AAA Protease isoenzymes and OMA1. J Cell Biol 187: 1023–1036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM: Inducible Proteolytic inactivation of OPA1 mediated by the OMA1 Protease in mammalian cells. J Cell Biol 187: 959–966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quirós PM, Ramsay AJ, Sala D, Fernández-Vizarra E, Rodríguez F, Peinado JR, et al.: Loss of mitochondrial Protease OMA1 alters Processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31: 2117–2133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker MJ, Lampe PA, Stojanovski D, Korwitz A, Anand R, Tatsuta T, et al.: Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. EMBO J 33: 578–593, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride H, Soubannier V: Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr Biol 20: R274–R276, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Löwer B, Wunderlich FT, et al.: Prohibitins control cell Proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev 22: 476–488, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatsuta T, Model K, Langer T: Formation of membrane-bound ring complexes by Prohibitins in mitochondria. Mol Biol Cell 16: 248–259, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuddeback SM, Yamaguchi H, Komatsu K, Miyashita T, Yamada M, Wu C, et al.: Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing Protein that associates with Bax. J Biol Chem 276: 20559–20565, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Pierrat B, Simonen M, Cueto M, Mestan J, Ferrigno P, Heim J: SH3GLB, a new endophilin-related Protein family featuring an SH3 domain. Genomics 71: 222–234, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, et al.: Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol 25: 9369–9382, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al.: Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9: 1142–1151, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi Y, Hori T, Cooper TK, Liao J, Desai N, Serfass JM, et al.: Bif-1 haploinsufficiency Promotes chromosomal instability and accelerates Myc-driven lymphomagenesis via suppression of mitophagy. Blood 121: 1622–1632, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karbowski M, Jeong SY, Youle RJ: Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol 166: 1027–1039, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, et al.: Immortalization and characterization of Proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int 50: 125–134, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Ross JA, Nagy ZS, Kirken RA: The PHB1/2 Phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J Biol Chem 283: 4699–4713, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Pabla N, Dong G, Jiang M, Huang S, Kumar MV, Messing RO, et al.: Inhibition of PKCδ reduces cisplatin-induced nephrotoxicity without blocking chemotherapeutic efficacy in mouse models of cancer. J Clin Invest 121: 2709–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al.: Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic Protease cascade. Cell 91: 479–489, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Brooks C, Cho SG, Wang CY, Yang T, Dong Z: Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol 300: C447–C455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z: hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem 286: 10411–10418, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green DR, Kroemer G: The Pathophysiology of mitochondrial cell death. Science 305: 626–629, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al.: Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youle RJ, Strasser A: The BCL-2 Protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Adams JM, Cory S: Bcl-2-regulated apoptosis: Mechanism and therapeutic Potential. Curr Opin Immunol 19: 488–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang CR, Blackstone C: Cyclic AMP-dependent Protein kinase Phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Cribbs JT, Strack S: Reversible Phosphorylation of Drp1 by cyclic AMP-dependent Protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, et al.: Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105: 15803–15808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rostovtseva TK, Boukari H, Antignani A, Shiu B, Banerjee S, Neutzner A, et al.: Bax activates endophilin B1 oligomerization and lipid membrane vesiculation. J Biol Chem 284: 34390–34399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osman C, Merkwirth C, Langer T: Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci 122: 3823–3830, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Richter-Dennerlein R, Korwitz A, Haag M, Tatsuta T, Dargazanli S, Baker M, et al.: DNAJC19, a mitochondrial cochaperone associated with cardiomyopathy, forms a complex with Prohibitins to regulate cardiolipin remodeling. Cell Metab 20: 158–171, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Song Z, Chen H, Fiket M, Alexander C, Chan DC: OPA1 Processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane Potential, and Yme1L. J Cell Biol 178: 749–755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu H, Miller WT: Role of the Brk SH3 domain in substrate recognition. Oncogene 23: 2216–2223, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z: Bax and Bak have critical roles in ischemic acute kidney injury in global and Proximal tubule-specific knockout mouse models. Kidney Int 84: 138–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonventre JV, Yang L: Cellular Pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al.: PGC1α drives NAD biosynthesis linking oxidative metabolism to renal Protection. Nature 531: 528–532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al.: PGC-1α Promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, et al.: Mitochondrial bioenergetics and structural network organization. J Cell Sci 120: 838–848, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Chen H, Chomyn A, Chan DC: Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem 280: 26185–26192, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al.: The role of dynamin-related Protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell 1: 515–525, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Griparic L, Kanazawa T, van der Bliek AM: Regulation of the mitochondrial dynamin-like Protein Opa1 by Proteolytic cleavage. J Cell Biol 178: 757–764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kasashima K, Ohta E, Kagawa Y, Endo H: Mitochondrial functions and estrogen receptor-dependent nuclear translocation of Pleiotropic human Prohibitin 2. J Biol Chem 281: 36401–36410, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wollweber F, von der Malsburg K, van der Laan M: Mitochondrial contact site and cristae organizing system: A central Player in membrane shaping and crosstalk. Biochim Biophys Acta Mol Cell Res 1864: 1481–1489, 2017 [DOI] [PubMed] [Google Scholar]
- 61.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, et al.: Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126: 163–175, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Gottlieb E: OPA1 and PARL keep a lid on apoptosis. Cell 126: 27–29, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.